Review of Treatment Techniques for Dredged Sediments in the Context of Valorization as Secondary Raw Materials
Abstract
1. Introduction
Utilization of Sediments
2. Research Methodology
“dredged sediment”, “sediment treatment”, “sediment reuse”, “sediment remediation”, “dredged material management”, and “construction applications of sediments”.
- Studies focused on treatment technologies (physical, chemical, biological, or combined) for dredged or water sediments.
- Research addressing the reuse potential of treated sediments (e.g., construction material, land reclamation, and soil amendment).
- Papers providing experimental data, field application results, or systematic evaluations of treatment methods.
Limitations of the Research Methodology
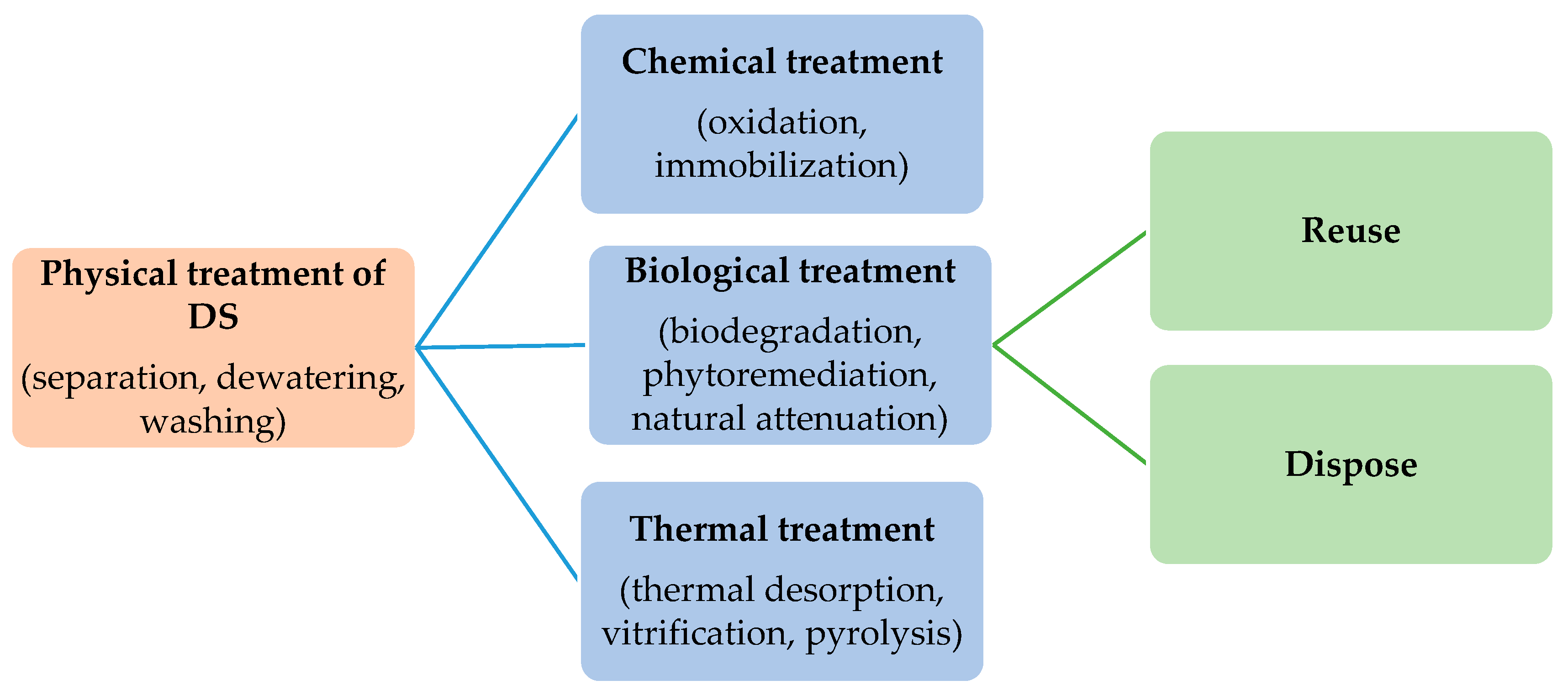
3. Treatment Techniques
3.1. Physical Treatment Process
3.1.1. Separation
3.1.2. Dewatering
3.1.3. Washing
3.2. Chemical Treatment Process
3.2.1. Chemical Oxidation
3.2.2. Immobilization
3.3. Biological Treatment Process
3.3.1. Biodegradation
3.3.2. Phytoremediation
3.3.3. Natural Attenuation
3.4. Thermal Treatment Process
3.4.1. Thermal Desorption (TD)
3.4.2. Vitrification
3.4.3. Pyrolysis
3.5. Artificial Intelligence Utilization in Sediment Remediation
4. Conclusions and Recommendations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Finch, G.; Marriage, G.; Pelosi, A.; Gjerde, M. Building envelope systems for the circular economy; Evaluation parameters, current performance and key challenges. Sustain. Cities Soc. 2021, 64, 9. [Google Scholar] [CrossRef]
- Iddio, E.; Wang, L.; Thomas, Y.; McMorrow, G.; Denzer, A. Energy efficient operation and modeling for greenhouses: A literature review. Renew. Sustain. Energy Rev. 2020, 117, 15. [Google Scholar] [CrossRef]
- Huo, T.F.; Ma, Y.L.; Yu, T.; Cai, W.G.; Liu, B.S.; Ren, H. Decoupling and decomposition analysis of residential building carbon emissions from residential income: Evidence from the provincial level in China. Environ. Impact Assess. Rev. 2021, 86, 12. [Google Scholar] [CrossRef]
- Chen, M.P.; Zhang, L.; Teng, F.; Dai, J.J.; Li, Z.; Wang, Z.Q.; Li, Y.T. Climate technology transfer in BRI era: Needs, priorities, and barriers from receivers’ perspective. Ecosyst. Health Sustain. 2020, 6, 1780948. [Google Scholar] [CrossRef]
- Shmlls, M.; Abed, M.; Fort, J.; Horvath, T.; Bozsaky, D. Towards closed-loop concrete recycling: Life cycle assessment and multi-criteria analysis. J. Clean. Prod. 2023, 410, 137179. [Google Scholar] [CrossRef]
- Martin, C.; Manu, E.; Hou, P.K.; Adu-Amankwah, S. Circular economy, data analytics, and low carbon concreting: A case for managing recycled powder from end-of-life concrete. Resour. Conserv. Recycl. 2023, 198, 107197. [Google Scholar] [CrossRef]
- Shi, C.; Zheng, K. A review on the use of waste glasses in the production of cement and concrete. Resour. Conserv. Recycl. 2007, 52, 234–247. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Ding, Y.N.; Jalali, S. Properties and durability of concrete containing polymeric wastes (tyre rubber and polyethylene terephthalate bottles): An overview. Constr. Build. Mater. 2012, 30, 714–724. [Google Scholar] [CrossRef]
- Tang, Q.; Ma, Z.M.; Wu, H.X.; Wang, W. The utilization of eco-friendly recycled powder from concrete and brick waste in new concrete: A critical review. Cem. Concr. Compos. 2020, 114, 103807. [Google Scholar] [CrossRef]
- Silva, R.; de Brito, J.; Dhir, R. Establishing a relationship between modulus of elasticity and compressive strength of recycled aggregate concrete. J. Clean. Prod. 2016, 112, 2171–2186. [Google Scholar] [CrossRef]
- Ferdous, W.; Manalo, A.; Siddique, R.; Mendis, P.; Yan, Z.G.; Wong, H.S.; Lokuge, W.; Aravinthan, T.; Schubel, P. Recycling of landfill wastes (tyres, plastics and glass) in construction—A review on global waste generation, performance, application and future opportunities. Resour. Conserv. Recycl. 2021, 173, 13. [Google Scholar] [CrossRef]
- Mo, K.H.; Alengaram, U.J.; Jumaat, M.Z.; Lee, S.C.; Goh, W.I.; Yuen, C.W. Recycling of seashell waste in concrete: A review. Constr. Build. Mater. 2018, 162, 751–764. [Google Scholar] [CrossRef]
- Almeshal, I.; Tayeh, B.A.; Alyousef, R.; Alabduljabbar, H.; Mohamed, A.M.; Alaskar, A. Use of recycled plastic as fine aggregate in cementitious composites: A review. Constr. Build. Mater. 2020, 253, 119146. [Google Scholar] [CrossRef]
- Siddique, R.; Singh, M.; Jain, M. Recycling copper slag in steel fibre concrete for sustainable construction. J. Clean. Prod. 2020, 271, 122559. [Google Scholar] [CrossRef]
- Fort, J.; Mildner, M.; Keppert, M.; Pommer, V.; Cerny, R. Experimental and Environmental Analysis of High-Strength Geopolymer Based on Waste Bricks and Blast Furnace Slag. Polymers 2023, 15, 3092. [Google Scholar] [CrossRef]
- Thomas, B.; Yang, J.; Mo, K.; Abdalla, J.; Hawileh, R.; Ariyachandra, E. Biomass ashes from agricultural wastes as supplementary cementitious materials or aggregate replacement in cement/geopolymer concrete: A comprehensive review. J. Build. Eng. 2021, 40, 102332. [Google Scholar] [CrossRef]
- Qian, L.P.; Xu, L.Y.; Alrefaei, Y.; Wang, T.; Ishida, T.; Dai, J.G. Artificial alkali-activated aggregates developed from wastes and by-products: A state-of-the-art review. Resour. Conserv. Recycl. 2022, 177, 105971. [Google Scholar] [CrossRef]
- Matos, A.M.; Sousa-Coutinho, J. Municipal solid waste incineration bottom ash recycling in concrete: Preliminary approach with Oporto wastes. Constr. Build. Mater. 2022, 323, 126548. [Google Scholar] [CrossRef]
- Yang, H.; Feng, Q.; Zhu, J.; Liu, G.; Dai, Y.; Zhou, Q.; Xia, S.; Wu, Z.; Zhang, Y. Towards sustainable futures: A review of sediment remediation and resource valorization techniques. J. Clean. Prod. 2024, 435, 140529. [Google Scholar] [CrossRef]
- Zhou, A.; Li, K.X.; Liu, T.J.; Zou, D.J.; Peng, X.; Lyu, H.X.; Xiao, J.D.; Luan, C.C. Recycling and optimum utilization of engineering sediment waste into low-carbon geopolymer paste for sustainable infrastructure. J. Clean. Prod. 2023, 383, 135549. [Google Scholar] [CrossRef]
- Baksa, P.; Cepak, F.; Lukman, R.K.; Ducman, V. An Evaluation of Marine Sediments in Terms of their usability in the Brick Industry: Case Study Port of Koper. J. Sustain. Dev. Energy Water Environ. Syst.-Jsdewes 2018, 6, 78–88. [Google Scholar] [CrossRef]
- Adongo, T.; Kyei-Baffour, N.; Abagale, F.; Agyare, W. Assessment of reservoir sedimentation of irrigation dams in northern Ghana. Lake Reserv. Manag. 2020, 36, 87–105. [Google Scholar] [CrossRef]
- Aldrees, A.; Taha, A.T.B.; Mohamed, A.M. Prediction of sustainable management of sediment in rivers and reservoirs. Chemosphere 2022, 309, 136369. [Google Scholar] [CrossRef] [PubMed]
- Albarano, L.; Costantini, M.; Zupo, V.; Lofrano, G.; Guida, M.; Libralato, G. Marine sediment toxicity: A focus on micro- and mesocosms towards remediation. Sci. Total Environ. 2020, 708, 134837. [Google Scholar] [CrossRef]
- Jeong, H.; Choi, J.Y.; Lim, J.; Shim, W.J.; Kim, Y.O.; Ra, K. Characterization of the contribution of road deposited sediments to the contamination of the close marine environment with trace metals: Case of the port city of Busan (South Korea). Mar. Pollut. Bull. 2020, 161, 111717. [Google Scholar] [CrossRef]
- Polrot, A.; Kirby, J.R.; Birkett, J.W.; Sharples, G.P. Combining sediment management and bioremediation in muddy ports and harbours: A review. Environ. Pollut. 2021, 289, 117853. [Google Scholar] [CrossRef]
- Crocetti, P.; Gonzalez-Camejo, J.; Li, K.; Foglia, A.; Eusebi, A.L.; Fatone, F. An overview of operations and processes for circular management of dredged sediments. Waste Manag. 2022, 146, 20–35. [Google Scholar] [CrossRef]
- Zhang, J.L.; Shang, Y.Z.; Liu, J.Y.; Lu, J.; Wei, S.T.; Wan, Z.W.; Luo, Q.S.; Chen, C.X.; Tong, L.; Wang, Q.; et al. Optimisation of reservoir operation mode to improve sediment transport capacity of silt-laden rivers. J. Hydrol. 2021, 594, 15. [Google Scholar] [CrossRef]
- Junakova, N.; Junak, J. Sustainable Use of Reservoir Sediment through Partial Application in Building Material. Sustainability 2017, 9, 852. [Google Scholar] [CrossRef]
- Martellotta, A.; Levacher, D.; Gentile, F.; Piccinni, A. Estimation of Silting Evolution in the Camastra Reservoir and Proposals for Sediment Recovery. J. Mar. Sci. Eng. 2024, 12, 250. [Google Scholar] [CrossRef]
- Akcil, A.; Erust, C.; Ozdemiroglu, S.; Fonti, V.; Beolchini, F. A review of approaches and techniques used in aquatic contaminated sediments: Metal removal and stabilization by chemical and biotechnological processes. J. Clean. Prod. 2015, 86, 24–36. [Google Scholar] [CrossRef]
- Debnath, A.; Singh, P.K.; Sharma, Y.C. Metallic contamination of global river sediments and latest developments for their remediation. J. Environ. Manag. 2021, 298, 113378. [Google Scholar] [CrossRef] [PubMed]
- Bortali, M.; Rabouli, M.; Yessari, M.; Hajjaji, A. Assessment of harbor sediment contamination for a path to valorize dredged material. Arab. J. Chem. 2023, 16, 105208. [Google Scholar] [CrossRef]
- De Gisi, S.; Todaro, F.; Mesto, E.; Schingaro, E.; Notarnicola, M. Recycling contaminated marine sediments as filling materials by pilot scale stabilization/solidification with lime, organoclay and activated carbon. J. Clean. Prod. 2020, 269, 122416. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Tsang, D.C.W.; Li, J.S.; Baek, K.; Hou, D.Y.; Ding, S.M.; Poon, C.S. Recycling dredged sediment into fill materials, partition blocks, and paving blocks: Technical and economic assessment. J. Clean. Prod. 2018, 199, 69–76. [Google Scholar] [CrossRef]
- Palaparthi, J.; Briggs, T.R.; Kali, P.K.; Comas, X. Evaluating offshore sediment resources for non-traditional coastal restoration projects. Ocean. Coast. Manag. 2022, 220, 106074. [Google Scholar] [CrossRef]
- Capasso, I.; Liguori, B.; Ferone, C.; Caputo, D.; Cioffi, R. Strategies for the valorization of soil waste by geopolymer production: An overview. J. Clean. Prod. 2021, 288, 125646. [Google Scholar] [CrossRef]
- Norén, A.; Fedje, K.K.; Strömvall, A.M.; Rauch, S.; Andersson-Sköld, Y. Integrated assessment of management strategies for metal-contaminated dredged sediments—What are the best approaches for ports, marinas and waterways? Sci. Total Environ. 2020, 716, 135510. [Google Scholar] [CrossRef]
- Mehdizadeh, H.; Guo, M.Z.; Ling, T.C. Ultra-fine sediment of Changjiang estuary as binder replacement in self-compacting mortar: Rheological, hydration and hardened properties. J. Build. Eng. 2021, 44, 103251. [Google Scholar] [CrossRef]
- Ferrans, L.; Schmieder, F.; Mugwira, R.; Marques, M.; Hogland, W. Dredged sediments as a plant-growing substrate: Estimation of health risk index. Sci. Total Environ. 2022, 846, 157463. [Google Scholar] [CrossRef]
- Brigham, R.D.; Pelini, S.; Xu, Z.H.; Vázquez-Ortega, A. Assessing the effects of lake-dredged sediments on soil health: Agricultural and environmental implications for northwestern Ohio. J. Environ. Qual. 2021, 50, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Kazberuk, W.; Szulc, W.; Rutkowska, B. Use Bottom Sediment to Agriculture-Effect on Plant and Heavy Metal Content in Soil. Agronomy 2021, 11, 1077. [Google Scholar] [CrossRef]
- Kiani, M.; Raave, H.; Simojoki, A.; Tammeorg, O.; Tammeorg, P. Recycling lake sediment to agriculture: Effects on plant growth, nutrient availability, and leaching. Sci. Total Environ. 2021, 753, 141984. [Google Scholar] [CrossRef] [PubMed]
- Braga, B.; de Carvalho, T.; Brosinsky, A.; Foerster, S.; Medeiros, P. From waste to resource: Cost-benefit analysis of reservoir sediment reuse for soil fertilization in a semiarid catchment. Sci. Total Environ. 2019, 696, 158–169. [Google Scholar] [CrossRef]
- Oliveira, B.R.F.; van Laarhoven, K.; Smit, M.P.J.; Rijnaarts, H.H.M.; Grotenhuis, T. Impact of compost and manure on the ripening of dredged sediments. J. Soils Sediments 2017, 17, 567–577. [Google Scholar] [CrossRef]
- Jamsawang, P.; Poorahong, H.; Yoobanpot, N.; Songpiriyakij, S.; Jongpradist, P. Improvement of soft clay with cement and bagasse ash waste. Constr. Build. Mater. 2017, 154, 61–71. [Google Scholar] [CrossRef]
- Yoobanpot, N.; Jamsawang, P.; Horpihulsuk, S. Strength behavior and microstructural characteristics of soft clay stabilized with cement kiln dust and fly ash residue. Appl. Clay Sci. 2017, 141, 146–156. [Google Scholar] [CrossRef]
- Güllü, H.; Canakci, H.; Al Zangana, I.F. Use of cement based grout with glass powder for deep mixing. Constr. Build. Mater. 2017, 137, 12–20. [Google Scholar] [CrossRef]
- Yoobanpot, N.; Jamsawang, P.; Simarat, P.; Jongpradist, P.; Likitlersuang, S. Sustainable reuse of dredged sediments as pavement materials by cement and fly ash stabilization. J. Soils Sediments 2020, 20, 3807–3823. [Google Scholar] [CrossRef]
- Loudini, A.; Ibnoussina, M.; Witam, O.; Limam, A.; Turchanina, O. Valorisation of dredged marine sediments for use as road material. Case Stud. Constr. Mater. 2020, 13, e00455. [Google Scholar] [CrossRef]
- Singh, D.; Kumar, R.; Nighot, N.S.; Rajput, A.; Prajapati, A.; Singh, B.K.; Kirgiz, M.S.; Srinivasaraonaik, B.; Mishra, R.K.; Khan, S.; et al. A comprehensive review on valorisation of octal by-product as supplementary admixtures in the production of fired and unfired bricks. Constr. Build. Mater. 2023, 408, 133641. [Google Scholar] [CrossRef]
- Adazabra, A.N.; Viruthagiri, G.; Atingabono, J. Developing fired clay bricks by incorporating scrap incinerated waste and river dredged sediment. Process Saf. Environ. Prot. 2023, 179, 108–123. [Google Scholar] [CrossRef]
- Bozic, M.; Zibret, L.; Kvock, D.; Pranjic, A.M.; Gregorc, B.; Ducman, V. Drava river sediment in clay brick production: Characterization, properties, and environmental performance. J. Build. Eng. 2023, 71, 106470. [Google Scholar] [CrossRef]
- Slimanou, H.; Eliche-Quesada, D.; Kherbache, S.; Bouzidi, N.; Tahakourt, A.K. Harbor Dredged Sediment as raw material in fired clay brick production: Characterization and properties. J. Build. Eng. 2020, 28, 101085. [Google Scholar] [CrossRef]
- Thomas, G.; Isabelle, C.; Frederic, H.; Catherine, P.; Marie-Anne, B. Demonstrating the influence of sediment source in dredged sediment recovery for brick and tile production. Resour. Conserv. Recycl. 2021, 171, 105653. [Google Scholar] [CrossRef]
- Bhairappanavar, S.; Liu, R.; Shakoor, A. Eco-friendly dredged material-cement bricks. Constr. Build. Mater. 2021, 271, 121524. [Google Scholar] [CrossRef]
- Nagaraj, H.B.; Rajesh, A.; Sravan, M.V. Influence of soil gradation, proportion and combination of admixtures on the properties and durability of CSEBs. Constr. Build. Mater. 2016, 110, 135–144. [Google Scholar] [CrossRef]
- Malkanthi, S.N.; Balthazaar, N.; Perera, A. Lime stabilization for compressed stabilized earth blocks with reduced clay and silt. Case Stud. Constr. Mater. 2020, 12, e00326. [Google Scholar] [CrossRef]
- Sekhar, D.C.; Nayak, S. Utilization of granulated blast furnace slag and cement in the manufacture of compressed stabilized earth blocks. Constr. Build. Mater. 2018, 166, 531–536. [Google Scholar] [CrossRef]
- Sitton, J.D.; Zeinali, Y.; Heidarian, W.H.; Story, B.A. Effect of mix design on compressed earth block strength. Constr. Build. Mater. 2018, 158, 124–131. [Google Scholar] [CrossRef]
- Sujatha, E.R.; Devi, S.S. Reinforced soil blocks: Viable option for low cost building units. Constr. Build. Mater. 2018, 189, 1124–1133. [Google Scholar] [CrossRef]
- Lam, C.M.; Yu, I.K.M.; Medel, F.; Tsang, D.C.W.; Hsu, S.C.; Poon, C.S. Life-cycle cost-benefit analysis on sustainable food waste management: The case of Hong Kong International Airport. J. Clean. Prod. 2018, 187, 751–762. [Google Scholar] [CrossRef]
- Beddaa, H.; Ouazi, I.; Ben Fraj, A.; Lavergne, F.; Torrenti, J.M. Reuse potential of dredged river sediments in concrete: Effect of sediment variability. J. Clean. Prod. 2020, 265, 121665. [Google Scholar] [CrossRef]
- Couvidat, J.; Benzaazoua, M.; Chatain, V.; Bouamrane, A.; Bouzahzah, H. Feasibility of the reuse of total and processed contaminated marine sediments as fine aggregates in cemented mortars. Constr. Build. Mater. 2016, 112, 892–902. [Google Scholar] [CrossRef]
- Meddah, M.S.; Zitouni, S.; Belâabes, S. Effect of content and particle size distribution of coarse aggregate on the compressive strength of concrete. Constr. Build. Mater. 2010, 24, 505–512. [Google Scholar] [CrossRef]
- Huang, W.; Chen, X.D.; Luan, J.J.; Ning, Y.J.; Ji, T.; Shi, Z.X. Deep insight into mechanical behaviour and microstructure mechanism of dredged sand in the lower reaches of the Yangtze River and manufactured sand concrete. J. Build. Eng. 2023, 68, 106105. [Google Scholar] [CrossRef]
- Ozer-Erdogan, P.; Basar, H.M.; Erden, I.; Tolun, L. Beneficial use of marine dredged materials as a fine aggregate in ready-mixed concrete: Turkey example. Constr. Build. Mater. 2016, 124, 690–704. [Google Scholar] [CrossRef]
- Kumar, G.S.; Deoliya, R. Recycled cement and recycled fine aggregates as alternative resources of raw materials for sustainable cellular light weight flowable material. Constr. Build. Mater. 2022, 326, 126878. [Google Scholar] [CrossRef]
- Spadaro, P.; Rosenthal, L. River and harbor remediation: “polluter pays”, alternative finance, and the promise of a “circular economy”. J. Soils Sediments 2020, 20, 4238–4247. [Google Scholar] [CrossRef]
- Zheng, Z.J.; Lin, M.Y.; Chiueh, P.T.; Lo, S.L. Framework for determining optimal strategy for sustainable remediation of contaminated sediment: A case study in Northern Taiwan. Sci. Total Environ. 2019, 654, 822–831. [Google Scholar] [CrossRef]
- Faure, A.; Coudray, C.; Anger, B.; Moulin, I.; Colin, H.; Izoret, L.; Théry, F.; Smith, A. Beneficial reuse of dam fine sediments as clinker raw material. Constr. Build. Mater. 2019, 218, 365–384. [Google Scholar] [CrossRef]
- Zhao, Z.F.; Benzerzour, M.; Abriak, N.E.; Damidot, D.; Courard, L.; Wang, D.X. Use of uncontaminated marine sediments in mortar and concrete by partial substitution of cement. Cem. Concr. Compos. 2018, 93, 155–162. [Google Scholar] [CrossRef]
- Ferone, C.; Liguori, B.; Capasso, I.; Colangelo, F.; Cioffi, R.; Cappelletto, E.; Di Maggio, R. Thermally treated clay sediments as geopolymer source material. Appl. Clay Sci. 2015, 107, 195–204. [Google Scholar] [CrossRef]
- Mostefa, F.; Bouhamou, N.E.; Aggoune, S.; Mesbah, H. Elaboration of geopolymer cement based on dredged sediment. J. Mater. Eng. Struct. 2019, 6, 39–51. [Google Scholar]
- Belayali, F.; Maherzi, W.; Benzerzour, M.; Abriak, N.E.; Senouci, A. Compressed Earth Blocks Using Sediments and Alkali-Activated Byproducts. Sustainability 2022, 14, 3158. [Google Scholar] [CrossRef]
- Fort, J.; Afolayan, A.; Mildner, M.; Hotek, P.; Keppert, M.; Cerny, R.; Huang, B.T. Assessment of Clayey Freshwater Sediments as Suitable Precursors for Alkaline Activation. Polymers 2024, 16, 175. [Google Scholar] [CrossRef]
- Caldeira, L.; Brito, A. Use of Soil-Rock Mixtures in Dam Construction. J. Constr. Eng. Manag. 2014, 140, 04014030. [Google Scholar] [CrossRef]
- Kos, K.; Gruchot, A.; Zawisza, E. Bottom Sediments from a Dam Reservoir as a Core in Embankments-Filtration and Stability: A Case Study. Sustainability 2021, 13, 1221. [Google Scholar] [CrossRef]
- Park, J.; Son, Y.; Noh, S.; Bong, T. The suitability evaluation of dredged soil from reservoirs as embankment material. J. Environ. Manag. 2016, 183, 443–452. [Google Scholar] [CrossRef]
- Wang, Y.K.; Wang, Z.H.; Chen, Y.Y.; Cao, T.C.; Yu, X.; Rui, P. Experimental study on bio-treatment effect of the dredged Yellow River silt based on soybean urease induced calcium carbonate precipitation. J. Build. Eng. 2023, 75, 106943. [Google Scholar] [CrossRef]
- Takahashi, H.; Sato, I.; Morikawa, Y.; Ozawa, A. Long-Term Durability of Cement-Treated Soil Used in Offshore Airport Island Construction. Appl. Sci. 2023, 13, 8081. [Google Scholar] [CrossRef]
- Wang, D.X.; Wiltshire, J. Special issue: Land reclamation and enhancement of soft marine sediment for building. Mar. Georesources Geotechnol. 2018, 36, 1. [Google Scholar] [CrossRef]
- Peng, Y.Z.; Peng, X.; Yang, M.; Shi, H.L.; Wang, W.C.; Tang, X.C.; Wu, Y. The performances of the baking-free bricks of non-sintered wrap-shell lightweight aggregates from dredged sediments. Constr. Build. Mater. 2020, 238, 117587. [Google Scholar] [CrossRef]
- Svensson, N.; Norén, A.; Modin, O.; Fedje, K.; Rauch, S.; Strömvall, A.; Andersson-Sköld, Y. Integrated cost and environmental impact assessment of management options for dredged sediment. Waste Manag. 2022, 138, 30–40. [Google Scholar] [CrossRef]
- Chen, Q.W.; Li, Q.Y.; Lin, Y.Q.; Zhang, J.Y.; Xia, J.; Ni, J.R.; Cooke, S.J.; Best, J.; He, S.F.; Feng, T.; et al. River Damming Impacts on Fish Habitat and Associated Conservation Measures. Rev. Geophys. 2023, 61, e2023RG000819. [Google Scholar] [CrossRef]
- Hauer, C.; Wagner, B.; Aigner, J.; Holzapfel, P.; Flödl, P.; Liedermann, M.; Tritthart, M.; Sindelar, C.; Pulg, U.; Klösch, M.; et al. State of the art, shortcomings and future challenges for a sustainable sediment management in hydropower: A review. Renew. Sustain. Energy Rev. 2018, 98, 40–55. [Google Scholar] [CrossRef]
- Çevikbilen, G.; Basar, H.M.; Karadogan, Ü.; Teymur, B.; Dagli, S.; Tolun, L. Assessment of the use of dredged marine materials in sanitary landfills: A case study from the Marmara sea. Waste Manag. 2020, 113, 70–79. [Google Scholar] [CrossRef]
- Nikafkar, N.; Alroaia, Y.V.; Heydariyeh, S.A.; Schleiss, A.J. Economic and commercial analysis of reusing dam reservoir sediments. Ecol. Econ. 2023, 204, 107668. [Google Scholar] [CrossRef]
- Pal, D.; Hogland, W. An overview and assessment of the existing technological options for management and resource recovery from beach wrack and dredged sediments: An environmental and economic perspective. J. Environ. Manag. 2022, 302, 113971. [Google Scholar] [CrossRef]
- Shi, Z.; Pi, K.; Huang, X.; Shi, Y.; Chen, Z.; Tang, R.; Hu, Z.; Gerson, A.; Liu, D. Enhanced dewater efficiency for river sediment by top-to-bottom water transmitting channels with different materials. Environ. Sci. Pollut. Res. 2020, 27, 29228–29238. [Google Scholar] [CrossRef]
- Liu, M.W.; Xu, G.R.; Li, G.B. Effect of the ratio of components on the characteristics of lightweight aggregate made from sewage sludge and river sediment. Process Saf. Environ. Prot. 2017, 105, 109–116. [Google Scholar] [CrossRef]
- Kim, J.O.; Choi, J.; Lee, S.; Chung, J. Evaluation of hydrocyclone and post-treatment technologies for remediation of contaminated dredged sediments. J. Environ. Manag. 2016, 166, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Bianco, F.; Race, M.; Papirio, S.; Esposito, G. A critical review of the remediation of PAH-polluted marine sediments: Current knowledge and future perspectives. Resour. Environ. Sustain. 2023, 11, 100101. [Google Scholar] [CrossRef]
- Olin-Estes, T.; Palermo, M. Recovery of dredged material for beneficial use: The future role of physical separation processes. J. Hazard. Mater. 2001, 85, 39–51. [Google Scholar] [CrossRef]
- Mulligan, C.; Yong, R.; Gibbs, B. An evaluation of technologies for the heavy metal remediation of dredged sediments. J. Hazard. Mater. 2001, 85, 145–163. [Google Scholar] [CrossRef]
- Veetil, D.; Mercier, G.; Blais, J.; Chartier, M.; Tran, L.; Taillard, V. Remediation of Contaminated Dredged Sediments Using Physical Separation Techniques. Soil Sediment Contam. 2014, 23, 932–953. [Google Scholar] [CrossRef]
- Zentar, R.; Miraoui, M.; Abriak, N.; Benzerzour, M. Natural Dewatering of Marine Dredged Sediments. Dry. Technol. 2011, 29, 1705–1713. [Google Scholar] [CrossRef]
- Yang, G.; Chen, M.; Yeh, C. Dewatering of a biological industrial sludge by electrokinetics-assisted filter press. Sep. Purif. Technol. 2011, 79, 177–182. [Google Scholar] [CrossRef]
- Azaiez, D.; Allariz, B.; Levacher, D. Study of Physical and Mechanical Relationships during the Natural Dewatering of River Sediments and a Kaolin. J. Mar. Sci. Eng. 2024, 12, 1354. [Google Scholar] [CrossRef]
- Hussan, A.; Levacher, D.; Mezazigh, S.; Jardin, L. Co-valorization of sediments incorporating high and low organic matter with alkali-activated GGBS and hydraulic binder for use in road construction. J. Build. Eng. 2023, 66, 105848. [Google Scholar] [CrossRef]
- Maletic, S.P.; Beljin, J.M.; Roncevic, S.D.; Grgic, M.G.; Dalmacija, B.D. State of the art and future challenges for polycyclic aromatic hydrocarbons is sediments: Sources, fate, bioavailability and remediation techniques. J. Hazard. Mater. 2019, 365, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Ferrarese, E.; Andreottola, G.; Oprea, I. Remediation of PAH-contaminated sediments by chemical oxidation. J. Hazard. Mater. 2008, 152, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Rivas, F. Polycyclic aromatic hydrocarbons sorbed on soils: A short review of chemical oxidation based treatments. J. Hazard. Mater. 2006, 138, 234–251. [Google Scholar] [CrossRef]
- Usman, M.; Faure, P.; Hanna, K.; Abdelmoula, M.; Ruby, C. Application of magnetite catalyzed chemical oxidation (Fenton-like and persulfate) for the remediation of oil hydrocarbon contamination. Fuel 2012, 96, 270–276. [Google Scholar] [CrossRef]
- Ahemad, M. Remediation of metalliferous soils through the heavy metal resistant plant growth promoting bacteria: Paradigms and prospects. Arab. J. Chem. 2019, 12, 1365–1377. [Google Scholar] [CrossRef]
- Qian, G.; Chen, W.; Lim, T.; Chui, P. In-situ stabilization of Pb, Zn, Cu, Cd and Ni in the multi-contaminated sediments with ferrihydrite and apatite composite additives. J. Hazard. Mater. 2009, 170, 1093–1100. [Google Scholar] [CrossRef]
- Mamindy-Pajany, Y.; Hurel, C.; Geret, F.; Roméo, M.; Marmier, N. Comparison of mineral-based amendments for ex-situ stabilization of trace elements (As, Cd, Cu, Mo, Ni, Zn) in marine dredged sediments: A pilot-scale experiment. J. Hazard. Mater. 2013, 252, 213–219. [Google Scholar] [CrossRef]
- Kazi, T.; Jamali, M.; Kazi, G.; Arain, M.; Afridi, H.; Siddiqui, A. Evaluating the mobility of toxic metals in untreated industrial wastewater sludge using a BCR sequential extraction procedure and a leaching test. Anal. Bioanal. Chem. 2005, 383, 297–304. [Google Scholar] [CrossRef]
- Chiang, Y.; Santos, R.; Ghyselbrecht, K.; Cappuyns, V.; Martens, J.; Swennen, R.; Van Gerven, T.; Meesschaert, B. Strategic selection of an optimal sorbent mixture for in-situ remediation of heavy metal contaminated sediments: Framework and case study. J. Environ. Manag. 2012, 105, 1–11. [Google Scholar] [CrossRef]
- Xenidis, A.; Stouraiti, C.; Papassiopi, N. Stabilization of Pb and As in soils by applying combined treatment with phosphates and ferrous iron. J. Hazard. Mater. 2010, 177, 929–937. [Google Scholar] [CrossRef]
- Ayilara, M.; Adeleke, B.; Adebajo, M.; Akinola, S.; Fayose, C.; Adeyemi, U.; Gbadegesin, L.; Omole, R.; Johnson, R.; Edhemuino, M.; et al. Remediation by enhanced natural attenuation; an environment-friendly remediation approach. Front. Environ. Sci. 2023, 11, 1182586. [Google Scholar] [CrossRef]
- Mani, D.; Kumar, C. Biotechnological advances in bioremediation of heavy metals contaminated ecosystems: An overview with special reference to phytoremediation. Int. J. Environ. Sci. Technol. 2014, 11, 843–872. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M. Phytoremediation of heavy metals-Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, M.; Islam, M.; Ahmed, Z.; Nayar, F. Phytoremediation of heavy metal contaminated buriganga riverbed sediment by Indian mustard and marigold plants. Environ. Prog. Sustain. Energy 2016, 35, 117–124. [Google Scholar] [CrossRef]
- Pal, D.; Maiti, S. Abatement of cadmium (Cd) contamination in sediment using tea waste biochar through meso-microcosm study. J. Clean. Prod. 2019, 212, 986–996. [Google Scholar] [CrossRef]
- Liu, D.; Jiang, W.; Liu, C.; Xin, C.; Hou, W. Uptake and accumulation of lead by roots, hypocotyls and shoots of Indian mustard [Brassica juncea (L.)]. Bioresour. Technol. 2000, 71, 273–277. [Google Scholar] [CrossRef]
- Dahmani-Muller, H.; van Oort, F.; Balabane, M. Metal extraction by Arabidopsis halleri grown on an unpolluted soil amended with various metal-bearing solids:: A pot experiment. Environ. Pollut. 2001, 114, 77–84. [Google Scholar] [CrossRef]
- Perelo, L. Review: In situ and bioremediation of organic pollutants in aquatic sediments. J. Hazard. Mater. 2010, 177, 81–89. [Google Scholar] [CrossRef]
- Rahmati, F.; Lajayer, B.; Shadfar, N.; van Bodegom, P.; van Hullebusch, E. A Review on Biotechnological Approaches Applied for Marine Hydrocarbon Spills Remediation. Microorganisms 2022, 10, 1289. [Google Scholar] [CrossRef]
- Amar, M.; Benzerzour, M.; Kleib, J.; Abriak, N.E. From dredged sediment to supplementary cementitious material: Characterization, treatment, and reuse. Int. J. Sediment Res. 2021, 36, 92–109. [Google Scholar] [CrossRef]
- Falciglia, P.P.; Lumia, L.; Giustra, M.G.; Gagliano, E.; Roccaro, P.; Vagliasindi, F.G.A.; Di Bella, G. Remediation of petrol hydrocarbon-contaminated marine sediments by thermal desorption. Chemosphere 2020, 260, 127576. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Dong, Y.; Feng, Y.; Li, Y.; Dong, Y. Thermal desorption for remediation of contaminated soil: A review. Chemosphere 2019, 221, 841–855. [Google Scholar] [CrossRef] [PubMed]
- Quina, M.; Bordado, J.; Quinta-Ferreira, R. Treatment and use of air pollution control residues from MSW incineration: An overview. Waste Manag. 2008, 28, 2097–2121. [Google Scholar] [CrossRef]
- Li, C.; Huang, Y.; Huang, K.; Lee, W. Characterization of slags and ingots from the vitrification of municipal solid waste incineration ashes. Ind. Eng. Chem. Res. 2003, 42, 2306–2313. [Google Scholar] [CrossRef]
- Xiao, Y.; Oorsprong, M.; Yang, Y.; Voncken, J. Vitrification of bottom ash from a municipal solid waste incinerator. Waste Manag. 2008, 28, 1020–1026. [Google Scholar] [CrossRef]
- Guo, B.; Liu, B.; Yang, J.; Zhang, S. The mechanisms of heavy metal immobilization by cementitious material treatments and thermal treatments: A review. J. Environ. Manag. 2017, 193, 410–422. [Google Scholar] [CrossRef]
- Vidonish, J.; Alvarez, P.; Zygourakis, K. Pyrolytic Remediation of Oil-Contaminated Soils: Reaction Mechanisms, Soil Changes, and Implications for Treated Soil Fertility. Ind. Eng. Chem. Res. 2018, 57, 3489–3500. [Google Scholar] [CrossRef]
- Kim, K.H.; Jeon, S.E.; Kim, J.K.; Yang, S.C. An experimental study on thermal conductivity of concrete. Cem. Concr. Res. 2003, 33, 363–371. [Google Scholar] [CrossRef]
- Ammami, M.T.; Song, Y.; Benamar, A.; Portet-Koltalo, F.; Wang, H. Electro-dewatering of dredged sediments by combined effects of mechanical and electrical processes: Influence of operating conditions. Electrochim. Acta 2020, 353, 136462. [Google Scholar] [CrossRef]
- Xing, L.; Wen, J.; Yan, C.Y.; Wang, Q.; Hu, X.H.; Xue, Z.Z. Improving the microenvironment of Cd-contaminated river sediments through humic substances washing and zeolite immobilization. Process Saf. Environ. Prot. 2021, 146, 779–788. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Wen, J.; Hu, Y.; Fang, Y.; Zhang, H.B.; Xing, L.; Wang, Y.X.; Zeng, G.M. Humic substances from green waste compost: An effective washing agent for heavy metal (Cd, Ni) removal from contaminated sediments. J. Hazard. Mater. 2019, 366, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Cauwenberg, P.; Verdonckt, F.; Maes, A. Flotation as a remediation technique for heavily polluted dredged material. 2. Characterisation of flotated fractions. Sci. Total Environ. 1998, 209, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Levacher, D.; Allariz, B.; Hussan, A. Dewatering and Transport in Sustainable Sediment Management: A Review. Sustainability 2024, 16, 9663. [Google Scholar] [CrossRef]
- Dermont, G.; Bergeron, M.; Richer-Laflèche, M.; Mercier, G. Remediation of metal-contaminated urban soil using flotation technique. Sci. Total Environ. 2010, 408, 1199–1211. [Google Scholar] [CrossRef]
- Peng, J.; Song, Y.; Yuan, P.; Cui, X.; Qiu, G. The remediation of heavy metals contaminated sediment. J. Hazard. Mater. 2009, 161, 633–640. [Google Scholar] [CrossRef]
- Yoo, J.; Lee, C.; Yang, J.; Baek, K. Extraction characteristics of heavy metals from marine sediments. Chem. Eng. J. 2013, 228, 688–699. [Google Scholar] [CrossRef]
- Kim, K.; Kim, D.; Yoo, J.; Baek, K. Electrokinetic extraction of heavy metals from dredged marine sediment. Sep. Purif. Technol. 2011, 79, 164–169. [Google Scholar] [CrossRef]
- Beolchini, F.; Fonti, V.; Rocchetti, L.; Saraceni, G.; Pietrangeli, B.; Dell’Anno, A. Chemical and biological strategies for the mobilisation of metals/semi-metals in contaminated dredged sediments: Experimental analysis and environmental impact assessment. Chem. Ecol. 2013, 29, 415–426. [Google Scholar] [CrossRef]
- Di Palma, L.; Mecozzi, R. Heavy metals mobilization from harbour sediments using EDTA and citric acid as chelating agents. J. Hazard. Mater. 2007, 147, 768–775. [Google Scholar] [CrossRef]
- Shih, Y.; Wu, P.; Chen, C.; Chen, C.; Dong, C. Nonionic and anionic surfactant-washing of polycyclic aromatic hydrocarbons in estuarine sediments around an industrial harbor in southern Taiwan. Chemosphere 2020, 256, 127044. [Google Scholar] [CrossRef]
- Lamichhane, S.; Krishna, K.; Sarukkalige, R. Surfactant-enhanced remediation of polycyclic aromatic hydrocarbons: A review. J. Environ. Manag. 2017, 199, 46–61. [Google Scholar] [CrossRef]
- Mulligan, C.; Yong, R.; Gibbs, B. Heavy metal removal from sediments by biosurfactants. J. Hazard. Mater. 2001, 85, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Dahrazma, B.; Mulligan, C. Investigation of the removal of heavy metals from sediments using rhamnolipid in a continuous flow configuration. Chemosphere 2007, 69, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Flotron, V.; Delteil, C.; Padellec, Y.; Camel, V. Removal of sorbed polycyclic aromatic hydrocarbons from soil, sludge and sediment samples using the Fenton’s reagent process. Chemosphere 2005, 59, 1427–1437. [Google Scholar] [CrossRef]
- Smara, M.; Khalladi, R.; Moulai-Mostefa, N.; Madi, K.; Mansour, D.; Lekmine, S.; Benslama, O.; Tahraoui, H.; Zhang, J.; Amrane, A. Efficiency of Hydrogen Peroxide and Fenton Reagent for Polycyclic Aromatic Hydrocarbon Degradation in Contaminated Soil: Insights from Experimental and Predictive Modeling. Processes 2024, 12, 621. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, Z.; Zeng, G.; Jiang, M.; Yang, Z.; Cui, F.; Zhu, M.; Shen, L.; Hu, L. Effects of sediment geochemical properties on heavy metal bioavailability. Environ. Int. 2014, 73, 270–281. [Google Scholar] [CrossRef]
- Gutsalenko, T.; Bourdot, A.; Billon, G.; Alaimo, V.; Wattez, T.; Frouin, L.; Chaouche, M. Effect of hydraulic binders? addition on trace metals stabilization in the S/S process of dredged sediments. J. Environ. Manag. 2022, 324, 116362. [Google Scholar] [CrossRef]
- Covelo, E.; Vega, F.; Andrade, M. Simultaneous sorption and desorption of Cd, Cr, Cu, Ni, Pb, and Zn in acid soils I. selectivity sequences. J. Hazard. Mater. 2007, 147, 852–861. [Google Scholar] [CrossRef]
- Li, L.; Peng, C.; Deng, L.; Zhang, F.; Wu, D.; Ma, F.; Liu, Y. Understanding the synergistic mechanism of PAM-FeCl3 for improved sludge dewaterability. J. Environ. Manag. 2022, 301, 113926. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Liu, B. An Ex-Situ Immobilization Experiment with Zn, Pb, and Cu in Dredged Marine Sediments from Bohai Bay, China. J. Mar. Sci. Eng. 2019, 7, 394. [Google Scholar] [CrossRef]
- Dai, W.; Lu, K.; Zhang, J.; Wang, S.; Liu, K.; Zhang, H. Immobilization of Pb2+ and Cd2+ in water by sodium alginate magnet-biochar composite and LCA evaluation. J. Water Process Eng. 2024, 67, 106259. [Google Scholar] [CrossRef]
- Abu-Tahon, M.; Housseiny, M.; Aboelmagd, H.; Daifalla, N.; Khalili, M.; Isichei, A.; Ramadan, A.; El-Saad, A.; Seddek, N.; Ebrahim, D.; et al. A holistic perspective on the efficiency of microbial enzymes in bioremediation process: Mechanism and challenges: A review. Int. J. Biol. Macromol. 2025, 308, 142278. [Google Scholar] [CrossRef] [PubMed]
- Odoh, C.; Zabbey, N.; Sam, K.; Eze, C. Status, progress and challenges of phytoremediation—An African scenario. J. Environ. Manag. 2019, 237, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, H.; Kim, J. Current Status of and Challenges for Phytoremediation as a Sustainable Environmental Management Plan for Abandoned Mine Areas in Korea. Sustainability 2023, 15, 2761. [Google Scholar] [CrossRef]
- Schwab, A. Bioremediation of Polyaromatic Hydrocarbons in Soils: A Review of Recent Progress. Curr. Pollut. Rep. 2024, 10, 710–721. [Google Scholar] [CrossRef]
- El-Sheekh, M.; Hamouda, R.; Nizam, A. Biodegradation of crude oil by Scenedesmus obliquus and Chlorella vulgaris growing under heterotrophic conditions. Int. Biodeterior. Biodegrad. 2013, 82, 67–72. [Google Scholar] [CrossRef]
- Peng, W.H.; Li, X.M.; Xiao, S.T.; Fan, W.H. Review of remediation technologies for sediments contaminated by heavy metals. J. Soils Sediments 2018, 18, 1701–1719. [Google Scholar] [CrossRef]
- Wei, Z.; Le, Q.; Peng, W.; Yang, Y.; Yang, H.; Gu, H.; Lam, S.; Sonne, C. A review on phytoremediation of contaminants in air, water and soil. J. Hazard. Mater. 2021, 403, 123658. [Google Scholar] [CrossRef]
- Tripathi, M.; Munot, H.; Shouche, Y.; Meyer, J.; Goel, R. Isolation and functional characterization of Siderophore-producing lead- and cadmium-resistant Pseudomonas putida KNP9. Curr. Microbiol. 2005, 50, 233–237. [Google Scholar] [CrossRef]
- Rafati, M.; Khorasani, N.; Moattar, F.; Shirvany, A.; Moraghebi, F.; Hosseinzadeh, S. Phytoremediation Potential of Populus Alba and Morus alba for Cadmium, Chromuim and Nickel Absorption from Polluted Soil. Int. J. Environ. Res. 2011, 5, 961–970. [Google Scholar]
- Calderon, J.; Kaunda, R.; Sinkala, T.; Workman, C.; Bazilian, M.; Clough, G. Phytoremediation and phytoextraction in Sub-Saharan Africa: Addressing economic and social challenges. Ecotoxicol. Environ. Saf. 2021, 226, 112864. [Google Scholar] [CrossRef] [PubMed]
- Romantschuk, M.; Lahti-Leikas, K.; Kontro, M.; Galitskaya, P.; Talvenmäki, H.; Simpanen, S.; Allen, J.; Sinkkonen, A. Bioremediation of contaminated soil and groundwater by in situ biostimulation. Front. Microbiol. 2023, 14, 1258148. [Google Scholar] [CrossRef] [PubMed]
- Kebede, G.; Tafese, T.; Abda, E.; Kamaraj, M.; Assefa, F. Factors Influencing the Bacterial Bioremediation of Hydrocarbon Contaminants in the Soil: Mechanisms and Impacts. J. Chem. 2021, 2021, 9823362. [Google Scholar] [CrossRef]
- Erkelens, M.; Adetutu, E.; Taha, M.; Tudararo-Aherobo, L.; Antiabong, J.; Provatas, A.; Ball, A. Sustainable remediation—The application of bioremediated soil for use in the degradation of TNT chips. J. Environ. Manag. 2012, 110, 69–76. [Google Scholar] [CrossRef]
- Guarino, C.; Spada, V.; Sciarrillo, R. Assessment of three approaches of bioremediation (Natural Attenuation, Landfarming and Bioagumentation—Assistited Landfarming) for a petroleum hydrocarbons contaminated soil. Chemosphere 2017, 170, 10–16. [Google Scholar] [CrossRef]
- Daâssi, D.; Almaghribi, F. Petroleum-contaminated soil: Environmental occurrence and remediation strategies. 3 Biotech 2022, 12, 139. [Google Scholar] [CrossRef]
- Kang, C.; Kim, D.; Khan, M.; Kumar, R.; Ji, S.; Choi, K.; Paeng, K.; Park, S.; Jeon, B. Pyrolytic remediation of crude oil-contaminated soil. Sci. Total Environ. 2020, 713, 136498. [Google Scholar] [CrossRef]
- Yadav, S.; Bhandari, S.; Bhatta, D.; Poudel, A.; Bhattarai, S.; Yadav, P.; Ghimire, N.; Paudel, P.; Paudel, P.; Shrestha, J.; et al. Biochar application: A sustainable approach to improve soil health. J. Agric. Food Res. 2023, 11, 100498. [Google Scholar] [CrossRef]
- Ren, J.; Song, X.; Ding, D. Sustainable remediation of diesel-contaminated soil by low temperature thermal treatment: Improved energy efficiency and soil reusability. Chemosphere 2020, 241, 124952. [Google Scholar] [CrossRef]
- Kim, K.; Kwon, H.A.; Park, J.; Lee, H.; Choi, Y. Thermal treatment of petroleum-contaminated marine sediment according to oxygen availability and temperature: Product quality as a potential plant-growth medium. Chemosphere 2023, 324, 138347. [Google Scholar] [CrossRef]
- Li, Y.; Chen, W.; Fang, S.; Xu, Z.; Weng, H.; Zhang, X. The Influence of Pyrolysis Temperature and Feedstocks on the Characteristics of Biochar-Derived Dissolved Organic Matter: A Systematic Assessment. Clean Technol. 2024, 6, 1314–1325. [Google Scholar] [CrossRef]
- Zhao, L.N.; Hu, M.; Muslim, H.; Hou, T.Y.; Bian, B.; Yang, Z.; Yang, W.B.; Zhang, L.M. Co-utilization of lake sediment and blue-green algae for porous lightweight aggregate (ceramsite) production. Chemosphere 2022, 287, 132145. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.J.; Guo, C.L.; Liao, C.J.; Liu, S.S.; Wick, L.Y.; Peng, D.; Yi, X.Y.; Lu, G.N.; Yin, H.; Lin, Z.; et al. Drivers and applications of integrated clean-up technologies for surfactant-enhanced remediation of environments contaminated with polycyclic aromatic hydrocarbons (PAHs). Environ. Pollut. 2017, 225, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Labianca, C.; Chen, L.; De Gisi, S.; Notarnicola, M.; Guo, B.L.; Sun, J.; Ding, S.M.; Wang, L. Sustainable ex-situ remediation of contaminated sediment: A review. Environ. Pollut. 2021, 287, 117333. [Google Scholar] [CrossRef]
- Usman, M.; Hanna, K.; Faure, P. Remediation of oil-contaminated harbor sediments by chemical oxidation. Sci. Total Environ. 2018, 634, 1100–1107. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Tsang, D.C.W.; Li, J.S.; Yeung, T.L.Y.; Ding, S.M.; Poon, C.S. Green remediation of contaminated sediment by stabilization/solidification with industrial by-products and CO2 utilization. Sci. Total Environ. 2018, 631-632, 1321–1327. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, A.; Pandita, S.; Bhardwaj, R.; Thukral, A.K.; Cerda, A. A review of ecological risk assessment and associated health risks with heavy metals in sediment from India. Int. J. Sediment Res. 2020, 35, 516–526. [Google Scholar] [CrossRef]
- Todaro, F.; De Gisi, S.; Notarnicola, M. Contaminated marine sediment stabilization/solidification treatment with cement/lime: Leaching behaviour investigation. Environ. Sci. Pollut. Res. 2020, 27, 21407–21415. [Google Scholar] [CrossRef]
- Feng, M.; Zhou, J.H.; Yu, X.L.; Mao, W.; Guo, Y.S.; Wang, H. Insights into biodegradation mechanisms of triphenyl phosphate by a novel fungal isolate and its potential in bioremediation of contaminated river sediment. J. Hazard. Mater. 2022, 424, 127545. [Google Scholar] [CrossRef]
- Pino-Herrera, D.O.; Pechaud, Y.; Huguenot, D.; Esposito, G.; van Hullebusch, E.D.; Oturan, M.A. Removal mechanisms in aerobic slurry bioreactors for remediation of soils and sediments polluted with hydrophobic organic compounds: An overview. J. Hazard. Mater. 2017, 339, 427–449. [Google Scholar] [CrossRef]
- Ashkanani, Z.; Mohtar, R.; Al-Enezi, S.; Smith, P.; Calabrese, S.; Ma, X.; Abdullah, M. AI-assisted systematic review on remediation of contaminated soils with PAHs and heavy metals. J. Hazard. Mater. 2024, 468, 133813. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, B.; Chen, W.; Yin, Y.; Huang, L.; Wei, L.; Awad, M.; Liu, Z. Predictive Machine Learning Model to Assess the Adsorption Efficiency of Biochar-Heavy Metals for Effective Remediation of Soil-Plant Environment. Toxics 2024, 12, 575. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Lim, J.; Igalavithana, A.; Hwang, G.; Sang, M.; Masek, O.; Ok, Y. AI-guided investigation of biochar’s efficacy in Pb immobilization for remediation of Pb contaminated agricultural land. Appl. Biol. Chem. 2024, 67, 82. [Google Scholar] [CrossRef]
- Luo, N. Methods for controlling heavy metals in environmental soils based on artificial neural networks. Sci. Rep. 2024, 14, 2563. [Google Scholar] [CrossRef]
- Chen, K.; Chen, S. Applying an artificial intelligence model using multidimensional spatial-temporal data to predict arsenic contamination of groundwater. Process Saf. Environ. Prot. 2022, 163, 362–367. [Google Scholar] [CrossRef]
- Popescu, S.; Mansoor, S.; Wani, O.; Kumar, S.; Sharma, V.; Sharma, A.; Arya, V.; Kirkham, M.; Hou, D.; Bolan, N.; et al. Artificial intelligence and IoT driven technologies for environmental pollution monitoring and management. Front. Environ. Sci. 2024, 12, 1336088. [Google Scholar] [CrossRef]


| Resource Recovery Process | Treatment Techniques | Purpose | Contents Removed | Pros | Cons | Source |
|---|---|---|---|---|---|---|
| Physical | Separation using centrifuge, screening, hydrocyclone. | Separation of small and fine contaminated particles. | Contaminated fine particles. | Efficiency. | Energy consumption. | [89,92] |
| Dewatering using filter presses, draining screens, natural evaporation and drainage. | Removal of free and interstitial water. Treatment of contaminated fraction. | Water and contaminants. | Reduces volume of DS. Natural dewatering is more cost-effective and simple. Energy efficient. | Climate-dependent; requires large area. | [129,172] | |
| Washing with water or chemicals—acids, bases, surfactants. | Neutralization of toxic contaminants and salts. | Heavy metals and salts. | Depends on the reagent used, dosage, pH, contact time. | High energy; water and chemical consumption. | [130,131,173] | |
| Chemical | Oxidation, uses hydrogen peroxide, Fenton reagent, ozone, potassium permanganate. | Neutralization of organic contaminants. | Organic contaminants. | Fenton reagents produce excellent performance when catalyzed. | Consumption of chemicals; poor heavy-metal removal. | [101,174,175] |
| Immobilization, uses cement, lime. | Reduction in heavy-metal mobility. | Heavy metals | Less expensive. | Possible escape of contaminants. | [31,120,176] | |
| Thermal | Thermal desorption, operates at 100 to 600 °C with a boundary temperature of 300 °C. | Removal of volatile organic compounds (VOCs) and metals. | Mostly organic contaminants. | Short treatment time with low secondary pollution. Suitable for highly contaminated DS, with low effect on the material’s properties. | Air pollution with environmental impact. | [121,177] |
| Pyrolysis, operates at low temperatures of 200 to 500 °C. | Burning off of organic contaminants and reductions in heavy metals in the absence of oxygen | VOCs and heavy metals. | Remediates DS without destroying the material’s properties. Low heat consumption. Treated DS are suitable for agriculture. | High energy consumption. | [127,167,170] | |
| Thermal vitrification, operates at high temperatures of 1000 to 1500 °C. | Contaminants immobilized by transforming the DS into stable or crystalline materials. | VOCs and heavy metals | High destruction efficiency as to organic contaminants. Immobilizes toxic elements. DS volume reduction. | High energy consumption. | [38,178] | |
| Biological | Bioremediation, uses living microorganisms—bacteria, fungi, algae, cyanobacteria. | Degradation of organic contaminants and heavy metals by microbial activity. | Targeted contaminants. | Low operation cost. Contaminants are digested by microbes. Effective for different types of heavy metals and organic pollutants | Degradation is slow and time-consuming; requires huge land area. | [157,179,180] |
| Natural attenuation, contaminants are reduced over time in natural state using native microbial population. | Self-amendment of contaminated DS. | Organic contaminants and some heavy metals. | The method can be improved by combining processes such as biostimulation or bioaugmentation. | Slow process, requires extensive monitoring; effective in a low-contamination situation. | [118,119,165] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afolayan, A.; Černý, R.; Fořt, J. Review of Treatment Techniques for Dredged Sediments in the Context of Valorization as Secondary Raw Materials. Buildings 2025, 15, 1639. https://doi.org/10.3390/buildings15101639
Afolayan A, Černý R, Fořt J. Review of Treatment Techniques for Dredged Sediments in the Context of Valorization as Secondary Raw Materials. Buildings. 2025; 15(10):1639. https://doi.org/10.3390/buildings15101639
Chicago/Turabian StyleAfolayan, Ayodele, Robert Černý, and Jan Fořt. 2025. "Review of Treatment Techniques for Dredged Sediments in the Context of Valorization as Secondary Raw Materials" Buildings 15, no. 10: 1639. https://doi.org/10.3390/buildings15101639
APA StyleAfolayan, A., Černý, R., & Fořt, J. (2025). Review of Treatment Techniques for Dredged Sediments in the Context of Valorization as Secondary Raw Materials. Buildings, 15(10), 1639. https://doi.org/10.3390/buildings15101639










