Abstract
This study explored mycelium-based composites (MBCs) as a sustainable alternative to conventional materials, focusing on the role of lignocellulosic substrates in optimizing their physical, mechanical, and biodegradability properties. It also addressed the valorization of agroforestry by-products, particularly European hazelnut shells (HZ) and radiata pine sawdust (SW), in an effort to reduce waste and minimize environmental impacts. The MBCs were obtained using two formulations (HZ100 and HZ75-SW25) of local agroforestry by-products bound together with natural growth of fungal mycelium from Ganoderma sp. We examined the physical and mechanical properties of these novel materials, including the density, shrinkage, water absorption, hydrophobicity, moduli of rupture and elasticity, and internal bond strength. Additionally, we assessed the biodegradability of the MBCs in soil to estimate the time required for complete degradation. The results clearly indicated differences in performance between the MBCs from HZ100 and HZ75-SW25. In general, HZ75-SW25 demonstrated superior mechanical performance compared to HZ100. Water absorption was low in both cases, suggesting a degree of hydrophobicity on the surface. The biodegradation results indicated that the fabricated MBCs could fully decompose in less than one year when buried in soil, confirming that these biocomposites are entirely biodegradable.
1. Introduction
A key societal challenge is the conversion the world’s economy into a sustainable model by lowering CO2 emissions and minimizing waste production through nature-based circular processes. The biorefinery approach allows the valorization of agricultural and forest waste streams (by-products), converting them into materials like plastics, polymers, and building materials with a lower environmental impact [1,2,3].
In this study, we selected European hazelnut shells (Corylus avellana) and pine wood sawdust (Pinus radiata) as base materials due to their regional availability, cost-effectiveness, and contrasting physicochemical properties. In Chile, both of these by-products are produced extensively by local industries, necessitating innovative valorization strategies to comply with increasingly stringent waste management regulations. Hazelnut shells, which are a by-product of nut agro-industrial processing, are characterized by their high lignin content [4] and low water retention capacity. In contrast, pine sawdust, a typical by-product of the forestry industry, contains a high proportion of holocellulose and demonstrates a greater water retention capacity. These distinct properties allow us to explore the synergistic effects of these materials on mycelial growth and the final characteristics of the resulting biomaterial.
Hazelnuts are widely cultivated in various countries due to their significant economic and environmental value [5,6,7]. As the demand for hazelnuts continues to rise, the area dedicated to their cultivation is expanding globally, resulting in a marked increase in the production of hazelnut by-products. It is worth noting that hazelnut kernels account for less than 50% of the total weight of the nut, while substantial quantities of by-products, including skins, shells, husks (green leaf cover), and leaves, are generated during the harvesting and processing stages [7]. This situation inevitably leads to excessive waste and exerts pressure on the environment. Therefore, it is crucial to conduct professional research on utilizing these by-products to alleviate this issue. Approximately 50% of a hazelnut’s weight comprises its shell, which is commonly discarded during production and processing.
Meanwhile, mycelium bio-composites (MBCs), formed on a cellulose- or lignocellulose-rich substrate colonized by fungal mycelium, have garnered significant interest over the past decade, transforming agricultural and forestry waste into valuable resources [8,9,10,11,12,13,14,15,16]. MBCs are also widely used in applied arts, design, and architecture [17,18].
In fact, according to the Scopus database, the number of publications listing the words “mycelium” and “composites” has grown exponentially since 2010 (Figure 1). These biomaterials have significant potential to contribute to the bioeconomy due to the remarkable ability of fungi to recycle organic materials. MBCs can serve as alternatives to petroleum-based products like plastics [14], insulation materials [9,11,12], and packaging [19] due to the biodegradability of mycelium products compared to non-biodegradable plastics such as polyethylene and polystyrene [20].
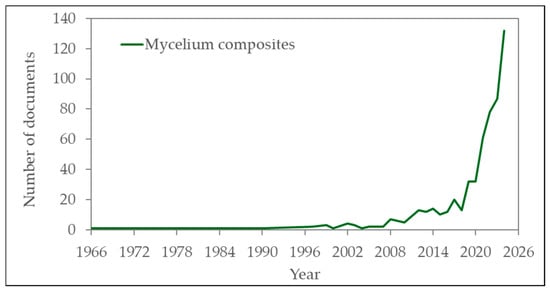
Figure 1.
The number of scientific publications with the words “mycelium” and “composites” since 1966, according to the Scopus database.
MBCs grown on lignocellulosic substrates produce an inert, rapidly growing matrix that acts as a natural binder, effectively adhering to any organic substrate by forming a dense network of hyphae. These materials outperform modern synthetic alternatives due to their biological origin, low production costs, and minimal energy consumption [17,21]. Additionally, it has been reported that using biomaterials can reduce costs by up to eighty times compared to using conventional construction materials such as cement, steel, gypsum, and plastic polymers. This approach can also reduce carbon emissions by nearly 800 million tons annually [21]. In this context, developing biomaterials composed of fungal mycelium from various fungal species does not require expensive or complicated processing methods [21,22]. The type of substrate used significantly influences mycelial growth rates because of its direct contact with the hyphae. This interaction enables the hyphae to extract the essential nutrients necessary for their development effectively [23].
MBC materials can display a wide range of structures and functionalities by varying the processing method and the substrates used in their development. This enables the creation of materials with tailored properties, such as improved fire resistance, structural support, or acoustic insulation, depending on the type of substrate employed [24]. While there are differences between the cold and hot processing of MBCs [10,25,26,27,28,29], it is important to select the appropriate temperature based on the size of the workpiece [10]. MycoCreate 2.0 was developed to broaden MBCs’ applications beyond packaging, showcasing that these biocomposites can also be utilized for building, due to their variable density, water resistance, and mechanical strength [18]. Additionally, 3D printing is emerging as an innovative technique for directly printing biocomposites without molds [30,31]. However, further research is necessary to address existing challenges and to scale up production. Furthermore, the quality of the resulting biomaterials is significantly influenced by the fungal species selected, as the presence of chitin in the mycelium enhances adhesion to the substrate [21]. Wood-degrading fungi are generally classified based on the type of decay, brown-rot, soft-rot, ou white-rot, depending on their enzymatic activities and environmental preferences [32]. Brown-rot and soft-rot fungi mainly degrade cellulose, causing minor changes in lignin, while white-rot fungi primarily focus on breaking down lignin. More than 70 species of fungi have been thoroughly investigated for their ability to produce MBCs [28].
Ganoderma lucidum has been utilized to develop MBCs on various substrates [13,33,34] due to its unique properties. It contains chitin in its hyphae, exhibits a rapid mycelial growth rate, and features a trimitic hyphal network system. Generally, monomitic species tend to have lower strength compared to dimitic and trimitic hyphal species [14,15,24]. This disparity occurs because monomitic species mainly consist of generative hyphae, while dimitic species include both generative and skeletal or ligative hyphae. In contrast, trimitic species consist of all three types of hyphae [24].
The aim of this study was to develop and characterize mycelium-based composites using hazelnut shells and sawdust as lignocellulosic substrates. This research focused on evaluating the impact of these substrates on fungal growth, as well as on the physical, mechanical, and biodegradability properties of the resulting biomaterials. A strain of the Ganoderma genus was chosen as the mycelium-producing fungus due to its well-known ability to degrade lignocellulose and create cohesive matrices. The results were analyzed from a practical perspective, emphasizing potential commercial applications for producing non-structural construction elements designed for indoor use and protected from exposure to the elements. While these elements are intended for sheltered environments, the study also examines their resilience to elevated humidity and temperature, recognizing that even isolated components like insulation panels may face more severe climatic conditions. Such applications include fire-retardant insulation panels, decorative design features, wall moldings, and other interior architectural elements prioritizing sustainability, biodegradability, and minimal environmental impact.
2. Materials and Methods
2.1. Source of Substrates and Characterization Processes
Two different types of agroforestry waste, namely European hazelnut (Corylus avellana) shells and wood sawdust (Pinus radiata), were used as the main substrates in this experiment. Both substrate types were sourced from agricultural and forestry areas located in Chile. Before being employed in testing, the substrates were cleaned manually and ground in an electric grinder to make the particles smaller. After that, the particles were sieved through multiple sieves, and those in the size range of 1–3.5 mm were collected and used for testing. Before being used, the water-holding capacity (WHC) of these substrates was measured to determine the exact amount of water needed to ensure mycelium development. This WHC, expressed as a percentage, is the relationship between the amount of water and the dry weight of the substrate.
The structural and non-structural chemical components of the substrates were analyzed following the protocols reported by The National Renewable Energy Laboratory (NREL). This analysis included the determination of extractable substances (NREL/TP 510-42619), structural carbohydrates (NREL/TP 510-42618), and both insoluble and soluble acid lignin (NREL/TP 510-42618). In addition, holocellulose was determined in accordance with ASTM D1104 and TAPPI T203.
2.2. Source of Mushroom Mycelia and Culture Conditions
The pure culture of Ganoderma sp. used in this study was provided by the Center of Innovation and Applied Science, Chile. This culture has been incorporated into the collections at the Wood Biodeterioration and Preservation Laboratory, which is part of the Department of Development in Forest Products, Chile. This species has been previously investigated and reported to have the potential to produce MBCs with various beneficial properties. The pure mycelium was cultivated on a solid medium consisting of 2% agar-agar and 3% malt extract, with a pH of 5.5. It was sterilized at 121 °C for 20 min prior to inoculation, and it was incubated at 25 ± 1 °C for seven days.
2.3. Primary Inoculation for Mycelial Growth
Substrates with a particle size of 1 mm were mixed with 1.5% agar to assess the mycelial growth area. One gram of each substrate powder was combined with 1.5% agar in 20 mL of Milli-Q water to prepare the agar–substrate medium. The resulting agar–substrate dispersions were autoclaved, poured into Petri plates (90 mm diameter), and inoculated with fresh mycelium. Five replicates were conducted for each combination of species and substrate. These inoculated Petri plates were then incubated in the dark at 25 °C and 60% humidity for 10 days, or until complete mycelium growth was observed. The fungal growth was assessed by measuring the diameter of the colony along four preset diametrical lines every day. Photographs were taken to record the change in growth and appearance. Five experiments were designed for the mixtures, using different proportions of hazelnut shells and sawdust, as indicated in Table 1.

Table 1.
Composition and ratios of substrates used in mycelial growth.
2.4. Mold Designs and Mycelium-Based Composite Production
2.4.1. Inoculum and Substrate Preparation
The liquid medium for the primary inoculation of substrates was prepared using 3% malt extract and autoclaved at 121 °C for 20 min. Additionally, the pH of the medium was adjusted to 5.5 using 1 M NaOH before sterilization. A portion of fresh, pure mycelium of Ganoderma was picked from the solid medium using an inoculation loop and placed into the flask with the liquid medium. The inoculated media were incubated for 10 to 12 days at 25 °C.
The substrates, with particle sizes ranging from 1 to 3.5 mm and a moisture content of 6.5 ± 0.5%, were placed in autoclavable transparent polypropylene bags (20 cm × 30 cm) and autoclaved at 121 °C for 20 min. Subsequently, each bag was then filled with the appropriate amount of water needed to achieve the required water-holding capacity (WHC) for each substrate. For the HZ75-SW25 mixture, the total water content was calculated using the weighted average of the WHC values of each component, based on their respective proportions in the mixture. The bags were inoculated by opening them, adding the liquid inoculum, and thoroughly mixing it with the substrate. Incubation took place in darkness at a temperature of 25 ± 1 °C for 14 days. The volume of liquid inoculum used corresponded to 5% (v/v) of the volume of the hydrated substrate.
2.4.2. Mold Preparation and Sterilization
Two types of molds were designed from plastic blocks to produce MBCs. Rectangular molds, measuring 5 cm × 5 cm × 20 cm, were used for mechanical testing to evaluate the moduli of rupture and elasticity. Meanwhile, square molds with dimensions of 5 cm × 5 cm × 5 cm were utilized to assess internal bond strength and conduct water absorption tests. Prior to use, all molds underwent sterilization by being sprayed with a 70% ethanol solution, followed by exposure to UVC light for 20 min in the inoculation chamber.
2.4.3. Development of Mycelium Bioblocks
The grown mycelium–substrate mass was then transferred to the molds and incubated for 14 days in darkness at 25 ± 1 °C. Later, the mycelium blocks were dried in a hot-air oven at 70 °C and tightly compressed with a 5 kg weight for 24 h.
2.5. Determination of Physical Properties
2.5.1. Density and Shrinkage
The bioblock densities were calculated and expressed based on the average density value of five bioblocks. The density of the samples was calculated using Equation (1).
The shrinkage percentage of the MBC samples was measured and calculated according to their volumes before drying and the volumes of the specimen pieces after drying. The following formula was used to calculate shrinkage: Shrinkage rate (%) = (V1 − V2/V1) × 100; here, V1 is the volume before drying and V2 is the volume after drying.
2.5.2. Water Absorption Measurements
The water absorption test for the obtained bioblocks (5 × 5 × 5 mm3) was conducted following the ASTM International D570 [35]. Initially, the dry weight of five mycelium bioblocks was recorded as the initial dry weight (Wi). The samples were then fully submerged in water at room temperature for 72 h. After immersion for 1, 3, 24, 48, and 72 h, the specimens were removed, gently wiped with a damp cloth to eliminate excess water, and weighed again to determine the final weight (Wf). The total water absorption was calculated using Equation (2).
2.5.3. Contact Angle Measurements
The water contact angle of the mycelium bioblocks was determined by applying 10 μL droplets of water onto their surface. A digital camera captured multiple images at room temperature, focusing on the droplet elevation and its interaction with the mycelium layer. The recorded images were then analyzed using the contact-angle measurement tool in ImageJ software version 1.54. A minimum of 30 measurements were taken for each sample to ensure accurate validation.
2.6. Determination of Mechanical Properties
The mechanical properties were evaluated using a three-point bending test conducted with an Instron universal testing machine, model 4411, at a crosshead speed of 10 mm/min and a span length of 140 mm. The modulus of rupture (MOR) and the modulus of elasticity (MOE) were calculated from a minimum of three replicates, each with dimensions of 20 mm (length) × 5 mm (width) × 5 mm (thickness). All tests were conducted at room temperature. The calculations for MOR and MOE were based on Equations (3) and (4), respectively:
where F is the maximum load (N), L is the span length (mm), b is the width of the specimen (mm), d is the depth (mm), and m is the slope of the linear portion of the load–deflection curve.
In addition, the internal bond (IB) strength was evaluated to determine the adhesion properties of the mycelium bioblocks. Three samples measuring 5 mm × 5 mm × 5 mm were tested at room temperature. The IB was calculated as the coefficient of the maximum force (N) applied perpendicularly to the surface area (mm2).
2.7. Exposure to Controlled Temperature and Humidity Conditions
The exposure to controlled environmental conditions was designed to simulate severe climatic conditions, specifically 35 °C with a relative humidity of 95%. The three bioblocks were subjected to these simulated conditions for 14 days in a climate chamber (VWR Shel Lab 9010, Sheldon Manufacturing Inc., Cornelius, OR, USA). After the exposure period, the specimens were allowed to acclimatize for one week at ambient temperature and relative humidity in the laboratory. Following this conditioning period, mechanical tests were performed to evaluate changes in mechanical properties. These tests included the modulus of rupture (MOR), modulus of elasticity (MOE), and internal bond (IB) strength, and they assessed the properties from day 0 to day 14.
2.8. Biodegradability
Biodegradation tests of the bioblocks were carried out according to the ASTM D5988-18 “Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials in Soil” [36]. The tests were performed in air-tight vessels containing 400 g of commercial compost soil (pH = 7.7 and C:N ≤ 25), produced by Anasac (Santiago, Chile). Cellulose served as the reference material. The pre-weighed sample bioblocks were buried in the soil, and the vessels were maintained in the darkness at 25 °C for six months. The carbon dioxide produced during microbial degradation was trapped in a 0.5 M potassium hydroxide (KOH) solution. This solution was titrated with standardized hydrochloric acid (HCl) every 7 days, except for the first three weeks, when measurements were taken twice a week. The endpoint of the titration was determined using phenolphthalein. To calculate the net CO2 emissions from the test materials, the average CO2 production from blank soil samples was subtracted from the CO2 generated in the vessels containing the test materials. The theoretical maximum amount of CO2 that could be produced from each sample (ThCO2) was calculated based on its total organic carbon (TOC) content, according to Equation (5):
where Msample is the dry mass of the test sample (mg), %C is the percentage of carbon in the sample, 12 is the atomic weight of carbon, and 44 is the molecular weight of CO2. Biodegradability (%) was then calculated according to Equation (6).
All measurements were performed in triplicate, and data are presented as average values with standard deviations.
3. Results
3.1. Characteristics of Substrates
The chemical composition of sawdust and hazelnut shells is presented in Table 2, where extractives, lignin, and holocellulose are quantified. Sawdust mainly comprised cellulose and hemicellulose (61.3% wt.), whereas hazelnut shells contained 44% wt. lignin and nearly 44% wt. holocellulose. The differences in standard deviations observed for these substrates’ composition parameters reflect the analytical techniques employed and the inherent variability in the materials. The low variability noted for lignin is attributed to UV-spectrophotometric methods, which provide high reproducibility. In contrast, the higher deviations observed in the extractives and holocellulose content are due to gravimetric techniques, which are more sensitive to handling and the natural heterogeneity of agroforestry residues. Other researchers have obtained similar chemical composition results [4,37,38]. Therefore, the substrates were suitable for developing mycelium bioblocks, since Ganoderma is a white-root fungus capable of degrading cellulose and lignin. In addition, the water-holding capacity of these substrates was quantified, as it directly affects mycelial growth, colonization, and the final properties of the material. This study determined the WHC of sawdust and European hazelnut (Corylus avellana) shells, yielding 184% and 28%, respectively. These results indicate a significant difference in moisture retention between the two substrates, which can influence water availability for mycelial expansion and nutrient uptake.

Table 2.
Characteristics of substrates.
3.2. In Vitro Mycelium Growth: Kinetics and Morphological Analysis
Several factors, including substrate composition, temperature, humidity, and pH, influence the growth of fungi [3]. To evaluate the influence of selected substrates on mycelium growth kinetics, Ganoderma sp. mycelium fibers were cultivated on different substrate mixtures composed of hazelnut shells and sawdust (100%, 75%, 50%, and 25%). The growth of fungal mycelium was monitored daily, with complete colonization of the Petri dishes occurring between 5 and 6 days, depending on the substrate mixture. Most Petri plates displayed a dense covering of thin mycelium fibers within five days of inoculation. In contrast, those using only sawdust (100% SW) achieved complete growth after six days (Figure 2).
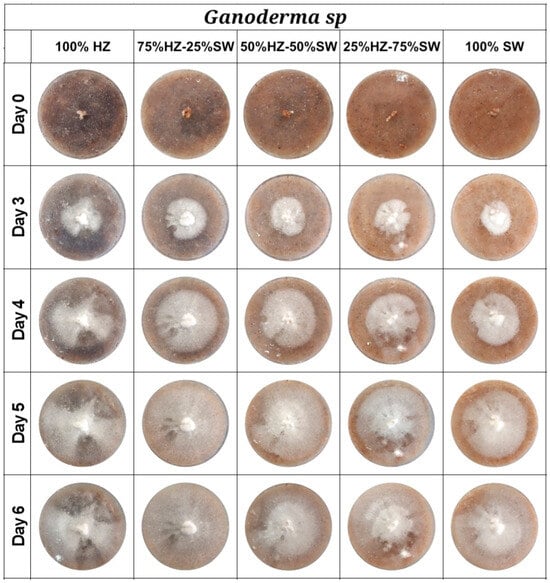
Figure 2.
Growth kinetics of Ganoderma sp. mycelium on different substrate mixtures for 6 days.
The radial growth rate (cm/day) was calculated and is detailed in Table 3 as the primary metric for comparing fungal development. Among the tested mixtures, the sawdust substrate exhibited the slowest growth, likely because Ganoderma sp. faced challenges in extracting nutrients from sawdust. Overall, the growth rate increased as the amount of hazelnut shell increased, reaching a maximum value of 1.15 cm per day on a substrate of 100% HZ. Therefore, to continue this investigation, the two mixtures of substrates with the highest HZ content were selected to fabricate MBCs: HZ75 and HZ100.

Table 3.
The growth rate of mycelium on different mixtures of substrates.
3.3. Characterization of Mycelium Bioblocks
A four-stage methodology was implemented for the fabrication of mycelium-based bioblocks. Mycelium was cultivated first on a liquid medium; then, this medium was inoculated into the two selected mixtures, incubated in polypropylene bags, and then transferred to two types of plastic molds. In the final stage, the mycelium substrate mass underwent heat treatment to halt further growth. The MBCs obtained with Ganoderma sp. mycelium in the substrates HZ100 and HZ75-SW25 retained the shape of the molds. However, they exhibited irregular surfaces and heterogeneity in the texture of the aerial mycelium (Figure 3).
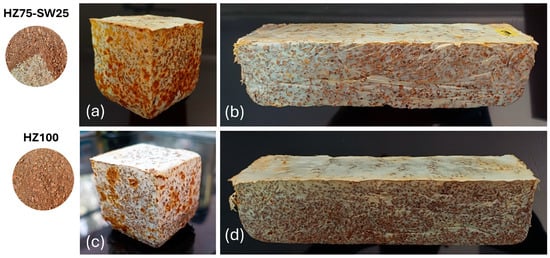
Figure 3.
Images of substrates and bioblocks: (a,b) represent MBC fabricated with a mixture of 75% hazelnut shells and 25% pine sawdust; (c,d) represent MBC fabricated entirely from 100% hazelnut shells. Samples (a,c) were used to test for internal bond strength and water absorption, while samples (b,d) were tested to calculate MOE and MOE.
A comparison of physical properties is presented in Table 4. The apparent density of the bioblocks ranged from 0.49 to 0.60 g/cm3 at a moisture content of 6%, where the highest value was obtained for the mycelium block composed of 100% hazelnut shells. The MBCs from the mixture HZ75-SW25 with an initial volume of 125 cm3 experienced a 20% reduction, while those with an initial volume of 500 cm3 showed a 15% decrease. In contrast, the bioblocks from HZ100 with an initial volume of 125 cm3 reduced their volume by 15%, whereas those with an initial volume of 500 cm3 exhibited a 14% reduction. Mycelium was found to exhibit a hydrophobic nature on the surface. The pure HZ substrate demonstrated high hydrophobicity at 105 degrees, while the combination of the substrates HZ75-SW25 showed hydrophobicity at nearly 99 degrees. However, the uneven growth of mycelial fibers on sample surfaces can expose portions of the porous substrate. These uncovered areas remain absorbent and can easily take up water. Figure 4 presents the water absorption behavior of the HZ75-SW25 and HZ100 bioblocks over a 72-h immersion period. The data indicate rapid initial uptake within the first few hours, particularly for the HZ75-SW25 samples, which reached 57% absorption after 24 h, 60% after 48 h, and 72% after 72 h. In contrast, HZ100 samples absorbed water more slowly, reaching 46% at 24 h, 52% at 48 h, and 58% at 72 h. This difference may be attributed to the composition and porosity of the substrates. As the biomaterials approach saturation, the curves suggest a decelerating trend in water absorption over time, typical of capillary-driven sorption. Error bars represent the standard deviation across three replicates, underscoring the consistency of the measurements. The inset image shows the experimental setup and confirms the structural integrity of the bioblocks throughout immersion.

Table 4.
Comparative analysis of physical properties of fabricated bioblocks.
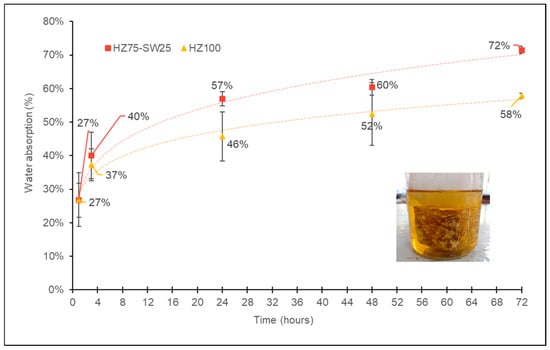
Figure 4.
Water uptake, expressed as a percentage, over an immersion period of 72 h, for the samples HZ75-SW25 (■) and HZ100 (▲). Data points represent the average of three replicates; error bars indicate the standard deviation, and dashed lines are included to guide the reader. The inset image represents the experimental setup and how the bioblocks remain intact throughout this period.
The mechanical performance of the bioblocks was determined before and after being exposed to severe conditions, and the results are presented in Table 5 for the modulus of rupture (MOR), modulus of elasticity (MOE), and internal bond (IB) strength. The initial mechanical properties of the MBC from the combination of the substrates HZ75 and SW25 were higher than those from bioblocks of pure HZ, which means the use of hazelnut shells for MBCs gave a biomaterial with reduced mechanical properties. The MOR for HZ75-SW25 was 56.03 ± 5.7 kPa, while for HZ100, it was 43.01 ± 2.9 kPa. Additionally, the MOE for HZ75-SW25 was 42.62 ± 2.8 kPa, compared to 33.86 ± 0.8 kPa for HZ100. Furthermore, the internal bond strength was 13.33 ± 1.1 Pa for HZ75-SW25, whereas it was 8.54 ± 0.23 Pa for HZ100. Later, the MBC samples were subjected to accelerated controlled environmental conditions to mimic weathering scenarios at 35 °C and a relative humidity of 95% for 14 days. Before retesting, the MBCs were conditioned for one week at 23 °C with a relative humidity of 12–14%. The same mechanical properties as before were measured, and the results are presented in Table 5. After exposure, the mechanical properties diminished in both cases. For HZ75-SW25, the MOR decreased from 56.03 ± 5.7 kPa to 48.94 ± 5.8 kPa, indicating a reduction of approximately 13% in the maximum stress capacity before fracture. In contrast, the MOE increased from 42.62 ± 2.8 kPa to 61.87 ± 3.1 kPa. This increase in stiffness can be attributed to a significant rise in the slope (m) of the elastic portion of the load–deflection curve, which increased from 0.318 to 0.454 N/mm. In contrast, the moment of inertia (I) of the samples remained relatively stable, changing from 41.88 mm4 to 40.12 mm4. This notable increase in stiffness suggests that the bioblocks became more resistant to initial deformation after exposure to environmental conditions. This enhancement is likely due to the presence of sawdust, which may have swelled in high humidity, resulting in rearrangement of the hyphal network and partial local densification. The internal bond strength dropped by 40%. In the case of MBCs fabricated with pure HZ, the mechanical properties exhibited significant degradation after exposure to extreme environmental conditions. The MOR decreased from 43.01 ± 2.9 kPa to 17.56 ± 4.5 kPa, indicating a reduction of 59% in the maximum stress capacity before fracture. Similarly, the MOE declined from 33.86 ± 0.8 kPa to 22.94 ± 13.3 kPa, reflecting a 32% loss in material stiffness. These reductions suggest significant weakening of the structural integrity, likely due to fiber degradation, moisture-induced damage, and potential disruption of the hyphal bonding network under conditions of high humidity and temperature. Interestingly, the internal bond strength increased from 8.5 ± 0.23 Pa to 11.2 ± 2.24 Pa following exposure. This improvement may be attributed to further consolidation or partial reorganization of the internal structure, leading to enhanced localized adhesion between the mycelium and the substrate particles. However, despite this localized strengthening, the overall mechanical performance of the pure HZ bioblocks was markedly diminished, underscoring the material’s susceptibility to environmental stress in the absence of composite reinforcement. Other researchers have reported similar findings, indicating that these materials typically lose strength when exposed to changing weather conditions due to their environmental sensitivity [13]. However, this challenge can be alleviated by applying a protective coating, as long as the materials used are sustainable and do not involve synthetic or non-biodegradable components.

Table 5.
Comparative analysis of mechanical properties of fabricated bioblocks.
3.4. Biodegradability of Mycelium Bioblocks
The biodegradation capacity of the MBCs was investigated by burying MBC samples in compost soil and monitoring the carbon dioxide produced over six months. The results, illustrating biodegradability over time, are displayed in Figure 5. Detailed data, including the mean and standard deviation, and the cumulative CO2 evolution chart, can be found in the Supplementary Materials (Table S1 and Figure S1, respectively). Cellulose was used as a reference material, represented by a solid blue circle in the figure. This material demonstrated 88% degradation after 188 days, achieving the highest degradation rate among the three samples tested. These results aligned with the validity requirements of the standard test method ASTM D5988 (biodegradation > 70% after six months), confirming that the soil was biologically active and the tests were valid. In comparison, the MBC sample made from HZ75-SW25, indicated by a solid red square, achieved 68% degradation by the end of the experiment. Meanwhile, the MBC sample derived from HZ100, a solid yellow triangle, demonstrated 58% degradation. The difference in biodegradation between the two MBC samples was attributed to their chemical composition, as the hazelnut shells contained 44% wt. of lignin. This biopolymer is less susceptible to biodegradation than cellulose or hemicellulose due to its complex structure and recalcitrant nature [39,40]. Additionally, studies have shown that the water content or accessibility of water within a material influences its biodegradation in soil [9]. The MBCs demonstrated a water absorption of 60% for HZ75-SW25 and 52% for SW100, with a consistent water absorption rate of 0.32 for both materials. Therefore, the accessibility of water may significantly impact the biodegradation process.
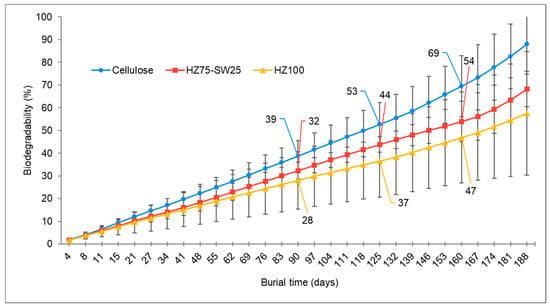
Figure 5.
Biodegradability, expressed as a percentage, of samples over a burial period of 188 days: cellulose (●), HZ75-SW25 (■), and HZ100 (▲). The data points represent the average of three replicates; error bars indicate the standard deviation. The data points for 90, 125, and 160 days have been labeled for clarity.
The biodegradation kinetics were assumed to follow a zero-order reaction. The results are presented in Table 6, which includes equations, velocities, and total degradation times. The data show that cellulose degraded faster than the MBC samples, exhibiting a degradation rate of 0.43% per day and total degradation occurring in 230 days under controlled conditions. In contrast, the MBC derived from the combination of the substrates HZ75-SW25 exhibited the highest degradation rate among the MBC blocks, with a velocity of 0.3324% per day and total degradation achieved after 294 days. Conversely, the MBC from HZ100 showed the lowest degradation velocity at 0.2782% per day, with total degradation completed after 349 days. Understanding biodegradation performance is crucial for developing biomaterials, as these bioproducts are expected to replace synthetic materials without causing contamination at the end of their life cycle. This relatively short time is translated into a low environmental impact.

Table 6.
Equations, velocity constants, and total degradation time of MBCs samples.
4. Discussion
Developing biomaterials from mycelium and agroforestry by-products presents a promising alternative for non-structural sustainable construction elements. Initially, five different formulations were evaluated based on varying proportions of European hazelnut shells and wood sawdust, focusing on the growth kinetics of the Ganoderma sp. fungus. From this evaluation, two formulations were selected: one consisting of 100% hazelnut shells (HZ100) and another comprising a mixture of 75% hazelnut shells and 25% pine sawdust (HZ75-SW25). The physicochemical characterization of these substrates and the assessment of mycelial growth indicated that the hazelnut shells enhanced the colonization of Ganoderma sp. This improvement was probably due to the production of laccase, peroxidases, and manganese peroxidases enzymes by Ganoderma sp., which efficiently metabolized the higher lignin content [41]. The high extractive content in the hazelnut shells may have further enhanced the availability of compounds in the medium that could act as inducers for expressing laccases and peroxidases. The formulation that incorporated sawdust may have promoted the adaptation of Ganoderma and enhanced the expression of genes related to cellulolysis, which could explain the initial slowdown in the colonization of the culture medium. Furthermore, the decrease in pH observed in the medium with sawdust also partially accounts for the reduced growth kinetics.
After the production of MBCs, one of the critical aspects analyzed for their application in construction was the volumetric shrinkage that occurs during drying. In this case, the formulation HZ75-SW25 exhibited greater shrinkage (19.8%) than the formulation HZ100, which showed a shrinkage of 14.9%. This suggests that the inclusion of sawdust may influence the dimensional stability of the biomaterial during drying. With its significant water retention capacity of 184.19%, sawdust is likely to contribute to increased volume loss as the remaining water evaporates. Nevertheless, this recurring challenge can be addressed and resolved at the production level.
Based on the apparent-density results, the formulation HZ100 exhibited a 0.6 g/cm3 density, while HZ75-SW25 had a lower density of 0.49 g/cm3. Here, we reported MBCs with similar density values to those in earlier studies, which range from 0.80 to 0.55 g/cm3 [14,25,42,43]. This indicates that, although the inclusion of sawdust led to a more porous structure and greater compaction during drying, it resulted in a material that was still less dense than the one made with 100% hazelnut shell. This approach allows the production of materials with varying densities, which can be tailored to meet the specific requirements of the final application while always considering the kinetics and mechanical properties associated with each formulation.
From the perspective of hydrophobicity after drying, the formulation HZ100 exhibited a contact angle of 105.14°, while HZ75-SW25 showed a slightly lower contact angle of 98.7°. These results indicate that both formulations possessed relatively hydrophobic surfaces. While both MBCs displayed these hydrophobic characteristics, evaluating water absorption is crucial for determining their durability. Over a 72-h period, the MBC fabricated with HZ100 absorbed less water than HZ75-SW25 (Figure 4). This observation suggests that the chemical composition of the shells, particularly their high lignin and extractive components, may contain hydrophobic domains that remain after the biomaterial has dried. The difference in water absorption may be related to the higher porosity of the material and the substrate composition, as sawdust demonstrated a significantly greater water retention capacity compared to hazelnut shells in the initial phase of the study (WHC). Nonetheless, the absorption rates for both formulations were quite similar, at 0.32, implying that the water uptake dynamics did not vary significantly between them. This similarity is likely due to the comparable growth of fungal biomass and porosities, which allowed for material transfer under similar conditions. The results of this study aligned with those of previous research, which indicates water absorption rates between 24% and 560% after immersion in water for 24 to 192 h [14,42,43,44,45]. However, we successfully reduced water absorption to below 72% after 72 h by combining hazelnut shells with sawdust. This outcome highlights the significant influence of substrate selection on material properties. Overall, these findings suggest that the initial use of these biomaterials should be limited to closed environments or at least kept away from direct contact with water. In outdoor applications, applying coatings could enhance their performance. For instance, a study was conducted to investigate the mechanical properties and water absorption of MBCs coated with various solutions, namely wet starch, chitosan, and epoxy resin [46]. These results demonstrated that MBCs coated with chitosan achieved the highest compressive strength, while those coated with epoxy resin exhibited the highest flexural strength and lowest water absorption, attributed to the hydrophobic nature of the resin. Furthermore, employing heat pressing as a post-treatment process resulted in a 200% increase in flexural strength compared to unpressed samples. Significant improvements in tensile properties were also noted in the heat-pressed samples [47].
In terms of mechanical properties, the HZ75-SW25 formulation demonstrated greater resistance compared to HZ100. The modulus of rupture (MOR) for the HZ100 formulation was 43.01 kPa, while the HZ75-SW25 reached 56.03 kPa. This difference indicates that the addition of sawdust enhances the material’s ability to withstand loads before fracturing. This can be attributed to the higher proportion of holocellulose, with sawdust containing 61.26%, compared to only 43.6% in hazelnut shells. This improved performance can primarily be explained by the greater abundance of fibrous components in sawdust, such as cellulose and hemicellulose. This leads to a matrix that fosters better physical interactions between the mycelium’s hyphae and the substrate fibers. Additionally, the higher presence of simple sugars in sawdust may stimulate increased production of extracellular polysaccharides (EPS), such as β-glucans and other exopolysaccharides [48]. These act as natural adhesives, promoting enhanced cohesion between the mycelial hyphae and the substrate particles.
The modulus of elasticity (MOE) displayed a similar trend, showing values of 33.86 kPa in the HZ100 formulation and 42.62 kPa in the HZ75-SW25 formulation. This suggests that the sawdust-based material was stiffer and less susceptible to deformation under loads. Additionally, the internal bond strength, which measures the resistance to separation between the layers of the material, was significantly higher in the HZ75-SW25 formulation at 13.33 Pa, compared to 8.54 Pa for the HZ100 formulation. This difference may be attributed to the higher water-holding capacity of sawdust, which likely enhanced initial mycelium expansion and improved adhesion between the substrate particles.
After exposure to severe weather conditions, these results indicate that the formulation labeled HZ100 experienced significant mechanical strength loss. Specifically, the MOR decreased by 59.2%, and the MOE declined by 32.2%. Interestingly, the internal bond strength improved, increasing from 8.54 Pa to 11.23 Pa. This suggests that, despite the structural degradation, the material retained some internal cohesion after being restabilized under standard conditions. On the other hand, the HZ75-SW25 formulation demonstrated improved mechanical stability. The MOR decreased by 12.7%, while the MOE increased from 42.62 kPa to 61.87 kPa. This increase in stiffness suggests that, after restabilization, the material recovered part of its initial structure, possibly due to the sawdust’s ability to form a complex and stronger mycelial network. However, the internal bonding strength decreased by 40%, which could reflect greater fragility in internal cohesion after exposure to extreme humidity. An MBC was fabricated with a mixture of sawdust and empty fruit bunches, and was subjected to similar weathering conditions over a period of 35 days [13]. The researchers found that applying a protective coating improved the material’s resistance. Consequently, these types of biomaterials have the potential to serve as an environmentally friendly substitute for particleboards.
The comparative analysis between these two formulations reveals the significant role of substrate composition in enhancing the environmental durability of MBCs. Incorporating wood sawdust in the HZ75-SW25 samples improved mechanical stability when exposed to environmental factors, likely due to the creation of additional binding sites and the reinforcement of the hyphal network. These results underscore the importance of optimizing substrates to enhance the long-term performance of MBCs, particularly under varying temperature and humidity conditions. Additional investigations are recommended to further understand the microstructural mechanism influencing mechanical behavior under environmental stresses, including the microscopic examination of hyphal structures.
The biodegradation results indicate that the formulation containing the highest amount of sawdust degraded more rapidly than the formulation made entirely of hazelnut shells. This difference can be attributed to the higher holocellulose content in sawdust (61.26% compared to 43.6%), which provided a more accessible substrate for microbial action. Additionally, the greater porosity and water retention capacity of the sawdust biomaterial likely promoted more effective microbial colonization, speeding up its decomposition. In contrast, the formulation made from 100% hazelnut shells exhibited moderate biodegradability. This could be linked to its higher content of lignin (44.2%), a highly resistant polymer to biological degradation. Consequently, this material may offer greater durability in applications that require a longer shelf life before biodegradation occurs.
MBCs still encounter various challenges, and several gaps need to be addressed. Architectural structures constructed from MBCs maintained their integrity for up to six weeks under outdoor exposure; however, signs of cracking, decay, infestation, and contamination with potentially harmful organisms became evident after four months [18,49,50]. Additionally, MBCs have been widely explored in insulation materials, packaging for small consumer products, and sustainable and acoustic architecture, where low mechanical strength is acceptable. Nevertheless, further research and development are necessary to adapt these composites for higher-strength applications. Although several studies have reported encouraging mechanical properties, additional efforts are needed to optimize and enhance the structural performance of MBCs. Here, we obtained potential results by varying the proportion of substrates, therefore tailoring properties which could apply to non-structural but high-strength applications such as interior wall panels, ceiling tiles, partition elements, isolation elements, and lightweight decorative structures in timber frame constructions. We are currently investigating the sound-absorbing properties of MBCs, along with their thermal capacity and fire resistance, since the porous structure of MBCs can effectively trap and dissipate sound waves, thus reducing noise levels [51], and also offer good thermal insulation [52]. Another challenge is related to changing the general public’s perception of these products and to fostering a more positive response toward them. Adopting a more holistic and globally inclusive view of the world, which recognizes the equality of all living species, could help to highlight the advantages and benefits of incorporating MBCs into daily life [53]. Given these challenges and the proposed solutions, future research and development are expected to span multiple interdisciplinary areas. The acceptance and esthetics of these bioproducts were analyzed because of the heterogeneity of their surfaces, which are characterized by porous and irregular textures and colors [54]. The findings indicated that MBC materials generally received a positive or not-negative assessment, demonstrating advantages over other materials like ceramic. This underscores that MBCs are perceived as more interesting, original, and environmentally friendly. Furthermore, a life cycle assessment (LCA) will demonstrate the environmental impacts of mycelium-based composites and identify ways to reduce their overall global warming potential [55,56].
5. Conclusions
The findings of this study indicate that mycelium-based composites (MBCs) created from agroforestry residues, namely hazelnut shells and pine sawdust with tailored properties, present a promising sustainable alternative to existing building materials. This research showed that the formulation containing 75% hazelnut shells and 25% pine sawdust (HZ75-SW25) exhibited superior mechanical properties, including a higher modulus of rupture and greater internal bond strength compared to composites made solely from hazelnut shells. While both formulations demonstrated hydrophobic surfaces and significant biodegradability, the inclusion of sawdust enhanced the structural integrity and accelerated the degradation process under controlled environmental conditions. These results, combined with insights from the introduction regarding the valorization of agroforestry by-products and the importance of sustainable material development, highlight the potential of these biocomposites to contribute to a circular economy and reduce the environmental impact of traditional non-structural construction materials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/buildings15111764/s1, Figure S1: Cumulative CO2 (mg) evolution of burial samples over 188 days: cellulose (●), HZ75-SW25 (■), and HZ100 (▲). Data points represent the average of three replicates; error bars indicate standard deviation. Data points have been labeled for clarity at 90, 125, and 160 days; Table S1: Mean and standard deviation values for the biodegradability (%) of burial samples over time.
Author Contributions
Conceptualization, C.F. and J.F.O.; methodology, C.F., J.F.O. and V.G.-M.; software, C.F. and J.F.O.; formal analysis, C.F., J.F.O., V.G.-M. and R.G.; investigation, C.F. and J.F.O.; resources, C.F. and J.F.O.; data curation, C.F.; writing—original draft preparation, C.F.; writing—review and editing, C.F. and J.F.O.; visualization, R.G.; supervision, C.F.; project administration, C.F. and J.F.O.; funding acquisition, C.F. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the research funding provided by ANID BASAL FB210015 CENAMAD and the projects VID RS06/24 and AYV082/24 (Vicerrectoría de Investigación y Desarrollo, Universidad de Chile). The APC was funded by ANID BASAL FB210015 CENAMAD.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank Alejandro Bozo for his support during the mechanical tests; Frutícola AGRICHILE S.A for kindly providing the hazelnut shells; ANID BASAL FB210015 CENAMAD and the projects VID RS06/24 and VID AYV082/24. The authors express their gratitude to Catalina Castillo and Guillermo Esparza for their valuable contributions during the execution of their undergraduate thesis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Livne, A.; Wösten, H.A.B.; Pearlmutter, D.; Gal, E. Fungal Mycelium Bio-Composite Acts as a CO2-Sink Building Material with Low Embodied Energy. ACS Sustain. Chem. Eng. 2022, 10, 12099–12106. [Google Scholar] [CrossRef]
- Sisti, L.; Gioia, C.; Totaro, G.; Verstichel, S.; Cartabia, M.; Camere, S.; Celli, A. Valorization of Wheat Bran Agro-Industrial Byproduct as an Upgrading Filler for Mycelium-Based Composite Materials. Ind. Crops Prod. 2021, 170, 113742. [Google Scholar] [CrossRef]
- Joshi, K.; Meher, M.K.; Poluri, K.M. Fabrication and Characterization of Bioblocks from Agricultural Waste Using Fungal Mycelium for Renewable and Sustainable Applications. ACS Appl. Bio Mater. 2020, 3, 1884–1892. [Google Scholar] [CrossRef]
- Hoşgün, E.Z.; Bozan, B. Effect of Different Types of Thermochemical Pretreatment on the Enzymatic Hydrolysis and the Composition of Hazelnut Shells. Waste Biomass Valorization 2020, 11, 3739–3748. [Google Scholar] [CrossRef]
- Bener, M.; Şen, F.B.; Önem, A.N.; Bekdeşer, B.; Çelik, S.E.; Lalikoglu, M.; Aşçı, Y.S.; Capanoglu, E.; Apak, R. Microwave-Assisted Extraction of Antioxidant Compounds from by-Products of Turkish Hazelnut (Corylus avellana L.) Using Natural Deep Eutectic Solvents: Modeling, Optimization and Phenolic Characterization. Food Chem. 2022, 385, 132633. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Crops and Livestock Products; Ministry of Agriculture-Czech Republic: Prague, Czechia, 2013. [Google Scholar]
- Allegrini, A.; Salvaneschi, P.; Schirone, B.; Cianfaglione, K.; Di Michele, A. Multipurpose Plant Species and Circular Economy: Corylus avellana L. as a Study Case. Front. Biosci. Landmark 2022, 27, 11. [Google Scholar] [CrossRef]
- Attias, N.; Livne, A.; Abitbol, T. State of the Art, Recent Advances, and Challenges in the Field of Fungal Mycelium Materials: A Snapshot of the 2021 Mini Meeting. Fungal Biol. Biotechnol. 2021, 8, 12. [Google Scholar] [CrossRef]
- Muñoz, H.; Molina, P.; Urzúa-Parra, I.A.; Vasco, D.A.; Walczak, M.; Rodríguez-Grau, G.; Chateau, F.; Sancy, M. Applicability of Paper and Pulp Industry Waste for Manufacturing Mycelium-Based Materials for Thermoacoustic Insulation. Sustainability 2024, 16, 8034. [Google Scholar] [CrossRef]
- Houette, T.; Maurer, C.; Niewiarowski, R.; Gruber, P. Growth and Mechanical Characterization of Mycelium-Based Composites towards Future Bioremediation and Food Production in the Material Manufacturing Cycle. Biomimetics 2022, 7, 103. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, F.; Still, B.; White, M.; Amstislavski, P. Physical and Mechanical Properties of Fungal Mycelium-Based Biofoam. J. Mater. Civ. Eng. 2017, 29, 04017030. [Google Scholar] [CrossRef]
- Pelletier, M.G.; Holt, G.A.; Wanjura, J.D.; Bayer, E.; McIntyre, G. An Evaluation Study of Mycelium Based Acoustic Absorbers Grown on Agricultural By-Product Substrates. Ind. Crops Prod. 2013, 51, 480–485. [Google Scholar] [CrossRef]
- Chan, X.Y.; Saeidi, N.; Javadian, A.; Hebel, D.E.; Gupta, M. Mechanical Properties of Dense Mycelium-Bound Composites under Accelerated Tropical Weathering Conditions. Sci. Rep. 2021, 11, 22112. [Google Scholar] [CrossRef] [PubMed]
- Appels, F.V.W.; Camere, S.; Montalti, M.; Karana, E.; Jansen, K.M.B.; Dijksterhuis, J.; Krijgsheld, P.; Wösten, H.A.B. Fabrication Factors Influencing Mechanical, Moisture- and Water-Related Properties of Mycelium-Based Composites. Mater. Des. 2019, 161, 64–71. [Google Scholar] [CrossRef]
- Aiduang, W.; Kumla, J.; Srinuanpan, S.; Thamjaree, W.; Lumyong, S.; Suwannarach, N. Mechanical, Physical, and Chemical Properties of Mycelium-Based Composites Produced from Various Lignocellulosic Residues and Fungal Species. J. Fungi 2022, 8, 1125. [Google Scholar] [CrossRef] [PubMed]
- Charpentier-Alfaro, C.; Benavides-Hernández, J.; Poggerini, M.; Crisci, A.; Mele, G.; Della Rocca, G.; Emiliani, G.; Frascella, A.; Torrigiani, T.; Palanti, S. Wood-Decaying Fungi: From Timber Degradation to Sustainable Insulating Biomaterials Production. Materials 2023, 16, 3547. [Google Scholar] [CrossRef]
- Sydor, M.; Bonenberg, A.; Doczekalska, B.; Cofta, G. Mycelium-Based Composites in Art, Architecture, and Interior Design: A Review. Polymers 2021, 14, 145. [Google Scholar] [CrossRef]
- Ghazvinian, A.; Khalilbeigi, A.; Mottaghi, E.; Gürsoy, B. The Design And Fabrication Of Mycocreate 2.0: A Spatial Structure Built With Load-Bearing Mycelium-Based Composite Components. J. Int. Assoc. Shell Spat. Struct. 2022, 63, 85–97. [Google Scholar] [CrossRef]
- Holt, G.A.; Mcintyre, G.; Flagg, D.; Bayer, E.; Wanjura, J.D.; Pelletier, M.G. Fungal Mycelium and Cotton Plant Materials in the Manufacture of Biodegradable Molded Packaging Material: Evaluation Study of Select Blends of Cotton Byproducts. J. Biobased Mater. Bioenergy 2012, 6, 431–439. [Google Scholar] [CrossRef]
- Jendrossek, D. Polyethylene and Related Hydrocarbon Polymers (“Plastics”) Are Not Biodegradable. New Biotechnol. 2024, 83, 231–238. [Google Scholar] [CrossRef]
- Alemu, D.; Tafesse, M.; Mondal, A.K. Mycelium-Based Composite: The Future Sustainable Biomaterial. Int. J. Biomater. 2022, 2022, 8401528. [Google Scholar] [CrossRef]
- Haneef, M.; Ceseracciu, L.; Canale, C.; Bayer, I.S.; Heredia-Guerrero, J.A.; Athanassiou, A. Advanced Materials From Fungal Mycelium: Fabrication and Tuning of Physical Properties. Sci. Rep. 2017, 7, 41292. [Google Scholar] [CrossRef] [PubMed]
- Bellettini, M.B.; Fiorda, F.A.; Maieves, H.A.; Teixeira, G.L.; Ávila, S.; Hornung, P.S.; Júnior, A.M.; Ribani, R.H. Factors Affecting Mushroom Pleurotus spp. Saudi J. Biol. Sci. 2019, 26, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Park, D.; Qin, Z. Material Function of Mycelium-Based Bio-Composite: A Review. Front. Mater. 2021, 8, 737377. [Google Scholar] [CrossRef]
- Ghazvinian, A.; Farrokhsiar, P.; Vieira, F.; Pecchia, J.; Gursoy, B. Mycelium-Based Bio-Composites for Architecture: Assessing the Effects of Cultivation Factors on Compressive Strength. Mater. Res. Innov. 2019, 2, 505–514. [Google Scholar]
- Jones, M.; Mautner, A.; Luenco, S.; Bismarck, A.; John, S. Engineered Mycelium Composite Construction Materials from Fungal Biorefineries: A Critical Review. Mater. Des. 2020, 187, 108397. [Google Scholar] [CrossRef]
- Alaneme, K.K.; Anaele, J.U.; Oke, T.M.; Kareem, S.A.; Adediran, M.; Ajibuwa, O.A.; Anabaranze, Y.O. Mycelium Based Composites: A Review of Their Bio-Fabrication Procedures, Material Properties and Potential for Green Building and Construction Applications. Alex. Eng. J. 2023, 83, 234–250. [Google Scholar] [CrossRef]
- Sydor, M.; Cofta, G.; Doczekalska, B.; Bonenberg, A. Fungi in Mycelium-Based Composites: Usage and Recommendations. Materials 2022, 15, 6283. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Long, L.; Sheng, Y.; Xu, J.; Qiu, H.; Li, X.; Wang, Y.; Wu, H. Preparation of a Kind of Novel Sustainable Mycelium/Cotton Stalk Composites and Effects of Pressing Temperature on the Properties. Ind. Crops Prod. 2019, 141, 111732. [Google Scholar] [CrossRef]
- Mohseni, A.; Vieira, F.R.; Pecchia, J.A.; Gürsoy, B. Three-Dimensional Printing of Living Mycelium-Based Composites: Material Compositions, Workflows, and Ways to Mitigate Contamination. Biomimetics 2023, 8, 257. [Google Scholar] [CrossRef]
- Luo, D.; Yang, J.; Peek, N. 3D-Printed Mycelium Biocomposites: Method for 3D Printing and Growing Fungi-Based Composites. 3D Print. Addit. Manuf. 2025, 12, 98–111. [Google Scholar] [CrossRef]
- Li, K.; Jia, J.; Wu, N.; Xu, Q. Recent Advances in the Construction of Biocomposites Based on Fungal Mycelia. Front. Bioeng. Biotechnol. 2022, 10, 1067869. [Google Scholar] [CrossRef] [PubMed]
- Tacer-Caba, Z.; Varis, J.J.; Lankinen, P.; Mikkonen, K.S. Comparison of Novel Fungal Mycelia Strains and Sustainable Growth Substrates to Produce Humidity-Resistant Biocomposites. Mater. Des. 2020, 192, 108728. [Google Scholar] [CrossRef]
- Vašatko, H.; Gosch, L.; Jauk, J.; Stavric, M. Basic Research of Material Properties of Mycelium-Based Composites. Biomimetics 2022, 7, 51. [Google Scholar] [CrossRef]
- ASTM D570-22; Standard Test Method for Water Absorption of Plastics. American Society for Testing and Materials: West Conshohocken, PA, USA, 2022.
- ASTM D5988-18; Standard Test Method for Determining Anaerobic Biodegradation of Plastic Materials Under High-Solids Anaerobic-Digestion Conditions. ASTM: West Conshohocken, PA, USA, 2018.
- Zhao, J.; Wang, X.; Lin, H.; Lin, Z. Hazelnut and Its By-Products: A Comprehensive Review of Nutrition, Phytochemical Profile, Extraction, Bioactivities and Applications. Food Chem. 2023, 413, 135576. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Li, S.; Jin, J.; Lin, L.; Zhou, Y.; Peng, L.; Fu, F. Effect of High-Intensity Microwave (HIMW) Treatment on Chemistry of Radiata Pine. Wood Sci. Technol. 2023, 57, 1077–1097. [Google Scholar] [CrossRef]
- Datta, R.; Kelkar, A.; Baraniya, D.; Molaei, A.; Moulick, A.; Meena, R.; Formanek, P. Enzymatic Degradation of Lignin in Soil: A Review. Sustainability 2017, 9, 1163. [Google Scholar] [CrossRef]
- Scheu, S. Cellulose and Lignin Decomposition in Soils from Different Ecosystems on Limestone as Affected by Earthworm Processing. Pedobiologia 1993, 37, 167–177. [Google Scholar] [CrossRef]
- Zhou, X.-W.; Cong, W.-R.; Su, K.-Q.; Zhang, Y.-M. Ligninolytic Enzymes from Ganoderma Spp: Current Status and Potential Applications. Crit. Rev. Microbiol. 2013, 39, 416–426. [Google Scholar] [CrossRef]
- Elsacker, E.; Vandelook, S.; Brancart, J.; Peeters, E.; De Laet, L. Mechanical, Physical and Chemical Characterisation of Mycelium-Based Composites with Different Types of Lignocellulosic Substrates. PLoS ONE 2019, 14, e0213954. [Google Scholar] [CrossRef]
- Teeraphantuvat, T.; Jatuwong, K.; Jinanukul, P.; Thamjaree, W.; Lumyong, S.; Aiduang, W. Improving the Physical and Mechanical Properties of Mycelium-Based Green Composites Using Paper Waste. Polymers 2024, 16, 262. [Google Scholar] [CrossRef]
- Aiduang, W.; Chanthaluck, A.; Kumla, J.; Jatuwong, K.; Srinuanpan, S.; Waroonkun, T.; Oranratmanee, R.; Lumyong, S.; Suwannarach, N. Amazing Fungi for Eco-Friendly Composite Materials: A Comprehensive Review. J. Fungi 2022, 8, 842. [Google Scholar] [CrossRef] [PubMed]
- Kuribayashi, T.; Lankinen, P.; Hietala, S.; Mikkonen, K.S. Dense and Continuous Networks of Aerial Hyphae Improve Flexibility and Shape Retention of Mycelium Composite in the Wet State. Compos. Part A Appl. Sci. Manuf. 2022, 152, 106688. [Google Scholar] [CrossRef]
- Sakunwongwiriya, P.; Taweepreda, W.; Luenram, S.; Chungsiriporn, J.; Iewkittayakorn, J. Characterization of Uncoated and Coated Fungal Mycelium-Based Composites from Water Hyacinth. Coatings 2024, 14, 862. [Google Scholar] [CrossRef]
- Elsacker, E.; Vandelook, S.; Damsin, B.; Van Wylick, A.; Peeters, E.; De Laet, L. Mechanical Characteristics of Bacterial Cellulose-Reinforced Mycelium Composite Materials. Fungal Biol. Biotechnol. 2021, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.-H.; Zhong, J.-J. Effect of Initial PH on Production of Ganoderic Acid and Polysaccharide by Submerged Fermentation of Ganoderma Lucidum. Process Biochem. 2002, 37, 769–774. [Google Scholar] [CrossRef]
- Dessi-Olive, J. Strategies for Growing Large-Scale Mycelium Structures. Biomimetics 2022, 7, 129. [Google Scholar] [CrossRef]
- Gan, J.K.; Soh, E.; Saeidi, N.; Javadian, A.; Hebel, D.E.; Le Ferrand, H. Temporal Characterization of Biocycles of Mycelium-Bound Composites Made from Bamboo and Pleurotus Ostreatus for Indoor Usage. Sci. Rep. 2022, 12, 19362. [Google Scholar] [CrossRef]
- Walter, N.; Gürsoy, B. A Study on the Sound Absorption Properties of Mycelium-Based Composites Cultivated on Waste Paper-Based Substrates. Biomimetics 2022, 7, 100. [Google Scholar] [CrossRef]
- Schritt, H.; Vidi, S.; Pleissner, D. Spent Mushroom Substrate and Sawdust to Produce Mycelium-Based Thermal Insulation Composites. J. Clean. Prod. 2021, 313, 127910. [Google Scholar] [CrossRef]
- Le Ferrand, H. Critical Review of Mycelium-Bound Product Development to Identify Barriers to Entry and Paths to Overcome Them. J. Clean. Prod. 2024, 450, 141859. [Google Scholar] [CrossRef]
- Bonenberg, A.; Sydor, M.; Cofta, G.; Doczekalska, B.; Grygorowicz-Kosakowska, K. Mycelium-Based Composite Materials: Study of Acceptance. Materials 2023, 16, 2164. [Google Scholar] [CrossRef] [PubMed]
- Weinland, F.; Lingner, T.; Schritt, H.; Gradl, D.; Reintjes, N.; Schüler, M. Life Cycle Assessment of Mycelium Based Composite Acoustic Insulation Panels. Clean. Circ. Bioecon. 2024, 9, 100106. [Google Scholar] [CrossRef]
- Volk, R.; Schröter, M.; Saeidi, N.; Steffl, S.; Javadian, A.; Hebel, D.E.; Schultmann, F. Life Cycle Assessment of Mycelium-Based Composite Materials. Resour. Conserv. Recycl. 2024, 205, 107579. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).