Modification Mechanism of Spinel Inclusions in Medium Manganese Steel with Rare Earth Treatment
Abstract
:1. Introduction
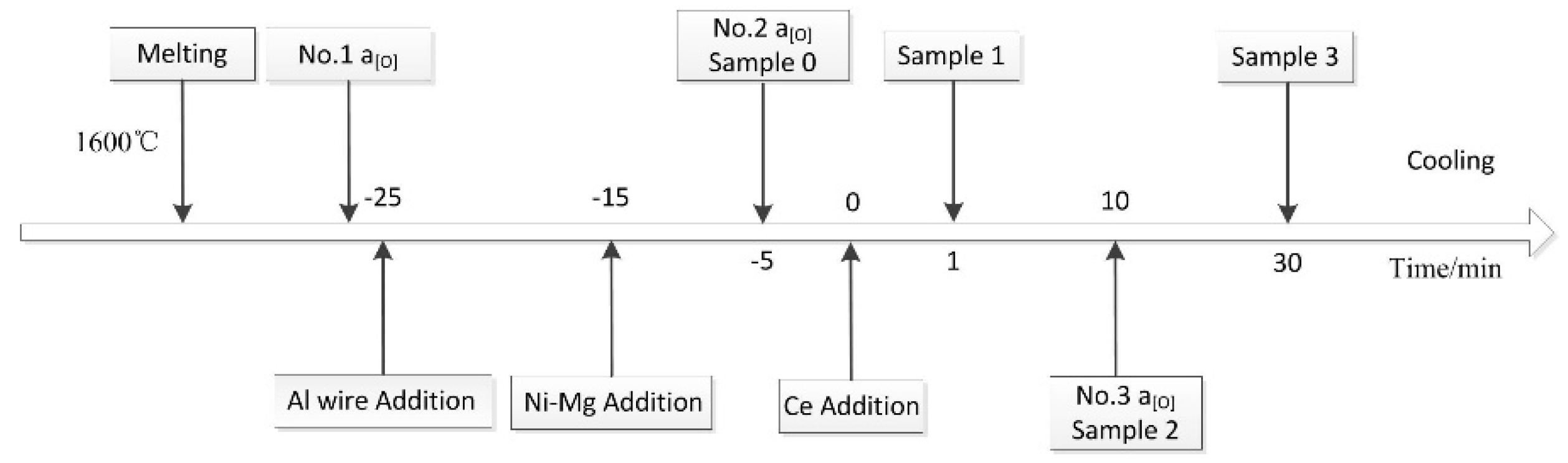
2. Experimental Procedure
3. Results
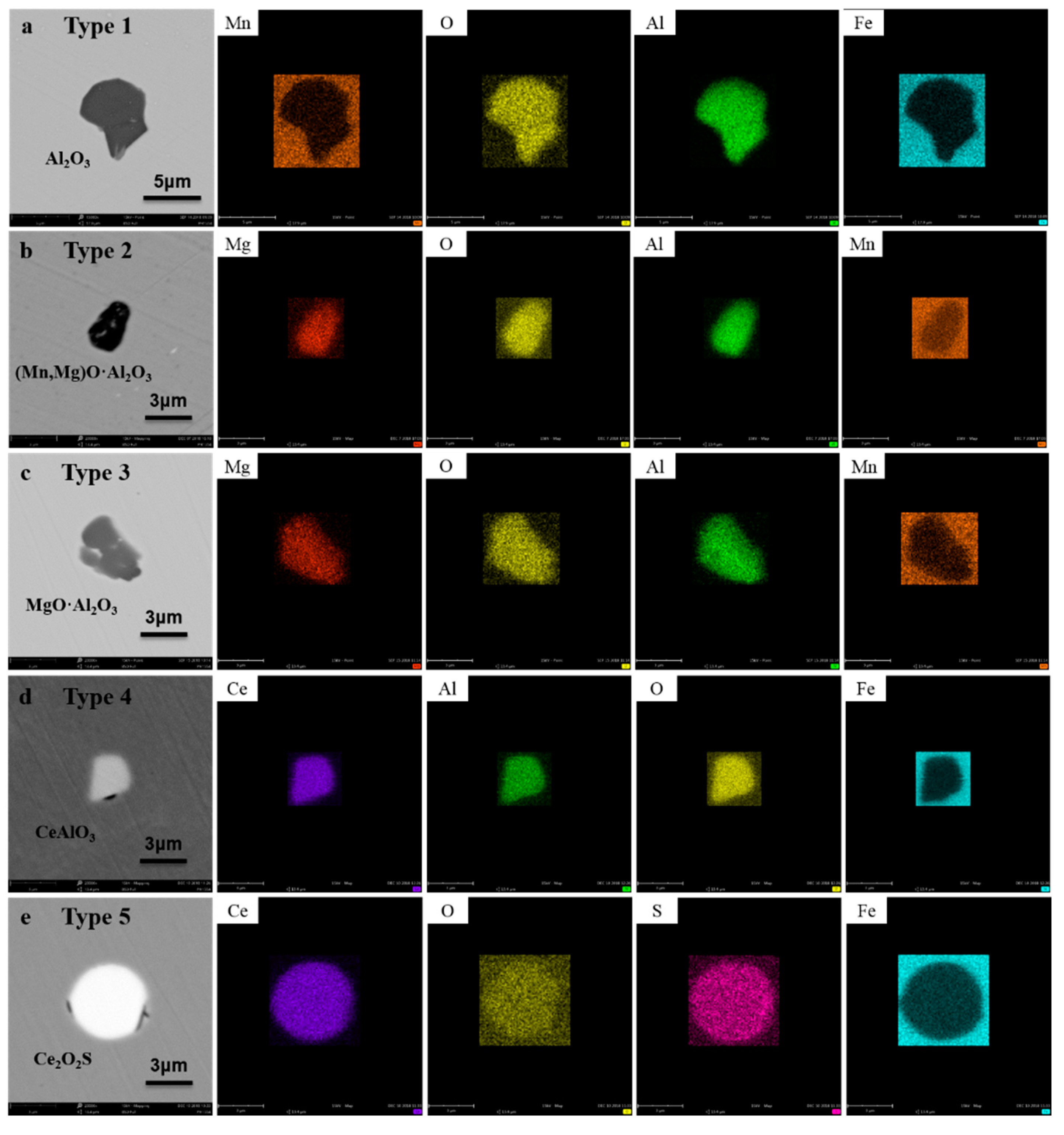
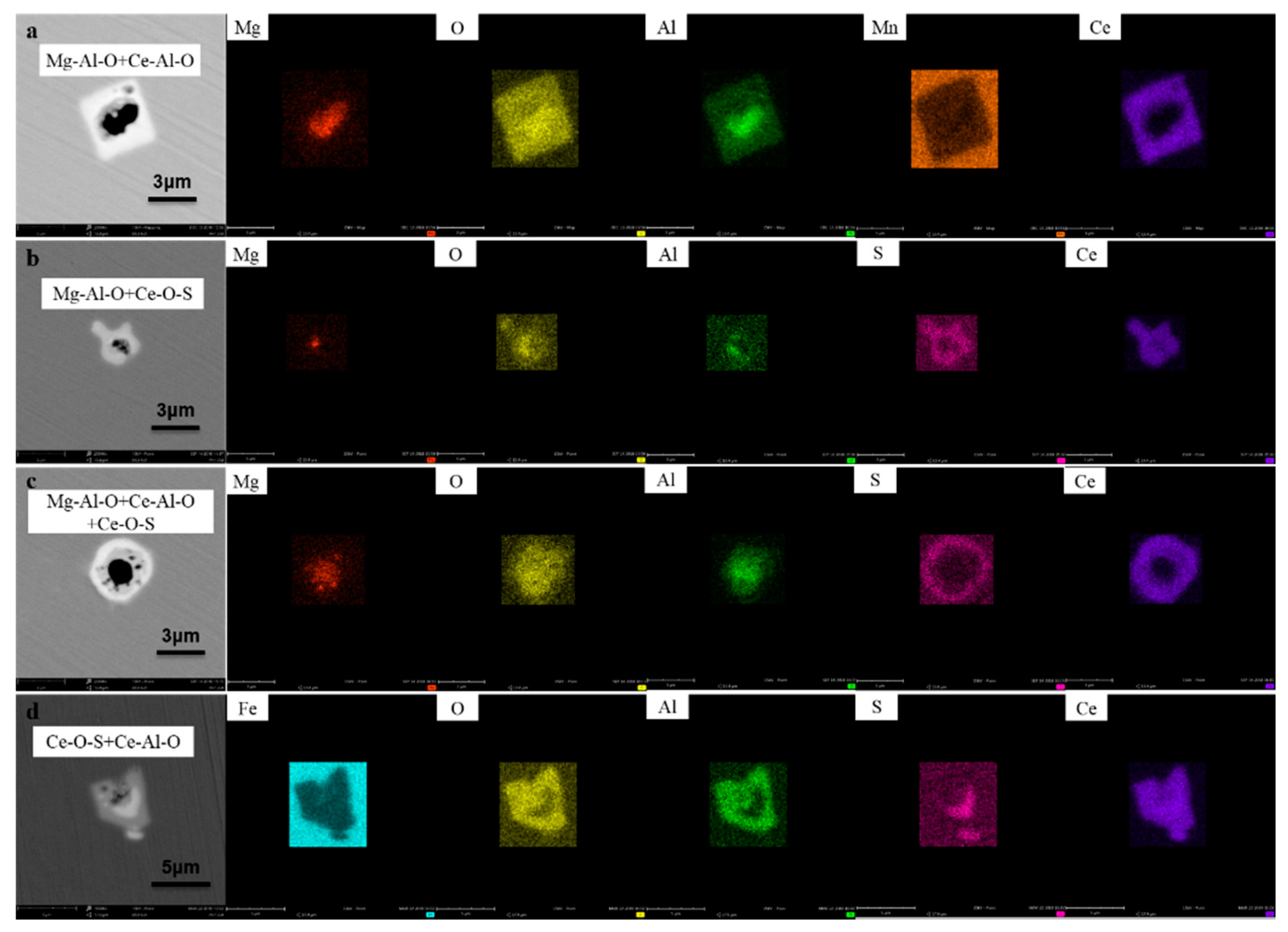
3.1. Inclusion Transformation
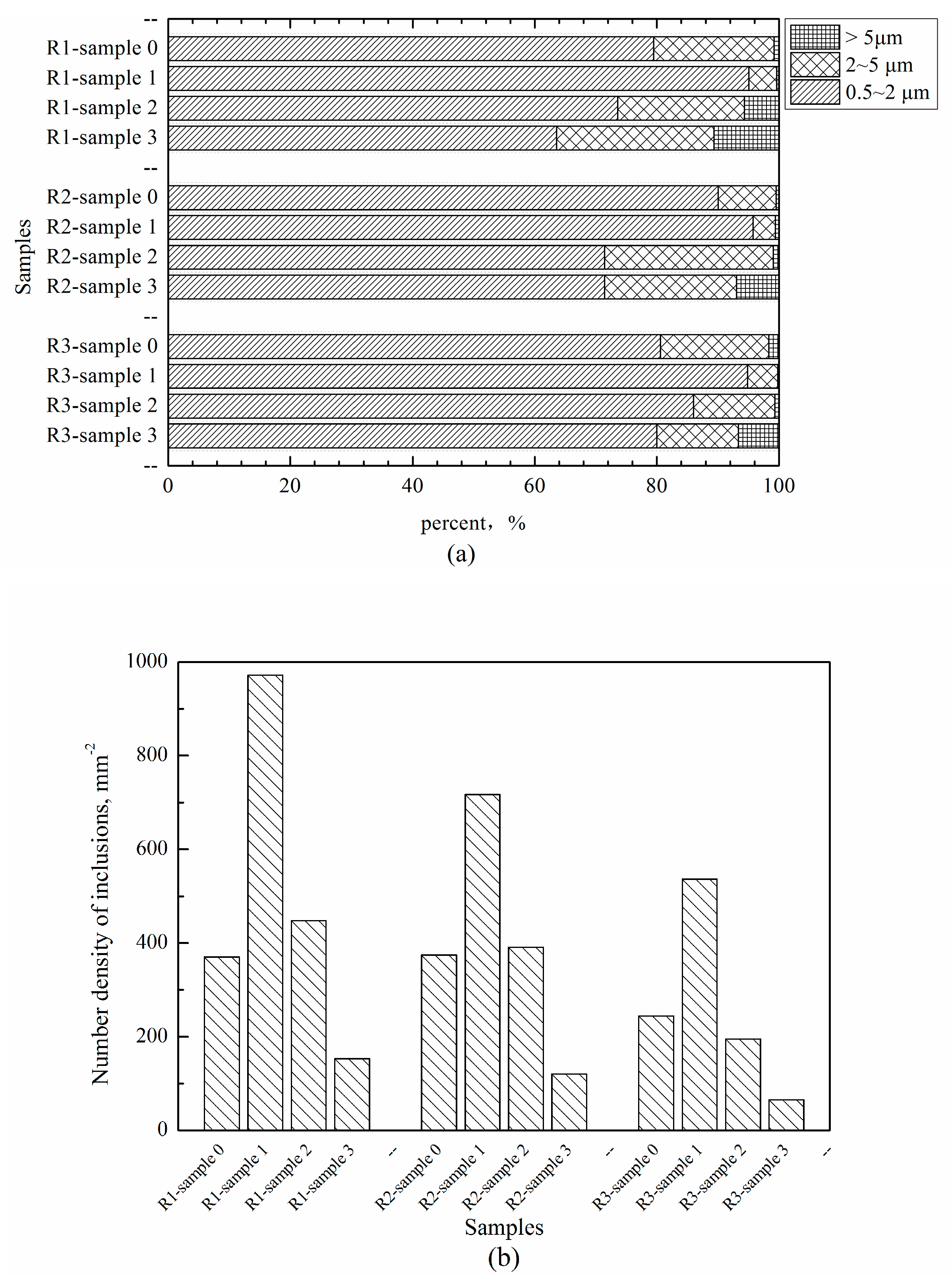
3.2. The Number Density and Size Change of Inclusions
4. Thermodynamics Analysis
5. Ce Content Required for Complete Modification of Spinel Inclusions
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kong, L.Z.; Deng, Z.Y.; Zhu, M.Y. Formation and evolution of non-metallic inclusions in medium Mn steel during secondary refining process. ISIJ Int. 2017, 57, 1537–1545. [Google Scholar] [CrossRef]
- Shao, X.J.; Ji, C.X.; Cui, Y.; Li, H.B.; Zhu, G.S.; Chen, B.; Liu, B.S.; Bao, C.L. Brief instruction of the effect of inclusions on fatigue behavior of steel. In Proceedings of the National Steelmaking Conference, Xi’an, China, 21 May 2014. [Google Scholar]
- Wang, L.; Zhang, P.J.; Lu, J.X. Quality and production technology of steel sheets for automobile. Iron Steel 1999, 34, 908–912. (In Chinese) [Google Scholar]
- Yang, S.F.; Li, J.S.; Wang, Z.F.; Li, J.; Lin, L. Modification of MgO·Al2O3 spinel inclusions in Al-killed steel by Ca-treatment. Int. J. Miner. Metall. Mater. 2011, 18, 18–23. [Google Scholar] [CrossRef]
- Pretorius, E.B.; Oltmann, H.G.; Cash, T. The effective modification of spinel inclusions by Ca treatment in LCAK steel. Iron Steel Technol. 2010, 7, 31–44. [Google Scholar]
- Ma, W.J.; Bao, Y.P.; Wang, M.; Zhao, L.H. Effect of Mg and Ca treatment on behavior and particle size of inclusions in bearing steels. ISIJ Int. 2014, 54, 536–542. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, G.G.; Xie, Y. Modification mechanism of cerium on the inclusions in drill steel. Acta Metall. 2018, 54, 1253–1261. [Google Scholar]
- Wang, L.J.; Liu, Y.Q.; Chou, K.C. Evolution mechanisms of MgO·Al2O3 inclusions by cerium in spring steel used in fasteners of high-speed railway. Trans. Iron Steel Inst. Jpn. 2015, 55, 970–975. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, G.G.; Li, S.J.; Dai, W.X. Effect of Cerium on the Behavior of Inclusions in H13 Steel. Steel Res. Int. 2018, 89, 1800371. [Google Scholar] [CrossRef]
- Hirata, H.; Isobe, K. Steel having finely dispersed inclusions. U.S. Patent 20060157162A1, 20 July 2006. [Google Scholar]
- Adabavazeh, Z.; Hwang, W.S.; Su, Y.H. Effect of adding cerium on microstructure and morphology of Ce-based inclusions formed in low-carbon steel. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.G.; Kay, D.A.R.; Vahed, A. The use of thermodynamics and phase equilibria to predict the behavior of the rare earth elements in steel. JOM 1974, 26, 14–23. [Google Scholar] [CrossRef]
- Fruehan, R.J. The free energy of formation of Ce2O2S and the nonstoichiometry of cerium oxides. Metall. Mater. Trans. B 1979, 10, 143–148. [Google Scholar] [CrossRef]
- Paek, M.K.; Jang, J.M.; Kang, Y.B.; Pak, J.J. Aluminum deoxidation equilibria in liquid iron: Part I. experimental. Metall. Mater. Trans. B 2015, 46, 1826–1836. [Google Scholar] [CrossRef]
- Vahed, A.; Kay, D.A.R. Thermodynamics of rare earths in steelmaking. Metall. Mater. Trans. B 1976, 7, 375–383. [Google Scholar] [CrossRef]
- Du, T. Thermodynamics of rare earth elements in iron-base solutions. J. Iron. Steel Res. 1994, 6, 6–12. [Google Scholar]
- Hong, T.; Debroy, T. Time-temperature-transformation diagrams for the growth and dissolution of inclusions in liquid steels. Scr. Mater. 2001, 44, 847–852. [Google Scholar] [CrossRef]
- Itoh, H.; Hino, M.; Ban, Y.S. Thermodynamics on the formation of non-metallic inclusion of spinel (MgO·Al2O3) in liquid steel. Tetsu-to-Hagané 1998, 84, 85–90. [Google Scholar] [CrossRef]
- Rohde, L.E.; Choudhury, A.; Wahlster, M. Neuere Untersuchungen über das Aluminium-Sauerstoff-Gleichgewicht in Eisenschmelzen. Archiv für das Eisenhüttenwesen 1971, 42, 165–174. [Google Scholar] [CrossRef]
- Seo, J.D.; Kim, S.H.; Lee, K.R. Thermodynamic assessment of the Al deoxidation reaction in liquid iron. Steel Res. Int. 1998, 69, 49–53. [Google Scholar] [CrossRef]
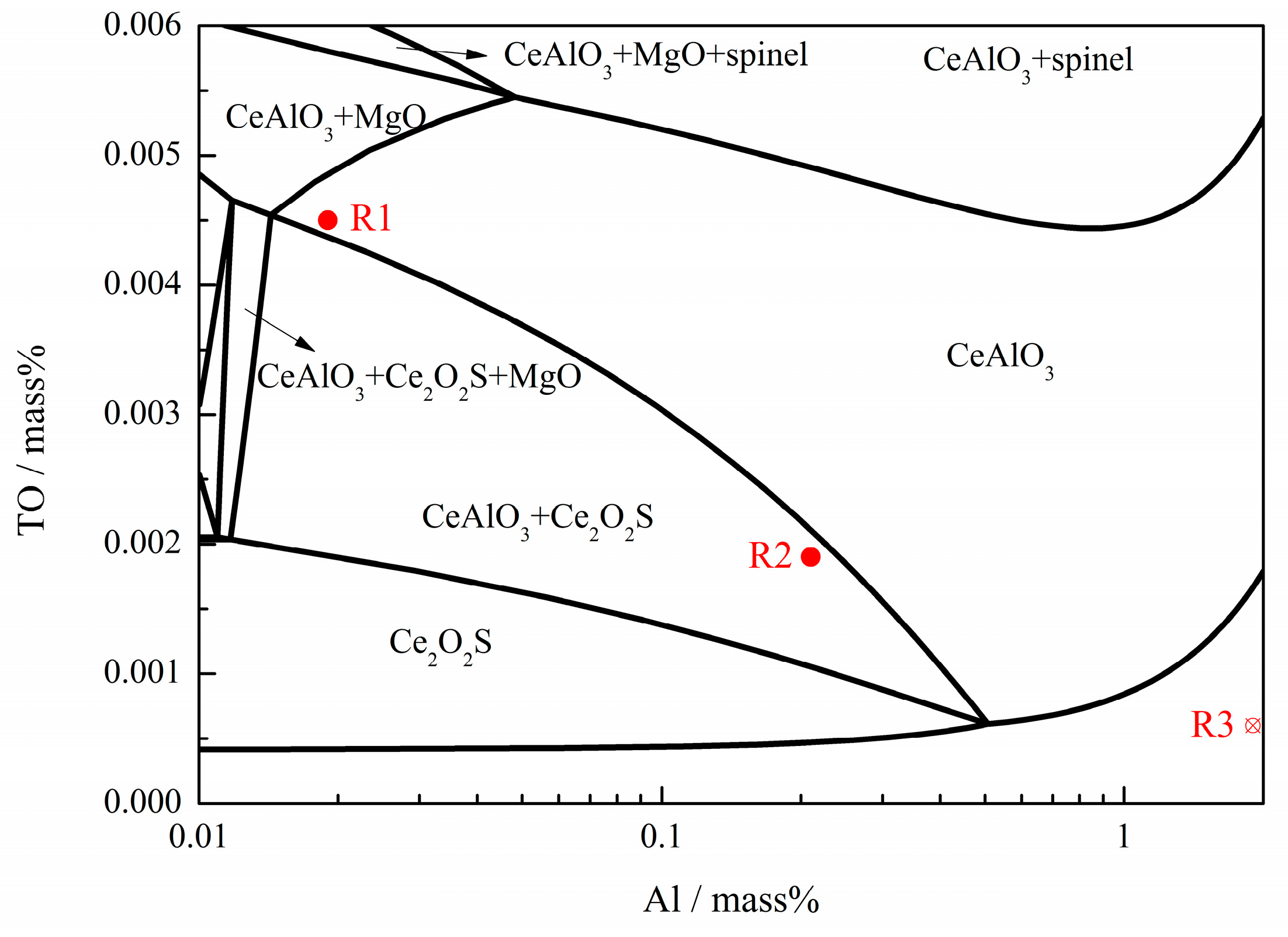
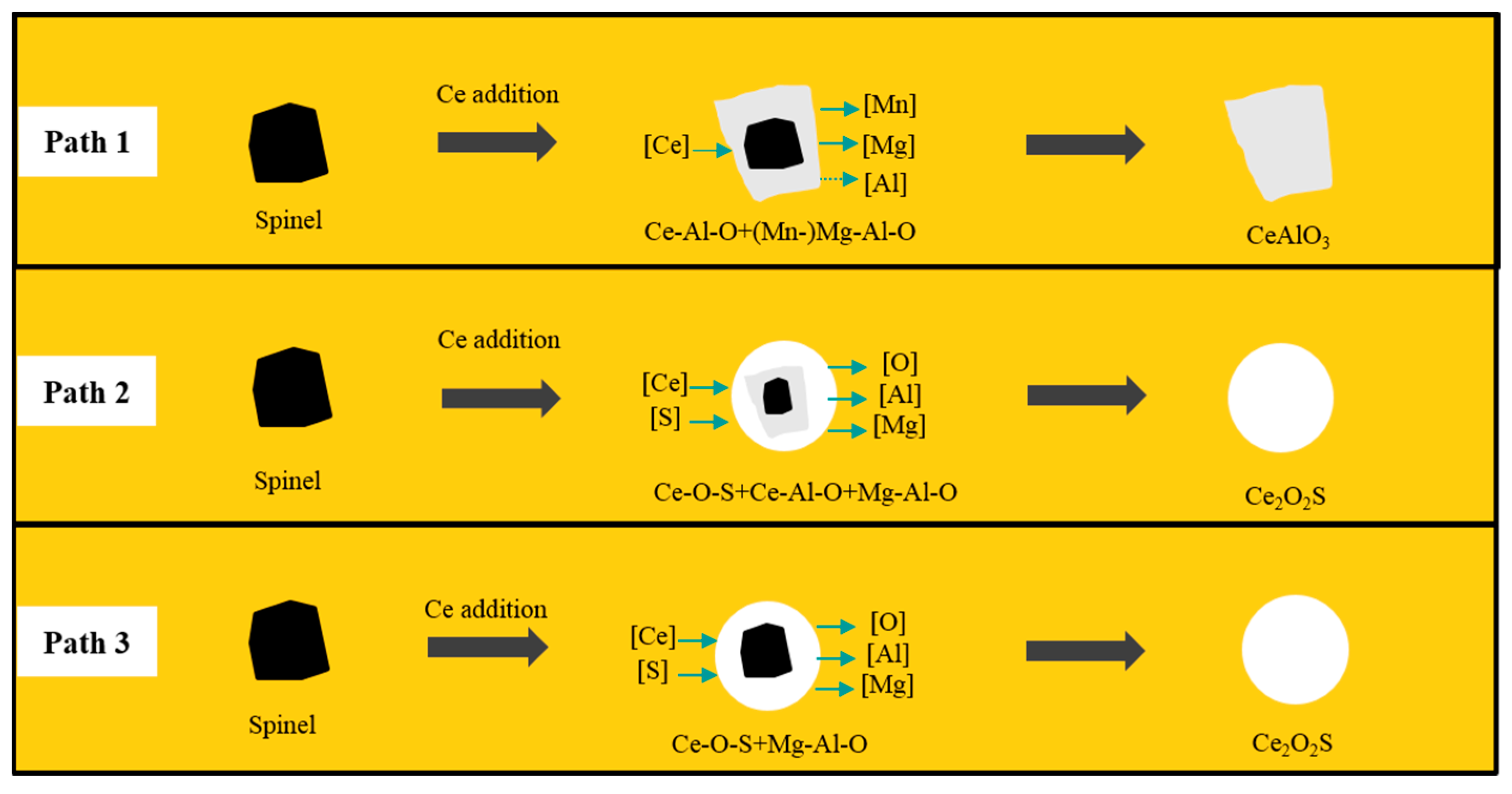
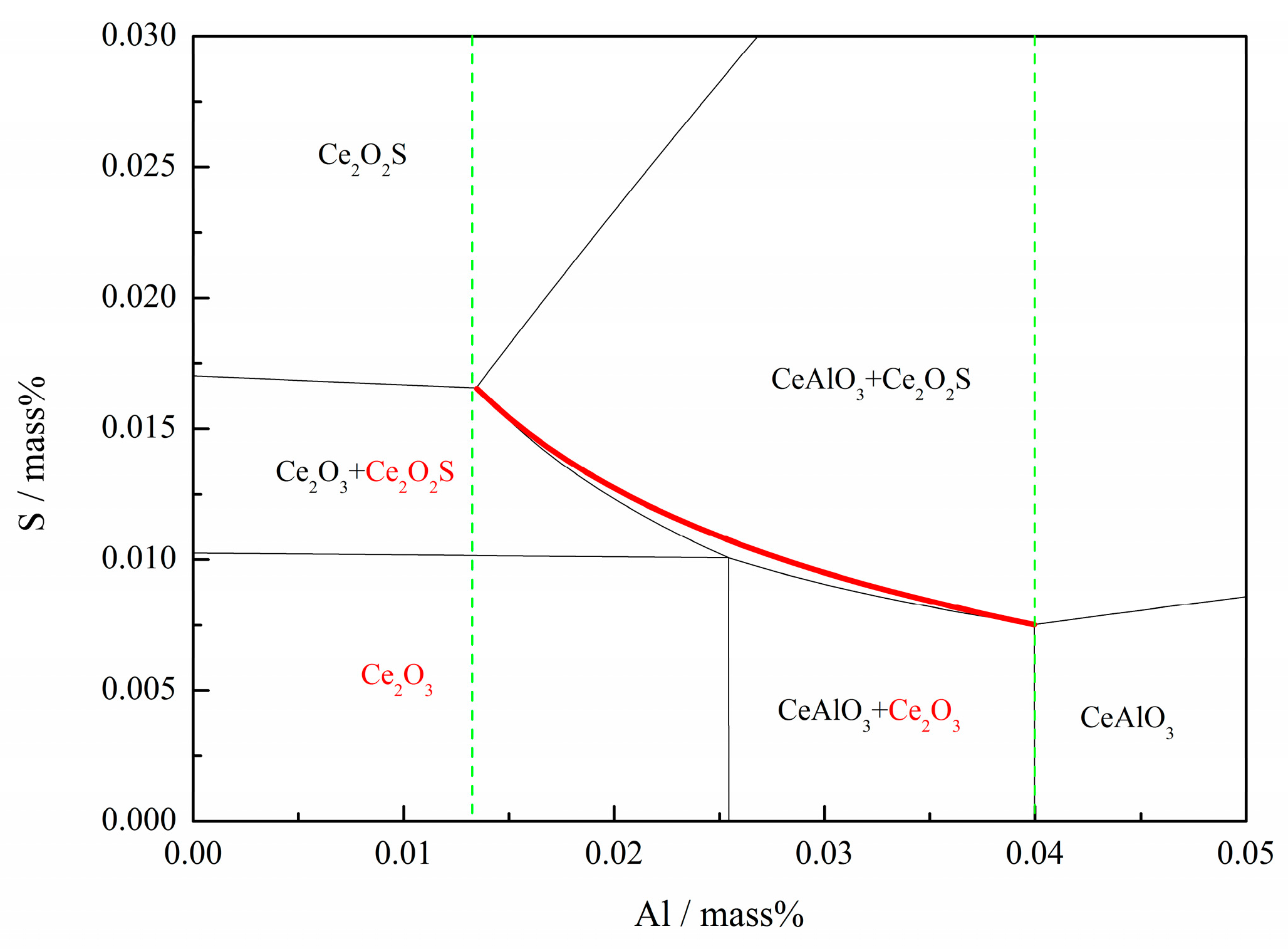
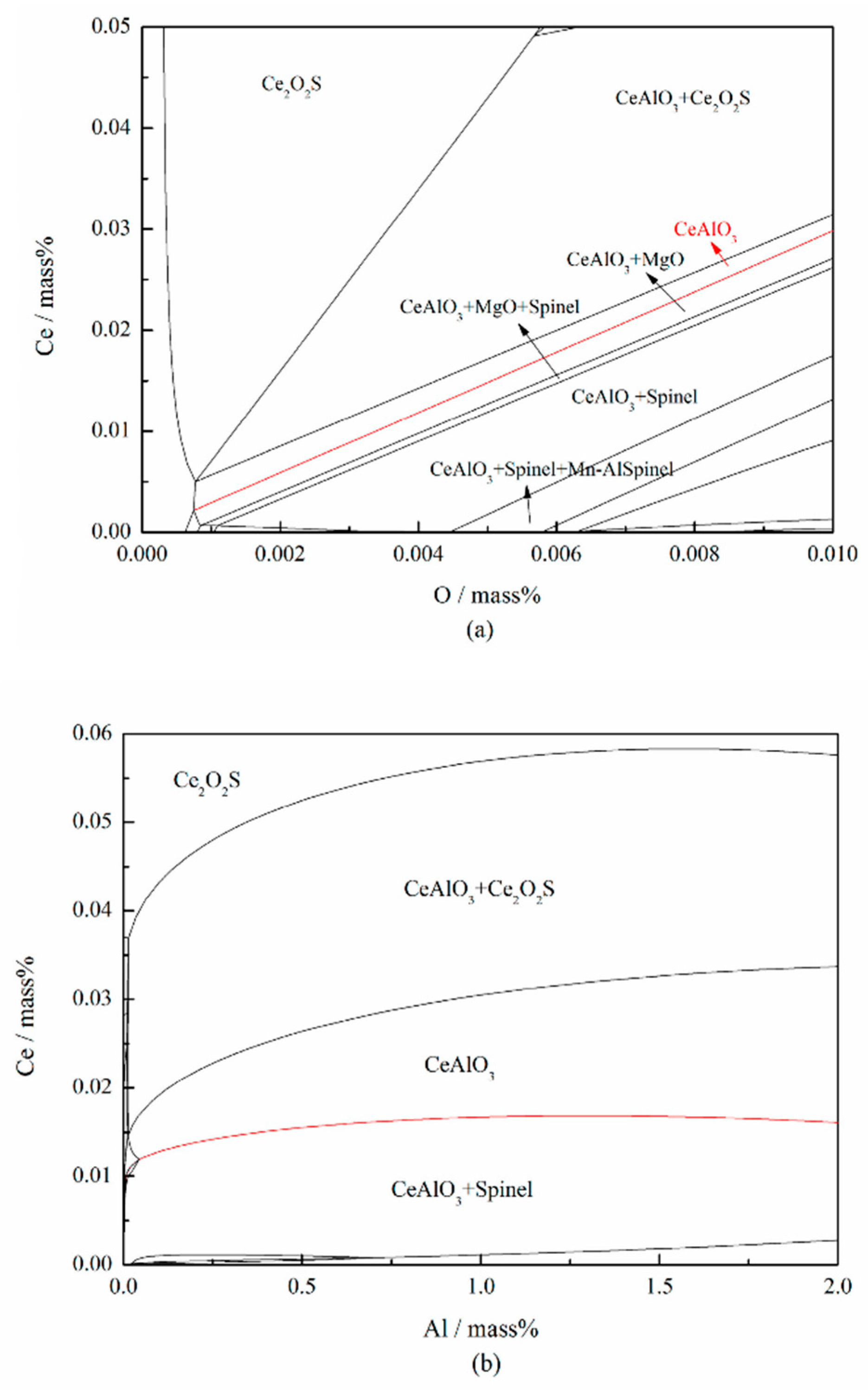
| Type | Fe | Ni | Mg | Al | C | Si | Mn | Ce | S | P | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pure iron | 99.9 | 0.01 | - | 0.001 | 0.0021 | 0.01 | 0.03 | - | 0.003 | 0.005 | 0.03 |
| Al wire | - | - | - | 99.99 | - | - | - | - | - | - | 0.01 |
| MnFe alloy | 12.57 | - | - | - | 1.5 | 1.5 | 84.2 | - | 0.03 | 0.2 | - |
| NiMg alloy | 0.93 | 80.12 | 17.98 | - | 0.78 | 0.19 | - | - | - | - | - |
| CeFe alloy | 79 | - | - | - | - | - | - | 19 | - | - | 2 |
| No. | C | Mn | Al | Mg | Ce | S | a[O] | TO | ||
|---|---|---|---|---|---|---|---|---|---|---|
| No. 1 | No. 2 | No. 3 | ||||||||
| R1 | 0.23 | 8.95 | 0.019 | 0.001 | 0.014 | 0.016 | 0.0149 | 0.0014 | 0.0005 | 0.0045 |
| R2 | 0.21 | 9.18 | 0.21 | 0.001 | 0.015 | 0.016 | 0.0124 | 0.0002 | 0.0002 | 0.0021 |
| R3 | 0.25 | 9.35 | 2.03 | 0.002 | 0.013 | 0.014 | 0.0149 | 0.0001 | 0.0001 | 0.0006 |
| Type | Chemical Formula of Inclusions | Samples in R1 Steel | Samples in R2 Steel | Samples in R3 Steel | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | ||
| 1 | Al2O3 | √ | √ | √ | |||||||||
| 2 | (Mn,Mg)O·Al2O3 | √√ | |||||||||||
| 3 | MgO·Al2O3 | √ | √√ | √ | |||||||||
| 4 | CeAlO3 | √√ | √√ | √√ | √√ | √ | √ | ||||||
| 5 | Ce2O2S | √√ | √ | √√ | √√ | √√ | √ | √ | √ | ||||
| a | Mg-Al-O + Ce-Al-O | √ | √ | √ | √ | ||||||||
| b | Mg-Al-O + Ce-O-S | √ | √ | √ | √ | ||||||||
| c | Mg-Al-O + Ce-Al-O + Ce-O-S | √ | √ | √ | |||||||||
| d | Ce-O-S + Ce-Al-O | √ | |||||||||||
| No. | Reaction Equations in Molten Steel | |
|---|---|---|
| 1 | 2[Al] + 3[O] = Al2O3(s) | −867,370 + 222.5T [20] |
| 2 | [Mg] + [O] = MgO(s) | −89,960 − 82.0T [19] |
| 3 | [Mn] + [O] = MnO(s) | −287,900 + 125T [17] |
| 4 | [Mn] + 2[Al] + 4[O] = MnO·Al2O3(s) | −1,545,000 + 524T [17] |
| 5 | [Mg] + 2[Al] + 4[O] = MgO·Al2O3(s) | −978,182 + 128.93T [18] |
| 6 | 2[Ce] + 2[O] + [S] = Ce2O2S(s) | −1,353,592.4 + 331.6T [16] |
| 7 | [Ce] + [Al] + 3[O] = CeAlO3(s) | −1,366,460 + 364T [15] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Liu, C. Modification Mechanism of Spinel Inclusions in Medium Manganese Steel with Rare Earth Treatment. Metals 2019, 9, 804. https://doi.org/10.3390/met9070804
Yu Z, Liu C. Modification Mechanism of Spinel Inclusions in Medium Manganese Steel with Rare Earth Treatment. Metals. 2019; 9(7):804. https://doi.org/10.3390/met9070804
Chicago/Turabian StyleYu, Zhe, and Chengjun Liu. 2019. "Modification Mechanism of Spinel Inclusions in Medium Manganese Steel with Rare Earth Treatment" Metals 9, no. 7: 804. https://doi.org/10.3390/met9070804
APA StyleYu, Z., & Liu, C. (2019). Modification Mechanism of Spinel Inclusions in Medium Manganese Steel with Rare Earth Treatment. Metals, 9(7), 804. https://doi.org/10.3390/met9070804





