1. Introduction
With rising population and diminishing water supplies, desalination is becoming a very viable option to meet freshwater demands [
1]. Current studies show that 40% of the world’s population faces water shortage [
2]. This number is expected to rise to 60% in the coming years [
3]. It is essentially important to meet water demands and bridge this gap while keeping in mind sustainability and environmental concerns [
4] (Gude, 2017). To meet these water demands desalination has been on the rise and has increased pressure on desalination plants. Despite the benefits, desalination produces a highly concentrated saline solution known as “reject brine”. This highly saline waste is a potential environmental detriment as it is very basic in nature and highly corrosive [
5]. The main source of reject brine production is desalination plants. For example, desalination plants supply approximately 42% of drinking water in the United Arab Emirates (UAE), amounting to over 7 million m
3 per day. This dependency on desalinated water is higher in Kuwait, accounting for approximately 90%, Oman (86%), and Saudi Arabia (70%) [
6]. The number of desalination plants worldwide has exceeded 21,000 as of 2022, nearly doubling the count from the past decade, and is expected to grow at a rate of 6% to 12% in the coming years [
6]. Desalination is predominant in the gulf countries and arid regions to meet the demands for potable water. The most conventional methods used for desalination are classified as reverse osmosis (RO), which is a pressure-driven membrane process in which saline water is passed through a semi-permeable membrane which filters the salts and impurities, multistage flash (MSF), which is a thermal desalination technique in which saline water is heated and quickly flashed into a stream in multiple stages under reduced pressure and this steam is condensed to yield freshwater, forward osmosis (FO), which is also a membrane process and also uses a concentration gradient to pass water through a semi-permeable membrane from a feed and it is converted into a concentrated solution from which freshwater can be recovered, and membrane distillation (MD), which is a thermally driven process in which saline water is heated and the vapor is passed through a hydrophobic membrane allowing only the water vapor to pass blocking salts and impurities, and this vapor is then condensed to yield freshwater [
7]. All these procedures produce a large amount of reject brine after the freshwater is extracted, hence making desalination plants the largest producers of reject brine. Other major industrial processes also contribute to the production of reject brine. If reject brine is not disposed of correctly, it can pose a severe risk to the environment as it contains high levels of salts, chemicals, scalants, coagulants, and other dissolved solids [
8].
Section 1.1,
Section 1.2 and
Section 1.3 delve deeper into global reject brine production, highlighting the regions with maximum reject brine production, the chemical and physical characteristics of reject brine, and the current disposal methods of reject brine and its negative impact on the environment, highlighting the need for sustainable alternatives.
1.1. Reject Brine Production
The production of reject brine largely depends on the type of desalination technology used and the quality of feed water [
9]. Depending on the source of feed water and desalination technology used, generally only 35% to 45% of pure water can be recovered and the rest is discharged as reject brine [
10]. According to recent studies an estimated 141.5 million m
3/day to 51.7 billion m
3/day of reject brine is produced globally as shown in
Table 1.
Table 1 lists the approximate daily quantities of reject brine produced globally and the percentages of these quantities in different regions of the world [
5]. These quantities are approximately 50% greater than the total daily volume of desalinated water produced globally [
5]. Most of the reject brine production is concentrated in the Middle East and North Africa (MENA) region, which produces around 99.4 million m
3/day of reject brine, representing around 70.2% of the total quantities of reject brine produced globally, followed by the East Africa and Pacific region with around 10.5% [
5,
11]. Reject brine, as indicated in several previous research efforts, has been proven to be toxic and detrimental to the environment [
5]. Therefore, it is important to understand its characteristics, the impact of the current methods of reject brine disposal and its effects on the environment, including its potential to harm marine ecosystems, disrupt aquatic life, and degrade water quality, and the risk of salinity concentration of the soil [
12].
1.2. Characteristics of Reject Brine
The definition of “brine” theoretically means any water with high levels of salinity. Reject brine is defined as the highly concentrated saline water which is discharged in the last stage of the desalination process [
13]. The desalination process generally uses many different types of chemicals for pre- and posttreatment operations. The chemicals used for these processes include sodium hypochlorite (NaOCl), which is used for the prevention of bacterial growth. Ferric chloride (FeCl
3) and aluminum chloride (AlCl
3) are used as flocculants for removing suspended particles. Anti-scalant additives such as sodium hexameta phosphate (NaPO
3)
6 is used to prevent scaling on membranes and pipes of the desalination facility. Acids such as sulfuric acid (H
2SO
4) are typically used to adjust the pH levels of feed water. Therefore, these chemicals are additionally present at varying concentrations along with other chemicals that are present in the reject brine that is discharged.
Table 2 shows the average characteristics and chemicals that are present in reject brine according to samples collected from the MENA region [
14].
Due to the presence of the chemicals shown in
Table 2, when reject brine is discharged directly into the sea, it can alter the salinity, pH levels, and temperature of seawater which can lead to the disruption of marine ecosystems [
14]. Reject brine characteristics also depend on the quality of feed water, recovery ratio (RR), and on the type of desalination process used [
15].
1.3. Reject Brine Disposal Methods
According to previous studies, it is estimated that, for every cubic meter of water desalinated, approximately the same amount of reject brine is produced.
Table 3 shows the most common practice of reject brine disposal is releasing it back into nature [
16]. This could have detrimental effects on the environment in the long run. Even with the advancement in the development of efficient desalination technology, very little improvement has been observed in the management and disposal of reject brine. Reject brine poses a major environmental challenge, whose disposal and management are becoming increasingly expensive [
17]. Reject brine disposal methods currently in practice are the “direct discharge” and the “evaporation treatment discharge”. The processes of the “direct discharge” method include surface discharge (into lakes, oceans, and other water bodies), deep well injection, and evaporation ponds. On the other hand, the processes of the “evaporation treatment discharge” method include solar ponds and wind-aided intensification of evaporation. However, the “direct discharge” is the most common method of reject brine disposal. Since reject brine contains high levels of additives and salts, this type of disposal has negative impacts on the water bodies and soil into which it is discharged. The effects of reject brine disposal using the methods mentioned earlier and their effects on the environment have been discussed in detail in
Section 1.3.1 and
Section 1.3.2.
1.3.1. Direct Discharge
Disposing of reject brine into close-by water bodies mainly rivers and oceans is the most common practice. This type of disposal has been deemed most practical and cost effective. Due to the cost effectiveness of this practice, the environmental impacts have largely been overlooked [
18]. Since reject brine contains high levels of chemicals, salts, and TDS and also has a higher temperature and salinity, it can seriously affect the marine ecosystem. The high level of chemicals can lead to reduction of oxygen available for marine life [
19]. Hydrogen, sulfide, and chloride present in reject brine can have serious negative impacts on marine life if not treated before being released. This is a concern especially for regions that depend on desalination for their water needs and can have long-term impacts with large amounts of reject brine being disposed into the ocean.
Inland desalination plants often discharge the produced reject brine into sewers, which is then transported into sewage treatment plant facilities [
20]. This is also considered as a practical and cost-effective solution for disposal. However, the presence of high salinity and dissolved solids and chemicals can be an issue for managing excessive salinity in wastewater treatment facilities [
21]. This practice can result in additional environmental concerns and have effects on the water bodies where the sewage is being disposed of. Deep well injection is another frequently chosen method for disposing of reject brine from inland desalination plants [
22]. This method is also cost effective; however, this is not viable in regions that are prone to earthquakes [
23]. If deep well injection is not monitored correctly, it can lead to contamination of groundwater as a result of seepage since the waste reject brine is pumped into deep underground wells in this process [
24]. Therefore, deep well injection is also considered as an environmental threat in the long run as it can degrade the quality of soil [
25].
1.3.2. Evaporation Treatment Discharge
Disposal of reject brine with the help of evaporation ponds has by far been considered the most cost effective and economical solution, especially in hot and arid regions where sunlight is abundant and evaporation rates are high [
15]. However, the evaporation ponds need to be constructed with a lining to avoid seepage into the ground. The design aspects of these ponds such as surface area and depth play a major role in speeding up the process. Other attempts have been made to increase the efficiency of evaporation with the help of mechanical wind-aided intensified evaporation [
26]. The use of mechanical-aided evaporation can increase the rate of evaporation by almost 50% under favorable climatic conditions. This technique is highly applicable in regions that have high winds [
27].
1.3.3. Environmental Impact of Current Reject Brine Disposal Methods
Considering the currently used reject brine disposal methods discussed in
Section 1.3.1 and
Section 1.3.2, it is important to highlight that the high levels of salts and chemicals in reject brine require safe disposal to minimize its effects on the environment [
28]. A number of previous studies have highlighted the impact of reject brine disposal on soil, ground water, and the marine environment [
11]. The high salinity of reject brine can cause harm to marine life and sea grass structure. Soil degradation and groundwater contamination are other concerns related to improper disposal of reject brine, which has the potential to damage vegetation due to the increase in the salinity of soil [
29]. Disposal of reject brine into unlined pits can lead to the mixing of saline water with freshwater due to seepage into nearby freshwater bodies [
30]. Keeping in mind the long-term impacts, it is essential to monitor the disposal operation and implement appropriate policies and regulations to monitor safe disposal practices [
31]. Another major problem with unmonitored disposal of reject brine on land is the replacement of calcium with sodium ions [
32]. This results in reduction of water infiltration and reduction in soil aeration which can lead to the change of soil structure over time and can also affect vegetation [
33]. Additionally, increased levels of Cl, Br, and Na can reduce plant productivity [
34]. Therefore, it is essential to develop sustainable options for reject brine management and application which can help in turning this toxic waste into a reusable resource. This study highlights some such innovative reject brine management and application methods.
The following subsection focuses on sustainable strategies to manage reject brine and explores potential innovative application techniques that aim towards mitigating environmental impact by emphasizing resource recovery and alternative reuse strategies across different industries, especially in construction.
1.4. Reject Brine Management and Applications
The present options available for management and applications of reject brine are limited and most have not achieved a practical solution to tackle the environmental challenges [
15]. Over recent years the management and application strategies for reject brine have gained considerable attention due to the negative effects of reject brine and increasing demand for sustainable waste management [
35]. It is essential to have correct management practices and application strategies to mitigate long-term environmental and financial consequences that arise due to improper and traditional disposal methods [
36]. The following
Section 1.4.1 and
Section 1.4.2 present an overview of sustainable reject brine management and the possibility of converting reject brine from a waste product into a valuable resource, highlighting the requirement for innovative solutions for reject brine disposal and advanced treatment and resource recovery to reduce environmental harm. It highlights specific applications of reject brine, such as its application and reuse in agriculture, energy, and resource recovery.
Section 1.4.3 explores the possibility of reusing reject brine in construction, discussing various possibilities of using reject brine for cement and concrete production, brick manufacturing, soil stabilization, and asphalt binders.
1.4.1. Reject Brine Management
Management of reject brine involves treatment to minimize the resulting consequences. Examples of such approaches are two-step reverse osmosis (RO), in which accelerated precipitation softening to remove scale-forming ions is integrated before a second RO stage to improve the rate of recovery and membrane protection with chemical precipitation between RO stages by using softening pretreatment and adjusting pH to reduce scaling and improving RO performance [
36], two-step RO with biological treatment, which uses a biological process for the removal of organic compounds before RO to reduce fouling and enhance efficiency [
37], and two-step nanofiltration and seeded slurry precipitation and reverse osmosis (SAPRO), which is a hybrid method which combines nanofiltration and seeded precipitation to reduce scaling [
38]. Emerging techniques include membrane distillation, which is a thermally driven process in which vapor is passed through a hydrophobic membrane and is used for brine concentration and high-salinity treatment, pervaporation, where water vapor is selectively diffused through a hydrophilic membrane, achieving almost complete salt rejection [
39], capacitive deionization, which incorporates electrically charged electrodes to remove ions [
40], and FO, where a concentrated draw solution is used to pull water across a membrane without applying pressure. These techniques are gaining importance but have a relatively high operating cost. Zero liquid discharge (ZLD) is another membrane-based technology that can recover usable solids and freshwater with the help of multistage thermal and membrane processes and is becoming popular in cases where reject brine has high amounts of metals or other toxins depending on the type of feed water and required capturing of the resulting solid products [
41]. In a ZLD system, often all solid components of reject brine can be recovered using an evaporation–crystallization technique which produces a solid residue (most commonly calcium sulfate salts) and the liquid effluent produces a distillate which generates freshwater [
42]. However, despite the high efficiency of the ZLD system it consumes a large amount of energy and is viable only for selected cases.
Another technique of reject brine management focuses on the valorization of reject brine by exploitation of minerals present in it [
15]. This method separates divalent salts by extraction using an organic solvent. It is a technically feasible process but has high economic factors and requires a solvent recovery system that requires energy consumption [
43]. Therefore, keeping in mind the adverse effects of reject brine on the environment and the limited available management techniques which are viable in all aspects, it is important to develop additional management and application solutions and technologies which can help in converting reject brine into a valuable resource while minimizing its environmental impact at the same time. Some of the available reject brine application strategies along with their pros and cons have been further discussed in the following subsections which highlight the possibility of sustainable reuse.
1.4.2. General Applications of Reject Brine
Reject brine can have several applications across various industries. Components present in reject brine such as dissolved salts, minerals, and other chemicals can be useful for different applications. This will not only be a sustainable approach towards reject brine disposal but will also promote resource reuse by converting reject brine into something valuable while reducing the environmental impact along with reducing cost.
Table 3 lists the most common applications of reject brine across different industries. These methods are economically viable and reduce environmental deterioration and are particularly useful in regions which face water scarcity. Therefore, over the past year these techniques have been gaining popularity. The following sections will discuss these techniques in detail with an emphasis on construction applications of reject brine. Application of reject brine can be found across various industries. This typically involves reusing reject brine for different applications and operations which result in better outcomes as compared to conventional methods. One of the major challenges facing the desalination process is the safe disposal of the reject brine produced, which is considered a major technical and economical concern [
44].
Table 3.
Different Applications of Reject Brine Across Various Industries.
Table 3.
Different Applications of Reject Brine Across Various Industries.
| Industries | Applications | Reference |
|---|
| Agriculture | Irrigation | [45] |
| Fish Farming |
| Energy | Generation of Electricity | [46] |
| Resource Recovery | Salt Production and Recovery | [47] |
| Minerals and Salt Mining | [48] |
| Construction | Cement and Concrete Production | [49] |
| Manufacturing Bricks/Blocks and Mortar | [50] |
| Soil Stabilization | [51] |
| Asphalt Binders | [52] |
The use of reject brine in agriculture (irrigation and fish farming) in arid and semi-arid regions is an integrated scheme that enables the cultivation of certain species of fish that are resistant to saline conditions and help with the irrigation of a wide range of saline-resistant crops, reducing the dependency on freshwater [
53]. Certain fish species have adapted well to high levels of salinity. In this system the reject brine that is discharged from desalination plants is mixed with some freshwater in the forward production stage [
35]. The water that exits these ponds is then mixed with organic fertilizers (manure from local livestock) and then pumped for the use of halophyte plantations. This method helps in turning unproductive lands and saline ponds into valuable commercial products. Therefore, the use of reject brine in irrigation and aquaculture helps in achieving an environmentally acceptable solution as well as a sustainable production scheme [
54].
Previous studies have shown that reject brine has electrical conductivity (EC) which ranges from 40,000 to 85,000 µS/cm, depending on the method of desalination used and the characteristics of feed water. When this mixes with freshwater, it results in an ion exchange driven by a steep salinity and conductivity gradient between the fluids [
55]. The number of salts present in reject brine depending on the type of feed water is directly proportional to its EC. Additionally, when reject brine mixes with freshwater it creates an electrical gradient which can be used to generate electricity also known as “blue energy”. Pressure-retarded osmosis (PRO) and electrodialysis (ED) are techniques used to harness blue energy [
56]. Generation of blue energy has the potential for producing sustainable power, especially in coastal regions where desalination is widely used [
56]. Furthermore, reject brine contains large amounts of dissolved salts which can be recovered and reused for several industrial applications with the help of techniques like distillation, membrane separation, ED, ion exchange, eutectic freezing, and chemical processes. The main salts that are recovered from these processes are MgCl
2, NaCl
2, Mg, Br, Na, SiO
2, SO
4, Cl, and several others [
57]. Some of the other components recovered from reject brine are metals and minerals, since reject brine has high concentrations of TDS. Extraction of metals and minerals from reject brine has proven to be cost effective when compared to traditional methods [
58].
1.4.3. Application of Reject Brine in Construction
Another very efficient reuse and application of reject brine is in construction. With the rapid growth of population and urbanization, the construction industry is growing rapidly. To meet this growing demand there is constant pressure on natural resources. Construction activities often produce large amounts of CO
2 and other harmful substances which leave a major carbon footprint. Therefore, incorporating reject brine in construction applications can reduce the constant need and demand for freshwater as well as help in a sustainable way to reuse and dispose of reject brine [
59]. Various uses of reject brine in construction have been evaluated in the following subsections, highlighting the potential to promote sustainable construction practices and reduce freshwater dependency by evaluating replacement of freshwater in cement and concrete production with reject brine without compromising on structural integrity and by evaluating the role of reject brine in brick and mortar manufacture by emphasizing strength and durability improvements. The following subsections highlight the use of reject brine to stabilize soil for construction purposes and the use of reject brine as asphalt binder. The following subsections demonstrate the potential diverse applications of reject brine in construction regarding it as a sustainable resource.
Use of Reject Brine for Cement and Concrete Production
With increasing industrialization and urbanization, the need and demand for concrete is ever increasing, with an approximate of 30 billion tons per year, amounting to approximately 4 tons per person per year and approximately 11 kg per person per day [
60]. Concrete is generally prepared with Portland cement (PC) and this requires large quantities of freshwater. Being an energy-intensive process, cement and concrete production is not an environmentally friendly process. The constant demand for water and the production of greenhouse gases have raised serious concerns [
61]. To address both of these concerns, the possibility of using reject brine for the mixing of concrete is gaining popularity. The use of reject brine for concrete preparation would serve two main purposes; first, it would reduce the dependency on and consumption of freshwater, as approximately 170 L of water is required per cubic meter of concrete produced [
62]. Second, the detrimental ecological and physiochemical effect of reject brine on receiving bodies would reduce as this could serve as a sustainable disposal alternative [
63]. A study conducted by Kaushik and Islam [
64] highlighted in an experiment the effect of saline water on the setting time of cement and found that both initial and final setting times of cement were significantly reduced. The initial setting time reduced from 113 min (plain water) to 60 min (3 times saline water concentration) and further reduced to 13 min (5 times saline water concentration) before slightly increasing to 16 min (10 times saline water concentration). The final setting time was similarly reduced from 175 min (plain water) to 74 min (five times saline water concentration). This experiment highlighted that the chloride ions present in saline water can accelerate cement hydration and reduce setting time. Another study conducted by Mori [
65] found that the difference in strength between concrete prepared with freshwater and saline water was relatively small. On the other hand, concrete mixed with saline water showed higher strength compared to concrete mixed with freshwater under experimental temperatures of less than 15 degrees Celsius [
66]. Previously, saline water mixed with concrete was avoided for steel-reinforced structures due to the possibility of corrosion. However, it was observed that the negative effects of chloride ions present in saline water were negligible. The main reason for corrosion was found to be due to sulfate attacks on structures that were exposed to harsh environmental conditions and locations [
67]. Results of experiments conducted by Fattah [
60] showed that there were no negative effects of adding reject brine on the compressive strength of concrete. Using reject brine in concrete showed a slight decrease in early strength, however, there was no change in compressive strength after 28 days as compared to freshwater [
68]. It has also been observed that concrete cured with reject brine mixed with freshwater had a good response to durability tests [
68].
However, there are a limited number of studies that have reported extensive results of using reject brine in concrete preparation [
69]. Several other studies have shown that partial replacement of freshwater with reject brine (up to 50%) yielded better results than 100% reject brine [
70]. This incorporation of reject brine in concrete mixing can be very sustainable and cost effective as it can save up to 1.7 kg to 3.4 kg of CO
2 per m
3 and reduce the dependency on freshwater. As reject brine is a waste product it is free of cost, which would in turn reduce cost and help achieve sustainable and circular economy goals [
59].
Manufacturing Bricks/Blocks and Mortar with Reject Brine
Similar to mixing concrete mentioned in the previous subsection, the production of concrete blocks also requires large amounts of water, creating pressure on the limited available resources [
71]. As concrete blocks are an essential component of buildings, with rapid urbanization their demand is also ever increasing. Previous studies have explored the use of reject brine in the production of concrete blocks to enable sustainable practices. A comprehensive life-cycle cost analysis showed that combining saline water and recycled concrete aggregate (RCA) can result in a life-cycle cost of USD 336.3/m
2, which is approximately 50% less than the conventional mixing cost of USD 690.4/m
2. This analysis was performed considering a 100-year service period, showing long-term economic/financial benefits [
72], making it a sustainable approach. Previous research efforts have explored the possibilities of mixing reject brine with other additives to produce concrete blocks. The mechanical properties of concrete blocks showed improvement when it was mixed with parts of reject brine with freshwater while preparing the mixture [
73]. The initial strength of the mix containing 50% reject brine was approximately 7% higher than the mix prepared with only freshwater [
70]. The cement paste used as mortar can also be prepared with reject brine as it contains components like clay, lime, silica, magnesia, alumina, etc. [
74]. These components provide stability, strength, and durability to ensure long-lasting service life. The presence of MgO in cement guarantees favorable setting time and strength. This improvement in early strength and hydration kinetics is useful while incorporating alternative sources of water like reject brine, as the ionic interaction can affect the chemistry of cement. The presence of MgO can remove the potential harmful effects by improving the strength of the concrete mix, which can help it last longer under harsh conditions [
75]. Reject brine mixed with cement and fly ash has also been used for making concrete paving blocks as it showed increased compressive strength with an increase in hydration period for the samples of controlled mix compositions [
76]. Previous research efforts have proven that reject brine can be potentially used as a freshwater replacement to manufacture sustainable construction materials.
Ground Stabilization Using Reject Brine
The ground is an essential part of any activity and is an essential component which is often neglected. Ground conditions play an important role in construction as it is the foundation of all structures constructed like roads, dams, buildings, etc. [
77]. From a previous experiment it was observed that the bearing capacity of the ground decreases with an increase in reject brine content observed over a period of 364 days [
78]. This reduction shows the importance of long-term effects of using reject brine in ground applications. The decrease in strength indicates that soluble salts from reject brine can alter ground structure and chemistry in the long run, leading to lower load-bearing capacity. This suggests the need for concentration monitoring and control when reject brine is used in a geotechnical context. However, other recent studies have shown that the addition of reject brine from desalination plants mixed with marine clay at varying percentages enhanced the steadiness of ground conditions. Brine sludge mixed with moist clay could be used as a substitute to stabilize soft clay, as the addition of reject brine was seen to improve the geotechnical properties of soil [
79]. Additionally, the use of reject brine in controlled quantities enhances the shear strength, bearing capacity, plastic limit, and liquid limit of clayey soil. Hydraulic properties were also observed to improve [
80]. This shows that reject brine can be used as a ground stabilizer, as it can modify the consistency and strength of fine-grained ground. These benefits can make it possible to reuse reject brine in infrastructures, leading to a sustainable approach which aligns with the principles of a circular economy. This will reduce the dependency on freshwater and help to reuse a waste product, providing a safe disposal alternative. Similarly, reject brine can be utilized for several such applications, including its use as a construction material, which adhere to sustainability and circular economy principles.
Using Reject Brine as Asphalt Binder
Another potential application of reject brine is its use as a binder in asphalt. However, research on the use of reject brine as a binder in asphalt is rather limited. Some previous studies have investigated the effects of saline solutions with asphalt, including deicing. However, there have been speculations concerning the harmful effects of salts on asphalt concrete mixes due to the presence of CaCl
2 which can negatively influence the mix properties, reducing the service life of pavements [
81]. Asphalt binders were found to have increased stiffness due to the exposure to salt which suggests the potential acceleration of aging and reduction of service life [
82]. However, these studies were conducted only at laboratory levels and have no real field results. On the other hand, research revealed that adding reject brine to asphalt binder used for road pavements can better withstand the freeze–thaw cycle in cold regions and withstand the destructive activity of salts. Several studies on saline solutions including reject brine have shown that its application in asphalt can help resist freeze–thaw cycles as the salts present reduce the freezing point of water present in asphalt, limiting the damage caused by expansion during freezing. Freeze–thaw resistance is particularly helpful in colder climatic conditions. The high salinity of reject brine can improve the workability and compaction of asphalt mixtures, as the salts present may reduce internal friction during the mixing process, allowing better packing and producing a more homogenous and solid asphalt structure with enhanced durability [
83]. Another study indicated that the salts present in reject brine enhance stiffness and reduce rutting and deformation under heavy loads [
84].
Another potential benefit of reject brine as an additive in asphalt is the reduction of material cost by repurposing waste. This reduces production expenses along with minimizing waste disposal [
85]. This incorporation further reduces environmental impacts associated with reject brine disposal [
86]. With growing interest in sustainability and in an effort to reduce the dependency on limited freshwater resources, reject brine has great potential for applications in construction. With controlled application techniques and correct utilization, reject brine incorporated in construction can enhance the mechanical properties and durability of cement composites, asphalt, soil, and other construction material and make the practice a sustainable approach along with reducing cost, making it an economically viable and environmentally feasible alternative to conventional methods.
In this study, several studies on reject brine management and application were evaluated to understand their global standing. The next section explains the adopted methodology used to evaluate and understand the current state of research and identify gaps and shortfalls.
2. Objectives and Methodology of the Review
This study uses comprehensive bibliographic analysis to provide a global assessment of research practices related to reject brine disposal and management and its applications with an emphasis on construction applications. The main aim is to provide an insight into the developments in the field of reject brine disposal and management over the years. The sharp increase in global demand for freshwater resulted in an increase in desalination plants and reject brine production. This study looks into a number of aspects of reject brine production and management and the status of research facility spread across the globe at different geographical locations. It also seeks to update the literature available on reject brine disposal and management practices and its applications in construction.
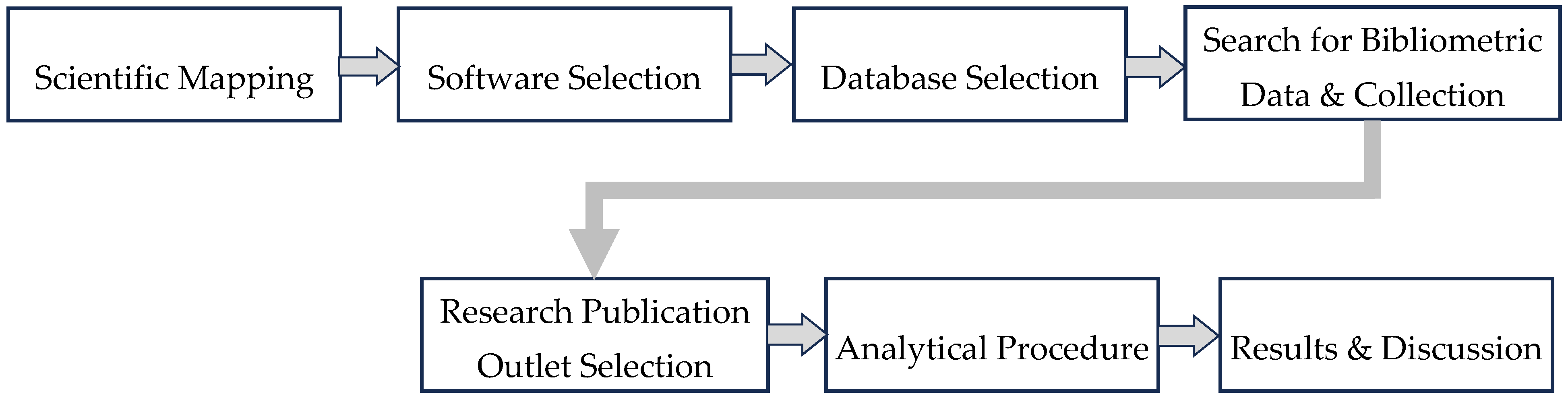
Figure 1 shows the precise methodology of the review adopted to conduct the bibliometric analysis. The academic work analyzed in this study ranges over the time period of 23 years (2000 to 2023) which was gathered from various databases like Scopus, Web of Science, and Google Scholar. This data was then categorized into five groups (contribution of countries, research outlet frequency, keyword analysis, co-citation, and co-occurrence). These categories were examined to understand the research landscape, evolving trends, and research gaps that require further investigation. Network maps were generated from the gathered data using VOSviewer software (version 1.6.20) to better understand the research landscape and identify the potential of using reject brine in construction.
Figure 1 shows the systematic review methodology which has also been used to analyze the results.
First step is the “Scientific Mapping”, which was used to define the scope of research and trends, followed by “Software Selection”, which was used to select tools for data analysis. In this case VOSviewer was the selected tool for data analysis. Next, the “Database Selection” step identified the relevant scientific repositories like Scopus and Web of Science, which were then used for bibliometric data and collection of key information like research frequency, keywords, and citations. The “Search for Bibliometric Data & Collection” step, on the other hand, is used to find and gather data about published academic literature and evaluate the impact, trends, and structure of these scholarly publications. The next step (Research Publication Outlet Selection) focused on important journals and sources leading to “Analytical Procedure”. In this part, the data was processed using methods like citation mapping and content analysis. Finally, the “Results and Discussion” step synthesized the findings to highlight the insights, conclusion, and recommendations. This structured methodology ensured a thorough and reliable review process.
The main aim of this analysis was to understand the reject brine disposal, management, and application techniques over the selected time period of 23 years. The analysis identified research trends, research gaps, and interrelations, while providing a comprehensive scientific mapping to support future research directions. Bibliometric maps were generated using VOSviewer to understand the complex network of co-citation, co-occurrence, keywords, and research outlets. The data was cross-checked to ensure credibility, including top journals, citation metric analysis, keyword grouping, and text-based co-occurrence patterns. The results obtained have been elaborately discussed in the next “Results” section to provide insights into past research efforts and emerging research trends, highlighting the importance of the growing interest in reject brine and the awareness of the negative impacts of its disposal.
3. Results
This section presents the results obtained from the bibliographic analysis, including the network maps, tables, and graphs to interpret trends and patterns. The results highlight the advancements in reject brine management, disposal, and application while identifying the research gaps and future research directions. The data was categorized into four parts: contribution of countries, journal frequency, keyword analysis, and co-citation analysis. The observed trends indicate fluctuating outputs over time, while contribution of countries highlights the global research influence. Key journals influencing academic work and keyword analysis highlight the most prominent research themes. Co-citation analysis shows the relation between influential works, offering a comprehensive understanding of current research trajectories.
As observed from
Table 4, the number of publications focusing on reject brine management, application, and disposal has been on a steady rise over the past decade from 2015. The number of publications has more than doubled, marking an overall increase of approximately 106.5%. This steady growth shows an increase in global interest in reject brine management, application, and disposal and wider application prospects. While a small decrease was observed in 2020, this could be attributed to the global slowdown due to the COVID-19 pandemic. However, in the years following the COVID-19 pandemic, the number of publications rapidly regained momentum with the numbers rising sharply. This growth trend highlights the increase in interest and awareness of the environment and other related challenges that reject brine poses and the increasing search for innovative solutions to tackle the same. Nevertheless, despite the overall growth in research activity, the application of reject brine in construction and building materials is still relatively unexplored. With the given promising potential of the use of reject brine to enhance sustainability and promote resource circularity, this particular research area offers considerable scope for future research and contribution to the domain.
3.1. Contribution of Countries
The current reject brine production is approximately 141.5 million m
3/day, which approximately equates to 51.7 billion m
3/year. This yearly production of reject brine is approximately 50% higher than the amount of desalinated water produced globally [
5]. It is observed that the majority of global reject brine production is concentrated in the MENA region. This is possibly due to the arid geographical and climatic conditions, making desalination the major source of freshwater [
5]. This could be the reason for the majority of contributors towards reject brine management and application research being from the MENA region.
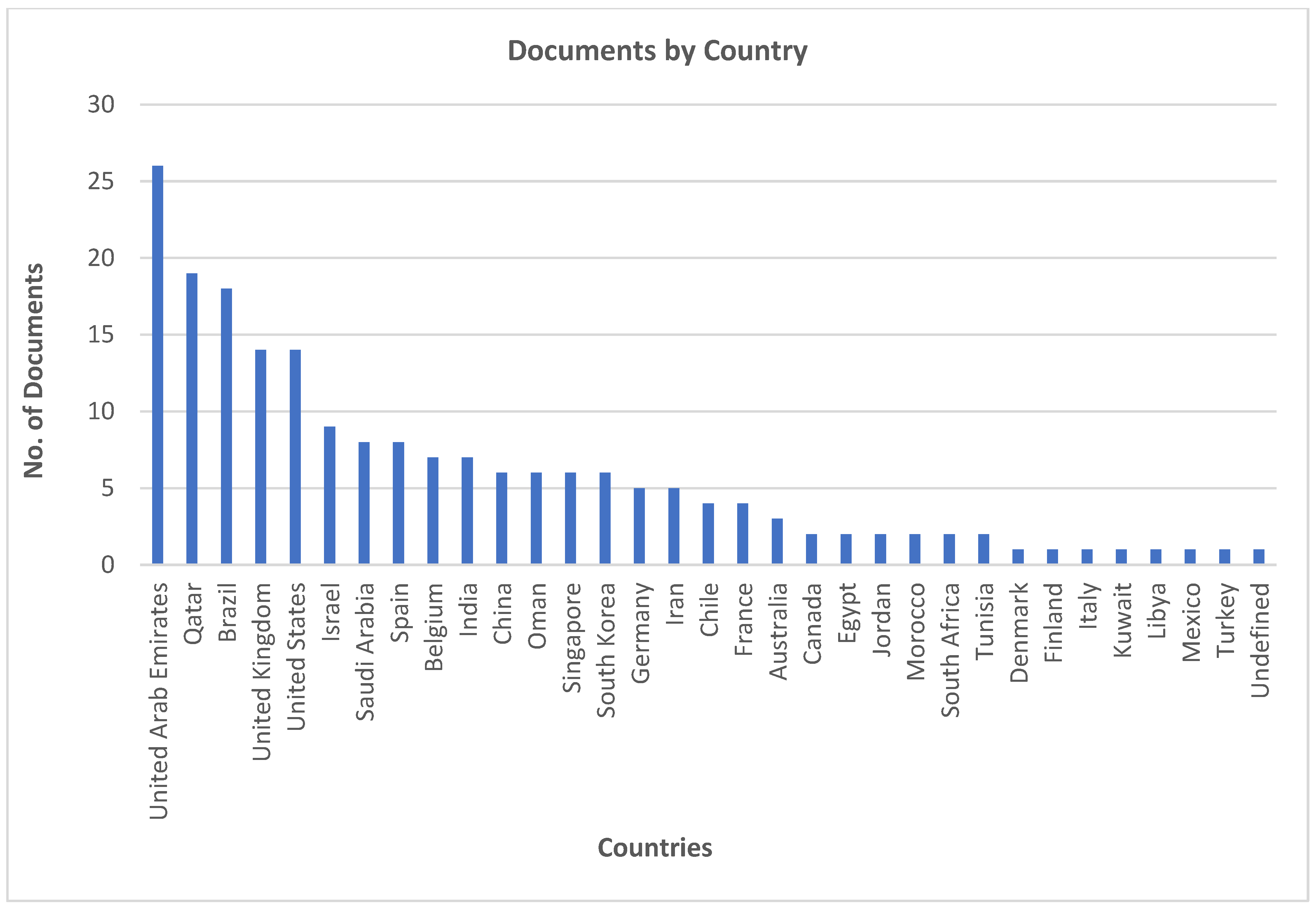
Figure 2 shows the countries with the most contributions to the selected research topic.
UAE is the major contributor with the largest number of publications, followed by Qatar. Both countries rely mainly on desalination to meet water demands and hence make significant investments towards improving reject brine management practices and finding suitable application techniques. Brazil is also a major contributor and follows Qatar on the list. Brazil does not depend only on desalination to meet water demands, but it rather investigated alternative uses of reject brine considering the ecological aspects. This is followed by the UK and USA who focus mainly on technological advancements and environmental impact with a major focus of policy regulations governing reject brine management. The next major contributors are Saudi Arabia and Israel. Both countries have advanced water management strategies and focus mainly on resource recovery and reject brine treatment. Mid-tier contributors are India, Belgium, Spain, and China who have fewer contributions to the selected research area as they do not solely depend on desalination to meet water demands and the amount of reject brine produced by each of these countries is considerably less compared to the major contributors. Other countries like Germany, France, Australia, Chile, Iran, etc. have very limited contributions to the field of reject brine management and application, indicating that they focus mainly on case-specific studies rather than focusing on the broader spectrum. Other countries with fewer contributions might indicate the limitation of scope and focus. In general, most of the contributors focus on treatment and management of reject brine to find a sustainable solution for application. Integration of reject brine in construction is still limited even in the major contributing countries despite having a vast scope and possibility.
3.2. Journal Frequency
Table 5 shows the top 10 journals which have the greatest impact towards research on reject-brine-related topics. In the analysis it was observed that some of the foundational topics like “desalination”, “water treatment”, and “wastewater management” were established in high-impact journals much earlier and recent studies have focused on specialized topics like reject brine management and application. Over the years an increasing interest has been observed in the journals in cleaner production and sustainable practices. Key publication outlets like the journal “
Desalination” provide a wide variety of technical solutions such as membrane technologies used for reject brine treatment. The journal “
Water Research” provides insight into a wide range of water treatment options and effects of reject brine disposal on the environment. The “
Journal of Environmental Management” and the “
Journal of Hazardous Materials” focus mainly on sustainability and innovative reject brine treatment to mitigate environmental hazards. The journal “
Separation and Purification Technology” and the “
Journal of Membranes” focus mainly on reject brine treatment technology, offering wide range of academic work on innovative and emerging technology useful for reject brine treatment. On the other hand, the journal “
Environmental Science and Technology” provides a variety of academic work highlighting the advancement of environmental research for reject brine treatment, while the journals “
Water Science and Technology” and “
Chemosphere” focus largely on interdisciplinary research on water systems and the environmental consequences of reject brine disposal. All the top 10 journals mentioned in
Table 5 are high-impact research outlets that address innovations and challenges faced by researchers in the area of reject brine management and disposal.
On the other hand,
Table 6 shows the top 10 journals that focus on the reuse and application of reject brine, including its application in construction, providing innovative academic work progress on incorporating waste reject brine into construction applications by converting it into a valuable resource. The journal “
Construction and Building Materials”, for example, provides an array of research that looks into the use of alternative construction materials, including waste reject brine, to produce different construction materials like concrete and bricks along with looking into the possibilities of using reject brine for ground stabilization and binders for asphalt to enhance its service life. The journal “
Construction and Building Materials”, one of the most influential research outlets, focuses on advances in construction materials and use of waste materials like reject brine for different construction applications. The “
Journal of Cleaner Production” and the journal “
Resources Conservation and Recycling” focus mainly on exploring the use of industrial waste like reject brine to enhance properties of concrete which can reduce the environmental impact of construction as well as reduce the harmful effects of toxic waste disposal. The “
Journal of Hazardous Materials” provides a variety of academic work which focuses on handling and disposal of hazardous materials including reuse and incorporation of reject brine in construction to mitigate environmental hazards. The journal “
Sustainable Cities and Society” provides extensive research work on sustainable development in urban areas by integrating materials like reject brine in construction of eco-friendly buildings and developing sustainable infrastructure. The “
Journal of Water Process Engineering” focuses on advancements on water research and treatment and reuse technologies by highlighting the potential of repurposing reject brine from desalination plants in construction.
“
Waste Management”, another high impact journal, pays special attention to repurposing industrial waste in construction by focusing on treatment, disposal, and recycling of toxic waste like reject brine, while the journal “
Cement and Concrete Research” is another prominent research outlet whose major focus is on the advancement of cement- and concrete-related materials. This journal provides relevant academic content on the use of alternative materials and waste products supporting innovations in construction materials. The journal “
Environmental Science and Pollution Research” focuses mainly on reusing reject brine, emphasizing sustainable solutions for pollution control and wastewater reuse in different areas of construction. The journal “
Studies in Construction Materials” provides practical case studies on construction materials including real-life innovative projects on the use of reject brine in concrete which assess its performance and durability. This journal mainly focuses on real-world projects that address sustainable material performance. All the top 10 journals listed in
Table 6 focus on the use of reject brine and support and influence research to address critical environmental challenges and development of sustainable construction practices that would align with a circular economy goal.
3.3. Keyword Analysis
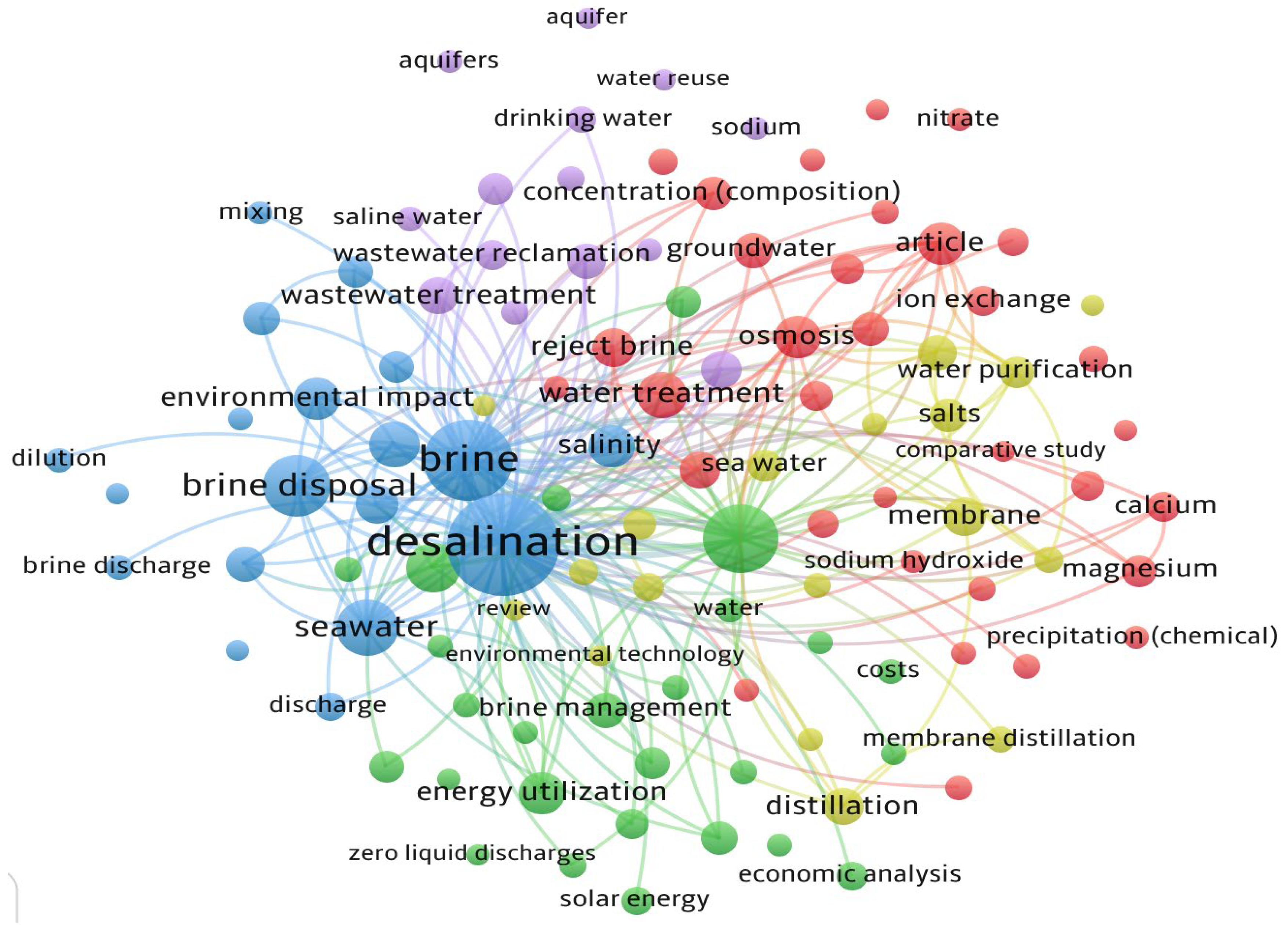
An important aspect of research is keyword analysis as it helps increase the visibility of academic work by assisting in finding relevant materials. Keyword analysis shows the topics of research conducted in the selected time period. Therefore, the inclusion of relevant keywords is essential to identify emerging technology and find the most prevalent research conducted. A comprehensive visual map was generated using VOSviewer to understand the connection between keywords and available research performed on reject brine management, disposal, and application.
Figure 3 shows, in color clusters, the network map of the most common keywords that appeared from 2000 to 2023. The blue cluster focuses on environmental aspects such as brine disposal, seawater, and environmental impact, while the green cluster emphasizes energy and management technologies, including distillation, solar energy, and zero liquid discharge (ZLD). The red cluster focuses on membrane technology and water chemistry, featuring terms like membranes, salts, ion exchange, calcium, and magnesium. The purple cluster relates to water reuse, groundwater, and aquifers, indicating links to hydrological impacts. Lastly, the yellow cluster focuses on economic or comparative studies and purification methods. As observed from
Figure 3, the central focus is on “desalination”, “brine”, and “brine disposal”. In this study a total of 151 keywords were identified, which appeared over 400 times. It was also observed that “desalination” has remained the most common word over time, whereas “brine” started appearing frequently only from the last decade. This indicates that research interests in reject brine have been on the rise in the last decade owing to the increase in demand for freshwater and increase in water desalination. The word “desalination” is represented by the largest node in
Figure 3 and is linked with different terms that represent different stages of water treatment, management, disposal, and application. The network links “brine discharge”, “energy utilization”, “economic factors”, and “cost”, providing an overall view of the diverse nature of research related to reject brine.
This covers aspects such as sustainability, treatment, and management technology. Each word in
Figure 3 is represented by a node and the size of the node indicates its frequency (the larger the node, the higher the frequency). Each node is connected to the other by links which show their co-occurrence or connection. The nodes “desalination” and “brine” are connected to all other smaller nodes like “water treatment”, “energy utilization”, “economic analysis”, etc., showing the interdependency and the primary issues related to reject brine generation and desalination that are serious environmental threats. Out of the 151 keywords identified from 2000 to 2023 that appeared over 400 times, 60 of the top and most commonly occurring keywords were identified, as represented in
Table 7.
These commonly occurring keywords represent the major problems that may occur due to reject brine. These include safe disposal, environmental impacts, treatment, etc. and the associated risk. The dominance of these keywords emphasizes inventive methods of mitigating risks and repurposing reject brine for beneficial purposes, which include construction applications. The observations indicate a growing interest in the alignment of circular economy principles and converting waste into a useful resource.
3.4. Co-Citation Analysis
Co-citation is a bibliometric tool which was used to analyze the research framework of the selected topic. This method of analysis was first used in 1973 [
146] and is defined as the frequency of citing two or more items of previous research efforts and can be applied on different elements like authors, sources, or keywords. In this study, co-citation analysis has been performed to identify sources that highlight the impact and significance of past research efforts on reject brine disposal, management, and application from 2000 to 2023. The identified sources/publications cover a wide range of available techniques for reject brine disposal and management, such as membrane technology and solar-driven methods. Topics such as ZLD and maximum liquid discharge (MLD) appear frequently, indicating a growing interest towards sustainable approach and waste minimization. High-impact publications that highlight the use of reverse osmosis (RO) and forward osmosis (FO) indicate evolving techniques used for reject brine management and water recovery. Most of the work focuses on environmental impacts and energy requirements associated with reject brine management, all of which aims towards reducing carbon footprints and optimizing energy utilization. As shown in
Table 8, most co-cited works emphasize innovative techniques, which can be integrated with desalination to reduce energy consumption and manage the produced reject brine. Specific studies on reject brine applications like removal of nitrate and perchlorate in rural water treatment suggest the need for and necessity of reject brine management in different spheres and contexts.
Twenty of the most cited publications have been represented in
Table 8. High citation counts of new and old research work show the evolving research landscape and growing interest in reject brine management, disposal, and application in various locations. The most cited work over the years helped identify the most dominating researched domain of the selected topic. Additionally, higher citations increase the visibility and acceptance of academic work. Analyzing this academic data can help in identifying the existing gaps in the literature and point out areas where further research is required.
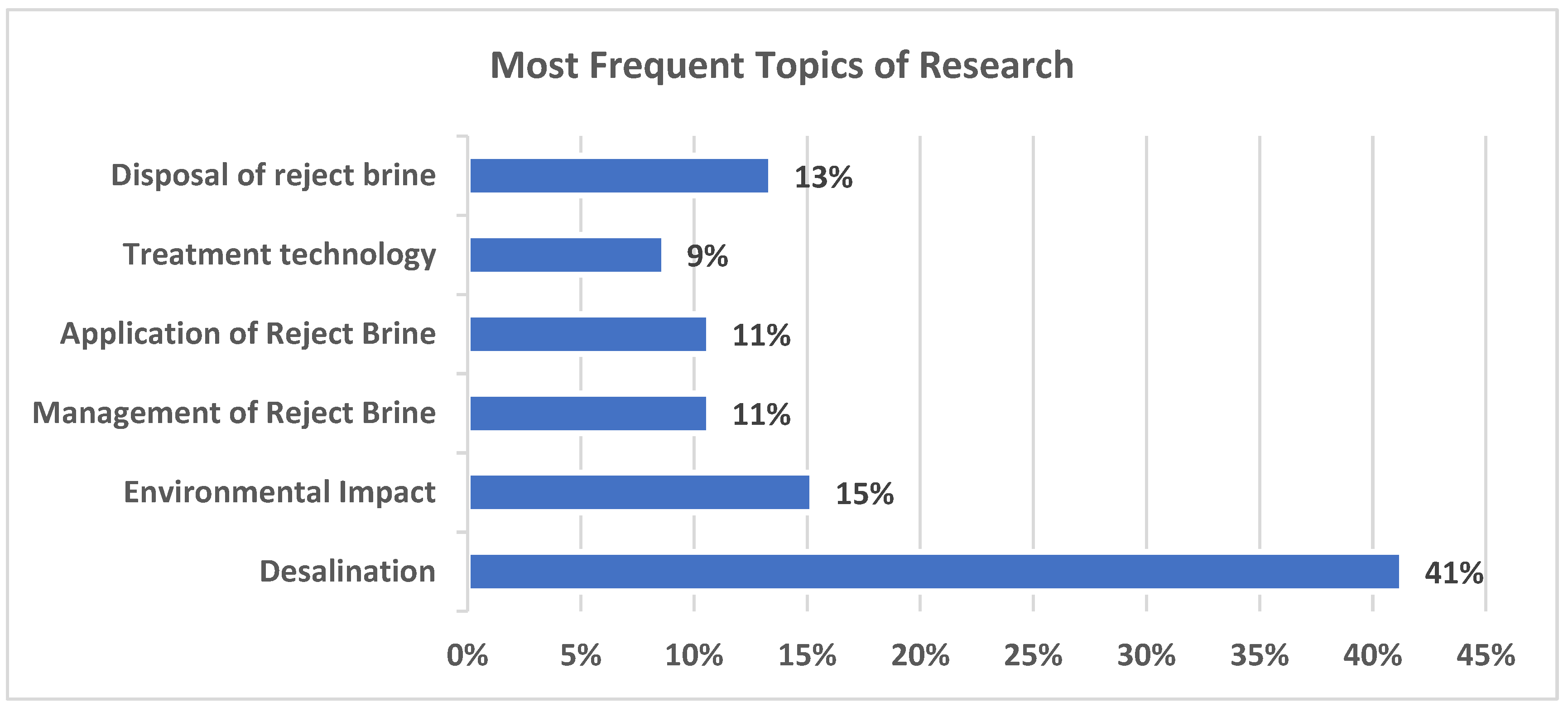
It was observed from the above analyses performed that the main focus of research related to reject brine had been concentrated on “desalination”. As shown in
Figure 4, approximately 41% of all research performed from 2000 to 2023 has been on “desalination”, indicating a high rate of interest in innovative solutions for optimizing technology for better water production. This interest is driven by increasing global water demand and scarcity of resources, making this a critical area for developing innovative and efficient techniques. The next most popular topic of research is the “environmental impact” of reject brine, which constitutes approximately 15% of the research performed over the selected time period. This indicated the awareness of ecological issues and environmental harm that is associated with the conventional disposal methods and needs serious efforts to develop sustainable disposal strategies. This is followed by “management of reject brine”, accounting for 11% of research performed during the selected time period. The major focus area is on efforts to mitigate negative environmental impacts and investigate the potential of reusing reject brine for different purposes. Research on “disposal of reject brine” accounts for 13% of the total research, which highlights the need for the development of safe disposal strategies to reduce the negative impacts. The “application of reject brine” accounts for only 11% of the total research performed from 2000 to 2023, which shows that there is limited research on the use of reject brine for industrial applications and converting the waste into a useful resource. The use of reject brine in construction applications is included in a very limited number of research publications (only 3.7% of the 11% for all reject brine applications). Finally, “treatment technology” accounts for 9% of the total research performed during the selected time period.
4. Discussion
The results of this study highlight the growing interest in reject brine management, disposal, and application globally. Especially with the growing demand for freshwater and increasing water scarcity, desalination operations have been growing exponentially. In this study, a comprehensive statistical analysis of scientific literature on reject brine management, disposal, and application from 2000 to 2023 that addresses a diverse range of technical, environmental, and socio-economic aspect has been provided. The results show a notable rise in research output from 2014, with a peak in 2022. This coincides with global awareness of environmental and economic challenges posed by reject brine, especially in dry and arid regions like the MENA region, which rely on desalination to meet their water demands [
5]. This region produces more than 70% of the global reject brine, making it a top contributor in the selected research field, given the growing awareness of environmental impacts caused by reject brine disposal [
11].
One of the key findings of this study was that research on desalination technologies accounts for up to 41% of the total research from 2000 to 2023. This underlines the need for desalination towards bridging the supply demand gap of water globally. Environmental impacts of reject brine discharge like degradation of marine habitat and soil degradation account for 15% of the total research work in the selected period. The hypersaline nature of reject brine from desalination plants and its negative impacts on nature have received increased attention in recent years [
52]. The high chemical content and salinity of reject brine pose a major environmental hazard, demanding new disposal and management solutions [
164,
165,
166]. However, despite technological advancements, economic costs and energy demands for reject brine management and treatment remain a constant barrier for widespread application [
52]. Traditional methods of disposal like ocean discharge or deep well injection are cost effective but have a negative impact on the environment [
18]. The need for sustainable alternatives is essential to minimize environmental harm. Methods like ZLD, mineral extraction, and energy recovery through salt gradient are very beneficial and aim to recover valuable resources from reject brine [
151]. However, the high energy requirement and high economic cost linked with these remain a hurdle towards the widespread adoption and complete realization of these techniques worldwide [
41]. This underlines the need for further research into energy-efficient and cost-effective solutions which can aid industrial applications.
Reject brine has promising potential applications across various industries like agriculture, energy, and construction which offer promising avenues for sustainable resource utilization. Using reject brine for irrigation and aquaculture to support cultivating saline-resistant crops and species of fish has shown promising results [
53]. Similarly, generating blue energy from salinity gradients using reject brine is an innovative method to harness renewable energy [
60]. However, these methods have only been tested at laboratory levels, and there is insufficient data available to scale them on an industrial level and in terms of cost effectiveness. More research is required to assess their long-term impacts and economic feasibility.
Using reject brine in the construction industry as a substitute for freshwater for concrete production and ground stabilization has shown positive results. Previous studies have shown that reject brine can be used in concrete as a partial replacement for freshwater without compromising the structural integrity of the material. This offers a sustainable alternative for freshwater, reducing the dependency on scarce resources [
59]. Furthermore, reject brine has been shown to enhance the geotechnical properties of soil, making it a suitable option for construction [
79]. Using reject brine for binders in asphalt has also shown positive results with potential for improving performance and durability of asphalt, especially under harsh environmental conditions. Incorporating reject brine into construction not only offers a sustainable alternative but also provides a safe alternative disposal method for the waste reject brine, indicating the transformative potential of reject brine in construction [
79]. However, limited information is available on the long-term performance and durability of construction materials that incorporate reject brine. Several research gaps related to reject brine remain unaddressed. For example, the long-term structural and environmental impacts of using reject brine as a construction material have not been assessed and analyzed completely. Experiments at laboratory levels have yielded promising results, however, these have not been applied to real-life scenarios which can validate the feasibility and durability of these materials under actual conditions. Moreover, the economic viability of using reject brine in construction needs to be explored [
72].
Several other research gaps were identified in the study that have pushed back the complete realization of reject brine as a sustainable resource. The long-term environmental impacts of reject brine used in agriculture and aquaculture are still not well understood particularly in terms of water and soil degradation [
30]. Similarly, the cost effectiveness of innovative technologies like ZLD and energy gradient is yet to be assessed [
57]. Another critical research gap exists in the area of integrating reject brine with renewable energy sources since reject brine treatment consumes high levels of energy, and this is a major concern amidst the growing global efforts to reduce carbon footprints.
While there has been significant progress in reject brine management and application, several research gaps still exist and there is a need to conduct further research to address these challenges. It is essential to develop cost-effective, energy-efficient, and environmentally sustainable solutions to achieve global water security goals with Sustainable Development Goals (SDGs). It is possible to convert reject brine from a waste product to a valuable resource to mitigate its environmental impacts and align it with circular economy goals. To achieve this, future research must focus on optimizing existing technologies and exploring innovative applications by assessing the long-term environmental and economic consequences of reject brine reuse.
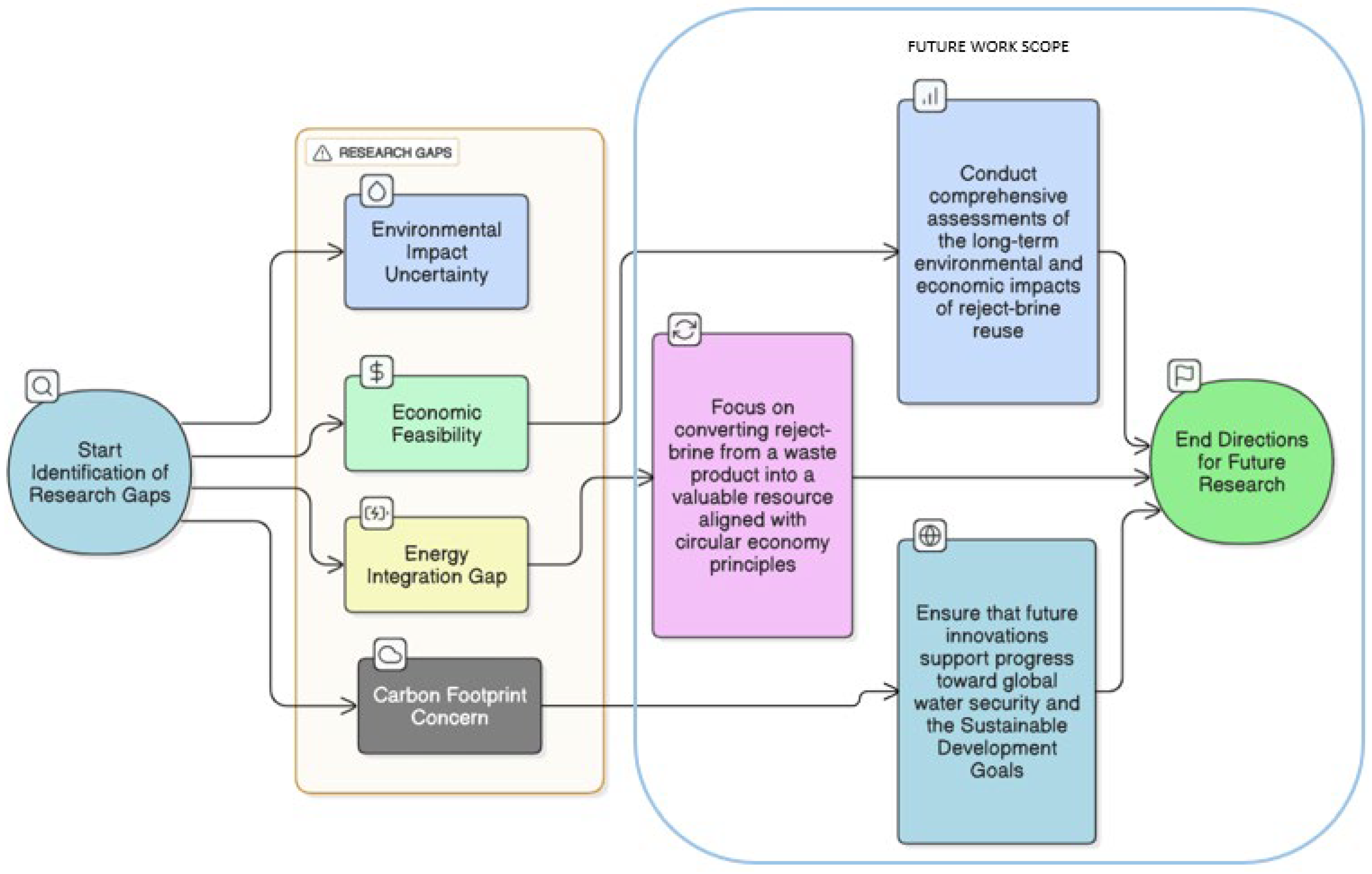
Figure 5 shows a visual summary of the major research gaps and future work directions identified in this study. As has been observed, environmental impact uncertainty, economic feasibility, energy integration issues, cost efficiency, and carbon footprint concerns continue to slow down the large-scale adoption of reject brine reuse solutions. By addressing these pertaining issues, a definitive research strategy can be drawn which not only focuses on converting reject brine into a valuable resource but can also align them with circular economy principles. Additionally, this study also emphasizes the need for critically analyzing long-term effects of reject brine to ensure achievement of global sustainable goals. Therefore, to address these drawbacks identified in existing reject brine research and ensure impactful future research, an organized method was implemented to identify and map existing research gaps. This combined approach of bibliometric analysis, keyword analysis, journal analysis, and contributions of countries highlights the unexplored areas, especially the limited applications of reject brine in industrial and construction applications.
Figure 5 shows the process of research gap identification along with a scope of future work, which has been discussed further in
Section 5.1 and
Section 5.2 in the Conclusions section to lead future research directions which can align with sustainability and circular economy.
5. Conclusions
With increasing demand for drinking water and increasing water scarcity, desalination is becoming the most availed option to bridge the demand–supply gap. Desalination can ensure access to safe drinking water to all by 2030 to achieve SDG 6 to safeguard water supply for current and future generations. In addition to SDG 6, a number of SDGs are linked with water resource management, like SDG 2 aiming at zero hunger, SDG 3 ensuring healthy life, SDG 8 promoting sustainable economic development, SDG 11 making human settlements and cities inclusive, and SDG 13 combating climate change. Therefore, these SDGs have water-related targets that must be achieved for the possible ultimate realization. Specific challenges related to desalination need to be addressed, such as high economic cost, and environmental concerns include the release of large amounts of reject brine into the environment which requires serious management. Reject brine management is economically expensive and technically difficult. This has resulted in most desalination plants discharging untreated reject brine directly into the environment. To address these challenges, research conducted over the past years has shown that there are several opportunities associated with reject brine, such as:
Salt and mineral recovery.
Use of reject brine in agriculture.
Use of reject brine in specific construction applications.
Therefore, there is a need to convert these research opportunities into practices to change the concerns related to reject brine into an economic and sustainable opportunity. This is particularly important in countries that depend on desalination to meet their water demands like the MENA region. Further research is essential to develop efficient and affordable solutions to understand the long-term effects of reject brine. The identified research gaps include the following:
Developing efficient and affordable solutions to understand the long-term effects of reject brine.
Expanding research into reuse of reject brine as a sustainable, cost-effective construction material.
Reducing reject brine disposal and its effects on the environment.
Bridging these gaps would ensure the development of a sustainable, cost-effective solution which would align with circular economy principles. With progress in research on reject brine management and application it will be possible to integrate innovative technology and policies which can transform reject brine from a waste product to a valuable resource. In order to direct future advancements, it is necessary to recognize the existing limitations and highlight strategic directions for future research work. The following subsections elaborate the key research gaps that continue to challenge the sustainable reuse of reject brine, followed by the potential areas which can lead towards future research prospects aimed at maximizing the value of reject brine within the principles of a circular economy.
5.1. Key Research Gaps and Challenges
In spite of the considerable advancement over the past years, there are various challenges that slow down the widespread adoption of reject brine reuse technology. A major research gap exists between understanding the long-term ecological impacts of reject brine application, especially on ground and soil quality and on biodiversity when used in agriculture. Additionally, a major gap remains in the comprehensive understanding of life-cycle assessments which can evaluate the sustainability of the present reject brine treatment technologies, mainly in the areas of high energy and operational demands. Furthermore, the economic viability of advanced reject brine management and treatment solutions like ZLD or hybrid processes remains theoretical without practical cost–benefit analyses. Integrating renewable energy into reject brine treatment still remains unexplored. Reuse of reject brine in construction applications is also still not investigated. Another overlooked area is the socio-political aspect of public perception and policy alignment which are necessary for full-scale realization. By addressing these research gaps reject brine can be transformed into a strategic asset rather than being a liability.
5.2. Identified Possible Future Research
The findings of this study have identified various possible directions for future research which can transform reject brine into a sustainable resource. Despite promising results in certain areas such as agriculture, energy generation, and construction, many applications still remain at an experimental stage and need further validation. One major area to investigate is the long-term performance of construction materials with the incorporation of reject brine. Recent studies have shown promising mechanical properties and durability in controlled environments; however, full-scale and real-world assessments are required to understand their performance under real environmental conditions. Another aspect is economic feasibility. Reject brine generally offers cost savings by replacing freshwater and reducing waste disposal. However, a comprehensive life-cycle cost analysis is still not available. Future research must evaluate both short- and long-term financial aspects across various sectors. Additionally, future studies need to focus on optimizing mix designs for the construction sector, keeping in mind the chemical variability of reject brine obtained from different sources. Different chemicals have different effects on material properties; this needs further investigation. Considering the energy-intensive nature of reject brine treatment technologies, further research is required to explore renewable energy integration to improve sustainability. Solar and wind energy can be very beneficial in regions with high energy potential. Finally, regulatory support is critical for widespread acceptance. By addressing these areas, future research can bridge the gaps and facilitate safe, cost-effective and large-scale reuse of reject brine, converting a major environmental hazard to an opportunity.