Modification of Non-Metallic Inclusions in Stainless Steel by Addition of CaSi
Abstract
1. Introduction
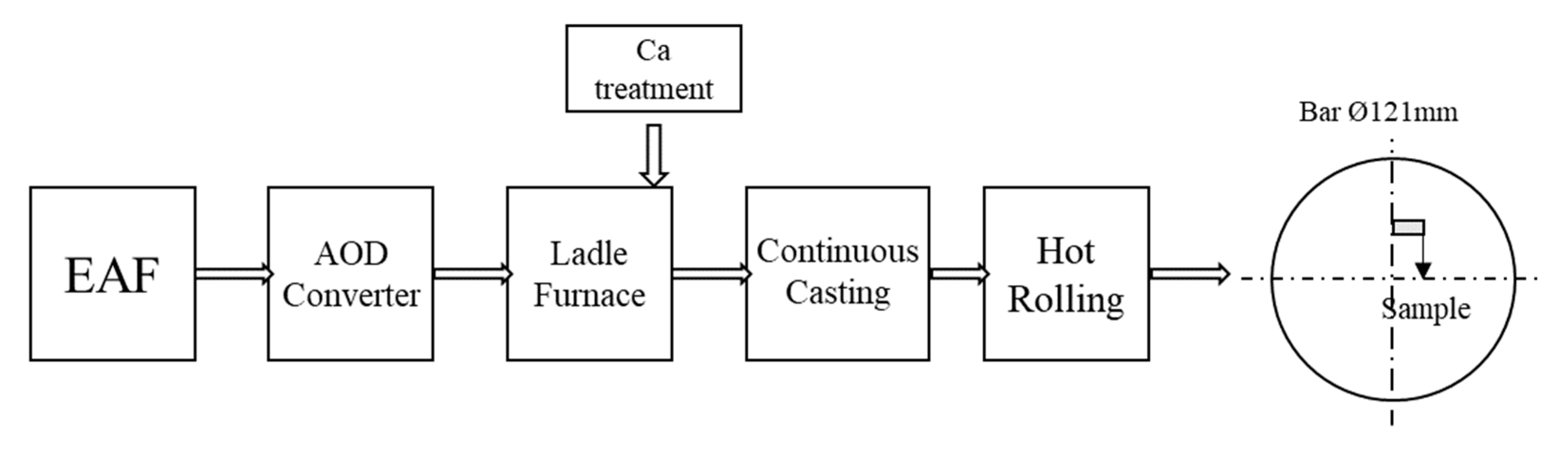
2. Materials and Methods
2.1. Materials
2.2. Electrolytic Extraction and Investigation of Inclusions
3. Results and Discussions
3.1. Characterization of Inclusions after Extraction in Different Electrolytes
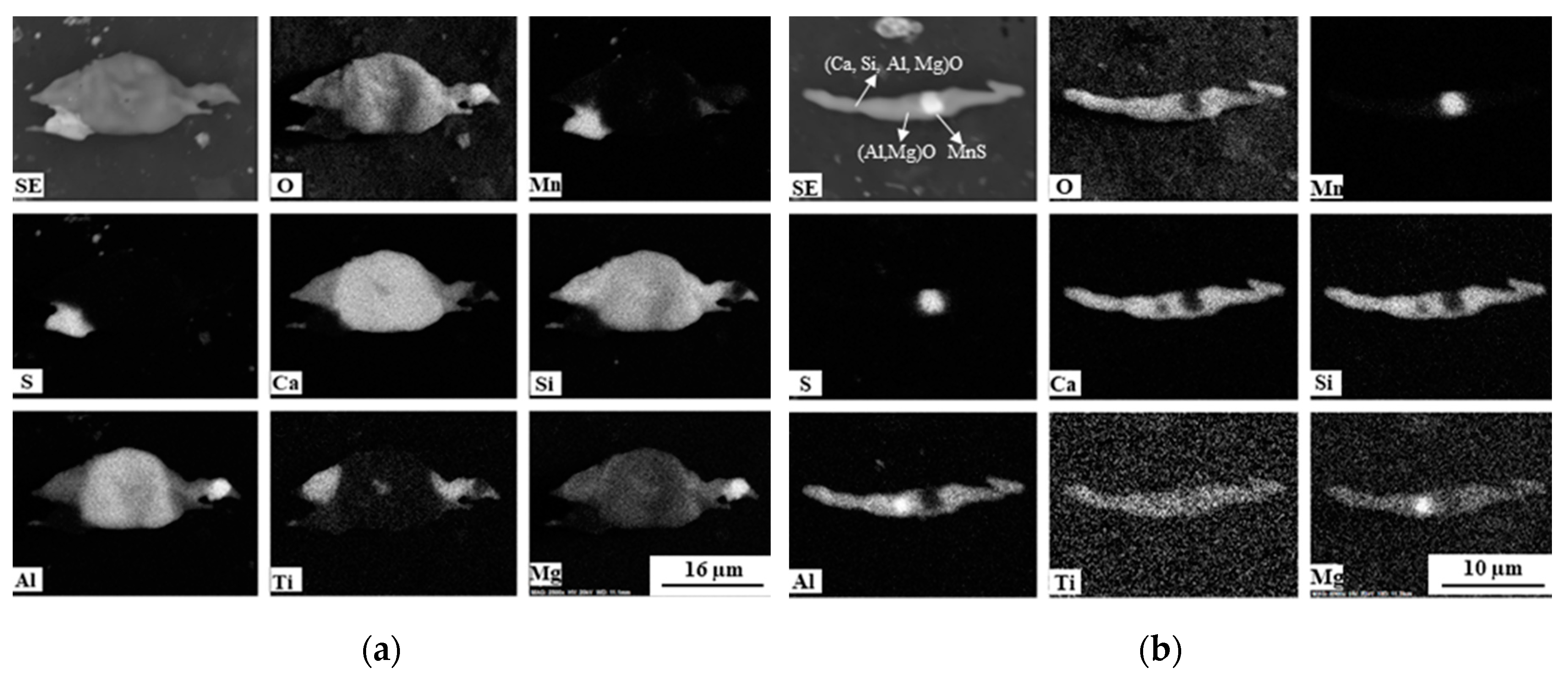
3.2. Classification of Non-Metallic Inclusions in 316R and 316Ca Steels
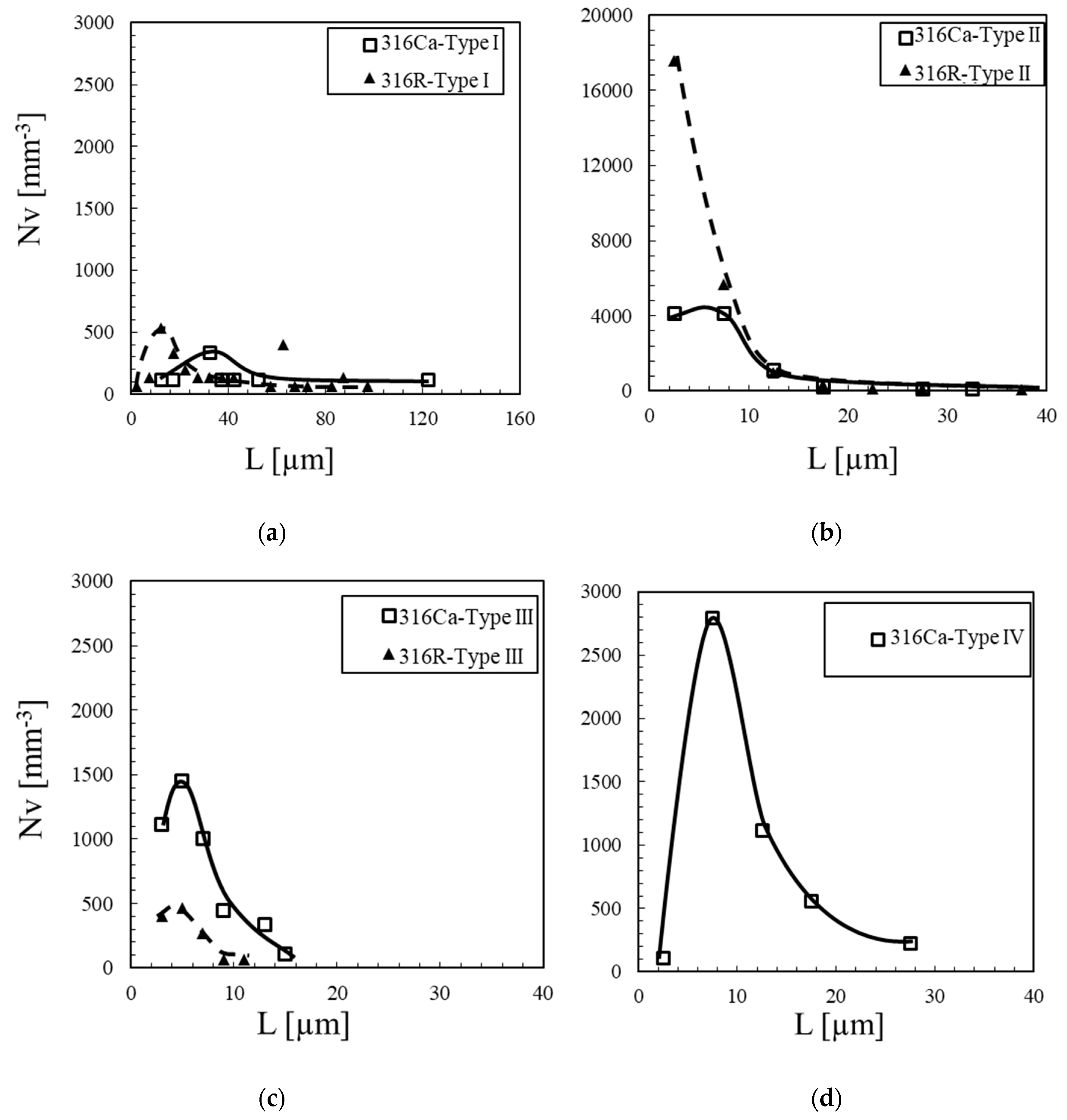
3.3. Particle Size Distribution of the Inclusions Observed in 316R and 316Ca Steels
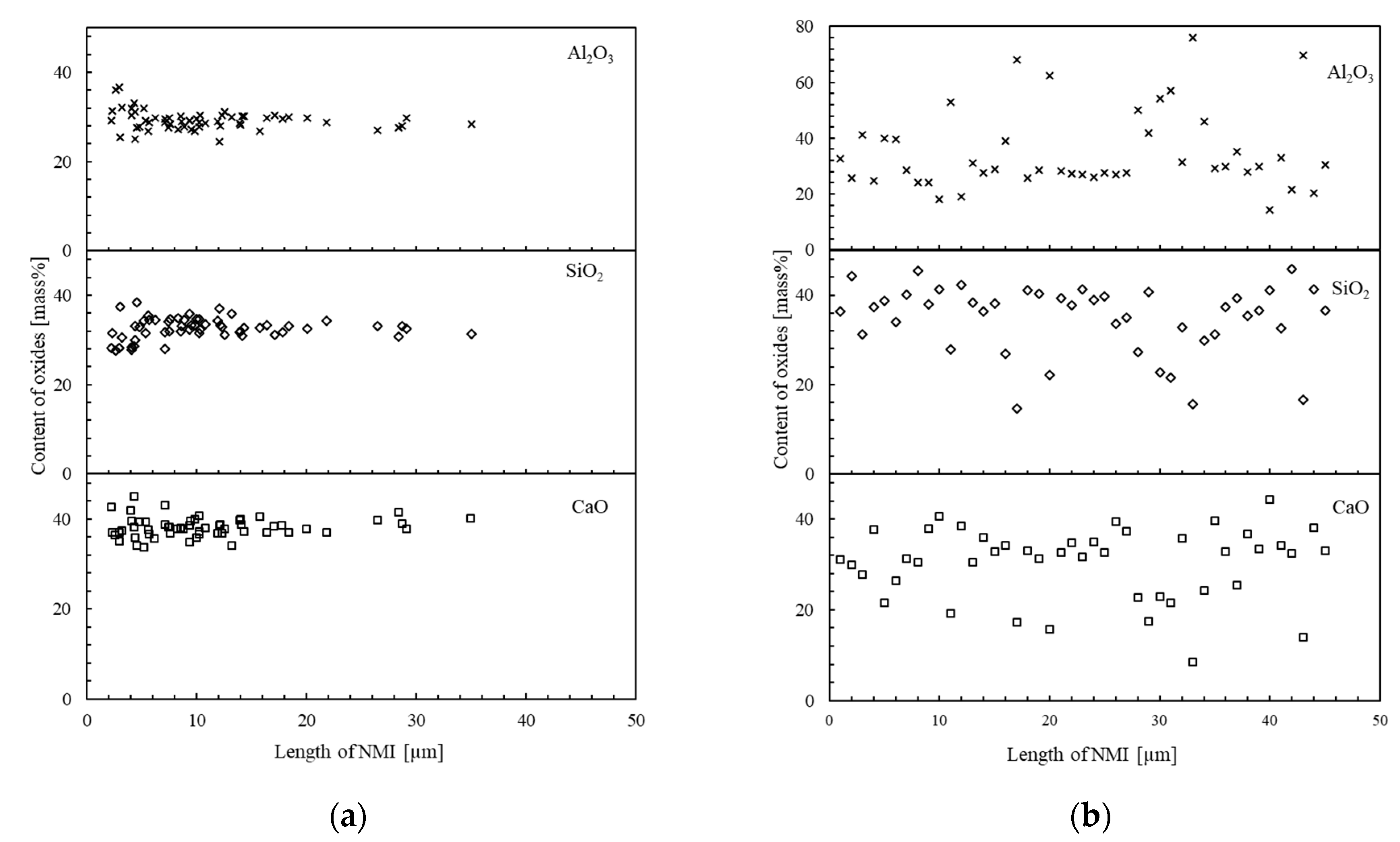
3.4. Mechanism of Inclusion Transformation
4. Conclusions
- Similar compositions of oxide inclusions in 316Ca steel were obtained after EE by using 2% TEA and 10% AA electrolytes.
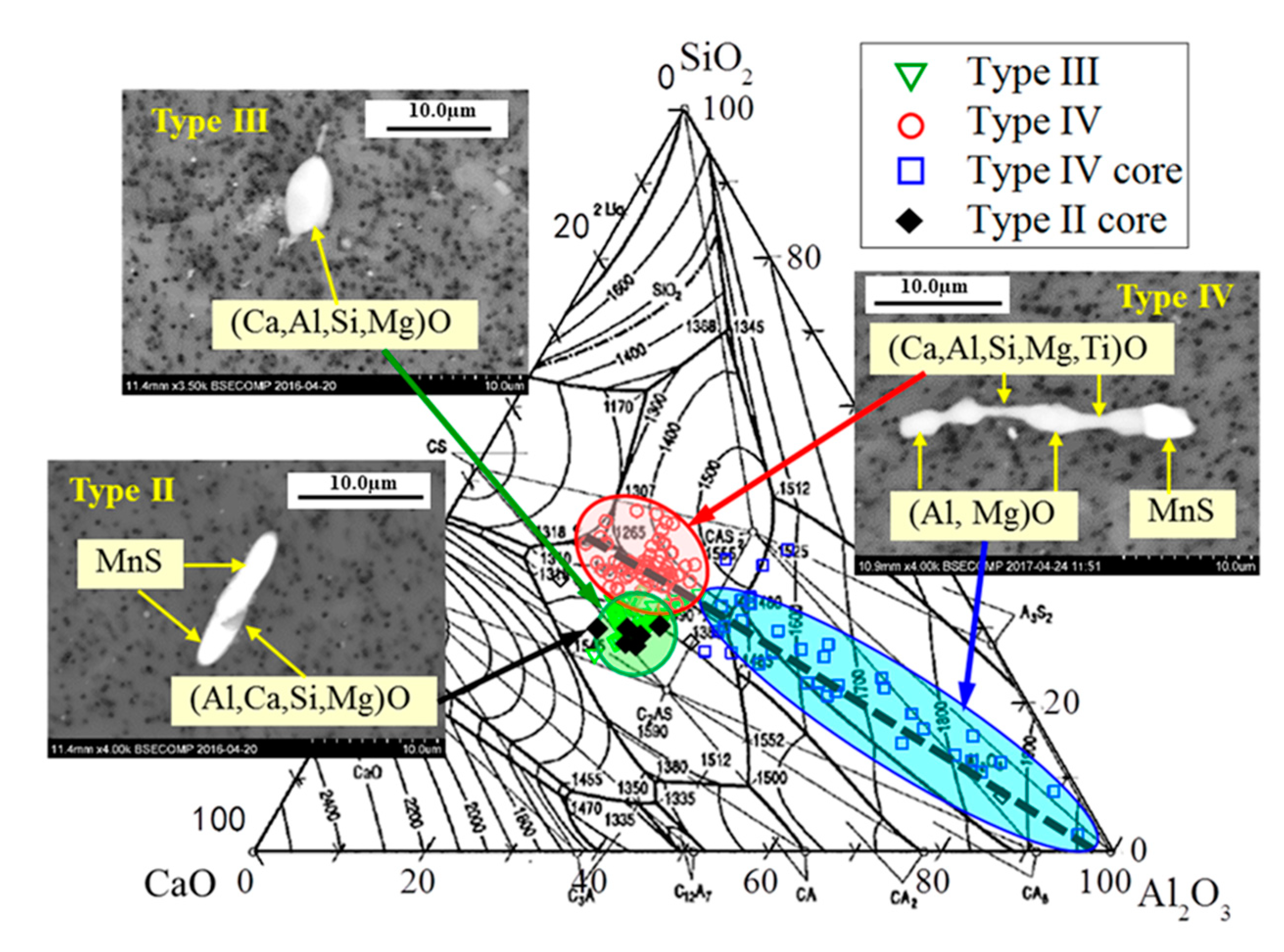
- The 316L steels contained four types of inclusions: (1) elongated MnS (Type I), (2) MnS sulfides with hard oxide cores (Type II), (3) undeformed irregular oxides (Type III), and (4) elongated oxides with a hard oxide core (Type IV).
- In the reference sample, the oxide composition was mainly Al2O3–MgO–MnO. However, after Ca treatment of 316L steel, about 46% of the observed inclusions were oxide inclusions (Types III and IV), which correlated to gehlenite and to a mixture of gehlenite and anorthite, which are favorable for the machinability of steel.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cyril, N.; Fatemi, A.; Cryderman, B. Effects of sulfur level and anisotropy of sulfide inclusions on tensile, impact, and fatigue properties of SAE 4140 steel. SAE Int. J. Manuf. Mater. 2008, 1, 218–227. [Google Scholar] [CrossRef]
- Ånmark, N.; Karasev, A.; Jönsson, P.G. The Influence of Microstructure and Non-Metallic Inclusions on the Machinability of Clean Steels. Steel Res. Int. 2016, 87, 1–8. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Bao, Y. Effects of Non-metallic Inclusions on Machinability of Free-Cutting Steels Investigated by Nano-Indentation Measurements. Metall. Mater. Trans. A 2015, 46, 281–292. [Google Scholar] [CrossRef]
- Qi, H.S.; Mills, B. On the formation mechanism of adherent layers on a cutting tool. Wear 1996, 198, 192–196. [Google Scholar] [CrossRef]
- Sidjanin, L.; Kovac, P. Fracture mechanisms in chip formation processes. Mater. Sci. Technol. 1997, 13, 439–444. [Google Scholar] [CrossRef]
- Abdelaziz, S.; Megahed, G.; El-Mahallawi, I.; Ahmed, H. Control of Ca addition for improved cleanness of low C, Al killed steel. Ironmak Steelmak 2009, 36, 432–441. [Google Scholar] [CrossRef]
- Verma, N.; Pistorius, P.C.; Fruehan, R.J.; Potter, M.; Lind, M.; Story, S. Transient Inclusion Evolution During Modification of Alumina Inclusions by Calcium in Liquid Steel: Part II. Results and Discussion. Metall. Mater. Trans. B 2011, 42, 720–729. [Google Scholar] [CrossRef]
- Lis, T. Modification of oxygen and sulphur inclusions in steel by calcium treatment. Metalurgija 2009, 48, 95–98. [Google Scholar]
- Guo, Y.; He, S.; Chen, G.; Wang, Q. Thermodynamics of Complex Sulfide Inclusion Formation in Ca-Treated Al-Killed Structural Steel. Metall. Mater. Trans. B 2016, 47, 2549–2557. [Google Scholar] [CrossRef]
- Liu, J.; Wu, H.; Bao, Y.; Wang, M. Inclusion variations and calcium treatment optimization in pipeline steel production. Int. J. Miner. Metall. Mater. 2011, 18, 527. [Google Scholar] [CrossRef]
- Bletton, O.; Duet, R.; Pedarre, P. Influence of oxide nature on the machinability of 316L stainless steels. Wear 1990, 139, 179–193. [Google Scholar] [CrossRef]
- Nordgren, A.; Melander, A. Tool wear and inclusion behaviour during turning of a calcium-treated quenched and tempered steel using coated cemented carbide tools. Wear 1990, 139, 209–223. [Google Scholar] [CrossRef]
- Fang, X.D.; Zhang, D. An investigation of adhering layer formation during tool wear progression in turning of free-cutting stainless steel. Wear 1996, 197, 169–178. [Google Scholar] [CrossRef]
- Ånmark, N.; Björk, T. Effects of the composition of Ca-rich inclusions on tool wear mechanisms during the hard-turning of steels for transmission components. Wear 2016, 368, 173–182. [Google Scholar] [CrossRef]
- Gutnichenko, O.; Bushlya, V.; Zhou, J.M.; Stahl, J.E. Tool wear and machining dynamics when turning high chromium white cast iron with pcBN tools. Wear 2017, 390–391, 253–269. [Google Scholar] [CrossRef]
- Kanbe, Y.; Karasev, A.; Todoroki, H.; Jönsson, P.G. Application of Extreme Value Analysis for Two- and Three-Dimensional Determinations of the Largest Inclusion in Metal Samples. ISIJ Int. 2011, 51, 593–602. [Google Scholar] [CrossRef]
- Karasev, A.; Suito, H. Analysis of size distributions of primary oxide inclusions in Fe-10 mass Pct Ni-M (M = Si, Ti, Al, Zr, and Ce) alloy. Metall. Mater. Trans. B 1999, 30, 259–270. [Google Scholar] [CrossRef]
- Kanbe, Y.; Karasev, A.; Todoroki, H.; Jönsson, P.G. Analysis of Largest Sulfide Inclusions in Low Carbon Steel by Using Statistics of Extreme Values. Steel Res. Int. 2011, 82, 313–322. [Google Scholar] [CrossRef]
- Inoue, R.; Kiyokawa, K.; Tomoda, K.; Ueda, S.; Ariyama, T. Three-dimensional estimation of multi-component inclusion particles in steel. In Proceedings of the 8th International Workshop on Progress in Analytical Chemistry and Materials Characterisation in the Steel and Metal Industries (CETAS’11), Luxembourg, 17–19 May 2011. [Google Scholar]
- Bletton, O.; Duet, R.; Henry, M.; Cogne, J.Y. Resulphurized Austenitic Stainless Steel with Improved Machinability. U.S. Patent 5,089,224, 18 February 1992. [Google Scholar]
- Todoroki, H.; Mizuno, K. Effect of Silica in Slag on Inclusion Compositions in 304 Stainless Steel Deoxidized with Aluminum. ISIJ Int. 2004, 44, 1350–1357. [Google Scholar] [CrossRef]


| Steel Grade | Ca-treated | C | Si | Mn | Cr | Ni | S a | O a | Ca a | Al a |
|---|---|---|---|---|---|---|---|---|---|---|
| (mass %) | ||||||||||
| 316R | No | 0.02 | 0.38 | 1.60 | 16.82 | 11.18 | 70 | 20 | - | 40 |
| 316Ca | Yes | 0.01 | 0.46 | 1.58 | 16.86 | 11.14 | 90 | 59 | 28 | 40 |
| Steel Grade | Sample | Observed Area, Aobs (mm2) | Dissolved Metal, Wdis (g) | Dissolved Depth (µm) | Number of Observed NMIs, n | Size Range (µm) |
|---|---|---|---|---|---|---|
| 316R | R | 0.898 | 0.1563 | ≈188 | 435 | 2–98 |
| 316Ca | A | 0.898 | 0.0935 | ≈85 | 180 | 3–124 |
| Typical SEM Images | 316R | 316Ca | ||||
|---|---|---|---|---|---|---|
| L (µm) | AR | Composition | L (µm) | AR | Composition | |
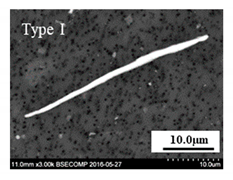 | 5–98 | 7–30 | MnS > 90% | 14–124 | 7–25 | MnS 90–100% and (Al, Ca)O < 10% |
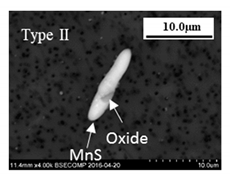 | 2–36 | 1–8 | MnS 30–90% and (Al, Mg, Mn)O 10–70% | 3–33 | 1–7 | MnS 30–90% and (Al, Ca, Si)O 10–70% |
 | 2–10 | 1–3 | Al2O3 48–75% MgO 2–24% MnO 9–29% TiOx 1–21% | 3–15 | 1–3 | CaO 33–41% Al2O3 24–31% SiO2 30–33% MgO 2–4% TiOx <1% |
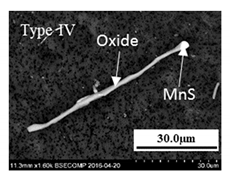 | none | - | - | 4–28 | 2–14 | Oxide (CaO 26–39% Al2O3 16–30% SiO2 28–39% MgO 0–5% TiOx 0–14%) and MnS |
| Samples | Inclusion Type | L (μm) | W (μm) | De (μm) | AR | fv × 104 | Nv (mm−3) | Frequency (%) |
|---|---|---|---|---|---|---|---|---|
| 316R | Type I | 33.2 ± 24.8 (5–98) | 2.6 ± 1.4 (1–7) | 6.0 ± 3.5 (1–16) | 7–30 | 6.7 | 2667 | 9.2 |
| Type II | 5.0 ± 3.8 (2–36) | 1.8 ± 0.7 (1–7) | 2.4 ± 1.1 (1–10) | 1–8 | 3.7 | 24,933 | 86.4 | |
| Type III | 5.4 ± 2.2 (2–10) | 3.6 ± 1.5 (2–8) | 4.0 ± 1.6 (2–9) | 1–3 | 0.7 | 1267 | 4.4 | |
| Type IV | - | - | - | - | - | - | ||
| 316Ca | Type I | 43.0 ± 32.4 (14–124) | 3.7 ± 1.1 (2–5) | 8.1 ± 3.2 (4–14) | 7–25 | 4.1 | 1003 | 5.0 |
| Type II | 7.0 ± 4.8 (3–33) | 2.6 ± 0.8 (1–5) | 3.6 ± 1.3 (2–10) | 1–7 | 3.5 | 9811 | 48.9 | |
| Type III | 6.2 ± 3.0 (3–15) | 3.3 ± 0.9 (2–6) | 4.1 ± 1.3 (2–8) | 1–3 | 2.1 | 4460 | 22.2 | |
| Type IV | 10.7 ± 5.2 (4–28) | 2.3 ± 0.6 (1–4) | 3.8 ± 1.1 (2–7) | 2–14 | 1.7 | 4794 | 23.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, H.; Karasev, A.; Sundqvist, O.; Jönsson, P.G. Modification of Non-Metallic Inclusions in Stainless Steel by Addition of CaSi. Metals 2019, 9, 74. https://doi.org/10.3390/met9010074
Du H, Karasev A, Sundqvist O, Jönsson PG. Modification of Non-Metallic Inclusions in Stainless Steel by Addition of CaSi. Metals. 2019; 9(1):74. https://doi.org/10.3390/met9010074
Chicago/Turabian StyleDu, Hongying, Andrey Karasev, Olle Sundqvist, and Pär G. Jönsson. 2019. "Modification of Non-Metallic Inclusions in Stainless Steel by Addition of CaSi" Metals 9, no. 1: 74. https://doi.org/10.3390/met9010074
APA StyleDu, H., Karasev, A., Sundqvist, O., & Jönsson, P. G. (2019). Modification of Non-Metallic Inclusions in Stainless Steel by Addition of CaSi. Metals, 9(1), 74. https://doi.org/10.3390/met9010074





