Abstract
Geopolymer foam concrete (GFC) is a green, lightweight material produced by introducing bubbles into the geopolymer slurry. The raw materials for GFC are primarily silicon–aluminum-rich minerals or solid waste. Lead–zinc tailings (LZTs), as an industrial solid waste with high silicon–aluminum content, hold significant potential as raw materials for building materials. This study innovatively utilized LZTs to prepare GFC, incorporating MK, GGBS, and alkali activators as silicon–aluminum-rich supplementary materials and using H2O2 as a foaming agent, successfully producing GFC with excellent properties. The effects of different LZT content on the pore structure and various macroscopic properties of GFC were comprehensively evaluated. The results indicate that an appropriate addition of LZT effectively optimizes the pore structure, resulting in uniform pore distribution and pore shapes that are more spherical. Spherical pores exhibit better geometric compactness. The optimal LZT content was determined to be 40%, at which the GFC exhibits the best compressive strength, thermal conductivity, and water resistance. At this content, the dry density of GFC is 641.95 kg/m3, the compressive strength reaches 6.50 MPa after 28 days, and the thermal conductivity is 0.176 (W/(m·K)). XRD and SEM analyses indicate that under the combined effects of geopolymerization and hydration reactions, N–A–S–H gel and C–S–H gel were formed. The preparation of GFC using LZTs shows significant potential and research value. This study also provides a feasible scheme for the recycling and utilization of LZTs.
1. Introduction
It is evident that contemporary mining and beneficiation operations worldwide, encompassing both developing and developed countries, result in the mass production of tailings on an annual basis [1,2,3]. In China, for instance, statistical data [4] reveal that since 2011, the utilization of clearly measured tailings has fallen short of 36% (Figure 1). Lead–zinc tailings, a specific type of tailings, are the residual solid waste that is left over after lead–zinc ore has undergone the processes of crushing and flotation. In comparison with other tailings, these tailings frequently contain heavy metals such as lead and zinc. The disposal of lead–zinc tailings consumes a significant amount of land resources and poses a serious threat to the local environment, particularly soil, groundwater, and surrounding ecosystems [5,6]. Consequently, there is an imperative for rigorous research to explore the diversified recycling and utilization of lead–zinc tailings.
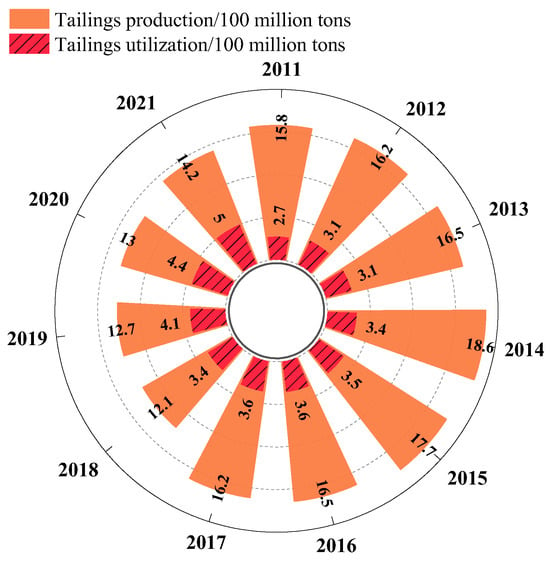
Figure 1.
Comprehensive utilization of tailings in China from 2011 to 2021.
In recent years, many scholars have conducted extensive research on the resource utilization of lead–zinc tailings and achieved a large number of research results. Li et al. [7] used F-grade fly ash and desulphurization gypsum as the main raw materials and modified the lead–zinc tailings by geopolymerization, which can produce a geopolymer material with dimensional stability and unconfined compressive strength that meets the requirements of building material standards. Luo et al. [8] used lead–zinc tailings and coal gangue to produce ceramsite. The bulk density and compressive strength of the ceramsite after high-temperature sintering with 30% lead–zinc tailings were 696 kg/m3 and 2.26 MPa, respectively. Ou et al. [9] used a lead–zinc waste sintering method to prepare a CaO-Al2O3-SiO2 system glass-ceramic. Orthogonal experiments were used to determine that the optimum lead–zinc scrap addition was 25%. Evidently, the utilization of lead–zinc tailings as raw materials for the fabrication of building materials exhibits considerable potential and furnishes an efficacious method for the recycling and utilization of such tailings [10,11].
As a common building material, foam concrete has excellent lightweight and thermal insulation properties [12]. It is widely used in prefabricated non-load-bearing components, wall filling, roof insulation, and other fields [13]. However, conventional foamed concrete primarily utilizes Portland cement as the cementitious material, and it should be noted that cement production results in the emission of a considerable amount of carbon dioxide during the entire production process. In contrast with Portland cement, geopolymers—a novel category of inorganic cementitious materials—exhibit considerable low-carbon advantages [14]. They are formed through a geological polymerization reaction, resulting in a three-dimensional network structure comparable to that of ceramics [15,16]. Geopolymer foam concrete (GFC) is a novel, eco-friendly, porous lightweight material that is prepared by introducing bubbles into the geopolymer slurry [17,18]. In comparison with conventional cement-based foamed concrete, GFC, prepared by alkali activation of a precursor material containing aluminosilicate, exhibits superior mechanical properties and remarkable heat and corrosion resistance [19]. Moreover, a further advantage of GFC is that its production process greatly reduces cement consumption and carbon emissions [20].
In addition, the raw materials for preparing GFC are mostly aluminosilicate minerals and solid wastes with high aluminosilicate content, such as metakaolin (MK), ground granulated blast furnace slag (GGBS), fly ash (FA), etc. Table 1 summarizes the information on GFC from the relevant literature [21,22,23,24,25,26,27,28,29,30,31,32], showing that most studies use MK and GGBS as raw materials for GFC preparation. Nevertheless, MK and GGBS are comparatively costly, compelling some researchers to substitute MK or GGBS with industrial solid wastes, including FA [22,23], waste glass powder [24], rice husk ash [25], and steel slag [32]. This results in samples that still exhibit the relatively desirable macroscopic properties. In summary, the utilization of industrial solid waste in the fabrication of GFC holds considerable potential and research value.

Table 1.
Relevant information of GFC studied in the literature.
Lead–zinc tailings are also a type of industrial solid waste with high silicon and aluminum content. However, there have been no reports on the use of these tailings to prepare GFC. Consequently, this study employs an innovative approach by utilizing lead–zinc tailings as a substitute for a portion of the active silicon-aluminum raw materials in the preparation of GFC. This approach has the potential to reduce the production cost of GFC to a certain extent [33]. Additionally, it can serve as a method of managing the disposal of tailings, thereby promoting the recycling and utilization of lead–zinc tailings [34,35].
In this experiment, alkaline activators were employed to chemically activate lead–zinc mine tailings, with MK and GGBS incorporated as supplementary aluminosilicate materials to enhance the geopolymerization reaction. In the formulation of GFC, hydrogen peroxide was employed as the foaming agent. Subsequently, the research systematically evaluated the influence of varying lead–zinc tailing dosages on key macroscopic properties, including fluidity, dry density, compressive strength, thermal conductivity, and drying shrinkage, among others. Using a stereoscopic microscope combined with Image-Pro-Plus software to study the pore structure of the samples, we analyzed the microscopic pore structure to identify the intrinsic mechanisms causing the differences in the macroscopic properties of GFC. Through a thorough comparison process, we ascertained the optimal dosage of lead–zinc tailings to prepare GFC, which exhibited exceptional performance and met the relevant specifications. In the end, the hydration products and microstructure of the samples were analyzed using an X-ray diffractometer (XRD) and a scanning electron microscope (SEM).
2. Materials and Methods
2.1. Raw Materials
GFC was prepared using lead–zinc tailings (LZTs), metakaolin (MK), and ground granulated blast furnace slag (GGBS) as aluminosilicate precursors. The LZT utilized in the experiment was obtained from a tailing pond situated at Shuikoushan, Hengyang City, China. The chemical composition of the LZT is characterized by a high SiO2 content of 72.47% and a CaO content of 10.74%. The MK used in the experiment was supplied by Hongle Mining Products Co., Ltd., located in Gongyi City, China, and was employed as an aluminum supplement to adjust the silicon-to-aluminum ratio of the system. The GGBS was obtained from Longze Water Purification Materials Co., Ltd. in Gongyi City, China, and has a CaO content of 34.00%. It is utilized as a calcium supplement to regulate the setting time of the geopolymer. The X-ray fluorescence (XRF) analysis of three types of geopolymer precursor materials is shown in Table 2. The phase composition of the LZT was determined through a combination of X-ray diffraction (XRD) analysis and the utilization of Jade 9.0 software. The results are displayed in Figure 2, which indicates that the primary mineral phases of LZT are quartz and hatrurite. Additionally, a laser particle size analyzer was utilized to assess the size distribution of the three raw materials, as depicted in Figure 3.

Table 2.
The main chemical composition (%) of raw materials.
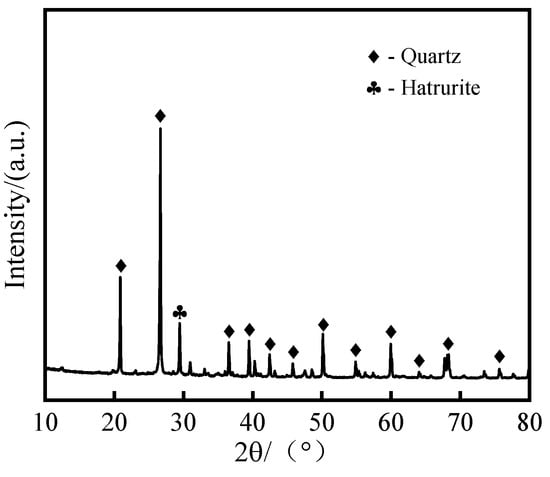
Figure 2.
XRD pattern of LZT.
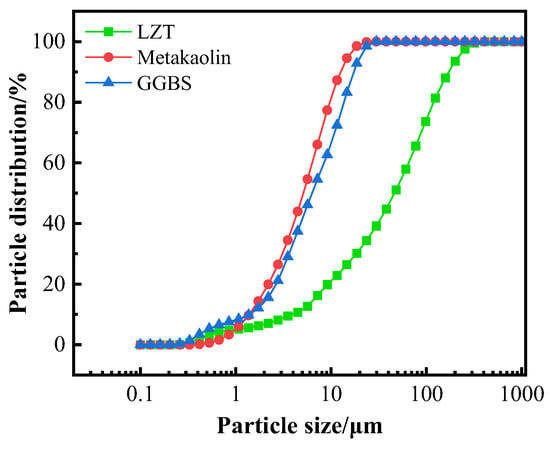
Figure 3.
Differential particle distribution of raw materials.
Sodium hydroxide and liquid sodium silicate are mixed in a precise ratio to create the alkali activator. The Na2SiO3 solution utilized in the experiment exhibited a modulus of 3.3, comprising 8.53% Na2O and 26.98% SiO2. The NaOH utilized in this study is analytically pure flaked sodium hydroxide, produced by Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. The water utilized in the experiment was obtained from the laboratory’s tap.
A 30% hydrogen peroxide solution is utilized as the chemical foaming agent. The foam stabilizer, calcium stearate analytical pure, was supplied by Tianjin Zhiyuan Chemical Reagent Co., Ltd., Tianjin, China. It is a white powder with a relative molecular mass of 607.03. The foam stabilizer functions as a surfactant, reducing surface free energy and enhancing the toughness of the bubbles. This, in turn, reduces bubble breakage and coalescence.
2.2. Heavy Metal Leaching Concentration Testing
The heavy metal concentration leaching test for LZT was conducted using the horizontal oscillation method, with the results and concentration limits listed in Table 3. As demonstrated in Table 3, the measured leaching concentrations of Pb and Zn in the tailings are both below the limits specified by Chinese national standards. Consequently, lead–zinc LZTs do not meet the criteria for designation as hazardous solid waste materials. Moreover, the alkaline solubility, gelation network bonding, solidification mechanism [36], and the physical encapsulation effect of geopolymer facilitate the effective solidification of heavy-metal elements in LZTs. Consequently, LZTs can be safely utilized as a raw material in the production of GFC, thereby achieving two objectives: the solidification of hazardous heavy metals and the enhancement of the utilization rate of tailings solid waste.

Table 3.
Leaching concentration (mg/L) of heavy metals from LZT.
2.3. Mix Proportion
In this experiment, consider that the addition of GGBS can shorten the initial setting time of the geopolymer slurry, thereby preventing the collapse of the molds of the expanded specimens. However, it has been demonstrated that the addition of GGBS in excess can lead to rapid hardening [23,37], resulting in incomplete decomposition of hydrogen peroxide. Consequently, the GGBS content was established at 15%. The Na2O content (alkali equivalent) directly determines the alkalinity of the system, thereby influencing the reaction mechanism of geopolymers [11]. An Na2O content that is too low limits the solubility of [SiO4]4− and [AlO4]5−, while an excessively high content can lead to alkali efflorescence on the geopolymer surface. Following preliminary exploratory experiments, the Na2O content was determined to be 7.5% in this experiment, with the alkali activator modulus maintained at 1.3.
Liquid sodium silicate, NaOH, and water were meticulously amalgamated in precise proportions to formulate the requisite solvent for the experiment, ensuring a constant liquid-to-solid ratio of 30% throughout the duration of the study. The liquid mass encompasses the mass of the liquid within the water glass and the mass of water, whereas the solid mass comprises the combined masses of the three silica–alumina materials, NaOH, and the solid content in the water glass. LZT content was selected at 30%, 40%, 50%, 60%, and 70% of the precursor raw material, and single-factor experiments were conducted to further investigate the effect of different LZT contents on GFC. The specific experimental mix design is delineated in Table 4.

Table 4.
Mix proportions of GFC samples (g).
2.4. Specimens Preparation
According to the test mix ratio, LZT, MK, GGBS, and calcium stearate are added to the mixer in sequence. To ensure uniform mixing of the raw materials, the mixture is blended at low speed for five minutes. Water and the prepared alkali activator are then added to the dry materials. The mixture undergoes low-speed mixing for 2 min, followed by high-speed mixing for 2 min to ensure homogeneity of the geopolymer slurry. Subsequently, the H2O2 foaming agent is incorporated, followed by a low-speed mixing process lasting 40 s. The slurry is finally poured into the corresponding molds. Additionally, the slurry only needs to be poured into the mold to 1/2–2/3 of its capacity and then left to expand naturally. This phenomenon can be attributed to the reaction of H2O2 with OH in an alkaline environment, which accelerates the decomposition rate of hydrogen peroxide [31,38]. The process of decomposition generates oxygen, which forms uniform bubbles within the geopolymer slurry [39]. This leads to the expansion of the slurry within the mold, resulting in the slurry filling the entire mold.
Upon reaching the initial setting time, the molded body ceases expansion. Cover with plastic wrap to prevent moisture loss. Subsequently, the sample should be placed at room temperature to facilitate the process of further curing. After 24 h, demold the sample and cut off the expanded portion of the mold. Test specimens should be stored in a curing chamber maintaining a temperature of 20 ± 2 °C and ≥90% relative humidity. Designated curing durations must be completed prior to testing. A flowchart of the methodology route used in this work is illustrated in Figure 4.
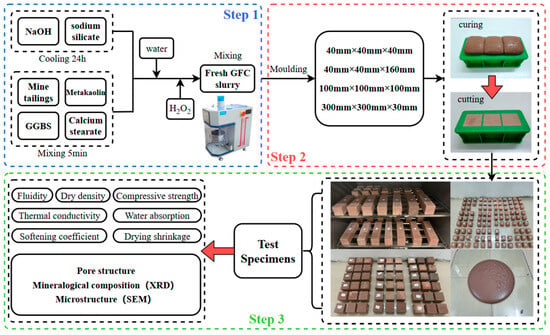
Figure 4.
A flowchart of the methodology route used in this work.
2.5. Testing Methods
2.5.1. Identification and Analysis of Pore Structure
Initially, the cross-section of the test specimen is ground and polished using sandpaper with grit sizes of 60, 200, and 1000, respectively. The residual powder on the cross-section is then removed using a soft-bristled brush. Subsequently, an Olympus stereomicroscope is employed to observe and photograph the cross-section of the test specimen with a fixed focal length and exposure parameters. The GFC cross-section photographs are then pre-processed using PS software, such as color correction, background removal, and contrast enhancement. Finally, the images were converted to 8-bit grayscale images using Image-Pro-Plus software and subjected to binarization processing. The macroporous structure of the pores was identified and statistically analyzed to obtain the average pore diameter (Feret diameter), porosity, and average roundness value (arithmetic mean of the roundness values of all pores) of the GFC. The roundness value is indicative of the degree of deviation of the pore shape from a spherical shape. The calculation formula for roundness is shown in Equation (1).
where R is the roundness value; P is the pore circumference (μm); and A is the pore area (μm2).
2.5.2. Macroscopic Properties
The dry density, water absorption rate, softening coefficient, and drying shrinkage rate of GFC were tested according to JG/T 266-2011 [40] specifications. Fluidity assessment followed JGJ/T 341-2014 [41] using cylindrical molds (h = 80 mm, d = 80 mm) with an electronic vernier caliper. Compressive strength was evaluated using a computer-controlled electro-hydraulic testing machine on 40 mm × 40 mm × 40 mm specimens under a 1.5 kN/s loading rate. In compliance with GB/T 10294-2008 [42], 300 mm × 300 mm × 30 mm specimens were prepared, and thermal conductivity was determined using an HFM436 thermal conductivity analyzer. Furthermore, each performance characteristics were assayed under uniform test conditions, and the mean value of three samples was considered the final test result.
2.5.3. XRD and SEM Characterization
X-ray diffraction (Rigaku SmartLab SE, Tokyo, Japan) was used to analyze the mineral phases of the raw materials and the hydration products of GFC. Standard cured test specimens were cut into small pieces of approximately 0.5 cm3, placed in anhydrous ethanol to stop hydration, dried before testing, and then ground flat with sandpaper. A scanning electron microscope (TESCAN MIRA LMS, Brno, Czech Republic) was used to observe the microstructure of the GFC specimens after gold spraying.
3. Results and Discussion
3.1. Pore Structure
As demonstrated in Figure 5, optical microscope images of the pore structure of GFC under varying LZT content are presented. The pores were identified and counted using Image-Pro-Plus software, as demonstrated in Figure 6. It has been observed that at a tailings content of 30%, the pore shapes are predominantly irregular polygons, with dense small pores distributed between the larger pores, and the distribution of pores with different diameters is uneven. In the case of LZTs with contents of 40% and 50%, the predominant pore morphology is that of closed pores. The pore shapes become more regular and spherical in shape, and the pore distribution becomes relatively uniform. In addition, the spacing between pores is relatively consistent, and there are no discernible areas of local pore density or sparsity. However, as the tailings content is increased, the pore diameter undergoes a significant increase, particularly at 70% tailings content, where the uniformity of pore distribution deteriorates and notable bonding phenomena occur between pores, leading to an increase in merged pores and interconnected pores formed by the combination of small pores.
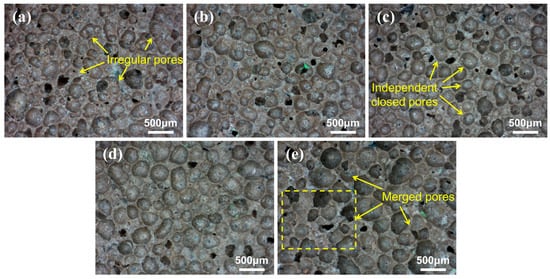
Figure 5.
Microscopic images of pore structures were captured at a fixed focal length, with labels (a–e) denoting representative cross-sectional images of the pore structure of GFC at varying LZT contents: (a) 30%; (b) 40%; (c) 50%; (d) 60%; (e) 70%.
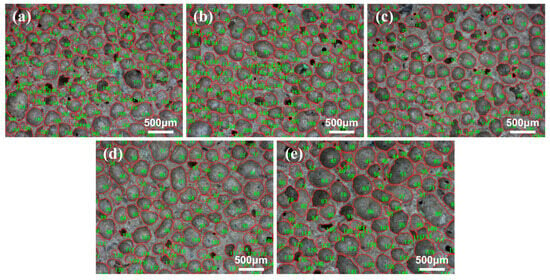
Figure 6.
Pore structure images identified and calculated by Image-Pro-Plus software. Labels (a–e) correspond to images of the pore structure of GFC under LZT contents ranging from 30% to 70%.
The data regarding the pore structure for each group consists of pores measured from three distinct cross-sections. The pore structure parameters of GFC under varying LZT content are illustrated in Figure 7. The study indicates that an increase in LZT content leads to an increase in porosity, with the average pore diameter increasing from 212.02 μm to 286.09 μm. This phenomenon is attributed to the enhanced flowability of GFC as LZT content rises, which decreases the relative viscosity of the slurry. This reduction in viscosity enables bubbles to surmount viscous resistance and expand more readily, leading to the formation of larger, independent pores [28]. Furthermore, the surface of tailings particles is characterized by a relative roughness, which, under the influence of friction, can lead to the rupture or merging of some bubbles into larger interconnected pores. This process results in an augmentation of both the average pore diameter and porosity.
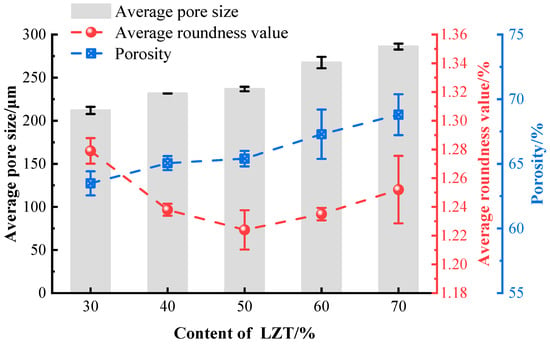
Figure 7.
Pore structure parameters of GFC with varying LZT content.
However, as the LZT dosage increases, the average roundness value of GFC initially decreases and then increases. At a tailings dosage of 30%, the average roundness value of the pores is 1.279, and the roundness of the pores in the formed samples is relatively poor, with most pores exhibiting irregular shapes. The study found that at a 50% LZT content, the average roundness value was the smallest, indicating that appropriately increasing the tailings content helps improve pore shape roundness. This phenomenon is primarily attributed to the optimization of the slurry rheological properties and the provision of additional nucleation sites for bubbles by the tailing particles [43]. Pores with shapes close to spherical have better geometric compactness, resulting in more uniform distribution of compressive stress on the pore walls when subjected to pressure [20,38,39]. Furthermore, an excessive addition of LZT results in a diminished stability of the bubble liquid membrane, thereby causing the average roundness value to gradually increase.
As shown in Figure 8, the effect of LZT content on pore size distribution is evident. The pore size distribution of GFC displays a tendency toward skewed distributions, with an evident trend of pore size distribution shifting toward larger pore sizes as the level of LZT content rises. As the LZT content increases from 30% to 70%, the percentage of pores in the 200–300 μm pore size range exhibits a corresponding increase, reaching 36.99%, 47.96%, 44.85%, 40.00%, and 24.22%, respectively. This is particularly evident at a 40% LZT content, where nearly 50% of the pores fall within this range, suggesting that the pore size distribution is relatively uniform at this content, exhibiting a lower dispersion degree in the pore size distribution. However, when the tailings content exceeded 60%, there was a substantial increase in the proportion of pores with diameters greater than 400 μm.
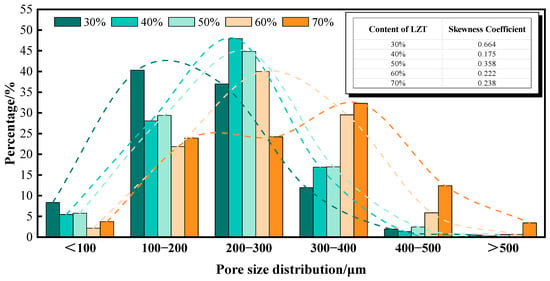
Figure 8.
Effect of LZT content on pore size distribution of GFC, the dashed line indicates the trend line of this distribution.
Figure 8 also shows the skewness coefficients of the pore size distribution under different LZT dosages. It is evident that all skewness coefficients are positive, indicating that the pore size distribution of GFC exhibits a positively skewed distribution. Furthermore, the tailings content exerts a substantial influence on the degree of skewness in the pore size distribution. At a 40% LZT content, the skewness coefficient is minimal, and the pore size distribution exhibits higher symmetry and is close to a normal distribution. This indicates that the pore size distribution exhibits optimal uniformity at a 40% LZT content. In general, high-performance foam concrete is characterized by a relatively uniform pore size distribution, with no significant presence of excessively large or small pores [44,45].
3.2. Fluidity and Dry Density
Figure 9 demonstrates the increased fluidity of GFC that occurs with the incorporation of LZT; however, the growth rate gradually decelerates. The larger average particle size of the tailings (see Figure 3) results in a lower water requirement for particle wetting, thereby achieving significantly improved fluidity under a constant liquid-solid ratio. However, the presence of a rougher surface on the tailings results in an increase in resistance to the flow of the slurry, thereby limiting the growth rate of flowability. Increasing the flowability of the slurry appropriately facilitates bubble encapsulation, thereby enhancing bubble stability. This is because higher fluidity corresponds to lower yield stress, reducing the resistance that bubbles must overcome when moving within the slurry [31]. This appropriate variation prevents local aggregation of bubbles, thereby reducing their rupture or merger. Consequently, this results in a more uniform pore distribution in the GFC. Additionally, the discrepancy in the stability of GFC bubbles, attributable to alterations in fluidity, can also be discerned through microscopic imagery of stomata, as illustrated in Figure 5.
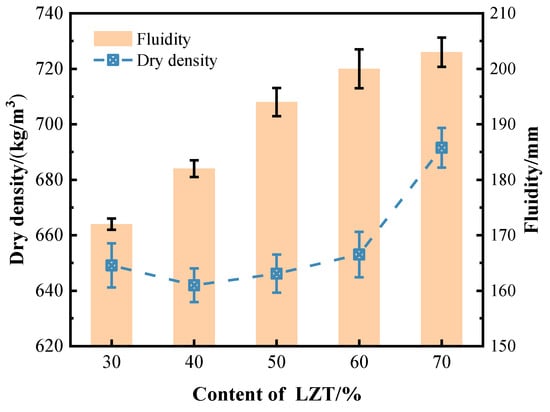
Figure 9.
Influence of various LZT content on the fluidity and dry density of GFC.
With rising LZT content, the dry density of GFC initially decreases and subsequently increases (Figure 9). As the LZT content ranges from 30% to 50%, the measured dry density of the samples is found to be relatively low, primarily due to the increase in the average pore diameter and porosity of the GFC samples (Figure 7). At a LZT content of 40%, the measured dry density of the GFC samples is the lowest, with a dry density of 641.95 kg/m3. As the LZT content continues to increase, although the average pore diameter and porosity of the GFC samples increase, the high density of LZT itself causes a large number of high-density tailings particles to fill the internal structure of the material, resulting in an overall increase in the total mass of GFC per unit volume. It is particularly evident that at a 70% LZT content, the augmentation in porosity diminishes the stability of the internal pore structure of GFC. Meanwhile, its substantial density may precipitate local collapse, resulting in the formation of a non-uniform structure with a dense bottom and a porous top. This phenomenon directly culminates in a substantial increase in dry density.
3.3. Compressive Strength
As displayed in Figure 10, the compressive strength of GFC demonstrates an initial increase and subsequent decrease with increasing LZT content. The findings indicate that, at a LZT content of 40%, the compressive strength of GFC samples at all ages achieves its maximum values, with the corresponding measurements recorded at 5.39 MPa, 5.53 MPa, and 6.50 MPa, respectively. It is noteworthy that the compressive strength of all samples at three days reached 70% or more of the 28-day strength. This is primarily attributed to the higher CaO content in the GGBS and LZT components. In comparison with Si-O and Al-O bonds, Ca-O bonds demonstrate a higher propensity for breakage [46]. The reaction of the precipitated Ca2+ with the silicoaluminate monomers is catalyzed by alkali activators, resulting in the formation of C–(A)–S–H gel. The spatial network structure, which is formed rapidly, significantly enhances the early strength of GFC.
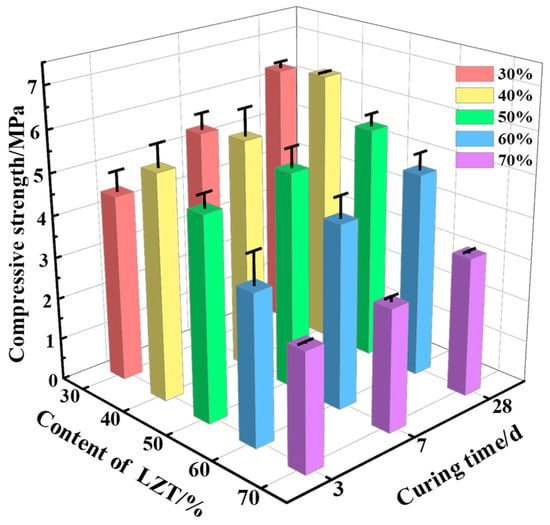
Figure 10.
Influence of different content of LZT on compressive strength of GFC.
A moderate increase in the LZT content has been shown to promote the development of GFC strength. The enhancement in strength from 30% to 40% can be attributed to the optimization of the pore structure. In light of the preceding analysis of the pore structure, the GFC containing 40% tailings exhibits uniform pore distribution. Its small average roundness value indicates that the pore shapes are closer to spherical. This results in a more uniform distribution of compressive stress on the pore walls under pressure, thereby effectively preventing stress concentration and crack initiation [38,39]. Additionally, a proportion of the tailing particles function as an inert skeleton, filling the material voids and enhancing the overall compactness of the GFC. Concurrently, the alkali exciter has the capacity to activate the active components in LZT, thereby facilitating the generation of geopolymer gels in conjunction with MK and GGBS. These gelling products have the potential to function as bonding and reinforcing agents [7], further bonding the unreacted tailings particles.
Nevertheless, when the LZT content surpasses 40%, a decline in the compressive strength of GFC samples at all ages is evident. When the LZT content is set at 50%, 60%, and 70%, the compressive strength of the specimens at 28 days is 5.55 MPa, 4.86 MPa, and 3.31 MPa, respectively. This represents a decrease of 14.62%, 25.23%, and 49.08%, respectively, compared to the optimal group. This phenomenon is primarily attributable to the change in the silicon-to-aluminum ratio (Si/Al) within the system, which serves as a pivotal factor in determining the structural morphology of the geopolymer [21,47]. As LZTs continue to replace MK, the amount of aluminum participating in the reaction decreases, directly leading to insufficient formation of geopolymer gel, which fails to effectively bind and strengthen the material. Moreover, due to their inert nature, the hydration reaction of tailings particles is not significant, resulting in reduced strength of the GFC. As mentioned earlier in Table 1 above, the GFC in this study exhibited higher compressive strength compared to some samples prepared from industrial solid waste [24,25,26,30,32]. This finding suggests that the preparation of GFC using LZTs has considerable potential.
3.4. Thermal Conductivity
Thermal conductivity is the primary indicator of the thermal insulation performance of foam concrete. Foam concrete with low thermal conductivity has the potential to markedly reduce the energy consumption of building operations. As demonstrated in Figure 11, the thermal conductivity initially decreases and subsequently increases with an increase in LZT content. It is noteworthy that at 40% LZT content, the thermal conductivity of GFC is the lowest, measuring at 0.176 W/(m·K). In general, the thermal conductivity of foam concrete is associated with its pore structure and dry density. At a tailings’ content of 40%, GFC exhibits the lowest dry density, with its pores predominantly comprising closed, nearly spherical gas pores. The presence of closed pores functions as minute insulating units, impeding heat conduction through continuous pathways and thereby effectively hindering rapid heat transfer within the material [19,48]. Furthermore, pores with smaller average roundness values possess relatively regular and stable geometric shapes, which renders them less susceptible to local heat bridges or heat short circuits during heat conduction [38]. As a result, gas convection heat transfer is suppressed, thereby reducing localized heat concentrations. However, as the LZT content increases, the higher dry density of GFC enhances thermal conductivity efficiency. Additionally, the numerous interconnected pores within the material form pathways for heat transfer, allowing heat to flow without being obstructed by numerous closed thermal insulation units. This results in a significant increase in the thermal conductivity coefficient.
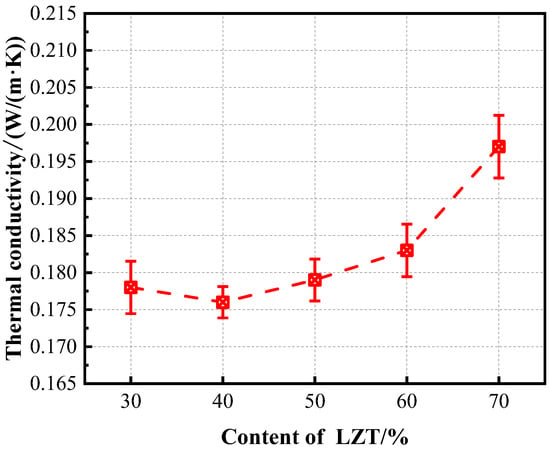
Figure 11.
Effect of LZT content on thermal conductivity of GFC.
The thermal conductivity of foam concrete is closely related to its dry density. In this study, thermal conductivity and dry density data from the relevant literature were collected and compared with the experimental results (see Figure 12). Linear regression analysis was performed on the dry density and thermal conductivity of GFC in this study, yielding the fitting equation y = 4.16 × 10−4x − 0.09, with R2 = 0.9695, indicating a significant linear relationship between the two variables. In addition, It has been observed that materials with lower dry density exhibit lower thermal conductivity [26,49,50,51,52]. This phenomenon is primarily attributed to the higher porosity present within the material or the presence of low-thermal-conductivity media, which contribute to the material’s excellent thermal insulation properties. However, lower dry density implies lower mechanical strength, and insufficient material strength may limit its application scope in actual engineering projects. The lead–zinc tailings geopolymer foam concrete prepared in this experiment exhibits both ideal thermal insulation properties and lightweight and high-strength characteristics. Furthermore, in comparison with cement-based foam concrete that possesses a similar dry density grade [45], the GFC under consideration in this experiment exhibits a reduced thermal conductivity.
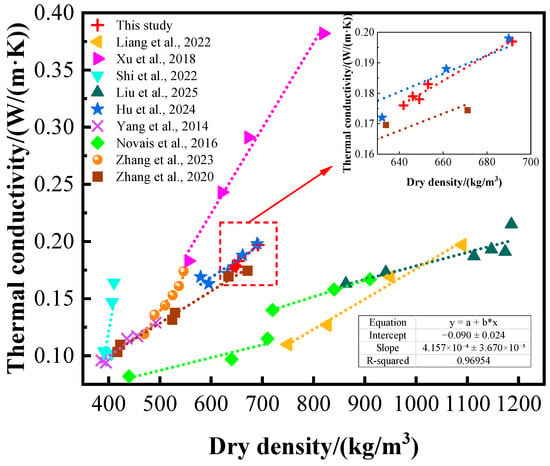
Figure 12.
Relationship between density and thermal conductivity (comparison between this study and the relevant literature). In which, Liang et al. [20], Xu et al. [23], Shi et al. [26], Liu et al. [43], Hu et al. [45], Yang et al. [49], Novais et al. [50], Zhang et al. [51], Zhang et al. [52].
3.5. Water Absorption and Softening Coefficient
As illustrated in Figure 13, an augmentation in LZT content gives rise to contrasting trends in the water absorption rate and softening coefficient of GFC. Specifically, the water absorption rate exhibited an increase that was correlated with rising LZT content, with the rate showing marked acceleration. The water absorption rate of foam concrete is indicative of the interconnected/open pores within the material [19,53]. When LZT content ranges between 30% and 50%, pores in GFC material are predominantly closed pores, thereby effectively impeding pathways for external water penetration. However, the presence of excessive LZT content has been demonstrated to induce degradation of the pore structure, resulting in an augmented number of interconnected and large pores within the GFC. This change subsequently leads to a substantial augmentation in the rate of water absorption.
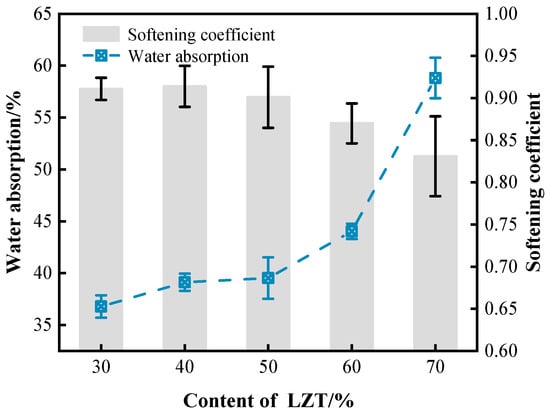
Figure 13.
Effect of LZT content on water absorption and softening coefficient of GFC.
The softening coefficient is a critical indicator of the water resistance of foam concrete materials. The softening coefficients of GFC materials were found to vary with different LZT content levels, exhibiting values of 0.911, 0.914, 0.901, 0.870, and 0.831, respectively. The disparities in water absorption rates were the underlying cause of the variations in softening coefficients. Furthermore, the softening coefficients of all GFC groups are relatively high, indicating that the material possesses strong resistance to water erosion. This phenomenon is primarily attributed to the dense gel network formed by the geopolymer gel in GFC and the micro-aggregate filling effect of LZT, which serves to minimize the impact of water erosion on the material [54,55].
3.6. Drying Shrinkage
Figure 14 displays the drying shrinkage of GFC as a function of LZT content. During the initial phases of specimen curing, the drying shrinkage of GFC undergoes rapid development. This phenomenon can be attributed to the predominant occurrence of dissolution and condensation reactions of the silicon-aluminum precursor raw materials in an alkaline environment during the initial stages of the process. This results in elevated water consumption, attributable to the progression of geopolymerization reactions [56,57]. The phenomenon of drying shrinkage principally entails the contraction of gel pores and capillaries, culminating in a decline in the water saturation of the internal capillaries as moisture is lost to the external environment. As the LZT content increased from 30% to 70%, the drying shrinkage of GFC persisted in its escalation, with the 56-day drying shrinkage rate rising from 2439 × 10−6 to 5613 × 10−6. On one hand, the larger average particle size of LZT relative to MK results in a smaller specific surface area, which leads to a reduced capacity for water adsorption. Consequently, more water remains in the system, awaiting evaporation and loss. On the other hand, an excess of LZT content has been shown to increase the number of interconnected pores within the GFC, thereby providing direct pathways for moisture migration. Additionally, an increase in LZT content results in a reduction in GFC strength, consequently diminishing its capacity to resist shrinkage stress. In comparison with the findings of other studies [26,58], the drying shrinkage of the specimens in this study was marginally higher. However, certain scholars have achieved reduced drying shrinkage or enhanced crack resistance by incorporating fibers such as polypropylene [59], polyvinyl alcohol [29], and basalt fibers [60] into the material. Therefore, future studies can optimize the mix ratio of this experiment by incorporating fibers to reduce the drying shrinkage rate of GFC.
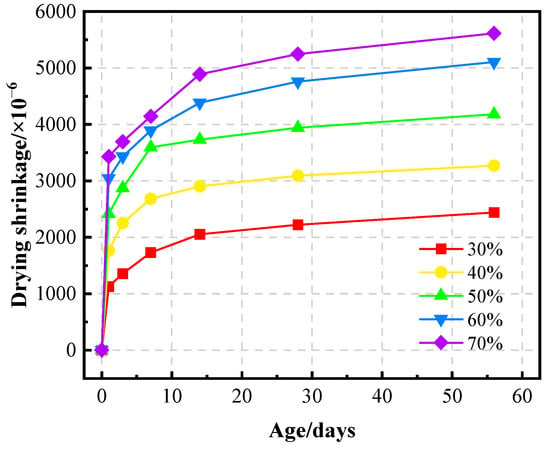
Figure 14.
Drying shrinkage of GFC samples with various content of LZT.
3.7. XRD and SEM Analysis
In accordance with the preceding analysis of the pore structure and physical-mechanical properties of GFC, the optimal dosage of LZT was ascertained to be 40%. The prepared samples were then cured for 28 days and subsequently subjected to XRD and SEM testing. The XRD patterns of the GFC samples are displayed in Figure 15. It has been observed that hydration products, including C–S–H gel and silicoaluminate minerals (tetragonal sodium zeolite), have formed. Furthermore, a broad, amorphous diffuse peak emerges in the 20–40° diffraction angle range, aligning with the diffraction peak characteristics of geopolymers [11,61]. This signifies the formation of amorphous silicoaluminate gel. Concurrently, the presence of well-crystallized, strongly reflective phase peaks of calcium iron garnet was observed. In addition, sharp characteristic peaks indicative of quartz crystals were identified. This phenomenon can be attributed to the relatively low activity of this component in LZT, which hinders its complete dissolution in an alkaline environment. Consequently, the majority of the component retains its crystalline structure. However, these minerals form a rigid framework with high strength, tightly bonded to the gel-like products, thereby enhancing the material’s mechanical properties.
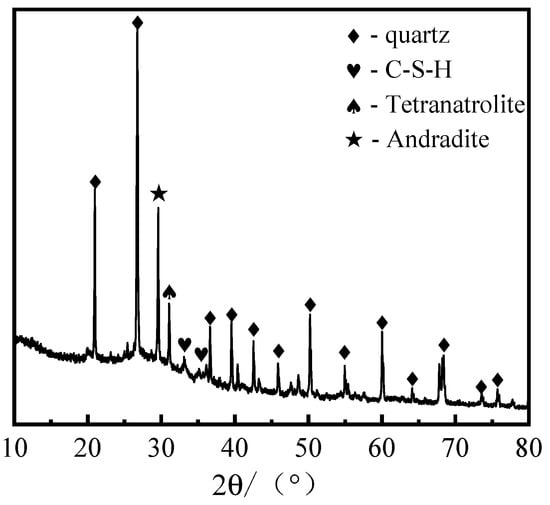
Figure 15.
XRD spectrum of sample cured for 28 days.
Figure 16 presents scanning electron microscopy (SEM) images of GFC. In Figure 16a, as the geopolymerization and hydration reactions progressed, a structurally dense gel phase was formed, primarily composed of amorphous sodium aluminum silicate gel (N–A–S–H gel) and flake-like C–S–H gel. The silica–alumina precursor is dissolved under the action of an alkaline activator. The active silica and alumina tetrahedra undergo geopolymerization, resulting in the formation of N–A–S–H gel. The formation of C–S–H gel is a consequence of hydration reactions between the silica tetrahedra within the system and CaO from LZT and GGBS. Furthermore, the figure indicates the presence of unreacted tailings particles, which correspond to the quartz phase diffraction peaks observed in the XRD spectrum. The micro-aggregate filling effect of LZT is responsible for the formation of the mineral skeleton of the GFC. And the gelatinous products exhibit a high degree of adhesion to the tailing particles [62], thereby forming a dense and stable microstructure. As shown in Figure 16b, amorphous N–A–S–H gel is observed to extensively coat the pore surfaces, resembling a thick protective layer. This process has been shown to enhance the stability and strength of the pore structure. Figure 16c displays that the pores in GFC predominantly manifest as closed pores, exhibiting shapes that approximate regular spherical forms. The presence of microcracks on the pore surfaces was observed, primarily due to damage incurred during the sample preparation process for SEM testing. Additionally, the presence of microcracks may be attributed to insufficient bonding or encapsulation of certain tailings particles by the gel products [43,63]. This phenomenon results in the initiation and subsequent expansion of the microcracks.
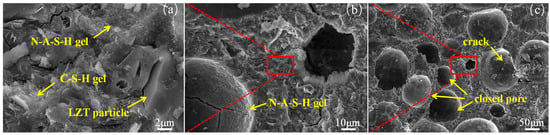
Figure 16.
SEM images of samples at different magnifications. (a) is the image obtained by zooming in on the red boxed area in (b), and similarly, (b) is the image obtained by zooming in on the red boxed area in (c).
4. Conclusions
This study innovatively utilized LZT to prepare geopolymer foam concrete (GFC), incorporating MK and GGBS as silicon–aluminum-based supplementary materials, and using H2O2 as a foaming agent, successfully producing GFC with excellent performance across all parameters. A thorough investigation was undertaken to ascertain the impact of varying LZT concentrations on the pore structure and macroscopic properties of GFC. Through microscopic pore structure analysis, the intrinsic mechanisms that give rise to these variations in macroscopic properties were elucidated. Based on the experimental results and analysis, the following main conclusions were drawn:
- The use of LZT for the preparation of GFC holds significant potential and research value. This investigation proposes a feasible scheme for the recycling and reuse of LZT. Furthermore, it provides a reference and further research directions for the preparation of foam concrete from such industrial solid waste.
- Increases in the LZT content result in a gradual rise in the average pore size and porosity of GFC, while the average roundness value initially declines and subsequently rises. At 40% and 50% LZT contents, the pore distribution of GFC is most uniform, with relatively small average roundness values, and most pores are closer to spherical in shape, exhibiting good geometric compactness.
- As the LZT content increases, the flowability, water absorption, and drying shrinkage of GFC gradually increase, while the dry density first decreases and then increases. Appropriately increasing the LZT content has a positive effect on the compressive strength of GFC. At a tailings’ content of 40%, the material’s compressive strength at 28 days reaches its maximum value of 6.50 MPa.
- A comparison of this GFC with foam concrete prepared in other studies reveals that the former possesses not only the characteristics of light weight and high strength, but also ideal thermal insulation properties. The thermal conductivity (K) of GFC is found to be 0.176 W/(m·K) when the optimal LZT content is utilized. This material can effectively reduce building energy consumption, indicating that it has promising application prospects in the field of building insulation.
- The silicon-aluminum precursor material was subjected to an alkaline activator, resulting in the formation of N–A–S–H gel and C–S–H gel. The gel products exhibited a strong bond with the unreacted tailings particles, and the interfaces between different phases were firmly bonded. This resulted in a microstructure with high compactness, which had a positive effect on the development of material strength.
- However, this study still has room for further exploration. The complex service environment places high demands on the durability of GFC, such as freeze resistance and dry–wet cycle performance, which require further in-depth research. Additionally, by further adjusting the amount of foaming agent, foaming method, and curing conditions, GFC with more diverse performance can be prepared.
Author Contributions
Methodology, Y.Y., M.L., and Q.H.; software, Y.Y. and C.L.; validation, Y.Y., M.L., and Q.H.; formal analysis, Y.Y. and M.L.; investigation, Y.Y., M.L., Q.H., and C.L.; resources, M.L. and Q.H.; data curation, Y.Y. and Q.H.; writing—original draft, Y.Y.; writing—review and editing, Y.Y., M.L., Q.H., and C.L.; supervision, M.L. and Q.H.; funding acquisition, Q.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Project 23A0334, supported by the Key Scientific Research Project of the Department of Education of Hunan Province.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that might influence the work reported here.
References
- Maruthupandian, S.; Chaliasou, A.; Kanellopoulos, A. Recycling mine tailings as precursors for cementitious binders-Methods, challenges and future outlook. Constr. Build. Mater. 2021, 312, 125333. [Google Scholar] [CrossRef]
- Marín, O.A.; Kraslawski, A.; Cisternas, L.A. Estimating processing cost for the recovery of valuable elements from mine tailings using dimensional analysis. Miner. Eng. 2022, 184, 107629. [Google Scholar] [CrossRef]
- Krishna, R.S.; Shaikh, F.; Mishra, J.; Lazorenko, G.; Kasprzhitskii, A. Mine tailings-based geopolymers: Properties, applications and industrial prospects. Ceram. Int. 2021, 47, 17826–17843. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Deng, R.; Xing, D.; Chen, Q. Research status and significance of comprehensive utilization of lead and zinc tailings in China. Geol. Explor. 2024, 60, 724–734, (In Chinese with English abstract). [Google Scholar]
- Li, R.; Yin, Z.; Lin, H. Research Status and Prospects for the Utilization of Lead–Zinc Tailings as Building Materials. Buildings 2023, 13, 150. [Google Scholar] [CrossRef]
- Dong, L.; Tong, X.; Li, X.; Zhou, J.; Wang, S.; Liu, B. Some developments and new insights of environmental problems and deep mining strategy for cleaner production in mines. J. Clean. Prod. 2019, 210, 1562–1578. [Google Scholar] [CrossRef]
- Li, D.; Andrea, O.R.; Alseny, B.; Li, F. Valorization of lead-zinc mine tailing waste through geopolymerization: Synthesis, mechanical, and microstructural properties. J. Environ. Manag. 2024, 349, 119501. [Google Scholar] [CrossRef]
- Luo, Z.; Guo, J.; Liu, X.; Mu, Y.; Zhang, M.; Zhang, M.; Tian, C.; Ou, J.; Mi, J. Preparation of ceramsite from lead-zinc tailings and coal gangue: Physical properties and solidification of heavy metals. Constr. Build. Mater. 2023, 368, 130426. [Google Scholar] [CrossRef]
- Ou, X.; Guo, Y.; Zhong, G.; Li, B.; Chen, Y.; Cao, X. Manufacture of the Glass-Ceramics from the Lead-Zinc Tailings by Sintering. Adv. Mater. Res. 2014, 955–959, 2818–2823. [Google Scholar] [CrossRef]
- Lin, H.; Li, R.; Li, S. Fabrication of Lead–Zinc Tailings Sintered Brick and Its Effect Factors Based on an Orthogonal Experiment. Materials 2024, 17, 2352. [Google Scholar] [CrossRef]
- Bah, A.; Feng, D.; Kedjanyi, E.A.G.; Shen, Z.; Bah, A.; Li, F. Solidification of (Pb–Zn) mine tailings by fly ash-based geopolymer I: Influence of alkali reagents ratio and curing condition on compressive strength. J. Mater. Cycles Waste Manag. 2022, 24, 351–363. [Google Scholar] [CrossRef]
- Shah, S.N.; Mo, K.H.; Yap, S.P.; Yang, J.; Ling, T.-C. Lightweight foamed concrete as a promising avenue for incorporating waste materials: A review. Resour. Conserv. Recycl. 2021, 164, 105103. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, X.; Wang, L.; Li, Y. Foam Concrete: A State-of-the-Art and State-of-the-Practice Review. Adv. Mater. Sci. Eng. 2020, 2020, 1–25. [Google Scholar] [CrossRef]
- Hassan, A.; Arif, M.; Shariq, M. Use of geopolymer concrete for a cleaner and sustainable environment—A review of mechanical properties and microstructure. J. Clean. Prod. 2019, 223, 704–728. [Google Scholar] [CrossRef]
- Hwalla, J.; El-hassan, H.; Assaad, J.J.; El-maaddawy, T. Performance of cementitious and slag-fly ash blended geopolymer screed composites: A comparative study. Case Stud. Constr. Mater. 2023, 18, e02037. [Google Scholar] [CrossRef]
- Yang, H.; Liu, L.; Yang, W.; Liu, H.; Ahmad, W.; Ahmad, A.; Aslam, F.; Joyklad, P. A comprehensive overview of geopolymer composites: A bibliometric analysis and literature review. Case Stud. Constr. Mater. 2022, 16, e00830. [Google Scholar] [CrossRef]
- Dhasindrakrishna, D.K.; Pasupathy, K.; Ramakrishnan, S.; Sanjayan, J.G. Progress, current thinking and challenges in geopolymer foam concrete technology. Cem. Concr. Compos. 2021, 116, 103886. [Google Scholar] [CrossRef]
- Kočí, V.; Černý, R. Directly foamed geopolymers: A review of recent studies. Cem. Concr. Compos. 2022, 130, 104530. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Mechanical, thermal insulation, thermal resistance and acoustic absorption properties of geopolymer foam concrete. Cem. Concr. Compos. 2015, 62, 97–105. [Google Scholar] [CrossRef]
- Liang, G.; Liu, T.; Li, H.; Dong, B.; Shi, T. A novel synthesis of lightweight and high-strength green geopolymer foamed material by rice husk ash and ground-granulated blast-furnace slag. Resour. Conserv. Recycl. 2022, 176, 105922. [Google Scholar] [CrossRef]
- Samson, G.; Cyr, M.; Gao, X.X. Thermomechanical performance of blended metakaolin GGBS alkali-activated foam concrete. Constr. Build. Mater. 2017, 157, 982–993. [Google Scholar] [CrossRef]
- Raj, S.; Ramamurthy, K. Physical, hydrolytic, and mechanical stability of alkali-activated fly ash-slag foam concrete. Cem. Concr. Compos. 2023, 142, 105223. [Google Scholar] [CrossRef]
- Xu, F.; Gu, G.; Zhang, W.; Wang, H.; Huang, X.; Zhu, J. Pore structure analysis and properties evaluations of fly ash-based geopolymer foams by chemical foaming method. Ceram. Int. 2018, 44, 19989–19997. [Google Scholar] [CrossRef]
- Ziejewska, C.; Grela, A.; Hebda, M. Influence of Waste Glass Particle Size on the Physico-Mechanical Properties and Porosity of Foamed Geopolymer Composites Based on Coal Fly Ash. Materials 2023, 16, 2044. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Zou, S.; Zhang, G. Experimental research on a feasible rice husk/geopolymer foam building insulation material. Energy Build. 2020, 226, 110358. [Google Scholar] [CrossRef]
- Shi, J.; Liu, Y.; Wang, E.; Wang, L.; Li, C.; Xu, H.; Zheng, X.; Yuan, Q. Physico-mechanical, thermal properties and durability of foamed geopolymer concrete containing cenospheres. Constr. Build. Mater. 2022, 325, 126841. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, T.; Li, C.; Wan, M.; Xuan, W.; Wang, X. Effect of Precursor Blending Ratio and Rotation Speed of Mechanically Activated Fly Ash on Properties of Geopolymer Foam Concrete. Buildings 2024, 14, 841. [Google Scholar] [CrossRef]
- Kamseu, E.; Ngouloure, Z.N.M.; Ali, B.N.; Zekeng, S.; Melo, U.C.; Rossignol, S.; Leonelli, C. Cumulative pore volume, pore size distribution and phases percolation in porous inorganic polymer composites: Relation microstructure and effective thermal conductivity. Energy Build. 2015, 88, 45–56. [Google Scholar] [CrossRef]
- Dhasindrakrishna, K.; Pasupathy, K.; Ramakrishnan, S.; Sanjayan, J. Rheology and elevated temperature performance of geopolymer foam concrete with varying PVA fibre dosage. Mater. Lett. 2022, 328, 133122. [Google Scholar] [CrossRef]
- Łach, M.; Kozub, B.; Bednarz, S.; Bąk, A.; Melnychuk, M.; Masłoń, A. The Influence of the Addition of Basalt Powder on the Properties of Foamed Geopolymers. Materials 2024, 17, 2336. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Li, X.; Tian, D.; Ma, M.; Wang, T. Optimized pore structure and high permeability of metakaolin/fly-ash-based geopolymer foams from Al– and H2O2–sodium oleate foaming systems. Ceram. Int. 2022, 48, 18348–18360. [Google Scholar] [CrossRef]
- Ahıskalı, A.; Ahıskalı, M.; Bayraktar, O.Y.; Kaplan, G.; Assaad, J. Mechanical and durability properties of polymer fiber reinforced one-part foam geopolymer concrete: A sustainable strategy for the recycling of waste steel slag aggregate and fly ash. Constr. Build. Mater. 2024, 440, 137492. [Google Scholar] [CrossRef]
- Wei, B.; Zhang, Y.; Bao, S. Preparation of geopolymers from vanadium tailings by mechanical activation. Constr. Build. Mater. 2017, 145, 236–242. [Google Scholar] [CrossRef]
- He, X.; Yuhua, Z.; Qaidi, S.; Isleem, H.F.; Zaid, O.; Althoey, F.; Ahmad, J. Mine tailings-based geopolymers: A comprehensive review. Ceram. Int. 2022, 48, 24192–24212. [Google Scholar] [CrossRef]
- Lazorenko, G.; Kasprzhitskii, A.; Shaikh, F.; Krishna, R.S.; Mishra, J. Utilization potential of mine tailings in geopolymers: Physicochemical and environmental aspects. Process Saf. Environ. Prot. 2021, 147, 559–577. [Google Scholar] [CrossRef]
- Ahmari, S.; Zhang, L. Durability and leaching behavior of mine tailings-based geopolymer bricks. Constr. Build. Mater. 2013, 44, 743–750. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, D.; Zhao, J.; Li, D.; Ng, S.; Rui, Y. Effect of calcium stearate based foam stabilizer on pore characteristics and thermal conductivity of geopolymer foam material. J. Build. Eng. 2018, 20, 21–29. [Google Scholar] [CrossRef]
- Yan, S.; Ren, X.; He, C.; Wang, W.; Zhang, M.; Xing, P. Microstructure evolution and properties of red mud/slag-based cenosphere/geopolymer foam exposed to high temperatures. Ceram. Int. 2023, 49, 34362–34374. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Li, X.; Ma, M.; Zhang, Z.; Ji, X. Slurry rheological behaviors and effects on the pore evolution of fly ash/metakaolin-based geopolymer foams in chemical foaming system with high foam content. Constr. Build. Mater. 2023, 379, 131259. [Google Scholar] [CrossRef]
- JG/T 266-2011; Foamed Concrete. Standards Press of China: Beijing, China, 2011.
- JGJ/T 341-2014; Technical Specification for Application of Foamed Concrete. China Building Industry Press: Beijing, China, 2014.
- GB/T 10294-2008; Thermal Insulation Determination of Steady State Thermal Resistance and Related Properties Guarded Hot Plate Apparatus. Standards Press of China: Beijing, China, 2008.
- Liu, Y.; Hu, N.; Yang, S.; Ye, Y.; Li, Q.; Tang, R.; Wu, Y. The hydration behavior and foaming mechanism of foamed concrete prepared by substantial amounts of phosphate tailings. Constr. Build. Mater. 2025, 472, 140799. [Google Scholar] [CrossRef]
- Xiao, L.G.; Liu, C.; Zhang, S.T.; Wang, W.B. Influences of admixtures on properties of foam concrete with iron tailings. Key Eng. Mater. 2014, 599, 61–65. [Google Scholar] [CrossRef]
- Hu, B.; Fu, C.; Li, S. Preparation and properties of high performance foamed concrete. J. Funct. Mater. 2024, 55, 2181–2186, (In Chinese with English abstract). [Google Scholar]
- Chen, X.; Zhang, J.; Lu, M.; Chen, B.; Gao, S.; Bai, J.; Yang, Y. Study on the effect of calcium and sulfur content on the properties of fly ash based geopolymer. Constr. Build. Mater. 2022, 314, 125650. [Google Scholar] [CrossRef]
- He, P.; Wang, M.; Fu, S.; Jia, D.; Zhou, Y. Effects of Si/Al ratio on the structure and properties of metakaolin based geopolymer. Ceram. Int. 2016, 42, 14416–14422. [Google Scholar] [CrossRef]
- Ji, Y.; Ren, Q.; Li, X.; Zhao, P.; Vandeginste, V. On Thermal Insulation Properties of Various Foaming Materials Modified Fly Ash Based Geopolymers. Polymers 2023, 15, 3254. [Google Scholar] [CrossRef]
- Yang, K.; Lee, K.; Song, J.; Gong, M. Properties and sustainability of alkali-activated slag foamed concrete. J. Clean. Prod. 2014, 68, 226–233. [Google Scholar] [CrossRef]
- Novais, R.M.; Ascensão, G.; Buruberri, L.H.; Senff, L.; Labrincha, J.A. Influence of blowing agent on the fresh- and hardened-state properties of lightweight geopolymers. Mater. Des. 2016, 108, 551–559. [Google Scholar] [CrossRef]
- Zhang, D.; Hao, W.; Yang, Q. Experimental Study on the Application of Recycled Concrete Waste Powder in Alkali-Activated Foamed Concrete. Materials 2023, 16, 5728. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H. Preparation and properties of rice husk ash foamed concrete. Bull. Silic. 2020, 39, 2795–2799, (In Chinese with English abstract). [Google Scholar]
- Zhao, Y.; Ye, J.; Lu, X.; Liu, M.; Lin, Y.; Gong, W.; Ning, G. Preparation of sintered foam materials by alkali-activated coal fly ash. J. Hazard. Mater. 2010, 174, 108–112. [Google Scholar] [CrossRef]
- Bilici, S.; Carvalheiras, J.; Labrincha, J.A.; Novais, R.M. Evaluation of the Nature and Concentration of the Surfactant on the Properties of Red Mud/Metakaolin Porous Geopolymers Foamed with Aluminium. Materials 2022, 15, 7486. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.; Chen, S.; Liu, Y.; Yan, D.; Sun, Z. Investigation on the effect of fiber wettability on water absorption kinetics of geopolymer composites. Ceram. Int. 2022, 48, 36678–36689. [Google Scholar] [CrossRef]
- Chen, W.; Li, B.; Wang, J.; Thom, N. Effects of alkali dosage and silicate modulus on autogenous shrinkage of alkali-activated slag cement paste. Cem. Concr. Res. 2021, 141, 106322. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, H.; Feng, P.; Zhang, P. A review on shrinkage-reducing methods and mechanisms of alkali-activated/geopolymer systems: Effects of chemical additives. J. Build. Eng. 2022, 49, 104056. [Google Scholar] [CrossRef]
- Zhang, M.; Qiu, X.; Shen, S.; Wang, L.; Zang, Y. Mechanical and Thermal Insulation Properties of rGFRP Fiber-Reinforced Lightweight Fly-Ash-Slag-Based Geopolymer Mortar. Sustainability 2023, 15, 7200. [Google Scholar] [CrossRef]
- Agustini, N.K.A.; Triwiyono, A.; Sulistyo, D.; Suyitno, S. Mechanical Properties and Thermal Conductivity of Fly Ash-Based Geopolymer Foams with Polypropylene Fibers. Appl. Sci. 2021, 11, 4886. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Hu, Y.; Li, Y.; Hu, P.; Zhao, Y. Physical and high temperature properties of basalt fiber-reinforced geopolymer foam with hollow microspheres. Constr. Build. Mater. 2024, 411, 134698. [Google Scholar] [CrossRef]
- Dai, B.-B.; Zou, Y.; He, Y.; Lan, M.; Kang, Q. Solidification Experiment of Lithium-Slag and Fine-Tailings Based Geopolymers. Sustainability 2023, 15, 4523. [Google Scholar] [CrossRef]
- Lemougna, P.N.; Adediran, A.; Yliniemi, J.; Ismailov, A.; Levanen, E.; Tanskanen, P.; Kinnunen, P.; Roning, J.; Illikainen, M. Thermal stability of one-part metakaolin geopolymer composites containing high volume of spodumene tailings and glass wool. Cem. Concr. Compos. 2020, 114, 103792. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Effect of GGBFS on setting, workability and early strength properties of fly ash geopolymer concrete cured in ambient condition. Constr. Build. Mater. 2014, 66, 163–171. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).