Electropolishing of Stainless Steel in Laboratory and Industrial Scale
Abstract
1. Introduction
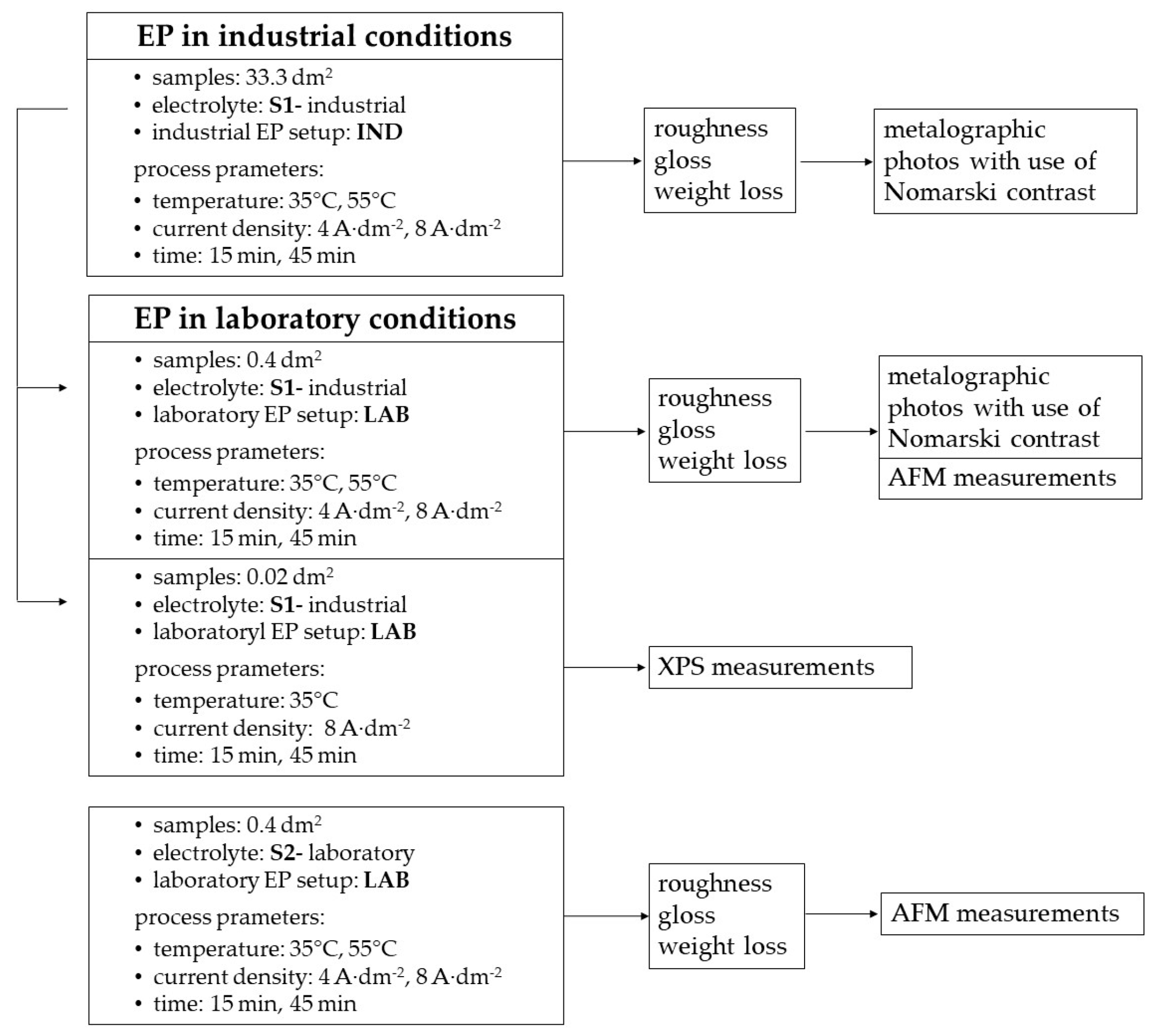
2. Materials and Methods
2.1. Experimental Setup
2.2. Materials
2.3. Roughness Tests
2.4. Gloss Tests
2.5. Atomic Force Microscopy
2.6. X-Ray Photoelectron Spectroscopy
3. Results
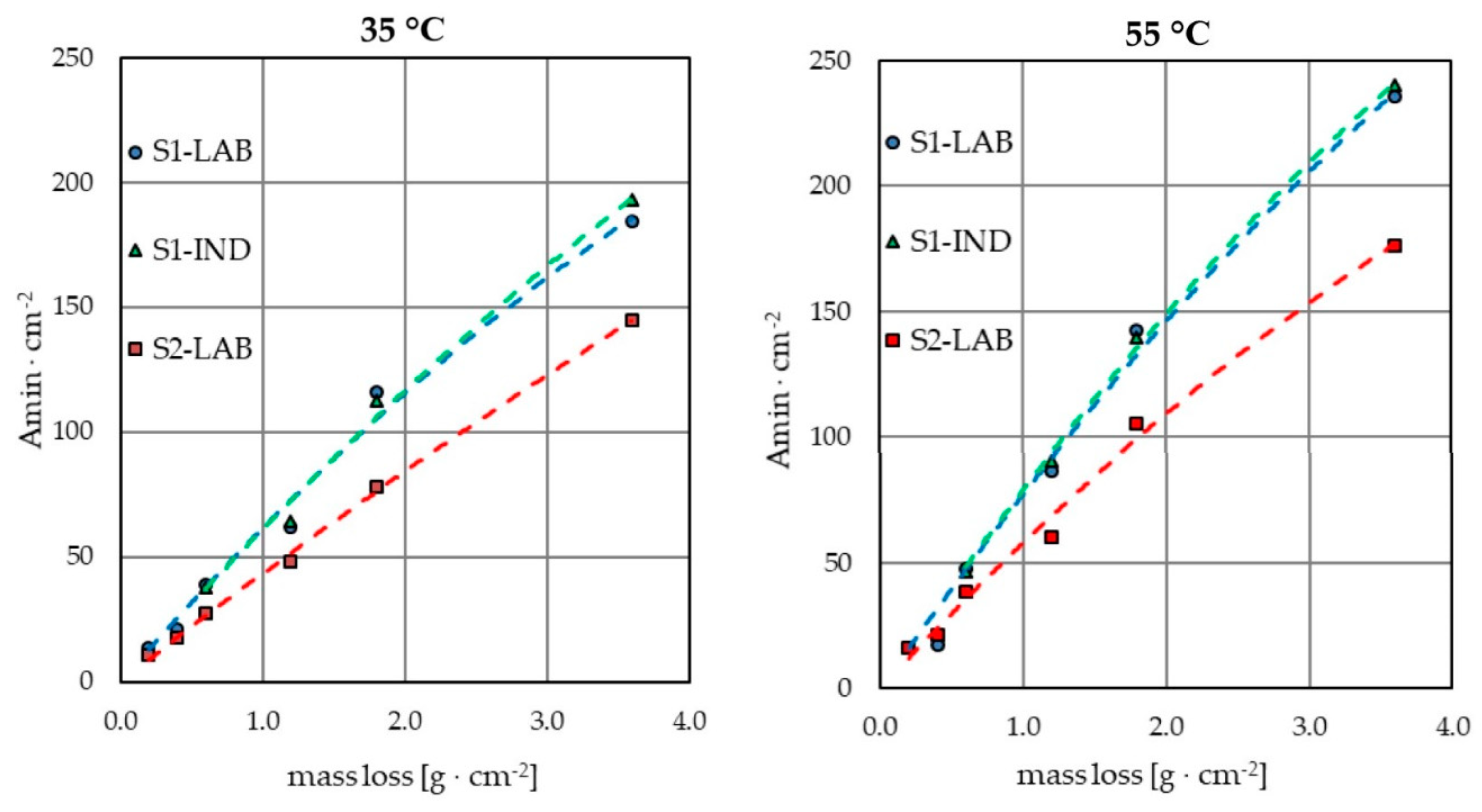
3.1. Electropolishing in Laboratory and Industrial Scale
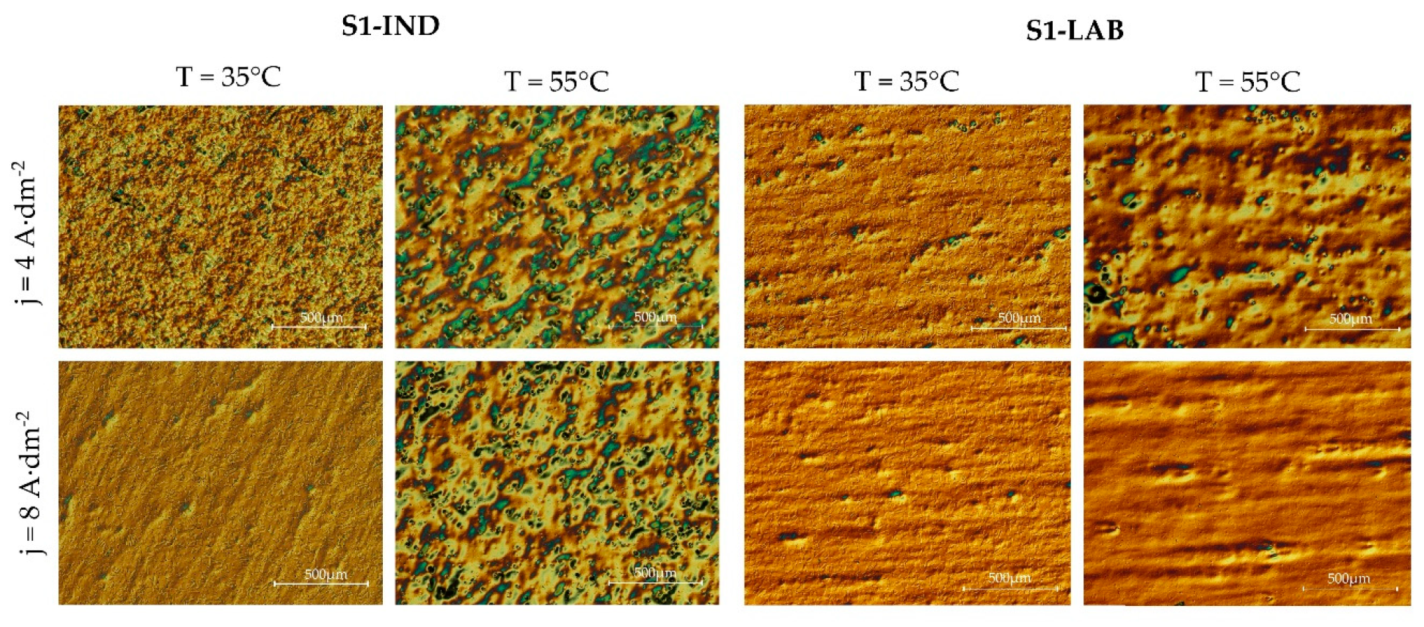
3.2. Selected Metallographic Images
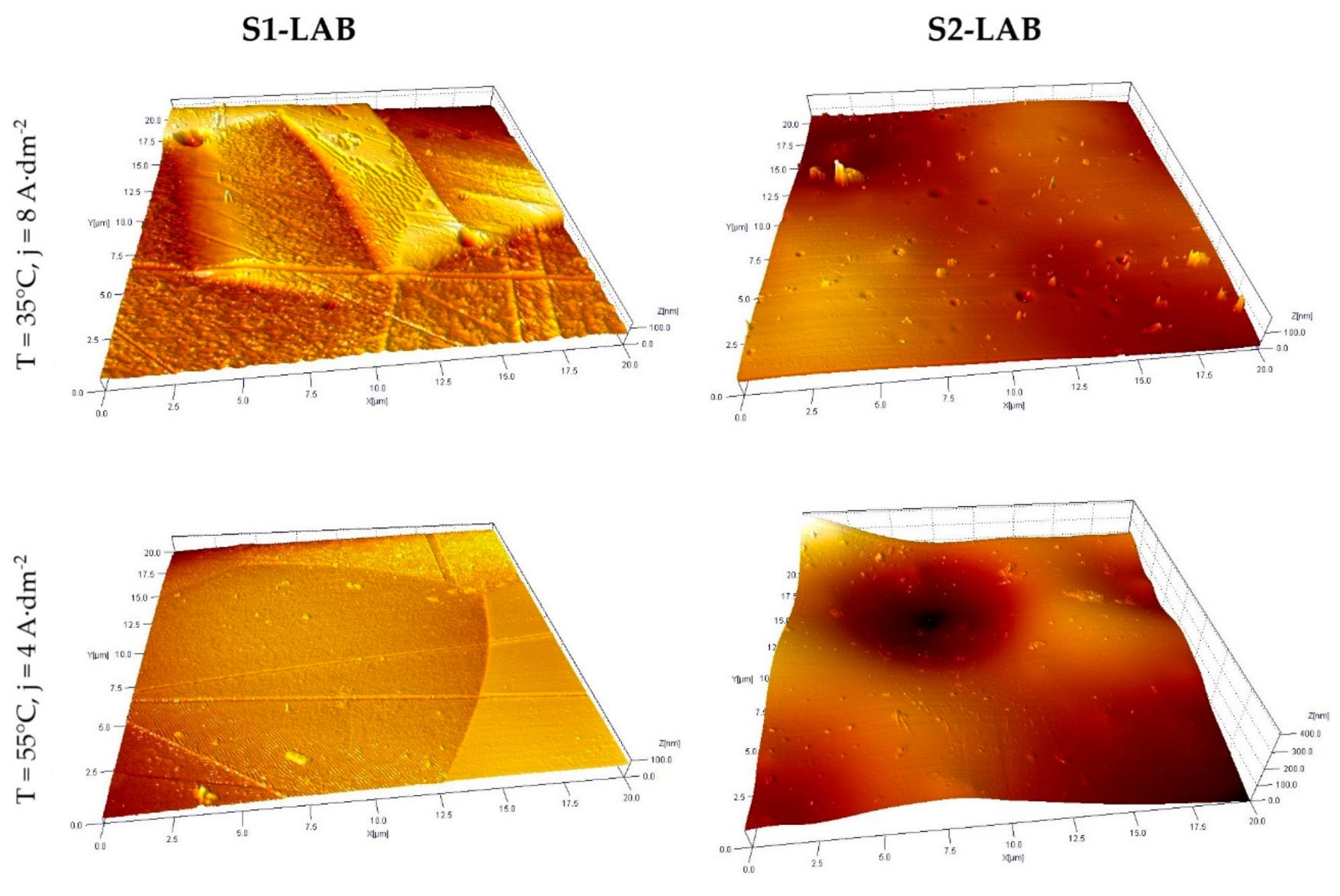
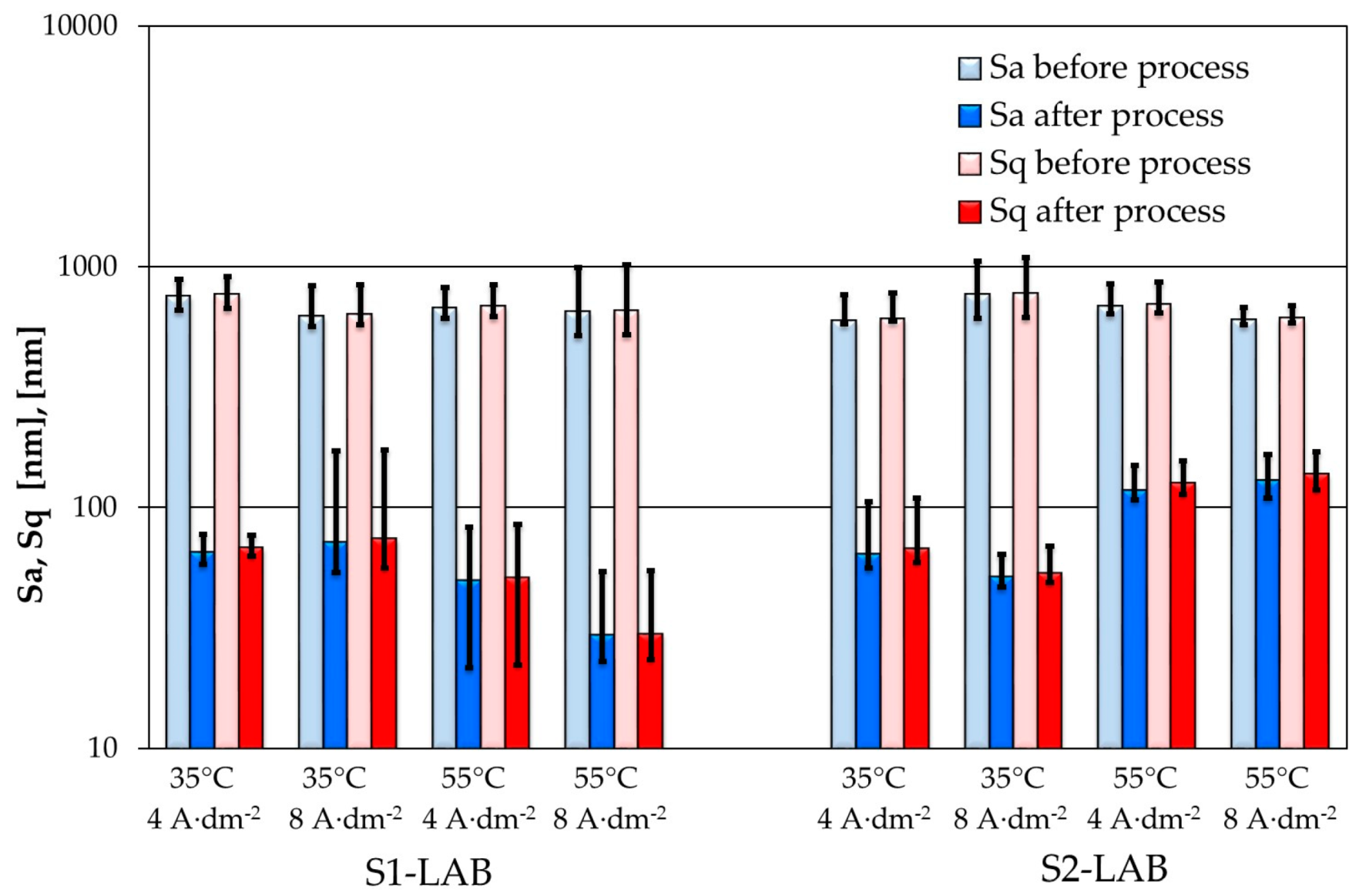
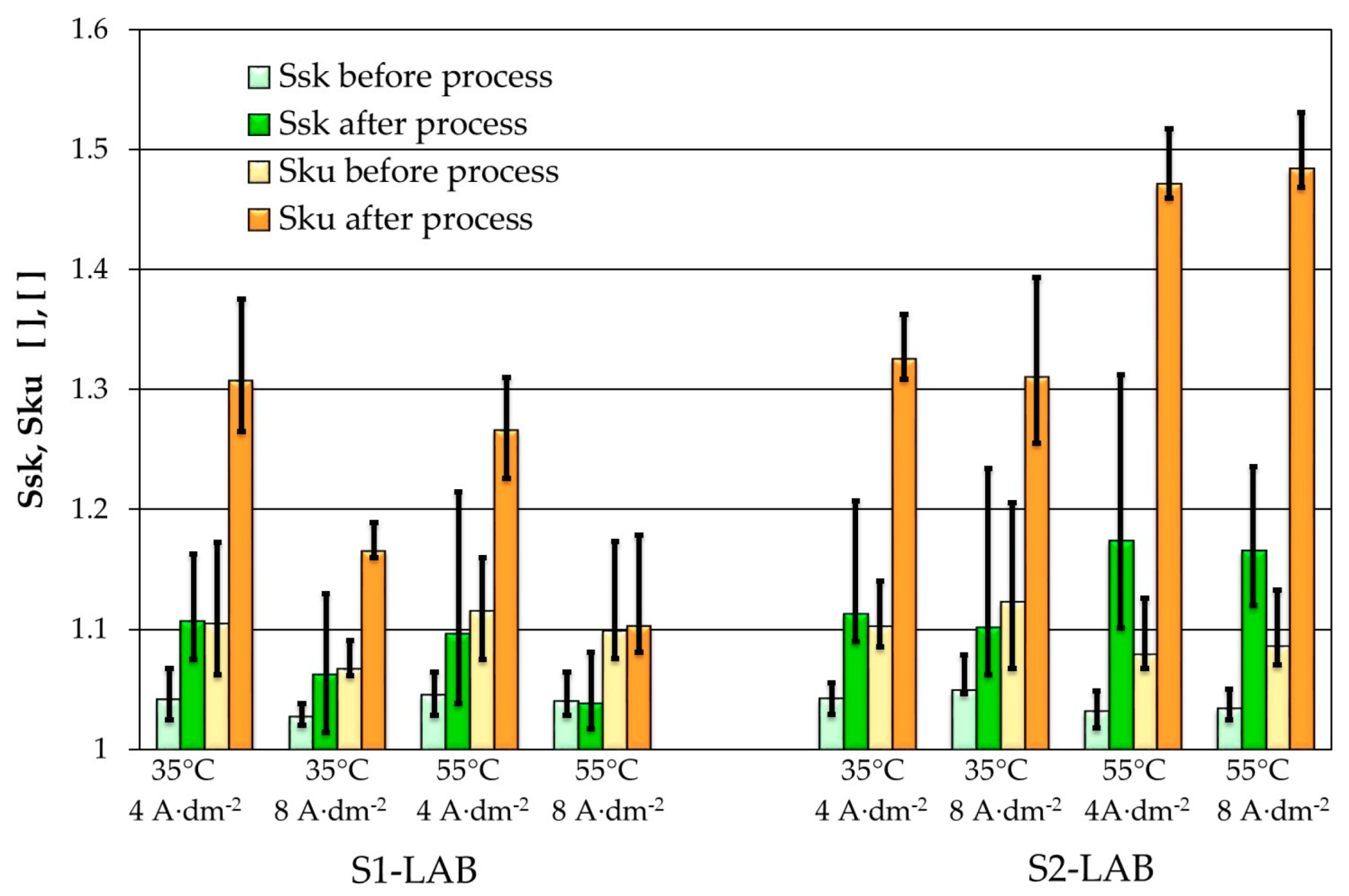
3.3. Selected AFM Images
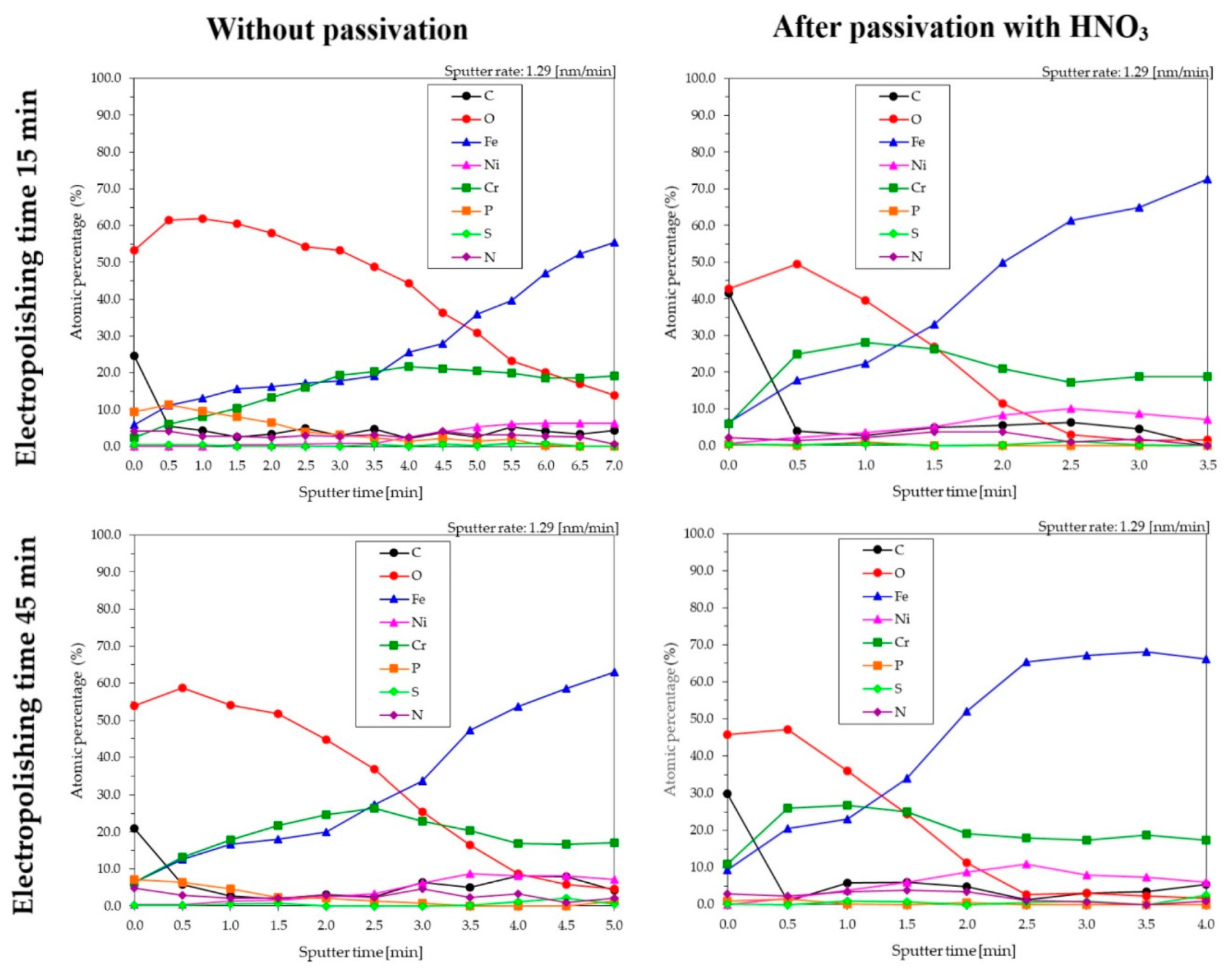
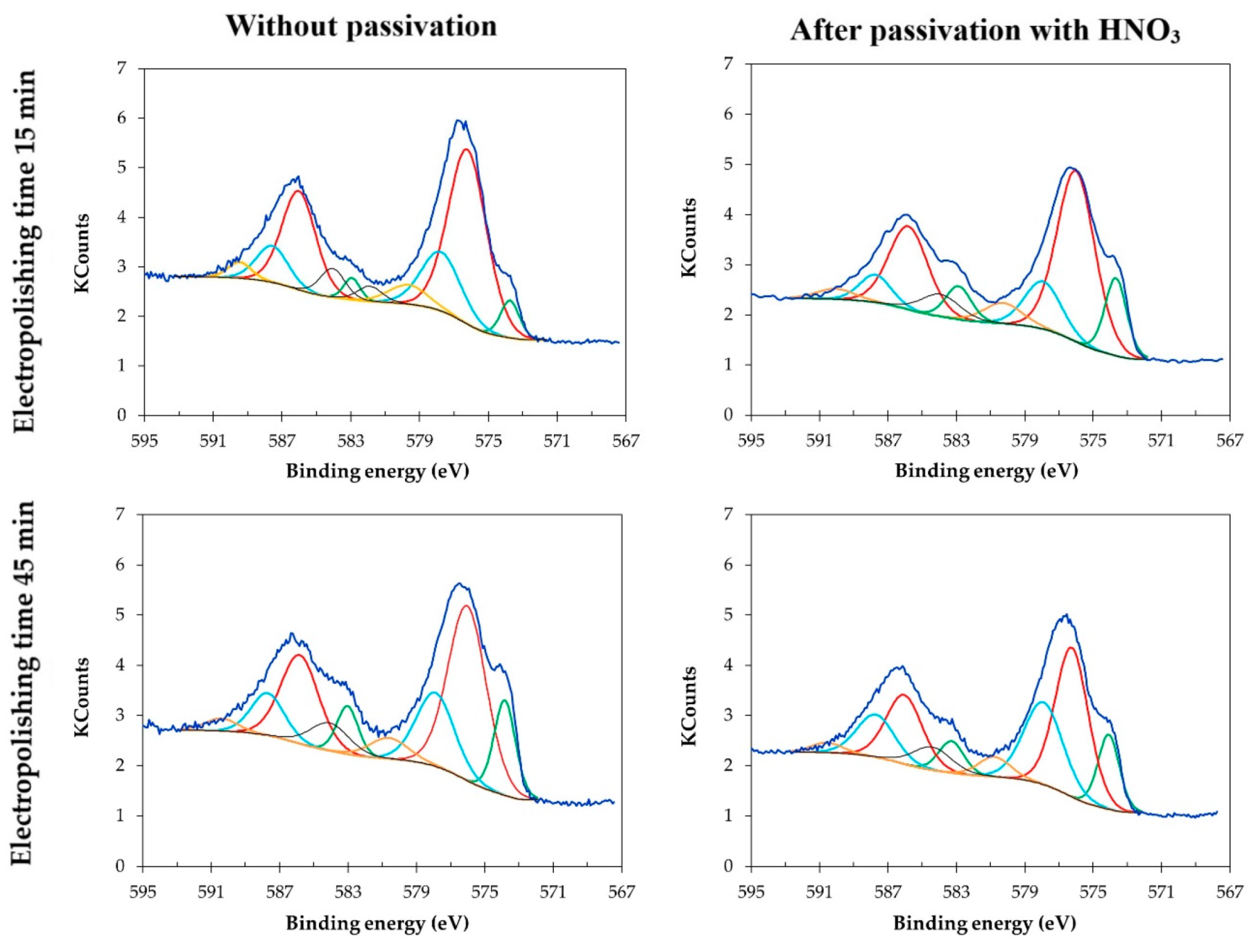
3.4. XPS for Samples Electropolished in the Industrial Bath
4. Conclusions
- Knowledge of the weight loss of samples as a result of electropolishing in laboratory conditions enables us to determine the estimated weight loss of elements in industrial conditions with an accuracy of even below 5%. This offers a possibility to estimate the rate of contamination of the industrial bath. It is also the basis for calculating the approximate load of contaminants that are generated during the process as a result of dissolution of the electropolished elements.
- The results of electropolishing in laboratory conditions with use of an industrial bath allow for initial selection of parameters that enable us to obtain satisfactory results of the process conducted in industrial conditions.
- The use of metallographic imaging with Nomarski contrast allows for the visual assessment of defects that emerge on electropolished surfaces, even for defects whose nature is difficult to be described by the gloss and roughness parameters.
- The time of electropolishing of the analyzed samples had only a slight influence on the composition of the passive layer. Passivation in nitric acid solution enables us to reduce the layer of contaminants on the surface of the sample.
- Improved morphological properties of sample surface were confirmed both with use of optical microscopy and atomic force microscopy. Data obtained with use of these techniques are complementary and, to a certain extent, mutually confirming.
- Having the abovementioned in mind, one can optimize the electropolishing process parameters in order to obtain expected morphological parameters of the surface in respect to the application of the specific product.
- The laboratory experiments must be performed in the way allowing to take into account the large-scale non-homogeneities of the process, typical for industrial production.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chatterjee, B. Science and industry of electropolishing (part 1). Galvanotechnik 2019, 110, 49–58. [Google Scholar]
- Han, W.; Fang, F. Fundamental aspects and recent developments in electropolishing. Int. J. Mach. Tools Manuf. 2019, 139, 1–23. [Google Scholar] [CrossRef]
- Rokosz, K. SEM/EDX, XPS, corrosion and surface roughness characterization of AISI 316L SS after electrochemical treatment in concentrated HNO3. Teh. Vjesn. Tech. Gaz. 2015, 22, 125–131. [Google Scholar] [CrossRef][Green Version]
- Rotty, C.; Doche, M.L.; Mandroyan, A.; Hihn, J.Y.; Montavon, G.; Moutarlier, V. Comparison of electropolishing behaviours of TSC, ALM and cast 316L stainless steel in H3PO4/H2SO4. Surf. Interfaces 2017, 6, 170–176. [Google Scholar] [CrossRef]
- Rokicki, R.; Hryniewicz, T.; Pulletikurthi, C.; Rokosz, K.; Munroe, N. Towards a better corrosion resistance and biocompatibility improvement of nitinol medical devices. J. Mater. Eng. Perform. 2015, 24, 1634–1640. [Google Scholar] [CrossRef]
- Marciniak, J.; Szewczenko, J.; Kajzer, W. Surface modification of implants for bone surgery. Arch. Metall. Mater. 2015, 60, 2123–2129. [Google Scholar] [CrossRef]
- Bohinc, K.; Dražić, G.; Abram, A.; Jevšnik, M.; Jeršek, B.; Nipič, D.; Kurinčič, M.; Raspor, P. Metal surface characteristics dictate bacterial adhesion capacity. Int. J. Adhes. Adhes. 2016, 68, 39–46. [Google Scholar] [CrossRef]
- Wolf, A.; Michele, V.; Schlüter, O.F.K.; Herbstritt, F.; Heck, J.; Mleczko, L. Precipitation in a micromixer—From laboratory to industrial scale. Chem. Eng. Technol. 2015, 38, 2017–2024. [Google Scholar] [CrossRef]
- Obalová, L.; Jirátová, K.; Karásková, K.; Chromčáková, Ž. N2O catalytic decomposition—From laboratory experiment to industry reactor. Catal. Today 2012, 191, 116–120. [Google Scholar] [CrossRef]
- Ziatdinov, M.K. Metallurgical SHS processes as a route to industrial-scale implementation: An autoreview. Int. J. Self Propag. High Temp. Synth. 2018, 27, 1–13. [Google Scholar] [CrossRef]
- Krymsky, V.; Shaburova, N. Applying of pulsed electromagnetic processing of melts in laboratory and industrial conditions. Materials 2018, 11, 954. [Google Scholar] [CrossRef] [PubMed]
- Assaad, J.J. Industrial versus laboratory clinker processing using grinding aids (scale effect). Adv. Mater. Sci. Eng. 2015, 2015, 1–12. [Google Scholar] [CrossRef][Green Version]
- Thomas, M.; Zdebik, D.; Białecka, B. Using sodium trithiocarbonate to precipitate heavy metals from industrial wastewater—From the laboratory to industrial scale. Polish J. Environ. Stud. 2018, 27, 1753–1763. [Google Scholar] [CrossRef]
- Pendyala, P.; Bobji, M.S.; Madras, G. Evolution of surface roughness during electropolishing. Tribol. Lett. 2014, 55, 93–101. [Google Scholar] [CrossRef]
- Maciąg, T.; Wieczorek, J.; Wȩcki, B. Parameters selection for electropolishing process of products made of copper and its alloys. Arch. Metall. Mater. 2017, 62, 1443–1447. [Google Scholar] [CrossRef]
- Jin, S.; Lu, X.; Lin, L.; Wu, A.T.; Zhao, K. Buffered electropolishing parameters on niobium sheet. Phys. Rev. Spec. Top. Accel. Beams 2010, 13, 061001. [Google Scholar] [CrossRef]
- Lochynski, P.; Łyczkowska, E.; Pawełczyk, A.; Szczygieł, B. Effect of bath exploitation on steel electropolishing process efficiency. Przem. Chem. 2012, 5, 846–848. [Google Scholar]
- Lochyński, P.; Charazińska, S.; Łyczkowska-Widłak, E.; Sikora, A.; Karczewski, M. Electrochemical reduction of industrial baths used for electropolishing of stainless steel. Adv. Mater. Sci. Eng. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Gadalińska, E.; Wronicz, W. Electropolishing procedure dedicated to in-depth stress measurements with x-ray diffractometry. Fatigue Aircr. Struct. 2016, 2016, 65–72. [Google Scholar] [CrossRef]
- Urlea, V.; Brailovski, V. Electropolishing and electropolishing-related allowances for powder bed selectively laser-melted Ti-6Al-4V alloy components. J. Mater. Process. Technol. 2017, 242, 1–11. [Google Scholar] [CrossRef]
- Huang, C.A.; Chang, J.H.; Zhao, W.J.; Huang, S.Y. Examination of the electropolishing behaviour of 73 brass in a 70% H3PO4 solution using a rotating disc electrode. Mater. Chem. Phys. 2014, 146, 230–239. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, S.G.; Choi, W.K.; Lee, E.S. A study of the improvement surface roughness and optimum machining characteristic of L-shaped tube STS 316L by electropolishing. Int. J. Adv. Manuf. Technol. 2016, 85, 2313–2324. [Google Scholar] [CrossRef]
- Lin, C.C.; Hu, C.C. Electropolishing of 304 stainless steel: Surface roughness control using experimental design strategies and a summarized electropolishing model. Electrochim. Acta 2008, 53, 3356–3363. [Google Scholar] [CrossRef]
- Rokosz, K.; Lahtinen, J.; Hryniewicz, T.; Rzadkiewicz, S. XPS depth profiling analysis of passive surface layers formed on austenitic AISI 304L and AISI 316L SS after high-current-density electropolishing. Surf. Coatings Technol. 2015, 276, 516–520. [Google Scholar] [CrossRef]
- Ur Rahman, Z.; Deen, K.M.; Cano, L.; Haider, W. The effects of parametric changes in electropolishing process on surface properties of 316L stainless steel. Appl. Surf. Sci. 2017, 410, 432–444. [Google Scholar] [CrossRef]
- Konica Minolta. Available online: https://sensing.konicaminolta.us/products/rhopoint-iq-s-20-60-85/ (accessed on 20 June 2019).
- Sikora, A.; Janus, P.; Sierakowski, A. The impact of the light exposure on the morphological properties of selected photoresists. Opt. Appl. 2019, 49, 177–185. [Google Scholar]
- Sikora, A. The new approach to the investigation of the roughness changes of the non-uniform materials irradiated with UV light and imaged by means of atomic force microscopy supported with precise repetitive scanning area positioning. Meas. Sci. Technol. 2017, 28, 034016. [Google Scholar] [CrossRef]
- Sikora, A.; Tomczuk, K. Impact of the LED-based light source working regime on the degradation of polymethyl methacrylate. Light. Res. Technol. 2019, 1–12. [Google Scholar] [CrossRef]
- Image Metrology. Available online: https://www.imagemet.com/ (accessed on 20 June 2019).
- De Vito, E.; Marcus, P. XPS study of passive films formed on molybdenum-implanted austenitic stainless steels. Surf. Interface Anal. 1992, 19, 403–408. [Google Scholar] [CrossRef]
- Latifi, A.; Imani, M.; Khorasani, M.T.; Joupari, M.D. Electrochemical and chemical methods for improving surface characteristics of 316L stainless steel for biomedical applications. Surf. Coatings Technol. 2013, 221, 1–12. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Han, E.H.; Ke, W. Influence of dissolved oxygen on oxide films of Alloy 690TT with different surface status in simulated primary water. Corros. Sci. 2011, 53, 3623–3635. [Google Scholar] [CrossRef]
- Guo, S.; Xu, L.; Zhang, L.; Chang, W.; Lu, M. Characterization of corrosion scale formed on 3Cr steel in CO2-saturated formation water. Corros. Sci. 2016, 110, 123–133. [Google Scholar] [CrossRef]
- Han, Y.; Mei, J.; Peng, Q.; Han, E.H.; Ke, W. Effect of electropolishing on corrosion of nuclear grade 316L stainless steel in deaerated high temperature water. Corros. Sci. 2016, 112, 625–634. [Google Scholar] [CrossRef]
- Machet, A.; Galtayries, A.; Zanna, S.; Klein, L.; Maurice, V.; Jolivet, P.; Foucault, M.; Combrade, P.; Scott, P.; Marcus, P. XPS and STM study of the growth and structure of passive films in high temperature water on a nickel-base alloy. Electrochim. Acta 2004, 49, 3957–3964. [Google Scholar] [CrossRef]
| Electropolishing Bath Setup | t | T | j | S1-IND | S1-LAB | S2-LAB | S1-IND | S1-LAB | S2-LAB |
|---|---|---|---|---|---|---|---|---|---|
| Ra | Gloss | ||||||||
| (min) | (°C) | (A·dm−2) | (µm) | (µm) | (µm) | (GU) | (GU) | (GU) | |
| the initial stage of use 0 mass.% Fe | 15 | 35 | 4 | 0.24 | 0.24 | 0.18 | 139 | 335 | 400 |
| 15 | 35 | 8 | 0.15 | 0.12 | 0.13 | 378 | 623 | 703 | |
| 15 | 55 | 4 | 0.46 | 0.32 | 0.40 | 223 | 631 | 470 | |
| 15 | 55 | 8 | 0.43 | 0.23 | 0.20 | 276 | 695 | 821 | |
| 45 | 35 | 4 | 0.31 | 0.15 | 0.16 | 305 | 930 | 907 | |
| 45 | 35 | 8 | 0.08 | 0.10 | 0.10 | 1029 | 1152 | 1181 | |
| 45 | 55 | 4 | 0.55 | 0.22 | 0.25 | 326 | 950 | 806 | |
| 45 | 55 | 8 | 0.44 | 0.14 | 0.12 | 359 | 1191 | 1218 | |
| advanced stage of use 3 mass.% Fe | 15 | 35 | 4 | 0.28 | 0.15 | 0.17 | 150 | 493 | 240 |
| 15 | 35 | 8 | 0.13 | 0.15 | 0.13 | 442 | 717 | 575 | |
| 15 | 55 | 4 | 0.36 | 0.24 | 0.28 | 269 | 613 | 320 | |
| 15 | 55 | 8 | 0.26 | 0.13 | 0.20 | 479 | 998 | 299 | |
| 45 | 35 | 4 | 0.24 | 0.12 | 0.16 | 420 | 950 | 582 | |
| 45 | 35 | 8 | 0.07 | 0.10 | 0.12 | 1022 | 1056 | 899 | |
| 45 | 55 | 4 | 0.52 | 0.42 | 0.22 | 258 | 589 | 364 | |
| 45 | 55 | 8 | 0.49 | 0.12 | 0.16 | 304 | 1196 | 388 | |
| Electropolishing Time (min) | Sputter Time (min) | Etch Depth (nm) | C | O | Fe | Ni | Cr | P | S | N | Suma | Cr/Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15 | 3.5 | 4.5 | 4.6 | 48.9 | 19.2 | 0.9 | 20.3 | 2.4 | 0.6 | 3.1 | 100 | 1.06 |
| 15 + pass * | 1.0 | 1.3 | 2.8 | 39.7 | 22.3 | 3.6 | 28.1 | 1.0 | 0.5 | 2.1 | 100 | 1.26 |
| 45 | 2.0 | 2.6 | 3.1 | 44.8 | 20.0 | 2.5 | 24.5 | 2.1 | 0.0 | 2.0 | 100 | 1.23 |
| 45 + pass * | 1.0 | 1.3 | 5.8 | 35.9 | 23.1 | 3.8 | 26.7 | 0.1 | 1.0 | 3.5 | 100 | 1.15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lochyński, P.; Charazińska, S.; Łyczkowska-Widłak, E.; Sikora, A. Electropolishing of Stainless Steel in Laboratory and Industrial Scale. Metals 2019, 9, 854. https://doi.org/10.3390/met9080854
Lochyński P, Charazińska S, Łyczkowska-Widłak E, Sikora A. Electropolishing of Stainless Steel in Laboratory and Industrial Scale. Metals. 2019; 9(8):854. https://doi.org/10.3390/met9080854
Chicago/Turabian StyleLochyński, Paweł, Sylwia Charazińska, Edyta Łyczkowska-Widłak, and Andrzej Sikora. 2019. "Electropolishing of Stainless Steel in Laboratory and Industrial Scale" Metals 9, no. 8: 854. https://doi.org/10.3390/met9080854
APA StyleLochyński, P., Charazińska, S., Łyczkowska-Widłak, E., & Sikora, A. (2019). Electropolishing of Stainless Steel in Laboratory and Industrial Scale. Metals, 9(8), 854. https://doi.org/10.3390/met9080854





