The Research of Low-Oxygen Control and Oxygen Behavior during RH Process in Silicon-Deoxidization Bearing Steel
Abstract
1. Introduction
2. Experimental
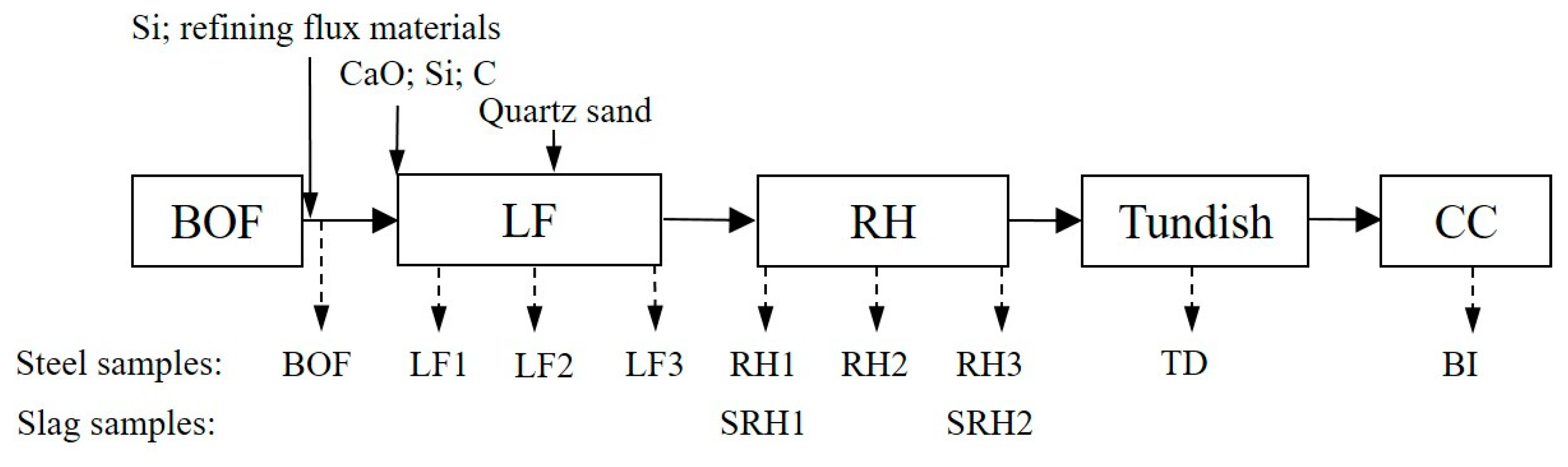
2.1. Production Process
2.2. Detection and Analysis Method
2.2.1. Inclusion and Slag Composition Detection
2.2.2. Off-Gas Analysis
3. Results
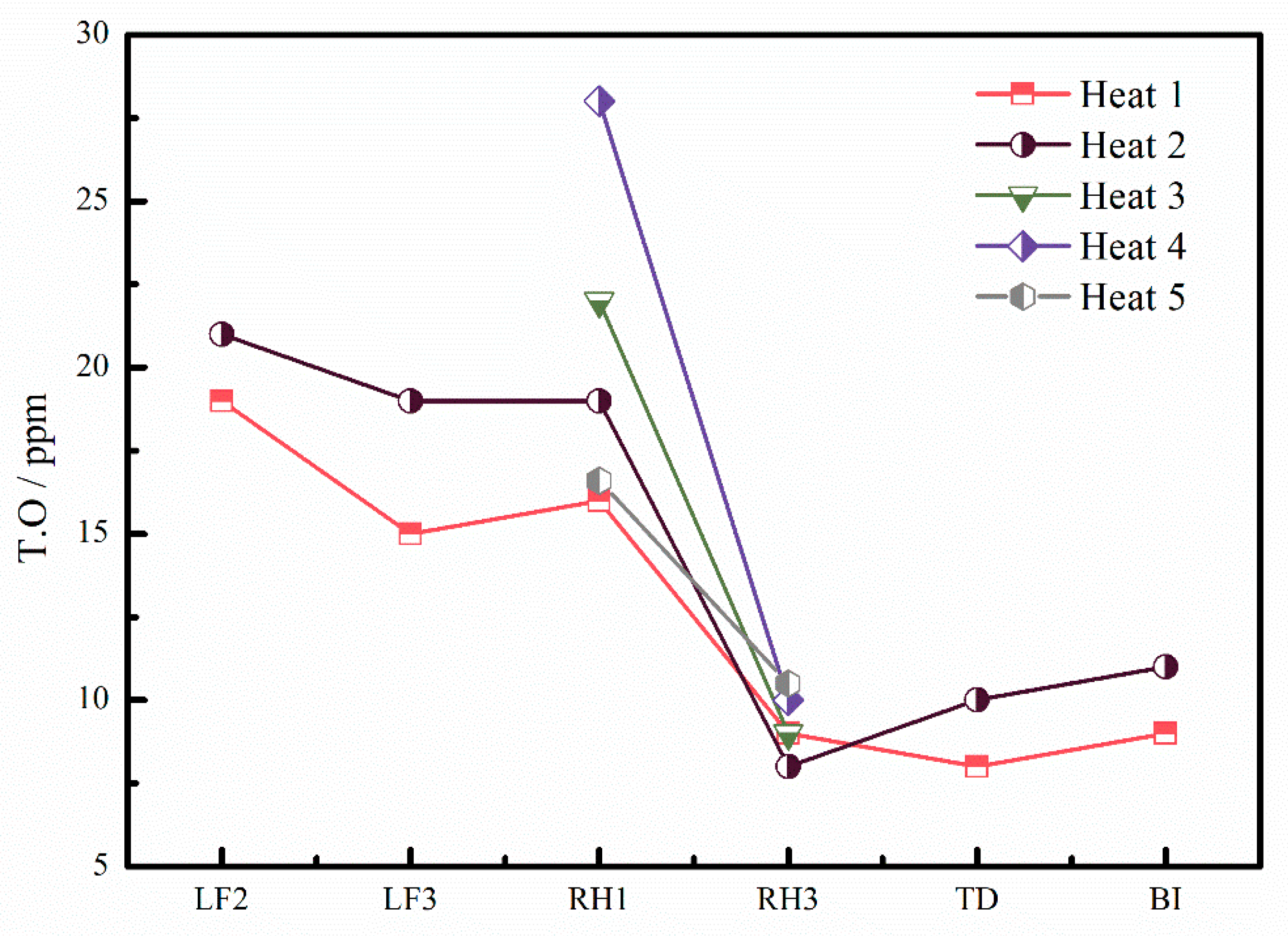
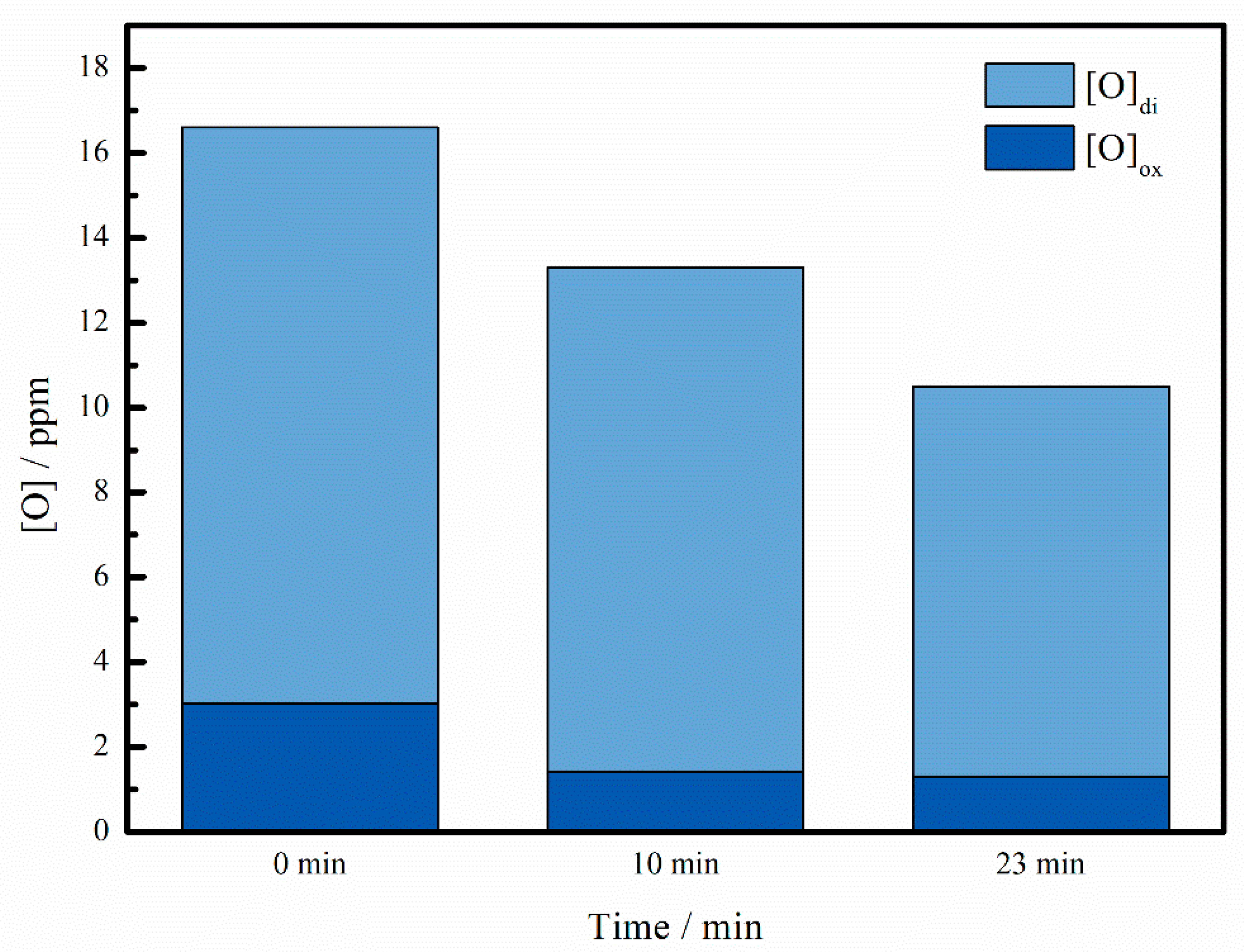
3.1. T.O Content Change
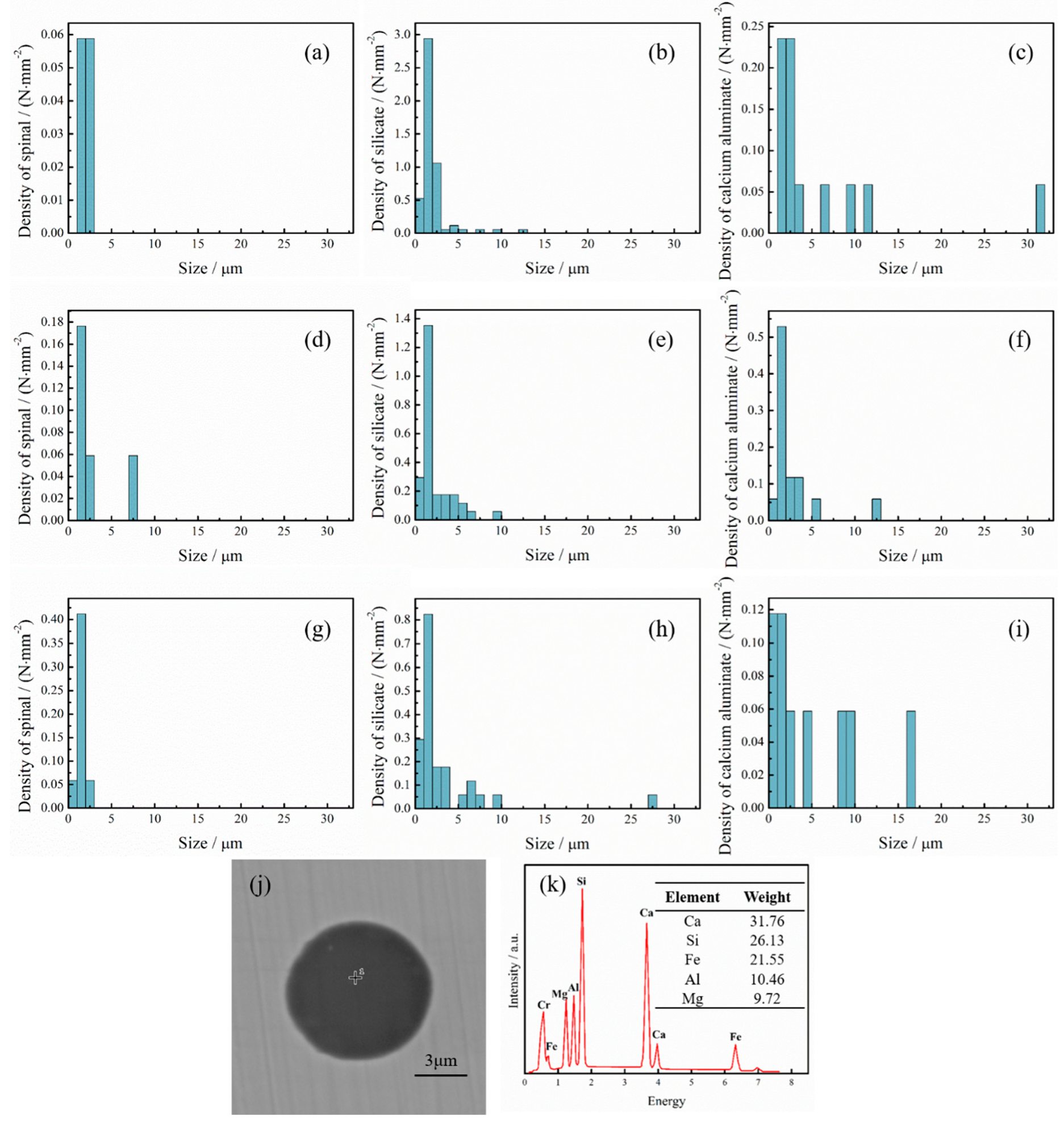
3.2. The Change of Oxides During RH Process
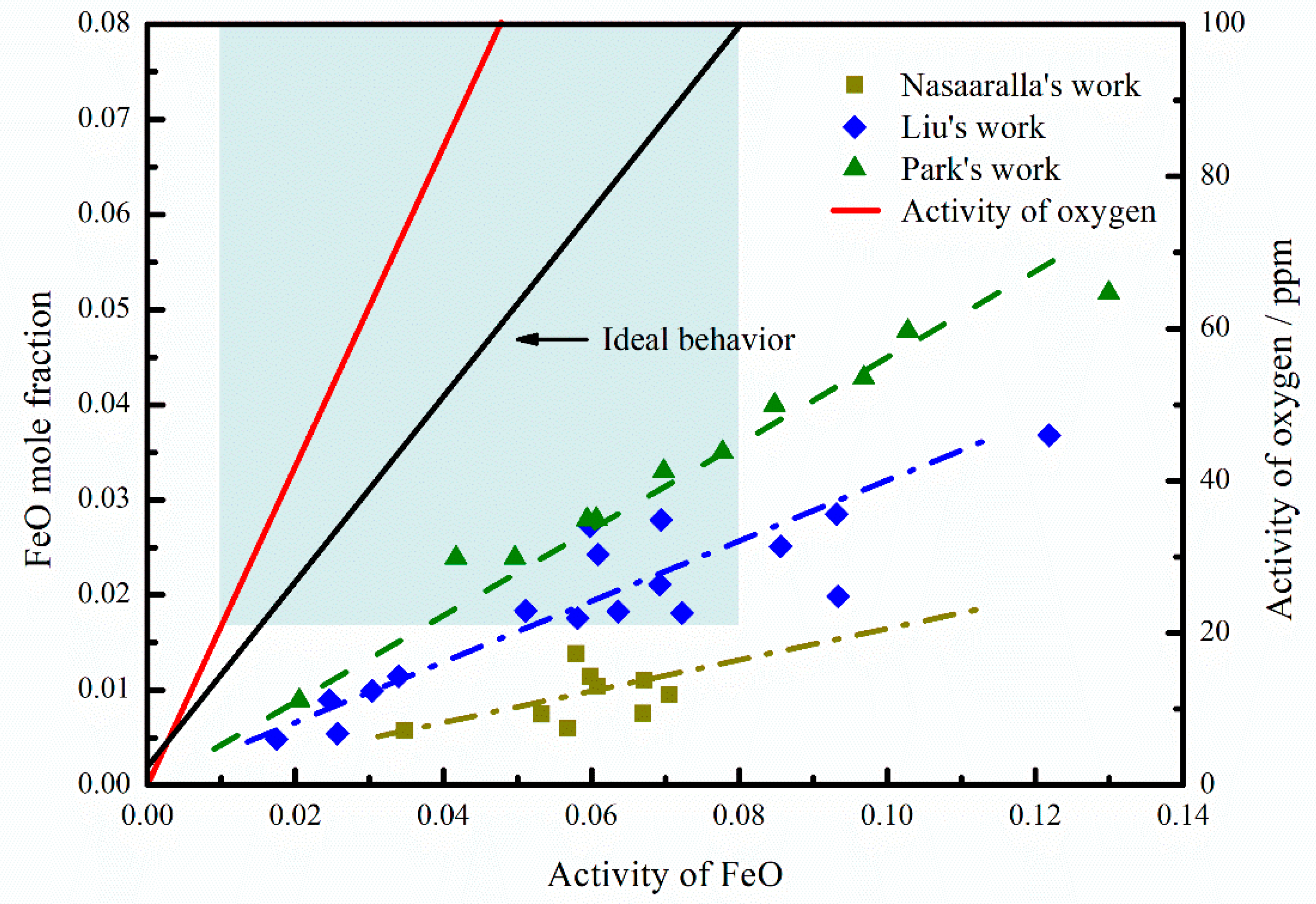
3.3. Change of FeO Content in Refining Slag
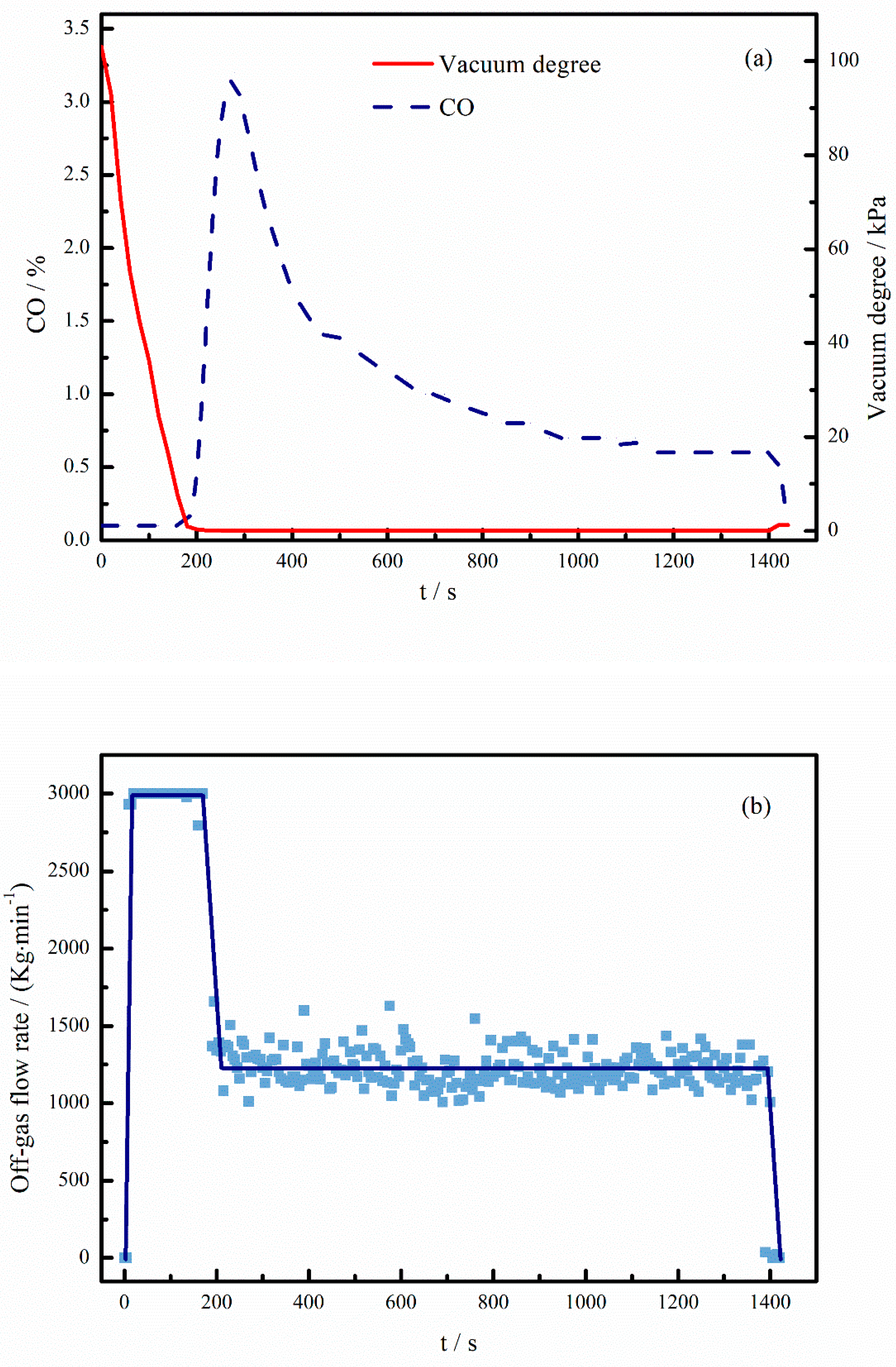
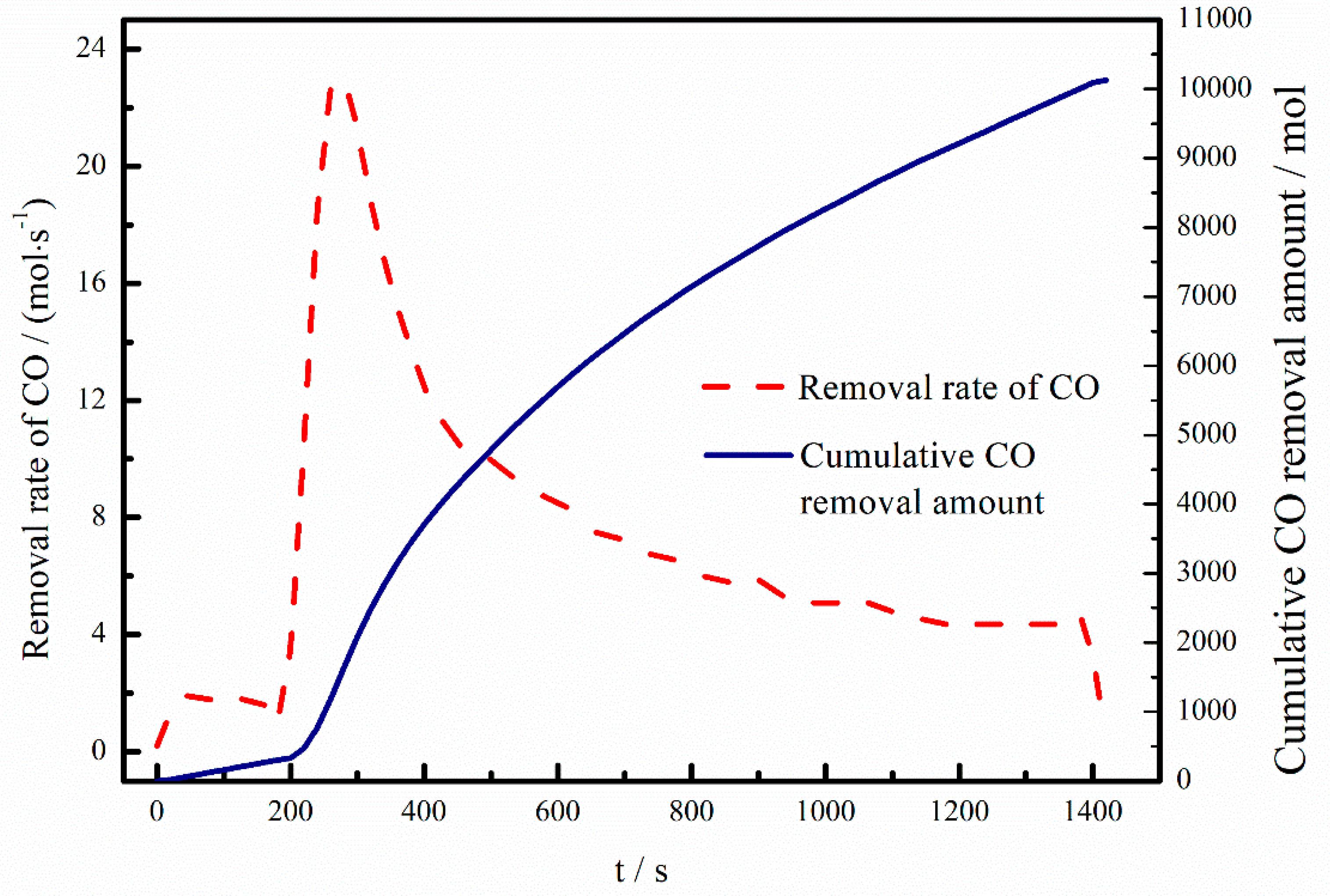
3.4. Component Analysis of Off-Gas
4. Discussion
4.1. The Relationship of Smelting Time and Content of Combined and Dissolved Oxygen
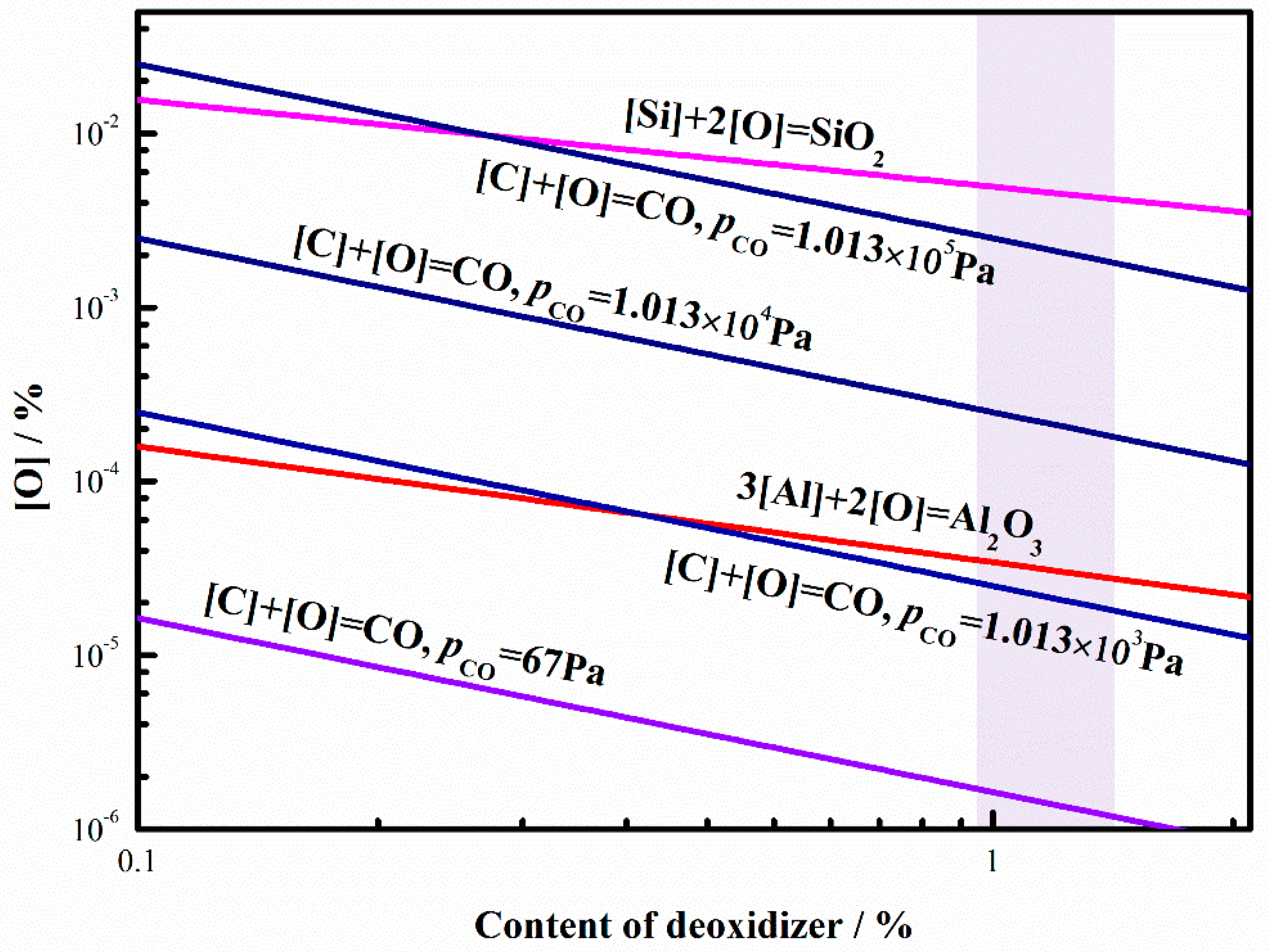
4.2. Carbon Deoxidization during the RH Process
4.2.1. The Deoxidization Ability of Carbon during Different Vacuum Condition
4.2.2. Effect of Oxidizing RH Slag on Deoxidization
4.3. Behavior of [O] during RH Process
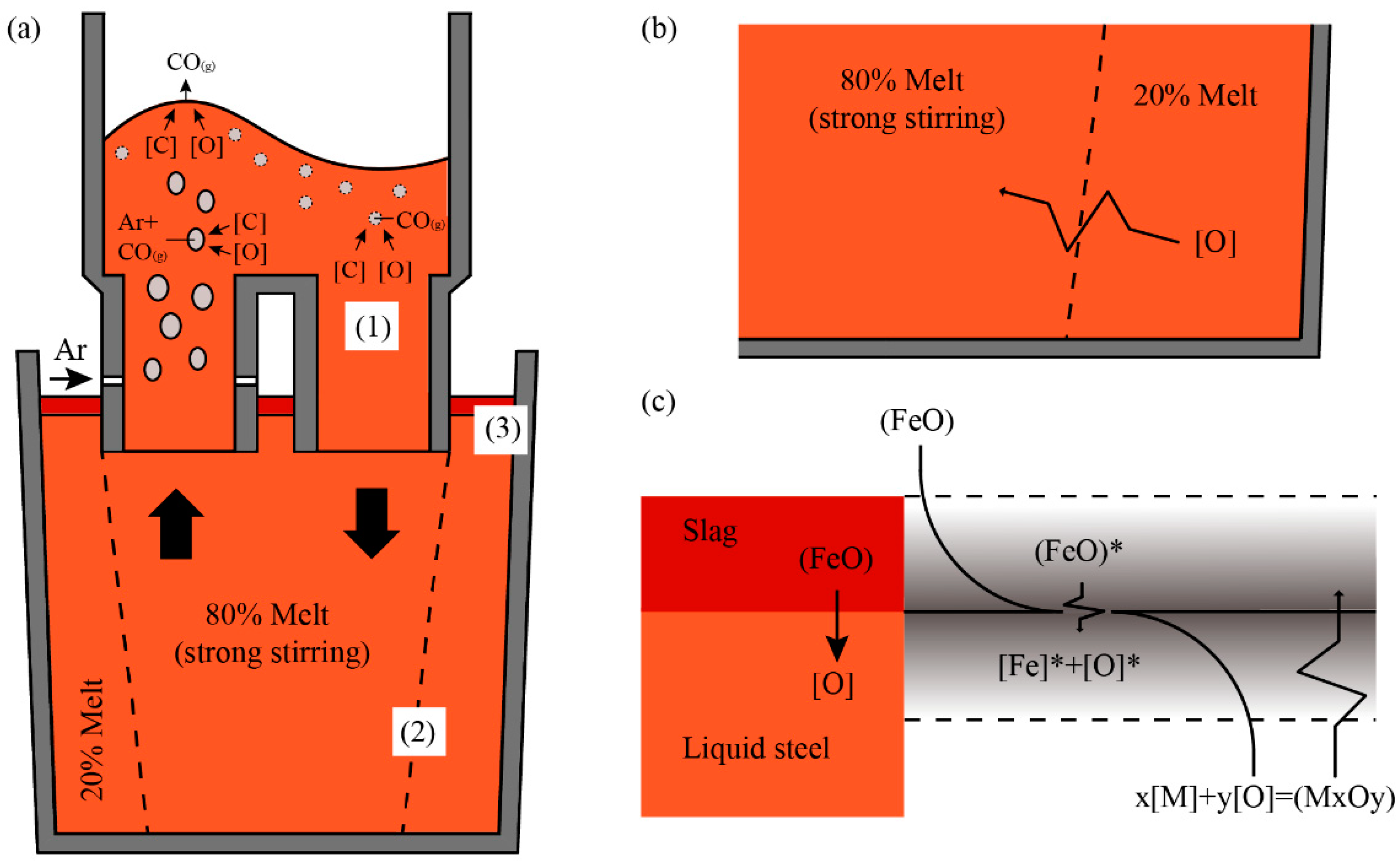
- (1)
- Deoxidization reaction mainly takes place in a vacuum chamber that involves the following three reaction sites: (a) Spontaneous CO bubbles formed in the bulk steel; (b) Ar bubbles from the Ar blowing in the upper snorkel; (c) bath surface exposed in the ultra-low-pressure environment. The reaction product, CO (g), will be exhausted due to the vacuum condition promoting the reaction.
- (2)
- There is part of the liquid steel in the ladle that is nearly immobile when the circulation of RH agitates liquid steel, which is called the dead zone in ladle. The oxygen content of the liquid steel out from the vacuum chamber is relatively lower than that in the dead zone in the ladle because of the deoxidization reaction that occurred in the vacuum chamber. The gradient of oxygen results in the oxygen mass transfer between dead zone and stirring zone.
- (3)
- The oxygen in the slag significantly affects the deoxidization effect in the RH process as explained above. The oxygen mass transfer between the ladle slag and liquid steel mainly occurred due to the reactions of Equations (17) and (18). Some researchers [21] used double-film theory to explain the re-oxidization process, which is shown in Figure 9c. According to the theory, the activity of oxygen at the boundary layer depends on the Fe–O equilibrium at the steel slag interface, while the concentration of oxygen in liquid steel depends on the M–O equilibrium. The M–O equilibrium cannot be established because of the deoxidization of RH process with the vacuum condition. Thus, a new M–O equilibrium can be established only when [M] in steel reacts with O in slag continuously, which results in the mass transfer of oxygen from ladle slag to liquid steel.
5. Conclusions
- (1)
- The content change of the combined oxygen during the RH process was calculated. Compared with the change of T.O, it is found that the decrease of the oxygen content is not mainly caused by the removal of oxides, but caused by the removal of dissolved oxygen during RH process.
- (2)
- Carbon deoxidization exists in the RH process in high-carbon steel with low oxygen content, and carbon is found to be a strong deoxidizer. In high vacuum degree, the deoxidizing capacity of carbon is even stronger than Al.
- (3)
- The oxygen is transferred from the top slag to the liquid steel during the deoxidization process, leading to the re-oxidization of liquid steel despite the low FeO content in top slag.
- (4)
- During the RH process, the change of the oxygen content mainly exists in three processes: 1) Deoxidization reaction in the vacuum chamber, 2) oxygen mass transfer process between liquid steel out from the vacuum chamber and in the ladle, and 3) oxygen mass transfer between the ladle slag and liquid steel. The changes in the oxygen content mainly depends on the mass transfer of the oxygen in the liquid steel.
Author Contributions
Funding
Conflicts of Interest
References
- Wang, S.; Lv, Q.; Wang, H.; Liang, M. Behavior of non-metallic inclusions in casting slab of bof bearing steel. J. Southeast Univ. Nat. Sci. Ed. 2006, 27, 192–195. [Google Scholar]
- Walker, P.F.F. Improving the reliability of highly loaded rolling bearings: The effect of upstream processing on inclusions. Mater. Sci. Tech.-Lond. 2014, 30, 385–410. [Google Scholar] [CrossRef]
- Kamiya, T.; Mizobe, K.; Kida, K. Effect of observation position of SUJ2 bar specimens on inclusions distribution. IOP Conf. Ser. Mater. Sci. Eng. 2018, 307, 012046. [Google Scholar] [CrossRef]
- Gu, C.; Bao, Y.; Gan, P.; Wang, M.; He, J. Effect of main inclusions on crack initiation in bearing steel in the very high cycle fatigue regime. Int. J. Min. Met. Mater. 2018, 25, 623–629. [Google Scholar] [CrossRef]
- Liu, L. New tendency and process innovation for special steels. Steelmaking 2017, 33, 1–11. [Google Scholar]
- Ma, W.J.; Lui, G.L.; Gao, P.; Luo, Y.Z.; Li, H.B.; Chen, B. Study on controlling inclusions and total oxygen of high-carbon steel. Mater. Sci. Tech.-Lond. 2016, 32, 1119–1125. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Duan, J.; Yang, W. Formation and controlling of type-D inclusions in bearing steel. Chin. J. Eng. 2018, 40, 31–35. [Google Scholar]
- Gu, C.; Bao, Y.; Gan, P.; Lian, J.; Münstermann, S. An experimental study on the impact of deoxidation methods on the fatigue properties of bearing steels. Steel Res. Int. 2018, 89, 1800129. [Google Scholar] [CrossRef]
- Geng, D.; Lei, H.; He, J. Numerical simulation of the multiphase flow in the Rheinsahl–Heraeus (RH) system. Metall. Mater. Trans. B 2010, 41, 234–247. [Google Scholar] [CrossRef]
- Wang, M.; Bao, Y.; Zhao, L.; Yang, Q.; Lin, L. Difference analysis in steel cleanness between two RH treatment modes for SPHC grade. ISIJ Int. 2015, 55, 1652–1660. [Google Scholar] [CrossRef]
- Ling, H.; Zhang, L. A mathematical model for prediction of carbon concentration during RH refining process. Metall. Mater. Trans. B 2018, 49, 2963–2968. [Google Scholar] [CrossRef]
- Van Ende, M.; Kim, Y.; Cho, M.; Choi, J.; Jung, I. A kinetic model for the Ruhrstahl-Heraeus (RH) degassing process. Metall. Mater. Trans. B 2011, 42, 477–489. [Google Scholar] [CrossRef]
- Ling, H.; Zhang, L. Investigation on the fluid flow and decarburization process in the RH process. Metall. Mater. Trans. B 2018, 49, 2709–2721. [Google Scholar] [CrossRef]
- Kuwabara, T.; Umezawa, K.; Mori, K.; Watanabe, H. Investigation of decarburization behavior in RH-reactor and its operation improvement. Trans. Iron Steel Inst. Jpn. 1988, 28, 305–314. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Kishimoto, Y.; Sakuraya, T.; Fujii, T.; Aratani, M.; Nishikawa, H. Effect of refining conditions for ultra low carbon steel on decarburization reaction in RH degasser. ISIJ Int. 1992, 32, 126–135. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, G.; Li, H.; Li, B.; Cui, Y.; Cui, A. Decarburization rate of RH refining for ultra low carbon steel. Int. J. Min. Met. Mater. 2010, 17, 22–27. [Google Scholar] [CrossRef]
- Wang, M.; Bao, Y.; Cui, H.; Wu, H.; Wu, W. The composition and morphology evolution of oxide inclusions in Ti-bearing ultra low-carbon steel melt refined in the RH process. ISIJ Int. 2010, 50, 1606–1611. [Google Scholar] [CrossRef]
- Kim, S.; Song, B. Thermodynamic aspects of steel reoxidation behavior by the ladle slag system of CaO-MgO-SiO2-Al2O3-FetO-MnO-P2O5. Metall. Mater. Trans. B 1999, 30, 435–442. [Google Scholar] [CrossRef][Green Version]
- Liu, S.; Fruehan, R.J.; Morales, A.; Ozturk, B. Measurement of FeO activity and solubility of MgO in smelting slags. Metall. Mater. Trans. B 2001, 32, 31–36. [Google Scholar] [CrossRef]
- Deng, Z.; Zhu, M. Deoxidation mechanism of Al-killed steel during industrial refining process. ISIJ Int. 2014, 54, 1498–1506. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, X.; Li, L.; Huang, F. Effect of oxidizing slag on cleanliness of if steel during ladle holding process. Steel Res. Int. 2015, 86, 1037–1045. [Google Scholar] [CrossRef]
| Component | T.O | C | Si | Cr | Mn | P | S | Al |
|---|---|---|---|---|---|---|---|---|
| Concentration | 0.0008 | 1 | 0.27 | 1.38 | 0.35 | 0.015 | 0.0065 | 0.002 |
| Stage | CaO | SiO2 | Al2O3 | MgO | FeO | MnO | R |
|---|---|---|---|---|---|---|---|
| SRH1 | 37.2 | 41.7 | 4.67 | 12.5 | 1.386 | 0.859 | 1.072 |
| SRH2 | 37.6 | 41.8 | 4.76 | 12.4 | 0.945 | 0.882 | 1.074 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, W.; Wang, M.; Bao, Y. The Research of Low-Oxygen Control and Oxygen Behavior during RH Process in Silicon-Deoxidization Bearing Steel. Metals 2019, 9, 812. https://doi.org/10.3390/met9080812
Xiao W, Wang M, Bao Y. The Research of Low-Oxygen Control and Oxygen Behavior during RH Process in Silicon-Deoxidization Bearing Steel. Metals. 2019; 9(8):812. https://doi.org/10.3390/met9080812
Chicago/Turabian StyleXiao, Wei, Min Wang, and Yanping Bao. 2019. "The Research of Low-Oxygen Control and Oxygen Behavior during RH Process in Silicon-Deoxidization Bearing Steel" Metals 9, no. 8: 812. https://doi.org/10.3390/met9080812
APA StyleXiao, W., Wang, M., & Bao, Y. (2019). The Research of Low-Oxygen Control and Oxygen Behavior during RH Process in Silicon-Deoxidization Bearing Steel. Metals, 9(8), 812. https://doi.org/10.3390/met9080812





