Achieving Optimum Compressive Strength for Geopolymers Manufactured at Both Low and High Si:Al Values
Abstract
1. Introduction
2. Experimental Details
3. Results and Discussion
3.1. Precursors
3.1.1. Particle Size Distribution
3.1.2. X-Ray Diffraction
3.2. Geopolymers
3.2.1. X-Ray Diffraction
3.2.2. Compressive Strength
4. Discussion
5. Conclusions
- Four fly ash-based geopolymers and two metakaolin-based geopolymers with a wide range of Si:Al ratios have revealed a novel feature of dual compressive strength peaks.
- In all cases investigated, the strength peaks of the geopolymers were present for Si:Al values greater and less than the Si:Al of the precursor aluminosilicate.
- This new information considerably expands the dynamic Si:Al range of geopolymers.
- In essence, a greater choice of product end use can now be considered: low Si:Al-based AAM for fire resistance or higher Si:Al-based geopolymer for structural applications.
- What is particularly important is that precursors that might previously have been classified as unusable due to an unacceptably high Si:Al can be prepared as AAMs with target Si:Al of 3 or as low as 1.3.
- Another key revelation is that between the two strength peaks, there is a valley where strength may be unacceptably low for targeted applications.
- As this valley may be very narrow, a small miscalculation in targeted Si:Al may result in a very low strength geopolymer. Thus, sample preparation must be undertaken precisely to ensure that the optimum strength is attained.
6. Future Studies
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- van Riessen, A.; Jamieson, E.; Gildenhuys, H.; Skane, R.; Allery, J. Using XRD to Assess the Strength of Fly-Ash- and Metakaolin-Based Geopolymer. Materials 2025, 18, 2093. [Google Scholar] [CrossRef]
- Kim, B.; Lee, S. Review on characteristics of metakaolin-based geopolymer and fast setting. J. Korean Ceram. Soc. 2020, 57, 368–377. [Google Scholar] [CrossRef]
- Scherb, S.; Köberl, M.; Beuntner, N.; Thienel, K.-C.; Neubauer, J. Reactivity of Metakaolin in Alkaline Environment: Correlation of Results from Dissolution Experiments with XRD Quantifications. Materials 2020, 13, 2214. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Liu, Z.; Tian, X.; Wu, J.; Wang, L. Review on the impact of metakaolin-based geopolymer’s reaction chemistry, nanostructure and factors on its properties. Constr. Build. Mater. 2024, 412, 134760. [Google Scholar] [CrossRef]
- Longhi, M.A.; Rodriguez, E.D.; Walkley, B.; Zhang, Z.; Kirchheim, A.P. Metakaolin-based geopolymers: Relation between formulation, physicochemical properties and efflorescence formation. Compos. Part B Eng. 2020, 182, 107671. [Google Scholar] [CrossRef]
- N’Cho, W.C.; Gharzouni, A.; Jouin, J.; Rossignol, S. Impact of different metakaolin mixtures on oligomer formation and geopolymer properties: Impurity effect. Open Ceram. 2023, 15, 100411. [Google Scholar] [CrossRef]
- Kuenzel, C.; Ranjbar, N. Dissolution mechanism of fly ash to quantify the reactive aluminosilicates in geopolymerisation. Resour. Conserv. Recycl. 2019, 150, 104421. [Google Scholar] [CrossRef]
- Singh, N.B. Fly Ash-Based Geopolymer Binder: A Future Construction Material. Minerals 2018, 8, 299. [Google Scholar] [CrossRef]
- Abdulkareem, M.; Komkova, A.; Havukainen, J.; Habert, G.; Horttanainen, M. Identifying Optimal Precursors for Geopolymer Composite Mix Design for Different Regional Settings: A Multi-Objective Optimization Study. Recycling 2023, 8, 32. [Google Scholar] [CrossRef]
- Muthukrishnan, S.; Ramakrishnan, S.; Sanjayan, J. Rapid early age strength development of in-line activated geopolymer for concrete 3D printing. Constr. Build. Mater. 2023, 406, 133312. [Google Scholar] [CrossRef]
- Segura, P.I.; Luukkonen, T.; Yliniemi, J.; Sreenivasan, H.; Damø, A.J.; Jensen, L.S.; Canut, M.; Kantola, A.M.; Telkki, V.-V.; Jensen, P.A. Comparison of one-part and two-part alkali-activated metakaolin and blast furnace slag. J. Sustain. Metall. 2022, 8, 1816–1830. [Google Scholar] [CrossRef]
- Lau, C.; Rowles, M.; Parnham, G.; Htut, T.; Ng, T.S. Investigation of geopolymers containing fly ash and ground-granulated blast-furnace slag blended by amorphous ratios. Constr. Build. Mater. 2019, 222, 731–737. [Google Scholar] [CrossRef]
- Barbosa, V.F.F.; MacKenzie, K.J.D.; Thaumaturgo, C. Synthesis and characterisation of materials based on inorganic polymers of alumina and silica: Sodium polysialate polymers. Int. J. Inorg. Mater. 2000, 2, 309–317. [Google Scholar] [CrossRef]
- Walkley, B.; Rees, G.J.; San Nicolas, R.; van Deventer, J.S.J.; Hanna JVProvis, J.L. New structural model of hydrous sodium aluminosilicate gels and the role of charge-balancing extra-framework Al. J. Phys. Chem. C. 2018, 122, 5673–5685. [Google Scholar] [CrossRef]
- McLellan, B.C.; Williams, R.P.; Lay, J.; van Riessen, A.; Corder, G.D. Costs and carbon emissions for geopolymer pastes in comparison to ordinary Portland cement. Aust. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef]
- Rickard, W.D.A.; Williams, R.; Temuujin, J.; van Riessen, A. Assessing the suitability of three Australian fly ashes as an aluminosilicate source for geopolymers in high temperature applications. Mater. Sci. Eng. A 2011, 528, 3390–3397. [Google Scholar] [CrossRef]
- Temuujin, J.; Lee, M.; Chen-Tan, N.; van Riessen, A. Characterisation of class F fly ash geopolymer pastes immersed in acid and alkaline solutions. Cem. Concr. Compos. 2011, 33, 1086–1091. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Kriven, W.M.; Leonelli, C.; Provis, J.L.; Boccaccini, A.R.; Attwell, C.; Ducman, V.S.; Ferone, C.; Rossignol, S.; Luukkonen, T.; van Deventer, J.S.J.; et al. Why geopolymers and alkali-activated materials are key components of a sustainable world: A perspective contribution. J. Am. Ceram. Soc. 2024, 107, 5159–5177. [Google Scholar] [CrossRef]
- Jamieson, E.; McLellan, B.; van Riessen, A.; Nikraz, H. Comparison of embodied energies of Ordinary Portland Cement with Bayer-derived Geopolymer products. J. Clean. Prod. 2015, 99, 112–118. [Google Scholar] [CrossRef]
- Wudil, Y.S.; Al-Fakih, A.; Al-Osta, M.A.; Gondal, M.A. Effective carbon footprint assessment strategy in fly ash geopolymer concrete based on adaptive boosting learning techniques. Environ. Res. 2025, 266, 120570. [Google Scholar] [CrossRef] [PubMed]
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Luhar, S.; Nicolaides, D.; Luhar, I. Fire Resistance Behaviour of Geopolymer Concrete: An Overview. Buildings 2021, 11, 82. [Google Scholar] [CrossRef]
- Amran, M.; Huang, S.-S.; Debbarma, S.; Rashid, R.S.M. Fire resistance of geopolymer concrete: A critical review. Constr. Build. Mater. 2022, 324, 126722. [Google Scholar] [CrossRef]
- Yang, W.; Zhu, P.; Liu, H.; Wang, X.; Ge, W.; Hua, M. Resistance to Sulfuric Acid Corrosion of Geopolymer Concrete Based on Different Binding Materials and Alkali Concentrations. Materials 2021, 14, 7109. [Google Scholar] [CrossRef]
- Alzeer, M.I.M.; MacKenzie, K.J.D.; Keyzers, R.A. Porous aluminosilicate inorganic polymers (geopolymers): A 26 new class of environmentally benign heterogeneous solid acid catalysts. Appl. Catal. A Gen. 2016, 524, 173–181. [Google Scholar] [CrossRef]
- Luukkonen, T.; Heponiemi, A.; Runtti, H.; Pesonen, J.; Yliniemi, J.; Lassi, U. Application of alkali-activated materials for water and wastewater treatment: A review. Rev. Env. Sci. Biotechnol. 2019, 18, 271–297. [Google Scholar] [CrossRef]
- Yu, Y.; Perumal, P.; Corfe, I.J.; Paul, T.; Illikainen, M.; Luukkonen, T. Combined granulation–alkali activation–direct foaming process: A novel route to porous geopolymer granules with enhanced adsorption properties. Mater. Des. 2023, 227, 111781. [Google Scholar] [CrossRef]
- Toniolo, N.; Bednarzig, V.; Roether, J.A.; Rost, H.; Boccaccini, A.R. Advancing processing technologies for designed geopolymers: 3D printing and mechanical machining. Interceram 2019, 68, 18–21. [Google Scholar] [CrossRef]
- Ricciotti, L.; Apicella, A.; Perrotta, V.; Aversa, R. Geopolymer materials for bone tissue applications: Recent advances and future perspectives. Polymers 2023, 15, 1087. [Google Scholar] [CrossRef]
- Sazama, P.; Bortnovsky, O.; Dědeček, J.; Tvarůžková, Z.; Sobalík, Z. Geopolymer based catalysts—New group of catalytic materials. Catal. Today 2011, 164, 92–99. [Google Scholar] [CrossRef]
- Sutter, L.L.; Hooton, H.D. Progress towards sustainability through performance-based standards and specifications. Cem. Concr. Res. 2023, 174, 107303. [Google Scholar] [CrossRef]
- Beers, D.; Corder, G.; Bossilkov, A.; van Berkel, R. Industrial symbiosis in the Australian minerals industry. J. Ind. Ecol. 2007, 11, 55–72. [Google Scholar] [CrossRef]
- van Riessen, A.; Rickard, W.D.A.; Williams, R.P.; van Riessen, G.A. Methods for Geopolymer Formulation Development and Microstructural Analysis. J. Ceram. Sci. Technol. 2017, 08, 421–431. [Google Scholar]
- Williams, R.; van Riessen, A. Determination of the reactive component of fly ashes for geopolymer production using XRF and XRD. Fuel 2010, 89, 3683–3692. [Google Scholar] [CrossRef]
- Rowles, M.; O’Connor, B. Chemical optimisation of the compressive strength of aluminosilicate geopolymers synthesised by sodium silicate activation of Metakaolinite. J. Mater. Chem. 2003, 13, 1161–1165. [Google Scholar] [CrossRef]
- Williams, R.; Hart, R.; van Riessen, A. Quantification of the extent of reaction of metakaolin based geopolymers using XRD, SEM and EDS. J. Am. Ceram. Soc. 2011, 94, 2663–2670. [Google Scholar] [CrossRef]
- Longhi, M.A.; Walkley, B.; Rodriguez, E.D.; Kirchheim, A.P.; Zhang, Z.; Wang, H. New selective dissolution process to quantify reaction extent and product stability in metakaolin-based geopolymers. Compos. Part B 2019, 176, 107172. [Google Scholar] [CrossRef]
- Vitola, L.; Pundiene, I.; Pranckeviciene, J.; Bajare, D. The Impact of the Amount of Water Used in Activation Solution and the Initial Temperature of Paste on the Rheological Behaviour and Structural Evolution of Metakaolin-Based Geopolymer Pastes. Sustainability 2020, 12, 8216. [Google Scholar] [CrossRef]
- Abbass, A.M.; Firdous, R.; Djobo, J.N.Y.; Stephan, D.; Elrahman, M.A. The role of chemistry and fineness of metakaolin on the fresh properties and heat resistance of blended fly ash-based geopolymer. SN Appl. Sci. 2023, 5, 136. [Google Scholar] [CrossRef]
- Subaer; van Riessen, A. Thermo-mechanical and microstructural characterisation of sodium-poly (sialate-siloxo) (Na-PSS) geopolymers. J. Mater. Sci. 2007, 42, 3117–3123. [Google Scholar] [CrossRef]
- Lee, S.; Moon, H.-S. Phase Transformation Sequence from Kaolinite to Mullite Investigated by an Energy-Filtering TEM. J. Am. Ceram. Soc. 1999, 82, 2841–2848. [Google Scholar] [CrossRef]
- Skane, R.; Schneider, P.A.; Jones, F.; van Riessen, A.; Jamieson, E.; Sun, X.; Rickard, W.D.A. Predicting the stability of geopolymer activator solutions for optimised synthesis through thermodynamic modelling. Chem. Eng. J. 2025, 515, 163543. [Google Scholar] [CrossRef]
- ASTM C39/C39M—21; Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens. ASTM International: West Conshohocken, PA, USA, 2021. [CrossRef]
- Krol, M.; Minkiewicz, J.; Mozgawa, W. IR spectroscopy studies of zeolites in geopolymeric materials derived from kaolinite. J. Mol. Struct. 2016, 1126, 200–206. [Google Scholar] [CrossRef]
- Wan, Q.; Rao, F.; Song, S.; García, R.E.; Estrella, R.M.; Patino, C.L.; Zhang, Y. Geopolymerization reaction, microstructure and simulation of metakaolin-based geopolymers at extended Si/Al ratios. Cem. Concrette Compos. 2017, 79, 45–52. [Google Scholar] [CrossRef]
- Lasaga, A.C. Chemical kinetics of water-rock interactions. J. Geophys. Res. Solid Earth 1984, 89, 4009–4025. [Google Scholar] [CrossRef]
- Brady, P.V.; Walther, J.V. Controls on silicate dissolution rates in neutral and basic pH solutions at 25 °C. Geochim. Cosmochim. Acta 1989, 53, 2823–2830. [Google Scholar] [CrossRef]
- Alonso, S.; Palomo, A. Alkaline activation of metakaolin and calcium hydroxide mixtures: Influence of temperature, activator concentration and solids ratio. Mater. Lett. 2001, 47, 55–62. [Google Scholar] [CrossRef]
- Khale, D.; Chaudhary, R. Mechanism of geopolymerization and factors influencing its development: A review. J. Mater. Sci. 2007, 42, 729–746. [Google Scholar] [CrossRef]
- Rattanasak, U.; Chindaprasirt, P. Influence of NaOH solution on the synthesis of fly ash geopolymer. Miner. Eng. 2009, 22, 1073–1078. [Google Scholar] [CrossRef]
- Lee, W.K.W.; van Deventer, J.S.J. Structural reorganisation of class F fly ash in alkaline silicate solutions. Colloids Surf. A Physiochem. Eng. Asp. 2002, 211, 49–66. [Google Scholar] [CrossRef]
- Iler, R.K. The Chemistry of Silica: Solubility, Polymerization, Colloid and Surface Properties, and Biochemistry; John Wiley & Sons: New York, NY, USA, 1979. [Google Scholar]
- Provis, J.L.; Van Deventer, J.S.J. (Eds.) Geopolymers: Structures, Processing, Properties and Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2009; Chapter 4; p. 51. [Google Scholar]
- Barton, J.L.; Milshtein, J.D.; Hinricher, J.J.; Brushett, F.R. Quantifying the impact of viscosity on mass-transfer coefficients in redox flow batteries. J. Power Sources 2018, 399, 133–143. [Google Scholar] [CrossRef]
- Brinker, C.J. Hydrolysis and condensation of silicates: Effects on structure. J. Non-Cryst. Solids 1988, 100, 31–50. [Google Scholar] [CrossRef]
- Rees, C.A.; Provis, J.L.; Lukey, G.C.; Van Deventer, J.S. The mechanism of geopolymer gel formation investigated through seeded nucleation. Colloids Surf. A Physicochem. Eng. Asp. 2008, 318, 97–105. [Google Scholar] [CrossRef]
- Heah, C.Y.; Kamarudin, H.; Al Bakri, A.M.; Bnhussain, M.; Luqman, M.; Nizar, I.K.; Ruzaidi, C.M.; Liew, Y.M. Study on solids-to-liquid and alkaline activator ratios on kaolin-based geopolymers. Constr. Build. Mater. 2012, 35, 912–922. [Google Scholar] [CrossRef]
- Chen, H.; Gao, Y.; Li, J.; Sun, C.; Sarkar, B.; Bhatnagar, A.; Bolan, N.; Yang, X.; Meng, J.; Liu, Z.; et al. Insights into simultaneous adsorption and oxidation of antimonite [Sb (III)] by crawfish shell-derived biochar: Spectroscopic investigation and theoretical calculations. Biochar 2022, 4, 37. [Google Scholar] [CrossRef]
- Chen-Tan, N.W.; van Riessen, A.; Ly, C.V.; Southam, D.C. Determining the Reactivity of a Fly Ash for Production of Geopolymer. J. Am. Ceram. Soc. 2009, 92, 881–887. [Google Scholar] [CrossRef]
- Le Chatelier’s Principle and Dynamic Equilibria. Chemistry LibreTexts. 2023. Available online: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Equilibria/Le_Chateliers_Principle/Le_Chatelier’s_Principle_and_Dynamic_Equilbria (accessed on 30 March 2025).
- Hajimohammadi, A.; Provis, J.L.; van Deventer, J.S. The effect of silica availability on the mechanism of geopolymerisation. Cem. Concr. Res. 2011, 41, 210–216. [Google Scholar] [CrossRef]
- Siyal, A.A.; Mohamed, R.M.S.R.; Shamsuddin, R.; Ridzuan, M.B. A comprehensive review of synthesis kinetics and formation mechanism of geopolymers. RSC Adv. 2024, 14, 446–462. [Google Scholar] [CrossRef] [PubMed]
- Hind, A.R.; Bhargava, S.K.; Grocott, S.C. The surface chemistry of Bayer process solids: A review. Colloids Surf. A Physicochem. Eng. Asp. 1999, 146, 359–374. [Google Scholar] [CrossRef]
- Puertas, F.; Fernández-Jiménez, A. Mineralogical and microstructural characterisation of alkali-activated fly ash/slag pastes. Cem. Concr. Compos. 2003, 25, 287–292. [Google Scholar] [CrossRef]
- Provis, J.L.; Fernández-Jiménez, A.; Kamseu, E.; Leonelli, C.; Palomo, A. Binder Chemistry—Low-Calcium Alkali-Activated Materials. In Alkali-Activated Materials: State-of-the-Art Report; RILEM TC 224-AAM; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- McCutchin, C.A.; Edgar, K.J.; Chen, C.L.; Dove, P.M. Silica–Biomacromolecule Interactions: Toward a mechanistic understanding of silicification. Biomacromolecules 2024, 26, 43–84. [Google Scholar] [CrossRef] [PubMed]
- Duxson, P.; Mallicoat, S.W.; Lukey, G.C.; Kriven, W.M.; van Deventer, J.S.J. The effect of alkali and Si/Al ratio on the development of mechanical properties of metakaolin-based geopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2007, 292, 8–20. [Google Scholar] [CrossRef]
- Rickard, W.; van Riessen, A.; Temuujin, J. Thermal analysis of geopolymer pastes synthesised from five fly ashes of variable composition. J. Non-Cryst. Solids 2012, 358, 1830–1839. [Google Scholar] [CrossRef]
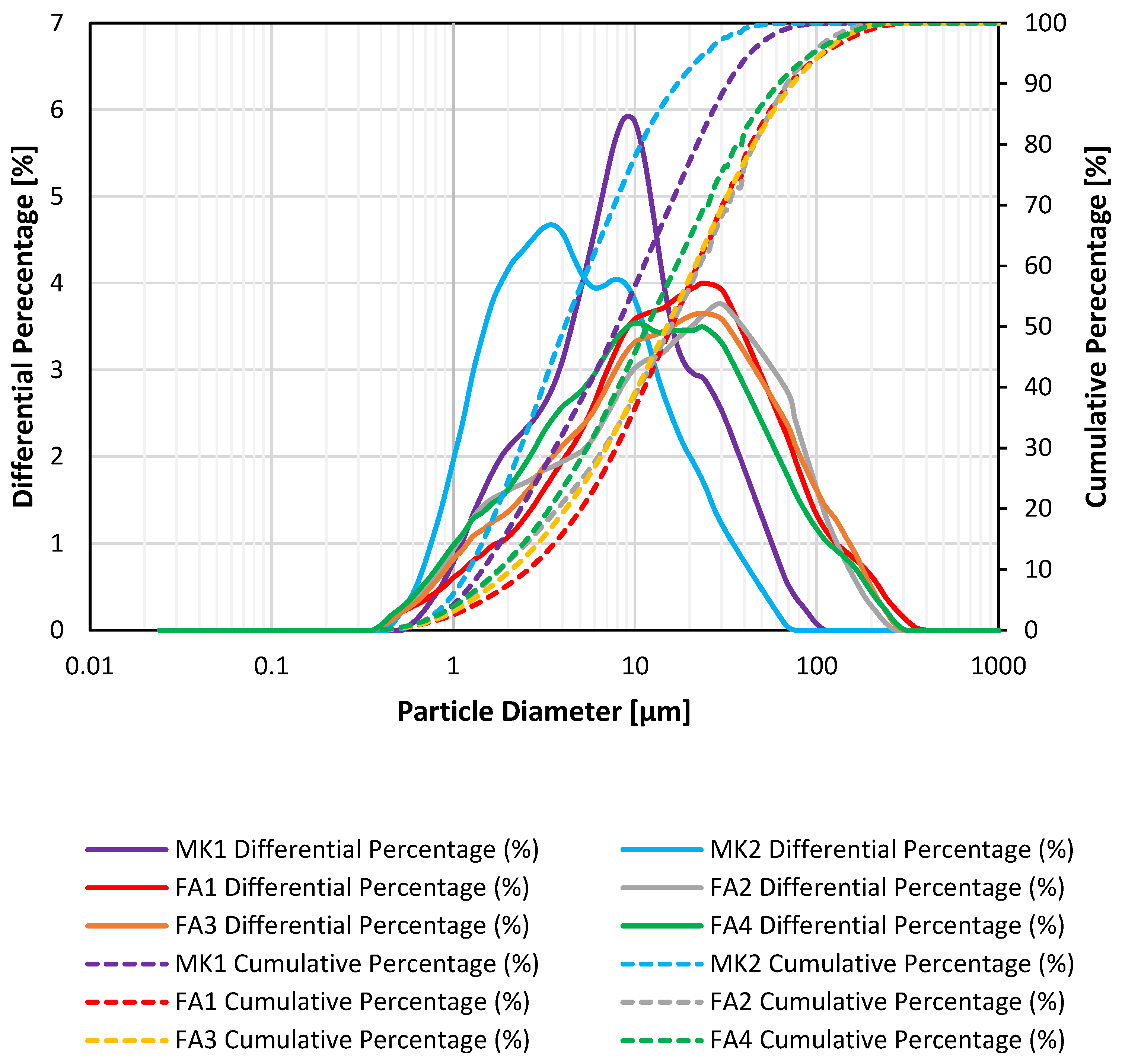

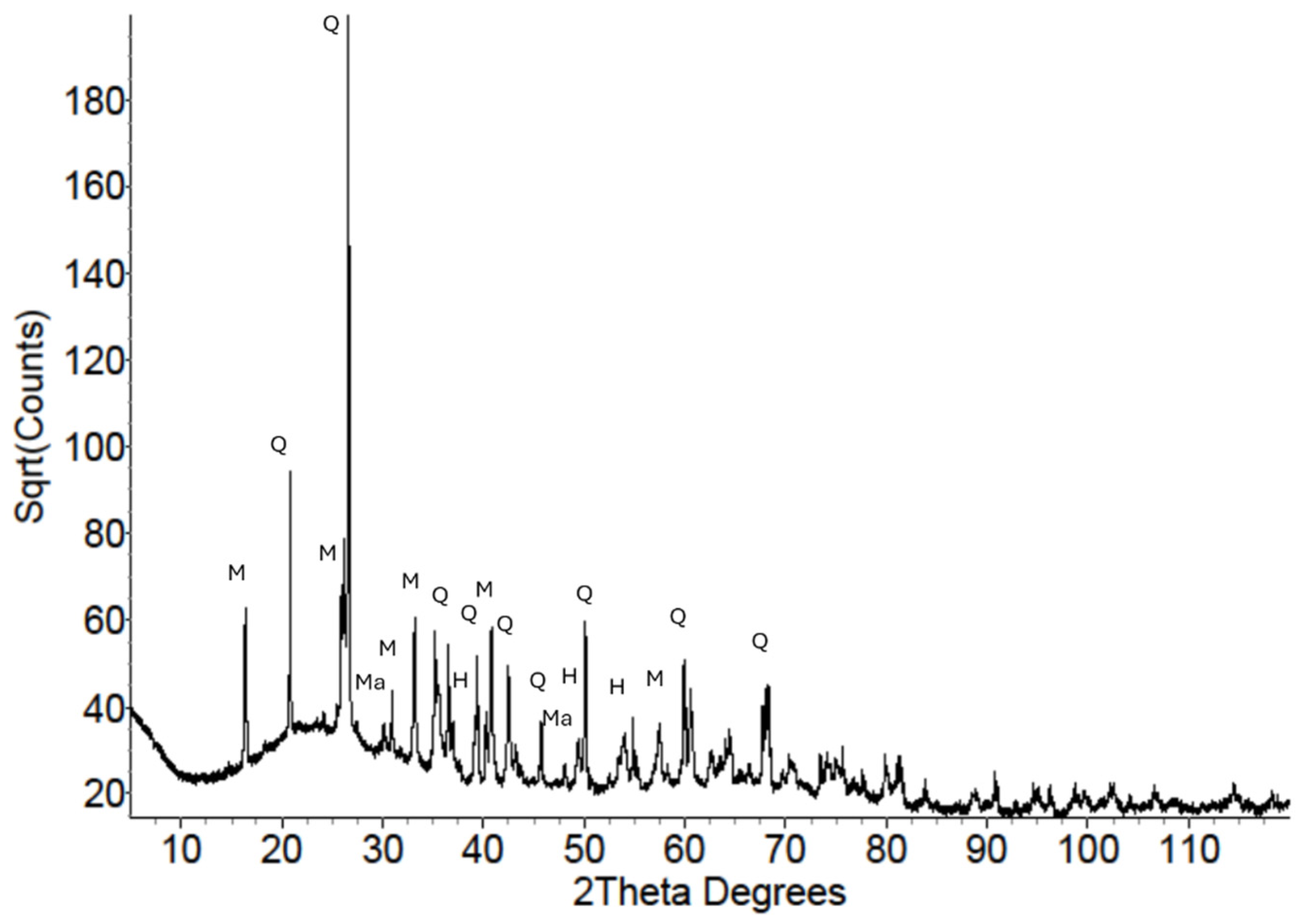
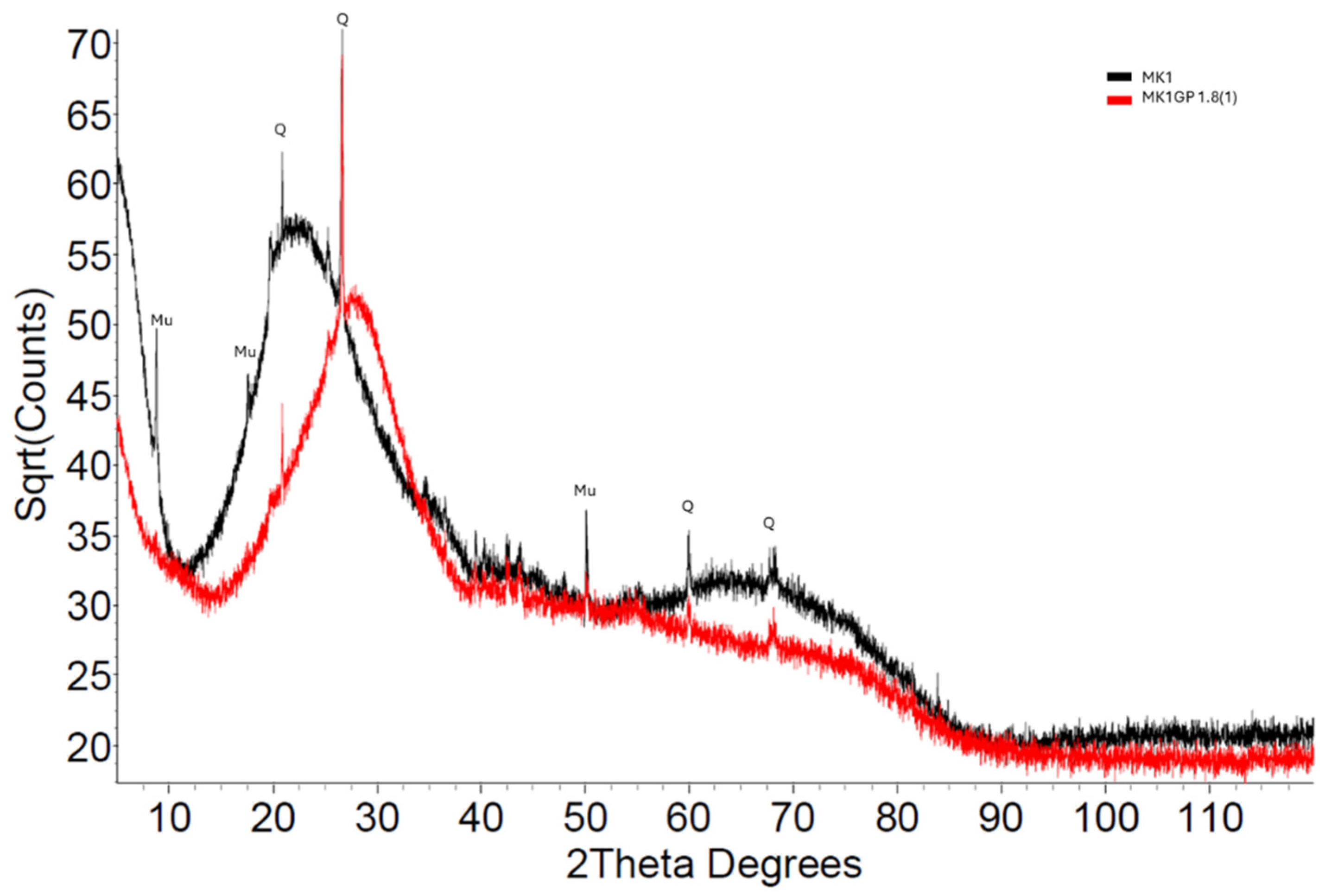
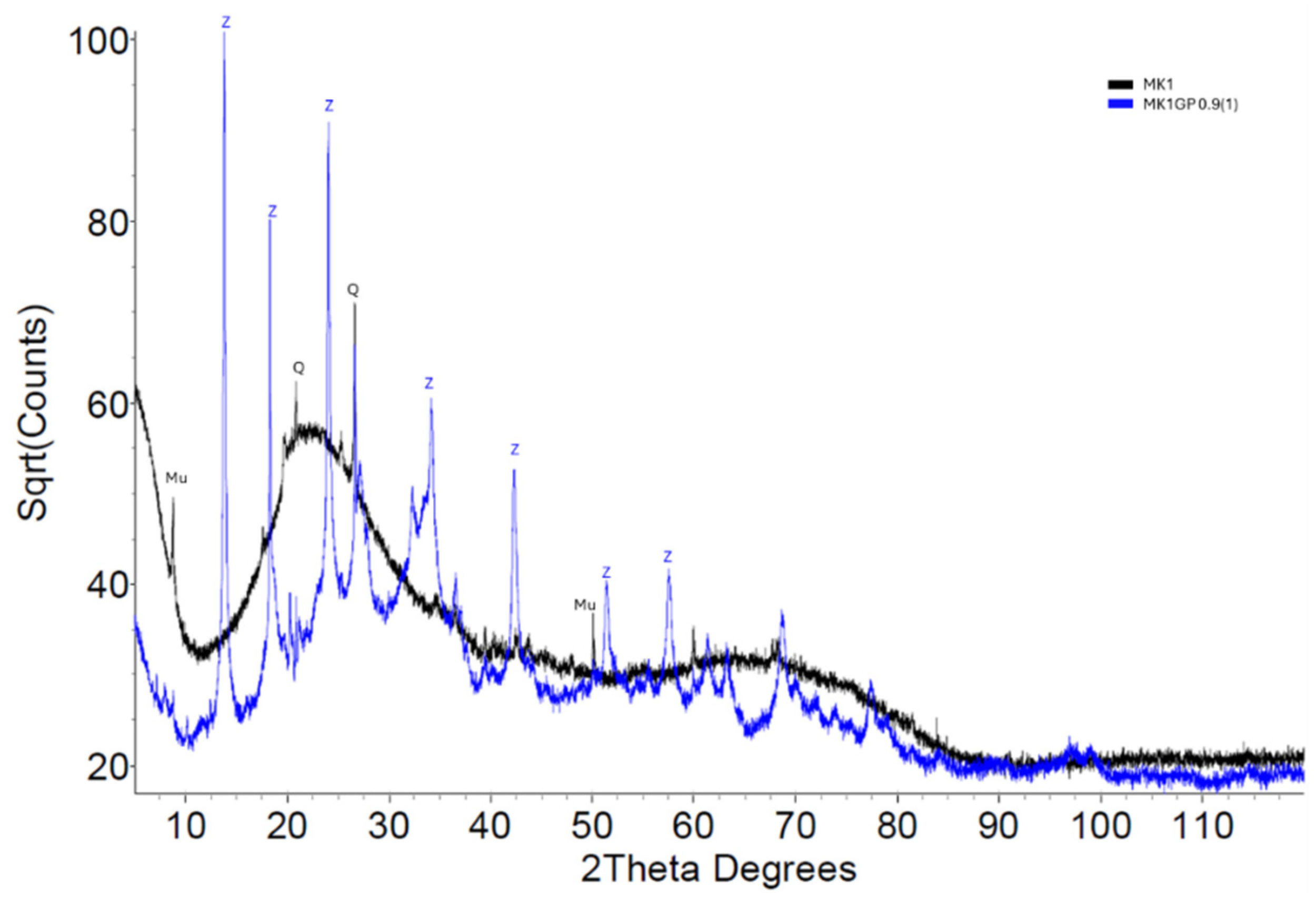



| Precursor Type | Precursor (Amorphous) Si:Al | Target Si:Al | Target Na:Al | Target H:Si | Total Water w/w% | Compressive Strength (MPa) |
|---|---|---|---|---|---|---|
| Metakaolin | 1.01 | 1.6 | 1.0 | 7.23 | 35 | 50(9) |
| Fly Ash | 4.91 | 1.5 | 1.0 | 5.80 | 18 | 35(4) |
| Analyte | MK1 | MK2 | FA1 | FA2 | FA3 | FA4 |
|---|---|---|---|---|---|---|
| Al2O3 | 44.81 | 41.99 | 29.72 | 29.8 | 25.74 | 24.96 |
| BaO | 0.01 | X | 0.3 | 0.27 | 0.2 | 0.45 |
| CaO | 0.01 | 0.1 | 1 | 0.76 | 0.67 | 1.78 |
| Cr2O3 | X | X | 0.02 | 0.02 | 0.02 | 0.02 |
| Fe2O3 | 0.06 | 1.09 | 8.87 | 9.54 | 8.06 | 16.72 |
| K2O | 0.29 | 0.25 | 0.5 | 0.48 | 0.46 | 0.61 |
| MgO | 0.06 | 0.2 | 0.66 | 0.74 | 0.8 | 1.31 |
| MnO | X | X | 0.05 | 0.05 | 0.05 | 0.1 |
| Na2O | 0.03 | 0.03 | 0.22 | 0.25 | 0.24 | 0.35 |
| P2O5 | 0.01 | 0.02 | 1.23 | 0.74 | 0.38 | 1.524 |
| SO3 | 0.05 | X | 0.26 | 0.12 | 0.18 | 0.33 |
| SiO2 | 53.27 | 53.4 | 53.94 | 53.98 | 60.42 | 50.1 |
| TiO2 | 0.28 | 1.13 | 1.83 | 1.82 | 1.85 | 1.39 |
| LOI (1000 °C) | 0.91 | 1.65 | 1.17 | 1.03 | 0.74 | 0.33 |
| Total | 99.8 | 99.86 | 99.94 | 99.84 | 99.86 | 100.23 |
| Si:Al (wt%) | 1.05 | 1.12 | 1.60 | 1.60 | 2.07 | 1.77 |
| Molar Si:Al | 1.01 | 1.08 | 1.54 | 1.54 | 1.99 | 1.70 |
| MK1 | MK2 | FA1 | FA2 | FA3 | FA4 | |
|---|---|---|---|---|---|---|
| Amorphous Content | 98.29 | 95.96 | 52 | 51 | 52 | 58 |
| Amorphous Molar Si:Al | 0.99 | 1.01 | 2.40 | 2.95 | 2.58 | 2.10 |
| Quartz 1 (SiO2) | 1.13 | 3.28 | 13 | 14 | 20 | 15 |
| Quartz 2 (SiO2) | - | - | 6 | 3 | 5 | 3 |
| Mullite (Al4+2xSi2-2xO10−x) | - | - | 25 | 26 | 21 | 14 |
| Hematite (Fe2O3) | - | - | 1 | 2 | 2 | 3 |
| Magnetite (Fe3O4) | - | - | 2 | 2 | 1 | 3 |
| Spinel (MgAl2O4) | - | - | 1 | 2 | - | 4 |
| Muscovite (KAl3Si3O10) | 0.47 | - | - | - | - | - |
| Anatase (TiO2) | - | 0.76 | - | - | - | - |
| MK1 | MK2 | FA1 | FA2 | FA3 | FA4 | |
|---|---|---|---|---|---|---|
| Al2O3 | 44.61 | 41.99 | 10.20 | 8.81 | 9.94 | 11.56 |
| SiO2 | 51.91 | 50.12 | 28.81 | 30.60 | 30.27 | 28.67 |
| CaO | 0.01 | 0.10 | 1.00 | 0.76 | 0.67 | 1.78 |
| Fe2O3 | 0.06 | 1.09 | 6.95 | 6.62 | 5.60 | 12.34 |
| K2O | 0.23 | 0.25 | 0.50 | 0.48 | 0.46 | 0.61 |
| Na2O | 0.03 | 0.03 | 0.22 | 0.25 | 0.24 | 0.35 |
| MgO | 0.06 | 0.20 | 0.38 | 0.17 | 0.80 | 0.18 |
| TiO2 | 0.28 | 0.37 | 1.83 | 1.82 | 1.85 | 1.39 |
| SiO2:Al2O3 | 1.16 | 1.19 | 2.83 | 3.47 | 3.04 | 2.48 |
| Si:Al | 1.03 | 1.05 | 2.49 | 3.07 | 2.69 | 2.19 |
| Precursor | Si:Al | H:Si |
|---|---|---|
| MK1 | 2.0 | 6.40 |
| MK1 | 1.8 | 6.79 |
| MK1 | 1.6 | 7.23 |
| MK1 | 1.4 | 7.73 |
| MK1 | 1.2 | 8.31 |
| MK1 | 1.0 | 8.98 |
| MK1 | 0.8 | 9.56 |
| MK1 | 0.6 | 10.20 |
| MK2 | 1.9 | 5.86 |
| MK2 | 1.8 | 5.02 |
| MK2 | 1.7 | 5.17 |
| MK2 | 1.6 | 5.34 |
| MK2 | 1.5 | 5.54 |
| MK2 | 1.4 | 5.76 |
| MK2 | 1.3 | 6.02 |
| MK2 | 1.2 | 6.32 |
| MK2 | 1.0 | 7.32 |
| MK2 | 0.8 | 8.28 |
| FA 1 | 3.4 | 4.31 |
| FA 1 | 3.2 | 4.48 |
| FA 1 | 3.0 | 4.68 |
| FA 1 | 2.8 | 4.98 |
| FA 1 | 2.5 | 5.33 |
| FA 1 | 2.4 | 5.48 |
| FA 1 | 2.0 | 5.65 |
| FA 1 | 1.8 | 5.77 |
| FA 1 | 1.6 | 5.91 |
| FA 1 | 1.4 | 6.09 |
| FA 1 | 1.2 | 6.34 |
| FA 1 | 1.0 | 6.69 |
| FA 1 | 0.8 | 7.20 |
| FA 2 | 4.0 | 4.16 |
| FA 2 | 3.8 | 4.34 |
| FA 2 | 3.5 | 4.54 |
| FA 2 | 3.0 | 5.10 |
| FA 2 | 2.5 | 5.23 |
| FA 2 | 2.0 | 5.44 |
| FA 2 | 1.5 | 5.78 |
| FA 2 | 1.0 | 6.47 |
| FA 3 | 3.4 | 3.39 |
| FA 3 | 3.2 | 3.53 |
| FA 3 | 3.0 | 3.69 |
| FA 3 | 2.8 | 3.87 |
| FA 3 | 2.6 | 4.07 |
| FA 3 | 2.4 | 4.27 |
| FA 3 | 2.2 | 4.35 |
| FA 3 | 2.0 | 4.43 |
| FA 3 | 1.8 | 4.53 |
| FA 3 | 1.6 | 4.65 |
| FA 3 | 1.3 | 4.86 |
| FA 3 | 0.8 | 5.66 |
| FA 4 | 3.2 | 4.16 |
| FA 4 | 2.9 | 4.43 |
| FA 4 | 2.7 | 4.65 |
| FA 4 | 2.5 | 4.91 |
| FA 4 | 2.3 | 5.21 |
| FA 4 | 2.1 | 5.55 |
| FA 4 | 1.9 | 5.66 |
| FA 4 | 1.7 | 5.79 |
| FA 4 | 1.5 | 5.95 |
| FA 4 | 1.3 | 6.16 |
| FA 4 | 1.1 | 6.45 |
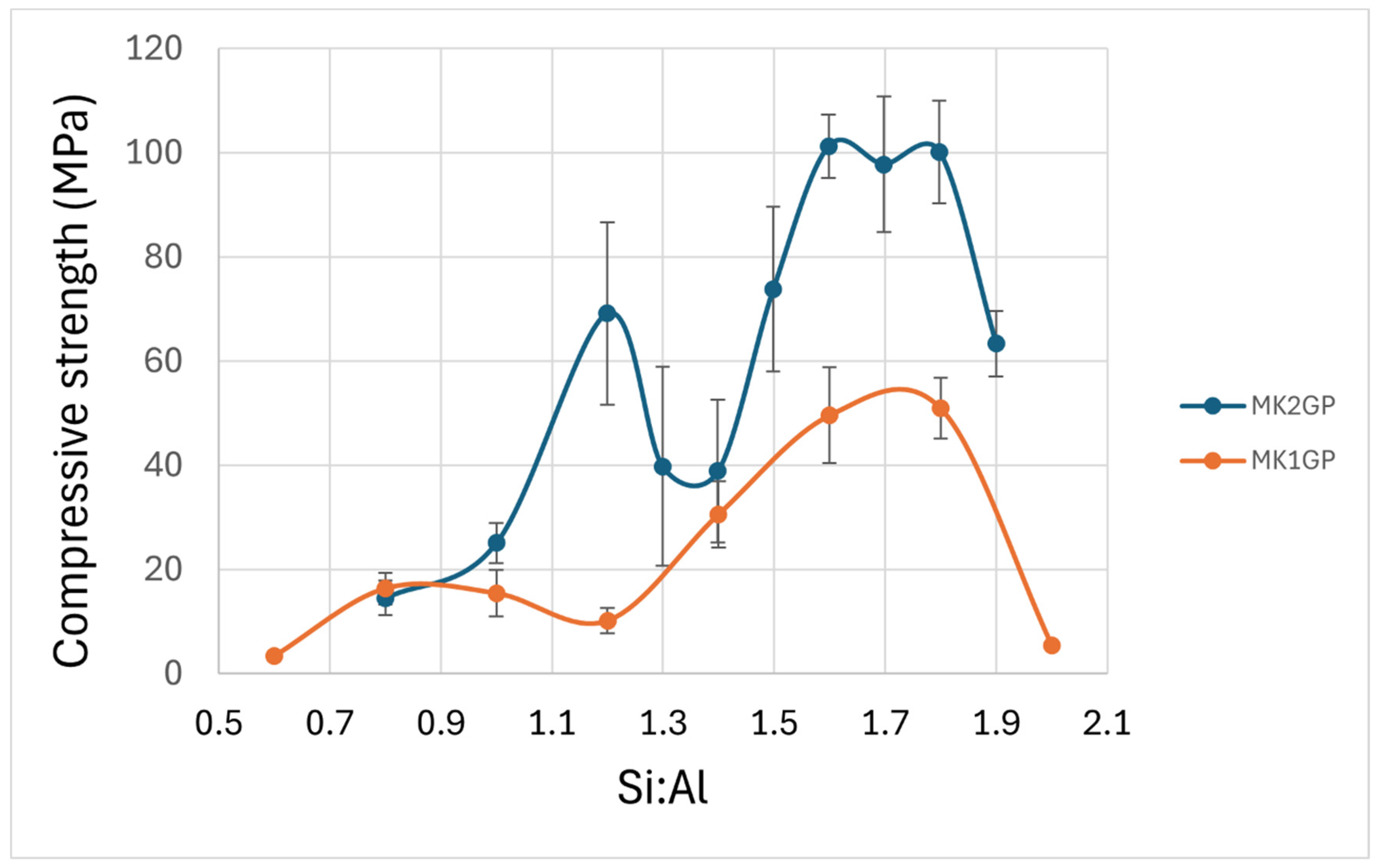
| Precursor | Peak-Low Si:Al | Maximum Compressive Strength (MPa) | Precursor Amorphous Si:Al | Peak-High Si:Al | Maximum Compressive Strength (MPa) | Difference Between High and Low Si:Al Peaks |
|---|---|---|---|---|---|---|
| MK1 | 0.8 | 16 (3) | 0.99 | 1.8 | 51 (6) | 1.0 |
| MK2 | 1.2 | 69 (18) | 1.01 | 1.6 | 101 (6) | 0.4 |
| FA1 | 1.2 | 43 (5) | 2.40 | 3.0 | 33 (6) | 1.8 |
| FA2 | 1.5 | 35 (3) | 2.95 | 3.5 | 13 (2) | 2.0 |
| FA3 | 1.3 | 51 (1) | 2.58 | 3.0 | 70 (9) | 1.7 |
| FA4 | 1.3 | 53 (4) | 2.10 | 2.7 | 55 (3) | 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Riessen, A.; Jamieson, E.; Gildenhuys, H.; Allery, J.; Skane, R. Achieving Optimum Compressive Strength for Geopolymers Manufactured at Both Low and High Si:Al Values. Buildings 2025, 15, 2822. https://doi.org/10.3390/buildings15162822
van Riessen A, Jamieson E, Gildenhuys H, Allery J, Skane R. Achieving Optimum Compressive Strength for Geopolymers Manufactured at Both Low and High Si:Al Values. Buildings. 2025; 15(16):2822. https://doi.org/10.3390/buildings15162822
Chicago/Turabian Stylevan Riessen, Arie, Evan Jamieson, Hendrik Gildenhuys, Jarrad Allery, and Ramon Skane. 2025. "Achieving Optimum Compressive Strength for Geopolymers Manufactured at Both Low and High Si:Al Values" Buildings 15, no. 16: 2822. https://doi.org/10.3390/buildings15162822
APA Stylevan Riessen, A., Jamieson, E., Gildenhuys, H., Allery, J., & Skane, R. (2025). Achieving Optimum Compressive Strength for Geopolymers Manufactured at Both Low and High Si:Al Values. Buildings, 15(16), 2822. https://doi.org/10.3390/buildings15162822






