Preparation of Vanadium Oxides from a Vanadium (IV) Strip Liquor Extracted from Vanadium-Bearing Shale Using an Eco-Friendly Method
Abstract
:1. Introduction
2. Thermodynamic Analysis
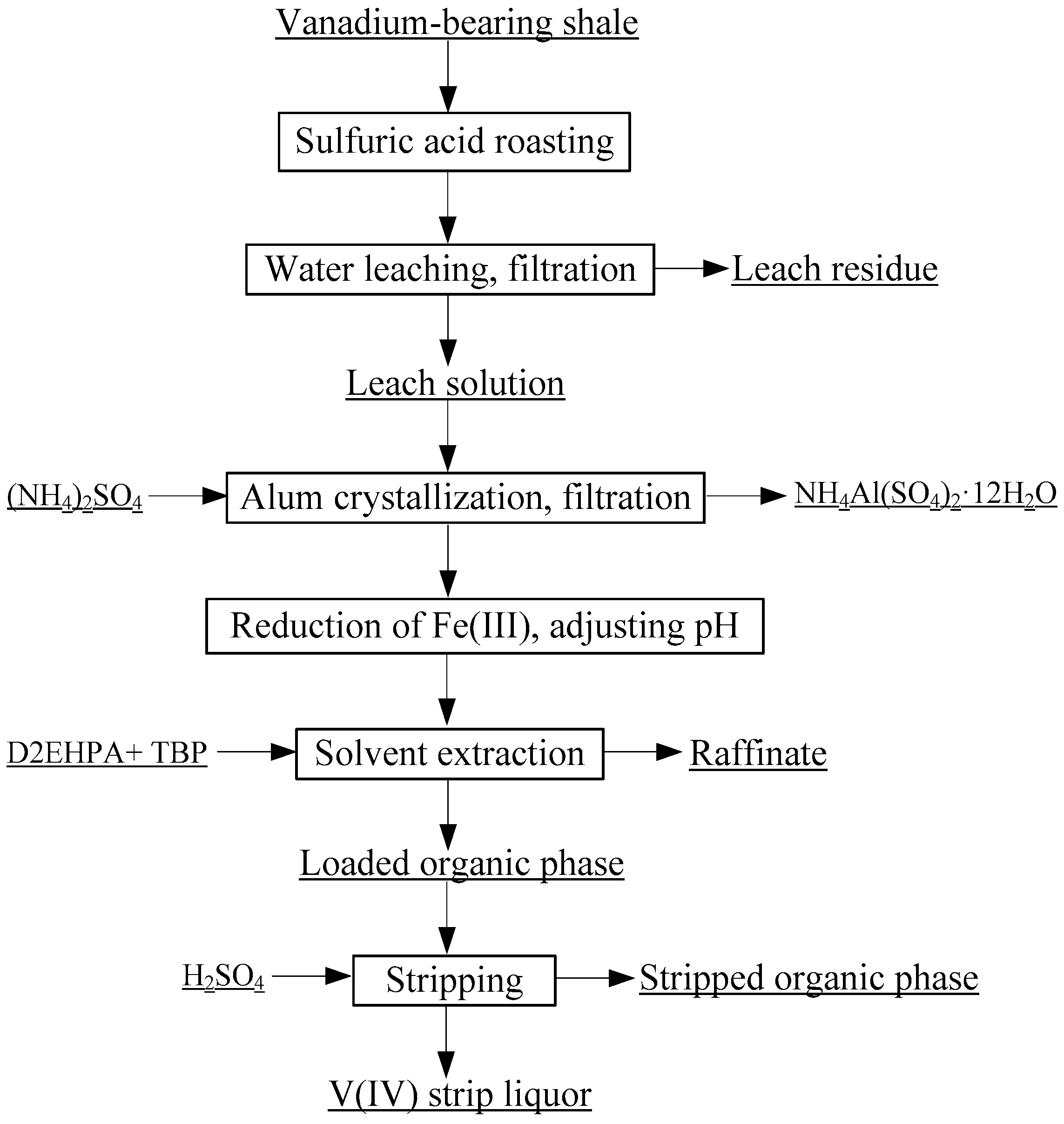
3. Materials and Methods
3.1. Materials and Analysis
3.2. Procedures
3.2.1. Source of the V(IV) Strip Liquor
3.2.2. Preparation of VO2 and V2O5
4. Results and Discussion
4.1. Effect of pH on Vanadium Precipitation
4.2. Effect of Time on Vanadium Precipitation
4.3. Effect of Temperature on Vanadium Precipitation
4.4. Removal of Na+ by Washing
4.5. Preparation of VO2 and V2O5 by Calcination
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Perles, T. Vanadium Market Fundamentals and Implications. In Proceedings of the Metal Bulletin 28th International Ferroalloys Conference, Berlin, Germany, 13 November 2012. [Google Scholar]
- Skyllaskazacos, M.; Cao, L.Y.; Kazacos, M.; Kausar, N.; Mousa, A. Vanadium electrolyte studies for the vanadium redox battery—A review. Cheminform 2016, 47, 1521–1543. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, Y.; Huang, J.; Liu, T.; Xue, N.; Shi, Q. Optimal location of vanadium in muscovite and its geometrical and electronic properties by DFT calculation. Minerals 2017, 7, 32. [Google Scholar] [CrossRef]
- Kear, G.; Shah, A.; Walsh, F. Development of the all-vanadium redox flow battery for energy storage: A review of technological, financial and policy aspects. Int. J. Energy Research 2012, 36, 1105–1120. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Bao, S. Preparation high purity V2O5 from a typical low-grade refractory stone coal using a pyro-hydrometallurgical process. Minerals 2016, 6, 69. [Google Scholar] [CrossRef]
- Zhang, Y.; Bao, S.; Liu, T.; Chen, T.; Huang, J. The technology of extracting vanadium from stone coal in China: History, current status and future prospects. Hydrometallurgy 2011, 109, 116–124. [Google Scholar] [CrossRef]
- Moskalyk, R.R.; Alfantazi, A.M. Processing of vanadium: A review. Miner. Eng. 2003, 16, 793–805. [Google Scholar] [CrossRef]
- Raja, B. Vanadium market in the world. Steelworld 2007, 13, 19–22. [Google Scholar]
- Wu, Y.; Zhang, G.; Chou, K. Preparation of high quality ferrovanadium nitride by carbothermal reduction nitridation process. J. Min. Metall. B Metall. 2017, 53, 383–390. [Google Scholar] [CrossRef]
- Sikong, L.; Kumbour, P. A new route for thermochromic vanadium dioxide synthesis. Digest J. Nanomaterials Biostructures 2015, 10, 135–140. [Google Scholar]
- Osmolovskaya, O.; Murin, I.; Smirnov, V.; Osmolovsky, M. Synthesis of vanadium dioxide thin films and nanopowders a brief review. Rev. Adv. Mater. Sci. 2014, 36, 70–74. [Google Scholar]
- Lu, Y.; Zhou, X. Synthesis and characterization of nanorod-structured vanadium oxides. Thin Solid Films 2018, 660, 180–185. [Google Scholar] [CrossRef]
- Abbasi, M.H.; Safarnoorallah, M. extraction of vanadium oxide from boiler fuel ash. J. Adv. Mater. Eng. 2014, 17, 165–175. [Google Scholar]
- Chen, F.; Zhang, Y.; Huang, J.; Liu, T.; Xue, N. Mechanism of enhancing extraction of vanadium from stone coal by roasting with MgO. Minerals 2017, 7, 33. [Google Scholar] [CrossRef]
- Li, X.; Xie, B. Extraction of vanadium from high calcium vanadium slag using direct roasting and soda leaching. Int. J. Miner. Metall. Mater. 2012, 19, 595–601. [Google Scholar] [CrossRef]
- Dash, H.; Rout, D.; Kar, B. Extraction of vanadium from vanadium bearing spent catalyst through heat treatment method. Int. J. Innovative Res. Sci. Eng. Technol. 2013, 2, 3201–3203. [Google Scholar]
- Jena, B.; Dresler, W.; Reilly, I. Extraction of titanium, vanadium and iron from titanomagnetite deposits at pipestone lake, Manitoba, Canada. Miner. Eng. 1995, 8, 159–168. [Google Scholar] [CrossRef]
- Deng, Z.; Wei, C.; Fan, G.; Li, M.; Li, C.; Li, X. Extracting vanadium from stone-coal by oxygen pressure acid leaching and solvent extraction. Trans. Non-Ferrous Metall. Soc. China 2010, 20, s118–s122. [Google Scholar] [CrossRef]
- Ning, P.; Lin, X.; Wang, X.; Cao, H. High-efficient extraction of vanadium and its application in the utilization of the chromium-bearing vanadium slag. Chem. Eng. J. 2016, 301, 132–138. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Zhang, H. Main factors affecting precipitation of vanadium with acidic ammonium salt and its countermeasures. Iron Alloy 2012, 4, 12–16. (In Chinese) [Google Scholar]
- Wu, X.; Ming, X. Experimental study of V2O3 production with V2O5 as raw material. Rare Met. Ceme. Carbides 2015, 43, 15–17. [Google Scholar]
- Xu, C.; Pang, M. Preparation of VO2 powder by deoxidizing V2O5. J. Mater. Sci. Eng. 2006, 24, 252–254. [Google Scholar]
- Surmacz-Górska, J.; Cichon, A.; Miksch, K. Nitrogen removal from wastewater with high ammonia nitrogen concentration via shorter nitrification and denitrification. Water Sci. Tech. 1997, 36, 73–78. [Google Scholar] [CrossRef]
- Lin, L.; Yuan, S.; Chen, J.; Xu, Z.; Lu, X. Removal of ammonia nitrogen in wastewater by microwave radiation. J. Hazard. Mater. 2009, 161, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Feng, Y.; Zou, X.; Luo, X. Study on microbial reduction of vanadium metallurgical waste water. Hydrometallurgy 2009, 99, 13–17. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Y.; Bao, S. Vacuum membrane distillation treatment of high concentration ammonia wastewater produced in vanadium extraction from stone coal. Min. Metall. Eng. 2012, 32, 103–106. [Google Scholar]
- Liu, B.; Zheng, S.; Wang, S.; Zhang, Y.; Du, H. The electrowinning of vanadium oxide from alkaline solution. Hydrometallurgy 2016, 165, 244–250. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, Y.; Bao, S.; Huang, J.; Zhang, L. A novel eco-friendly vanadium precipitation method by hydrothermal hydrogen reduction technology. Minerals 2017, 7, 182. [Google Scholar] [CrossRef]
- Wang, M.; Huang, S.; Chen, B.; Wang, X. A review of processing technologies for vanadium extraction from stone coal. Trans. Inst. Min. Metall. 2018. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, C.; Li, P.; Li, S. A new process of extracting vanadium from stone coal. Int. J. Miner. Metall. Mater. 2010, 17, 381–388. [Google Scholar] [CrossRef]
- Li, X.; Wei, C.; Deng, Z.; Li, M.; Li, C.; Fan, G. Selective solvent extraction of vanadium over iron from a stone coal/black shale acid leach solution by D2EHPA/TBP. Hydrometallurgy 2011, 105, 359–363. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Bao, S.; Shen, C. Separation and recovery of vanadium from a sulfuric-acid leaching solution of stone coal by solvent extraction using trialkylamine. Sep. Purif. Technol. 2016, 164, 49–55. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.; Wang, M.; Jiang, C.; Xiang, X.; Zhang, X. Separation of V(IV) and Fe(III) from the acid leach solution of stone coal by D2EHPA/TBP. Hydrometallurgy 2015, 153, 38–45. [Google Scholar] [CrossRef]
- Zhou, X.; Wei, C.; Li, M.; Qiu, S.; Li, X. Thermodynamics of vanadium-sulfur-water systems at 298 K. Hydrometallurgy 2011, 106, 104–112. [Google Scholar] [CrossRef]
- Dean, J. Langes’s Handbook of Chemistry, 13th ed.; McGraw-Hill, Inc.: New York, NY, USA, 1985. [Google Scholar]
- Wang, M.; Wang, X.; Li, B. A Novel Technology of Vanadium Extraction from Stone Coal. In Rare Metal Technology 2015; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 187–192. [Google Scholar]
- National Standard of People’s Republic of China, Vanadium pentoxide; YB/T 5304-2011; Standards Press of China: Beijing, China, 2005. (In Chinese)
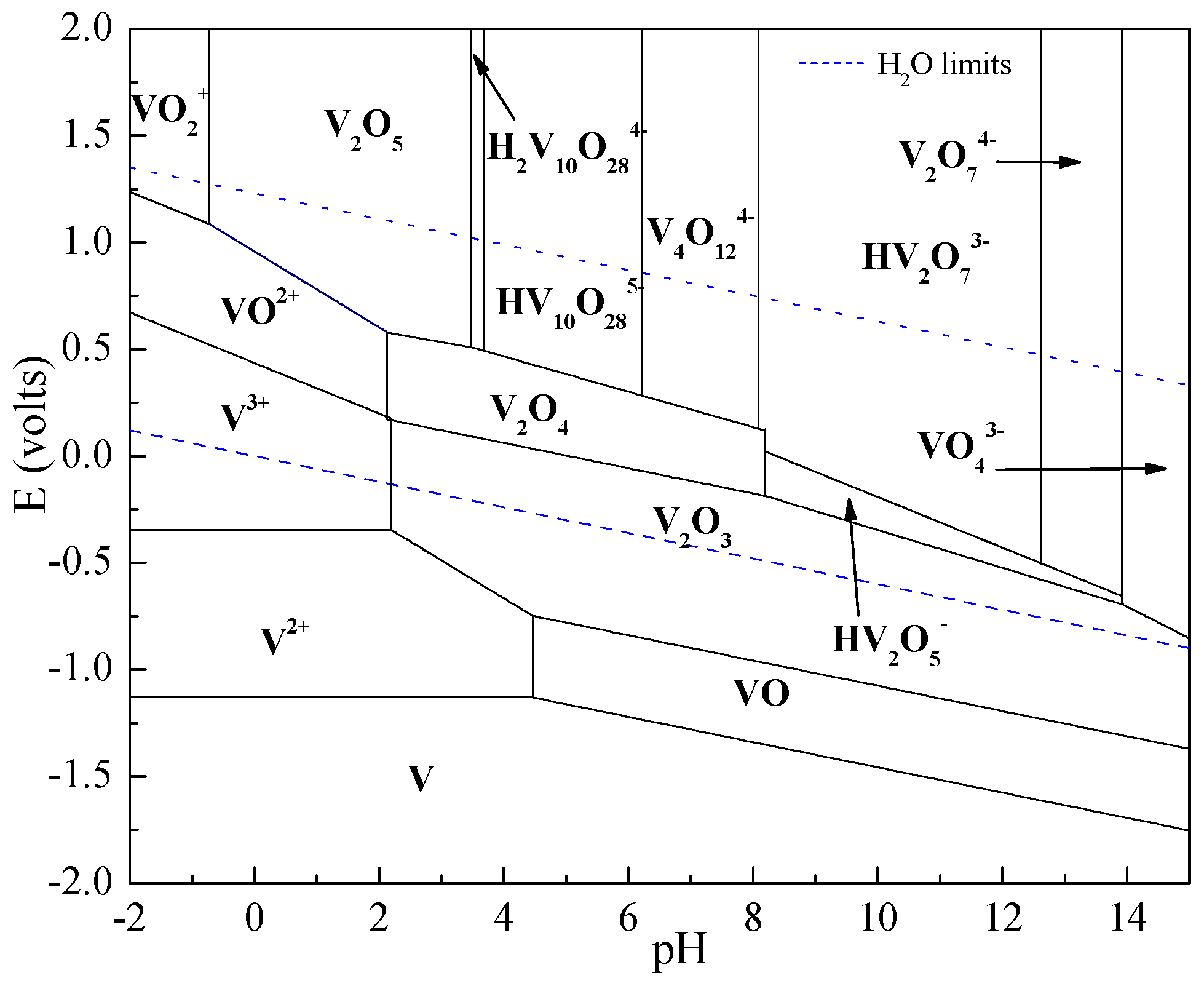
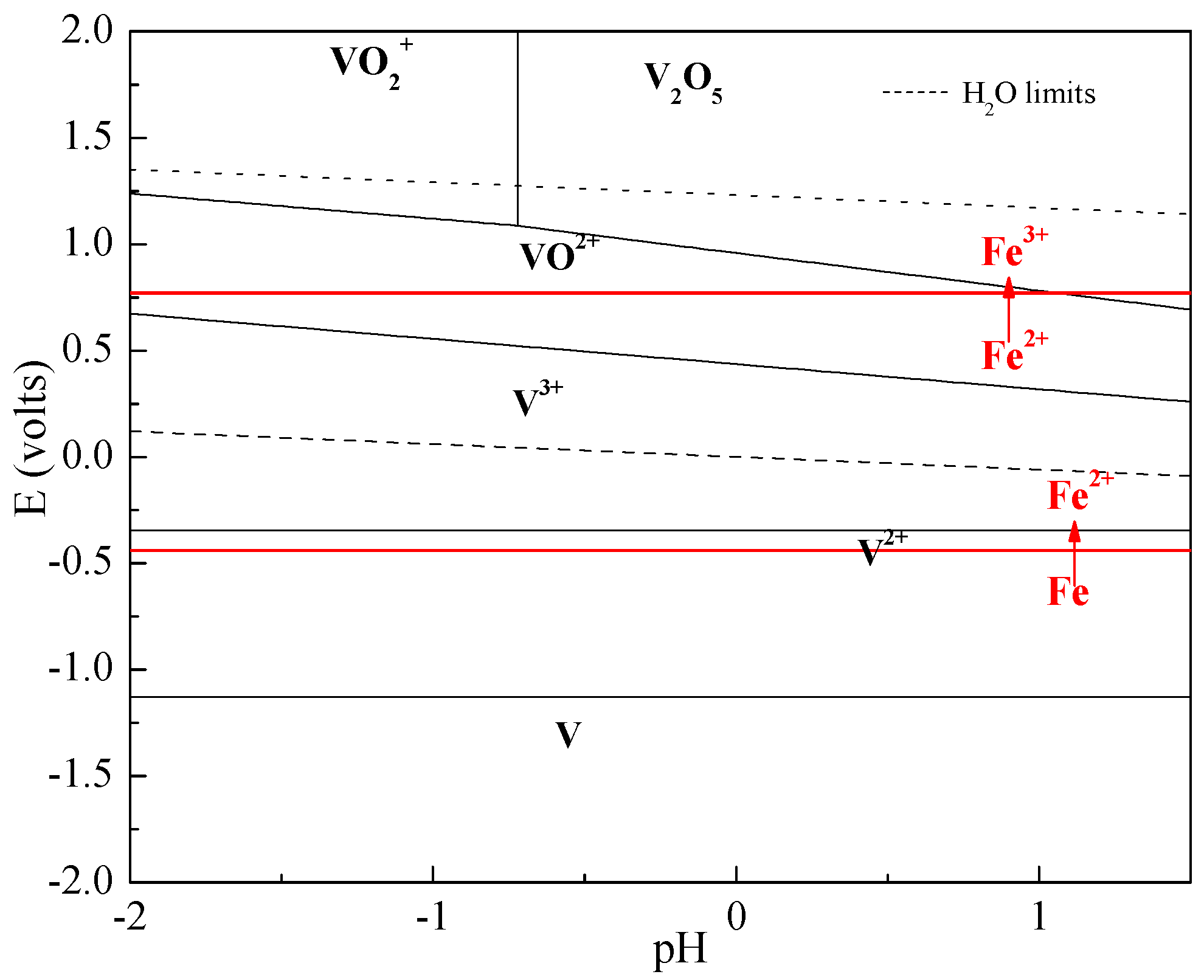
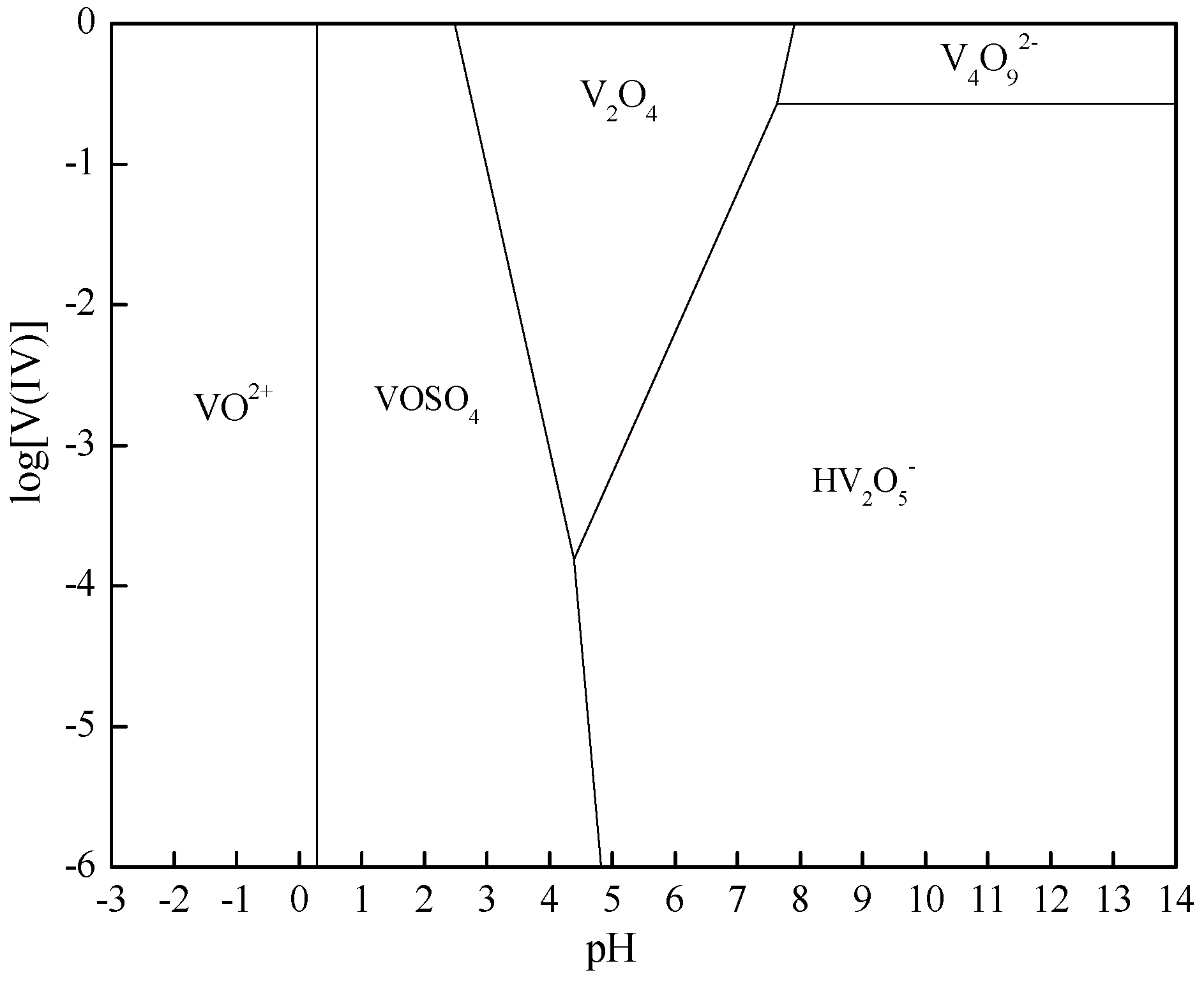

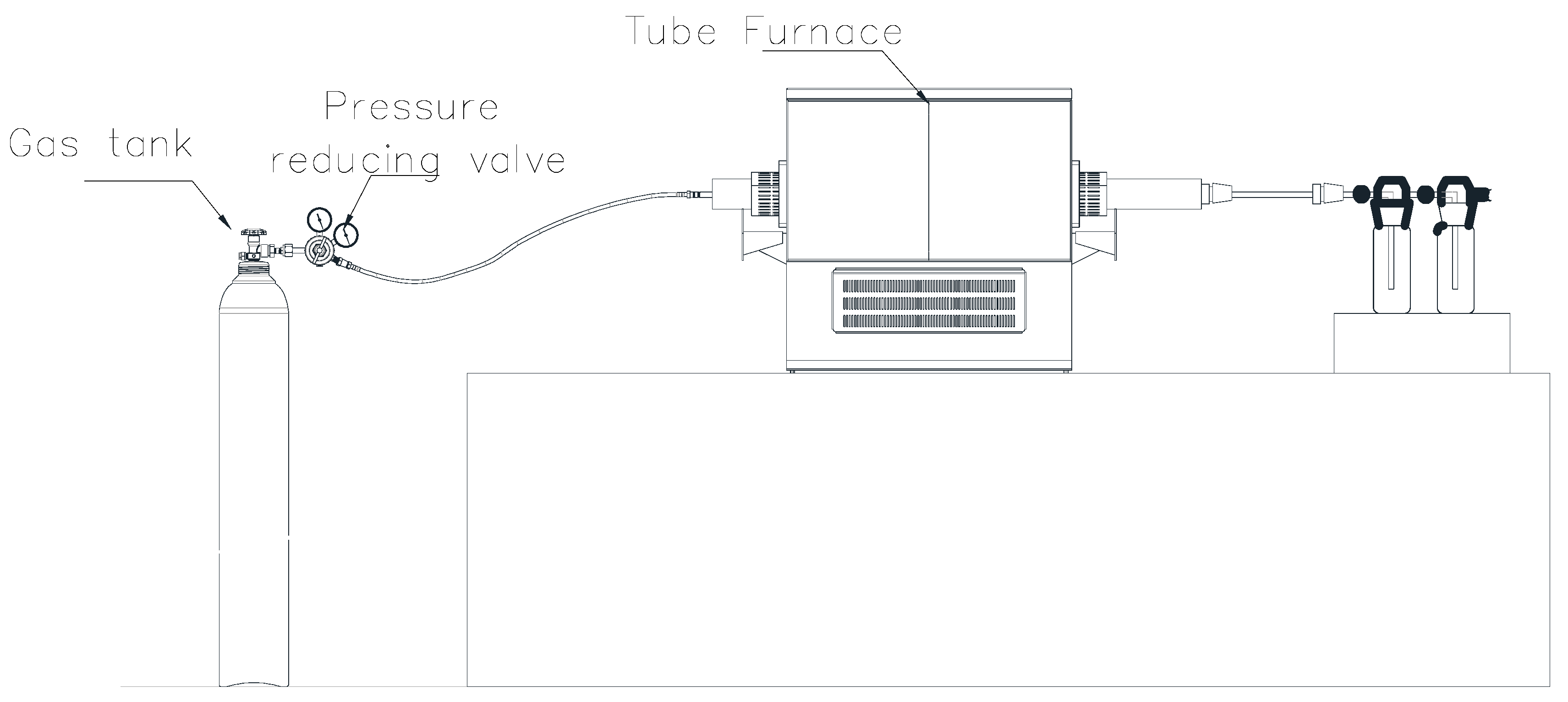
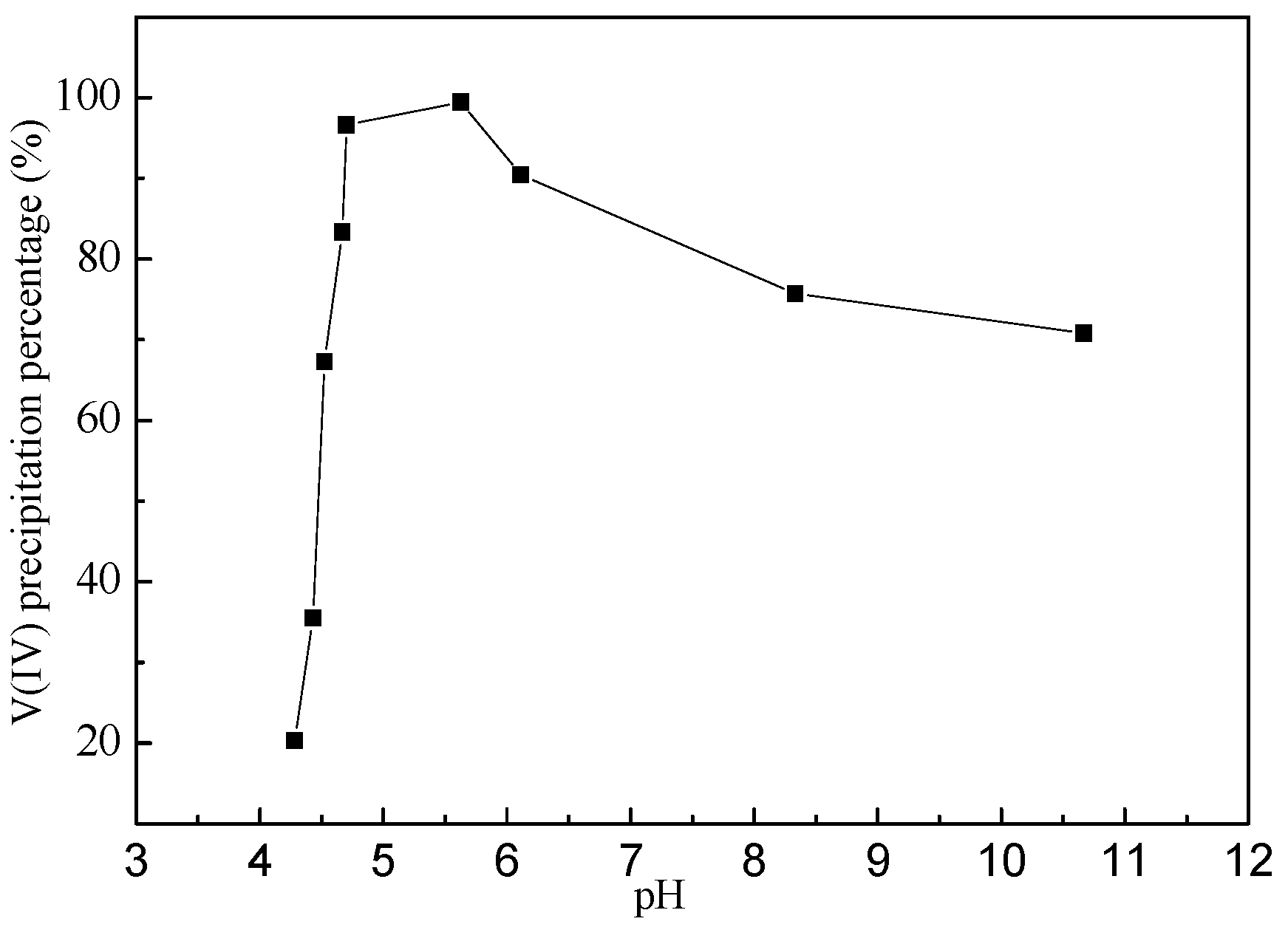
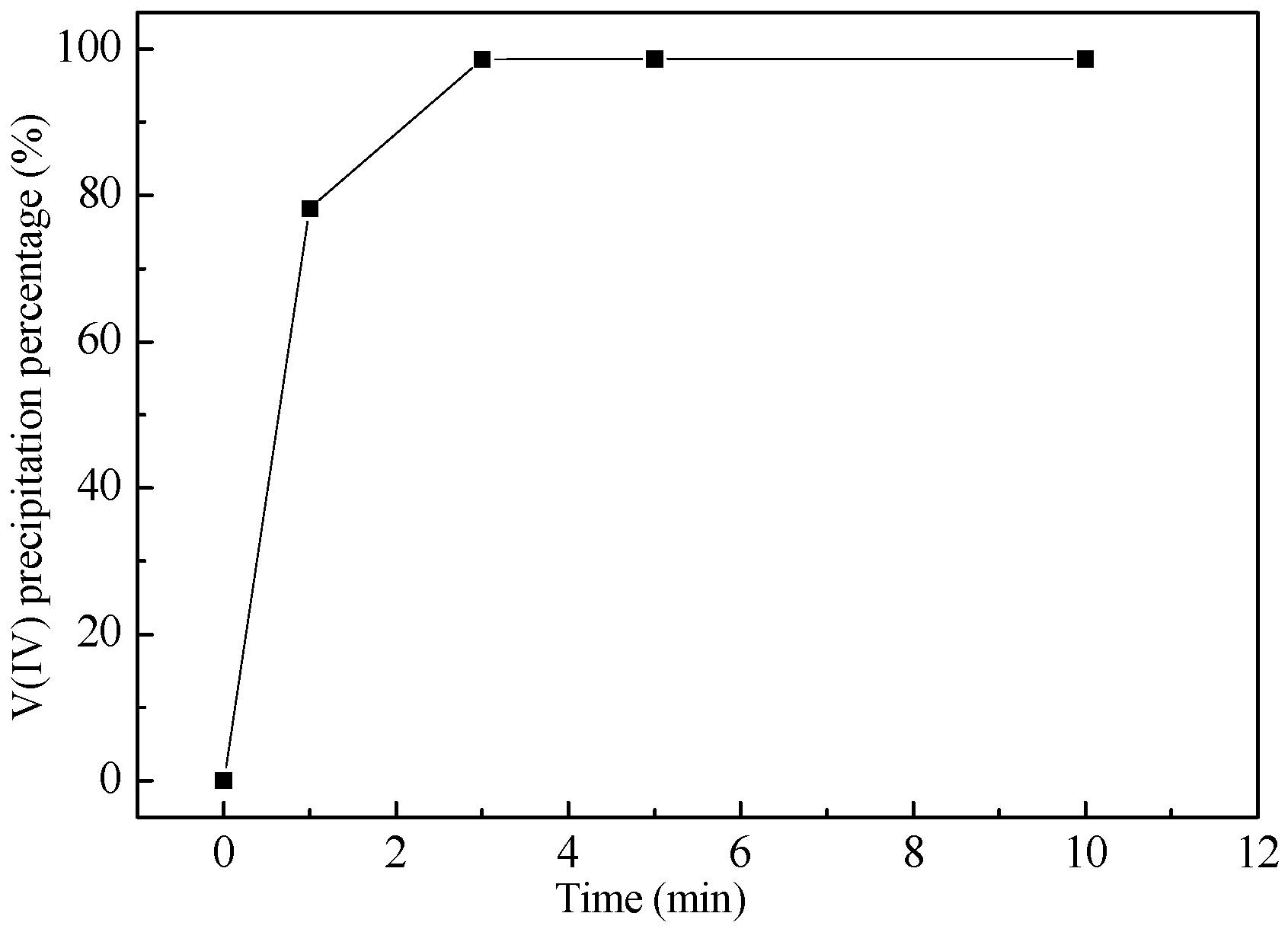

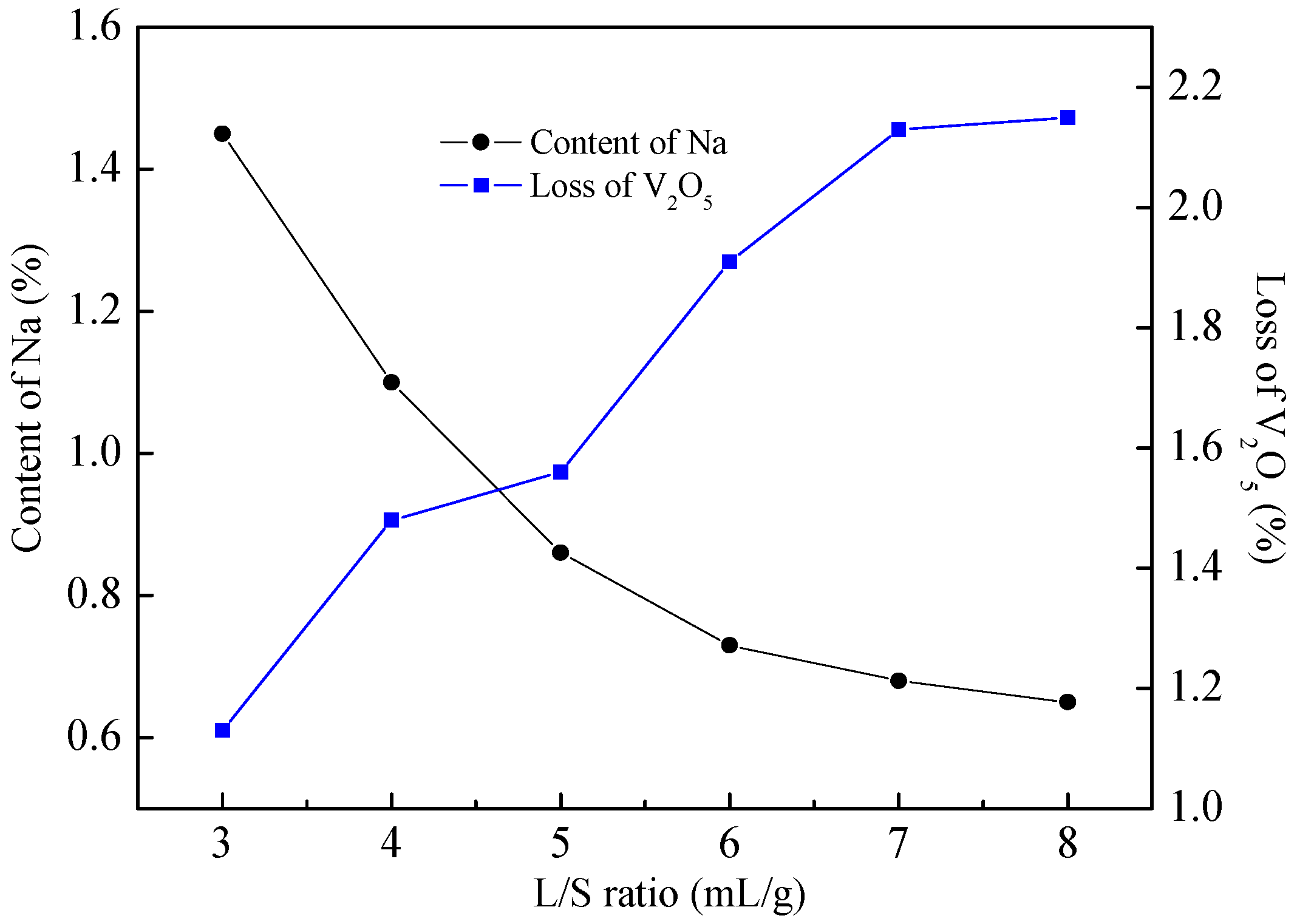

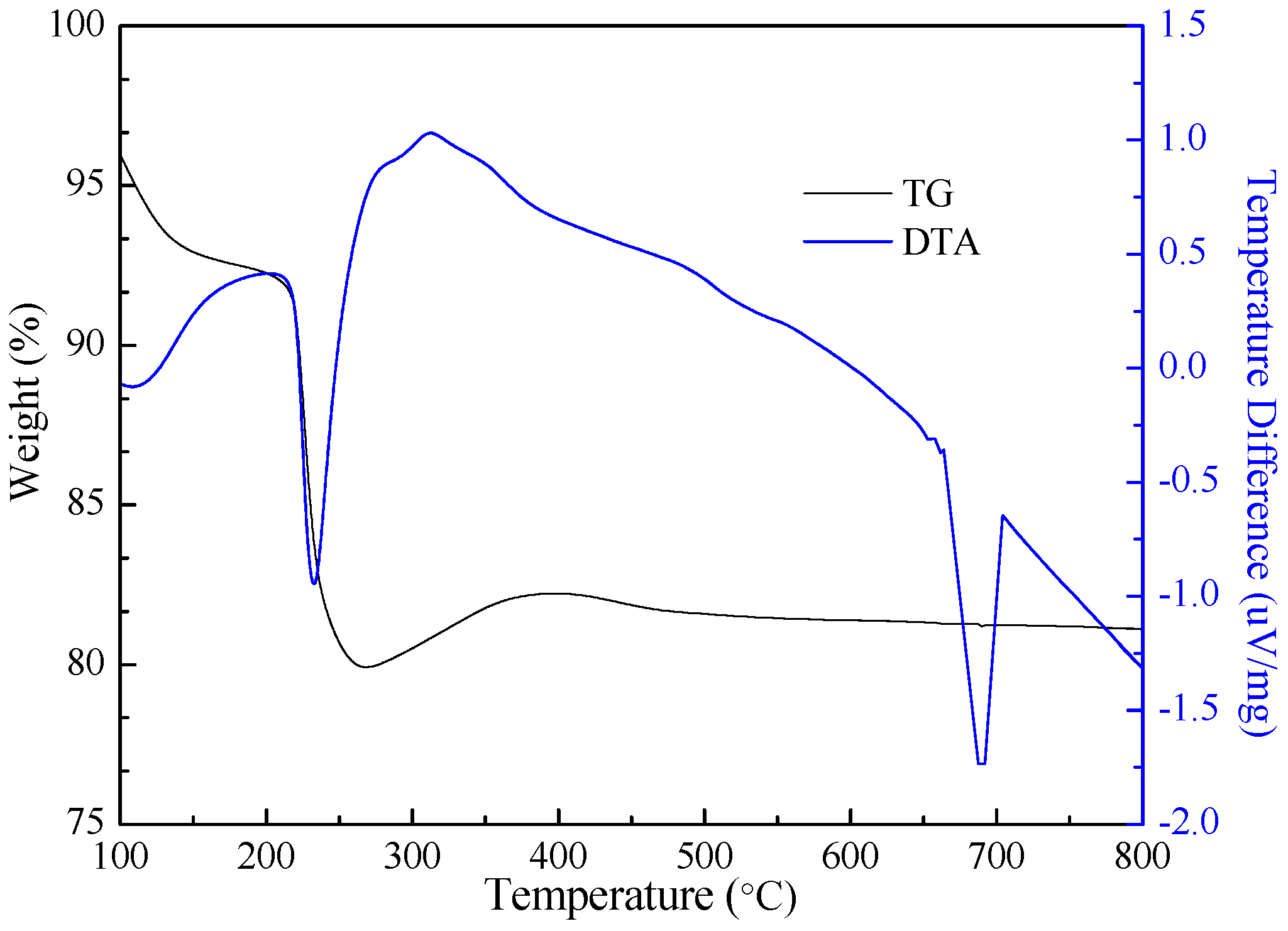
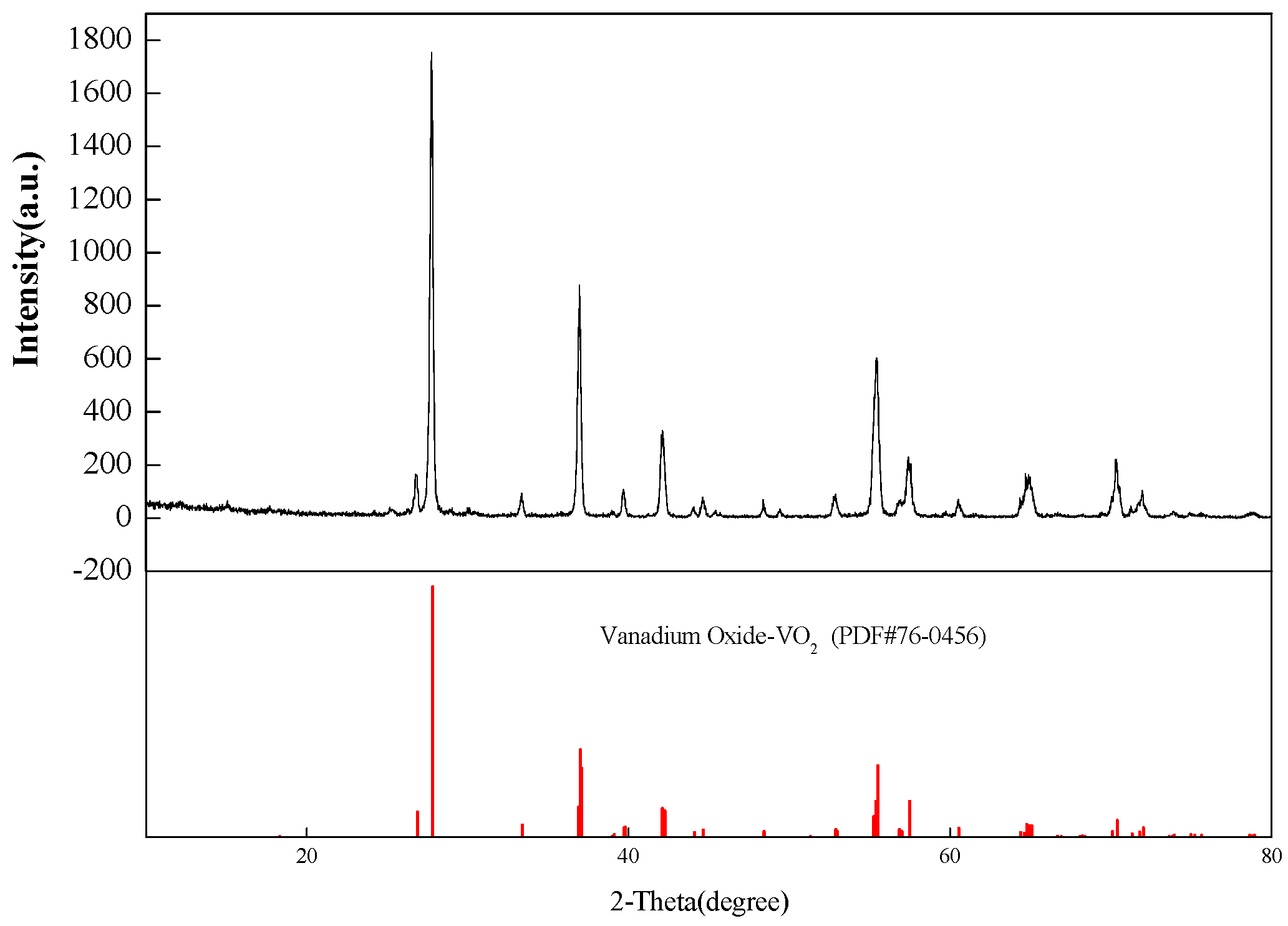
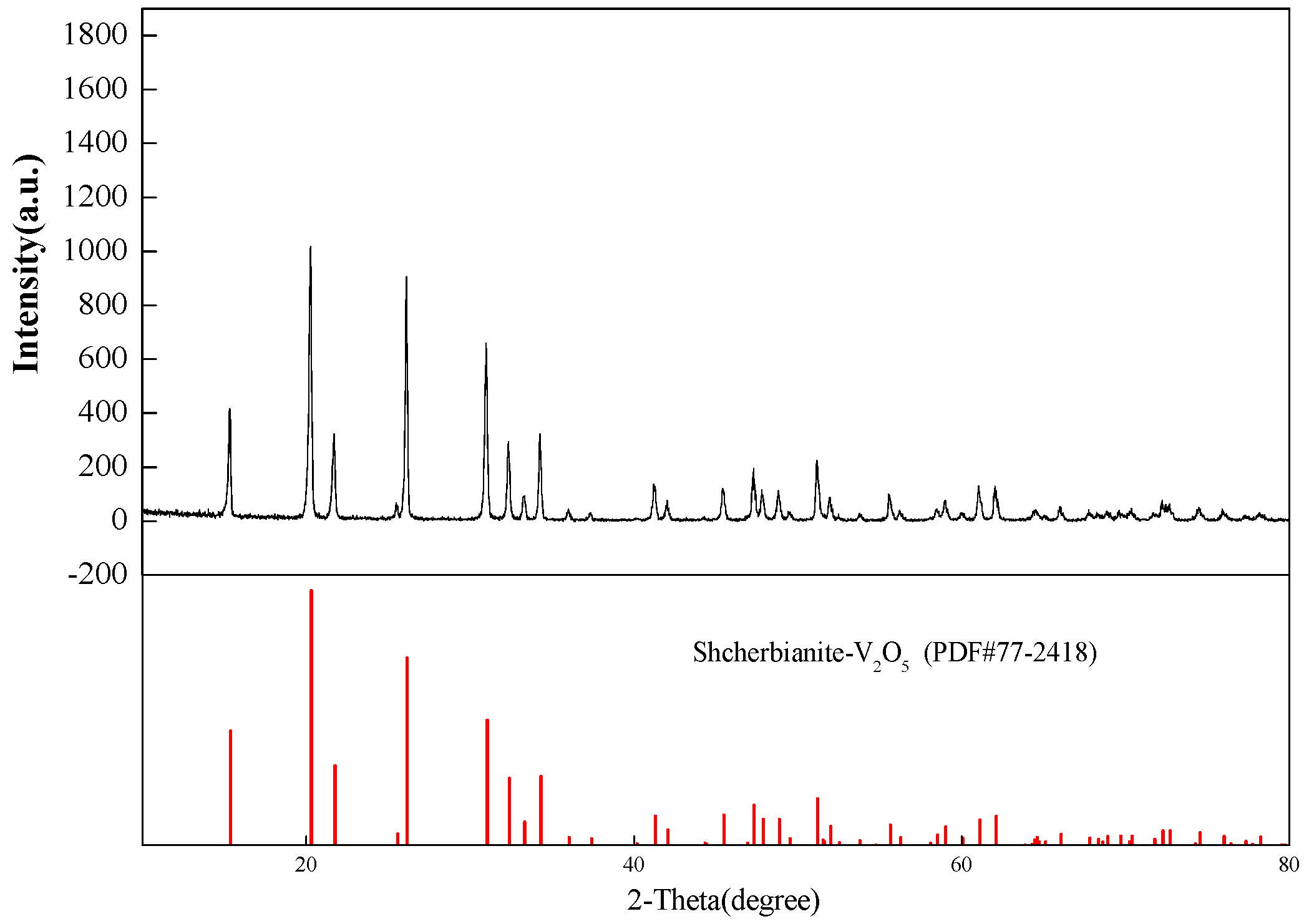
| Chemical Reactions | ΔrGθ (KJ·mol−1) |
|---|---|
| VOSO4 + H+ = VO2+ + HSO4− | −1.61 |
| HV2O5− + 2SO42− + 5 H+ = 2VOSO4 + 3H2O | −114.92 |
| HV2O5− = V4O92− + H2O | −1512.22 |
| V2O4 + 2SO42− + 4H+ = 2VOSO4 + 2H2O | −68.1 |
| HV2O5− + H+ = V2O4 + H2O | −46.82 |
| V4O92− + 2H+ = 2V2O4 + H2O | −90.38 |
| Concentration (g/L) | V | Fe(II) | Fe(III) | Al | Mg | K | Na | Ca | SO42− |
|---|---|---|---|---|---|---|---|---|---|
| Acid leach solution | 2.31 | 2.71 | 6.84 | 25.79 | 1.09 | 7.44 | 1.01 | 1.11 | 185 |
| V(IV) strip liquor | 22.1 | 0 | 0.009 | 0.01 | 0.008 | 0.006 | <0.001 | 0.005 | 98 |
| Element | VO2 | V2O5 | Fe | Al | S | P | Si | Na2O + K2O |
|---|---|---|---|---|---|---|---|---|
| VO2 (wt.%) | 98.82 | - | 0.26 | 0.005 | 0.003 | 0.03 | 0.001 | 1.1 |
| V2O5 (wt.%) | - | 98.70 | 0.25 | 0.006 | 0.003 | 0.03 | 0.002 | 1.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Wang, X.; Stopic, S.; Wang, M.; Kremer, D.; Wotruba, H.; Friedrich, B. Preparation of Vanadium Oxides from a Vanadium (IV) Strip Liquor Extracted from Vanadium-Bearing Shale Using an Eco-Friendly Method. Metals 2018, 8, 994. https://doi.org/10.3390/met8120994
Ma Y, Wang X, Stopic S, Wang M, Kremer D, Wotruba H, Friedrich B. Preparation of Vanadium Oxides from a Vanadium (IV) Strip Liquor Extracted from Vanadium-Bearing Shale Using an Eco-Friendly Method. Metals. 2018; 8(12):994. https://doi.org/10.3390/met8120994
Chicago/Turabian StyleMa, Yiqian, Xuewen Wang, Srecko Stopic, Mingyu Wang, Dario Kremer, Hermann Wotruba, and Bernd Friedrich. 2018. "Preparation of Vanadium Oxides from a Vanadium (IV) Strip Liquor Extracted from Vanadium-Bearing Shale Using an Eco-Friendly Method" Metals 8, no. 12: 994. https://doi.org/10.3390/met8120994
APA StyleMa, Y., Wang, X., Stopic, S., Wang, M., Kremer, D., Wotruba, H., & Friedrich, B. (2018). Preparation of Vanadium Oxides from a Vanadium (IV) Strip Liquor Extracted from Vanadium-Bearing Shale Using an Eco-Friendly Method. Metals, 8(12), 994. https://doi.org/10.3390/met8120994







