Abstract
Chalcocite is the most important and abundant secondary copper ore in the world with a rapid dissolution of copper in an acid-chloride environment. In this investigation, the methodology of surface optimization will be applied to evaluate the effect of three independent variables (time, concentration of sulfuric acid and chloride concentration) in the leaching of pure chalcocite to extract the copper with the objective of obtaining a quadratic model that allows us to predict the extraction of copper. The kinetics of copper dissolution in regard to the function of temperature is also analyzed. An ANOVA indicates that the linear variables with the greatest influence are time and the chloride concentration. Also, the concentration of chloride-time exerts a significant synergic effect in the quadratic model. The ANOVA indicates that the quadratic model is representative and the R2 value of 0.92 is valid. The highest copper extraction (67.75%) was obtained at 48 h leaching under conditions of 2 mol/L H2SO4 and 100 g/L chloride. The XRD analysis shows the formation of a stable and non-polluting residue; such as elemental sulfur (S0). This residue was obtained in a leaching time of 4 h at room temperature under conditions of 0.5 mol/L H2SO4 and 50 g/L Cl−.
1. Introduction
Most of the copper minerals on the planet correspond to sulfur minerals and a smaller amount of oxidized minerals. A report by COCHILCO [1] mentions that the world copper production is currently 19.7 million tons. Seventy-five percent of this total comes from the pyrometallurgical processing of copper sulfide minerals processed in smelting plants [2], and 25% by the hydrometallurgical route [3].
There is a need to generate a new momentum that overcomes a certain stagnation in the growth capacity of the mining industry. Even in its role as a surplus generator, large-scale mining faces great challenges. These include an increase in costs due to various factors; such as the deterioration of laws and other elements associated with the aging of deposits and input costs to be compatible with sustainable development demands [4].
Sulfur minerals have been treated for decades with flotation and pyrometallurgical processes [5], which result in major environmental problems; such as tailings dams and the generation of acid drainage (sulfuric acid and oxides of iron) by the oxidation of sulfur minerals with a high presence of pyrite. This sulfide is one of the most common and abundant minerals in the world and is associated with hydrothermal mineralization [6]. On the other hand, foundries produce large emissions of sulfur dioxide (SO2), which together, with NOx and CO2, can cause large problems; such as acid rain and increasing local pollution, therefore, the abatement of waste gases is an important task for the protection of the environment [7,8,9]. As a result, new hydrometallurgical alternatives are being developed in the mining industry, because they are more ecological and economic processes to recover copper [10,11].
Chalcocite is the most abundant copper sulfide mineral after chalcopyrite [5,12], and it is the copper sulfide the most easily treated by hydrometallurgical routes [13]. Several investigations have been carried out for the leaching of this mineral with the use of multiple additives and in different media such as; bioleaching [14,15,16,17,18], ferric sulfate solution [19], chloride media [20,21,22,23], pressure leaching for chalcocite [24] and synthetic chalcocite (white metal) [25].
When operating in sulphated or chloride media, the oxidative dissolution of the chalcocite occurs in two stages [13,19,20,23,25].
Cu2S + 2Fe3+ = Cu2+ + 2Fe2+ + CuS
CuS + 2Fe3+ = Cu2+ + 2Fe2+ + S0
The first stage of leaching of the chalcocite is much faster than the second stage. This is controlled by diffusion of the oxidant on the surface of the ore at low values of activation energy (4–25 kJ mol−1) [19]. The second stage is slower and can be accelerated depending on the temperature [13,26].
The investigations shown in Table 1 were obtained as a result of high extractions (90%), but these results were obtained with the application of high temperatures and/or with the addition of ferric or cupric ions as an oxidizing agent. In addition, the previous investigations were made with mixtures of copper sulfides, with the presence of gangue or with the use of synthetic chalcocite. It is emphasized that the present investigation will include a leaching of pure chalcocite in a chlorinated medium, without the addition of oxidizing agents (Fe3 +, Cu2 +, etc.) and at room temperature.

Table 1.
Comparison of previous investigations of chalcocite with the use of Cl−.
A chalcocite leaching is performed with the injection of O2 at ambient pressure in a H2SO4-NaCl solution, where the leaching agents are Cu2+, CuCl+, CuCl2 and Cu, which are generated during leaching in a Cu2+/Cl− system. The general reaction of chalcocite leaching is as follows:
While the chalcocite leaching reactions occur in two stages, guiding us to Equation (3), the following occurs:
The resulting products expected from this chalcocite leaching should be soluble copper; such as Cu and a solid residue of elemental sulfur (S0) with covellite residues or copper polysulfides (CuS2) that still contain valuable metals.
The Cu is the predominant soluble specie due to the complexation of Cu (I) with the presence of Cl− at room temperature, in a system of high concentrations of chloride (greater than 1 M). This Cu is stable in a range of potentials between 0–500 mV and pH < 6–7 (depending on the chloride concentration in the system) [20,28].
The shortage of fresh water in arid areas is an economic, environmental and social problem [29]. The use of sea water has become increasingly important for mining in Chile, not only because of its positive effects on leaching processes due to its chloride content, but also as a strategic and indispensable resource. For example, some metallic and non-metallic mining companies in the north of Chile have deposits rich in copper, gold, silver, iron and minerals from salt lakes, which are found in hyper-arid zones and at high altitudes, which emphasizes the necessity of this resource [30]. In addition, it is important to mention that the Chilean authorities have indicated that large-scale mining projects involving the use of water from aquifers will not be authorized [31]. An attractive alternative is the use of waste water from desalination plants. These companies produce drinking water for the population, however, their disposal product pollutes the oceans, for this reason, it is necessary to think of possible alternatives to recycle this resource and at the same time optimize extraction processes in local mining.
In the present investigation, a statistical analysis will be carried out using the methodology of surface optimization (design of the central composite face) to sensitize independent parameters (time, sulfuric acid concentration and chloride concentration) in the leaching of a pure mineral of chalcocite in chlorinated media. In addition, the effect on chloride concentration in the system will be evaluated when operating with potable water, seawater and reusing waste water.
2. Materials and Methods
2.1. Chalcocite
The pure chalcocite mineral used for the present investigation was collected manually directly from the veins by expert geologists from Mina Atómica, located in the region of Antofagasta, Chile.
The pure chalcocite samples were checked by X-ray diffraction (XRD) analysis, using an automatic and computerized X-ray diffractometer Bruker model Advance D8 (Bruker, Billerica, MA, USA). Figure 1 shows the results of the XRD analysis, indicating the presence of 99.9% chalcocite. The chemical analysis was performed by atomic emission spectrometry via induction-coupled plasma (ICP-AES), the sample of chalcocite was digested using aqua regia and HF. Table 2 shows the chemical composition of the samples. The samples for XRD and ICP-OES were ground in a porcelain mortar to reach a size range between −147 + 104 μm. The procedures described were performed in the applied geochemistry laboratory of the department of geological sciences of the Universidad Católica del Norte.
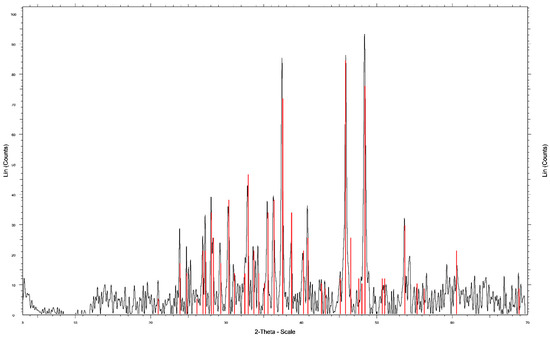
Figure 1.
X-ray diffractogram for the chalcocite mineral.

Table 2.
Chemical analysis of the chalcocite ore.
2.2. Leaching and Leaching Tests
The sulfuric acid used for the leaching tests was grade P.A., Merck brand, purity 95–97%, density 1.84 kg/L and molecular weight of 98.08 g/mol, though the tests also work with the use of sea water and waste water from the “Aguas Antofagasta” Desalination Plant. Table 3 shows the chemical composition of waste water.

Table 3.
Chemical analysis of waste water.
Leaching tests were carried out in a 50 mL glass reactor with a 0.01 S/L ratio. A total of 200 mg of chalcocite ore in a size range between −147 + 104 μm and the addition of NaCl at different concentrations were maintained in agitation and suspension with the use of a 5-position magnetic stirrer (IKA ROS, CEP 13087-534, Campinas, Brazil) at a speed of 600 rpm and the temperature was controlled using an oil-heated circulator (Julabo, St. Louis, MO, USA). The temperature range tested in the experiments was 25 °C. Also, the tests were performed in duplicate and measurements (or analyzes) were carried out on 5 mL aliquot and diluted to a range of dilutions using atomic absorption spectrometry with a coefficient of variation ≤5% and a relative error between 5 to 10%. Measurements of pH and oxidation-reduction potential (ORP) of leach solutions were made using a pH-ORP meter (HANNA HI-4222, St. Louis, MO, USA). The ORP solution was measured in a combination ORP electrode cell of a platinum working electrode and a saturated Ag/AgCl reference electrode.
2.3. Experimental Design
The effects of independent variables on Cu extraction rates from leaching of chalcocite were studied using the response surface optimization method [32,33,34,35]. The central composite face (CCF) design and a quadratic model were applied to the experimental design for Cu2S leaching.
Twenty-seven experimental tests were carried out to study the effects of time, chloride and H2SO4 concentration as independent variables. Minitab 18 software (version 18, Pennsylvania State University, State College, PA, USA) was used for modeling and experimental design, which allowed the study of the linear and quadratic effects of the independent variables. The experimental data were fitted by multiple linear regression analysis to a quadratic model, considering only those factors that helped to explain the variability of the model. The empirical model contains coefficients of linear, quadratic, and two-factor interaction effects.
The general form of the experimental model is represented by:
Where, is time, is Chloride, is H2SO4 concentration, and is the variable coefficients.
Table 4 presents the ranges of parameter values used in the experimental model. The variable values are codified in the model. The following Equation (8) is used for transforming a real value (Zi) into a code value (Xi) according to the experimental design:

Table 4.
Experimental parameters for the central design of the composite face.
and are the highest and lowest levels of a variable, respectively [36].
A factorial design was applied involving three factors, each one having three levels thus 27 experimental tests were carried out in Table 5, evaluating the effect of time and H2SO4 and chloride concentration.

Table 5.
Experimental configuration and Cu extraction.
The statistical R2, R2adj, p-values and Mallows’s Cp indicate whether the model obtained is adequate to describe Cu extraction under a given domain. The R2 coefficient is a measure of the goodness of fit, which measures the proportion of total variability of the dependent variable with respect to its mean, which is explained by the regression model. The p-values represent statistical significance, which indicates whether there is a statistically significant association between the response variable and the terms. The predicted R2 was used to determine how well the model predicts the response for new observations. Finally, Mallows’s Cp is a precise measure in the model, estimating the true parameter regression [36].
3. Results
3.1. ANOVA
An ANOVA analysis (Table 6) showed F-value and p-value for the model.

Table 6.
ANOVA (analysis of variance) Cu extraction.
In the contour plot in Figure 2, it is observed that Cu extraction increases at long times, high chloride concentration, and high H2SO4 concentration.
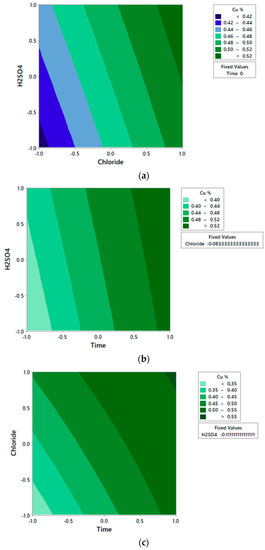
Figure 2.
Experimental contour plot of independent variables of Chloride and H2SO4 concentration (a); Time and H2SO4 concentration (b); and Time and Chloride concentration (c) on the dependent variable Cu extraction.
Table 6 shows ANOVA analysis. There is no significant effect (p > 0.05) of the interactions concentration of chloride concentration of H2SO4 and time-concentration of H2SO4 in copper extraction, complying with the theory that the increase in sulfuric acid concentration does not have a great influence on the leaching of chalcocite above 0.02 mol/L [19,22]. Rather, it is only the time-concentration interaction of chloride that must be considered in the model. Additionally, the effects of curvature of the variable chloride concentration and H2SO4 concentration do not contribute significantly to explaining the variability of the model. On the other hand, the linear effects of chloride time and concentration contribute to explaining the experimental model, as shown in the contour plot of Figure 2.
Figure 3 and Figure 4 show that time, chloride and H2SO4 concentration, as well as the interaction of time-H2SO4 and Cl-H2SO4 affected Cu extraction.
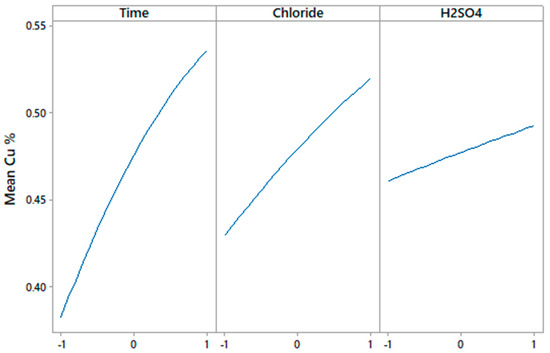
Figure 3.
Linear effect plot for Cu extraction.
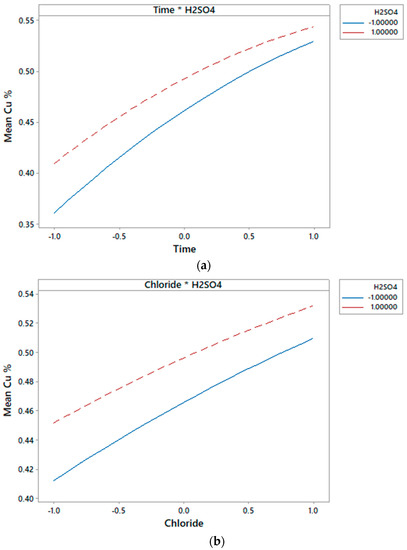
Figure 4.
Interaction effect plot of independent variables Time and H2SO4 concentration (a); and Chloride and H2SO4 concentration (b) on the dependent variable Cu extraction.
In Figure 3, the linear effects demonstrate what has been said by several authors [13,20,37], with respect to the effect of the concentration of chloride present in the leaching media and the effect of sulfuric acid concentration. The concentration of chloride has a great impact on the dissolution of copper from a sulfide; such as chalcocite. According to Velásquez-Yévenes et al. [38], the chloride ions present in the media increase the rate of oxidation of cuprous ions, while Cheng and Lawson [20,39] proposed that the effect of chloride ions promotes the formation of long sulfide crystals that allow the reactants to penetrate the sulfide layer, since in their tests they noticed that, in the absence of chloride ions, the kinetics of dissolution decreased considerably and that covellite did not dissolve. This with time was supported in the research of Nicol and Basson [37], without the presence of chloride ions or with a very low concentration of ions, the potential needed to dissolve covellite is very high.
Figure 4 shows the mean Cu extraction at different combinations of factor levels. In the interaction time-chloride, the lines are not parallel, and the plot indicates that there is an interaction between the factors. On the other hand, the interaction between time-H2SO4 and chloride-H2SO4 is low.
Equation (9) presents the Cu extraction model over the range of experimental conditions after eliminating the non-significant coefficients.
Where and are codified variables that respectively represent time, chloride and H2SO4 concentration.
An ANOVA test indicated that the quadratic model adequately represented Cu extraction from Cu2S under the established parameter ranges. The model did not require adjustment and it was validated by the R2 value (0.92) and R2adj value (0.90). The ANOVA analysis showed that the factors indicated influence Cu extraction from Cu2S (FRegression (22.73) > FT,95% confidence level = F5,21 (2.68)). On the other hand, the p-value of the model (Equation (9)) is lower than 0.05, indicating that the model is statistically significant.
The Mallows’s Cp = 3.62 (constant + 5 predictors) indicated that the model was accurate and did not present bias in estimating the true regression coefficients. This value of Cp of Mallows allows comparison with other models and establishes that the model found is the one that is most adjustable, due to the Cp closest to the number of constants and predictors.
In addition, all variance inflation factors (VIF) values are close to one, which ensures that there is no multicollinearity.
It also allows for prediction with an acceptable future forecast margin of error of R2pred = 0.8684.
Finally, from the adjustment of the ANOVA analysis, it was found that the factors considered, after analysis of the main components, explained the variation in the response. The difference between the R2 and R2pred of the model was minimal, thus reducing the risk that the model was over adjusted. That means, the probability that the model fits only in the sample data is lower. The ANOVA analysis indicated that time, chloride concentration, H2SO4 concentration and the interaction of time-chloride are the factors that explain to a greater extent the behavior of the system for the sampled data set.
Table 5 shows that the increase in sulfuric acid concentration does not affect copper dissolution, obtaining similar results under similar conditions of leaching, only a minimum amount of sulfuric acid is needed in the leaching system. This result is consistent with other investigations, since according to Cheng and Lawson [20], a concentration of sulfuric acid of 0.02 mol/L, is sufficient to perform a leaching of chalcocite and its subsequent phases as it is the djurleite, digenite ore [22]. After this value its effect is null.
On the other hand, it is shown that at a leaching time of 12 h, the values of copper extraction do not vary regardless of the concentration of chloride and sulfuric acid. This could be explained with Equations (4) and (5); Equation (4) is the rapid reaction of the transformation of chalcocite to covellite, in which a low activation energy is required to achieve its transformation [13]. When covellite is formed (Equation (5)), it needs more energy (about 72 kJ approximately) to achieve its dissolution and later become a copper polysulfide (CuS2), what it requires is even more demanding conditions to achieve its complete dissolution [37].
3.2. Effect on the Chloride Concentration
It has been known since the 1970s that it is beneficial to work with chloride ions in the leaching of sulfide minerals [23,40]. In Figure 5a, when operating at higher chloride concentrations, higher copper recoveries are obtained. When operating with the highest chloride concentrations (100 g/L), the highest recovery (68.82%) is obtained at 48 h. However, a large difference in copper recoveries cannot be seen when operating at chloride concentrations between 20 and 50 g/L. At 48 h and 20 g/L, a recovery of 63.58% of Cu was obtained and for chloride concentrations of 50 g/L, 65.45% was obtained. On the other hand, in Figure 5b it is observed that with the use of waste water (39.16 g/L of Cl−), results similar to those presented in Figure 5a were obtained in a Cl− concentration of 50 g/L, so it is noted that the presence of calcium ions, fluorine, magnesium and calcium carbonate did not affect the dissolution of copper from the chalcocite. In the tests carried out with seawater, which has approximately a concentration of 20 g/L Cl−, obtained copper extractions of up to 63.4% at 48 h with a concentration of 0.5 mol/L of sulfuric acid. In previous investigations [13,20], it has been determined that leaching is independent of a chloride concentration between 0.5 and 2 mol/L, but a greater kinetic of dissolution is observed in the first minutes and then the difference decreases as a function of time and behavior similar to that of Figure 5.

Figure 5.
Effect of chloride concentration on Cu extraction from chalcocite (T = 25°C. H2SO4 = 0.5 mol/L); (a) Cl− added by NaCl; (b) Cl− added by waste water and seawater.
Figure 6 shows a residue analysis performed under the conditions of 50 g/L Cl− and 0.5 mol/L H2SO4, in a leaching time of 4 h. The result of this XRD is useful to understand the behavior of the chalcocite in a short time and in low reagent conditions, and to observe which mineralogical species are forming. The results show a high formation of synthetic covellite (77.34 wt %), early formation of elemental sulfur (20.20 wt %) and a remaining chalcocite (4.46 wt %), which still does not dissolve. From this, it follows that the transformation of chalcocite to covellite is faster than the transformation of covellite to elemental sulfur, which is similar to that observed in Equations (4) and (5), also, according to Figure 5, the slope of the curve is decreasing slowly, which means less kinetics of copper dissolution as a function of time. In the investigation of Senanayake [28], it is reported that the dissolution of chalcocite in a chloride-iron-water system at 25 °C occurs at potentials greater than 500 mV with a pH < 4, while in the research of Miki et al. [13] it is reported that the chalcocite dissolution occurs rapidly at a potential of 500 mV but stops when it reaches 50% copper extraction. When the potential increases to 550 mV, this extraction increases again because once it reaches 50% copper extraction, the mineral present is mainly covellite, which has a dissolution kinetics lower than the chalcocite and that needs potentials greater than 600 mV to dissolve.
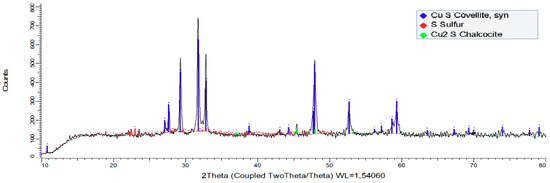
Figure 6.
X-ray diffractogram for solid residues (chalcocite mineral) after being leached at 25 °C in a time of 4 h with 0.5 mol/L H2SO4 and 50 g/L Cl−.
4. Conclusions
The present investigation shows the experimental results necessary to dissolve Cu from a chalcocite mineral in chloride media. The findings of this study were:
- The linear variables with the greatest influence in the model are: time, chloride concentration and sulfuric acid concentration, respectively.
- Under normal pressure and temperature conditions, only the chloride-time concentration exerts a significant synergistic effect on the extraction of copper from a chalcocite mineral.
- The ANOVA analysis indicates that the presented quadratic model is adequate to represent the copper extractions and the value of R2 (0.92) validates it.
- The highest copper extraction is achieved under conditions of low concentration of sulfuric acid (0.5 mol/L), high concentrations of chloride (100 g/L) and a prolonged leaching time (48 h) to obtain an extraction of 67.75% copper.
- The XRD analysis shows the formation of a stable and non-polluting residue; such as elemental sulfur (S0). This residue was obtained in a leaching time of 4 h at room temperature under conditions of 0.5 mol/L H2SO4 and 50 g/L Cl−.
Author Contributions
N.T. and K.P. contributed in project administration, investigation and wrote paper, W.B. contributed in the data curation and software, M.C. and E.T. contributed in validation and supervision and R.S. and P.H. performed the experiments, review and editing.
Funding
This research received no external funding.
Acknowledgments
The authors are grateful for the contribution of the Scientific Equipment Unit- MAINI of the Universidad Católica del Norte for aiding in generating data by automated electronic microscopy QEMSCAN® and for facilitating the chemical analysis of the solutions. We are also grateful to the Altonorte Mining Company for supporting this research and providing slag for this study, and we thank to Marina Vargas Aleuy and María Barraza Bustos of the Universidad Católica del Norte for supporting the experimental tests.
Conflicts of Interest
The authors declare they have no conflict of interest.
References
- Comisión Chilena del Cobre. Sulfuros Primarios: Desafíos y Oportunidades. Registro Propiedad Intelectual N° 2833439. 2017. Available online: https://www.cochilco.cl/Listado Temtico/sulfuros primarios_desafíos y oportunidades.pdf (accessed on 25 April 2019).
- Navarra, A.; Oyarzun, F.; Parra, R.; Marambio, H.; Mucciardi, F. System dynamics and discrete event simulation of copper smelters. Miner. Metall. Process. 2017, 34, 96–106. [Google Scholar] [CrossRef]
- International Copper Study Group. The World Copper Factbook 2017; International Copper Study Group: Lisbon, Portugal, 2017. [Google Scholar]
- CESCO. La Minería como plataforma para el desarrollo: Hacia una relación integral y sustentable de la industria minera en Chile. Available online: http://www.cesco.cl/wp-content/uploads/2018/06/Resumen-Position-Paper.pdf (accessed on 12 July 2019).
- Schlesinger, M.E.; King, M.J.; Sole, K.C.; Davenport, W.G. Extractive Metallurgy of Copper, 5th ed.; Elsevier: Amsterdam, Netherlands, 2011. [Google Scholar]
- Oyarzun, R.; Oyarzún, J.; Lillo, J.; Maturana, H.; Higueras, P. Mineral deposits and Cu-Zn-As dispersion-contamination in stream sediments from the semiarid Coquimbo Region, Chile. Environ. Geol. 2007, 53, 283–294. [Google Scholar] [CrossRef]
- Dijksira, R.; Senyard, B.; Shah, U.; Lee, H. Economical abatement of high-strength SO2off-gas from a smelter. J. South. African Inst. Min. Metall. 2017, 117, 1003–1007. [Google Scholar] [CrossRef]
- Serbula, S.M.; Milosavljevic, J.S.; Radojevic, A.A.; Kalinovic, J.V.; Kalinovic, T.S. Extreme air pollution with contaminants originating from the mining—Metallurgical processes. Sci. Total Environ. 2017, 586, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Zagoruiko, A.N.; Vanag, S.V. Reverse-flow reactor concept for combined SO2 and co-oxidation in smelter off-gases. Chem. Eng. J. 2014, 238, 86–92. [Google Scholar] [CrossRef]
- Baba, A.A.; Balogun, A.F.; Olaoluwa, D.T.; Bale, R.B.; Adekola, F.A.; Alabi, A.G.F. Leaching kinetics of a Nigerian complex covellite ore by the ammonia-ammonium sulfate solution. Korean J. Chem. Eng. 2017, 34, 1133–1140. [Google Scholar] [CrossRef]
- Pradhan, N.; Nathsarma, K.C.; Rao, K.S.; Sukla, L.B.; Mishra, B.K. Heap bioleaching of chalcopyrite: A review. Miner. Eng. 2008, 21, 355–365. [Google Scholar] [CrossRef]
- Mindat. Copper: The mineralogy of Copper. Available online: https://www.mindat.org/element/Copper (accessed on 8 July 2019).
- Miki, H.; Nicol, M.; Velásquez-Yévenes, L. The kinetics of dissolution of synthetic covellite, chalcocite and digenite in dilute chloride solutions at ambient temperatures. Hydrometallurgy 2011, 105, 321–327. [Google Scholar] [CrossRef]
- Leahy, M.J.; Davidson, M.R.; Schwarz, M.P. A model for heap bioleaching of chalcocite with heat balance: Mesophiles and moderate thermophiles. Hydrometallurgy 2007, 85, 24–41. [Google Scholar] [CrossRef]
- Lee, J.; Acar, S.; Doerr, D.L.; Brierley, J.A. Comparative bioleaching and mineralogy of composited sulfide ores containing enargite, covellite and chalcocite by mesophilic and thermophilic microorganisms. Hydrometallurgy 2011, 105, 213–221. [Google Scholar] [CrossRef]
- Palencia, I.; Romero, R.; Mazuelos, A.; Carranza, F. Treatment of secondary copper sulphides (chalcocite and covellite) by the BRISA process. Hydrometallurgy 2002, 66, 85–93. [Google Scholar] [CrossRef]
- Xingyu, L.; Biao, W.; Bowei, C.; Jiankang, W.; Renman, R.; Guocheng, Y.; Dianzuo, W. Bioleaching of chalcocite started at different pH: Response of the microbial community to environmental stress and leaching kinetics. Hydrometallurgy 2010, 103, 1–6. [Google Scholar] [CrossRef]
- Ruan, R.; Zhou, E.; Liu, X.; Wu, B.; Zhou, G.; Wen, J. Comparison on the leaching kinetics of chalcocite and pyrite with or without bacteria. Rare Met. 2010, 29, 552–556. [Google Scholar] [CrossRef]
- Niu, X.; Ruan, R.; Tan, Q.; Jia, Y.; Sun, H. Study on the second stage of chalcocite leaching in column with redox potential control and its implications. Hydrometallurgy 2015, 155, 141–152. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Lawson, F. The kinetics of leaching chalcocite in acidic oxygenated sulphate-chloride solutions. Hydrometallurgy 1991, 27, 249–268. [Google Scholar] [CrossRef]
- Herreros, O.; Quiroz, R.; Viñals, J. Dissolution kinetics of copper, white metal and natural chalcocite in Cl2/Cl− media. Hydrometallurgy 1999, 51, 345–357. [Google Scholar] [CrossRef]
- Herreros, O.; Viñals, J. Leaching of sulfide copper ore in a NaCl-H2SO4-O2 media with acid pre-treatment. Hydrometallurgy 2007, 89, 260–268. [Google Scholar] [CrossRef]
- Senanayake, G. Chloride assisted leaching of chalcocite by oxygenated sulphuric acid via Cu(II)-OH-Cl. Miner. Eng. 2007, 20, 1075–1088. [Google Scholar] [CrossRef]
- Muszer, A.; Wódka, J.; Chmielewski, T.; Matuska, S. Covellinisation of copper sulphide minerals under pressure leaching conditions. Hydrometallurgy 2013, 137, 1–7. [Google Scholar] [CrossRef]
- Ruiz, M.C.; Abarzúa, E.; Padilla, R. Oxygen pressure leaching of white metal. Hydrometallurgy 2007, 86, 131–139. [Google Scholar] [CrossRef]
- Petersen, J.; Dixon, D. Principles, mechanisms and dynamics of chalcocite heap bioleaching. In Microbial Processing of Metal Sulfides; Springer: Dordrecht, The Netherlands, 2007; pp. 193–218. [Google Scholar]
- Ruiz, M.C.; Honores, S.; Padilla, R. Leaching kinetics of digenite concentrate in oxygenated chloride media at ambient pressure. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 1998, 29, 961–969. [Google Scholar] [CrossRef]
- Senanayake, G. A review of chloride assisted copper sulfide leaching by oxygenated sulfuric acid and mechanistic considerations. Hydrometallurgy 2009, 98, 21–32. [Google Scholar] [CrossRef]
- Tundisi, J.G. Water resources in the future: problems and solutions. Estud. Avançados 2008, 22, 7–16. [Google Scholar] [CrossRef]
- Cisternas, L.A.; Gálvez, E.D. The use of seawater in mining. Miner. Process. Extr. Metall. Rev. 2018, 39, 18–33. [Google Scholar] [CrossRef]
- MCH. Agua en la Minería. Agua en la Minería. 2018. Available online: https://www.mch.cl/columnas/agua-la-mineria/# (accessed on 3 June 2019).
- Aguirre, C.L.; Toro, N.; Carvajal, N.; Watling, H.; Aguirre, C. Leaching of chalcopyrite (CuFeS2) with an imidazolium-based ionic liquid in the presence of chloride. Miner. Eng. 2016, 99, 60–66. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Dean, A.; Voss, D.; Draguljic, D. Response Surface Methodology. In Design and Analysis of Experiments; Springer Texts in Statistics: Cham, Switzerland, 2017; pp. 565–614. [Google Scholar]
- Toro, N.; Herrera, N.; Castillo, J.; Torres, C.; Sepúlveda, R. Initial Investigation into the Leaching of Manganese from Nodules at Room Temperature with the Use of Sulfuric Acid and the Addition of Foundry Slag—Part I. Minerals 2018, 8, 565. [Google Scholar] [CrossRef]
- Montgomery, D.C. Cap. 3, 6, 7 and 10. In Design and Analysis of Experiments, 8th ed.; Wiley: New York, NY, USA, 2012. [Google Scholar]
- Nicol, M.; Basson, P. The anodic behaviour of covellite in chloride solutions. Hydrometallurgy 2017, 172, 60–68. [Google Scholar] [CrossRef]
- Velásquez-Yévenes, L.; Nicol, M.; Miki, H. The dissolution of chalcopyrite in chloride solutions: Part 1. The effect of solution potential. Hydrometallurgy 2010, 103, 108–113. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Lawson, F. The kinetics of leaching covellite in acidic oxygenated sulphate-chloride solutions. Hydrometallurgy 1991, 27, 269–284. [Google Scholar] [CrossRef]
- Dutrizac, J.E. The leaching of sulphide minerals in chloride media. Hydrometallurgy 1992, 29, 1–45. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).