Evaluation of Phase Change Materials for Pre-Cooling of Supply Air into Air Conditioning Systems in Extremely Hot Climates
Abstract
:1. Introduction
2. Experimental Analysis
2.1. Phase Change Material (PCM) Selection and Characterization
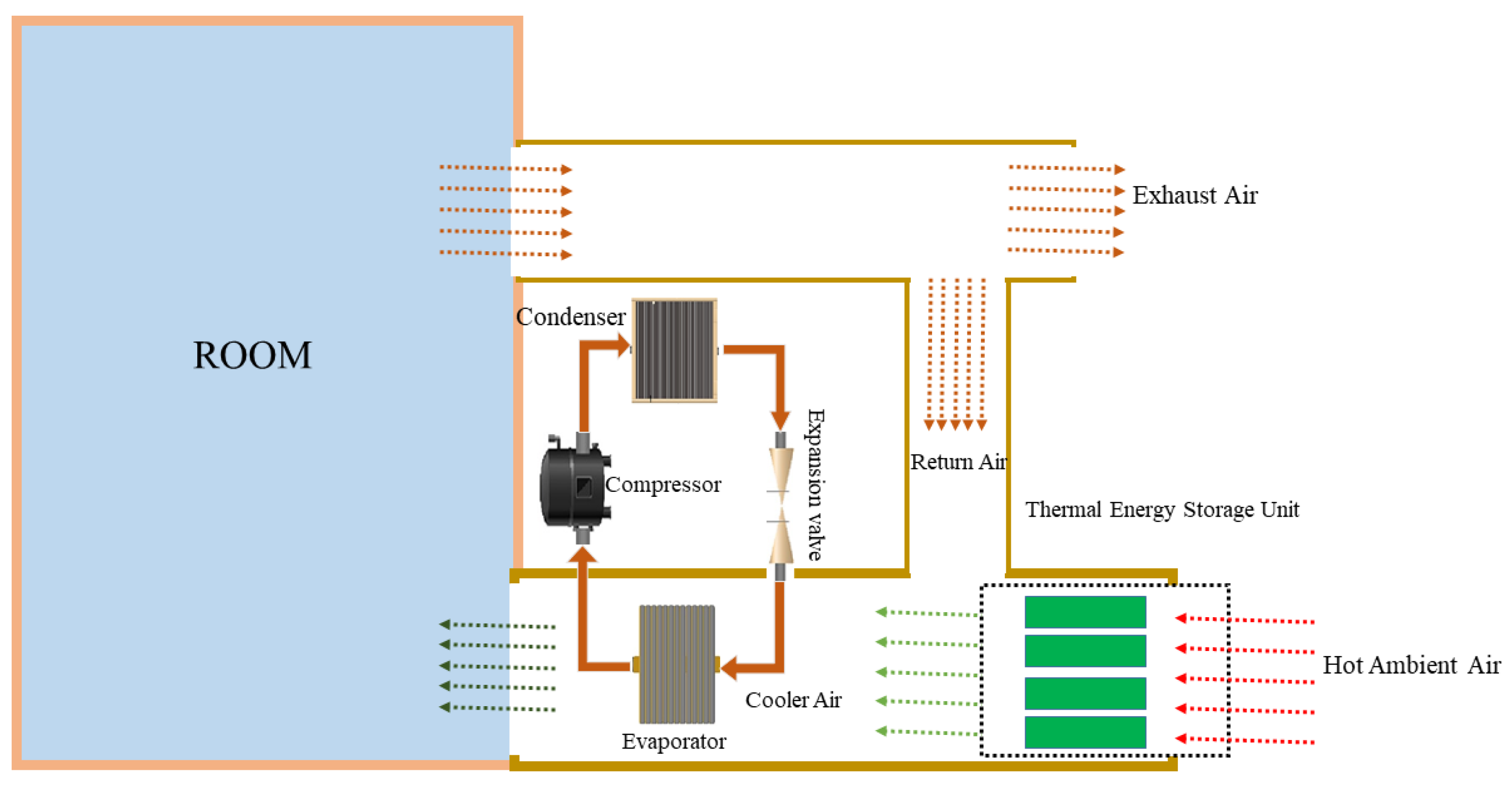
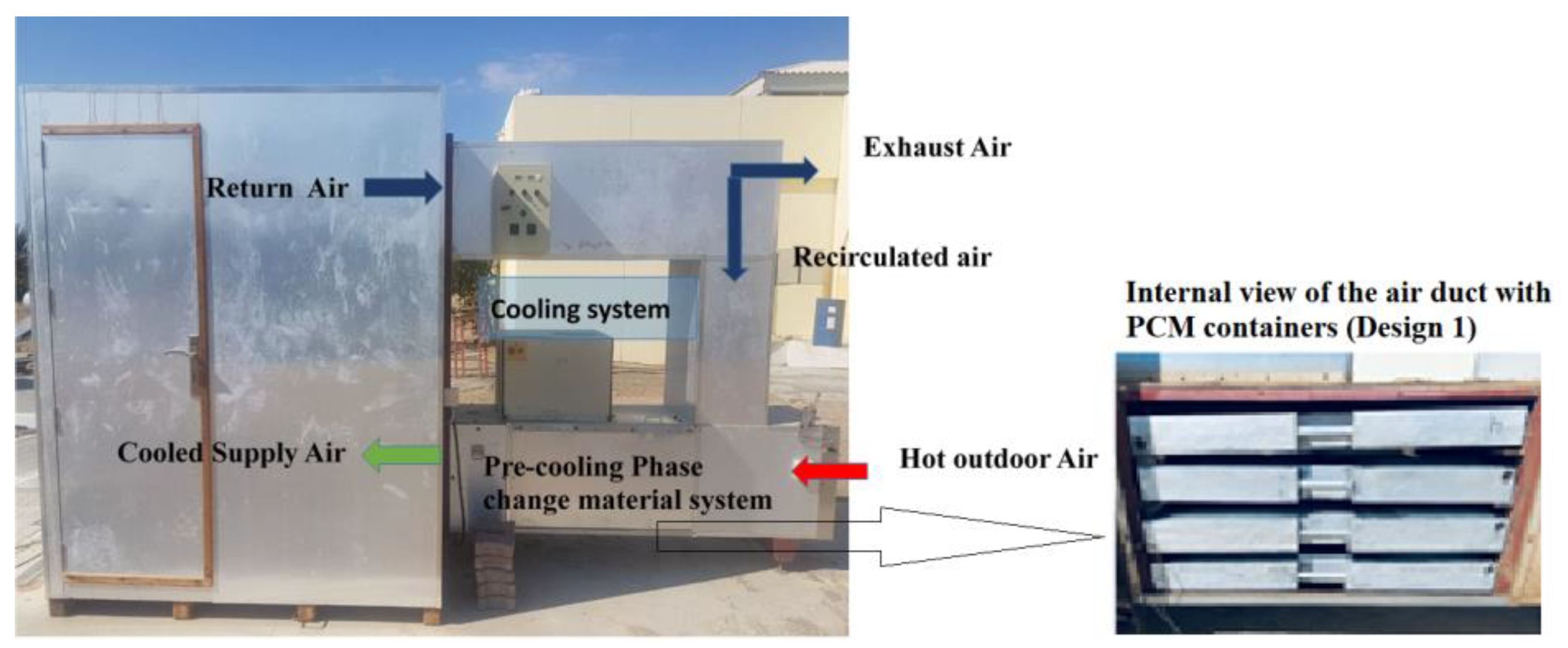
2.2. Experimental System
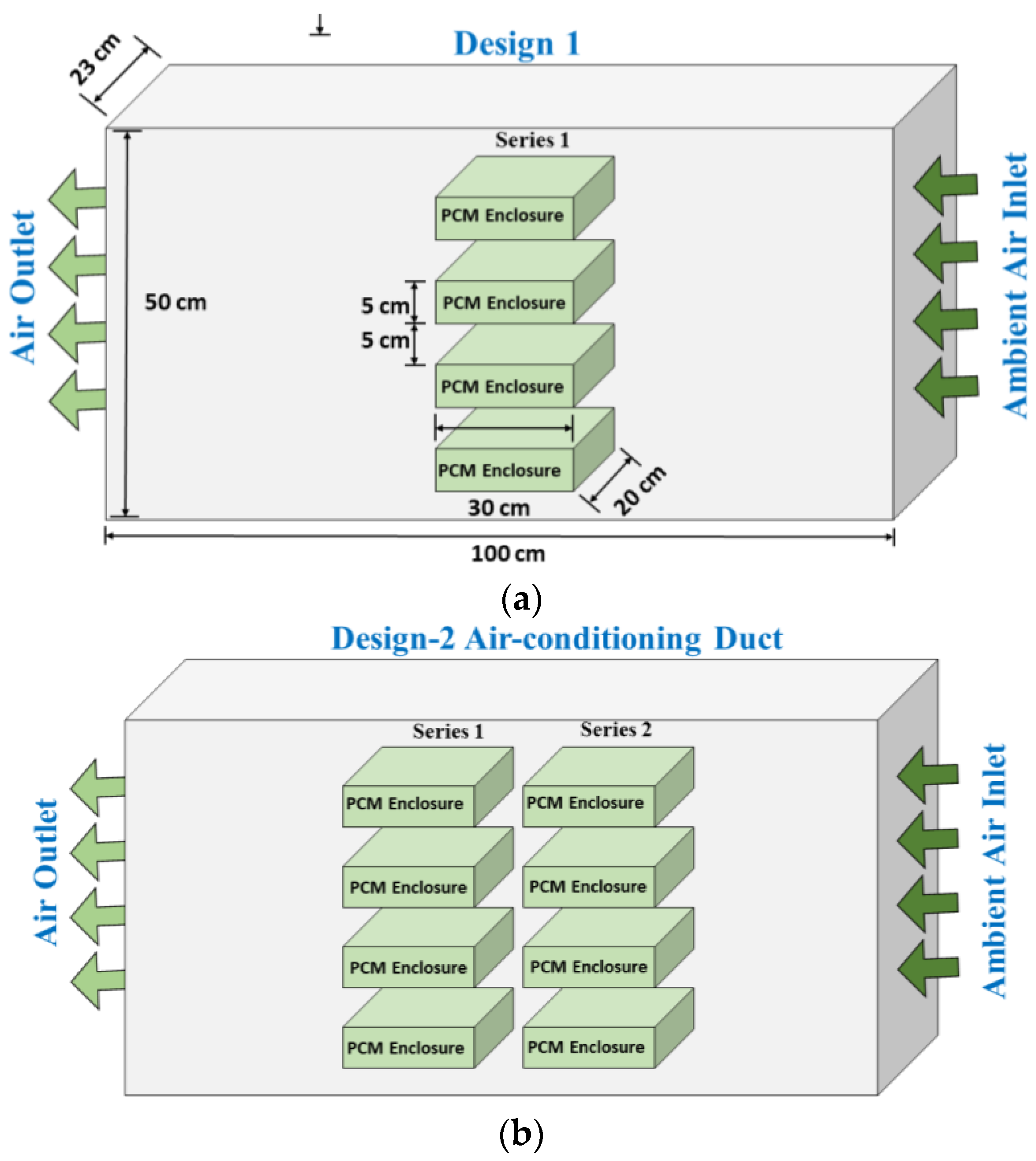
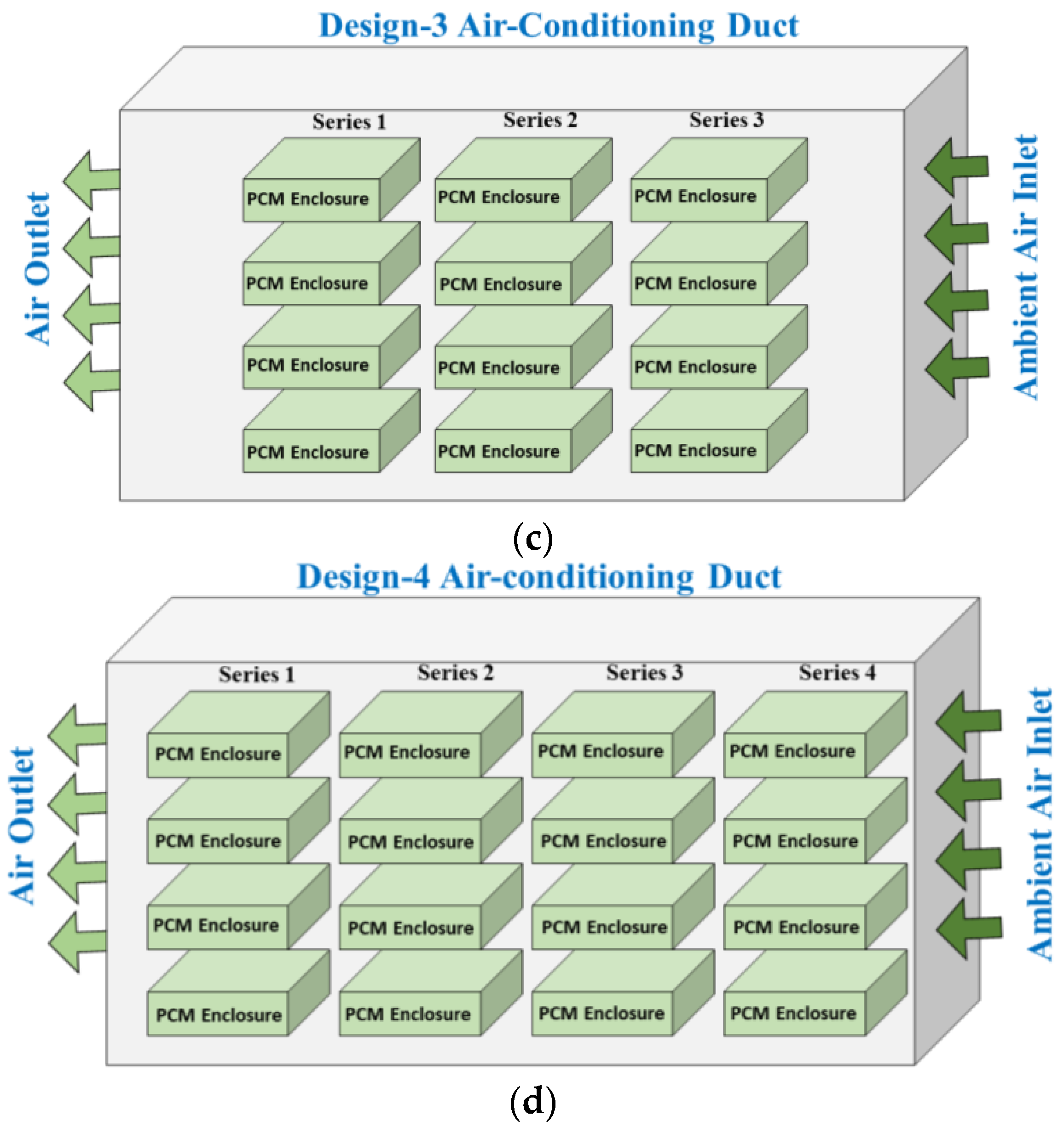
2.3. PCM-Based TES Unit
3. Numerical Modeling
Initial and Boundary Conditions
4. Results and Discussion
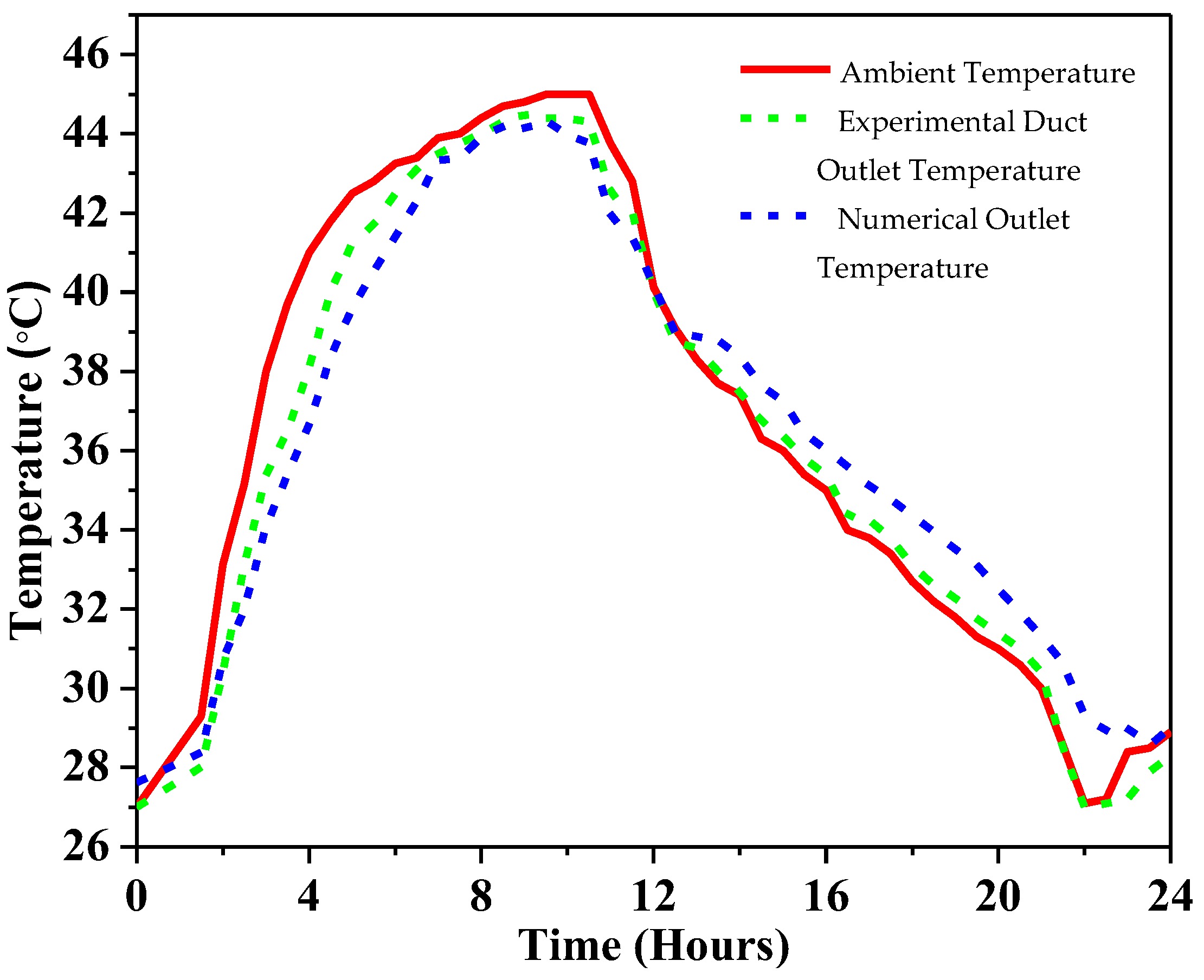
4.1. Experimental Validation
4.2. Numerical Results
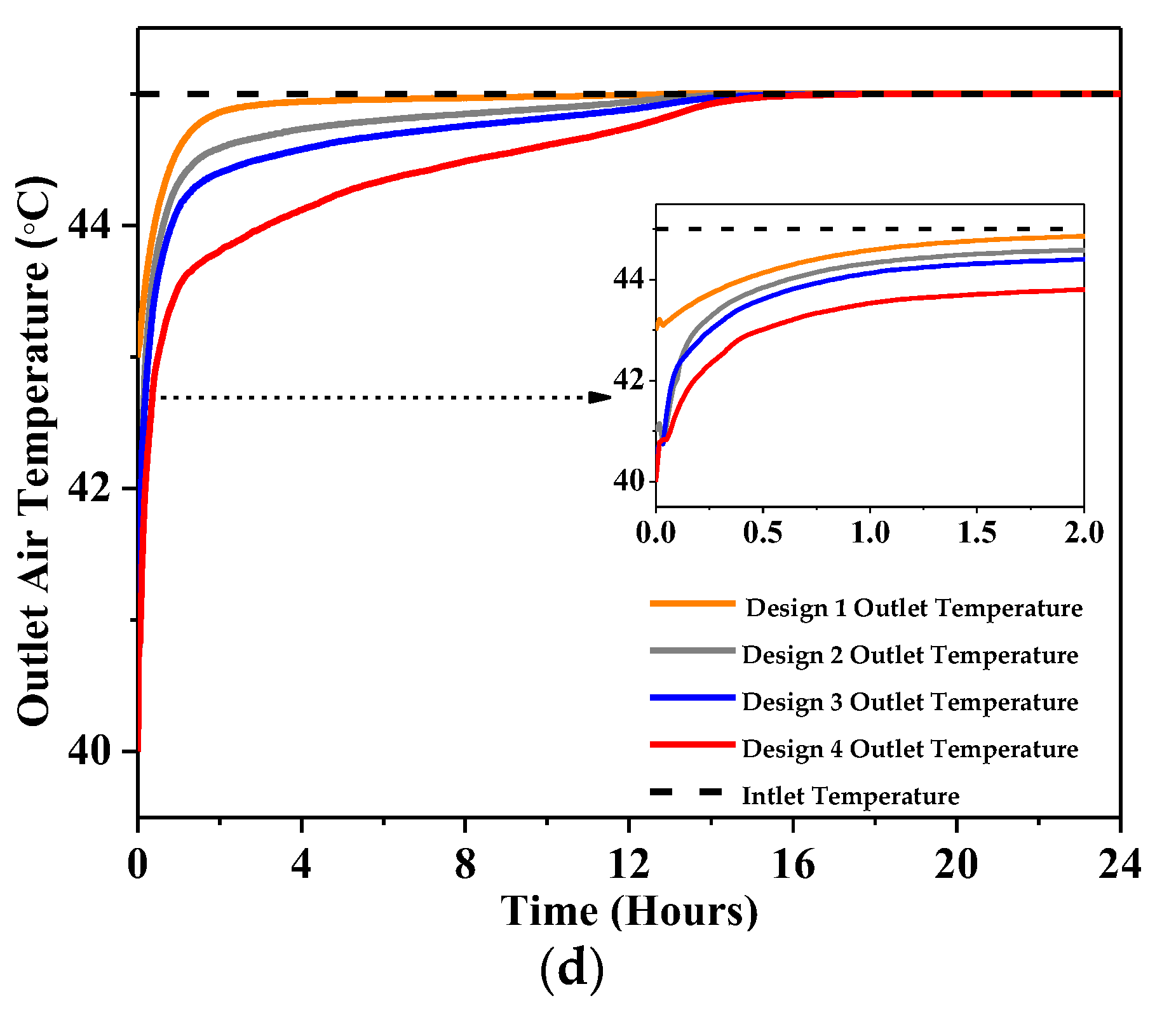
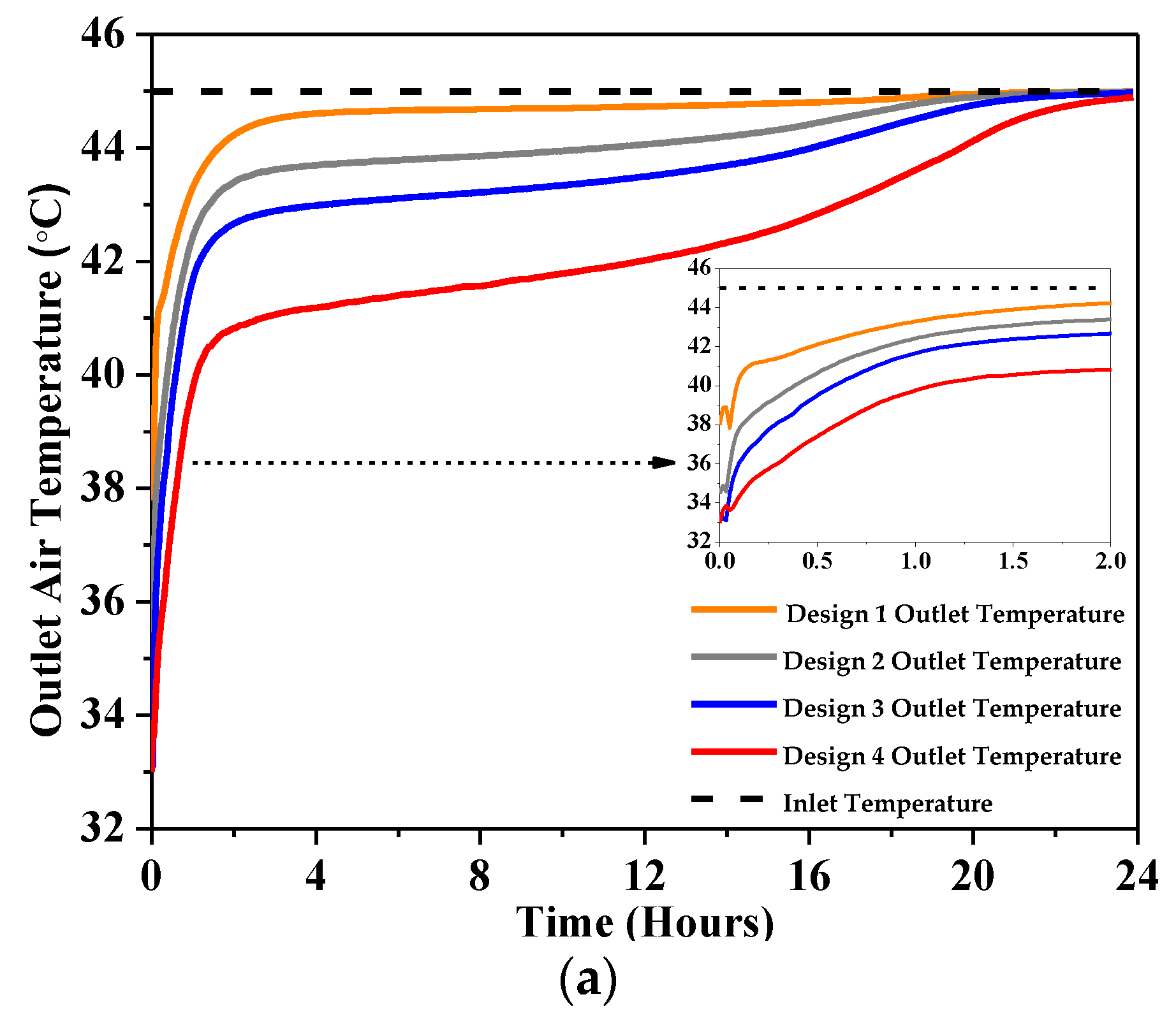
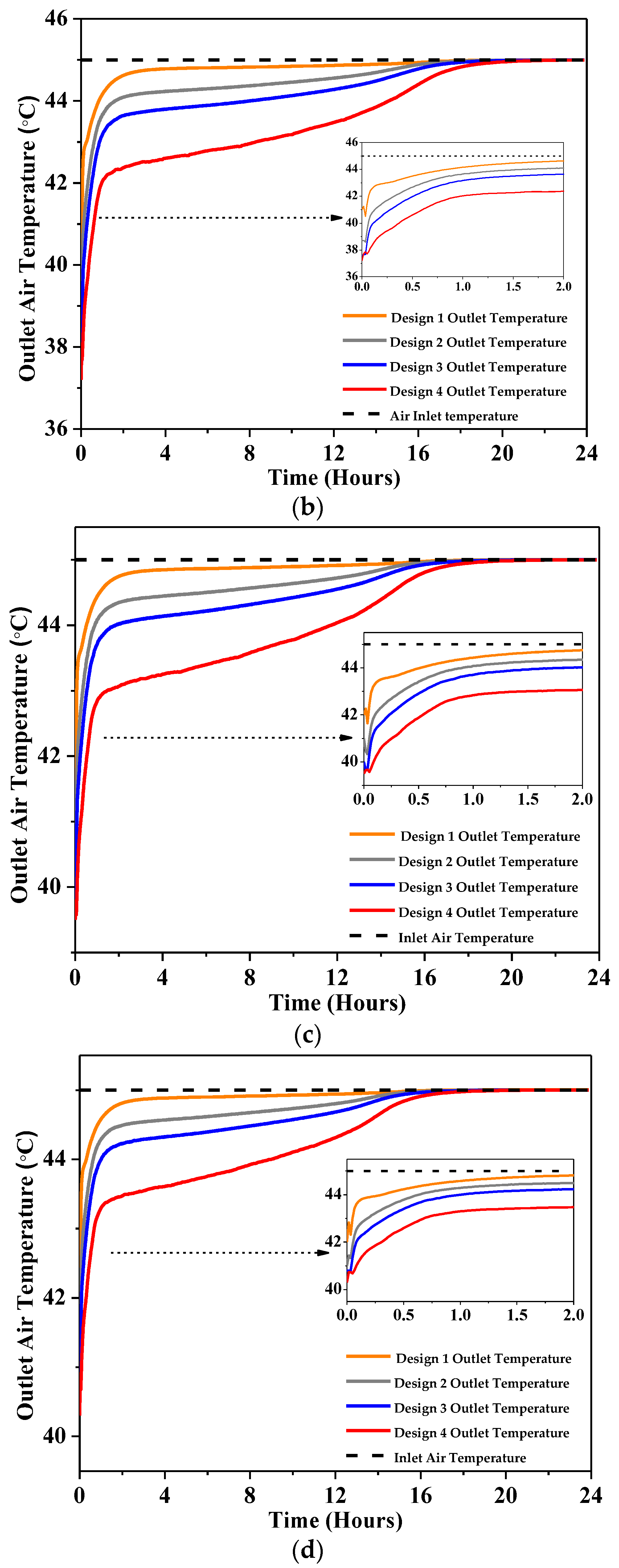
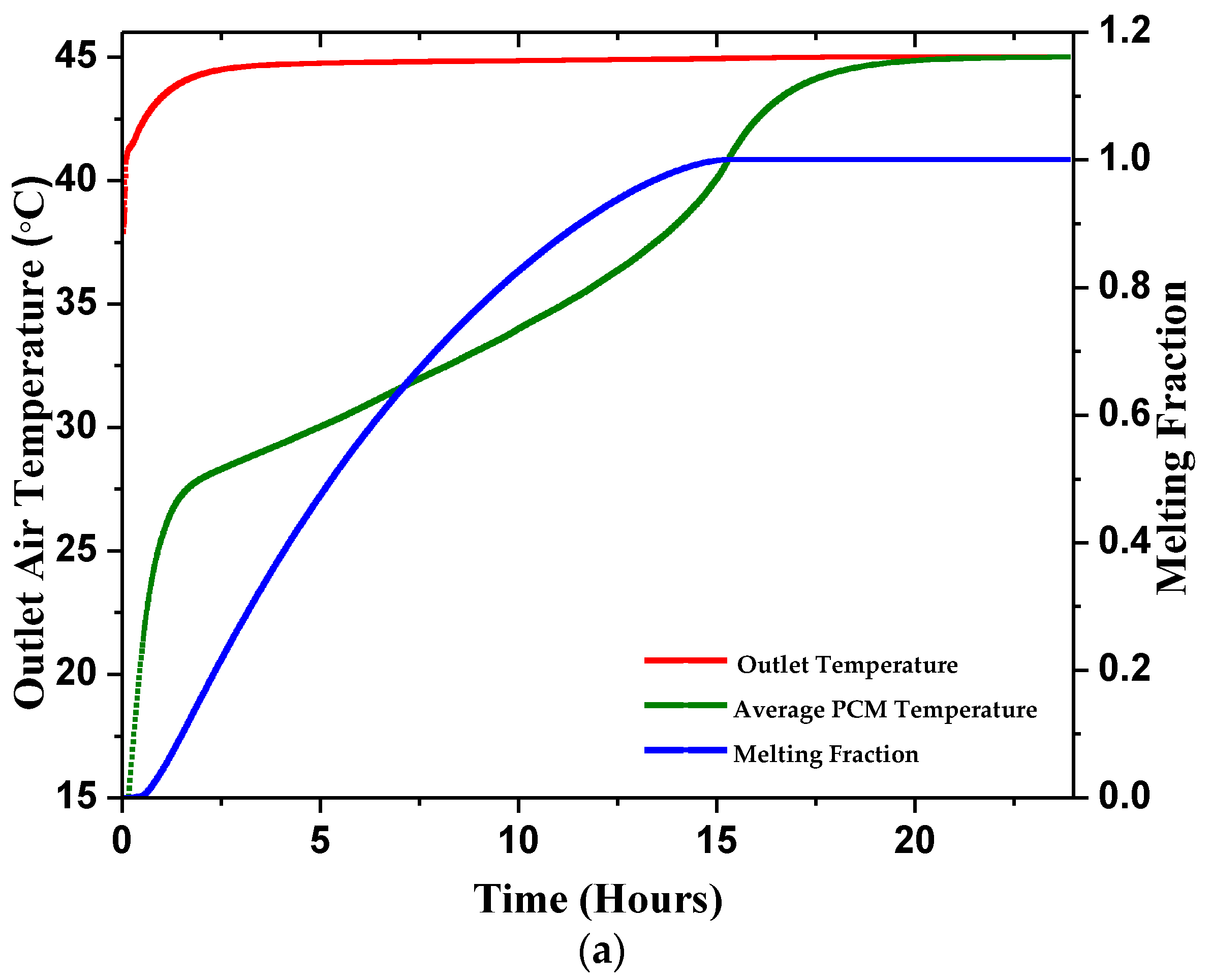
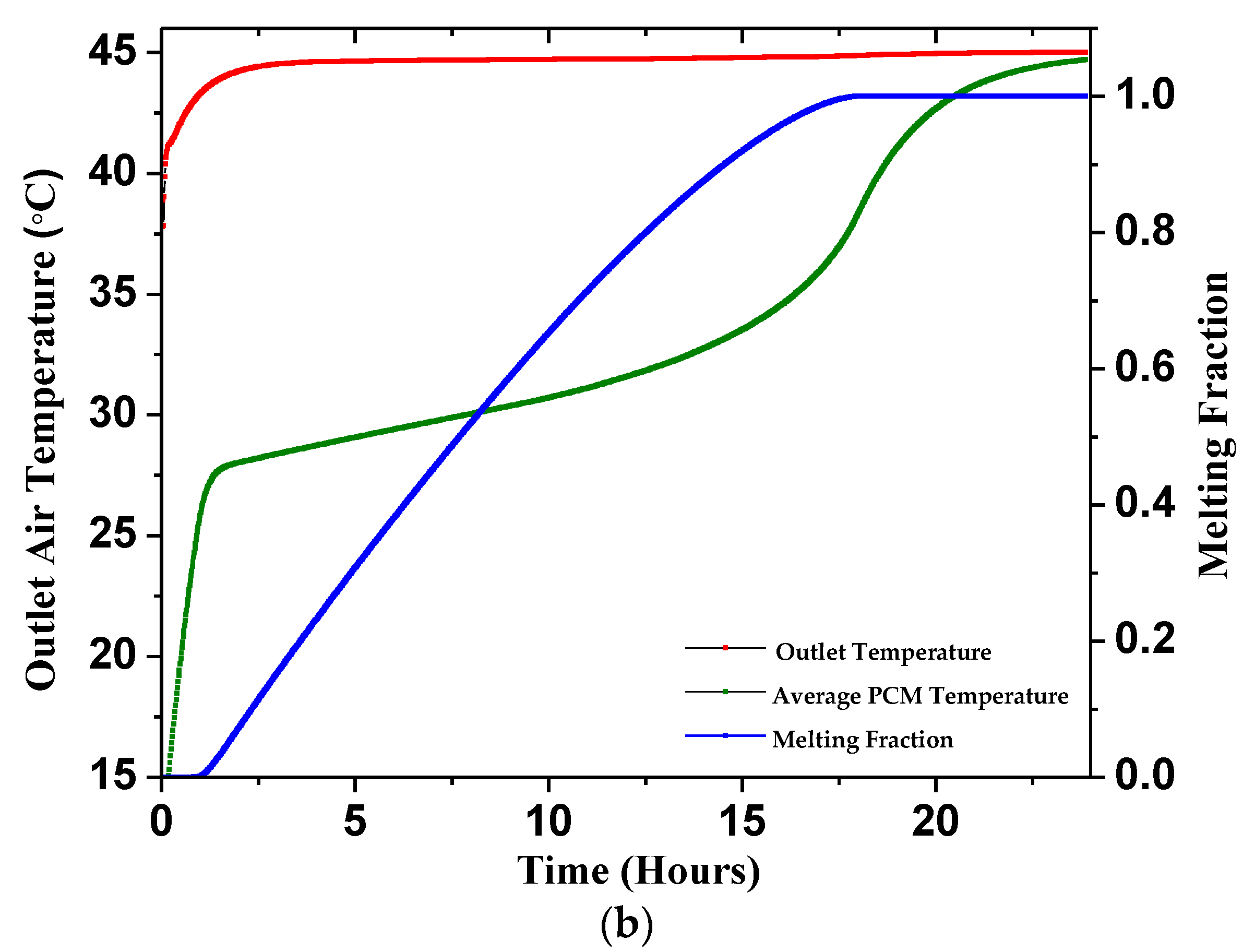
4.2.1. Outlet Temperature
4.2.2. Average PCM Temperature
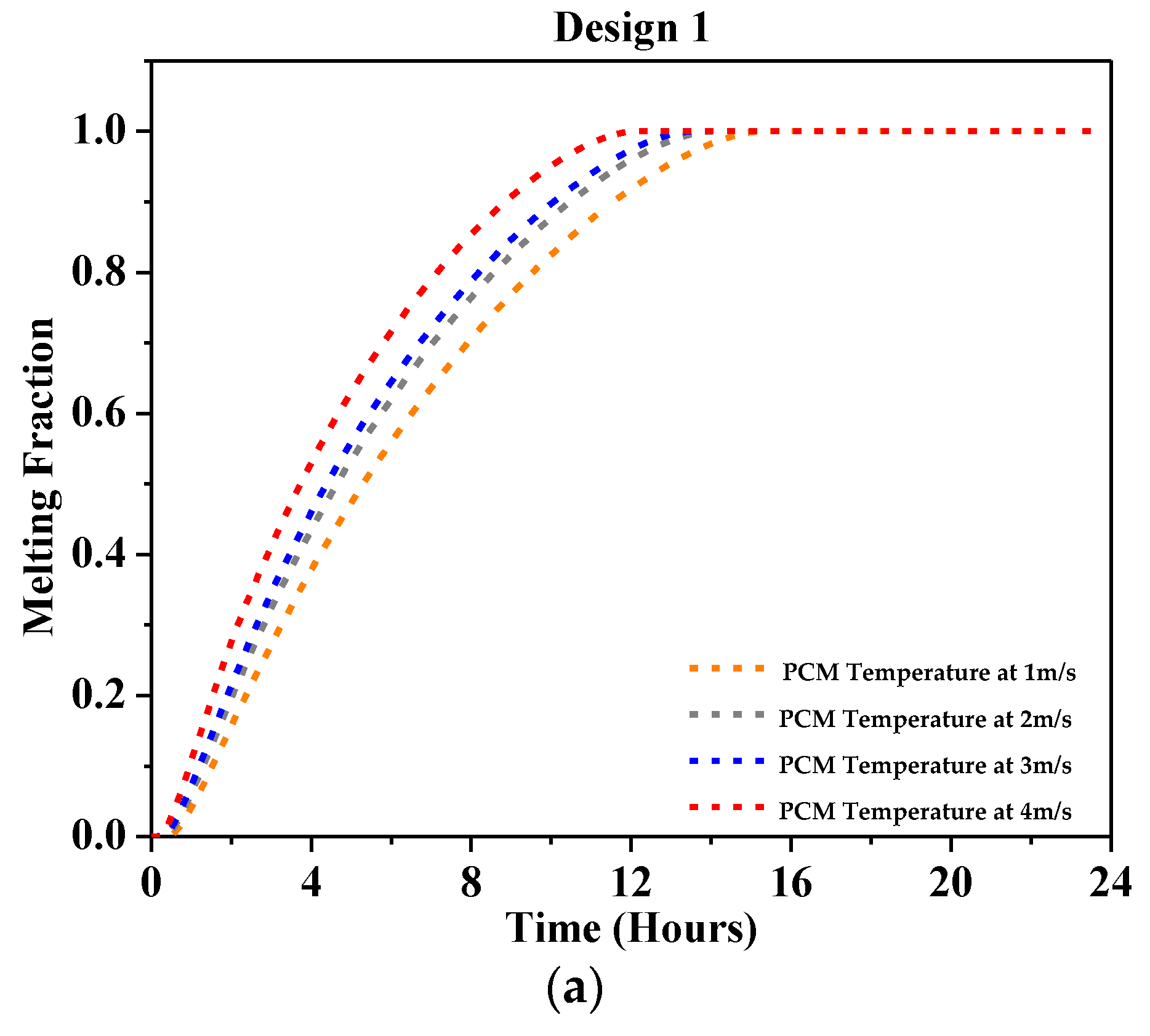
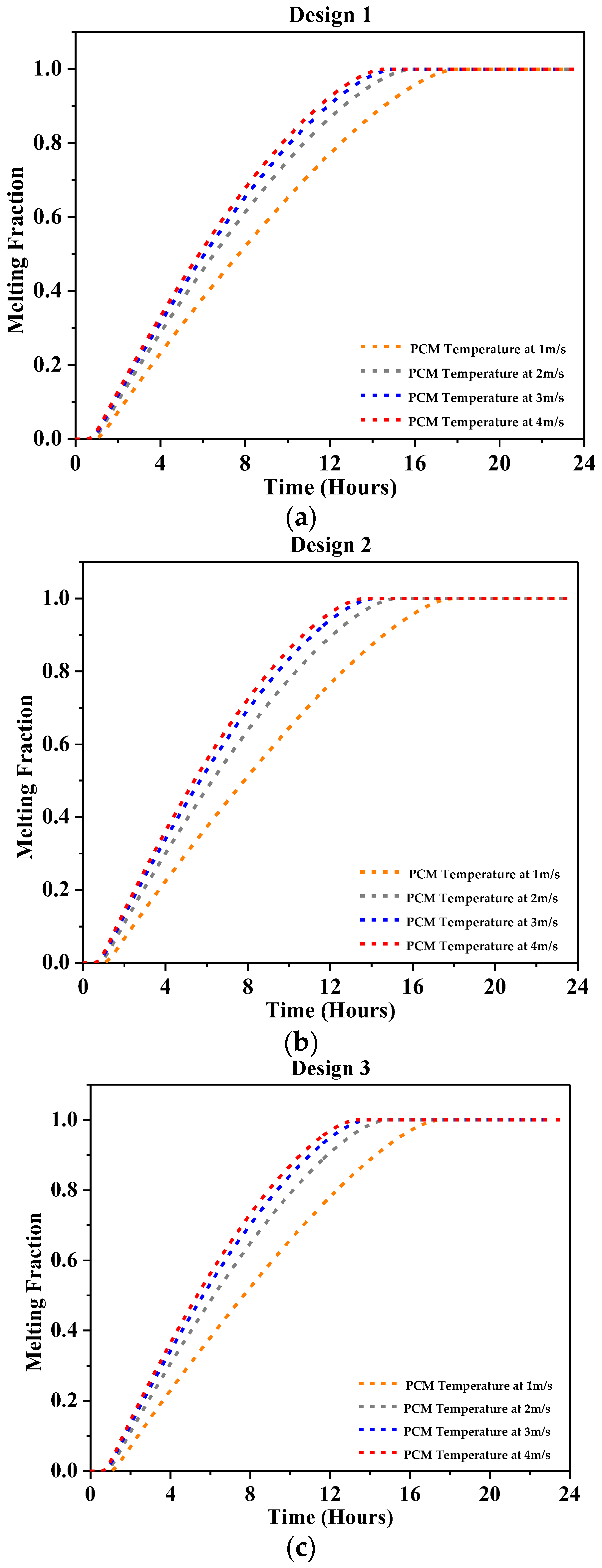
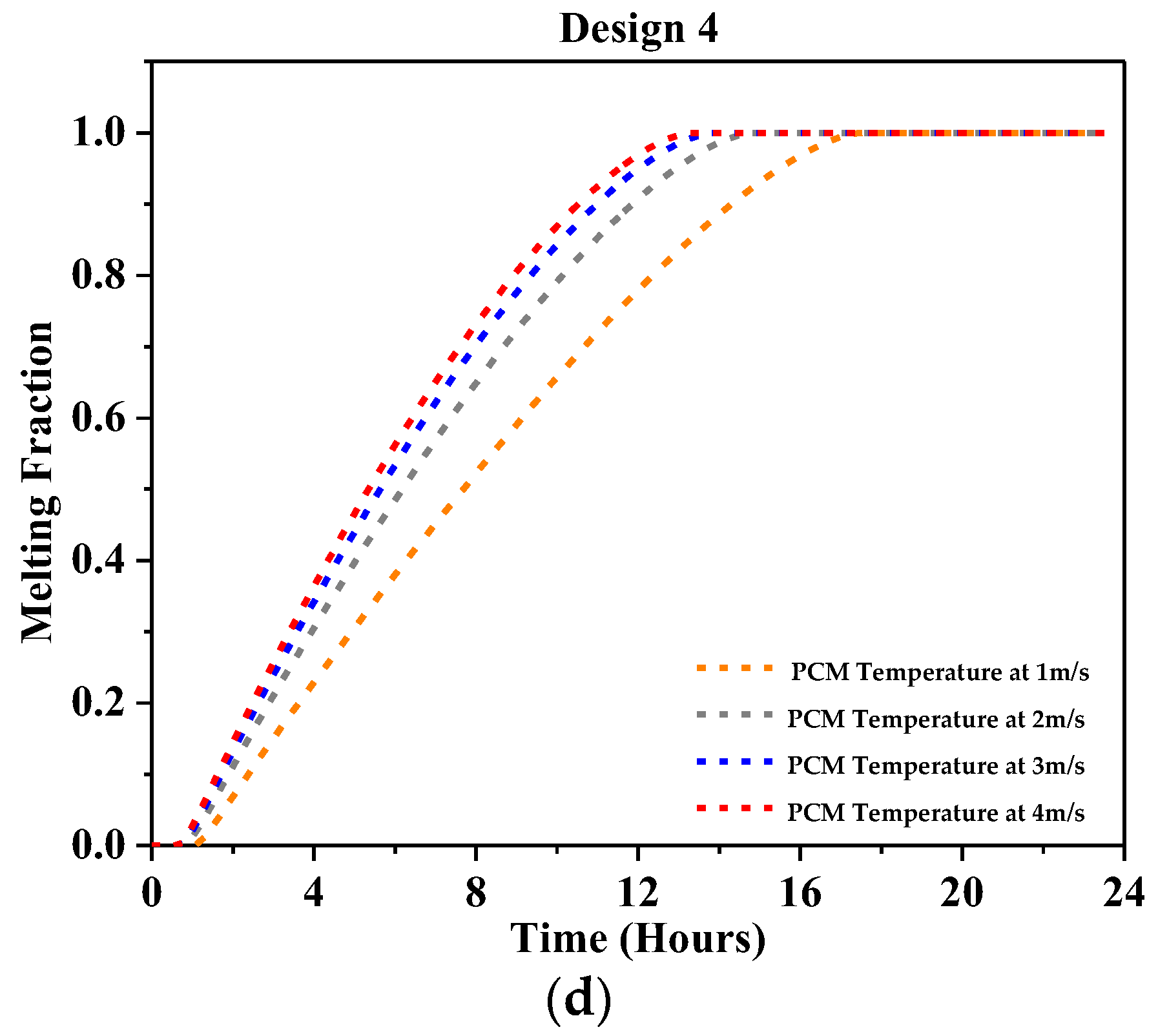
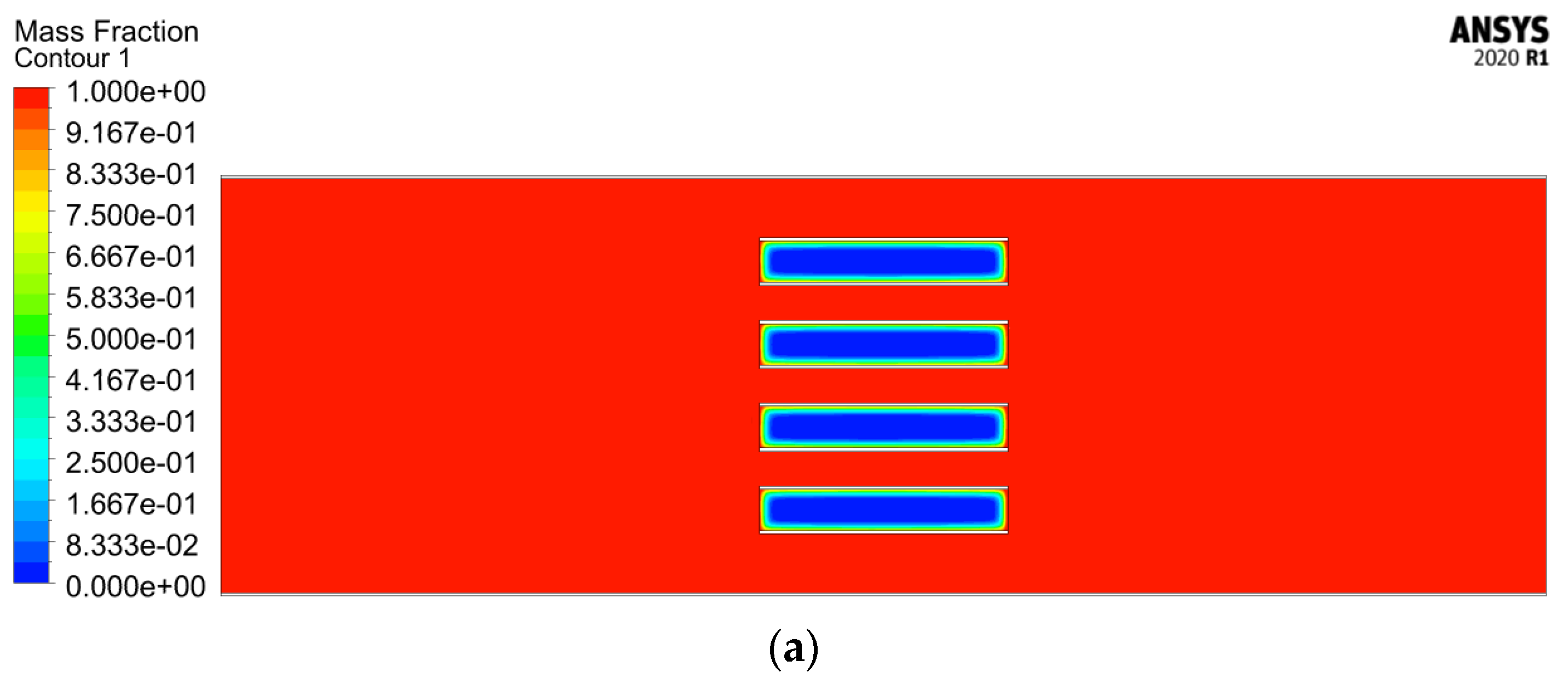
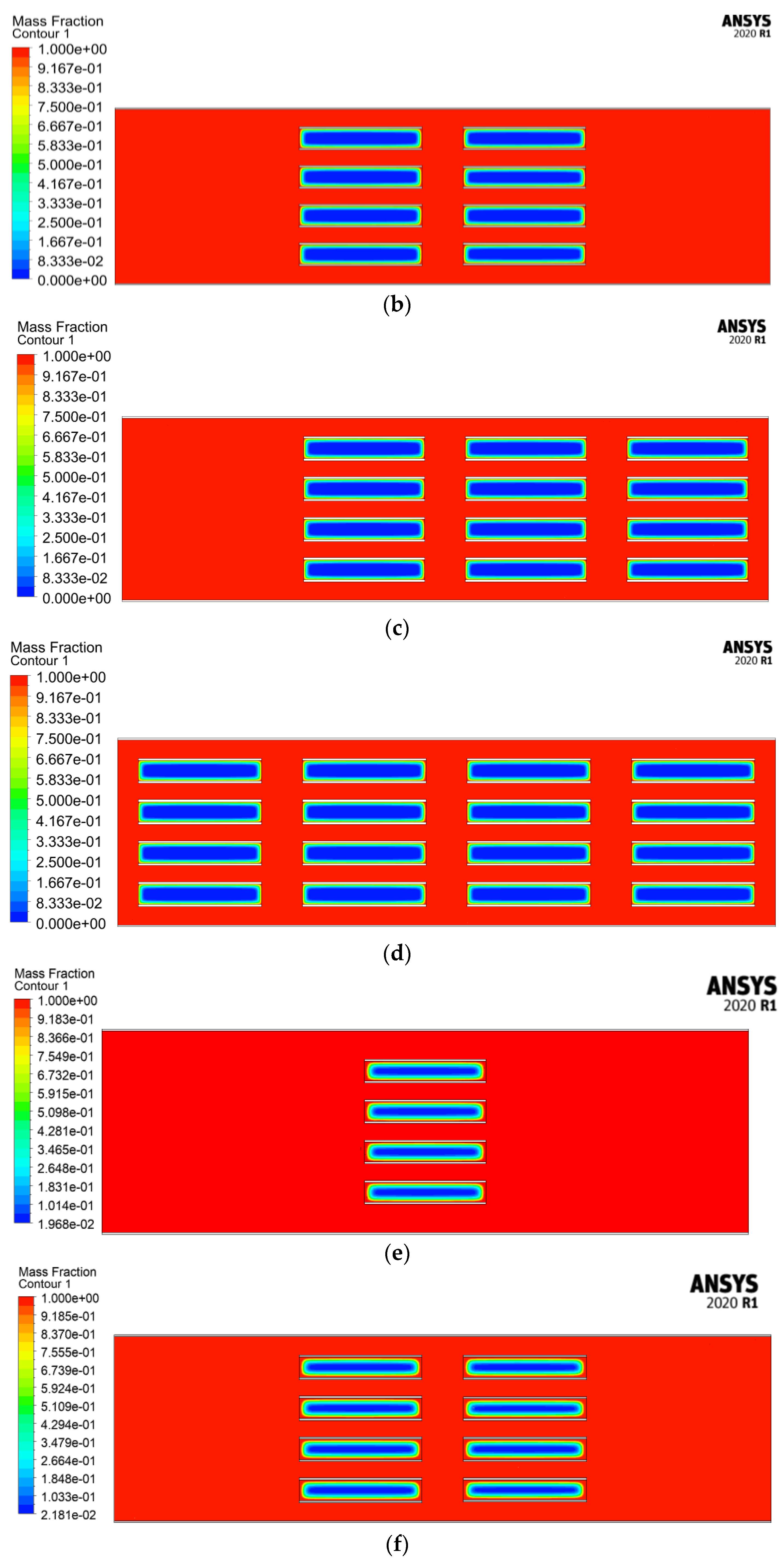
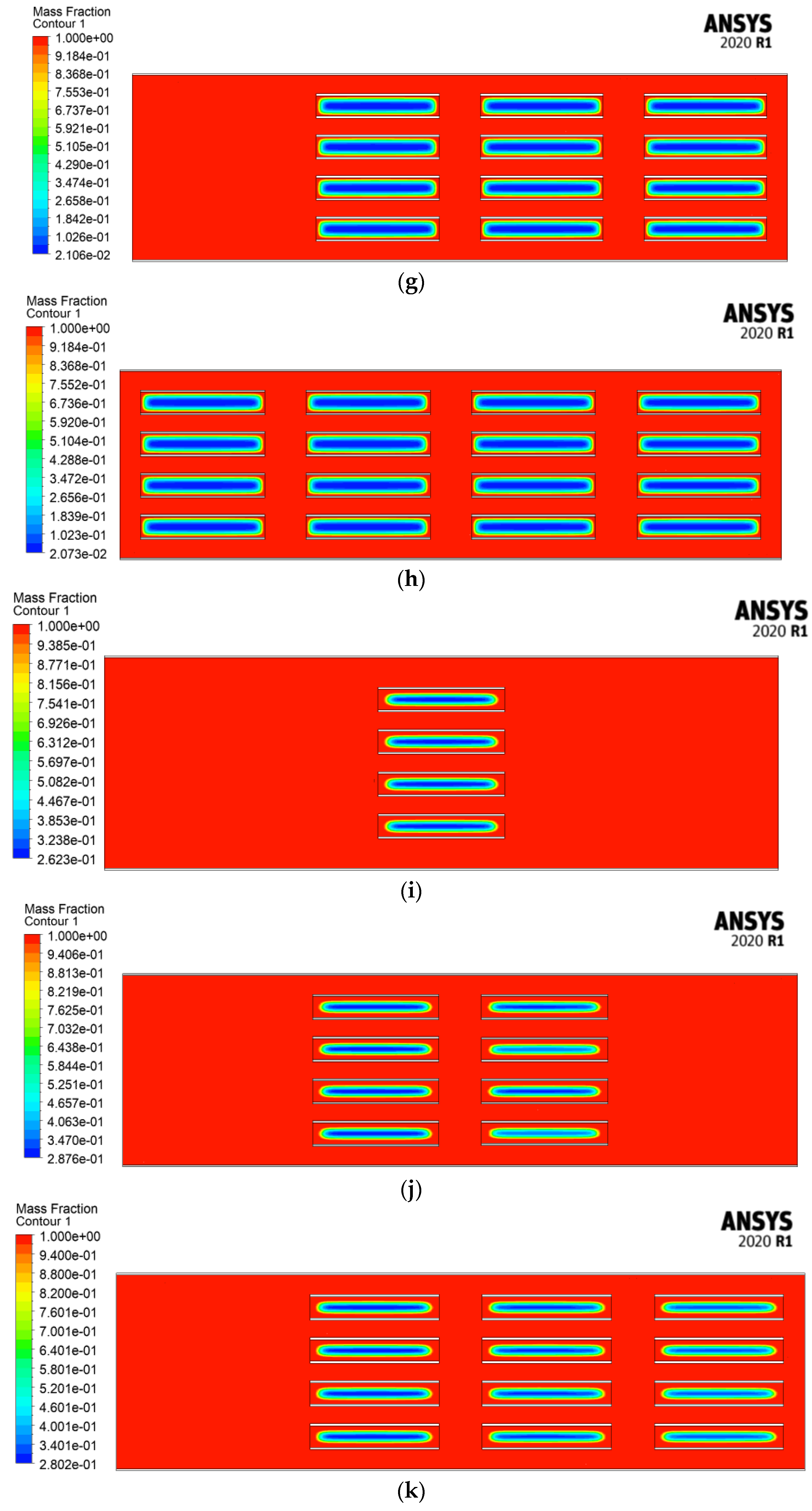
4.2.3. Melting Fraction
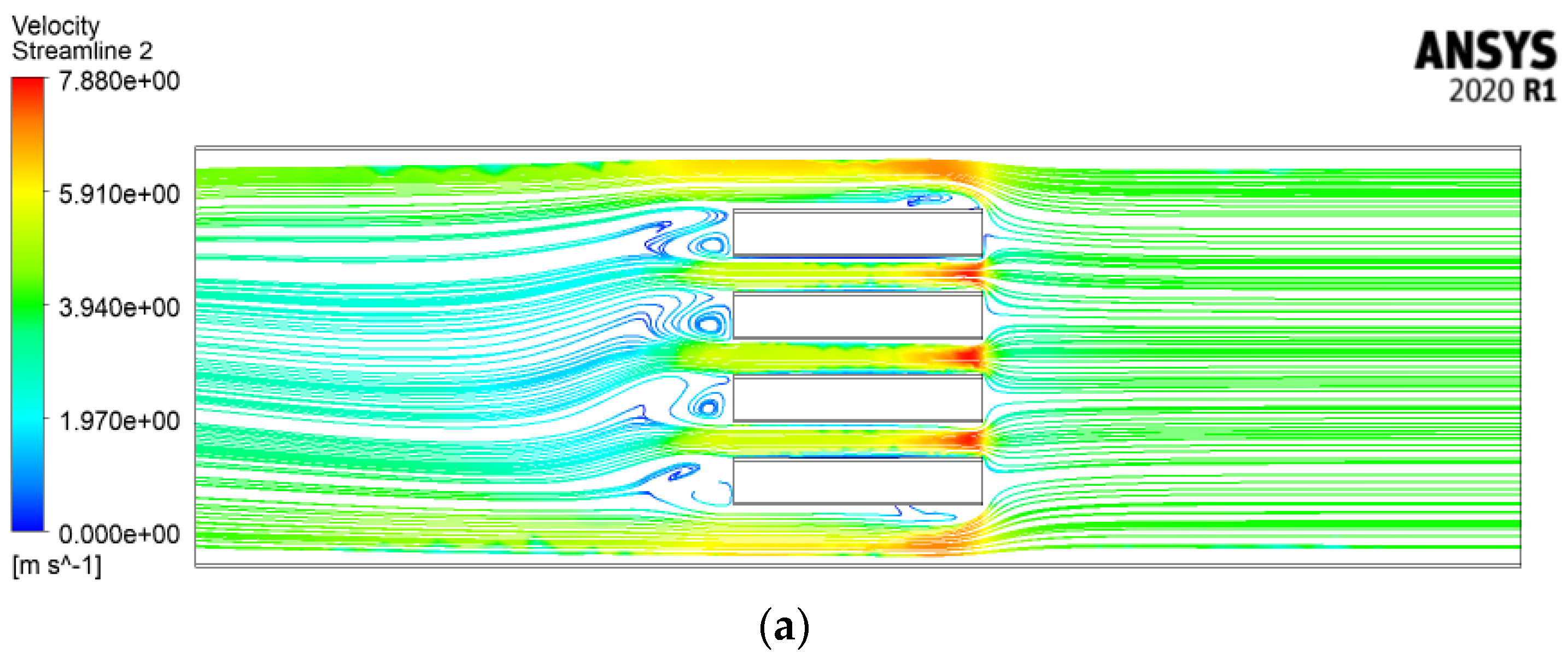
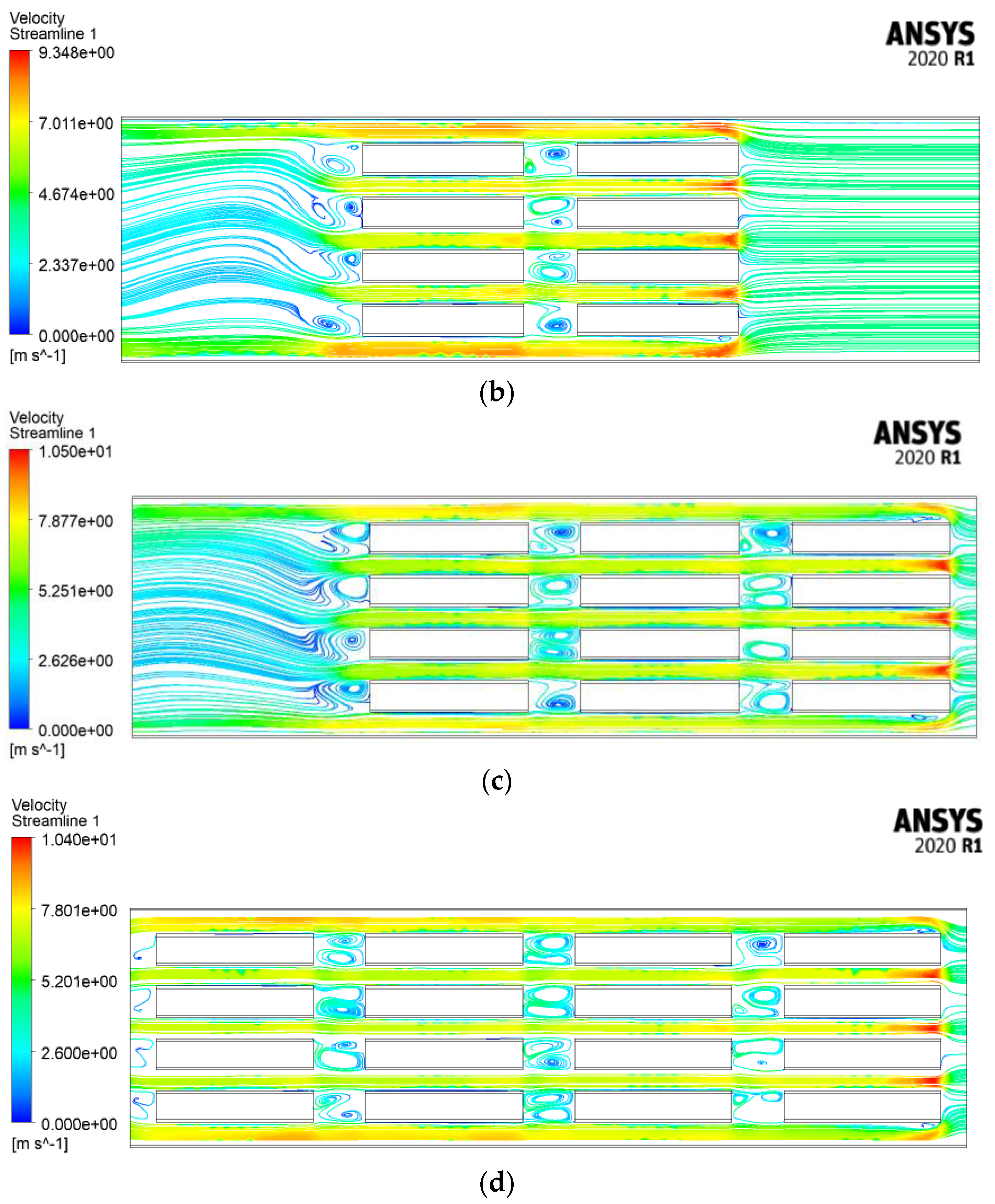
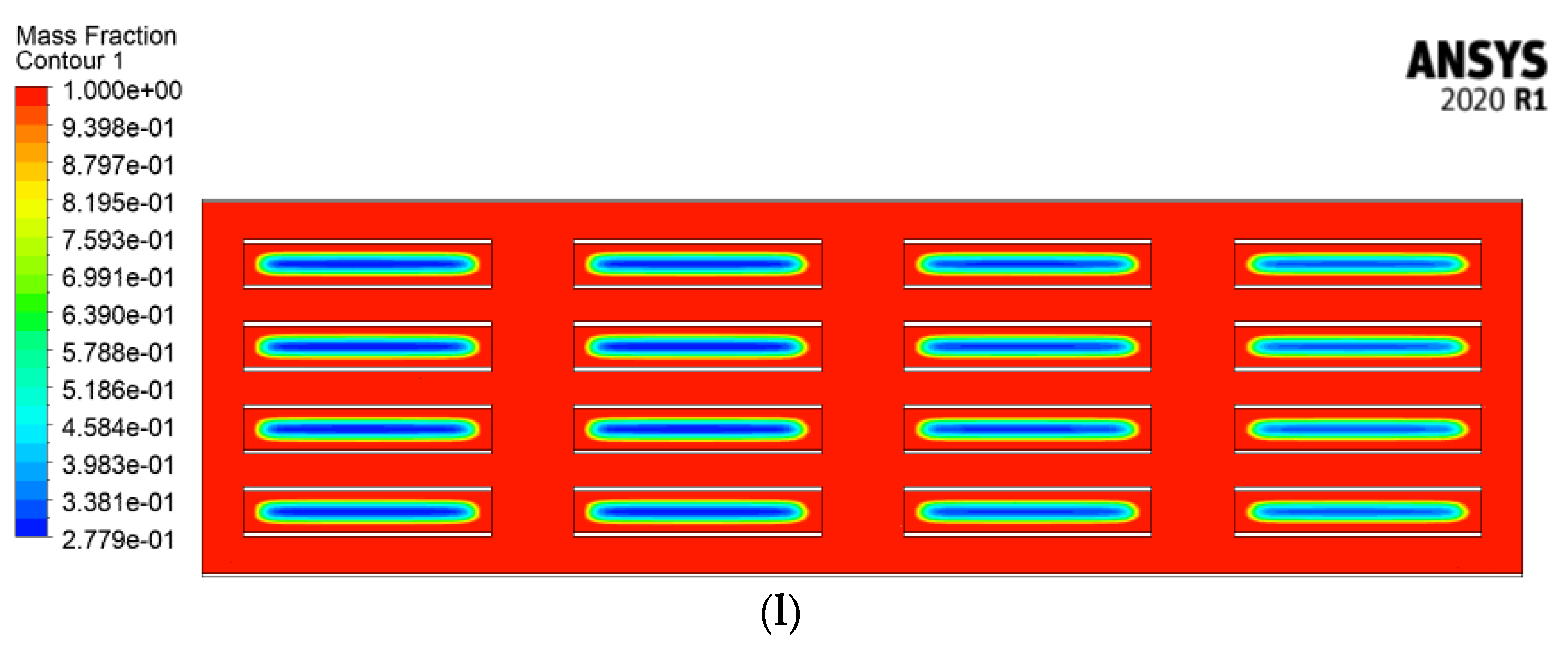
4.2.4. Velocity Streamlines and Melting Fraction Contours
5. Conclusions
- At 1 m/s, a significant outlet air temperature drop was reported, about 7 °C for Design 1, 10 °C for Design 2, 12 °C for Design 3, and 12.2 °C for Design 4 using RT-31.
- At 2 m/s, the peak air temperature dropped by a maximum of 4 °C for Design 1, 7 °C for Design 2, and 8 °C for Design 3 and Design 4, as reported using RT-31.
- At 3 m/s, the peak outlet temperature dropped by 6.5 °C at most with Design 4 and 3 °C at least with Design 1 as reported using RT-31.
- At 4 m/s, the highest temperature drop showed a 5 °C drop in peak air temperature at most with Design 4 and 2.5 °C at least with Design 1 was reported using RT-31.
- Similar highest temperature drop trends were reported using CaCl2·6H2O compared to paraffin, which was able to sustain the temperature drop for more time.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gagliano, A.; Patania, F.; Nocera, F.; Ferlito, A.; Galesi, A. Thermal performance of ventilated roofs during the summer period. Energy Build. 2012, 49, 611–618. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Chàfer, M. Technological options and strategies towards zero energy buildings contributing to climate change mitigation: A systematic review. Energy Build. 2020, 219, 110009. [Google Scholar] [CrossRef]
- Masood, U.; Haggag, M.; Hassan, A.; Laghari, M. A Review of Phase Change Materials as a Heat Storage Medium for Cooling Applications in the Built Environment. Buildings 2023, 13, 1595. [Google Scholar] [CrossRef]
- Hasan, A.; Al-Sallal, K.A.; Alnoman, H.; Rashid, Y.; Abdelbaqi, S. Effect of phase change materials (PCMs) integrated into a concrete block on heat gain prevention in a hot climate. Sustainability 2016, 8, 1009. [Google Scholar] [CrossRef]
- Velmurugan, K.; Kumarasamy, S.; Wongwuttanasatian, T.; Seithtanabutara, V. Review of PCM types and suggestions for an applicable cascaded PCM for passive PV module cooling under tropical climate conditions. J. Clean. Prod. 2021, 293, 126065. [Google Scholar] [CrossRef]
- Hasan, A.; Hejase, H.; Abdelbaqi, S.; Assi, A.; Hamdan, M.O. Comparative effectiveness of different phase change materials to improve cooling performance of heat sinks for electronic devices. Appl. Sci. 2016, 6, 226. [Google Scholar] [CrossRef]
- Vanaga, R.; Blumberga, A.; Freimanis, R.; Mols, T.; Blumberga, D. Solar facade module for nearly zero energy building. Energy 2018, 157, 1025–1034. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castellón, C.; Nogués, M.; Medrano, M.; Leppers, R.; Zubillaga, O. Use of microencapsulated PCM in concrete walls for energy savings. Energy Build. 2007, 39, 113–119. [Google Scholar] [CrossRef]
- Experimental Investigation of PCM Transient Performance in Free Cooling of the Fresh Air of Air Conditioning Systems-ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S2352710219316882?casa_token=g7ablpICE6gAAAAA:6Nrl-B3yqM1LoHvOHoUDVfu-YJ5T1EBy_NIgMqWZqZY-rCHtQZkx_PhEyTcnPJ353bYXgTu6gC8z (accessed on 17 September 2023).
- Energy Saving in Building with PCM Cold Storage-Stritih-2007-International Journal of Energy Research-Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1002/er.1318 (accessed on 17 September 2023).
- Liu, S.; Iten, M.; Shukla, A. Numerical study on the performance of an air—Multiple PCMs unit for free cooling and ventilation. Energy Build. 2017, 151, 520–533. [Google Scholar] [CrossRef]
- Gado, M.G.; Hassan, H. Energy-saving potential of compression heat pump using thermal energy storage of phase change materials for cooling and heating applications. Energy 2023, 263, 126046. [Google Scholar] [CrossRef]
- Chaiyat, N.; Kiatsiriroat, T. Energy reduction of building air-conditioner with phase change material in Thailand. Case Stud. Therm. Eng. 2014, 4, 175–186. [Google Scholar] [CrossRef]
- Huang, B.; Zheng, Z.; Lu, G.; Zhai, X. Design and experimental investigation of a PCM based cooling storage unit for emergency cooling in data center. Energy Build. 2022, 259, 111871. [Google Scholar] [CrossRef]
- Akbari, A.D.; Talati, F.; Mahmoudi, S.M.S. New solution method for latent energy storage and thermoeconomic optimization for an air conditioning system. Int. J. Refrig. 2020, 109, 12–24. [Google Scholar] [CrossRef]
- Said, M.A.; Hassan, H. Parametric study on the effect of using cold thermal storage energy of phase change material on the performance of air-conditioning unit. Appl. Energy 2018, 230, 1380–1402. [Google Scholar] [CrossRef]
- Nagano, K.; Takeda, S.; Mochida, T.; Shimakura, K.; Nakamura, T. Study of a floor supply air conditioning system using granular phase change material to augment building mass thermal storage—Heat response in small scale experiments. Energy Build. 2006, 38, 436–446. [Google Scholar] [CrossRef]
- A Comparative Study on PCM and Ice Thermal Energy Storage Tank for Air-Conditioning Systems in Office Buildings-ScienceDirect. Available online: https://www.sciencedirect.com/science/article/abs/pii/S1359431115013551?via%3Dihub (accessed on 17 September 2023).
- Vakilaltojjar, S.M.; Saman, W. Analysis and modelling of a phase change storage system for air conditioning applications. Appl. Therm. Eng. 2001, 21, 249–263. [Google Scholar] [CrossRef]
- Mosaffa, A.H.; Farshi, L.G.; Ferreira, C.A.I.; Rosen, M.A. Advanced exergy analysis of an air conditioning system incorporating thermal energy storage. Energy 2014, 77, 945–952. [Google Scholar] [CrossRef]
- Mosaffa, A.H.; Farshi, L.G. Exergoeconomic and environmental analyses of an air conditioning system using thermal energy storage. Appl. Energy 2016, 162, 515–526. [Google Scholar] [CrossRef]
- Maccarini, A.; Hultmark, G.; Bergsøe, N.C.; Afshari, A. Free cooling potential of a PCM-based heat exchanger coupled with a novel HVAC system for simultaneous heating and cooling of buildings. Sustain. Cities Soc. 2018, 42, 384–395. [Google Scholar] [CrossRef]
- Hai, T.; Sajadi, S.M.; Zain, J.M.; El-Shafay, A.S.; Sharifpur, M. The effect of using tubes filled with phase change materials in the air conditioning system of a residential building. J. Build. Eng. 2022, 49, 104079. [Google Scholar] [CrossRef]
- Farah, S.; Liu, M.; Saman, W. Numerical investigation of phase change material thermal storage for space cooling. Appl. Energy 2019, 239, 526–535. [Google Scholar] [CrossRef]
- Nie, B.; She, X.; Du, Z.; Xie, C.; Li, Y.; He, Z.; Ding, Y. System performance and economic assessment of a thermal energy storage based air-conditioning unit for transport applications. Appl. Energy 2019, 251, 113254. [Google Scholar] [CrossRef]
- Hasan, A.; McCormack, S.J.; Huang, M.J.; Norton, B. Characterization of phase change materials for thermal control of photovoltaics using Differential Scanning Calorimetry and Temperature History Method. Energy Convers. Manag. 2014, 81, 322–329. [Google Scholar] [CrossRef]
- Lewis, R.W.; Morgan, K.; Thomas, J.R.; Seetharamu, I.N. The Finite Element Method in Heat Transfer Analysis; John Wiley & Sons: Hoboken, NJ, USA, 1999. [Google Scholar]
- Reese, T.G.; Heid, O.; Weisskoff, R.M.; Wedeen, V.J. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 2003, 49, 177–182. [Google Scholar] [CrossRef]
- Bang, D.; Lee, J.H.; Lee, E.S.; Lee, S.; Choi, J.S.; Kim, Y.K.; Cho, B.K.; Koh, J.K.; Won, Y.H.; Kim, N.I.; et al. Epidemiologic and clinical survey of Behcet’s disease in Korea: The first multicenter study. J. Korean Med. Sci. 2001, 16, 615–618. [Google Scholar] [CrossRef]
- Lamberg, P.; Lehtiniemi, R.; Henell, A.M. Numerical and experimental investigation of melting and freezing processes in phase change material storage. Int. J. Therm. Sci. 2004, 43, 277–287. [Google Scholar] [CrossRef]

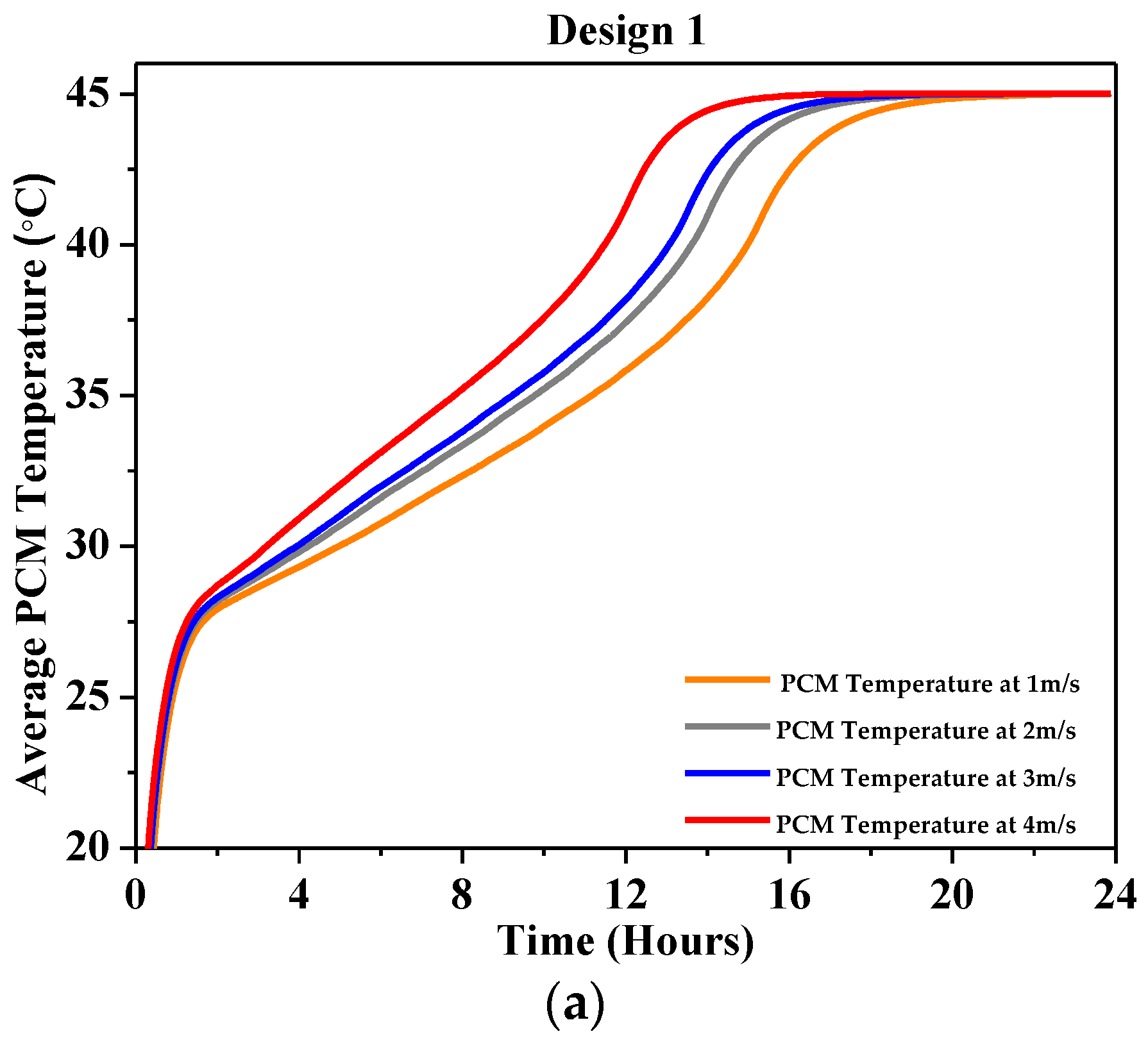
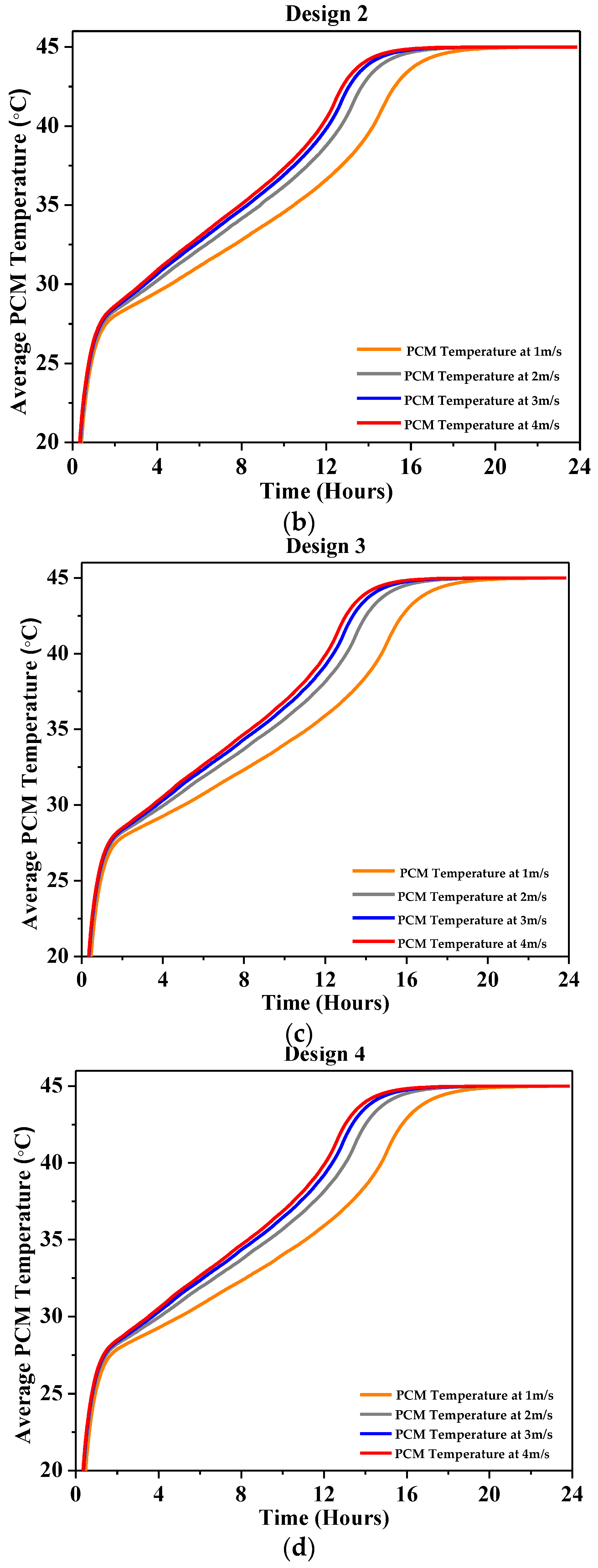
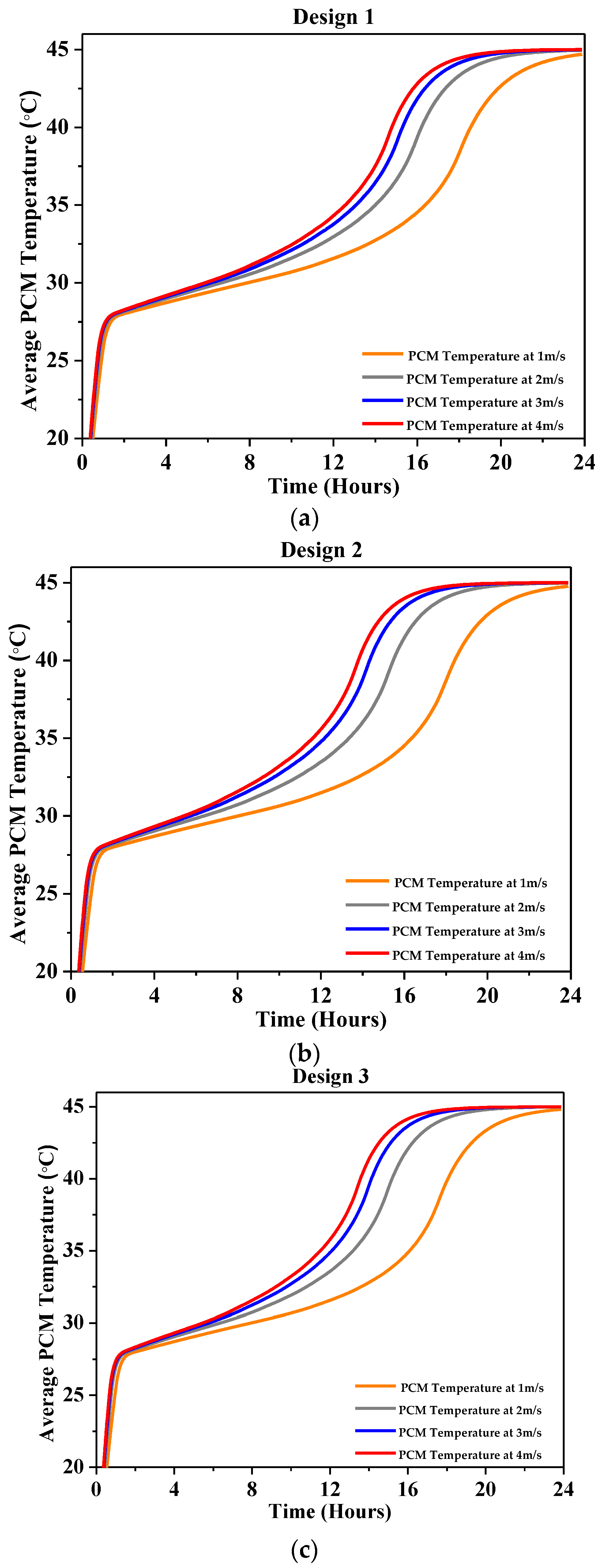
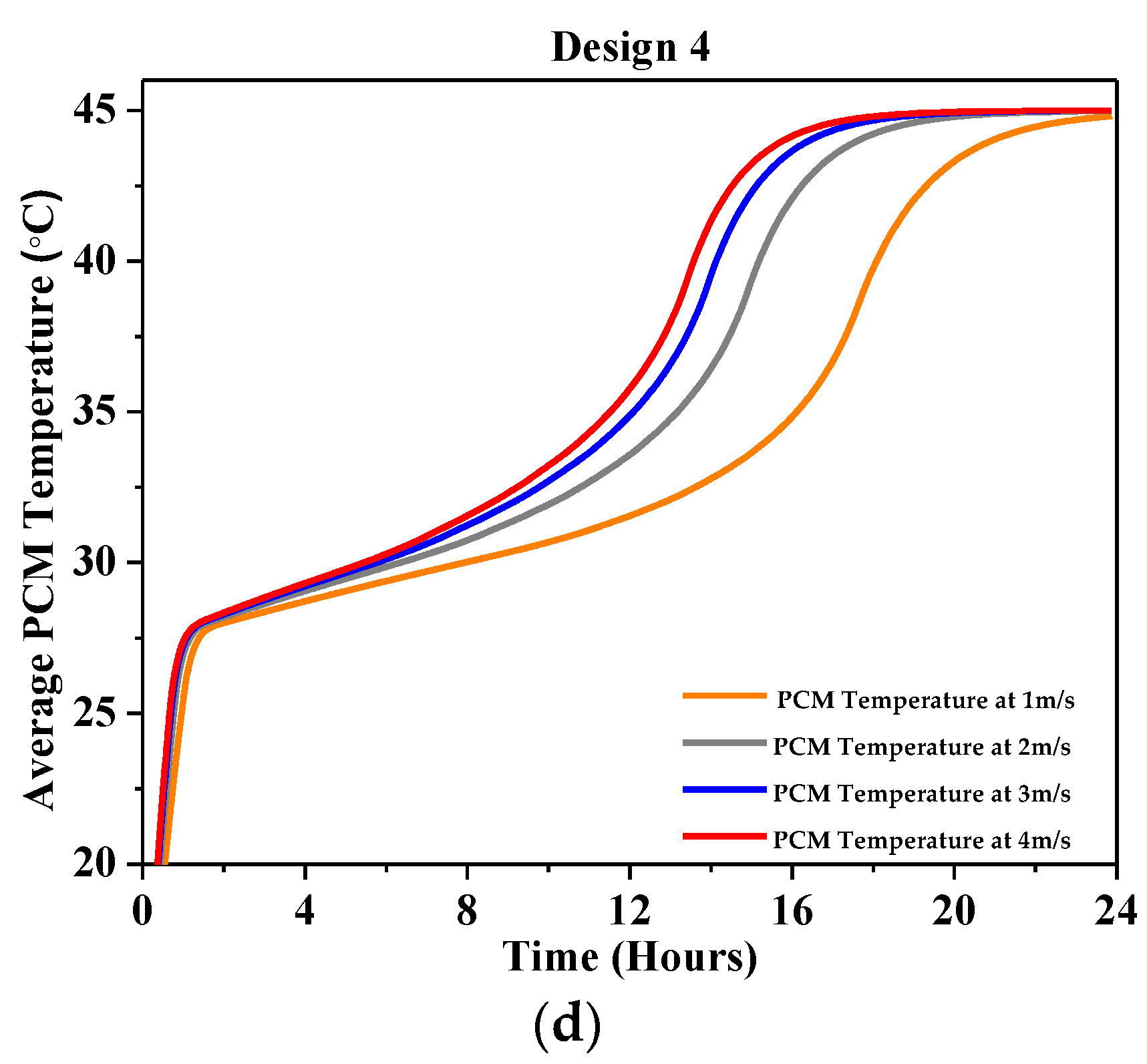

| Characteristics | Description |
|---|---|
| Area of the window | 10% of the gross wall area—uniformly distributed |
| Area of the floor | 300 m2 |
| Solar absorbance value | 0.50 for the external walls and roof |
| Window | 6 mm single green-tinted glazing |
| Occupancy density | 6 people |
| Equip. power density | 7 W/m2 |
| Lighting power density | 4.5 W/m2 |
| Property | RT-31 | CaCl2·6H2O | Aluminum | Air |
|---|---|---|---|---|
| Melting temperature range (°C) | 27–33 | 27.7–32.5 | 660 | - |
| Latent heat of fusion (kJ kg−1) | 158 | 187 | - | - |
| Specific heat (kJ kg−1 K−1) | 2 | 1.4 (solid), 2.2 (liquid) | 8.7 | 1.007 |
| Density (kg m−3) | 900 | 1710 (solid), 1530 (liquid) | 2710 | 1.14 |
| Thermal conductivity (W m−1 K−1) | 0.2 | 1.09 (solid), 0.53 (liquid) | 237 | 0.028 |
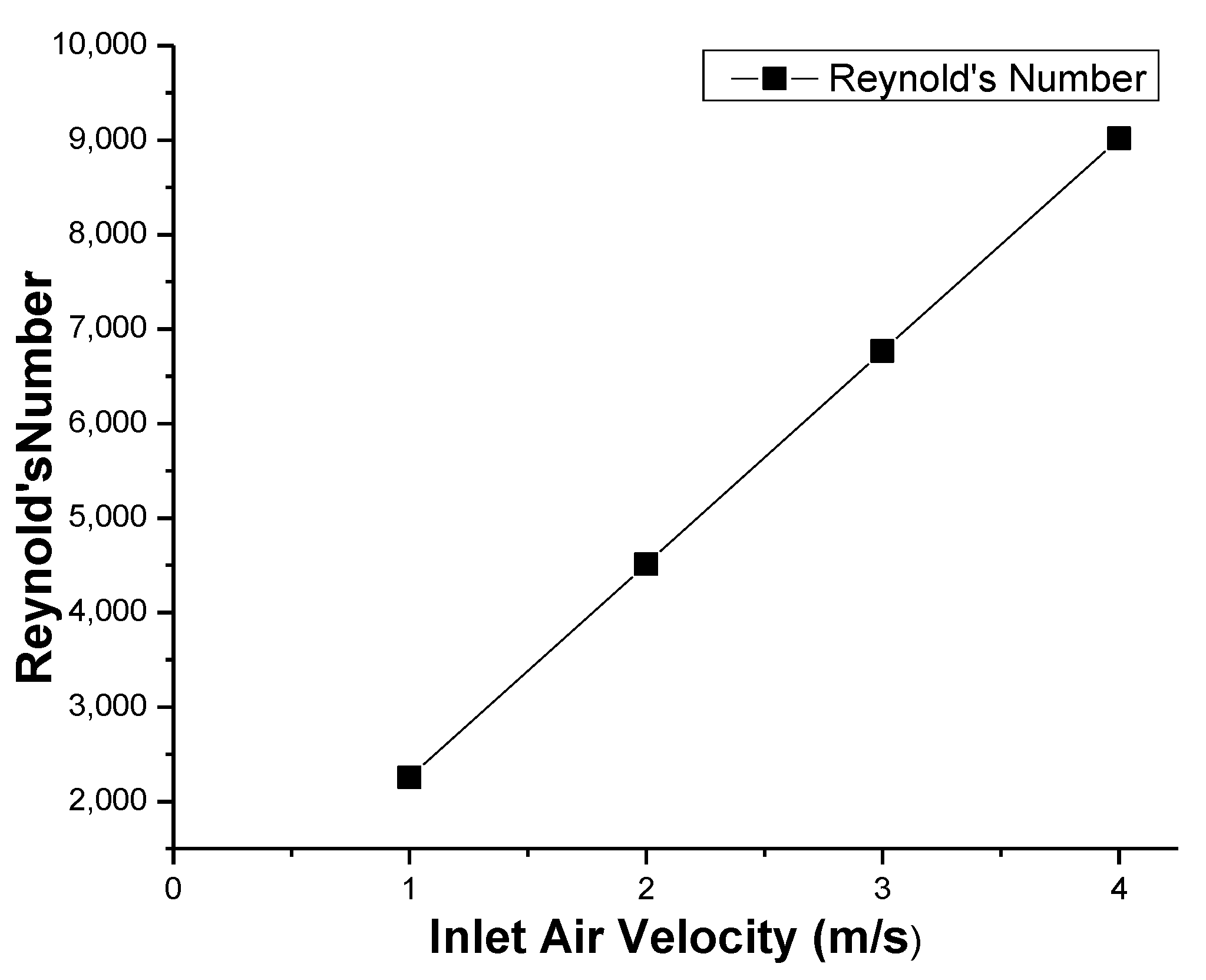
| Velocity (m s−1) | - | - | 1, 2, 3, 4 m/s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masood, U.; Haggag, M.; Hassan, A.; Laghari, M. Evaluation of Phase Change Materials for Pre-Cooling of Supply Air into Air Conditioning Systems in Extremely Hot Climates. Buildings 2024, 14, 95. https://doi.org/10.3390/buildings14010095
Masood U, Haggag M, Hassan A, Laghari M. Evaluation of Phase Change Materials for Pre-Cooling of Supply Air into Air Conditioning Systems in Extremely Hot Climates. Buildings. 2024; 14(1):95. https://doi.org/10.3390/buildings14010095
Chicago/Turabian StyleMasood, Usman, Mahmoud Haggag, Ahmed Hassan, and Mohammad Laghari. 2024. "Evaluation of Phase Change Materials for Pre-Cooling of Supply Air into Air Conditioning Systems in Extremely Hot Climates" Buildings 14, no. 1: 95. https://doi.org/10.3390/buildings14010095
APA StyleMasood, U., Haggag, M., Hassan, A., & Laghari, M. (2024). Evaluation of Phase Change Materials for Pre-Cooling of Supply Air into Air Conditioning Systems in Extremely Hot Climates. Buildings, 14(1), 95. https://doi.org/10.3390/buildings14010095







