Early-Strength Controllable Geopolymeric CLSM Derived by Shield Tunneling Muck: Performance Optimization and Hydration Mechanism of GGBFS–CS Systems
Abstract
1. Introduction
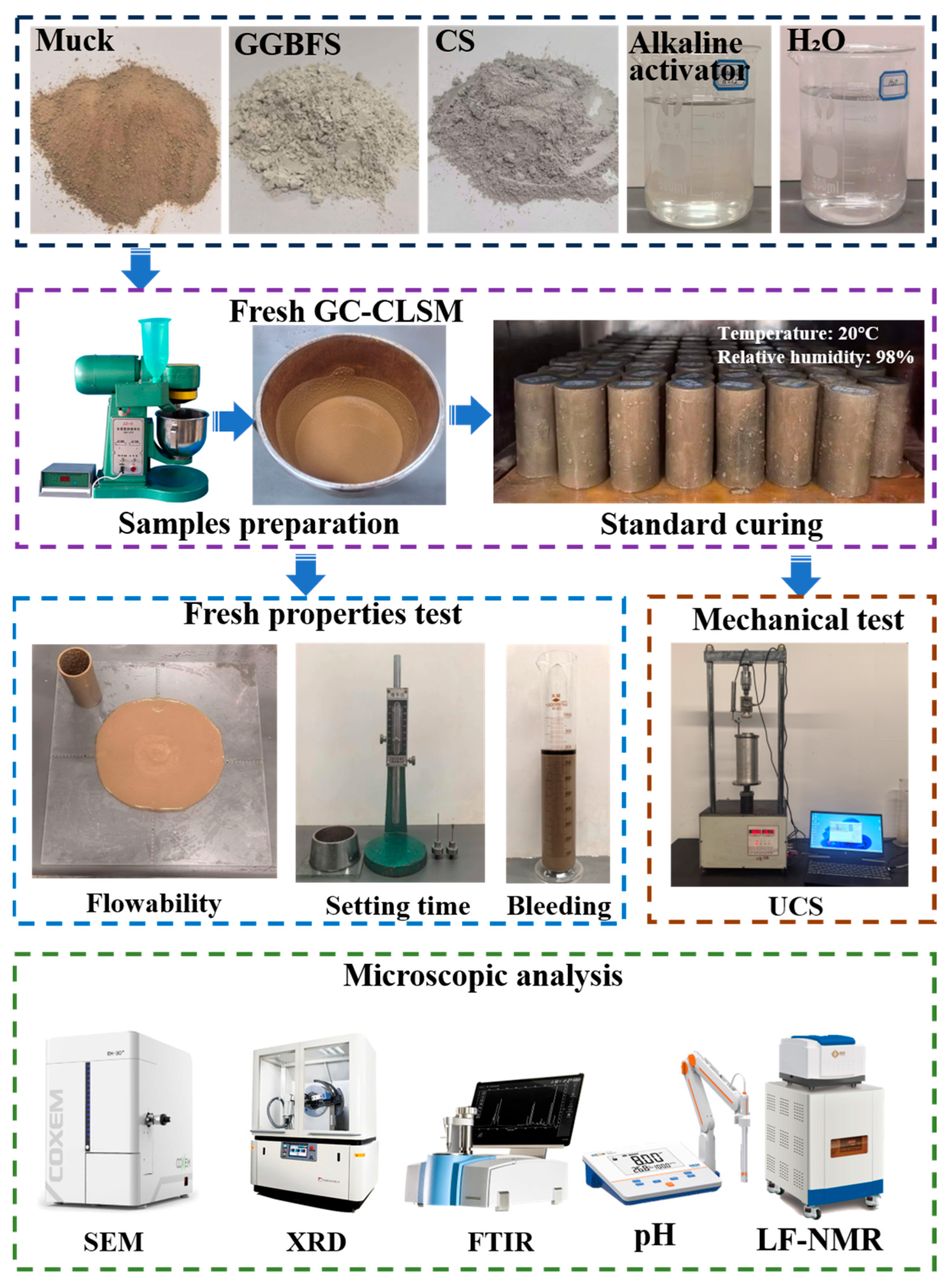
2. Materials and Methods
2.1. Materials
2.2. Fresh Properties of GC-CLSM
2.3. Mechanical Property Testing
2.4. Microstructural Characterization
2.5. GC-CLSM Preparation and Mix Design
3. Results
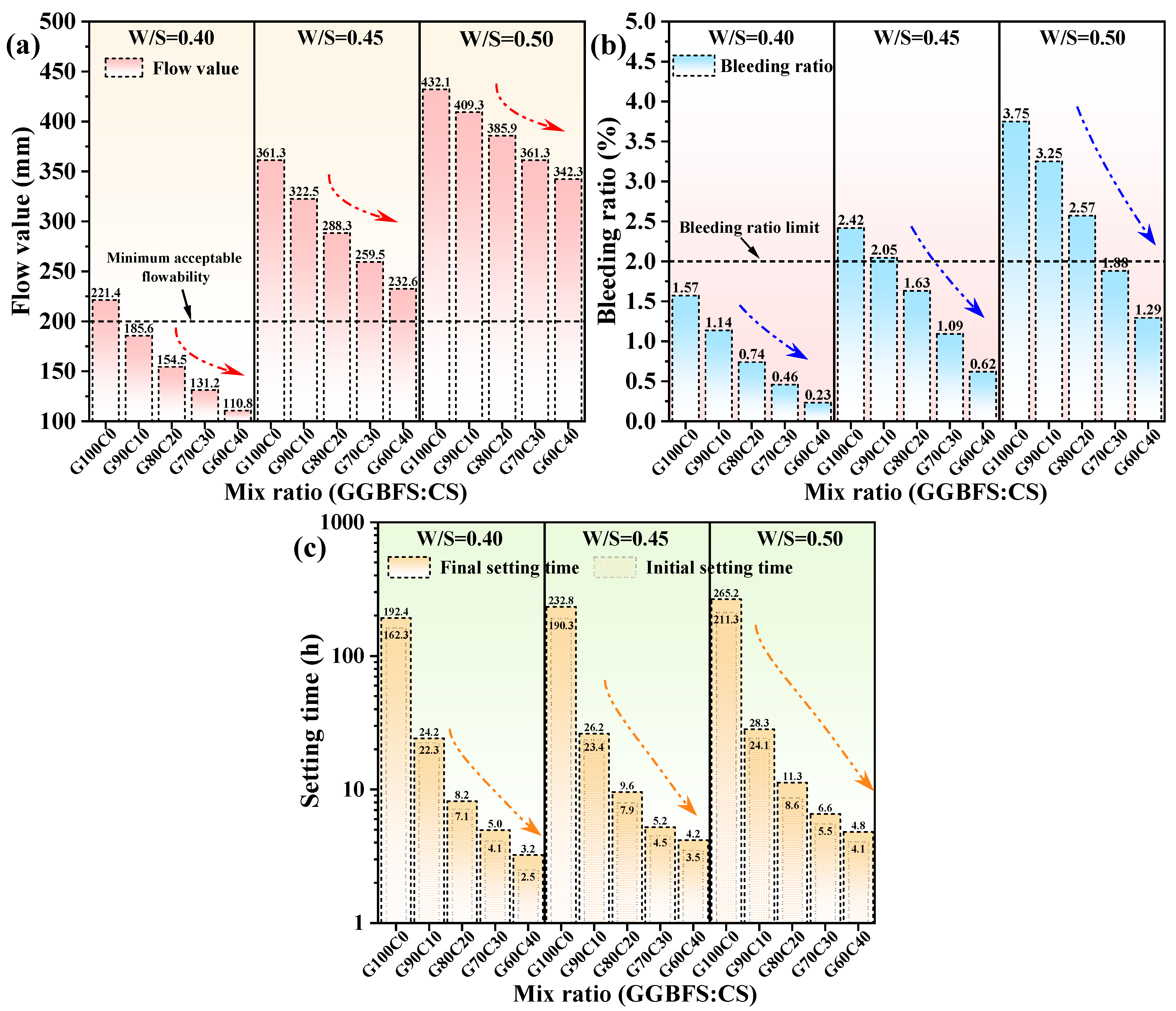
3.1. Fresh Properties
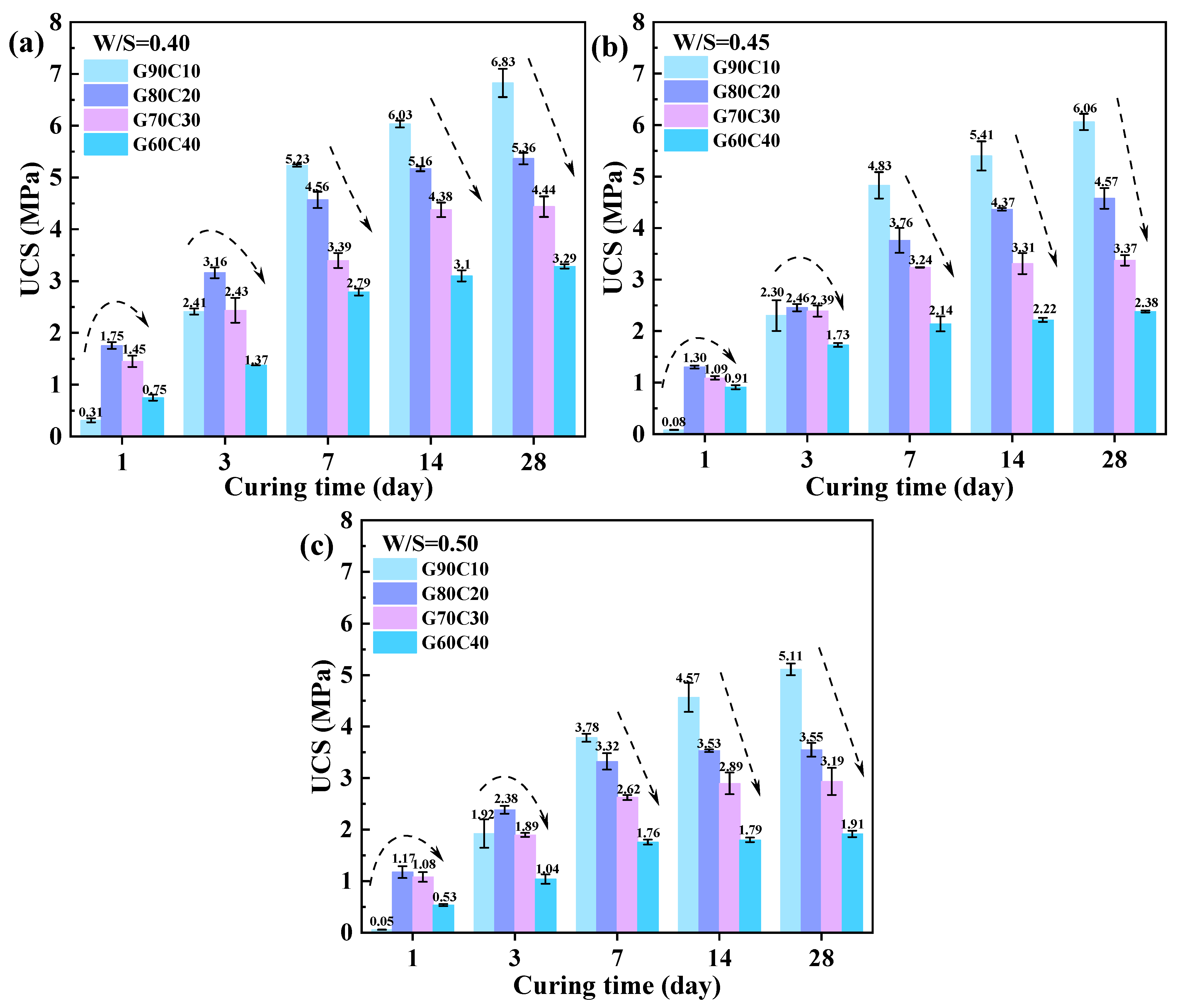
3.2. Mechanical Properties
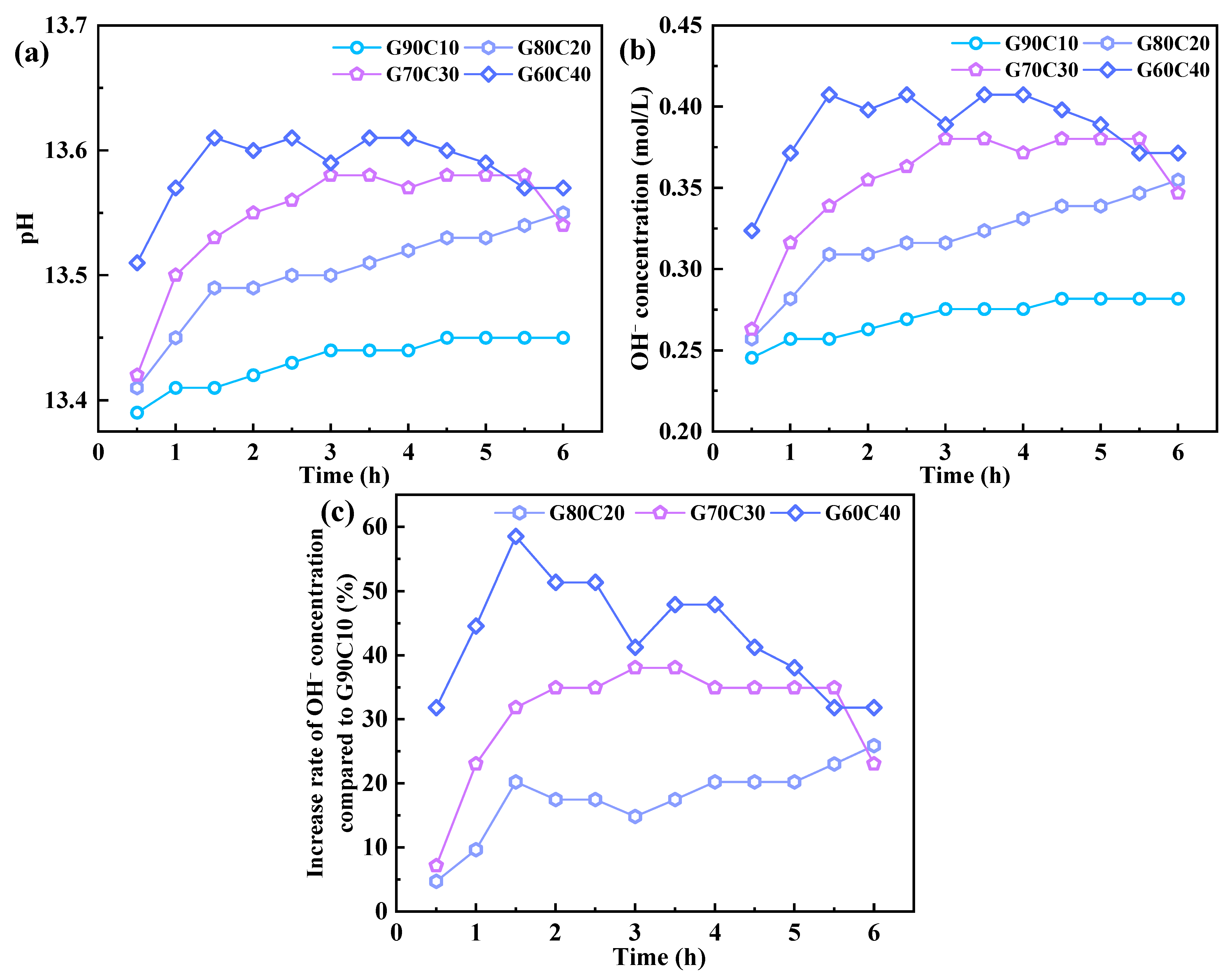
3.3. pH of Pore Water
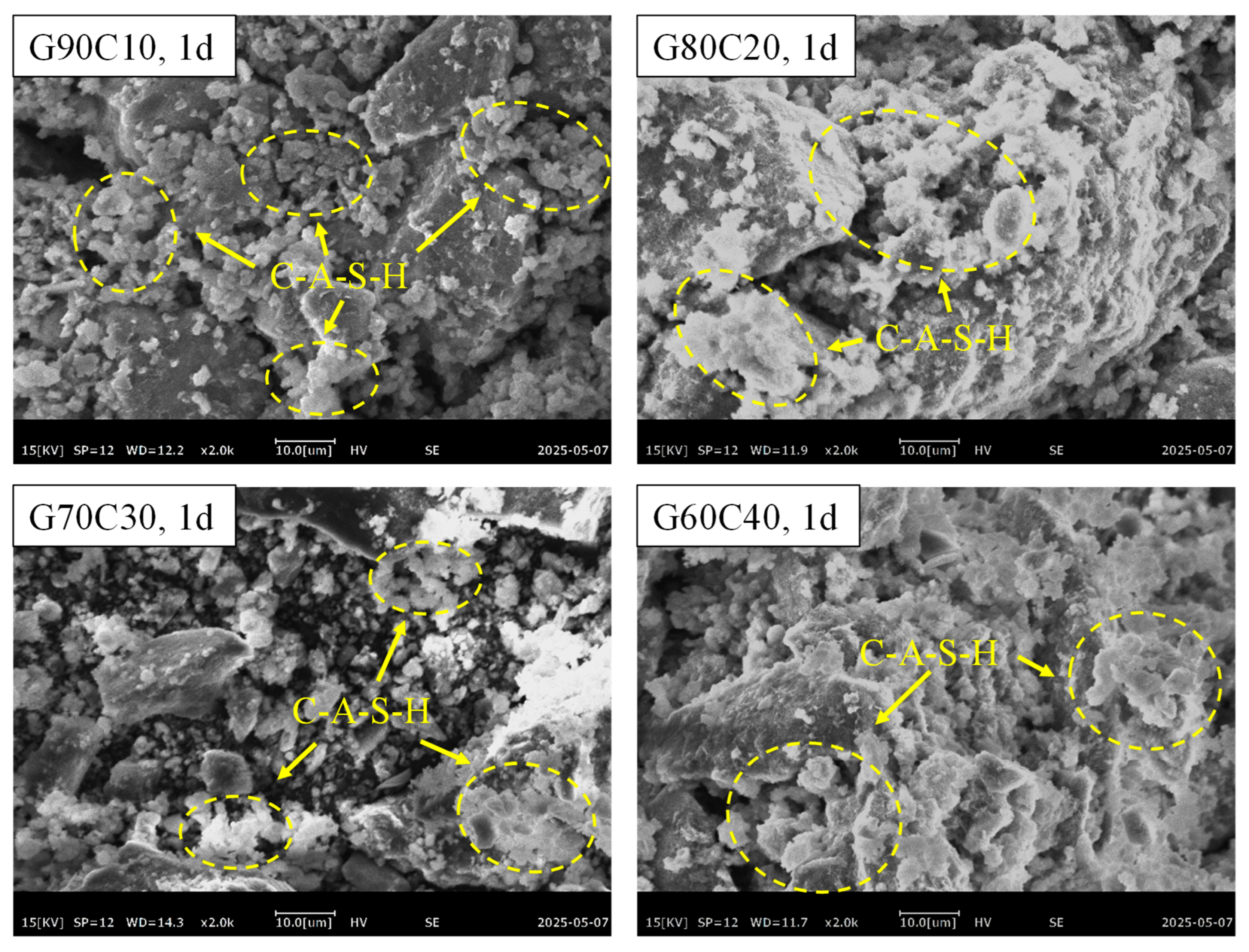
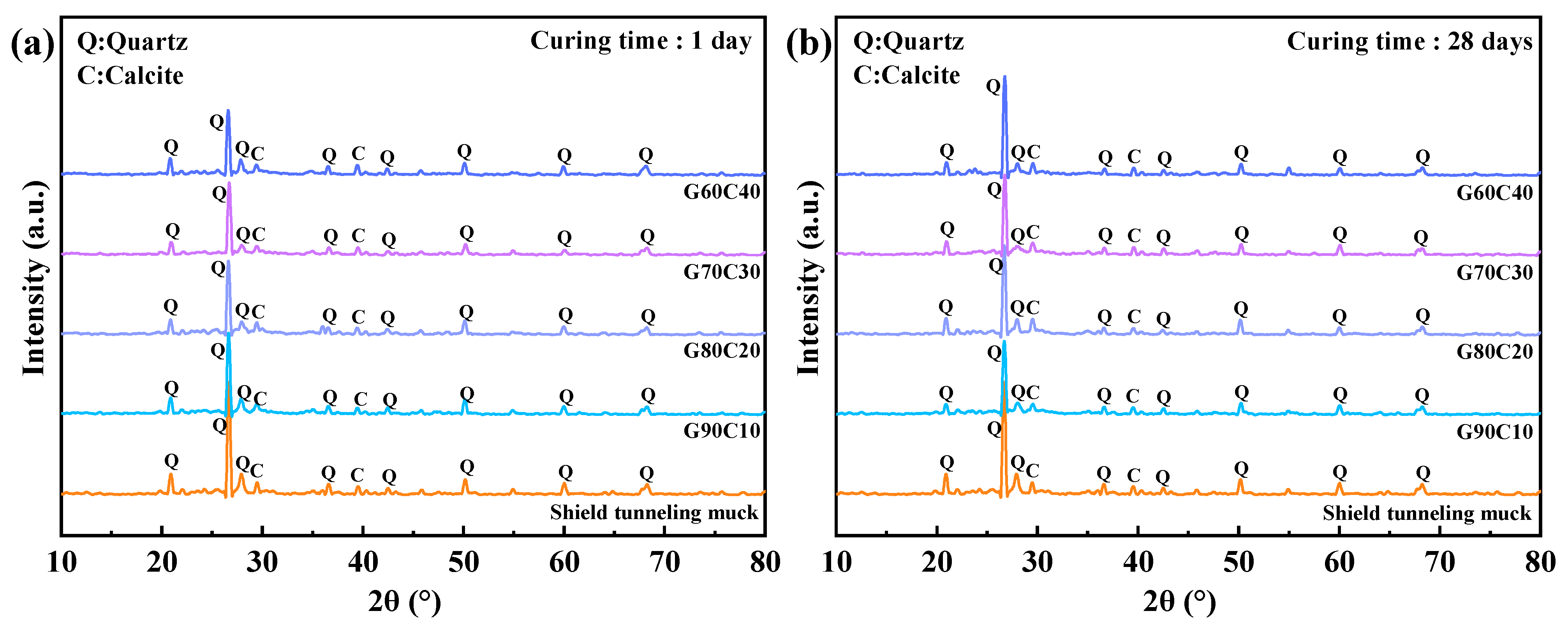
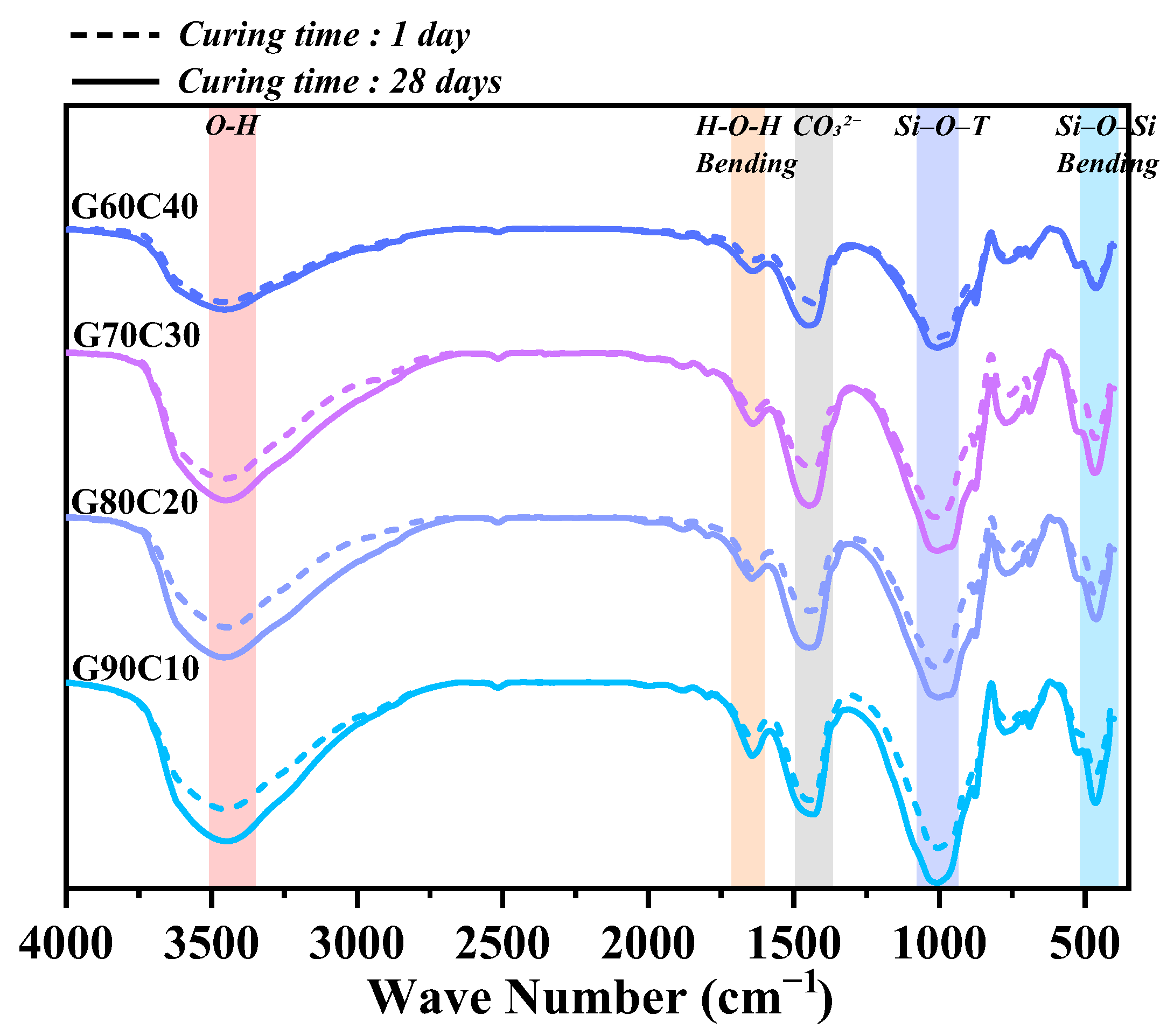
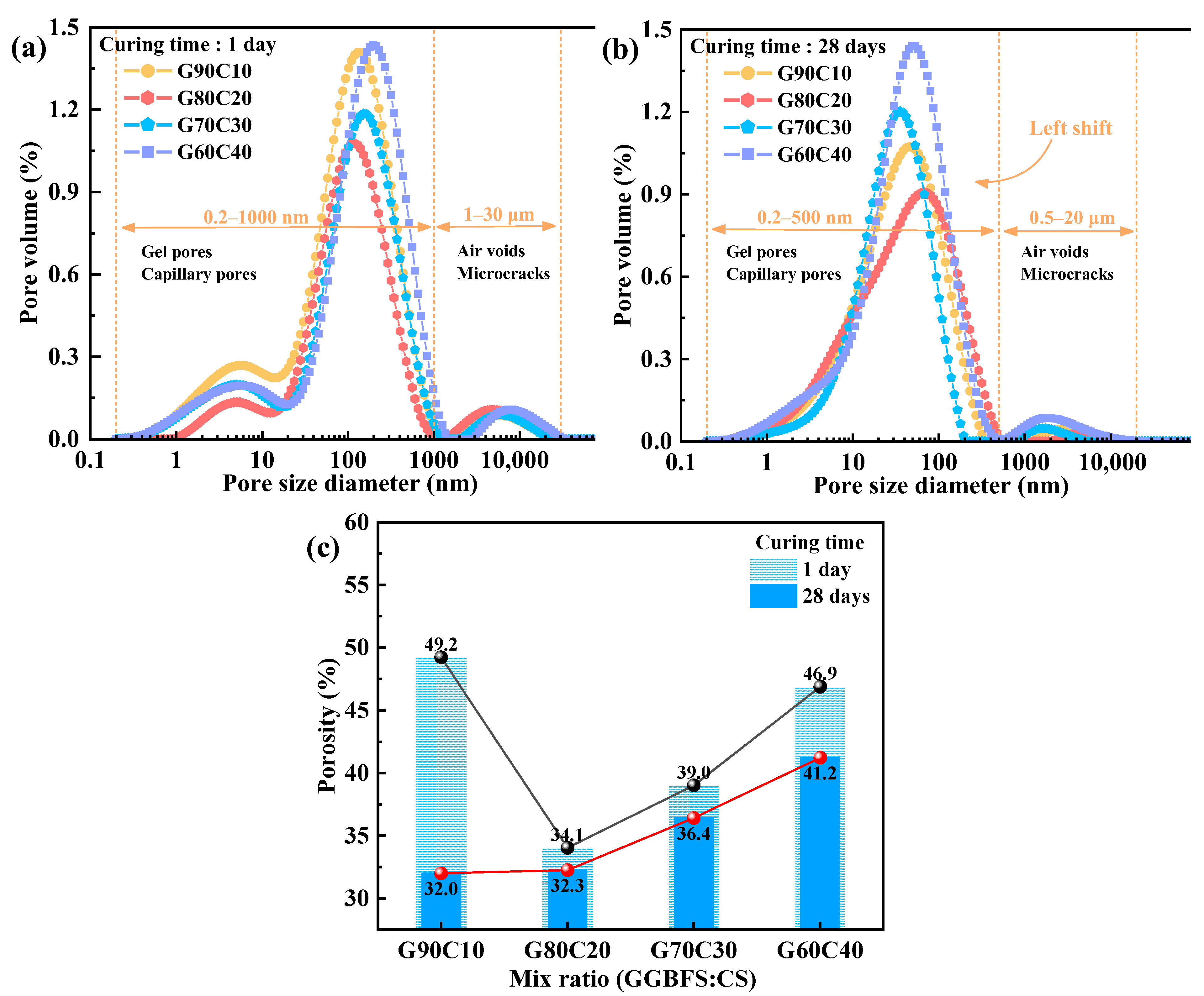
3.4. Microstructural Characteristics
4. Discussion
4.1. Effect of the CS-to-GGBFS Ratio on Early-Strength Development
4.2. Impact of CS Content on Strength-Controlling Mechanisms
4.3. Carbon Emission Analysis of GC-CLSM
5. Conclusions
- (1)
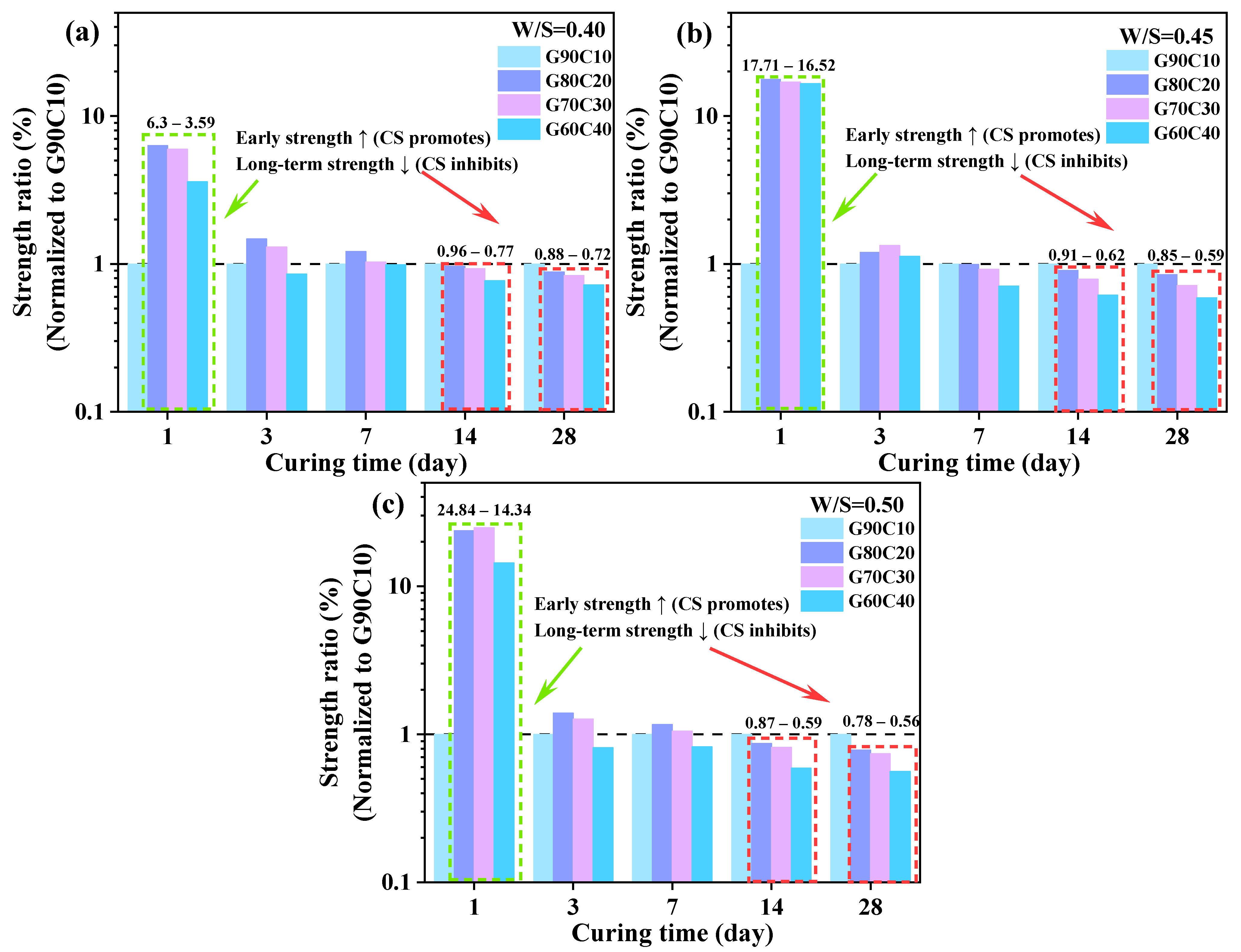
- The proposed GC-CLSM system exhibited excellent early-age strength performance, meeting practical engineering needs such as rapid backfilling and temporary load-bearing. The optimal GGBFS:CS ratio was determined to be 80:20, at which the GC-CLSM achieved a 1-day UCS of 1.17–1.75 MPa and a 28-day UCS of 3.55–5.36 MPa, demonstrating both rapid strength development and satisfactory long-term performance.
- (2)
- The GGBFS:CS ratio is critical in balancing early and long-term strength development. A moderate CS content (e.g., G80C20) significantly enhanced the early strength—achieving 6.3 to 23.6 times that of the low-CS reference—while incurring an acceptable 28-day strength loss of approximately 20%. In contrast, excessive CS addition (e.g., G60C40) still provided notable early-strength gains but resulted in substantial 28-day strength reductions, with losses of up to 50%.
- (3)
- The underlying mechanism of strength regulation is attributed to the synergy between alkalinity and Ca2+ supply. Increased CS content elevates pore-solution OH− concentration and provides readily available Ca2+, accelerating GGBFS dissolution and promoting rapid C–A–S–H gel formation. However, excessive CS leads to loosely packed gel and non-uniform pore structures, as confirmed by SEM, EDS, FTIR spectroscopy, and LF-NMR, which impair long-term strength development.
- (4)
- The GC-CLSM system developed in this study achieved a substantial reduction in carbon emissions—by approximately 68.6% to 70.3%—compared with conventional cement-based CLSM, highlighting its strong potential for sustainable construction applications.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CLSM | Controlled low-strength material |
| OPC | Ordinary Portland cement |
| UCS | Unconfined compressive strength |
| GGBFS | Ground granulated blast-furnace slag |
| CS | Carbide slag |
| SEM | Scanning electron microscopy |
| XRD | X-ray diffraction |
| XRF | X-ray fluorescence |
| FTIR | Fourier transform infrared |
| LF-NMR | Low-field nuclear magnetic resonance |
Appendix A
| Mix Ratio (GGBFS:CS) | Flow Value (mm) | ||
|---|---|---|---|
| W/S = 0.40 | W/S = 0.45 | W/S = 0.50 | |
| G100C0 | 221.4 | 361.3 | 432.1 |
| G90C10 | 185.6 | 322.5 | 409.3 |
| G80C20 | 154.5 | 288.3 | 385.9 |
| G70C30 | 131.2 | 259.5 | 361.3 |
| G60C40 | 110.8 | 232.6 | 342.3 |
| Mix Ratio (GGBFS:CS) | Bleeding Ratio (%) | ||
|---|---|---|---|
| W/S = 0.40 | W/S = 0.45 | W/S = 0.50 | |
| G100C0 | 1.57 | 2.42 | 3.75 |
| G90C10 | 1.14 | 2.05 | 3.25 |
| G80C20 | 0.74 | 1.63 | 2.57 |
| G70C30 | 0.46 | 1.09 | 1.88 |
| G60C40 | 0.23 | 0.62 | 1.29 |
| Mix Ratio (GGBFS:CS) | Setting Time (h) | |||||
|---|---|---|---|---|---|---|
| W/S = 0.40 | W/S = 0.45 | W/S = 0.50 | ||||
| Initial | Final | Initial | Final | Initial | Final | |
| G100C0 | 162.3 | 192.4 | 190.3 | 232.8 | 211.3 | 265.2 |
| G90C10 | 22.3 | 24.2 | 23.4 | 26.2 | 24.1 | 28.3 |
| G80C20 | 7.1 | 8.2 | 7.9 | 9.6 | 8.6 | 11.3 |
| G70C30 | 4.1 | 5.0 | 4.5 | 5.2 | 5.5 | 6.6 |
| G60C40 | 2.5 | 3.2 | 3.5 | 4.2 | 4.1 | 4.8 |
| Time (h) | pH | |||
|---|---|---|---|---|
| G90C10 | G80C20 | G70C30 | G60C40 | |
| 0.5 | 13.39 | 13.41 | 13.42 | 13.51 |
| 1 | 13.41 | 13.45 | 13.5 | 13.57 |
| 1.5 | 13.41 | 13.49 | 13.53 | 13.61 |
| 2 | 13.42 | 13.49 | 13.55 | 13.6 |
| 2.5 | 13.43 | 13.5 | 13.56 | 13.61 |
| 3 | 13.44 | 13.5 | 13.58 | 13.59 |
| 3.5 | 13.44 | 13.51 | 13.58 | 13.61 |
| 4 | 13.44 | 13.52 | 13.57 | 13.61 |
| 4.5 | 13.45 | 13.53 | 13.58 | 13.6 |
| 5 | 13.45 | 13.53 | 13.58 | 13.59 |
| 5.5 | 13.45 | 13.54 | 13.58 | 13.57 |
| 6 | 13.45 | 13.55 | 13.54 | 13.57 |
References
- Wang, W.; Wang, N.; Bao, Y.; Wang, Q.; Chang, X.; Sha, F.; Chen, X. Research and Implementation of Eco-Grouting Material Derived from Shield Tunneling Muck. Case Stud. Constr. Mater. 2024, 21, e03852. [Google Scholar] [CrossRef]
- Sharghi, M.; Jeong, H. The Potential of Recycling and Reusing Waste Materials in Underground Construction: A Review of Sustainable Practices and Challenges. Sustainability 2024, 16, 4889. [Google Scholar] [CrossRef]
- Wu, T.; Gao, Y.; Huang, C.; Jin, A.; Qu, X.; Ji, M.; Zhou, Y.; Li, J. In Situ Resource Reutilization of Earth Pressure Balance (EPB) Shield Muck for the Generation of Novel Synchronous Grouting Materials. Constr. Build. Mater. 2024, 421, 135737. [Google Scholar] [CrossRef]
- Zhao, H.; Mao, Y.; Jiang, M.; Dong, X.; Wang, T.; Xue, Y. Sustainable Production of Lightweight Ceramics from Shield Tunnel Muck and Phosphorus Tailings Enhanced by CaO/SiO2 Ratios. Constr. Build. Mater. 2025, 482, 141738. [Google Scholar] [CrossRef]
- Cheng, B.; Chang, R.; Yin, Q.; Li, J.; Huang, J.; Chen, H. A PSR-AHP-GE Model for Evaluating Environmental Impacts of Spoil Disposal Areas in High-Speed Railway Engineering. J. Clean. Prod. 2023, 388, 135970. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, H.; Han, Z.; Huang, S.; Wu, S.; Tian, Z.; Chen, X.; Wang, C.; Dong, Z. Mechanism of Geopolymeric Solidification in Shield-Tunnelling Slurry from Diverse Sources: The Role of Bentonite Adsorption in Influencing the Reaction Process. Constr. Build. Mater. 2024, 431, 136627. [Google Scholar] [CrossRef]
- Devaraj, V.; Mangottiri, V.; Balu, S. Sustainable Utilization of Industrial Wastes in Controlled Low-Strength Materials: A Review. Environ. Sci. Pollut. Res. 2022, 30, 14008–14028. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Wen, P.; Zeng, W.; Wang, X. A Comprehensive Review on Mechanical Properties of Green Controlled Low Strength Materials. Constr. Build. Mater. 2023, 363, 129611. [Google Scholar] [CrossRef]
- Ibrahim, M.; Rahman, M.K.; Najamuddin, S.K.; Alhelal, Z.S.; Acero, C.E. A Review on Utilization of Industrial By-Products in the Production of Controlled Low Strength Materials and Factors Influencing the Properties. Constr. Build. Mater. 2022, 325, 126704. [Google Scholar] [CrossRef]
- Parhi, S.K.; Dwibedy, S.; Panda, S.; Panigrahi, S.K. A Comprehensive Study on Controlled Low Strength Material. J. Build. Eng. 2023, 76, 107086. [Google Scholar] [CrossRef]
- Blanco, A.; Pujadas, P.; Cavalaro, S.H.P.; Aguado, A. Methodology for the Design of Controlled Low-Strength Materials. Application to the Backfill of Narrow Trenches. Constr. Build. Mater. 2014, 72, 23–30. [Google Scholar] [CrossRef]
- Li-Jeng, H.; Her-Yung, W.; Yeong-Nain, S.; Duc-Hien, L. Earth Pressure and Settlement Analysis of Trench Ducts Backfilled with Controlled Low Strength Materials. Procedia Eng. 2016, 142, 174–181. [Google Scholar] [CrossRef]
- Zhao, G.; Pan, X.; Yan, H.; Tian, J.; Han, Y.; Guan, H.; Liu, B.; Wang, S. Utilization of High Fine-Grained Shield Tunnel Spoil in CLSM and Effect of Foam Agent Content on Properties. Constr. Build. Mater. 2024, 423, 135836. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, J.; Xie, Y.; Fu, J.; Wang, S.; Yin, J. Utilization of Tunnel Spoils as a Lightweight Filling Material for the Voids behind Tunnel Excavation Contour. J. Clean. Prod. 2022, 372, 133559. [Google Scholar] [CrossRef]
- Zhang, T.; Jianming, Y.; Wang, W.; Chen, P.; Chen, C.; Wu, Z.; Wei, J.; Yu, Q. Efficient Utilization of Waste Shield Slurry and CDW Fines to Prepare Eco-Friendly Controlled Low-Strength Material. J. Clean. Prod. 2024, 444, 141343. [Google Scholar] [CrossRef]
- Cheng, D.; Reiner, D.M.; Yang, F.; Cui, C.; Meng, J.; Shan, Y.; Liu, Y.; Tao, S.; Guan, D. Projecting Future Carbon Emissions from Cement Production in Developing Countries. Nat. Commun. 2023, 14, 8213. [Google Scholar] [CrossRef]
- Sousa, V.; Bogas, J.A.; Real, S.; Meireles, I. Industrial Production of Recycled Cement: Energy Consumption and Carbon Dioxide Emission Estimation. Environ. Sci. Pollut. Res. 2022, 30, 8778–8789. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Wang, D.; Xia, C.; Tang, J. China’s Provincial Process CO2 Emissions from Cement Production during 1993–2019. Sci. Data 2022, 9, 165. [Google Scholar] [CrossRef]
- Zhao, G.; Guan, H.; Yan, H.; Ruan, Y.; Han, Y.; Pan, X.; Tian, J.; Liu, B. An Innovative Sustainable Solution: Recycling Shield-Discharge Waste Soil as Fine Aggregate to Produce Eco-Friendly Geopolymer-Based Flowable Backfill Materials. Environ. Technol. Innov. 2024, 36, 103857. [Google Scholar] [CrossRef]
- Jeremiah, J.J.; Abbey, S.J.; Booth, C.A.; Kashyap, A. Geopolymers as Alternative Sustainable Binders for Stabilisation of Clays—A Review. Geotechnics 2021, 1, 439–459. [Google Scholar] [CrossRef]
- Sun, X.; Liu, W.; Chen, X.; Wu, S.; Chen, G.; Bi, Y.; Chen, Z. Sustainable Solutions: Transforming Waste Shield Tunnelling Soil into Geopolymer-Based Underwater Backfills. J. Clean. Prod. 2024, 445, 141363. [Google Scholar] [CrossRef]
- Zhao, G.; Pan, X.; Yan, H.; Tian, J.; Han, Y.; Guan, H.; Ruan, Y.; Liu, B.; Chen, F. Optimization and Characterization of GGBFS-FA Based Alkali-Activated CLSM Containing Shield-Discharged Soil Using Box-Behnken Response Surface Design Method. Case Stud. Constr. Mater. 2024, 21, e03800. [Google Scholar] [CrossRef]
- Zhang, S.; Jiao, N.; Ding, J.; Guo, C.; Gao, P.; Wei, X. Utilization of Waste Marine Dredged Clay in Preparing Controlled Low Strength Materials with Polycarboxylate Superplasticizer and Ground Granulated Blast Furnace Slag. J. Build. Eng. 2023, 76, 107351. [Google Scholar] [CrossRef]
- Park, S.M.; Lee, N.K.; Lee, H.K. Circulating Fluidized Bed Combustion Ash as Controlled Low-Strength Material (CLSM) by Alkaline Activation. Constr. Build. Mater. 2017, 156, 728–738. [Google Scholar] [CrossRef]
- Chompoorat, T.; Thepumong, T.; Nuaklong, P.; Jongvivatsakul, P.; Likitlersuang, S. Alkali-Activated Controlled Low-Strength Material Utilizing High-Calcium Fly Ash and Steel Slag for Use as Pavement Materials. J. Mater. Civ. Eng. 2021, 33, 04021178. [Google Scholar] [CrossRef]
- Jiang, M.; Qian, Y.; Sun, Q. Preparation of Controlled Low-Strength Materials from Alkali-Excited Red Mud-Slag-Iron Tailings Sand and a Study of the Reaction Mechanism. Environ. Sci. Pollut. Res. 2022, 30, 22232–22248. [Google Scholar] [CrossRef]
- Mahamaya, M.; Jain, S.; Das, S.K.; Paul, R. Engineering Properties of Cementless Alkali Activated CLSM Using Ferrochrome Slag. J. Mater. Civ. Eng. 2023, 35, 4022441. [Google Scholar] [CrossRef]
- Zhou, Y.; Huo, M.; Zhang, L.; Guan, Q. Strength Development and Solidification Mechanism of Soils with Different Properties Stabilized by Cement-Slag-Based Materials. Case Stud. Constr. Mater. 2024, 21, e04034. [Google Scholar] [CrossRef]
- Ling, T.-C.; Kaliyavaradhan, S.K.; Poon, C.S. Global Perspective on Application of Controlled Low-Strength Material (CLSM) for Trench Backfilling—An Overview. Constr. Build. Mater. 2018, 158, 535–548. [Google Scholar] [CrossRef]
- Kaliyavaradhan, S.K.; Ling, T.-C.; Guo, M.-Z. Upcycling of Wastes for Sustainable Controlled Low-Strength Material: A Review on Strength and Excavatability. Environ. Sci. Pollut. Res. 2022, 29, 16799–16816. [Google Scholar] [CrossRef]
- ASTM D6103; Standard Test Method for Flow Consistency of Controlled Low Strength Material (CLSM). ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM C940; Standard Test Method for Expansion and Bleeding of Freshly Mixed Grouts for Preplaced-Aggregate Concrete in the Laboratory. ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM C403; Standard Test Method for Time of Setting of Concrete Mixtures by Penetration Resistance. ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM D2166/D2166M-13; Standard Test Method for Unconfined Compressive Strength of Cohesive Soil. ASTM International: West Conshohocken, PA, USA, 2013.
- Vollpracht, A.; Lothenbach, B.; Snellings, R.; Haufe, J. The Pore Solution of Blended Cements: A Review. Mater. Struct. 2016, 49, 3341–3367. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, M.; Sheikh, I.R.; Chen, C.; He, J.; Chen, F.; Chen, X.; Guan, J.; Wu, S.; Dong, Z. Geocell-Reinforced Pourable Geopolymeric Clayey Waste Slurry: Performance as a Roadway Layer under Cyclic Loading. Transp. Geotech. 2025, 54, 101610. [Google Scholar] [CrossRef]
- Kapeluszna, E.; Kotwica, Ł.; Różycka, A.; Gołek, Ł. Incorporation of Al in C-A-S-H Gels with Various Ca/Si and Al/Si Ratio: Microstructural and Structural Characteristics with DTA/TG, XRD, FTIR and TEM Analysis. Constr. Build. Mater. 2017, 155, 643–653. [Google Scholar] [CrossRef]
- Pardal, X.; Pochard, I.; Nonat, A. Experimental Study of Si–Al Substitution in Calcium-Silicate-Hydrate (C-S-H) Prepared under Equilibrium Conditions. Cem. Concr. Res. 2009, 39, 637–643. [Google Scholar] [CrossRef]
- Hong, S.-Y.; Glasser, F.P. Alkali Sorption by C-S-H and C-A-S-H Gels. Cem. Concr. Res. 2002, 32, 1101–1111. [Google Scholar] [CrossRef]
- Jha, A.K.; Kumar, D.; Sivapullaiah, P.V. Influence of Fly Ash on Geotechnical Behaviour of Red Mud: A Micro-Mechanistic Study. Geotech. Geol. Eng. 2020, 38, 6157–6176. [Google Scholar] [CrossRef]
- Razeghi, H.R.; Safaee, F.; Geranghadr, A.; Ghadir, P.; Javadi, A.A. Investigating Accelerated Carbonation for Alkali Activated Slag Stabilized Sandy Soil. Geotech. Geol. Eng. 2024, 42, 575–592. [Google Scholar] [CrossRef]
- Antunes Boca Santa, R.A.; Bernardin, A.M.; Riella, H.G.; Kuhnen, N.C. Geopolymer Synthetized from Bottom Coal Ash and Calcined Paper Sludge. J. Clean. Prod. 2013, 57, 302–307. [Google Scholar] [CrossRef]
- Li, Z.; Ikeda, K. Compositions and Microstructures of Carbonated Geopolymers with Different Precursors. Materials 2024, 17, 1491. [Google Scholar] [CrossRef]
- Harirchi, P.; Yang, M. Exploration of Carbon Dioxide Curing of Low Reactive Alkali-Activated Fly Ash. Materials 2022, 15, 3357. [Google Scholar] [CrossRef]
- Wang, S.; Su, J.; Wu, Z.; Ma, W.; Li, Y.; Hui, H. Silty Clay Stabilization Using Metakaolin-Based Geopolymer Binder. Front. Phys. 2021, 9, 769786. [Google Scholar] [CrossRef]
- Yan, D.; Xie, L.; Qian, X.; Ruan, S.; Zeng, Q. Compositional Dependence of Pore Structure, Strengthand Freezing-Thawing Resistance of Metakaolin-Based Geopolymers. Materials 2020, 13, 2973. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ruan, S.; Zeng, Q.; Liu, Y.; Zhang, M.; Tian, Y.; Yan, D. Pore Structure of Geopolymer Materials and Its Correlations to Engineering Properties: A Review. Constr. Build. Mater. 2022, 328, 127064. [Google Scholar] [CrossRef]
- Liu, B.; Yang, J.; Li, D.; Xing, F.; Fang, Y. Effect of a Synthetic Nano-CaO-Al2O3-SiO2-H2O Gel on the Early-Stage Shrinkage Performance of Alkali-Activated Slag Mortars. Materials 2018, 11, 1128. [Google Scholar] [CrossRef] [PubMed]
- Mastoi, A.K.; Hameed, S.; Ali, M.; Traore, A.F. Machine Learning–Based Prediction of Unconfined Compressive Strength and Contaminant Leachability in Dredged Contaminated Sediments for Land Reclamation Projects. Environ. Sci. Pollut. Res. 2025, 32, 8160–8182. [Google Scholar] [CrossRef]
- Nikolić, I.; Drinčić, A.; Djurović, D.; Karanović, L.; Radmilović, V.V.; Radmilović, V.R. Kinetics of Electric Arc Furnace Slag Leaching in Alkaline Solutions. Constr. Build. Mater. 2016, 108, 1–9. [Google Scholar] [CrossRef]
- Snellings, R. Solution-Controlled Dissolution of Supplementary Cementitious Material Glasses at PH 13: The Effect of Solution Composition on Glass Dissolution Rates. J. Am. Ceram. Soc. 2013, 96, 2467–2475. [Google Scholar] [CrossRef]
- Yang, G.; Li, C.; Xie, W.; Yue, Y.; Kong, C.; Li, X. Effect of Carbide Slag and Steel Slag as Alkali Activators on the Key Properties of Carbide Slag-Steel Slag-Slag-Phosphogypsum Composite Cementitious Materials. Front. Mater. 2024, 11, 1353004. [Google Scholar] [CrossRef]
- Song, S.; Jennings, H.M. Pore Solution Chemistry of Alkali-Activated Ground Granulated Blast-Furnace Slag11This Paper Was Originally Submitted to Advanced Cement Based Materials. The Paper Was Received at the Editorial Office of Cement and Concrete Research on 12 November 1998 and Accepted in Final Form on 16 November 1998. Cem. Concr. Res. 1999, 29, 159–170. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Rose, V.; Mejía de Gutierrez, R. Evolution of Binder Structure in Sodium Silicate-Activated Slag-Metakaolin Blends. Cem. Concr. Compos. 2011, 33, 46–54. [Google Scholar] [CrossRef]
- Gebregziabiher, B.S.; Thomas, R.; Peethamparan, S. Very Early-Age Reaction Kinetics and Microstructural Development in Alkali-Activated Slag. Cem. Concr. Compos. 2015, 55, 91–102. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L. Durability of Alkali-Activated Materials: Progress and Perspectives. J. Am. Ceram. Soc. 2014, 97, 997–1008. [Google Scholar] [CrossRef]
- Lamaa, G.; Duarte, A.P.C.; Silva, R.V.; de Brito, J. Carbonation of Alkali-Activated Materials: A Review. Materials 2023, 16, 3086. [Google Scholar] [CrossRef]
- Zhang, X.; Long, K.; Liu, W.; Li, L.; Long, W.-J. Carbonation and Chloride Ions’ Penetration of Alkali-Activated Materials: A Review. Molecules 2020, 25, 5074. [Google Scholar] [CrossRef] [PubMed]




| Materials | SiO2 (%) | Al2O3 (%) | CaO (%) | Fe2O3 (%) | TiO2 (%) | SO3 (%) | MnO (%) | V2O5 (%) | CrO3 (%) | Rb2O (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Shield tunneling muck | 70.33 | 12.66 | 8.81 | 3.99 | 0.76 | 0.01 | 0.07 | 0.03 | 0.02 | 0.01 |
| GGBFS | 31.76 | 14.42 | 48.66 | 0.36 | 1.40 | 2.38 | 0.46 | - | - | - |
| CS | 3.49 | 1.78 | 93.16 | 0.52 | 0.09 | 0.89 | - | - | 0.02 | - |
| No. | Sample | GGBFS:CS Ratio | GGBFS (%) | CS (%) | CS/GGBFS Ratio (%) | Alkaline Activator (%) | W/S (%) |
|---|---|---|---|---|---|---|---|
| 1 | G100C0 | 100:0 | 20 | 0 | 0 | 5 | 40 |
| 2 | G90C10 | 90:10 | 18 | 2 | 11.11 | 5 | 40 |
| 3 | G80C20 | 80:20 | 16 | 4 | 25 | 5 | 40 |
| 4 | G70C30 | 70:30 | 14 | 6 | 42.86 | 5 | 40 |
| 5 | G60C40 | 60:40 | 12 | 8 | 66.67 | 5 | 40 |
| 6 | G100C0 | 100:0 | 20 | 0 | 0 | 5 | 45 |
| 7 | G90C10 | 90:10 | 18 | 2 | 11.11 | 5 | 45 |
| 8 | G80C20 | 80:20 | 16 | 4 | 25 | 5 | 45 |
| 9 | G70C30 | 70:30 | 14 | 6 | 42.86 | 5 | 45 |
| 10 | G60C40 | 60:40 | 12 | 8 | 66.67 | 5 | 45 |
| 11 | G100C0 | 100:0 | 20 | 0 | 0 | 5 | 50 |
| 12 | G90C10 | 90:10 | 18 | 2 | 11.11 | 5 | 50 |
| 13 | G80C20 | 80:20 | 16 | 4 | 25 | 5 | 50 |
| 14 | G70C30 | 70:30 | 14 | 6 | 42.86 | 5 | 50 |
| 15 | G60C40 | 60:40 | 12 | 8 | 66.67 | 5 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhang, J.; Sun, X.; Dong, S.; Wu, S. Early-Strength Controllable Geopolymeric CLSM Derived by Shield Tunneling Muck: Performance Optimization and Hydration Mechanism of GGBFS–CS Systems. Buildings 2025, 15, 2373. https://doi.org/10.3390/buildings15132373
Liu J, Zhang J, Sun X, Dong S, Wu S. Early-Strength Controllable Geopolymeric CLSM Derived by Shield Tunneling Muck: Performance Optimization and Hydration Mechanism of GGBFS–CS Systems. Buildings. 2025; 15(13):2373. https://doi.org/10.3390/buildings15132373
Chicago/Turabian StyleLiu, Jiguo, Jun Zhang, Xiaohui Sun, Shutong Dong, and Silin Wu. 2025. "Early-Strength Controllable Geopolymeric CLSM Derived by Shield Tunneling Muck: Performance Optimization and Hydration Mechanism of GGBFS–CS Systems" Buildings 15, no. 13: 2373. https://doi.org/10.3390/buildings15132373
APA StyleLiu, J., Zhang, J., Sun, X., Dong, S., & Wu, S. (2025). Early-Strength Controllable Geopolymeric CLSM Derived by Shield Tunneling Muck: Performance Optimization and Hydration Mechanism of GGBFS–CS Systems. Buildings, 15(13), 2373. https://doi.org/10.3390/buildings15132373





