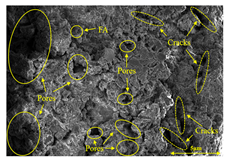
Abstract
The climate in extremely cold regions is becoming increasingly unstable, resulting in more frequent freeze-thaw cycles. These cycles significantly degrade the mechanical properties of soft soil foundations, reducing their bearing capacity and ultimately compromising the safety and lifespan of construction and infrastructure. To mitigate these effects, soil stabilization technology is commonly employed to reinforce soft soil in cold regions. However, evaluating the durability of stabilized soft soil, particularly its resistance to freezing in extremely cold environments, remains a critical challenge. This study investigates the use of industrial waste raw materials, such as slag and fly ash (FA), in combination with a solid alkali activator (NaOH), to develop one-part alkali-activated cementitious materials (ACMs) for soft soil stabilization. The effects of different raw material ratios, freeze-thaw temperatures, and the number of freeze-thaw cycles on the freezing resistance of one-part alkali-activated slag/FA (OP-ASF) stabilized soft soil were examined. Mass loss, unconfined compressive strength (UCS), and pH value were conducted to assess soil deterioration and structural integrity under freeze-thaw conditions. Additionally, microstructure analysis was conducted using scanning electron microscopy with energy dispersive X-ray spectrometry (SEM-EDS) and X-ray diffraction (XRD) to analyze hydration product formation and internal structure characteristics. Image-pro plus (IPP) was also employed for structure looseness evolution, providing deeper insights into the freezing resistance mechanisms of OP-ASF stabilized soft soil. The results indicated that as the freezing temperature decreases and the number of freeze-thaw cycles increases, both mass loss and UCS loss become more pronounced. When the ratio of slag to fly ash was optimized at 80:20, OP-ASF stabilized soft soil exhibited the highest freezing resistance, characterized by the lowest mass loss and UCS loss, along with the highest UCS and pH value. Furthermore, structure looseness remained at its lowest across all freeze-thaw temperatures and cycles, highlighting the beneficial role of slag and FA in OP-ASF. These findings contribute to the advancement of sustainable and durable construction materials by demonstrating the potential of one-part alkali-activated slag/fly ash for stabilizing soft soils in seasonally frozen regions.
1. Introduction
The durability of soft soil is significantly influenced by a range of external forces and long-term environmental factors that alter its physical and mechanical properties [1,2,3]. Such alterations destabilize the internal structure of the soil, leading to a decrease in bearing capacity and stability, which is especially critical in building engineering. Many seasonally frozen regions (e.g., in China, Russia, and Canada) in the Northern Hemisphere exhibit a mean annual ground temperature (MAGT) below 0 °C (as shown in Figure 1). In such regions, winter subzero temperatures trigger soil freezing, followed by summer thawing. Repeated freeze-thaw cycles are a major factor contributing to soil degradation, progressively weakening its mechanical properties over time [4,5,6]. The volume expansion and contraction of soil, combined with water migration during freeze-thaw cycles, leads to a significant reduction in the strength and stiffness of soft soil [7,8]. This degradation threatens the long-term safety of infrastructure, particularly roads, airport runways, and bridge foundations, which are highly susceptible to freeze-thaw damage [9,10]. Therefore, understanding the behavior of soft soil under freeze-thaw conditions is essential for the design, maintenance, and enhancement of infrastructure in cold regions [11].
Figure 1.
Mean annual ground temperature across the Northern Hemisphere.
Numerous studies have investigated the detrimental effects of freeze-thaw cycles on soil behavior. For instance, Zhang et al. [12] observed increased mass loss in microbial-stabilized engineering residue with rising freeze-thaw cycles, while Sorensen et al. [13] reported freeze-thaw cycles significantly degrade the mechanical properties of soil, noting that warming measures failed to mitigate such damage. To enhance the freezing resistance, stabilization technology is essential, particularly under freeze-thaw conditions. Traditional soil stabilization technology using Ordinary Portland Cement (OPC) [14,15], lime [16], gypsum [17], or their combinations [18,19] has been widely explored in construction and building engineering to improve the durability of soft soil. For instance, Lu et al. [20] studied the hydrothermal deformation characteristics of OPC stabilized soft soil under freeze-thaw cycles, finding that environmental temperature and the number of cycles significantly influence the unfrozen water content. Mansour et al. [21] studied the role of gypsum content in lime-stabilized soil, concluding that increasing the ratio of gypsum to lime up to a threshold level of 1.5 had no significant impact on soil performance. These binders enhance soil properties by reducing water content through hydration reactions and densifying soil structures via hydration products [22]. However, their production generates substantial greenhouse gas (e.g., sulfur dioxide [SO2] and nitrogen oxide [NOX]), raising sustainability concerns [23,24,25,26].
In contrast, alkali-activated cementitious materials (ACMs), which offer a more sustainable alternative, are synthesized from potential silicate-rich industrial waste materials (i.e., slag, red mud, and silica fume) [27,28,29,30] or natural pozzolanic materials (i.e., fly ash [FA]) [31,32]. Activated by alkaline activators (e.g., NaOH, KOH, Ca(OH)2, or water glass), ACMs form a gel-like substance with high mechanical performance at ambient temperatures, outperforming traditional binders in durability and carbon footprint, making them a preferred choice for construction and building engineering. Nevertheless, the traditional two-part method, which relies on liquid alkaline activators, poses challenges in cold regions due to transportation and storage difficulties [33]. A promising solution is the one-part ACM method, which utilizes solid activators for on-site practicality.
This study systematically investigated the mechanical properties and microstructure evolution of one-part alkali-activated slag/fly ash (OP-ASF) stabilized soft soil under freeze-thaw cycles. By analyzing the effects of different slag to FA ratios, freeze-thaw cycle temperatures, and freeze-thaw cycles, the study sought to elucidate the freeze-thaw resistance. Scanning electron microscopy with energy dispersive X-ray spectrometry (SEM-EDS) and X-ray diffraction (XRD) were used to examine the degradation mechanisms of OP-ASF stabilized soft soil, while Image-Pro Plus (IPP) software (version 6.0) was used to analyze the structure looseness index. The findings advance the optimization of ACM stabilization technology, offering insights to enhance the durability of infrastructure in cold regions.
2. Materials and Methods
2.1. Soft Soil
The soft soil used in this study was sourced from the East China coast. Its primary physical properties (e.g., natural moisture content, plastic limit, liquid limit, etc.) are shown in Figure 2. Figure 3 provides detailed chemical composition data obtained through X-ray fluorescence (XRF) analysis. The soft soil samples were prepared from remold soil with a 50% moisture content (as shown in Figure 1). Initially, the soft soil was dried in an oven at 105 °C for 24 h to remove all moisture. After drying, the soft soil was crushed and sieved using a 2.0 mm square-hole sieve. The particles that passed through the 2 mm sieve were used to prepare the remolded soil by adding a specified amount of water to match the natural moisture content of the in situ soft soil, as described by Min et al. [34].
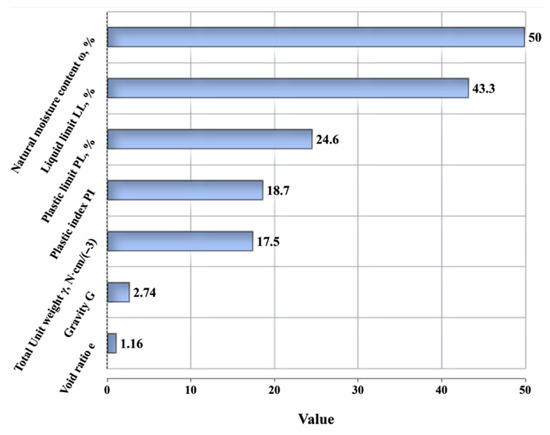
Figure 2.
Primary physical properties of soft soil.
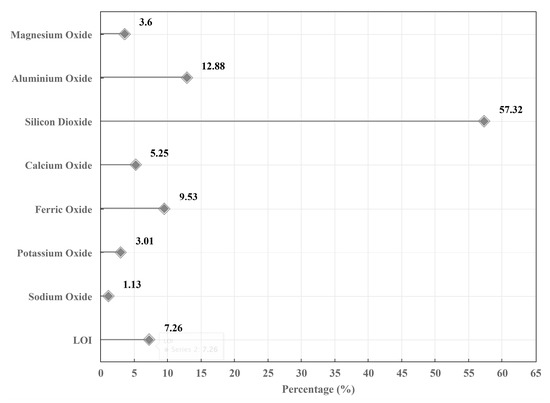
Figure 3.
Chemical composition of soft soil from XRF.
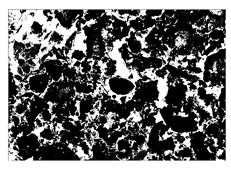
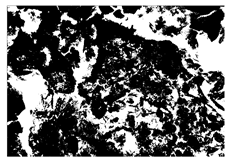
2.2. Alkali-Activated Raw Materials
Alkali-activated cementitious materials (ACMs) consist primarily of alkali-activated aluminosilicate substances. In this study, slag and fly ash (FA) were used as the main raw materials to prepare the precursor of ACM. Slag, a solid white powder, was obtained from a steel factory in Suqian, China, while FA, a solid black powder classified as F-class by ASTM C618 standard [35], was sourced from a coal power plant in Jiangyin, China. The particle size distribution of slag and FA is shown in Figure 4. The chemical compositions of slag and FA, analyzed by X-ray fluorescence (XRF) analysis, are detailed in Figure 5, respectively. Figure 6 and Figure 7 also display the micromorphology and mineral phases of slag and FA, as analyzed by scanning electron microscopy (SEM) and X-ray diffraction (XRD), respectively. It should be noted that the chemical and mineralogical characteristics of slag and FA can vary significantly by region due to differences in industrial processing (e.g., steel production methods, coal combustion types). Such variability may affect the reactivity and performance of alkali-activated materials. To accommodate local raw material differences and maintain consistent mechanical and durability performance, supplementary pozzolanic materials such as silica fume or metakaolin can be incorporated based on XRF results to compensate for insufficient reactive components (e.g., CaO, SiO2, or Al2O3). As the alkali activator for this study, commercially available solid NaOH flakes with a purity of 99% were employed.
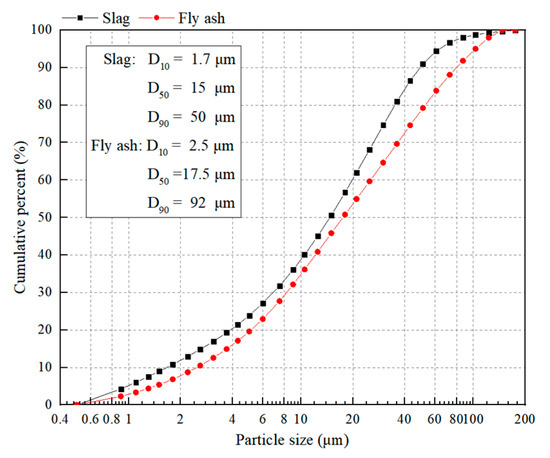
Figure 4.
Particle size distribution of slag and FA.
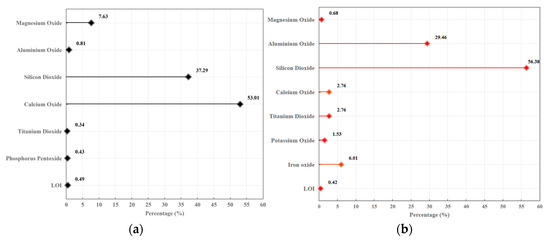
Figure 5.
Chemical composition from XRF analysis: (a) slag; (b) FA.
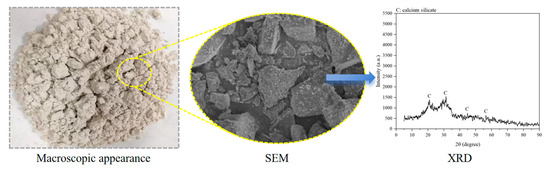
Figure 6.
Macroscopic and microscopic properties of slag.
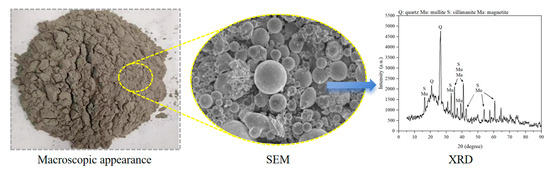
Figure 7.
Macroscopic and microscopic properties of FA.
2.3. ACM Stabilization Technology and F-T Condition
For one-part ACM stabilization technology, the mix proportions are presented in Table 1. The dry binder, consisting of a precursor mixture (slag + FA) and a solid alkaline activator (NaOH), made up 0.2 of the mass of dry soft soil, with NaOH accounting for 0.15 of the mass of the precursor [36]. The ratios of slag to fly used as precursors were 100:0, 90:10, 80:20, and 70:30, designated as SF100, SF90, SF80, and SF70, respectively. The water-to-solid mixture ratio (water: precursor [slag and FA] + solid alkaline activator [NaOH]) for the experiment was set at 0.7, as outlined by Li et al. [37].

Table 1.
Mix proportions of one-part ACM stabilization technology.
Following the preparation process shown in Figure 8, the stabilized soft soil samples, which had a diameter of 39.8 mm and a height of 80 mm, underwent 28 days of standard curing. After 1 day, OP-ASF stabilized soft soil samples were demolded, wrapped in cling film, and stored in a standard curing box (temperature: 20 ± 1 °C, humidity: above 95%) for 24 days. After this period, the samples were soaked in water for 4 days to achieve full saturation, allowing the internal pores to fill with water. During this soaking period, the water temperature was monitored daily, and warm water was added as needed to maintain the temperature at 20 ± 1 °C. Freeze-thaw cycles were performed according to Procedure B of the ASTM C666/C666M-03 standard test method [38]. During the freezing process, OP-ASF stabilized soft soil samples were placed in a cold box and completely frozen by cold air. Thawing was carried out at room temperature (20 ± 1 °C). Each freeze-thaw cycle consisted of 12 h of freezing followed by 12 h of thawing. Based on freezing conditions observed in cold regions, the experiment utilized freezing temperatures of −5, −10, and −20 °C, with a thawing room temperature of 20 ± 1 °C. The number of freeze-thaw cycles was set to 1, 2, 3, 4, 5, and 10, with four ratios of slag to FA applied at each stage. These experimental conditions were designed to evaluate the physical and mechanical properties of OP-ASF stabilized soft soil under freeze-thaw cycles. A detailed overview of the F-T cycle programs is provided in Table 2 below.
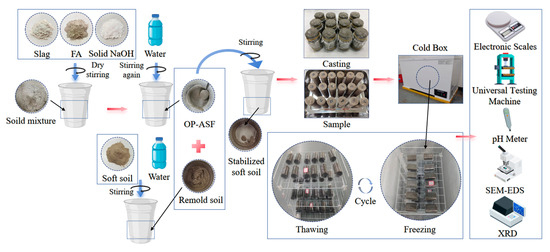
Figure 8.
Preparation process of OP-ASF stabilized soft soil sample.

Table 2.
F-T cycle programs.
2.4. Test Design
The design of each test, including the mass loss test, unconfined compressive strength (UCS) test, pH value test, and SEM-EDS analysis for OP-ASF stabilized soft soil under freeze-thaw cycles, is presented in Table 3. Symbol descriptions for mass loss and UCS tests are also provided. Before and after freeze-thaw cycles, the mass loss of each sample was measured and recorded. Afterward, a universal test machine (YS.WCX-II) was employed to determine the UCS values of the stabilized soft soil samples, both before and after freeze-thaw cycles. Soil particles finer than 1.0 mm were collected to perform the pH value test using a portable pH meter, following the ASTM D4972-19 standard [39]. Each reported value represents the average of three samples. For XRD and SEM-EDS analysis, the samples were immersed in absolute ethanol for 24 h immediately after the final freeze-thaw cycle to terminate ongoing hydration reactions. After ethanol treatment, samples were vacuum-dried at 40 °C for 48 h. The dried samples were then gently ground into fine powders for XRD testing, while fractured surfaces were used for SEM-EDS analysis. All samples were stored in airtight containers with desiccants to prevent moisture uptake and carbonation prior to microstructure analysis.

Table 3.
Test design.
3. Results
3.1. Mass Loss Results
3.1.1. Effect of Slag to Fly Ash Ratio
The mass loss curves for OP-ASF stabilized soft soil with different ratios of slag to fly ash (SF100, SF90, SF80, and SF70) were shown in Figure 9a–c. The mass loss curves of all ratios exhibited similar trends under varying freeze-thaw cycles. However, the final mass losses varied depending on the ratio of slag to FA. At the same freezing temperature and number, the stabilized soft soil sample SF80 had the lowest final mass loss, whereas the sample SF70 had the highest. Specifically, the final mass losses of the OP-ASF stabilized soft soil sample SF80 at various temperatures were 11.56%, 20.99%, and 31.38% for −5 °C, −10 °C, and −20 °C, respectively. On the other hand, the stabilized soft soil sample SF70 consistently showed a final mass loss of 30% at each freezing temperature. The results highlighted that the presence of a higher slag content led to gentler slopes in the mass loss curves, suggesting that higher slag ratios favor the reduction of mass loss. This was likely due to the increased formation of alkali-activated gels that contribute to improved bonding and reduced cohesion loss during freeze-thaw cycles. In contrast, when the proportion of fly ash increased excessively, it introduced more pores and cracks, allowing a larger volume of water within the soil to freeze. As the water froze and expanded, it resulted in the disruption of the soil structure, causing a sharper increase in mass loss, especially in SF70.
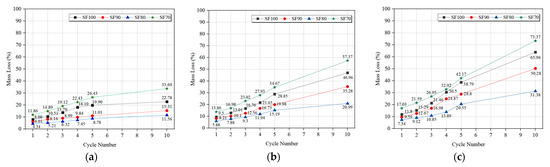
Figure 9.
Mass loss curves of OP-ASF stabilized soft soil: (a) −5 °C; (b) −10 °C; (c) −20 °C.
3.1.2. Effect of Cycle Temperature and Number
Figure 10a–d presents the mass loss curves of OP-ASF stabilized soft soil samples after multiple freeze-thaw cycles at varying temperatures. The figures revealed that as the number of freeze-thaw cycles increased, the mass losses also grew steadily. For instance, at a freezing temperature of −5 °C, the mass loss of the stabilized soft soil sample SF100 rose from 8.00% after one cycle to 19.90% after five cycles, eventually reaching 22.78%. Similarly, the mass loss of SF90 at −5 °C showed an increase from 6.01% after one cycle to 11.01% after five cycles, eventually reaching 15.31% after 10 cycles, respectively. In Figure 10c, the stabilized soft soil sample SF80 displayed an increase in mass loss from 4.34% after one cycle to 8.78% after five cycles, ending at 11.56%. Lastly, the SF70 sample at −5 °C saw mass loss rise from 11.86% after one cycle to 26.43% after five cycles, reaching 33.69% after 10 cycles.
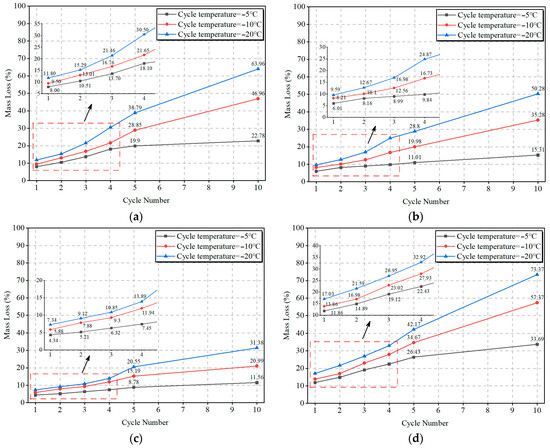
Figure 10.
Mass loss curves of OP-ASF stabilized soft soil: (a) SF100; (b) SF90; (c) SF80; (d) SF70.
When the ratio of slag to FA remained constant, the impact of lower freezing temperatures became evident. As shown in Figure 10a, final mass loss at freezing temperatures of −10 °C and −20 °C surged to 46.96% and 63.96%, respectively, for SF100, confirming that low temperatures accelerate mass loss. Similarly, SF90 samples reached 35.28% and 50.28% in Figure 10b, respectively. For SF80, final mass loss rose to 20.99% and 31.38% under the same conditions. SF70 exhibited the most severe mass loss increase at lower temperatures, with final mass loss of 57.37% and 73.37% in Figure 10d, confirming that as freezing temperature decreased, the mass loss of OP-ASF stabilized soft soil samples increased significantly.
The comparison of mass loss at different temperatures and cycles indicated that colder temperatures and more cycles would lead to more extensive damage to the stabilized soft soil, promoting greater deterioration due to freeze-thaw cycles. These results reinforce the conclusion that both the slag to fly ash ratio and the freeze-thaw cycle conditions significantly affect the mass loss behavior of stabilized soft soil.
3.2. UCS Results
3.2.1. Effect of Slag to Fly Ash Ratio
After a certain number of freeze-thaw cycles, the UCS value of OP-ASF stabilized soft soil samples was tested. The UCS values for OP-ASF stabilized soft soil before (i.e., 0) and after (i.e., 1 to 10) freeze-thaw cycles are shown in Figure 11. Before freezing, the UCS values of OP-ASF stabilized soft soil samples SF100, SF90, SF80, and SF70 after 28 days of standard curing were 2.19, 2.29, 2.69, and 2.05 MPa, respectively. Among these, the SF80 sample demonstrated the highest UCS, while SF70 exhibited the lowest. The UCS trends after freeze-thaw cycles for stabilized soft soil samples with four different slag to fly ash ratios were similar. The UCS of samples with a slag to fly ash ratio of 80:20 was larger than that of those with other slag to fly ash ratios, likely because a small amount of fly ash would promote the long-term increase in strength of OP-ASF stabilized soft soil. Before entering the freeze-thaw cycle, the stabilized samples underwent a 4-day soaking stage, during which water entered through surface cracks, internal pores, and capillaries. During freezing, as water entered the sample, the cold environment caused the outer layer of water to freeze, expanding the ice volume within the pores and increasing the pore count. These expanded pores formed cracks on the sample surface, allowing it to absorb more water during freezing and form additional ice in the next cycle. According to the mechanism of water migration in frozen soil [44,45], in a low-temperature environment, the surface temperature of the sample was lowest, drawing water from the interior to the surface and supplying more moisture for ice crystal formation. This process created additional cracks and pores, forming a porous structure within the sample that weakened structural support, ultimately reducing the compressive strength of OP-ASF stabilized soft soil.
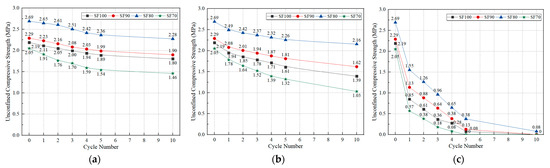
Figure 11.
UCS value of OP-ASF stabilized soft soil with different slag to FA ratios before and after freeze-thaw cycles: (a) −5 °C; (b) −10 °C; (c) −20 °C.
3.2.2. Effect of Cycle Temperature and Number
Figure 12a–d present the UCS values and UCS loss of OP-ASF stabilized soft soil under different freeze-thaw cycles. As shown in the figures, the UCS of these stabilized soft soils decreased significantly with an increasing number of freeze-thaw cycles. Following 28 days of standard curing, the UCS of sample SF80 was higher than that of samples with other ratios. In Figure 12a, under a freezing temperature of −5 °C, the UCS of OP-ASF stabilized soft soil with 100% slag decreased from 2.19 MPa to 1.56 MPa after 10 freeze-thaw cycles, representing a UCS loss of 29.05%. At lower freezing temperatures, UCS losses were even more pronounced, with increases of 17.84% and 53.11%, respectively, when compared to −5 °C conditions. Similarly, in Figure 12b, the initial UCS of the sample with a slag to fly ash ratio of 90:10 was 2.29 MPa, dropping to 1.65 MPa after 10 freeze-thaw cycles, with a UCS loss of 27.77%. When frozen at −10 °C and −20 °C, the UCS losses increased by 16.15% and 56.18%, respectively, compared to those at −5 °C. In Figure 12c, under −5 °C conditions, the UCS value of the stabilized soft soil sample with slag to fly ash ratio of 80:20 decreased from 2.69 MPa to 2.00 MPa after 10 freeze-thaw cycles, with a UCS loss of 25.75%. Freezing at −10 °C and −20 °C increased the UCS loss by 14.40% and 56.95%, respectively, compared to −5 °C. Finally, in Figure 12d, the UCS of the sample with 70:30 slag to FA ratio fell from 2.05 MPa to 1.40 MPa after 10 cycles at −5 °C, with a UCS loss of 31.97%. Lower freezing temperatures of −10 °C and −20 °C raised the UCS loss by 23.87% and 44.16%, respectively, compared to −5 °C. These UCS trends mirror those in the mass loss results, suggesting freeze-thaw cycles degrade both the surface and internal structures of stabilized soft soil, leading to a loss of compressive strength. The higher UCS loss observed at lower freezing temperatures further supported the need for optimizing the slag to fly ash ratio and freeze-thaw cycle conditions to enhance soil stabilization.
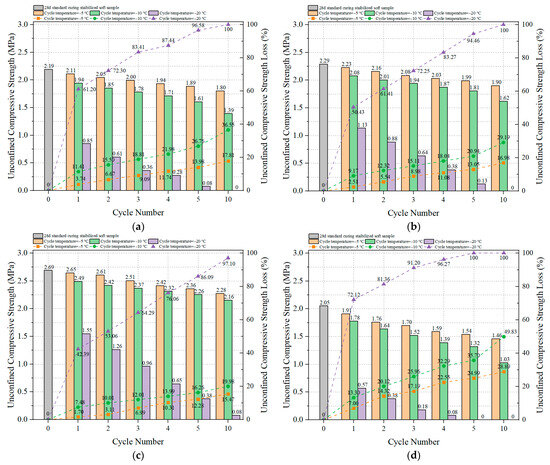
Figure 12.
UCS and UCS loss of OP-ASF stabilized soft soil: (a) SF100; (b) SF90; (c) SF80; (d) SF70.
3.2.3. pH Value Results
Figure 13a–d display the pH value of OP-ASF stabilized soft soil samples after varying freeze-thaw cycles. The figure indicated that the pH values of all stabilized soft soil samples decrease as freeze-thaw cycles increase, with lower freezing temperatures corresponding to lower pH values. The pH of samples frozen at −5 °C showed the highest values, while those frozen at −20 °C had the lowest values. In Figure 13a, the pH value of the stabilized soft soil sample with 100% slag decreased from 12.58 after one freeze-thaw cycle at −5 °C to 12.22 after 10 cycles. Additionally, at lower freezing temperatures (i.e., −10 °C and −20 °C), the initial pH values for SF100 were 12.50 and 12.33, decreasing to 12.13 and 11.93 by the end of the cycles. Figure 13b shows the sample with a 90:10 slag to fly ash ratio, where the pH value dropped from 12.80 after one freeze-thaw cycle at −5 °C to 12.35 after 10 cycles. At −10 °C and −20 °C, the initial pH values of 12.66 and 12.46 fell to 12.23 and 12.08, respectively. In Figure 13c, the pH of the sample SF80 decreased from 12.90 after one freeze-thaw cycle at −5 °C to 12.40 after 10 cycles. At −10 °C and −20 °C, the pH dropped from 12.81 and 12.62 to 12.34 and 12.14, respectively. Lastly, Figure 13d illustrates the sample with a 70:30 ratio of slag to fly ash, where the pH value decreased from 12.48 after one cycle at −5 °C to 12.11 after 10 cycles. At −10 °C and −20 °C, the initial pH values of 12.40 and 12.28 decreased to 12.00 and 11.79. These pH results aligned with the expected behavior of alkali-activated materials under freeze-thaw conditions. The decrease in pH was likely associated with continued pozzolanic reactions and the formation of secondary mineral phases (XRD results) such as tamarugite, which consumes alkali ions. Tamarugite, composed of sulfates, sulfites, and sodium sourced from pore water and ionic migration, tends to form and accumulate with ongoing cycles. This ongoing mineral evolution leads to a gradual reduction in system alkalinity. Nonetheless, despite the observed decline, the samples generally maintained a moderately alkaline environment, indicating the structural resilience of OP-ASF stabilized soft soil under freeze-thaw conditions.
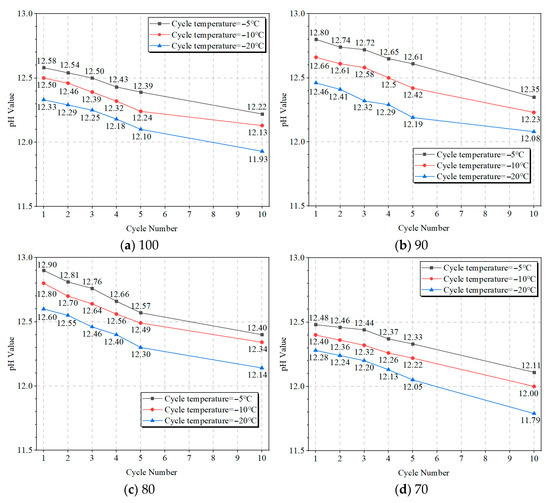
Figure 13.
pH value of OP-ASF stabilized soft soil: (a) SF100; (b) SF90; (c) SF80; (d) SF70.
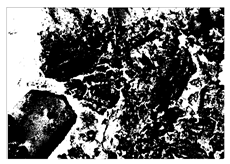
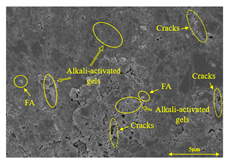
3.3. Microstructure Analysis
SEM-EDS and IPP Results
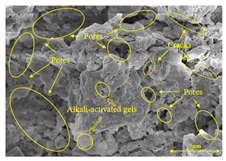
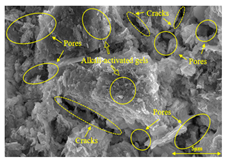
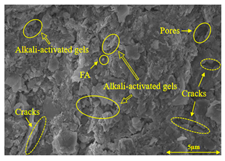
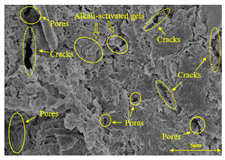
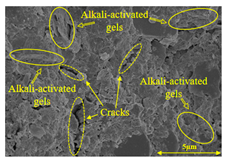
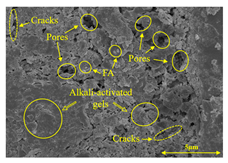
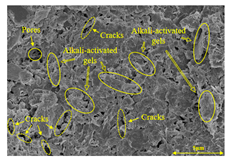
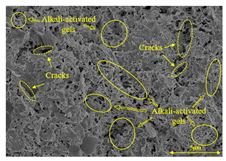
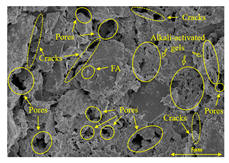
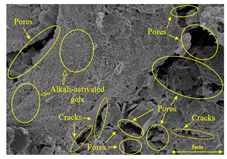
To gain further insights into the above macroscopic results of OP-ASF stabilized soft soil, SEM-EDS was employed to analyze the microstructure of the samples. SEM images revealed the development of alkali-activated gels, cracks, and pores, which were essential in explaining the observed mass loss and decreased UCS under varying freeze-thaw conditions. EDS analysis of OP-ASF stabilized soft soil is presented in Figure 14, and the element content of alkali-activated gels is shown in Table 4. The EDS results are consistent with previous research [34,46,47,48], highlighting that the alkali-activation process of slag and FA differed from traditional cement systems. The resulting gels primarily included net-like calcium silicate hydrates [C-S-H], slat-like calcium aluminate (aluminosilicate) hydrates [C-A-(S)-H], and amorphous sodium aluminosilicate hydrates [N-A-S-H], which SEM-EDS analysis verified [49].
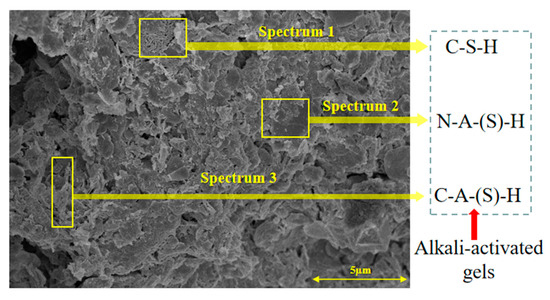
Figure 14.
EDS analysis of OP-ASF stabilized soft soil.

Table 4.
Element content of alkali-activated gels in EDS analysis.
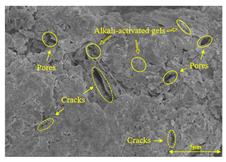
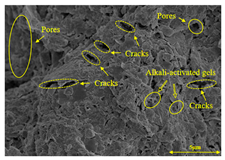
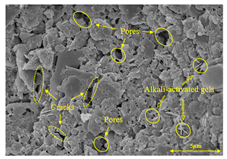
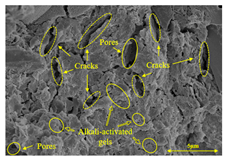
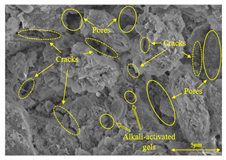
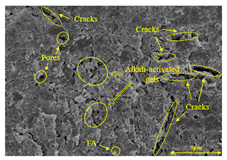
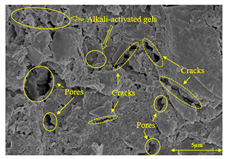
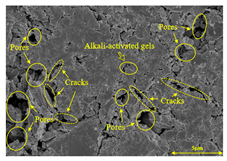
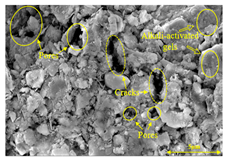
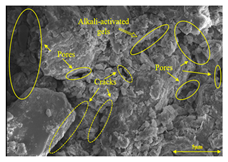
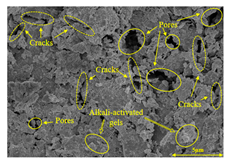
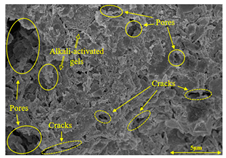
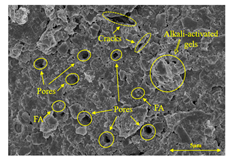
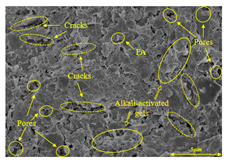
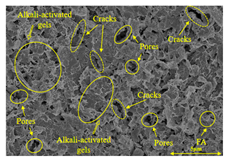
To compare the microstructure of OP-ASF stabilized soft soil samples (SF100, SF90, SF80, and SF70), SEM-EDS results under different freeze-thaw temperatures and numbers are presented in Table 5. Alkali-activated gels and structural features such as cracks and pores are highlighted in the images. Significant differences in microstructure were evident under varying freeze-thaw conditions. At a freezing temperature of −5 °C, minimal damage was observed after one and five freeze-thaw cycles, with only a few pores present. After 10 cycles, surface pores begin to develop but remain relatively sparse. At −10 °C, as the number of freeze-thaw cycles increased, small cracks and pores appeared on the surface, progressively reducing the UCS of the stabilized soft soil. At −20 °C, severe surface deterioration occurred after just five cycles, with numerous cracks and pores forming, leading to a porous, weakened internal structure and significant strength loss. Notably, the SF70 sample (slag to FA ratio 70:30) lost all strength after 10 cycles, while SF100 and SF90 samples showed increasing damage, larger pores, and more pronounced cracks. Conversely, SF80 maintained some structural integrity, even after 10 cycles at −20 °C. More alkali-activated gels (i.e., C-S-H, C-A-(S)-H, and N-A-S-H) in SF80 samples played a vital role in establishing a dense structure within the stabilized sample, thereby reducing the impact of freeze-thaw cycles. The results from SEM-EDS correlated with the trends observed in UCS and mass loss, confirming that an optimal balance of slag and fly ash is crucial for enhancing the freezing resistance of stabilized soft soil.

Table 5.
SEM-EDS and IPP results of OP-ASF stabilized soft soil.
The analysis of internal structure characteristics is essential for understanding the mechanical properties of stabilized soft soil. Advances in imaging and analysis tools, such as Image-Pro Plus (IPP), have significantly improved the accuracy of microstructure measurements, including pores and cracks. For instance, Yao et al. [50] employed IPP to analyze the micropore structure of soil samples subjected to freeze-thaw cycles, obtaining data on porosity, average pore length, and average pore width. Similarly, Zhang et al. [51] applied IPP to evaluate the parameters such as the equivalent diameter, circularity, directional frequency, and fractal dimension of the soil microstructure. In this study, IPP was also utilized to perform binary processing on SEM images of the stabilized soft soil. This enabled calculation of the internal structure looseness index for OP-ASF stabilized soft soil under varying freeze-thaw conditions. The development of cracks and pores would induce a progressive increase in structure looseness. While multiple methods exist to calculate the changes in soil structure, such as pores and cracks, this study emphasized analyzing the trend of internal structure changes over multiple freeze-thaw cycles. According to the SEM image analysis, grayscale value adjustment was applied to enhance phase differentiation. In the processed images, the darker regions (Pb) corresponded to stabilized soil particles or alkali-activated gels phases, whereas the brighter regions (Pw) represented cracks and pores within the microstructure. All test results were obtained under identical material compositions and consistent experimental conditions, ensuring a direct correlation between microstructure evolution and macroscopic performance. These trends offered valuable insights into the durability and structure integrity of stabilized soil samples under such conditions. The formula used in this study to calculate the structure looseness of OP-ASF stabilized soft soil through IPP is provided in Equation (1).
where L represents the internal structure looseness index of OP-ASF stabilized soft soil; Pb and Pw are black area percentage and white area percentage, respectively; n denotes the total number of pixels in the image; A is the area of each pixel; G stands for the maximum grayscale value of the image; gi is the grayscale of the i-th pixel.
The structure looseness index of OP-ASF stabilized soft soil under freeze-thaw cycles is shown in Figure 15. As illustrated, the structure looseness of stabilized soft soil samples gradually increased with the number of freeze-thaw cycles, reaching its maximum after 10 cycles. For the SF70 sample, the structure looseness index reached its peak among all the ratios of slag to FA, measuring 0.200, 0.234, and 0.297 at freezing temperatures of −5 °C, −10 °C, and −20 °C, respectively. In contrast, the SF80 samples all exhibited the lowest looseness index across all freezing temperatures, with values of 0.122, 0.129, and 0.284. The figure also revealed that the structure looseness index increases with decreasing freezing temperatures. Under a constant freeze-thaw cycle number of 10, the structure looseness index of the SF100 sample increased from 0.174 at −5 °C to 0.224 at −10 °C, eventually reaching its maximum value of 0.293 at −20 °C. A similar trend was observed for the SF90 sample, the structure looseness index of which rose from 0.164 at −5 °C to 0.180 at −10 °C, peaking at 0.295 at −20 °C. The increase in structure looseness was directly related to the deterioration in the mechanical properties of the stabilized soft soil. A higher structure looseness not only reduced the UCS of soft soil but also increased its mass loss during freeze-thaw cycles, highlighting the importance of controlling pore and crack formation in freeze-thaw conditions. SEM-EDS results further revealed that the SF80 sample contained a greater amount of alkali-activated gels filling the pores and cracks, whereas the SF70 sample exhibited fewer alkali-activated gels. This explains why the freeze resistance of the SF80 sample is superior to other ratios, while the SF70 sample performs the worst under freeze-thaw cycles. These observations aligned with the conclusions drawn from USC and mass loss results.
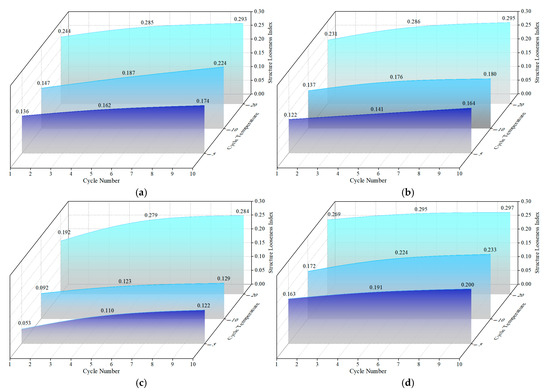
Figure 15.
Structure looseness index of OP-ASF stabilized soft soil under freeze-thaw cycles: (a) SF100; (b) SF90; (c) SF80; (d) SF70.
In conclusion, the study demonstrates that the slag to fly ash ratio, freeze-thaw cycles, and temperature conditions play a significant role in determining the performance of OP-ASF stabilized soft soil. By optimizing the raw material composition and freeze-thaw conditions, it is possible to enhance the durability and structural integrity of stabilized soils used in engineering applications.
3.4. XRD Results
To investigate the main phase changes in OP-ASF stabilized soft soil during freeze-thaw cycles, XRD analysis was conducted. Figure 16 displays the XRD patterns of OP-ASF stabilized soft soil, using SF80 as a representative sample, after one, five, and 10 freeze-thaw cycles at a freezing temperature of −20 °C. The primary mineral phases identified include quartz, gismondine, tamarugite, albite, and anorthite. The results indicated that the mineral composition remained essentially unchanged throughout the freeze-thaw cycles. No new crystalline phases were detected within the 2θ range of 20–35°, which corresponded to the characteristic region of alkali-activated gels. This suggested that OP-ASF stabilized soft soil exhibited high mineralogical stability under freeze-thaw conditions, and its amorphous gel structure remained intact and resistant to damage.
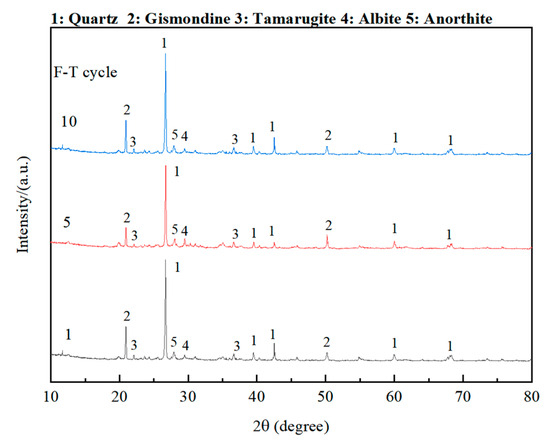
Figure 16.
XRD results for OP-ASF stabilized soft soil (SF80) under different numbers of freeze-thaw cycles.
3.5. Freezing Resistance Mechanism
Based on the above experimental results and microscopic analysis, the freezing resistance mechanism of OP-ASF stabilized soft soil can be divided into three stages, as shown in Figure 17.
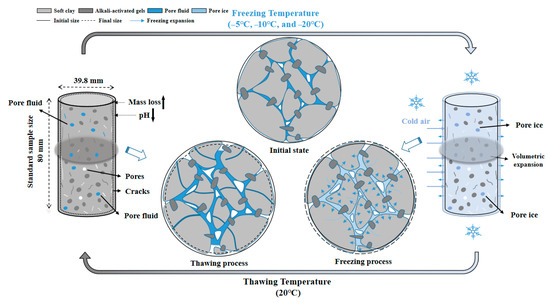
Figure 17.
Freezing resistance mechanism of OP-ASF stabilized soft soil.
(I) Initial state
Before freeze-thaw cycles, OP-ASF stabilized soft soil had a dense and compact structure with minimal internal structure looseness. At this stage, the ACM bound tightly with the soft soil particles, forming a stable, reinforced structure. This provided excellent stabilization, enabling the soft soil to effectively resist external stress and environmental impacts. The high strength of the stabilized soil ensured that it maintained its structural integrity under normal conditions.
(II) Freezing process
During the freezing process, pore fluid within the soft soil froze, forming pore ice. As the temperature dropped, this pore ice generated frost heave forces that caused the volume of soil to expand. This expansion led to an increase in the structure looseness index as cracks began to form within the stabilized soft soil. Over time, the sustained frost heave forces loosened the internal structure, resulting in a reduction in the mechanical properties of the stabilized soft soil. The freezing process significantly challenged the structure of the stabilized soft soil, potentially leading to progressive damage over multiple freeze-thaw cycles.
(III) Thawing state
As the stabilized soft soil thawed, the previously formed cracks became deeper, and new cracks could develop, eventually leading to the formation of complete pores throughout the stabilized soft soil. The ongoing freeze-thaw cycles caused a continuous increase in the structure looseness index, weakening the mechanical properties of the soft soil, including its mass loss and UCS. Water repeatedly entered the soil, leading to further degradation of its structure and a reduction in pH. However, despite these detrimental effects, one-part ACM in the stabilized soft soil continued to exert a strong stabilization effect, which helped maintain a certain level of freezing resistance. This indicates that OP-ASF stabilized soft soil can maintain significant durability, even under freeze-thaw conditions.
4. Conclusions
This study adopted slag and FA as industrial waste raw materials to prepare alkali-activated cementitious materials through a one-part method. The mechanical properties and microstructure changes of OP-ASF stabilized soft soil under different slag and FA ratios, freeze-thaw temperatures, and freeze-thaw cycles were analyzed through mass loss, UCS, and pH value tests. The freezing resistance mechanism of stabilized soft soil was also derived based on the experimental results. The main conclusions were drawn as follows:
- The freezing resistance of OP-ASF stabilized soft soil varies with the ratio of slag to FA. When the ratio of slag to FA is 80:20, the stabilized soil exhibits the best performance in terms of mass loss, UCS, and pH value. A ratio of 70:30 leads to the worst freezing resistance, as excessive FA increases structure looseness, promotes water diffusion, and forms pores during freeze-thaw cycles.
- The mass loss and UCS loss increase with the number of freeze-thaw cycles. After 10 cycles, both reach their highest values. Additionally, as the freezing temperature decreases, the mass loss and UCS loss increase, with the maximum mass loss occurring at −20 °C. The pH value also decreases over time, indicating a weakening of the alkalinity in the stabilized soft soil, especially at lower temperatures.
- SEM-EDS analysis revealed that the SF80 sample (with a slag to FA ratio of 80:20) contained more alkali-activated gels filling the pores and cracks, which contributed to better performance. These findings highlight the importance of structure looseness in determining the mechanical properties of the stabilized soil.
- The freezing resistance mechanism of OP-ASF stabilized soft soil involves the formation of alkali-activated gels that fill pores and cracks, establishing a dense structure. This prevents pore fluid within the soil from freezing, thereby reducing the impact of multiple freeze-thaw cycles.
Author Contributions
L.L.: Conceptualization, Data acquisition, Investigation, Methodology; Writing—original draft preparation. X.Z.: Conceptualization, Supervision, Validation. M.Y.: Data acquisition, Investigation, Methodology. J.W.: Methodology, Project administration, Investigation, Validation, Formal analysis, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number No. 42377201 and 42402261. The article processing charge was funded by he National Natural Science Foundation of China, grant number No. 42402261.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The assistance of Jinqiuye Zhang for the experimental tests is highly appreciated.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, J.; Shan, Y.; Ni, P.; Cui, J.; Li, Y.; Zhou, J. Mechanics, durability, and microstructure analysis of marine soil stabilized by an eco-friendly calcium carbide residue-activated coal gangue geopolymer. Case Stud. Constr. Mater. 2024, 20, e02687. [Google Scholar] [CrossRef]
- Qin, J.; Yi, Y. Use of gasification fly ash, sodium carbonate, and ground granulated blast-furnace slag for soft clay stabilization. Constr. Build. Mater. 2024, 426, 136072. [Google Scholar] [CrossRef]
- Yu, W.; Zhu, Z.; Zhang, D.; Sun, H.; Li, Y. Research on strength and deformation properties of high moisture soft soils stabilized by GGBS, cement, fly ash, and sodium silicate. Constr. Build. Mater. 2024, 448, 138244. [Google Scholar] [CrossRef]
- Du, C.; Yang, Q. Freeze-thaw behavior of calcium carbide residue-plant ash stabilized marine soft clay. Cold Reg. Sci. Technol. 2022, 193, 103432. [Google Scholar] [CrossRef]
- Rikhtehgar, Y.A.; Teymür, B. Effect of magnesium chloride solution as an antifreeze agent in clay stabilization during freeze-thaw cycles. Appl. Sci. 2024, 14, 4140. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Z.; Liu, S.; Wu, K.; Yuan, Z.; He, H. Durability against dry-wet and freeze-thaw cycles of carbon sequestration foamed concrete utilizing abandoned soil and waste serpentine. J. Build. Eng. 2024, 95, 110194. [Google Scholar] [CrossRef]
- Yang, H.; Lei, B.; Xie, L.; Hu, C.; Liu, J. Experimental study on water and salt migration and the aggregate insulating effect in coarse-grained saline soil subgrade under freeze-thaw cycles. Appl. Sci. 2024, 14, 8970. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhao, M.; Lan, B.; Liu, G.; Lu, Z.; Yao, H. Study on stability of soil slope in seasonally frozen region based on water transfer and strength deterioration under freeze-thaw cycles. J. Cold Reg. Eng. 2025, 39, 04024035. [Google Scholar] [CrossRef]
- Samaptika, M.; Nagendra, R.; Prasad, S.S.; Parveen, S. Strength and durability of fly ash, GGBS and cement clinker stabilized dispersive soil. Cold Reg. Sci. Technol. 2021, 191, 103358. [Google Scholar]
- Salih, R.S.; Shafiqu, M.S.Q. The effect of adding mixture of gypsum-lime with geopolymer on the properties of swelling soil. Civ. Environ. Eng. 2024, 20, 978–992. [Google Scholar] [CrossRef]
- Yilmaz, F.; Fidan, D. Influence of freeze-thaw on strength of clayey soil stabilized with lime and perlite. Geomech. Eng. 2018, 14, 301–306. [Google Scholar]
- Zhang, M.; Feng, C.; Xu, P.; Chen, C. The effect of freeze-thaw cycles and wind erosion on the characteristics of microbial solidified engineering residue. Constr. Build. Mater. 2024, 418, 135374. [Google Scholar] [CrossRef]
- Sorensen, P.O.; Finzi, A.C.; Marc-André, G.; Reinmann, A.B.; Templer, P.H. Winter soil freeze-thaw cycles lead to reductions in soil microbial biomass and activity not compensated for by soil warming. Soil Biol. Biochem. 2018, 116, 39–47. [Google Scholar] [CrossRef]
- Nasiri, H.; Khayat, N.; Nazarpour, A. Utilization of the oil-contaminated soil as a sustainable resource in rural road construction and rehabilitation in oil-producing countries. J. Clean Prod. 2024, 483, 144175. [Google Scholar] [CrossRef]
- Xue, Z.; Zhang, W.; Zhao, X.; Meng, F.; Qin, F.; Xiao, G.; Chen, J. Utilization of cement deep mixing pile for soft soil foundation: A malaysian case study. Front. Mater. 2024, 11, 1484228. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, R.; Yan, Z.; Zhang, G.; Wu, M.; Yu, T. Study on mixing cement-waste by-product to stabilize soft marine clay. Int. J. Geosynth. Ground Eng. 2023, 9, 20. [Google Scholar] [CrossRef]
- Abdolvand, Y.; Sadeghiamirshahidi, M. Soil stabilization with gypsum: A review. J. Rock Mech. Geotech. Eng. 2024, 16, 5278–5296. [Google Scholar] [CrossRef]
- Barcelos, P.J.; Rodríguez, S.R.A.; Bargiela, R.; Mariano, E.; Gloyshina, V.O.; Jones, L.D.; Rosolem, A.C. Lime, gypsum, and nitrogen as drivers to increase the abundance of soil fungi and N-cycling microorganisms in integrated agricultural systems. Appl. Soil Ecol. 2024, 202, 105549. [Google Scholar] [CrossRef]
- Sajad, S.; Hossein, A.V.; Mehdi, M. Effects of freeze-thaw cycles on the characteristics of the expansive soils treated by nanosilica and Electric Arc Furnace (EAF) slag. Cold Reg. Sci. 2021, 182, 103216. [Google Scholar]
- Lu, J.; Tan, L.; Yang, H.; Wan, X.; Wang, Y.; Yan, Z. Experimental study on the hydro-thermal-deformation characteristics of cement-stabilized soil exposed to freeze-thaw cycles. Front. Earth Sci. 2023, 10, 1041249. [Google Scholar] [CrossRef]
- Mansour, E.; John, K.; Jonathan, O. Role of gypsum content on the long-term performance of lime-stabilised soil. Materials 2022, 15, 5099. [Google Scholar]
- Scrivener, K.L.; Matschei, T.; Georget, F.; Juilland, P.; Mohamed, A.K. Advances in hydration and thermodynamics of cementitious systems. Cem. Concr. Res. 2023, 174, 107332. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.B. Resistance of alkali-activated slag concrete to alkali-aggregate reaction. Cement Concr. Res. 2001, 31, 331–334. [Google Scholar] [CrossRef]
- Du, Y.J.; Bo, Y.L.; Jin, F.; Liu, C.Y. Durability of reactive magnesia-activated slag-stabilized low plasticity clay subjected to drying-wetting cycle. Eur. J. Environ. Civ. Eng. 2016, 20, 215–230. [Google Scholar] [CrossRef]
- Du, Y.J.; Wei, M.L.; Jin, F. Laboratory investigation on strength properties of cement stabilized zinc-contaminated clay. Eng. Geol. 2013, 167, 20–26. [Google Scholar] [CrossRef]
- Tao, D.; Chen, S.; Parekh, B.; Hepworth, M.T. An investigation of a thermochemical process for conversion of gypsum and pyrite wastes into useful products. Adv. Environ. Res. 2001, 5, 277–284. [Google Scholar] [CrossRef]
- Hou, W.; Ye, F.; Yu, Y.; Chen, D.; Wang, J.; Liu, F.; Feng, D.; Liang, S. Experimental study on the strength characteristics and seawater degradation resistance of sandy silt solidified with alkali-activated slag. Constr. Build. Mater. 2025, 458, 139610. [Google Scholar] [CrossRef]
- Hunar, M.D.; Yavuz, O.B.; Ali, Ö.; Gökhan, K. Sustainable use of waste tire rubbers in eco-friendly and lightweight alkali-activated slag-silica fume mortars. J. Mater. Civ. Eng. 2024, 36, 04024432. [Google Scholar]
- Xiao, R.; Nie, Q.; Dai, X.; Wan, Z.; Zhong, J.; Ma, Y.; Huang, B. Developing alkali-activated controlled low-strength material (CLSM) using urban waste glass and red mud for sustainable construction. J. Build. Eng. 2024, 98, 111202. [Google Scholar] [CrossRef]
- Yang, T.; Yang, S.; Sun, Z.; Wang, S.; Pang, R. Deterioration mechanism of alkali-activated slag and fly ash blended recycled aggregate concrete under freeze-thaw cycle. J. Build. Eng. 2025, 99, 111555. [Google Scholar] [CrossRef]
- Kamakshi, T.A.; Ramagiri, K.K.; Subramaniam, K.V.L. Fly ash-based aqueous nanosilica enhanced activator for efficient production of room temperature-cured concrete with two-part alkali-activated binders. J. Mater. Civ. Eng. 2024, 36, 04024312. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Lin, J.; Li, Y.; Lu, Z.; Jiang, J. Target dispersion of pozzolanic materials nearby hydration-released calcium hydrates to improve the pozzolanic reaction degree. Cement Concr. Res. 2024, 153, 105741. [Google Scholar] [CrossRef]
- Zhao, Q.; Ma, C.; Huang, B.; Lu, X. Development of alkali activated cementitious material from sewage sludge ash: Two-part and one-part geopolymer. J. Clean Prod. 2023, 384, 135547. [Google Scholar] [CrossRef]
- Min, Y.; Wu, J.; Li, B.; Zhang, J. Effects of Fly ash content on the strength development of soft clay stabilized by one-part geopolymer under curing stress. J. Mater. Civ. Eng. 2021, 33, 04021274. [Google Scholar] [CrossRef]
- ASTM C618-15; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use as a Mineral Admixture in Concrete. ASTM International: West Conshohocken, PA, USA, 2015.
- Zheng, X.; Wu, J. Early strength development of soft clay stabilized by one-part ground granulated blast furnace slag and fly ash-based geopolymer. Front. Mater. 2021, 8, 616430. [Google Scholar] [CrossRef]
- Li, L.; Zheng, X.; Wu, J.; Zhang, J.; Li, P.; Wei, X. Performance of the one-part geopolymer stabilized soft clay under acids attack. J. Clean. Prod. 2024, 452, 142183. [Google Scholar] [CrossRef]
- ASTM C666/C666M-03; Standard Test Method for Resistance of Concrete to Rapid Freezing and Thawing. ASTM International: West Conshohocken, PA, USA, 2003.
- ASTM D4972-19; Standard Test Methods for pH of Soils. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM C267-20; Standard Test Methods for Chemical Resistance of Pastes, Grouts, and Monolithic Surfacings and Polymer Concretes. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM C109/C109M-21; Standard Test Method for Compressive Strength of Hydraulic Cement Pastes (Using 2-in. or [50-mm] Cube Specimens). ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM C1365-18; Standard Test Method for Determination of the Proportion of Phases in Portland Cement and Portland-cement Clinker Using X-Ray Powder Diffraction Analysis. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM C1723-16; Standard Guide for Examination of Hardened Concrete Using Scanning Electron Microscopy. ASTM International: West Conshohocken, PA, USA, 2016.
- Akagawa, S. Experimental study of frozen fringe characteristics. Cold Reg. Sci. Technol. 1988, 15, 209–223. [Google Scholar] [CrossRef]
- Harlan, R.L. Analysis of coupled heat-fluid transport in partially frozen soil. Water Resour. Res. 1973, 9, 1314–1323. [Google Scholar]
- Adesanya, E.; Ohenoja, K.; Maria, D.A.; Kinnunen, P.; Illikainen, M. Alternative alkali-activator from steel-making waste for one-part alkali-activated slag. J. Clean. Prod. 2020, 274, 123020. [Google Scholar] [CrossRef]
- Min, Y.; Wu, J.; Li, B.; Zhang, M.; Zhang, J. Physicochemical and mechanical behavior of the one-Part Geopolymer paste exposed to hydrochloric and sulfuric acids. J. Mater. Civ. Eng. 2023, 35, 04022456. [Google Scholar] [CrossRef]
- Sturm, P.; Gluth, G.J.G.; Brouwers, H.J.H.; Kühne, H.C. Synthesizing one-part geopolymers from rice husk ash. Constr. Build. Mater. 2016, 124, 961–966. [Google Scholar] [CrossRef]
- Richard, C.; Ravi, A.P.; Frank, D. Activation kinetic model and mechanisms for alkali-activated slag cements. Constr. Build. Mater. 2022, 323, 126577. [Google Scholar]
- Yao, C.; Shen, A.; Lyu, Z.; Zeng, G. Mitigating freeze-thaw deterioration of pavement concrete under multi-field coupling by superabsorbent polymers. Constr. Build. Mater. 2024, 414, 134726. [Google Scholar] [CrossRef]
- Zhang, X.; Ding, Z.; He, S.; Zhang, G.; Sun, M.; Xia, T. An experimental study on the microstructure evolution of soil under lateral consolidation compression. Appl. Sci. 2022, 16, 8331. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).