1. Introduction
Low-pressure carburizing (LPC) is a method of strengthening the surface of the steel. A vacuum of 1–10 hPa is created in the vacuum furnace chamber with the subsequent introduction of small amounts of carburizing gas at a temperature within the range of 900–1050 °C. Non-treated steel is post-cured in this environment for the time required to obtain the desired carbon profile. Due to the high temperature, the carbon transfer is more efficient in the case of the conventional carburizing method, thus leading to a shorter carburizing time. Yada and Watanabe reported an LPC process that did not pollute the environment, did not cause intergranular oxidation, and consumed less energy [
1]. Additionally, Kula et al. demonstrated carburized steel after quenching and tempering to be multiple times harder than non-treated steel and, as such, to be industrially applicable in those processes where resistance to wear under working conditions is of significance [
2].
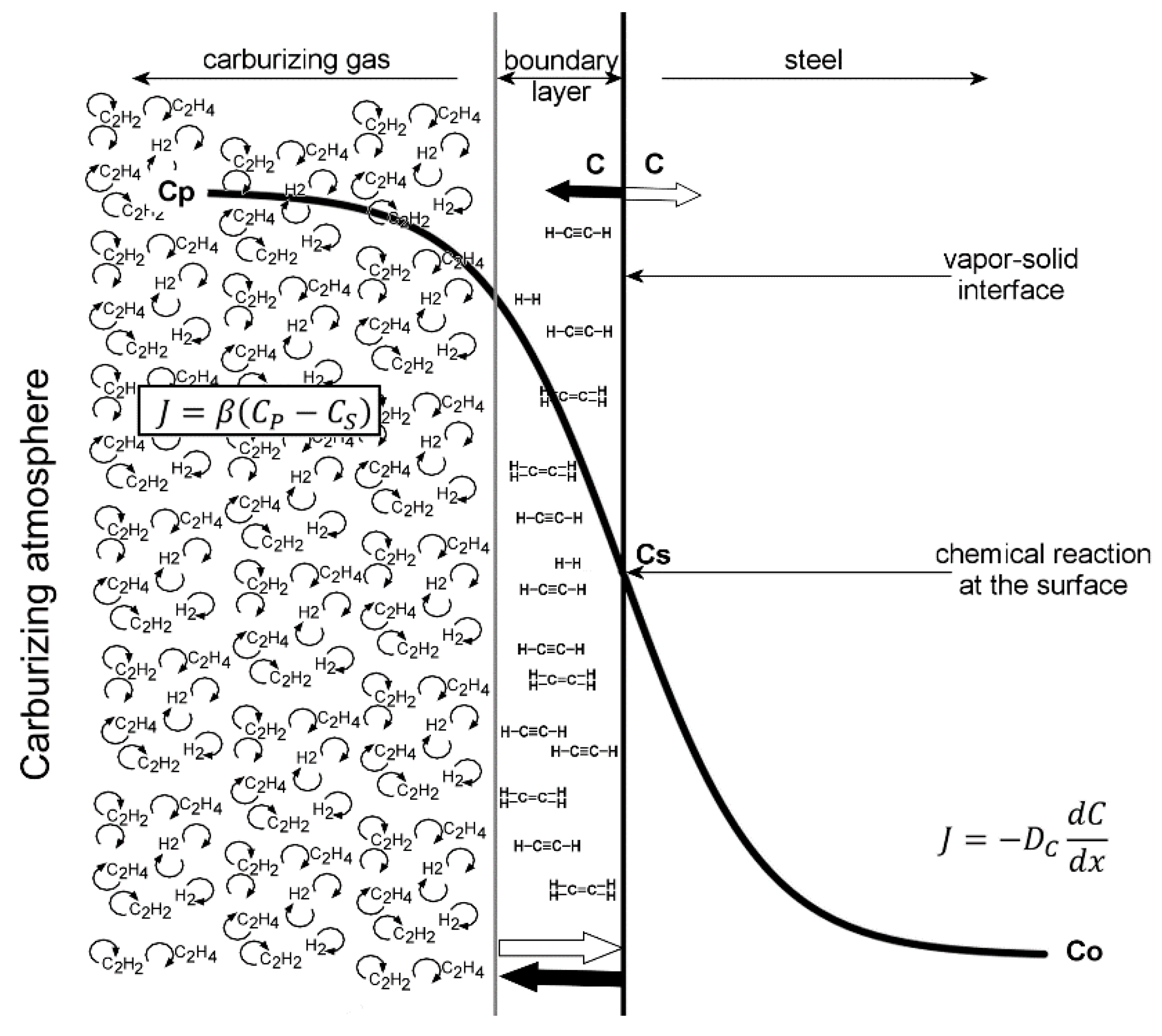
The process of low-pressure carburizing is organized into cyclically repeating boost (saturation) and diffusion segments, where a boost segment is a characteristic of an intense surface and diffusion phenomena taking place in the part treated, while a diffusion segment solely consists of diffusion processes, with no carbon being delivered to the surface. During the boost segments, light hydrocarbons (methane, propane, acetylene, ethylene)—being a source of carbon—are dosed into the furnace chamber. Bensabath et al. described the phenomena of the decomposition of these compounds at high temperatures. Additionally, they indicated that hydrocarbon pyrolysis under low-pressure gas carburizing conditions might lead to gas phase reactions, which produce polycyclic aromatic hydrocarbons (PAHs) [
3]. Mendiara et al. showed that increasing reaction temperature and the initial C
2H
2 concentration favors a formation of soot [
4]. However, an optimization of the furnace performance is still carried out by using an empirical formula like the Harris equation [
5] as a detailed vacuum carburizing prediction method has not yet been established.
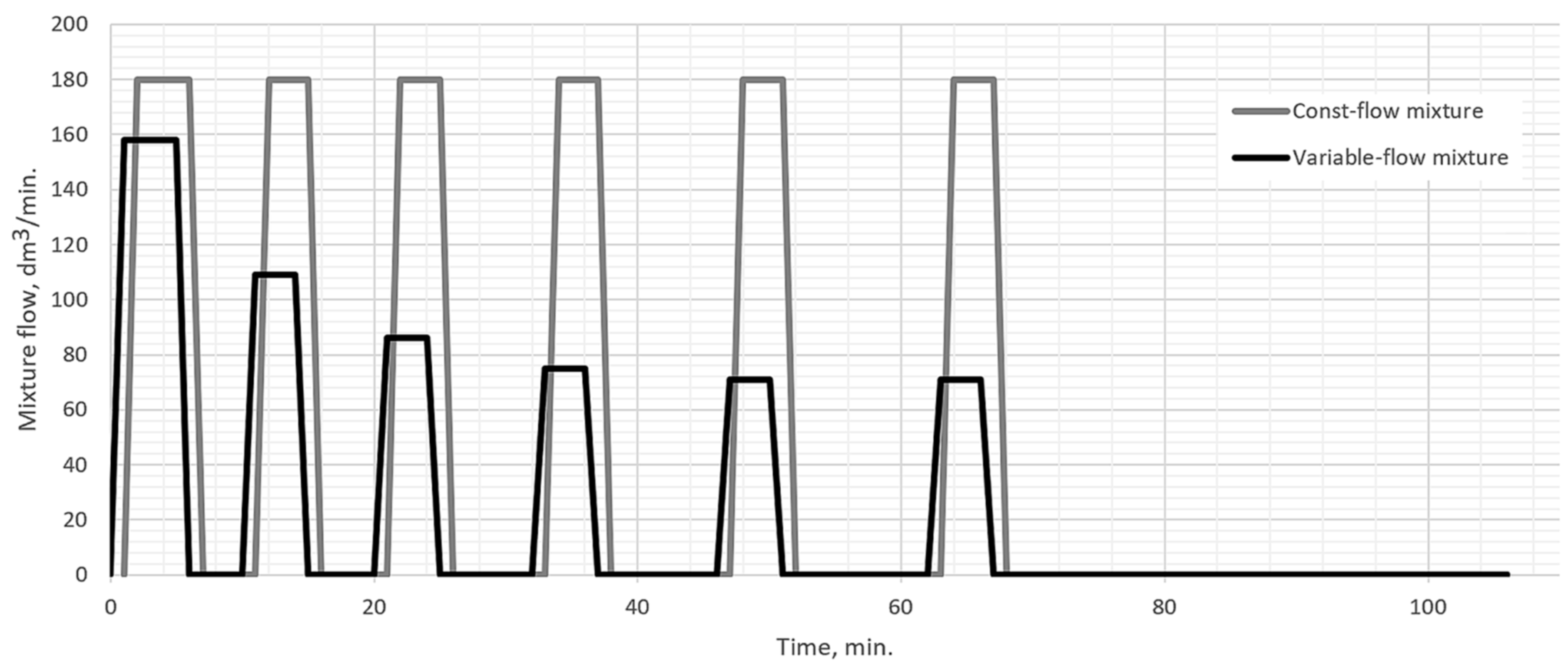
Antes have proved that the correct selection of the carburizing mixture flow rate in the carburization chamber during the boost segment is of key importance for that segment efficiency [
6]. An insufficient volume of carburizing gas in the carburization chamber results in a non-uniform carburized layer and, in consequence, a rejection of a batch of parts at the production stage. On the other hand, as Herring presented, an excess volume of such gas results in a deposition of carbon derivative substances accumulating in the furnace chamber, thus necessitating service break for the duration of its cleaning [
7]. Therefore, a correct calculation of the amount of carburizing gas required for uniform carburization of parts, taking into account the process temperature and input (batch) size, remains problematic.
The EU 1558781 and US 7550049 patent publications describe a method of carburizing steel products applied in vacuum furnaces in an anaerobic atmosphere under reduced pressure, where the parts are saturated with carbon through continuous dosage of a carburizing atmosphere consisting of a mix of ethylene—or propane or acetylene—with hydrogen in the volume ratio of 1.5–10 for 5–40 min, flowing with constant efficiency throughout the whole duration of the carburization phase [
8,
9]. The EU 1558780 and US 7513958 patent publications describe a mixture for carburizing steel in a vacuum, containing a carbon carrier in the form of a mixture of ethylene and acetylene in a volume ratio of 0.1–2.0 and a modifier in the form of hydrogen in an amount of 0.7–1.5 times the carbon carrier volume, or in the form of ammonia in an amount of 0.7–5.0 times the carbon carrier volume, which is also dosed with constant efficiency during the saturation phase [
10,
11]. Additionally, Hermanowicz and Smolik report that it is possible to apply an impulse method of low-pressure carburizing, consisting of dosing carburizing gases during the saturation phase and then pumping the reaction products out during the diffusion phase [
12].
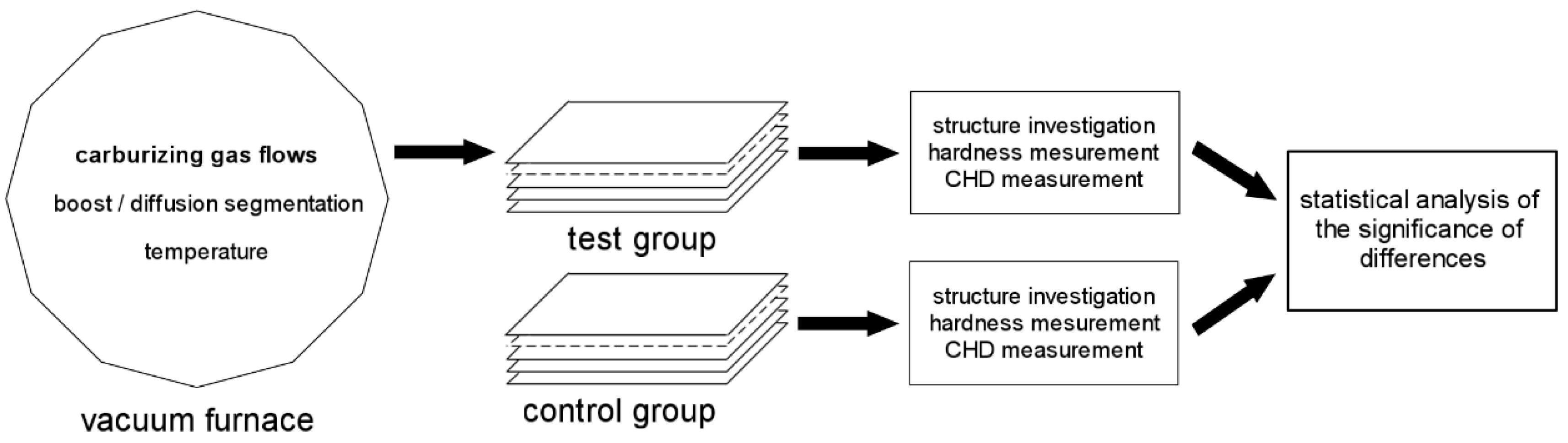
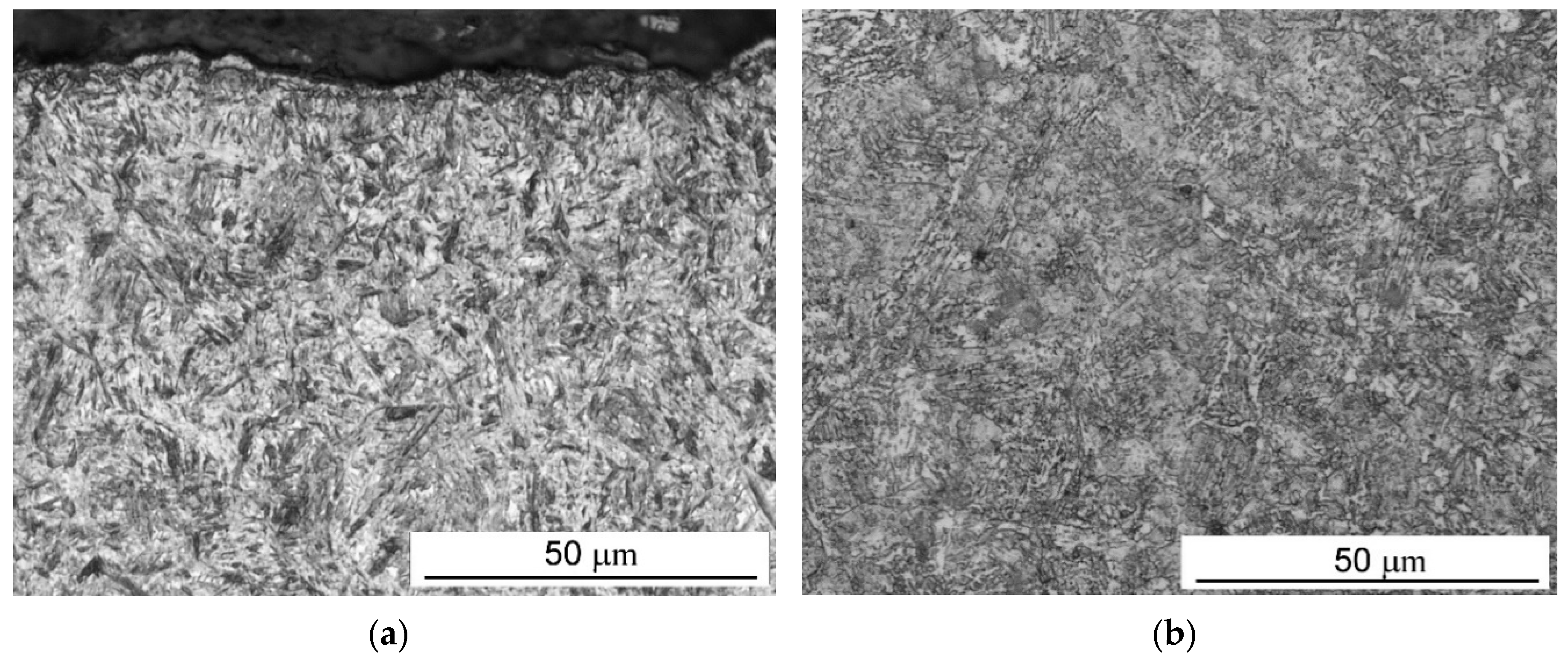
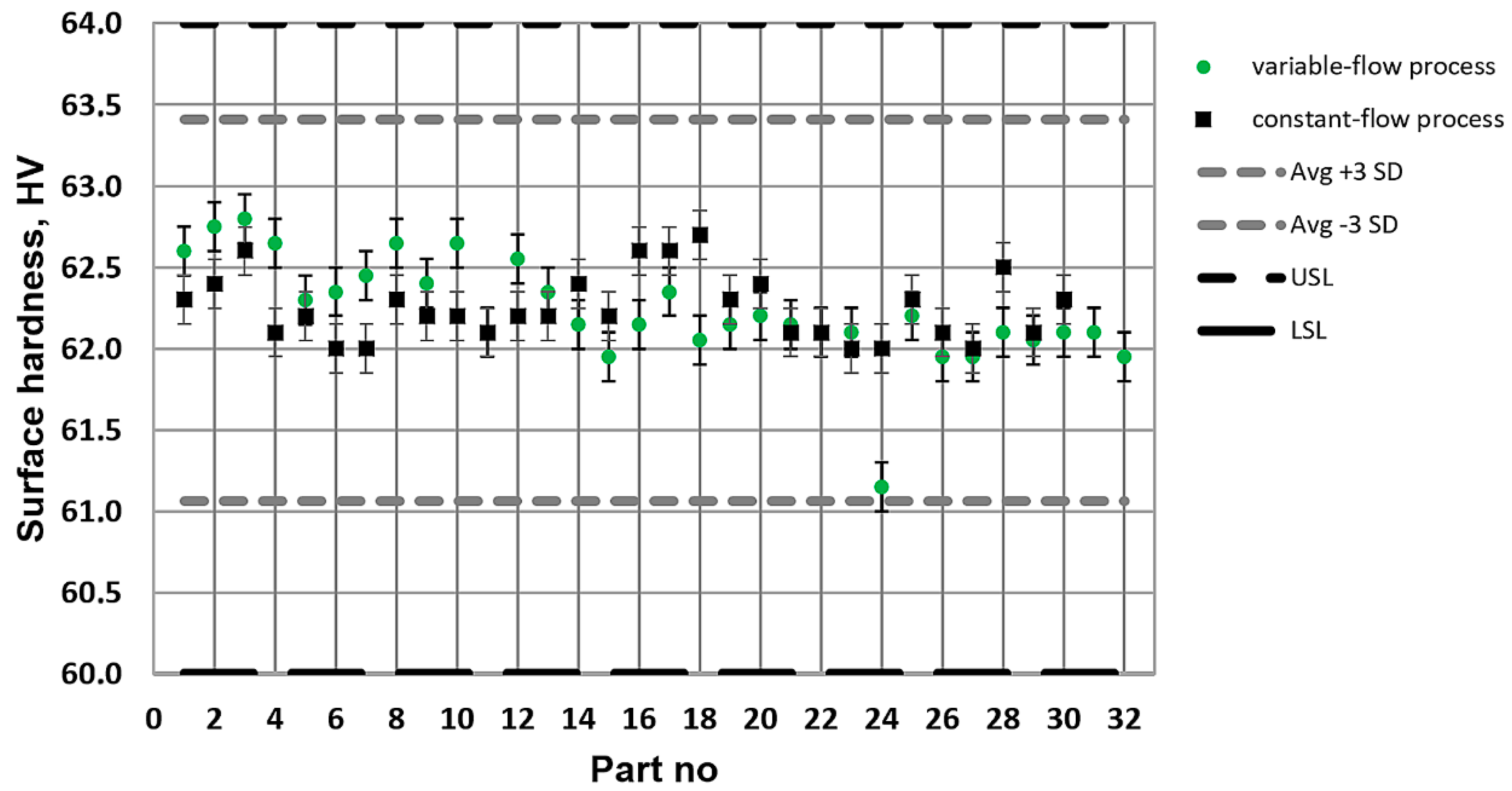
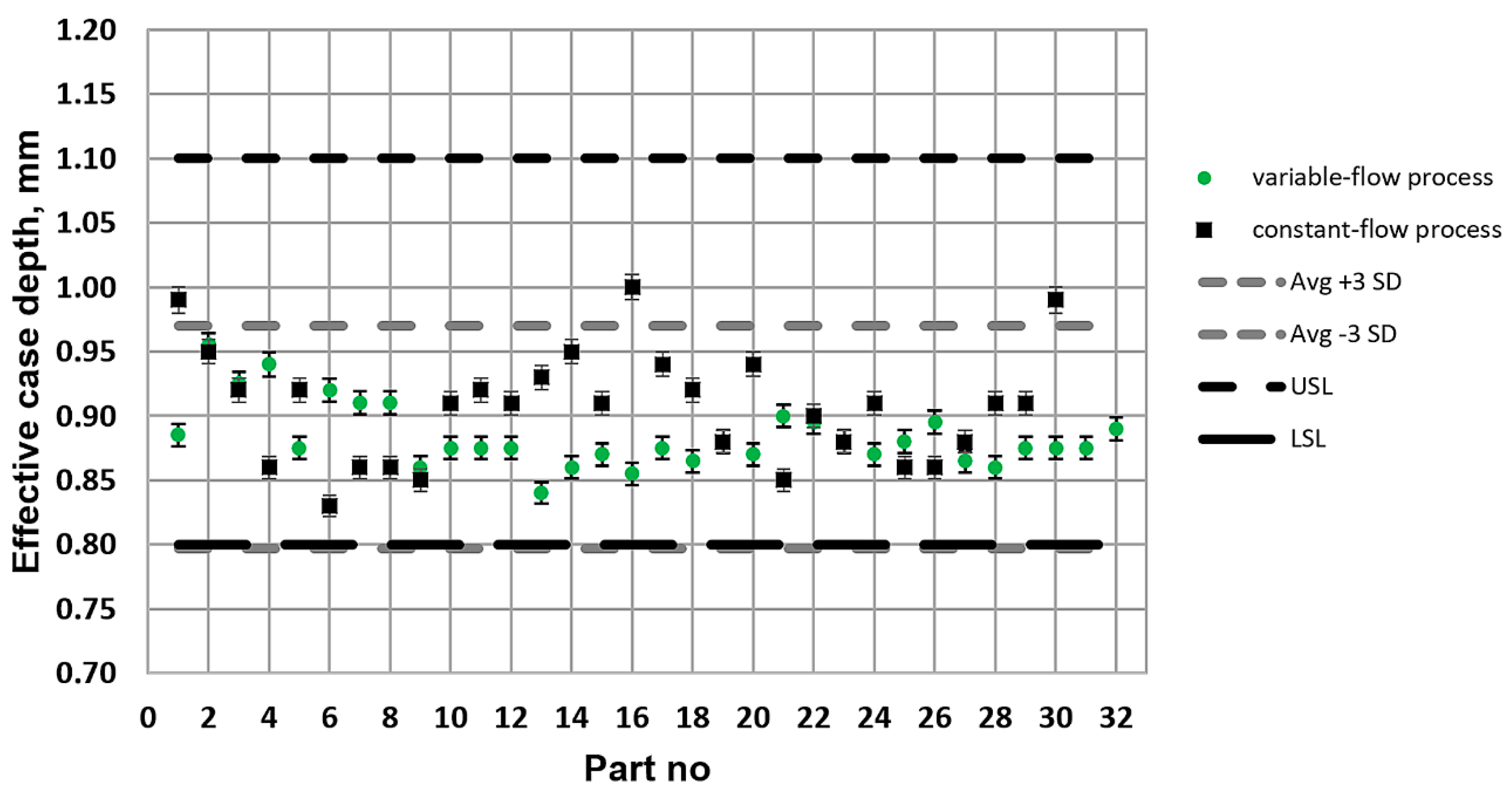
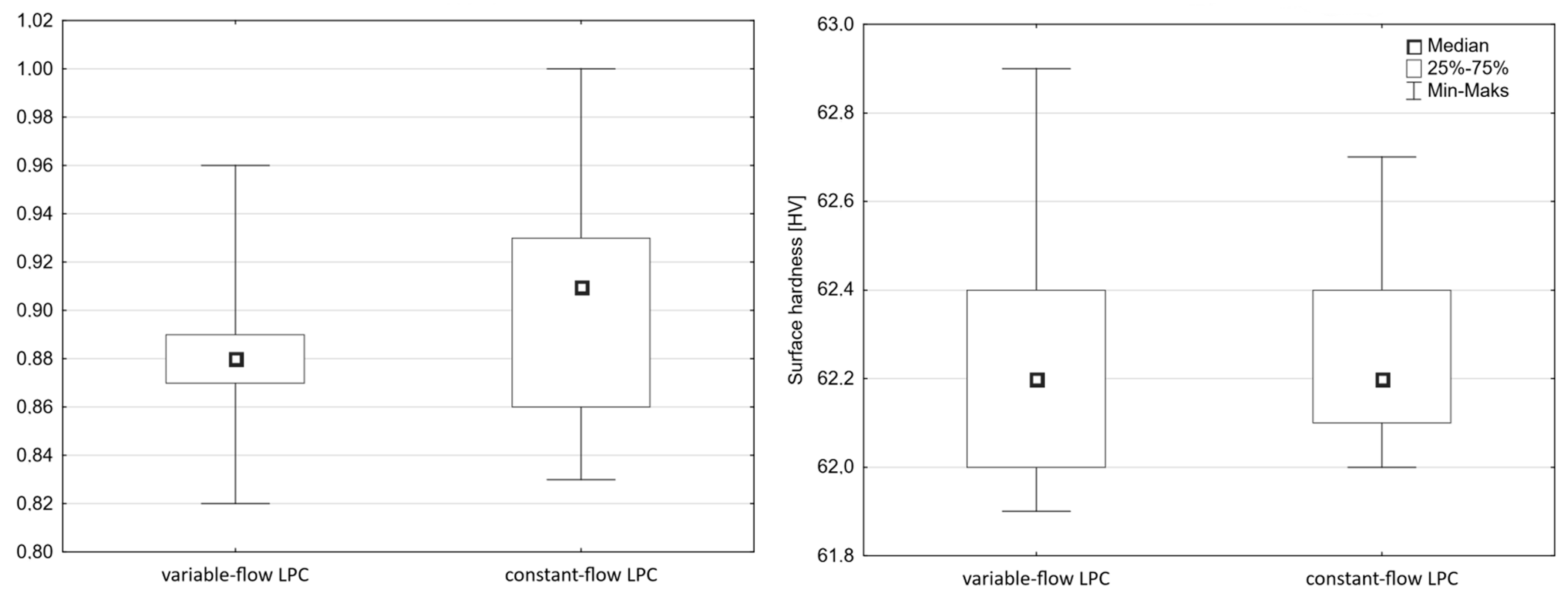
What is characteristic of all the low-pressure carburization methods presented is an introduction of carburizing gas or gas mixture with constant efficiency (constant flow), without taking into account the actual demand resulting from absorption of carbon in the material carburized (which fluctuates during the carburization process). At the same time, the dependency described by Fick’s first law indicates that the demand for carbon during a process decreases proportionally to an increase in carbon content in the material carburized. Therefore, this study aimed to establish whether the volume reduction of carburizing gases to the level given by Fick’s laws would have an impact on the properties of the hardened case obtained in regular low-pressure carburization processes. The following null hypotheses were tested: no differences in the properties of the surface layer (microstructure, surface hardness, case hardness depth) will be found between specimens that were carburized in a variable-flow carburizing process and those that were treated in a constant-flow carburizing process.
Author Contributions
Conceptualization, E.W.-K. and M.K.; Data curation, M.S.; Formal analysis, M.K.; Investigation, M.S. and A.B.; Methodology, E.W.-K. and M.K.; Project administration, P.K.; Resources, M.S. and A.B.; Software, E.W.-K.; Supervision, P.K.; Validation, M.K. and P.K.; Visualization, A.B.; Writing—original draft, E.W.-K.; Writing—review & editing, P.K.
Funding
This research was funded by the Polish National Centre for Research and Development, grant number POIR.04.01.04-00-0087/15.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Yada, K.; Watanabe, O. Reactive flow simulation of vacuum carburizing by acetylene gas. Comput. Fluids 2013, 79, 65–76. [Google Scholar] [CrossRef]
- Kula, P.; Pietrasik, R.; Dybowski, K. Vacuum carburizing—Process optimization. J. Mater. Process. Technol. 2005, 164–165, 876–881. [Google Scholar] [CrossRef]
- Bensabath, T.; Monnier, H.; Glaude, P.-A. Detailed kinetic modeling of the formation of toxic polycyclic aromatic hydrocarbons (PAHs) coming from pyrolysis in low-pressure gas carburizing conditions. J. Anal. Appl. Pyrolysis 2016, 122, 342–354. [Google Scholar] [CrossRef][Green Version]
- Mendiara, T.; Domene, M.P.; Millera, A.; Bilbao, R.; Alzueta, M.U. An experimental study of the soot formed in the pyrolysis of acetylene. J. Anal. Appl. Pyrolysis 2005, 74, 486–493. [Google Scholar] [CrossRef]
- Harris, F.E. Case depth—An attempt at a practical definition. Met. Prog. 1943, 44, 265–272. [Google Scholar]
- Antes, H.W. Calculating the gas flow rate for vacuum carburization. Heat Treat. Prog. 2005, 8, 51–53. [Google Scholar]
- Herring, D.H. A case for acetylene based low pressure carburizing of gears. Therm. Process. Gear Solut. 2012, 9, 40–45. [Google Scholar]
- Kula, P.; Olejnik, J.; Heilman, P. Method for Under-Pressure Carburizing of Steel Workpieces. EU Patent EU 1558781, 25 October 2006. [Google Scholar]
- Kula, P.; Olejnik, J.; Heilman, P. Method for Under-Pressure Carburizing of Steel Workpieces. U.S. Patent US 7550049, 23 June 2009. [Google Scholar]
- Kula, P.; Olejnik, J.; Heilman, P. Hydrocarbon Gas Mixture for the Under Pressure Carburizing of Steel. EU Patent EU 1558780, 15 August 2007. [Google Scholar]
- Kula, P.; Olejnik, J.; Heilman, P. Hydrocarbon Gas Mixture for the Under-Pressure Carburizing of Steel. U.S. Patent US 7513958, 7 April 2009. [Google Scholar]
- Hermanowicz, P.; Smolik, J. Vacuum carburizing of low carbon steel DC4 in atmosphere composed by aliphatic hydrocarbons. Problemy Eksploatacji 2008, 1, 21–34. (In Polish) [Google Scholar]
- Turpin, T.; Dulcy, J.; Gantois, M. Carbon diffusion and phase transformations during gas carburizing of high-alloyed stainless steels: Experimental study and theoretical modeling. Metall. Mater. Trans. A 2005, 36, 2751–2760. [Google Scholar] [CrossRef]
- Karabelchtchikova, O. Fundamentals of Mass Transfer in Gas Carburizing. Ph.D. Thesis, Worcester Polytechnic Institute, Worcester, MA, USA, 18 December 2007. [Google Scholar]
- Wei, Y.; Wang, G.; Sisson, R.D.; Bernard, B.; Poor, R. Intelligent heat treating: Simulation of carburizing process. In Proceedings of the 26th ASM Heat Treating Society Conference, Cincinnati, OH, USA, 31 October–2 November 2011; ASM International: Cincinnati, OH, USA, 2011; pp. 91–98. [Google Scholar]
- Kula, P.; Dybowski, K.; Wolowiec, E.; Pietrasik, R. Boost-diffusion vacuum carburizing—Process optimisation. Vacuum 2014, 99, 175–179. [Google Scholar] [CrossRef]
- Korecki, M.; Wołowiec-Korecka, E.; Sut, M. Low-Pressure Carburizing Method (LPC). Poland Patent P.424224, 1 August 2018. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).