Abstract
With the vigorous development of the world’s mineral resources, the global ecological environment has been severely damaged. The tailings cemented filling technology is an important way to realize the green and low-carbon development of the mining industry. However, sulfur-containing tailings from metal mines can destroy the stability and strength of cemented tailings backfill. Therefore, it is imperative to reduce the harm of the sulfur-containing tailings to the strength of cemented tailings backfill. Firstly, based on the research results of sulfur-containing tailings cemented backfill in recent years, this paper reviews the influence of sulfur-containing tailings on the strength of cemented backfill. Accordingly, the mechanism of strength failure of cemented backfill caused by sulfur-containing tailings is further studied, and the erosion failure of sulfide and sulfate is deeply discussed and analyzed. In addition, three control measures are proposed, including adjusting the combination of filling materials and optimizing the filling ratio, controlling the oxidation conditions in the filling process, and adding ad-mixtures as a supplement. Finally, the main conclusions and outlooks of this review are summarized. The purpose of this review is to provide guidance to improve the strength and durability of the cemented sulfur tailings backfill, effectively treating metal tailings, and to propose some ideas for the further improvement and development of the tailings cemented filling technology.
1. Introduction
Mineral resources are the material basis for sustainable economic and social development, also the precious natural wealth of a country or a region. However, mining has generated a large number of tailings which caused severe pollution and significant harm to the environment [1]. The global tailings rules meeting held in March 2019 established global tailings management standards and supported governments to incorporate tailings management standards into their national legislation and policies [2]. Tailings and environmental issues have been in the spotlight in the world nowadays, and achieving green and sustainable mining has become a common theme in the mining industry [3].
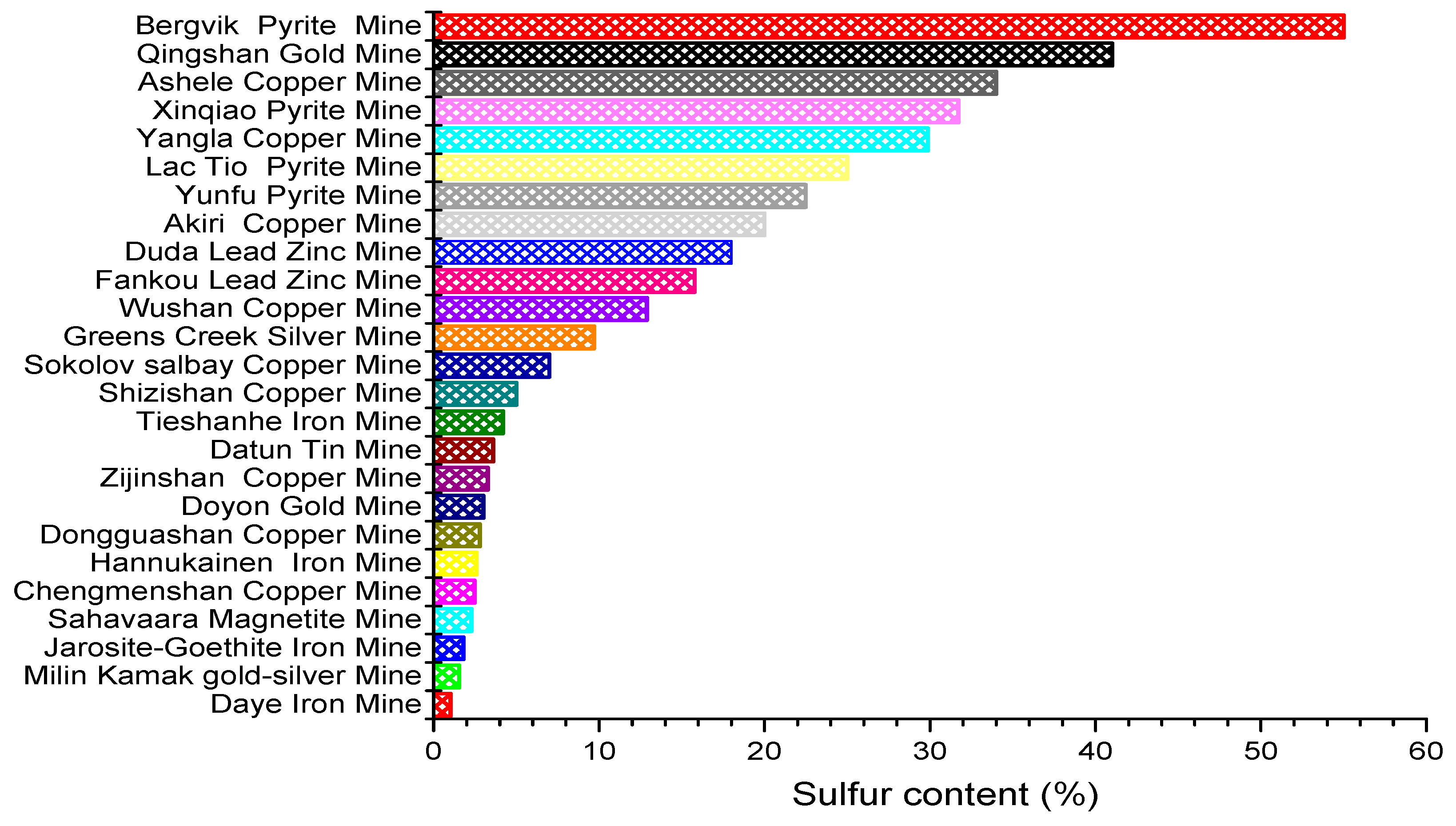
As a new technology to realize the green sustainable development of mine resources, cemented tailings filling technology has been widely promoted and applied in tailings treatment. [4,5]. Scholars have found that sulfur content in tailings will have a great impact on the strength of cemented tailings backfill. China’s Code for Design of Nonferrous Metal Mining (GB50771-2012) has strict regulations on the sulfur content of tailings cemented backfill, which should not exceed 8% [6]. Most metal tailings are faced with the problem of high sulfur content (Figure 1), so it is crucial to study sulfur-containing tailings in cemented backfill. Only by mastering the failure mechanism of sulfur-containing tailings on the strength of the cemented tailings backfill can we prevent mining accidents, improve technical defects and efficiently treat metal tailings [7].
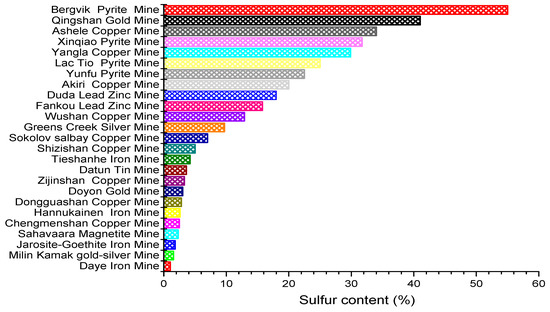
Figure 1.
Sulfur content in some metal mines [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30].
The innovation of this paper is to clarify the type and mechanism of sulfide erosion and sulfate erosion, define three specific failure modes of sulfate erosion, including expansion failure, cohesive failure, and stress failure, and put forward the corresponding control measures. In addition, the influence of sulfur content and the age of cemented sulfur tailings backfill on its strength has revealed that the principle of improving the strength of cemented backfill by sulfur-containing tailings in the primary stage of filling is expounded. We hope this review can provide a comprehensive understanding of the strength of cemented sulfur tailings backfills and help and guide in the practical application of metal tailings treatment.
2. Influence of Sulfur-Containing Tailings on the Strength of Cemented Tailings Backfill
According to relevant studies, sulfur-containing tailings as a backfill material can significantly affect the strength and mechanical properties of cemented backfills; the sulfur content in tailings and the age of cemented roles in increasing or decreasing the strength of cemented tailings backfill.
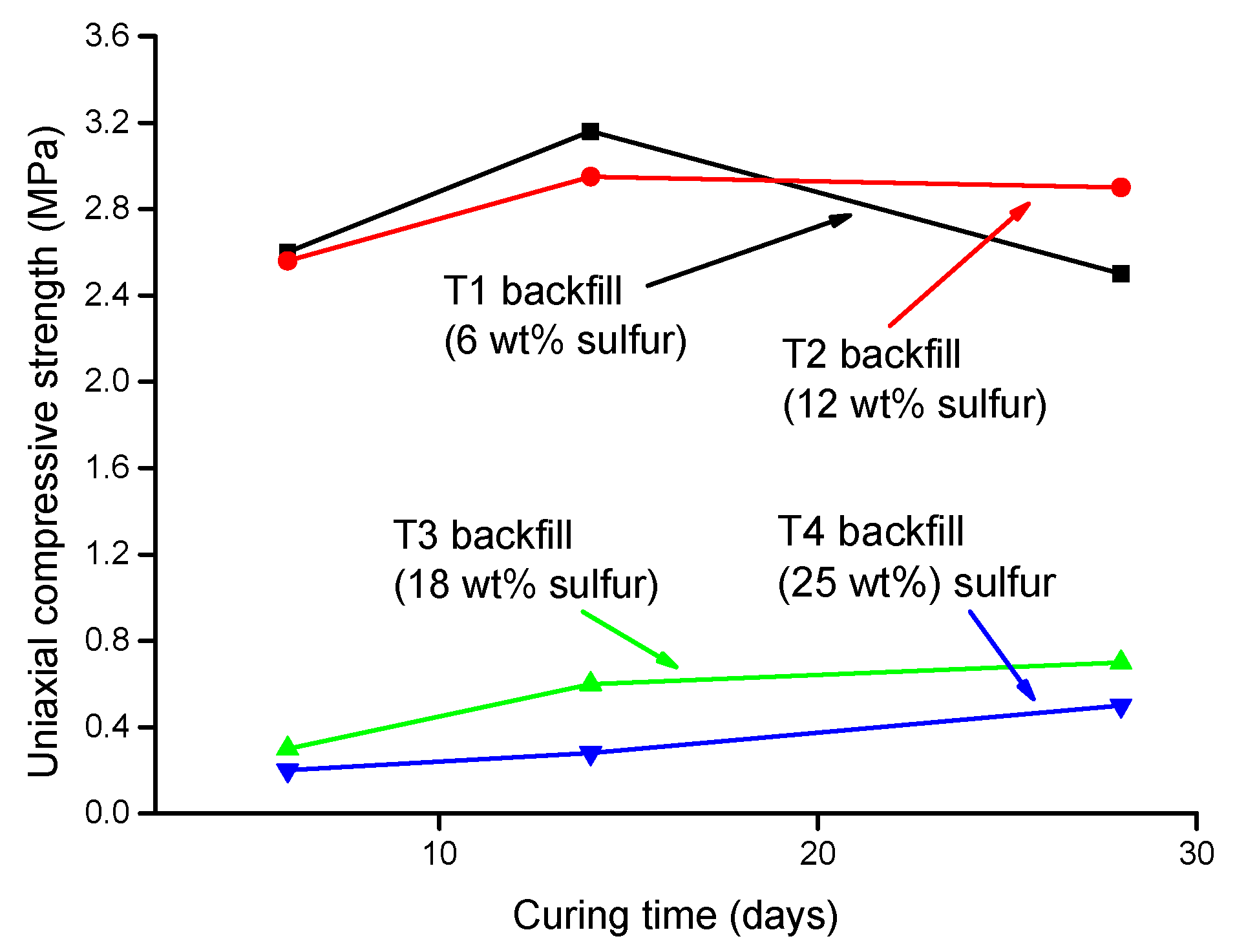
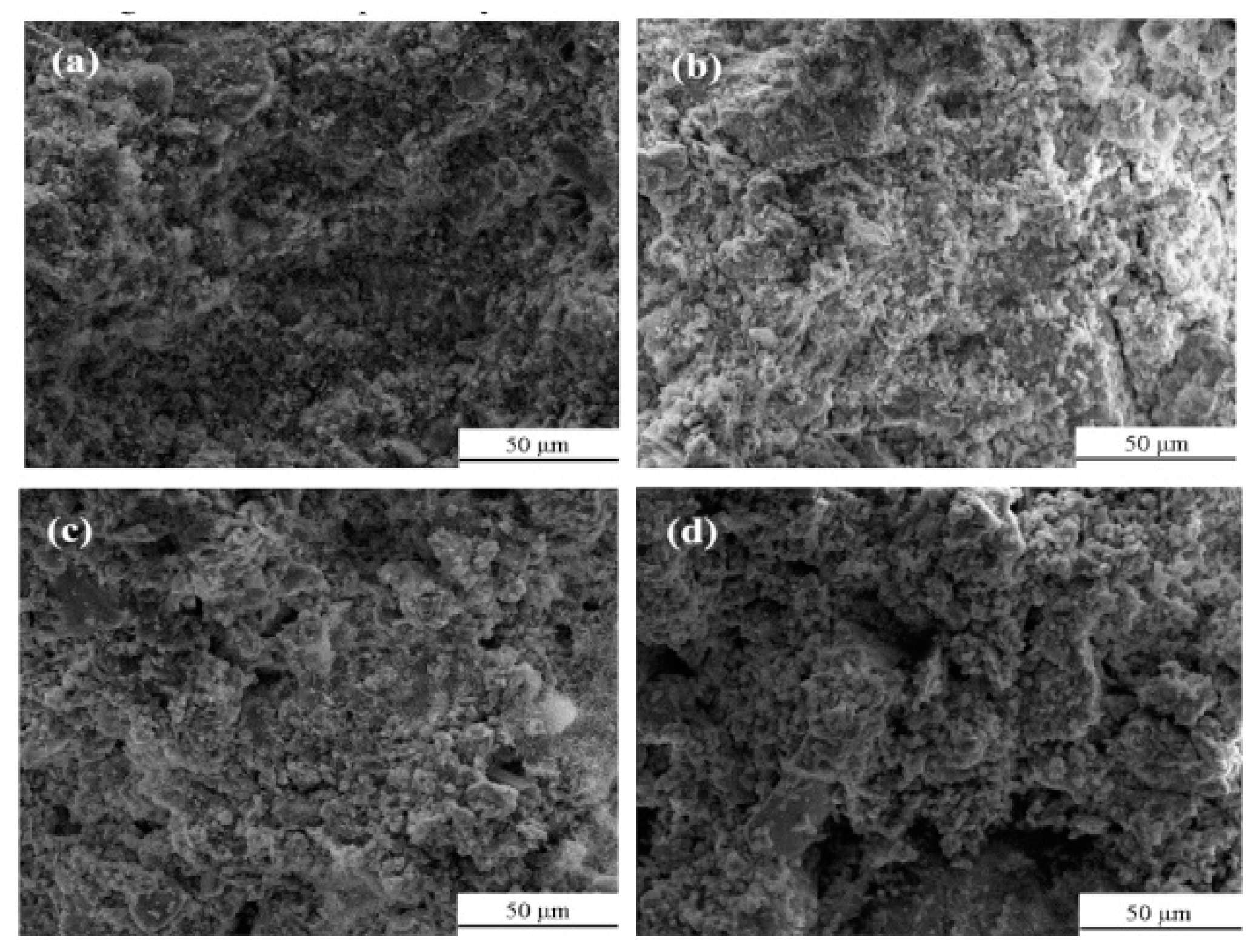
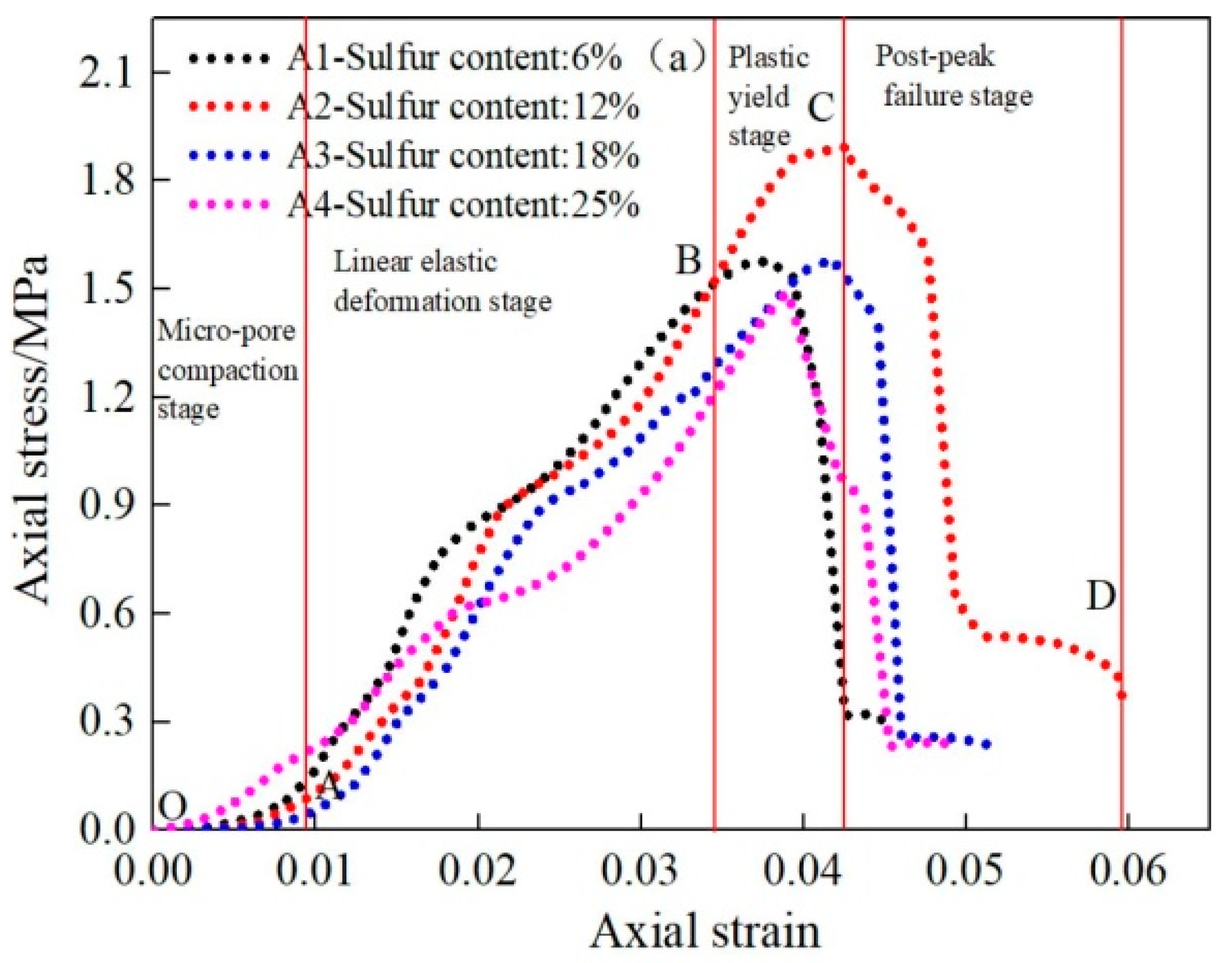
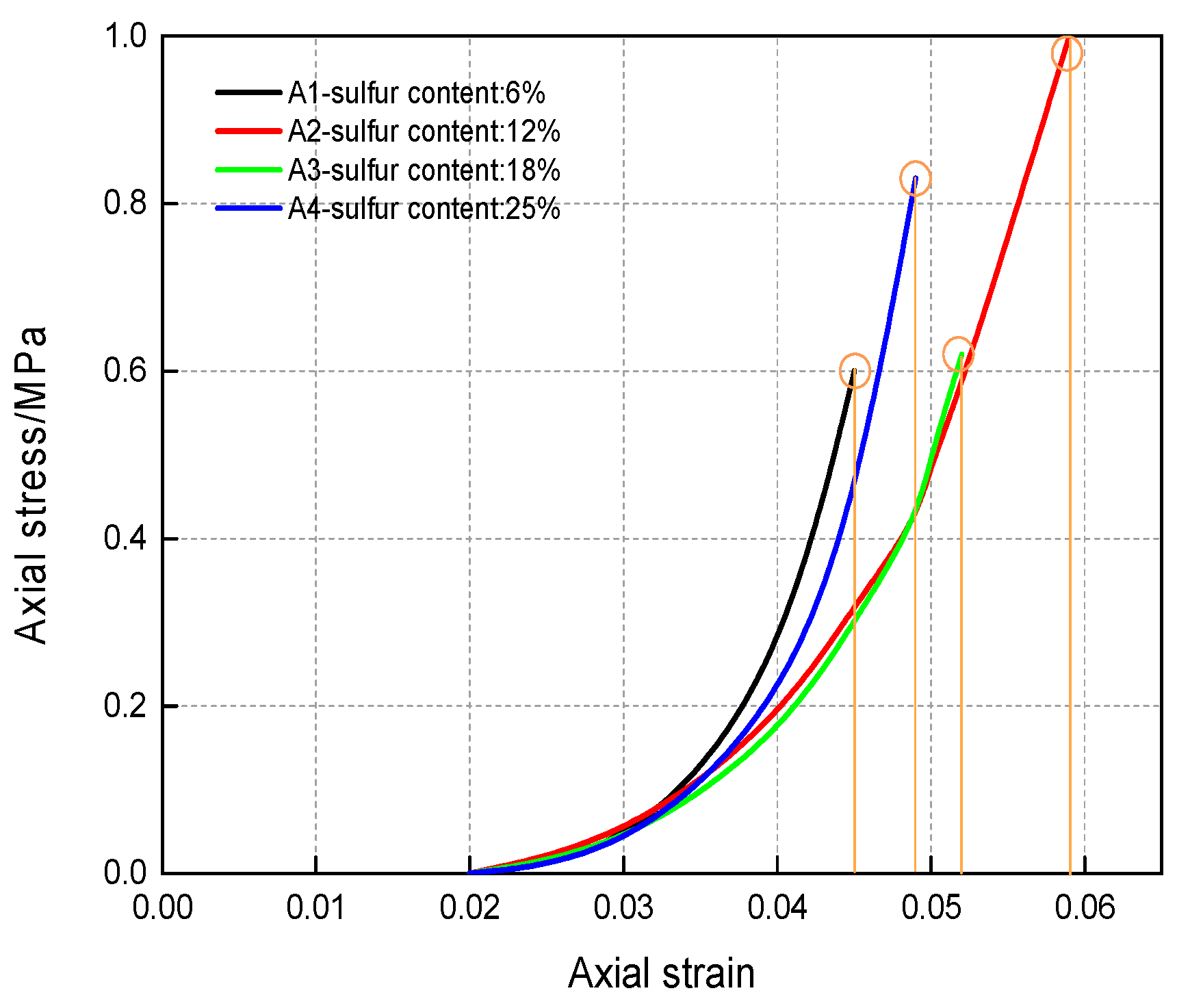
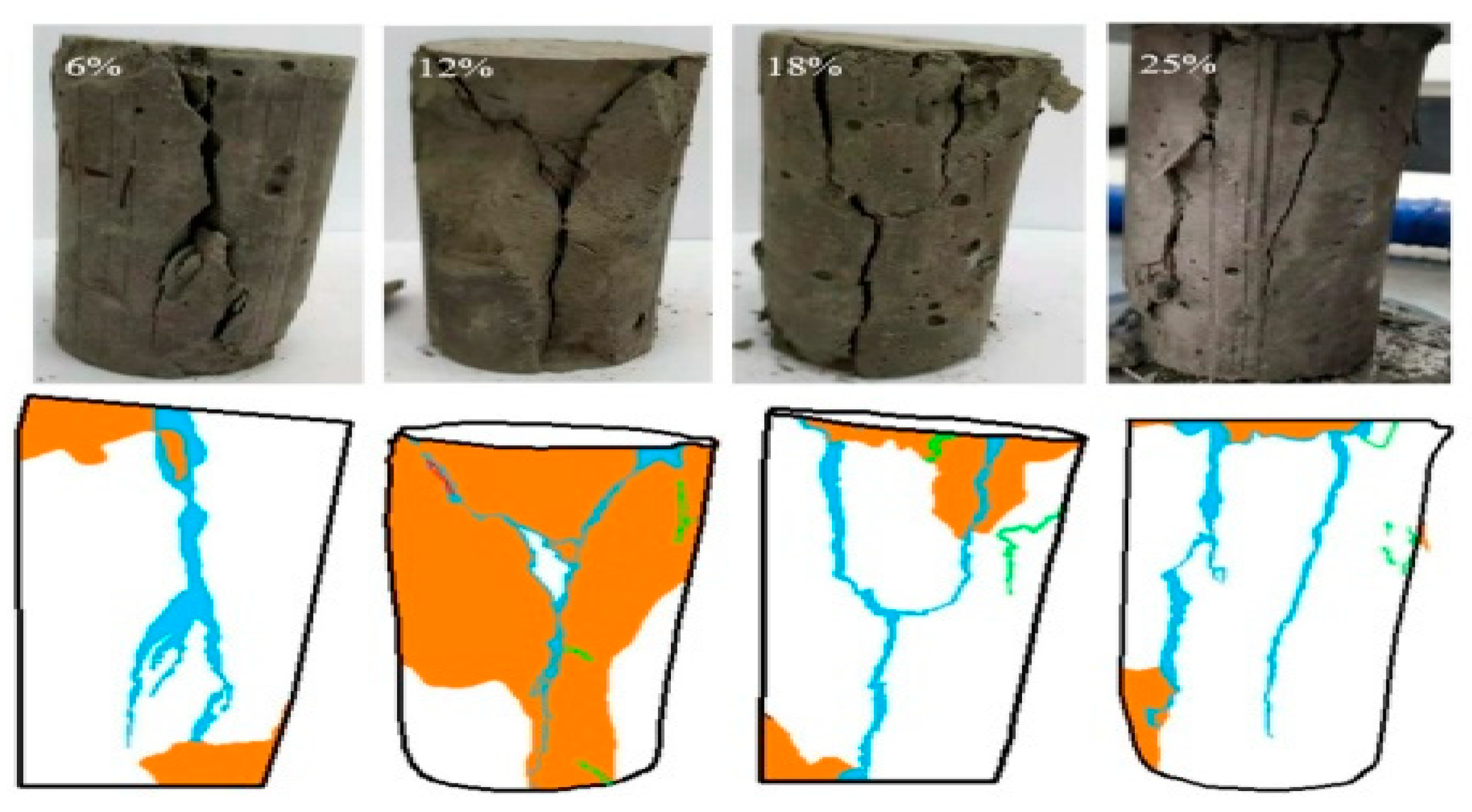
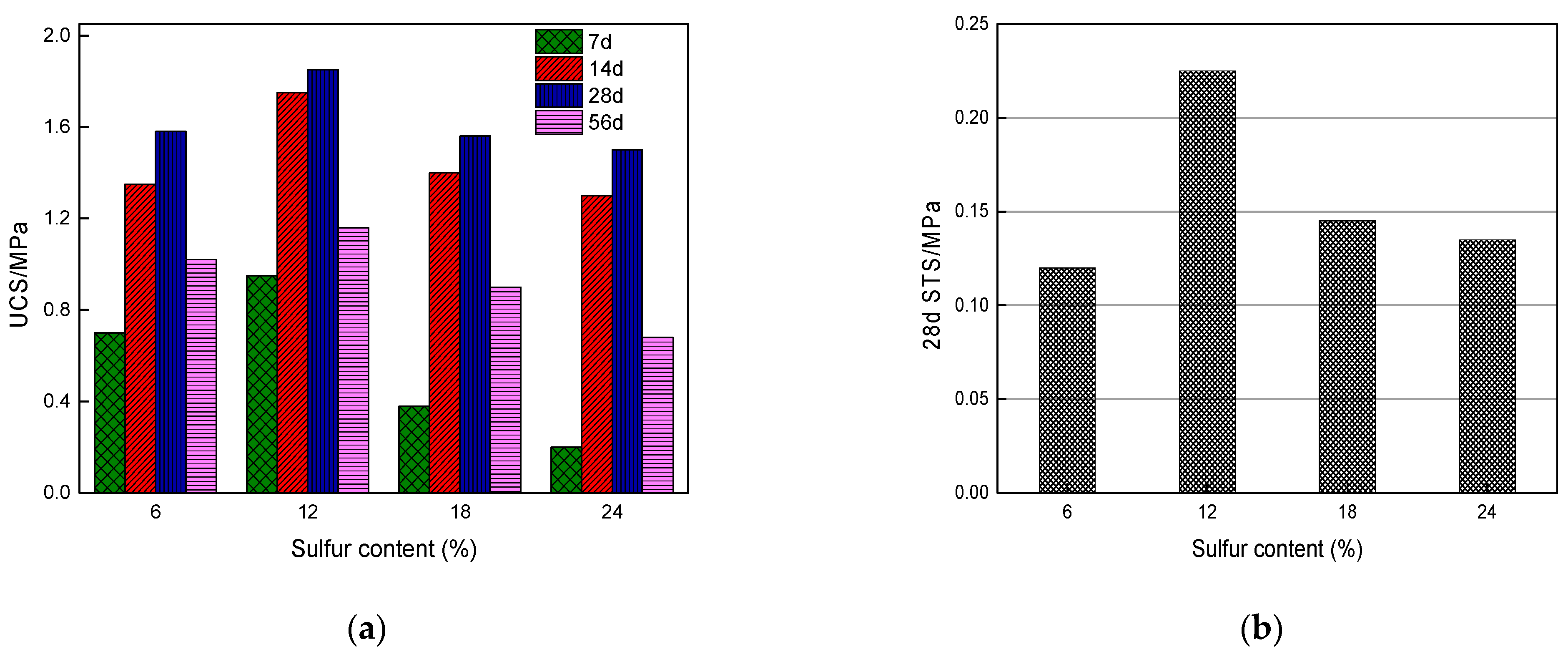
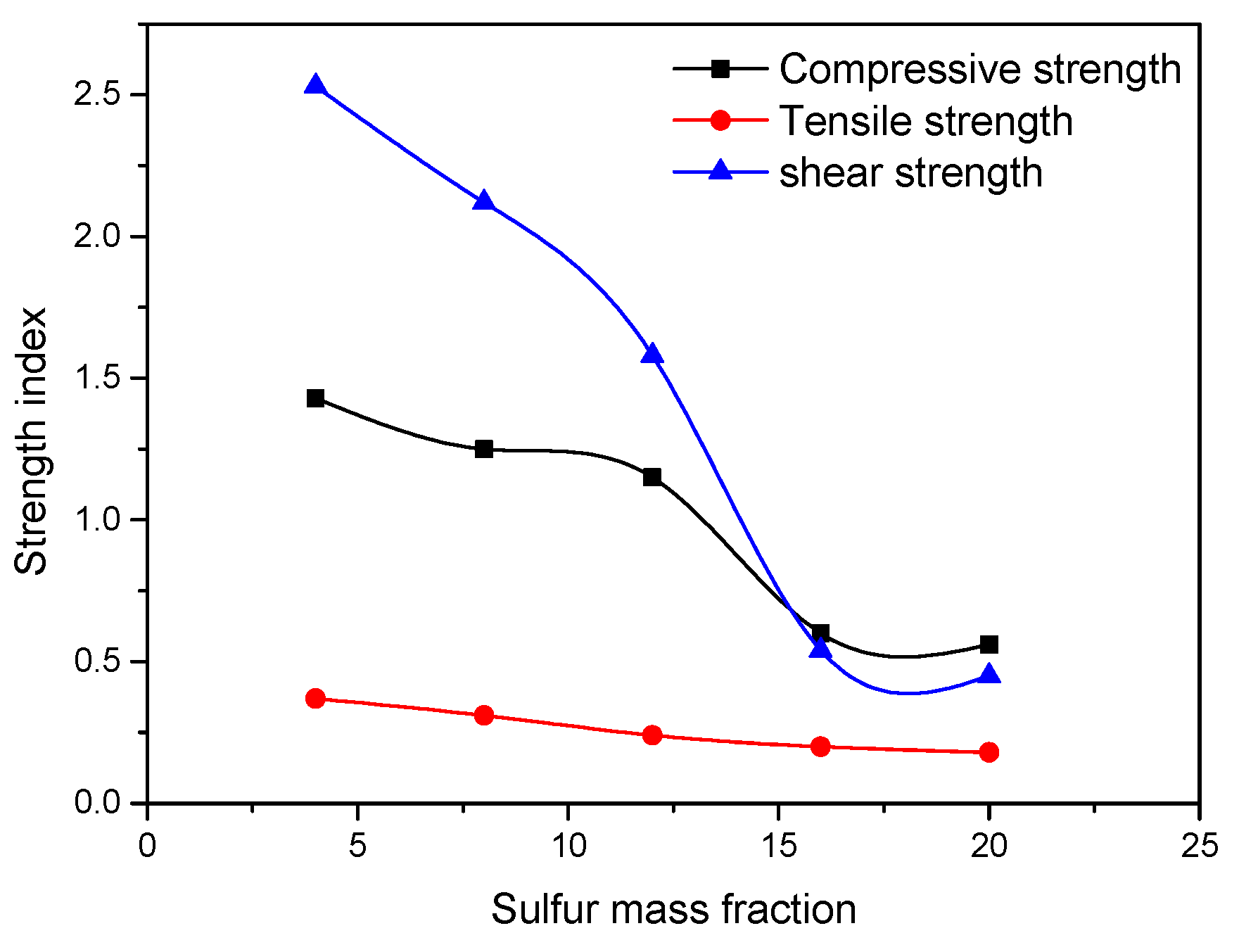
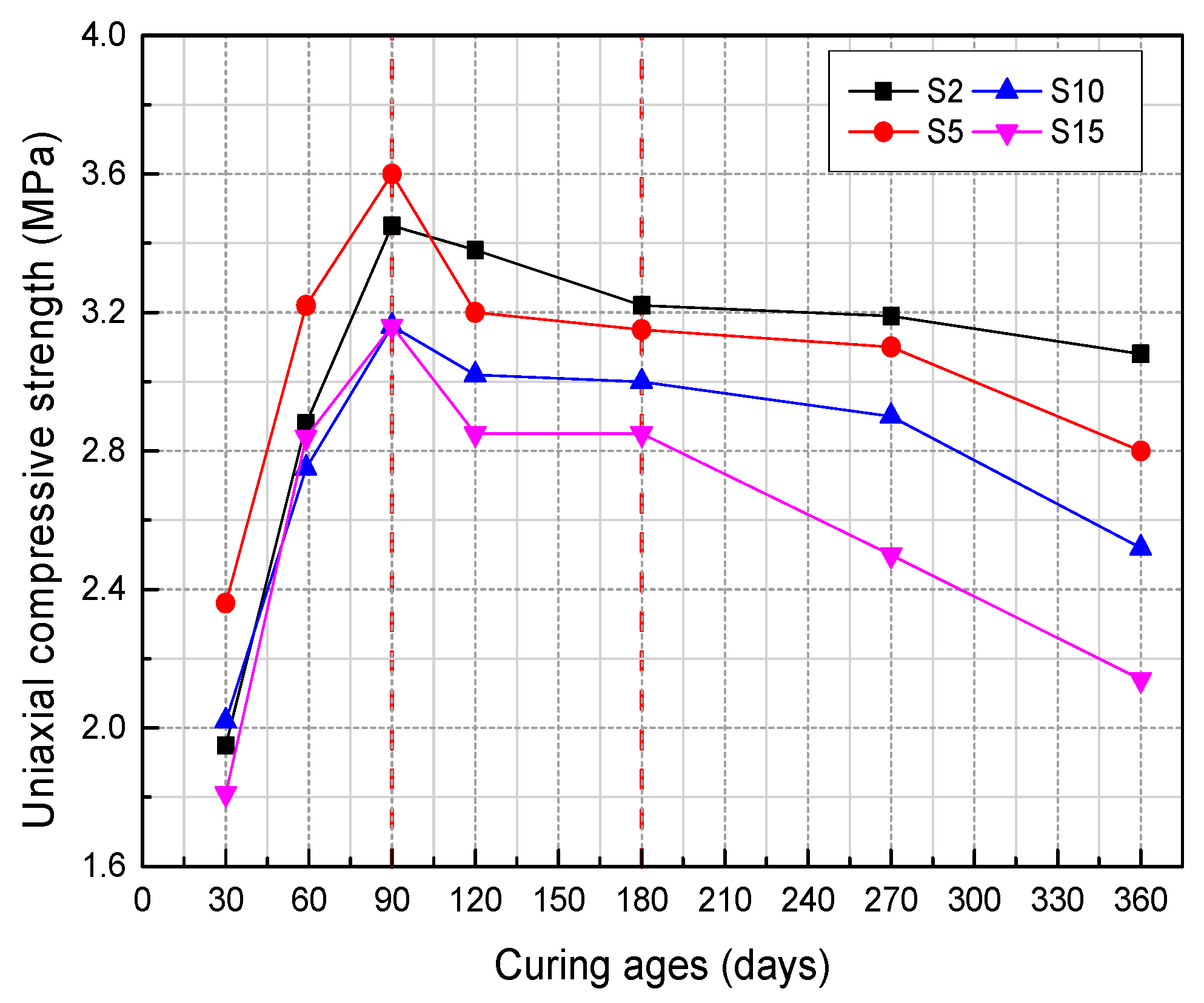
Li et al. (2019) [31] studied the influence of various sulfide content in tailings on the cemented backfills strength deterioration and tested the uniaxial compressive strength of the samples with other sulfur content (6 wt%, 12 wt%, 18 wt%, and 25 wt%, namely, T1, T2, T3, and T4) (Figure 2). After curing for some time, the cemented tailings backfill’s strength changed with sulfur content and curing time. It was found that after SEM microstructure analysis of the samples cured for 28 days (Figure 3), the porosity and texture looseness of the cemented tailings backfill with different sulfide contents can be significantly distinguished. That is to say, the cemented tailings backfill with other sulfur content has significant differences in strength and stability. Hou et al. (2022) [32] obtained the stress–strain curve of the cemented sulfur tailings backfill (CSTB) by the uniaxial compression tests (Figure 4). The peak stress, residual stress, and peak strain of CSTB with different sulfur contents were different under uniaxial loading in Figure 4. Moreover, Hou et al. [32,33] investigated the damage evolution of CSTB to reflect the mechanical performance of CSTB under load. Finally, The change in the damage growth rate of CSTB clearly shows the difference in compressive strength and load-bearing capacity (Figure 5). Damage crack evolution characteristics of CSTB (Figure 6) reflect the strength degradation of CSTB at different sulfur content. (the orange, blue and green parts represent the fracture surface range, large-scale failure crack and microcrack of CSTB, respectively). Yin et al. (2021) [34] investigated the relationship between the sulfur content of CSTB at different ages and uniaxial compressive strength (UCS), (see Figure 7a), and the histogram of 28 days splitting tensile strength (STS) and sulfur content of CSTB (Figure 7b). As shown in Figure 7a, the UCS value ages increased when the sulfur content of samples aged 7 d, 14 d, 28 d and 56 d increased from 6% to 12%. As the sulfur content increased from 12% to 25%, the UCS of CSTB of different age groups decreased. However, the magnitude of the rise and fall in UCS is different. Moreover, Figure 7b showed when sulfur content increased from 6% to 12% and then to 25%, the 28 d STS of CSTB increased first and then decreased. If the sulfur content is 12%, the UCS of CSTB at different ages and 28 d STS of CSTB reached the maximum. Zhan et al. (2018) [35] investigated the effect of sulfur content on the different strength indexes (compressive strength, tensile strength, shear strength) of CSTB by uniaxial compression tests, Brazilian splitting tests and shear tests (Figure 8). When the sulfur content is 4%, the compressive strength and the shear strength of CSTB reach the maximum of 1.43 MPa and 2.53 MPa, respectively, the tensile strength is 0.37 Mpa. When the sulfur content is 4%~16%, the compressive strength and shear strength decreased to 0.6 MPa and 0.54 Mpa, respectively, at a higher rate, and the tensile strength decreased to 0.31 MPa at a slower rate. When the sulfur content is 16%~20%, the compressive strength, the tensile strength, and the shear strength of CSTB have no obvious change, which is stable at 0.6 Mpa, 0.18 MPa and 0.45 MPa, respectively. Therefore, it can be concluded that the tailing’s sulfur content has a significant impact on the strength of cemented backfill. Jiang et al. (2017) [36] explored the impact of the sulfur content of tailings on the performance of cemented tailings backfill (CTB), adjusted the sulfur content of tailings to 6.1%, 12%, 18% and 25%, respectively, and measured the change in uniaxial compressive strength. When the sulfur content is less than 12%, the strength of CTB aged 7 d and 14 d decreased with increasing sulfur content, but the strength of CTB aged 28 d increased. When the sulfur content exceeds 12%, the strength of the CTB aged 7 d, 14 d and 28 d decreased with increasing sulfur content. The lowering of CTB strength is the fastest, while sulfur content is 12 to 18%. When the sulfur content is 25%, compared with the CTB with a sulfur content of 6.1 %, the strength of the CTB at the age of 28 d decreases by 69.3%. When sulfur content is 6% and 12%, CTB’s strength of 28 d is 21.1% and 3.6%, respectively, lower than that of the CTB at the age of 14 d. Therefore, it can be concluded that the strength of CSTB is influenced not only by sulfur content but also by age. Moreover, the critical value of sulfur content of CSTB is different at different ages. Dong et al. (2019) [37] studied the strength changes of four different sulfur-content cemented paste backfill (CPB) samples within 28~360 days (Figure 9). Figure 9 shows that the strength of CPB specimens has been increasing until 90 d and the increasing rate of CPB is different. The strength of the S5 (5 wt%) was the largest for 90 days, and the sulfur content of S5 was in the middle of the four specimens. After 90 days, the strength of CPB samples began to decline. From 90 to 360 days, the strength of S2, S5, S10 and S15 decreased by 11.8%, 12.3%, 20.7% and 32.7%, respectively. Therefore, there is a critical value in the sulfur content of CPB, and the strength of the CPB can be maximized within a specific curing time. After a period of curing, the strength of CPB continues to decline.
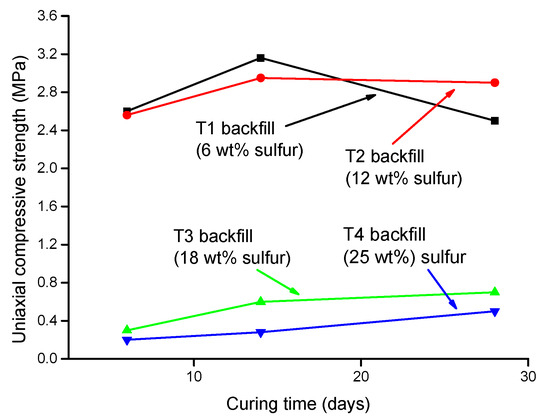
Figure 2.
Variation of UCS fillings with different sulfur content [31].
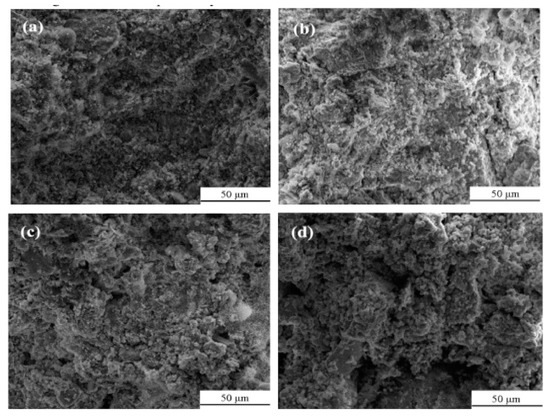
Figure 3.
SEM images of tailing samples with different sulfide content [31].
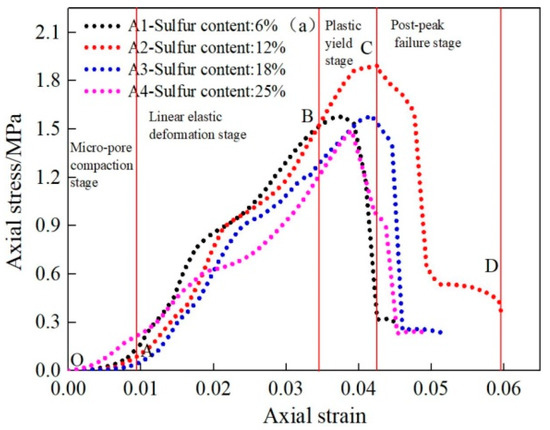
Figure 4.
Stress–strain curve of CSTB under uniaxial loading [32].
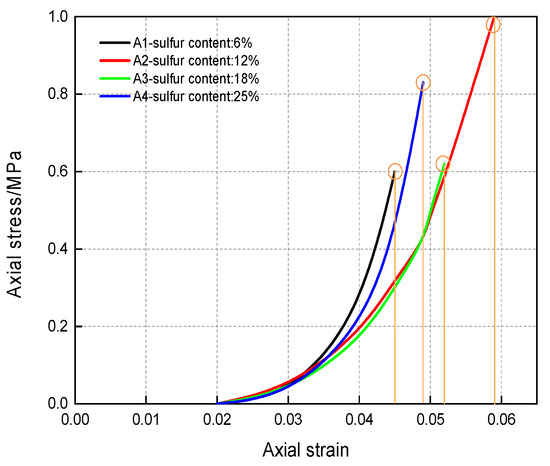
Figure 5.
Damage Value and Axial Strain Curve of CSTB [32].
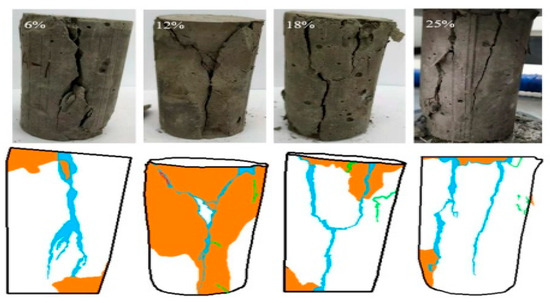
Figure 6.
Failure crack evolution characteristics of CSTB under uniaxial loading [32].
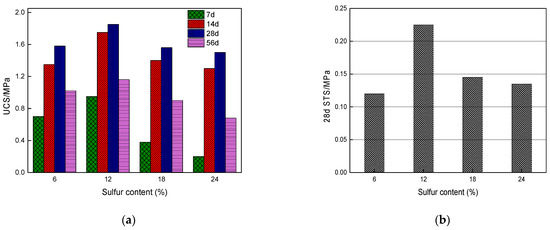
Figure 7.
Relationship between sulfur content and UCS and 28 d STS of CSTB samples: (a) Uniaxial compressive strength; (b) 28 d Splitting tensile strength [34].
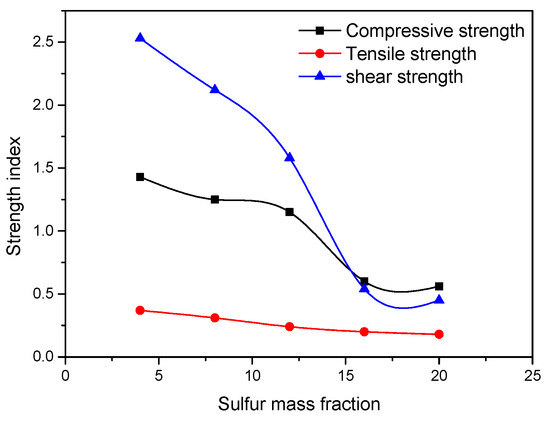
Figure 8.
Strength index curve [35].
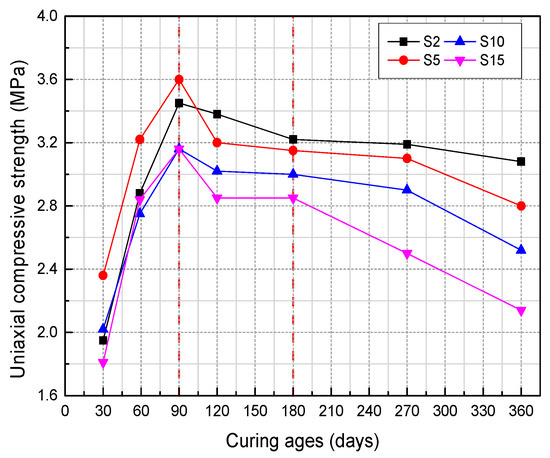
Figure 9.
The UCS of samples with different sulfur content [37].
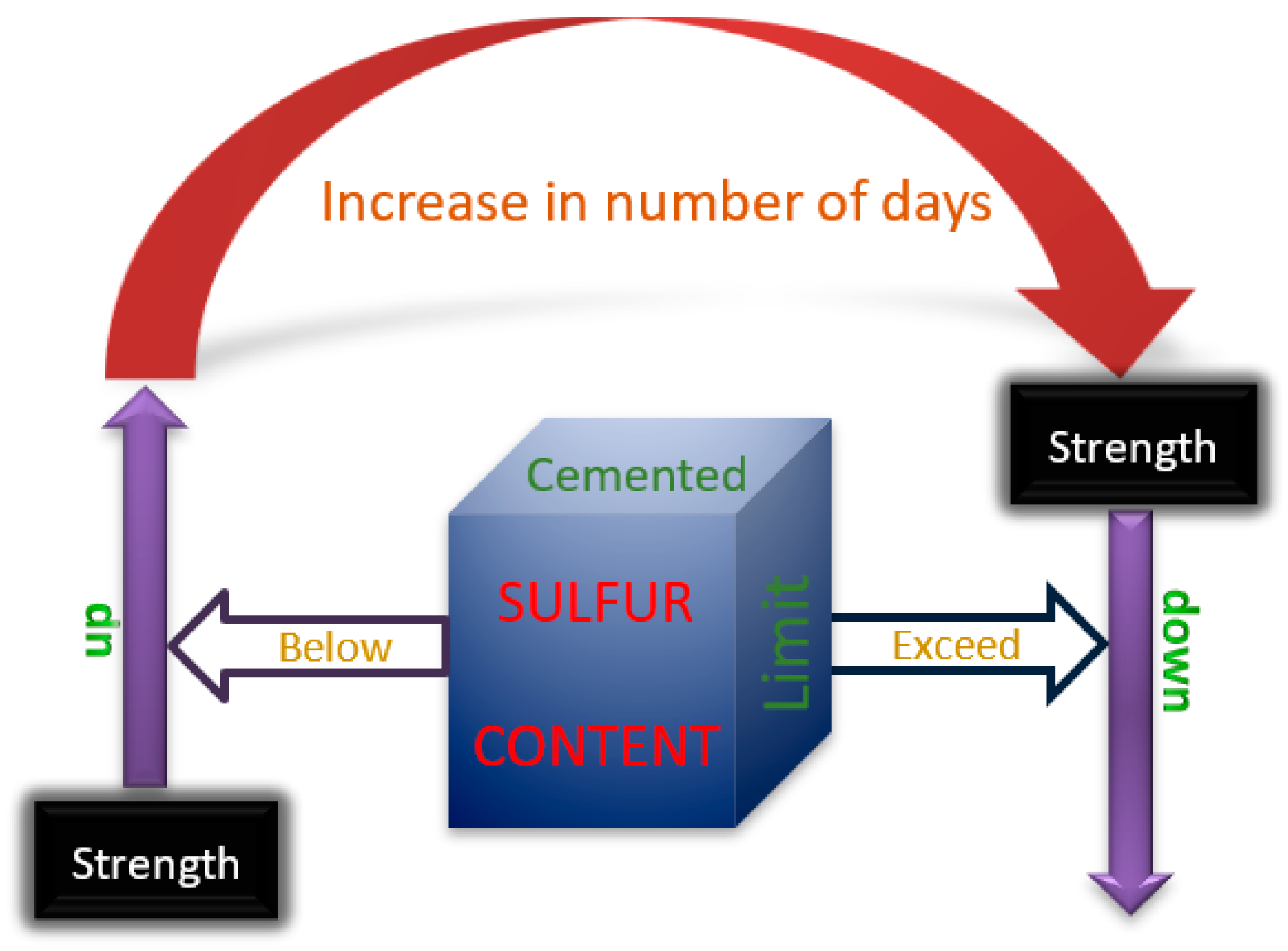
In summary, as shown in Figure 10, the strength will decrease when the sulfur content in the cemented tailings backfill is too high to exceed the critical value. When the sulfur content is less than the critical value, the strength of the cemented tailings backfill will increase at an early age. The critical value of sulfur content in tailings and the aging time of the cemented backfill, which influence the increasing and falling, are currently uncertain and need further study.
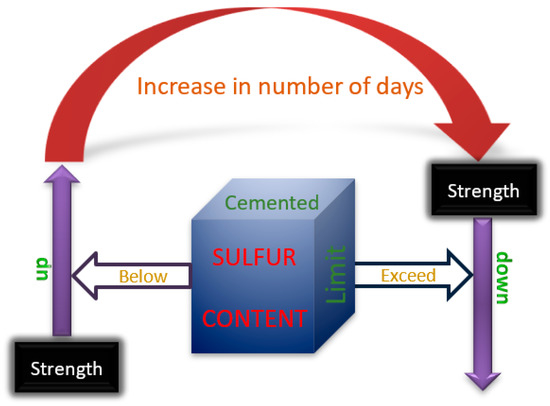
Figure 10.
Relationship between critical value of sulfur content and age on strength of cemented tailings backfill.
From a long-term perspective, sulfur-containing tailings have an adverse effect on the strength of the cemented backfill. Therefore, understanding the failure mechanism of sulfur-containing tailings on the strength of cemented backfill is very important.
3. Failure Mechanism of Sulfur Tailings on Strength of the Cemented Tailings Backfill
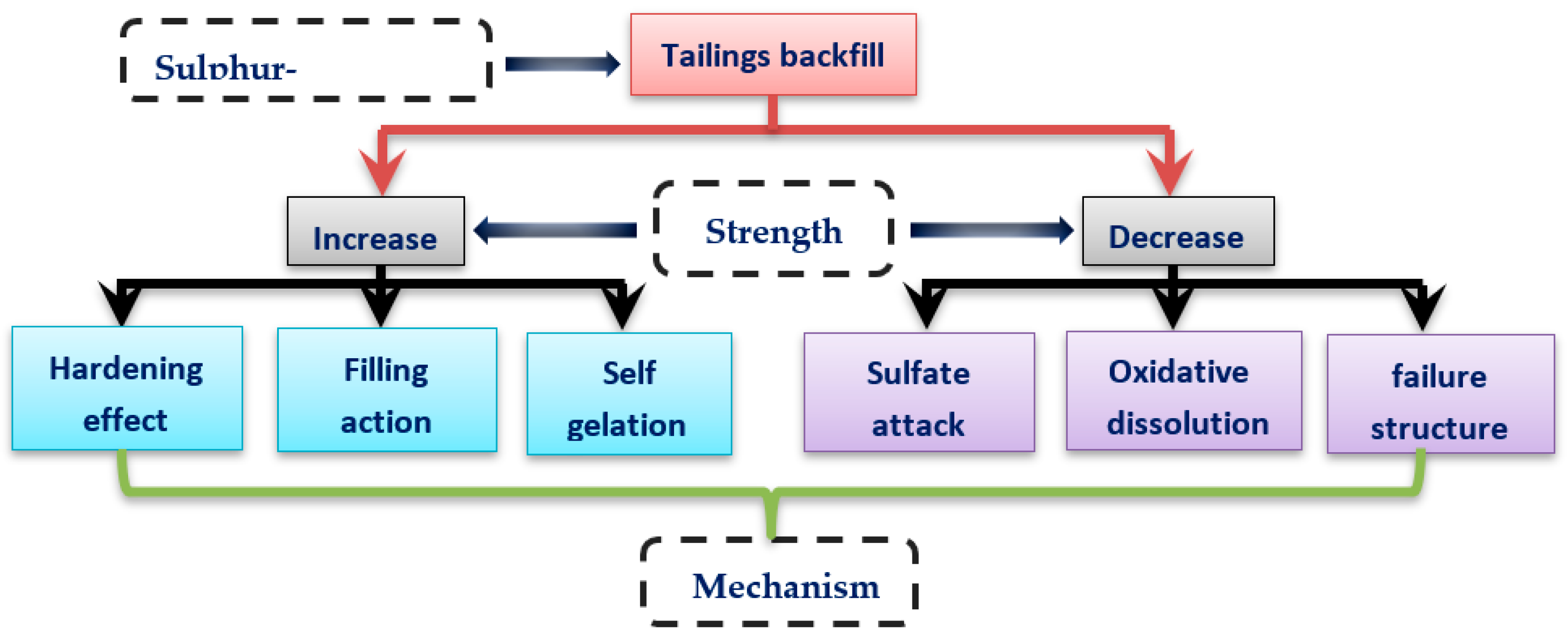
The influence of sulfur content in tailings on the strength of the cemented backfill is mainly reflected in two aspects: increase and decrease (Figure 11). In response to the reduction of backfill strength, it is a prerequisite to study the damage mechanism of sulfur-containing tailings to the strength of cemented backfill for regulation and prevention. When sulfur-containing tailings are used as filling materials, the sulfur content should be controlled. Because of the interaction of sulfur-containing minerals (mainly pyrite-type sulfide minerals) with air and water, sulfide minerals are oxidized to generate sulfate ions, which have a destructive effect on cementitious materials. The sulfur content is usually considered to have an adverse effect on the later cemented tailing backfill strength. Moreover, if the cement sulfur filler contains sulfate and other oxides, the adverse influence on the strength of cement backfill is significant [30].
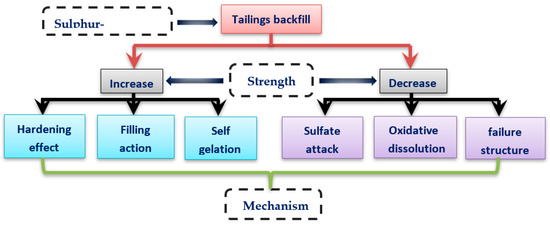
Figure 11.
Mechanism of sulfide tailings on the strength of cemented tailings backfill.
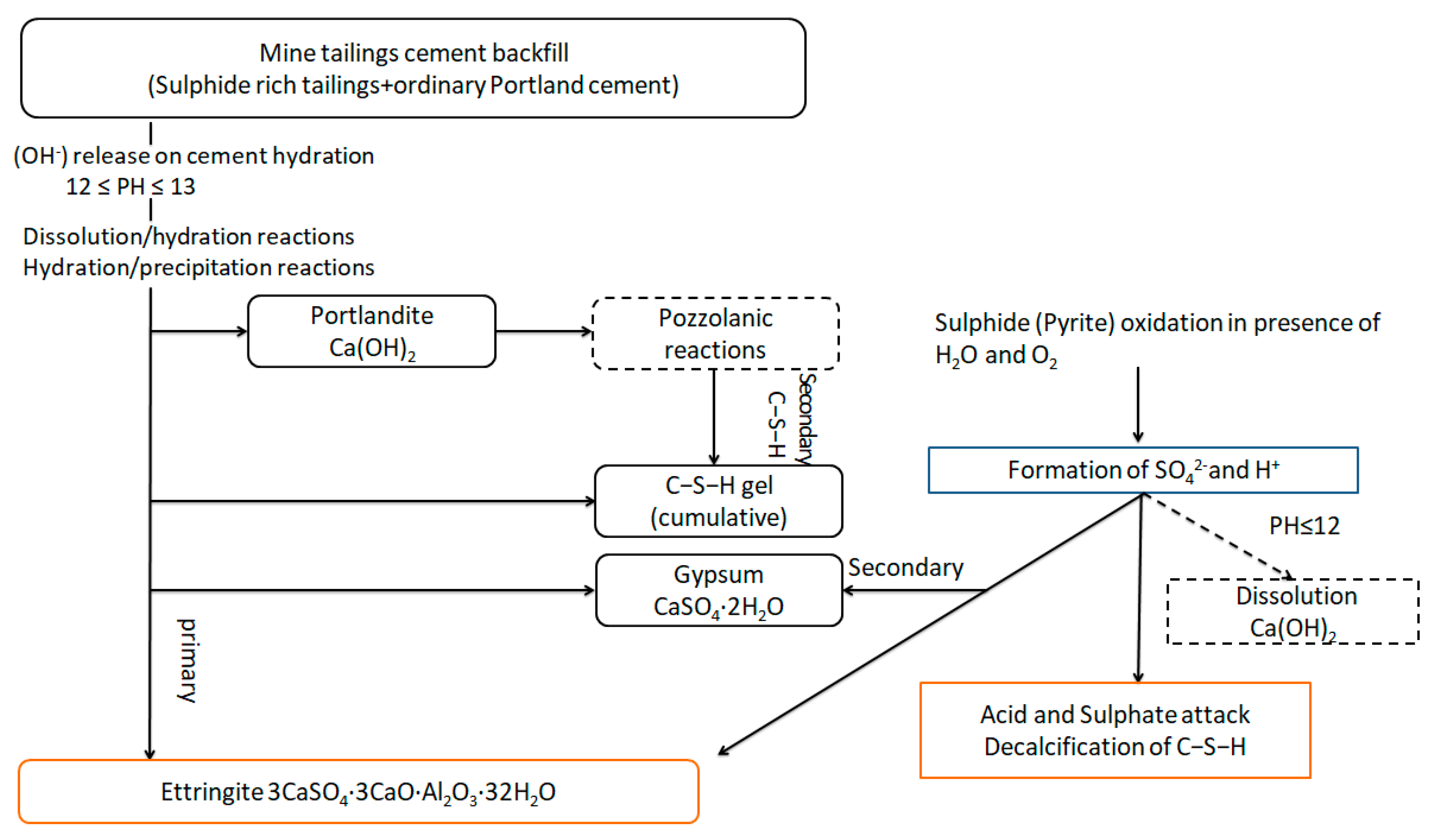
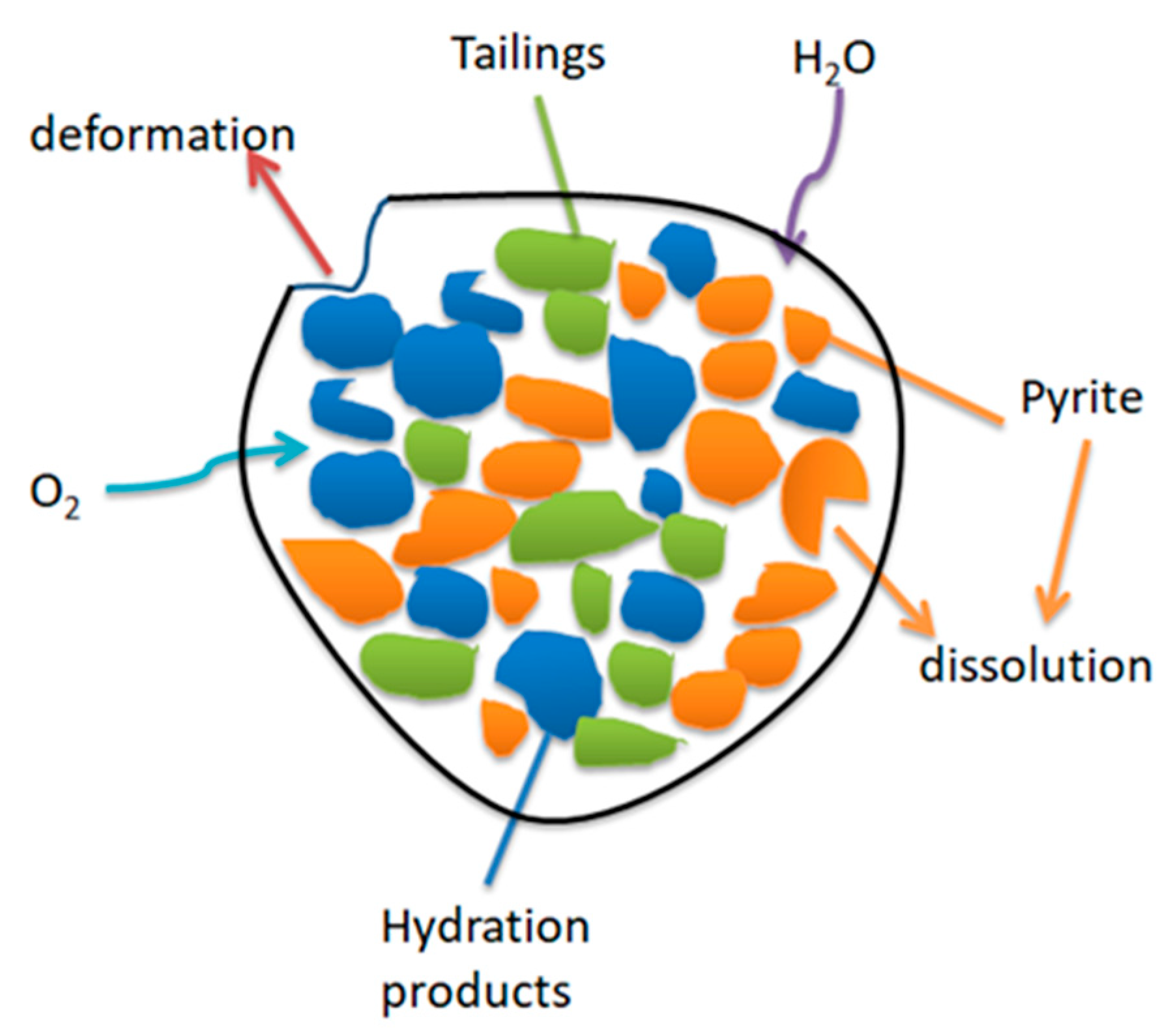
The hydration reaction of silicate cement as the main component of cementitious filler can continue for many years. In response, a large amount of Ca(OH)2, C-S-H, and other products are generated. Finally, a tight, mutually cemented hardening system is formed. The sulfide erosion and sulfate erosion of the cementitious filler by the sulfur-containing tailings destroy this stable system and consume a large number of hydration products, which damage the cementitious filler’s internal structure and deteriorate its strength. Eventually, cracks appear, and the cemented tailings backfill is decomposed (Figure 12) [30].
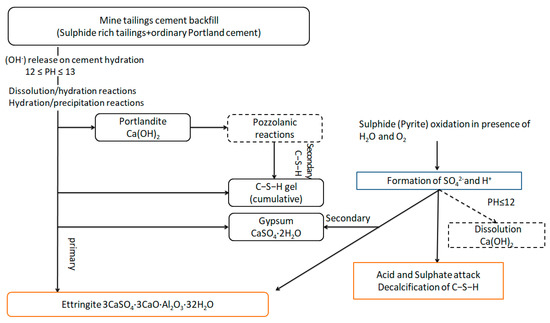
Figure 12.
Schematic diagram of the decomposition process in cemented tailings backfill [30].
3.1. Sulfides Erosion
Once starting, sulfide erosion is highly severe. On the one hand, SO42− generated by the oxidation of sulfides, will combine with other metal ions to produce ettringite, gypsum, magnesium sulfate, calcium silicon carbide, and other salt compounds, resulting in sulfate erosion damage. On the other hand, during the oxidation reaction of sulfides, the large amounts of H+ generated will react with alkaline hydration products to reduce the alkalinity of the cemented tailings that backfill the internal system [38,39]. To ensure calcium equilibrium in the design and maintain the PH, components with cementing ability, such as hydrated calcium silicate, are no longer stable, and decalcification reactions occur. At the same time, H+ will cause the dissolution of the cement structure. The characteristic of this damage is that there are almost no large cracks on the surface of samples and the samples will not swell [40,41].
When the pyrite is entirely dissolved by oxidation, the solid phase morphology of the particles changes, and the internal microstructure is deformed (Figure 13). Taking the tailings with 15% sulfur content as an example, the pyrite content in the tailings accounts for about 1/4 of the total mass. It can be expected that with the extension of time, about 1/4 of the particles inside the specimens will eventually dissolve, and the cemented tailings backfill skeleton will be missing or deformed, thus reducing the pressure-bearing capacity [42].
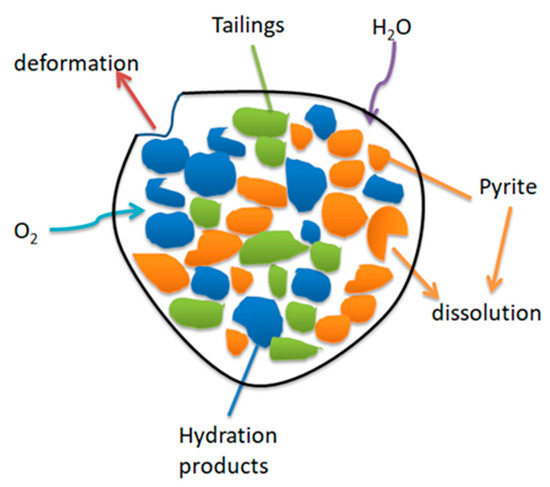
Figure 13.
Failure of cemented sulfur tailings backfill skeleton [37].
3.2. Sulfate Attack
The sulfate source of cemented sulfur tailings backfill erosion is classified as external and internal. External sulfate ions are mainly provided by the external environment, such as river seawater immersion and soil corrosion. Sulfate ions enter the interior from the surface, the erosion from the outside to the inside [43]. The internal sulfate ions are mainly provided by the oxidation of sulfide minerals in tailings.
3.2.1. Ettringite Type Sulfate Erosion
When the cemented sulfur tailings backfill is in an alkaline environment (PH > 12), the main erosion product of sulfate is ettringite. The initial reaction of cement hydration produces a large amount of Ca(OH)2. Sulfate will react chemically with Ca(OH)2 and 3CaO•Al2O3 to make secondary ettringite (see the following Equations (1)–(3)) [44,45]. When Ca(OH)2 is consumed, SO42− will react again with hydrated calcium silicate (C-S-H), causing decalcification of C-S-H to produce Ca(OH)2 [46,47].
3CaO•Al2O3 + 3Ca2+ +3SO42− + 26H2O→3CaO•Al203•3CaSO4•32H2O
3CaO•Al2O3•Ca(OH)2 + xH2O + 2Ca2+ + 3SO42− + (31 − x)H2O→CaO•Al2O3•3CaSO4•32H2O
3CaO•Al2O3•CaSO4 + xH2O + 2Ca2+ + SO42− + (32 − x)H2O→3CaO•Al2O3•3CaSO4•32H2O
3.2.2. Gypsum Type Sulfate Erosion
When the PH value in the cemented sulfur tailings backfill is lower (PH < 10.5) and the sulfate concentration in the capillary pores is more significant than 1000 mg/L, the main erosion product is gypsum, both Ca(OH)2 and C-S-H in the hydration products will react with sulfate and form gypsum. The generation of gypsum consumes a large number of Ca(OH)2, and gypsum crystals volume expanded by 1.24 times, which continues to grow after filling the interstices of the cemented tailings backfill [48,49].
3.2.3. Magnesium Sulfate Erosion
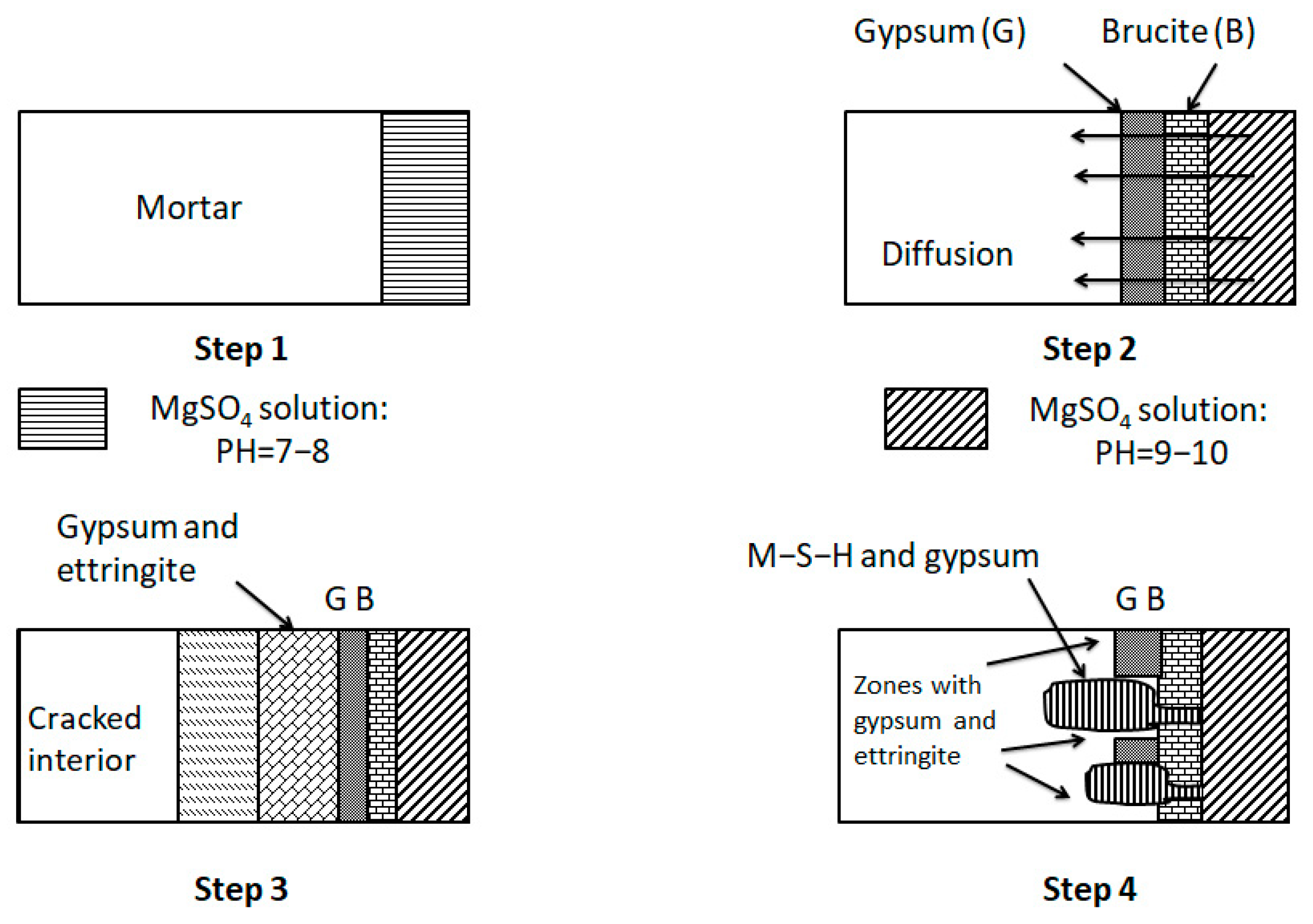
The erosion mechanism of magnesium sulfate is shown in Figure 14. When the solution PH is between 9–10, a layer of Mg(OH)2 and gypsum will be formed on the mortar surface immediately. As the magnesium sulfate solution continues to penetrate and diffuse inside the mortar, magnesium hydroxide (Mg(OH)2) will eventually decompose in several places, thus establishing flow channels. C-S-H starts to be attacked and transformed into M-S-H. The mortar’s final failure is due to the deprivation of strength and integrity caused by the conversion of C-S-H to M-S-H [50,51].
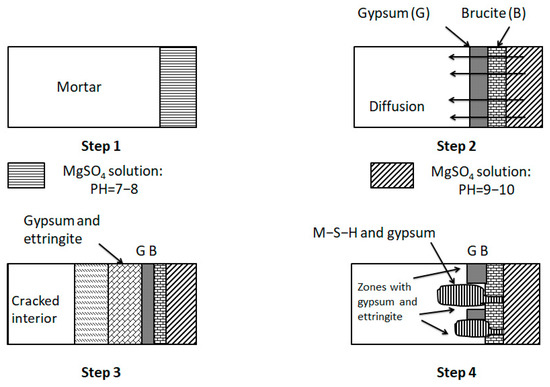
Figure 14.
Mechanism of magnesium sulfate attack [50].
3.2.4. TSA- Type Sulfate Erosion
TSA-type (thaumasite form of sulfate attack) sulfate erodes the cemented tailings backfill even more severely than the above-mentioned three kinds of erosion mechanisms. The appearance of thaumasite is white needle crystal with length of 2~6 μm, which will fill the mortar cracks and cause serious erosion of mortar. [52]. The TSA-type erosion process is divided into four stages [47].
- •SO42− diffuses from the outside to the inside of the filling, while Ca(OH)2 in the pore solution gradually leaches out to the outside.
- Gypsum, as an intermediate product, is transformed into ettringite.
- Ca(OH)2 is consumed, C-S-H decomposes, and gypsum production.
- Generation of calcium silicon carbide.
3.3. The Sulfate Erosion Mechanism
The main types and mechanisms of sulfate attack are shown in Table 1. According to Table 1, a specific classification and the means of sulfate erosion damage are summarized.

Table 1.
Main types of sulfate attack.
3.3.1. Expansion Damage
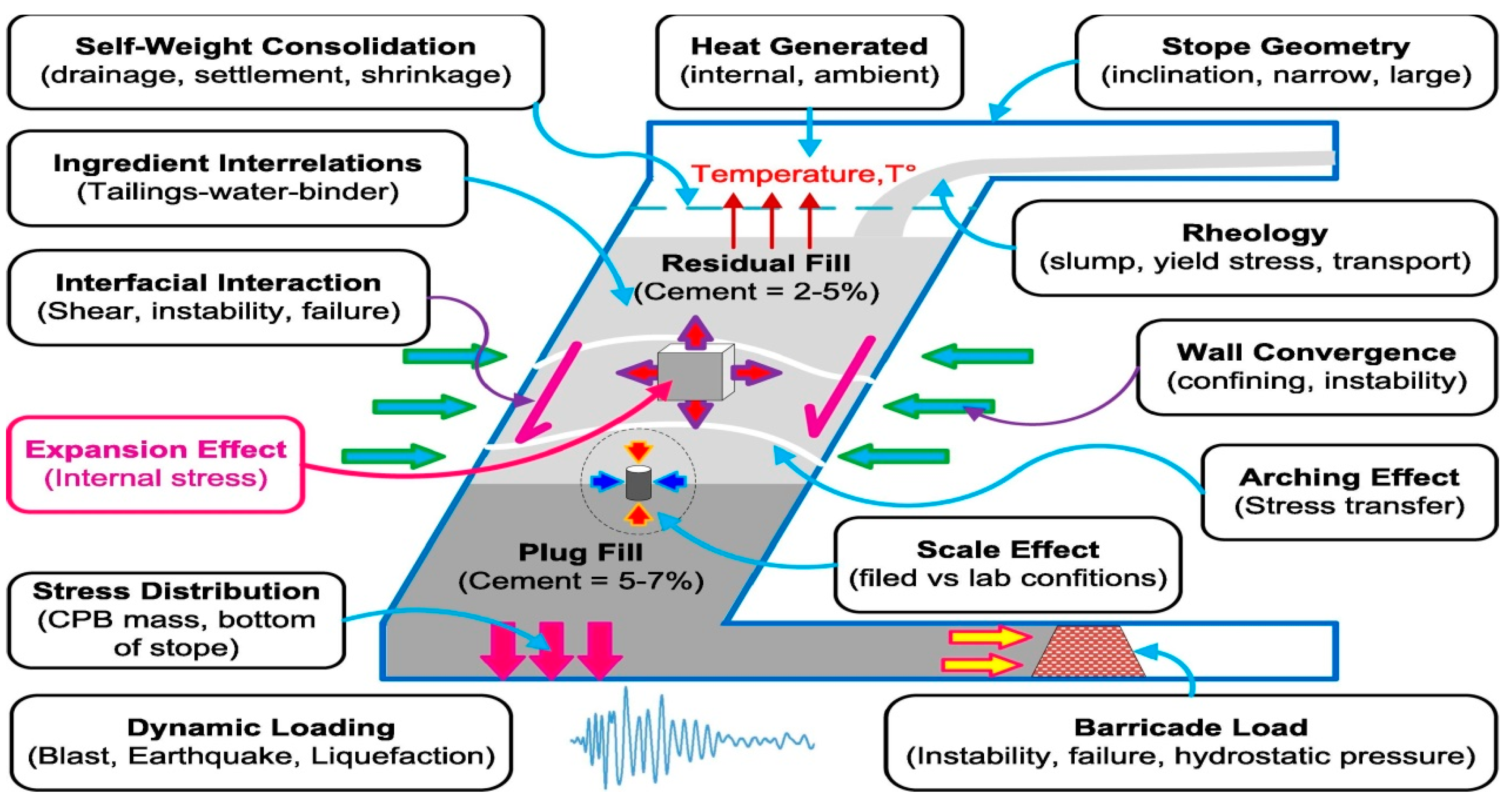
The quality and properties of the cemented fillings are greatly influenced by internal and external factors (Figure 15) [30]. The damage to the cemented tailings backfill is mainly based on internal factors, and the expansion damage is the most obvious one among the internal factors.
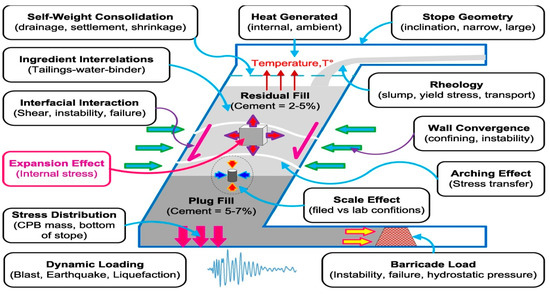
Figure 15.
Internal and external factors affecting the strength of cemented sulfur tailings backfill [30].
Expansion damage is mainly due to sulfate erosion in the cemented tailings backfill to produce products having expansion characteristics, such as ettringite and gypsum, causing crystalline expansion-type erosion damage. These crystalline minerals are more significant in volume than the original solid-phase components, causing swelling, cracking, spalling, and disintegration of the cemented backfill, destroying the cemented backfill.
As a result, the strength of cemented backfill is significantly reduced [46,47].
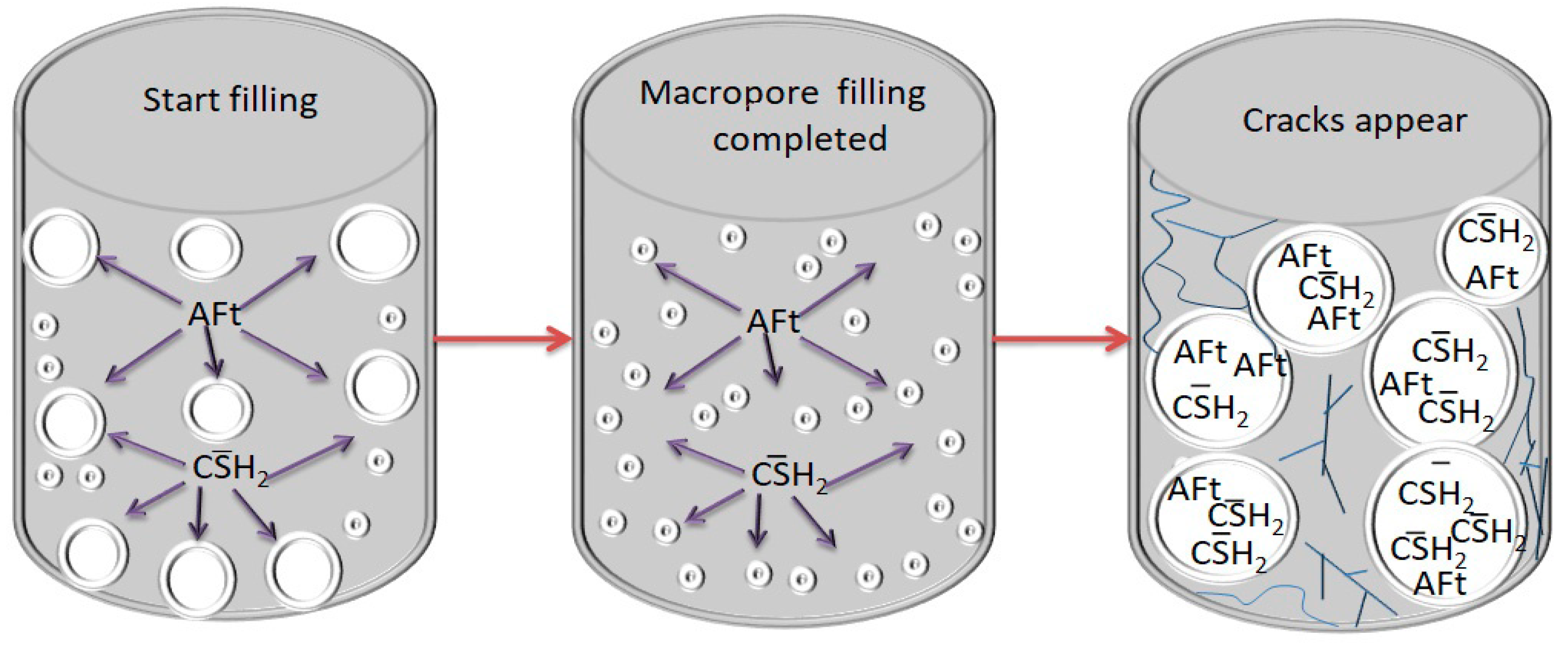
The ettringite and gypsum will fill into the original pores of the cemented backfill at the beginning, reducing the number of large pores and increasing cemented backfill strength. However, when the number of expansive crystals generated is too much, the original pore fissures can no longer fully accommodate a large number of expansive crystals. The swelling action expands the pore spaces and generates new large pores, causing internal structural deterioration and strength reduction of the cemented backfill. Large pore fissures will allow more sulfate and expansive crystals to enter the interior of the cemented backfill for erosion to occur, generating more amounts of expansive products and intensifying erosion. Thus a repeated vicious cycle is formed, and this process leads to more severe damage to the cemented backfill (see Figure 16) [52,53].
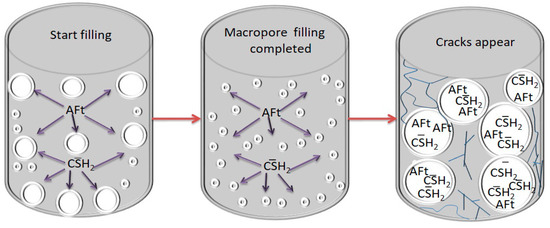
Figure 16.
Expansion failure process.
3.3.2. Bonding Performance Damage
Cement hydration produces C-S-H, which has good bonding properties. However, C-S-H will convert into hydrated magnesium silicate (M-S-H) (See Equations (4)–(6)). M-S-H has a poor bonding property and a lower strength [54]. As M-S-H generation increases and erosion deepens, some parts of the PC mortar sample were deteriorated deeply, and were surrounded by accompanying gypsum deposits [51]. The low solubility of the generated Mg(OH)2 will accelerate the reaction, facilitating the consumption of Ca(OH)2. This causes the pH value to decrease, thus promoting the decomposition of C-S-H and generating more M-S-H, significantly reducing cemented backfill strength and eventually leading to loss of concrete strength [55].
Ca(OH)2 + MgSO4 + 2H2O→CaSO4•2H2O + Mg(OH)2
3CaO•2SiO2•3H2O + MgSO4 + 8H2O→3(CaSO4•2H2O) + 3Mg(OH)2 + 2SiO2•3H2O
2SiO44− + 4Mg2+ + 3H2O→3MgO•2SiO2•2H2O + Mg(OH)2
The generation of calcium silicon carbide in silicate cement consumes large amounts of C-S-H and Ca(OH)2 (see Equation (7)). The cementation of the cement is weakened, causing the strength to decrease. Cement is transformed into a loose material with no cementation and strength [52].
C3S2H3 + CH + 2CC + 2MS + 28H→2C3SCSH15 + 2MH
The Ca(OH)2 and C-S-H can contribute to maintaining and enhancing the cemented backfill strength, which is continuously dissolved and decomposed. The reduction of cementation products leads to the loss of bond properties between aggregates and reduces the strength of the cemented backfill [53,56].
3.3.3. Stress Failure
On the one hand, crystallization pressure is produced when sulfate is supersaturated in pores of cemented tailings backfill through capillary action [56]. On the other hand, the change of crystalline water contained in sodium salt (Na2SO4•10H2O) and magnesium salt (MgSO4•H2O, MgSO4•6H2O) causes volume expansion. Especially in the conversion process of anhydrous mannite into mannite, the volume expands by about 315%, which makes the tensile stress of the cemented backfill increase. If the tensile stress due to expansion exceeds the ultimate tensile stress of cemented backfill, cracks will begin to appear inside the cemented tailings backfill. The tensile and compressive stress can work together to make the structure crack, and strength significantly reduces in the cemented tailings backfill [42,53].
In summary, sulfate erosion is a complex and gradually varying process resulting from the comprehensive impact of internal and external factors.
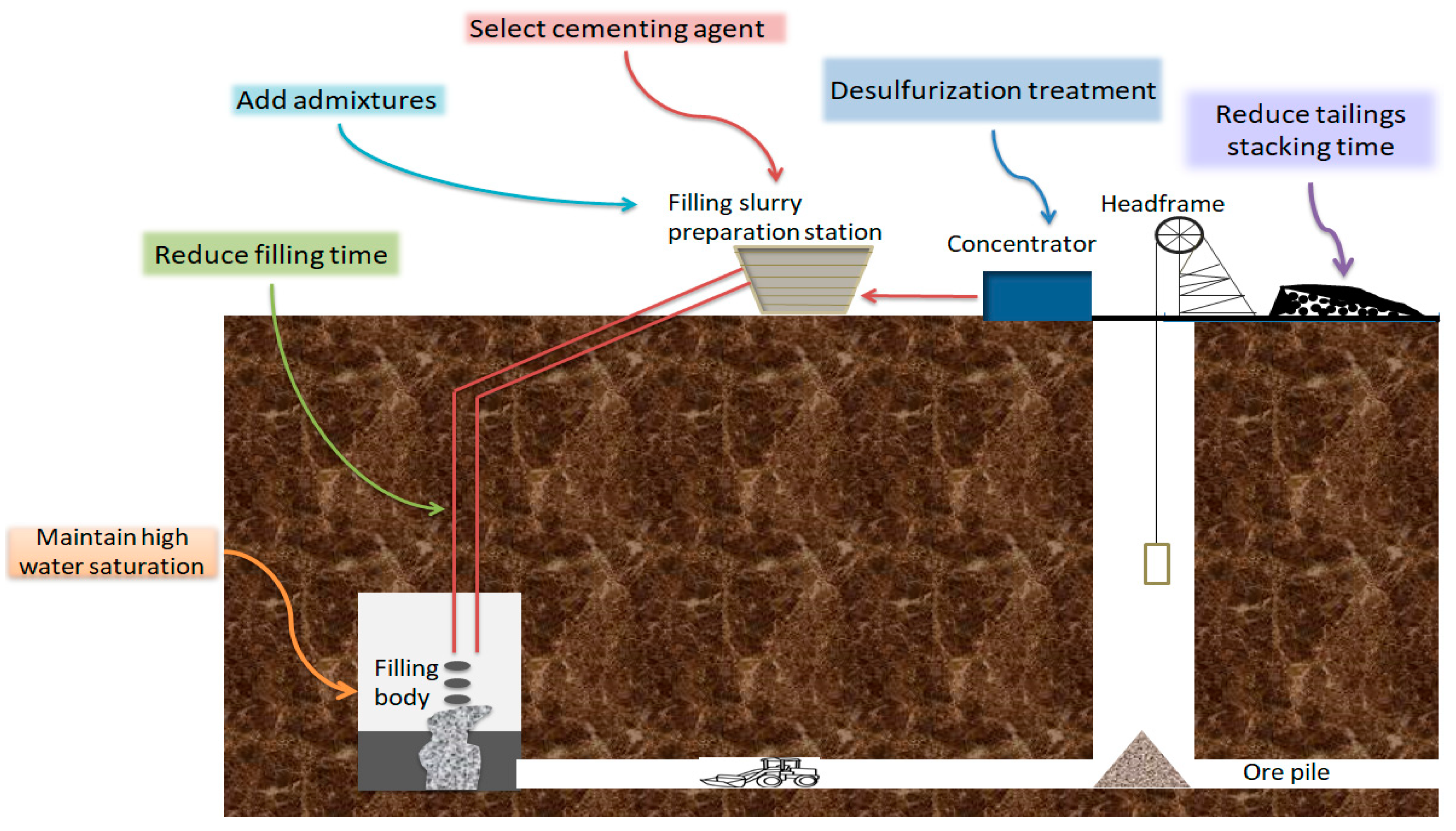
4. Control of Cemented Sulfur Tailings Backfill
To effectively reduce the harm of sulfur tailings, improve the cemented tailings filling technology. The author starts by adjusting the combination of filling materials, optimizing the filling ratio, and controlling the filling process (Figure 17).
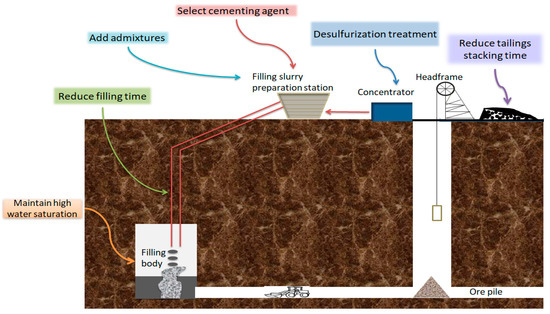
Figure 17.
Regulation of filling process using sulfur tailings.
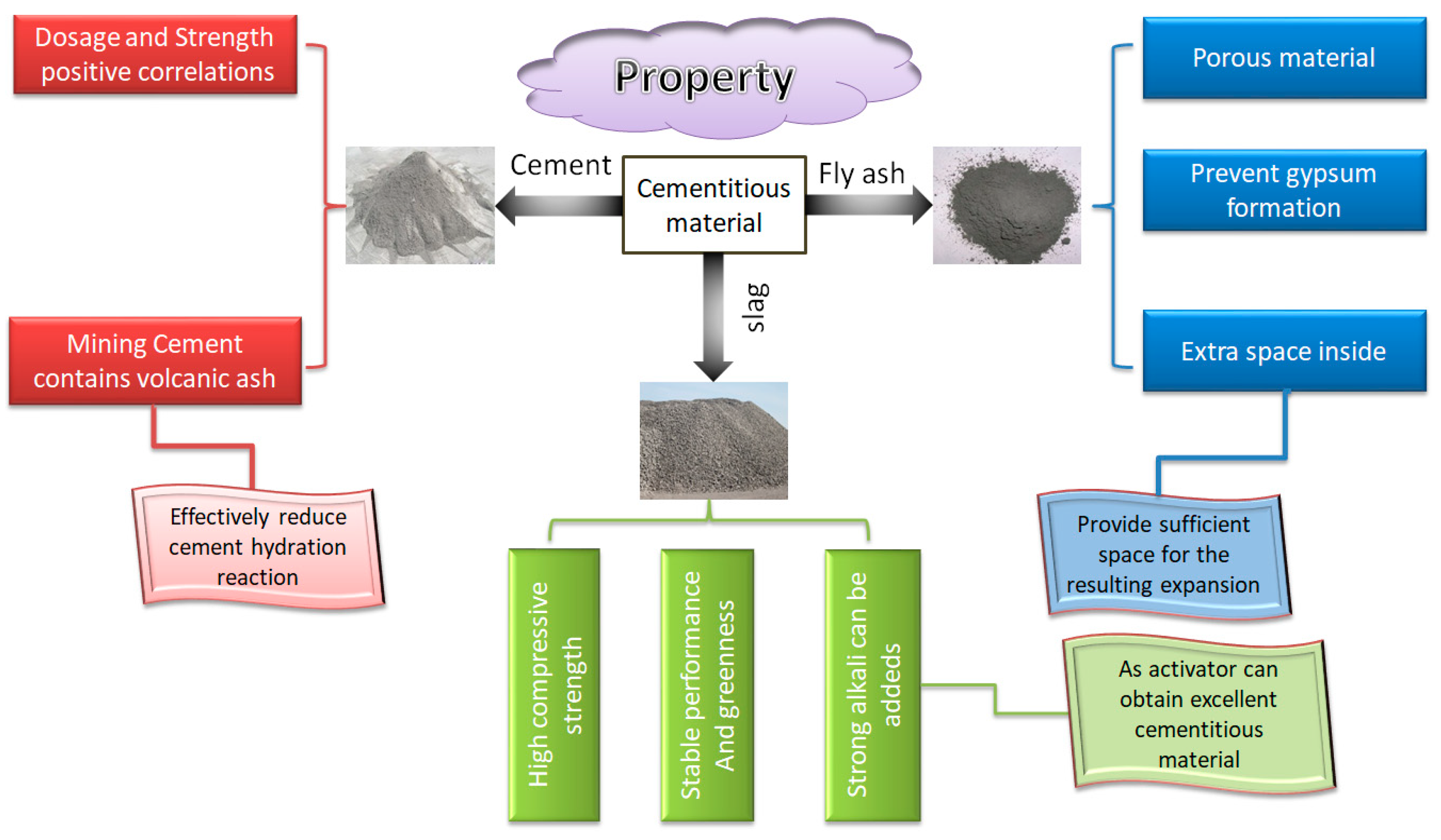
4.1. Blending Cementitious Materials
Cementing material is an essential component of cemented sulfur tailings backfill. Appropriate cementing materials can effectively reduce the adverse effects of sulfur-containing tailings on the cemented tailings backfill. Cement, fly ash and slag are the most commonly used cementitious materials in the industry. Their properties and advantages are shown in Figure 18.
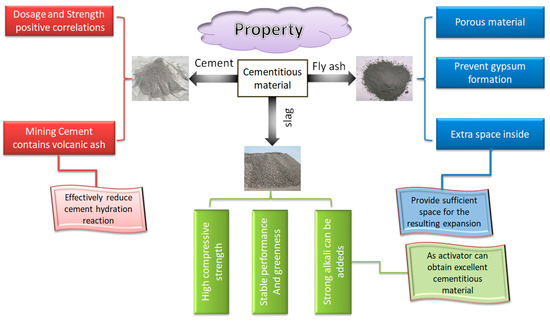
Figure 18.
Properties of three kinds of cementitious material.
4.1.1. Selection of Cement
Recent studies have shown that the cement type has a significant impact on the strength of cemented backfill and filling quality. Li et al. (2004) [57] compared the strength of cemented paste filling (CPF) using mining cement (cement with volcanic ash as the main component) and general-purpose cement as cementitious materials. They found that the former’s strength is higher. The strength of the cemented backfill prepared by mining cement is 40% higher than that of general-purpose cement. The reason for this lies in the presence of volcanic ash in the mining cement, which reduces the hazards posed by the chemical reaction between sulfate and cement in the cemented sulfur tailings backfill [58].
According to the different sulfur content of tailings, the most favorable cement type is selected to increase the strength of the filling material and save the filling cost.
4.1.2. Application of Fly Ash
Fly ash derives from burned carbon particles, which contain various oxides such as SiO2, Al2O3 and CaO. The SEM images (Figure 19) are shown that fly ash is composed of spherical particles of different sizes.

Figure 19.
SEM image observed for fly ash cenosphere [59].
Fly ash has suitable activation property and gelation properties. It can not only improve the fluidity of filling slurry and improve the later-stage strength of cemented backfill but also can reduce the cost of cementitious materials [60]. Its function principle mainly has the following two points:
- SiO2 and Al2O3 in the fly ash react with Ca(OH)2 formed by hydration of 3CaO•SiO2 and 2CaO•SiO2 in cement to form stable mCaO•SiO2•nH2O, xCaO•Al2O3•yH2O and CaO •Al2O3•2SiO2•4H2O, which prevents the reaction of sulfate ions with Ca(OH)2 and reduces the damage of sulfate on the cemented sulfur tailings backfill [39,59].
- Fly ash is a porous material, which can provide additional space for the expansive cemented sulfur tailings backfill, ensure that the cemented sulfur tailings backfill does not collapse due to expansion in the late stage, and maintain the integrity and strength of the cemented tailings backfill. Experimental results show that fly ash can effectively avoid the decrease of cemented backfill strength with high-sulfur tailings [60].
In summary, fly ash can be used as a cementitious material instead of part of cement and can be mixed with cement to maximize its effect.
4.1.3. Properties of Slag Cementitious Materials
Slag comes from industrial waste discharged during ironmaking. Slag cementitious material is a kind of green cementitious material with stable performance. In mine filling, slag cementing material is chosen as the cementing agent to for -m a filling body with high compressive strength. According to recent studies, cementitious materials with excellent properties can be prepared by adding a suitable amount of strong alkali into slag as activator material [61,62].
4.2. Control of Oxidation Conditions
Control measures for common oxidation conditions are shown in Table 2.

Table 2.
Sulfide oxidation mitigation measures.
The oxidation of sulfide aggravates the erosion of sulfate. Slowing down the sulfide oxidation is a crucial measure to solve the strength loss of cemented sulfur tailings backfill.
- Desulfurization treatment. According to the Ercikdi et al. (2013) experiments [63], the strength of the colluvial filler treated with desulfurization of sulfur-rich tailings was higher than that of the undesulfurized sulfur-containing tailings. Therefore, high-sulfur tailings can also be desulfurized to reduce the sulfur content of sulfur-containing tailings to improve the cemented tailing’s backfill strength. However, the cost of the desulfurization process is high [64].
- Slow down sulfide oxidation. The oxidation of sulfide aggravates the erosion of sulfate. Slowing down the oxidation of sulfide is a crucial measure to solve the problem of strength decline of tailings filled with sulfur. Reducing the stacking time of tailings in the air before making filling paste can reduce the amount of sulfate produced by sulfide oxidation and fundamentally lessen the effect of sulfate on the long-term strength of cemented tailings backfill [65,66]. The filling with sulfur-containing tailings should be completed in a relatively short time, and the inlet duct should be closed as much as possible after the completion of filling to reduce the oxidation of the cemented tailings backfill. In addition, maintaining the higher water saturation of the cemented tailings backfill limits the entry of oxygen, which is an important measure to inhibit the production of sulfate in the cemented tailings backfill and prevent sulfate erosion [40].
4.3. Adding Admixture
Adding an appropriate amount of admixture to the cemented sulfur tailings backfill can slow down the speed of strength failure and extend the service life of the cemented tailings backfill. Common admixtures and effects are shown in Table 3.

Table 3.
Admixture Types and Effects.
The following is a detailed analysis of the action principle of the additives listed in Table 2.
- Adding silicon powder. Silicon powder is an excellent concrete admixture. Du et al. (2004) [67] conducted sulfate attack experiments and found that the sulfate resistance of mortar is proportional to the silicon fumes content. By observing the difference between silica powder mortar and ordinary mortar at the microscopic level, it is found that the microscopic pore structure of cement stone adding silica powder as the additional material was significantly denser than that of ordinary net mortar. Silica powder can seal the pores of cement structure, reduce the number of micropores and make the overall system more compact, thus slowing down the reaction of sulfate and cement hydration. The sulfate erosion is diminished, thus improving the strength and stability of the cemented backfill.
- Adding sodium silicate. The hydrolysis of sodium silicate in an aqueous solution generates silica sol and NaOH (see Equation (8)), both of which accelerate the generation of C-S-H, thus shortening the coagulation time of the filler and improve the early strength of the cemented tailings backfill [68].
Na2O•SiO2 + 2H2O = SiO2·H2O + 2OH− + 2Na+
- Adding citric acid. Although citric acid increases the coagulation time of the cemented backfill, it is helpful to improve the late strength of cemented backfill. The reason is that the secondary expansion phase can fill the void between C-S-H and make the interior of cemented tailings backfill more compact. According to the conclusion obtained from experiments, adding 0.3% citric acid can achieve a less retarding effect on the high-sulfur tailings backfill [36]. At the same time, the strength of cemented tailings backfills aged 28d increased by 27.3%. Thus, the filling requirements can be met, and it is the best measure to optimize the performance of cemented sulfur tailings backfill.
- Adding polypropylene fiber. Adding polypropylene fiber into sulfur tailings cemented backfill can significantly improve its mechanical properties. The interface between polypropylene fiber and cemented backfill will produce a bonding effect, which can resist the strength degradation of the backfill and improve its durability. Moreover, polypropylene fiber forms a unique spatial skeleton structure, which can play the role of physical reinforcement and improve the overall compressive strength of cemented backfill [32,33].
- Adding high-efficiency water-reducing agent. High-efficiency water-reducing agent is an organic polymer molecule that adheres to cement particles and tailings and disperses them by internal electrostatic and spatial forces, thus changing the rheological properties of cemented tailings backfill. Moreover, by reducing the water content, the expected strength of the sulfur-containing tailings of the cemented filling body can be achieved, and the durability can be improved [69,70].
5. Conclusions and Outlooks
This review makes an in-depth analysis and discussion of the effect of sulfur-containing tailings on the strength of cemented filling. The types and principles of sulfide and sulfate erosion, which are the main factors affecting the strength of cemented sulfur tailings backfill, are reviewed. The main conclusions and outlook can be summarized as follows.
Sulfur-containing tailings can improve the cemented backfill strength in a specific time. On the one hand, because of the self-gluing effect of sulfide in sulfur-containing tailings, sulfide particles and filling particles can bond together, which significantly improves the strength of the cemented sulfur tailings backfill. On the other hand, sulfate as an early strength agent can promote the hydration and hardening of the cement.
A sulfide attack is more severe once it starts. On the one hand, with the progress of the sulfide oxidation, the cementitious components such as C-S-H are decomposed, and the cemented sulfur tailings backfill is softened. On the other hand, sulfide particles such as pyrite dissolve, and the skeleton of the cemented sulfur tailings backfill is missing or deformed, reducing the bearing capacity.
The sulfate erosion in sulfur-containing tailings is the main reason for the strength failure of the cemented tailings backfill. The mechanism of action is mainly the expansion damage produced by the crystallization of sulfate, which increases the internal tensile stress and external compressive stress of the cemented sulfur tailings backfill, making the cracks in cemented tailings backfill increase and the strength significantly reduced. Moreover, the process of sulfate generation leads to the loss of bonding properties between the aggregates and a decrease in the strength of the cemented tailings backfill.
The control of cemented filling of sulfur tailings mainly starts by adjusting the combination of filling materials and optimizing the filling ratio. Through the deployment of cementing materials, the oxidation conditions of sulfur-containing tailings are controlled in the filling process, and a certain amount of additives are added appropriately.
Overall, the discussion in this paper is based on the existing results but lacks experimental demonstration. The strength and mechanical properties of the cemented tailings backfill are significantly affected by the sulfur-containing tailings. The strength of the cemented tailings backfill will be enhanced in the early stage. However, the strength of the cemented sulfur tailings backfill will gradually decrease again in the later stage with the increased aging time. The critical value of sulfur content in tailings and the aging time affecting the strength of cemented sulfur tailings backfill are still uncertain and need further study.
Author Contributions
J.W.; conceptualization, methodology, software, formal analysis, writing—original draft, writing—review and editing, M.X.; visualization, investigation, conceptualization, methodology, data curation, writing—original draft, writing—review and editing, X.Y.; methodology, conceptualization, validation, supervision, writing—review and editing, X.Y.; methodology, conceptualization, validation, supervision, writing—review and editing, H.J.; visualization, investigation, data curation, F.C.; conceptualization, methodology, writing—review and editing, L.Y.; writing—review and editing, J.Y.; writing –review and editing, Y.F.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 51834001).
Data Availability Statement
All data have been presented in this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, W.; Yin, S.; Zhou, G.; Li, Z.; Song, Q. Copper recovery from tailings through bioleaching and further application of bioleached tailings residue: Comprehensive utilization of tailings. J. Clean. Prod. 2022, 332, 130129. [Google Scholar] [CrossRef]
- The Global Industry Standard on Tailings Management (GISTM). Global Tailings Review. 2020. Available online: https://globaltailingsreview.org/global-industry-standard (accessed on 5 August 2020).
- Kongar-Syuryun, C.; Ivannikov, A.; Khayrutdinov, A.; Tyulyaeva, Y. Geotechnology using composite materials from man-made waste is a paradigm of sustainable development. Mater. Today Proc. 2021, 38, 2078–2082. [Google Scholar] [CrossRef]
- Dong, L.; Deng, S.; Wang, F. Some developments and new insights for environmental sustainability and disaster control of tailings dam. J. Clean. Prod. 2020, 269, 122270. [Google Scholar] [CrossRef]
- Ermolovich, E.; Ivannikov, A.; Khayrutdinov, M.; Kongar-Syuryun, C.; Tyulyaeva, Y. Creation of a Nanomodified Backfill Based on the Waste from Enrichment of Water-Soluble Ores. Materials 2022, 15, 3689. [Google Scholar] [CrossRef] [PubMed]
- MHURD (Ministry of Housing and Urban-Rural Development of the PRC). Code for Design of Nonferrous Metal Mining; China Planning Press: Beijing, China, 2012. (In Chinese)
- Hefni, M.; Ahmed, H.; Omar, E.; Ali, M. The Potential Re-Use of Saudi Mine Tailings in Mine Backfill: A Path towards Sustainable Mining in Saudi Arabia. Sustainability 2021, 13, 6204. [Google Scholar] [CrossRef]
- Arvidson, B.; Klemetti, M.; Knuutinen, T.; Kuusisto, M.; Man, Y.; Hughes-Narborough, C. Flotation of pyrrhotite to produce magnetite concentrates with a sulphur level below 0.05% w/w. Miner. Eng. 2013, 50–51, 4–12. [Google Scholar] [CrossRef]
- Bendz, D.; Tiberg, C.; Kleja, D.B. Mineralogical characterization and speciation of sulfur, zinc and lead in pyritecinder from Bergvik, Sweden. Appl. Geochem. 2021, 131, 105010. [Google Scholar] [CrossRef]
- Onuaguluchi, O.; Eren, O. Recycling of copper tailings as an additive in cement mortars. Constr. Build. Mater. 2012, 37, 723–727. [Google Scholar] [CrossRef]
- Craig, A.; Shkarupin, A.; Amos, R.; Lindsay, M.; Blowes, D.; Ptacek, C.J. Reactive transport modelling of porewater geochemistry and sulfur isotope fractionation in organic carbon amended mine tailings. Appl. Geochem. 2021, 127, 104904. [Google Scholar] [CrossRef]
- Du, H.; Zheng, J.; Tian, L.; Liang, H.; Guo, J.; Li, Y. Microfabrics, in-situ trace element and sulfur isotope compositions of pyrite from the Jinjiwo copper deposit in Chengmenshan ore field, northern Yangtze Block: Syngenetic stratabound mineralization and hydrothermal remobilization. Ore Geol. Rev. 2020, 127, 103830. [Google Scholar] [CrossRef]
- Jiao, H.; Zhang, W.; Wang, Y.; Chen, X.; Yang, L.; Rong, Y. Study on Strength Reduction Law and Meso-Crack Evolution of Lower Layered Cemented Tailings Backfill. J. Renewable Mater. 2023, 11(3), 1513–1529. [Google Scholar] [CrossRef]
- Song, M.; Li, J.; Yu, X.; Song, Y.; Ding, Z.; Li, S. Metallogenic characteristics and tectonic setting of the Jiaodong gold deposit, China. Solid Earth Sci. 2021, 6, 385–405. [Google Scholar] [CrossRef]
- McEvoy, J.; Thibault, Y.; Beauchemin, S. Iron and Sulphur management options during Ni recovery from (bio) leaching of pyrrhotite tailings Part 2: Strategies for Sulphur fixation during biomass-induced magnetization of goethite and jarosite. Miner. Eng. 2020, 150, 106266. [Google Scholar] [CrossRef]
- Mashifana, T.; Sithole, T. Clean production of sustainable backfill material from waste gold tailings and slag. J. Clean. Prod. 2021, 308, 127357. [Google Scholar] [CrossRef]
- Hawkins, T.; Smith, M.; Herrington, R.; Maslennikov, V.; Boyce, A.; Jeffries, T.; Creaser, R.A. The geology and genesis of the iron skarns of the Turgai belt, northwestern Kazakhstan. Ore Geol. Rev. 2017, 85, 216–246. [Google Scholar] [CrossRef]
- Wu, Z.; Zou, L.; Chen, J.; Lai, X.; Zhu, Y. Column bioleaching characteristic of copper and iron from Zijinshan sulfide ores by acid mine drainage. Int. J. Miner. Process. 2016, 149, 18–24. [Google Scholar] [CrossRef]
- Li, J.; Vasconcelos, P.; Zhou, M.; Deng, X.; Cohen, B.; Bi, S.; Zhao, X.; Selby, D. Longevity of magmatic–hydrothermal systems in the Daye Cu–Fe–Au District, eastern China with implications for mineral exploration. Ore Geol. Rev. 2014, 57, 375–392. [Google Scholar] [CrossRef]
- Sabeva, R.; Mladenova, V.; Mogessie, A.; Ore, P. Hydrothermal alteration, fluid inclusions, and sulfur stable isotopes of the Milin Kamak intermediate sulfidation epithermal Au-Ag deposit in Western Srednogorie, Bulgaria. Ore Geol. Rev. 2017, 88, 400–415. [Google Scholar] [CrossRef]
- Toubri, Y.; Plante, B.; Demers, I.; Fillion, M. Probing cleaner production opportunities of the Lac Tio pyrite-enriched tailings generated to alleviate sulfur dioxide emissions. J. Clean. Prod. 2022, 357, 132027. [Google Scholar] [CrossRef]
- Vincent, V.I.; Li, H.; Girei, M.B.; Förster, M.W.; Ahmed, H.A.; Ntekim, E.E. In situ trace elements and sulfur isotope analysis of sulfides from the Akiri Cu ± (Ag) deposit, Benue Trough, North-central Nigeria: Implications for ore genesis. Geochemistry 2021, 81, 125801. [Google Scholar] [CrossRef]
- Jiang, Y.; Qiu, H.; Xu, Y. Hydrothermal fluids, argon isotopes and mineralization ages of the Fankou Pb–Zn deposit in south China: Insights from sphalerite 40Ar/39Ar progressive crushing. Geochim. Cosmochim. Acta 2012, 84, 369–379. [Google Scholar] [CrossRef]
- Wen, C.; Shao, Y.; Li, B.; Dick, J.; Lai, J.; Huang, G.; Luo, X. Fluid evolution of the Wushan skarn-dominant copper deposit in the Middle-Lower Yangtze River metallogenic belt, Eastern China. Ore Geol. Rev. 2019, 12, 103035. [Google Scholar] [CrossRef]
- Yang, F.; Wu, C.; Cui, Y.; Lu, G. Apparent activation energy for spontaneous combustion of sulfide concentrates in storage yard. Trans. Nonferrous Met. Soc. China Metal Mine. 2011, 21, 395–401. [Google Scholar] [CrossRef]
- Xu, L.; Xie, Q.; Zhou, Y.; Wang, J.; Chen, T.; Xu, X.; Xie, J. Recognizing the evolution of the stratabound polymetallic massive sulfide deposits in Tongling mineralization cluster, east China through colloform pyrite. Ore Geol. Rev. 2022, 146, 104915. [Google Scholar] [CrossRef]
- Zheng, Y.; Long, L.; Yu, P.; Zhang, X.; Hu, Z.; Wu, Y. Metal endowment and geodynamic evolution of the Late Paleozoic SEDEX deposits in South China: The Yunfu giant iron-sulfide deposit, Yunkai Domain. Ore Geol. Rev. 2022, 145, 104918. [Google Scholar] [CrossRef]
- Li, B.; Wang, X.; Tang, G.; Liu, Y.; Zou, G. S-Pb isotopes and tectono-geochemistry of the Lunong ore block, Yangla large Cu deposit, SW China: Implications for mineral exploration. Ore Geol. Rev. 2021, 136, 104249. [Google Scholar] [CrossRef]
- Liao, X.; Chen, Y.; Chen, J. Application of macromolecular organic polymer S-7261A in arsenic removal by flotation of refractory mixed copper ore. Miner. Eng. 2022, 182, 107560. [Google Scholar] [CrossRef]
- Yin, S.; Shao, Y.; Wu, A.; Wang, Y.; Chen, X. Expansion and strength properties of cemented backfill using sulphidic mill tailings. Constr. Build. Mater. 2018, 165, 138–148. [Google Scholar] [CrossRef]
- Li, H.; Wu, A.; Wang, H. Evaluation of short-term strength development of cemented backfill with varying sulphide contents and the use of additives. J. Environ. Manag. 2019, 239, 279–286. [Google Scholar] [CrossRef]
- Yin, S.; Hou, X.; Chen, X.; Zhang, M.; Du, H.; Gao, C. Mechanical behavior, failure pattern and damage evolution of fiber-reinforced cemented sulfur tailings backfill under uniaxial loading. Constr. Build. Mater. 2022, 332, 127248. [Google Scholar] [CrossRef]
- Fang, K.; Fall, M. Shear Behaviour of Rock–Tailings Backfill Interface: Effect of Cementation, RockType, and Rock Surface Roughness. Geotech. Geol. Eng. 2021, 39, 1753–1770. [Google Scholar] [CrossRef]
- Yin, S.; Hou, Y.; Chen, X.; Zhang, M. Mechanical, flowing and microstructural properties of cemented sulfur tailings backfill: Effects of fiber lengths and dosage. Constr. Build. Mater. 2021, 309, 125058. [Google Scholar] [CrossRef]
- Zhan, F.; Fu, Y.; Yang, S. Study on the effect of sulfur on the strength of cemented tailings backfill. Ind. Miner. Process. 2018, 47, 42–45. [Google Scholar] [CrossRef]
- Jiang, G.; Wu, A.; Li, H. Properties of high sulfur tailings backfill and effects of chemical admixture-s on it. Metal Mine. 2017, 171–175. [Google Scholar] [CrossRef]
- Dong, Q.; Liang, B.; Jia, L.; Jiang, L. Effect of sulfide on the long-term strength of lead-zinc tailings cemented paste backfill. Constr. Build. Mater. 2019, 200, 436–446. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.; Tian, M. Effect of Sulfide on Strength of Filling Body. In Proceedings of the 8th International Symposium on Mining with Backfill, Beijing, China, 19–21 September 2004; pp. 176–177. [Google Scholar]
- Liu, Y.; Hou, D. Strength test of sulfur-containing tailings backfill. Mod. Min. 2013, 29, 195–196+199. [Google Scholar]
- Wang, B.; Zhang, H.; Dong, X.; Gao, Q. Effect of sulfide oxidation on long-term strength of backfill. Ind. Miner. Process. 2007, 29–31. [Google Scholar] [CrossRef]
- Liu, P.; Chen, Y.; Wang, W.; Yu, Z. Effect of physical and chemical sulfate attack on performance degradation of concrete under different conditions. Chem. Phys. Lett. 2020, 745, 137254. [Google Scholar] [CrossRef]
- Dong, Q. Study of Evolution and Mechanism of the Strength to Sulfur Content Lead-Zinc Tailings Cemented Paste Backfll. Master’s Thesis, Liaoning Technical University, Fuxin, China, 2017. [Google Scholar]
- Carmona-Quiroga, P.; Blanco-Varela, M. Barium carbonate and supplementary cementitious materials to counteract thaumasite sulfate attack in mortars: Effect of aggregate composition. Constr. Build. Mater. 2021, 282, 122583. [Google Scholar] [CrossRef]
- Wang, H.; Dowd, P.; Xu, C. A reaction rate model for pyrite oxidation considering the influence of water content and temperature. Miner. Eng. 2019, 134, 345–355. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, J.; Yang, J.; Zou, Y.; Wang, Z. Understanding of the deterioration characteristic of conc-rete exposed to external sulfate attack: Insight into mesoscopic pore structures. Constr. Build. Mater. 2020, 260, 119932. [Google Scholar] [CrossRef]
- Wang, J.; Niu, D.; Ma, R. Investigation of sulfate attack resistance of shotcrete under dry-wet cycles. J. Wuhan Univ. Technol.-Mater. 2016, 31, 1329–1335. [Google Scholar] [CrossRef]
- Yang, J.; Wang, P.; Li, H. Sulfate attack resistance of air-entrained silica fume concrete under dry-wet cycle condition. J. Wuhan Univ. Technol.-Mater. 2016, 31, 857–864. [Google Scholar] [CrossRef]
- Nevile, A. The confused world of sulfate attack on concrete. CBI Rapp. 2004, 34, 1275–1296. [Google Scholar] [CrossRef]
- Shen, C.; Huang, L.; Chen, M.; Lü, Q. Experimental study on Influencing Factors of sulfate corrosion of concrete. J. Water Resour. Archit. Eng. 2010, 8, 38–42+100. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, D.M.; Olek, J. Mechanism of sulfate attack: A fresh look: Part 2. Proposed mechanisms. CBI Rapp. 2003, 33, 341–346. [Google Scholar] [CrossRef]
- Skaropoulou, A.; Tsivilis, S.; Kakali, G.; Sharp, J.H.; Swamy, R.N. Long term behavior of Portland limestone cement mortars exposed to magnesium sulfate attack. Cem. Concr. Compos. 2009, 31, 628–636. [Google Scholar] [CrossRef]
- Torres, S.M.; Sharp, J.H.; Swamy, R.N.; Lynsdale, C.J.; Huntley, S.A. Long term durability of Portland-limestone cement mortars exposed to magnesium sulfate attack. Cem. Concr. Compos. 2003, 25, 947–954. [Google Scholar] [CrossRef]
- Zhutovsky, S.; Nayman, S. Modeling of crack-healing by hydration products of residual cement in concrete. Constr. Build. Mater. 2022, 340, 127682. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, M.; Olek, J. Sulfate attack research—Whither now? Cem. Concr. Res. 2001, 31, 845–851. [Google Scholar] [CrossRef]
- Bernard, E.; Dauzères, A.; Lothenbach, B. Magnesium and calcium silicate hydrates, Part II: Mg-exchange at the interface “low-pH” cement and magnesium environment studied in a C-S-H and M-S-H model system. Appl. Geochem. 2018, 89, 210–218. [Google Scholar] [CrossRef]
- Patsikas, N.; Katsiotis, N.; Pipilikaki, P.; Papageorgiou, D.; Chaniotakis, E.; Beazi-Katsioti, M. Durability of mortars of white cement against sulfate attack in elevated temperatures. Constr. Build. Mater. 2012, 36, 1082–1089. [Google Scholar] [CrossRef]
- Li, J.; Villaescusa, E.; Tyler, D.; McGrath, T. Factors Affecting Filling Quality of Underground Mine. In Proceedings of the 8th International Symposium on Mining with Backfill, Beijing, China, 19–21 September 2004; pp. 195–199. [Google Scholar]
- Sari, M.; Yilmaz, E.; Kasap, T.; Guner, N.U. Strength and microstructure evolution in cemented mine backfill with low and high pH pyritic tailings: Effect of mineral admixtures. Constr. Build. Mater. 2022, 328, 127109. [Google Scholar] [CrossRef]
- Nong, Z.; Park, S.S.; Lee, S.B.; Jiang, P.M. Cyclic resistance of fly ash influenced by anisotropic stress condition, sand contents, and gravel content. Soils Found. 2022, 62, 101157. [Google Scholar] [CrossRef]
- Zhang, Q.; Kang, Q.; Xiao, F.; Pan, C.; Chen, X. Key Technology of Cemented Backfilling with High Viscosity Sulfur-content Tailings. Met. Mine 2010, 39–42+67. [Google Scholar]
- Miraki, H.; Shariatmadari, N.; Ghadir, P.; Jahandari, S.; Tao, Z.; Siddique, R. Clayey soil stabilization using alkali-activated volcanic ash and slag. J. Rock. Mech. Geotech. 2021, 14, 576–591. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, Z.; Liu, X.; Sheng, M.; Li, H.; Xu, X.; Ai, L.; Yan, Q.; Yang, Y. Preparation of composite micro-slag based on the application of tailings slag in cement and concrete. Constr. Build. Mater. 2022, 322, 126515. [Google Scholar] [CrossRef]
- Ercikdi, B.; Baki, H.; Muhammet, İ. Effect of desliming of sulphide-rich mill tailings on the long-term strength of cemented paste backfill. J. Environ. Manag. 2013, 115, 5–13. [Google Scholar] [CrossRef]
- Skandrani, A.; Demers, I.; Kongolo, M. Desulfurization of aged gold-bearing mine tailings. Miner. Eng. 2019, 138, 195–203. [Google Scholar] [CrossRef]
- Jiao, H.; Wu, Y.; Wang, H.; Chen, X.; Li, Z.; Wang, Y.; Zhang, B.; Liu, J. Micro-scale mechanism of sealed water seepage and thickening from tailings bed in rake shearing thickener. Miner. Eng. 2021, 173, 107043. [Google Scholar] [CrossRef]
- Zhang, Z.; Jin, X.; Luo, W. Long-term behaviors of concrete under low-concentration sulfate attack subjected to natural variation of environmental climate conditions. Cem. Concr. Res. 2019, 116, 217–230. [Google Scholar] [CrossRef]
- Du, Y.; Niu, J.; Li, Y.; Ma, S. Effect of silica fume admixture on sulfate resistance of mortar. J. Water Resour. Water Eng. 2004, 60–62. [Google Scholar] [CrossRef]
- Sugiyama, D. Chemical alteration of calcium silicate hydrate (C–S–H) in sodium chloride solution. Cem. Concr. Res. 2008, 38, 1270–1275. [Google Scholar] [CrossRef]
- Ercikdi, B.; Cihangir, F.; Kesimal, A.; Deveci, H.; Alp, B. Utilization of water reducing admixtures in cemented paste backfill of sulphide-rich mill tailings. J. Hazard. Mater. 2010, 179, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Saedi, A.; Darban, A.K.; Zanjani, J.A. A review of additives used in the cemented paste tailings: Environmental aspects and application. J. Environ. Manag. 2021, 289, 112501. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).