Study on Mechanical Properties of Heap Deposited Fly Ash Based Geopolymers with Different Alkaline Activator Properties
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
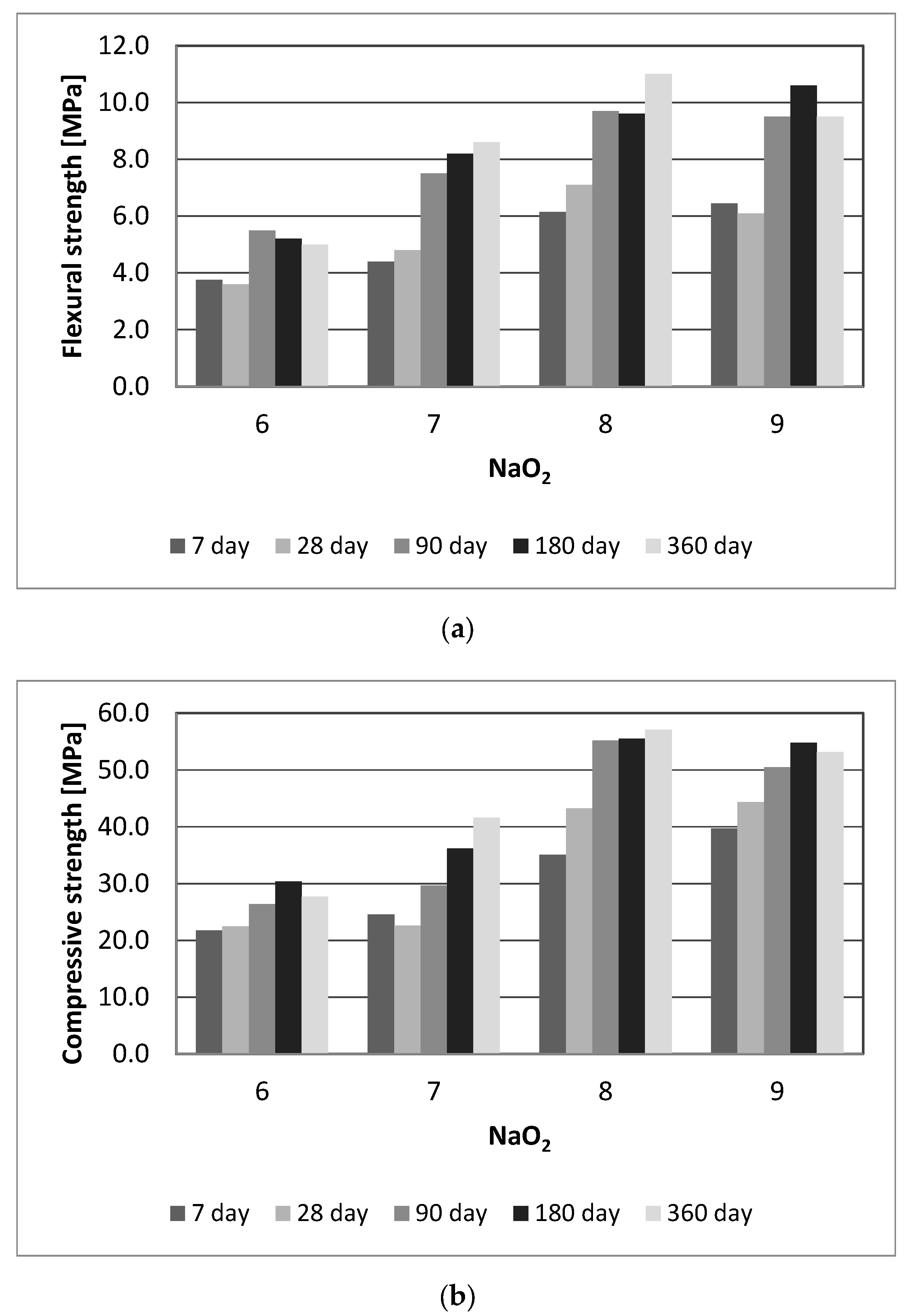
3.1. Effect of Na2O Amount
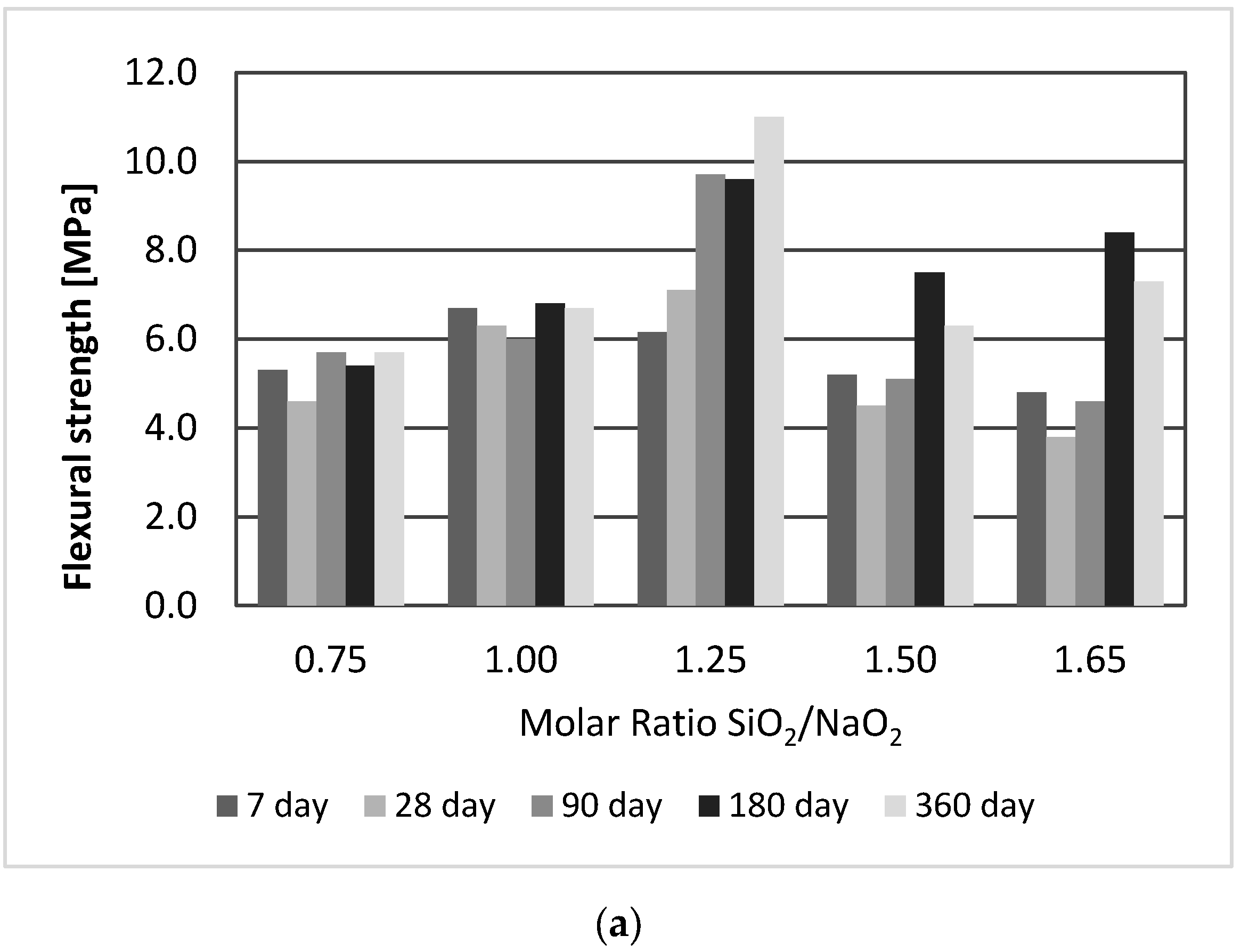
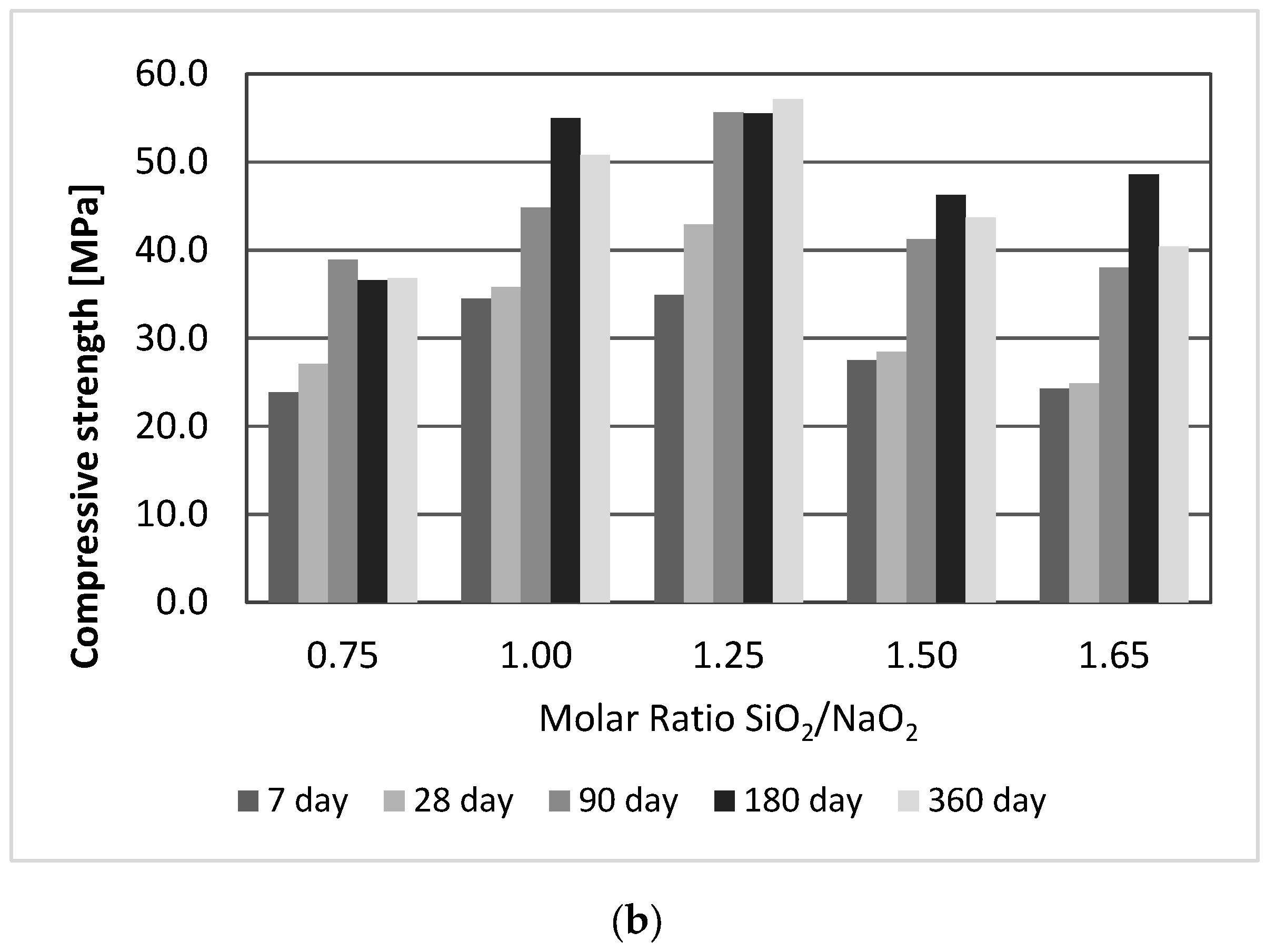
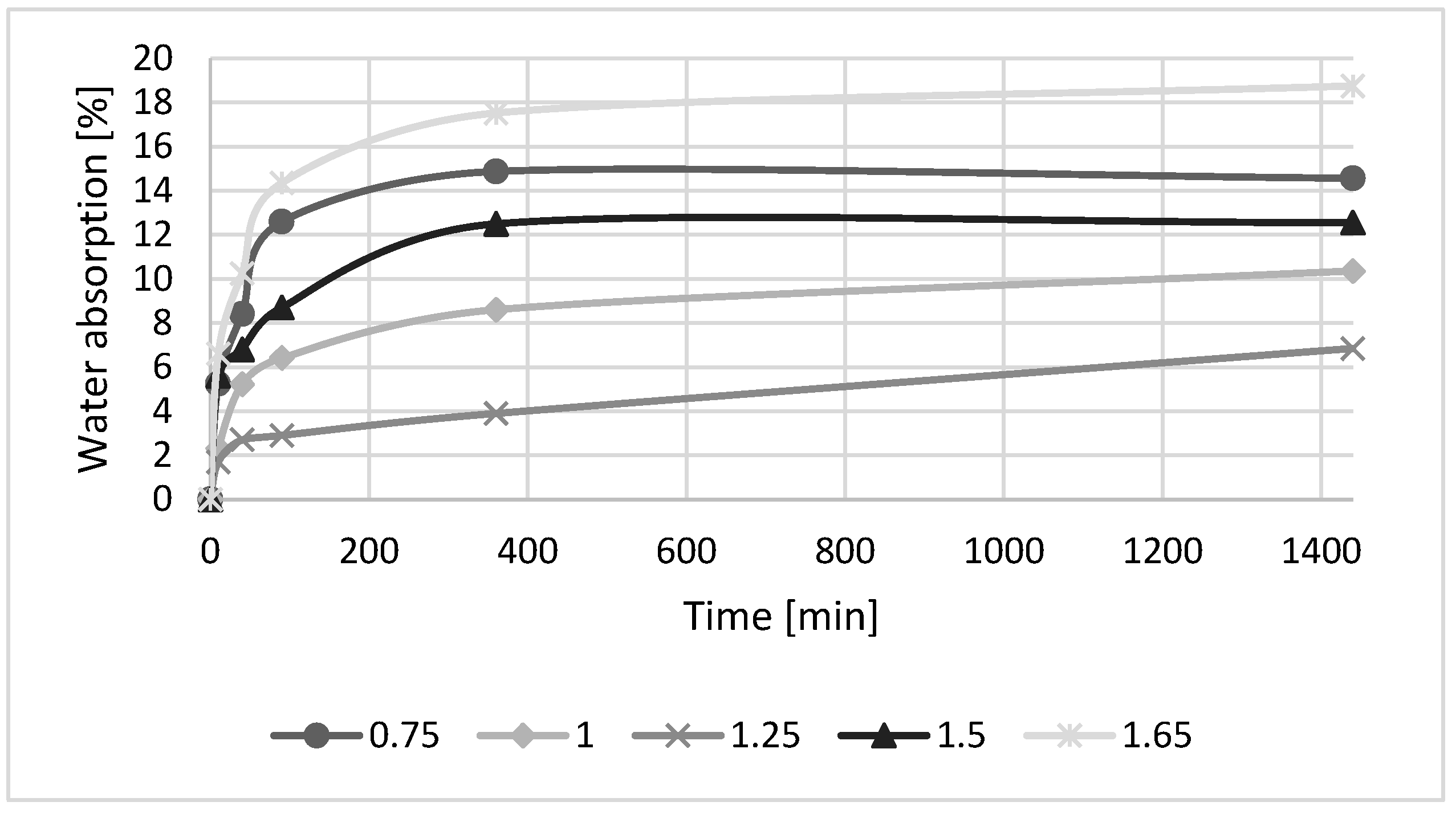
3.2. Effect of SiO2/Na2O Molar Ratio
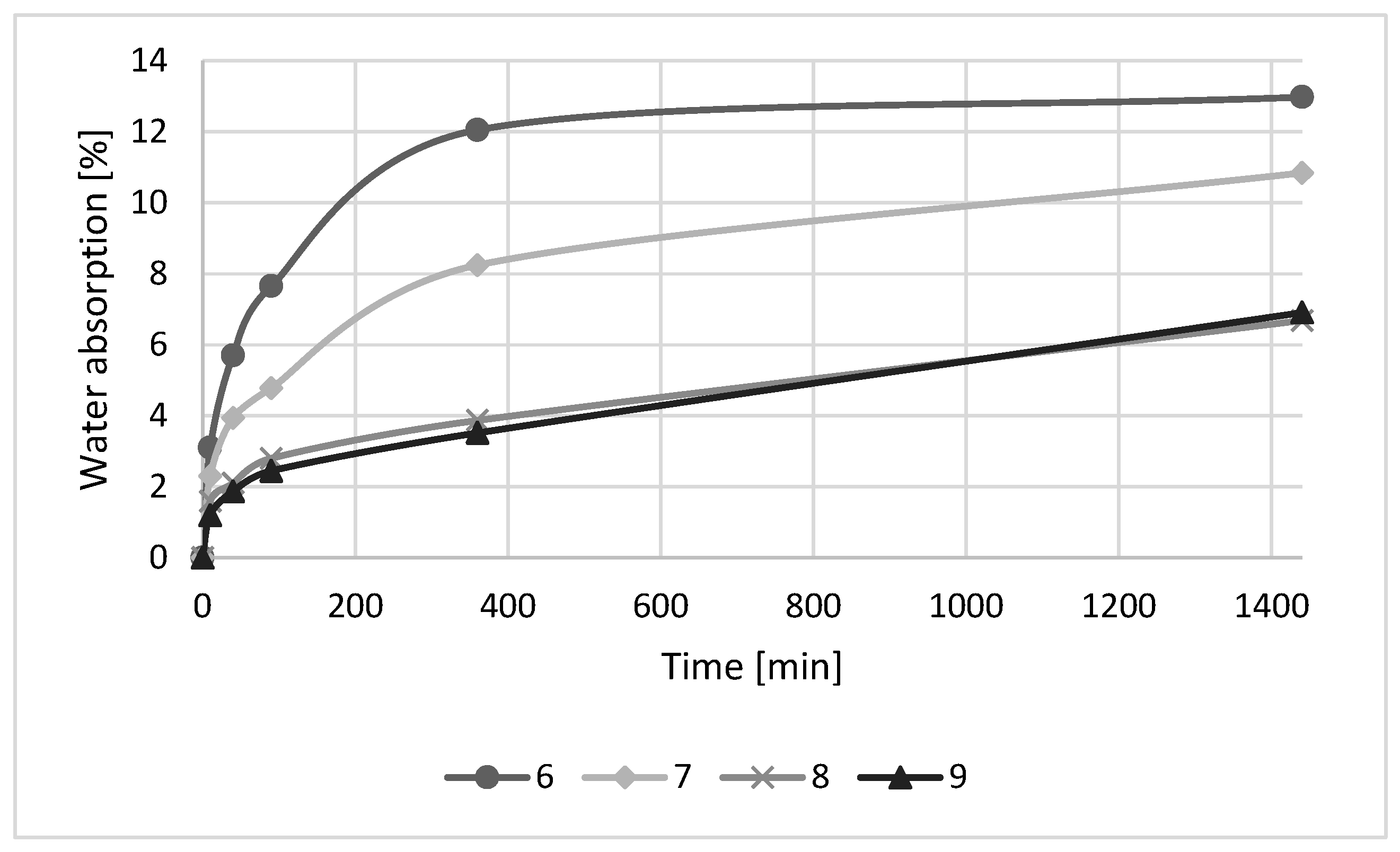
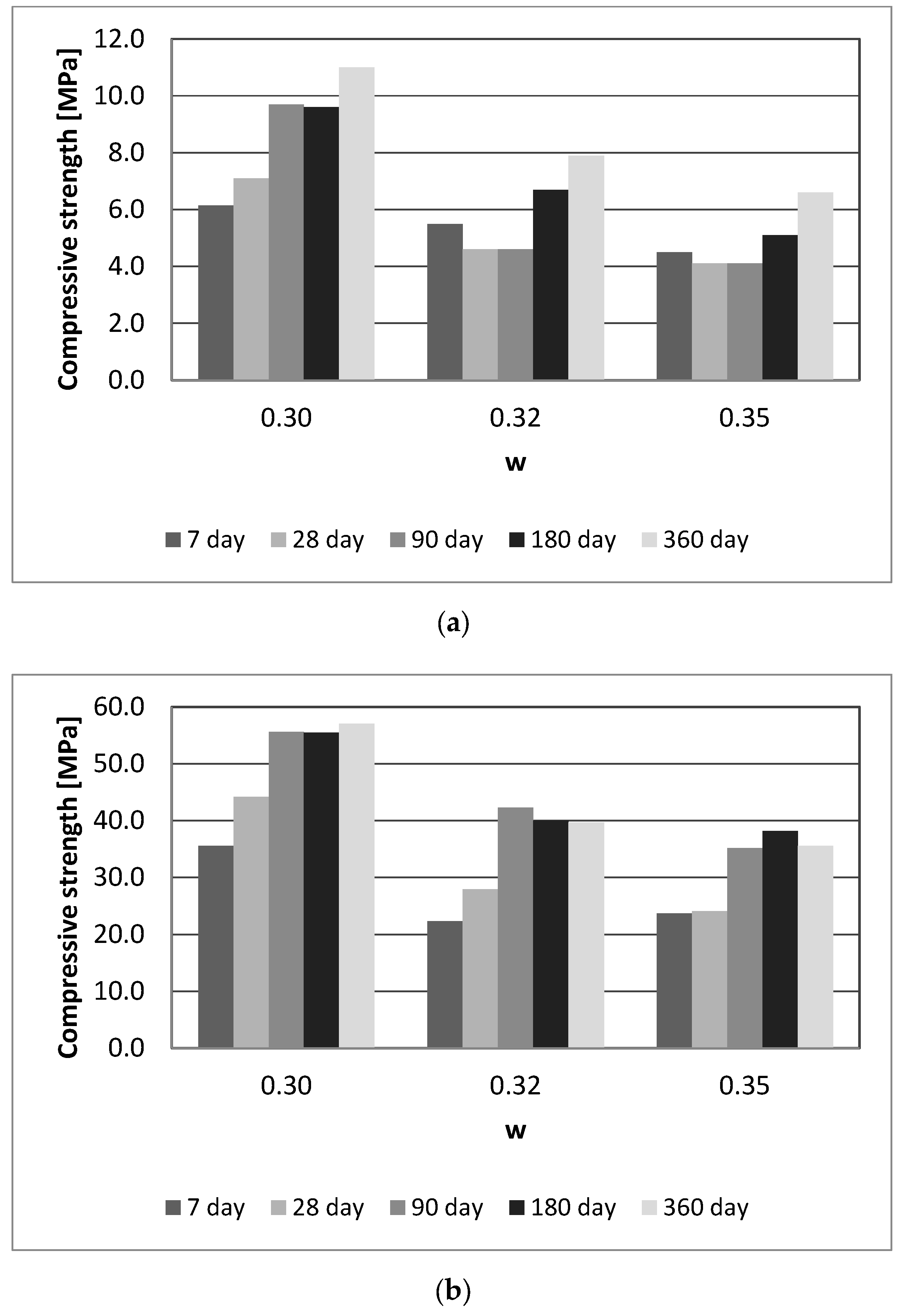
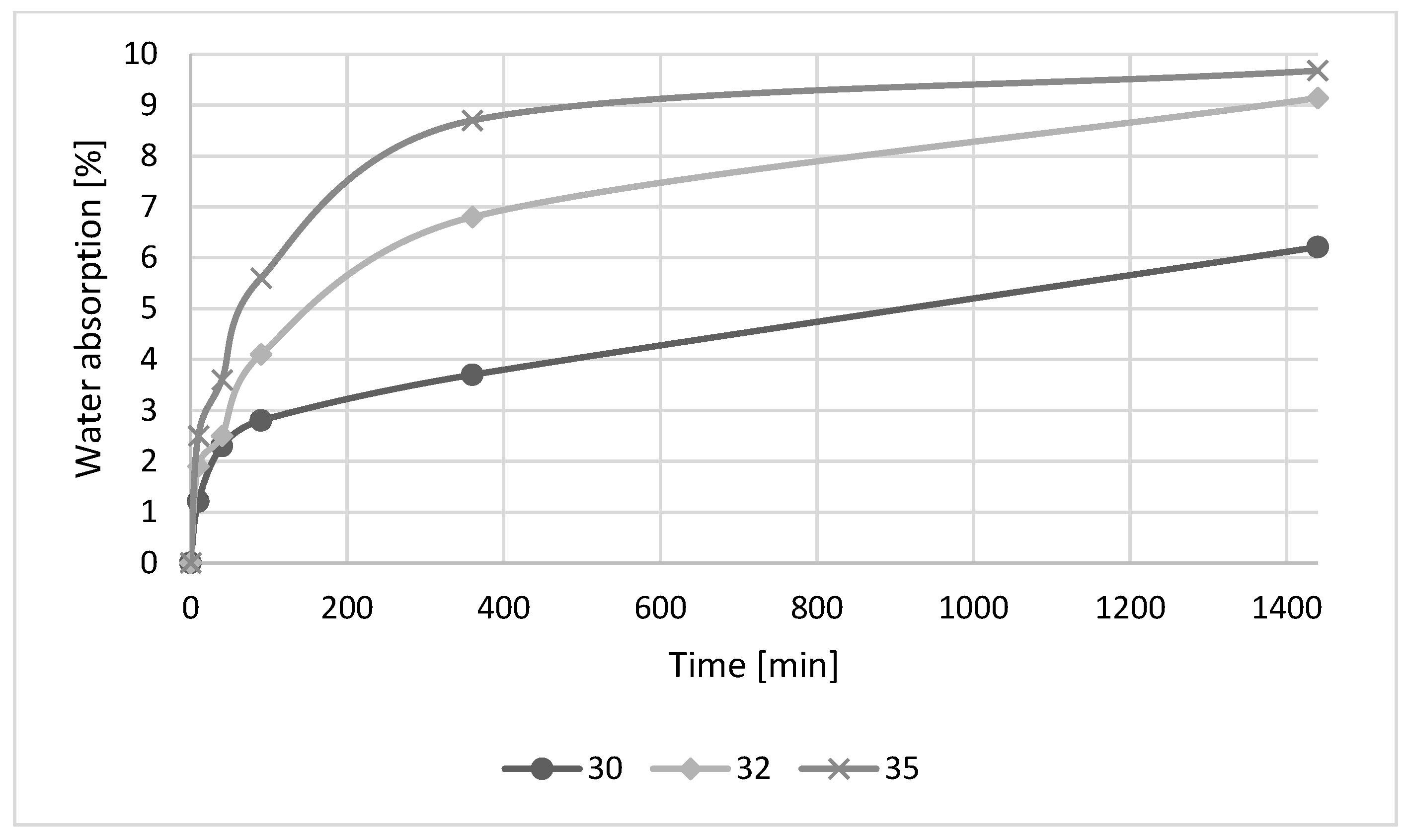
3.3. Effect of HDFA/Water Ratio
4. Conclusions
- The mechanical strengths of the geopolymers improved as the volume of Na2O in the mixture increased. After 360 days, the compressive strengths have the highest values, but after 90 days, the strength did not improve much.
- The geopolymer strengths improved as the SiO2/Na2O molar ratio increased to 1.25, but a further increase was accompanied by a decline in strength. After 180 days, several samples showed a decrease in strength.
- The mechanical strength of the geopolymers was decreased as the volume of water in the mixture was increased.
- A water absorption experiment revealed that an increase in the amount of water in the geopolymer mixture has a negative effect on the development of the strength of the geopolymer samples. The mechanical strengths of geopolymer samples have an impact on final water absorption.
- The geopolymer has the greatest mechanical properties when it was prepared with an alkaline activator solution with a SiO2/Na2O molar ratio of 1.25, 8% Na2O in the mixture, and 30% water in the mixture. This alkaline activator solution’s formulation was ideal for the HDFA geopolymers from our investigation. This mixture was used to produce geopolymer, which was cured for 6 h with 80 °C. It had a flexural strength of 11 MPa and a compressive strength of 57.1 MPa after 360 days, and its water absorption was 6.68%.
Author Contributions
Funding
Conflicts of Interest
References
- European Commission. 2050 Long-Term Strategy. Available online: https://ec.europa.eu/clima/eu-action/climate-strategies-targets/2050-long-term-strategy_en (accessed on 25 May 2022).
- European Commission. A European Green Deal. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en (accessed on 25 May 2022).
- Sachs, J.; Schmidt-Traub, G.; Kroll, C.; Lafortune, G.; Fuller, G.; Woelm, F. Sustainable Development Report 2020: The Sustainable Development Goals and COVID-19 Includes the SDG Index and Dashboards; University Press Cambridge: Cambridge, UK, 2021. [Google Scholar] [CrossRef]
- Nodehi, M.; Taghvaee, V.M. Alkali-activated materials and geopolymer: A review of common precursors and activators addressing circular economy. Circ. Econ. Sust. 2022, 2, 165–196. [Google Scholar] [CrossRef]
- John, S.K.; Nadir, Y.; Girija, K. Effect of source materials, additives on the mechanical properties and durability of fly ash and fly ash-slag geopolymer mortar: A review. Constr. Build. Mater. 2021, 280, 122443. [Google Scholar] [CrossRef]
- Lee, N.K.; Jang, J.G.; Lee, H.K. Shrinkage characteristics of alkali-activated fly ash/slag paste and mortar at early ages. Cem. Concr. Compos. 2014, 53, 239–248. [Google Scholar] [CrossRef]
- Gunasekara, C.; Law, D.W.; Setunge, S.; Sanjayan, J.G. Zeta potential, gel formation and compressive strength of low calcium fly ash geopolymers. Constr. Build. Mater. 2015, 95, 592–599. [Google Scholar]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Khale, D.; Chaudhary, R. Mechanism of geopolymerization and factors influencing its development: A review. J. Mater. Sci. 2007, 42, 729–746. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Van Deventer, J.S. The role of inorganic polymer technology in the development of ‘green concrete’. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Palomo, A.; Grutzeck, M.W.; Blanco, M.T. Alkali-activated fly ashes: A cement for the future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers and geopolymeric materials. J. Therm. Anal. 1989, 35, 429–441. [Google Scholar] [CrossRef]
- Wong, C.L.; Mo, K.H.; Alengaram, U.J.; Yap, S.P. Mechanical strength and permeation properties of high calcium fly ash-based geopolymer containing recycled brick powder. J. Build. Eng. 2020, 32, 101655. [Google Scholar] [CrossRef]
- Baran, P.; Nazarko, M.; Włosińska, E.; Kanciruk, A.; Zarębska, K. Synthesis of geopolymers derived from fly ash with an addition of perlite. J. Clean. Prod. 2021, 293, 126112. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R. Mechanical activation of fly ash: Effect on reaction, structure and properties of resulting geopolymer. Ceram. Int. 2011, 37, 533–541. [Google Scholar] [CrossRef]
- Barbosa, V.F.; MacKenzie, K.J. Thermal behaviour of inorganic geopolymers and composites derived from sodium polysialate. Mater. Res. Bull. 2003, 38, 319–331. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, J.; Kaur, M. Synthesis of fly ash based geopolymer mortar considering different concentrations and combinations of alkaline activator solution. Ceram. Int. 2018, 44, 1534–1537. [Google Scholar] [CrossRef]
- Patankar, S.V.; Ghugal, Y.M.; Jamkar, S.S. Mix design of fly ash based geopolymer concrete. Adv. Struct. Eng. 2015, 3, 1619–1634. [Google Scholar] [CrossRef]
- Dvořák, K.; Hájková, I. The Effect of High-Speed Grinding Technology on the Properties of Fly Ash. Mater. Tehnol. 2016, 50, 683–687. [Google Scholar] [CrossRef]
- Gollakota, A.R.; Volli, V.; Shu, C.M. Progressive utilisation prospects of coal fly ash: A review. Sci. Total Environ. 2019, 672, 951–989. [Google Scholar] [CrossRef]
- Sisol, M.; Kudelas, D.; Marcin, M.; Holub, T.; Varga, P. Statistical evaluation of mechanical properties of slag based alkali-activated material. Sustainability 2019, 11, 5935. [Google Scholar] [CrossRef]
- Marcin, M.; Sisol, M.; Brezani, I. Effect of slag addition on mechanical properties of fly ash based geopolymers. Procedia Eng. 2016, 151, 191–197. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer cement. A review. Geopolymer Inst. 2013, 21, 1–11. [Google Scholar]
- Ryu, G.S.; Lee, Y.B.; Koh, K.T.; Chung, Y.S. The mechanical properties of fly ash-based geopolymer concrete with alkaline activators. Constr. Build. Mater. 2013, 47, 409–418. [Google Scholar] [CrossRef]
- Görhan, G.; Kürklü, G. The influence of the NaOH solution on the properties of the fly ash-based geopolymer mortar cured at different temperatures. Compos. Eng. 2014, 58, 371–377. [Google Scholar] [CrossRef]
- Aliabdo, A.A.; Abd Elmoaty, M.; Salem, H.A. Effect of water addition, plasticizer and alkaline solution constitution on fly ash based geopolymer concrete performance. Constr. Build. Mater. 2016, 121, 694–703. [Google Scholar] [CrossRef]
- Pavithra, P.E.; Reddy, M.S.; Dinakar, P.; Rao, B.H.; Satpathy, B.K.; Mohanty, A.N. A mix design procedure for geopolymer concrete with fly ash. J. Clean. Prod. 2016, 133, 117–125. [Google Scholar] [CrossRef]
- Xie, T.; Ozbakkaloglu, T. Behavior of low-calcium fly and bottom ash-based geopolymer concrete cured at ambient temperature. Ceram. Int. 2015, 41, 5945–5958. [Google Scholar] [CrossRef]
- Reddy, M.S.; Dinakar, P.; Rao, B.H. A review of the influence of source material’s oxide composition on the compressive strength of geopolymer concrete. Microporous Mesoporous Mater. 2016, 234, 12–23. [Google Scholar] [CrossRef]
- Shi, C.; Qu, B.; Provis, J.L. Recent progress in low-carbon binders. Cem. Concr. Res. 2019, 122, 227–250. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Yan, F. Synthesis and mechanical properties of metakaolinite-based geopolymer. Colloids Surf. Physicochem. Eng. Asp. 2005, 268, 1–6. [Google Scholar] [CrossRef]
- Ozer, I.; Soyer-Uzun, S. Relations between the structural characteristics and compressive strength in metakaolin based geopolymers with different molar Si/Al ratios. Ceram. Int. 2015, 41, 10192–10198. [Google Scholar] [CrossRef]
- Yuan, J.; He, P.; Jia, D.; Yang, C.; Yan, S.; Yang, Z.; Zhou, Y. Effect of curing temperature and SiO2/K2O molar ratio on the performance of metakaolin-based geopolymers. Ceram. Int. 2016, 42, 16184–16190. [Google Scholar] [CrossRef]
- da Silva Rocha, T.; Dias, D.P.; França, F.C.C.; de Salles Guerra, R.R.; de Oliveira, L.R.D.C. Metakaolin-based geopolymer mortars with different alkaline activators (Na+ and K+). Constr. Build. Mater. 2018, 178, 453–461. [Google Scholar] [CrossRef]
- Lee, W.H.; Lin, K.L.; Chang, T.H.; Ding, Y.C.; Cheng, T.W. Sustainable development and performance evaluation of marble-waste-based geopolymer concrete. Polymers 2020, 12, 1924. [Google Scholar] [CrossRef] [PubMed]
- Le, V.S.; Louda, P.; Tran, H.N.; Nguyen, P.D.; Bakalova, T.; Ewa Buczkowska, K.; Dufkova, I. Study on Temperature-Dependent Properties and Fire Resistance of Metakaolin-Based Geopolymer Foams. Polymers 2020, 12, 2994. [Google Scholar] [CrossRef] [PubMed]
- La Scalia, G.; Saeli, M.; Adelfio, L.; Micale, R. From lab to industry: Scaling up green geopolymeric mortars manufacturing towards circular economy. J. Clean. Prod. 2021, 316, 128164. [Google Scholar] [CrossRef]
- De Azevedo, A.R.G.; Teixeira Marvila, M.; Barbosa de Oliveira, L.; Macario Ferreira, W.; Colorado, H.; Rainho Teixeira, S.; Mauricio Fontes Vieira, C. Circular economy and durability in geopolymers ceramics pieces obtained from glass polishing waste. Int. J. Appl. Ceram. Technol. 2021, 18, 1891–1900. [Google Scholar] [CrossRef]
- Behun, M.; Kascak, P.; Hrabcak, M.; Behunova, A.; Knapcikova, L.; Sofranko, M. Investigation of sustainable geopolymer composite using automatic identification technology. Sustainability 2020, 12, 6377. [Google Scholar] [CrossRef]
- Kanuchova, M.; Drabova, M.; Sisol, M.; Mosej, J.; Kozakova, L.; Skvarla, J. Influence of mechanical activation of fly ash on the properties of geopolymers investigated by XPS method. Environ. Prog. Sustain. Energy 2016, 35, 1338–1343. [Google Scholar] [CrossRef]
- Assi, L.N.; Deaver, E.E.; Ziehl, P. Effect of source and particle size distribution on the mechanical and microstructural properties of fly Ash-Based geopolymer concrete. Constr. Build. Mater. 2018, 167, 372–380. [Google Scholar] [CrossRef]
- Temuujin, J.; Williams, R.P.; Van Riessen, A. Effect of mechanical activation of fly ash on the properties of geopolymer cured at ambient temperature. J. Mater. Process. Technol. 2009, 209, 5276–5280. [Google Scholar] [CrossRef]
- Paruthi, S.; Husain, A.; Alam, P.; Khan, A.H.; Hasan, M.A.; Magbool, H.M. A review on material mix proportion and strength influence parameters of geopolymer concrete: Application of ANN model for GPC strength prediction. Constr. Build. Mater. 2022, 356, 129253. [Google Scholar] [CrossRef]
- Adak, D.; Mandal, S. Strength and durability performance of fly ash-based process-modified geopolymer concrete. J. Mater. Civ. Eng. 2019, 31, 04019174. [Google Scholar] [CrossRef]
- Komljenović, M.; Baščarević, Z.; Bradić, V. Mechanical and microstructural properties of alkali-activated fly ash geopolymers. J. Hazard. Mater. 2010, 181, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Rashidian-Dezfouli, H.; Rangaraju, P.R. Comparison of strength and durability characteristics of a geopolymer produced from fly ash, ground glass fiber and glass powder. Mater. De Constr. 2017, 67, e136. [Google Scholar] [CrossRef]
- Cho, Y.-K.; Yoo, S.-W.; Jung, S.-H.; Lee, K.-M.; Kwon, S.-J. Effect of Na2O content, SiO2/Na2O molar ratio, and curing conditions on the compressive strength of FA-based geopolymer. Constr. Build. Mater. 2017, 145, 253–260. [Google Scholar] [CrossRef]
- Leong, H.Y.; Ong, D.E.L.; Sanjayan, J.G.; Nazari, A. The effect of different Na2O and K2O ratios of alkali activator on compressive strength of fly ash based-geopolymer. Constr. Build. Mater. 2016, 106, 500–511. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Mid-infrared spectroscopic studies of alkali-activated fly ash structure. Microporous Mesoporous Mater. 2005, 86, 207–214. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, R.; Gong, L.; Li, Y.; Cao, W.; Cheng, X. Development of porous fly ash-based geopolymer with low thermal conductivity. Mater. Des. 2015, 65, 529–533. [Google Scholar] [CrossRef]
- Prochon, P.; Zhao, Z.; Courard, L.; Piotrowski, T.; Michel, F.; Garbacz, A. Influence of Activators on Mechanical Properties of Modified Fly Ash Based Geopolymer Mortars. Materials 2020, 13, 1033. [Google Scholar] [CrossRef]
- Culkova, K.; Khouri, S.; Straka, M.; Rosova, A. Ecological and economic savings of fly ash using as geopolymer. Rocz. Ochr. Srodowiska 2018, 20, 73–88. [Google Scholar]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
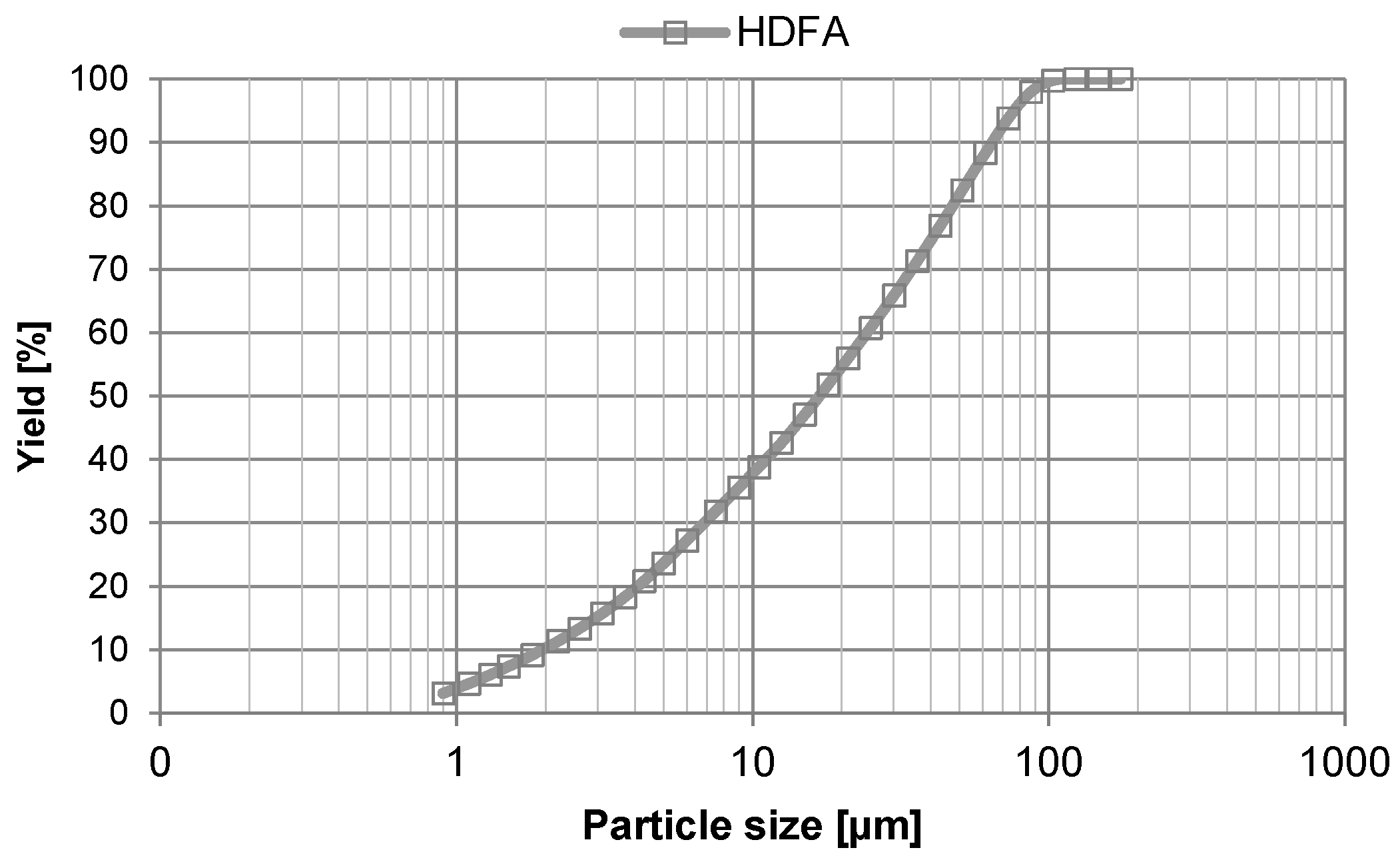

| wt.% | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Other | LOI |
|---|---|---|---|---|---|---|---|
| HDFA | 45.63 | 15.28 | 8.74 | 3.62 | 1.68 | 2.68 | 23.87 |
| wt.% | Amorphous | Hematite | Quartz | Mullite |
|---|---|---|---|---|
| HDFA | 83.52 | 1.57 | 6.42 | 8.46 |
| Material | Na2O | SiO2/Na2O | W |
|---|---|---|---|
| HDFA | 6 | 1.25 | 30 |
| HDFA | 7 | 1.25 | 30 |
| HDFA | 8 | 1.25 | 30 |
| HDFA | 9 | 1.25 | 30 |
| HDFA | 8 | 0.75 | 30 |
| HDFA | 8 | 1 | 30 |
| HDFA | 8 | 1.25 | 30 |
| HDFA | 8 | 1.5 | 30 |
| HDFA | 8 | 1.65 | 30 |
| HDFA | 8 | 1.25 | 30 |
| HDFA | 8 | 1.25 | 32 |
| HDFA | 8 | 1.25 | 35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sisol, M.; Marcin, M.; Dvořák, K.; Suďová, M.; Ivanková, V. Study on Mechanical Properties of Heap Deposited Fly Ash Based Geopolymers with Different Alkaline Activator Properties. Buildings 2022, 12, 1780. https://doi.org/10.3390/buildings12111780
Sisol M, Marcin M, Dvořák K, Suďová M, Ivanková V. Study on Mechanical Properties of Heap Deposited Fly Ash Based Geopolymers with Different Alkaline Activator Properties. Buildings. 2022; 12(11):1780. https://doi.org/10.3390/buildings12111780
Chicago/Turabian StyleSisol, Martin, Michal Marcin, Karel Dvořák, Michaela Suďová, and Viera Ivanková. 2022. "Study on Mechanical Properties of Heap Deposited Fly Ash Based Geopolymers with Different Alkaline Activator Properties" Buildings 12, no. 11: 1780. https://doi.org/10.3390/buildings12111780
APA StyleSisol, M., Marcin, M., Dvořák, K., Suďová, M., & Ivanková, V. (2022). Study on Mechanical Properties of Heap Deposited Fly Ash Based Geopolymers with Different Alkaline Activator Properties. Buildings, 12(11), 1780. https://doi.org/10.3390/buildings12111780









