Abstract
Intumescent coating is able to provide effective fire protection with both practicality and aesthetics. In this study, expansion performance and thermal physical property experiments are firstly carried out to obtain the basic parameters at different temperatures. Then, the thermal response model of the concrete-filled steel tubular (CFST) structure under the protection of intumescent coating in a fire is established. Finally, based on the experimental data and thermal response model, the effects of initial thickness, expansion rate, intra-pore emissivity and reaction heat on the structure temperature are discussed in detail. The results of this study can provide guidance on intumescent coating formulation design, as well as fire protection design.
1. Introduction
Concrete-filled steel tubular (CFST) structures are widely used in modern buildings due to their excellent mechanical and construction performance [1,2]. However, the mechanical performance of a CFST structure degrades significantly with increasing temperatures, and the structure cannot maintain its strength and stiffness at high temperatures [3]. Therefore, effective fire protection measures must be taken to reduce the temperature-rise rate of the CFST structure. Intumescent coating is able to provide efficient fire protection with relatively less thickness, and it is a common fireproofing material for CFST structures [4]. The intumescent coating will swell at high temperatures, and a porous carbon layer will be formed with relatively more thickness [5]. The porous carbon layer directs the path of external heat transfer into the protected structure, and the thermal conductivity of the porous carbon layer is extremely low [6]. Meanwhile, the physical and chemical changes of intumescent coatings will absorb a large amount of heat [7]. In summary, intumescent coating is able to reduce the temperature-rise rate of the protected structure with both practicality and aesthetics [8,9,10].
In the past decades, researchers conducted extensive studies on the thermal insulation performance of intumescent coatings. The studies can be divided into two categories. One type focused on the material design and formulation for intumescent coatings and developed new components and formulations to improve the physical and chemical performance of intumescent coatings at high temperatures. Vabdersall [11] put forward that the intumescent coating’s thermal performance was dependent on the quantity ratio of carbon, nitrogen and phosphorus. Reshentnikov [12,13,14] found that the best thermal insulation performance was achieved when the ratio of polyphosphate to polyol was 7:3. Studies showed that expandable graphite expanded at high temperatures and formed porous carbon layers with high porosity [15,16,17,18]. Researchers found that the proper addition of fillers improved the intumescent coating’s thermal insulation performance. For instance, the use of magnesium hydroxide, titanium, zirconium, fire-resistant fibers and nanomaterials were able to improve the physical and chemical performance at high temperatures [18,19,20,21,22,23,24,25].
The other type of study focused on the thermal response behavior of intumescent coatings at high temperatures, including pyrolysis, expansion and heat-transfer mechanisms. This type of study provided theoretical guidance for the design and formulation of intumescent coatings. In the early years, semi-empirical models were developed to describe the expansion process [26,27,28,29,30]. Blasi and Colomba [31,32] established a mathematical model to characterize the pyrolysis behavior of the intumescent coating; the model described the expansion process with some empirical parameters. Some researchers [33,34,35] established comprehensive models by considering a combination of the pyrolysis, expansion and heat-transfer process. Li et al. [36,37] adopted the constant equivalent thermal resistance to indicate the thermal insulation performance of the intumescent coating. Cirpici et al. [38] used the bubble growth model to describe the expansion behavior of the intumescent coating. Zhang et al. [39] studied the couple thermal transfer mechanisms of the porous char layer, including thermal conduction, convection and radiation.
In this paper, expansion performance and thermal physical property experiments at high temperatures are carried out to obtain the thickness, thermal conductivity and density of intumescent coating. Then, a numerical model is established to describe the thermal response of CFST structures under the protection of intumescent coatings in a fire. Based on the experimental data and numerical model, this study examines the thermal response characteristics of the structure. The effect of initial thickness, expansion rate, intra-pore emissivity and reaction heat are discussed in detail. This results of this study can be applied to provide guidance on fire protection design.
2. Experiments
2.1. High-Temperature Expansion Performance Experiment
A high-temperature expansion performance experiment of a typical intumescent coating was carried out to obtain the variation in thickness with temperature. The intumescent coating used in the experiment was produced by Wuli Coating Co. The substrate was a surface-treated Q235 steel plate with a size of 120 mm × 120 mm × 3 mm. The initial thickness of the intumescent coating was 3 (±0.1) mm. The specimen was placed on the experimental table for dry maintenance. The instrument used for the experiment was the SX2-10-13A high-temperature electric furnace produced by Chongqing Xinbang Electric Furnace Company. The electric furnace was composed of two parts: the furnace body and the temperature-control system, as shown in Figure 1. The maximum range of the furnace is 1573 K, and a variety of temperature-rise rates can be set to meet the required test conditions. The main processes were as follows.

Figure 1.
Electric furnace.
- (a)
- The specimen is placed on the refractory brick and surrounded by a certain amount of sponge to prevent heat dissipation, as shown in Figure 2.
 Figure 2. Specimen in the furnace.
Figure 2. Specimen in the furnace. - (b)
- The temperature of the furnace is set according to the working conditions and maintained for 1800 s to ensure full expansion at this temperature.
- (c)
- After cooling, the expanded specimen is taken out and the thickness is measured by vernier caliper.
The expansion thickness and the expansion rate, which is defined as the ratio of the expansion thickness to the initial thickness, are shown in Table 1. In order to display the variation trend with the change in temperature more clearly, the data in the table are plotted as curves, as shown in Figure 3. It can be seen that in the initial stage (293 K to 573 K), the expansion thickness and rate remained unchanged with the increase in temperature; this is because the components of the intumescent coating have not yet decomposed. When the temperature reached 573 K, the expansion thickness and rate increased with the increase in temperature, which indicates that the decomposition reaction of the foaming agent and other components started to take place. Then, the expansion thickness and rate continued to increase and reached the maximum value at a certain temperature. Finally, the expansion thickness and rate started to decrease; this is due to the intumescent coating’s oxidation at high temperatures.

Table 1.
Thickness and expansion rate (d3-373 K indicates the initial thickness is 3 mm and the experiment temperature is 373 K).
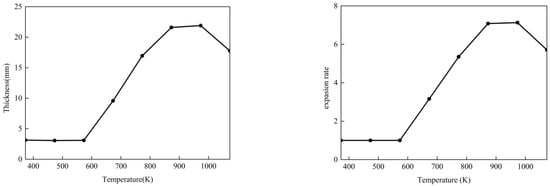
Figure 3.
Thickness and expansion rate variation with temperature.
2.2. Thermal Conductivity Experiment
The instrument used for the thermal conductivity experiment was the C-Therm thermal conductivity meter, as shown in Figure 4, which is based on the principle of “Modified Transient Plane Source”. The thermal conductivity of solids, liquids, powders and colloids could be determined quickly and accurately. The thermal conductivity could be measured from 0 to 500 W/(m·K), and the temperatures were from 273 K to 423 K.

Figure 4.
C-Therm conductivity meter.
According to the results of the high-temperature expansion performance experiment, the state of the intumescent coating at different temperatures varied greatly, as shown in Figure 5. Therefore, the experiment of thermal conductivity was divided into a low-temperature stage and a high-temperature stage. In the low-temperature stage (273 K–573 K), the intumescent coating was in a dense block state, and we tested the thermal conductivity directly. However, in the high-temperature stage (673 K–1073 K), the intumescent coating reached the expansion temperature and was in a porous carbon layer state. We first pressed the porous carbon layer into powder, then tested the powder coating’s thermal conductivity. The specimens are shown in Figure 6.

Figure 5.
State of the intumescent coating at different temperatures.
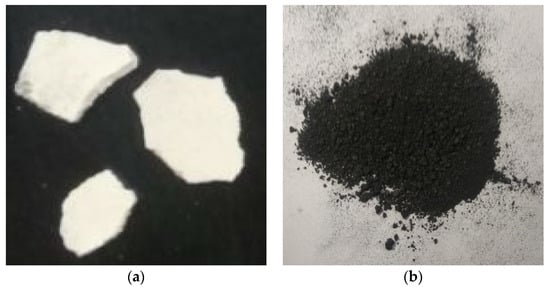
Figure 6.
Specimen for the thermal conductivity experiment. (a) Block state; (b) powder state.
Figure 7 displays the thermal conductivities of the intumescent coating before expansion under different temperatures. It can be seen that the thermal conductivity increased with the increasing temperature. The equation for the thermal conductivity and the temperature was obtained by linear fitting, as Equation (1). The thermal conductivity at different temperatures (273–573 K) can be calculated by the following equation.
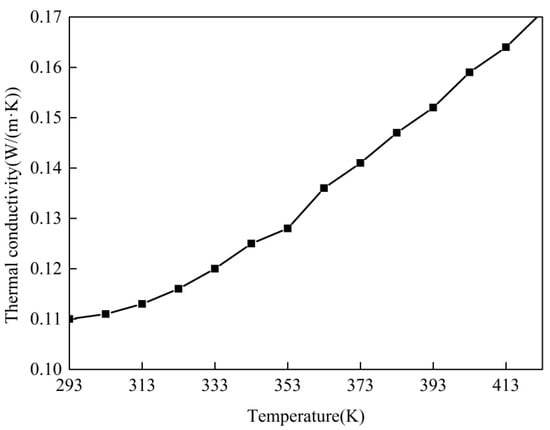
Figure 7.
Variations of the thermal conductivity before expansion with temperature.
Figure 8 displays the thermal conductivities of the intumescent coating after expansion under different temperatures. It can be seen that the thermal conductivity increased with the increasing temperature. By linear fitting, multiple equations for the thermal conductivity with temperature were obtained, as Equations (2)–(6). The thermal conductivity of the powder coating at different temperatures (673–1073 K) can be calculated by the following equations:
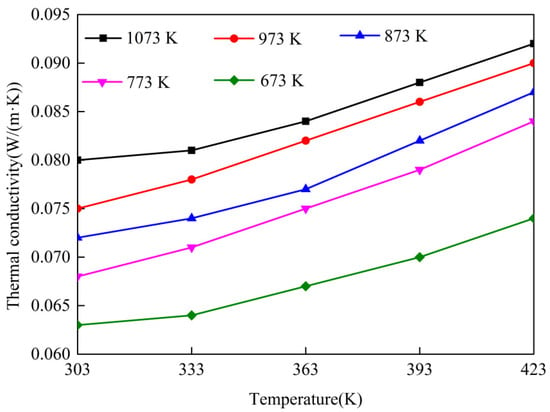
Figure 8.
Variations of the thermal conductivity after expansion with temperature.
2.3. Density and Porosity Experiment
The density experiment of the intumescent coating was similar to the thermal conductivity experiment. In the low-temperature stage (273–573 K), a direct method was adopted for testing. In the high-temperature stage (673–1073 K), there were many pores in the carbon layer, the density was characterized into true density and apparent density and an indirect method was adopted for testing. The specimen is shown in Figure 9. The true density denotes the expanded carbon density without the pores, and the apparent density means the expanded carbon density within the pores. Lastly, the porosity can be calculated by Equation (7), which stands for the relationship of the true density to the apparent density:
where ρ and ρ1 denote the apparent density and true density in g/cm3, and ϕ is the porosity.

Figure 9.
Specimen in the high-temperature stage.
The measured density and the calculated porosity are shown in Table 2. It can be seen that in the low-temperature stage (373–573 K), the true density decreased from 0.791 g/cm3 to 0.723 g/cm3. In this stage, the water and volatility in the intumescent coating escaped and the mass of the coating decreased, whereas the volume remained unchanged. In the high-temperature stage (673–1073 K), the apparent density decreased from 0.297 g/cm3 to 0.074 g/cm3, and the true density decreased from 0.611 g/cm3 to 0.240 g/cm3. The coating started to expand, the mass decreased, and the volume continued to increase. The apparent density and true density of the expanded coating both decreased with the increasing temperature. In addition, the porosity of the intumescent coating increased from 0.514 to 0.722 in the temperature range (673–973 K), whereas it decreased from 0.722 to 0.692 in the temperature range (973–1073 K). The is due to the intumescent coating’s oxidation at high temperatures.

Table 2.
Measured density and calculated porosity.
3. Numerical Simulation
3.1. Physical Model
A typical CFST structure was selected as the research object. According to GB 50936-2014 [40], the outer diameter of the CFST structure should not be less than 168 mm and the wall thickness should not be less than 3 mm. In this study, the outer diameter of the steel pipe was taken as 180 mm and the wall thickness was 3 mm. Figure 10 shows the physical model of the CFST structure under the protection of the intumescent coating. According to GB 14907-2018 [41], the thickness of the intumescent coating should not be less than 1.5 mm.
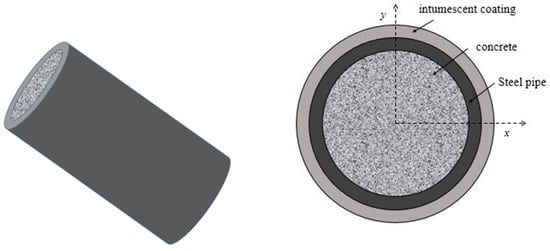
Figure 10.
Physical model of the CFST structure under the protection of intumescent coating.
3.2. Governing Equation
The thermal response of the CFST structure under the protection of the intumescent coating in the fire is a transient heat-transfer process, and the numerical simulation follows the three-dimensional unsteady heat conduction differential equation:
where T means temperature in K, c is the thermal capacity in J/(kg·K), ρ is the true density from the density experiment in kg/m3, x, y, z denote the coordinate system in m and t represents the time in s.
The temperature change along the axial direction of the CFST structure was much smaller than that along the radial direction of the structure, so the temperature change along the axial direction of the structure can be ignored:
Substituting Equation (9) into (8), the governing equation for the thermal response of the CFST structure under the protection of the intumescent coating is obtained as follows:
The solution to Equation (10) requires the thermal property parameters of the intumescent coating and the CFST structure. The thermal property parameters of the intumescent coating in this study were obtained through the experiments, and the thermal property parameters of the CFST were taken based on GB 50936-2014 [40].
3.3. Initial and Boundary Conditions
The initial condition is as follows:
where T0 denotes the ambient temperature with a value of 293 K.
Solving the above heat-transfer governing equation also requires boundary conditions. When the CFST structure is under fire, it is usually understood that external heat is transferred to the structure by means of thermal convection and radiation, as shown in Figure 11. The boundary conditions can be expressed as:
where q, qconv and qrad represent, respectively, the total heat flux, convective heat flux and radiative heat flux.
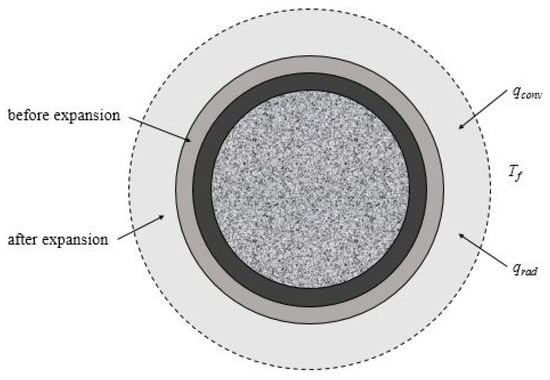
Figure 11.
Boundary conditions of the numerical model.
The convective heat flux is defined as:
where hf is the convective heat-transfer coefficient in W/(m2⋅K), the value of hf is in the range from 10 W/(m2⋅K) to 20 W/(m2⋅K) and 15 W/(m2⋅K) is adopted in the numerical simulation according to Gillet [42]. Tf is the gas temperature, and Ts is the surface temperature of the intumescent coating.
The radiative heat flux is defined as [43]:
where εsys denotes the system emissivity with a value of 0.75, and σ is the Boltzmann constant with a value of 5.67 × 10−8 W/(m2·K4).
According to the boundary conditions, the gas temperature Tf is the main external factor affecting the heat transfer into the structure. Therefore, it is important to describe the temperature-rise history of the gas temperature accurately. Various fire scenarios have some differences in the temperature-rise models. The fire scenarios generally have two forms: (1) fire dominated by hydrocarbon substances, which is usually caused by petroleum; (2) fire dominated by fibrous substances, which is usually caused by wood, paper and textiles.
For the fibrous substances fire, the ISO 834 model is a commonly used model and can be expressed as:
Figure 12 shows the gas temperature curve used in the simulations; it can be seen from the figure that the gas temperature increased with time.
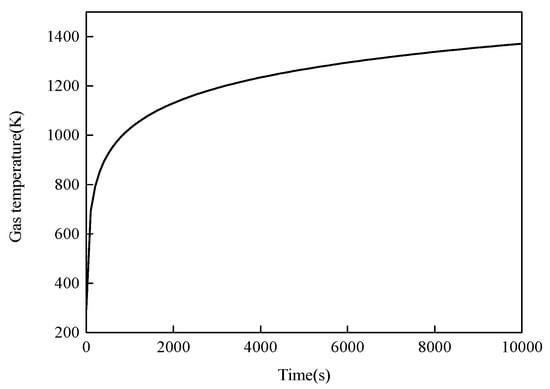
Figure 12.
Gas temperature curve used in the simulations.
3.4. Treatments of the Nonlinear Problem in the Numerical Simulation
3.4.1. Heat-Absorption Effect in the Pyrolysis
The intumescent coating pyrolysis will absorb part of the external heat and thus reduces the temperature-rise rate of the protected structure, so the heat-absorption effect should be considered in the numerical simulation. The pyrolysis is treated as a special phase-change process, and the equivalent heat capacity method is used to solve the nonlinear problem in the phase change.
The sensible heat capacity(cp,e) is defined as:
where cp,s and cp,l represent, respectively, the sensible heat capacity before and after phase change; the value is 1000 J/(kg⋅K) and 2000 J/(kg⋅K). L is the latent heat; the value is 100 kJ/kg. Ts and Tl denote, respectively, the temperature before and after phase change, with values of 573 K and 873 K.
3.4.2. Nonlinear Size Effect in the Expansion
In the high-temperature expansion performance experiment, the thickness at different temperatures was obtained. Although the thickness of the coating changed with temperature, a fixed region was still used for the numerical simulation by considering the efficiency and feasibility. The change in the intumescent coating’s thickness is converted into the change in the thermal conductivity equivalently, which can simplify the numerical simulation and reduce the calculation cost. The equivalent conversion processes are as follows:
- (a)
- Using Equation (1) to calculate the thermal conductivity before expansion and using Equations (2)–(6) to calculate the thermal conductivity after expansion, the results are shown in Table 3.
 Table 3. Thermal conductivity of the intumescent coating.
Table 3. Thermal conductivity of the intumescent coating. - (b)
- An equivalent thermal conductivity model of the intumescent coatings is adopted to calculate the thermal conductivity [44]:

Table 4.
Equivalent thermal conductivity of the intumescent coating.
- (c)
- Assuming that the thickness of the intumescent coating remains unchanged, the thickness change is equivalently converted into the thermal conductivity change by Equation (18). As a result, the input values in the simulation calculation are obtained, which can be seen in Table 5:
 Table 5. The input value in the numerical simulation.
Table 5. The input value in the numerical simulation.
3.5. Results and Discussion
3.5.1. Finite Element Model and Feature Point
The governing equation with initial and boundary conditions was solved numerically using the commercial software Comsol Multiphysics (Version 5.5, Comsol Inc., Stockholm, Sweden). The quarter-symmetric model was adopted for lower cost. The built-in grid module was used for grid generation, and the grid structure with a legend of quality is shown in Figure 13. The closer the value to 1, the better the quality of the grid. The total number of grids was 20,334 and the average value was 0.95. The time step in the calculation was 0.01 s. Figure 14 shows the location of the feature point 1, which was used for numerical analysis. In this section, numerical simulations are carried out to investigate the temperature variation of point 1 with time. The effects of initial thickness (0, 2 mm, 3 mm, 4 mm, 5 mm), expansion rate (0.1 n, 0.2 n, n, 5 n, 10 n), intra-pore emissivity (0, 0.2, 0.4, 0.6, 0.8, 1) and reaction heat (10 kJ/kg, 100 kJ/kg, 1000 kJ/kg, 5000 kJ/kg, 10,000 kJ/kg, 20,000 kJ/kg) are studied in detail.
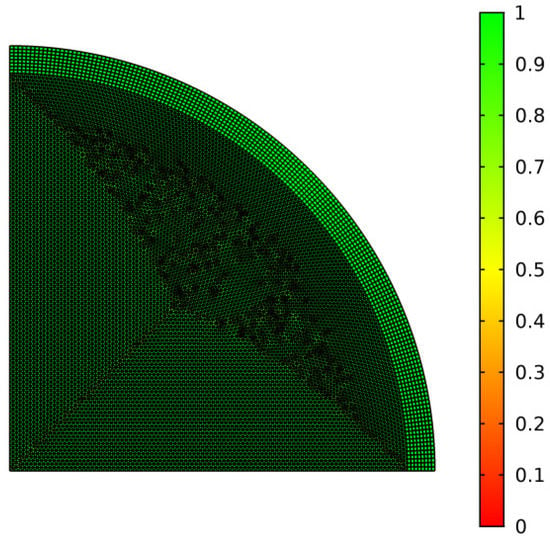
Figure 13.
Grid structure of the model.
Figure 14.
Location of the feature point.
3.5.2. The Effect of Initial Thickness
Figure 15 shows the temperature variation of point 1 with different initial thicknesses. Table 6 and Table 7 present, respectively, the temperature and the temperature-rise rate of point 1 with different initial thicknesses at different times. It can be seen that temperature of point 1 was the highest when the initial thickness was 0. This indicates that the intumescent coating has a thermal insulation effect on the protected structure and can thus reduce the temperature and temperature-rise rate of the protected structure. In addition, the greater the thickness, the lower the temperature of point 1. This means that the intumescent coating’s thermal insulation performance increases with the increasing initial thickness. Furthermore, the effect of the initial thickness on the thermal insulation performance is also related to the time interval, and it was found that the best effect occurred in the range from 2000–4000 s.
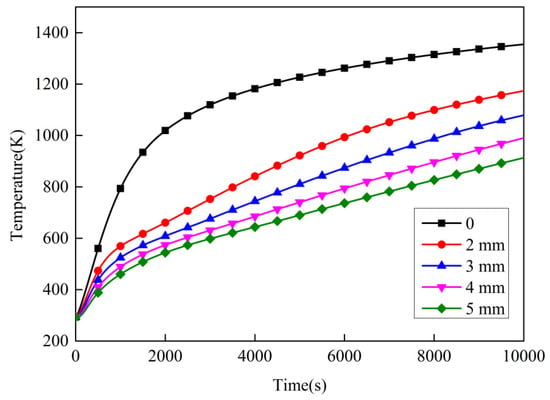
Figure 15.
Temperature variation over time with different initial thicknesses.

Table 6.
Temperature with different initial thicknesses at different times.

Table 7.
Temperature-rise rate with different initial thicknesses at different times.
3.5.3. The Effect of Expansion Rate
Figure 16 shows the temperature variation of point 1 with different expansion rates. Table 8 and Table 9 present, respectively, the temperature and temperature-rise rate of point 1 with different expansion rates at different times. The expansion rate n was obtained from the expansion performance experiment. It can be seen that the greater the expansion rate, the lower the temperature of point 1. This indicates that the intumescent coating’s thermal insulation performance increased with the increasing expansion rate. This is because the increasing expansion rate led to a longer path for heat transfer. In addition, the curves are very close to each other when the expansion rates were 5 n and 10 n. This means that thermal insulation performance no longer significantly improved when the expansion rate increased to a certain level. In addition, more expansion may lead to a more fragile char that may get blown off the surface compared with a thinner, more durable char. However, this paper focuses on the heat-transfer mechanism, and the numerical model does not include the effects of the durability of the char itself.
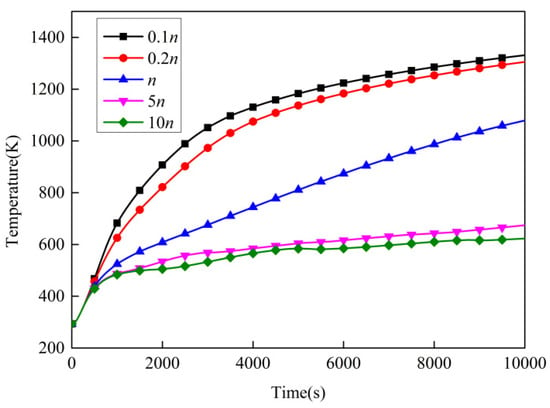
Figure 16.
Temperature variation over time with different expansion rates.

Table 8.
Temperature with different expansion rates at different times.

Table 9.
Temperature-rise rate with different expansion rates at different times.
3.5.4. The Effect of Intra-Pore Emissivity
Figure 17 shows the temperature variation of point 1 with different intra-pore emissivity. Table 10 and Table 11 present, respectively, the temperature and temperature-rise rate of point 1 with different intra-pore emissivity at different times. The intra-pore emissivity of 0 means that radiation in the carbon layer was not considered. From the figure, the greater the intra-pore emissivity, the higher the temperature of point 1. This indicates that the intumescent coating’s thermal insulation performance decreased with the increasing intra-pore emissivity. The reason for this is that the increase in intra-pore emissivity enhances the intro-pore thermal radiation, thereby leading to an increase in equivalent thermal conductivity. Comparing the temperature variation at an intra-pore emissivity of 0 and 1, the temperature difference reached 326.3 K at t = 10,000 s. This means that thermal radiation in the carbon layer formed by the intumescent coating at high temperatures should be considered.
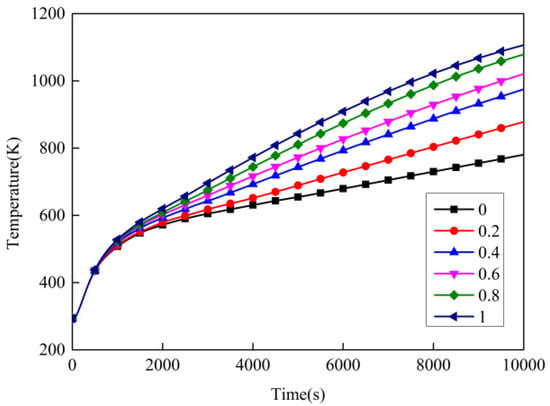
Figure 17.
Temperature variation over time with different intra-pore emissivity.

Table 10.
Temperature with different intra-pore emissivity at different times.

Table 11.
Temperature-rise rate with different intra-pore emissivity at different times.
3.5.5. The Effect of Reaction Heat
In the calculations, the latent heat L represents the reaction heat, but L does not appear in the thermal governing equation (Equation (8)). Therefore, the equivalent heat capacity method (Equation (16)) was used to transition. Figure 18 shows the temperature variation of point 1 with different reaction heats. Table 12 and Table 13 present, respectively, the temperature and temperature-rise rate of point 1 with different reaction heats at different times. The reaction heat represents the intumescent coating heat-absorb effect due to physical and chemical changes. From the figure, the greater the reaction heat, the lower the temperature of point 1. This indicates that the intumescent coating’s thermal insulation performance increased with the increasing reaction heat. The reason for this is that the heat-absorb effect led to a decrease in temperature rate. However, the effect of the reaction heat on the thermal insulation’s performance is limited. Form the figure and tables, the maximum temperature difference is 68.9 K at t = 1151 s when the reaction heat increases from 10 kJ/kg to 10,000 kJ/kg.
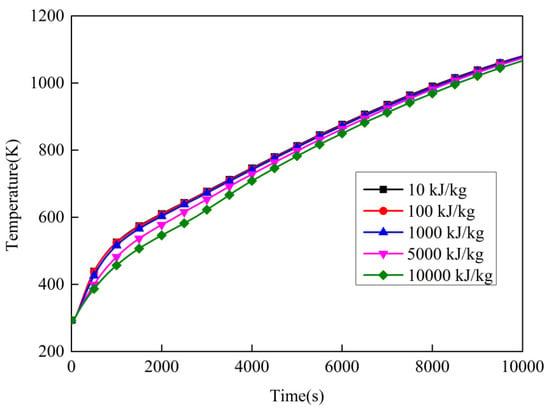
Figure 18.
Temperature variation over time with different reaction heats.

Table 12.
Temperature with different reaction heats at different times.

Table 13.
Temperature-rise rate with different reaction heats at different times.
4. Conclusions
Expansion performance and thermal physical property experiments were carried out to obtain the basic data of intumescent coating at high temperatures. The thermal response model of the CFST structure under the protection of intumescent coating in a fire was established, and the effects of initial thickness, expansion rate, intra-pore emissivity and reaction heat on the structure’s temperature were discussed based on the numerical simulation. The following conclusions could be drawn:
- (1)
- The thickness of the intumescent coating stayed the same at low temperatures, then increased rapidly with the increasing temperature, finally decreasing due to oxidation at high temperatures. The thermal conductivity increased with the increasing temperature, and the calculation equations at different temperature were obtained.
- (2)
- The initial thickness had a positive effect on the intumescent coating’s thermal insulation performance. As the initial thickness increased, the thermal insulation performance improved.
- (3)
- The expansion rate had a remarkable effect on the intumescent coating’s thermal insulation performance, but the effect was limited when the expansion rate increased to a certain level. In the material formulation design, appropriately increasing the quantity of the foaming agent could effectively improve the thermal insulation performance.
- (4)
- The intra-pore emissivity had a negative effect on the intumescent coating’s thermal insulation performance. Thermal radiation in the porous carbon layer strengthened the coupled heat transfer. In the material-formulation design, specific fillers could be used to decrease the intra-pore emissivity, thereby improving the thermal insulation performance.
- (5)
- The reaction heat hd a slightly positive effect on the intumescent coating’s thermal insulation performance. In the material formulation design, modifying the components could strengthen the heat-absorption effect, thus improving the thermal insulation’s performance.
Author Contributions
Conceptualization, Y.H. and M.L.; methodology, L.Z.; software, L.Z.; validation, Y.H., L.Z. and M.L.; formal analysis, L.Z.; investigation, Y.H.; resources, L.Z.; data curation, L.Z.; writing—original draft preparation, L.Z.; writing—review and editing, L.Z.; visualization, L.Z.; supervision, Y.H.; project administration, M.L.; funding acquisition, M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, X.; Fan, F.; Lai, J. Strength behavior of circular concrete-filled steel tube stub columns under axial compression: A review. Constr. Build. Mater. 2022, 322, 126144. [Google Scholar] [CrossRef]
- Le, K.B.; Cao, V.V. Numerical Study of Circular Concrete Filled Steel Tubes Subjected to Pure Torsion. Buildings 2021, 11, 397. [Google Scholar]
- Shao, Z.; Zha, X.; Wan, C. Design method of fire-resistance capacity of reinforced-concrete-filled steel tube column under axial compression. Fire Saf. J. 2022, 129, 103572. [Google Scholar] [CrossRef]
- Eremina, T.; Korolchenko, D. Fire Protection of Building Constructions with the Use of Fire-Retardant Intumescent Compositions. Buildings 2020, 10, 185. [Google Scholar] [CrossRef]
- de Silva, D.; Nuzzo, I.; Nigro, E.; Occhiuzzi, A. Intumescent Coatings for Fire Resistance of Steel Structures: Current Approaches for Qualification and Design. Coatings 2022, 12, 696. [Google Scholar] [CrossRef]
- Anees, S.M.; Dasari, A. A review on the environmental durability of intumescent coatings for steels. J. Mater. Sci. 2018, 53, 124–145. [Google Scholar]
- Griffin, G.J.; Bicknell, A.D.; Brown, T.J. Studies on the Effect of Atmospheric Oxygen Content on the Thermal Resistance of Intumescent, Fire-Retardant Coatings. J. Fire Sci. 2005, 23, 303–328. [Google Scholar] [CrossRef]
- Yasir, M.; Ahmad, F.; Yusoff PS, M.M.; Ullah, S.; Jimenez, M. Latest trends for structural steel protection by using intumescent fire protective coatings: A review. Surf. Eng. 2019, 36, 334–363. [Google Scholar] [CrossRef]
- Lucherini, A.; Maluk, C. Intumescent coatings used for the fire-safe design of steel structures: A review. J. Constr. Steel Res. 2019, 162, 105712. [Google Scholar] [CrossRef]
- Puri, R.G.; Khanna, A.S. Intumescent coatings: A review on recent progress. J. Coat. Technol. Res. 2017, 14, 1–20. [Google Scholar]
- Vandersall, H.L. Intumescent coating systems, their development and chemistry. J. Fire Flammabl. 1971, 2, 97–140. [Google Scholar]
- Reshetnikov, I.; Antonov, A.; Rudakova, T.; Aleksjuk, G.; Khalturinskij, N. Some aspects of intumescent fire retardant systems. Polym. Degrad. Stab. 1996, 54, 137–141. [Google Scholar]
- Reshetnikov, I.S.; Garashchenko, A.N.; Strakhov, V.L. Experimental investigation into mechanical destruction of intumescent chars. Polym. Adv. Technol. 2000, 11, 392–397. [Google Scholar] [CrossRef]
- Reshetnikov, I.S.; Yablokova, M.Y.; Potapova, E.V.; Khalturinskij, N.A.; Chernyh, V.Y.; Mashlyakovskii, L.N. Mechanical stability of intumescent chars. J. Appl. Polym. Sci. 1998, 67, 1827–1830. [Google Scholar] [CrossRef]
- Wang, G.; Yang, J. Influences of expandable graphite modified by polyethylene glycol on fire protection of waterborne intumescent fire resistive coating. Surf. Coat. Technol. 2010, 204, 3599–3605. [Google Scholar] [CrossRef]
- Wang, Z.; Han, E.; Ke, W. Influence of expandable graphite on fire resistance and water resistance of flame-retardant coatings. Corros. Sci. 2007, 49, 2237–2253. [Google Scholar] [CrossRef]
- Ullah, S.; Ahmad, F. Effects of zirconium silicate reinforcement on expandable graphite based intumescent fire retardant coating. Polym. Degrad. Stab. 2014, 103, 49–62. [Google Scholar] [CrossRef]
- Weihua, F.; Yufei, W.; Bo, T.; Sheng, Z.; Xiaoyu, G.; Hongfei, L.; Jun, S. Suppression on heat and smoke diffusion by zirconium nitride (ZrN) in intumescent flame retardant epoxy coatings. Prog. Org. Coat. 2020, 146, 105714. [Google Scholar]
- Yew, M.C.; Sulong, N.R.; Yew, M.K.; Amalina, M.A.; Johan, M.R. Influences of flame-retardant fillers on fire protection and mechanical properties of intumescent coatings. Prog. Org. Coat. 2015, 78, 59–66. [Google Scholar]
- Yang, Z.; Xiao, G.; Chen, C.; Chen, C.; Wang, M.; Zhong, F.; Zeng, S.; Lin, L. Synergistic decoration of organic titanium and polydopamine on boron nitride to enhance fire resistance of intumescent waterborne epoxy coating. Colloids Surf. A Physicochem. Eng. Asp. 2021, 621, 126561. [Google Scholar] [CrossRef]
- Puri, R.G.; Khanna, A.S. Effect of cenospheres on the char formation and fire protective performance of water-based intumescent coatings on structural steel. Prog. Org. Coat. 2016, 92, 8–15. [Google Scholar] [CrossRef]
- Yasir, M.; Amir, N.; Ahmad, F.; Ullah, S.; Jimenez, M. Effect of basalt fibers dispersion on steel fire protection performance of epoxy-based intumescent coatings. Prog. Org. Coat. 2018, 122, 229–238. [Google Scholar] [CrossRef]
- Ahmad, F.; Zulkurnain, E.S.B.; Ullah, S.; Al-Sehemi, A.G.; Raza, M.R. Improved fire resistance of boron nitride/epoxy intumescent coating upon minor addition of nano-alumina. Mater. Chem. Phys. 2020, 256, 123634. [Google Scholar] [CrossRef]
- Chaisaenrith, P.; Taksakulvith, P.; Pavasupree, S. Effect of nano titanium dioxide in intumescent fireproof coating on thermal performance and char morphology. Mater. Today Proc. 2021, 47, 3462–3467. [Google Scholar] [CrossRef]
- Yew, M.C.; Yew, M.K.; Saw, L.H.; Ng, T.C.; Durairaj, R.; Beh, J.H. Influences of nano bio-filler on the fire-resistive and mechanical properties of water-based intumescent coatings. Prog. Org. Coat. 2018, 124, 33–40. [Google Scholar] [CrossRef]
- Cagliostro, D.E.; Riccitiello, S.R.; Clark, K.J.; Shimizu, A.B. Intumescent coating modeling. J. Fire Flammabl. 1975, 6, 205–221. [Google Scholar]
- Anderson, C.E.; Wauters, D.K. A thermodynamic heat transfer model for intumescent systems. Int. J. Eng. Sci. 1984, 22, 881–889. [Google Scholar] [CrossRef]
- Charles, E.; Anderson, J.R.; Dziuk, J.R.; Mallow, W.A.; Buckmaster, J. Intumescent Reaction Mechanisms. J. Fire Sci. 1985, 3, 161–194. [Google Scholar]
- Anderson, C.E.; Ketchum, D.E.; Mountain, W.P. Thermal Conductivity of Intumescent Chars. J. Fire Sci. 1988, 6, 390–410. [Google Scholar] [CrossRef]
- Buckmaster, J.; Anderson, C.; Nachman, A. A model for intumescent paints. Int. J. Eng. Sci. 1986, 24, 263–276. [Google Scholar] [CrossRef]
- Di Blasi, C.; Branca, C. Mathematical model for the nonsteady decomposition of intumescent coatings. AIChE J. 2001, 47, 2359–2370. [Google Scholar]
- Di Blasi, C. Modeling the effects of high radiative heat fluxes on intumescent material decomposition. J. Anal. Appl. Pyrolysis 2004, 71, 721–737. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.C.; Bailey, C.; Taylor, A.P. Global modelling of fire protection performance of intumescent coating under different cone calorimeter heating conditions. Fire Saf. J. 2012, 50, 51–62. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.C.; Bailey, C.G.; Taylor, A.P. Global modelling of fire protection performance of an intumescent coating under different furnace fire conditions. J. Fire Sci. 2013, 31, 51–72. [Google Scholar] [CrossRef]
- Staggs, J.E.J.; Crewe, R.J.; Butler, R. A theoretical and experimental investigation of intumescent behaviour in protective coatings for structural steel. Chem. Eng. Sci. 2012, 71, 239–251. [Google Scholar] [CrossRef]
- Li, G.Q.; Zhang, C.; Lou, G.B.; Wang, Y.C.; Wang, L.L. Assess the Fire Resistance of Intumescent Coatings by Equivalent Constant Thermal Resistance. Fire Technol. 2012, 48, 529–546. [Google Scholar] [CrossRef]
- Li, G.Q.; Han, J.; Lou, G.B.; Wang, Y.C. Predicting intumescent coating protected steel temperature in fire using constant thermal conductivity. Thin-Walled Struct. 2016, 98, 177–184. [Google Scholar] [CrossRef]
- Cirpici, B.K.; Wang, Y.C.; Rogers, B.D.; Bourbigot, S. A theoretical model for quantifying expansion of intumescent coating under different heating conditions. Polym. Eng. Sci. 2016, 56, 798–809. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.; Li, M. Combined Heat Transfer Mechanisms in the Porous Char Layer Formed from the Intumescent Coatings under Fire. Coatings 2021, 11, 200. [Google Scholar] [CrossRef]
- GB 50936-2014; Technical Code for Concrete-Filled Steel Tubular Structures. Architecture &. Building Press: Beijing, China, 2014. (In Chinese)
- GB 14907-2018; Fire Resistive Coating for Steel Structure. Standards Press of China: Beijing, China, 2018. (In Chinese)
- Gillet, M.; Autrique, L.; Perez, L. Mathematical model for intumescent coatings growth: Application to fire retardant systems evaluation. J. Phys. D Appl. Phys. 2007, 40, 883. [Google Scholar]
- Wu, S. Numerical Simulation Research on Thermal Response of Structure with Explosive under Fire Circumanstance. China Acad. Eng. Phys. 2014. Available online: https://wenku.baidu.com/view/5263ad978f9951e79b89680203d8ce2f006665a0?fr=xueshu_top (accessed on 31 May 2022).
- Carson, J.K.; Lovatt, S.J.; Tanner, D.J.; Cleland, A.C. Thermal conductivity bounds for isotropic, porous materials. Int. J. Heat Mass Transf. 2005, 48, 2150–2158. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).