1. Introduction
Concrete is one of the most widely used materials in the world, and due to its high application rate in the construction industry, it has significant impacts on the environment. Cement production alone reportedly accounts for an estimated 5–8% of global CO
2 emissions [
1]. Over the past two decades, researchers have increasingly explored the means and methods to reduce the environmental impacts of the cement and concrete industry by leveraging several strategies, including clinker substitution, using alternative fuels and improving the energy efficiency of cement production kilns, carbon capture at the source, and design optimization to prolong the service life of concrete structures and reduce the required periodic maintenance. Among these strategies, clinker substitution has been the cornerstone of much ongoing research, and there is a growing interest in identifying materials that can be used as a partial replacement for cement in concrete. Agricultural residues, particularly corn stover, represent a promising yet untapped resource in sustainable material development. Corn stover refers to the non-grain part of the corn plant (i.e., stalks, cobs, leaves, and husk) that is typically harvested for animal feed, biofuel production, and soil amendments. In some regions, however, due to the high magnitude of harvest, the excess corn stover can result in blocking sunlight and promoting fungal diseases [
2,
3,
4], all of which can negatively affect soil health [
2]. Therefore, open burning the stover in the field has been a quick remedy for many years if baling the stover is not possible [
4,
5,
6]. In 2024, the United States and China together produced more than 470 million metric tons of corn stover, and the global production of corn stover is estimated to exceed 1.1 billion metric tons [
7,
8]. Previous research shows that corn stover ash (CSA) has pozzolanic properties and can be added to concrete to replace a portion of cement [
9,
10,
11,
12]. Shakouri et al. [
9] showed that substituting cement with acid-pretreated CSA by 5% and 20% increased the compressive strength of concrete by 7.1% and 9.6% at 112 days. Other researchers have used corn stover fiber (CSF) in concrete and observed up to a 42.9% increase in tensile strength and 16.3% increase in flexural strength compared to plain concrete [
11,
13,
14]. Research has also shown that incorporating 4% CSF into concrete lowers its thermal conductivity significantly, reaching a minimum of 0.0402 W/mK, which is comparable to other high-performance insulation materials [
15]. Additionally, the incorporation of CSA and CSF can significantly improve the bulk electrical resistivity and thermal insulating properties of concrete [
9,
11,
12,
16]. Despite the reported benefits associated with the application of CSA and CSF in concrete, research shows that the properties of both CSA and CSF can vary from one region to another and show different properties depending on the pretreatment regimen used. Shakouri et al. [
12] assessed the reactivity and chemical composition of various pretreated CSA sourced from various corn-producing states in the U.S. and reported significant regional variability in chemical composition and reactivity due to differences in soil composition, farming practices, and fertilizers used. One method to reduce the variability in the properties of CSA is through pretreatment. In addition, Shakouri et al. [
12] assessed the effect of corn stover pretreatment with water and various acids, including nitric, hydrochloric, sulfuric, and citric acid, and reported that acid treatment can significantly reduce the heterogeneity in the chemical composition of CSA, with citric acid being the most environmentally friendly option and nitric acid being the most effective in reducing the alkaline metals in the produced CSA.
While these approaches are effective for ash, improving the performance of corn fibers in cementitious systems requires a different strategy, and it often involves treatment with an alkaline solution to dissolve lignin and hemicellulose from the fibers, which are known to reduce the bond between the fiber and the cement matrix [
15,
17]. Additionally, alkaline treatment can decrease the degradability of the resulting biopolymer composites, which is crucial for maintaining their mechanical performance [
18,
19].
Although pretreatment can enhance the consistency and performance of CSA and CSF, inconsistencies in chemical composition and reactivity can still pose challenges to achieving reliable improvements in the mechanical properties of concrete, thus limiting their adoption in applications where the structural properties of concrete are important. This limitation, however, can present an opportunity in non-structural concrete elements, such as concrete masonry units (CMUs) used in partition walls, infill blocks, landscaping, and other CMU-based products, where compressive strength is not the primary concern, and sustainability and thermal performance are prioritized over load-bearing capacity [
14,
15,
20,
21].
To make CMU production more environmentally friendly, a portion of cement is often replaced with fly ash or other coal combustion byproducts (e.g., cinder). The resulting product, also known as “cinder block”, is relatively lightweight, weaker than conventional CMUs, and more susceptible to cracking [
22]. This issue can be mitigated by incorporating CSA and CSF, which have been shown to improve the strength, roughness, and crack resistance of concrete [
9,
17,
23]. It is important to note, however, that the majority of previous studies reporting improvements in mechanical properties from the incorporation of CSA and CSF have focused on conventional concrete mixtures. In contrast, CMUs are typically made using zero-slump mixtures with finer aggregate gradation and lower cement content [
24]. Instead of being vibrated internally like conventional concrete, they are compacted using external vibration and pressure, often with an egg-layer machine [
24]. This distinction is critical, as the dry nature of CMU mixes can limit fiber dispersion and weaken the bond between the fibers and the cementitious matrix. In addition, the low water content in CMU mixes can inhibit cement hydration and potentially limit the pozzolanic activity of CSA. Given that there are no data available on the performance of CSA and CSF in dry-cast masonry applications, this study aims to evaluate their combined effect on the mechanical, durability, and thermal properties of CMUs, and to determine whether such an approach can offer a viable and sustainable alternative to conventional CMU blocks.
2. Materials and Methods
2.1. Materials
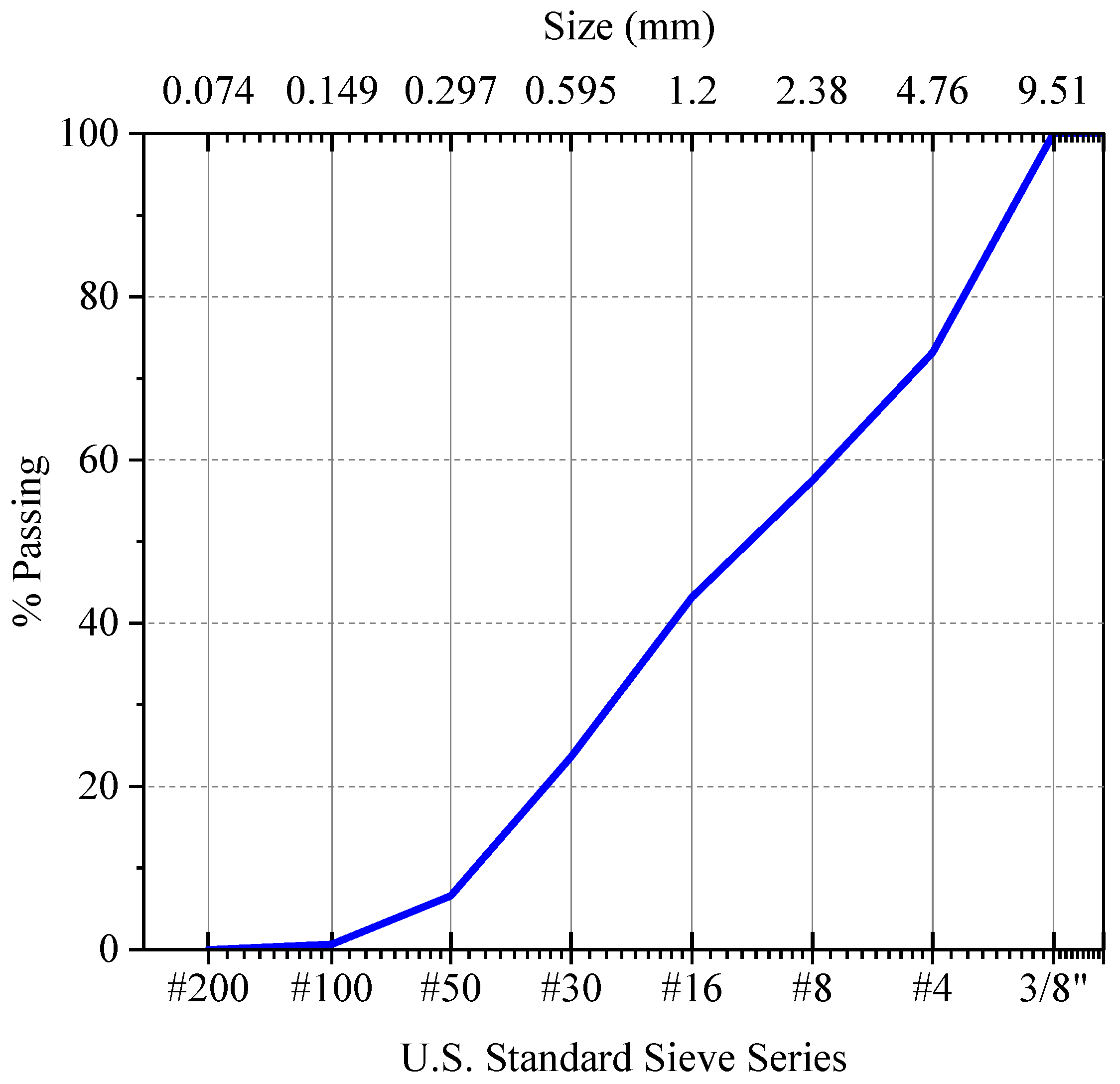
Corn stover was collected locally from Greeley, CO, USA. Washed sand, with a bulk specific gravity of 2.75, a fineness modulus of 3.9, and the gradation shown in
Figure 1, along with ordinary Portland cement Type I/II meeting the ASTM C150 requirements [
25] and with the composition shown in
Table 1, were used to make the mortar.
2.1.1. CSA Preparation
In this study, the as-received corn stover was first soaked in water for a week, then rinsed and soaked for another week in 0.1 M citric acid at a liquid-to-stover mass ratio of 10:1. Previous research by the PI indicates that raw corn stover typically contains a high alkali content, and the most effective way to reduce these alkalis is through acid-soaking [
9,
16,
26]. After completing the acid-soaking process, the stover was washed again and dried in an oven at 100 °C until no changes in mass in two consecutive days were observed. The acidic treatment solution was neutralized using baking soda prior to disposal. A cyclonic ashing furnace was then used to burn the dried stover, a process that typically results in ash with high carbon content. The yield after burning in the cyclonic ashing furnace was 20.8%. To reduce the carbon content of the CSA further, the resulting ash from the open-burning process underwent additional thermal processing at 550 °C in a muffle furnace, which produced a yield of 5.5%. The reduced-carbon CSA was then ground in a jar mill for 30 min to produce particles with a median size of 30 microns and density of 2.35 g/cm
3.
2.1.2. CSF Preparation
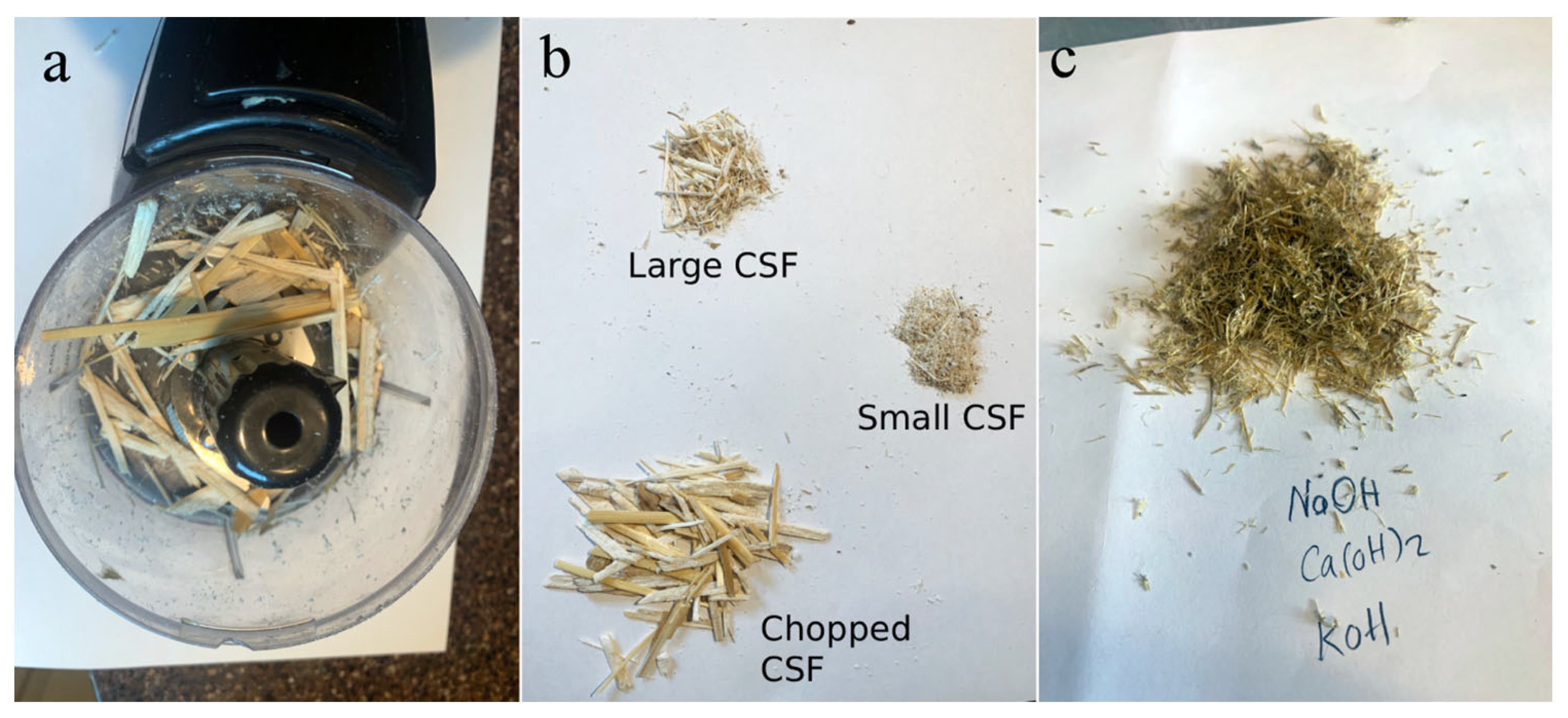
To produce CSF, only the corn stalks were selected, while the leaves and branches were excluded. After manually removing the pith, only the outer rind of the stalks was retained for further processing. The cleaned stalks were then chopped into smaller pieces using a 300 W coffee grinder. Grinding duration ranged from one to five minutes, depending on the toughness of the material. Once ground, the particles were passed through No. 16 (1.18 mm) and No. 30 (0.6 mm) sieves to classify the fibers into coarse and fine fractions. The long CSF had an average length and thickness of 10.7 mm and 0.9 mm, respectively, while the shorter CSF had an average length and thickness of 3 mm and 0.4 mm, respectively.
Figure 2 illustrates the process of CSF preparation.
In this study, CSF reinforcements were first soaked in water for 24 h, followed by immersion in three different alkaline solutions, namely, 0.5 M NaOH, 0.5 M KOH, and a SCPS for 24 h to dissolve lignin and hemicellulose from the fibrous part of the fiber. The SCPS was prepared by dissolving 7.6 g of NaOH, 10.64 g of KOH, and 2 g of Ca(OH)
2 in one liter of distilled water, following the guidelines specified in ASTM C1876 [
27].
2.2. Sample Preparation
2.2.1. Preliminary Study for Selecting CSF Size, Replacement Level, and Treatment Method
To select the fiber size, replacement level, and alkalinization method of CSF reinforcement used in CMU blocks, a preliminary study consisting of two trials was conducted. For both trials, 50 mm mortar cubes were prepared per guidelines provided in ASTM C109 [
28]. Each set of three cubes was made using 500 g of OPC, 1375 g of sand, and 242 g of water, as specified in ASTM C109. At this stage, no CSA was included in the mixtures. In the first trial, as shown in
Table 2, short and long CSF reinforcements treated with SCPS were incorporated at replacement levels of 0%, 1%, 3%, and 5% by volume. Three replicates were prepared and tested for each replacement level.
Based on the results of the first trial, a second trial was conducted using CSF treated with 0.5 M NaOH and 0.5 M KOH at the best performing fiber size and replacement level identified in the first trial (see
Table 3), to determine which treatment solution was more effective. Similar to Trial 1, three replicates of 50 mm cubes were cast using various treated CSF types and cured in a controlled environment maintained at 23 °C and 95% relative humidity for 7 days before compressive strength testing.
Further details on the compressive strength results from the preliminary study, and their influence on the design of the main study, will be provided in the results section.
2.2.2. CMU Mixture with CSA and CSF
Building on the findings of the preliminary study, the final concrete mixture for CMU blocks was developed by incorporating the short CSF at 1% replacement level by volume. The initial design of the mixture was based on the fineness modulus method proposed in [
24], which was further refined through several trial batches. The control mix hereafter referred to as CMU only contained OPC, whereas in the test mix (hereafter referred to as Corncrete), 10% of cement by mass (i.e., equivalent to 12% of the volume) was replaced with CSA. The final control mix had a cement-to-aggregate mass ratio of 1:8, with the water content adjusted to 12% of the total weight of cement and aggregates. In the Corncrete mixture containing CSA and CSF, the water content was increased to 14% of the total weight of cement and aggregates to account for the water absorption of CSF.
Table 4 summarizes the mix design proportions adjusted for one cubic meter of concrete.
Various specimens with different sizes were prepared to evaluate different properties. For compressive strength testing, cylinders of 75 mm × 150 mm were cast, with three replicates for each mixture. Additionally, two cylinders of 100 mm × 200 mm were produced for Rapid Chloride Permeability Testing (RCPT), three 75 mm × 150 mm cylinders for fire resistance assessment, and six 100 mm × 200 mm cylinders for water absorption measurements.
All specimens underwent curing in a saturated lime solution for 91 days to ensure proper hydration and strength development.
2.3. Methods
2.3.1. Chemical and Physical Characterization of CSA and CSF
The oxide composition of CSA was analyzed using X-ray fluorescence (XRF). The pozzolanic activity of CSA was assessed using the modified R
3 test [
29,
30], which quantifies the heat released during the reaction between CSA and calcium hydroxide in an alkaline environment. To prepare the samples, one part CSA (by mass) was mixed with three parts reagent-grade dry Ca(OH)
2. This mixture was then mixed with a 0.5 M potassium hydroxide (KOH) solution at a liquid-to-solid mass ratio of 0.9. To ensure homogeneity of the mixture, the materials were mixed for four minutes using an overhead paste mixer set to 300 RPM. Approximately 6 g of the paste was placed into special glass ampules, which were sealed and then placed inside an isothermal calorimeter. The heat of hydration generated from the reaction between CSA and alkaline solution was recorded for 10 days, while the calorimeter isothermal chamber was maintained at 50 ± 0.05 °C. After ten days, the glass ampule was taken out of the calorimeter and a small sample (approximately 20 mg) was taken from the center of the paste and analyzed using thermogravimetric analysis (TGA) to quantify the residual calcium hydroxide. The TGA process involved recording mass loss while heating up the sample from ambient to 500 °C at a rate of 20 °C/min in an inert nitrogen atmosphere. After that, the mass loss of calcium hydroxide which typically occurs between 350 °C and 500 °C [
29] was quantified using the tangential method [
31]. This mass loss indicates the residual calcium hydroxide in the sample. The decreased calcium hydroxide content relative to the control sample implies a higher degree of pozzolanic reaction in the CSA-containing mix, and conversely, higher CH levels would indicate reduced pozzolanic activity. The degree of reactivity (DoR) of CSA was estimated using the empirical method proposed by Bharadwaj et al. [
32]. The method defines DoR as the ratio of the cumulative heat release during the modified R
3 test to the theoretical heat release of a fully reactive material with a similar composition, determined through thermodynamic simulations [
33,
34].
The dimensional analysis of CSF was carried out using digital calipers to measure the length and thickness of randomly selected fibers. The density of both CSF and CSA was determined using Le Chatelier’s flask method following the guidelines in ASTM C188 [
35,
36]. In this method, kerosene was used as the displacement medium for measuring the volume of the solid particles. Water absorption of CSF was measured by submerging pre-weighed oven-dried fiber samples in water for 24 h. After immersion, the fibers were surface-dried and weighed to calculate the percentage of water absorption.
2.3.2. Compressive Strength Measurement
The compressive strength of 50 mm mortar cubes in the preliminary study was measured after 7 days. The compressive strengths of 76 mm × 152 mm cylinders from the final mix were measured at 28 and 91 days of curing.
2.3.3. Water Absorption and Bulk Density Measurement
The water absorption, voids content, and bulk density of the CMU and Corncrete specimens were determined per ASTM C642 [
37]. For each mix, three 100 × 50 mm sections were cut from the 100 × 200 mm cylinders. These specimens were first dried in an oven at 110 ± 5 °C for 24 h until their mass stabilized and the oven-dry mass (
Mdry) of each specimen was recorded. After drying, the specimens were soaked in water at room temperature (23.0 ± 2.0 °C) for 48 h. After this immersion period, the saturated surface-dry mass (
Mssd) of each specimen was recorded. Following this, the specimens were boiled in water for 5 h, then allowed to cool in the water for 14 h, after which the saturated mass after boiling (
Mboil) was measured. To determine the apparent volume of the specimens, each sample was suspended in water (i.e., after submersion and boiling steps discussed above), and its submerged mass (
Msub) was measured. Equations (1) through (4) were used to measure density, absorption, and volume of permeable voids in the specimens.
where
ρ is the density of water (1000 kg/m
3).
2.3.4. Rate of Water Absorption Measurement
The water absorption rate for CMU and Corncrete specimens was measured in accordance with the procedure outlined in ASTM C1585 [
38]. This test aims to quantify the water ingress by measuring the incremental mass gain of the specimens over a period of up to 9 days. Sorptivity is a critical parameter for evaluating concrete durability, as it provides an indirect measure of the permeable porosity within the material, which influences long-term performance in aggressive environments [
37]. For each mix, three 100 × 50 mm sections were cut from the middle of the 100 × 200 mm cylinders after curing for 91 days in a saturated limewater solution. These specimens were conditioned by saturation in a vacuum chamber, followed by placement in an environmental chamber at 50 °C and 80% relative humidity for 72 h. After conditioning, the specimens were wrapped in plastic and stored at 23.0 ± 2.0 °C for 15 days to achieve moisture equilibrium before testing. For sorptivity testing, the bottom 2 mm of each specimen was submerged in water in accordance with ASTM C1585. Mass measurements were taken at specific intervals as outlined in the ASTM C1585 standard. Sorptivity for each mix was calculated using Equation (5), based on the recorded mass changes over time.
Here, I indicates absorption (mm), mt is the change in mass at a given time t (g), a refers to the surface area exposed to water (mm2), and ρw is the water density, assumed to be 0.001 g/mm3. The initial rate of water absorption, also known as sorptivity, was calculated by fitting a linear regression line to the data from the first six hours of testing. A second linear regression was applied to the data collected beyond the six-hour mark to determine the secondary absorption rate.
2.3.5. Bulk and Surface Electrical Resistivity Measurement
The bulk and surface electrical resistivity of 100 × 200 mm cylindrical specimens prepared for the RCPT test were measured using a four-electrode concrete resistivity meter with a 38 mm probe spacing. Measurements were taken every three days over a total duration of 91 days, with each measurement performed at a frequency of 40 Hz. For surface resistivity (ρs), measurements were taken at eight different locations on the surface of the specimens, and the average value was used for analysis.
To determine the bulk resistivity (
ρb) of the specimens, the specimens were placed between two metallic plates, with a layer of conductive foam between the mortar and the plates. The plates were then connected to the resistivity meter to measure the resistance of the cubes. This resistance value was subsequently adjusted using Equation (1) to account for the probe spacing and the geometric configuration of the specimens.
where
ρ is the average resistivity (Ω.cm) of four sides of the cube,
R is the resistance (kΩ.cm),
a is the probe spacing (cm), which was 3.8 cm in this study,
A is the cross-section area of the cubic specimens (i.e., 25 cm
2), and
L is the depth of the cubes (i.e., 5 cm). The term
A/L in Equation (6) is often called geometry constant,
k.
2.3.6. Rapid Chloride Permeability Measurement
To conduct the RCPT test, two cylindrical specimens measuring 100 × 200 mm (diameter × length) were prepared from each mixture and cured in a saturated lime solution for a duration of 91 days. Subsequently, two specimens sized 100 × 50 mm were cut from the middle section of each cylinder using a wet masonry saw. The actual dimensions of the specimens were recorded using a caliper for correcting the final results. Following cutting, epoxy was applied to all but the end faces, and the specimens were left to dry overnight. After the epoxy cured, the specimens were placed in a desiccator partially filled with distilled water and subjected to vacuum saturation for 18 ± 1 h at an absolute pressure of 50 mm Hg (6650 Pa), following ASTM C1202 [
39]. Following saturation, specimens were mounted between acrylic cells filled with 0.30 N NaOH and 3% NaCl. Current was monitored at one-minute intervals over six hours using Perma from Giatech. Based on the total charge passed, the concrete was classified following ASTM C1202 [
39], which defines five levels of chloride permeability from negligible to high.
2.3.7. Mass Loss and Residual Strength Measurement After Exposure to Fire
The fire resistance of the Corncrete and CMU specimens was evaluated using the ASTM E119 [
40] standard, which requires subjecting specimens to elevated temperatures over time following a prescribed time–temperature curve as shown in
Figure 3. The test starts at room temperature and increases rapidly, reaching approximately 927 °C within the first hour. As the test progresses, the temperature continues to rise, leveling off near 1260 °C after 8 h.
Three 75 mm × 150 mm cylinders from each mix were dried in an oven at 105 °C. The specimens were then cooled down to room temperature, weighed, and placed inside a programmable muffle furnace that simulated the heating rate specified in ASTM E119. After 8 h of exposure to extreme heat, the specimens were taken out and allowed to cool down overnight. The next day, the weight of the specimens was measured, and the difference in masses was expressed as percent mass loss. Following the mass loss assessment, the compressive strength of these specimens was measured and reported as the percent residual compressive strength after exposure to extreme heat.
2.4. Stakeholder Feedback Collection
To gain a comprehensive understanding of the potential and challenges associated with Corncrete production, interviews and correspondence were conducted with key stakeholders from commodity boards in Colorado and Nebraska. These stakeholders included representatives with expertise in agriculture, environmental compliance, and renewable materials. Semi-structured interviews were used to allow for in-depth discussions, focusing on their perspectives regarding the environmental, logistical, and economic implications of producing corn stover ash at scale.
Each interview was designed to gather qualitative insights on several key areas: (1) environmental impacts and regulatory concerns, (2) logistical challenges in sourcing and processing corn stover, (3) economic feasibility and potential barriers to scaling CSA production, and (4) specific feedback on the pretreatment processes involved, such as acid treatment and disposal. Notes from these interviews were then reviewed and analyzed to identify recurring themes and concerns, which were used to inform the limitations and opportunities discussed in this study.
3. Results
3.1. Chemical and Physical Properties of CSA and CSF
3.1.1. Chemical Composition and Reactivity of CSA
Table 5 presents the oxide composition of CSA both prior to and following treatment with 0.1 M citric acid. The treatment of raw stover with 0.1 M citric acid led to considerable changes in the chemical composition of the resulting ash. The SiO
2 content increased by 28.4%, from 59.50% before treatment to 76.40% after treatment. This suggests that the citric acid treatment effectively concentrated the silica by removing other components. Al
2O
3 also increased by 22.2%, from 5.40% to 6.60%. In contrast, several other oxides showed reductions. CaO decreased by 9.8% (from 4.10% to 3.70%), P
2O
5 by 56.3% (from 1.60% to 0.70%), Fe
2O
3 by 23.5% (from 1.70% to 1.30%), and MgO by 65.5% (from 2.90% to 1.00%). The most substantial decrease was observed in K
2O, which dropped by 86.9% (from 9.90% to 1.30%), while Cl content decreased by 66.7% (from 0.30% to 0.10%). The reduction in K
2O is significant, as raw corn stover typically contains a high concentration of this compound.
Previous research by the author indicates that excessive K
2O can lead to rapid setting and premature compressive strength gain [
9,
11,
39,
40]. Additionally, the significant reduction in K
2O not only minimizes the risk of detrimental reactions between reactive silicious aggregates with alkalis but also contributes to a purer ash composition that is better suited for pozzolanic reactions. The treatment of the stover with citric acid resulted in a 53.1% reduction in loss on ignition (LOI) of CSA, which indicates the removal of volatiles and organic material during the treatment process
3.1.2. CSA Reactivity
Table 6 shows the results of the modified R
3 test and the DoR estimated using the PRT method. The results show a significant improvement in the reactivity of CSA after treatment with 0.1 M citric acid. The heat release increased from 230.67 J/g in the untreated CSA to 312.58 J/g in the treated sample, indicating 35.5% enhancement in the exothermic reactions during hydration. This suggests that the treatment increases the pozzolanic activity of the CSA, allowing it to participate more effectively in cementitious reactions.
Table 6 also shows that the consumption of Ca(OH)
2 in treated CSA increased significantly, from 69.30 g/100 g SCM to 108.53 g/100 g SCM, further supporting the conclusion that the treatment with 0.1 M citric acid boosts pozzolanic activity by enabling the CSA to react more readily with available calcium hydroxide.
The DoR, as estimated by the PRT method, increased from 28.60% to 38.79% after treatment, demonstrating that a larger portion of the CSA becomes chemically reactive following the treatment process. This increased reactivity, along with the combined improvements in SiO2 and Al2O3 content, the reduction in K2O, and the lower LOI, all contribute to the enhanced heat release and overall performance of the treated CSA, making it more effective in concrete applications.
3.1.3. Physical Properties of CSF
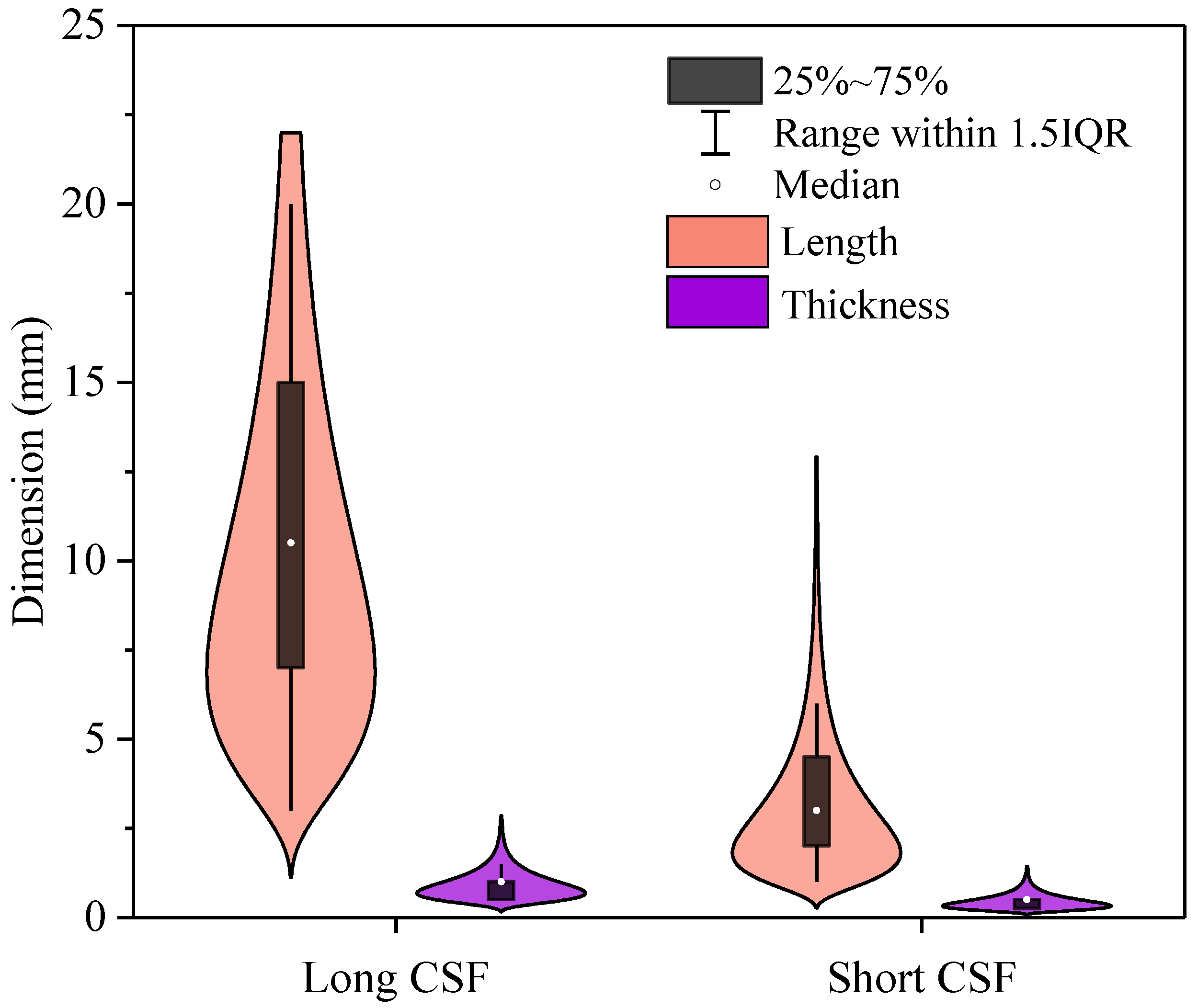
The violin plot in
Figure 4 illustrates the size distribution of both length and thickness for long and short corn straw fibers. For the long CSF, the distribution of lengths is broad, with values ranging from approximately 5 mm to 20 mm. The median length is around 12 mm, and the average length is 10.7 mm with a standard deviation of 4.9 mm, indicating some variability in the fiber lengths. In contrast, the thickness of long CSF is relatively consistent, with a median of about 0.2 to 0.3 mm, and the average thickness being 0.9 mm with a standard deviation of 0.4 mm
The short CSF, on the other hand, demonstrates a much narrower length distribution with low variability. The average length is 3 mm with a standard deviation of 1.6 mm, and the range of lengths typically falls between 2 mm and 4 mm. The thickness distribution for short CSF is also narrow, with an average thickness of 0.4 mm and a standard deviation of 0.2 mm.
Table 7 shows the effect of treatment solutions on the physical properties of CSF. According to
Table 7, short CSF treated in 0.5 M NaOH had a density of 0.74 g/cm
3, and that treated with SCPS slightly lower at 0.70–0.72 g/cm
3. Similarly, long CSF showed densities of 0.74 g/cm
3 and 0.7 g/cm
3 for 0.5 M NaOH and SCPS treatments, respectively. These findings suggest that all types of alkaline solutions used for pretreating the fibers performed consistently in removing lignin and hemicellulose, resulting in similar densities.
Fiber absorption test results showed that absorption in both short and long CSFs ranged from 14.3% to 18%. Regardless of fiber lengths, no significant differences in absorption were found between CSFs treated with NaOH and KOH solutions. However, the results indicate that treating short and long CSFs with SCPS increased absorption by approximately 14% in short fibers and 26% in long fibers. The most likely reason for the higher water absorption in CSFs treated with the combination of KOH, NaOH, and Ca(OH)
2 is that calcium suppresses hornification [
41]. Hornification happens during the drying stage when cellulose chains stick back together, which closes up pores and makes the fiber less able to absorb water. Calcium seems to slow down this process by interfering with how the cellulose chains reattach, helping the fibers keep their open, porous structure [
41]. Furthermore, Ca
2+ readily forms stable, water-attracting complexes with carboxyl groups in hemicellulose and lignin, which are far more chemically reactive than cellulose [
42]. These complexes increase the hydrophilicity of the fiber matrix and facilitate greater moisture uptake. Alkaline calcium pretreatments have also been shown to increase surface roughness and fibrillation, thereby providing more water-accessible regions for water uptake [
43].
3.2. Preliminary Study Results
3.2.1. Trial 1: Effect of Fiber Size and Replacement Level
The preliminary results from Trial 1 are shown in
Table 8 and indicate that specimens from Mix 1-1 and Mix 1-4, which incorporated short and long CSFs, respectively at 1% by volume, achieved compressive strength of 25 MPa and 21.MPa, respectively. These values exceed the minimum requirement for CMUs as specified by ASTM C90 [
11], which mandates a minimum net area compressive strength of 13.8 MPa for the lightweight, medium-weight, and normal weight CMUs. The results also show that, regardless of fiber length, increasing fiber replacement levels to 3% and 5% resulted in a significant reduction in compressive strengths. For example, Mix 1-2 (short CSF at 3%) and Mix 1-6 (long CSF at 5%) exhibited compressive strengths of only 8.1 MPa and 5.6 MPa, respectively, which are 41% and 59% below the 13.8 MPa requirement by ASTM C90. This reduction in strength is likely due to the dry nature of the CMU mix, which makes it difficult for the fibers to distribute evenly throughout the mixture. In low-water mixtures like these, the fibers tend to clump together, leading to agglomeration. These fiber clusters can create weak spots in the matrix, ultimately reducing the overall compressive strength of the concrete [
44,
45]. The effectiveness of fiber length also plays a role in compressive strength. Previous investigations show that shorter fibers generally enhance compressive strength [
46,
47] and improve fiber distribution [
46], while longer fibers tend to increase flexural strength and ductility [
48,
49].
The strength activity index (SAI) values, which are the ratio of compressive strength of test specimens to control specimens, further support the observed trends in
Table 8. Mix 1-1, with an SAI of 82.9%, suggests that the short fibers at 1% replacement maintained a high level of strength relative to the control. Mix 1-4, with an SAI of 70.1%, also showed reasonable strength but was less effective than Mix 1-1. Meanwhile, the SAIs for higher fiber replacement levels (e.g., 26.8% for Mix 1-2 and 18.5% for Mix 1-6) were significantly lower, reflecting the weakening effect of higher fiber content on compressive strength. Based on the findings from Trial 1, short fibers used at 1% by volume appear to be the most effective choice for reinforcing CMUs among the tested sizes and replacement levels.
3.2.2. Trial 2: Effect of Alkalization Solution
In Trial 2, the effect of different alkalization solutions (NaOH, KOH, and SCPS) on the compressive strength of specimens reinforced with short fibers at 1% by volume was investigated. The results are summarized in
Table 9 and indicate that the compressive strength of the mixes varied depending on the alkalization solution used to treat the corn stover fibers. Mix 2-3, treated with synthetic concrete pore solution, achieved the highest compressive strength at 25.0 MPa, closely followed by Mix 2-2, treated with 0.5 M KOH, which produced a compressive strength of 24.0 MPa. Mix 2-1, treated with 0.5 M NaOH, exhibited the lowest compressive strength at 21.0 MPa. The lower standard deviation observed for the SCPS-treated mix (0.5 MPa) indicates more consistent results, while the KOH-treated mix showed slightly higher variability (1.4 MPa).
These results suggest that the synthetic concrete pore solution, composed of NaOH, KOH, and Ca(OH)
2, is more effective for treating CSF compared to solutions containing solely NaOH or KOH. This is due to the unique chemical composition of SCPS, where the calcium ions (Ca
2+) play a crucial role in treating biofibers by forming stable complexes with pectin, a major component of many natural fibers [
50]. This interaction, known as the “egg-box” model, enhances the adsorption of calcium onto the fiber surface, which can enhance the mechanical interlocking between fibers and the cement matrix, thus improving the mechanical properties of specimens. Studies have shown that while NaOH and KOH individually improve tensile strength and remove impurities, the addition of Ca(OH)
2 provides a unique advantage by enhancing the fiber’s structural integrity and interaction with the cement matrix [
51,
52].
3.3. Performance Evaluation of Corncrete vs. CMU
3.3.1. Compressive Strength
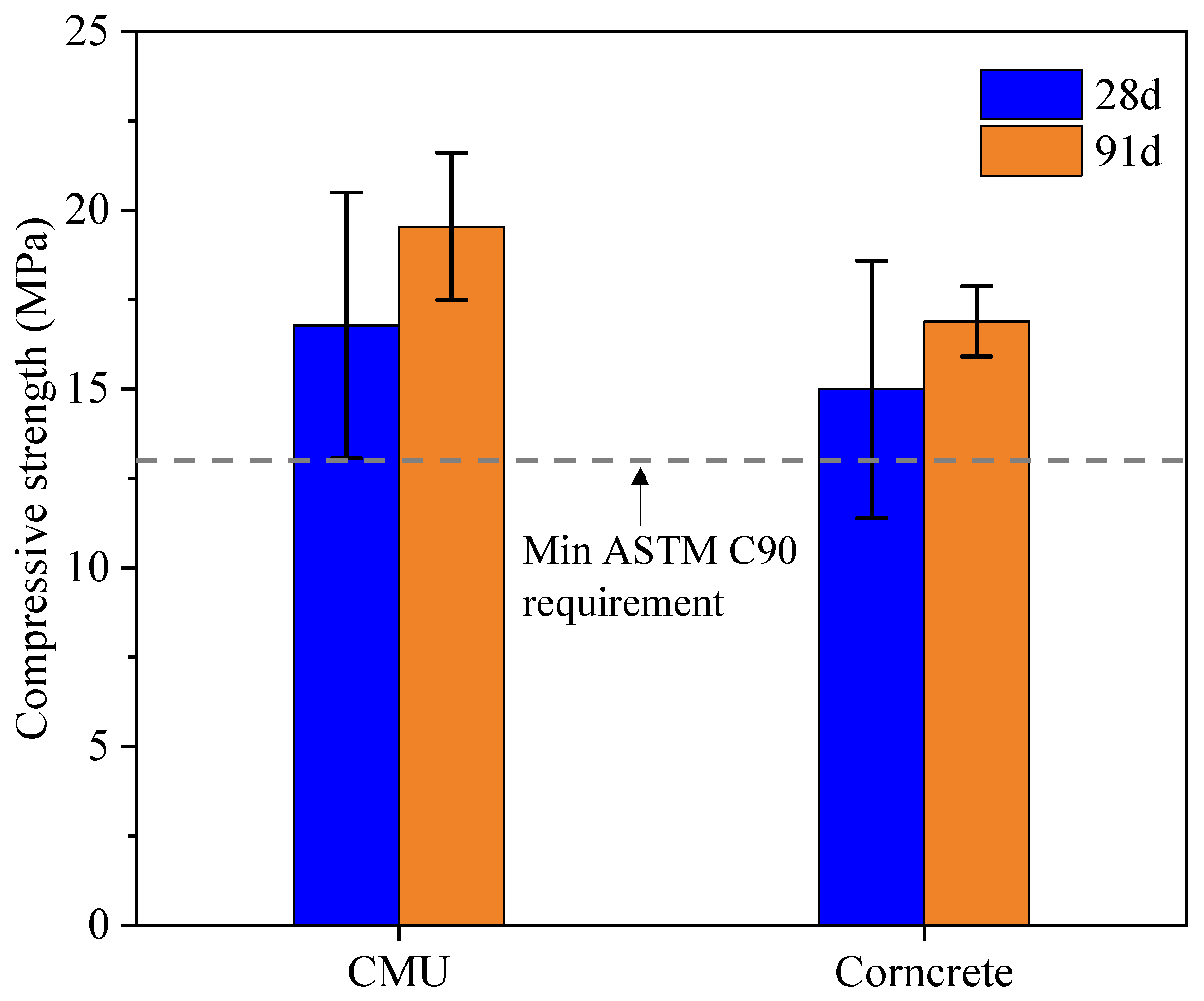
Figure 5 shows the compressive strength results of CMU and Corncrete specimens at 28 days and 91 days. The dashed line in this figure represents the minimum strength threshold required by ASTM C90. At 28 days, CMU specimens achieved a compressive strength of approximately 17 MPa, while Corncrete specimens exhibited slightly lower strength, around 15 MPa. By 91 days, both materials experienced a noticeable increase in strength, with CMU reaching 19.5 MPa and Corncrete achieving 16.9 MPa. This increase is typical as hydration progresses, and both materials continue to develop strength over time. The lower compressive strength observed in Corncrete specimens in this study differs from the findings of Shakouri et al. [
9], who reported strength gains with acid-treated CSA in the concrete used in their study. The underlying reason behind this discrepancy is that the mix used in Shakouri et al. [
9] had a higher cement content and a lower water-to-cement ratio, creating more favorable conditions for CSA reactivity. In contrast, the Corncrete mix in this study had low workability with reduced cement and no coarse aggregate, which may limit pozzolanic activity. The addition of corn straw fiber may also contribute to strength reduction, as poor dispersion in dry mixes can cause fiber agglomeration and weak zones in the matrix.
Although CMU specimens in this study showed higher compressive strength at 28 and 91 days, the overlapping error bars suggest that the difference is not statistically significant. This interpretation was confirmed by Welch’s t-tests, which showed no significant difference between Corncrete and control CMUs at 28 days (p = 0.53) or 91 days (p = 0.11). These results indicate that Corncrete, which contains 1% CSF by volume and 10% cement replacement with CSA, performed similarly to traditional CMU in terms of strength, although the 91-day trend suggests potential long-term improvement that warrants further investigation with larger sample sizes.
3.3.2. Physical Properties
Table 10 shows the physical properties of specimens made with CMU and Corncrete mixtures. Corncrete had a bulk density of 1950 kg/m3, which is 4.41% lower than that of CMU at 2040 kg/m3. Bulk density is defined as the mass of the material divided by its total volume, including both solids and connected pore spaces. In contrast, the apparent density of Corncrete was 1.2 percent higher than that of CMU. Apparent density refers to the mass of the solid material divided by its volume, excluding any open or connected pores. Accordingly, it can be concluded that the solid phases in Corncrete are denser than those in CMU. The combination of lower bulk density and higher apparent density suggests that Corncrete contains more internal voids and connected pore spaces. This interpretation is further supported by the higher volume of permeable voids reported for Corncrete in
Table 10.
According to
Table 10, Corncrete exhibits an increase of 7.99% in water absorption. This is primarily due to the inclusion of 1% corn fiber by volume, which has a water absorption capacity of 17.2%. While the fibers may have been partially saturated after 91 days of curing in limewater, their presence still significantly increases the porosity of Corncrete specimens. These fibers create additional pathways for water to infiltrate the matrix, contributing to the higher absorption even if the fibers themselves are saturated.
Corncrete, with a bulk density of 1950 kg/m3, is considered a medium-weight unit (1680–2000 kg/m3 as per ASTM C90). In contrast, the CMU, with a bulk density of 2040 kg/m3, is classified as a normal-weight unit. For water absorption, Corncrete has an absorption value of 195.86 kg/m3, which is below the maximum limit of 240 kg/m3 for medium-weight units, while the CMU mix, with 183.74 kg/m3, complies with the maximum 208 kg/m3 limit for normal-weight units. Thus, both specimens satisfy ASTM C90 requirements for their respective density classifications and water absorption limits.
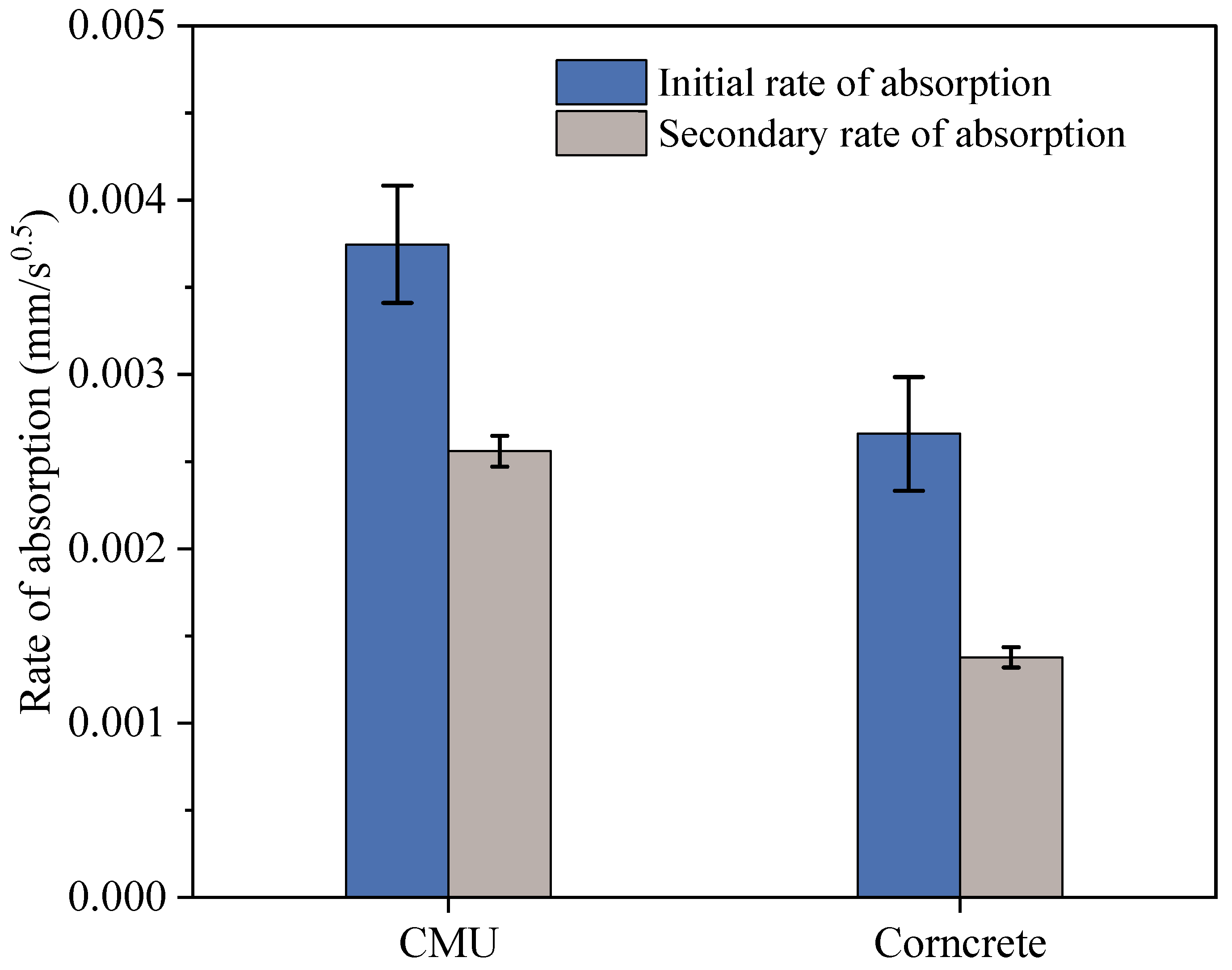
3.3.3. Rate of Absorption of Water
Figure 6 presents the initial and secondary rates of water absorption for CMU and Corncrete specimens, with the initial rate corresponding to the first six hours and the secondary rate representing the average absorption over the remaining nine days, as per ASTM C1585. The results show that CMU has both a higher initial and secondary absorption rate compared to Corncrete. Specifically, the initial rate of absorption for CMU is 0.00375 mm/s0.5, while Corncrete has a lower initial rate of 0.002556 mm/s0.5, representing a 32% lower absorption rate for Corncrete in the first six hours. Welch’s
t-test confirmed that this difference was statistically significant (
p = 0.010). Similarly, during the secondary absorption phase, CMU absorbs water at a rate of 0.00266 mm/s0.5, whereas Corncrete has a secondary rate of 0.00138 mm/s0.5, which is 48% lower than CMU’s rate during the remaining nine days. This difference was highly significant according to Welch’s
t-test (
p < 0.001).
Both materials exhibit a reduction in the rate of absorption over time. This is typical of concrete [
53], where the initial rate is higher due to the quick filling of surface and near-surface pores. As time progresses and more water is absorbed, the rate slows because the deeper pores fill more slowly due to reduced capillary action and less available pore space.
It is important to note that while Corncrete has a higher volume of permeable voids (22.85%) than CMU (18.3%), this does not necessarily mean it absorbs water faster. Studies on biofiber-reinforced concrete show that adding biofibers increases the total amount of pores, but the shape and path of those pores become more tortuous and less connected [
54]. This slows down how quickly water can move through the material. For example, Jin et al. [
55] investigated concrete incorporating treated corn straw fibers and found that although the total porosity increased, the internal structure became more compact and less connected. This was attributed to the rough surface and better bonding of the treated fibers, which created more tortuous pathways and reduced water mobility. Similarly, Jiang et al. [
56] observed that wheat straw fibers, when untreated, absorbed large amounts of water. However, after modification, the fibers became more hydrophobic and less permeable, which limited the rate of water penetration into the composite.
3.3.4. Bulk Electrical Resistivity
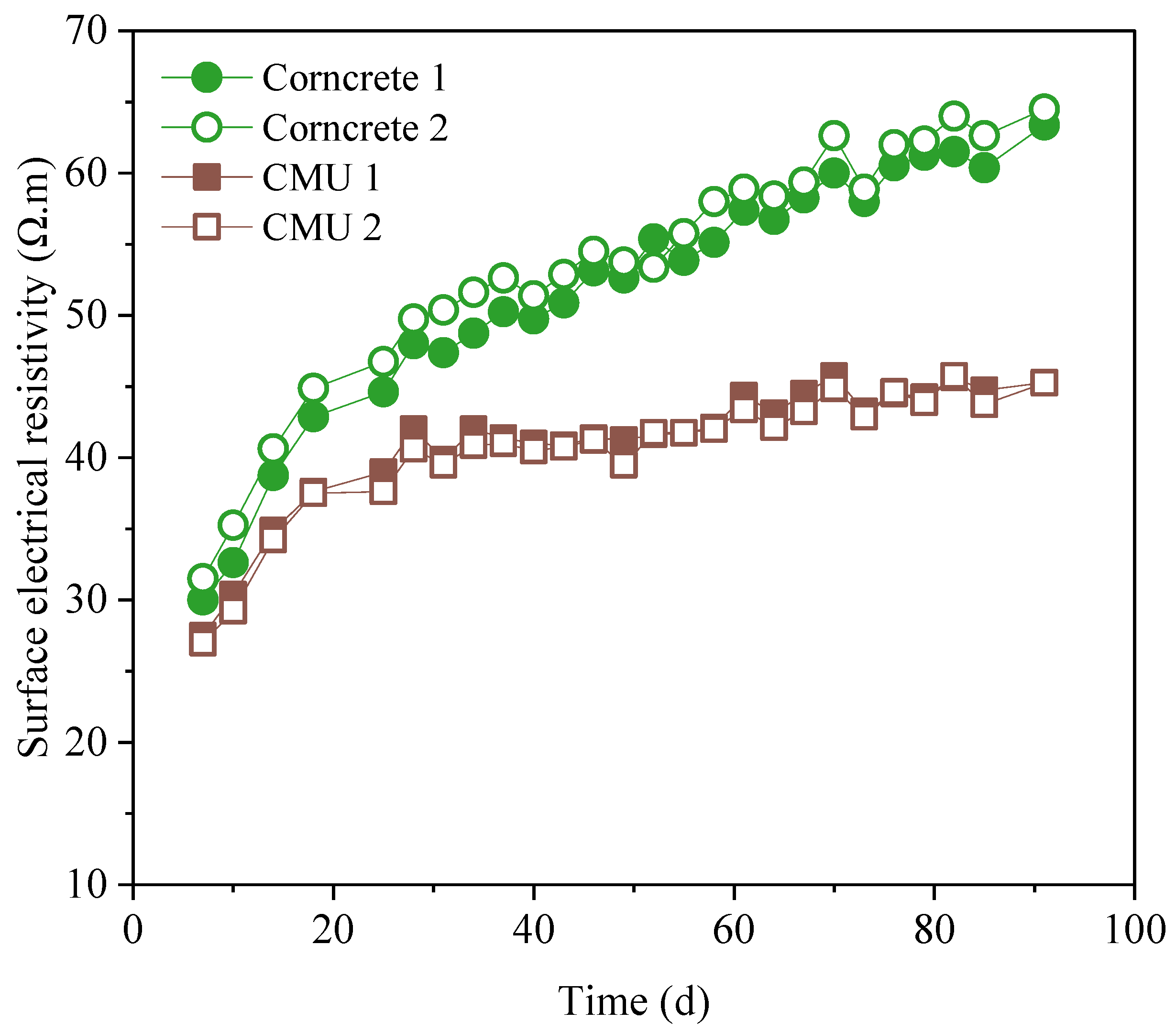
Figure 7 illustrates the progression of bulk electrical resistivity values for each mix throughout the testing period. Bulk electrical resistivity is a crucial indicator of a concrete’s durability, particularly its resistance to ion penetration, which is often associated with the concrete’s permeability and potential for reinforcement corrosion [
57,
58]. This is particularly important in reinforced CMUs, where lower permeability helps prevent the ingress of corrosive agents, such as chlorides, that can lead to reinforcement corrosion. The bulk electrical resistivity trends observed in
Figure 7 show that Corncrete specimens consistently exhibited higher resistivity values compared to CMU specimens over time. Both Corncrete and CMU exhibited an increasing resistivity trend as curing progresses, which is typical as the concrete continues to hydrate, reducing porosity and limiting the movement of ions through the material [
59]. However, Corncrete maintained a distinct gap in resistivity values, with Corncrete 1 and 2 reaching above 40 Ω·m by 91 days, while CMU specimens peaked around 30 Ω·m.
In general, concrete resistivity is strongly influenced by the ionic concentration of the pore solution, porosity, and the tortuosity of the pore network, with lower ion concentrations and more tortuous pore paths leading to higher resistivity values [
60,
61,
62]. While Corncrete shows a higher total water absorption (9.74%) compared to CMU (9.02%) as reported in
Table 10, this alone does not imply lower resistivity. In fact, as discussed in the previous section, the slower rate of water absorption observed in Corncrete suggests it is likely due to a more tortuous pore network, where water must follow longer and more complex paths to move through the material. According to established models of electrical conduction in porous media, including Archie’s law [
63], resistivity increases when pore paths are more tortuous, even if overall porosity is higher. This explains why Corncrete, despite its greater void volume, exhibits higher resistivity: its pore structure presents greater resistance to ion transport due to the complexity of internal pathways. Another reason that may help explain the higher electrical resistivity in Corncrete specimens is that the pozzolanic activity of CSA consumes calcium hydroxide and reduces the ionic concentration of calcium and other alkalis in the pore solution [
9,
11,
16,
61]. This lower ion content results in a less conductive pore solution, which increases resistivity [
64].
Figure 8 illustrates the progression of surface electrical resistivity values for each mix throughout the testing period. Similar to the bulk resistivity results (
Figure 7), Corncrete consistently demonstrates higher surface resistivity values compared to CMU. By the end of the 91-day testing period, Corncrete reaches values above 60 Ω·m, while CMU stays around 40 Ω·m.
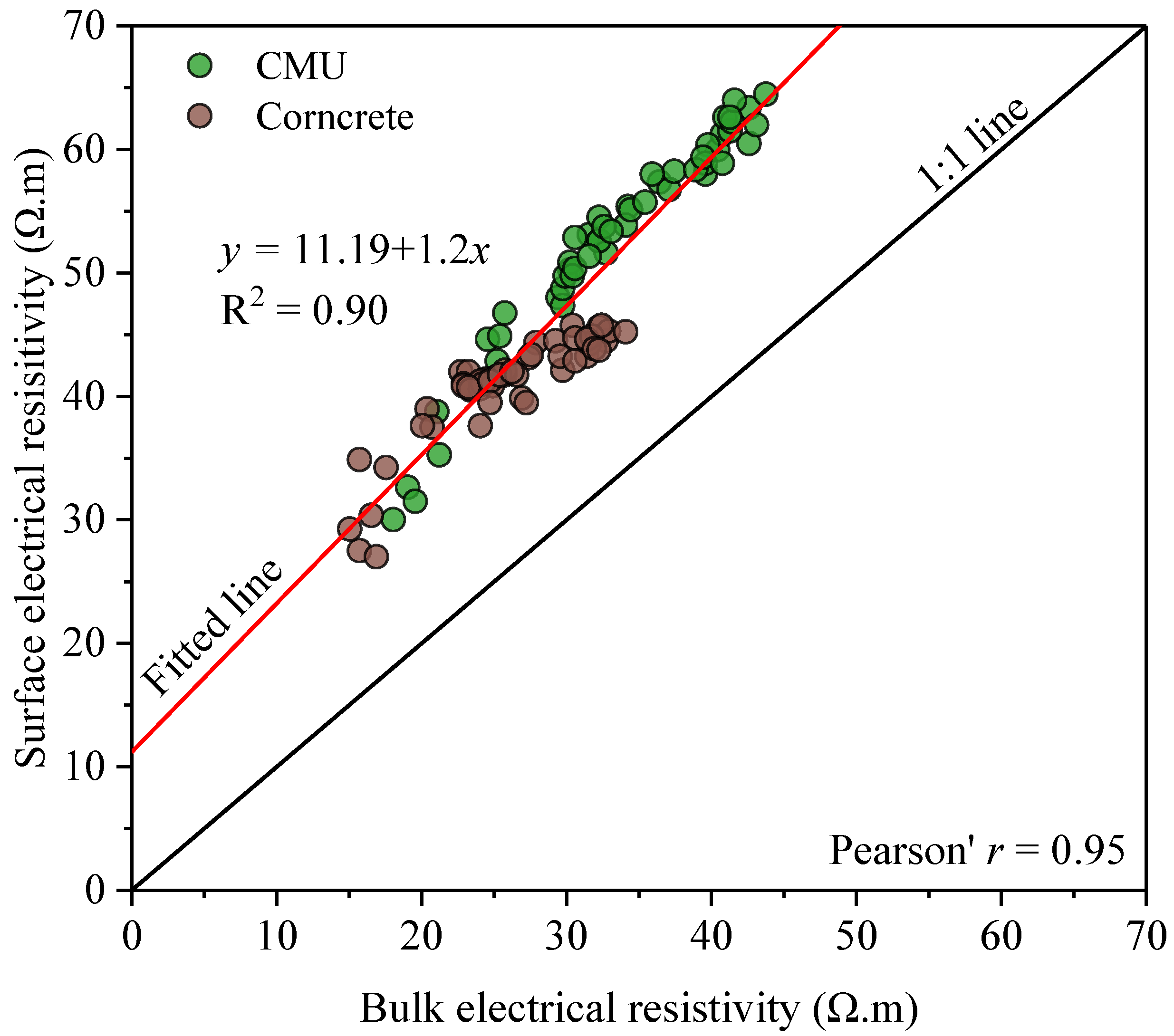
Figure 9 shows the relationship between the measured bulk and surface resistivity of the specimens. The results show a strong positive correlation with a Pearson’s
r of 0.95 and an R
2 of 0.90, which is in agreement with other published works [
65,
66].
The regression equation in
Figure 9 indicates that surface resistivity is generally higher than bulk resistivity by a factor of approximately 1.2, which is typical as the surface tends to hydrate more quickly and densify sooner than the bulk of concrete [
65].
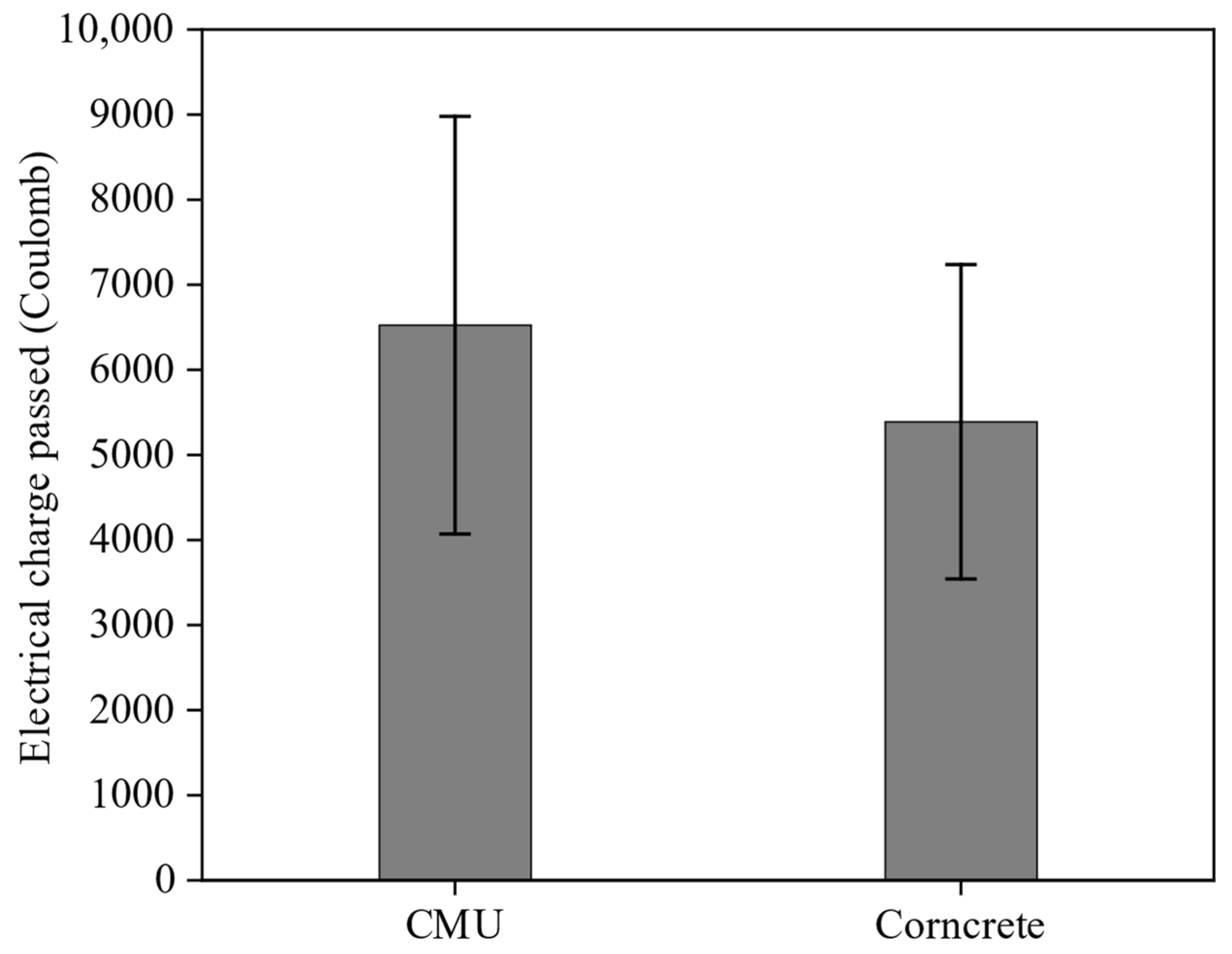
3.3.5. RCPT
Figure 10 shows the results of the RCPT tests, which measure how well concrete resists chloride ion penetration by recording the total electrical charge that passes through each specimen over a six-hour period. The results show that Corncrete had a lower charge passed of 5389 Coulombs, which is 13.7% less than the 6245.5 Coulombs recorded for CMU. This suggests that Corncrete has slightly better resistance to chloride ion penetration. Welch’s
t-test, however, indicated that the difference was not statistically significant (
p = 0.51). According to ASTM C1202, both materials fall into the category of high chloride ion penetrability, as both values exceed 4000 Coulombs.
The RCPT results are consistent with the water absorption and bulk resistivity findings shown in
Figure 5 and
Figure 6. Corncrete exhibited a lower rate of water absorption during both the initial and secondary phases, along with a higher electrical bulk resistivity compared to CMU. These characteristics suggest that Corncrete is more effective at limiting the movement of chlorides through its internal structure, even though the reduction in charge passed during the RCPT test was not statistically significant.
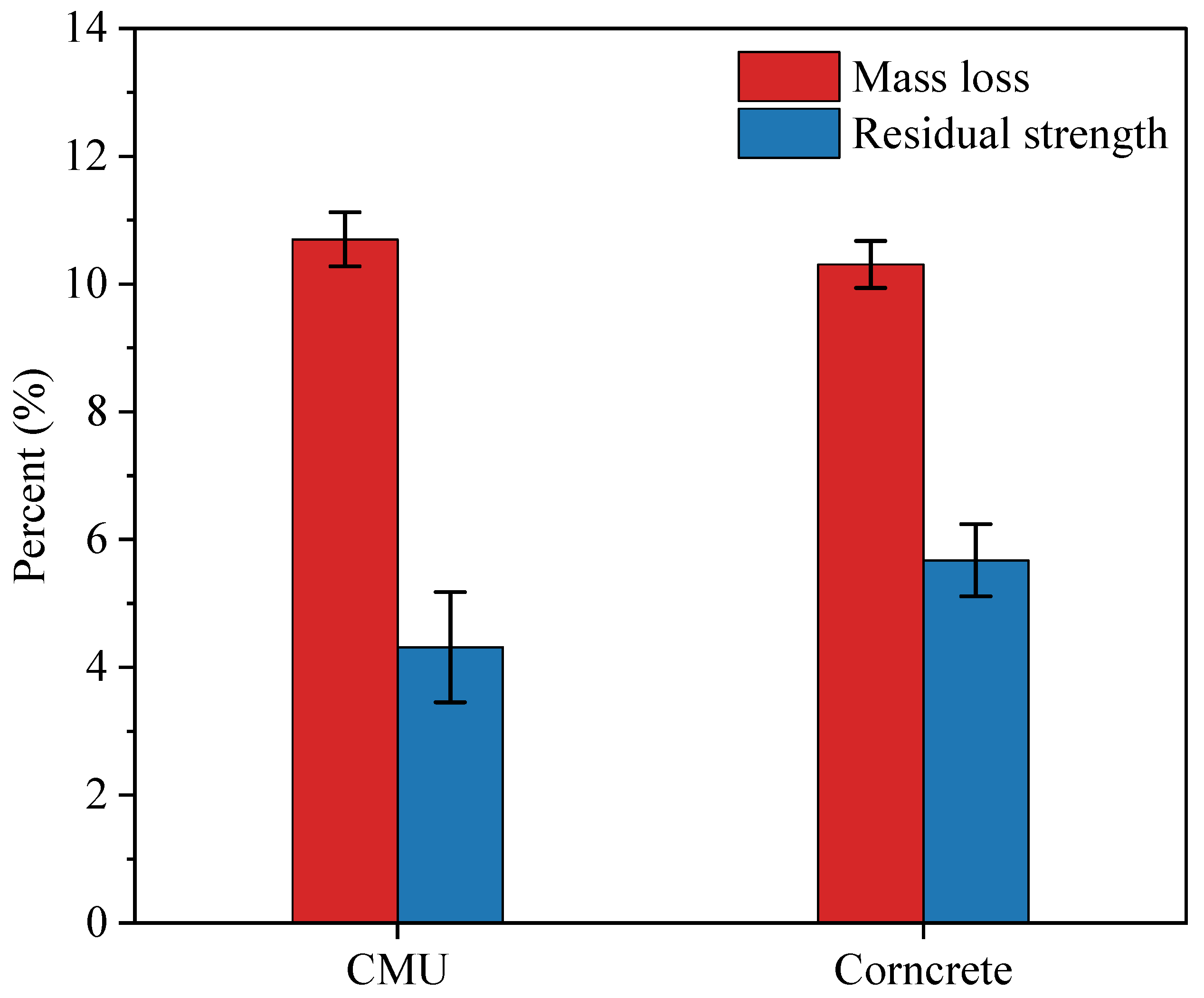
3.3.6. Mass Loss and Residual Strength
Figure 11 illustrates the percent mass loss and residual strength of CMU and Corncrete specimens after exposure to elevated temperatures in accordance with ASTM E119. The results show that, at the end of the experiment, CMU specimens experienced a mass loss of 10.7%, while Corncrete specimens showed a slightly lower mass loss of 10.39%. Welch’s
t-test indicated that this difference was not statistically significant (
p = 0.24), suggesting that both materials performed similarly in terms of mass loss. In terms of residual strength, CMU specimens retained an average of 4.38% of their original strength, while Corncrete specimens retained 5.78%. While Corncrete shows marginally better performance, the overlapping 95% confidence intervals and Welch’s
t-test (
p = 0.071) indicate that the difference is not statistically significant. Nonetheless, the trend suggests that Corncrete may provide improved residual strength under thermal exposure, which warrants further investigation with larger sample sizes.
Both materials experienced significant mass and strength loss, primarily due to the decomposition of the calcium silicate hydrate phase, which begins to break down at 180–200 °C and undergoes nearly complete decomposition at 700 °C and above. The degradation of C-S-H severely weakens the structural integrity of the concrete matrix, which explains the observed strength loss.
Although Corncrete contains only one percent corn fiber by volume, it is important to consider how these fibers behave under elevated temperatures. As temperature rises, the components of natural fibers degrade at different stages. Hemicellulose is the first to break down, typically between 200 and 300 degrees Celsius, due to its low thermal stability. Cellulose follows, decomposing between 300 and 400 degrees Celsius, while lignin, which has a more complex and thermally stable structure, degrades more gradually over a wider temperature range, often extending beyond 400 degrees Celsius [
67]. This stepwise degradation causes the fibers to lose their structure, releasing gases and forming voids [
66,
68]. These voids, especially around the fiber–paste interface, contribute to microcracking and reduce the integrity of the surrounding cement matrix. Simultaneously, the cement paste undergoes chemical changes, with calcium silicate hydrate and portlandite beginning to decompose at temperatures above 400 degrees Celsius [
69]. By the time the material reaches 1200 degrees Celsius, both Corncrete and conventional CMU experience extensive degradation of their internal structure. At this stage, the fibers have fully decomposed and no longer contribute to strength, which explains the comparable performance of the two materials under extreme thermal exposure.
4. Key Stakeholders Feedback, Limitations, and Future Opportunities
One of the key advantages of Corncrete over conventional CMUs is partial substitution of cement with CSA, which requires a relatively lower production temperature than cement clinker. To better understand the feasibility of commercializing this product and assessing the corn stover supply chain in the U.S., the authors performed interviews and had several correspondences with key stakeholders from commodity boards in Colorado and Nebraska. These discussions focused on both the agricultural and environmental challenges associated with producing CSA and CSF, which need to be addressed before moving forward with the commercialization of this product.
One key limitation raised by several board members was to the environmental impacts of the pretreatment processes involved in CSA and CSF production. One major concern was that that the process of burning or incinerating corn stover to produce CSA and discarding acid waste may present a potential conflict with EPA air quality and environmental regulations [
70]. Others raised a valid concern that the pretreatment methods, such as acid-soaking, drying, burning, and neutralizing acid, can be complex and place an extra burden on farmers, with little benefit if they have to perform them on their own. On the agricultural side, some commodity board members expressed concern that removing corn stover for CSA production could negatively impact soil health in areas where the harvest is not excessive. This was particularly problematic given the current role of corn stover in many farms as a natural soil amendment. A few stakeholders were concerned that open burning of corn stover could negatively increase farmers’ carbon intensity (CI) scores. In many regions, the CI score measures the greenhouse gas emissions associated with producing a crop like corn, with lower scores indicating more climate-friendly practices [
71,
72]. For example, corn farmers with low CI scores in the U.S. can benefit from premium payments from biofuel producers who benefit from clean fuel incentives like the 45Z tax credit [
73].
Beyond agricultural and environmental issues, the economic viability of Corncrete production presents significant challenges alongside the environmental and agricultural concerns. The necessary pretreatment processes, including acid-soaking, drying, and burning, grinding, and neutralizing the acid solution demand substantial energy inputs that drive up manufacturing costs. Without demonstrating clear cost savings compared to traditional concrete blocks, Corncrete may struggle to gain traction in competitive construction markets. However, the growing wave of sustainability legislation could provide Corncrete with a crucial market opportunity. Policies like the Buy Clean Act [
74], which is already adopted in multiple states in the U.S., specifically favor construction materials with lower carbon footprints. Since Corncrete replaces part of the cement content with agricultural waste, resulting in significantly reduced CO
2 emissions, it fits perfectly with these new environmental mandates. The Act requires state-funded projects to prioritize low-carbon options, which might give Corncrete an advantage in public infrastructure work.
Making Corncrete commercially viable will require focused improvements in two key areas. The first involves developing more environmentally sustainable processing methods. Investments in cleaner pretreatment technologies could significantly reduce emissions and eliminate current regulatory concerns. Alternative approaches that avoid traditional burning and acid treatments would also help meet EPA standards while maintaining production efficiency.
The second challenge centers on creating viable economic incentives for agricultural partners. Since corn stover provides valuable soil benefits, farmers need compelling reasons to participate in feedstock collection programs. Potential solutions could include market-based compensation models such as carbon credit systems or direct subsidies to offset potential yield impacts. Such mechanisms would help balance agricultural priorities with industrial material needs.
5. Incremental Cost Analysis
To evaluate the economic implications of substituting 10% of cement with CSA in CMU production, a preliminary cost analysis was conducted based on standard CMU composition, raw material prices, and reagent requirements specific to the preparation methods employed in this study. A density of 1900 kg·m−3 was assumed for medium-weight CMUs, consistent with reported ranges. Cement content was estimated at ~12% of total mass, equivalent to 228 kg per cubic meter. In the Corncrete mixture, 10% of this cement (≈22.8 kg) was replaced with CSA, corresponding to ~21.5 kg CSA·m−3 in the final mix.
The U.S. Geological Survey recently reported the average mill value of Portland cement at USD 140–150 per metric ton [
75]. For comparison, fly ash typically retails for USD 25–60 per ton [
76]. Corn stover feedstock delivered to a processing facility is more costly, with values of USD 60–80 per ton reported in techno-economic assessments [
77,
78]. Corn stover yields only 4.8–7.3% ash [
79], which implies that large quantities of feedstock are required to generate sufficient CSA. Based on the 5.5% yield achieved in this study after cyclonic ashing and thermal beneficiation, approximately 391 kg of stover are needed per cubic meter of Corncrete.
Bulk industrial reagent prices needed for CSA and CSF preparation were assumed as follows: citric acid, USD 810·t
−1 [
80]; sodium bicarbonate, USD 338–369·t
−1 [
81]; sodium hydroxide, USD 368–788·t
−1 [
82,
83]; potassium hydroxide, USD 563·t
−1 [
84]; and calcium hydroxide, USD 125·t
−1 [
85]. Corn stover fibers were incorporated at 1% by volume (7.2 kg·m
−3), but their contribution to reagent cost was negligible compared to citric acid treatment.
Transportation was also included, as logistics represent 10–20% of construction material costs [
86] and ~28% of U.S. greenhouse gas emissions [
87]. Trucking was priced at USD 0.11 per ton-mile, with sensitivity between 30 and 350 miles.
In addition to raw material and reagent costs, the mechanical and thermal processing steps required to produce CSA and CSF were also considered. Cutting the stalks into fibers and classifying them through sieving was estimated to consume ~0.3–0.5 kWh·kg−1 of fiber, equivalent to 2–4 kWh per cubic meter of Corncrete, or approximately USD 0.20–0.40 at an electricity price of USD 0.10·kWh−1. Ball milling of CSA, necessary to reduce the ash to a median size of ~30 µm, typically consumes 0.5–1 kWh·kg−1; for the 21.5 kg CSA used per cubic meter, this represents 11–22 kWh, or about USD 1.10–2.20. The drying step, carried out at 100 °C until constant mass, was estimated to require 2–3 MJ·kg−1 of water removed; for 391 kg stover at 10% moisture, this corresponds to 80–120 MJ (22–33 kWh), costing roughly USD 2–3 per cubic meter. Ashing in a cyclonic furnace followed by thermal polishing at 550 °C adds the largest single energy burden, approximated at 2–3 MJ·kg−1 stover, or 780–1170 MJ per cubic meter, equivalent to USD 8–18 even at efficient industrial energy rates of USD 0.01–0.02·MJ−1. Finally, water consumption during acid-soaking is significant, as the process requires 10 L per kilogram of stover, totaling ~3.9 m3 of water per cubic meter of Corncrete. At municipal rates of USD 1–2 per m3, this translates into an additional USD 4–8 per cubic meter.
The incremental cost of Corncrete relative to CMU, ∆
Cnet, was calculated as
where
Ccem,saved= is the cement cost saved (10% replacement, mrep = 22.8 kg.m−3, Pc = USD 160 t−1);
Cstover = is the cost of corn stove feedstock (mCSA = 21.5 kg CSA·m−3, yield Y = 0.055, Pstover = USD 60 t−1);
Ccit = is the cost of citric acid (0.1 M, 10 L·kg−1 soak, Mcit = 192.12 g·mol−1, Pcit = USD 810 t−1);
is the neutralization cost with sodium bicarbonate ( = 84.01·mol−1, = USD 338–369 t−1);
CSCPS = is the pretreatment solution cost for fibers (72 L; NaOH, KOH, Ca(OH)2 at industrial bulk prices);
Ctransport = is the stover transport cost (80 km, 0.068 USD ⋅t−1⋅km−1).
Processing cost were computed as follows:
Ccut = EcutPelec is cutting/sieving energy cost (Ecut = 2–4 kWh·m−3);
Cmill = EmillPelec is ball milling cost (Emill = 0.5–1 kWh·kg−1 CSA, 21.5 kg·m−3);
Cdry = EdryPelec is drying cost (Edry = 22–33 kWh·m−3);
Cash = QashPth is ashing cost (Qash = 780–1170 MJ·m−3 at Pth = USD 0.01–0.02 MJ−1);
Cwater = VwPw is soaking water cost (Vw = 3.9 m3·m−3 at Pw = USD 1–2·m−3).
The incremental cost of Corncrete production in Equation (7) was normalized by the volume of concrete per block, in this case 0.0087 m3).
Table 11 outlines how the additional cost of producing Corncrete compares with conventional CMU under three boundary conditions that capture the uncertainty in raw material prices and processing requirements. In the most optimistic scenario, identified as the low case, the calculations assume cheaper reagent prices, lower electricity demands for chopping, grinding, and drying, a reduced thermal load for ashing, and inexpensive water rates. By contrast, the high case reflects the opposite extreme, where reagents are priced at the upper end of reported ranges, equipment consumes more electricity, the ashing process requires greater fuel input, and water charges are doubled. The mid-case represents a balanced estimate that lies between these two limits, relying on average market prices and intermediate energy and water use. Taken together, these scenarios indicate that the added cost of Corncrete falls between roughly USD 132 and USD 157 per cubic meter of material, or about USD 1.15–1.37 per standard block.
The analysis makes clear that the main contributors to this premium are the acid pretreatment and neutralization procedures, and the high energy intensity of the ashing step. This suggests that economic viability will hinge on strategies that lower chemical consumption and energy use, or on sourcing CSA from alternative streams such as biomass energy residues, where the ash is already available as a byproduct.
6. Conclusions
This study comprehensively evaluated the performance of Corncrete, a sustainable alternative to conventional concrete masonry units, through systematic assessment of its physical, mechanical, durability, and thermal properties. The results demonstrate that partial replacement of cement with corn stover ash and incorporation of alkalinized corn stover fibers yield a material with comparable structural performance to traditional CMUs, while offering distinct advantages in terms of sustainability and durability.
The results indicated that Corncrete meets ASTM specifications for medium-weight units, exhibiting marginally lower bulk density and higher porosity. Mechanical test results showed that the compressive strength of Corncrete and CMU blocks was not statistically different, albeit Corncrete had, on average, 13.3% lower compressive strength. Notably, Corncrete demonstrated enhanced durability, with significantly higher bulk and surface electrical resistivities and reduced chloride permeability. Thermal resistance testing revealed comparable fire performance, with both materials exhibiting similar mass loss and residual strength after exposure to extreme temperatures (1200 °C).
Alongside testing physical and durability properties, this study also examined how Corncrete compares to standard CMUs in terms of cost. Preliminary analysis shows that the current laboratory process, which involves soaking in citric acid, neutralizing with sodium bicarbonate, and subjecting the material to high-temperature ashing, adds a significant premium. The higher cost arises not from an inherent limitation of Corncrete but from the way the ash is produced at this stage. In practical terms, the chemical pretreatment and energy required for ashing make the blocks more expensive than conventional units. This represents a process challenge rather than a material drawback. If CSA can be obtained as a waste ash from existing operations, or if the pretreatment steps are simplified and reliance on costly reagents is reduced, the additional cost could be substantially lowered.
The current CSA preparation route also raises environmental concerns linked to emissions and energy, which could conflict with air quality regulations. In addition, the agricultural implications of large-scale stover removal require careful consideration, since corn stover contributes to soil health and carbon sequestration. Future research should therefore focus on optimizing CSA production methods to reduce both cost and environmental impacts, developing sustainable harvesting protocols that preserve soil quality, incorporating durability assessments such as freeze–thaw resistance, accelerated aging, and chemical attack, and validating long-term performance through comprehensive field studies.
7. Patents
Shakouri, M. Agricultural Waste Ash as Cementitious Material and Methods of Making the Same. U.S. Patent Application No. 18/096,509. Publication date: 13 July 2023. This patent covers processes for utilizing agricultural waste ash as a cementitious material and provides methods for its preparation and application in concrete systems. The innovation directly relates to the present study, as it underpins the development of alternative cementitious binders from renewable waste sources and demonstrates the potential for practical implementation.