Abstract
Black copper oxides are amorphous materials of copper-bearing phases of manganese. They are complex mineral compounds with difficult to recognize mineralogy and have slow dissolution kinetics in conventional hydrometallurgical processes. This study evaluates the effects of various leaching media on copper dissolution from black copper minerals. Leach of a pure black copper sample from Lomas Bayas Mine and another from a regional mine were characterized by inductively coupled plasma atomic emission spectroscopy (ICP-AES), X-ray diffraction (XRD), scanning electron microscopy (SEM), Qemscan and mechanically prepared for acid leaching under standard, oxidizing and reducing conditions through the addition of oxygen, iron sulfate or sulfur dioxide, respectively. Standard and high potential leaching (770 mV (SHE)) results in a copper dissolution rate of 70% and manganese dissolution rate of 2%. The addition of potential reducing agents (FeSO4 or SO2) decreases the redox potential to 696 and 431 mV, respectively, and favors the dissolution of manganese, thus increasing the overall copper extraction rate. The addition of SO2 results in the lowest redox potential and the highest copper extraction rates of 86.2% and 75.5% for the Lomas Bayas and regional samples, respectively, which represent an increase of 15% over the copper extract rates under standard and oxidizing conditions.
1. Introduction
There are numerous copper porphyry ore deposits with oxidization zones in the central Chilean Andes that have generated enriched surface oxide ores and sometimes resulted in the development of neighboring bodies of exotic copper mineralization. These “exotic” deposits vary widely in size and grade. The Mina Sur deposit contains of 3.63 million tons of fine Cu [1]. Black copper ore is most often found in exotic deposits [2], including in those at Mina Sur, Spence, Lomas Bayas and Centinela [3]. Black copper ore is a dark colored mineral compound that is difficult to recognize due to its mineralogical complexity and the presence of polymetallic connections and characterized by a resistance to dissolution and slow dissolution kinetics in hydrometallurgical systems conventionally applied to copper oxide ores [4]. Black copper ores are heterogeneous mixtures of amorphous materials, traditionally referred as copper wad (CuO·MnO2·7H2O), copper pitch (MnO(OH)CuSiO2·nH2O) and other copper-bearing manganese phases [5]. However, samples from Mina Sur show that there is no significant difference between copper pitch and copper wad. Furthermore, their chemical composition varies widely, showing black copper content of mainly Cu (1% to 54%), Si, Mn, Fe and Al, with trace quantities of Ca, Na, K, Mg, S, P, Cl, Mo, Co, Ni, As, Zn, Pb, U and V [4].
Resources associated with black copper ores are generally not incorporated into extraction circuits or are left untreated, either in stock, leach pads or residues [6]. Little research has been performed on black copper ores because research has largely been aimed at the treatment of copper sulfides [7]. Consequently, black copper ore is now becoming an important subject of geological and metallurgical interest because it is found close to or in existing mines and extraction facilities, and represents an important resource that can extend operational life. In particular, the Lomas Bayas mine has mineral deposits with the presence of black copper ore. Lomas Bayas operates with a head grade of 0.30% total copper (0.26% soluble and 0.04% insoluble copper). Mineralogical analyses estimate that 30% of the insoluble copper is black copper.
There have been few studies on the treatment of black copper. Its non-crystalline character, variable composition, with Cu, Mn, Fe, Al and Si as major elements, and its resistance to dissolution are similar to the characteristics of marine polymetallic nodules and manganese minerals. Minerals associated with manganese have been studied from a metallurgical point of view and provide a precedent for leaching black copper [8,9]. Manganese dioxide minerals like pyrolusite (MnO2) and oxides with Mn and Cu content in marine nodules are stable under acidic or alkaline oxidizing conditions. Manganese can be extracted through the medium of sulfuric acid under reductive conditions, incorporating SO2 [10,11,12], oxalic acid [13], hydrogen peroxide [14,15], iron sulfate, FeSO4 [12,16,17], iron metal [17] or through the incorporation of pyrite in hydrochloric acid media [18].
Among the few works on the metallurgical behavior of black copper minerals [19] is a study on the classification of copper types associated with copper wad and the leaching behavior of minerals that contain copper wad mixed with secondary sulfurs, which results in copper yields of over 90%. Sulfuric acid leaching of copper wad and chalcocite is proposed according to the following reaction:
2(CuO.MnO2.7H2O) + 6H2SO4 + Cu2S → 4CuSO4 + 2MnSO4 + S + 20H2O
There is an intermediary reaction that generates and consumes iron ions, which favors the overall reaction [19]. Copper, nickel, cobalt, zinc and other metals can be extracted from leach solutions by several proven techniques, among them direct electrowinning, solvent extraction–electrowinning, crystallization, cementation with iron (for copper), and sulfide precipitation. The choice of one method over another depends on a number of factors, including solution chemistry, the pregnant leach solution (PLS) flow rate and concentration, the shape of the metal product, input availability and costs, and capital costs. In general, high metal mass flows (solution flow and grade) are necessary to justify the capital expenditures of solvent extraction and electrowinning. The metallurgical efficiency and cost effectiveness of these processes can be reduced as feed metal concentrations decline. The present study investigated the effects of different methods of acid leaching on the dissolution of copper from black copper ore.
2. Materials and Methods
To determine the behavior of black copper ore under acid leaching with different modifying potential agents, a program of eight agitation leach test was performed. Two mineral samples were used, the first from Minera Lomas Bayas (LB) and the second from an Antofagasta Region mine (RA). A standard test was performed in an acid medium, followed by a test in oxidizing medium through the addition of oxygen. Finally, two tests were carried out in reducing media, one in iron sulfate (FeSO4) and the other in SO2. Both mineral samples were subjected to the same four tests. Feed samples and leaching residues were characterized.
2.1. Experimental Procedure
2.1.1. Sample Characterization
The feed samples used were identified as LB and RA. Samples were crushed and milled to a particle size 100% below 295 μm. The chemical composition was determined using inductively coupled plasma atomic emission spectroscopy (ICP-AES). The mineralogy of the samples was determined by X-ray diffraction (XRD). For XRD analysis, the sample was ground in an agate mortar to a size of less than 45 μm and analyzed in an automatic and computerized X-ray diffractometer (Siemens model D5600, Bruker, Billerica, MA, USA), with an analysis time of one hour. The ICDD (International Center for Diffraction Data) database was used to identify the species present. Qemscan analysis was performed using a Model Zeiss EVO Series, with Bruker AXS XFlash 4010 detectors and iDiscover 5.3.2.501 software. Qemscan analyses of exotic Cu deposits yielded more accurate and precise Cu mineralogy and deportment [20].
The morphology was characterized by scanning electron microscopy (SEM) using JEOL 6360-LV equipment with a coupled energy-dispersive X-ray spectroscopy (EDS) microanalysis system and operated at 30 kV under high vacuum conditions. Mineral samples were metallized with a thin carbon layer to improve their conductivity.
2.1.2. Leaching Experiments
Eight leaching tests were conducted in the present work, four for each of the two samples: under standard conditions, with an oxidizing media, with SO2 gas and iron sulfate (FeSO4) used separately as reductive media, all the tests were performed in duplicate. The results of the standard leaching test were compared to those with an oxidizing or reductive medium. The standard conditions were a particle size below 295 µm, a temperature of 25 °C, a leaching time of 240 min, 10 g/L of H2SO4 (pH = 0.9), 300 min−1 of mechanical agitation and 4.8 g of mineral added to 250 mL solution. The oxidizing condition is achieved by adding technical grade oxygen (1 L/min) under standard conditions. The reductive conditions are generated by adding iron sulfate (1.2 g/L) or SO2 (1 L/min) under standard conditions. O2 or SO2 gasses are injected with a porous frit. The aliquots obtained at different leaching times (15, 30, 60, 120 and 240 min) were filtered (0.2 μm) and the metal concentrations (Cu2+, Mn2+ and Fet) in the filtrate were determined using inductively coupled plasma atomic emission spectroscopy (PerkinElmer, model Optima 2000 DV). The solid residues after the leaching experiment were analyzed by X-ray diffraction to determine the behavior of solids during leaching under different experimental conditions. SEM analysis was also applied. Figure 1 shows a representation of the system.
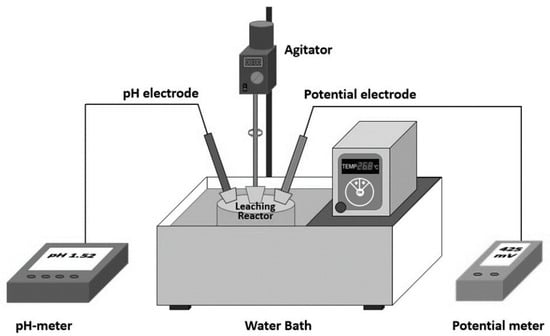
Figure 1.
Experimental setup.
3. Results and Discussion
3.1. Samples Characterization
The feed samples, identified as LB and RA, were characterized by inductively coupled plasma atomic emission spectroscopy (ICP-AES), X-ray diffraction (XRD), and Qemscan analysis. Table 1 shows the ICP-AES results, with an evident difference in copper content.

Table 1.
Chemical composition of black oxide samples.
The XRD analysis of the RA and LB samples yielded spectrums with high noise levels, characteristic of amorphous or low crystallinity minerals like black copper minerals. An analysis of the low concentration compounds detected the presence of copper silicates and crystalline types of manganese. Sample RA contained birnessite, K0,46Mn1,54Mn0,46O4(H2O); plancheite, Cu8(Si4O11)2(OH)4H2O; crednerite, CuMnO2; pyrite, (FeS2); pyrochroite, Mn(OH)2 and lithiodionite, CuNaKSi4O10, while sample LB contained albite, NaAlSi3O8; bixbyte, FeMnO3; chlorite, (Mg,Fe)6(Si,Al)4O10(OH)8; quartz, SiO2; illite, (K,H3O)Al2Si3AlO10(OH)2; microcline, KAlSi3O8; molysite, FeCl3; montmorillonite, Na0.3(Al,Mg)2Si4O10(OH)2·xH2O; natrojarosite, NaFe3(SO4)2(OH)6 and nontronite, Na0.3Fe2Si4O10(OH)2·4H2O.
The EDS (Figure 2) and semi-quantitative analyses of sample LB (Table 2) indicated the presence of oxidized Si, Mn, Cu, and Fe. SEM analysis of sample LB showed characteristic particles, permitting a qualitative analysis of not only the elements present, but also of the associations among them. It was possible to determine the presence of oxidized phases formed by the association of Cu-Mn-Fe and K, as well as quartz and iron oxides.
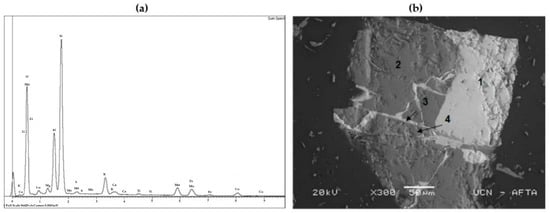
Figure 2.
EDS analysis (a) and scanning electron microscopy (SEM) image (X 300) of sample LB (b). Association of (1) Cu-Mn-Fe-K-O (2) Si-O (3) Fe-O (4) Al-Fe-O.

Table 2.
Semi-quantitative analysis of sample LB.
Qemscan analysis showed that the black copper in the two samples was of the copper wad type, without other types present like copper pitch or copper oxides (Fe-Cu) (Figure 3). Copper wad is the term given to a subgroup of black copper, specifically hydroxides of Cu and Mn, with traces of other elements such as Co, Ca, Fe, Al and Mg. More than 90% of sample RA was copper bearing minerals. The 10% of gangue was composed principally of feldspar and kaolinite. Sample LB was approximately 20% copper-bearing minerals, while the gangue was mainly quartz, feldspar, muscovite/sericite and kaolinite.
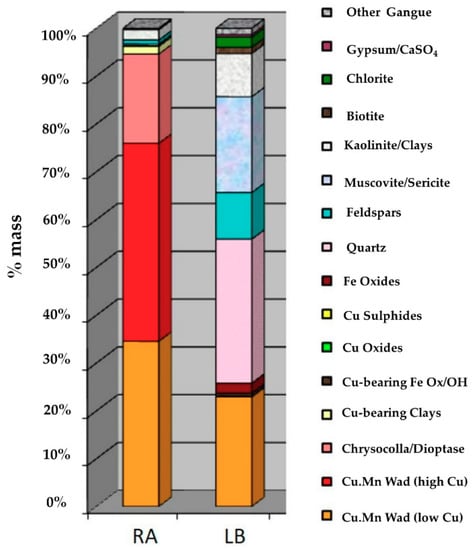
Figure 3.
Mineralogical analysis by Qemscan of samples RA and LB.
Figure 4 shows the distribution of copper in the samples. Low-content copper wad was 70% of the copper in sample RA, while the remaining 30% was mainly chrysocolla/dioptase. Low-content copper wad accounted for 95% of the copper in sample LB.
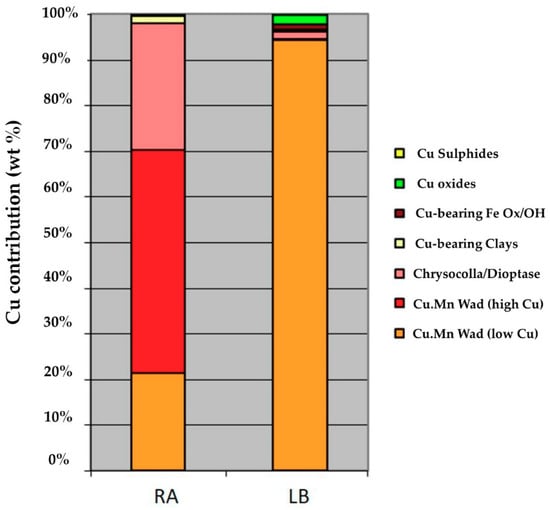
Figure 4.
Cu distribution in samples RA and LB.
Figure 5 shows the copper wad associations in the samples, 42% of which was associated with chrysocolla/dioptase, while 53% was free from association (sample RA). About 45% of sample LB was free from association, while about 20% was associated with feldspar, muscovite/sericite and kaolinite/clays.
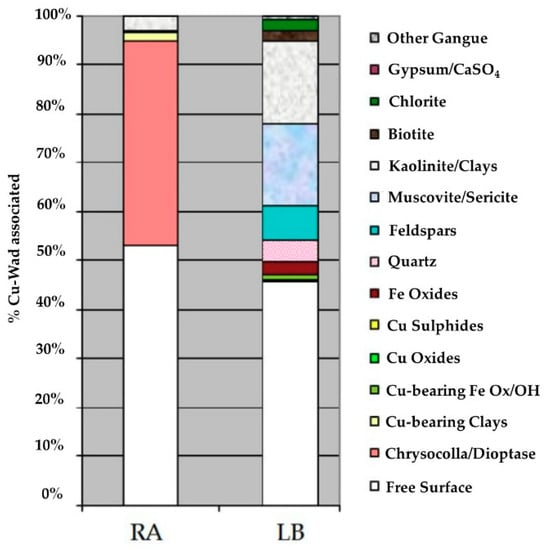
Figure 5.
Cu wad associated in samples RA and LB.
3.2. Leaching Test
Figure 6, Figure 7, Figure 8 and Figure 9 show the leaching kinetics with samples RA and LB in acid media under standard conditions and with the addition of oxidizing (O2) or reducing agents (FeSO4 or SO2). The Cu extraction rate with the RA sample was around 70% under standard conditions (♦) with a high redox potential of 773 mV due to the small quantity of iron in the sample. The rate was not greatly altered by the addition of the oxidant (▲) (777 mV, practically the same as the rate under standard condition), which was due to the highly oxidized nature of black copper mineral and it’s not affected for this condition. When reducing agents are added, the extraction increases by 15%, showing a greatest effect the SO2 addition reaching up to 86% of copper extraction (■). The maximum extraction rates were reached in every test in less than 120 min (Figure 6).
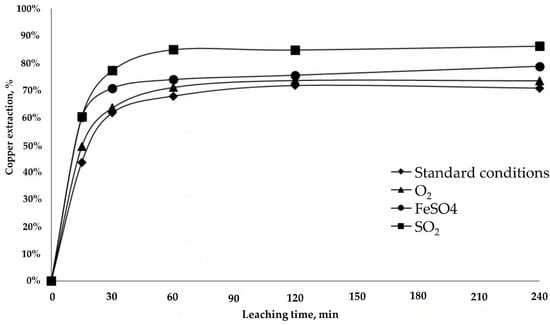
Figure 6.
Copper extraction from the RA sample.
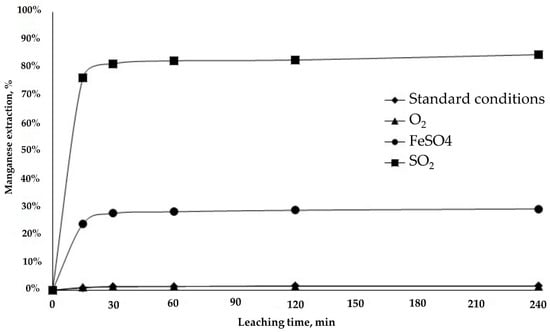
Figure 7.
Manganese extraction from RA sample.
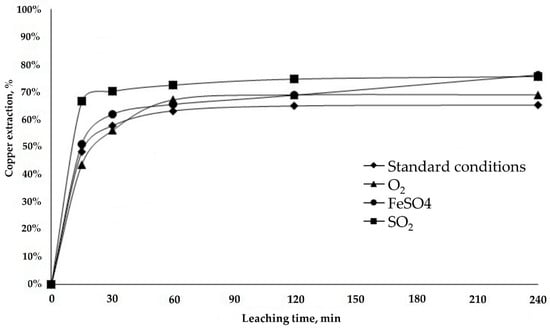
Figure 8.
Copper extraction from LB sample.
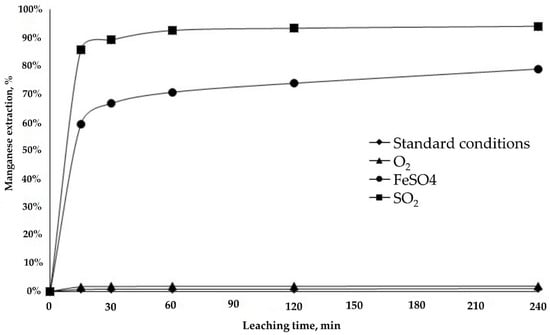
Figure 9.
Manganese extraction from LB sample.
The Mn extraction rate for the RA sample was practically zero under standard and oxidizing conditions (♦ and ▲). This was confirmed by tests performed by [21], who obtained a manganese dissolution rate of around 1% in an acid medium at room temperature, indicating that manganese oxides are relatively insoluble in a conventional acid medium [12,21].
The addition of the reducing agents had notable effects, in particular SO2 (■), which increased Mn extraction to 80% in less than 30 min (Figure 7). These results concur with those of [12], who indicated that SO2 is a useful reagent. The benefit of SO2 is due to its rapid and selective dissolution, yielding manganese extraction rates from MnO2 of over 95% under moderate leaching conditions.
The Mn dissolution rate increased as a consequence of the addition of a reducing agent (solution potential of 431 mV (SHE)), which exposed the copper to acid and increased overall Cu dissolution from sample RA. Figure 7 shows that the test with SO2 had an average solution potential of 430 mV (SHE), which generated a favorable reducing acid medium for dissolving black copper ore.
Ferrous sulfate allowed a manganese extraction of 30% in less than 30 min. The dissolution of Mn with this reducing agent is affected by the concentration of acid according to the following reactions, [12].
With ferrous sulfate solution and small amount of sulfuric acid,
With ferrous sulfate solution and excess of sulfuric acid,
The Mn dissolution with ferrous sulfate is temperature and sulfuric acid concentration dependent. In a study performed by [12] The authors exposed Mn dissolution above 90% at 90 °C and 147 g/L H2SO4. Furthermore, [22] achieved a 90% Mn dissolution at 60 °C and at a molar ratio H2SO4/MnO2 of 2.0. This can explain the low Mn dissolution (less than 80%) for RA and LB samples. In this study, 10 g/L H2SO4 was used at room temperature.
The copper extraction rates were similar for the two samples. Under standard (♦) and oxidant (▲) conditions, the copper extraction rates were between 65% and 67%, and increased by 10% with the reducing agents (■) (●) (Figure 8). The smaller increase in extraction is associated with the mineralogy of this sample, which contains more low-grade copper wad than does the RA sample, the latter being 30% chrysocolla.
The Mn extraction rate is associated directly with the presence of reducing agents in the leaching process, and its dissolution favors Cu extraction (Figure 9). Mn extraction increased from 2% to 95% with the reducing agents. The use of SO2 stands out by the rapid dissolution of Cu and the Mn that it produces, reaching its maximum value at 30 min of leaching (■).
According to the results and the literature, black copper resists leaching under standard and oxidizing conditions. The addition of a reducing agent increases copper and manganese extraction rates because of the rupture of the Cu-Mn matrix, which increases copper extraction from copper wad. Our results concur with those of [12], who indicated that SO2 plays an important role in the dissolution of Mn from MnO2 in accordance with the following reaction
In addition to this reaction, there are intermediate and secondary reactions that depend on the pH of the medium [12].
The result obtained opens the possibility of recovering copper from minerals and residues with black copper content. This process can be through primary or secondary leaching, modifying the redox potential of the irrigation solution with a reducing agent decreasing its value to 460 mV.
3.3. Residues Characterization
The solid residues from the tests under standard conditions and with FeSO4 as a reducing agent were characterized by SEM and mapping analysis. The elements shown by mapping analysis are: copper (red), manganese (green) and silica (blue).
Figure 10a shows an abundance of manganese and silica, reflecting the low manganese extraction rate under standard leaching conditions (2% of Mn extracted). The presence of copper is still evident considering the high level in the initial sample (22.8% Cu). Figure 10a shows the Cu extraction rate at 70%, with no direct relationship with Mn extraction, due to the presence of chrysocolla or dioptase in the feed. Figure 10b shows a significant decrease of Mn in the residue when FeSO4 is used as a reducing agent (Figure 10b). The above had a direct effect in terms of increasing the copper extraction rate under these conditions (80%), thus giving way to the dissolution of species associated in the Cu–Mn wad that did not react. Figure 11 shows the approach to a particle from the leaching residue obtained using FeSO4. There is a notable presence of manganese that did not react. A low presence of copper is also evident. It thus seems that there are Cu–Mn phases (with a low presence of copper) without reacting.
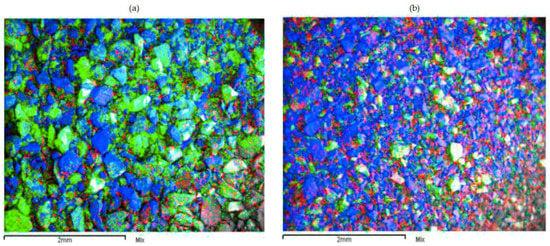
Figure 10.
SEM image shows RA residues obtained under standard conditions (a) and with FeSO4 as a reducing agent (b). Manganese (green), copper (red) and silica (blue).
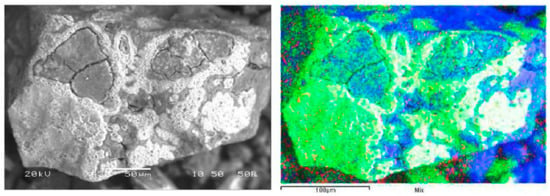
Figure 11.
SEM image shows RA residues obtained under reducing conditions using FeSO4. Manganese (green), copper (red) and silica (blue).
Figure 12 shows the comparative results of SEM mapping analysis for residues obtained from the LB sample under standard leaching conditions (12a) and under reducing conditions using FeSO4 (12b). An abundance of manganese and silica can be observed in Figure 12a, mainly resulting from Cu–Mn wad and quartz, respectively. This abundance is due to almost no Mn dissolution under standard leaching conditions (1% of Mn dissolved). The residue obtained under reducing conditions using FeSO4 was considerably less in the presence of manganese, but remained largely unchanged in the presence of quartz (12b). The low presence of manganese was due to the 80% dissolution rate obtained under these conditions. Finally, the presence of copper was due to the fact that 30% of copper could not be dissolved.
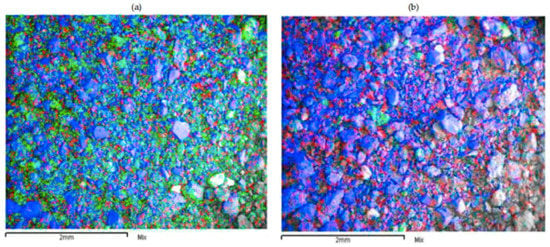
Figure 12.
SEM image shows LB residues obtained under standard conditions (a) and with FeSO4 as a reducing agent (b). Manganese (green), copper (red) and silica (blue).
4. Conclusions
Despite the difference in grade, the black copper from both the RA and LB samples can be classified as copper wad and behaved similarly in the leaching tests under all studied conditions.
The lowest copper extraction rates are under standard leaching conditions, and are in the range of 70%, while manganese dissolution rates are less than 2%. The redox potential is high and in the order of 770 mV.
The results from leaching under oxidizing conditions (produced by adding oxygen) are analogous to those obtained under standard conditions, with an extraction of 2% for manganese and 73% for copper. Therefore, the addition of an oxidant does not improve the black copper dissolution rate.
The addition of reducing agents (FeSO4 or SO2) decreases the redox potential to 696 and 431 mV, respectively, and favors the dissolution of manganese. This increases the overall copper extraction rate in minerals with high copper wad content. The use of SO2 resulted in the highest extraction rates of 86.2% for sample RA and 75.5% for LB, which is 15% higher than the rates obtained under standard conditions.
Author Contributions
Conceptualization, O.B., M.H., and Y.Z.; methodology, O.B., M.H., and E.M.; validation, O.B., M.H., and Y.Z.; formal analysis, O.B., E.M., V.Q., and D.N.; investigation, O.B., and M.H.; resources, O.B., M.H., and Y.Z.; data curation, E.M., V.Q., and D.N.; writing—original draft preparation, O.B., E.M., and V.Q.; writing—review and editing, O.B., E.M., V.Q.; visualization, E.M., V.Q.; supervision, O.B., M.H., D.N., and Y.Z.; project administration, O.B., and M.H.; funding acquisition, O.B., M.H., and Y.Z.
Funding
This research was funded by CORFO INNOVA (No. 08CM01-09).
Acknowledgments
The authors are grateful for the financial support provided by the CORFO INNOVA Program and Glencore Lomas Bayas to the project “Geometallurgical Modelling and Design of a Specific Process for the Recovery of Ores with Black Copper Content”.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, the collection, analysis, or interpretation of data, the writing of the manuscript, or the decision to publish the results.
References
- Münchmeyer, C. Exotic deposits-products of lateral migration of supergene solutions from porphyry copper deposits. In Andean Copper Deposits: New Discoveries, Mineralization, Styles and Metallogeny; Society of Economic Geologists, Special Publication: Tulsa, OK, USA, 1996; pp. 43–58. [Google Scholar]
- Pinget, M.; Dold, B.; Fontboté, L. Exotic mineralization at Chuquicamata, Chile: Focus on the copper wad enigma. In Proceedings of the 10th Swiss Geoscience Meeting, Bern, Swiss, 16–17 November 2012; pp. 88–89. [Google Scholar]
- Riquelme, R.; Tapia, M.; Campos, E.; Mpodozis, C.; Carretier, S.; González, R.; Muñoz, S.; Fernández-Mort, A.; Sanchez, C.; Marquardt, C. Supergene and exotic Cu mineralization occur during periods of landscape stability in the Centinela Mining District, Atacama Desert. Basin Res. 2018, 30, 395–425. [Google Scholar] [CrossRef]
- Pincheira, M.; Dagnini, A.; Kelm, U.; Helle, S. Copper Pitch Y Copper Wad: Contraste Entre Las Fases Presentes En Las Cabezas Y En Los Ripios En Pruebas De Mina sur, Chuquicamata. In Proceedings of the X Congreso Geológico Chileno, Concepción, Chile, 6–10 October 2003; p. 10. [Google Scholar]
- Mote, T.I.; Becker, T.A.; Renne, P.; Brimhall, G.H. Chronology of exotic mineralization at El Salvador, Chile, by 40Ar 39Ar dating of copper wad and supergene alunite. Econ. Geol. 2001, 92, 351–366. [Google Scholar] [CrossRef]
- Helle, S.; Pincheira, M.; Jerez, O.; Kelm, U. Sequential extraction to predict the leaching potential of refractory black copper ores. In Proceedings of the XV Balkan Mineral Processing Congress, Sozopol, Bulgaria, 12–16 June 2013; pp. 109–111. [Google Scholar]
- Velásquez-Yévenes, L.; Quezada-Reyes, V. Influence of seawater and discard brine on the dissolution of copper ore and copper concentrate. Hydrometallurgy 2018, 180, 88–95. [Google Scholar] [CrossRef]
- Mukherjee, A.; Raichur, A.M.; Modak, J.M.; Natarajan, K.A. Bioprocessing of polymetallic Indian Ocean nodules using a marine isolate. Hydrometallurgy 2004, 73, 205–213. [Google Scholar] [CrossRef]
- Acharya, R.; Ghosh, M.K.; Anand, S.; Das, R.P. Leaching of metals from Indian ocean nodules in SO2-H2O-H2SO4-(NH4)2SO4 medium. Hydrometallurgy 1999, 53, 169–175. [Google Scholar] [CrossRef]
- Senanayake, G. A mixed surface reaction kinetic model for the reductive leaching of manganese dioxide with acidic sulfur dioxide. Hydrometallurgy 2004, 73, 215–224. [Google Scholar] [CrossRef]
- Naik, P.K.; Sukla, L.B.; Das, S.C. Aqueous SO2 leaching studies on Nishikhal manganese ore through factorial experiment. Hydrometallurgy 2000, 54, 217–228. [Google Scholar] [CrossRef]
- Sinha, M.K.; Purcell, W. Reducing agents in the leaching of manganese ores: A comprehensive review. Hydrometallurgy 2019, 187, 168–186. [Google Scholar] [CrossRef]
- Sahoo, R.N.; Naik, P.K.; Das, S.C. Leaching of manganese from low-grade manganese ore using oxalic acid as reductant in sulphuric acid solution. Hydrometallurgy 2001, 62, 157–163. [Google Scholar] [CrossRef]
- Jiang, T.; Yang, Y.; Huang, Z.; Qiu, G. Simultaneous leaching of manganese and silver from manganese-silver ores at room temperature. Hydrometallurgy 2003, 69, 177–186. [Google Scholar] [CrossRef]
- Jiang, T.; Yang, Y.; Huang, Z.; Zhang, B.; Qiu, G. Leaching kinetics of pyrolusite from manganese-silver ores in the presence of hydrogen peroxide. Hydrometallurgy 2004, 72, 129–138. [Google Scholar] [CrossRef]
- Das, S.C.; Sahoo, P.K.; Rao, P.K. Extraction of manganese from low-grade manganese ores by FeSO4 leaching. Hydrometallurgy 1982, 8, 35–47. [Google Scholar] [CrossRef]
- Bafghi, M.S.; Zakeri, A.; Ghasemi, Z.; Adeli, M. Reductive dissolution of manganese ore in sulfuric acid in the presence of iron metal. Hydrometallurgy 2008, 90, 207–212. [Google Scholar] [CrossRef]
- Kanungo, S.B. Rate process of the reduction leaching of manganese nodules in dilute HCl in presence of pyrite. Part II: Leaching behavior of manganese. Hydrometallurgy 1999, 52, 331–347. [Google Scholar] [CrossRef]
- Helle, S.; Kelm, U.; Barrientos, A.; Rivas, P.; Reghezza, A. Improvement of mineralogical and chemical characterization to predict the acid leaching of geometallurgical units from Mina Sur, Chuquicamata, Chile. Miner. Eng. 2005, 18, 1334–1336. [Google Scholar] [CrossRef]
- Menzies, A.; Campos, E.; Hernández, V.; Sola, S.; Riquelme, R. Understanding Exotic-Cu Mineralisation Part II: Characterization of “Black Copper”ore (Cobre Negro). In Proceedings of the 13th SGA Biennial Meeting, Nancy, France, 24–27 August 2015; pp. 3–6. [Google Scholar]
- Han, K.; Fuerstenau, D. Acid leaching of ocean floor manganese at elevated temperature. Int. J. Miner. Process. 1975, 2, 163–171. [Google Scholar] [CrossRef]
- Zakeri, A.; Bafghi, M.S.; Shahriari, S.; Das, S.C.; Sahoo, P.K.; Rao, P.K. Dissolution kinetics of manganese dioxide ore in sulfuric acid in the presence of ferrous ion. Hydrometallurgy 2007, 8, 22–27. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).