Kinetics of Chalcopyrite Leaching by Hydrogen Peroxide in Sulfuric Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Procedure
2.2. Methods
2.3. Material
3. Results and Discussion
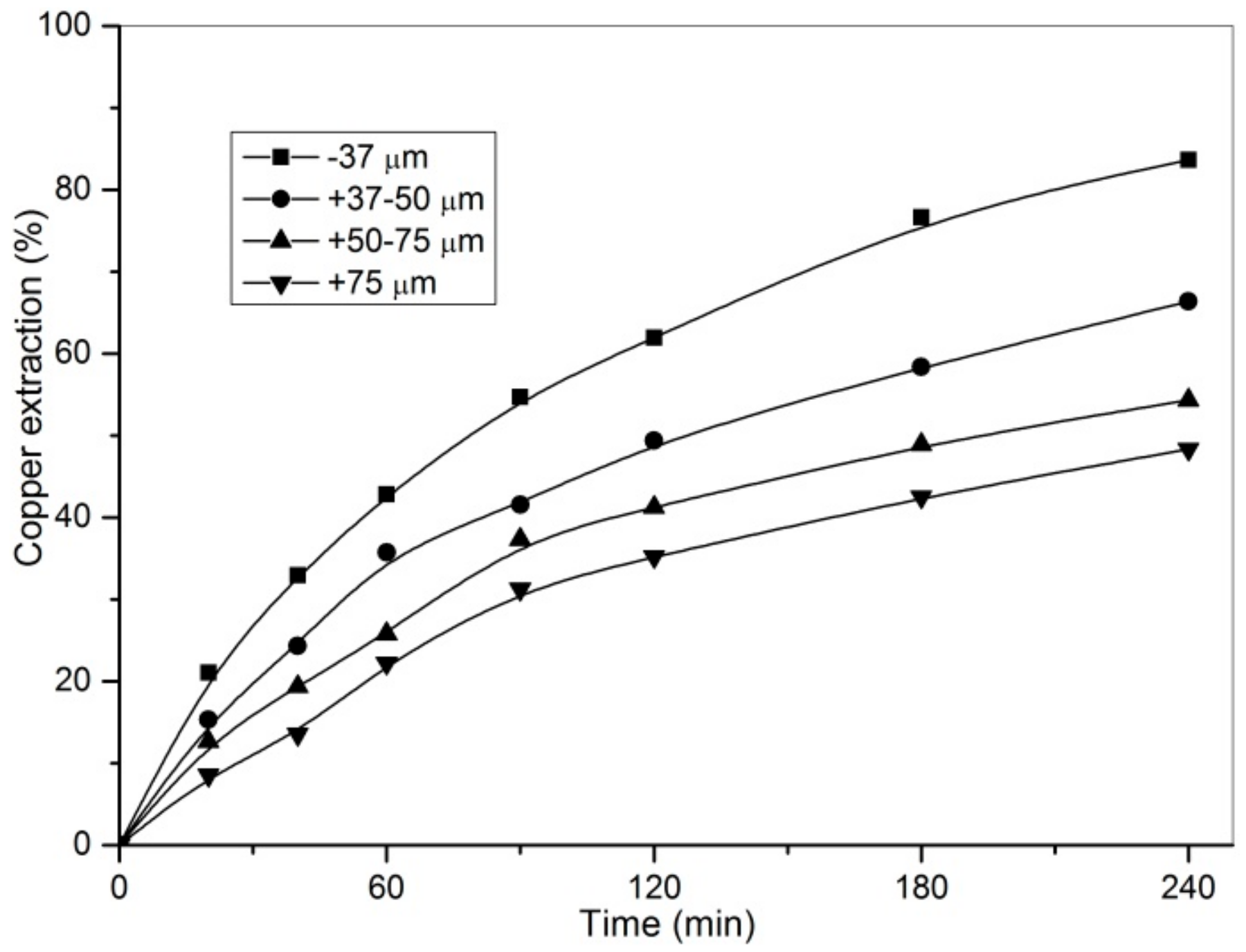
3.1. Effect of Particle Size
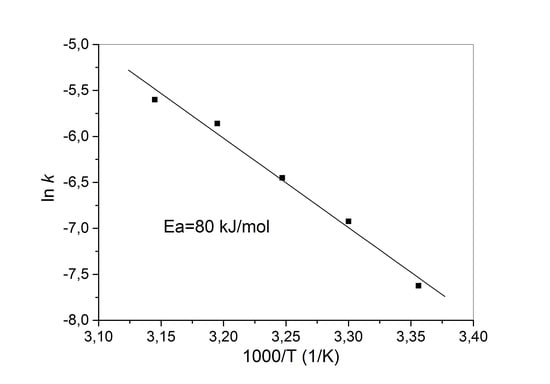
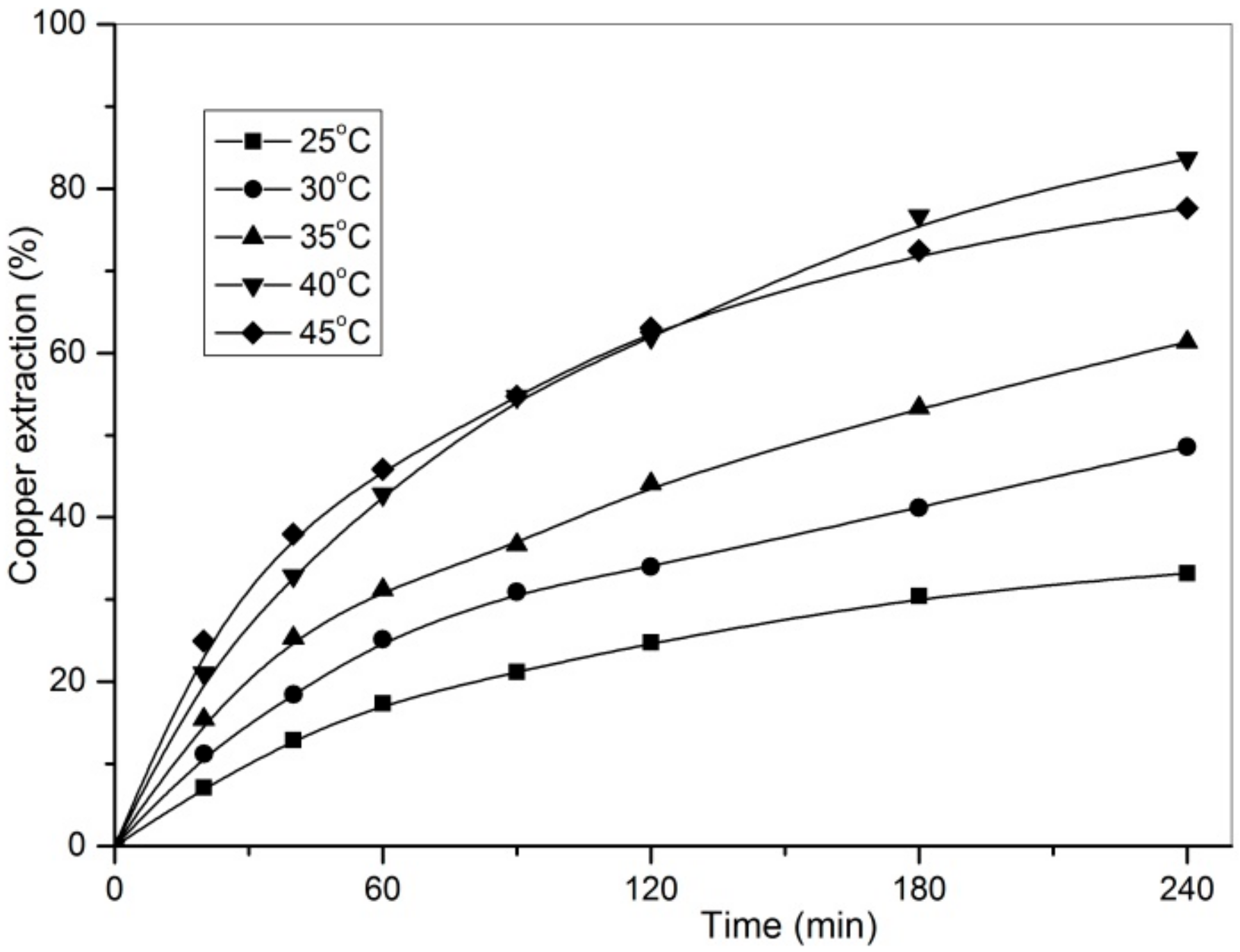
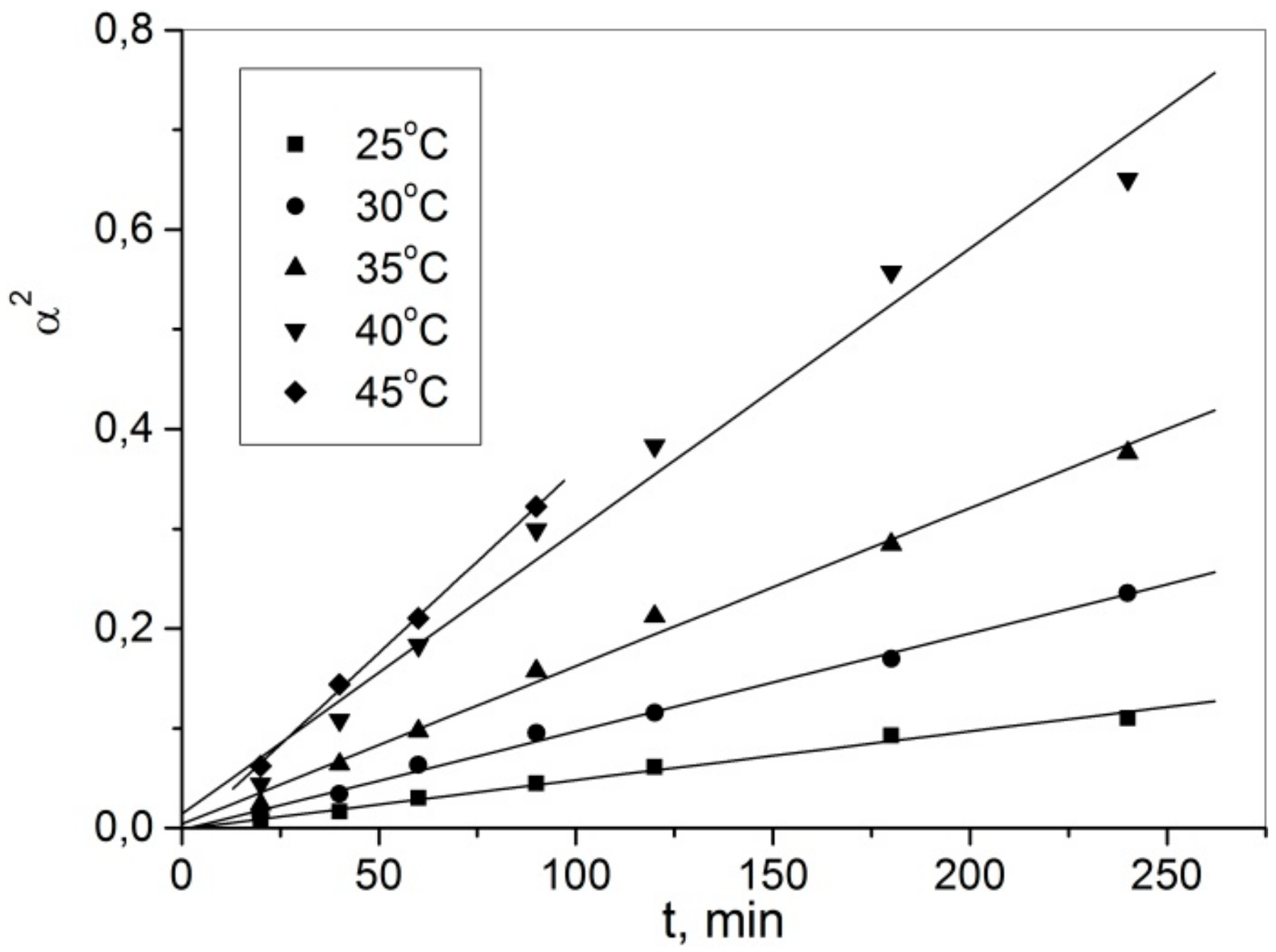
3.2. Effect of Temperature and Leaching Time
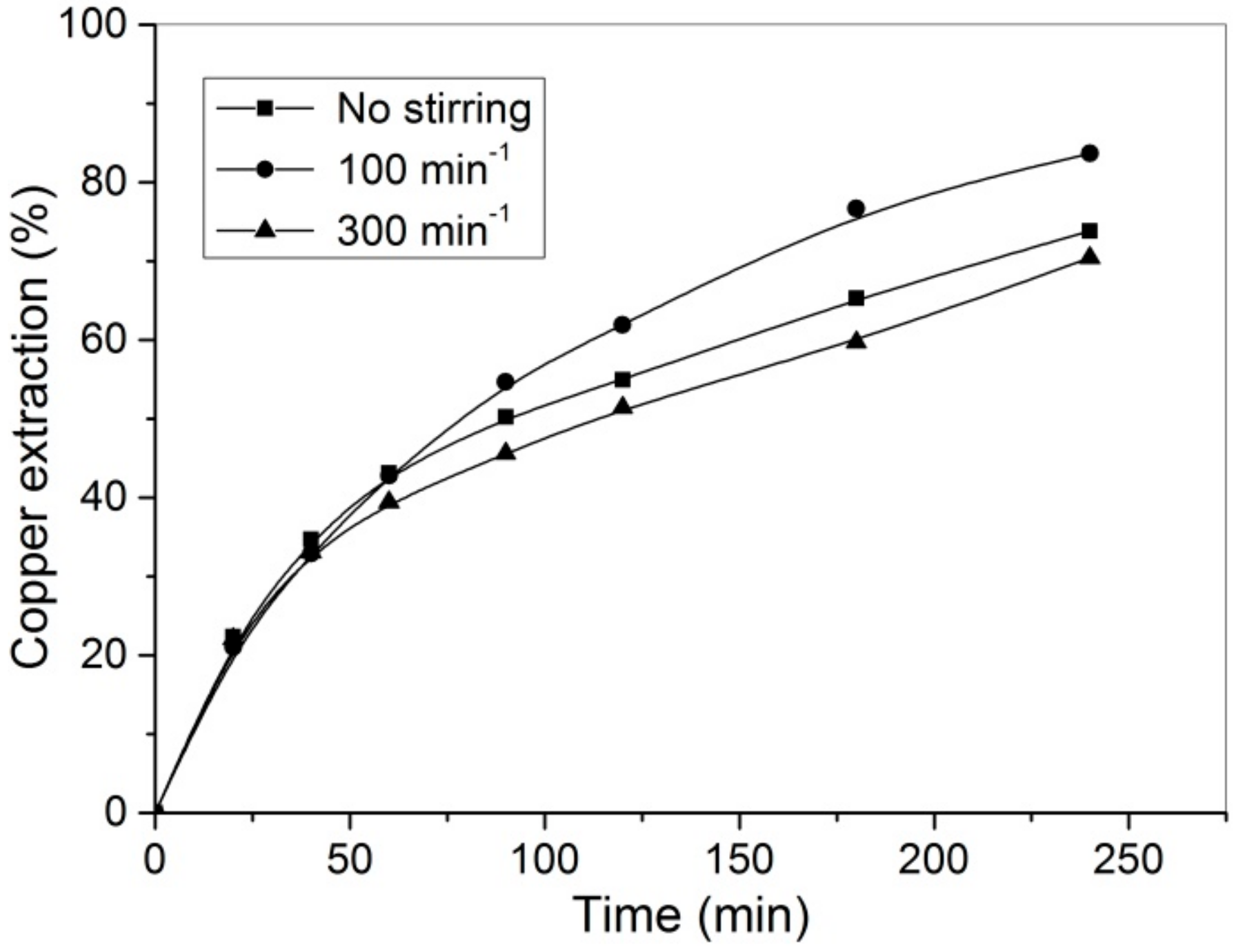
3.3. Effect of Stirring Speed
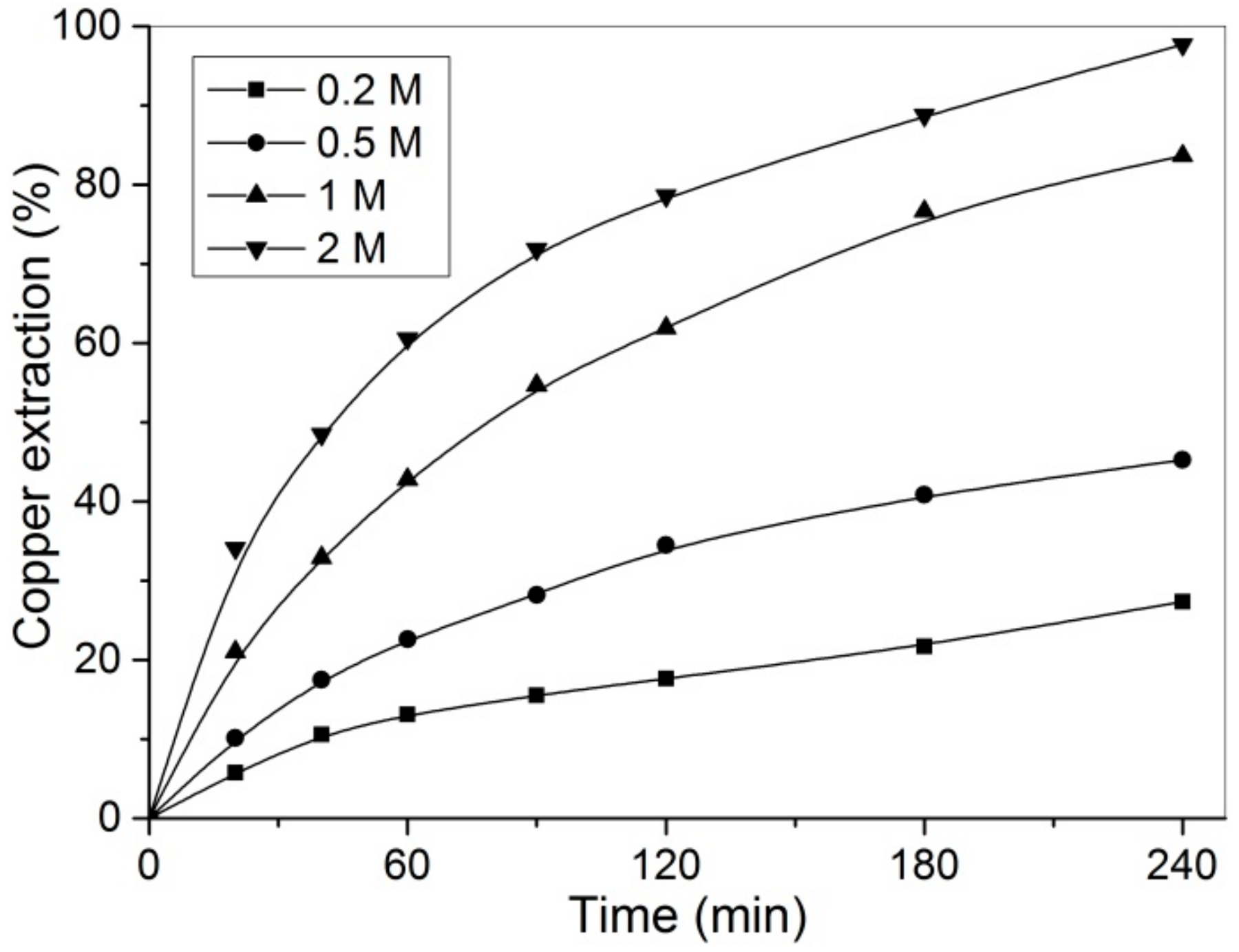
3.4. Effect of Hydrogen Peroxide Concentration
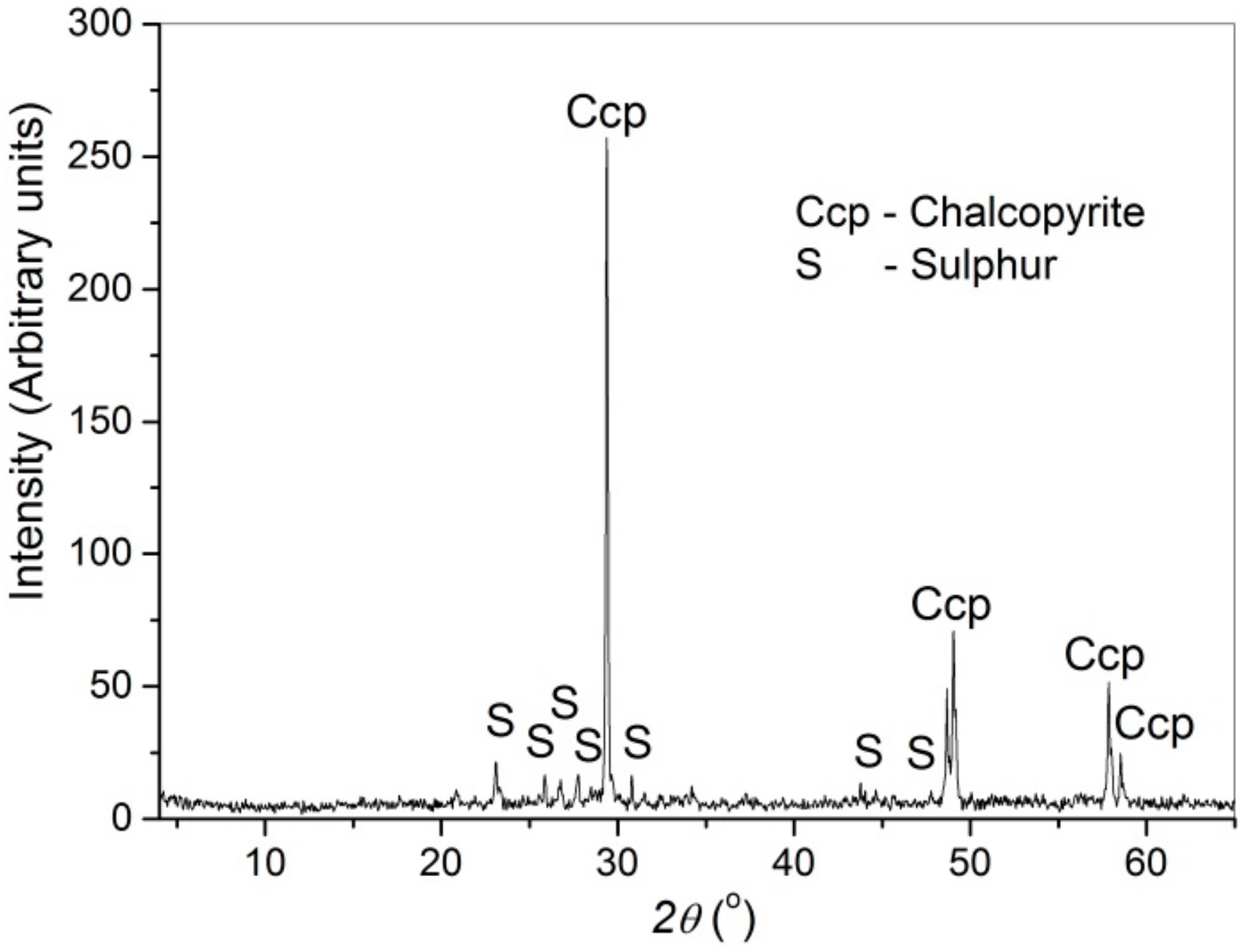
3.5. Characterization of Leach Residue
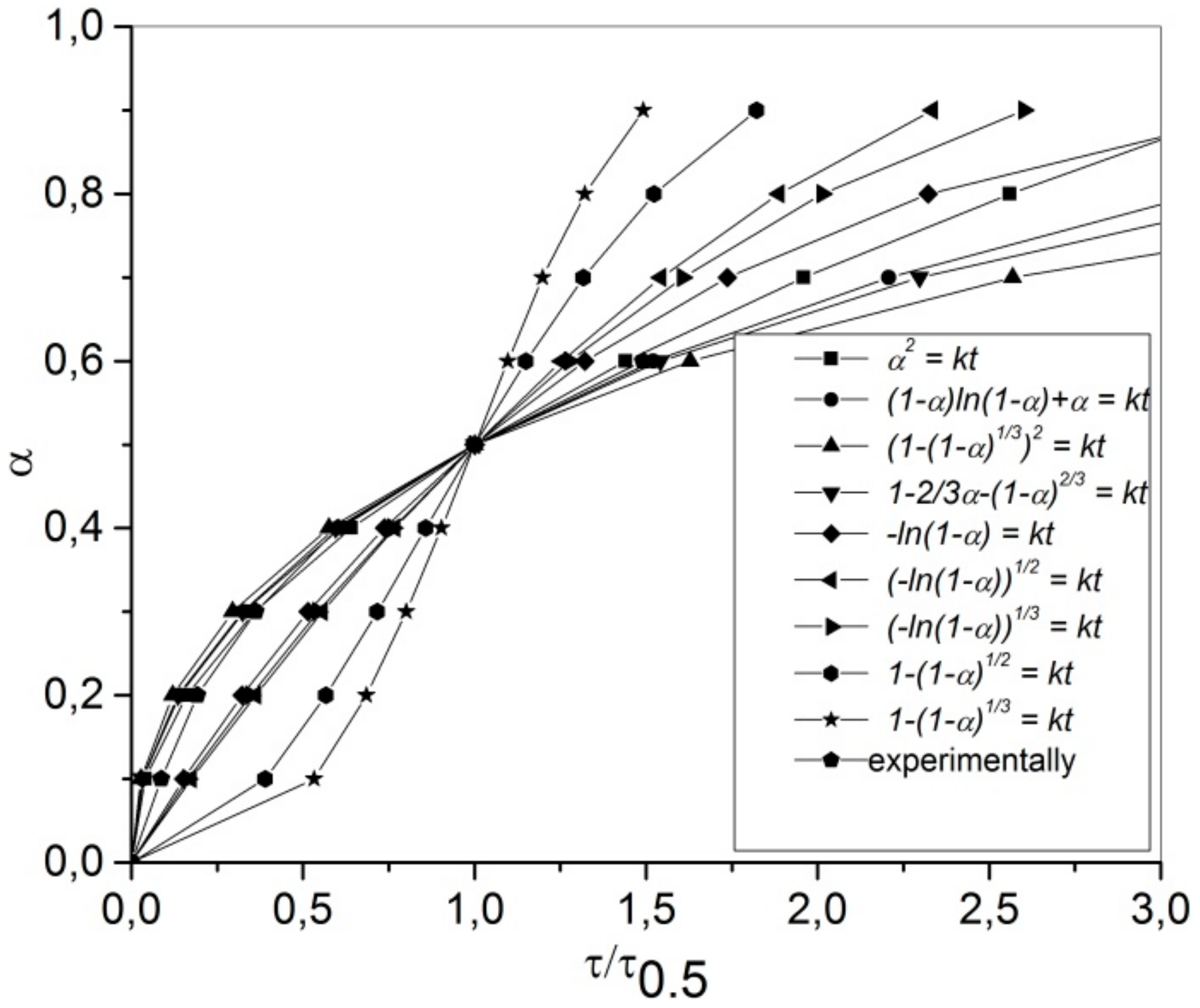
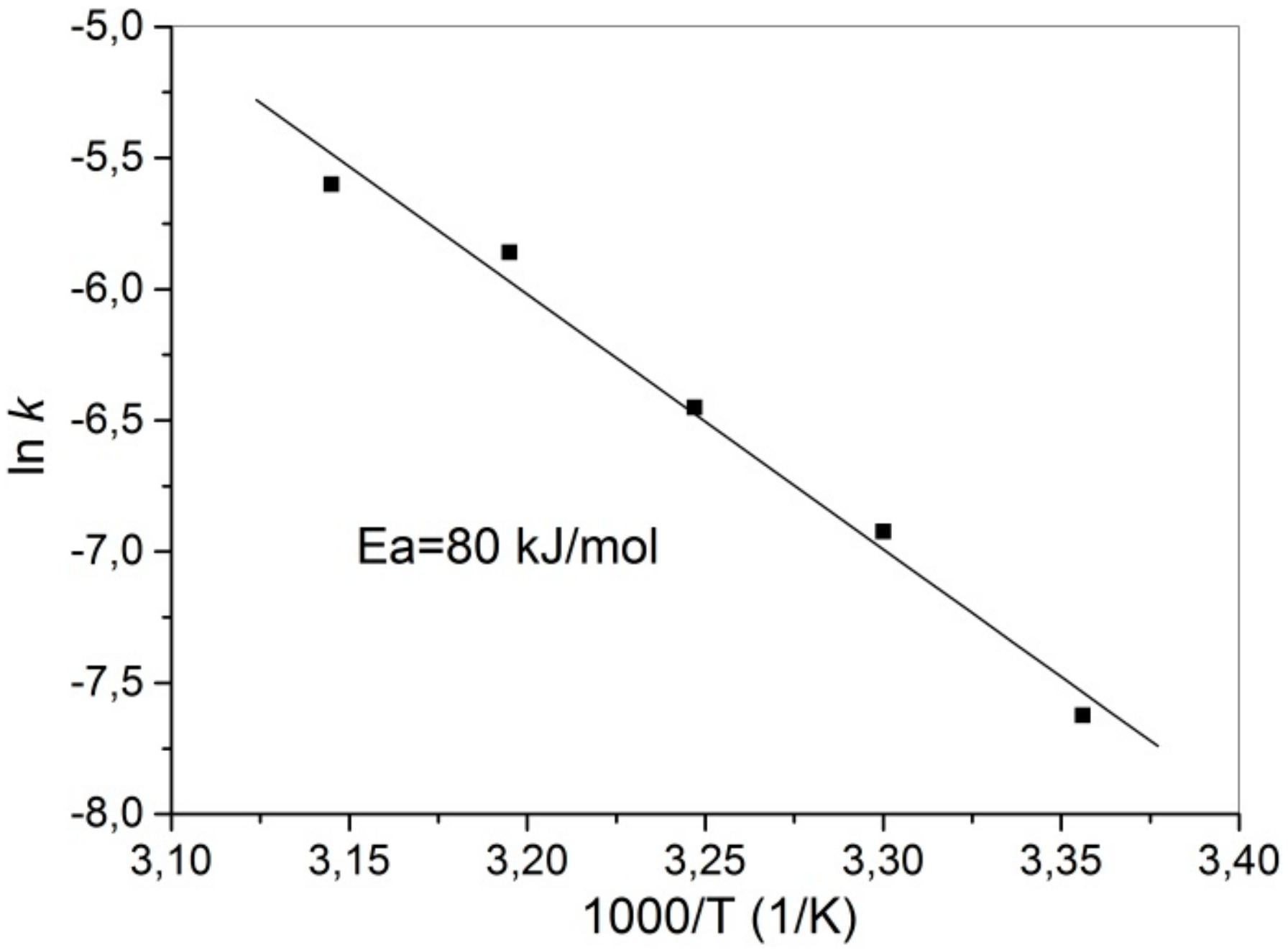
3.6. Leaching Kinetics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Watling, H.R. Chalcopyrite hydrometallurgy at atmospheric pressure: 1. Review of acidic sulfate, sulfate-chloride and sulfate-nitrate process options. Hydrometallurgy 2013, 140, 163–180. [Google Scholar] [CrossRef]
- Li, Y.; Kawashima, N.; Li, J.; Chandra, A.P.; Gerson, A.R. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Adv. Colloid Interface Sci. 2013, 197, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Dutrizac, J.E. The dissolution of chalcopyrite in ferric sulfate and ferric chloride media. Metall. Mater. Trans. B 1981, 12, 371–378. [Google Scholar] [CrossRef]
- Hackl, R.P.; Dreisinger, D.B.; Peters, E.; King, J.A. Passivation of chalcopyrite during oxidative leaching in sulfate madia. Hydrometallurgy 1995, 39, 25–48. [Google Scholar] [CrossRef]
- Maurice, D.; Hawk, J.A. Simultaneous autogenous milling and ferric chloride leaching of chalcopyrite. Hydrometallurgy 1999, 51, 371–377. [Google Scholar] [CrossRef]
- Klauber, C. A critical review of the surface chemistry of acidic ferric sulfate dissolution of chalcopyrite with regards to hindered dissolution. Int. J. Miner. Process. 2008, 86, 1–17. [Google Scholar] [CrossRef]
- Cordoba, E.M.; Munoz, J.A.; Blazquez, M.L.; Gonzalez, F.; Ballester, A. Leaching of chalcopyrite with ferric iron: Part I: General aspects. Hydrometallurgy 2008, 93, 81–87. [Google Scholar] [CrossRef]
- Yang, C.; Jiao, F.; Qin, W. Leaching of chalcopyrite: An emphasis on effect of copper and iron ions. J. Cent. South Univ. 2018, 25, 2380–2386. [Google Scholar] [CrossRef]
- Nikoloski, A.N.; O’Malley, G.P. The acidic ferric sulfate leaching of primary copper sulfides under recycle solution conditions observed in heap leaching. Part 1. Effect of standard conditions. Hydrometallurgy 2018, 178, 231–239. [Google Scholar] [CrossRef]
- Lundstrom, M.; Aromaa, J.; Forsen, O.; Hyvarinen, O.; Barker, M.H. Leaching of chalcopyrite in cupric chloride solution. Hydrometallurgy 2005, 77, 89–95. [Google Scholar] [CrossRef]
- Winand, R. Chloride hydrometallurgy. Hydrometallurgy 1991, 27, 285–316. [Google Scholar] [CrossRef]
- Skrobian, M.; Havlik, T.; Ukasik, M. Effect of NaCl concentration and particle size on chalcopyrite leaching in cupric chloride solution. Hydrometallurgy 2005, 77, 109–114. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, C.; Kou, J.; Zhao, H.; Wei, D.; Xing, Y. Enhancing the Leaching of Chalcopyrite Using Acidithiobacillus ferrooxidans under the Induction of Surfactant Triton X-100. Minerals 2019, 9, 11. [Google Scholar] [CrossRef]
- Martins, F.L.; Patto, G.B.; Leão, V.A. Chalcopyrite bioleaching in the presence of high chloride concentrations. J. Chem. Technol. Biotechnol. 2019, 94, 2333–2344. [Google Scholar] [CrossRef]
- Sandstrom, A.; Petersson, S. Bioleaching of a complex sulfide ore with moderate thermophilic and extreme thermophilic microorganisms. Hydrometallurgy 1997, 46, 181–190. [Google Scholar] [CrossRef]
- Dreisinger, D. Copper leaching from primary sulfides: options for biological and chemical extraction of copper. Hydrometallurgy 2006, 83, 10–20. [Google Scholar] [CrossRef]
- Watling, H.R. The bioleaching of sulfide minerals with emphasis on copper sulfides-A review. Hydrometallurgy 2006, 84, 81–108. [Google Scholar] [CrossRef]
- Pradhan, N.; Nathsarma, K.C.; Srinivasa Rao, K.; Sukla, L.B.; Mishra, B.K. Heap bioleaching of chalcopyrite: A review. Miner. Eng. 2008, 21, 355–365. [Google Scholar] [CrossRef]
- Sokić, M.; Marković, B.; Živković, D. Kinetics of chalcopyrite leaching by sodium nitrate in sulfuric acid. Hydrometallurgy 2009, 95, 273–279. [Google Scholar] [CrossRef]
- Sokić, M.; Matković, V.; Marković, B.; Štrbac, N.; Živković, D. Passivation of Chalcopyrite During the Leaching with Sulfuric Acid Solution in Presence of Sodium Nitrate. Hemijska Industrija 2010, 64, 343–350. [Google Scholar]
- Anderson, C.G. Treatment of copper ores and concentrates with industrial nitrogen species catalized pressure laching and non-cyanide precious metal recovery. JOM 2003, 55, 32–36. [Google Scholar] [CrossRef]
- Anderson, C.G.; Harrison, K.D.; Krys, L.E. Theoretical considerations of sodium nitrite oxidation and fine grinding in refractory precious metals concentrate pressure leaching. Trans. Soc. Min. Metall. Explor. 1996, 299, 4–11. [Google Scholar] [CrossRef]
- Antonijević, M.; Janković, Z.; Dimitrijević, M. Investigation of the kinetics of chalcopyrite oxidation by potassium dichtomate. Hydrometallurgy 1994, 35, 187–201. [Google Scholar] [CrossRef]
- Aydogan, S.; Ucar, G.; Canbazoglu, M. Dissolution kinetiks of chalcopyrite in acidic potassium dichromate solution. Hydrometallurgy 2006, 81, 45–51. [Google Scholar] [CrossRef]
- Devi, N.B.; Madhuchhanda, M.; Rath, P.C.; Srinivasa Rao, K.; Paramguru, R.K. Simultaneous leaching of a deep-sea manganese nodule and chalcopyrite in hydrochloric acid. Metall. Mater. Trans. B 2001, 32B, 777–784. [Google Scholar] [CrossRef]
- Havlik, T.; Laubertova, M.; Miskufova, A.; Kondas, J.; Vranka, F. Extraction of copper, zinc, nickel and cobalt in acid oxidative leaching of chalcopyrite at the presence of deep-sea manganese nodules as oxidant. Hydrometallurgy 2005, 77, 51–59. [Google Scholar] [CrossRef]
- Sandstrom, A.; Schukarev, A.; Paul, J. XPS characterization of chalcopyrite chemically and bioleached at high and low redox potential. Miner. Eng. 2005, 18, 505–515. [Google Scholar] [CrossRef]
- Kariuki, S.; Moore, C.; McDonald, A.M. Chlorate based oxidative hydrometallurgical extraction of copper and zinc from copper concentrate sulfide ores using mild acidic conditions. Hydrometallurgy 2009, 96, 72–76. [Google Scholar] [CrossRef]
- Zhong, S.; Li, Y. An improved understanding of chalcopyrite leaching kinetics and mechanisms in the presence of NaCl. J. Mater. Res. Technol. 2019, 8, 3487–3494. [Google Scholar] [CrossRef]
- Padilla, R.; Pavez, P.; Ruiz, M.C. Kinetics of copper dissolution from sulfidized chalcopyrite at high pressures in H2SO4–O2. Hydrometallurgy 2008, 91, 113–120. [Google Scholar] [CrossRef]
- McDonald, R.G.; Muir, D.M. Pressure oxidation leaching of chalcopyrite. Part I. Comparison of high and low temperature reaction kinetics and products. Hydrometallurgy 2007, 86, 191–205. [Google Scholar] [CrossRef]
- Akcil, A.; Ciftci, H. Metals recovery from multimetal sulfide concentrates (CuFeS2–PbS–ZnS): combination of thermal process and pressure leaching. Int. J. Miner. Process. 2003, 71, 233–246. [Google Scholar] [CrossRef]
- Cháidez, J.; Parga, J.; Valenzuela, J.; Carrillo, R.; Almaguer, I. Leaching chalcopyrite concentrate with oxygen and sulfuric acid using a low-pressure reactor. Metals 2019, 9, 189. [Google Scholar] [CrossRef]
- Benavente, O.; Hernández, M.C.; Melo, E.; Núñez, D.; Quezada, V.; Zepeda, Y. Copper Dissolution from Black Copper Ore under Oxidizing and Reducing Conditions. Metals 2019, 9, 799. [Google Scholar] [CrossRef]
- Carillo Pedroza, F.R.; Sanchez-Castillo, M.A.; Soria-Aguilar, M.J.; Martinez-Luevanos, A.; Gutierrez, E.C. Evaluation of of acid leaching of low grade chalcopyrite using ozone by statistical analysis. Can. Metall. Q. 2010, 49, 219–226. [Google Scholar] [CrossRef]
- Gomez, C.; Roman, E.; Blazquez, M.L.; Ballester, A. SEM and AES studies of chalcopyrite bioleaching in the presence of catalytic ions. Miner. Eng. 1997, 10, 825–835. [Google Scholar] [CrossRef]
- Hiroyoshi, N.; Arai, M.; Miki, H.; Tsunekawa, M.; Hirajima, T. A new reaction model for the catalytic effect of silver ions on chalcopyrite leaching in sulfuric acid solution. Hydrometallurgy 2002, 63, 257–267. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.; Kingman, S.W. Microwave-assisted leaching – A review. Hydrometallurgy 2004, 73, 189–203. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.; Kingman, S.; Hankins, N.; Somerfield, C.; Bradshaw, S.; Louw, W. The influence of microwaves on the leaching kinetics of chalcopirite. Miner. Eng. 2005, 18, 1259–1268. [Google Scholar] [CrossRef]
- Adebayo, A.O.; Ipinmorti, K.O.; Ajayi, O.O. Dissolution kinetics of chlacopyrite with hydrogen peroxide in sulfuric acid medium. Chem. Biochem. Eng. Q. 2003, 17, 213–218. [Google Scholar]
- Antonijević, M.; Dimitrijević, M.; Janković, Z. Leaching of pyrite with hydrogen peroxide in sulfuric acid. Hydrometallurgy 1997, 46, 71–83. [Google Scholar] [CrossRef]
- Antonijević, M.M.; Janković, Z.D.; Dimitrijević, M.D. Kinetics of chalcopyrite dissolution by hydrogen peroxide in sulfuric acid. Hydrometallurgy 2004, 71, 329–334. [Google Scholar] [CrossRef]
- Misra, M.; Fuerstenau, M.C. Chalcopyrite leaching at moderate temperature and ambient pressure in the presence of nanosize silica. Miner. Eng. 2005, 18, 293–297. [Google Scholar] [CrossRef]
- Olubambi, P.A.; Potgieter, J.H. Investigations on the machanisms of sulfuric acid leaching of chlacopyrite in the presence of hydrogen peroxide. Miner. Process. Extr. Metall. Rev. 2009, 30, 327–345. [Google Scholar] [CrossRef]
- Turan, M.D.; Altundogan, H.S. Leaching of chalcopyrite concentrate with hydrogen peroxide and sulfuric acid in an autoclave system. Metall. Mater. Trans. B 2013, 44B, 809–819. [Google Scholar] [CrossRef]
- Agacayak, T.; Aras, A.; Aydogan, S.; Erdemoglu, M. Leaching of chlacopyrite concentrate in hydrogen peroxide solution. Physicochem. Prob. Miner. Process. 2014, 50, 657–666. [Google Scholar]
- Wu, J.; Ahn, J.; Lee, J. Comparative leaching study on conichalcite and chalcopyrite under different leaching systems. Korean J. Met. Mater. 2019, 57, 245–250. [Google Scholar] [CrossRef]
- Petrović, S.J.; Bogdanović, G.D.; Antonijević, M.M. Leaching of chalcopyrite with hidrogen peroxide in hydrochloric acid solution. Trans. Nonferrous Met. Soc. China 2018, 28, 1444–1455. [Google Scholar] [CrossRef]
- Ahn, J.; Wu, J.; Lee, J. Investigation on chalcopyrite leaching with methanesulfonic acid (MSA) and hydrogen peroxide. Hydrometallurgy 2019, 187, 54–62. [Google Scholar] [CrossRef]
- Hu, J.; Tian, G.; Zi, F.; Hu, X. Leaching of chalcopyrite with hydrogen peroxide in 1-hexil-3-methil-imidazolium hydrogen sulfate ionic liquid aqueous solution. Hydrometallurgy 2017, 169, 1–8. [Google Scholar] [CrossRef]
- Mahajan, V.; Misra, M.; Zhong, K.; Fuerstenau, M.C. Enhanced leaching of copper from chalcopyrite in hydrogen peroxide-glycol system. Miner. Eng. 2007, 20, 670–674. [Google Scholar] [CrossRef]
- Ruiz-Sanchez, A.; Lapidus, G.T. Study of chalcopyrite leaching from a copper concentrate with hydrogen peroxide in aqueous ethylene glycol media. Hydrometallurgy 2017, 169, 192–200. [Google Scholar] [CrossRef]
- Solís Marcial, O.J.; Nájera Bastida, A.; Bañuelos, J.E.; Valdés Martínez, O.U.; Luevano, L.A.; Serrano Rosales, B. Chalcopyrite Leaching Kinetics in the Presence of Methanol. Int. J. Chem. Reactor Eng. 2019, in press. [Google Scholar] [CrossRef]
- Lin, H.K.; Luong, H.V. Column leaching for simultaneous heap and in-situ soli remediation with metallic Fenton reaction. J. Miner. Mater. Charact. Eng. 2004, 3, 33–39. [Google Scholar]
- Dutrizac, J. Ferric ion leaching of chalcopyrites from different localities. Metall. Mater. Trans. B 1982, 13B, 303–309. [Google Scholar] [CrossRef]
- Sokić, M.; Radosavljević, S.; Marković, B.; Matković, V.; Štrbac, N.; Kamberović, Ž.; Živković, D. Influence of chalcopyrite structure on their leaching by sodium nitrate in sulfuric acid. Metall. Mater. Eng. 2014, 20, 53–60. [Google Scholar]
- Craus, W.; Nolze, G. POWDER CELL-a program for the representation and manipulation of crystal structures and calculation of the resulting X-ray powder patterns. J. Appl. Crystallogr. 1996, 29, 301–303. [Google Scholar] [CrossRef]
- Sharp, J.H.; Brindley, G.W.; Achar, B.N.N. Numerical data for some commonly used solid state reaction equations. J. Am. Ceram. Soc. 1996, 49, 379–382. [Google Scholar] [CrossRef]
- Sokić, M.; Marković, B.; Matković, V.; Živković, D.; Štrbac, N.; Stojanović, J. Kinetics and mechanism of sphalerite leaching by sodium nitrate in sulfuric acid solution. J. Min. Metall. Sect. B. 2012, 48, 185–195. [Google Scholar] [CrossRef]
- Ammou-chokroom, M.; Canbazoglu, M.; Steinmetz, D. Oxydation ménagée de la chalcopyrite en solution acide: analyse cinétique des réactions. II. Modèles diffusionnels. Bulletin de Minéralogie 1997, 100, 161–177. [Google Scholar] [CrossRef]
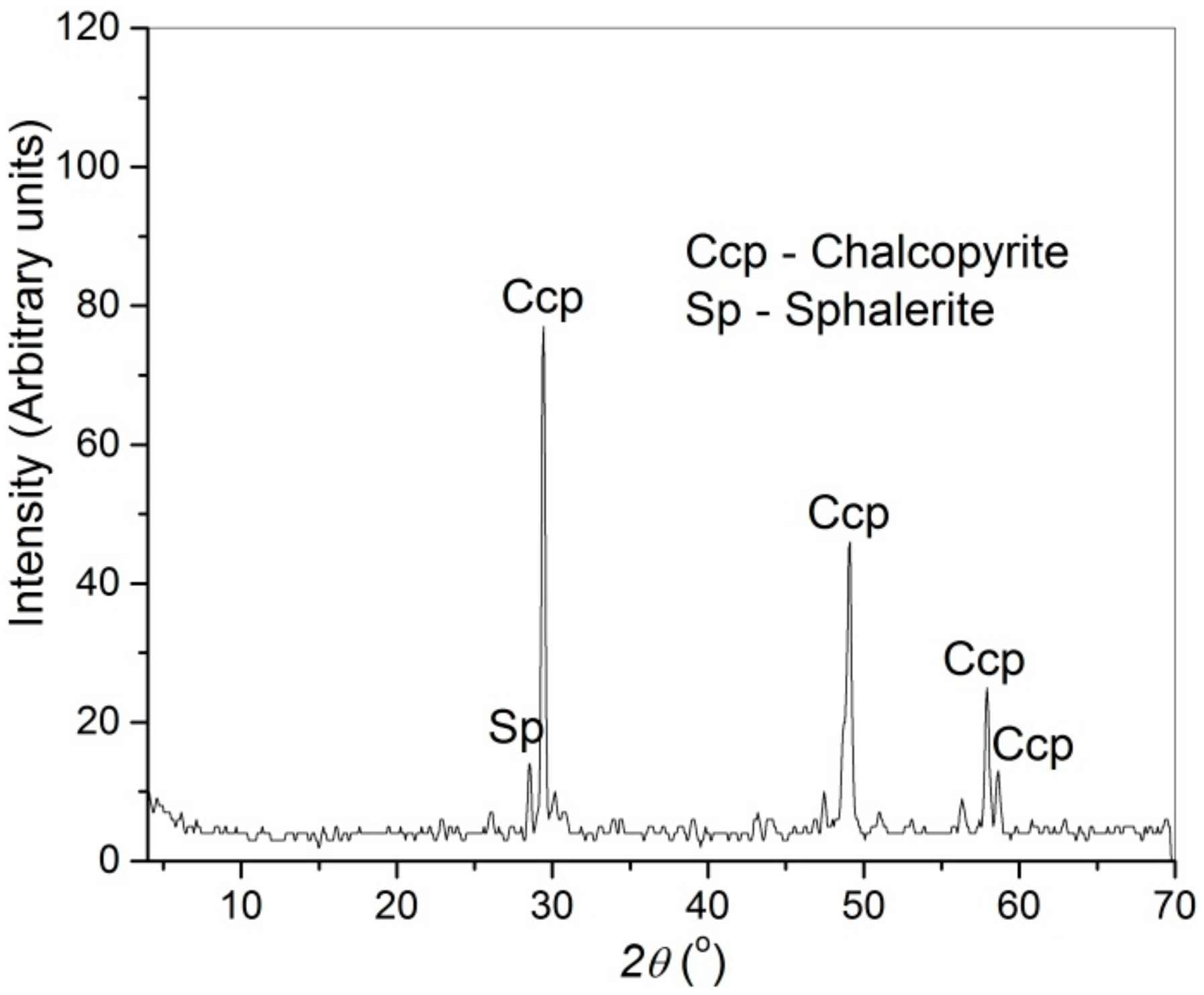
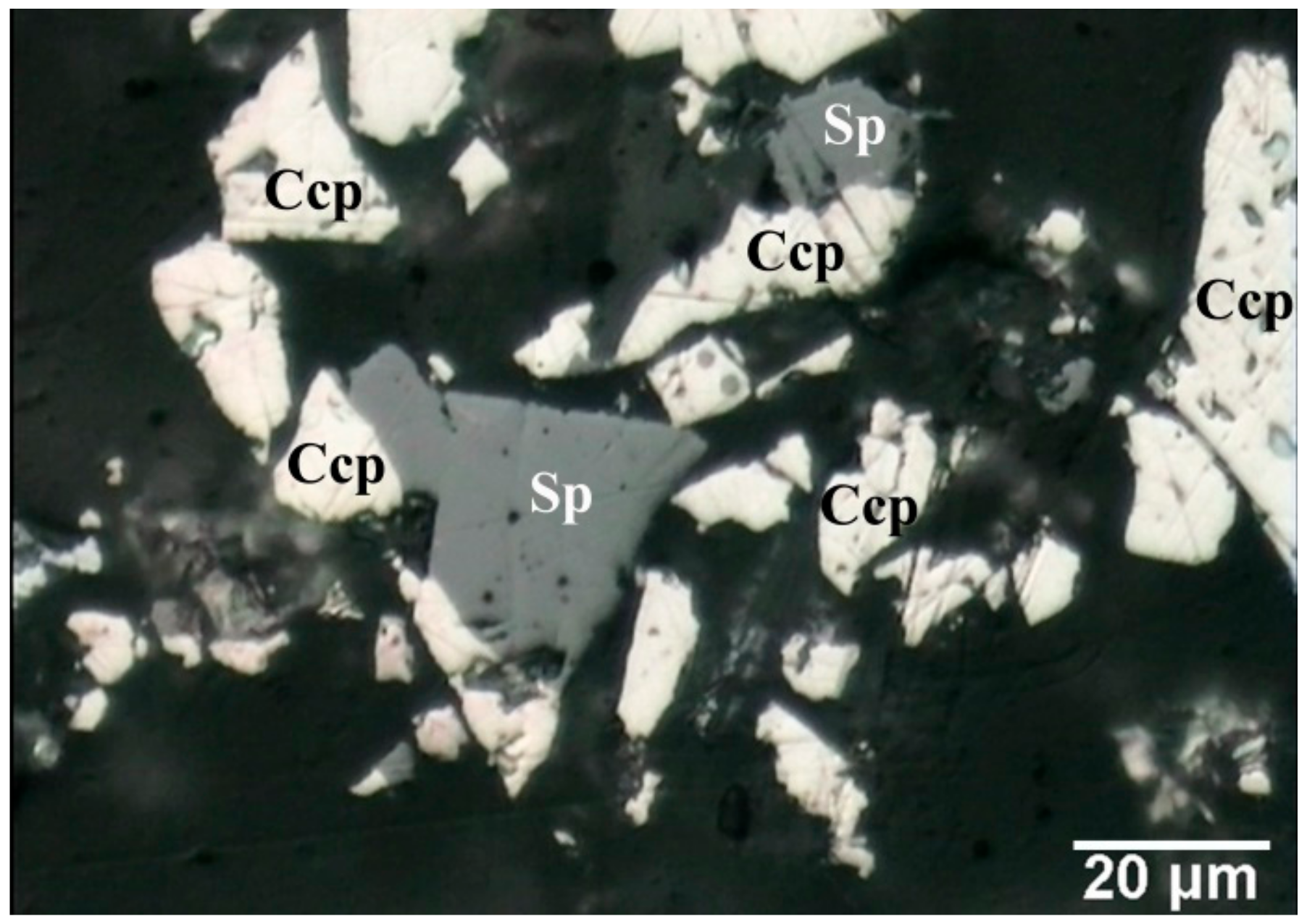

| Class, μm | Mass Fraction, % | Content (%) | ||||
|---|---|---|---|---|---|---|
| Cu | Zn | Pb | Fe | S | ||
| +75 | 7.32 | 23.38 | 3.43 | 4.08 | 22.25 | 28.31 |
| −75 + 50 | 21.15 | 26.55 | 4.28 | 1.70 | 24.43 | 30.19 |
| −50 + 37 | 5.18 | 26.95 | 4.36 | 1.85 | 24.75 | 30.92 |
| −37 | 66.35 | 27.08 | 4.15 | 2.28 | 25.12 | 31.12 |
| Mineral | Mass % |
|---|---|
| Chalcopyrite | 78.247 |
| Sphalerite | 6.249 |
| Galena | 2.633 |
| Pyrrhotite | 1.161 |
| Pyrite | 0.099 |
| Arsenopyrite | 0.211 |
| Covellite | 0.081 |
| Native bismuth | 0.031 |
| Limonite | 0.118 |
| Gangue | 11.170 |
| Total: | 100.000 |
| Mass of the Leach Residue (g) | Content (%) | ||||
|---|---|---|---|---|---|
| Cu | Zn | Pb | Fe | S | |
| 0.38 | 21.80 | 0.14 | 4.95 | 20.12 | 41.34 |
| Mineral | Mass % |
|---|---|
| Chalcopyrite | 63.002 |
| Sphalerite | 0.222 |
| Galena | 0.417 |
| Pyrrhotite | 1.215 |
| Anglesite | 6.754 |
| Elemental sulfur | 18.887 |
| Quartz | 9.503 |
| Total: | 100.000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokić, M.; Marković, B.; Stanković, S.; Kamberović, Ž.; Štrbac, N.; Manojlović, V.; Petronijević, N. Kinetics of Chalcopyrite Leaching by Hydrogen Peroxide in Sulfuric Acid. Metals 2019, 9, 1173. https://doi.org/10.3390/met9111173
Sokić M, Marković B, Stanković S, Kamberović Ž, Štrbac N, Manojlović V, Petronijević N. Kinetics of Chalcopyrite Leaching by Hydrogen Peroxide in Sulfuric Acid. Metals. 2019; 9(11):1173. https://doi.org/10.3390/met9111173
Chicago/Turabian StyleSokić, Miroslav, Branislav Marković, Srđan Stanković, Željko Kamberović, Nada Štrbac, Vaso Manojlović, and Nela Petronijević. 2019. "Kinetics of Chalcopyrite Leaching by Hydrogen Peroxide in Sulfuric Acid" Metals 9, no. 11: 1173. https://doi.org/10.3390/met9111173
APA StyleSokić, M., Marković, B., Stanković, S., Kamberović, Ž., Štrbac, N., Manojlović, V., & Petronijević, N. (2019). Kinetics of Chalcopyrite Leaching by Hydrogen Peroxide in Sulfuric Acid. Metals, 9(11), 1173. https://doi.org/10.3390/met9111173