Abstract
Project delays remain a persistent challenge in the construction industry, having significant financial implications and contributing to disputes between project participants. Forensic Delay Analysis (FDA) has emerged as a specialised function that identifies the root causes of such delays, quantifies their duration, and assigns responsibility to the appropriate parties. While FDA is a widely practised process, it has yet to fully exploit the potential of emerging technologies. This study explores the integration of both existing and emerging technologies for enhancing FDA processes. A Design Science Research (DSR) approach is adopted, with data collection methods that involve the use of the literature, archival materials, case studies and survey methods. The research demonstrates how the use of technologies, such as database management systems (DBMSs), building information modelling (BIM), artificial intelligence (AI) and games engines, can improve the analytical efficiency, data management, and presentation of findings through a case study. The study showcases the transformative potential of these interventions in streamlining FDA processes, ultimately leading to more accurate and efficient resolution of construction disputes. The proposed process is exemplified by the development of a prototype: the Forensic Information Modelling Visualiser (FIMViz). The FIMViz is a practical tool that has received positive evaluation by FDA experts. The prototype and the enhanced FDA process model that underpins it demonstrate significant advancement in FDA practices, promoting improved decision-making and collaboration between project participants. Further development is needed, but the results could ultimately streamline the FDA process and minimise the uncertainties in FDA outcomes, thus reducing the incidence of costly disputes to the wider economic benefit of the industry generally.
1. Introduction
Construction project delays are commonplace and often lead to disputes and serious financial losses [1], accentuating the need to understand and apportion their causes and impacts. Forensic Delay Analysis (FDA) has emerged as a specialism that focuses on identifying, analysing, and apportioning the causes and impacts of project delays, and then presenting evidence in the resulting disputes [2]. But despite its importance, existing FDA workflows have limitations that affect the accuracy, cost, promptness, and reliability of the delay analysis outcomes, which have been described by Gibbs [3] as relying on manual processes and static data structures that are time-consuming, error-prone, and limited in their ability to capture the dynamic complexities of construction projects. They employ methodologies that are often lengthy, costly, complex, and challenging to communicate, and they rely heavily on judgement, interpretation, and selective presentation of findings [3]. A paper by Grzeszczyk et al. [4] reveals the different analysis perspectives that pervade the FDA-related literature by identifying eight common themes. The theme of ‘project characteristics’ investigates differing project contexts (e.g., geographical, delivery-related). The ‘contractual or legal references’ theme is concerned with specific jurisdictions, case law, and forms of contract. The ‘reliability’ and ‘reported’ flaws themes overlap somewhat, identifying problems such as subjectivity, low credibility, and limited data. Two themes, ‘protocol preference’ and ‘schedule determinism’, relate to the value, use and shortcomings of procedural guidelines (in the case of the former) and scheduling techniques (in the case of the latter). In the final pair of themes, the ‘reported remedies’ grouping involves proposals for the enhancement of existing techniques and methods, while the ‘innovation’ theme is predominantly focused on advances in information technology.
In response, the work described here addresses the ‘reliability’, ‘reported flaws’ and ‘innovation’ analytical themes. Matters such as specific project characteristics, different jurisdictions or forms of contract, and the reliability or otherwise of scheduling techniques are excluded, though they will be reflected upon in the final section. Fundamentally, the work described here addresses the need to develop more efficient and accurate approaches to FDA processes and proposes an enhanced FDA workflow in which digital technologies are integrated.
The foremost challenge to effective FDA lies in finding the evidence. The records at the disposal of the delay analyst are frequently incomplete and inadequate, consisting of unstructured and inconsistent data, thus making the identification, sourcing, retrieval, and validation of relevant information one of the most time-consuming and costly aspects of claims preparation [5]. Alkass et al. [6] estimated this to take around 70% of the analyst’s effort, a figure that has been empirically verified [7]. Thus, the first serious major challenge to the FDA process concerns the quality, availability, and transparency of evidence, and addressing these data issues is vital for enhancing the overall effectiveness and reliability of the FDA process. The second challenge involves the lack of consensus on how to analyse the evidence. The choice from amongst a variety of delay analysis techniques, as described by Parry [8], is complex and diverse, and it can be influenced by a variety of factors [9]. Guidelines provided by professional organisations, such as the Association for the Advancement of Cost Engineering (AACE) [10] in the USA and the UK’s Society of Construction Law (SCL) [11], have added some clarity, but the proper application of any preferred methods remains highly dependent on the adequacy of the supporting information, which is in itself problematic. The third major challenge arises in the final stage of the process: communication of findings. As highlighted by Gibbs [4], difficulties emerge when presenting complex evidence (e.g., programmes, specialist terminologies and technical information), particularly when a dispute has escalated as far as arbitration or litigation.
The advent of the digital technologies associated with Construction 4.0 presents new opportunities to enhance FDA practices. Construction 4.0, as derived from the term Industry 4.0 [12], is described by Sawney et al. ([13] p. 3) as an idea ‘based on a confluence of trends and technologies (both digital and physical) that promise to reshape the way that built environment assets are designed and constructed’. However, uptake by the construction sector has been limited [14] and FDA has shown no apparent benefits. The key areas of interest in this context are database management systems (DBMSs), building information modelling (BIM) and the tools it enables, and the utilisation of artificial intelligence (AI) and game engines. DBMSs can improve data management and retrieval efficiency by structuring unorganised project records. BIM-based software tools can produce a digital representation of projects that incorporate various data sources. AI can automate data clustering, keyword extraction, and classification, improving the accuracy and speed of analysis and reducing human error. Game engines can provide advanced visualisation tools that can present analytical findings in an intuitive and interactive manner. By integrating these technologies, FDA can be transformed into a more efficient, accurate, and comprehensive process. These advances offer immense potential for enhancing FDA processes, thereby leading to improved efficiency and accuracy. This study proposes the enhancement of FDA by leveraging emerging technologies. The approach involves integrating DBMSs, BIM, AI, and games engine technologies into the FDA process in order to streamline data collection, enable its seamless transfer through the process, improve the accuracy and efficiency of delay analysis, and enhance the communication of findings to project participants. A case study is presented to demonstrate the practical application of the proposed approach, highlighting its potential to transform FDA practices in the construction industry.
The remainder of this article comprises four sections. Section 2 describes the research methodology adopted (as shown in Figure 1). Section 3 explains the development of an enhanced model resulting from the incorporation of digital technologies into the FDA process. Section 4 describes the operationalisation of the model to produce the FIMViz prototype. Finally, Section 5 reflects upon the evaluation of the enhanced FDA process model and the FIMViz prototype by FDA experts.
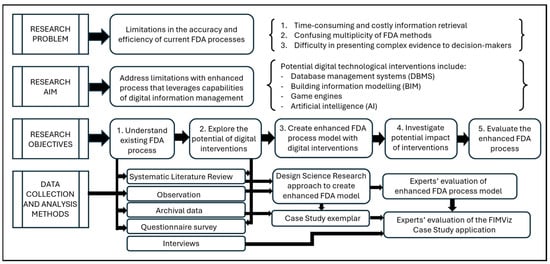
Figure 1.
Research methodology flowchart.
2. Methodology
Responding to the research problem, the aim and objectives were set accordingly and data collection followed. The methodological process is shown in Figure 1.
2.1. Objectives 1 and 2: Understand the Existing FDA Process and Explore the Potential of Digital Interventions
As shown in Figure 1, to understand the existing FDA process and its limitations, and to explore the potential of digital interventions in addressing these limitations, four data collection methods were developed. These are presented in turn, as follows.
2.1.1. Systematic Literature Review
A systematic literature review (SLR) was conducted using the Scopus, Web of Science, Science Direct, and Google Scholar databases. This comprised three areas of interest: Forensic Delay (FDA), building information modelling (BIM), and artificial intelligence/machine learning (AI/ML) in the construction industry. Accordingly, a range of search entry parameters, including abbreviations, spelling variations, and truncations, were established. Based on these parameters, searches were performed using the format required for each database. The search was limited to conference proceedings, dissertations, journal articles, interviews, and reports. Titles, abstracts and keywords were interrogated using three combined query strings that related to each of the areas of interest (BIM + FDA, BIM + AI/ML, FDA + AI/ML), as shown in Table 1. As an example of this, Figure 2 shows the query strings used for interrogating the Scopus database for the BIM + FDA combination.

Table 1.
Relevant publications accessed, with duplicates removed and filtered.

Figure 2.
Sample query string for BIM + FDA in the Scopus database.
The combined searches returned a total of 2797 items. The targeted publication timescale had been left ‘open’, though very few (<4%) matches were found to be published prior to 2006. The results were then filtered in two stages. In the first (Stage 1), publications that were clear duplicates were removed. Stage 2 followed a manual review of each abstract, after which non-relevant publications were removed. Table 1 shows the results.
Manual analysis of the 276 identified publications provided the means of addressing the first two research objectives, namely, to establish an understanding of FDA practice and its current limitations (Objective 1) and to establish the potential of digital interventions in addressing FDA’s limitations (Objective 2). An outline of the key findings relating to each objective is given in Section 3.
2.1.2. Observation
The first objective was to establish an understanding of FDA practice and its current limitations. Information obtained from the SLR discussed in the previous section was supplemented by detailed observation, at intervals over a three-year period, of the work of a company specialising in the management of construction project claims.
2.1.3. Archival Data
The same company also afforded the opportunity to access archival data from past projects, and this added further to the understanding of the conventional FDA process and its limitations, as well as providing realistic data that would later be used to populate a visualised simulation of the enhanced process.
2.1.4. Questionnaire Survey
An online questionnaire was administered via email or LinkedIn to 50 individuals, pre-selected using the criteria of domain knowledge and contextual expertise, which resulted in 30 valid responses. The questionnaire contained 17 main questions. The ‘Demographic’ themes included professional background and experience, dispute involvement, practice locality, and familiarity with guidance documents and emerging technologies. A majority (n = 22) of respondents identified as ‘forensic delay analysts’ (the remainder being six ‘subject matter experts’, three ‘construction project managers’, and two ‘legal experts’). The main objective of the survey was to verify the conventional FDA process model, the details of which follow the description of the model in Section 3. The questions in this part of the survey asked participants to compare the proposed model of the conventional FDA process and its main stages with the actual FDA processes and stages with which they were familiar. Each question required a multiple choice, single answer response in the form of 5-point Likert scale (Not at all-Very little-Somewhat-Mostly-Exactly). Space was al-lowed for respondents to elaborate on their answers. A final question gauged the respondents’ interest in participating in a future interview.
2.2. Objective 3: Creation of an Enhanced FDA Process and Its Operationalisation as a Working Prototype
The third research objective was to create an enhanced FDA process model that benefited from certain digital interventions. To produce the model and its exemplar case study, a DSR approach was adopted. As described by Hevner et al. [15], as well as by other information systems researchers, DSR is targeted at practical problem-solving and is particularly suitable for research that aims to produce actionable and innovative outcomes that are underpinned by a series of design–test–evaluate iterations as their development continues. In this study, the approach translated into three steps: (i) to design an improved FDA process that beneficially accommodates relevant emerging technologies; (ii) to operationalise and test the model by creating a case-based example that incorporates the relevant technologies; and (iii) to evaluate the improved process model, conceptually and through assessment of the case-based example.
2.3. Objectives 4 and 5: Investigate the Potential Impact of Interventions and Evaluate the Enhanced FDA Process
For evaluating the enhanced FDA process and its digital interventions, a series of one-to-one semi-structured interviews were held with experts. The main objective of the interviews was to evaluate the enhanced FDA process model its operationalisation in the FIMViz prototype, set as Objectives 4 and 5. Of the 30 respondents who completed the questionnaire, 19 agreed to participate in follow-up interviews. Ultimately, 11 participants were interviewed individually. They were provided in advance with details of the full enhanced FDA process model, and during the interview, each participant was shown a video recording of the FIMViz prototype. (Descriptions of the enhanced FDA model and of the FIMViz prototype are provided in Section 3).
The interviews were conducted via an internet-based web conferencing tool and each interview lasted between one and two hours. All the interviewees accepted the ethical considerations and agreed to the interview being recorded. After a preliminary discussion of the enhanced FDA process model, a short video of the FIMViz application was shown to prompt the ultimate evaluation of the digitally enhanced FDA process. The expert evaluations are presented in Section 5.
3. Two FDA Process Models: ‘Conventional’ and ‘Enhanced’
As a starting point and based on the first part of the systematic literature review (relating to the conventional FDA process and methodology), a conventional (‘as-is’) model of the FDA process was established and supported by observation of, and interviews with, staff in an FDA consulting organisation. Next, the results of the BIM and AI/ML parts of the systematic review that related to the potential of digital technologies were used as the basis for creating an ‘enhanced’ FDA process model.
3.1. A Model of the Conventional FDA Process
Within the literature, a seminal work was that of Alkass et al. [6], which involved an empirical investigation of FDA methodologies. Subsequent researchers have generally agreed that the FDA process comprises three main steps: (i) the retrieval of information, (ii) its analysis, and (iii) its presentation [4,16,17]. Others such as Perera et al. [9], Arditi et al. [18], Marzouk et al. [19], and Yang et al. [20], have reviewed commonly used FDA methods and sought to optimise the choice between them. These sources, verified by the observation of FDA analysts at work, enabled the generation of a generalised ‘as-is’ process model that represents the conventional FDA workflows. This simple model is shown in Figure 3.
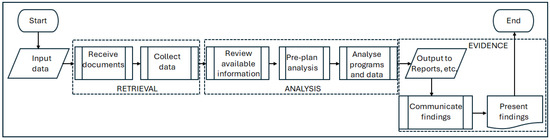
Figure 3.
Simple model of the conventional FDA process.
In Figure 3, FDA activities are grouped into three stages: retrieval, analysis, and evidence. The following explanations include the limitations within each stage that emerged from the literature review.
3.1.1. Retrieval of Information
As already noted, the retrieval of relevant information is FDA’s most time-consuming and costly task [5]. Ali et al. [21] estimated that this represents 70% of the entire FDA effort. This figure is broadly corroborated by Atanasov et al. [7], who, in a quantitative review of production hours in twelve separate project delay analyses, found that in some instances, up to 90% of the analysts’ time was spent on retrieving, validating, and processing project records. Establishing the quality, availability, transparency, and validity of relevant information is perhaps the most serious challenge to the FDA process. Furthermore, the availability of information has an impact on the methodology chosen for its analysis, which is the next stage in the process.
3.1.2. Analysis of Information
It is only after review of the available information that a suitable delay analysis method can be selected. Braimah [2] and Arditi and Pattanakitchamroon [18] independently developed recommendations to help delay analysts to choose appropriately from the available analysis methodologies, and Parry [8] produced a formula for their selection. Despite an apparent multiplicity of analysis methods [8], two influential guidance documents, the AACE’s International Recommended Practice No. 29R-03, ‘Forensic Schedule Analysis’ [10] and the SCL’s ‘Delay and Disruption Protocol’ [11], have mitigated this variability, and six prevalent approaches can be identified [22]. It should be noted that the proper application of any preferred method of analysis remains highly dependent on the presence of adequate supporting information in the retrieval stage [21,23,24]. Fundamentally, all the methods rely on the availability, as a minimum, of an as-planned (‘baseline’) construction programme and its as-built counterpart (ideally with progressive versions), but sometimes even these basic elements are incomplete [5]. Hence, the second challenge to the FDA process is the selection of a method of analysis that can be adequately supported by the available information.
3.1.3. Production and Communication of Evidence
The final stage of the FDA process in Figure 3 is the production and communication of evidence. This stage has three subdivisions, namely preparing reports, communicating the results, and presenting the results. The prepared reports, initially at least, are likely to be highly technical and specialised, relying on detailed works programmes and the use of the critical path logic [22]. However, subsequent communication and (if required) presentation to a third-party evaluator (e.g., in adjudication, arbitration, or litigation) will require a comprehensible narrative. As stated by Mr. Justice Akenhead in Cleveland Bridge UK Ltd. v Severfield-Rowen Structures Ltd., ‘In all … delay cases it is necessary to show that the claiming party was actually delayed by the factors of which it complains …’ [25]. The complexity of the technical and specialised FDA outputs represents a major obstacle to achieving this [26] and thus constitutes the third of the notable limitations of the conventional FDA process and the key challenges in terms of enhancing it.
3.2. Evaluation of the Conventional FDA Process: Results of the Questionnaire
Earlier, in Section 2, the administration of an online questionnaire was described, with its main objective being to verify the conventional FDA process model. The results of this survey supported the use of this as a starting point for the study (see Research Objective 1 in Figure 1). Twenty-eight of the thirty respondents recognised that the model matched the actual FDA processes they were familiar with.
3.3. Enhancing the Conventional FDA Process Through the Introduction of Digital Technology
Referring to the research methodology flowchart shown in Figure 1, the second research objective was to explore the potential of digital interventions in overcoming the limitations of the conventional FDA process, and this too was addressed in the SLR described in Section 2. The results of this exploration can be visualised as the framework of an enhanced FDA process model, as shown in Figure 4. This figure is based on the conventional FDA process model previously featured as Figure 3, with the potential technological interventions and benefits superimposed. Following this, support in the literature for the digital interventions is presented and the rationale for their use is explained.

Figure 4.
Framework of the enhanced FDA process model and its technological interventions.
As the enabler of the digitalisation of building information [27], BIM is of central importance to the framework, with benefits for all stages of the FDA process. BIM, in combination with DBMSs, can make the storage and retrieval of information more effective, support the analysis stage, and improve the communication of findings, particularly when used in conjunction with the superior visualisation and immersive features of game engines. The proposed use of AI is not integral to the enhanced FDA process model, but it can enhance the early stages of the process by using predictive capabilities for the automation of data collection, structuring, classification, and retrieval for analysis. The rationale for these proposed digital interventions and their support in the literature is now briefly considered.
3.3.1. Database Management Systems (DBMSs)
The use of DBMSs in the context of building information is not new. A common application has been for asset maintenance and life cycle management [28], though research on the use of DBMSs for claims management [5,6,29] has also featured, including the integration of DBMSs and BIM as a project information repository [19,21,30]. The challenges that relate to the retrieval of relevant FDA information have already been noted, and the adoption of a relational database management system (RDBMS) in the FDA process mitigates these challenges by organising and structuring project data. The RDBMS provides a comprehensive, accurate, and easily accessible framework for storing and linking project records, such as schedules, cost reports, and daily logs. In addition to providing data integrity, consistency, and version control, it offers enhanced data searchability and thus has important potential at both the retrieval and analysis stages of the FDA process.
3.3.2. BIM Tools
Since its emergence at the beginning of the 21st century, the advantages of BIM have been widely recognised [31,32,33] and its adoption has been well documented [34,35,36]. The initial manifestation of BIM (3D BIM) was as a digital model that contained design intent that could be visualised; to the three spatial dimensions others were added to reflect BIM’s increasing functionality [37,38]. Most noteworthy in the context of FDA are the software tools based on 4D BIM, which combines a time dimension with the 3D model [39]. The potential of BIM tools (especially those based on 4D BIM) for improving FDA performance in the collection, processing and presentation of data has been widely considered [4,16,19,21,30,31,39,40,41]. These tools offer advantages in the retrieval, analysis, and evidence stages. They can provide an accessible, centralised repository for data collection in the FDA process, enabling the extraction of forensic evidence regarding delay events, the rapid simulation of events and ‘what-if’ scenarios, and improved visualisation of complex project data.
3.3.3. Game Engine Technology
It was noted earlier that the effective communication of complex, technical FDA evidence can present a problem. There is long-standing acceptance of the superiority of visual over auditory information in terms of the cognition and memory in the receiver [42]. Although construction schedules can be visualised in 4D BIM, better results can be obtained using game engine technology, which can extend the BIM capabilities by creating realistic and immersive simulations of delay events and other forensic evidence [43]. This can be achieved by transferring building project data directly from 4D BIM to a chosen game engine [44,45,46] to improve the communication of FDA findings by presenting complex data in an intuitive, visually engaging format. Game engines can provide both analytical and presentational support to the process.
3.3.4. AI Techniques
The benefits of AI techniques such as machine learning (ML) and natural language processing (NLP) are, in a sense, supplementary to the enhanced FDA process. As already noted, AI is not integral to the process enhancements, but it can automate data collection, structuring, analysis, and other tasks (such as classification and consistent naming of folders and data files), which would significantly increase the efficiency and effectiveness of FDA workflows [47,48]. AI can provide analytical support to the process with key benefits, including increased efficiency, increased accuracy and reduced human error, and predictive capabilities [49,50]. Since AI techniques offer potential for the more efficient and effective collection, structuring, classification, and analysis of data, as well as offering predictive capabilities, they are potentially applicable to all three stages of the FDA process.
4. Operationalisation of the Model in the FIMViz Prototype
As described above, establishing the viability of incorporating advanced technical enhancements into the FDA process took place in two stages. The first involved the operationalisation of the framework shown in Figure 4 to create the ‘Forensic Information Modelling Visualiser’ (FIMViz) prototype, which in turn would accept real-world project data, extracted from a contemporary construction project, to produce a case-based simulation. The second stage involved subjecting the enhanced FDA process model and the FIMViz prototype to expert evaluation. The FIMViz was developed to operationalise the data workflow of the enhanced FDA process model, to test the viability and integration of its innovative interventions, and to assess the impact of each.
Figure 5 shows a simplified data workflow between the four technology-based interventions (DBMS, BIM, game engine, AI) in the enhanced FDA process and in the FIMViz prototype. The following sections deal with the operationalisation, within the FIMViz prototype, of the four digital interventions.
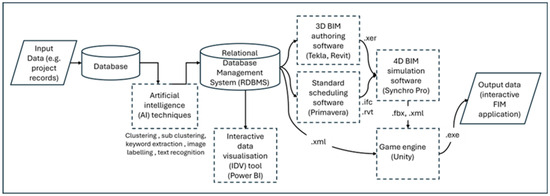
Figure 5.
Simplified view of the data workflow adopted to generate the FIMViz prototype.
4.1. RDBMS Intervention
The RDBMS is used to establish the relationships between various project records. Key data, such as contract documents, construction progress records, and BIM data, are linked using identifiers like the task IDs and room numbers, which are then used to generate relational data for analysis. In cases where the client lacks an internal RDBMS or is unwilling to provide access, the delay analysts can manually create these relationships. In creating the FIMViz prototype, the RDBMS intervention involved manually linking project records from the client, as access to the client’s database was not available. This process was automated using AI techniques discussed in Section 4.4. The RDBMS also facilitated the extraction of data into formats such as .ifc and .xer, which were used in the BIM and 4D simulations. Microsoft Access [51] was selected as a data organising tool and also as a bridging tool to transfer data from one software tool to another. For instance, it was used to generate forensic data by using unique identifiers to establish relational links between the database and the BIM models. This relational data was eventually transferred to the game engine, as discussed in Section 4.3. In summary, the RDBMS enhanced the FDA process by reducing the time spent on data retrieval and improving the accuracy of the analysis, ensuring that relevant data could be filtered and analysed efficiently. It should be noted that although the case is made here for integrating all four of the proposed digital technologies into the FDA process, each would offer significant benefits if adopted singly; for example, the adoption of an RDBMS could, on its own, enhance the effectiveness, efficiency, and collaborative potential of the FDA process.
4.2. BIM Intervention
On reviewing the BIM data available in the case study, deficiencies and missing data were found. Modelling was incomplete in some cases and relational links with project records were lacking. In creating the FIMViz simulation, this necessitated further effort to model additional elements and to build relational links with other project records. These inadequacies were to be expected, given the model authors’ unawareness of its potential use for FDA, and the suitability of the models can be expected to improve as familiarity increases. These additional model generations and modifications were carried out in the original BIM authoring software, namely Revit [52] and Tekla Structures [53]. For the 4D simulation aspect, Synchro 4D Pro [54] was chosen due to the researchers’ familiarity with the software and its seamless data exchange capabilities with the BIM authoring software. The resulting 4D simulation can be output either as media files for presenting the findings in the form of images in reports and static presentations, or animated videos. Alternatively, the final output can be exported (in a ‘.fbx’ file format) to a game engine, as it was in this case to enable the interactive FIMViz application (see Section 4.3). In summary, the BIM intervention in the FDA process was designed, and applied in the FIMViz tool, to facilitate analysis and to produce more meaningful outputs for the presentation of findings. The use of 4D simulations allow analysts to visualise delays more effectively, enabling more accurate and rapid analysis and more reliable and convincing communication.
4.3. Game Engine Intervention
A game engine tool allows users to interact with BIM data in a more immersive and interactive way. Additionally, the game engine’s element query feature was used to fetch the metadata attached to the model elements and display it, providing users with easy access to important information about specific model elements. For this layer of the FDA intervention, the Unity game engine [55] was chosen due to familiarity with the software itself, its scripting language, ‘C#’, and its integration with additional development tools. The process of integrating the game engine consisted of three main stages: (1) importing data into the game engine, (2) creating a FIMViz application in the game engine, and (3) enabling the export of the application as an output of this process. The following paragraphs describe these three stages.
Stage (1): Importing data into the game engine. Despite the attraction of the benefits offered by the game engine, the process of importing data from BIM authoring platforms is far from straightforward [56]. The data to be imported originates from two sources, namely BIM files and the RDBMS. The most common approach for the transfer of BIM data across BIM authoring tools is to use the industry foundation class file format (.ifc). Although Unity does not allow direct import of this file format, the exchange is possible via plug-ins and intermediaries; for example, Bille et al. [56] used 3ds Max [57] as an intermediary bridging tool to Unity. In this present study, a more accessible approach (that did not require the use of 3ds Max or any plug-in) was used by employing Synchro 4D Pro [54] as a transition tool between the BIM authoring software and the game engine. Here instead, ‘filmbox’ (.fbx) and ‘extensible markup language’ (.xml) files, which are natively supported by game engines, were used. The .fbx file format was used to store the geometric and animation data, and the .xml file format for the BIM metadata. Both files were linked within the Unity game engine using the unique identifiers that were common in both files. A variety of tools have recently been released that optimise the data exchange between BIM authoring tools and game engines. These remained untested in the current study, but their claims and potential have been discussed in [58] (pp. 115–117).
Stage (2): Creating a FIMViz application in the game engine. This required the creation of a design map that includes all the necessary elements for analysis, such as scenes, canvas (i.e., user interface, UI), 3D elements, cameras, lights, controllers, players, components, and software development kits. Using the design map as a basis, individual elements of the tool were designed. This included creating different scenes, such as an opening scene, welcoming scene, main menu scene, interactive scenes with different views (e.g., baseline versus as-built comparison view), Gantt chart scenes, interactive information pop-up window scenes, and documentation scenes for direct access to the project database (e.g., for additional information about a specific element or delay). The user interface (UI) elements of each scene in the tool were also designed and linked with 3D elements, 4D simulation parameters, cameras, and other necessary elements of the game engine (e.g., components, players, and scripted tools). The scripting was performed using the C# language in Visual Studio 2019 [59] supported by Unity.
Stage (3): Enabling the export from the application. After completing the design, an interactive tool, the FIMViz, was built as a standalone PC application compatible with most desktop platforms (Unity, like many game engines, caters for a wide range of platforms).
4.4. AI Intervention
The focus of AI adoption was the information retrieval stage of the FDA process. Various AI approaches were explored. Techniques, such as the k-means clustering algorithm [60] and GPT-3.5 models [61], were used. The programming was conducted using Python 3.9 [62] and employed a variety of modules, libraries, and tools, brief descriptions of which are given in Table 2.

Table 2.
Descriptions of the AI modules, libraries, and tools used.
Figure 6 presents a simplified data workflow that depicts the AI intervention in the information retrieval stage of the FDA process.
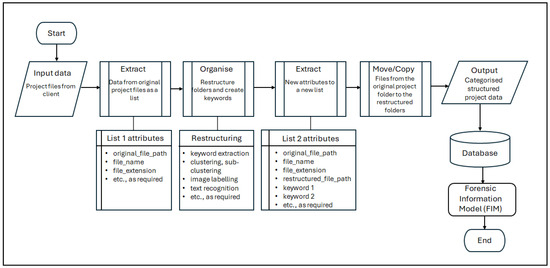
Figure 6.
Simplified view of the data workflow adopted for the AI intervention in the information retrieval stage of the FDA process.
The FDA process typically begins with the receipt of project records from the commissioning party. In practice, these records are frequently unorganised and voluminous. To overcome this, the first task is to import the necessary libraries and set the API key to interact with the GPT-3.5 models. The data is then extracted from the original project folder as a list in a ‘comma-separated-values’ (.csv) format containing attributes, such as original_file_path, file_name, file_extension, etc. These attributes are used to organise the files by restructuring these folders and creating useful keywords to benefit the data analysis task. The scripting tool reads these files to apply AI approaches, such as keyword extraction, clustering, sub-clustering, image labelling, text recognition, etc. In the tool, the Python 3.9 programming language, GPT-3.5 models, k-means (clustering algorithm) with the scikit-learn library, and other tools are used.
Keywords are extracted from the file paths, file names of the project records, etc. The extract_keywords function uses the GPT-3.5 models to extract from the attributes keywords (such as original_file_path and file_name) that are useful for understanding the context or the content of the file. The get_file_name_embedding and get_file_path_embedding functions generate ‘embeddings’ (i.e., mathematical representations of the textual data) for file names and file path names. If GPT-3.5 models fail to generate embeddings, a CountVectorizer can be used as a fallback. Keywords are used to group these files with different AI approaches, clustering and sub-clustering. The function cluster_files_recursive employs the k-means algorithm to cluster these files based on their keywords. It also allows for sub-clustering, providing a hierarchical structure. These techniques create new restructured file paths and extract meaningful keywords from these files to benefit the subsequent steps of the FDA process.
As an output of the above techniques, a new list that has attributes such as the original file path, file name, file extension, restructured file path, keyword 1, keyword 2, etc., can be extracted. Using these attributes, the files (i.e., project records) are copied from the original project folder to the restructured path. This can be a move operation (to relieve server storage space) or a copy operation. The new restructured folder system complies with the list of project records required for the analysis and is the same folder structure (which includes the required project records) used in the DBMS intervention to the FDA process.
In summary, AI interventions can reduce the time spent on data organisation and retrieval, significantly improving the efficiency of the FDA process. The project data, now organised and structured, is then used to populate the database and link with the BIM models to create an interactive FIMViz application for further analysis. For instance, in the case study, the restructured file paths of the project records are used as links. When the user clicks on a related element in the interactive environment, a new window pops up with further details about this specific element (i.e., further evidence relating to a delay event).
4.5. Operation of the Film FIMViz Prototype
The FIMViz prototype was created to integrate and operationalise the interventions within the enhanced FDA process model to demonstrate its functionality. The following sections explain how this was achieved using data from a contemporary project, and the accompanying screenshots (Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12 and Figure 13) illustrate the resulting case-based simulation.

Figure 7.
FIMViz simulation: ‘main scene’.
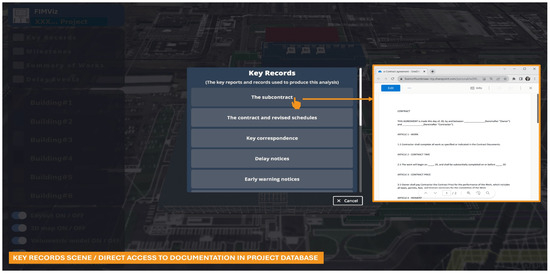
Figure 8.
FIMViz ‘key records’ scene/direct access to documentation in the project database.
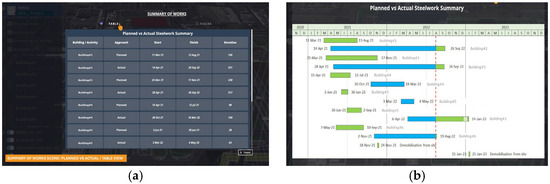
Figure 9.
FIMViz ‘summary of the works’ scenes (planned vs. actual) in the (a) table and (b) Gantt views.

Figure 10.
FIMViz ‘delay events’ scene/design-related delay event.

Figure 11.
FIMViz ‘delay events’ scene/handover-related delay event (planned vs. actual 4D view).

Figure 12.
FIMViz ‘delay events’ scene/handover-related delay event in the (a) table and (b) Gantt views [with highlighting added].

Figure 13.
FIMViz interactive scene/specific building (planned vs. actual) showing the (a) phase setting toggles and (b) interactive information windows.
The ‘main scene’ shown in Figure 7 is taken from information provided in the executive summary of an actual FDA report and presents a 3D environment of the project in basic geometric form. The layout buttons allow access to executive summary sections (such as the key records, milestones, summary of works, and a list of delay events) or to detailed views of each building. In the FIMViz prototype, these are all colour-coded to match the volumetric model. Additionally, the lower-left corner of the screen includes toggles for functionalities, such as on-or-off switching of the layout, 3D map, volumetric model, and steel model.
Figure 8, a sub-scene of the tool, offers direct access to the ‘key records’, allowing users to open relevant project documents by clicking on individual elements.
Figure 9 illustrates the ‘summary of the works’ sub-scene, comparing the planned and actual progress in a summary, through both table (Figure 9a) and Gantt chart (Figure 9b) views.
Figure 10 displays a ‘delay events’ sub-scene, highlighting a specific design-related delay event along with its corresponding timeline. The ‘more’ button in the lower right provides access to supporting evidence from the project database for this delay event.
Figure 11 shows a sub-sub-scene of ‘delay events’ featuring a specific handover-related delay event. It displays an interactive 4D view comparing visualising the planned and actual progress in the handover of steel erection phases. Each phase of steel erection requires a working laydown area ahead of it. For example, Phase 3 erection requires the availability of the area (Erection area—Phase 3) as well as the working area (Laydown area—Phase 4) to be available. Figure 11 illustrates this plan (on the left) and the actual situation (on the right), with delayed availabilities of both areas.
Further details of the same handover-related delay event are shown in tabular and Gantt chart formats in Figure 12. The tabular (Figure 12a) and Gantt chart (Figure 12b) formats offer alternative views of the planned and actual handover dates. The delay to the availability of Phase 3 and its impact on the completion of Phase 2 of the main frame steelwork are indicated by highlighting.
Figure 13 offers a detailed analysis of an individual building (this example being the data centre building). These interactive scenes allow users to compare the planned versus actual progress side-by-side. Users can interact with geometric elements by clicking on them to view an information window on a specific element or by toggling each phase on or off.
In summary, the FIMViz is a prototype tool in which the enhanced FDA process model was operationalised. This served several purposes. First, by the input of contemporary real-world construction project data, a case-based simulation was produced to satisfy the internal validity of the model’s data flow and the successful incorporation of different digital technology that could help overcome the limitations of conventional FDA. Secondly, the interactive nature of the FIMViz tool allows users to explore delay scenarios in detail, with the aim of enhancing the retrieval of information, the accuracy of its analysis and the effectiveness of its communication. Finally, the FIMViz tool also played a role in the next stage of the study: the evaluation by experts.
5. Evaluation of the Enhanced Conceptual Model and the FIMViz
The successful exchange of data between the digital technologies within the FIMViz and its ability to generate credible FDA outputs was itself a first step in verifying the applicability of the enhanced conceptual model. The next step was to subject the model and the FIMViz prototype to expert evaluation. The purpose of this part of the study was to evaluate the credibility and applicability of the conceptual model and its realisation in the FIMViz prototype. The background to the interviews and their administration has been described earlier, in Section 2.
There were eleven one-to-one web-based interviews of 1–2 h duration with individuals who had agreed (in their earlier questionnaire responses) to participate. Each interview took place in a Teams [63] meeting environment. The interviewer’s screen was shared with the interviewee to facilitate a structured and focused discussion. Responses to closed-ended questions were collected using both Qualtrics Surveys [64] and Mentimeter [65] to ensure accuracy and consistency. For open-ended questions, the recording and transcription feature of Teams was utilised as the primary data collection tool, eliminating the need for note taking during the sessions.
A majority (n = 8) identified themselves as ‘forensic delay analysts’; five had ‘over 15 years’ of experience and four between ‘11 and 15’ years in FDA. Of the 11 interviewees, 4 were ‘highly familiar’ with both the AACE (2011) and SCL (2017) methodologies; 4 were ‘highly familiar’; and 3 ‘familiar’ with SCL (2017) only.
The interviewees were first asked about the enhanced FDA process model (a detailed copy of which had previously been provided) and its proposed digital interventions. Next, they were shown a short video presentation of the FIMViz tool that operationalised the model. Finally, they were asked to evaluate the tool and re-evaluate the enhanced FDA process model in the light of what they had seen.
5.1. Evaluation of the Model
In evaluating the model as a whole, five interviewees were in ‘complete agreement’ and a further five ‘mostly agreed’ with the model’s effectiveness in covering all aspects of an ideal FDA process, in mitigating the challenges and shortcomings of conventional FDA, and in representing an ideal FDA workflow. Following the introductory questions, the focus was on the detail within each of the model’s stages. Using a five-point scale (exactly-mostly-somewhat-very little-not at all) interviewees reported to what extent they agreed with what was presented in each stage of the enhanced model. (As noted above, a detailed description of each stage of the FDA process model, together with any digital interventions, had previously been provided to each interviewee).
The first of these stages concerns information retrieval. Of the challenges to effective FDA highlighted in the literature (see Section 3.1) the most severe is the identification, sourcing, retrieval, and validation of relevant information [3,4]. When asked whether the detailed processes they were shown corresponded with an ideal FDA workflow, four interviewees responded ‘mostly’ and seven ‘exactly’, indicating strong confidence in the detailed content of this stage of the enhanced model. They were then asked about sources of information. In the literature, the information that is relevant to FDA is identified as programmes (or ‘schedules’), contract documents, and records (of progress, resources, costs) as well as administrative correspondence [5,6,7]. Additionally, given its fundamental importance to the proposed digital enhancements in this study, interviewees were asked about BIM data as an information source. Interviewees were requested to rank each of these seven information sources according to two criteria: ‘desirability’ (i.e., how important they were to the FDA process) and ‘availability’ (i.e., how readily available they usually were). Programmes and contract documents were reported to be the most ‘desirable’ and normally ‘available’ sources indicating their integral role in the FDA process. However, progress records were desirable but often not available, indicating that efforts to improve their availability could significantly benefit FDA processes. Correspondence and administration records were seen as reasonably available and moderately desirable. Resource records are ‘somewhat less available’ but maintain a moderate level of desirability, indicating that, while they may be important for the FDA process, accessing them may be difficult. Cost records were considered ‘somewhat accessible’ and ‘moderately desirable’. Interviewees did not anticipate any significant future change in their views about the availability or desirability of these six information sources. By contrast, ‘BIM data’ was considered currently to be ‘moderately desirable’ but not readily available, though five (out of the eleven) interviewees expected this to change as BIM maturity improves, with the likelihood that in future BIM data would become both more desirable and more avail-able.
The next stage in the model to be evaluated was the analysis of delay. Six interviewees considered that the detailed workflows in this stage of the enhanced model aligned ‘exactly’, and three ‘mainly’, with what they considered to be an ideal FDA workflow. In terms of overcoming the challenges to effective FDA, the second major challenge (see Section 3.1) was the lack of consensus on how to analyse the evidence [2,8,9,18]. Attempts have been made by professional organisations [10,11,22] to address the confusion and subjectivity highlighted earlier [8,9] though these protocols are not in themselves panaceas [4,5]. Interviewees were first asked about their familiarity with the delay analysis protocols published by AACE [10] and SCL [11]. Four of the eleven interviewees felt ‘highly familiar’ with both protocols. Of the remainder, four were ‘highly familiar’ and three were ’familiar’ with the SCL document.
The highest level of interviewees’ agreement was observed in the communications of the findings stage in the enhanced FDA process model, where nine interviewees agreed that the model ‘exactly’ corresponds to what an ideal FDA workflow would include. In terms of its detailed content, interviewees were asked to assess, on a four-tier rating (critical-important-optional-unwanted) the different formats for presenting findings that were inherent in the enhanced FDA process. These methods include reports (e.g., in .pdf format), presentations (e.g., PowerPoint slides), animations (e.g., videos), and 3D/4D interactions (e.g., BIM-based applications such as Synchro [55]). The resulting prioritization was that reports were considered ‘critical’ for presenting FDA findings (with ten out of eleven responses); the next in order of importance being presentations (which two interviewees thought ‘critical’, and eight ‘important’), animations (four responses of ‘important’). Only one interviewee considered 3D/4D interactions ‘important’ while nine considered them ‘optional’. Interestingly, however, this view was to change after the viewing of the FIMViz application video (see Section 5.2, below).
5.2. Evaluation of the FIMViz Tool and Re-Evaluation of the Enhanced Process Model
Having viewed the FIMViz application video, interviewees were asked whether their perspectives had been influenced or whether their views had altered in any way. Five of the interviewees acknowledged the usefulness of the application, yet they did not anticipate any change in the views they had previously held. However, six interviewees anticipate changes in their attitude to the use of animations and 3D interactions. One predicted that these methods would gain importance and become easier to implement due to technological advancements. One, however, expressed concerns about the practicality of these methods, given the time constraints inherent in FDA processes. Three other interviewees expected 3D interactions to shift from being optional to important. One interviewee expected 3D interactions to change from being ‘unwanted’ to ‘important’, echoing similar sentiments about the impact of technological advancement.
Interviewees were then questioned as to whether they thought the FIMViz application improved the existing FDA process and mitigated its assumed challenges and shortcomings. Almost all the interviewees recognised its potential. Five of the eleven interviewees thought that the application ‘mostly’ improves the existing process and effectively addresses many of the assumed challenges and shortcomings, and for the remaining six it ‘somewhat’ did so.
Further insights into the effectiveness of the FIMViz application included three respondents who specifically highlighted its effectiveness for both analysis and presentation purposes, mentioning its capability to query or relate different datasets within the same environment and its useful presentation mode feature. Conversely, two interviewees perceived its effectiveness solely for presentation. One interviewee highlighted the challenge of users reaching the requisite stage of technical ability. Another raised time and budget constraints, noting the significant investment required. A third interviewee observed that it might be challenging for tech-adverse users, such as traditionalist arbitrators or adjudicators, to utilise it or interpret its output. From a similar perspective, another interviewee questioned the practical use of the application in settings like courtrooms.
5.3. Evaluation Summary
The findings suggest that the model is generally accurate, reliable, and relevant to the intended purpose. However, certain aspects of the model require attention. The model can benefit from being tested on a larger and more diverse dataset to ensure it adequately represents the various complexities of real-world FDA scenarios. Improvements that were suggested included the incorporation of additional features, such as advanced data analytics capabilities (already considered in the model’s AI features, though these were not included in the FIMViz presentation) and enhanced user interface designs. Such features could enhance the model’s ability to process complex data more efficiently and make it more user-friendly, thereby increasing its utility in practical applications.
Overall, the evaluation was a success. The findings from the evaluation also provided valuable insights into the model’s strengths and weaknesses. Suggestions on the model’s improvement included testing on large, diverse project datasets to ensure it adequately represents the various complexities of real-world FDA scenarios, and incorporating additional features, such as advanced data analytics capabilities and enhanced user interface designs. These features can enhance the model’s ability to process complex data more efficiently and make it more user-friendly, thereby increasing its utility in practical applications. These insights will be instrumental in refining the model further, making it even more effective and useful for professionals in the field.
6. Discussion and Conclusions
This study took place in response to a perceived problem in terms of the limitations inherent in the conventional FDA process, its inaccuracy and consequent contentious nature, and its inefficiency. All these problems were widely discussed in the literature. Using a systematic review, the conventional FDA process was modelled and verified by a survey of experts. Returning to the literature, the potential of digital interventions (DBMS, BIM, AI, and game engine) was explored. Using a DSR approach, an enhanced FTA process was modelled that included these digital interventions. The enhanced model was then used to create a working prototype, the FIMViz, which integrated the digital interventions into a working example of a full, simulated FDA. The enhanced model and the prototype were then subjected to expert evaluation. The model was found to be highly accurate in mapping out and representing an ideal FDA process. It captured the critical elements and phases of FDA with precision, ensuring that the model reflects real-world scenarios and challenges faced by practitioners. The model was found to be relevant to its intended purpose of streamlining and enhancing the efficiency of the FDA process. It addressed key issues and bottlenecks in the existing process, offering a more streamlined and effective approach to delay analysis. The model was found to be reliable, underpinned by its methodologically sound design and the positive feedback received during validation. This indicated that the model’s processes and outcomes were consistent with best practices in terms of FDA. The uniformity and consistency of the feedback from various experts in the field of FDA enhanced the model’s credibility as a reliable process.
6.1. Achievements of the Study
This study has demonstrated how emerging digital technologies can be integrated into the FDA process. By addressing the limitations of existing FDA practices, the research has shown the viability and practicality of incorporating these technologies into an enhanced FDA process that can improve the efficiency, accuracy, and communication of delay analysis. The use of DBMSs creates a streamlined, structured repository of project data with precise data linkage between BIM models and other delay records, reducing manual retrieval and processing times. The addition of AI-driven automation presents opportunities for automated data structuring and information retrieval. Individually, BIM and 4D tools offer a detailed understanding of project timelines, while game engines add an interactive dimension that enhances the spatial perception. Together, they empower practitioners to simulate delay impacts in real time, compare planned versus actual progress, and communicate findings more effectively to project participants through engaging, intuitive interfaces.
6.2. Contribution and Potential Impact
The contribution of this work to the overall body of professional knowledge has been to demonstrate the integration of available digital technologies (DBMSs, BIM, AI, and game engines) into the FDA process. Some of these technologies (for example, BIM) are already established in the construction management context, but they have not, hitherto, been applied in an integrated way to FDA processes. A framework was created that explains how these technologies can combine to provide an enhanced FDA process model with more efficient workflows and more accurate analyses that can be communicated more effectively. Whilst each intervention can singly address specific limitations of current FDA practices, their combined implementation demonstrates improvement from both the analytical and presentational perspectives. As a result of this work, researchers will gain further insight into the FDA process and how the application of emerging technical innovations can overcome the shortcomings that are currently encountered.
Practitioners, particularly those involved in the field of forensic delay analysis, should be its main beneficiaries. The combined application of these innovations extends the scope of FDA from a static, uncertain, document-driven process to one that is dynamic, reliable, and data-driven, thus reducing the potential for uncertainty, disagreement, and unnecessary dispute. Furthermore, these technical interventions have been realised in the development of the FIMViz application, an accessible, easily navigable, and practical tool that leverages these technologies and, having been subjected to expert evaluation, confirms the transformative potential of this integrated approach.
Beyond its importance to those directly involved in delay analysis, this work also has practical implications for the many professionals involved in the planning and execution of construction projects, where the integration of BIM, 4D simulation, and game engines can provide a dynamic platform for visualising and simulating delay scenarios. The findings emphasise the importance of adopting integrated digital systems for more efficient, transparent, and reliable schedule management. By enabling interactive analysis of project data, practitioners can better anticipate and understand delay impacts, communicate findings effectively, and adopt collaboration across project participants.
6.3. Limitations and Further Work
Inevitably, this work has its limitations. It was noted in Section 1 that a variety of analytical perspectives can be applied to FDA and the present study has not followed all of them. The classification of these different perspectives [4] reveals certain real-world variables against which any FDA advances should be tested. These include the context of individual projects (geography, type, size, delivery), and their differing contractual or legal arrangements. Therefore the enhanced model can benefit from being tested against a wider range of projects and diverse contexts to ensure it adequately represents the various complexities of real-world FDA scenarios.
The proposed AI interventions in the enhanced FDA process were limited to its early stages (information retrieval) but could in future be extended. Although AI and ML were successfully integrated in the creation of the FIMViz prototype (as discussed in Section 4), their functionality did not feature explicitly in the audio–visual presentation of the FIMViz to the expert evaluators. Consequently, there was no expert evaluation of the AI intervention, although it was part of the enhanced process model. The improvements that were suggested by the expert evaluators included the incorporation additional features, such as advanced data analytics capabilities (as noted, the model’s AI features were not explicit in the FIMViz presentation) and enhanced user interface designs. Such features could enhance the model’s ability to process complex data more efficiently and make it more user-friendly, thereby increasing its utility in practical applications.
Future research should aim to broaden the applicability of this study across a wider range of construction projects and delay scenarios. Additionally, further exploration of the integration of other emerging technologies, such as extended reality and blockchain, into FDA processes could yield valuable insights. Advanced AI techniques combined with simulation-based optimisation present an opportunity for future research efforts to automate scenario creation for identifying potential delay events and implementing preventive actions, thus extending the applicability of FDA from a purely forensic, post hoc tool to one with a predictive risk management capability.
Author Contributions
Conceptualisation, S.E.B., D.G. and A.P.; methodology, S.E.B., D.G. and K.R.; software, S.E.B. and K.R.; validation, S.E.B. and A.P.; formal analysis, S.E.B.; investigation, S.E.B.; resources, S.E.B. and A.P.; data curation, S.E.B.; writing—original draft preparation, S.E.B.; writing—review & editing, S.E.B. and D.G.; visualisation, S.E.B.; supervision, D.G. and K.R.; project administration, D.G. and A.P.; funding acquisition, D.G. and A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was based on work completed as part of a PhD studentship for which funding was received from the European Regional Development Agency, IIIP NE studentship agreement with Project Advisors International (UK) Ltd. and Northumbria University (IIPNE3UNN005).
Data Availability Statement
Materials and data that support the findings of this study are available from the corresponding author upon reasonable request: this includes a video-based narrated demonstration of the FIMViz simulation. Some of the data, models, or code generated or used during the study are proprietary and/or confidential: this includes the raw data used in creating the FIMViz simulation.
Acknowledgments
The authors would like to express their gratitude to the owners and staff of Project Advisors International for their support with this project.
Conflicts of Interest
The authors confirm that the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| 3D | Three-dimensional (applied to BIM models with geometric information only) |
| 4D | Four-dimensional (applied to BIM models with additional time schedule information) |
| 5D | Five-dimensional (applied to BIM models with additional cost-related information) |
| AACE | Association for the Advancement of Cost Engineering |
| AI | Artificial Intelligence |
| API | Application Programming Interface |
| BIM | Building Information Modelling |
| DBMS | Database Management System |
| DSR | Design Science Research |
| FDA | Forensic Delay Analysis |
| FIM | Forensic Information Model |
| FIMViz | Forensic Information Modelling Visualiser |
| GPT | Generative Pre-trained Transformer (as in GPT-3.5) |
| ML | Machine Learning |
| NLP | Natural Language Processing |
| PC | Personal Computer |
| RDBMS | Relational Database Management System(s) |
| SCL | Society of Construction Law |
| UI | User interface |
References
- Arcadis. Global Construction Disputes Report. 2022. Available online: https://www.arcadis.com/en-us/insights/perspectives/north-america/united-states/2023/construction-disputes-report-2023 (accessed on 27 March 2025).
- Braimah, N. Construction Delay Analysis Techniques -A Review of Application Issues and Improvement Needs. Buildings 2013, 3, 506–531. [Google Scholar] [CrossRef]
- Gibbs, D.-J. Development of Building Information Models (BIM) to Support Innovative Time Management and Delay Analysis. Ph.D. Thesis, Loughborough University, Loughborough, UK, 2017. [Google Scholar]
- Grzeszczyk, G.; Sainati, T.; Unterhitzenberger, C. The evolution of forensic delay analysis: A literature review investigating changes and progress in project management approaches to delay measurement. J. Legal Aff. Dispute Resolut. Eng. Constr. 2024, 16, 03123001. [Google Scholar] [CrossRef]
- Vidogah, W.; Ndekugri, I. Improving management of claims: Contractors’ perspective. J. Manag. Eng. 1997, 13, 37–44. [Google Scholar] [CrossRef]
- Alkass, S.; Mazerolle, M.; Tribaldos, E.; Harris, F. Computer Aided Construction Delay Analysis and Claims Preparation. Constr. Manag. Econ. 1995, 13, 335–352. [Google Scholar] [CrossRef]
- Atanasov, V.; Greenwood, D.; Robson, S. The management of disputes as an element of construction transaction costs: An empirical study. In Proceedings of the 36th Annual ARCOM Conference, Lincoln, UK, 7–8 September 2020. [Google Scholar]
- Parry, A. The Improvement of Delay Analysis in the UK Construction Industry. Ph.D. Thesis, Northumbria University, Newcastle upon Tyne, UK, 2015. [Google Scholar]
- Perera, N.A.; Sutrisna, M.; Yiu, T.W. Decision-Making Model for Selecting the Optimum Method of Delay Analysis in Construction Projects. J. Manag. Eng. 2016, 32, 04016009. [Google Scholar] [CrossRef]
- Association for the Advancement of Cost Engineering International (AACE). International Recommended Practice No. 29R-03, Forensic Schedule Analysis. 2011. Available online: http://web.aacei.org/resources/recommended-practices (accessed on 27 March 2025).
- Society of Construction Law Delay and Disruption Protocol, 2nd ed.; Society of Construction Law: London, UK, 2017; Available online: https://www.scl.org.uk/sites/default/files/documents/SCL_Delay_Protocol_2nd_Edition_Final.pdf (accessed on 27 March 2025).
- Schwab, K. The Fourth Industrial Revolution: What it means, how to respond. In Handbook of Research on Strategic Leadership in the Fourth Industrial Revolution; Edward Elgar Publishing: Cheltenham, UK, 2024; pp. 29–34. [Google Scholar]
- Sawhney, A.; Riley, M.; Irizarry, J. Construction 4.0 an Innovation Platform for the Built Environment; Routledge: London, UK, 2020. [Google Scholar]
- Turner, C.J.; Oyekan, J.; Stergioulas, L.; Griffin, D. Utilizing industry 4.0 on the construction site: Challenges and opportunities. IEEE Trans. Ind. Inform. 2020, 17, 746–756. [Google Scholar] [CrossRef]
- Hevner, A.R.; March, S.T.; Park, J.; Ram, S. Design science in information systems research. MIS Q. 2004, 28, 75–105. [Google Scholar] [CrossRef]
- Chou, H.Y.; Yang, J.B. Preliminary evaluation of BIM-based approaches for schedule delay analysis. Mat. Sci. Eng. 2017, 245, 062048. [Google Scholar] [CrossRef]
- Chen, J.Q. AI-enabled digital forensic evidence examination. In Advances in Information and Communication: Proceedings of the 2020 Future of Information and Communication Conference (FICC); Arai, K., Kapoor, S., Bhatia, R., Eds.; Springer International Publishing: New York, USA, 2020; Volume 1, pp. 832–841. [Google Scholar]
- Arditi, D.; Pattanakitchamroon, T. Analysis methods in time-based claims. J. Constr. Eng. Manag. 2008, 134, 242–252. [Google Scholar] [CrossRef]
- Marzouk, M.; Othman, A.; Enaba, M.; Zaher, M. Using BIM to Identify Claims Early in the Construction Industry: Case Study. J. Leg. Aff. Disput. Resolut. Eng. Constr. 2018, 10, 05018001. [Google Scholar] [CrossRef]
- Yang, J.; Arditi, D.; Lee, D.-E.; Mutis, I. Delay Analysis Selection Model for a Construction Project. KSCE J. Civ. Eng. 2022, 26, 4926–4941. [Google Scholar] [CrossRef]
- Ali, B.; Zahoor, H.; Nasir, A.R.; Maqsoom, A.; Khan, R.W.A.; Mazher, K.M. BIM-Based Claims Management System: A Centralized Information Repository for Extension of Time Claims. Autom. Constr. 2020, 110, 102937. [Google Scholar] [CrossRef]
- HKA. A Statistical Review of Delay Analysis Methods Used Over the Last Decade. 2022. Available online: https://www.hka.com/article/a-statistical-review-of-delay-analysis-techniques-used-over-the-last-decade/#:~:text=Discussion,the%20nature%20of%20the%20project (accessed on 27 March 2025).
- Bubshait, A.; Cunningham, M. Comparison of Delay Analysis Methodologies. J. Constr. Eng. Manag. 1998, 124, 315–322. [Google Scholar] [CrossRef]
- Kumaraswamy, M.; Yogeswaran, K. Substantiation and Assessment of Claims for Extensions of Time. Int. J. Proj. Manag. 2003, 21, 27–38. [Google Scholar] [CrossRef]
- Cleveland Bridge UK Ltd. V Severfield-Rowen Structures Ltd. [2012] EWHC 3652 (TCC). Available online: https://www.casemine.com/judgement/uk/5a8ff7d960d03e7f57eb2754 (accessed on 19 May 2025).
- Gibbs, D.-J.; Emmitt, S.; Ruikar, K.; Lord, W. Recommendations on the creation of computer-generated exhibits for construction delay claims. Constr. Law J. 2014, 30, 236–248. [Google Scholar]
- Heaton, J.; Parlikad, A.K.; Owens, D.; Pawsey, N. BIM as an Enabler for Digital Transformation. In Proceedings of the International Conference on Smart Infrastructure and Construction (ICSIC): Driving Data-Informed Decision-Making; ICE Publishing: London, UK, 2019; pp. 49–54. [Google Scholar]
- Le, H.T.T.; Likhitruangsilp, V.; Yabuki, N. A BIM-integrated relational database management system for evaluating building life-cycle costs. Eng. J. 2020, 24, 75–86. [Google Scholar] [CrossRef]
- SSJA Al-Sabah, S.M.; Fereig, D.J. Hoare, A database management system to document and analyse construction claims. Adv. Eng. Softw. 2003, 34, 477–491. [Google Scholar] [CrossRef]
- Al Shami, K. Investigating the use of building information modeling (BIM) in managing construction claims. PM World J. 2018, 7, 1–17. [Google Scholar]
- Eastman, C.M.; Eastman, C.; Teicholz, P.; Sacks, R.; Liston, K. BIM Handbook: A Guide to Building Information Modeling for Owners, Managers, Designers, Engineers and Contractors; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Bryde, D.; Broquetas, M.; Volm, J.M. The project benefits of building information modelling (BIM). Int. J. Proj. Manag. 2013, 31, 971–980. [Google Scholar] [CrossRef]
- Demian, P.; Walters, D. The advantages of information management through building information modelling. Constr. Manag. Econ. 2014, 32, 1153–1165. [Google Scholar] [CrossRef]
- Jung, W.; Lee, G. The status of BIM adoption on six continents. J. Civ. Environ. Eng. 2015, 9, 444–448. [Google Scholar]
- Abbasnejad, B.; Nepal, M.P.; Ahankoob, A.; Nasirian, A.; Drogemuller, R. Building Information Modelling (BIM) adoption and implementation enablers in AEC firms: A systematic literature review. Archit. Eng. Des. Manag. 2021, 17, 411–433. [Google Scholar] [CrossRef]
- Khudhair, A.; Li, H.; Ren, G.; Liu, S. Towards future BIM technology innovations: A bibliometric analysis of the literature. Appl. Sci. 2021, 11, 1232. [Google Scholar] [CrossRef]
- Charef, R.; Alaka, H.; Emmitt, S. Beyond the third dimension of BIM: A systematic review of literature and assessment of professional views. J. Build. Eng. 2018, 19, 242–257. [Google Scholar] [CrossRef]
- Koutamanis, A. Dimensionality in BIM: Why BIM cannot have more than four dimensions? Autom. Constr. 2020, 114, 103153. [Google Scholar] [CrossRef]
- Lopez, R.; Chong, H.-Y.; Wang, X.; Graham, J. Technical review: Analysis and appraisal of four-dimensional building information modeling Usability in construction and engineering projects. Constr. Eng. Manag. 2016, 142, 1–6. [Google Scholar] [CrossRef]
- Guévremont, M.; Hammad, A. Ontology for Linking Delay Claims with 4D Simulation to Analyze Effects-Causes and Responsibilities. J. Leg. Aff. Disput. Resolut. Eng. Constr. 2021, 13, 04521024. [Google Scholar] [CrossRef]
- Valavanoglou, A.; Heck, D. Building Information Modeling and Forensic Analysis of Delay and Disruption. Proc. Int. Struct. Eng. Constr. 2020, 3, 527–532. [Google Scholar] [CrossRef]
- Anderson, J.R.; Qin, Y.; Jung, K.J.; Carter, C.S. Information-processing modules and their relative modality specificity. Cogn. Psychol. 2007, 54, 185–217. [Google Scholar] [CrossRef]
- Balali, V.; Noghabaei, M.; Heydarian, A.; Han, K. Improved stakeholder communication and visualizations: Real-time interaction and cost estimation within immersive virtual environments. In Construction Research Congress; American Society of Civil Engineers: Reston, VA, USA, 2018; pp. 522–530. [Google Scholar]
- Osorio-Sandoval, C.A.; Tizani, W.; Pereira, E.; Ninić, J.; Koch, C. Framework for BIM-based simulation of construction operations implemented in a game engine. Buildings 2022, 12, 1199. [Google Scholar] [CrossRef]
- Mariaca Clavel, I.S.; Florez-Perez, L.; Galera Zarco, C. Game-Based Interactive Framework for Construction Scheduling: Bridging the Gap between Traditional Scheduling Tools. Simulation Methods and 4D Bim. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=5129656, (accessed on 27 May 2025).
- Edwards, G.; Li, H.; Wang, B. BIM based collaborative and interactive design process using computer game engine for general end-users. Vis. Eng. 2015, 3, 4. [Google Scholar] [CrossRef]
- Abdirad, H.; Mathur, P. Artificial Intelligence for BIM Content Management and Delivery: Case Study of Association Rule Mining for Construction Detailing. Adv. Eng. Inform. 2021, 50, 101414. [Google Scholar] [CrossRef]
- Kim, H.; Soibelman, L.; Grobler, F. Factor selection for delay analysis using Knowledge Discovery in Databases. Autom. Constr. 2008, 17, 550–560. [Google Scholar] [CrossRef]
- Asadi, A.; Alsubaey, M.; Makatsoris, C. A Machine Learning Approach for Predicting Delays in Construction Logistics. Int. J. Adv. Logist. 2015, 4, 115–130. [Google Scholar] [CrossRef]
- Gondia, A.; Siam, A.; El-Dakhakhni, W.; Nassar, A.H. Machine Learning Algorithms for Construction Projects Delay Risk Prediction. J. Constr. Eng. Manag. 2020, 146, 04019085. [Google Scholar] [CrossRef]
- Microsoft. Microsoft Access [Computer Programme]. 2025. Available online: https://www.microsoft.com/en-gb/microsoft-365/access (accessed on 27 May 2025).
- Autodesk Revit (Version 2024) [Computer Programme]. Available online: https://www.autodesk.com/products/revit/overview?term=1-YEAR&tab=subscription (accessed on 27 May 2025).
- Trimble. Tekla Structures (Version 2022) [Computer Programme]. Available online: https://www.tekla.com/products/tekla-structures (accessed on 27 May 2025).
- Bentley Systems. Synchro 4D Pro (Version 6.5.2) [Computer Programme]. Available online: https://virtuosity.bentley.com/product/synchro-4d/ (accessed on 27 March 2025).
- Unity Technologies. Unity (Version 2023.1.16) [Computer Programme]. 2023. Available online: https://unity.com/ (accessed on 27 May 2025).
- Bille, R.; Smith, S.P.; Maund, K.; Brewer, G. Extending Building Information Models into Game Engines. In Proceedings of the Conference on Interactive Entertainment, Newcastle, Australia, 2–4 December 2014. [Google Scholar]
- Autodesk 3ds Max (Version 2024) [Computer Programme]. Available online: https://www.autodesk.eu/products/3ds-max/overview?term=1-YEAR&tab=subscription (accessed on 27 May 2025).
- Boyacioglu, S.E. A Digitally Driven System for Visualising the Forensic Delay Analysis of Construction Projects. Ph.D. Thesis, Northumbria University, Newcastle upon Tyne, UK, 2024. [Google Scholar]
- Microsoft. Visual Studio 2019 (Version 16.11.20) [Computer Programme]. Available online: https://visualstudio.microsoft.com/ (accessed on 27 May 2025).
- Wu, J.; Wu, J. Cluster analysis and K-means clustering: An introduction. In Advances in K-Means Clustering: A Data Mining Thinking; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2012; pp. 1–16. [Google Scholar]
- OpenAI GPT-3.5. OpenAI. Available online: https://platform.openai.com/docs/models (accessed on 27 May 2025).
- Python Software Foundation. Python (Version 3.9.6) [Computer Programme]. Available online: https://www.python.org/ (accessed on 27 May 2025).
- Microsoft Teams (Version 1.6.00.28557) [Computer Programme]. Available online: https://www.microsoft.com/en/microsoft-teams/group-chat-software (accessed on 27 May 2025).
- Qualtrics Surveys (Version 7.23) [Computer Programme]. Available online: https://www.qualtrics.com. (accessed on 27 May 2025).
- Mentimeter for PowerPoint (Version 4.3) [Computer Programme]. Available online: https://www.mentimeter.com/integrations/powerpoint (accessed on 27 May 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).