1. Introduction
The state of play in technology and the current requirements on the production of iron and steel call for up-to-date automation. It is also important for addressing new technological and research challenges in the field of processing ferriferous fine-grained materials. These processes require mathematical and physical modelling, a sophisticated approach, and up-to-date facilities. The monitoring, automation, and utilization of modern analytical tools is very important. It pertains to the sintering process as well, where there are many manual operations dependant on the human factor. In operating conditions, the sintering process takes place continuously on sintering belts. It is a non-linear technological processes affected by a high number of factors that are difficult to evaluate. Therefore, the individual parameters (mainly the composition of input materials, amount of fuel, temperatures in sintered layer, and pressures of sucked air) of the sintering process are mostly examined and analysed in laboratory conditions. Results from these experiments can provide verifiable conclusions for the industry [
1]. In the world, the production of agglomerate in laboratory conditions is carried out on static sintering equipment. It is necessary to state that the conditions of sintering on laboratory sintering equipment slightly differ from the conditions of sintering on a sintering belt both in terms of temperatures and gas flow. The possible application of the models in practice requires the mathematical prediction of the results of laboratory experiments using regression dependences [
2]. The creation of models, simulations, and predictions is very important today. For these models we can define physical‑chemical and thermal processes that alter the structure and composition of input sintering raw materials. The result is a blast furnace agglomerate with the required properties produced efficiently and environmentally.
In Slovakia, a laboratory sintering pan (LSP) was used and was fully equipped with measuring devices and analysers to sufficiently simulate the conditions on the sintering belt in relation to temperature conditions in the sintered layer, production of CO, CO
2, NO
x, SO
x, and particulate matter (PM) in the sintering process, as well as to the quality of the agglomerate [
3].
The laboratory sintering pan was located at the Institute of Metallurgy, Faculty of Materials, Metallurgy and Recycling, Technical University of Košice (UMET FMMR TUKE) (see
Figure 1). Thermodynamic and mathematical models focused primarily on the mass and thermal balance of the agglomerate production are also a part of this physical model of sintering [
3].
The sintering process takes place in a heterogeneous system of the gas–liquid–solid phase, while the gas phase ensures the fuel combustion, heat transfer, and oxidation-reduction processes. The process of sintering does not occur simultaneously across the layer. It occurs gradually in a narrow zone (the so-called combustion zone), which moves towards the grate. The heat in each elementary layer of the charge during sintering is given by the exothermic effect of burning fuel and this process is very important for the creation of sintering melt [
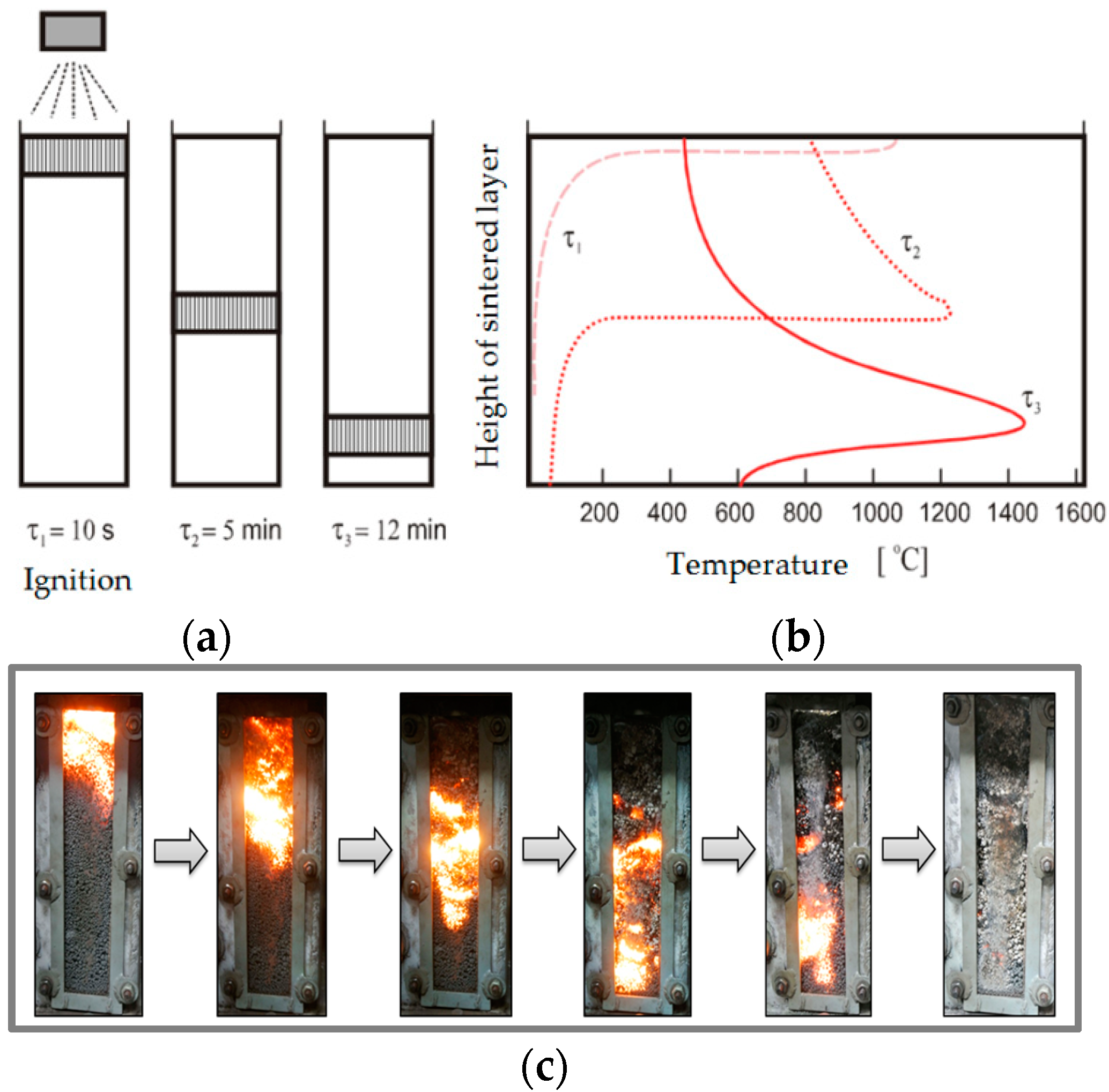
4]. Due to the progress of the combustion zone and the major heat transfer by convection, heat accumulation occurs in the elementary layers towards the sintering grates. The static sintering of the sintering layer in
Figure 2 shows the temperature distribution in the static sintered layer. After the ignition of an agglocharge, the combustion zone gradually moves downwards to the lower layers of the charge, increasing the maximum temperatures in the sintered layer [
5].
The agglomeration process has been modeled worldwide in several studies. Zhou and colleagues show the predicted melting and solidification heats and melt fraction as functions of time [
6]. These processes are very important for forming the final agglomerate structure. Young developed a 1-D mathematical model which studied in detail the heat and mass transfer processes in a sintering bed [
7]. Yamaoka and Kawaguchi [
8] discussed 3D variations on sinter properties produced on a pot apparatus experimental facility and presented a mathematical model based on the transport phenomena to simulate the experimental conditions in the sintering process. The melt formation temperature, liquid phase content, and liquid viscosity under different sintering temperatures were simulated to study the influence of these thermodynamic melt formation characteristics on the liquid phase fluidity of iron ore in the sintering process [
9]. Most modelling processes worldwide deal with heat generation and transfer, melt formation, and sinter cooling.
In the iron and steel production processes, it is very important to reduce fuel [
10]. The minimization of fuel consumption in the sintering process is ensured by the mathematical regression model aimed at calculating the mass and thermal balance of the agglomerate production [
6,
8]. The mass and thermal balance is based on measurements. However, certain assumptions need to be considered, as the sintering process takes place not only in the thermodynamic but also in the kinetic mode [
5]:
With normal fuel consumption (up to 6%), CO
2 and CO are formed in the combustion of carbon at the ratio of CO
2/CO = 4. With the high fuel consumption (above 7%), the CO
2/CO ratio is reduced to 2.5–3. The amount of heat produced in the combustion of carbon is:
The heat of agglomeration charge ignition reaches the value from 14,654 up to 16,747 kJ per 100 kg of the agglomerate.
A total amount of 95% of the organic and sulphide sulphur is removed. A total ammount of 60% of the sulphate sulphur is removed. The thermal balance for the removal of sulphur is:
The amount of heat used to evaporate the moisture is:
The following volumes are used to decompose carbonates, i.e., to remove 1 kg of CO2:
The amount of heat for the thermal dissociation of oxides is 18,288 kJ/kg of O2 (up to the final dissociation of Fe2O3 to FeO).
The heat of the hot final agglomerate varies between 33,494 and 60,242 kJ per 100 kg of agglomerate.
The creation of a computational model of the sintering process mass balance is based on the knowledge of processes taking place in the sintered layer, as well as the principles of conservation of mass and thermodynamic stability of compounds [
11,
12]. The effects of the composition of the sintering mixture on the formation of the melt phase under specified sintering conditions were modelled by Chen [
11]. The volume and properties of the melt formed during sintering depend heavily on temperature, which determines the fuel requirement for sintering.
It follows that the combustion of solid fuel represents the greatest thermal effect and is used mainly for the dissociation reactions and constitutes the heat of agglomerate and flue gas [
5].
Mathematical models are the basis of computational and theoretical methods of determining the parameters of sintering equipment, their operating modes, and predicting the properties of the sintering product [
12,
13]. The main criterion of the agglomeration process is that the quality produced of the agglomerate is with high reducibility while maintaining the ecological nature of the production [
14]. A thermodynamic analysis is employed in the creation of mathematical models to define the chemical processes and study the mass and thermal balance [
11].
Thermodynamic calculations are thus essential when determining the characteristics of a technological process and they enable one to clarify the formation of the major sintering products. By changing the basic condition of the thermodynamic system, it is feasible to find the optimum operating conditions of the sintering process and minimise the consumption of raw materials and energy.
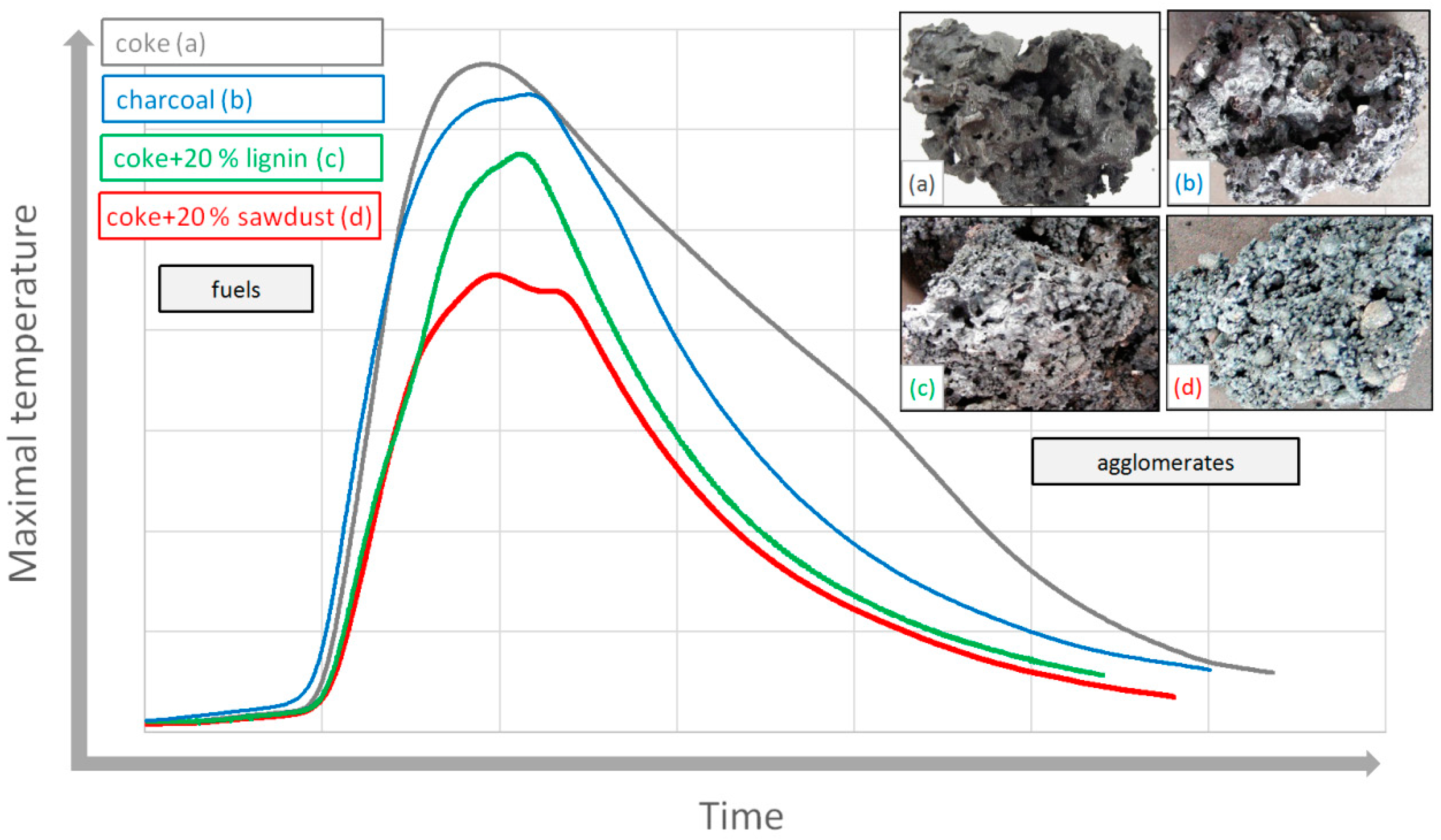
Table 1 shows the thermal balance of the sintering process, it follows that the greatest thermal effect to sintering layer is due to fuel combustion. For the production of agglomerate, energy from a coke can be technologically replaceable with biomass [
15]. The use of biomass within the high-temperature sintering process contributes to the overall lower production of emissions compared to coke.
The literature review shows that there are several separate models for specific monitored parameters worldwide, but they do not run online. Therefore, the development of a more comprehensive model would represent an innovative solution in the field of sintering process automation. This paper is aimed at describing the mathematical-thermodynamic model that could be used to predict the thermodynamic conditions of carbonaceous fuel combustion (including biomass) in the sintering process.
2. Materials and Methods
This paper proposes an application of the mathematical model and a physical simulation of the sintering process in laboratory conditions. For mathematical modelling the basic chemical reactions with standard Gibbs energy and mass and thermal balance were calculated. Thermodynamic data was obtained from the software HSC Chemistry [
16] (HSC means H—enthalpy, S—entropy, C—heat capacity). HSC Chemistry offers powerful calculation methods for studying the effects of different variables on the chemical system at equilibrium. The aim is to get the simplest approach (using this software to calculate equilibrium) which allows one to predict the output parameters (amounts, chemistry, mineralogical composition, and total heat) based on the initial composition analysis. Thermochemical calculations are based on enthalpy H, entropy S, heat capacity Cp or Gibbs energy G values for chemical species. They can all be mathematically derived from experimental observations. The following text gives a brief and simplified but illustrative idea of thermochemical quantities and methods.
The absolute values of enthalpy
H of substances cannot be measured, but enthalpy differences (d
H) between two temperatures (d
T) can be determined with a calorimeter. Heat capacity
Cp at constant pressure
p (specific heat) can be calculated from the data using Equation (10):
Equation (10) allows the calculation of enthalpy (11):
where Δ
Hf (298.15 K) is the enthalpy of formation at 298.15 K and Δ
Htr is the enthalpy of transformation of the species. The enthalpy of compounds also includes their enthalpy of formation Δ
Hf from elements.
The thermodynamic enthalpy and Gibbs energy functions for chemical reactions used in the model are calculated as the difference between the products and reactants using Equations (12) and (13):
where the following abbreviations have been used:
= the enthalpy change of reaction,
= the Gibbs energy change of reaction,
= the enthalpy change of the species i (product or reactant) in the temperature of T (K),
= the Gibbs energy change of the species i (product or reactant) in the temperature of T (K),
= stoichiometric coefficient of a species in reaction.
The composition of the equilibrium mixture is expressed by the initial composition and the extent of reaction (or degree of conversion), Equation (14):
where the following abbreviations have been used:
= number of moles of the substance in the system before the reaction,
= number of moles of substance i involved in the reaction, (ζ + for products, − for reactants),
= number of moles of the substance in equilibrium.
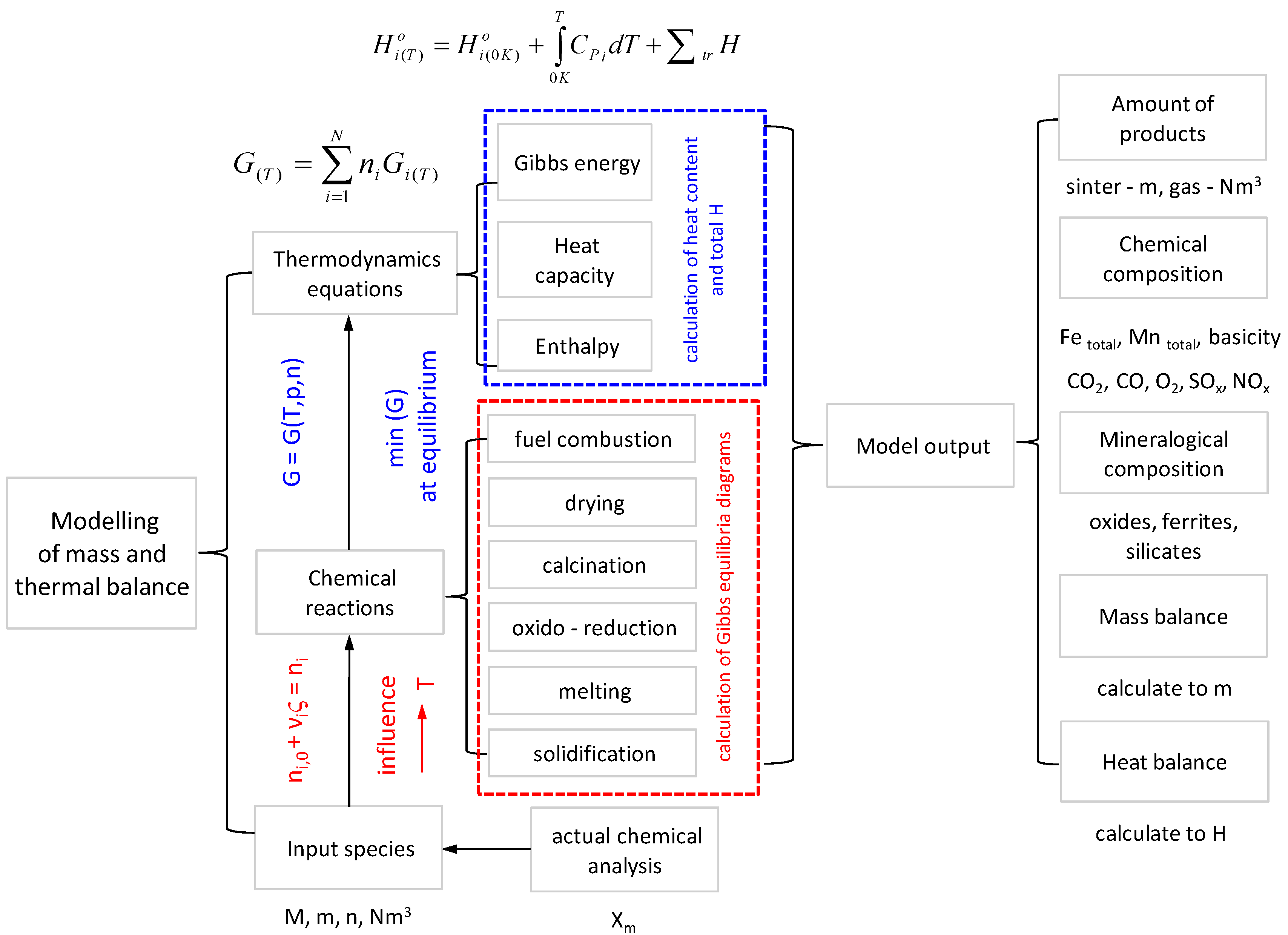
The thermodynamic equations from the program HSC Chemistry were used to obtain the necessary data for the respective chemical reactions taking place in the sintering process. These include fuel combustion, drying, calcination, oxido-reduction reactions, etc., (see
Figure 3). All the thermochemical data required in HSC and its modules can be calculated from the basic data in its databases using Equations (10)–(14). In order to incorporate the output mineralogical composition of the agglomerate into the program, Gibbs equilibrium diagrams had to be calculated first and suitable phases for the output were predicted on the basis of stoichiometric calculations. The first step was to specify the chemical reaction system, with its phases and species, and give the amounts of raw materials (M, m, n, Nm
3). The program calculates the amounts of products at equilibrium in isothermal and isobaric conditions. The basic idea of the heat balance module is to specify the IN and OUT species, temperatures, and amounts and the Heat Balance module automatically calculates the heat and material balances [
16]. The Heat Content is used to describe the energy which may be released when the compound is cooled down from the given temperature to 298.15 K. The enthalpies given in the Total H contain the values of the Heat Content as well as the heat of formation reactions. These values are used to calculate heat balances. In order to calculate a heat balance, one must first convert the (elemental-X
m) chemical analysis of the raw materials and the products into input and output substances (species). Sometimes this step is the most difficult, so one may choose to carry out this procedure using the HSC Species Converter module. It is important to check the element balance by selecting Calculate/Element Balance, in order to avoid incorrect material and heat balances [
16].
Figure 3 shows the global method with modelling the mass and thermal balance of the sintering process.
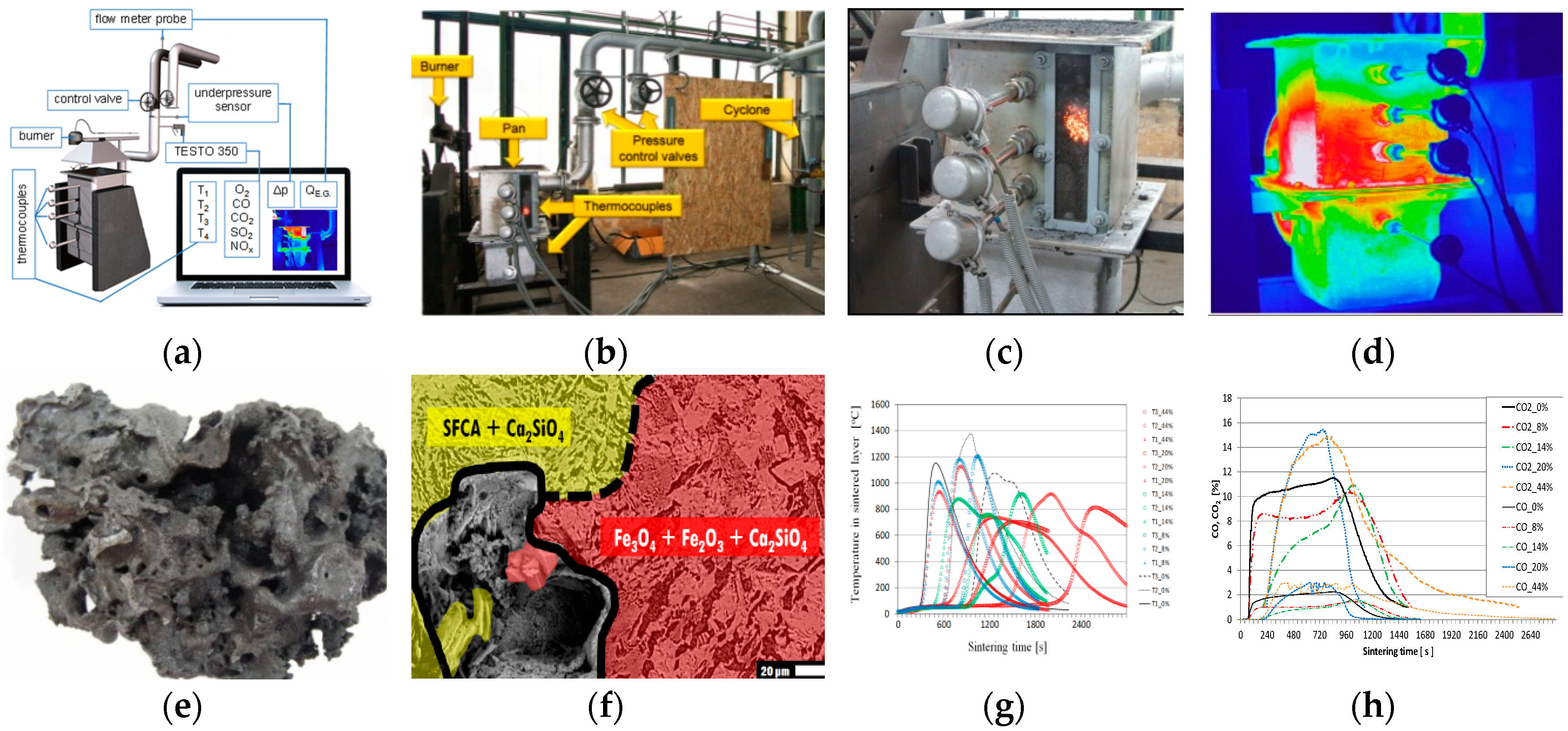
Using the thermodynamic program HSC 9 (see
Figure 4b), all outputs were calculated according to the created models. The experiments were carried out in a laboratory sintering pan (LSP), which simulates the conditions on an agglomeration belt, as well as the quality of the agglomerate (see
Figure 1a and
Figure 4a). For the high temperature range in the sintered layer, three thermocouples of the PtRh10-Pt type were used. The flue gas temperature was read at two levels by the NiCr-Ni type thermocouple. The chemical composition and temperature of the flue gas were analysed by the TESTO 350 device. The differential pressure was measured by the Anubar type probe. Laboratory experiments on the laboratory sintering pan were performed using ferriferous raw materials-aggloore from the Ukraine (content of Fe
TOT = 60.39%) and concentrate from the Ukraine (content of Fe
TOT = 67.95%) (see
Table 2). The concentrate/iron ore ratio in the mixtures was 1:1. In the agglomeration process, standard coke breeze with a grain size <3 mm was used as fuel (see
Table 3). Biomass was a technical hydrolyzed lignin.
Lignin is a by-product of ethanol production by the distillation of wood. The lignin was subjected to an experimental study for its use as a partial replacement of the agglomeration coke for the production of an iron ore agglomerate. Details about lignin are presented in the literature [
17]. The other mixture components (dolomite, calcite, and lime) had a commercial standard chemical composition. These inputs were included in prepared agglomeration mixtures with basicity within the interval of 1.5–1.9. The produced Fe agglomerates had Fe
TOT content within the interval of about 48–53 wt%. Chemical analysis of the samples was determined using XRF spectrometer ARL 9900S (Thermo Fisher Scientific, Waltham, MA, USA). The minerals species were detected using XRD spectrometer SEIFERT XRD 3003/PTS (General Electric Company, Boston, MA, USA). Diffraction records were evaluated by DIFFRAC.EVA (Search-Match, KARLSRUHE & VIERSEN, Bruker, Billerica, MA, USA) with PDF2 and TOPAS software (version 4, Bruker, Billerica, MA, USA) using the Rietveld method.