Synthesis of Magnesium Carbonate via Carbonation under High Pressure in an Autoclave
Abstract
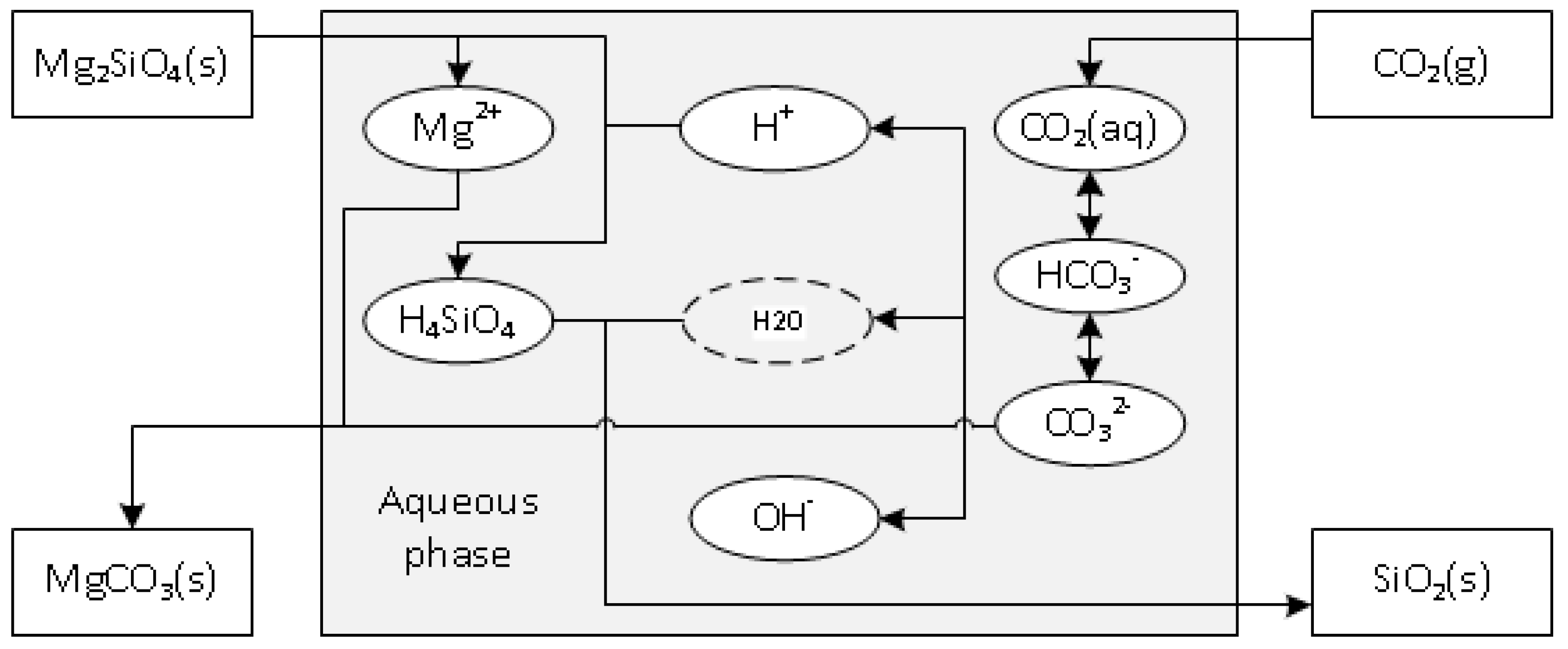
1. Introduction
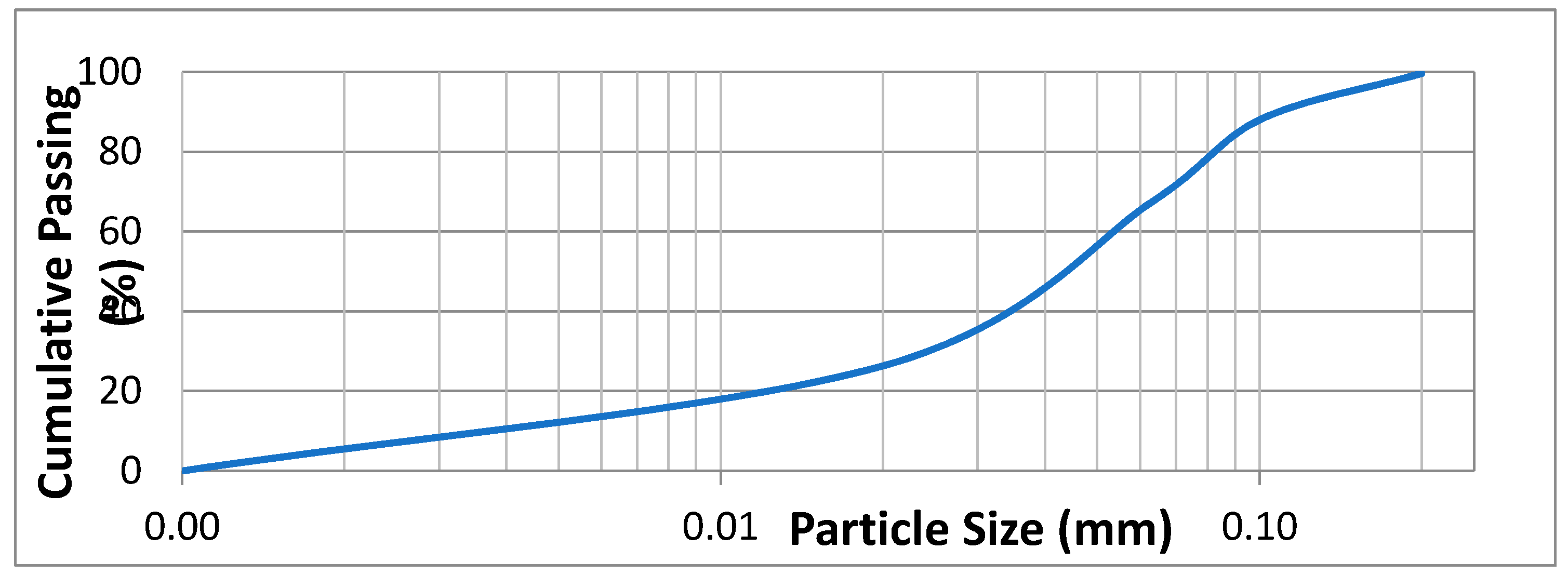
2. Experimental Section
3. Results and Discussion
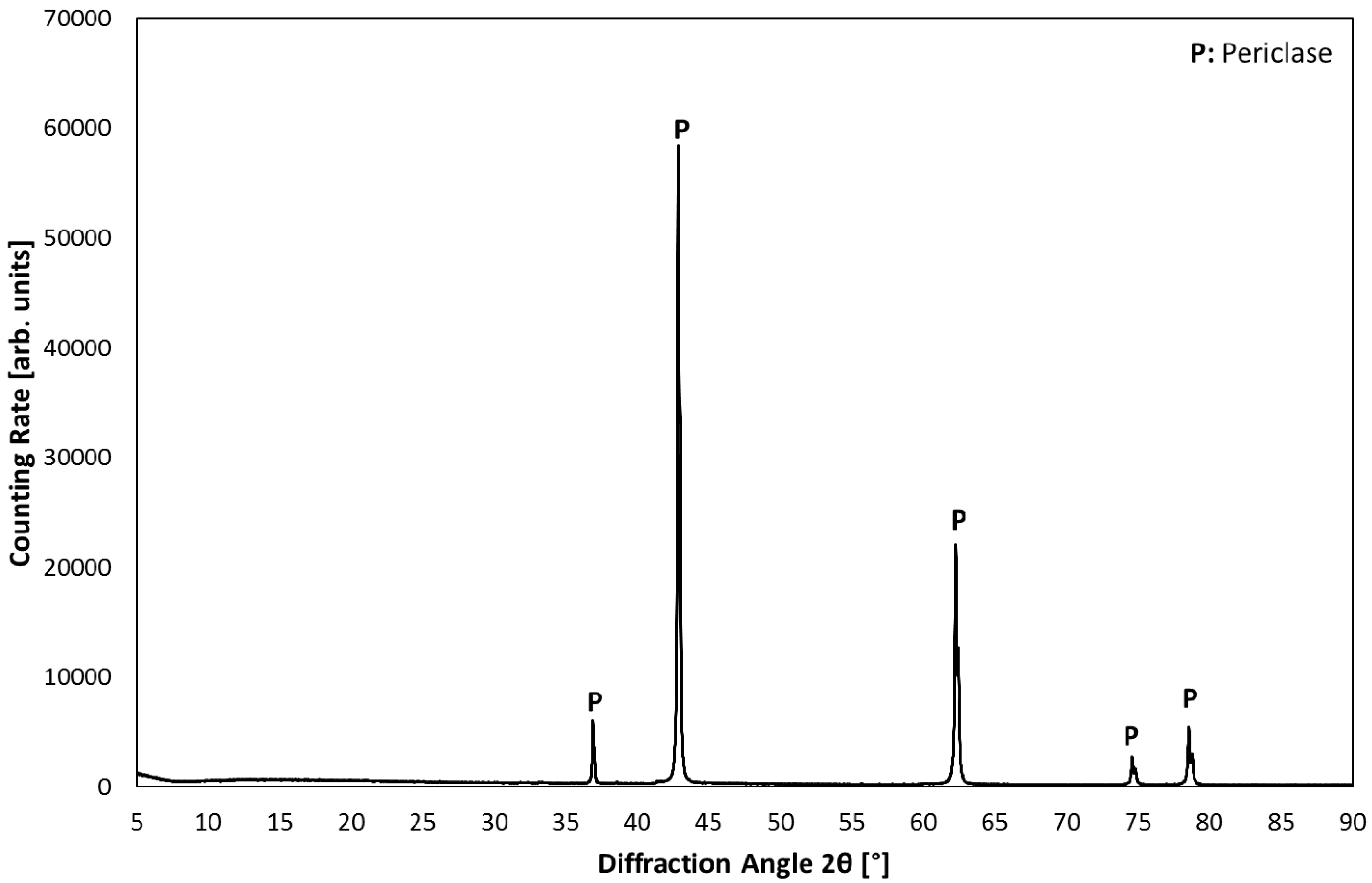
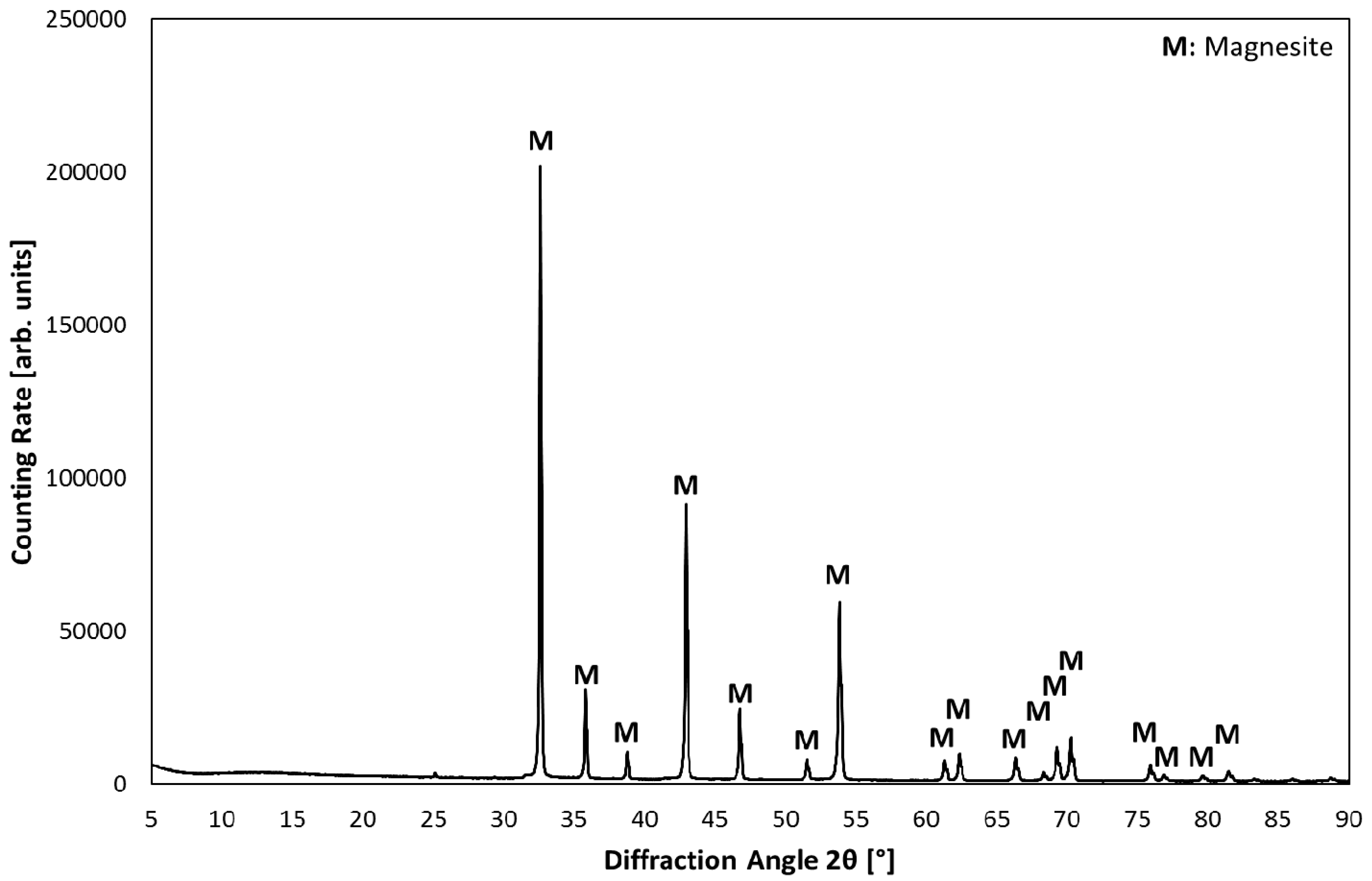
3.1. Product Characterization–XRD Analysis of Product after Carbonation
3.2. Analysis of Water Solution after Carbonation of an Olivine
3.3. Carbonation Extent
4. Conclusion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Nduagu, E.; Björklöf, T.; Fagerlund, J.; Mäkilä, E.; Salonen, J.; Geerlings, H.; Zevenhoven, R. Production of magnesium hydroxide from magnesium silicate for the purpose of CO2 mineralization–Part 2: Mg extraction modeling and application to different Mg silicate rocks. Miner. Eng. 2012, 30, 87–94. [Google Scholar] [CrossRef]
- Huijgen, W.J.J.; Comans, R.N.J. Carbon Dioxide Sequestration by Mineral Carbonation. Literature Review 2003–2004; Technol Report; Energy Research Centre of the Netherlands: Petten, The Netherlands, December 2005. [Google Scholar]
- Santos, R.; Verbeeck, W.; Knops, P.; Rijnsburger, K.; Pontikes, Y.; Gerven, T. Integrated mineral carbonation reactor technology for sustainable carbon dioxide sequestration: “CO2 Energy Reactor”. Energy Procedia 2013, 37, 5884–5891. [Google Scholar] [CrossRef]
- Georgakopoulos, E.D. Iron and steel slag valorization through carbonation and supplementary processes. Ph.D. Thesis, Cranfield University, Cranfield, UK, December 2016. [Google Scholar]
- Santos, R.; Audenaerde, A.; Chiang, Y.; Iacobescu, R.; Knops, P.; Gerven, T. Nickel Extraction fom olivine: Effect of carbonation pretreatment. Metals 2015, 5, 1620–1644. [Google Scholar] [CrossRef]
- Chang, E.; Pan, S.; Chen, Y.; Tan, C.; Chiang, P. Accelerated carbonation of steelmaking slags in a high-gravity rotating packed bed. J. Hazard. Mater. 2012, 227, 97–106. [Google Scholar] [CrossRef]
- Aminu, M.; Nabavi, S.; Rochelle, C.; Manovic, V. A review of developments in carbon dioxide storage. Appl. Energy 2017, 208, 1389–1419. [Google Scholar] [CrossRef]
- Azdarpour, A.; Asadullah, M.; Mohammadian, E.; Hamidi, H.; Junin, R.; Karaei, M. A review on carbon dioxide mineral carbonation through pH-swing process. Chem. Eng. J. 2015, 279, 615–630. [Google Scholar] [CrossRef]
- Chang, E.; Pan, S.; Chen, Y.; Chu, H.; Wang, C.; Chiang, P. CO2 sequestration by carbonation of steelmaking slags in an autoclave reactor. J. Hazard. Mater. 2011, 195, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, O.; Junin, R.; Tyrer, M.; Mohsin, R. Mineral carbonation of red gypsum for CO2 sequestration. Energy Fuels 2014, 28, 5953–5958. [Google Scholar] [CrossRef]
- Bremen, A.M.; Mhamdi, A.; Mitsos, A. Mineral carbonation modeling and investigation on relevant parameters. In Proceedings of the Internal Project Meeting, Aachen, Germany, 13 November 2017. [Google Scholar]
- Bremen, A.M.; Mhamdi, A.; Mitsos, A. Mineral carbonation model status update. In Proceedings of the Internal Project Meeting, Aachen, Germany, 7 September 2018. [Google Scholar]
- Béarat, H.; McKelvy, M.; Chizmeshya, A.; Gormley, D.; Nunez, R.; Carpenter, R.; Squires, K.; Wolf, G. Carbon sequestration via aqueous olivine mineral carbonation: Role of Passivating layer formation. Environ. Sci. Technol. 2006, 40, 4802–4808. [Google Scholar] [CrossRef] [PubMed]
- Eikeland, E.; Bank, A.; Tyrsted, C.; Jensen, A.; Iversen, B. Optimized carbonation of magnesium silicate mineral for CO2 storage. Appl. Mater. Interfaces 2015, 7, 5258–5264. [Google Scholar] [CrossRef] [PubMed]
- Daval, D.; Sissmann, O.; Menguy, N.; Salsi, G.; Gyot, F.; Martinez, I.; Corvisier, J.; Machouk, I.; Knauss, K.; Hellmann, R. Influence of anorphous silica lyer formation on the dissolution rate of olivine at 90 °C and elevated pCO2. Chem. Geol. 2011, 284, 193–209. [Google Scholar] [CrossRef]
- Oelkers, E. An experimental study of forsterite dissolution rates as afunction of temperature and aqueous Mg and Si concentrations. Chem. Geol. 2001, 175, 485–494. [Google Scholar] [CrossRef]
- Hänchen, M.; Prigiobbe, V.; Storti, G.; Seward, T.M.; Mazzotti, M. Dissolution kinetics of forsteritic olivine at 90–150 °C including effect of the presence of CO2. Geochim. Cosmochim. Acta 2006, 70, 4403–4416. [Google Scholar] [CrossRef]
- Turri, L.; Muhr, H.; Rijnsburger, K.; Knops, P.; Lapicque, F. CO2 sequestration by high pressure reaction with olivine in a rocking batch autoclave. Chem. Eng. Sci. 2017, 171, 27–31. [Google Scholar] [CrossRef]
- Olajire, A. A review of mineral carbonation technology in sequestration of CO2. J. Pet. Sci. Eng. 2013, 109, 364–392. [Google Scholar] [CrossRef]
- Qafoku, O.; Hu, J.; Hess, N.; Hu, M.; Ilton, E.; Feng, J.; Arey, B.; Felmy, A. Formation of submicron magnesite during reaction of natural forsterite in H2O-saturated supercritical CO2. Geochim. Cosmochim. Acta 2014, 134, 197–209. [Google Scholar] [CrossRef]
- Saldi, G.; Daval, D.; Morvan, G.; Knauss, K. The role of Fe and redox conditions in olivine carbonation rates: An experimental study of the rate limiting reactions at 90 and 150 °C in open and closed systems. Geochim. Cosmochim. Acta 2013, 118, 157–183. [Google Scholar] [CrossRef]
- Botha, A.; Strydom, C. Preparation of a magnesium hydroxy carbonate from magnesium hydroxide. Hydrometallurgy 2001, 62, 175–183. [Google Scholar] [CrossRef]


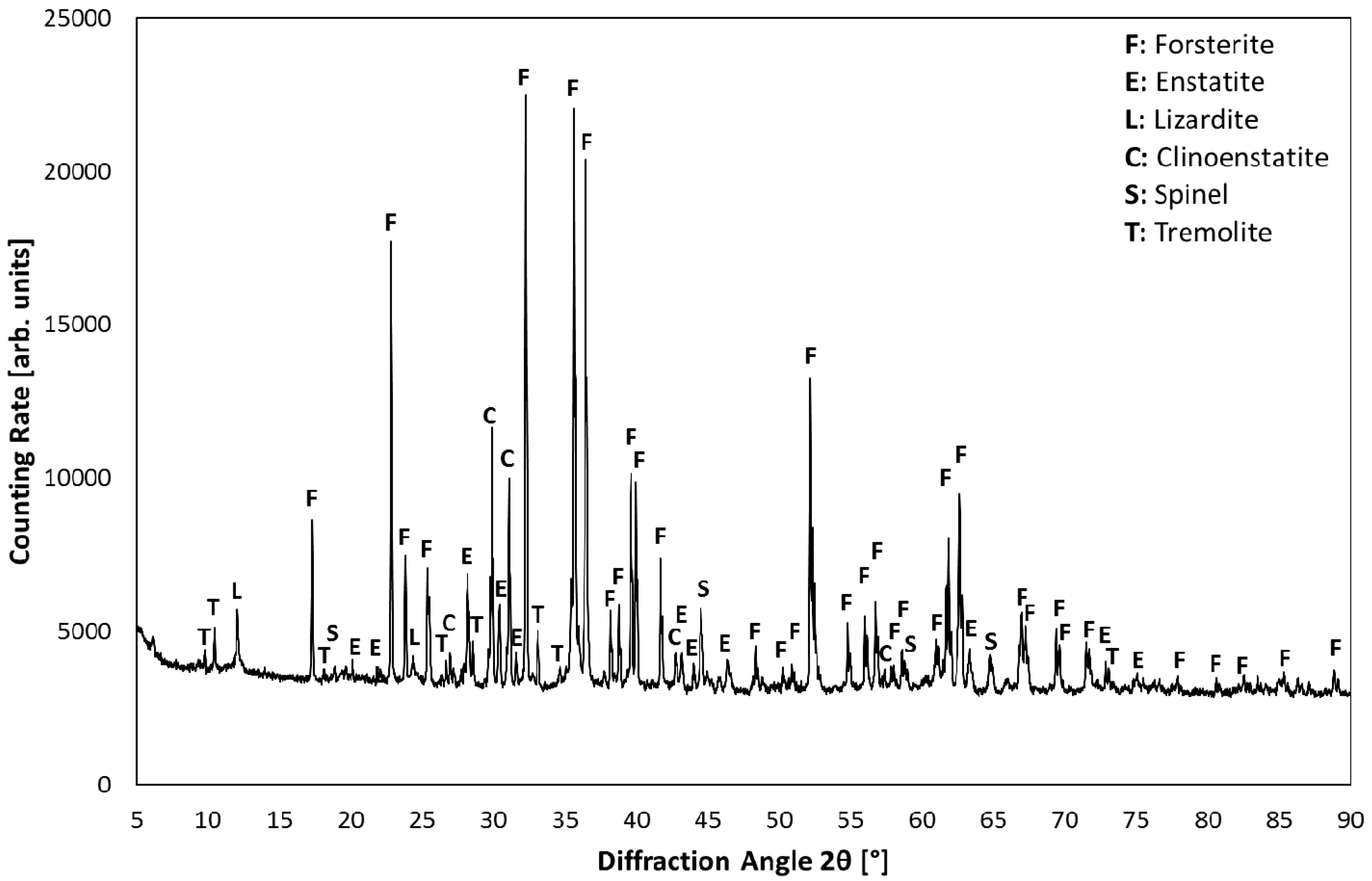
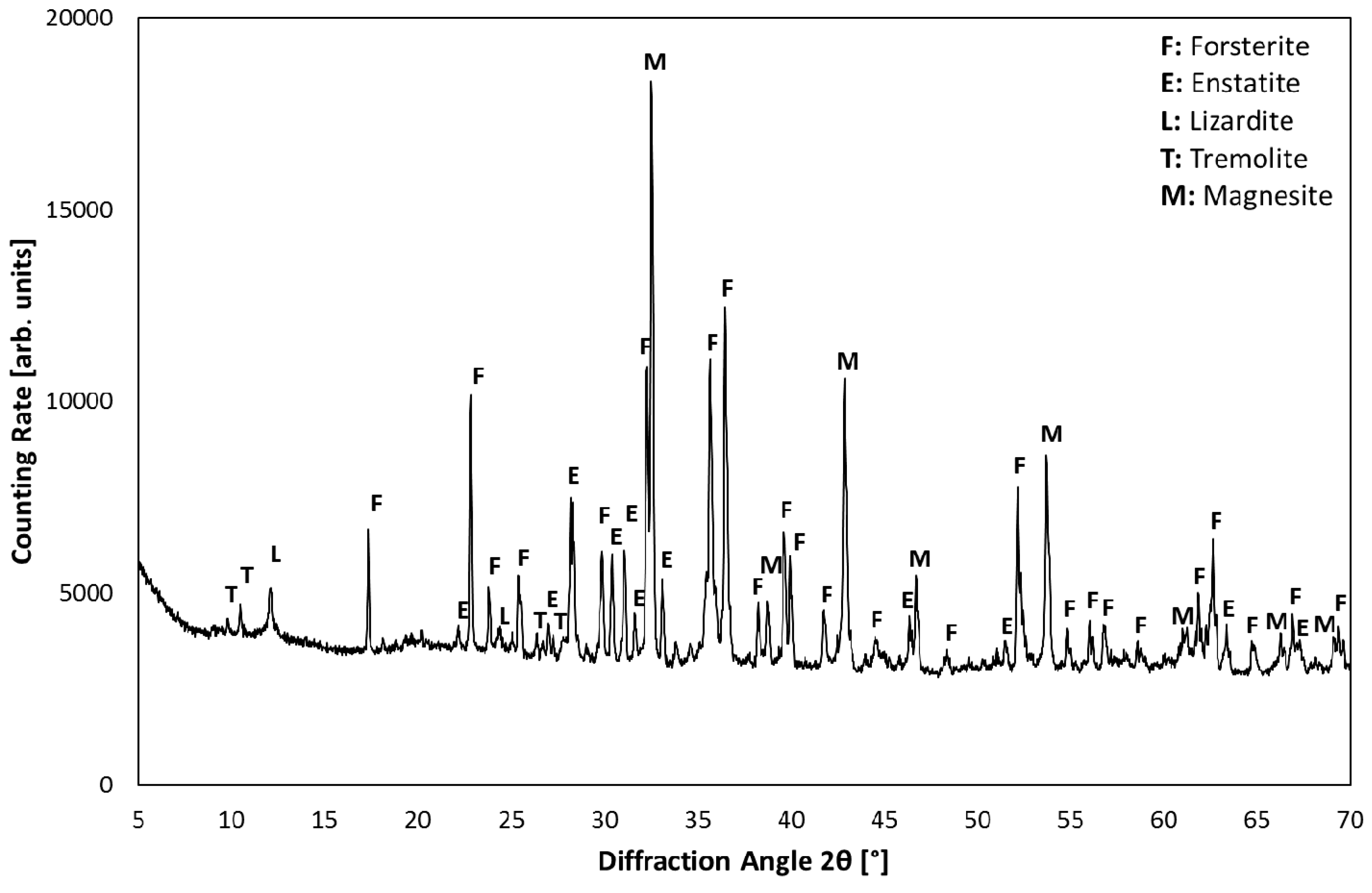
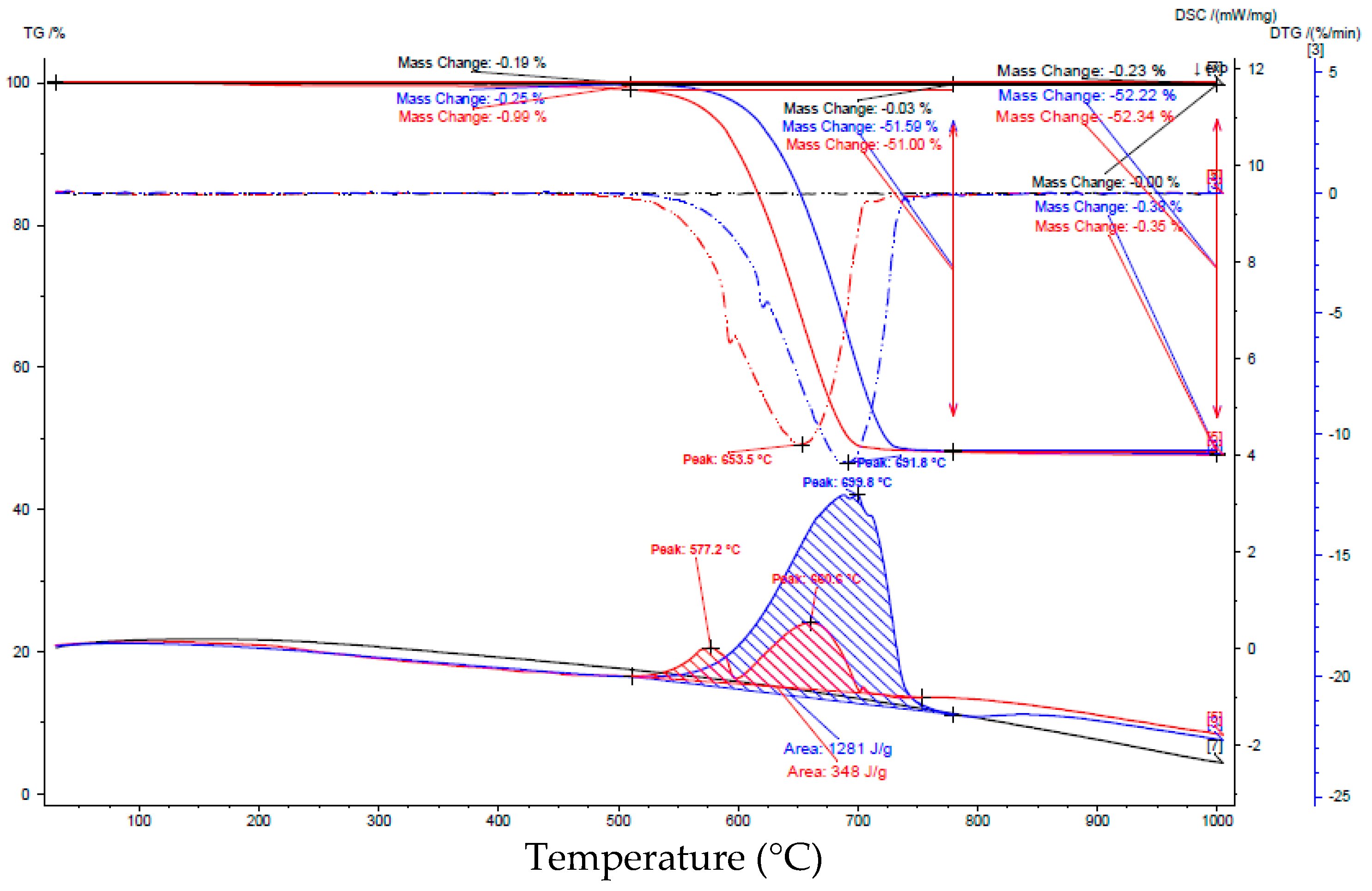
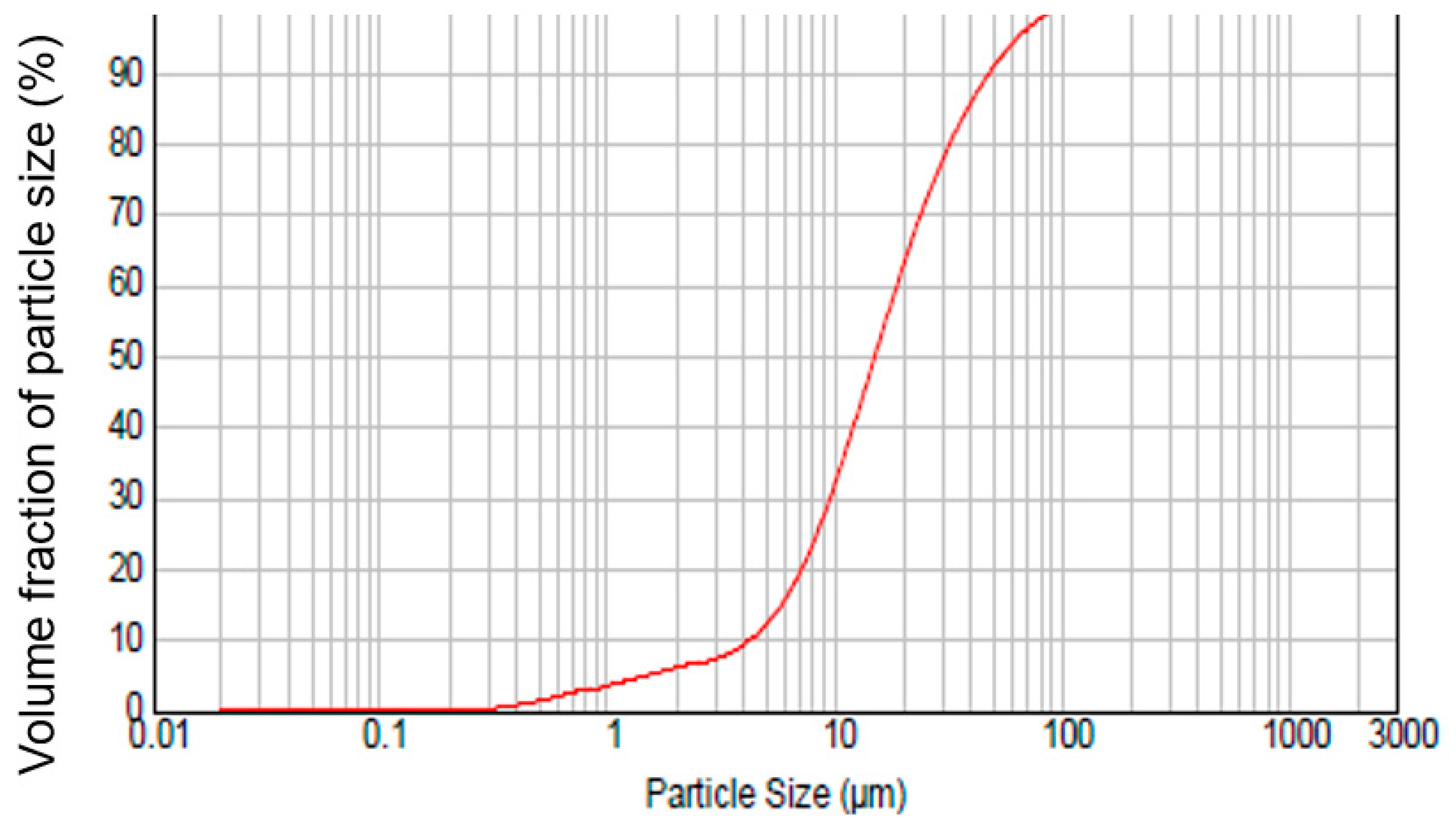
| Components | Olivine | Magnesia |
|---|---|---|
| SiO2 | 46.43 | 0.32 |
| Al2O3 | 2.55 | 0.20 |
| Fe2O3 | 10.88 | 0.58 |
| TiO2 | 0.11 | 0.05 |
| CaO | 2.16 | 0.75 |
| MgO | 35.57 | 97.56 |
| K2O | 0.39 | 0.02 |
| Na2O | 0.17 | 0.10 |
| MnO | 0.17 | 0.24 |
| Cr2O3 | 0.45 | 0.00 |
| P2O5 | 0.00 | 0.00 |
| ZrO2 | 0.02 | 0.03 |
| SO3 | 0.00 | 0.00 |
| BaO | 0.00 | 0.00 |
| ZnO | 0.08 | 0.08 |
| NiO | 0.89 | 0.09 |
| Co3O4 | 0.08 | 0.00 |
| CuO | 0.06 | 0.00 |
| Total | 100.00 | 100.00 |
| Exp. No | S/L (g/mL H2O) | Fraction Size (µm) | Concentration of Additives in Water, (mol/L) | Material |
|---|---|---|---|---|
| 1 | 10/150 (0.066) | 100–200 | No | Olivine, Italy (35.57 wt% MgO) |
| 2 | 10/150 (0.066) | <20 | No | Olivine, Italy (35.57 wt% MgO) |
| 3 | 30/150 (0.2) | 20–63 | 0.64 NaHCO3 0.06 H2C2O4 0.003 C6H8O6, | Olivine, Italy (35.57 wt% MgO) |
| 4 | 30/150 (0.2) | 20–63 | No | Synthetic magnesia (97.56 wt% MgO) |
| Exp. No. | Si | Mg | Fe | Ni | Cr | Co | Al |
|---|---|---|---|---|---|---|---|
| 2 (pH-6.7) | 166 | 229 | 0.11 | 0.73 | <1 | <1 | <1 |
| 3 (pH-7.3) | 195 | 705 | 67 | 10 | <1 | <1 | <1 |
| 4 (pH-2.32) | <1 | 211 | <1 | <1 | <1 | <1 | <1 |
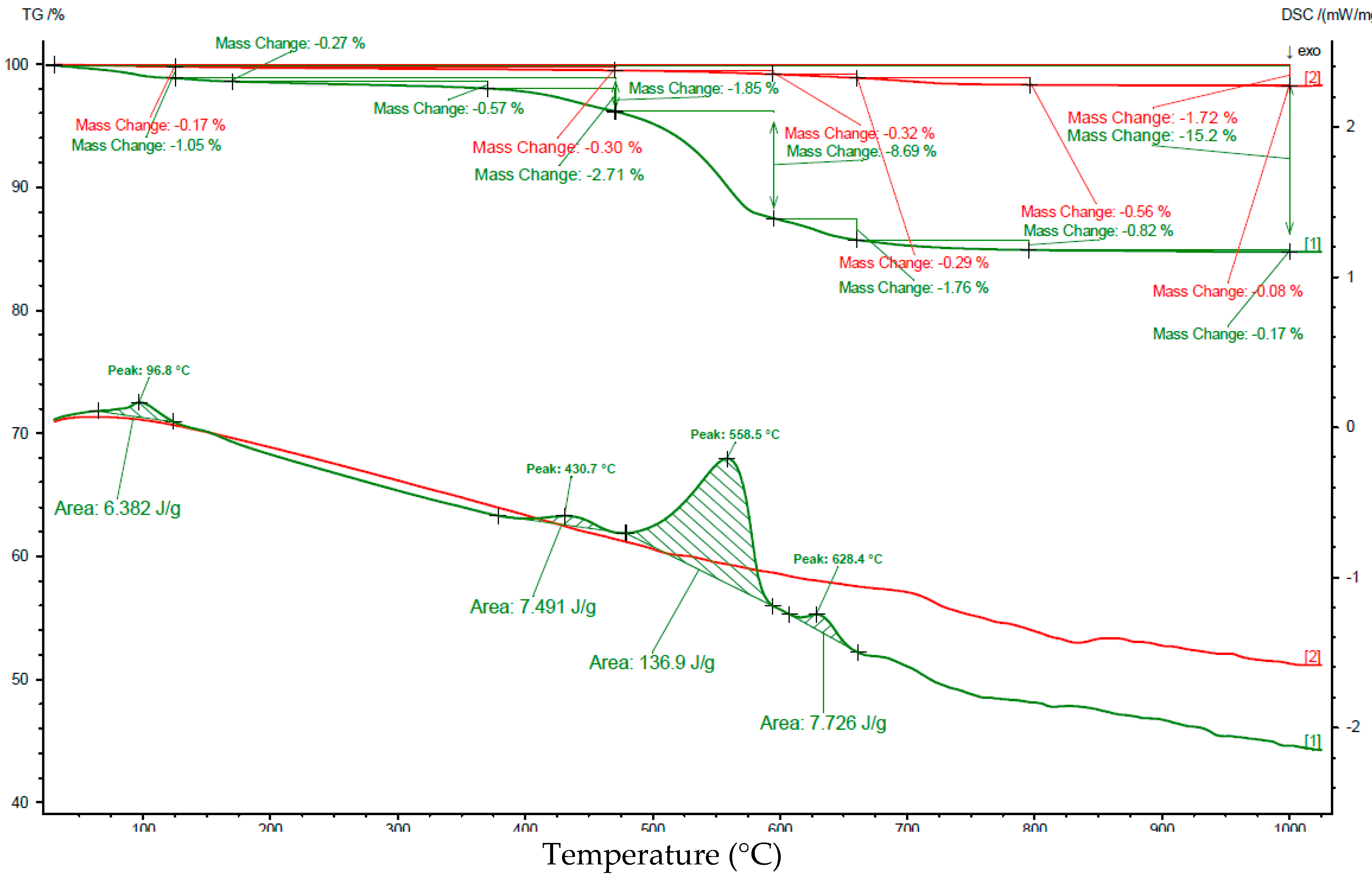
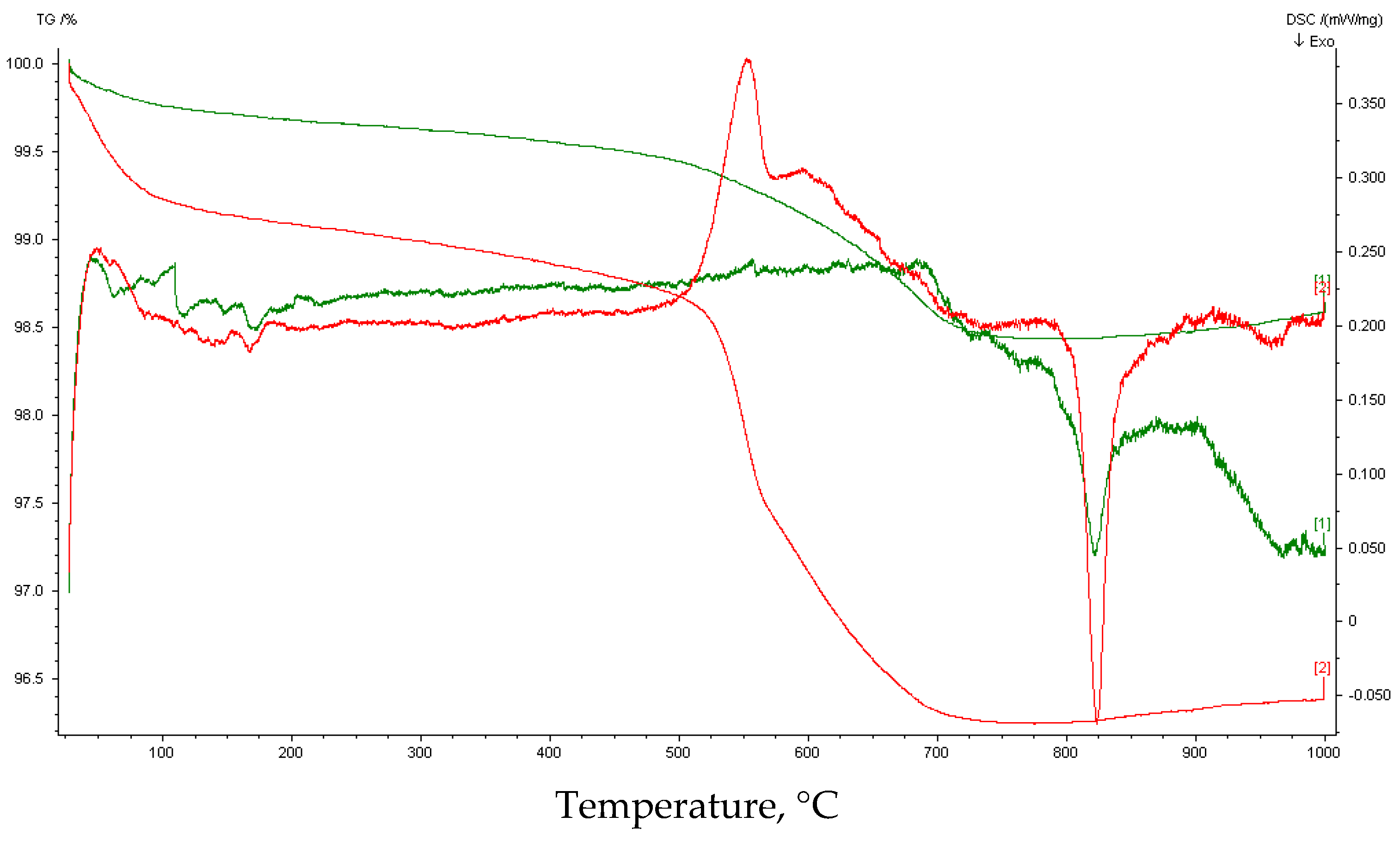
| Interval | Before Carbonation | After Carbonation | ||
|---|---|---|---|---|
| Interval [°C] | Loss of weight [%] | Peak area [J/g] | Loss of weight [%] | Peak area [J/g] |
| 25–125 | 0.17 | no peak | 1.05 | 6.38 |
| 125–385 | 0.22 | 0.93 | No peak | |
| 385–470 | 0.08 | 1.77 | 8.36 | |
| 470–595 | 0.32 | 8.69 | 137 | |
| 595–660 | 0.29 | 0.75 | 7.73 | |
| 660–725 | 0.44 | 4.40 | 0.62 | 4.10 |
| 725–1000 | 0.20 | No peak | 0.36 | No peak |
| 25–1000 | 1.7 | 15.2 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stopic, S.; Dertmann, C.; Modolo, G.; Kegler, P.; Neumeier, S.; Kremer, D.; Wotruba, H.; Etzold, S.; Telle, R.; Rosani, D.; et al. Synthesis of Magnesium Carbonate via Carbonation under High Pressure in an Autoclave. Metals 2018, 8, 993. https://doi.org/10.3390/met8120993
Stopic S, Dertmann C, Modolo G, Kegler P, Neumeier S, Kremer D, Wotruba H, Etzold S, Telle R, Rosani D, et al. Synthesis of Magnesium Carbonate via Carbonation under High Pressure in an Autoclave. Metals. 2018; 8(12):993. https://doi.org/10.3390/met8120993
Chicago/Turabian StyleStopic, Srecko, Christian Dertmann, Giuseppe Modolo, Philip Kegler, Stefan Neumeier, Dario Kremer, Hermann Wotruba, Simon Etzold, Rainer Telle, Diego Rosani, and et al. 2018. "Synthesis of Magnesium Carbonate via Carbonation under High Pressure in an Autoclave" Metals 8, no. 12: 993. https://doi.org/10.3390/met8120993
APA StyleStopic, S., Dertmann, C., Modolo, G., Kegler, P., Neumeier, S., Kremer, D., Wotruba, H., Etzold, S., Telle, R., Rosani, D., Knops, P., & Friedrich, B. (2018). Synthesis of Magnesium Carbonate via Carbonation under High Pressure in an Autoclave. Metals, 8(12), 993. https://doi.org/10.3390/met8120993









