Experimental Research on the Properties and Formulation of Fly Ash Based Geopolymer Grouting Material
Abstract
:1. Introduction
2. Experimental Program
2.1. Materials
2.2. Specimen Preparation and Testing Programs
3. Results and Discussion
3.1. Compressive Strength
3.2. Drying Shrinkage
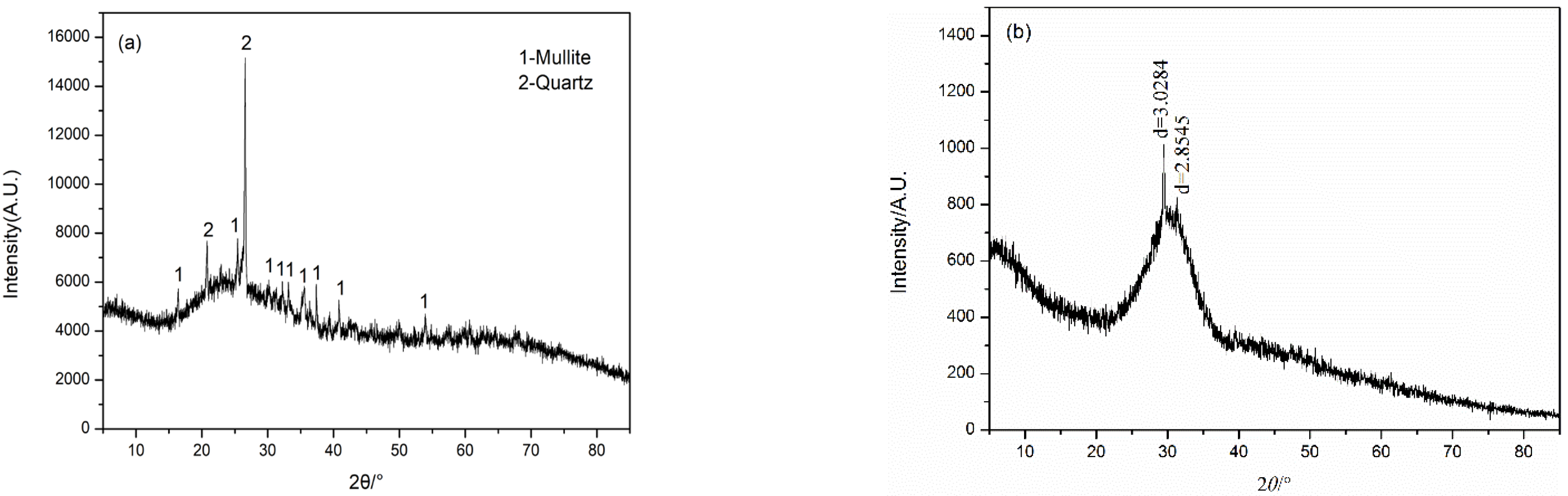
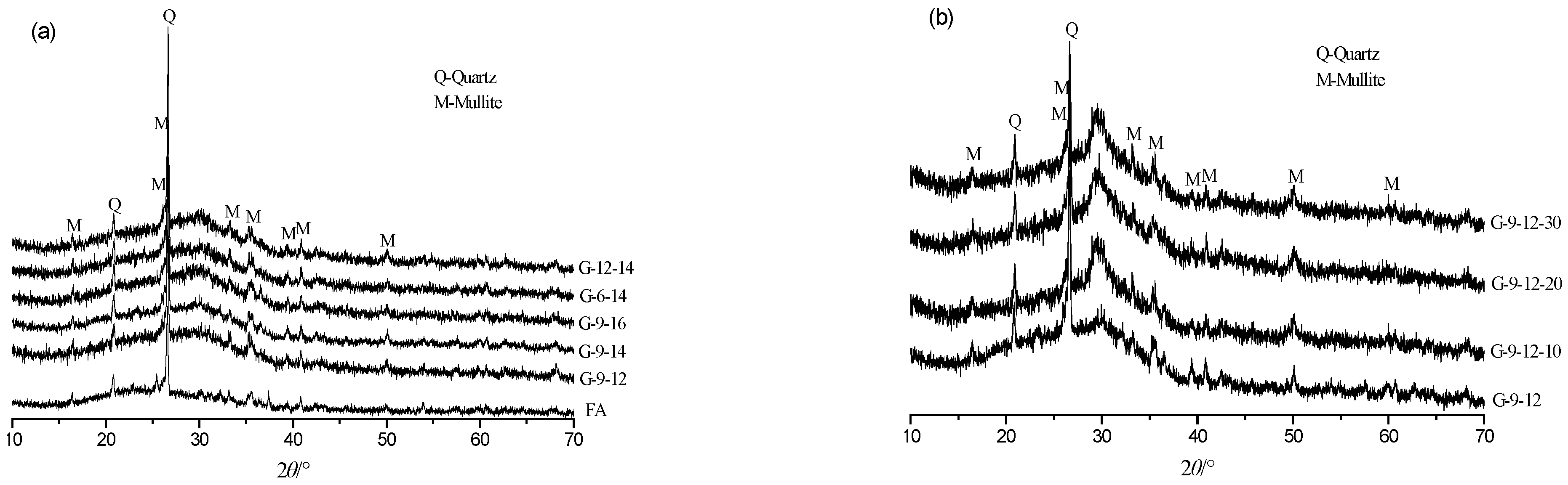
3.3. XRD Analysis
3.4. MIP Analysis
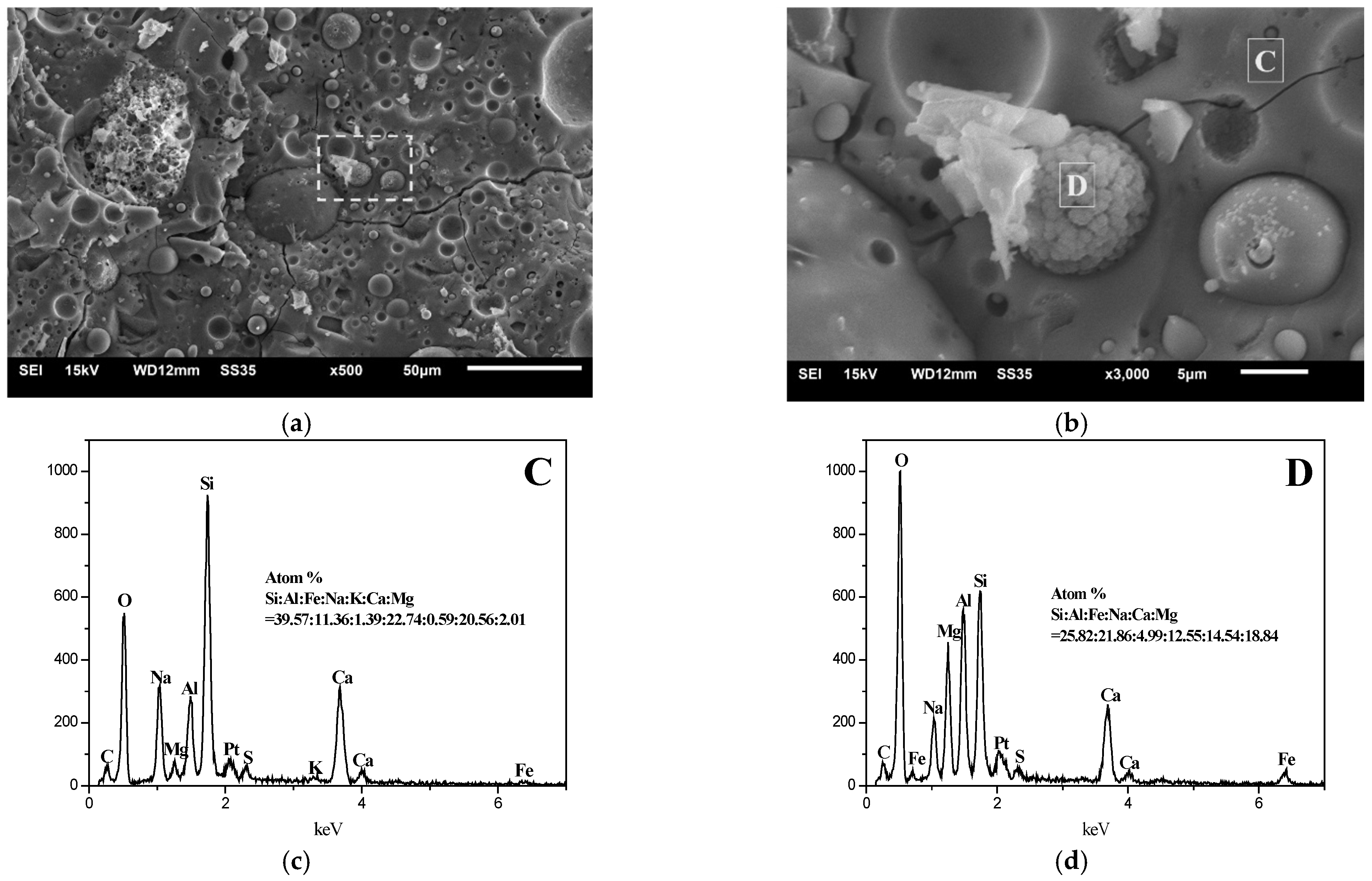
3.5. SEM–EDS Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mo, H.; Alengaram, U.J.; Jumaat, M.Z. Structural performance of reinforced geopolymer concrete members: A review. Constr. Build. Mater. 2016, 120, 251–264. [Google Scholar] [CrossRef]
- Chen, C.; Habert, G.; Bouzidi, Y.; Jullien, A. Environmental impact of cement production: Detail of the different processes and cement plant variability evaluation. J. Clean. Prod. 2010, 18, 478–485. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, X.; Liu, X.; Guan, X.; Zhang, C.; Niu, Y. Flexible and stretchable polyurethane/waterglass grouting material. Constr. Build. Mater. 2017, 138, 240–246. [Google Scholar] [CrossRef]
- Güllü, H. On the viscous behavior of cement mixtures with clay, sand, lime and bottom ash for jet grouting. Constr. Build. Mater. 2015, 93, 891–910. [Google Scholar] [CrossRef]
- Canakci, H.; Güllü, H.; Dwle, M. Effect of glass powder added grout for deep mixing of marginal sand with clay. Arab. J. Sci. Eng. 2018, 43, 1583–1595. [Google Scholar] [CrossRef]
- Gao, P.W.; Zhong, J.J.; Deng, Y.S.; Xu, X.F.; Li, M.Y.; Zhang, J. Preparation and microstructure analysis of high performance lightweight grouting materials. J. East China Jiaotong Univ. 2021, 38, 29–35. [Google Scholar]
- Davidovits, J. Geopolymer: Inorganic polymeric new materials. J. Ther. Anal. 1991, 37, 1633–1639. [Google Scholar] [CrossRef]
- Duxson, P.; Fernandez-Jimenez, A.; Provis, J.L. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Luckey, G.C. The role of inorganic polymer technology in the development of ‘green concrete’. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Liang, G.W.; Li, H.X.; Zhu, H.J.; Liu, T.J.; Chen, Q.; Guo, H.H. Reuse of waste glass powder in alkali-activated metakaolin/fly ash pastes: Physical properties, reaction kinetics and microstructure. Resour. Conserv. Recycl. 2021, 173, 105721. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Li, L. A review: The comparison between alkali-activated slag (Si + Ca) and metakaolin (Si + Al) cements. Cem. Concr. Res. 2010, 40, 1341–1349. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications; Geopolymer Institute: Saint-Quentin, France, 2008. [Google Scholar]
- Provis, J.L.; Duxson, P.; Deventer, J.S.J.V. The role of mathematical modelling and gel chemistry in advancing geopolymer technology. Chem. Eng. Res. Des. 2005, 83, 853–860. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Geopolymers and Related Alkali-Activated Materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Myers, R.J.; Bernal, S.A.; San Nicolas, R. Generalized structural description of calcium-sodium aluminosilicate hydrate gels: The cross-linked substituted tobermorite model. Langmuir 2013, 29, 5294–5306. [Google Scholar] [CrossRef]
- Güllü, H.; Cevik, A.; Al-Ezzi, K.M.A. On the rheology of using geopolymer for grouting: A comparative study with cement-based grout included fly ash and cold bonded fly ash. Constr. Build. Mater. 2019, 196, 594–610. [Google Scholar] [CrossRef]
- Aboulayt, A.; Jaafri, R.; Samouh, H. Stability of a new geopolymer grout: Rheological and mechanical performances of metakaolin-fly ash binary mixtures. Constr. Build. Mater. 2018, 181, 420–436. [Google Scholar] [CrossRef]
- Roviello, G.; Menna, C.; Tarallo, O.; Ricciotti, L. Preparation, structure and properties of hybrid materials based on geopolymers and polysiloxanes. Mater. Des. 2015, 87, 82–94. [Google Scholar] [CrossRef]
- Fernandez-Jimenez, A.; Palomo, A. Composition and microstructure of alkali activated fly ash binder: Effect of the activator. Cem. Concr. Res. 2005, 35, 1984–1992. [Google Scholar] [CrossRef]
- Criado, M.; Fernandez-Jimenez, A.; de la Torre, A.G.; Aranda, M.A.G.; Palomo, A. An XRD study of the effect of the SiO2/Na2O ration on the alkali activation of fly ash. Cem. Concr. Res. 2007, 37, 671–679. [Google Scholar] [CrossRef]
- Criado, M.; Fernandez-Jimenez, A.; Palomo, A. Alkali activation of fly ash: Effect of the SiO2/Na2O ratio PartⅠ: FTIR study. Microporous Mesoporous Mater. 2007, 106, 180–191. [Google Scholar] [CrossRef]
- Criado, M.; Fernandez-Jimenez, A.; Palomo, A.; Sobrados, I.; Sanz, J. Effect of the SiO2/Na2O ratio on the alkali activation of fly ash. PartⅡ: 29Si MAS-NMR Survey. Microporous Mesoporous Mater. 2008, 109, 525–534. [Google Scholar] [CrossRef]
- Lloyd, R.R.; Provis, J.L.; Devevter, J.S.J.V. Microscopy and microanalysis of inorganic polymer cements. 2: The gel binder. J. Mater. Sci. 2009, 44, 620–631. [Google Scholar] [CrossRef]
- Yang, T.; Zhu, H.; Zhang, Z.H. Effect of fly ash microsphere on the rheology and microstructure of alkali-activated fly ash/slag pastes. Cem. Concr. Res. 2018, 109, 198–207. [Google Scholar] [CrossRef]
- Amin, M.M.; Mohammad, B.; Alireza, J. Feasibility of Alkali-Activated Slag Pasteas Injection Material for Rehabilitation of Concrete Structures. J. Mater. Civ. Eng. 2018, 30, 04018252. [Google Scholar]
- Xu, J.; Kang, A.H.; Wu, Z.G.; Xiao, P.; Li, B.; Lu, Y.M. Reseach on the formulation and properties of a high-performance geopolymer grouting material based on slag and fly ash. KSCE J. Civ. Eng. 2021, 25, 3437–3447. [Google Scholar] [CrossRef]
- Xu, J.; Kang, A.H.; Wu, Z.G.; Xiao, P.; Gong, Y.F. Effect of high-calcium basalt fiber on the workability, menchanical properties and microstructure of slag-fly ash geopolymer grouting material. Constr. Build. Mater. 2021, 302, 124089. [Google Scholar] [CrossRef]
- Xiang, J.X.; Liu, L.P.; Cui, X.M.; He, Y.; Zheng, G.J.; Shi, C.J. Effect of fuller-fine sand on rheological, drying shrinkage, and microstructural properties of metakaolin-based geopolymer grouting materials. Cem. Concr. Compos. 2019, 104, 103381. [Google Scholar] [CrossRef]
- GB/T 17671-2020; Methods of Testing Cements-Determination of Strength. Standards Press of China: Beijing, China, 2020.
- JC/T 603-2004; Standard Test Method for Drying Shrinkage of Mortar. Standards Press of China: Beijing, China, 2004.
- Li, Z.M.; Lu, T.S.; Liang, X.H.; Dong, H.; Ye, G. Mechanisms of autogenous shrinkage of alkali-activated slag and fly ash pastes. Cem. Concr. Res. 2020, 135, 106107. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C. Understanding the relationship between geopolymer compositing, microstructure and mechanical properties. Colloids Surf. A 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Lloyd, R.R.; Provis, J.L.; Smeaton, K.J. Spatial distribution of pores in fly ash-based inorganic polymer gels visualised by Wood’s metal intrusion. Microporous Mesoporous Mater. 2009, 126, 32–39. [Google Scholar] [CrossRef]
- Provis, J.L.; Myers, R.J.; White, C.E. X-ray microtomography shows pore structure and tortuosity in alkali-activated binders. Cem. Concr. Res. 2012, 42, 855–864. [Google Scholar] [CrossRef]





| Material | SiO2 | Al2O3 | CaO | MgO | Na2O | K2O | MnO | P2O5 | TiO2 | SO3 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| FA | 50.62 | 21.97 | 7.18 | 9.17 | 2.12 | 1.33 | - | - | 0.79 | 2.16 | 0.77 |
| GBFS | 33.00 | 14.82 | 0.23 | 40.80 | - | - | 0.32 | 0.01 | 0.66 | - | 2.31 |
| No. | Mix | Composite Powder | Modified Sodium Silicate a | W/S b | ||||
|---|---|---|---|---|---|---|---|---|
| FA (wt%) | GBFS (wt%) | Ms | Na2O (wt%) | SiO2 (wt%) | H2O (wt%) | |||
| 1 | G-6-1.4 | 100 | 0 | 1.4 | 6.0 | 8.4 | 93.6 | 0.45 |
| 2 | G-9-1.2 | 100 | 0 | 1.2 | 9.0 | 10.8 | 89.8 | 0.45 |
| 3 | G-9-1.4 | 100 | 0 | 1.4 | 9.0 | 12.6 | 99.5 | 0.45 |
| 4 | G-9-1.6 | 100 | 0 | 1.6 | 9.0 | 14.4 | 101.0 | 0.45 |
| 5 | G-12-1.4 | 100 | 0 | 1.4 | 12.0 | 16.8 | 105.4 | 0.45 |
| 6 | G-9-1.2-10 | 90 | 10 | 1.2 | 9.0 | 10.8 | 89.8 | 0.45 |
| 7 | G-9-1.2-20 | 80 | 20 | 1.2 | 9.0 | 10.8 | 89.8 | 0.45 |
| 8 | G-9-1.2-30 | 70 | 30 | 1.2 | 9.0 | 10.8 | 89.8 | 0.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Q.; Gao, P.; Li, K.; Dong, G.; Jin, G.; Sun, X.; Zhao, J.; Chen, L. Experimental Research on the Properties and Formulation of Fly Ash Based Geopolymer Grouting Material. Buildings 2022, 12, 503. https://doi.org/10.3390/buildings12050503
Zeng Q, Gao P, Li K, Dong G, Jin G, Sun X, Zhao J, Chen L. Experimental Research on the Properties and Formulation of Fly Ash Based Geopolymer Grouting Material. Buildings. 2022; 12(5):503. https://doi.org/10.3390/buildings12050503
Chicago/Turabian StyleZeng, Qingwei, Peiwei Gao, Kuan Li, Guoqing Dong, Guanglai Jin, Xuewei Sun, Jingwei Zhao, and Lifeng Chen. 2022. "Experimental Research on the Properties and Formulation of Fly Ash Based Geopolymer Grouting Material" Buildings 12, no. 5: 503. https://doi.org/10.3390/buildings12050503
APA StyleZeng, Q., Gao, P., Li, K., Dong, G., Jin, G., Sun, X., Zhao, J., & Chen, L. (2022). Experimental Research on the Properties and Formulation of Fly Ash Based Geopolymer Grouting Material. Buildings, 12(5), 503. https://doi.org/10.3390/buildings12050503





