Leaching of Pure Chalcocite in a Chloride Media Using Sea Water and Waste Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Chalcocite
2.2. Leaching and Leaching Tests
2.3. Experimental Design
3. Results
3.1. ANOVA
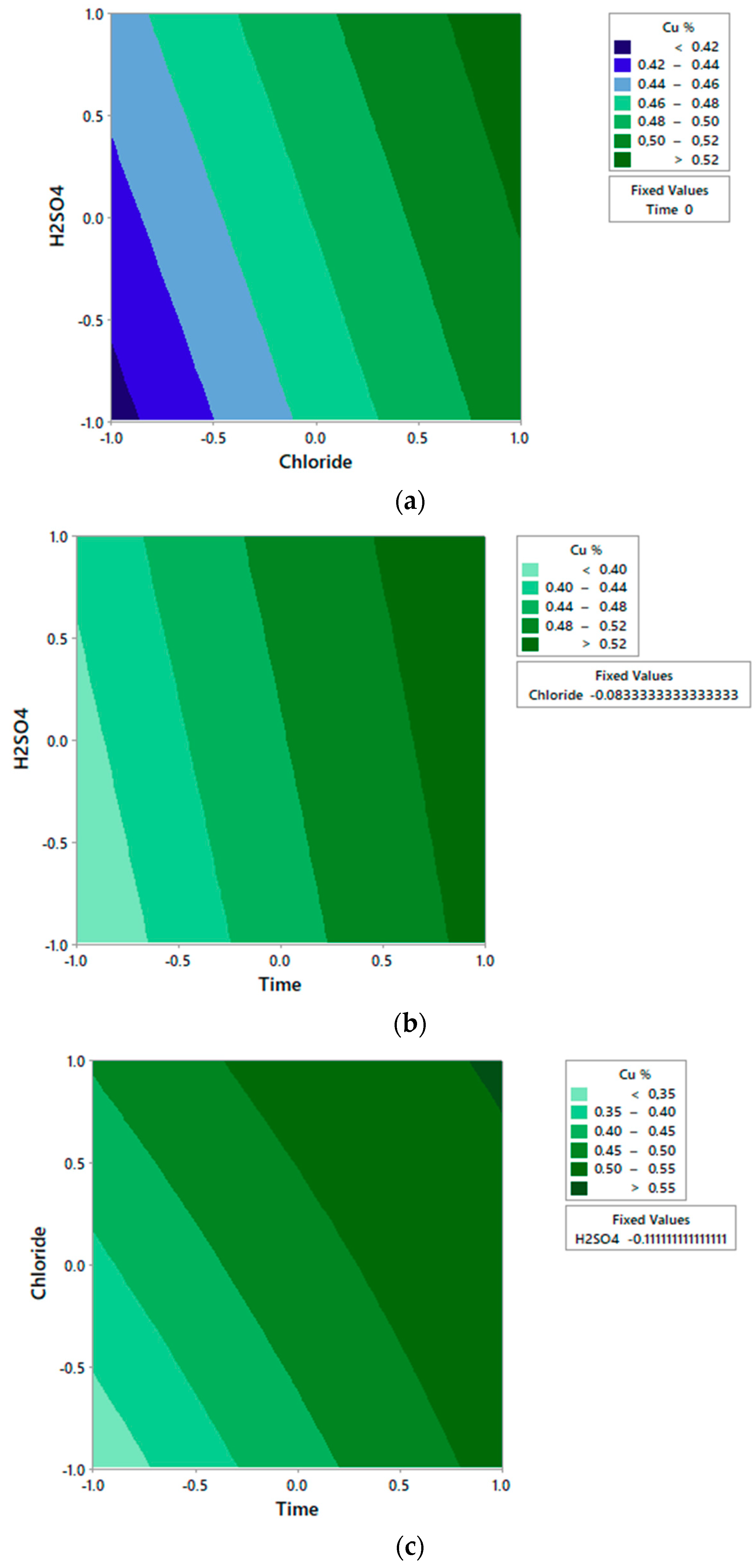
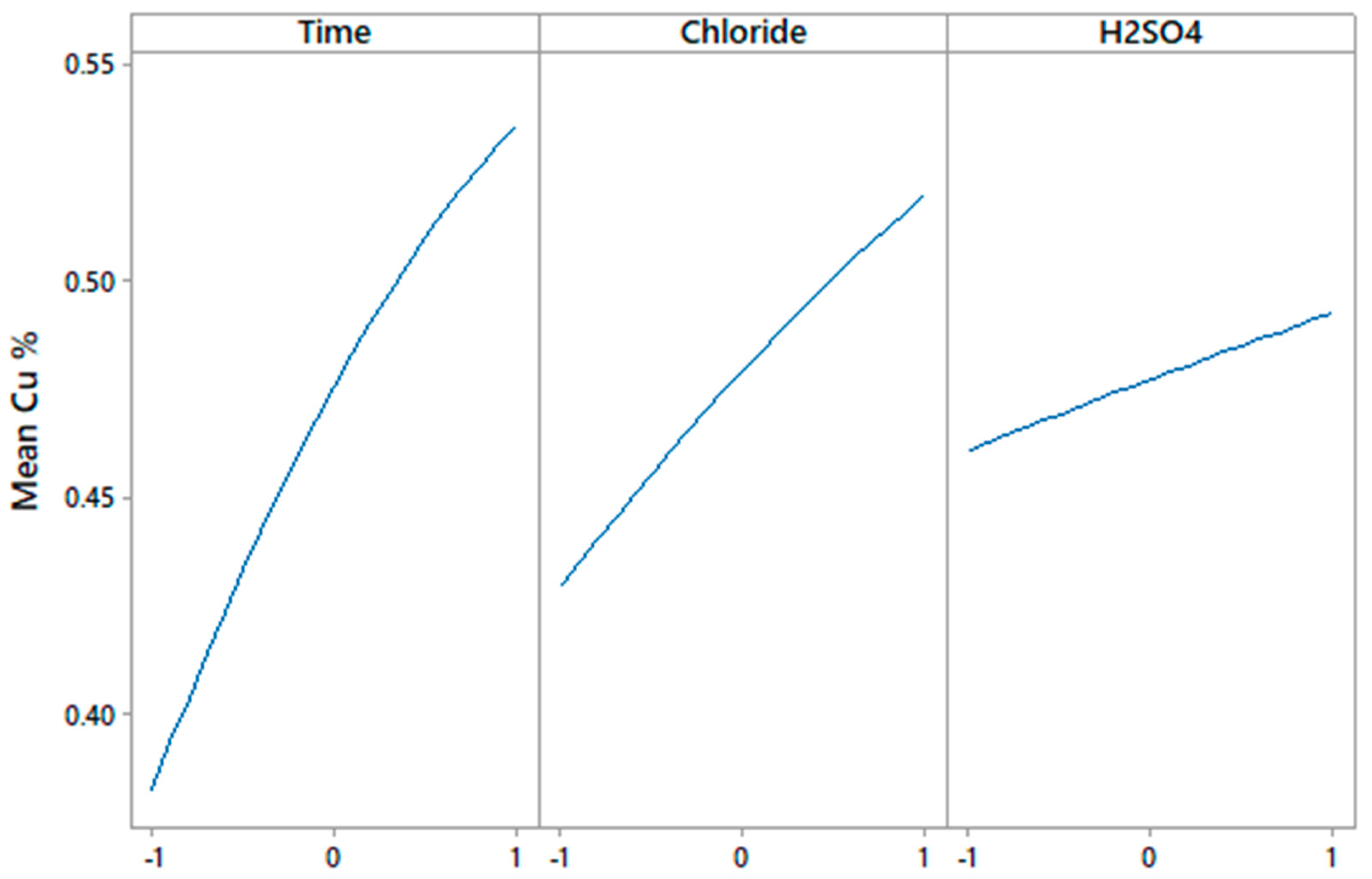
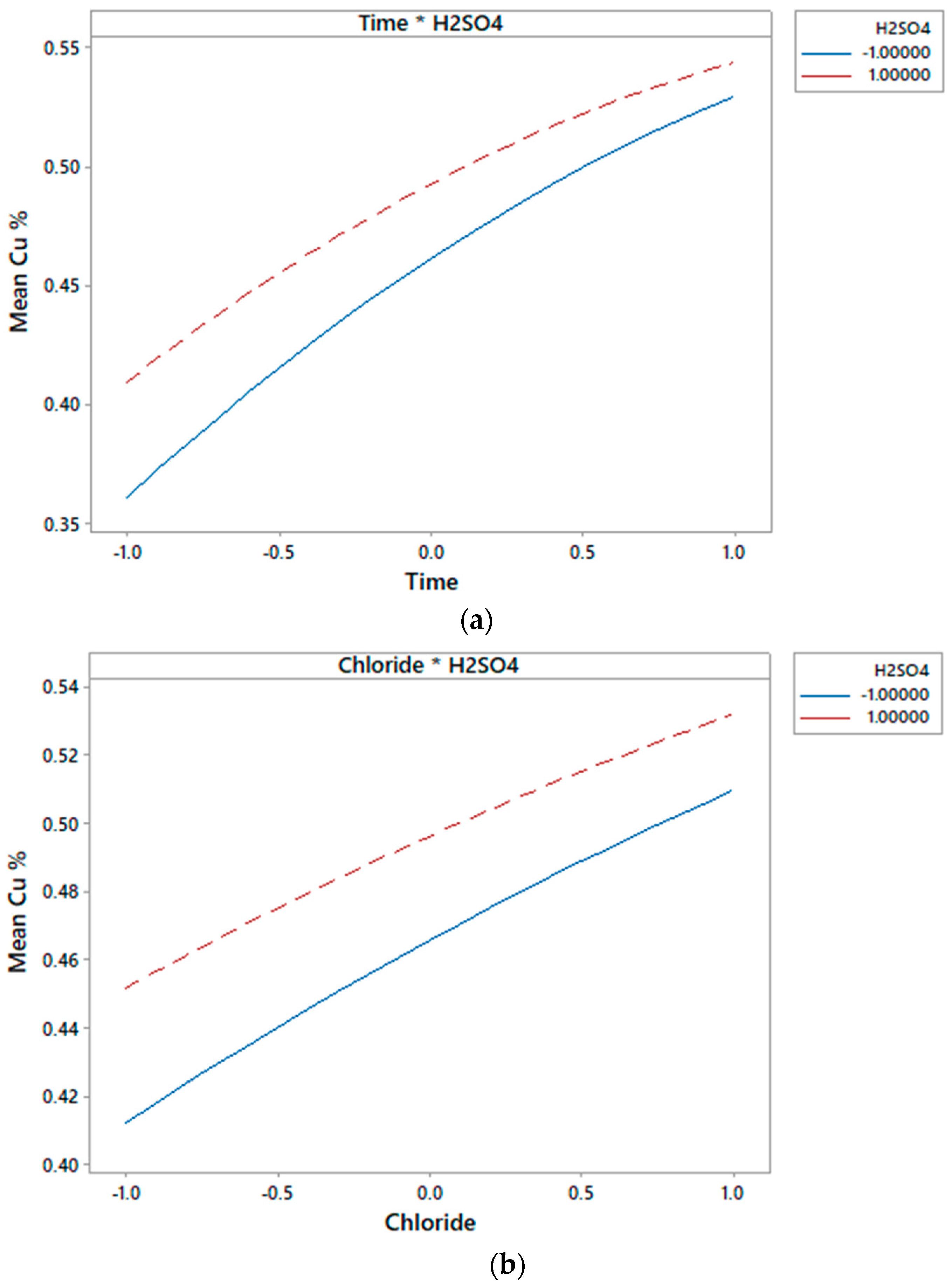
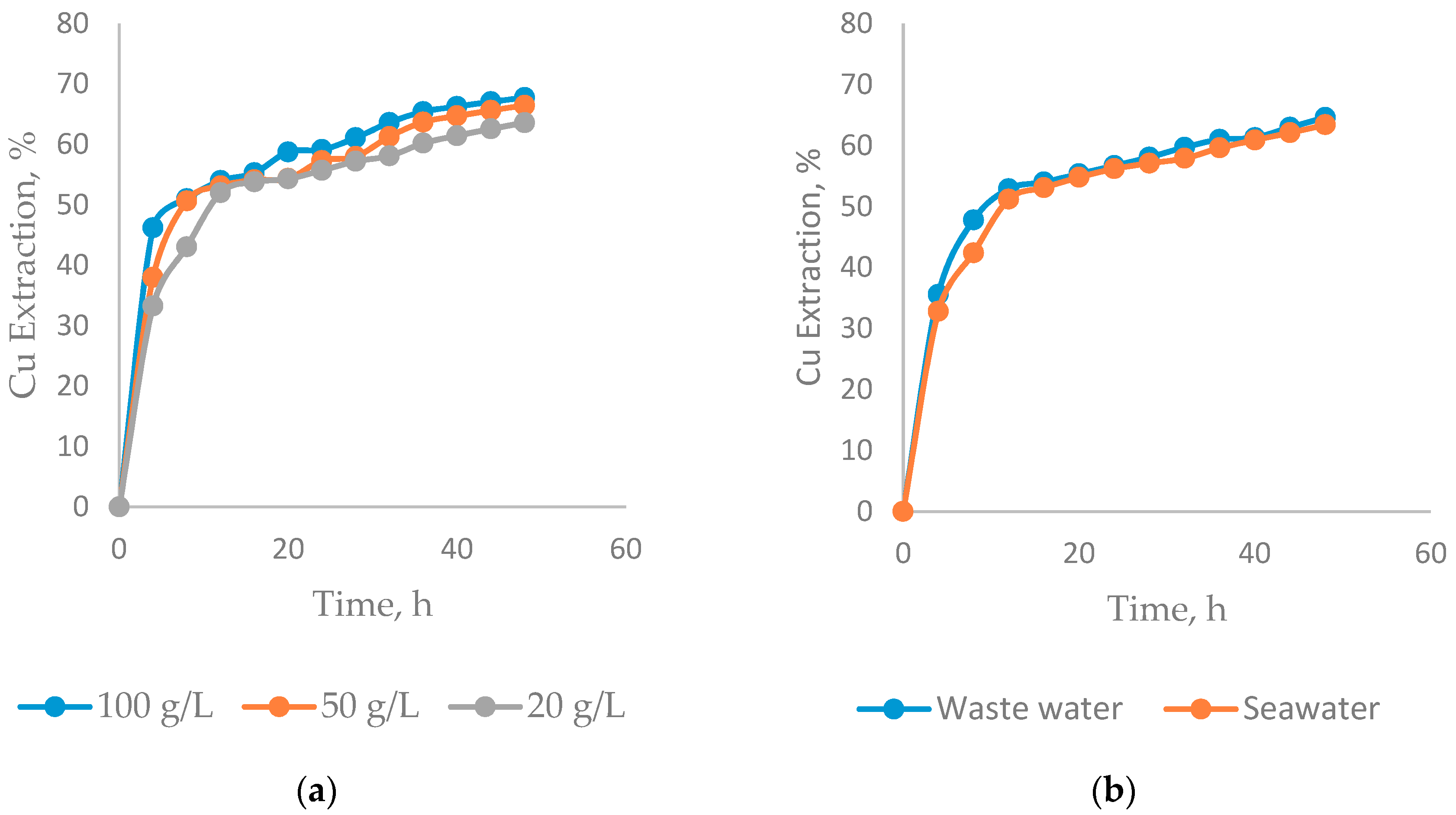
3.2. Effect on the Chloride Concentration
4. Conclusions
- The linear variables with the greatest influence in the model are: time, chloride concentration and sulfuric acid concentration, respectively.
- Under normal pressure and temperature conditions, only the chloride-time concentration exerts a significant synergistic effect on the extraction of copper from a chalcocite mineral.
- The ANOVA analysis indicates that the presented quadratic model is adequate to represent the copper extractions and the value of R2 (0.92) validates it.
- The highest copper extraction is achieved under conditions of low concentration of sulfuric acid (0.5 mol/L), high concentrations of chloride (100 g/L) and a prolonged leaching time (48 h) to obtain an extraction of 67.75% copper.
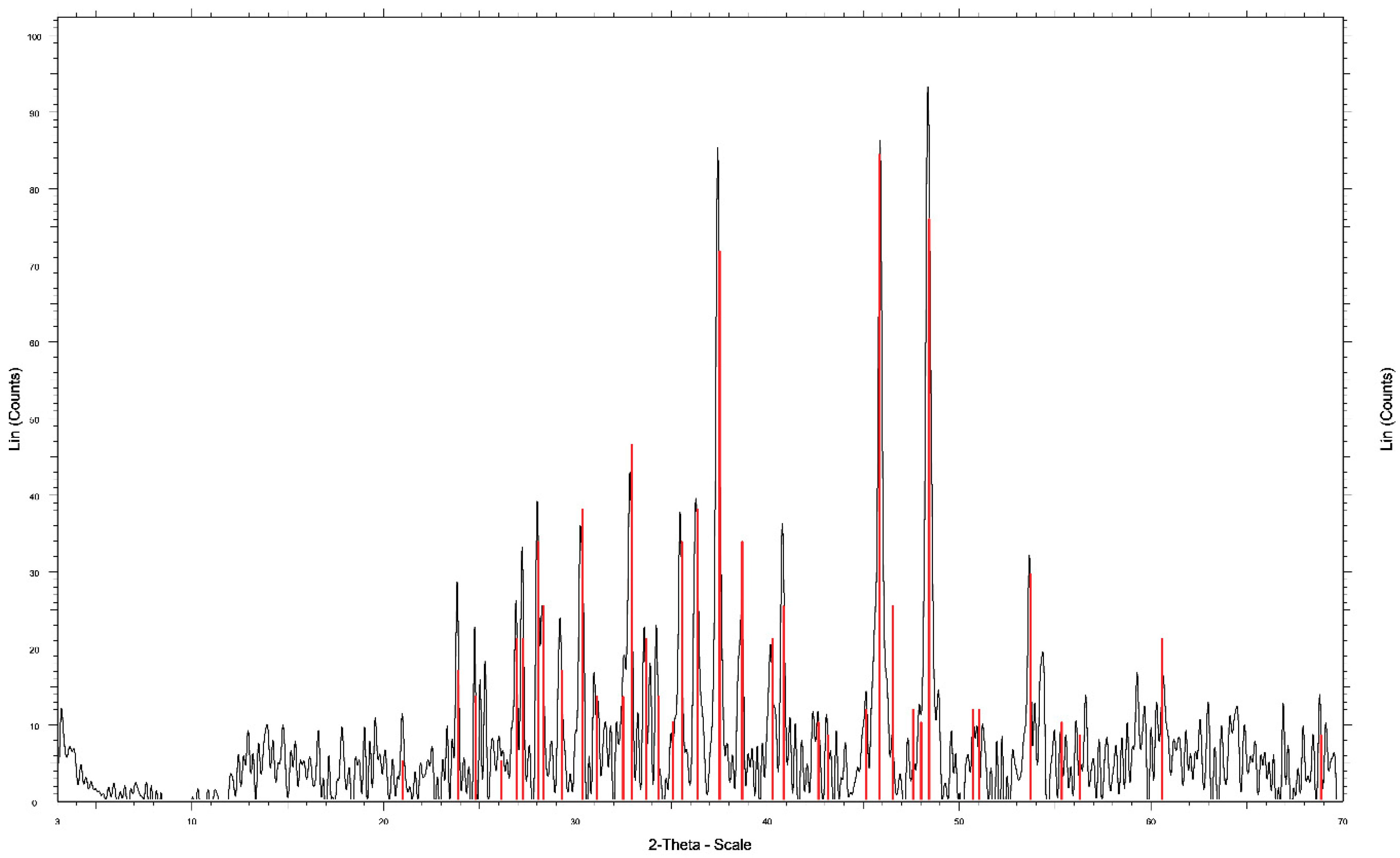
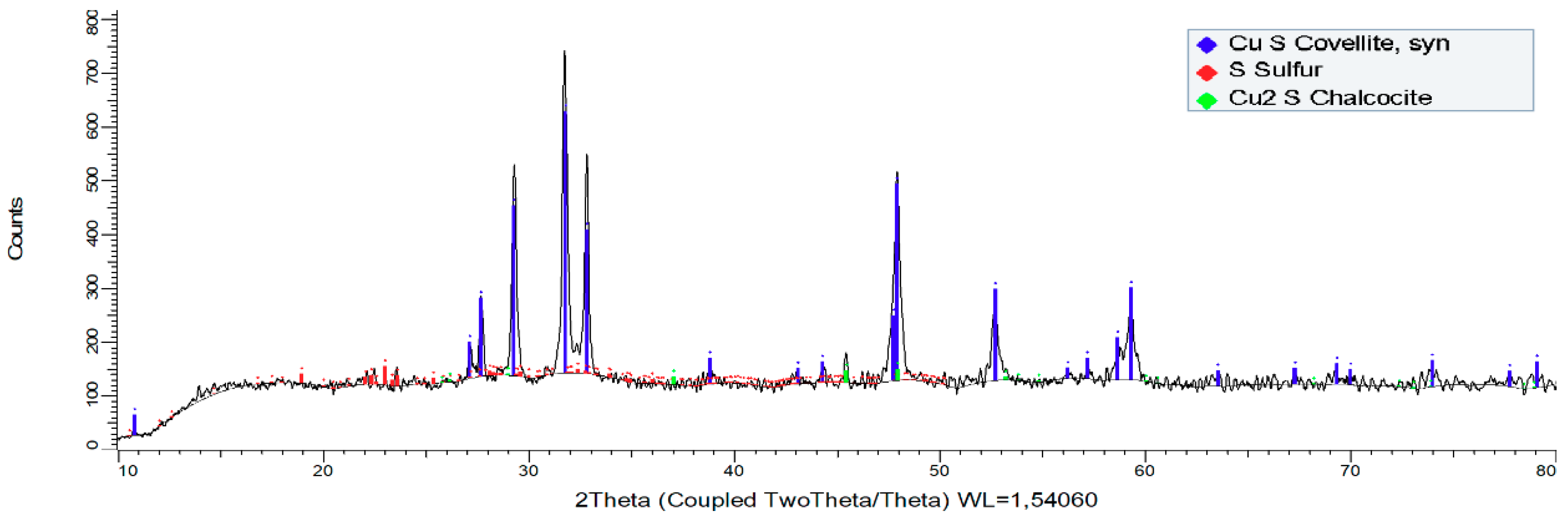
- The XRD analysis shows the formation of a stable and non-polluting residue; such as elemental sulfur (S0). This residue was obtained in a leaching time of 4 h at room temperature under conditions of 0.5 mol/L H2SO4 and 50 g/L Cl−.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Comisión Chilena del Cobre. Sulfuros Primarios: Desafíos y Oportunidades. Registro Propiedad Intelectual N° 2833439. 2017. Available online: https://www.cochilco.cl/Listado Temtico/sulfuros primarios_desafíos y oportunidades.pdf (accessed on 25 April 2019).
- Navarra, A.; Oyarzun, F.; Parra, R.; Marambio, H.; Mucciardi, F. System dynamics and discrete event simulation of copper smelters. Miner. Metall. Process. 2017, 34, 96–106. [Google Scholar] [CrossRef]
- International Copper Study Group. The World Copper Factbook 2017; International Copper Study Group: Lisbon, Portugal, 2017. [Google Scholar]
- CESCO. La Minería como plataforma para el desarrollo: Hacia una relación integral y sustentable de la industria minera en Chile. Available online: http://www.cesco.cl/wp-content/uploads/2018/06/Resumen-Position-Paper.pdf (accessed on 12 July 2019).
- Schlesinger, M.E.; King, M.J.; Sole, K.C.; Davenport, W.G. Extractive Metallurgy of Copper, 5th ed.; Elsevier: Amsterdam, Netherlands, 2011. [Google Scholar]
- Oyarzun, R.; Oyarzún, J.; Lillo, J.; Maturana, H.; Higueras, P. Mineral deposits and Cu-Zn-As dispersion-contamination in stream sediments from the semiarid Coquimbo Region, Chile. Environ. Geol. 2007, 53, 283–294. [Google Scholar] [CrossRef]
- Dijksira, R.; Senyard, B.; Shah, U.; Lee, H. Economical abatement of high-strength SO2off-gas from a smelter. J. South. African Inst. Min. Metall. 2017, 117, 1003–1007. [Google Scholar] [CrossRef]
- Serbula, S.M.; Milosavljevic, J.S.; Radojevic, A.A.; Kalinovic, J.V.; Kalinovic, T.S. Extreme air pollution with contaminants originating from the mining—Metallurgical processes. Sci. Total Environ. 2017, 586, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Zagoruiko, A.N.; Vanag, S.V. Reverse-flow reactor concept for combined SO2 and co-oxidation in smelter off-gases. Chem. Eng. J. 2014, 238, 86–92. [Google Scholar] [CrossRef]
- Baba, A.A.; Balogun, A.F.; Olaoluwa, D.T.; Bale, R.B.; Adekola, F.A.; Alabi, A.G.F. Leaching kinetics of a Nigerian complex covellite ore by the ammonia-ammonium sulfate solution. Korean J. Chem. Eng. 2017, 34, 1133–1140. [Google Scholar] [CrossRef]
- Pradhan, N.; Nathsarma, K.C.; Rao, K.S.; Sukla, L.B.; Mishra, B.K. Heap bioleaching of chalcopyrite: A review. Miner. Eng. 2008, 21, 355–365. [Google Scholar] [CrossRef]
- Mindat. Copper: The mineralogy of Copper. Available online: https://www.mindat.org/element/Copper (accessed on 8 July 2019).
- Miki, H.; Nicol, M.; Velásquez-Yévenes, L. The kinetics of dissolution of synthetic covellite, chalcocite and digenite in dilute chloride solutions at ambient temperatures. Hydrometallurgy 2011, 105, 321–327. [Google Scholar] [CrossRef]
- Leahy, M.J.; Davidson, M.R.; Schwarz, M.P. A model for heap bioleaching of chalcocite with heat balance: Mesophiles and moderate thermophiles. Hydrometallurgy 2007, 85, 24–41. [Google Scholar] [CrossRef]
- Lee, J.; Acar, S.; Doerr, D.L.; Brierley, J.A. Comparative bioleaching and mineralogy of composited sulfide ores containing enargite, covellite and chalcocite by mesophilic and thermophilic microorganisms. Hydrometallurgy 2011, 105, 213–221. [Google Scholar] [CrossRef]
- Palencia, I.; Romero, R.; Mazuelos, A.; Carranza, F. Treatment of secondary copper sulphides (chalcocite and covellite) by the BRISA process. Hydrometallurgy 2002, 66, 85–93. [Google Scholar] [CrossRef]
- Xingyu, L.; Biao, W.; Bowei, C.; Jiankang, W.; Renman, R.; Guocheng, Y.; Dianzuo, W. Bioleaching of chalcocite started at different pH: Response of the microbial community to environmental stress and leaching kinetics. Hydrometallurgy 2010, 103, 1–6. [Google Scholar] [CrossRef]
- Ruan, R.; Zhou, E.; Liu, X.; Wu, B.; Zhou, G.; Wen, J. Comparison on the leaching kinetics of chalcocite and pyrite with or without bacteria. Rare Met. 2010, 29, 552–556. [Google Scholar] [CrossRef]
- Niu, X.; Ruan, R.; Tan, Q.; Jia, Y.; Sun, H. Study on the second stage of chalcocite leaching in column with redox potential control and its implications. Hydrometallurgy 2015, 155, 141–152. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Lawson, F. The kinetics of leaching chalcocite in acidic oxygenated sulphate-chloride solutions. Hydrometallurgy 1991, 27, 249–268. [Google Scholar] [CrossRef]
- Herreros, O.; Quiroz, R.; Viñals, J. Dissolution kinetics of copper, white metal and natural chalcocite in Cl2/Cl− media. Hydrometallurgy 1999, 51, 345–357. [Google Scholar] [CrossRef]
- Herreros, O.; Viñals, J. Leaching of sulfide copper ore in a NaCl-H2SO4-O2 media with acid pre-treatment. Hydrometallurgy 2007, 89, 260–268. [Google Scholar] [CrossRef]
- Senanayake, G. Chloride assisted leaching of chalcocite by oxygenated sulphuric acid via Cu(II)-OH-Cl. Miner. Eng. 2007, 20, 1075–1088. [Google Scholar] [CrossRef]
- Muszer, A.; Wódka, J.; Chmielewski, T.; Matuska, S. Covellinisation of copper sulphide minerals under pressure leaching conditions. Hydrometallurgy 2013, 137, 1–7. [Google Scholar] [CrossRef]
- Ruiz, M.C.; Abarzúa, E.; Padilla, R. Oxygen pressure leaching of white metal. Hydrometallurgy 2007, 86, 131–139. [Google Scholar] [CrossRef]
- Petersen, J.; Dixon, D. Principles, mechanisms and dynamics of chalcocite heap bioleaching. In Microbial Processing of Metal Sulfides; Springer: Dordrecht, The Netherlands, 2007; pp. 193–218. [Google Scholar]
- Ruiz, M.C.; Honores, S.; Padilla, R. Leaching kinetics of digenite concentrate in oxygenated chloride media at ambient pressure. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 1998, 29, 961–969. [Google Scholar] [CrossRef]
- Senanayake, G. A review of chloride assisted copper sulfide leaching by oxygenated sulfuric acid and mechanistic considerations. Hydrometallurgy 2009, 98, 21–32. [Google Scholar] [CrossRef]
- Tundisi, J.G. Water resources in the future: problems and solutions. Estud. Avançados 2008, 22, 7–16. [Google Scholar] [CrossRef]
- Cisternas, L.A.; Gálvez, E.D. The use of seawater in mining. Miner. Process. Extr. Metall. Rev. 2018, 39, 18–33. [Google Scholar] [CrossRef]
- MCH. Agua en la Minería. Agua en la Minería. 2018. Available online: https://www.mch.cl/columnas/agua-la-mineria/# (accessed on 3 June 2019).
- Aguirre, C.L.; Toro, N.; Carvajal, N.; Watling, H.; Aguirre, C. Leaching of chalcopyrite (CuFeS2) with an imidazolium-based ionic liquid in the presence of chloride. Miner. Eng. 2016, 99, 60–66. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Dean, A.; Voss, D.; Draguljic, D. Response Surface Methodology. In Design and Analysis of Experiments; Springer Texts in Statistics: Cham, Switzerland, 2017; pp. 565–614. [Google Scholar]
- Toro, N.; Herrera, N.; Castillo, J.; Torres, C.; Sepúlveda, R. Initial Investigation into the Leaching of Manganese from Nodules at Room Temperature with the Use of Sulfuric Acid and the Addition of Foundry Slag—Part I. Minerals 2018, 8, 565. [Google Scholar] [CrossRef]
- Montgomery, D.C. Cap. 3, 6, 7 and 10. In Design and Analysis of Experiments, 8th ed.; Wiley: New York, NY, USA, 2012. [Google Scholar]
- Nicol, M.; Basson, P. The anodic behaviour of covellite in chloride solutions. Hydrometallurgy 2017, 172, 60–68. [Google Scholar] [CrossRef]
- Velásquez-Yévenes, L.; Nicol, M.; Miki, H. The dissolution of chalcopyrite in chloride solutions: Part 1. The effect of solution potential. Hydrometallurgy 2010, 103, 108–113. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Lawson, F. The kinetics of leaching covellite in acidic oxygenated sulphate-chloride solutions. Hydrometallurgy 1991, 27, 269–284. [Google Scholar] [CrossRef]
- Dutrizac, J.E. The leaching of sulphide minerals in chloride media. Hydrometallurgy 1992, 29, 1–45. [Google Scholar] [CrossRef]
| Investigation | Leaching Agent | Parameters Evaluated | Reference | Cu Ext (%) |
|---|---|---|---|---|
| The kinetics of leaching chalcocite (synthetic) in acidic oxygenated sulphate-chloride solutions | NaCl, H2SO4, HCl, HNO3 and Fe3+ | Oxygen flow, stirring speed, temperature, sulfuric acid concentration, ferric ions concentration, chloride concentration and particle size. | [20] | 97 |
| The kinetics of dissolution of synthetic covellite, chalcocite and digenite in dilute chloride solutions at ambient temperatures | HCl, Cu2+ and Fe3+ | Potential effect, chloride concentration, acid concentration, temperature, dissolved oxygen and pyrite effect. | [13] | 98 |
| Leaching kinetics of digenite concentrate in oxygenated chloride media at ambient pressure | CuCl2, HCl and NaCl | Effect of stirring speed, oxygen flow, cupric ion concentration, chloride concentration, acid concentration and temperature effect. | [27] | 95 |
| Leaching of sulfide copper ore in a NaCl–H2SO4–O2 media with acid pre-treatment | NaCl and H2SO4 | Chloride concentration, effect of agitation with compressed air, percentage of solids and particle size. | [22] | 78 |
| Component | Cu | S0 |
|---|---|---|
| Mass (%) | 79.83 | 20.17 |
| Compound | Concentration (g/L) |
|---|---|
| Fluorine (F−) | 0.01 |
| Calcium (Ca2+) | 0.80 |
| Magnesium (Mg2+) | 2.65 |
| Bicarbonate (HCO3−) | 1.10 |
| Chloride (Cl−) | 39.16 |
| Calcium carbonate (CaCO3) | 13.00 |
| Experimental Parameters | Low | Medium | High |
|---|---|---|---|
| Time (h) | 4 | 8 | 12 |
| Concentration | 20 | 50 | 100 |
| Cl− (g/L) | |||
| Concentration | 0.5 | 1 | 2 |
| H2SO4 (mol/L) | |||
| Codifications | −1 | 0 | 1 |
| Exp. No. | Time (h) | Cl− (g/L) | H2SO4 (mol/L) | Cu Ext. (%) |
|---|---|---|---|---|
| 1 | 4 | 20 | 0.5 | 31.63 |
| 2 | 4 | 20 | 1 | 33.25 |
| 3 | 4 | 20 | 2 | 37.00 |
| 4 | 4 | 50 | 0.5 | 32.25 |
| 5 | 4 | 50 | 1 | 33.38 |
| 6 | 4 | 50 | 2 | 38.00 |
| 7 | 4 | 100 | 0.5 | 44.75 |
| 8 | 4 | 100 | 1 | 44.88 |
| 9 | 4 | 100 | 2 | 46.19 |
| 10 | 8 | 20 | 0.5 | 35.75 |
| 11 | 8 | 20 | 1 | 38.75 |
| 12 | 8 | 20 | 2 | 43.00 |
| 13 | 8 | 50 | 0.5 | 48.13 |
| 14 | 8 | 50 | 1 | 49.50 |
| 15 | 8 | 50 | 2 | 50.63 |
| 16 | 8 | 100 | 0.5 | 51.50 |
| 17 | 8 | 100 | 1 | 53.00 |
| 18 | 8 | 100 | 2 | 54.88 |
| 19 | 12 | 20 | 0.5 | 52.25 |
| 20 | 12 | 20 | 1 | 52.75 |
| 21 | 12 | 20 | 2 | 52.63 |
| 22 | 12 | 50 | 0.5 | 53.13 |
| 23 | 12 | 50 | 1 | 53.13 |
| 24 | 12 | 50 | 2 | 53.00 |
| 25 | 12 | 100 | 0.5 | 53.25 |
| 26 | 12 | 100 | 1 | 53.88 |
| 27 | 12 | 100 | 2 | 55.63 |
| Source | F-Value | p-Value |
|---|---|---|
| Regression | 22.73 | 0 |
| Time | 123.15 | 0 |
| Cl− | 45.25 | 0 |
| H2SO4 | 5.44 | 0.03 |
| Time × Time | 2.06 | 0.17 |
| Cl− × Cl− | 0.13 | 0.72 |
| H2SO4 × H2SO4 | 0.00 | 0.97 |
| Time × Cl− | 10.27 | 0.01 |
| Time × H2SO4 | 1.18 | 0.29 |
| Cl− × H2SO4 | 0.31 | 0.59 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toro, N.; Briceño, W.; Pérez, K.; Cánovas, M.; Trigueros, E.; Sepúlveda, R.; Hernández, P. Leaching of Pure Chalcocite in a Chloride Media Using Sea Water and Waste Water. Metals 2019, 9, 780. https://doi.org/10.3390/met9070780
Toro N, Briceño W, Pérez K, Cánovas M, Trigueros E, Sepúlveda R, Hernández P. Leaching of Pure Chalcocite in a Chloride Media Using Sea Water and Waste Water. Metals. 2019; 9(7):780. https://doi.org/10.3390/met9070780
Chicago/Turabian StyleToro, Norman, Williams Briceño, Kevin Pérez, Manuel Cánovas, Emilio Trigueros, Rossana Sepúlveda, and Pía Hernández. 2019. "Leaching of Pure Chalcocite in a Chloride Media Using Sea Water and Waste Water" Metals 9, no. 7: 780. https://doi.org/10.3390/met9070780
APA StyleToro, N., Briceño, W., Pérez, K., Cánovas, M., Trigueros, E., Sepúlveda, R., & Hernández, P. (2019). Leaching of Pure Chalcocite in a Chloride Media Using Sea Water and Waste Water. Metals, 9(7), 780. https://doi.org/10.3390/met9070780









