Effects of C$H2 and CH on Strength and Hydration of Calcium Sulphoaluminate Cement Prepared from Phosphogypsum
Abstract
1. Introduction
2. Experimental Program
2.1. Materials
2.2. Test Methods
3. Results and Discussion
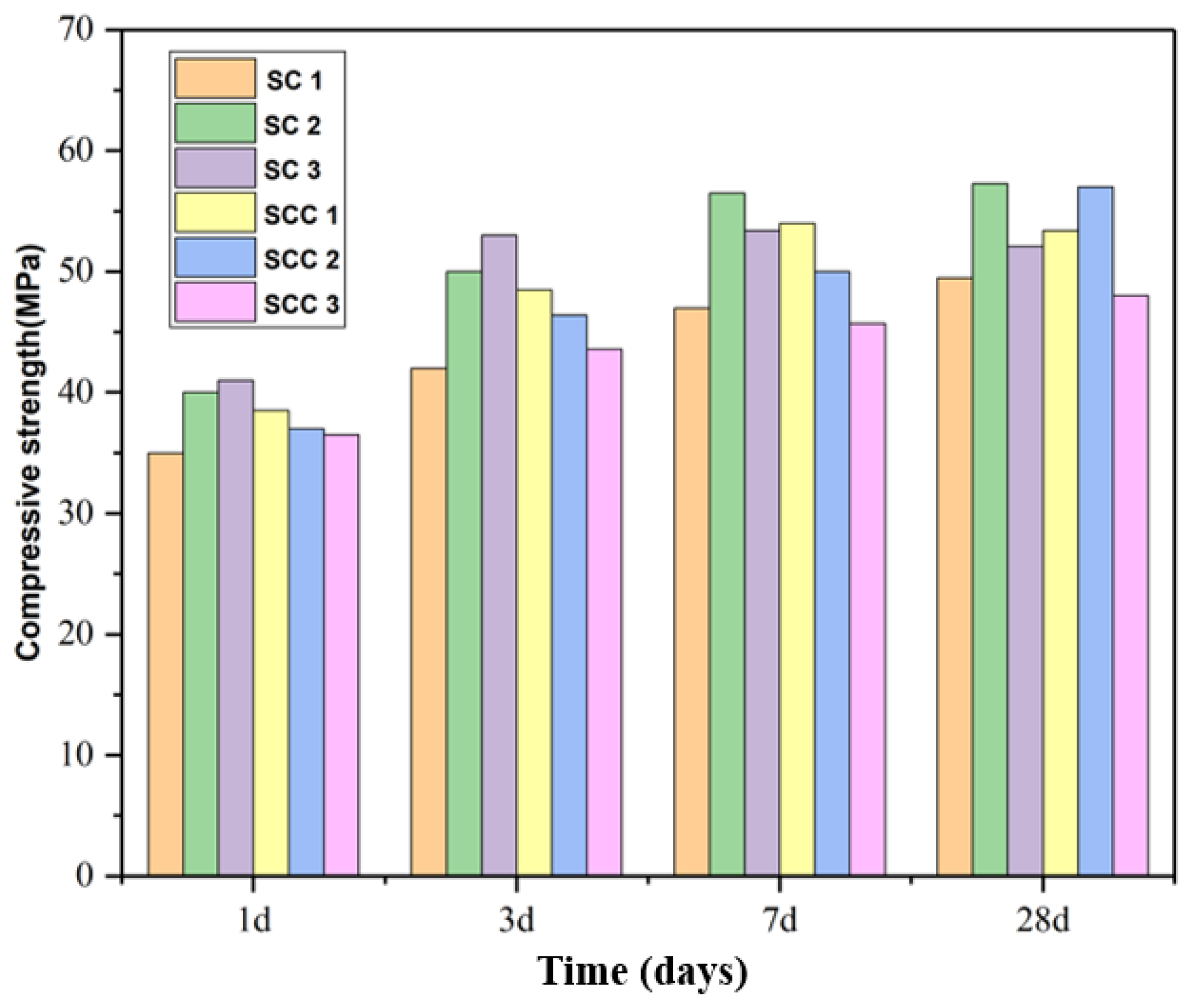
3.1. Compressive Strength
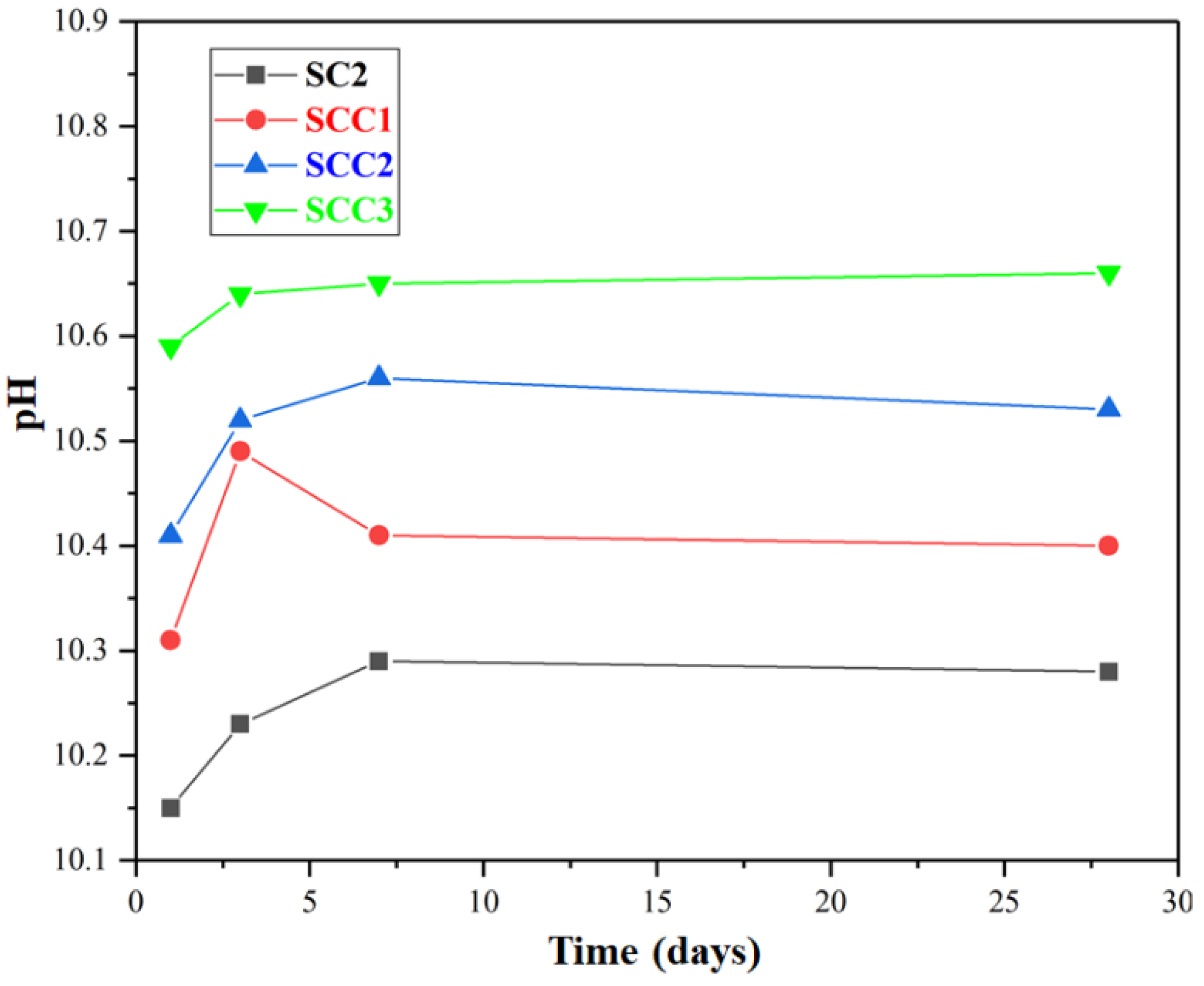
3.2. pH Measurement
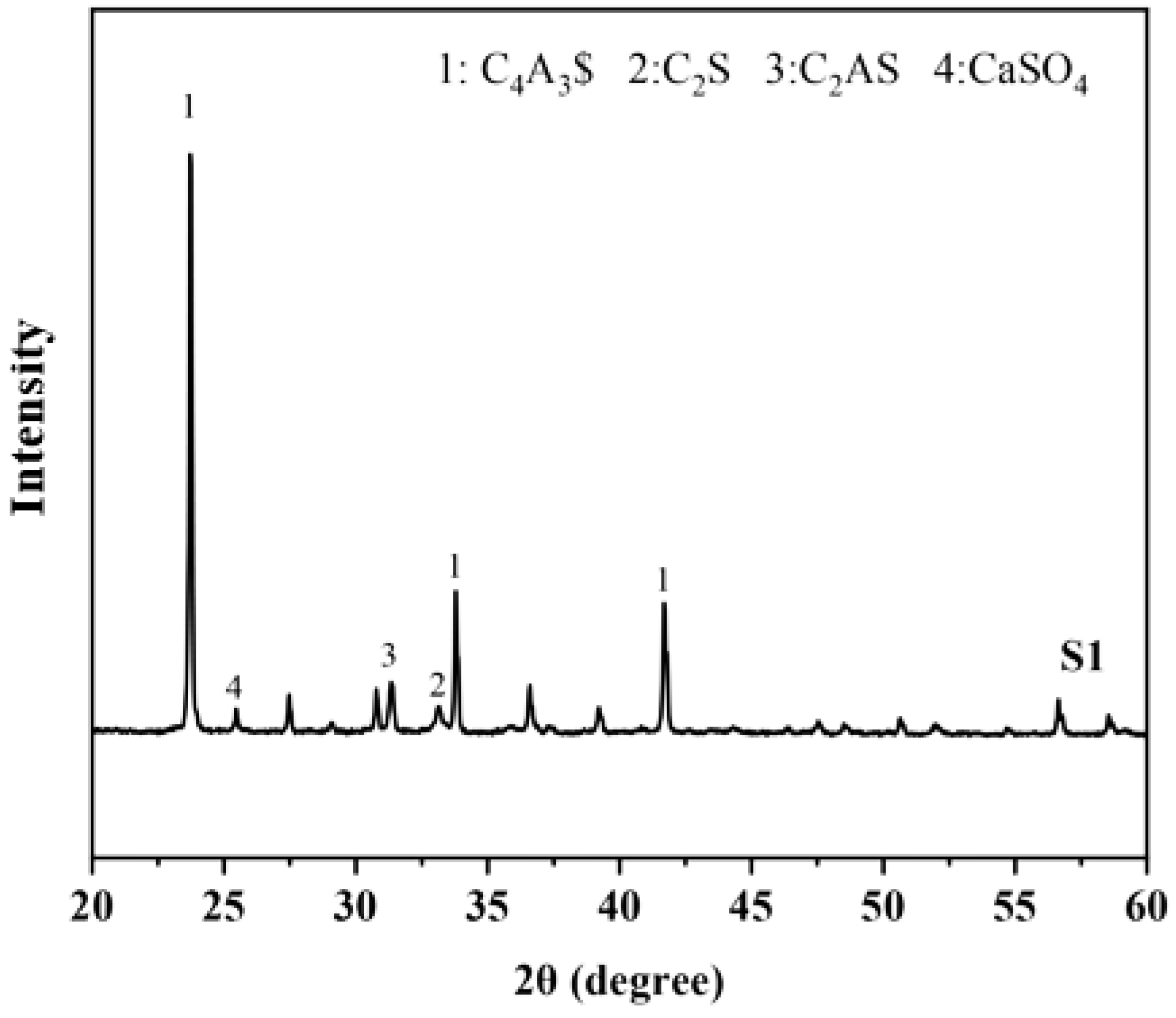
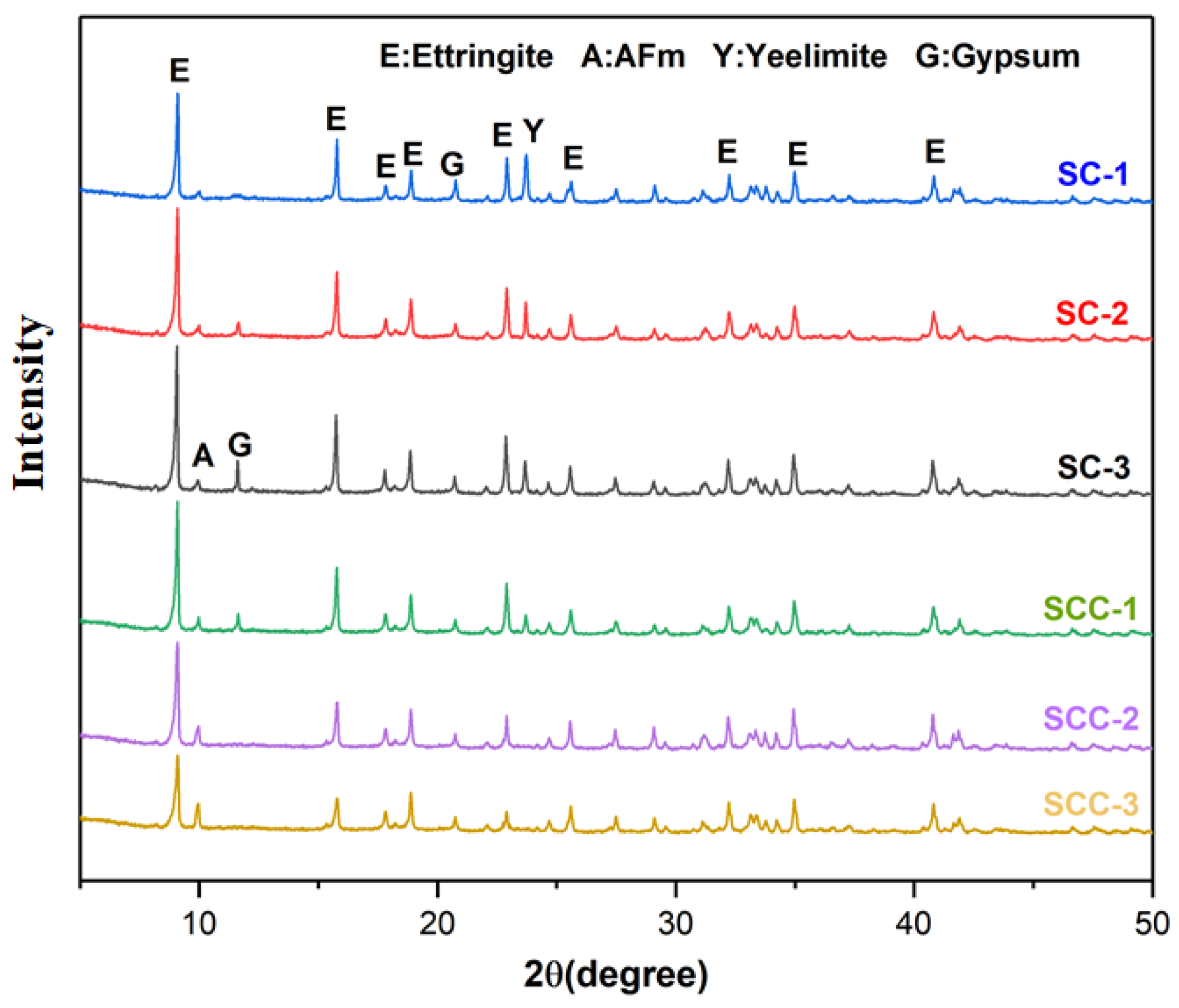
3.3. X-ray Diffraction Analysis
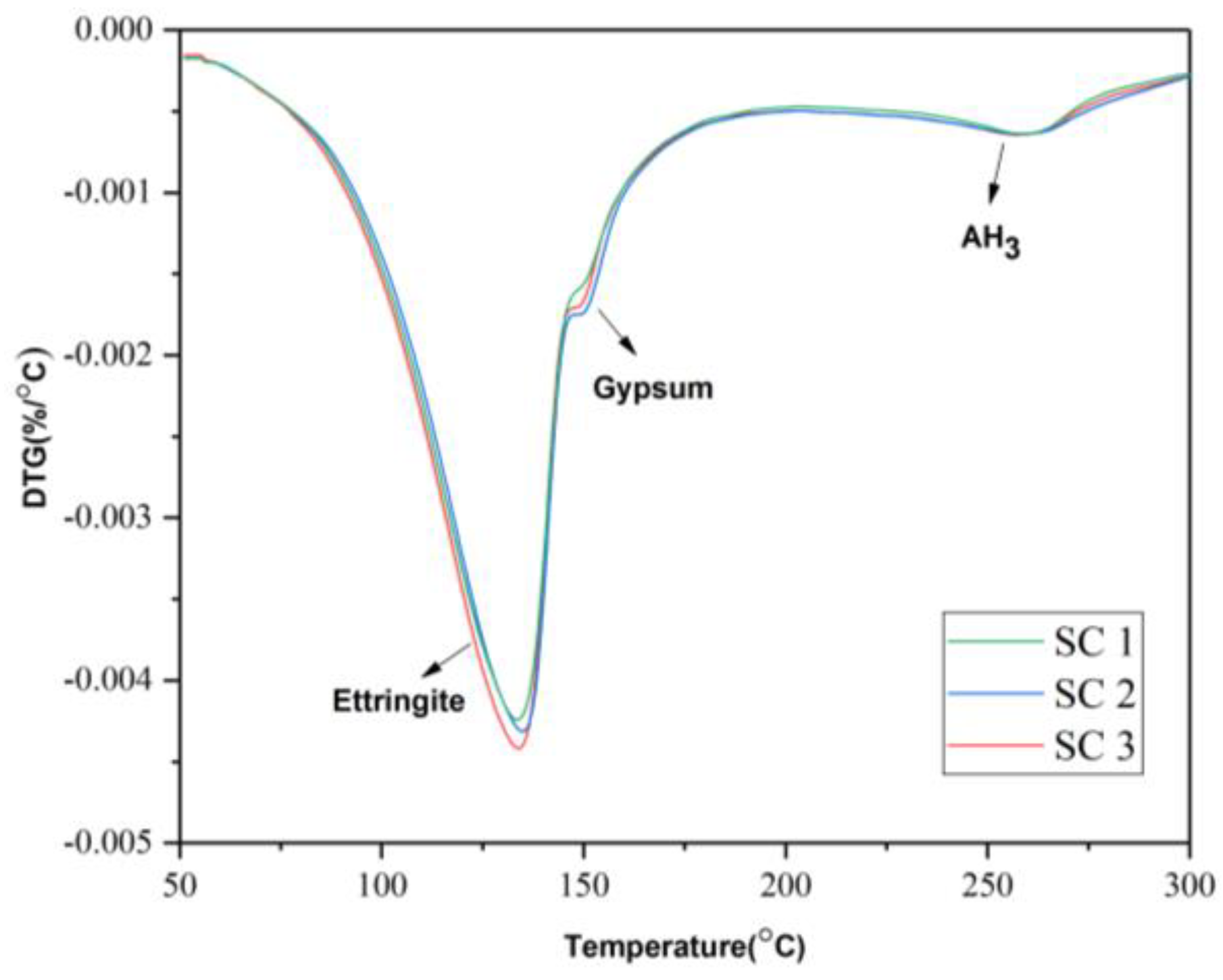
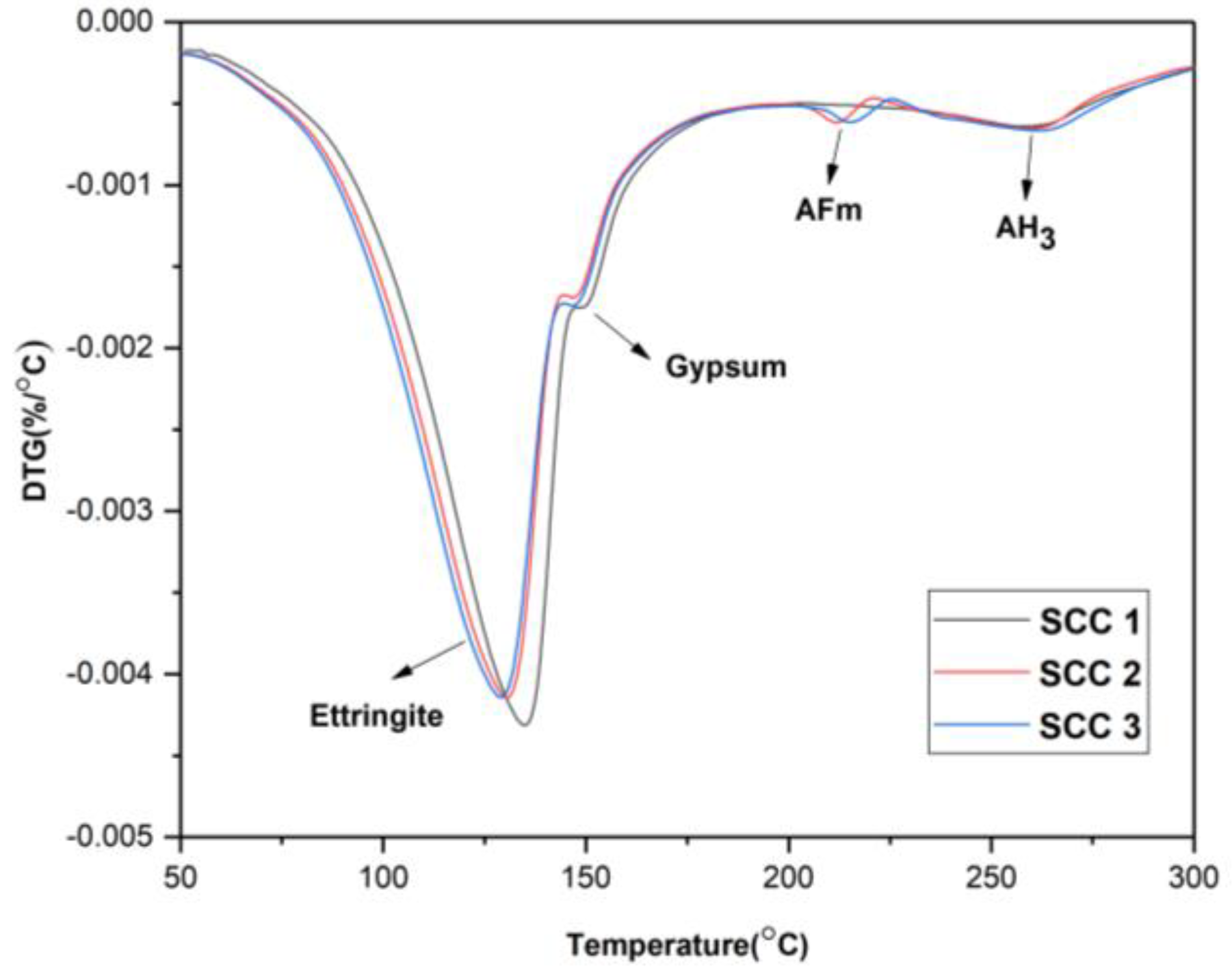
3.4. Thermogravimetric Analysis
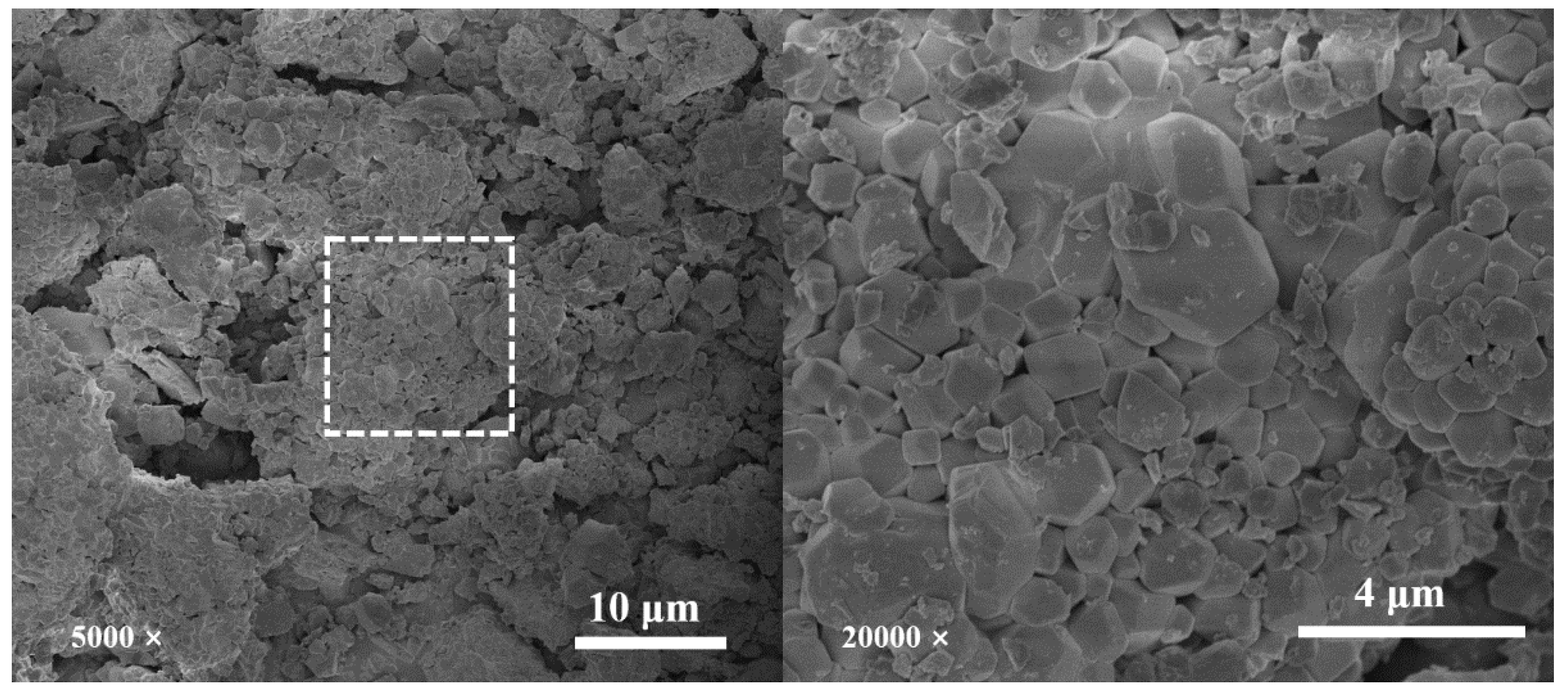
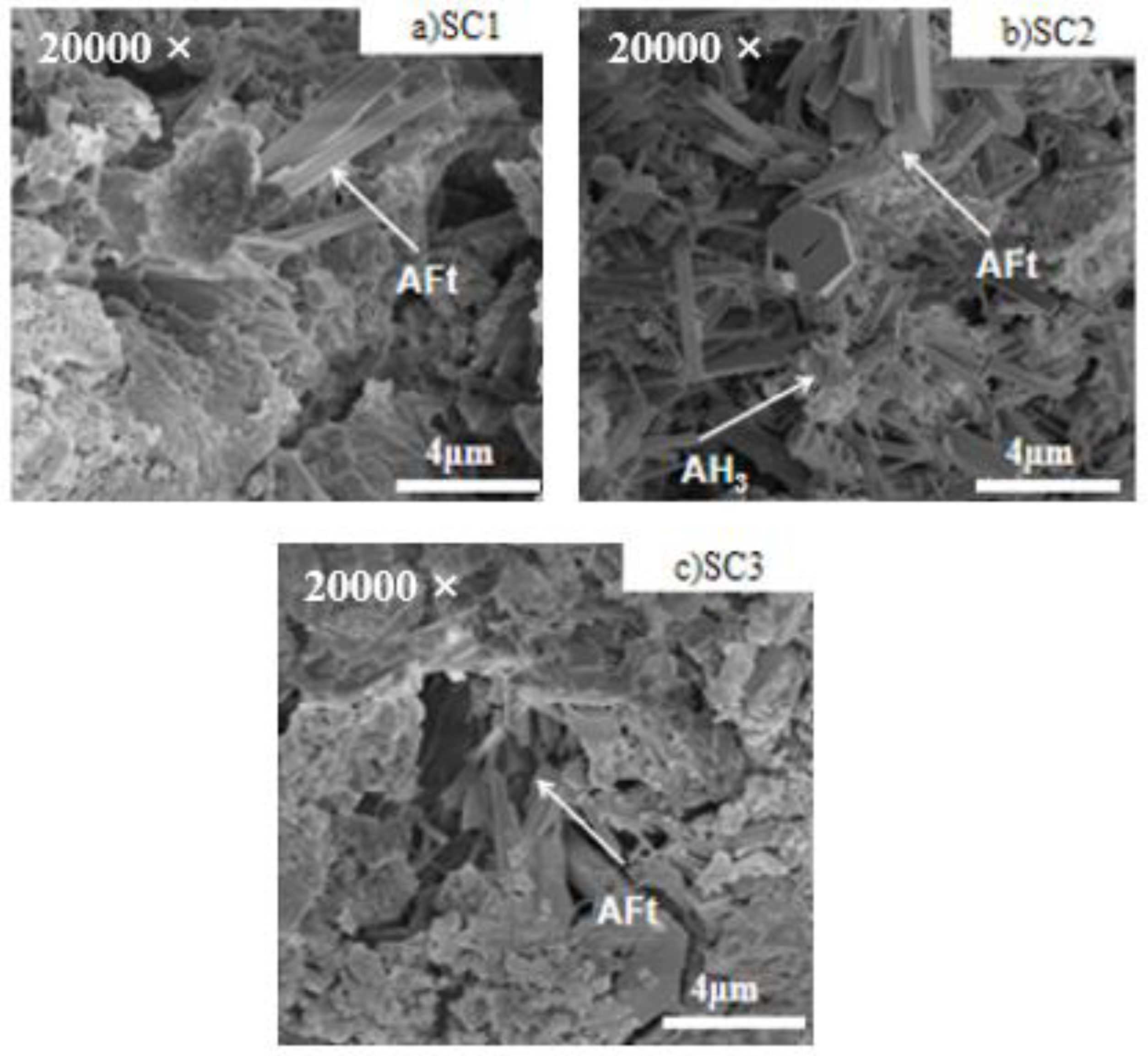
3.5. Microstructural Analysis
3.6. Porosity Analysis
4. Conclusions
- (1)
- When C$H2 was added to the cementitious material, the early strength of the cement increased as the amount of C$H2 increased. The optimum admixture of C$H2/C4A3$ was 1.5 mol. Adding CH decreased the cement’s compressive strength and increased the paste’s pH, which facilitated the development of the later strength. The optimum dose of CH/C4A3$ was 0.5 mol.
- (2)
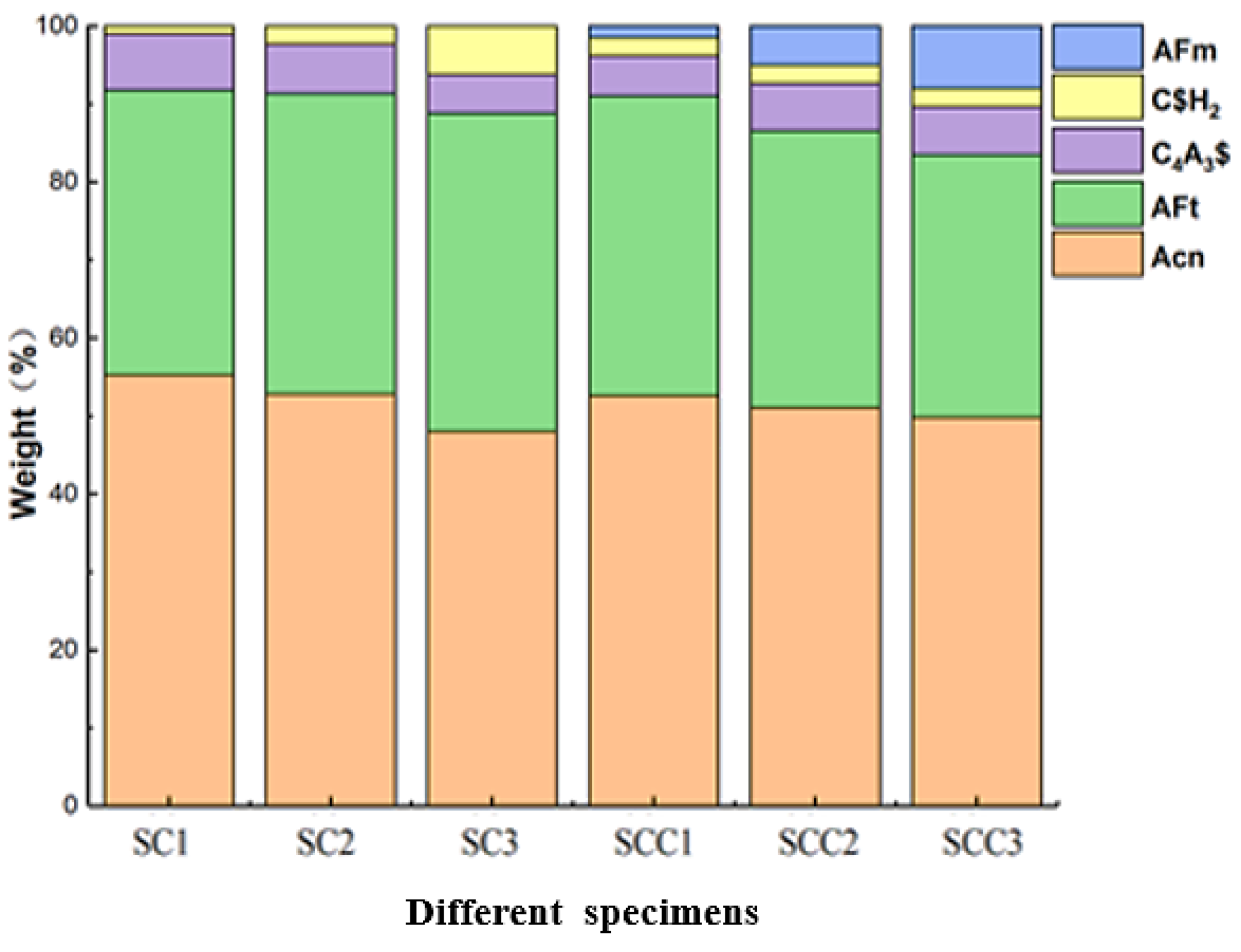
- The addition of C$H2 promoted the early hydration of C4A3$ and formed more AFt. The amount of C$H2 incorporation and the AFt content were positively correlated. The incorporation of CH changed the hydration process. It inhibited the early hydration of C4A3$, and the hydration products appeared as AFm. With the increase in CH, the amount of AFt production became less, and the AFm content increased.
- (3)
- The addition of C$H2 influenced the microstructure of the matrix. With the increase in C$H2, it was observed that the AFt crystals developed well, and the microscopic morphology changed from needle-rod to columnar or block. Excessive C$H2 caused AFt crystals to be stacked, not tightly cemented with AH3. At the same time, the matrix contained unreacted C$H2, leading to an increase in pore size and porosity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rashad, A.M. Phosphogypsum as a construction material. J. Clean. Prod. 2017, 166, 732–743. [Google Scholar] [CrossRef]
- Islam, G.S.; Chowdhury, F.H.; Raihan, M.T.; Amit, S.K.S.; Islam, M.R. Effect of Phosphogypsum on the Properties of Portland Cement. Procedia Eng. 2017, 171, 744–751. [Google Scholar] [CrossRef]
- Cuadri, A.; Navarro, F.; García-Morales, M.; Bolivar, J.P. Valorization of phosphogypsum waste as asphaltic bitumen modifier. J. Hazard. Mater. 2014, 279, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Rashad, A.M. Potential use of phosphogypsum in alkali-activated fly ash under the effects of elevated temperatures and thermal shock cycles. J. Clean. Prod. 2015, 87, 717–725. [Google Scholar] [CrossRef]
- Pérez-López, R.; Macías, F.; Cánovas, C.R.; Sarmiento, A.M.; Pérez-Moreno, S.M. Pollutant flows from a phosphogypsum disposal area to an estuarine environment: An insight from geochemical signatures. Sci. Total Environ. 2016, 553, 42–51. [Google Scholar] [CrossRef]
- Ma, B.; Lu, W.; Su, Y.; Li, Y.; Gao, C.; He, X. Synthesis of α-hemihydrate gypsum from cleaner phosphogypsum. J. Clean. Prod. 2018, 195, 396–405. [Google Scholar] [CrossRef]
- Papaslioti, E.-M.; Pérez-López, R.; Parviainen, A.; Sarmiento, A.M.; Nieto, J.M.; Marchesi, C.; Delgado-Huertas, A.; Garrido, C.J. Effects of seawater mixing on the mobility of trace elements in acid phosphogypsum leachates. Mar. Pollut. Bull. 2018, 127, 695–703. [Google Scholar] [CrossRef]
- Cánovas, C.R.; Macías, F.; López, R.P.; Nieto, J.M. Mobility of rare earth elements, yttrium and scandium from a phosphogypsum stack: Environmental and economic implications. Sci. Total Environ. 2018, 618, 847–857. [Google Scholar] [CrossRef]
- Papastefanou, C.; Stoulos, S.; Ioannidou, A.; Manolopoulou, M. The application of phosphogypsum in agriculture and the radiological impact. J. Environ. Radioact. 2006, 89, 188–198. [Google Scholar] [CrossRef]
- Shukla, V.; Ramachandran, T.; Chinnaesakki, S.; Sartandel, S.; Shanbhag, A. Radiological impact of utilization of phosphogypsum and fly ash in building construction in India. Int. Congr. Ser. 2005, 1276, 339–340. [Google Scholar] [CrossRef]
- Pérez-López, R.; Nieto, J.M.; López-Coto, I.; Aguado, J.L.; Bolívar, J.P.; Santisteban, M. Dynamics of contaminants in phosphogypsum of the fertilizer industry of Huelva (SW Spain): From phosphate rock ore to the environment. Appl. Geochem. 2010, 25, 705–715. [Google Scholar] [CrossRef]
- Kadirova, Z.; Hojamberdiev, M.; Bo, L.; Hojiyev, R.; Okada, K. Ion uptake properties of low-cost inorganic sorption materials in the CaO–Al2O3–SiO2 system prepared from phosphogypsum and kaolin. J. Clean. Prod. 2014, 83, 483–490. [Google Scholar] [CrossRef]
- Cui, K.; Chang, J.; Feo, L.; Chow, C.L.; Lau, D. Developments and Applications of Carbon Nanotube Reinforced Cement-Based Composites as Functional Building Materials. Front. Mater. 2022, 9. [Google Scholar] [CrossRef]
- Wu, S.; Yao, Y.; Yao, X.; Ren, C.; Li, J.; Xu, D.; Wang, W. Co-preparation of calcium sulfoaluminate cement and sulfuric acid through mass utilization of industrial by-product gypsum. J. Clean. Prod. 2020, 265, 121801. [Google Scholar] [CrossRef]
- Rosales, J.; Pérez, S.M.; Cabrera, M.; Gázquez, M.J.; Bolivar, J.P.; de Brito, J.; Agrela, F. Treated phosphogypsum as an alternative set regulator and mineral addition in cement production. J. Clean. Prod. 2020, 244, 118752. [Google Scholar] [CrossRef]
- Nizevičienė, D.; Vaičiukynienė, D.; Vaitkevičius, V.; Rudžionisb, Ž. Effects of waste fluid catalytic cracking on the properties of semi-hydrate phosphogypsum. J. Clean. Prod. 2016, 137, 150–156. [Google Scholar] [CrossRef]
- Li, B.; Li, L.; Chen, X.; Ma, Y.; Zhou, M. Modification of phosphogypsum using circulating fluidized bed fly ash and carbide slag for use as cement retarder. Constr. Build. Mater. 2022, 338, 127630. [Google Scholar] [CrossRef]
- Xu, W.; Li, Q.; Haruna, S. The Effect of Calcium Formate, Sodium Sulfate, and Cement Clinker on Engineering Properties of Fly Ash-Based Cemented Tailings Backfill. Adv. Mater. Sci. Eng. 2019, 2019, 5370360. [Google Scholar] [CrossRef]
- Isteri, V.; Ohenoja, K.; Hanein, T.; Kinoshita, H.; Kletti, H.; Rößler, C.; Tanskanen, P.; Illikainen, M.; Fabritius, T. Ferritic calcium sulfoaluminate belite cement from metallurgical industry residues and phosphogypsum: Clinker production, scale-up, and microstructural characterisation. Cem. Concr. Res. 2022, 154, 106715. [Google Scholar] [CrossRef]
- Shen, Y.; Qian, J.; Huang, Y.; Yang, D. Synthesis of belite sulfoaluminate-ternesite cements with phosphogypsum. Cem. Concr. Compos. 2015, 63, 67–75. [Google Scholar] [CrossRef]
- Huang, Y.; Qian, J.; Liu, C.; Liu, N.; Shen, Y.; Ma, Y.; Sun, H.; Fan, Y. Influence of phosphorus impurities on the performances of calcium sulfoaluminate cement. Constr. Build. Mater. 2017, 149, 37–44. [Google Scholar] [CrossRef]
- Huang, Y.; Qian, J.; Kang, X.; Yu, J.; Fan, Y.; Dang, Y.; Zhang, W.; Wang, S. Belite-calcium sulfoaluminate cement prepared with phosphogypsum: Influence of P2O5 and F on the clinker formation and cement performances. Constr. Build. Mater. 2019, 203, 432–442. [Google Scholar] [CrossRef]
- Kuryatnyk, T.; da Luz, C.A.; Ambroise, J.; Pera, J. Valorization of phosphogypsum as hydraulic binder. J. Hazard. Mater. 2008, 160, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Madan, C.S.; Panchapakesan, K.; Reddy, P.V.A.; Joanna, P.S.; Rooby, J.; Gurupatham, B.G.A.; Roy, K. Influence on the Flexural Behaviour of High-Volume Fly-Ash-Based Concrete Slab Reinforced with Sustainable Glass-Fibre-Reinforced Polymer Sheets. J. Compos. Sci. 2022, 6, 169. [Google Scholar] [CrossRef]
- Sánchez-Herrero, M.J.; Fernández-Jiménez, A.; Palomo, A. C4A3Š hydration in different alkaline media. Cem. Concr. Res. 2013, 46, 41–49. [Google Scholar] [CrossRef]
- Winnefeld, F.; Barlag, S. Calorimetric and thermogravimetric study on the influence of calcium sulfate on the hydration of ye’elimite. J. Therm. Anal. Calorim. 2010, 101, 949–957. [Google Scholar] [CrossRef]
- Hargis, C.W.; Kirchheim, A.P.; Monteiro, P.J.; Gartner, E.M. Early age hydration of calcium sulfoaluminate (synthetic ye’elimite, C4A3$) in the presence of gypsum and varying amounts of calcium hydroxide. Cem. Concr. Res. 2013, 48, 105–115. [Google Scholar] [CrossRef]
- Huang, Y.; Qian, J.; Lu, L.; Zhang, W.; Wang, S.; Wang, W.; Cheng, X. Phosphogypsum as a component of calcium sulfoaluminate cement: Hazardous elements immobilization, radioactivity and performances. J. Clean. Prod. 2020, 248, 119287. [Google Scholar] [CrossRef]
- Cui, K.; Lau, D.; Zhang, Y.; Chang, J. Mechanical properties and mechanism of nano-CaCO3 enhanced sulphoaluminate cement-based reactive powder concrete. Constr. Build. Mater. 2021, 309, 125099. [Google Scholar] [CrossRef]
- Cui, K.; Chang, J. Hydration, reinforcing mechanism, and macro performance of multi-layer graphene-modified cement composites. J. Build. Eng. 2022, 57, 104880. [Google Scholar] [CrossRef]
- Le Saoût, G.; Kocaba, V.; Scrivener, K. Application of the Rietveld method to the analysis of anhydrous cement. Cem. Concr. Res. 2011, 41, 133–148. [Google Scholar] [CrossRef]
- Hargis, C.W.; Telesca, A.; Monteiro, P.J.M. Calcium sulfoaluminate (Ye’elimite) hydration in the presence of gypsum, calcite, and vaterite. Cem. Concr. Res. 2014, 65, 15–20. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Kang, F. Real-time monitoring of humidity inside concrete structures utilizing embedded smart aggregates. Constr. Build. Mater. 2022, 331, 127317. [Google Scholar] [CrossRef]
- Yu, C.; Cui, C.; Zhao, J.; Zheng, J. A novel approach to utilizing dredged materials at the laboratory scale. Constr. Build. Mater. 2021, 313, 125568. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Boehm-Courjault, E.; Zajac, M.; Ben Haha, M.; Scrivener, K. Hydration reactions and stages of clinker composed mainly of stoichiometric ye’elimite. Cem. Concr. Res. 2019, 116, 120–133. [Google Scholar] [CrossRef]
- Cui, K.; Liang, K.; Chang, J.; Lau, D. Investigation of the macro performance, mechanism, and durability of multiscale steel fiber reinforced low-carbon ecological UHPC. Constr. Build. Mater. 2022, 327, 126921. [Google Scholar] [CrossRef]
- Xue, W.; Liu, W.; Gao, C.; Fan, H.; Zhang, H. Experimental study of permeability evolution in modified desulfurization gypsum under complete stress–strain process. J. Clean. Prod. 2022, 359, 132185. [Google Scholar] [CrossRef]
- Zhang, L.; Qian, X.; Yu, C.; Fang, M.; Qian, K.; Lai, J. Influence of evaporation rate on pore size distribution, water loss, and early-age drying shrinkage of cement paste after the initial setting. Constr. Build. Mater. 2019, 226, 299–306. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Kang, F.; Zhang, J. Monitoring and evaluation of the repair quality of concrete cracks using piezoelectric smart aggregates. Constr. Build. Mater. 2022, 317, 125775. [Google Scholar] [CrossRef]
- Xue, W.; Fan, H.; Liu, X.; Shen, L. Effects of early-age temperature and salt ion corrosion on the macroproperty deterioration of concrete and corresponding micromechanism. Arch. Civ. Mech. Eng. 2022, 22, 1–16. [Google Scholar] [CrossRef]
- Xue, W.; Liu, X.; Jing, W.; Yao, Z.; Gao, C.; Li, H. Experimental study and mechanism analysis of permeability sensitivity of mechanically damaged concrete to confining pressure. Cem. Concr. Res. 2020, 134, 106073. [Google Scholar] [CrossRef]
- Li, J.; Chang, J.; Wang, T.; Zeng, T.; Li, J.; Zhang, J. Effects of phosphogypsum on hydration properties and strength of calcium aluminate cement. Constr. Build. Mater. 2022, 347, 128398. [Google Scholar] [CrossRef]

| Materials | SO3 | CaO | SiO2 | Al2O3 | Fe2O3 | TiO2 | MgO | P2O5 | F |
|---|---|---|---|---|---|---|---|---|---|
| PG | 53.04 | 41.48 | 3.32 | 0.24 | 0.40 | 0.07 | - | 0.81 | 0.64 |
| Limestone | 0.24 | 89.68 | 3.75 | 0.94 | 0.77 | - | 4.62 | - | - |
| Bauxite | 6.25 | 0.47 | 9.82 | 72.72 | 5.19 | 5.33 | 0.22 | - | - |
| Sample | Limestone | Phosphogypsum | Bauxite |
|---|---|---|---|
| S | 39.44 | 22.67 | 37.89 |
| Sample | Phase Content (wt%) | Rwp (%) | ||||
|---|---|---|---|---|---|---|
| C4A3$ | C2S | C4AF | C$ | C2AS | ||
| S | 73.00 | 13.54 | 5.42 | 5.26 | 2.78 | 12.76 |
| Sample | The Molar Ratio of C$H2 to C4A3$ | The Molar Ratio of CH to C4A3$ | wt% | ||
|---|---|---|---|---|---|
| Clinker/C4A3$ | C$H2 | CH | |||
| SC1 | 1.0 | 0 | 82.10/59.93 | 17.90 | 0 |
| SC2 | 1.5 | 0 | 76.90/56.14 | 23.10 | 0 |
| SC3 | 2.0 | 0 | 70.90/51.76 | 29.10 | 0 |
| SCC1 | 1.5 | 0.25 | 103.97/75.90 | 22.70 | 1.40 |
| SCC2 | 1.5 | 0.50 | 102.6/74.90 | 22.48 | 2.62 |
| SCC3 | 1.5 | 0.75 | 101.34/73.98 | 22.19 | 3.83 |
| Pore Type | Aperture/nm | Causes |
|---|---|---|
| Gel pore | 1–102 | Pores of gel-like substances in hydration products |
| Capillary pore | 102–104 | Pores between the larger size crystals in the hydration product |
| Introduced pore | 104–106 | Pores introduced by air-entraining agents or aluminium powder during hydration |
| Forming pores | 106–107 | Pore space due to the poor pounding of the paste |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Chang, J.; Zhang, P.; Wang, T. Effects of C$H2 and CH on Strength and Hydration of Calcium Sulphoaluminate Cement Prepared from Phosphogypsum. Buildings 2022, 12, 1692. https://doi.org/10.3390/buildings12101692
Zhang J, Chang J, Zhang P, Wang T. Effects of C$H2 and CH on Strength and Hydration of Calcium Sulphoaluminate Cement Prepared from Phosphogypsum. Buildings. 2022; 12(10):1692. https://doi.org/10.3390/buildings12101692
Chicago/Turabian StyleZhang, Jixin, Jun Chang, Ping Zhang, and Tong Wang. 2022. "Effects of C$H2 and CH on Strength and Hydration of Calcium Sulphoaluminate Cement Prepared from Phosphogypsum" Buildings 12, no. 10: 1692. https://doi.org/10.3390/buildings12101692
APA StyleZhang, J., Chang, J., Zhang, P., & Wang, T. (2022). Effects of C$H2 and CH on Strength and Hydration of Calcium Sulphoaluminate Cement Prepared from Phosphogypsum. Buildings, 12(10), 1692. https://doi.org/10.3390/buildings12101692







