1. Introduction
Construction project delays are commonplace and often lead to disputes and serious financial losses [
1], accentuating the need to understand and apportion their causes and impacts. Forensic Delay Analysis (FDA) has emerged as a specialism that focuses on identifying, analysing, and apportioning the causes and impacts of project delays, and then presenting evidence in the resulting disputes [
2]. But despite its importance, existing FDA workflows have limitations that affect the accuracy, cost, promptness, and reliability of the delay analysis outcomes, which have been described by Gibbs [
3] as relying on manual processes and static data structures that are time-consuming, error-prone, and limited in their ability to capture the dynamic complexities of construction projects. They employ methodologies that are often lengthy, costly, complex, and challenging to communicate, and they rely heavily on judgement, interpretation, and selective presentation of findings [
3]. A paper by Grzeszczyk et al. [
4] reveals the different analysis perspectives that pervade the FDA-related literature by identifying eight common themes. The theme of ‘project characteristics’ investigates differing project contexts (e.g., geographical, delivery-related). The ‘contractual or legal references’ theme is concerned with specific jurisdictions, case law, and forms of contract. The ‘reliability’ and ‘reported’ flaws themes overlap somewhat, identifying problems such as subjectivity, low credibility, and limited data. Two themes, ‘protocol preference’ and ‘schedule determinism’, relate to the value, use and shortcomings of procedural guidelines (in the case of the former) and scheduling techniques (in the case of the latter). In the final pair of themes, the ‘reported remedies’ grouping involves proposals for the enhancement of existing techniques and methods, while the ‘innovation’ theme is predominantly focused on advances in information technology.
In response, the work described here addresses the ‘reliability’, ‘reported flaws’ and ‘innovation’ analytical themes. Matters such as specific project characteristics, different jurisdictions or forms of contract, and the reliability or otherwise of scheduling techniques are excluded, though they will be reflected upon in the final section. Fundamentally, the work described here addresses the need to develop more efficient and accurate approaches to FDA processes and proposes an enhanced FDA workflow in which digital technologies are integrated.
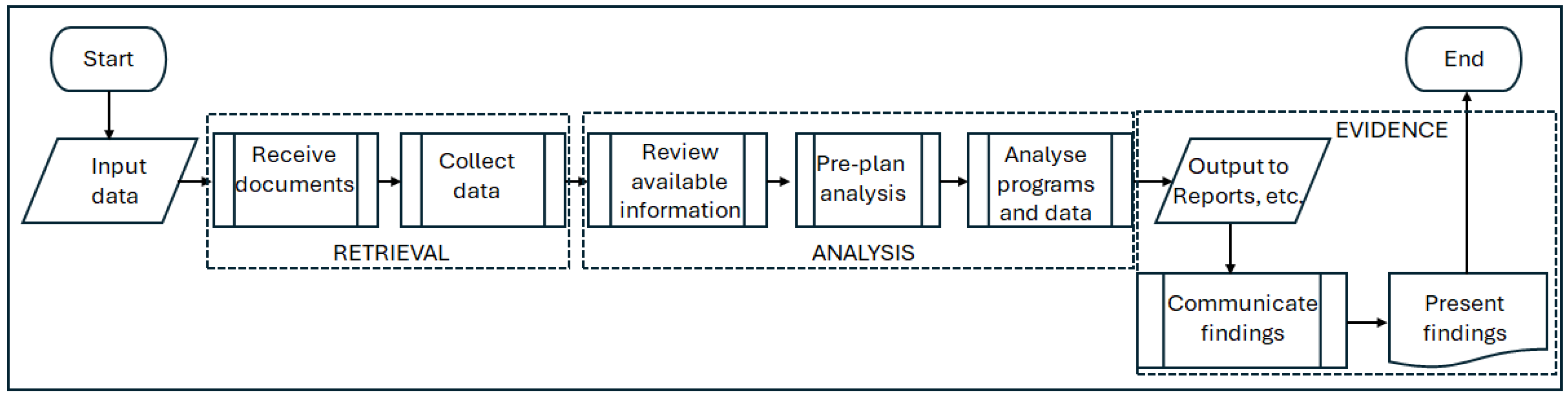
The foremost challenge to effective FDA lies in finding the evidence. The records at the disposal of the delay analyst are frequently incomplete and inadequate, consisting of unstructured and inconsistent data, thus making the identification, sourcing, retrieval, and validation of relevant information one of the most time-consuming and costly aspects of claims preparation [
5]. Alkass et al. [
6] estimated this to take around 70% of the analyst’s effort, a figure that has been empirically verified [
7]. Thus, the first serious major challenge to the FDA process concerns the quality, availability, and transparency of evidence, and addressing these data issues is vital for enhancing the overall effectiveness and reliability of the FDA process. The second challenge involves the lack of consensus on how to analyse the evidence. The choice from amongst a variety of delay analysis techniques, as described by Parry [
8], is complex and diverse, and it can be influenced by a variety of factors [
9]. Guidelines provided by professional organisations, such as the Association for the Advancement of Cost Engineering (AACE) [
10] in the USA and the UK’s Society of Construction Law (SCL) [
11], have added some clarity, but the proper application of any preferred methods remains highly dependent on the adequacy of the supporting information, which is in itself problematic. The third major challenge arises in the final stage of the process: communication of findings. As highlighted by Gibbs [
4], difficulties emerge when presenting complex evidence (e.g., programmes, specialist terminologies and technical information), particularly when a dispute has escalated as far as arbitration or litigation.
The advent of the digital technologies associated with Construction 4.0 presents new opportunities to enhance FDA practices. Construction 4.0, as derived from the term Industry 4.0 [
12], is described by Sawney et al. ([
13] p. 3) as an idea ‘based on a confluence of trends and technologies (both digital and physical) that promise to reshape the way that built environment assets are designed and constructed’. However, uptake by the construction sector has been limited [
14] and FDA has shown no apparent benefits. The key areas of interest in this context are database management systems (DBMSs), building information modelling (BIM) and the tools it enables, and the utilisation of artificial intelligence (AI) and game engines. DBMSs can improve data management and retrieval efficiency by structuring unorganised project records. BIM-based software tools can produce a digital representation of projects that incorporate various data sources. AI can automate data clustering, keyword extraction, and classification, improving the accuracy and speed of analysis and reducing human error. Game engines can provide advanced visualisation tools that can present analytical findings in an intuitive and interactive manner. By integrating these technologies, FDA can be transformed into a more efficient, accurate, and comprehensive process. These advances offer immense potential for enhancing FDA processes, thereby leading to improved efficiency and accuracy. This study proposes the enhancement of FDA by leveraging emerging technologies. The approach involves integrating DBMSs, BIM, AI, and games engine technologies into the FDA process in order to streamline data collection, enable its seamless transfer through the process, improve the accuracy and efficiency of delay analysis, and enhance the communication of findings to project participants. A case study is presented to demonstrate the practical application of the proposed approach, highlighting its potential to transform FDA practices in the construction industry.
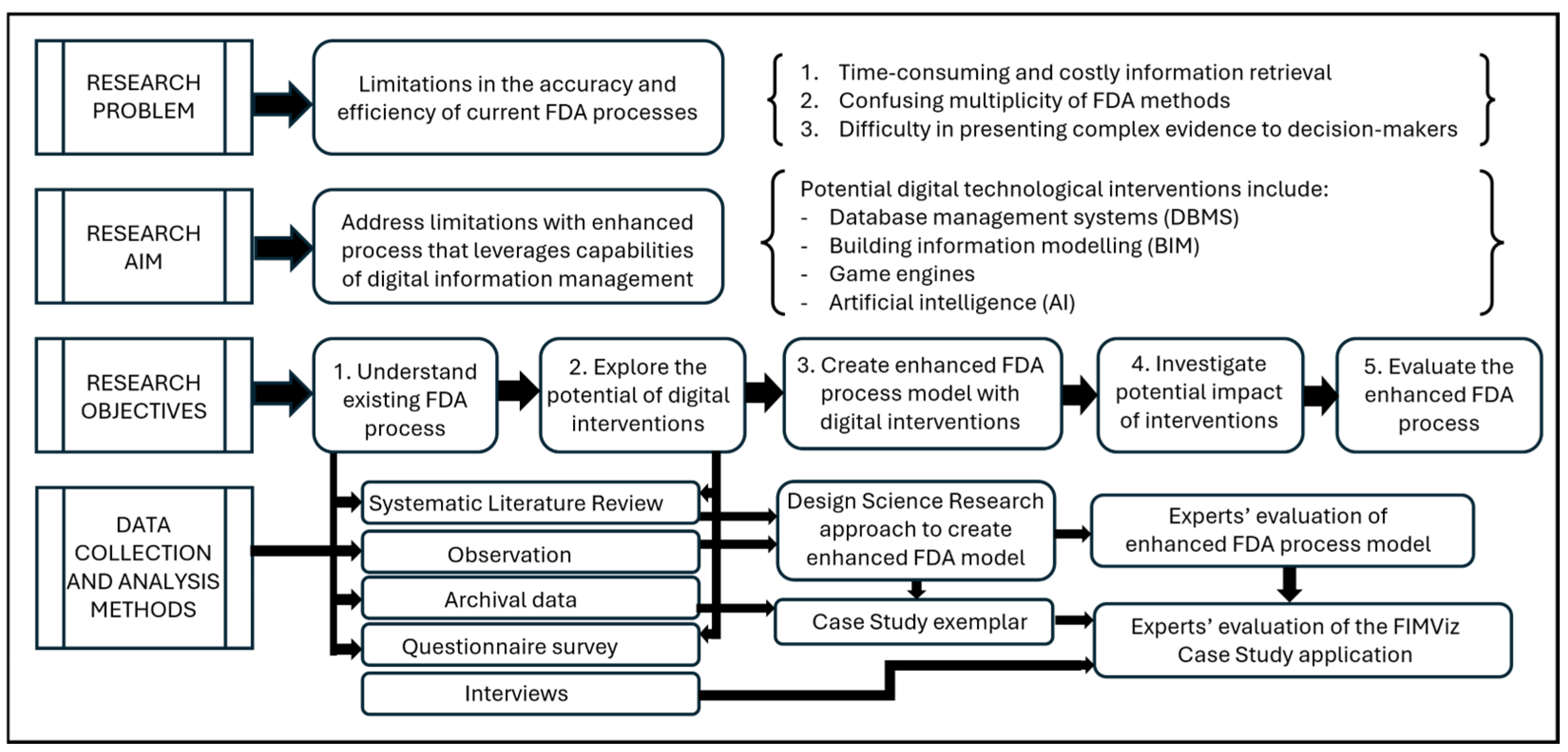
The remainder of this article comprises four sections.
Section 2 describes the research methodology adopted (as shown in
Figure 1).
Section 3 explains the development of an enhanced model resulting from the incorporation of digital technologies into the FDA process.
Section 4 describes the operationalisation of the model to produce the FIMViz prototype. Finally,
Section 5 reflects upon the evaluation of the enhanced FDA process model and the FIMViz prototype by FDA experts.
4. Operationalisation of the Model in the FIMViz Prototype
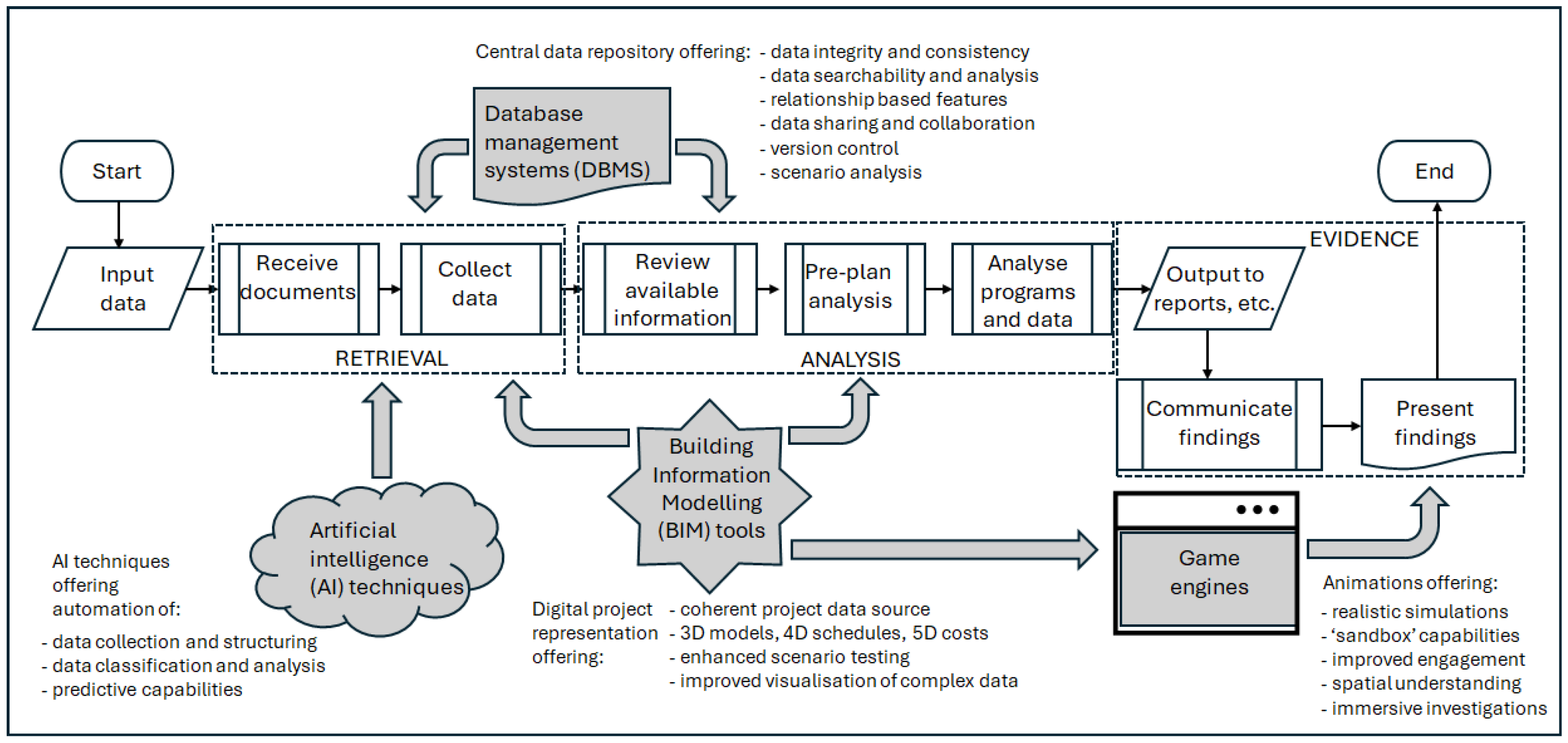
As described above, establishing the viability of incorporating advanced technical enhancements into the FDA process took place in two stages. The first involved the operationalisation of the framework shown in
Figure 4 to create the ‘Forensic Information Modelling Visualiser’ (FIMViz) prototype, which in turn would accept real-world project data, extracted from a contemporary construction project, to produce a case-based simulation. The second stage involved subjecting the enhanced FDA process model and the FIMViz prototype to expert evaluation. The FIMViz was developed to operationalise the data workflow of the enhanced FDA process model, to test the viability and integration of its innovative interventions, and to assess the impact of each.
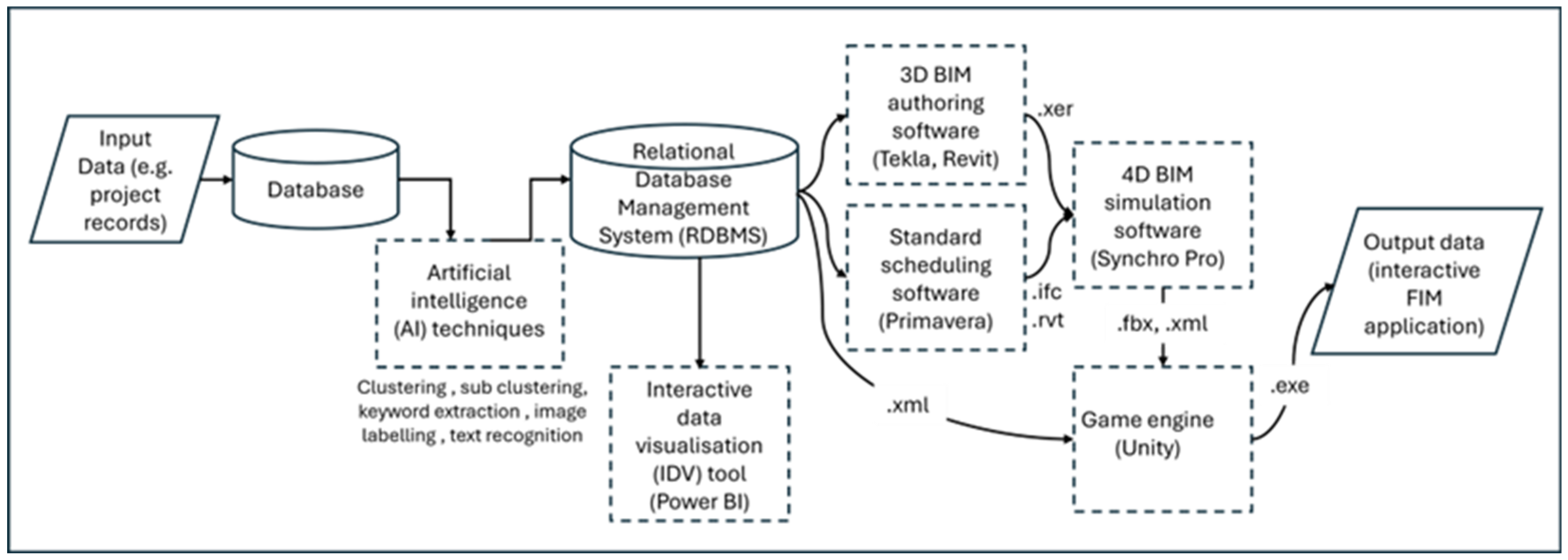
Figure 5 shows a simplified data workflow between the four technology-based interventions (DBMS, BIM, game engine, AI) in the enhanced FDA process and in the FIMViz prototype. The following sections deal with the operationalisation, within the FIMViz prototype, of the four digital interventions.
4.1. RDBMS Intervention
The RDBMS is used to establish the relationships between various project records. Key data, such as contract documents, construction progress records, and BIM data, are linked using identifiers like the task IDs and room numbers, which are then used to generate relational data for analysis. In cases where the client lacks an internal RDBMS or is unwilling to provide access, the delay analysts can manually create these relationships. In creating the FIMViz prototype, the RDBMS intervention involved manually linking project records from the client, as access to the client’s database was not available. This process was automated using AI techniques discussed in
Section 4.4. The RDBMS also facilitated the extraction of data into formats such as .ifc and .xer, which were used in the BIM and 4D simulations. Microsoft Access [
51] was selected as a data organising tool and also as a bridging tool to transfer data from one software tool to another. For instance, it was used to generate forensic data by using unique identifiers to establish relational links between the database and the BIM models. This relational data was eventually transferred to the game engine, as discussed in
Section 4.3. In summary, the RDBMS enhanced the FDA process by reducing the time spent on data retrieval and improving the accuracy of the analysis, ensuring that relevant data could be filtered and analysed efficiently. It should be noted that although the case is made here for integrating all four of the proposed digital technologies into the FDA process, each would offer significant benefits if adopted singly; for example, the adoption of an RDBMS could, on its own, enhance the effectiveness, efficiency, and collaborative potential of the FDA process.
4.2. BIM Intervention
On reviewing the BIM data available in the case study, deficiencies and missing data were found. Modelling was incomplete in some cases and relational links with project records were lacking. In creating the FIMViz simulation, this necessitated further effort to model additional elements and to build relational links with other project records. These inadequacies were to be expected, given the model authors’ unawareness of its potential use for FDA, and the suitability of the models can be expected to improve as familiarity increases. These additional model generations and modifications were carried out in the original BIM authoring software, namely Revit [
52] and Tekla Structures [
53]. For the 4D simulation aspect, Synchro 4D Pro [
54] was chosen due to the researchers’ familiarity with the software and its seamless data exchange capabilities with the BIM authoring software. The resulting 4D simulation can be output either as media files for presenting the findings in the form of images in reports and static presentations, or animated videos. Alternatively, the final output can be exported (in a ‘.fbx’ file format) to a game engine, as it was in this case to enable the interactive FIMViz application (see
Section 4.3). In summary, the BIM intervention in the FDA process was designed, and applied in the FIMViz tool, to facilitate analysis and to produce more meaningful outputs for the presentation of findings. The use of 4D simulations allow analysts to visualise delays more effectively, enabling more accurate and rapid analysis and more reliable and convincing communication.
4.3. Game Engine Intervention
A game engine tool allows users to interact with BIM data in a more immersive and interactive way. Additionally, the game engine’s
element query feature was used to fetch the metadata attached to the model elements and display it, providing users with easy access to important information about specific model elements. For this layer of the FDA intervention, the Unity game engine [
55] was chosen due to familiarity with the software itself, its scripting language, ‘C#’, and its integration with additional development tools. The process of integrating the game engine consisted of three main stages: (1) importing data into the game engine, (2) creating a FIMViz application in the game engine, and (3) enabling the export of the application as an output of this process. The following paragraphs describe these three stages.
Stage (1): Importing data into the game engine. Despite the attraction of the benefits offered by the game engine, the process of importing data from BIM authoring platforms is far from straightforward [
56]. The data to be imported originates from two sources, namely BIM files and the RDBMS. The most common approach for the transfer of BIM data across BIM authoring tools is to use the industry foundation class file format (.ifc). Although Unity does not allow direct import of this file format, the exchange is possible via plug-ins and intermediaries; for example, Bille et al. [
56] used 3ds Max [
57] as an intermediary bridging tool to Unity. In this present study, a more accessible approach (that did not require the use of 3ds Max or any plug-in) was used by employing Synchro 4D Pro [
54] as a transition tool between the BIM authoring software and the game engine. Here instead, ‘filmbox’ (.fbx) and ‘extensible markup language’ (.xml) files, which are natively supported by game engines, were used. The .fbx file format was used to store the geometric and animation data, and the .xml file format for the BIM metadata. Both files were linked within the Unity game engine using the unique identifiers that were common in both files. A variety of tools have recently been released that optimise the data exchange between BIM authoring tools and game engines. These remained untested in the current study, but their claims and potential have been discussed in [
58] (pp. 115–117).
Stage (2): Creating a FIMViz application in the game engine. This required the creation of a design map that includes all the necessary elements for analysis, such as scenes, canvas (i.e., user interface, UI), 3D elements, cameras, lights, controllers, players, components, and software development kits. Using the design map as a basis, individual elements of the tool were designed. This included creating different scenes, such as an opening scene, welcoming scene, main menu scene, interactive scenes with different views (e.g., baseline versus as-built comparison view), Gantt chart scenes, interactive information pop-up window scenes, and documentation scenes for direct access to the project database (e.g., for additional information about a specific element or delay). The user interface (UI) elements of each scene in the tool were also designed and linked with 3D elements, 4D simulation parameters, cameras, and other necessary elements of the game engine (e.g., components, players, and scripted tools). The scripting was performed using the C# language in Visual Studio 2019 [
59] supported by Unity.
Stage (3): Enabling the export from the application. After completing the design, an interactive tool, the FIMViz, was built as a standalone PC application compatible with most desktop platforms (Unity, like many game engines, caters for a wide range of platforms).
4.4. AI Intervention
The focus of AI adoption was the information retrieval stage of the FDA process. Various AI approaches were explored. Techniques, such as the k-means clustering algorithm [
60] and GPT-3.5 models [
61], were used. The programming was conducted using Python 3.9 [
62] and employed a variety of modules, libraries, and tools, brief descriptions of which are given in
Table 2.
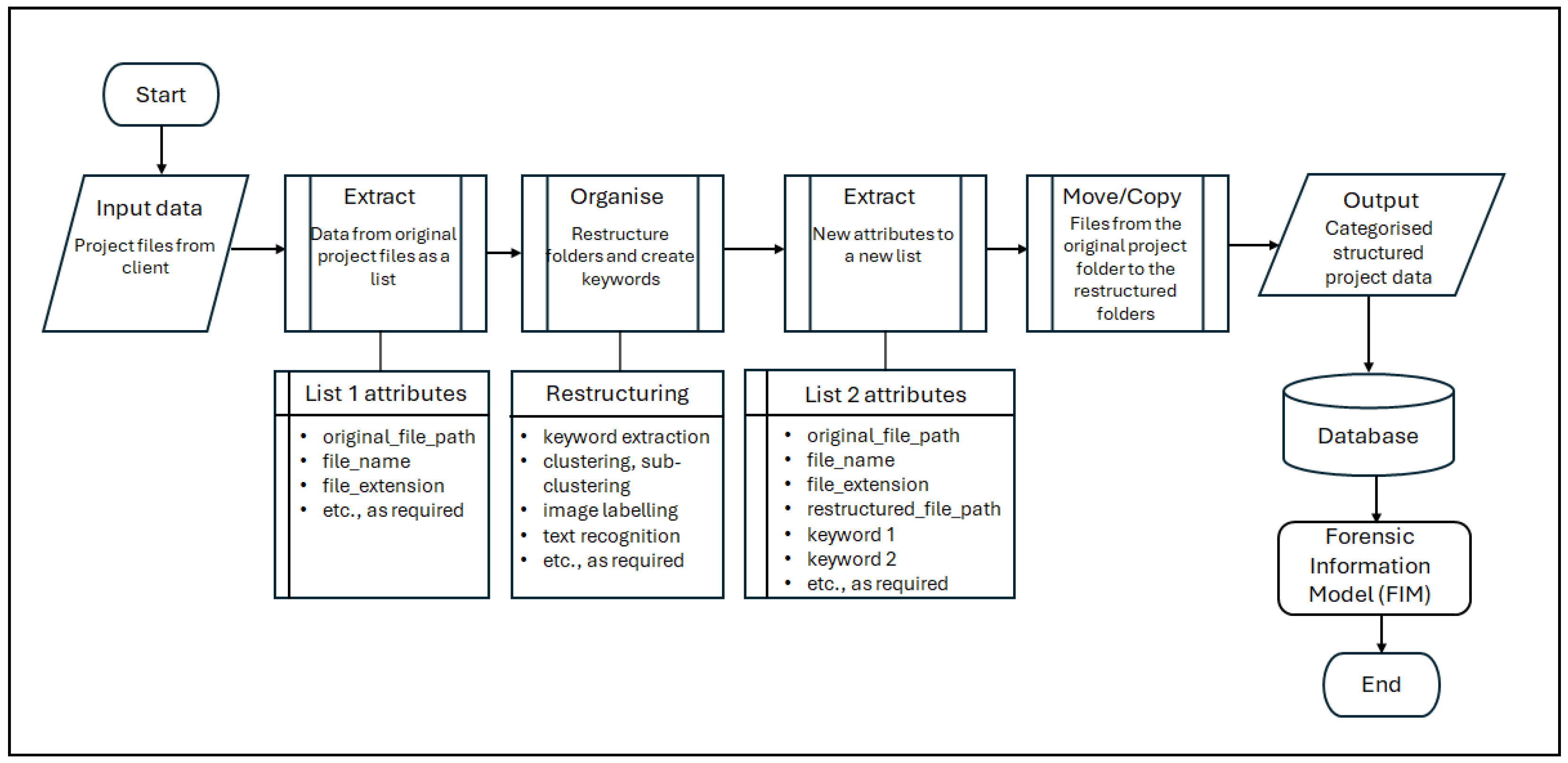
Figure 6 presents a simplified data workflow that depicts the AI intervention in the information retrieval stage of the FDA process.
The FDA process typically begins with the receipt of project records from the commissioning party. In practice, these records are frequently unorganised and voluminous. To overcome this, the first task is to import the necessary libraries and set the API key to interact with the GPT-3.5 models. The data is then extracted from the original project folder as a list in a ‘comma-separated-values’ (.csv) format containing attributes, such as original_file_path, file_name, file_extension, etc. These attributes are used to organise the files by restructuring these folders and creating useful keywords to benefit the data analysis task. The scripting tool reads these files to apply AI approaches, such as keyword extraction, clustering, sub-clustering, image labelling, text recognition, etc. In the tool, the Python 3.9 programming language, GPT-3.5 models, k-means (clustering algorithm) with the scikit-learn library, and other tools are used.
Keywords are extracted from the file paths, file names of the project records, etc. The extract_keywords function uses the GPT-3.5 models to extract from the attributes keywords (such as original_file_path and file_name) that are useful for understanding the context or the content of the file. The get_file_name_embedding and get_file_path_embedding functions generate ‘embeddings’ (i.e., mathematical representations of the textual data) for file names and file path names. If GPT-3.5 models fail to generate embeddings, a CountVectorizer can be used as a fallback. Keywords are used to group these files with different AI approaches, clustering and sub-clustering. The function cluster_files_recursive employs the k-means algorithm to cluster these files based on their keywords. It also allows for sub-clustering, providing a hierarchical structure. These techniques create new restructured file paths and extract meaningful keywords from these files to benefit the subsequent steps of the FDA process.
As an output of the above techniques, a new list that has attributes such as the original file path, file name, file extension, restructured file path, keyword 1, keyword 2, etc., can be extracted. Using these attributes, the files (i.e., project records) are copied from the original project folder to the restructured path. This can be a move operation (to relieve server storage space) or a copy operation. The new restructured folder system complies with the list of project records required for the analysis and is the same folder structure (which includes the required project records) used in the DBMS intervention to the FDA process.
In summary, AI interventions can reduce the time spent on data organisation and retrieval, significantly improving the efficiency of the FDA process. The project data, now organised and structured, is then used to populate the database and link with the BIM models to create an interactive FIMViz application for further analysis. For instance, in the case study, the restructured file paths of the project records are used as links. When the user clicks on a related element in the interactive environment, a new window pops up with further details about this specific element (i.e., further evidence relating to a delay event).
4.5. Operation of the Film FIMViz Prototype
The FIMViz prototype was created to integrate and operationalise the interventions within the enhanced FDA process model to demonstrate its functionality. The following sections explain how this was achieved using data from a contemporary project, and the accompanying screenshots (
Figure 7,
Figure 8,
Figure 9,
Figure 10,
Figure 11,
Figure 12 and
Figure 13) illustrate the resulting case-based simulation.
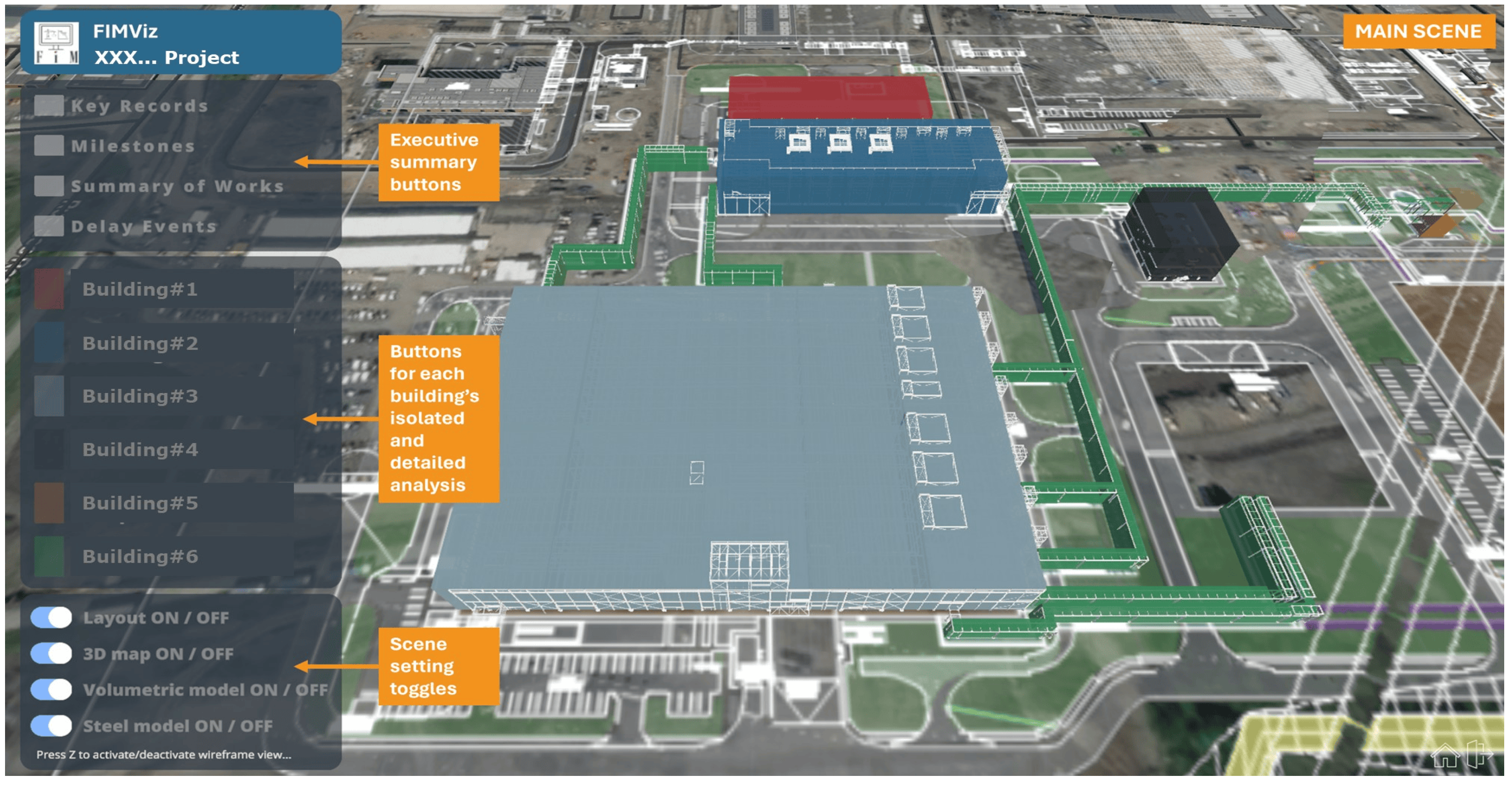
The ‘main scene’ shown in
Figure 7 is taken from information provided in the executive summary of an actual FDA report and presents a 3D environment of the project in basic geometric form. The layout buttons allow access to executive summary sections (such as the key records, milestones, summary of works, and a list of delay events) or to detailed views of each building. In the FIMViz prototype, these are all colour-coded to match the volumetric model. Additionally, the lower-left corner of the screen includes toggles for functionalities, such as on-or-off switching of the layout, 3D map, volumetric model, and steel model.
Figure 8, a sub-scene of the tool, offers direct access to the ‘key records’, allowing users to open relevant project documents by clicking on individual elements.
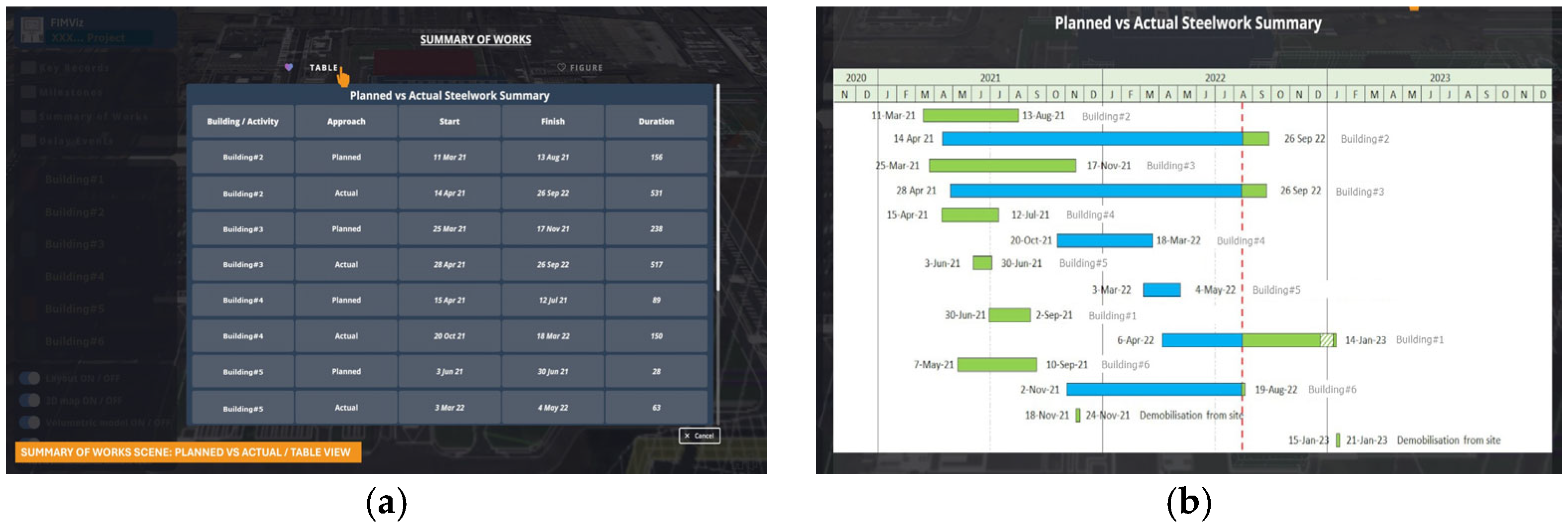
Figure 9 illustrates the ‘summary of the works’ sub-scene, comparing the planned and actual progress in a summary, through both table (
Figure 9a) and Gantt chart (
Figure 9b) views.
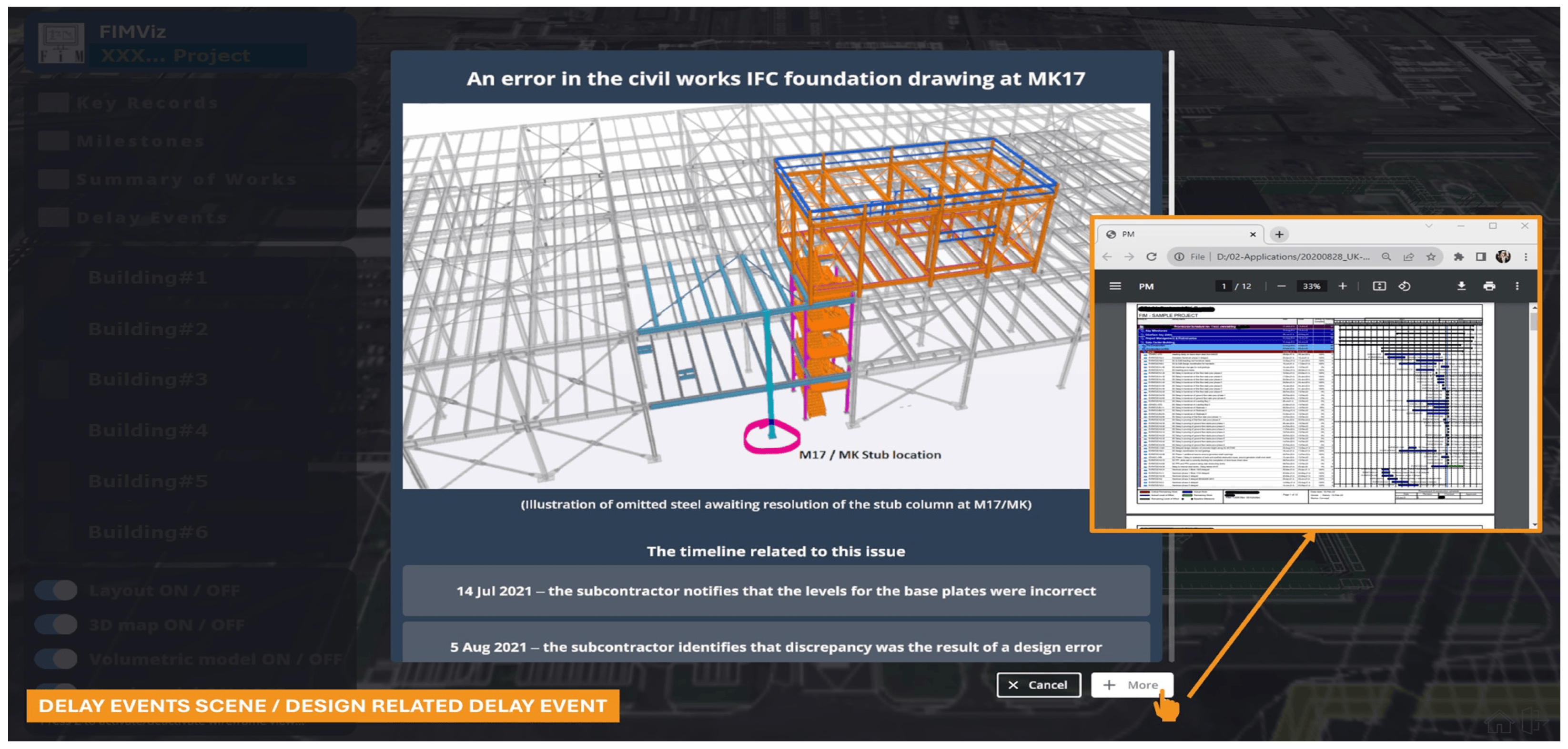
Figure 10 displays a ‘delay events’ sub-scene, highlighting a specific design-related delay event along with its corresponding timeline. The ‘more’ button in the lower right provides access to supporting evidence from the project database for this delay event.
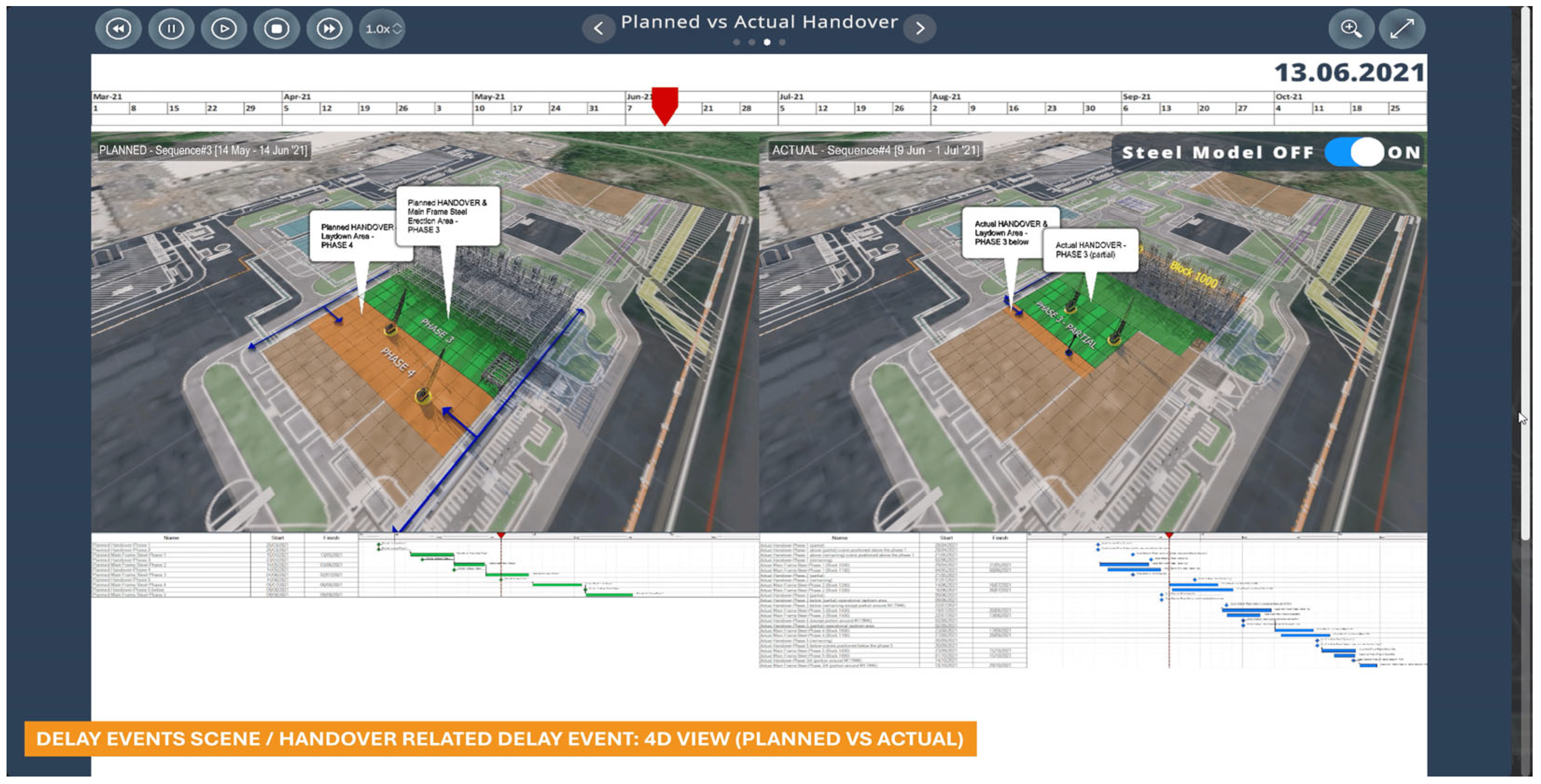
Figure 11 shows a sub-sub-scene of ‘delay events’ featuring a specific handover-related delay event. It displays an interactive 4D view comparing visualising the planned and actual progress in the handover of steel erection phases. Each phase of steel erection requires a working laydown area ahead of it. For example, Phase 3 erection requires the availability of the area (Erection area—Phase 3) as well as the working area (Laydown area—Phase 4) to be available.
Figure 11 illustrates this plan (on the left) and the actual situation (on the right), with delayed availabilities of both areas.
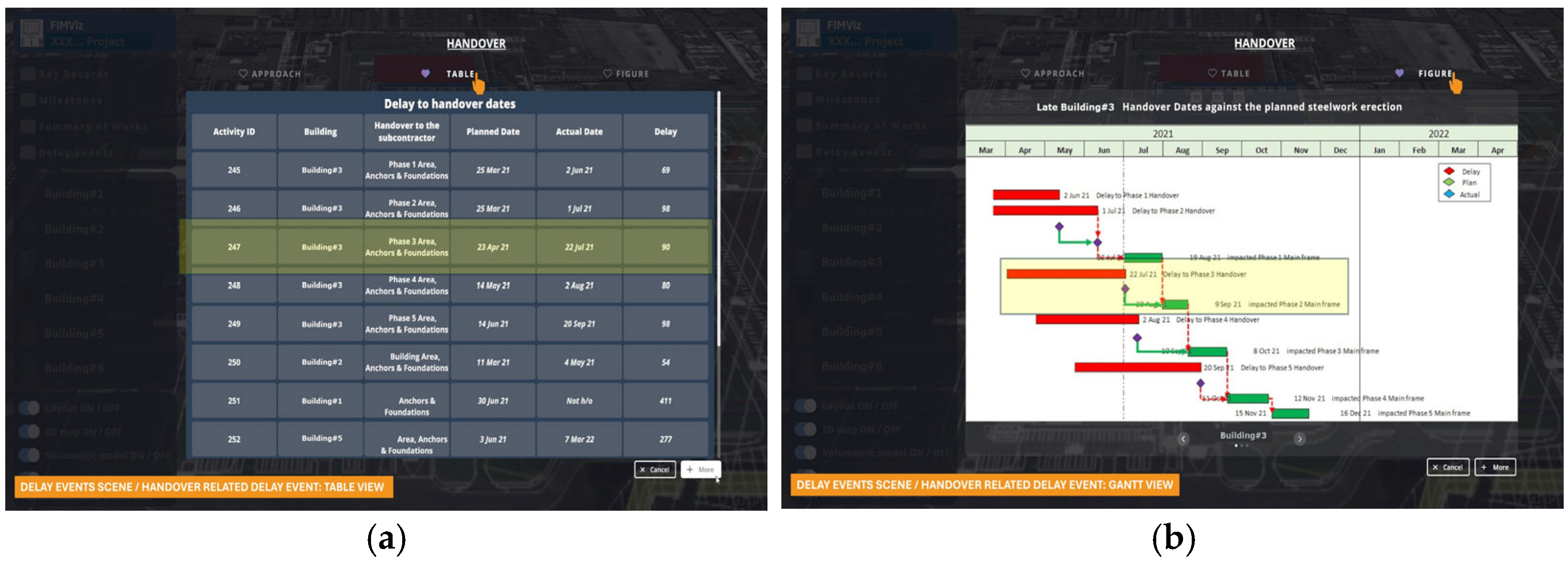
Further details of the same handover-related delay event are shown in tabular and Gantt chart formats in
Figure 12. The tabular (
Figure 12a) and Gantt chart (
Figure 12b) formats offer alternative views of the planned and actual handover dates. The delay to the availability of Phase 3 and its impact on the completion of Phase 2 of the main frame steelwork are indicated by highlighting.
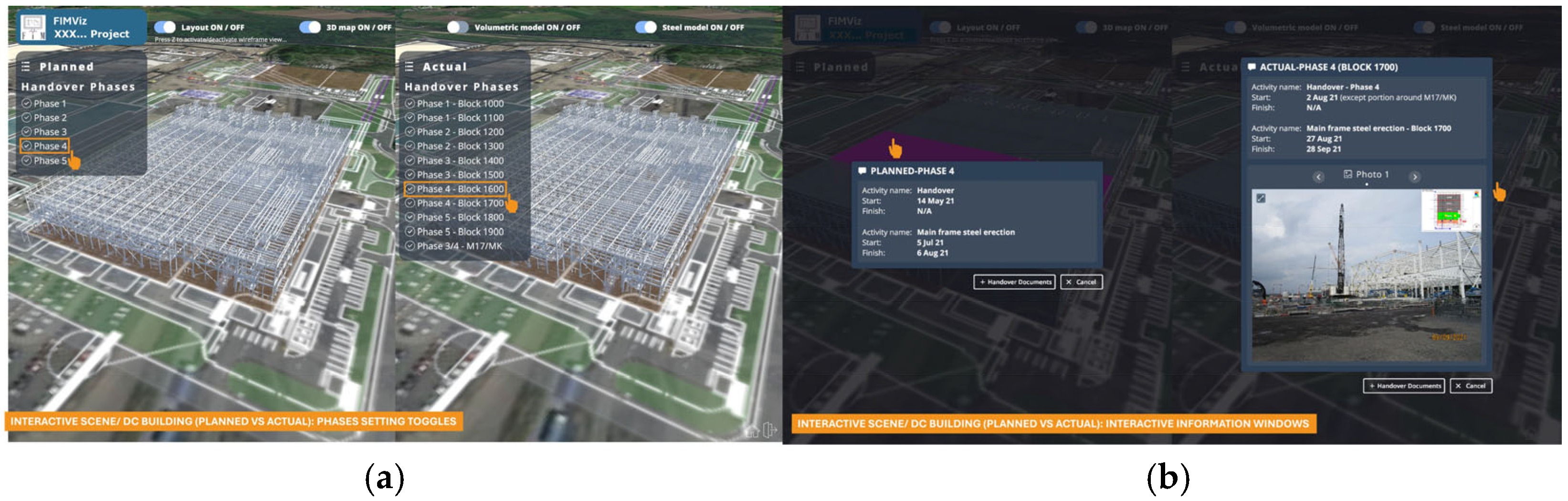
Figure 13 offers a detailed analysis of an individual building (this example being the data centre building). These interactive scenes allow users to compare the planned versus actual progress side-by-side. Users can interact with geometric elements by clicking on them to view an information window on a specific element or by toggling each phase on or off.
In summary, the FIMViz is a prototype tool in which the enhanced FDA process model was operationalised. This served several purposes. First, by the input of contemporary real-world construction project data, a case-based simulation was produced to satisfy the internal validity of the model’s data flow and the successful incorporation of different digital technology that could help overcome the limitations of conventional FDA. Secondly, the interactive nature of the FIMViz tool allows users to explore delay scenarios in detail, with the aim of enhancing the retrieval of information, the accuracy of its analysis and the effectiveness of its communication. Finally, the FIMViz tool also played a role in the next stage of the study: the evaluation by experts.
5. Evaluation of the Enhanced Conceptual Model and the FIMViz
The successful exchange of data between the digital technologies within the FIMViz and its ability to generate credible FDA outputs was itself a first step in verifying the applicability of the enhanced conceptual model. The next step was to subject the model and the FIMViz prototype to expert evaluation. The purpose of this part of the study was to evaluate the credibility and applicability of the conceptual model and its realisation in the FIMViz prototype. The background to the interviews and their administration has been described earlier, in
Section 2.
There were eleven one-to-one web-based interviews of 1–2 h duration with individuals who had agreed (in their earlier questionnaire responses) to participate. Each interview took place in a Teams [
63] meeting environment. The interviewer’s screen was shared with the interviewee to facilitate a structured and focused discussion. Responses to closed-ended questions were collected using both Qualtrics Surveys [
64] and Mentimeter [
65] to ensure accuracy and consistency. For open-ended questions, the recording and transcription feature of Teams was utilised as the primary data collection tool, eliminating the need for note taking during the sessions.
A majority (n = 8) identified themselves as ‘forensic delay analysts’; five had ‘over 15 years’ of experience and four between ‘11 and 15’ years in FDA. Of the 11 interviewees, 4 were ‘highly familiar’ with both the AACE (2011) and SCL (2017) methodologies; 4 were ‘highly familiar’; and 3 ‘familiar’ with SCL (2017) only.
The interviewees were first asked about the enhanced FDA process model (a detailed copy of which had previously been provided) and its proposed digital interventions. Next, they were shown a short video presentation of the FIMViz tool that operationalised the model. Finally, they were asked to evaluate the tool and re-evaluate the enhanced FDA process model in the light of what they had seen.
5.1. Evaluation of the Model
In evaluating the model as a whole, five interviewees were in ‘complete agreement’ and a further five ‘mostly agreed’ with the model’s effectiveness in covering all aspects of an ideal FDA process, in mitigating the challenges and shortcomings of conventional FDA, and in representing an ideal FDA workflow. Following the introductory questions, the focus was on the detail within each of the model’s stages. Using a five-point scale (exactly-mostly-somewhat-very little-not at all) interviewees reported to what extent they agreed with what was presented in each stage of the enhanced model. (As noted above, a detailed description of each stage of the FDA process model, together with any digital interventions, had previously been provided to each interviewee).
The first of these stages concerns information retrieval. Of the challenges to effective FDA highlighted in the literature (see
Section 3.1) the most severe is the identification, sourcing, retrieval, and validation of relevant information [
3,
4]. When asked whether the detailed processes they were shown corresponded with an ideal FDA workflow, four interviewees responded ‘mostly’ and seven ‘exactly’, indicating strong confidence in the detailed content of this stage of the enhanced model. They were then asked about sources of information. In the literature, the information that is relevant to FDA is identified as programmes (or ‘schedules’), contract documents, and records (of progress, resources, costs) as well as administrative correspondence [
5,
6,
7]. Additionally, given its fundamental importance to the proposed digital enhancements in this study, interviewees were asked about BIM data as an information source. Interviewees were requested to rank each of these seven information sources according to two criteria: ‘desirability’ (i.e., how important they were to the FDA process) and ‘availability’ (i.e., how readily available they usually were). Programmes and contract documents were reported to be the most ‘desirable’ and normally ‘available’ sources indicating their integral role in the FDA process. However, progress records were desirable but often not available, indicating that efforts to improve their availability could significantly benefit FDA processes. Correspondence and administration records were seen as reasonably available and moderately desirable. Resource records are ‘somewhat less available’ but maintain a moderate level of desirability, indicating that, while they may be important for the FDA process, accessing them may be difficult. Cost records were considered ‘somewhat accessible’ and ‘moderately desirable’. Interviewees did not anticipate any significant future change in their views about the availability or desirability of these six information sources. By contrast, ‘BIM data’ was considered currently to be ‘moderately desirable’ but not readily available, though five (out of the eleven) interviewees expected this to change as BIM maturity improves, with the likelihood that in future BIM data would become both more desirable and more avail-able.
The next stage in the model to be evaluated was the analysis of delay. Six interviewees considered that the detailed workflows in this stage of the enhanced model aligned ‘exactly’, and three ‘mainly’, with what they considered to be an ideal FDA workflow. In terms of overcoming the challenges to effective FDA, the second major challenge (see
Section 3.1) was the lack of consensus on how to analyse the evidence [
2,
8,
9,
18]. Attempts have been made by professional organisations [
10,
11,
22] to address the confusion and subjectivity highlighted earlier [
8,
9] though these protocols are not in themselves panaceas [
4,
5]. Interviewees were first asked about their familiarity with the delay analysis protocols published by AACE [
10] and SCL [
11]. Four of the eleven interviewees felt ‘highly familiar’ with both protocols. Of the remainder, four were ‘highly familiar’ and three were ’familiar’ with the SCL document.
The highest level of interviewees’ agreement was observed in the communications of the findings stage in the enhanced FDA process model, where nine interviewees agreed that the model ‘exactly’ corresponds to what an ideal FDA workflow would include. In terms of its detailed content, interviewees were asked to assess, on a four-tier rating (critical-important-optional-unwanted) the different formats for presenting findings that were inherent in the enhanced FDA process. These methods include reports (e.g., in .pdf format), presentations (e.g., PowerPoint slides), animations (e.g., videos), and 3D/4D interactions (e.g., BIM-based applications such as Synchro [
55]). The resulting prioritization was that reports were considered ‘critical’ for presenting FDA findings (with ten out of eleven responses); the next in order of importance being presentations (which two interviewees thought ‘critical’, and eight ‘important’), animations (four responses of ‘important’). Only one interviewee considered 3D/4D interactions ‘important’ while nine considered them ‘optional’. Interestingly, however, this view was to change after the viewing of the FIMViz application video (see
Section 5.2, below).
5.2. Evaluation of the FIMViz Tool and Re-Evaluation of the Enhanced Process Model
Having viewed the FIMViz application video, interviewees were asked whether their perspectives had been influenced or whether their views had altered in any way. Five of the interviewees acknowledged the usefulness of the application, yet they did not anticipate any change in the views they had previously held. However, six interviewees anticipate changes in their attitude to the use of animations and 3D interactions. One predicted that these methods would gain importance and become easier to implement due to technological advancements. One, however, expressed concerns about the practicality of these methods, given the time constraints inherent in FDA processes. Three other interviewees expected 3D interactions to shift from being optional to important. One interviewee expected 3D interactions to change from being ‘unwanted’ to ‘important’, echoing similar sentiments about the impact of technological advancement.
Interviewees were then questioned as to whether they thought the FIMViz application improved the existing FDA process and mitigated its assumed challenges and shortcomings. Almost all the interviewees recognised its potential. Five of the eleven interviewees thought that the application ‘mostly’ improves the existing process and effectively addresses many of the assumed challenges and shortcomings, and for the remaining six it ‘somewhat’ did so.
Further insights into the effectiveness of the FIMViz application included three respondents who specifically highlighted its effectiveness for both analysis and presentation purposes, mentioning its capability to query or relate different datasets within the same environment and its useful presentation mode feature. Conversely, two interviewees perceived its effectiveness solely for presentation. One interviewee highlighted the challenge of users reaching the requisite stage of technical ability. Another raised time and budget constraints, noting the significant investment required. A third interviewee observed that it might be challenging for tech-adverse users, such as traditionalist arbitrators or adjudicators, to utilise it or interpret its output. From a similar perspective, another interviewee questioned the practical use of the application in settings like courtrooms.
5.3. Evaluation Summary
The findings suggest that the model is generally accurate, reliable, and relevant to the intended purpose. However, certain aspects of the model require attention. The model can benefit from being tested on a larger and more diverse dataset to ensure it adequately represents the various complexities of real-world FDA scenarios. Improvements that were suggested included the incorporation of additional features, such as advanced data analytics capabilities (already considered in the model’s AI features, though these were not included in the FIMViz presentation) and enhanced user interface designs. Such features could enhance the model’s ability to process complex data more efficiently and make it more user-friendly, thereby increasing its utility in practical applications.
Overall, the evaluation was a success. The findings from the evaluation also provided valuable insights into the model’s strengths and weaknesses. Suggestions on the model’s improvement included testing on large, diverse project datasets to ensure it adequately represents the various complexities of real-world FDA scenarios, and incorporating additional features, such as advanced data analytics capabilities and enhanced user interface designs. These features can enhance the model’s ability to process complex data more efficiently and make it more user-friendly, thereby increasing its utility in practical applications. These insights will be instrumental in refining the model further, making it even more effective and useful for professionals in the field.
6. Discussion and Conclusions
This study took place in response to a perceived problem in terms of the limitations inherent in the conventional FDA process, its inaccuracy and consequent contentious nature, and its inefficiency. All these problems were widely discussed in the literature. Using a systematic review, the conventional FDA process was modelled and verified by a survey of experts. Returning to the literature, the potential of digital interventions (DBMS, BIM, AI, and game engine) was explored. Using a DSR approach, an enhanced FTA process was modelled that included these digital interventions. The enhanced model was then used to create a working prototype, the FIMViz, which integrated the digital interventions into a working example of a full, simulated FDA. The enhanced model and the prototype were then subjected to expert evaluation. The model was found to be highly accurate in mapping out and representing an ideal FDA process. It captured the critical elements and phases of FDA with precision, ensuring that the model reflects real-world scenarios and challenges faced by practitioners. The model was found to be relevant to its intended purpose of streamlining and enhancing the efficiency of the FDA process. It addressed key issues and bottlenecks in the existing process, offering a more streamlined and effective approach to delay analysis. The model was found to be reliable, underpinned by its methodologically sound design and the positive feedback received during validation. This indicated that the model’s processes and outcomes were consistent with best practices in terms of FDA. The uniformity and consistency of the feedback from various experts in the field of FDA enhanced the model’s credibility as a reliable process.
6.1. Achievements of the Study
This study has demonstrated how emerging digital technologies can be integrated into the FDA process. By addressing the limitations of existing FDA practices, the research has shown the viability and practicality of incorporating these technologies into an enhanced FDA process that can improve the efficiency, accuracy, and communication of delay analysis. The use of DBMSs creates a streamlined, structured repository of project data with precise data linkage between BIM models and other delay records, reducing manual retrieval and processing times. The addition of AI-driven automation presents opportunities for automated data structuring and information retrieval. Individually, BIM and 4D tools offer a detailed understanding of project timelines, while game engines add an interactive dimension that enhances the spatial perception. Together, they empower practitioners to simulate delay impacts in real time, compare planned versus actual progress, and communicate findings more effectively to project participants through engaging, intuitive interfaces.
6.2. Contribution and Potential Impact
The contribution of this work to the overall body of professional knowledge has been to demonstrate the integration of available digital technologies (DBMSs, BIM, AI, and game engines) into the FDA process. Some of these technologies (for example, BIM) are already established in the construction management context, but they have not, hitherto, been applied in an integrated way to FDA processes. A framework was created that explains how these technologies can combine to provide an enhanced FDA process model with more efficient workflows and more accurate analyses that can be communicated more effectively. Whilst each intervention can singly address specific limitations of current FDA practices, their combined implementation demonstrates improvement from both the analytical and presentational perspectives. As a result of this work, researchers will gain further insight into the FDA process and how the application of emerging technical innovations can overcome the shortcomings that are currently encountered.
Practitioners, particularly those involved in the field of forensic delay analysis, should be its main beneficiaries. The combined application of these innovations extends the scope of FDA from a static, uncertain, document-driven process to one that is dynamic, reliable, and data-driven, thus reducing the potential for uncertainty, disagreement, and unnecessary dispute. Furthermore, these technical interventions have been realised in the development of the FIMViz application, an accessible, easily navigable, and practical tool that leverages these technologies and, having been subjected to expert evaluation, confirms the transformative potential of this integrated approach.
Beyond its importance to those directly involved in delay analysis, this work also has practical implications for the many professionals involved in the planning and execution of construction projects, where the integration of BIM, 4D simulation, and game engines can provide a dynamic platform for visualising and simulating delay scenarios. The findings emphasise the importance of adopting integrated digital systems for more efficient, transparent, and reliable schedule management. By enabling interactive analysis of project data, practitioners can better anticipate and understand delay impacts, communicate findings effectively, and adopt collaboration across project participants.
6.3. Limitations and Further Work
Inevitably, this work has its limitations. It was noted in
Section 1 that a variety of analytical perspectives can be applied to FDA and the present study has not followed all of them. The classification of these different perspectives [
4] reveals certain real-world variables against which any FDA advances should be tested. These include the context of individual projects (geography, type, size, delivery), and their differing contractual or legal arrangements. Therefore the enhanced model can benefit from being tested against a wider range of projects and diverse contexts to ensure it adequately represents the various complexities of real-world FDA scenarios.
The proposed AI interventions in the enhanced FDA process were limited to its early stages (information retrieval) but could in future be extended. Although AI and ML were successfully integrated in the creation of the FIMViz prototype (as discussed in
Section 4), their functionality did not feature explicitly in the audio–visual presentation of the FIMViz to the expert evaluators. Consequently, there was no expert evaluation of the AI intervention, although it was part of the enhanced process model. The improvements that were suggested by the expert evaluators included the incorporation additional features, such as advanced data analytics capabilities (as noted, the model’s AI features were not explicit in the FIMViz presentation) and enhanced user interface designs. Such features could enhance the model’s ability to process complex data more efficiently and make it more user-friendly, thereby increasing its utility in practical applications.
Future research should aim to broaden the applicability of this study across a wider range of construction projects and delay scenarios. Additionally, further exploration of the integration of other emerging technologies, such as extended reality and blockchain, into FDA processes could yield valuable insights. Advanced AI techniques combined with simulation-based optimisation present an opportunity for future research efforts to automate scenario creation for identifying potential delay events and implementing preventive actions, thus extending the applicability of FDA from a purely forensic, post hoc tool to one with a predictive risk management capability.