Abstract
Heritage buildings face risks related to the degradation of exhibited or stored artefacts, up to their destruction over time, as well as the health of workers and visitors. The main causes are microclimatic parameters (temperature, humidity, brightness, particles suspension, pollutants, degree of ventilation or air circulation), biological (bacteria, fungi, molds and insects) and anthropogenic ones (improper maintenance of the building and overcrowding of rooms). In accordance with these, the present study considers a quantitative and qualitative analysis of the air quality and the degree of microbiological contamination of the surfaces and the air inside a synagogue in the municipality of Oradea, Romania. The microbiological study highlighted the presence of some potentially harmful genera of fungi (Alternaria sp., Penicillium sp., Aspergillus sp., Botrytis sp. and Cladosporium sp.) in the indoor air and on the surfaces inside the synagogue; suggesting an average degree of fungal contamination, with possible risk to individual health, especially in children and people with allergic status or allergic respiratory diseases. Statistical analysis concerning the occupational exposure to airborne microbes poses health risks to employees and visitors. Multivariate regression analysis results emphasize that higher symptoms scores were independently associated with experiencing a too low indoor air temperature; these symptoms would disappear within one to two hours after leaving the space. Air pollutants have become part of everyday life; therefore, consistent monitoring of indoor environments offers an effective approach to prevent or minimize the adverse health risk to building occupants in spaces such as heritage buildings.
1. Introduction
Heritage buildings, holding heritage objects such as museums, churches, libraries, and archives [1,2,3], face risks related to their degradation or even their destruction over time. Thus, it is necessary to understand the variability of fungal spores in relation to the type of artwork [4] and the potential risk of human illness, caused by biological factors (e.g., bacteria, fungi, molds, and insects) and microclimatic factors (e.g., air, humidity, temperature, light, particles in suspension, and pollutants) [5]. Poor construction and maintenance practices can also contribute to the creation of pollutants [6]. Risks associated with these pollutants can cause adverse health effects on respiratory, neurological, reproductive, dermatological and cardiovascular systems [7,8]. It is also important to understand the level of risk exposure from poor indoor air quality (IAQ) in relation to the time spent in the indoor environment as humans spend a considerable amount of time indoors [9,10,11].
Biological pollutants are caused by microclimatic parameters in the building which in turn result in early biodeterioration of building materials [11]. High concentrations of biological pollutants such as fungal spores result in high concentrations of particulate matter and cardon dioxide in the indoor spaces [11]. Nanomaterials used for conservation or other purposes in this kind of public spaces also expose their occupants to risk from chemical pollutants [10,11], because nanomaterials are a source of chemical pollutants despite the important role they play in the conservation efforts of heritage buildings and artifacts [12]. However, these nanomaterials which include substances such as biocides, consolidates, materials which function as hydrophobic protectives, mechanical resistance improvers, and flame-retardants can be very toxic to the buildings’ occupants and the natural environment [12]. Humans interact with these substances through ways such as the nasal passage, olfactory system, ophthalmic (eyes), oral (mouth) and dermal (skin) [13]. This results in damage to internal organs and systems including the brain, circulatory and cardiovascular systems. Other sources of chemical pollutants such as nitrogen oxide (Nox), sulfur oxide (Sox), and volatile and semi-volatile organic compounds (VOCs) also contribute to poor IAQ deriving from both natural and anthropic actions on the exterior of the building [14]. These oxides and VOCs also pose a risk to the heritage building and its historical contents by contributing to their deterioration by damaging the materials [14].
Thus, the causes and factors involved should be identified as early as possible and optimal conservation procedures should be applied [15,16,17,18]. Fungal spores have a ubiquitous spread, and the contamination of different rooms is easily achieved through air, especially in buildings with public access and frequently visited by tourists. Although many species of yeasts and molds are saprophytic, those isolated from these institutions are of particular interest due to their pathogenic potential, being capable of triggering or worsening an allergic status, sick-building syndrome and respiratory symptoms [19].
Regarding cultural heritage settings, objects made from organic materials (e.g., textiles, wood and paintings) are particularly susceptible to microbial growth due to their properties [15,20,21,22]. Studies have identified specific fungal genera that commonly contaminate heritage collections, the most common including Aspergillus sp., Penicillium sp., and Cladosporium sp. [20,23], which secrete enzymes, organic acids, and pigments that deteriorate, exhibit materials overtime [20,23,24]. Occupational exposure to microbial contaminants during conservation and restoration work further endangers the health of staff, and visitors [25]. While past research provides valuable insights, gaps remain in fully elucidating the relationships between indoor and outdoor environmental conditions, building operations, art conservation practices and microbial communities within heritage spaces. The synagogue contains an extensive collection of ritual objects, textiles, woodwork, paintings, and documents covering over 150 years. However, no previous studies have investigated indoor and surface microbial contamination at the site or have identified strategies to mitigate its impact.
This research study aims to generate a holistic understanding of microbial contamination risk at Neologic Synagogue Sion Oradea to inform the preservation of its cultural collections and ensure a healthy environment for staff and visitors.
The main research objectives and the specific research objectives of this study are: (1) Assess current environmental conditions in a larger unfolding study, including the relevant building operations at Neologic Synagogue Sion Oradea to identify risk factors for microbial growth. (2) Characterize the microbial communities on indoor air and exhibit materials within the Synagogue. (3) Develop evidence-based recommendations for optimizing environmental control, exhibition practices, and collection care policies to mitigate microbial contamination and deterioration at the synagogue while minimizing intervention impact.
By using a multidisciplinary research approach on Neologic Synagogue Sion Oradea, this study seeks to fill knowledge gaps regarding microbial contamination within an underexplored regional heritage institution. The results can potentially inform targeted strategies for collection preservation, occupant safety, and building performance optimization tailored to the specific challenges facing this and similar heritage sites.
The issue of microclimate and air pollution in the interiors of museums, churches, or other heritage buildings has aroused the interest of many scientists [5,21,26,27,28,29,30]. The connection between sick building syndrome and indoor air pollutants has been extensively studied [31,32]. Therefore, poor indoor air quality from microbial and other pollutants can lead to health issues for occupants. Numerous studies have examined the microbial decomposition of cultural heritage and works of art [16,19,33,34]. Objects of cultural heritage such as textiles, paintings, books, manuscripts, and wooden sculptures can be ideal substrates for microbial growth, which are highly influenced by temperature and humidity [17,26,35]. Therefore, microbial deterioration of cultural heritage objects depends on environmental conditions, substrate properties, and access to the sites of microbial colonization in venues and at-risk artifacts. Regarding the fungal contamination of museum and church textile collections in Slovenia: study of [36] have emphasized and analyzed fungal contamination (e.g., Penicillium sp., Aspergillus sp., and Cladosporium sp.). Similar studies found textile collections in a Jordanian museum contaminated with Aspergillus sp., Penicillium sp., Chaetomium sp. and Alternaria sp. species due to poor environmental conditions [26]. Fungal contamination of artefacts collections depends on temperature, humidity and other environmental conditions. Additionally, molecular analysis of microflora on textiles is crucial in this vein. Some studies have used molecular techniques to analyze microflora on textile materials [37]. New molecular analysis techniques can identify the microorganisms involved in the biodeterioration of cultural heritage artifacts to understand better the deterioration processes. Nanopore sequencing found biological contamination from Aspergillus sp., a critical factor in the biodeterioration of 18th and 19th-century oil paintings [17]. A study on Museum Prince’s Mohamed Ali, Giza, Egypt aimed to assess the degree of fungal contamination in the air and in the dust deposited on the surfaces of some works of art in this museum, the dominant species identified being Alternaria sp., Aspergillus sp. and Cladosporium sp. [4].
Aerobiological studies in museums [38,39], libraries [1], universities/schools [40], and archives [30] highlight the risk of fungi degrading exhibits. A Belgrade cultural heritage facility study found 74 fungal species, including potential human pathogens, allergens, and mycotoxin producers, posing risks to objects and humans [41]. Therefore, airborne microorganisms in cultural heritage institutions can pose dangers of deterioration/biodeterioration to collections and health issues for occupants. Importantly, occupational exposure in conservation–restoration sites is also essential in this vein. A study of occupational exposure in four Portuguese conservation–restoration sites found significant microbial contamination. Textile objects, paintings, and wood carvings were examined. Textile decorations, especially Aspergillus species, were most vulnerable to fungal contamination [42]. Hence, occupational exposure to microorganisms during the conservation and restoration of cultural heritage artifacts can pose health hazards to workers. Similar studies such as Di Carlo et al. [17] analyzed microbial loads in three Sicilian locations, highlighting risks to human health and artifacts from bacteria and fungi. Fungi are ubiquitous and a severe threat indoors, as noted by Khan [24]. Wysocka [28] studied microclimate, air quality, user discomfort, and mold growth in a church. Cappitelli et al. [15] discussed removing unwanted microorganisms through chemical, physical and biological methods. When traditional methods fail or have limited effects, using microorganisms can help restore works of art, as studied by Pyzik [43]. Numerous studies examined microclimate and air quality in Romanian heritage buildings such as museums [3,44,45,46,47], churches [48] archives and schools [49] which identified nine fungal genera, mostly Rhizopus sp., Aspergillus sp., Fusarium sp., and Penicillium sp., in 19th-century heritage buildings. There are studies that associate the presence of fungi, the development of molds inside buildings with the appearance and development of allergies and respiratory diseases. An example is a study conducted in a residential apartment complex in Seoul, South Korea, which revealed a diversity of molds that contaminated the microaeroflora and that have allergen risk: Cladosporium sp., Rhodotorula sp., Alternaria sp., Cryptococcus sp. and Crivellia sp. [50]. Another study demonstrated the association between humidity and the presence of asthma, wheezing, a long-lasting cough in children in schools where there were humidity problems compared to schools where there were no such problems. The humidity inside the rooms favors the development of molds as they are: Fusarium roseum, Aspergillus fumigatus, Phoma herbarum and Rhodotorula rubra [51]. A study carried out at Sultan Idris University found fungal contamination of the central cooling system, of the rooms especially at the corners, on the furniture, etc., which increases the risk of allergies among the occupants of the building (when the exposure time is at least 40 h per week). Because of colonization with microorganisms, a fungal allergy leads to the exacerbation of bronchial asthma and allergic rhinitis, especially among employees, which over time also contributes to the reduction in productivity, by increasing diseases resulting in absenteeism. Aspergillus fumigatus and Penicillium canescens were the most common fungi identified, with the greatest potential to cause allergies, which can also cause sensitization among the atopic population and imply worsening of the condition of symptomatic people with extended exposure to these fungi [52].
In Romania, [25] studied a Art Nouveau Museum Casa Darvas-La Roche, indoor microclimate, finding high to very high fungal contamination (Alternaria sp., Aspergillus sp., Penicillium sp., Cladosporium sp. and Botrytis sp.) posing moderate biodeterioration and health risks to exhibits and, respectively, people. Studies of microbial communities in wooden churches include those of Lupan et al. [53] analyzing a 17th-century church, and Marcu et al. [54], finding a 1710s wooden church with a very high air and surface contact (an interior microclimate risking parishioners, visitors, and deterioration/biodeterioration of objects).
Based on the previous arguments, we suggest three key hypotheses to achieve the study objectives. First it deals with poor indoor air quality due to high levels of microbes and other pollutants in the Neologic Synagogue Sion Oradea can negatively impact the health of employees and visitors. Microbial deterioration of objects and materials within the building depends on environmental conditions, substrate properties, and access to sites of microbial colonization within the synagogue and for at-risk artifacts. Fungal contamination of religious collections (textiles and leather) within building depends on environmental factors such as temperature, humidity, particulate matters, luminosity and other parameters.
2. Materials and Methods
2.1. The Case Study—Sion Synagogue
The Sion Synagogue has one of the most impressive architectures and interior decorations from the buildings in the municipality of Oradea. The methods of the Italian Renaissance are artistically employed, many times in original ways. The edifice is far from street alignment which, in its close vicinity displays the same Italian Renaissance look. A great central tower covered by a heightened cupola is sustained by four pendants standing on four large arches. Each corner features four thin columns linking each arch to the ground. Due to their fragility, they give the impression that the vaults are suspended straight from the sky and into the tower. In fact, the massive ground floor is suspended through them. The floor features pillars and arcades, and is bordered by the attic, which springs in between the railings of the first floor. The eastern wall, with its Ark and decorations—among which the adornment of the organ reigns over the entire light colored against a dark background structure surrounding it—resembles the Orthodox iconostasis. The vertical and horizontal curves of the arches, of the arcades and of the vaults concatenate, intermingle and complete each other. They are highlighted and relieved by pastel painted profiles and geometric decorations. All the shapes, surfaces, lines, and colors blend together harmoniously with the rest of the building. On the outside, the nearby buildings, with their similar allure and height, serve to integrate the synagogue in the architectural ambiance of the area. As a whole, the Sion Synagogue is a great edifice, not burdened by its own monumentality. When entering the large sanctuary, one may feel a surge of holiday spirit.
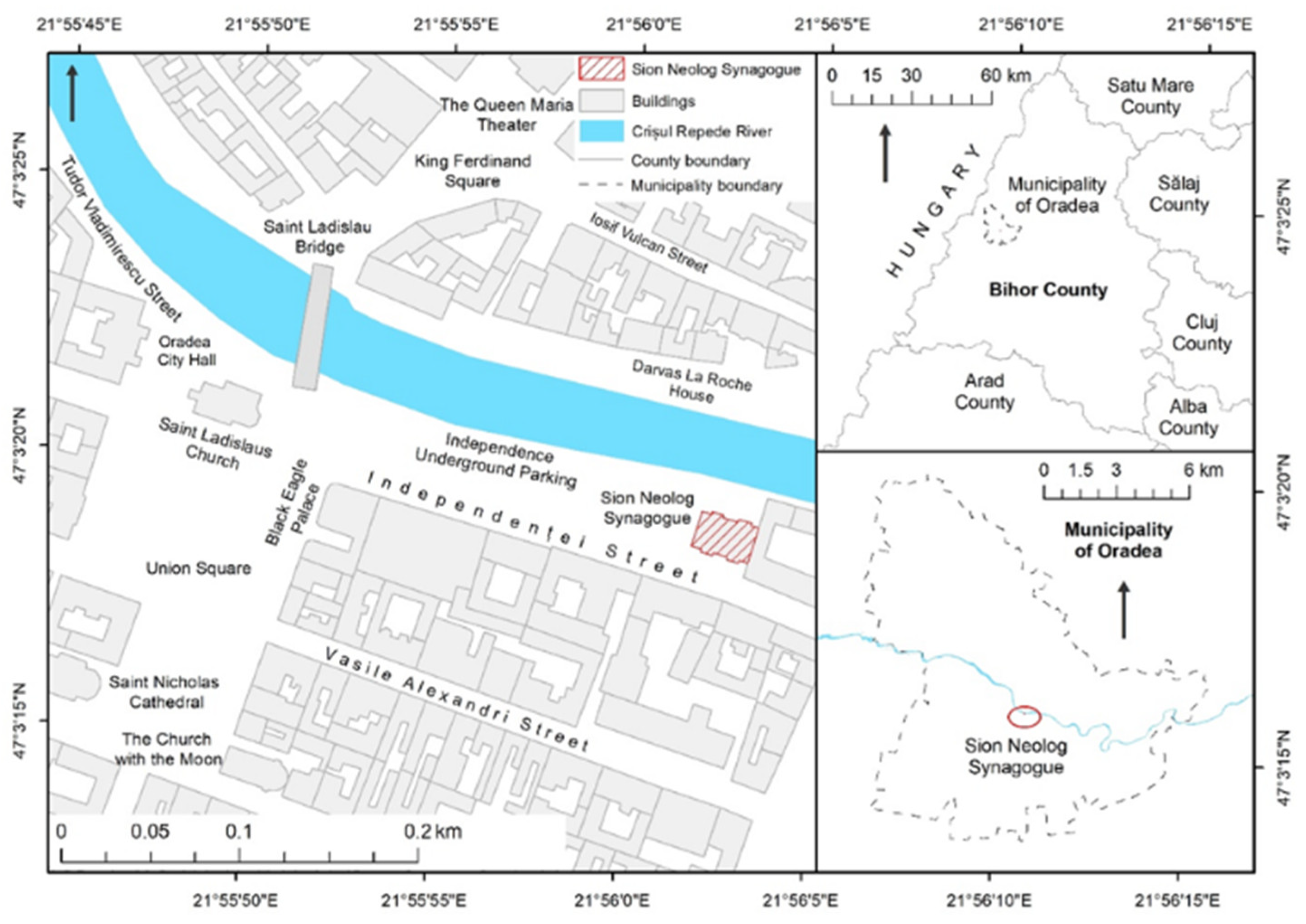
House, represent one of the symbolic architectural elements for the Jewish community in the municipality of Oradea, Romania (Figure 1).
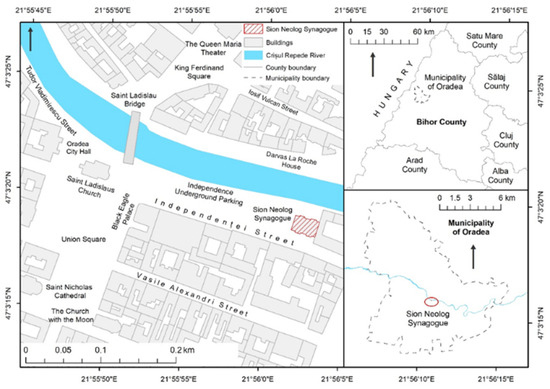
Figure 1.
Localization of Sion Sinagogue in Oradea city and Bihor County level.
2.2. Determining the Quality of the Internal Microclimate
For the assessment of the microclimate quality within the synagogue, the following parameters were monitored over a period of 28 days (from 25 October to 21 November 2022, period during which samples for microbiological analyses were also collected): temperature (T), relative humidity (RH) and CO2 concentration. Their analysis was conducted in terms of average, maximum, and minimum values, hourly and daily (Figure 2).
Figure 2.
Outline of the present study.
The research regarding the determination of the degree of fungal contamination of the ambient air and surfaces in the Neologue Synagogue Sion Oradea were carried out in the laboratories of the Faculty of Environmental Protection, Faculty of Medicine and Pharmacy of University of Oradea, Betania Clinics Oradea and Public Health Direction, Oradea, Romania.
Materials used for the procedures of seeding, isolation and identification of fungal species were as follows: 30 Petri dishes with sterile Sabouraud culture medium with the addition of Chloramphenicol; Petri dishes with sterile Czapek Dox culture medium for replanting and subcultures, as needed; 10 tubes with sterile swabs for harvesting surfaces; microbiological hood; incubator (thermostat); densitometer; Integral System Yeast Plus kit for identifying yeasts and testing their sensitivity to antifungals; glass slides and slides; KOH solution; optical microscope.
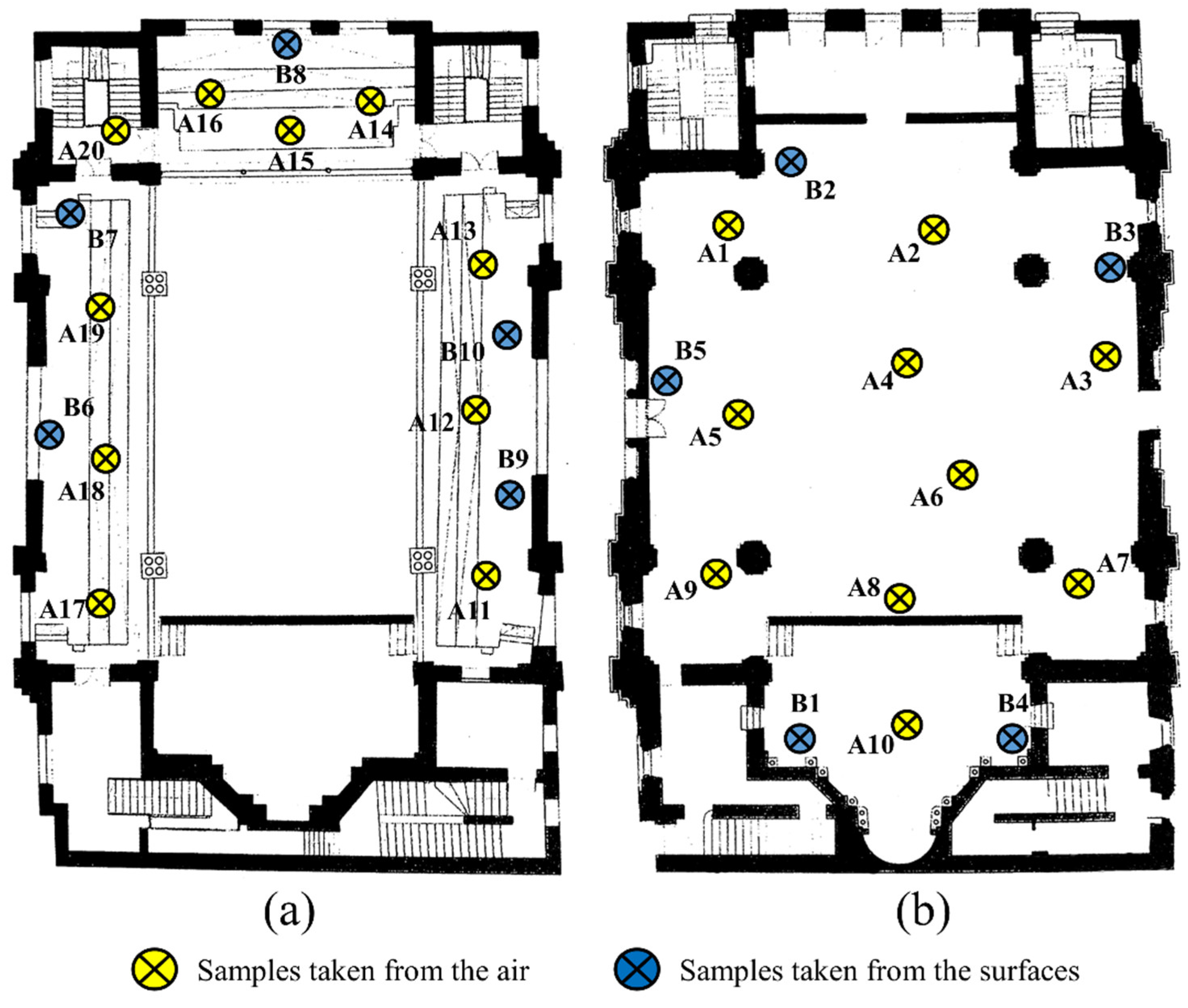
The samples taken to determine the fungal load of the air in the synagogue were 20, were marked with A. Sampling was carried out by the Koch sedimentation method [55], the method of choice for the detection of particles that passively settle due to gravity. A1–A10 taken from the large hall on the ground floor, A11–A20 on the first floor. The samples taken to determine the contamination of the surfaces, marked with B, were in number 10 and the sampling was carried out with the help of sterile swabs from 10 distinct surfaces of 10 cm × 10 cm. The actual working technique of Koch sedimentation involved exposure of culture media in Petri dishes at a height of at least 1 m above ground level for a duration of approximately 30 min (Figure 3). The seeded plates were transported under suitable conditions to the laboratory, where the following stages of examination and identification were carried out. Plates were incubated and monitored for 21 days at 21 ± 2 °C. The final identification of the fungi was achieved following the evaluation of macroscopic, microscopic and biochemical reactions, with the help of the Integral System Yeast Plus identification system. Integral System Yeast Plus is a system provided with 24 wells containing dehydrated biochemical substrates for the biochemical identification of the most clinically important fungi and the evaluation of their sensitivity to a set of antifungals. The system is inoculated with the suspension of yeast bodies and incubated at 36 ± 1 °C for a minimum of 48 h [56].
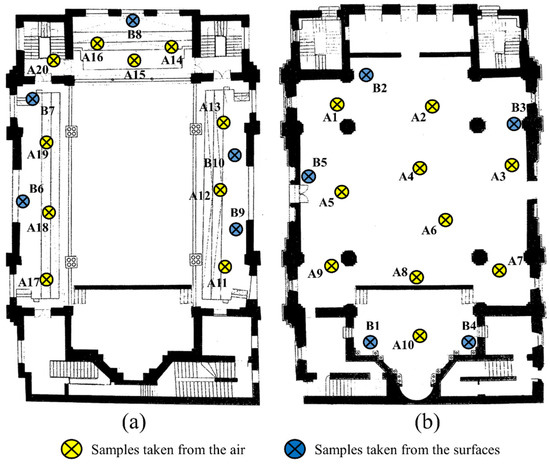
Figure 3.
The places for taking microbiological samples from the air and from the surfaces inside the Neological Sion Synagogue (a) the 1st floor of the synagogue; (b) the ground floor of the synagogue).
The final identification of the fungi was carried out following the formation of a numerical code. The 12 biochemical tests are divided into four groups, each containing three tests and each test is indicated with a positive value of one, two or four. By summing in each group the positive reaction values by color change, a code of four digits that allow identification of the microorganism under examination using the code table. Reading is performed automatically using the Integral System Yeast Plus application. Fungal colonies became visible macroscopically at 24 h on eight plates harvested from air (A) and on all 10 harvested from surfaces. After the first 72 h they entered a maturation process, changing their diameter, shape, color and texture, some species completely invading the culture media. The method of quantitative calculation of the degree of fungal contamination was carried out by counting the colonies developed on the culture medium (expressed in CFU/mL) and equating the result relative to the volume of air using the following calculation formulas:
- -
- formula from Polish standard PN 89/Z-04008/08 [57]
- -
- Omelianski formula [58]
Calculation formula by PN 89/Z-04008/08
where
- n = no of used Petri plates;
- S = Petri plates surface, in cm2 (for a diameter of 9 cm, S = 3.14 × R2 = 63.5 cm2);
- t = exposed time of the plate.
Omelianski formula is based on the observation that on a surface of 100 cm2 exposed to air for a certain period of time, a number of microorganisms equal to that contained in 10 dm3 of air settles.
where
- n = number of colonies developed on the plate;
- S = surface of the Petri plate (for a diameter of 9 cm, S = 3.14 × R2 = 63.5 cm2);
- k = air exposure time coefficient (in minutes, k = 1 for 5 min; k = 2 pentru 10 min; k = 3 for 15 min, etc.).
2.3. Determination and Analysis of Visitors’ Perception of Air Quality
The main variables of interest were measured using valid and reliable indicators. The symptoms experienced by the participants were assessed using an 11-item checklist of common symptoms related to poor indoor air quality, and each symptom was rated on a four-point Likert scale from ‘never’ to ‘very frequently’. An overall symptoms score was calculated for each participant by summing up the scores of all reported symptoms. The perceptions of the indoor environment quality were assessed using eight items pertaining to factors such as temperature, humidity, ventilation, odors, dust, and molds. Each item was rated on a four-point Likert scale from ‘never’ to ‘very frequently’.
The current study adopted a quantitative cross-sectional design to explore the prevalence and associated factors of symptoms experienced by staff members while performing their activities in a designated space. The study sample comprised 139 participants recruited through convenience sampling. Using a standardized questionnaire, we conducted a survey targeting at least 139 visitors during peak times. Visitors were randomly approached, and participation was voluntary. The survey consisted of close-ended, four-point Likert scale, and open-ended questions covering visitors’ perceptions of environmental conditions, comfort level, interest in exhibits, and how any issues may have impacted their experience. A sample size of at least 139 survey responses provided enough variation in demographic profiles and visitor experiences to derive meaningful conclusions. The objective sampling data and subjective survey data were then correlated to identify specific indoor environmental issues that most influenced visitor experience at the synagogue. Visitors were selected randomly to avoid bias. Criteria of at least 139 visitors ensured a representative sample while remaining feasible within the study timeline. This study found statistically significant associations between the overall symptom score of 571 participants and their exposures to certain factors within the study timeline. These factors included too low temperature of the indoor air, dry air, unpleasant smells, dust, and visible mold. Several physical stressors also showed statistically significant associations. However, some other factors demonstrated only marginal significance, not full statistical significance. Yet, there remained a marginal and potential significance for factors such as too high temperature of the indoor air, high humidity, and closed/unventilated air. While most associations were statistically significant, some factors only approached but did not fully reach significance. Nevertheless, the findings still indicated possible links between certain indoor environmental exposures and symptoms for participants. Further research may help clarify the roles of these marginally significant factors.
The survey data proved useful in identifying how microbial contamination and suboptimal environmental conditions negatively impacted visitors’ comfort, interest in exhibits, and overall impressions—providing important insights to mitigate these issues through targeted strategies.
The data were collected using an anonymous self-administered questionnaire that gathered information about the visitors’ socio-demographic characteristics, clinical history, experiences, and perceptions regarding indoor environment quality and health symptoms. Descriptive statistics were used to summarize the participants’ characteristics and responses. The differences in the symptoms score based on the participants’ characteristics and perceptions were tested using the Kruskal–Wallis test. Spearman’s correlation was used to assess the correlation between the symptoms score and continuous variables. Multivariate linear regression analysis was performed to determine the symptom score predictors. The data analysis was carried out using the IBM SPSS version 25.
To assess the overall health of the respondents, we asked the participants about the symptoms encountered while unfolding their activities in space. These included 12 symptoms, and the responses to each symptom was collected on a four-point Likert scale from 0 = Never to 3 = Very frequent. An overall health score was created by summing up the coded values of the 12 items. Therefore, the score ranged between 0 and 36, where higher scores indicated higher frequencies of experiencing the symptoms. Factors associated with the encountered symptoms score were assessed using inferential analyses, including a Wilcoxon rank sum test for variables with two categories (e.g., gender), a Kruskal–Wallis rank sum test for variables with three or more categories (e.g., self-rating of the air quality in the space) and a Spearman’s correlation test for numerical variables (e.g., age). To assess the independent predictors of participants’ overall health, the significantly associated variables from the inferential analyses were subsequently used as independent variables in a generalized linear regression model, where the overall symptoms score was incorporated as a dependent variable. Results of the regression analysis were presented as beta coefficients and their respective 95% confidence intervals (95% CIs). A p value of <0.05 indicated statistical significance. Internal consistency was examined using Cronbach’s alpha coefficient to measure how well the survey items designed to gauge a single construct (e.g., overall health score) produced similar scores. An alpha value of ≥0.7 typically indicates acceptable reliability. For the 12-item overall health score, we obtained a Cronbach’s alpha of 0.81, demonstrating good internal consistency among the symptom items. This validates interpreting the total score as a global indicator of health status. Additionally, we assessed inter-rater reliability for survey responses involving subjective ratings, such as self-reported air quality perceptions. A random sample of 20 questionnaires was independently coded by two raters. An interclass correlation coefficient (ICC) of 0.75 or greater is generally considered satisfactory. The ICCs for variables involving subjective assessments, such as perceived indoor environment quality, all exceeded 0.75. This provides assurance the raters applied scoring criteria in a reliable manner. Together, these reliability tests enhance confidence that our questionnaire elicited consistent and dependable data appropriate for identifying key predictors through multivariate analyses as detailed in the methods section.
3. Results and Discussions
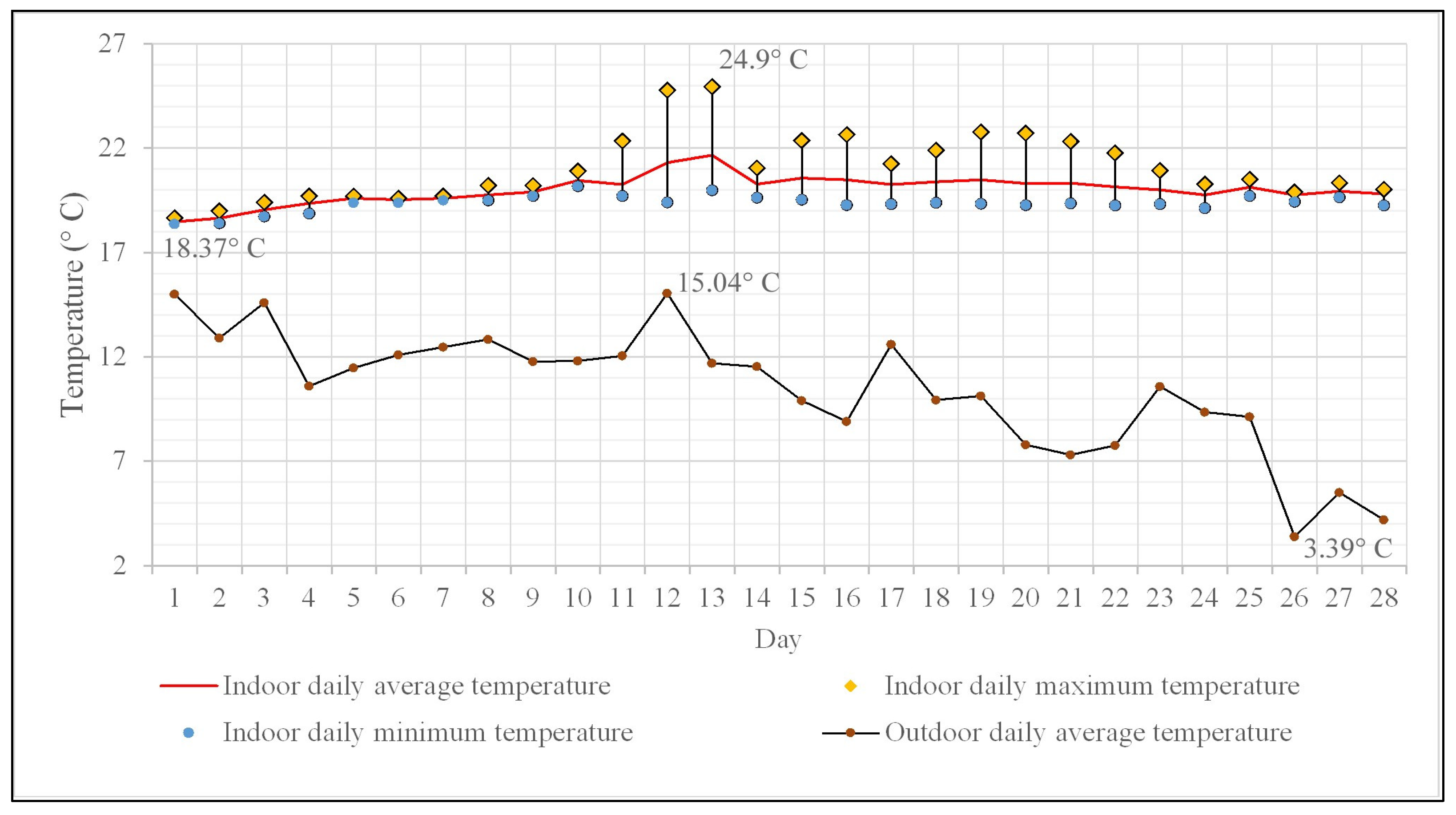
The daily average temperature values for the monitoring period of 28 days range between 18 and 21 °C (Figure 4), with only two days exceeding the average value of 21 °C. Regarding the frequency distribution of daily average temperature values, it is worth mentioning that the value of 20 °C practically divides the dataset into two almost equal parts (14 values above 20 °C and 13 values below 20 °C). The daily maximum temperature values range from 18.6 °C to 24.9 °C, and in terms of percentage distribution within this range, 67.8% of them exceed 20 °C. The daily minimum temperature values recorded remain between 18.3 °C and 20.1 °C, hence the differences between the daily maximum and minimum temperature values are small (50% of the differences have subunitary values), indicating that there are no significant diurnal temperature fluctuations.
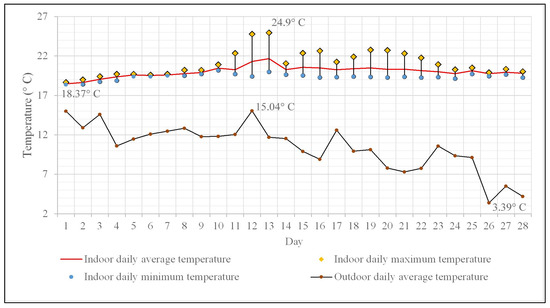
Figure 4.
Indoor and outdoor daily mean temperature variation. Indoor minimum and maximum temperature variation.
The average outdoor temperature over the same time interval was 10.4 °C, according to the data collected and processed from the National Network for Monitoring Air Quality—Romania [59], with fluctuations ranging between 15 °C (in the first half of the monitored period) and 3 °C (Figure 4). The differences between the average daily indoor and outdoor temperatures reach up to 16 °C towards the end of the monitoring period, which is completely normal, as it pertains to the second half of November.
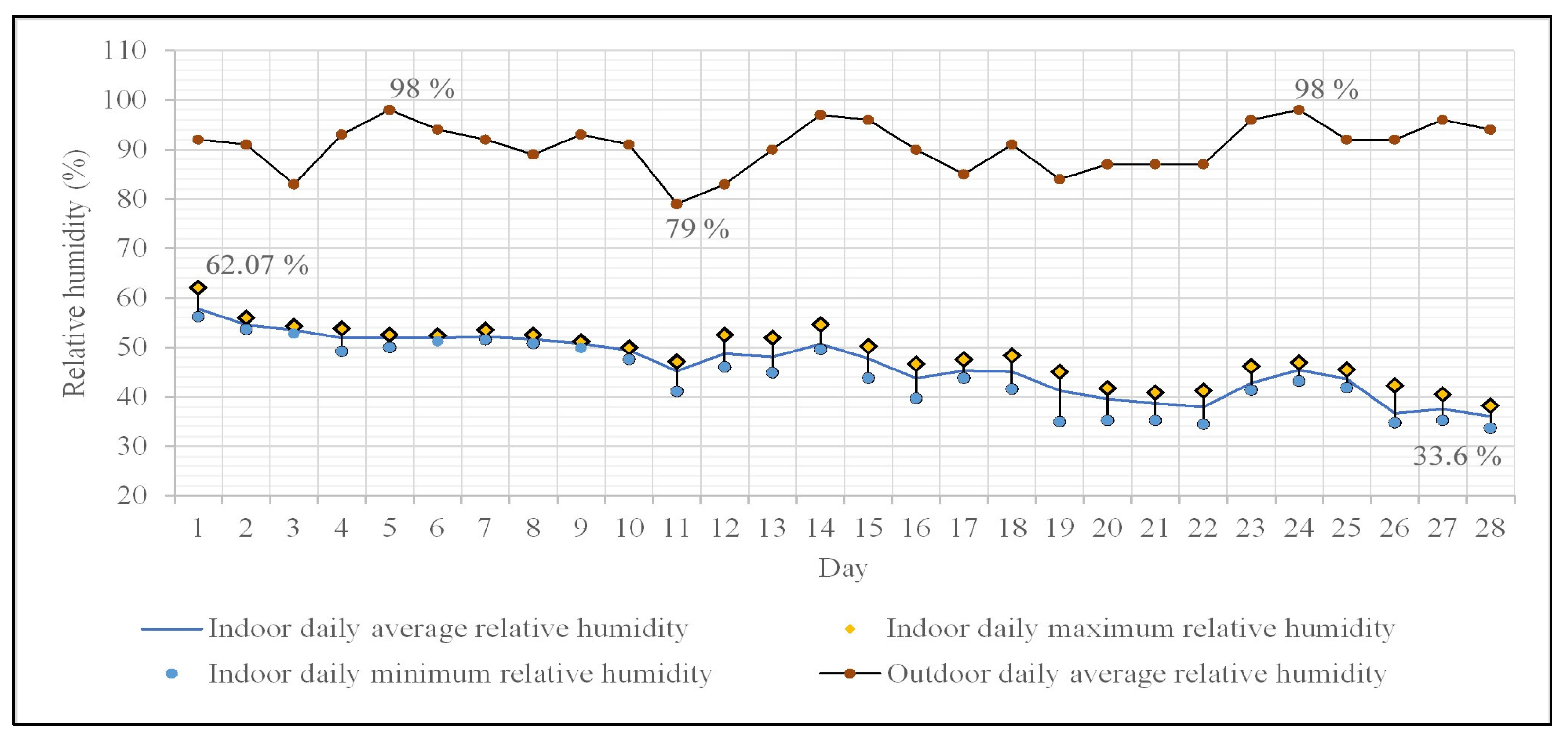
The daily average relative humidity (RH) ranges between 36% and 57.85% (Figure 5), with an overall average value of 46.4% for the entire monitoring period. Values above 40% dominate the frequency distribution for the daily average RH. The daily maximum values for RH exhibit a wide range, between 38.2% and 62%, with a similar pattern observed for the minimum values (33.6–56.2%). The daily variations of RH are maintained between 1–10%, with a predominance of differences between maximum and minimum exceeding 4 percents.
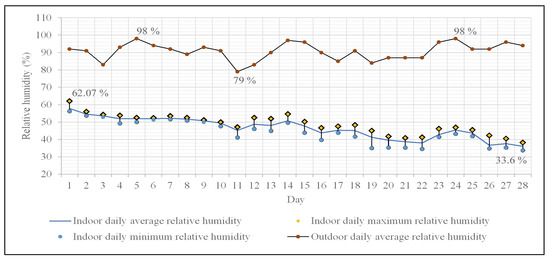
Figure 5.
Indoor and outdoor daily average relative humidity variation. Indoor minimum and maximum relative humidity variation.
The average daily RH values in Oradea (outdoors) [59] were quite high, ranging between 79% and 98%, with a predominance of values over 90% (90.7% being the average of the data series), highlighting a clear correlation with the lower temperatures during this period of the year.
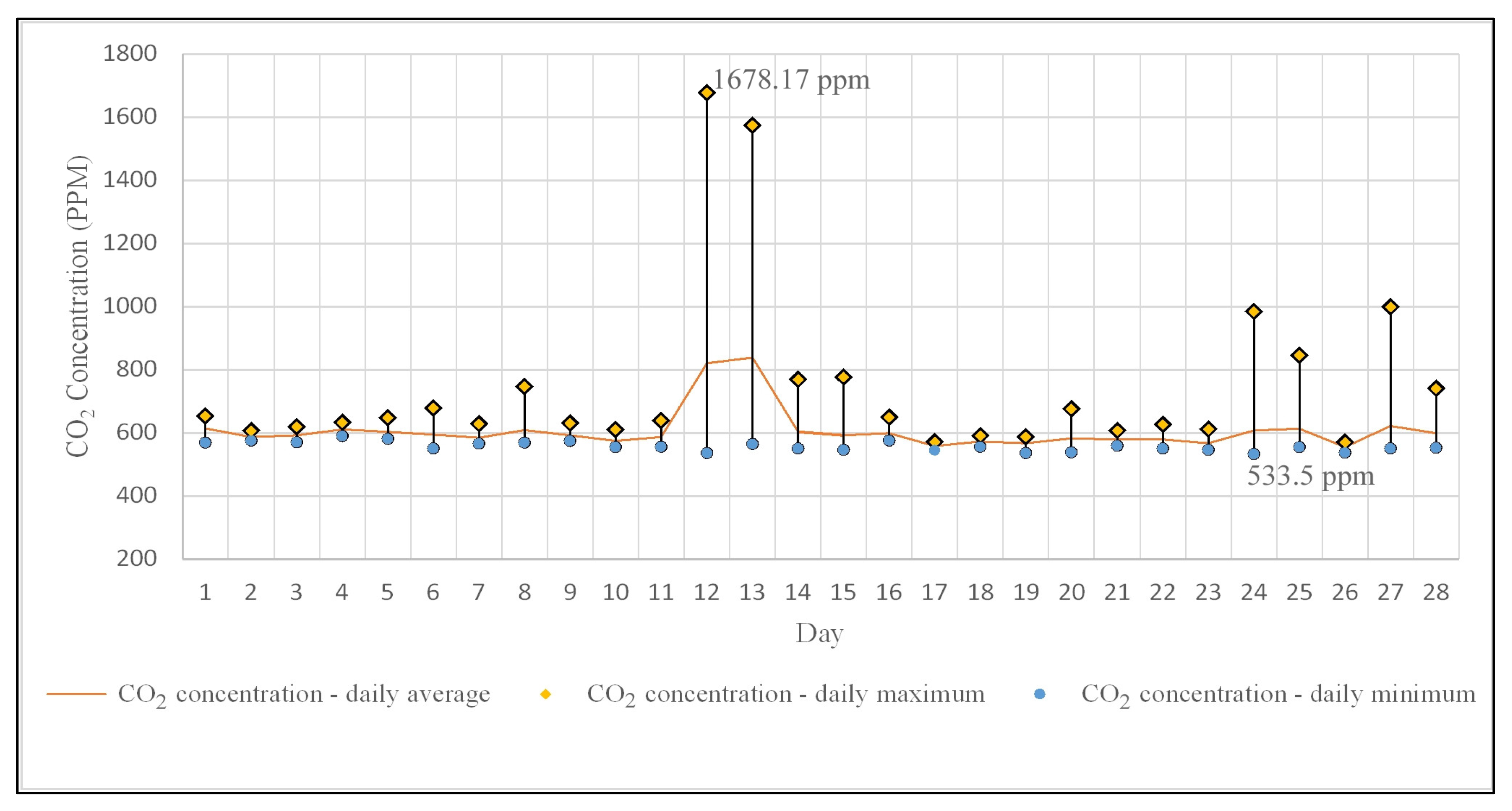
The CO2 concentration recorded daily average values ranging from 837 ppm to 554 ppm (Figure 6), with a mean value of 607 ppm. Over 90% of the daily average CO2 concentrations are below 800 ppm, with 60% of them being close to the value of 600 ppm. Regarding the maximum daily values, there were two instances of hourly intervals (each do not exceed 8 h) when the concentration exceeded 1000 ppm. However, this value, as specified by the American Society of Heating, Refrigerating, and Air Conditioning Engineers, Inc. [60], must be understood as an indicator of outdoor air ventilation rate per person, without any direct association with public or individual health effects. Moreover, all monitored microclimate parameters comply with standards or recommendations related to indoor air quality and comfort for human occupancy [61,62,63,64,65,66]. The situation changes when the values of these microclimate parameters (mainly relative humidity and temperature) are analyzed as factors in the development of fungi and bacteria in enclosed spaces, as existing studies indicate that temperature values of 18–20 °C, and especially relative humidity values of 50–60%, positively influence their growth [67,68,69].
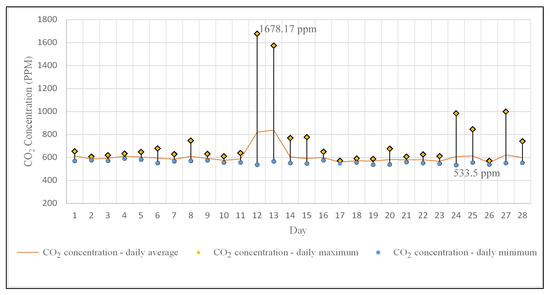
Figure 6.
Daily average, maximum, and minimum variation in indoor CO2 concentration.
Currently, there are no regulations or standards concerning the fungal load of the air; therefore, certain indicative norms established following research are used to assess the degree of contamination of the air in the rooms. [70]. Table 1 shows the system for evaluating the degree of fungal contamination using the Koch method, and Table 2 shows the first results, respectively, 72 h after sowing.

Table 1.
Evaluation of the degree of fungal contamination of the air in a room according to sanitary norms applied to non-industrial institutions [58].

Table 2.
Fungal contamination degree of the air.
The calculation method used in the present study is as follows: the arithmetic mean of the colonies on the 20 Sabouraud plates for each room, the interpolation in the Omelianski calculation formula and that according to the PN 89/Z-04008/08 standard and the classification in the degree of contamination according to Table 1. The results obtained are presented in Table 2.
The last plate reading was performed 16 days after sampling. The degree of invasion is minimal (≤1/4 of the plate), moderate (between 1/4 and 3/4 of the plate), increased (≥3/4 of the plate), respectively, complete invasion. Thus, five different species of fungi (Aspergillus sp., Botrytis sp., Penicillium sp., Cladosporium sp. and Alternaria sp.) and two species of yeasts (Rhodotorula sp. and Candida sp.) were identified (Table 3).

Table 3.
Fungal species identified and the degree of plate invasion.
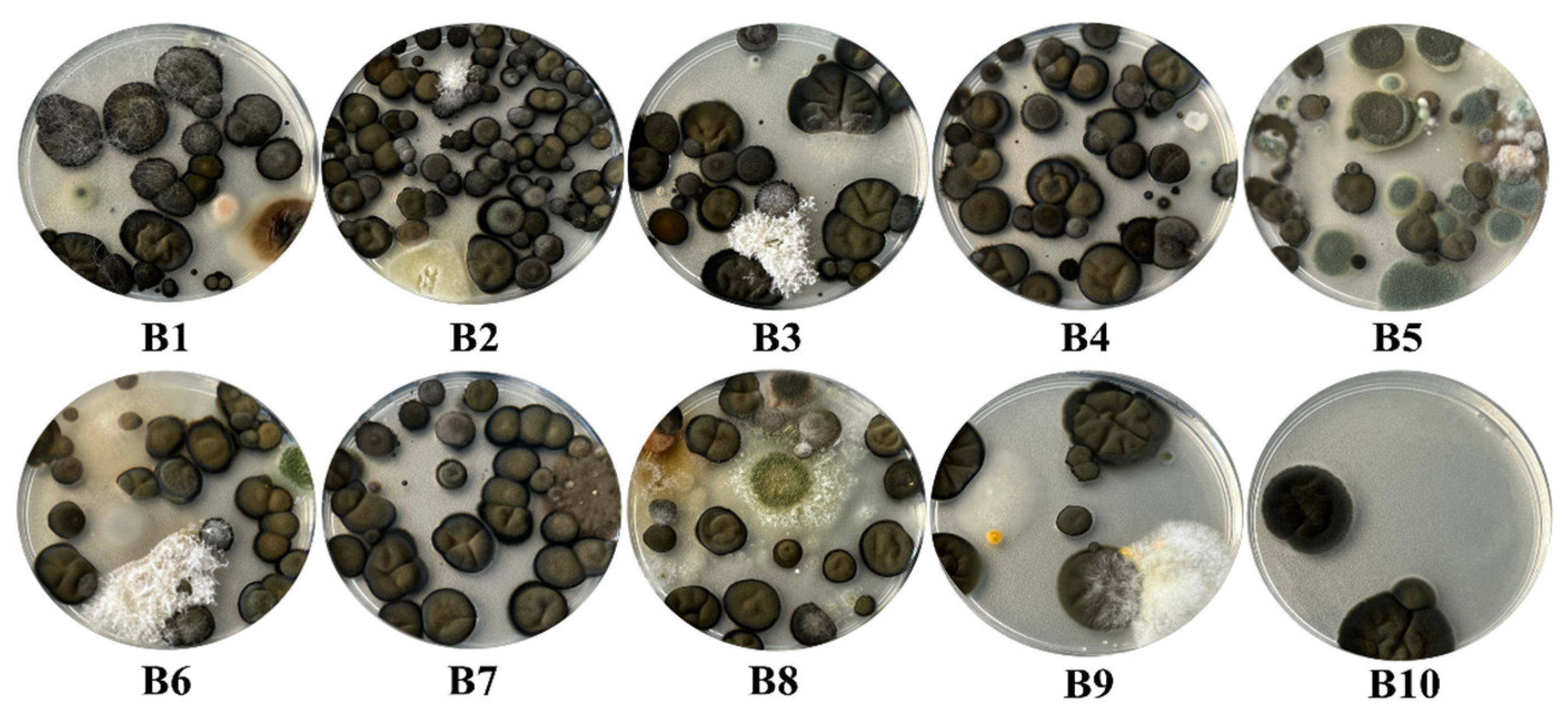
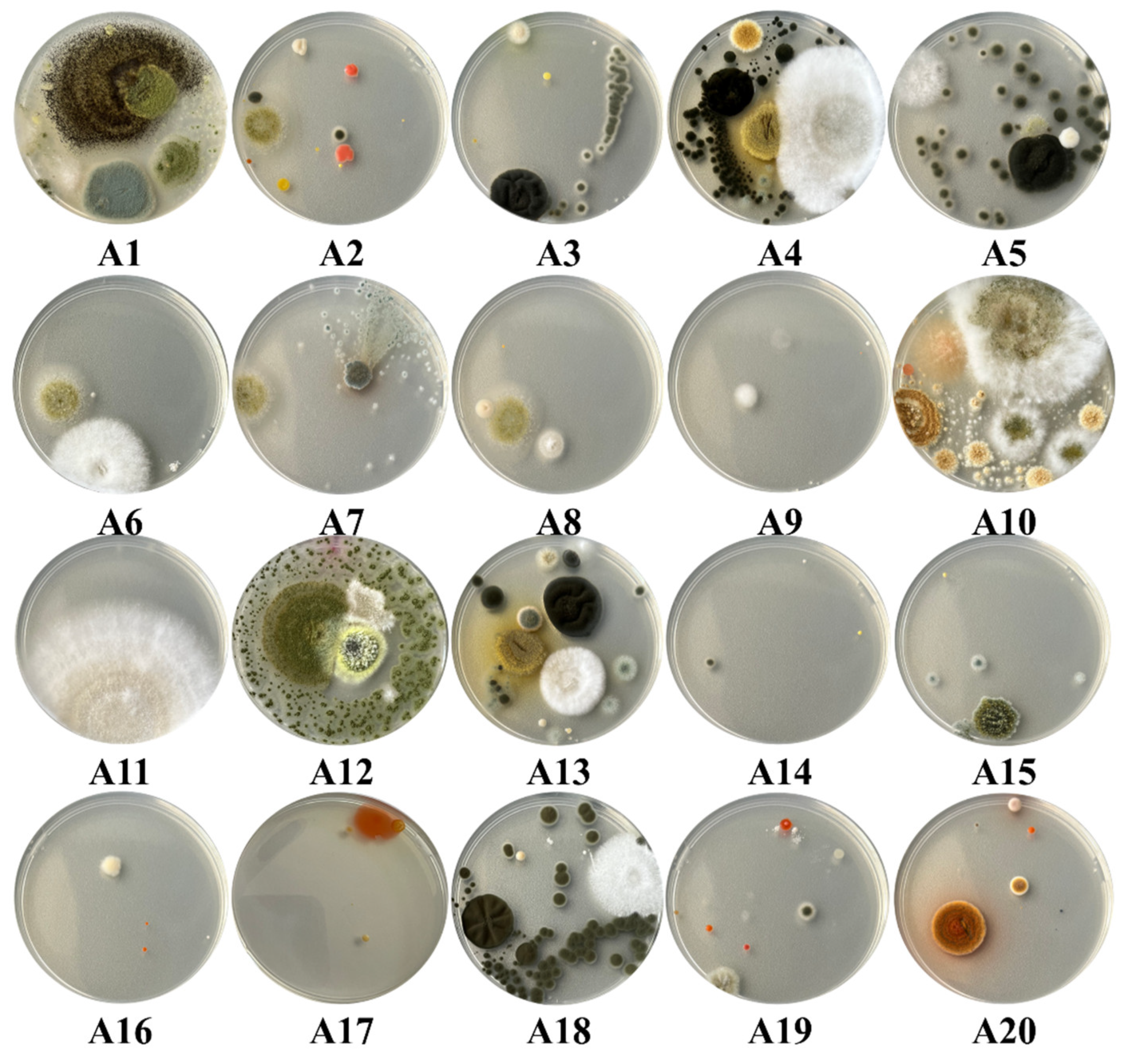
Among the 30 plaques used in the current study, 11 had a complete invasion, 1 had an increased invasion with fungi, 12 had a moderate invasion, while only 6 had a minimal invasion of the plaque. Within the samples collected from the surfaces, a total number of 28 species of fungi were identified, all plates being characterized by an increased invasion with fungi (Figure 7). Regarding the samples collected from the air, they stand out through a great diversity of fungi and yeasts identification, as well as regarding the degree of invasion with fungal colonies. Thus, a number of 48 species of fungi and 9 species of yeasts were identified in these samples. Regarding the degree of invasion of the Petri plates, 11 of the samples determined fully invasion of them, 1 had an increased invasion, 2 a moderate invasion, while 6 were distinguished by a low invasion (Figure 8).
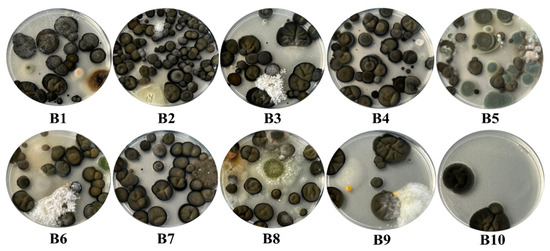
Figure 7.
The degree of fungal invasion of the Petri plates in the case of the samples taken from the surfaces inside the Neological Sion Synagogue.
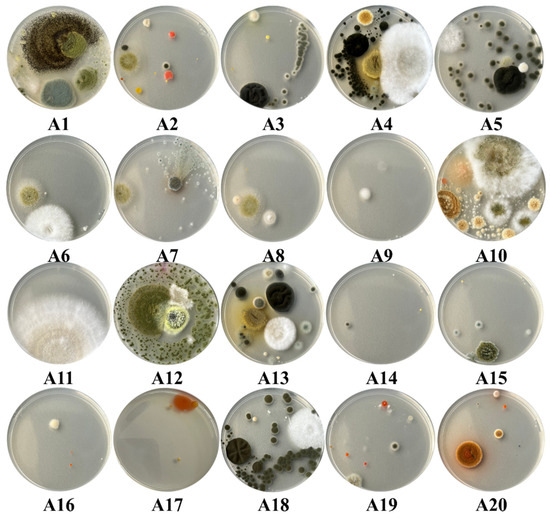
Figure 8.
The degree of fungal invasion of the Petri plates in the case of the samples taken from the air inside the Neological Sion Synagogue.
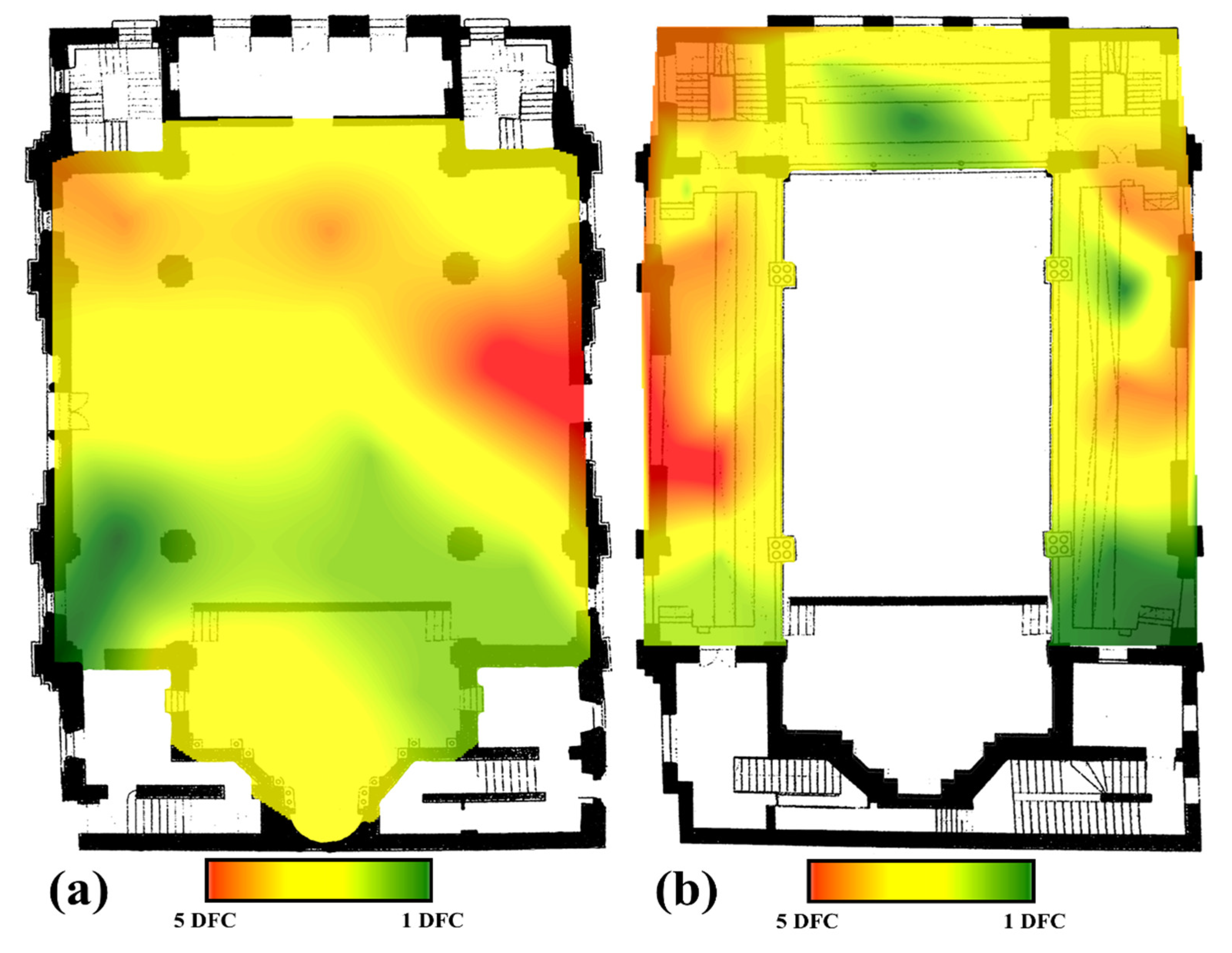
Regarding the spatial distribution of the fungal colonies in the air, within the two analyzed floors of the Neological Sion Synagogue Oradea, it can be observed that their distribution does not follow a well-established pattern. In the case of the ground floor of the building, the maximum number of fungal colonies (5) were identified in the northern part of the room and partially in the western and southwestern parts, while the rest of the room registered between one and three fungal colonies (Figure 9a). In the case of the 1st floor, the maximum number of fungal colonies (5) is identified in a single point located in the southern part of the room, while in some areas of the northern and southwestern parts, 4 fungal colonies were identified; but as in the previous case, most of the floor registers between one and three colonies (Figure 9b).
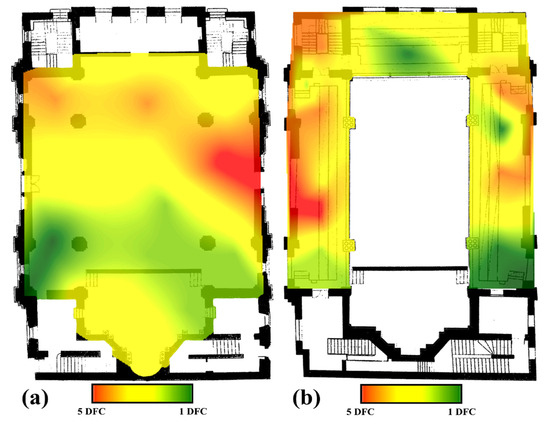
Figure 9.
The spatial distribution of the fungal colonies in the indoor air of the Neological Sion Synagogue. (a) the 1st floor of the synagogue; (b) the ground floor of the synagogue).
Candida albicans was identified with the help of the Integral System Yeast Plus system, which is also provided with wells that allow sensitivity testing by the microdilution method to the following antifungals: nystatin, amphotericin B, flucytosine, econazole, ketoconazole, clotrimazole, miconazole, itraconazole, voriconazole, fluconazole. Candida albicans turned out to be sensitive to all the antifungals mentioned above. The code obtained for the cream colony yeast according to Integral System Yeast Plus: Candida albicans—7242. The orange colony yeasts could not be identified using the Integral System Yeast Plus system because no color reactions occurred; therefore, no code was generated.
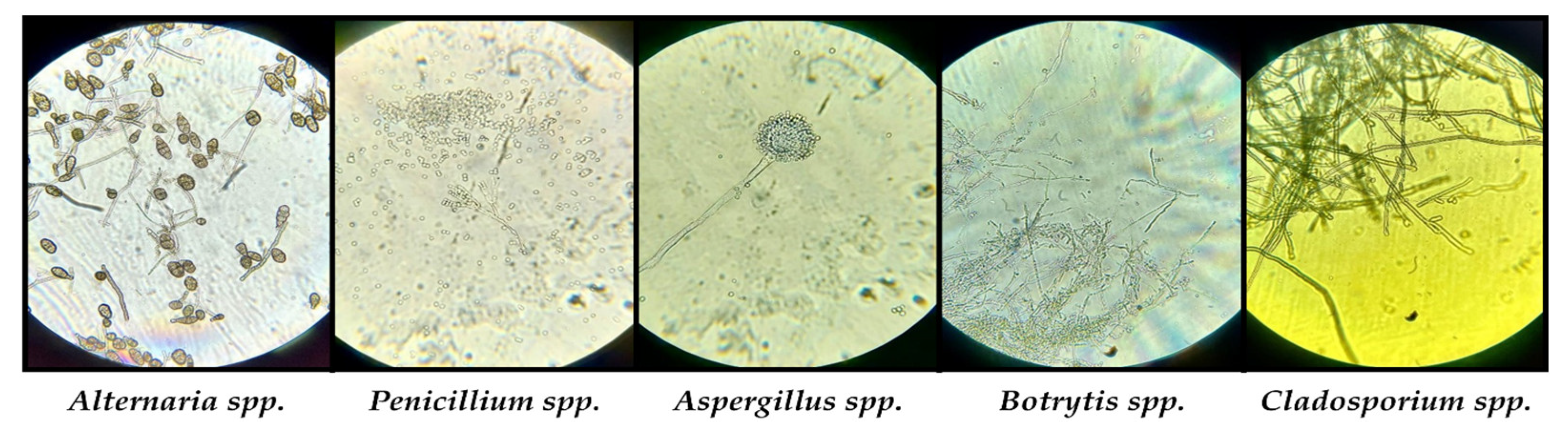
The orange colony belongs to the genus Rhodotorula according to macroscopic morphology and microscopic characteristics. It is a ubiquitous yeast with 46 species, of which only 3 species are considered human pathogenic in immunocompromised subjects. Information on genera and species of molds was obtained from macroscopic and microscopic examination of viable fungal cultures. According to the macroscopic (size, shape, contour, color, consistency, tendency to invade the plate) and microscopic (hyphae, pseudohyphae, spores, blastospores, sporocystospores, sporocysts, conidiophores, metulae, phialides, conidiophores, etc.) appearance of the colony, we were able to establish the types of molds developed on culture media [71,72]. Identified genera were: Alternaria sp., Penicillium sp., Aspergillus sp., Botrytis sp. and Cladosporium sp. (Figure 10).
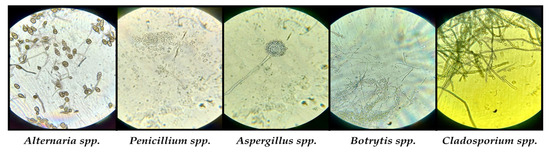
Figure 10.
Microscopic visualization of the identified fungal species.
Microorganisms that have reached the interior of the rooms will be deposited on damp walls, at the level of windows, carpets or in any other areas with a high degree of humidity. Spores or conidia and other structures of fungi enter the human body in different ways: nasal, skin [73]. The periodic study of the microflora of the air is necessary because the risk of biodeterioration and human illness increases proportionally with the number of pathogenic germs and with the presence of certain pathogenic or potentially pathogenic species and with the presence of favorable conditions for their development (humidity, temperature, certain material types from which objects are made).
Exposure and sensitization to fungal allergens can promote the development and worsening of allergic diseases. Allergic manifestations induced by fungi are recognized and include asthma, rhinitis, conjunctivitis, allergic sinusitis, pneumonia, urticaria, atopic dermatitis [52,73]. Allergic reactions can occur through all four types of hypersensitivity reactions I–IV. The mechanism by which the allergic reaction occurs depends on the species of fungi. The most common is immediate or type I hypersensitivity through the intervention of IgE and is manifested by bronchial asthma, rhinitis, sinusitis, urticaria, bronchoconstriction. Fungal allergens in contact with the human body determine the synthesis of IgE, the IgE antigen complexes are fixed on the surface of mast cells and basophils with the release of mediators responsible for allergic manifestations. Symptoms usually appear 10–30 min after contact with the allergen.
Type II reactions are less common and occur for example with Aspergillus sp. and Candida sp. The antigen involved in this type of reaction is most commonly mannan present in fungal cell walls. They are cytotoxic reactions that involve the formation of IgG and IgM antibodies, cytotoxic lymphocytes, natural killer (NK) cells or activated macrophages directed at the antigen that is incorporated into the blood cells or the cell membrane [73]. Examples of type III hypersensitivity to fungal antigens are alveolitis and bronchopulmonary aspergillosis (allergic bronchopulmonary aspergillosis_ABPA).
In type III hypersensitivity, immune complexes are formed between fungal antigens (small amounts of fungi in the case of APBA or prolonged exposure to Aspergillus fumigatus conidia) and antibodies (8) Clinical symptoms in type III hypersensitivity appear approximately 4–6 h after exposure to the allergen.
Type IV hypersensitivity reactions develop 24–48 h after antigen contact sensitized CD4+ lymphocytes. The antigen can be represented by various fungal cell structures or haptens produced by some fungi. Clinical manifestations are represented by contact dermatitis or urticaria, granulomatous cases with characteristic inflammatory infiltrates. Fungal spores present in indoor and outdoor air, although harmless in small quantities, can generate various allergic reactions in susceptible individuals. High concentrations of spores from the genera Alternaria sp. and Cladosporium sp. can cause exacerbation of bronchial asthma, especially at the end of summer. Spores of the genus Penicillium sp., Aspergillus sp. or Trichoderma sp. can produce similar effects. The concentration of spores or conidia of certain fungi in indoor air can be independent of the season [73].
Evidence shows that in the majority of the cases the so-called dust allergy and seasonal hay fever are caused by fungal spores, which are the principal cause of these conditions. This is because to the fact that fungal spores are present in large quantities in the dust inside the rooms, which is a real reservoir for fungi, providing them with optimal nutrients for development. Allergic reactions induced by mold are often similar to those associated with the influenza virus. The most common manifestations associated with mold exposure: runny nose, conjunctivitis, cough, congestion of the upper respiratory tract, chest pain or hives, noting the intensification of symptoms in patients with atopic dermatitis.
Fungi can also secrete a lot of volatile organic compounds (VOCs), which are one of the many products of their primary or secondary metabolism. The secretion of these chemical compounds is favored by closed, poorly ventilated rooms with a high degree of humidity and leads to the appearance of “sick building syndrome” (SBS) (8). Symptoms associated with VOC secretion most often manifest as discomfort, headache, eye pain, pharyngitis, dry cough, dizziness, blurred vision, difficulty concentrating, sensitivity to smell, fatigue, apathy, or even a greater tendency for colds. These symptoms disappear quickly, usually when exiting such a building or room [73].
Fungi also produce mycotoxins (aflatoxins, ochratoxin A, zearalenone, trichothecenes, fumonisins) which are metabolic products and can cause acute and chronic conditions in the human body. Mycotoxins are produced especially in conditions of increased temperature and humidity, especially inside poorly ventilated rooms. Chronic exposure to small amounts of mycotoxins increases the incidence of cancer, the most affected organs being the liver and kidney. Equally dangerous are trichothecenes, which appear to have gastrointestinal affinity and zearalenone, which has a significant negative effect on the reproductive system [73].
Rhodotorula mucilaginosa is among the most commonly encountered yeast strains. Natural enolase from R. mucilaginosa is a protein that has an allergenic role and is involved in allergies. Due to the similar structure between the allergenic enolases of Candida albicans, Penicillium citrinum, Aspergillus fumigatus, Cladosporium herbarum, Alternaria alternata there is allergenic/antigenic cross-reactivity [74]. Extrinsic allergic alveolitis is known following exposure to Rhodotorula rubra, with an increased titer of Rhodotorula-specific precipitating antibodies in patient sera [75].
Aspergillus sp., for example Aspergillus fumigatus, is a possible etiological agent of allergic mycotic bronchopneumonia characterized by asthma, peripheral blood eosinophilia, immediate skin reactivity to Aspergillus antigen, precipitation of antibodies against Aspergillus antigen, increased total IgE. The pathogenesis is characterized by the colonization of the airways with fungi that triggers a strong humoral and cellular immune response to the proteolytic enzymes secreted by the fungi resulting in increased levels of IgE and IgG antibodies. Allergenic proteins (n = 23) have been recorded from A. fumigatus and most of these allergenic proteins are cross-reactive allergens. Examples of allergic proteins: peroxisomal proteins, MnSOD, cyclophilins, thioredoxins, enolases, heat shock proteins, ribosomal proteins P1, ribosomal proteins P2 [76,77].
The findings of this investigation provide compelling evidence of the high prevalence of symptoms associated with poor indoor environmental quality among staff members during their routine activities in the designated space at Neologic Synagogue Sion Oradea. More specifically, over one-quarter of study participants reported frequent experiences of symptoms, including fatigue, headache, and sneezing. This high proportion of occupants experiencing symptoms highlights the need to identify factors contributing to occupants’ discomfort in order to develop appropriate interventions to mitigate the issues.
This study found statistically significant associations between the overall symptom score and exposures to several physical stressors: an indoor temperature perceived as too low, dry air, unpleasant odors, and the presence of dust in space. Previous research extensively documented these factors as major determinants of suboptimal indoor environmental quality that negatively impact occupants’ health and well-being. Prolonged exposure to such stressors in occupational settings can potentially lead to sick-building syndrome and reduced work productivity. At Neologia Synagogue Sion Oradea, efforts should be made to optimize thermal comfort, indoor air quality, and cleanliness through appropriate temperature and humidity control, increased ventilation, source control of odors and dust, and thorough routine cleaning. Regular heating, ventilation, and air conditioning systems maintenance is also critical to ensure effective environmental management. Together, these interventions aim to minimize physical stressors and their associated health symptoms among staff in order to improve occupant comfort, health, and performance. An interesting finding was that the tendency for symptoms to disappear within one to two hours after leaving the designated space at Neologic Synagogue Sion Oradea was associated with lower overall symptom scores among occupants. This suggests that occupants’ symptoms were directly related to their presence in the space and were possibly triggered by factors in the indoor environment. Once occupants left the space, their symptoms began to subside, indicating a strong relationship between indoor environmental conditions and the experienced symptoms. The relatively quick dissipation of symptoms after exiting the space further elucidates the role of the indoor environment in triggering occupants’ health issues.
This finding has important implications for identifying and managing risk factors at Neologic Synagogue Sion Oradea to improve occupant comfort and productivity. Interventions aimed at optimizing the physical indoor environment, such as those previously mentioned, can help minimize trigger factors that cause occupants symptoms during occupancy hours. However, targeted strategies may also be needed outside of typical work hours if certain environmental issues persist when the space is unoccupied. A holistic, multidisciplinary approach that considers occupant behaviors and indoor environmental dynamics throughout the day is recommended to fully address the indoor environment-related health issues reported by Neologic Synagogue Sion Oradea staff. Overall, this study provides critical insights into the high prevalence of indoor environment-related symptoms among staff members while performing their routine activities in the designated space. The findings call for urgent actions to improve indoor environmental conditions and safeguard the well-being of the occupants. Further studies should explore effective mitigation and management strategies that can be implemented to address the identified issues.
The current study analyzed data of 139 staff members. More than a half of them were females (53.7%), and 39.4% of them were active smokers. Only 7.2% wore contact lenses. Regarding the clinical history, 15.9% were receiving medications, and 10.1% of the respondents had been diagnosed with a health problem. More details about the characteristics of respondents are provided in Table 4.

Table 4.
Characteristics of the respondents.
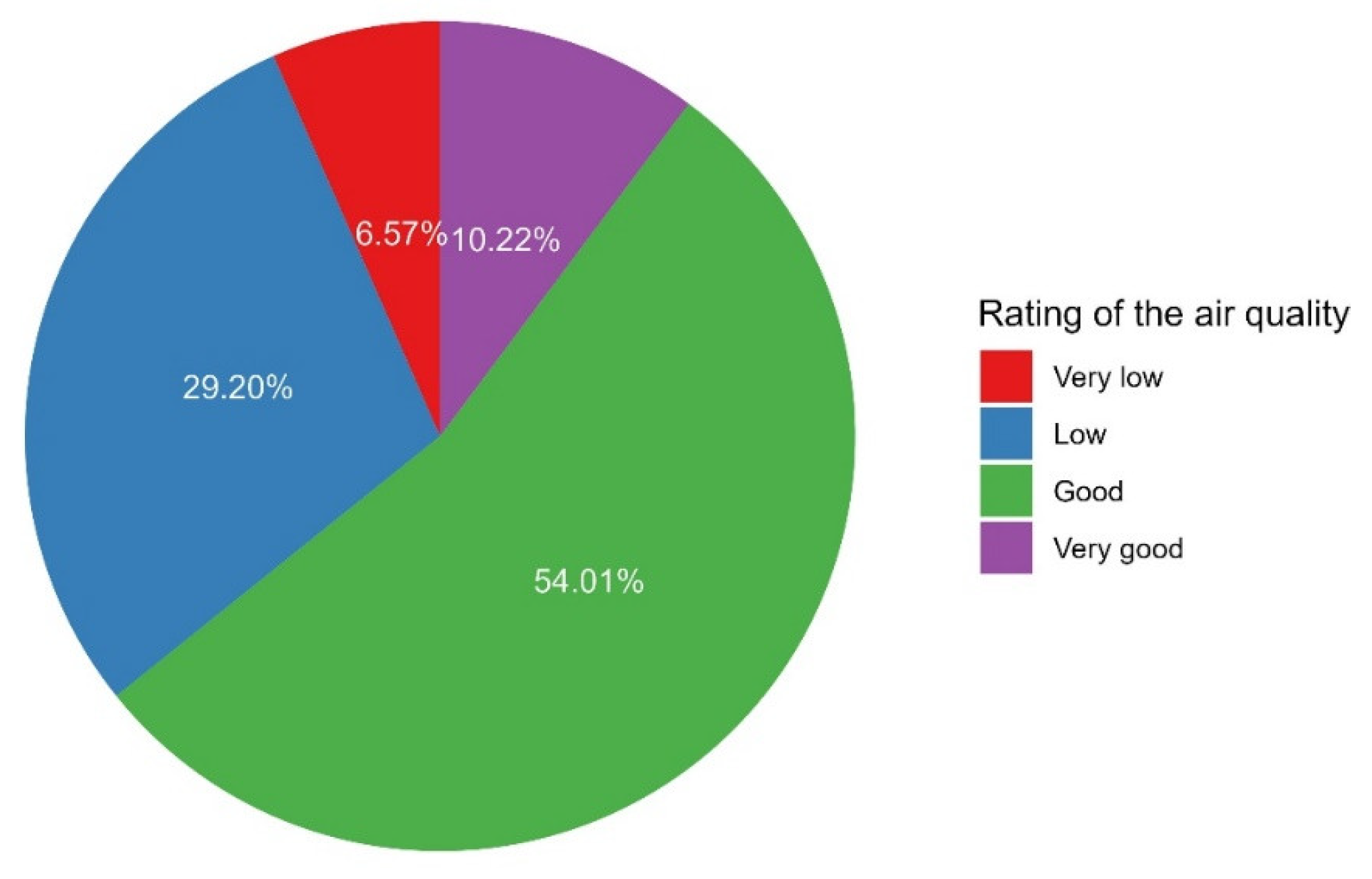
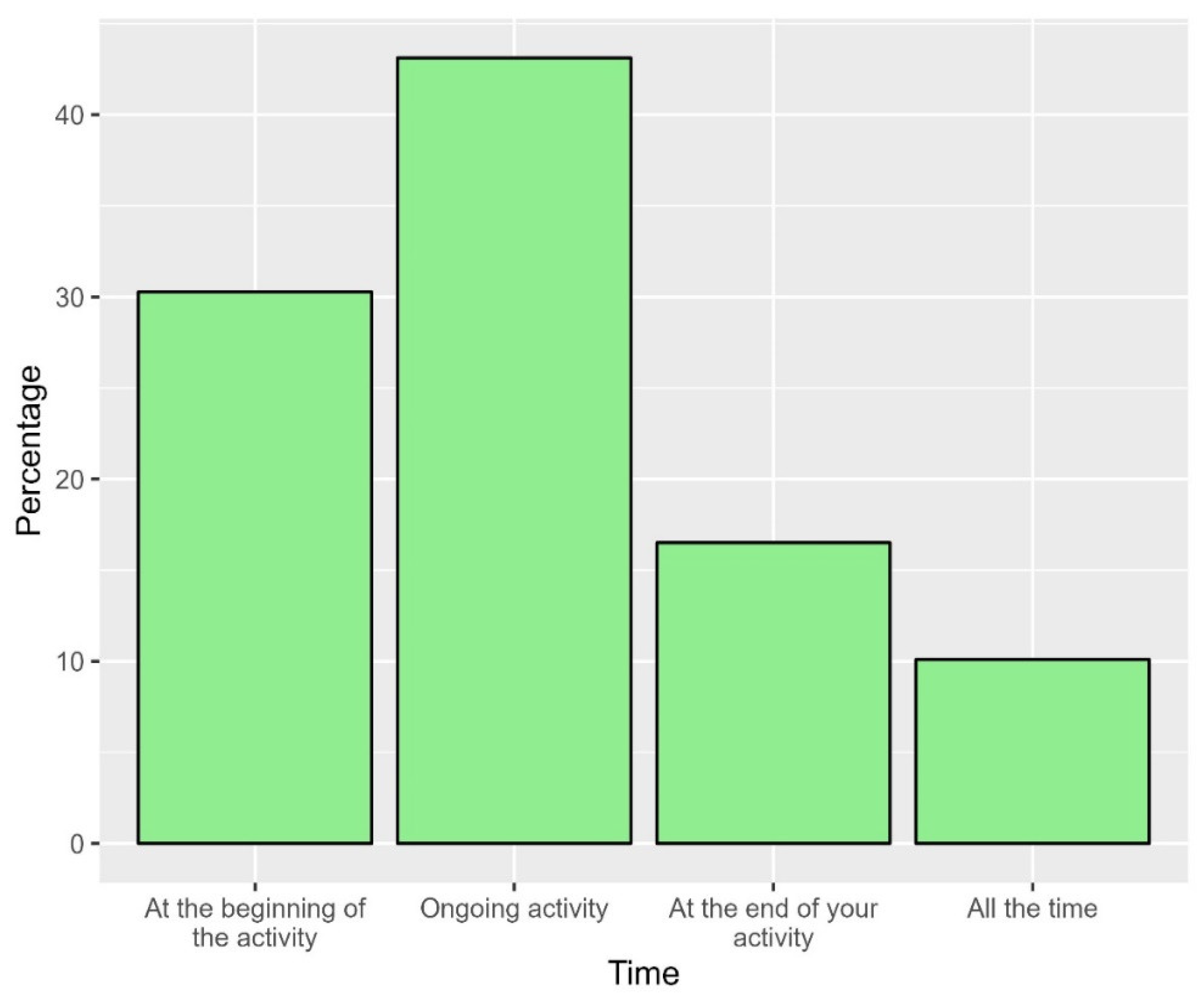
Approximately two-thirds of the participants indicated that the air quality was good to very good (64.2%, Figure 11). In a participant noticed a problem in the air quality of the space, the most frequent timing of such a problem was during the ongoing activities (43.1%) and at the beginning of the activity (30.3%, Figure 12).
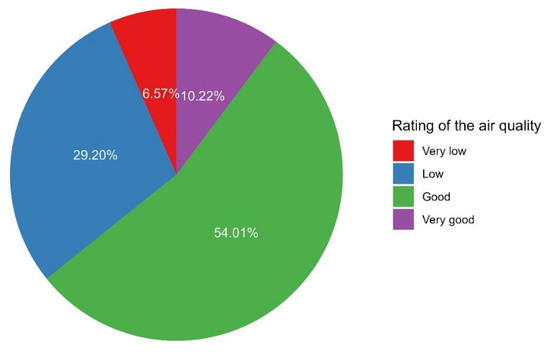
Figure 11.
The proportions of participants’ ratings of the air quality.
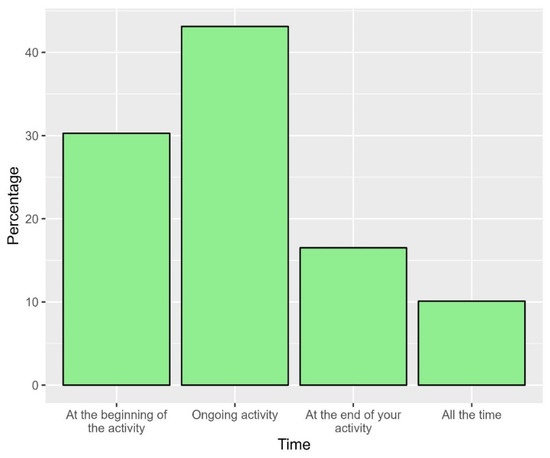
Figure 12.
The proportions of time at which the air quality would be compromised in the space.
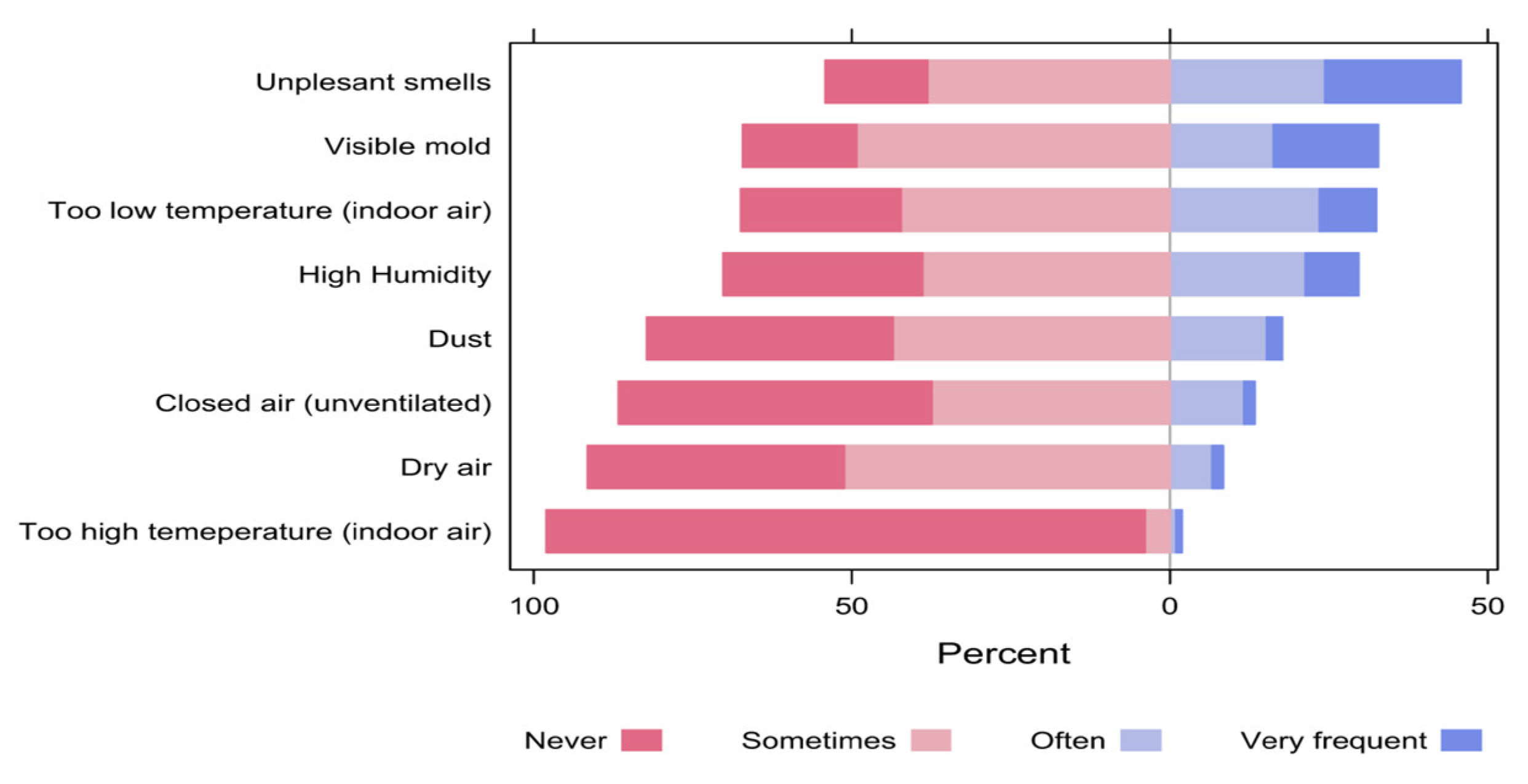
When the participants were asked about the factors that might bother them in the space, the most common bothering factors (responded as often or very frequent) included unpleasant smells (45.8%), visible mold (32.8%) and too low temperature of the indoor air (32.5%). On the contrary, the least bothering factors were too high temperature of the indoor air (2.0%), dry air (8.4%) and closed unventilated air (13.4%, Figure 13).
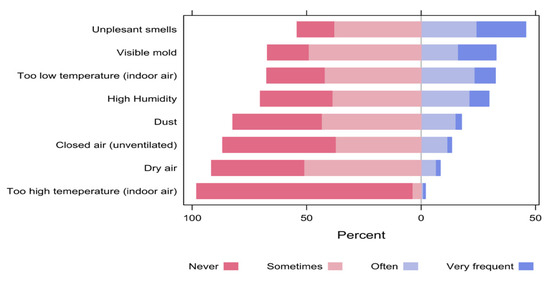
Figure 13.
The percentages of participants’ responses regarding the factors that might bother them in the space.
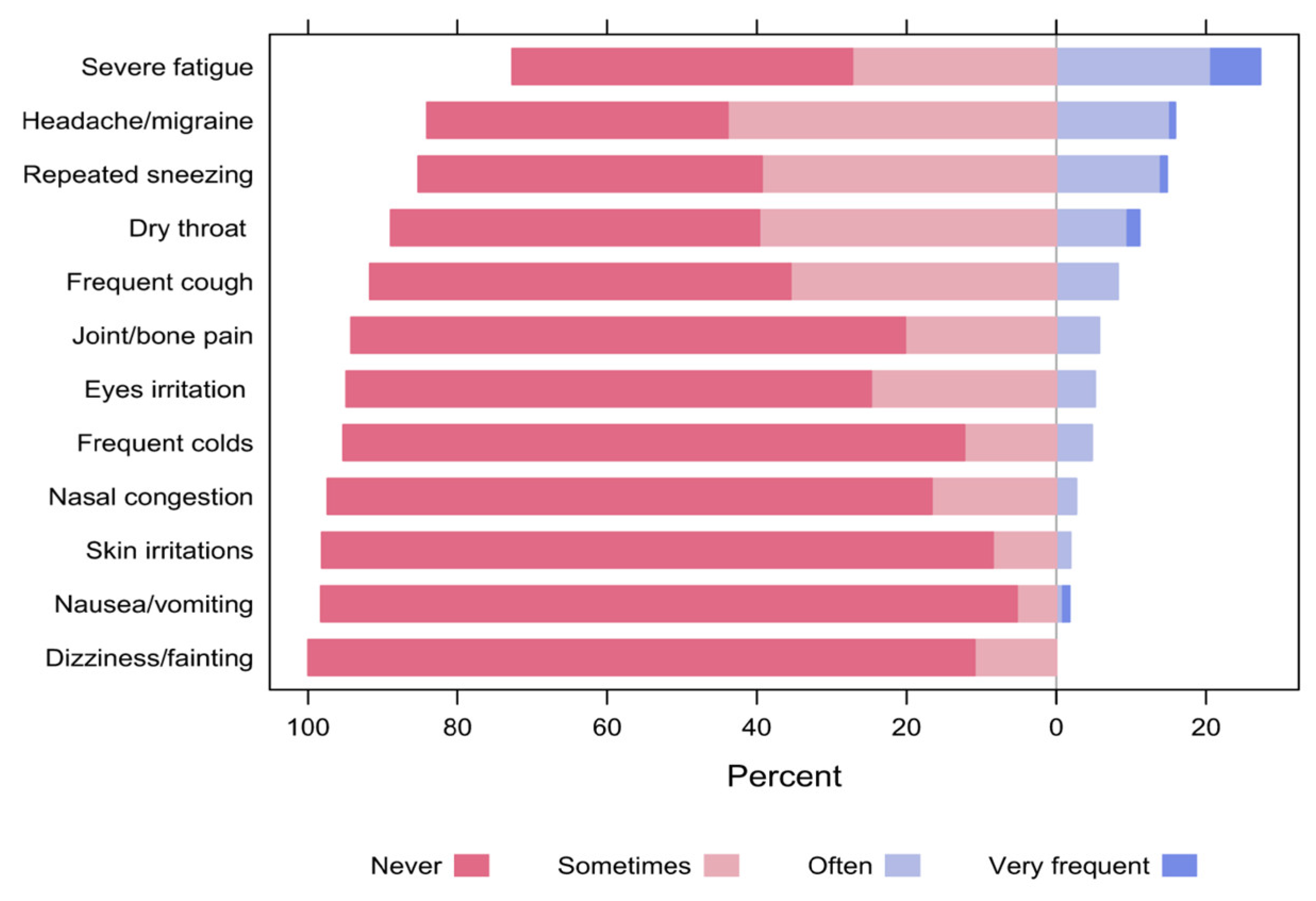
In general, almost one-quarter of the respondents reported that they often or very frequently experienced severe fatigue (27.3%), whereas 16.0% and 14.7% of them reported headache/migraine and repeated sneezing, respectively. Conversely, none of the participants often or frequently reported dizziness or fainting (0%), and only 1.8% of them reported nausea or vomiting (Figure 14). Notably, the majority of respondents (84.1%) declared that most of the experienced symptoms disappear within 1 to 2 h after leaving the space.
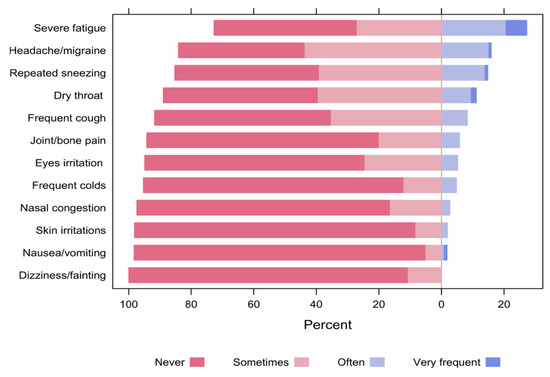
Figure 14.
The percentages of participants’ responses regarding symptoms experienced while unfolding the activities in the space.
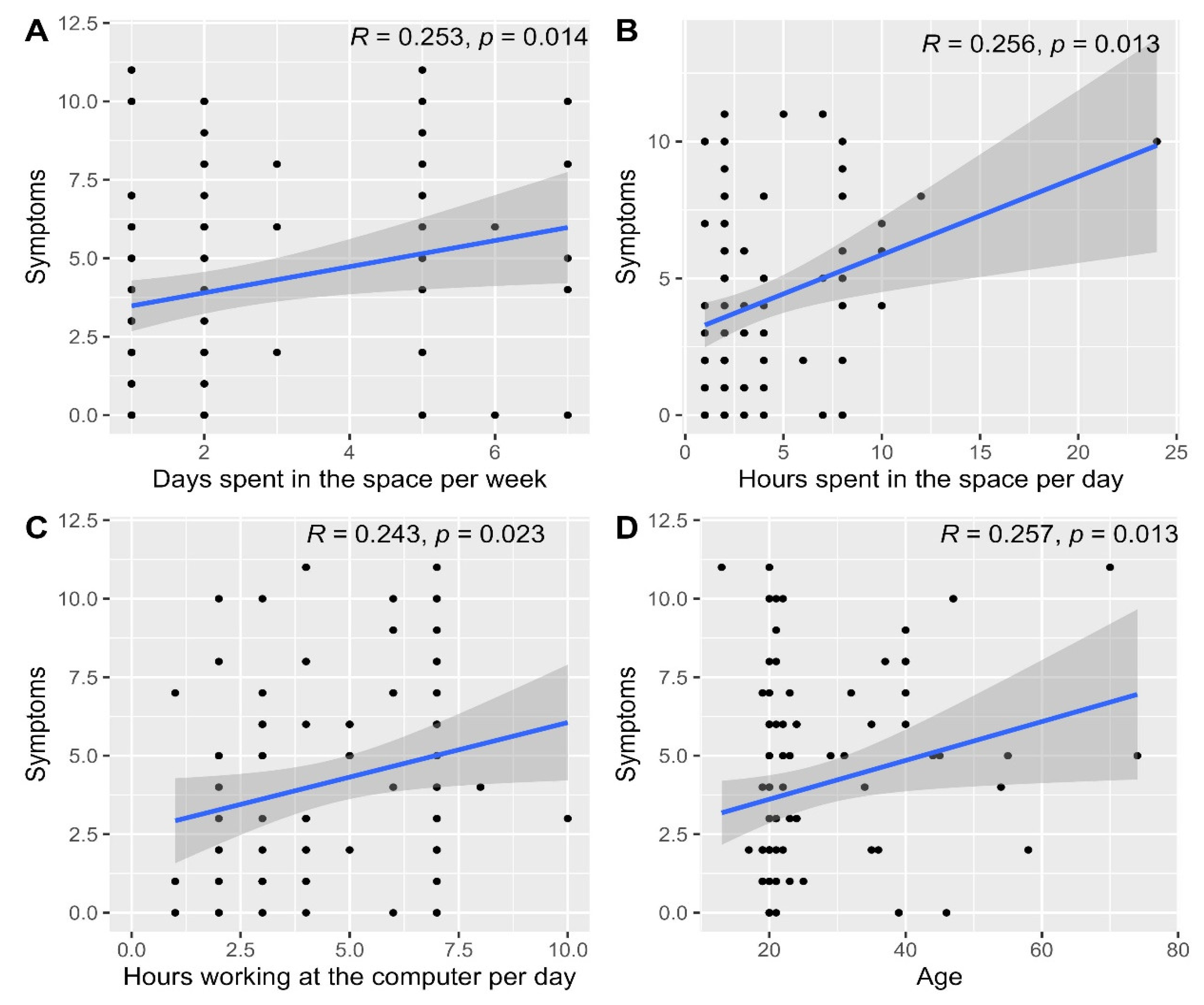
The median (IQR) symptoms score was 3.0 (1.0 to 6.0) with a minimum of 0 and a maximum of 11.0. The overall symptoms score was significantly different based on receiving medications (p = 0.033, Table 5) and having symptoms that disappeared within 1 to 2 h after leaving the space (p = 0.013), as well as being bothered by the too low temperature of the indoor air (p = 0.012), dry air (p = 0.006), unpleasant smells (p = 0.041) and dust (p = 0.038, Table 6). Furthermore, the overall symptoms score correlated significantly with the number of days spent in the space per week (p = 0.014, Figure 15A), the number of hours spent in the space per day (p = 0.013, Figure 15B), hours working on a computer per day (p = 0.023, Figure 15C) and participants’ ages (p = 0.013, Figure 15D).

Table 5.
The statistical differences in the symptoms score based on participants’ characteristics and their self-rating of the air quality.

Table 6.
The statistical differences in the symptoms score based on participants’ characteristics and their self-rating of the air quality.
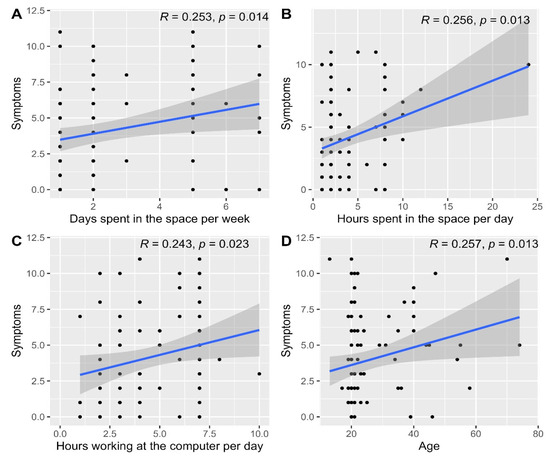
Figure 15.
Scatterplots depicting the correlations between the overall symptoms score and the number of days spent in the space per week (A), hours spent in the space per day (B), hours working on a computer per day (C) and age (D).
Results of the multivariate regression analysis showed that higher symptoms scores were independently associated with experiencing a too low temperature of the indoor air frequently (beta = 3.47, 95%CI, 0.58 to 6.36, p = 0.024), whereas symptoms were less likely to be experienced by participants who perceived that the symptoms would disappear within 1 to 2 h after leaving the space (beta = −2.70, 95%CI, −5.20 to −0.21, p = 0.040, Table 7).

Table 7.
Results of the multivariate linear regression analysis for the predictors of the symptoms score.
4. Conclusions
According to the data obtained and the analysis carried out, from a microbiological point of view, we can conclude that the ambient air in the Neologue Sion Synagogue Oradea under the average degree of fungal contamination may represent a possible risk for individual health, especially for children and people with allergic status or allergic respiratory diseases. The findings from the present investigation provide robust empirical support for the causal link between exposures to suboptimal indoor climatic conditions and the manifestation of occupants’ complaints in indoor spaces, including heritage sites such as Neologic Synagogue Sion Oradea. The outcomes reveal that deviations from optimal levels of key physical indoor environment attribute such as temperature, humidity, ventilation rates, and cleanliness can trigger a range of nonspecific health symptoms among occupants undertaking routine functional activities within the designated area. Therefore, meticulous maintenance of thermal comfort, sufficient air exchange, appropriate moisture content, and thorough elimination of contaminants from indoor surfaces and the air are imperative for ensuring the well-being, satisfaction, and productivity of occupants within heritage buildings that strive to optimize performance while preserving cultural heritage.
These findings have theoretical implications as well. This study lends support to the growing body of research applying the epidemiological paradigm of the “sick building syndrome” to heritage buildings. By quantifying associations between objectively measured indoor environmental exposures and subjectively reported occupant symptoms, the results help establish heritage sites as a valid context for investigating indoor environmental health issues. From a methodological perspective, the integrated empirical approach combining both objective instrumentation and subjective questionnaires enhances understanding of indoor environmental quality in heritage buildings. This answers recent calls in the literature for multidisciplinary, mixed-methods research to characterize the complex interplay between buildings, occupants, and environmental conditions. Future studies can build on this model to further elucidate determinants of indoor environmental health across diverse heritage properties. The outcomes also carry practical implications. For instance, heritage site managers can use the identified symptom predictors to prioritize remedial actions and target resources. Specifically, interventions that regulate temperature, humidity, ventilation and cleanliness may yield disproportionately large benefits given their independent impact on occupant comfort and health. Preventive maintenance programs addressing these modifiable risk factors are likely to maximize indoor environmental quality for a given investment. In addition, presenting the findings to occupants raises awareness of issues impacting comfort and well-being. This empowering knowledge can motivate behavioral changes such as utilizing available adaptive opportunities. Over the long-term, cultivating an informed, engaged stakeholder community may strengthen indoor environmental governance through participatory monitoring and shared problem-solving. Altogether, rigorously investigating indoor environmental quality in heritage buildings enhances scientific understanding while providing actionable strategies to balance preservation responsibilities with occupant stewardship. Looking ahead, continued application of multidisciplinary lenses will deepen insight into this critical intersection of building science, public health and cultural heritage protection.
Notably, the findings of this study provide important insights into the fungal populations present in the indoor environment of Neologue Sion Synagogue Oradea and their implications for occupant health. A range of medically relevant fungi were identified through culture-based techniques, including Candida albicans and genera such as Aspergillus sp., Penicillium sp., Cladosporium sp. and Alternaria sp. which contain opportunistic pathogens. Further molecular diagnostic tools could elucidate the specific species and strains present, revealing more about their pathogenic potential. While colony counts did not exceed contamination thresholds on their own, the mix of fungi detected warrants consideration given immune-compromised occupants. Particularly, Candida albicans demonstrated sensitivity to all antifungal agents tested, reflecting its potential treatability. However, fungi such as Aspergillus sp. and emerging resistance are concerns. Continued monitoring of fungal profiles and drug sensitivities over time could help track shifts requiring augmented IEQ or clinical management strategies. Additionally, genomic characterization of the identified fungi may provide insights into origins such as outdoors, water damage or systemic host colonization to better focus remediation efforts. The association between suboptimal thermal conditions, ventilation and perceived indoor air quality and increased occupant symptoms underscores the need for integrated indoor environmental quality maintenance protocols. Implementing preventative measures such as regular repairs, filtration upgrades and targeted cleanings can alleviate exposures to dusts, molds and other contaminants exacerbating symptoms. Occupant empowerment through education on self-reporting issues and proper building operation also warrants attention. Considering the heritage building context, a balanced approach prioritizing both preservation of culturally significant infrastructure and human health is prudent. Further collaboration between building scientists, clinicians, conservation specialists and occupants may yield innovative, minimally invasive solutions meeting all stakeholders’ needs. Overall, rigorous investigation of the indoor environment-health nexus sheds light on modifiable risk factors and informs evidence-based strategies for sustainably safeguarding cultural heritage and those who interact with these valued sites.
The results also highlight the value of objective measurements and subjective assessments of indoor climatic factors to corroborate the experience of symptoms among occupants. Such integrated approaches enable targeted interventions that rectify modifiable risk elements related to the indoor physical environment. For heritage sites with occupied zones, a preventive strategy centered on systematic surveillance of critical indoor environment attributes and timely adjustments when necessary, will likely engender the most effective indoor environmental quality management strategy. Alongside an awareness campaign that educates occupants about symptom causation, reporting, and mitigation, a holistic indoor environmental quality improvement program tailored to the specific conditions of the heritage site promises to minimize occupant discomfort while safeguarding cultural heritage. From a managerial standpoint, the findings suggest the need to implement an in-door environmental quality management program with proper monitoring and control mechanisms. It is crucial for building and facility managers to regularly and systematically monitor factors such as temperature, humidity, ventilation rates, air quality, and cleanliness in occupied spaces. Prompt actions must be taken whenever issues are identified to mitigate occupants’ discomfort and health impacts. Engaging occupants in monitoring and reporting processes through awareness campaigns can assist in developing effective management strategies. For instance, at Neologic Synagogue Sion Oradea, management could implement regular indoor environmental quality surveys of staff to identify emerging issues. Based on survey feedback, targeted interventions could be developed and implemented to optimize environmental conditions and minimize symptoms among occupants. An informed, engaged, and involved workforce is integral to achieving and sustaining high levels of indoor environmental quality.
5. Future Study
Development of multidisciplinary studies including some molecular analyses on microflora in indoor air, on objects and materials within the Neologic Synagogue Sion Oradea can identify and give details regarding the microorganism’s active involvement in the bio/deterioration processes, because it also affects the health of staff and visitors. Further studies are necessary to understand the complex correlations between building design, materials’ properties, environmental conditions, conservation practices that drive microbial colonization within heritage buildings.
Author Contributions
Conceptualization, D.C.I., L.B. and A.C.P.; methodology, L.B., T.H.H., T.C. and V.G.; software, G.V.H., P.D. and M.Z.; validation, T.M. and J.B.; writing—original draft preparation, D.C.I., A.I., L.B., T.C., G.V.H. and P.D.; writing—review and editing, T.H.H., V.G., A.C.P. and J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia [GRANT4,030].
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study may be obtained on request from the corresponding author.
Acknowledgments
The research has been funded with the support of the University of Oradea. The research undertaken was made possible by the equal scientific involvement of all the authors concerned.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Harkawy, A.; Górny, R.L.; Ogierman, L.; Wlazło, A.; Ławniczek-Wałczyk, A.; Niesler, A. Bioaerosol Assessment in Naturally Ventilated Historical Library Building with Restricted Personnel Access. Ann. Agric. Environ. Med. 2011, 18, 323–329. [Google Scholar] [PubMed]
- Ilieș, D.C.; Oneț, A.; Marcu, F.M.; Gaceu, O.R.; Timar, A.; Baias, Ș.; Ilieș, A.; Herman, G.V.; Costea, M.; Țepelea, M.; et al. Investigations on air quality in the historic wooden church in Oradea city, Romania. J. Environ. Eng. Landsc. Manag. 2018, 17, 2731–2739. [Google Scholar] [CrossRef]
- Ilieș, A.; Caciora, T.; Marcu, F.; Berdenov, Z.; Ilieș, G.; Safarov, B.; Hodor, N.; Grama, V.; Shomali, M.A.A.; Ilies, D.C.; et al. Analysis of the Interior Microclimate in Art Nouveau Heritage Buildings for the Protection of Exhibits and Human Health. Int. J. Environ. Res. Public Health 2022, 19, 16599. [Google Scholar] [CrossRef]
- Awad, A.H.A.; Saeed, Y.; Shakour, A.A.; Abdellatif, N.M.; Ibrahim, Y.H.; Elghanam, M.; Elwakeel, F. Indoor Air Fungal Pollution of a Historical Museum, Egypt: A Case Study. Aerobiologia 2020, 36, 197–209. [Google Scholar] [CrossRef]
- Azmi, A.E.; Rashid, A.A.; Razak, A.A. An Assessment of Indoor Air Quality (IAQ) in Foundry Laboratory. IOP Conf. Ser. Earth Environ. Sci. 2022, 1019, 012046. [Google Scholar] [CrossRef]
- Bungau, C.C.; Bungau, C.; Toadere, M.T.; Prada-Hanga, I.F.; Bungau, T.; Popescu, D.E.; Prada, M.F. Solutions for an Ecological and Healthy Retrofitting of Buildings on the Campus of the University of Oradea, Romania, Built Starting from 1911 to 1913. Sustain. Sci. Pract. Policy 2023, 15, 6541. [Google Scholar] [CrossRef]
- Gilbey, S.E.; Reid, C.M.; Zhao, Y.; Soares, M.J.; Huxley, R.R.; Rumchev, K.B. Residential Indoor Exposure to Fine and Ultrafine Particulate Air Pollution in Association with Blood Pressure and Subclinical Central Haemodynamic Markers of Cardiovascular Risk among Healthy Adults Living in Perth, Western Australia. Air Qual. Atmos. Health 2023, 16, 221–232. [Google Scholar] [CrossRef]
- Gruszecka-Kosowska, A. Assessment of the Kraków Inhabitants’ Health Risk Caused by the Exposure to Inhalation of Outdoor Air Contaminants. Stoch. Environ. Res. Risk Assess. 2018, 32, 485–499. [Google Scholar] [CrossRef]
- Leech, J.A.; Nelson, W.C.; Burnett, R.T.; Aaron, S.; Raizenne, M.E. It’s about Time: A Comparison of Canadian and American Time-Activity Patterns. J. Expo. Anal. Environ. Epidemiol. 2002, 12, 427–432. [Google Scholar] [CrossRef]
- Azuma, K.; Jinno, H.; Tanaka-Kagawa, T.; Sakai, S. Risk Assessment Concepts and Approaches for Indoor Air Chemicals in Japan. Int. J. Hyg. Environ. Health 2020, 225, 113470. [Google Scholar] [CrossRef]
- Brambilla, A.; Candido, C.; Gocer, O. Indoor Air Quality and Early Detection of Mould Growth in Residential Buildings: A Case Study. UCL Open Environ. 2022, 4, e049. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Villalba, L.S.; Salcines, C.; Fort, R. Application of Inorganic Nanomaterials in Cultural Heritage Conservation, Risk of Toxicity, and Preventive Measures. Nanomaterials 2023, 13, 1454. [Google Scholar] [CrossRef]
- Elsaesser, A.; Howard, C.V. Toxicology of Nanoparticles. Adv. Drug Deliv. Rev. 2012, 64, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Grøntoft, T.; Marincas, O. Indoor Air Pollution Impact on Cultural Heritage in an Urban and a Rural Location in Romania: The National Military Museum in Bucharest and the Tismana Monastery in Gorj County. Herit. Sci. 2018, 6, 73. [Google Scholar] [CrossRef]
- Cappitelli, F.; Cattò, C.; Villa, F. The Control of Cultural Heritage Microbial Deterioration. Microorganisms 2020, 8, 1542. [Google Scholar] [CrossRef]
- Paiva de Carvalho, H.; Mesquita, N.; Trovão, J.; Fernández Rodríguez, S.; Pinheiro, A.C.; Gomes, V.; Alcoforado, A.; Gil, F.; Portugal, A. Fungal Contamination of Paintings and Wooden Sculptures inside the Storage Room of a Museum: Are Current Norms and Reference Values Adequate? J. Cult. Herit. 2018, 34, 268–276. [Google Scholar] [CrossRef]
- Carlo, E.D.; Di Carlo, E.; Chisesi, R.; Barresi, G.; Barbaro, S.; Lombardo, G.; Rotolo, V.; Sebastianelli, M.; Travagliato, G.; Palla, F. Fungi and Bacteria in Indoor Cultural Heritage Environments: Microbial-Related Risks for Artworks and Human Health. Environ. Ecol. Res. 2016, 4, 257–264. [Google Scholar] [CrossRef]
- Estrada, A.R.; Torres, E.M.; Vázquez, M.A.A.; Piñero, J.L.H.; Lucio, M.A.G.; Martínez, S.M.S. Fungal Spores in Four Catholic Churches in the Metropolitan Area of Monterrey, Nuevo León State, Mexico--First Study. Ann. Agric. Environ. Med. 2015, 22, 221–226. [Google Scholar] [CrossRef][Green Version]
- Chinn, R.Y.; Sehulster, L. Guidelines for Environmental Infection Control in Health-Care Facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm. Rep. 2003, 52, 1–42. [Google Scholar]
- Ilies, D.C.; Gaceu, O.; Baias, S.; Georgita, M.; Ilies, A.; Caciora, T.; Indrie, L.; Albu, A.; Herman, G.V.; Baidog, A.; et al. Microclimatic Characteristics and Air Quality inside the National Archives of Bihor County, Romania. J. Environ. Eng. Landsc. Manag. 2021, 20, 459–466. [Google Scholar] [CrossRef]
- Ferdyn-Grygierek, J.; Kaczmarczyk, J.; Blaszczok, M.; Lubina, P.; Koper, P.; Bulińska, A. Hygrothermal risk in museum buildings located in moderate climate. Energies 2020, 13, 344. [Google Scholar] [CrossRef]
- Piñar, G.; Poyntner, C.; Lopandic, K.; Tafer, H.; Sterflinger, K. Rapid Diagnosis of Biological Colonization in Cultural Artefacts Using the MinION Nanopore Sequencing Technology. Int. Biodeterior. Biodegrad. 2020, 148, 104908. [Google Scholar] [CrossRef]
- Leijonhufvud, G.; Broström, T. Standardizing the Indoor Climate in Historic Buildings: Opportunities, Challenges and Ways Forward. J. Archit. Conserv. 2018, 24, 3–18. [Google Scholar] [CrossRef]
- Haleem Khan, A.A.; Mohan Karuppayil, S. Fungal Pollution of Indoor Environments and Its Management. Saudi J. Biol. Sci. 2012, 19, 405–426. [Google Scholar] [CrossRef]
- Ilies, D.C.; Caciora, T.; Ilies, A.; Berdenov, Z.; Hossain, M.A.; Grama, V.; Dahal, R.K.; Zdrinca, M.; Hassan, T.H.; Herman, G.V.; et al. Microbial Air Quality in the Built Environment—Case Study of Darvas-La Roche Heritage Museum House, Oradea, Romania. Buildings 2023, 13, 620. [Google Scholar] [CrossRef]
- Abdel-Kareem, O. Monitoring, controlling and prevention of the fungal deterioration of textile artifacts in the museum of Jordanian heritage. Mediterr. Archaeol. Archaeom. 2010, 10, 85–96. [Google Scholar]
- Prihatmanti, R.; Bahauddin, A. Indoor air quality in adaptively reused heritage buildings at a UNESCO World Heritage Site, Penang, Malaysia. J. Constr. Dev. Ctries. 2014, 19, 69–91. [Google Scholar]
- Wysocka, M. Analysis of Indoor Air Quality in a Naturally Ventilated Church. E3S Web Conf. 2018, 49, 00134. [Google Scholar] [CrossRef]
- Ameen, A.; Mattsson, M.; Boström, H.; Lindelöw, H. Assessment of Thermal Comfort and Air Quality in Office Rooms of a Historic Building: A Case Study in Springtime in Continental Climate. Buildings 2023, 13, 156. [Google Scholar] [CrossRef]
- Borrego, S.; Perdomo, I. Airborne Microorganisms Cultivable on Naturally Ventilated Document Repositories of the National Archive of Cuba. Environ. Sci. Pollut. Res. Int. 2016, 23, 3747–3757. [Google Scholar] [CrossRef]
- Schwab, C.J.; Straus, D.C. The roles of Penicillium and Aspergillus in sick building syndrome. Adv. Appl. Microbiol. 2004, 55, 215–240. [Google Scholar]
- Nur Fadilah, R.; Juliana, J. Indoor air quality (IAQ) and sick buildings syndrome (SBS) among office workers in new and old building in Universiti Putra Malaysia, Serdang. Health Environ. J. 2012, 3, 98–109. [Google Scholar]
- Sterflinger, K.; Piñar, G. Microbial Deterioration of Cultural Heritage and Works of Art—Tilting at Windmills? Appl. Microbiol. Biotechnol. 2013, 97, 9637–9646. [Google Scholar] [CrossRef]
- Romero, S.M.; Giudicessi, S.L.; Vitale, R.G. Is the Fungus Aspergillus a Threat to Cultural Heritage? J. Cult. Herit. 2021, 51, 107–124. [Google Scholar] [CrossRef]
- Mašková, L.; Smolík, J.; Ďurovič, M. Characterization of Indoor Air Quality in Different Archives—Possible Implications for Books and Manuscripts. Build. Environ. 2017, 120, 77–84. [Google Scholar] [CrossRef]
- Kavkler, K.; Gunde-Cimerman, N.; Zalar, P.; Demšar, A. Fungal Contamination of Textile Objects Preserved in Slovene Museums and Religious Institutions. Int. Biodeterior. Biodegrad. 2015, 97, 51–59. [Google Scholar] [CrossRef]
- Lech, T.; Ziembinska-Buczynska, A.; Krupa, N. Analysis of microflora present on historical textiles with the use of molecular techniques. Int. J. Conserv. Sci. 2015, 6, 137–144. [Google Scholar]
- Niesler, A.; Górny, R.L.; Wlazło, A.; Łudzeń-Izbińska, B.; Ławniczek-Wałczyk, A.; Gołofit-Szymczak, M.; Meres, Z.; Kasznia-Kocot, J.; Harkawy, A.; Lis, D.O.; et al. Microbial Contamination of Storerooms at the Auschwitz-Birkenau Museum. Aerobiologia 2010, 26, 125–133. [Google Scholar] [CrossRef]
- Lazaridis, M.; Katsivela, E.; Kopanakis, I.; Raisi, L.; Panagiaris, G. Indoor/outdoor Particulate Matter Concentrations and Microbial Load in Cultural Heritage Collections. Herit. Sci. 2015, 3, 34. [Google Scholar] [CrossRef]
- Nunes, I.; Mesquita, N.; Cabo Verde, S.; Bandeira, A.M.L.; Carolino, M.M.; Portugal, A.; Botelho, M.L. Characterization of an Airborne Microbial Community: A Case Study in the Archive of the University of Coimbra, Portugal. Int. Biodeterior. Biodegrad. 2013, 79, 36–41. [Google Scholar] [CrossRef]
- Savković, Ž.; Stupar, M.; Unković, N.; Ivanović, Ž.; Blagojević, J.; Popović, S.; Vukojević, J.; Grbić, M.L. Diversity and Seasonal Dynamics of Culturable Airborne Fungi in a Cultural Heritage Conservation Facility. Int. Biodeterior. Biodegrad. 2021, 157, 105163. [Google Scholar] [CrossRef]
- Viegas, C.; Cervantes, R.; Dias, M.; Gomes, B.; Pena, P.; Carolino, E.; Twarużek, M.; Kosicki, R.; Soszczyńska, E.; Viegas, S.; et al. Unveiling the Occupational Exposure to Microbial Contamination in Conservation-Restoration Settings. Microorganisms 2022, 10, 1595. [Google Scholar] [CrossRef] [PubMed]
- Pyzik, A.; Ciuchcinski, K.; Dziurzynski, M.; Dziewit, L. The Bad and the Good-Microorganisms in Cultural Heritage Environments-An Update on Biodeterioration and Biotreatment Approaches. Materials 2021, 14, 177. [Google Scholar] [CrossRef] [PubMed]
- Lincu, A.; Ilieș, M.; Ilieș, D.C.; Herman, G.V.; Baias, S.; Gozner, M.; Costea, M.; Mihincău, D. Conservating the traditional cellars of Salacea, Bihor County, Romania. GeoJ. Tour. Geosites 2018, 23, 748–758. [Google Scholar]
- Mihincău, D.C.; Ilies, D.C.; Koroleva, Y.; Herman, G.V. The study of indoor microclimate on wooden churches to be included among Oradea’s representative sights. GeoJ. Tour. Geosites 2019, 26, 737–750. [Google Scholar] [CrossRef]
- Indrie, L.; Oana, D.; Ilieş, M.; Ilieş, D.C.; Lincu, A.; Ilieş, A.; Baias, Ș.; Herman, G.; Onet, A.; Costea, M.; et al. Indoor air quality of museums and conservation of textiles art works. Case study: Salacea Museum House, Romania. Ind. Textila 2019, 70, 88–93. [Google Scholar]
- Ilieș, D.C.; Marcu, F.; Caciora, T.; Indrie, L.; Ilieș, A.; Albu, A.; Costea, M.; Burtă, L.; Baias, Ș.; Ilieș, M.; et al. Investigations of Museum Indoor Microclimate and Air Quality. Case Study from Romania. Atmosphere 2021, 12, 286. [Google Scholar] [CrossRef]
- Ilieș, D.C.; Buhaș, R.; Ilieș, A.; Gaceu, O.; Oneț, A.; Buhaș, S.; Marcu, F.; Oneț, C. Indoor air quality issues. Case study: The multipurpose sports hall of the University of Oradea. Environ. Eng. Manag. J. 2018, 17, 2999–3005. [Google Scholar]
- Sirghi, A.C.; Gheorghe, I.R.I.N.A.; Sarbu, I.; Marutescu, L.; Stoian, G.; Zhiyong, Z.; Chifiriuc, M.C. Identification of fungal strains isolated from buildings of cultural importance in Romania and antagonistic relationships amongst them. Biotechnol. Lett 2018, 24, 1008–1014. [Google Scholar] [CrossRef]
- An, C.; Yamamoto, N. Fungal Compositions and Diversities on Indoor Surfaces with Visible Mold Growths in Residential Buildings in the Seoul Capital Area of South Korea. Indoor Air 2016, 26, 714–723. [Google Scholar] [CrossRef]
- Taskinen, T.; Meklin, T.; Nousiainen, M.; Husman, T.; Nevalainen, A.; Korppi, M. Moisture and Mould Problems in Schools and Respiratory Manifestations in Schoolchildren: Clinical and Skin Test Findings. Acta Paediatr. 1997, 86, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.S.; Kttafah, G.H.; Nasuruddin, M.H. Allergenic Potential and Cross-Reactivity of Fungal Species Isolated from the Indoor Environment. J. Teknol. 2022, 84, 47–57. [Google Scholar] [CrossRef]
- Lupan, I.; Ianc, M.B.; Kelemen, B.S.; Carpa, R.; Rosca-Casian, O.; Chiriac, M.T.; Popescu, O. New and Old Microbial Communities Colonizing a Seventeenth-Century Wooden Church. Folia Microbiol. 2014, 59, 45–51. [Google Scholar] [CrossRef]
- Marcu, F.; Hodor, N.; Indrie, L.; Dejeu, P.; Ilieș, M.; Albu, A.; Sandor, M.; Sicora, C.; Costea, M.; Ilieș, D.C.; et al. Microbiological, Health and Comfort Aspects of Indoor Air Quality in a Romanian Historical Wooden Church. Int. J. Environ. Res. Public Health 2021, 18, 9908. [Google Scholar] [CrossRef]
- Puchianu, G.; Necula, V.; Enache, D.V. Research on Active and Passive Monitoring Aeromicroflora in the Milk Units Processing. Ann. Acad. Rom. Sci. Ser. Math. Appl. 2016, 5, 91–103. [Google Scholar]
- Insert kit Integral System Yeasts plus; Liofilchem: Roseto degli Abruzzi, Italia, 2012.
- Puchianu, G.; Necula, V.; Enache, D.V.; Daneș, M. Researches regarding the active and passive monitoring of aeromicroflora in milling and bread manufacturing. Rom. Biotechnol. Lett. 2020, 25, 1465–1472. [Google Scholar] [CrossRef]
- Cernei, E.R.; Maxim, D.C.; Mavru, R.; Indrei, L.L. Bacteriological analysis of air (aeromicroflora) from the level of dental offices in IAŞI County. Rom. J. Oral Rehabil. 2013, 5, 53–68. [Google Scholar]
- National Network for Monitoring Air Quality. Available online: https://www.calitateaer.ro/public/monitoring-page/reports-reports-page/?__locale=ro (accessed on 20 August 2023).
- ASHRAE Position Document on Indoor Carbon Dioxide; American Society of Heating, Refrigeration and Air Conditioning Engineers: Atlanta, GA, USA, 2022; Available online: https://www.ashrae.org/file%20library/about/position%20documents/pd_indoorcarbondioxide_2022.pdf (accessed on 26 April 2023).
- ANSI/ASHRAE Standard 55-2017; Thermal Environmental Conditions for Human Occupancy. American Society of Heating, Refrigeration and Air Conditioning Engineers: Atlanta, GA, USA, 2017. Available online: https://hogiaphat.vn/upload/docs/ASHRAE55-version2017.pdf (accessed on 21 April 2023).
- ANSI/ASHRAE Standard 62.1-2016; Ventilation for Acceptable Indoor Air Quality. American Society of Heating, Refrigerating and Air-Conditioning Engineers: Atlanta, GA, USA, 2013. Available online: https://upgreengrade.ir/admin_panel/assets/images/books/25223276727.pdf (accessed on 21 April 2023).
- ANSI/ASHRAE Addendum a to ANSI/ASHRAE Standard 55-2020; Thermal Environmental Conditions for Human Occupancy. American Society of Heating, Refrigeration and Air Conditioning Engineers: Atlanta, GA, USA, 2021. Available online: https://www.ashrae.org/file%20library/technical%20resources/standards%20and%20guidelines/standards%20addenda/55_2020_a_20210430.pdf (accessed on 23 April 2023).
- ANSI/ASHRAE Addendum s to ANSI/ASHRAE Standard 62.1-2016; Ventilation for Acceptable Indoor Air Quality. American Society of Heating, Refrigeration and Air Conditioning Engineers: Atlanta, GA, USA, 2019. Available online: https://www.ashrae.org/File%20Library/Technical%20Resources/Standards%20and%20Guidelines/Standards%20Addenda/62.1-2016/62_1_2016_s_20190726.pdf (accessed on 23 April 2023).
- Khovalyg, D.; Kazanci, O.B.; Halvorsen, H.; Gundlach, I.; Bahnfleth, W.P.; Toftum, J.; Olesen, B.W. Critical Review of Standards for Indoor Thermal Environment and Air Quality. Energy Build. 2020, 213, 109819. [Google Scholar] [CrossRef]
- Arsad, F.S.; Hod, R.; Ahmad, N.; Baharom, M.; Ja’afar, M.H. Assessment of Indoor Thermal Comfort Temperature and Related Behavioural Adaptations: A Systematic Review. Environ. Sci. Pollut. Res. Int. 2023, 30, 73137–73149. [Google Scholar] [CrossRef]
- Pasanen, A.-L.; Kasanen, J.-P.; Rautiala, S.; Ikäheimo, M.; Rantamäki, J.; Kääriäinen, H.; Kalliokoski, P. Fungal Growth and Survival in Building Materials under Fluctuating Moisture and Temperature Conditions. Int. Biodeterior. Biodegrad. 2000, 46, 117–127. [Google Scholar] [CrossRef]
- Wolkoff, P. Indoor Air Humidity, Air Quality, and Health—An Overview. Int. J. Hyg. Environ. Health 2018, 221, 376–390. [Google Scholar] [CrossRef]
- Wu, H.; Wong, J.W.C. Temperature versus Relative Humidity: Which Is More Important for Indoor Mold Prevention? J. Fungi 2022, 8, 696. [Google Scholar] [CrossRef] [PubMed]
- Commission of European Communities. Biological Particles in Indoor Environments, European Collaborative Action—Indoor Air Quality and Its Impact on Man; Report No 12; CEC: Luxembourg, 1993. [Google Scholar]
- Buiuc, D.; Neguţ, M. Tratat de Microbiologie Clinică; Editura Medicală: București, Romania, 2008. [Google Scholar]
- Atlas Micologie. Available online: https://issuu.com/emilyluciaa/docs/atlas_micolog_a_2019 (accessed on 3 September 2023).
- Dyląg, M. Fungi Present in Home and Their Impact on Human Health-A Short Review. Insights Biol. Med. 2017, 1, 16–25. [Google Scholar] [CrossRef][Green Version]
- Chang, C.-Y.; Chou, H.; Tam, M.F.; Tang, R.-B.; Lai, H.-Y.; Shen, H.-D. Characterization of Enolase Allergen from Rhodotorula Mucilaginosa. J. Biomed. Sci. 2002, 9, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Yeeh, Y. Rhodotorula. In Encyclopedia of Food Microbiology; Robinson, R.K., Ed.; Elsevier: Oxford, UK, 1999; pp. 1900–1905. ISBN 9780122270703. [Google Scholar]
- Fukutomi, Y.; Tanimoto, H.; Yasueda, H.; Taniguchi, M. Serological Diagnosis of Allergic Bronchopulmonary Mycosis: Progress and Challenges. Allergol. Int. 2016, 65, 30–36. [Google Scholar] [CrossRef]
- Jurcă, M.C.; Bembea, M.; Kozma, K.; Şandor, M.I.; Negrean, R.A.; Dobjanschi, L.; Cuc, E.A.; Petcheşi, C.D.; Jurcă, A.D. Empty sella associated with growth hormone deficiency and polydactyly. Rom. J. Morphol. Embryol. = Rev. Roum. Morphol. Embryol. 2018, 59, 381–384. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).