Influence of Al Content on the Inclusion-Microstructure Relationship in the Heat-Affected Zone of a Steel Plate with Mg Deoxidation after High-Heat-Input Welding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Steel Preparation
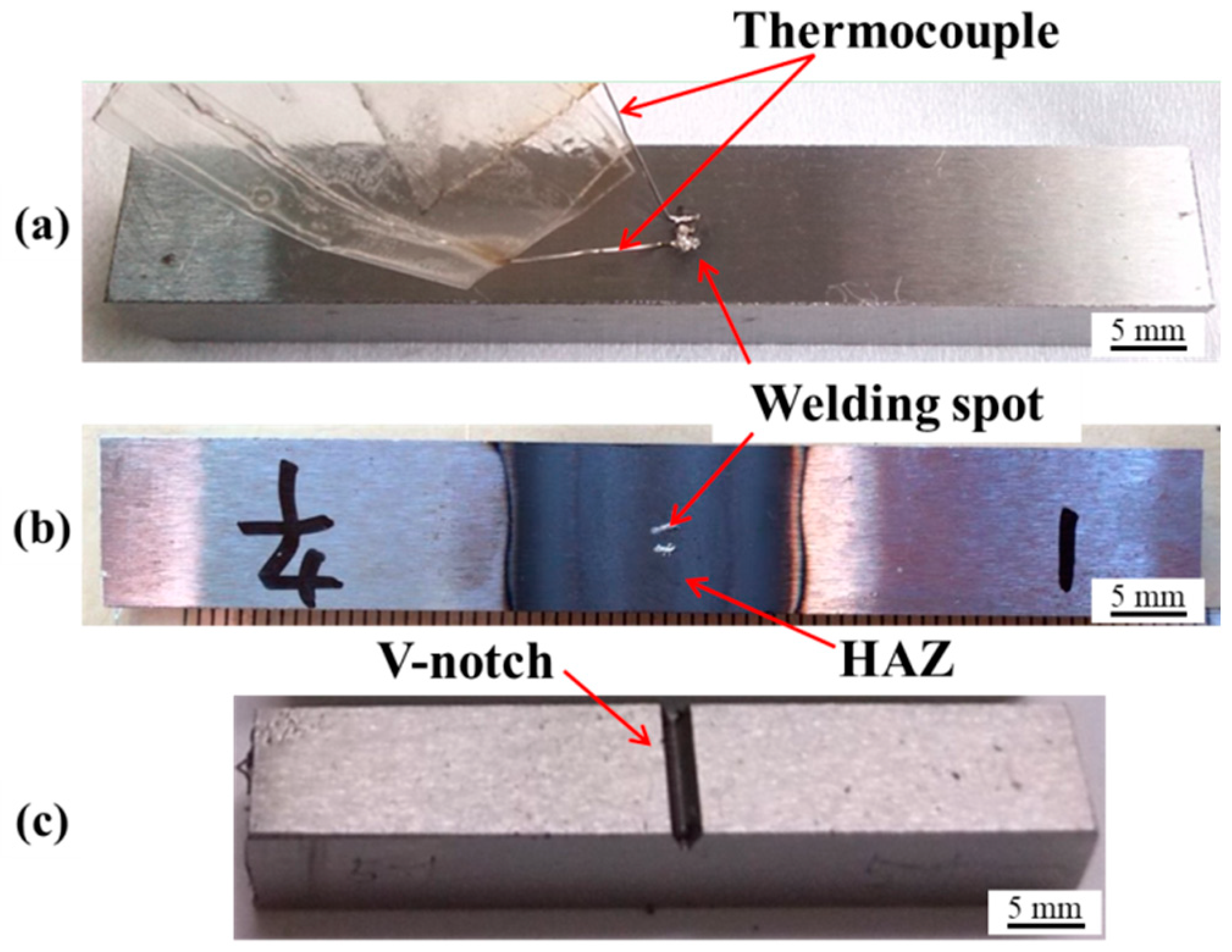
2.2. Simulated High-Heat-Input Welding Experiments
2.3. Characterization of Inclusions and Microstructures
3. Experimental Results
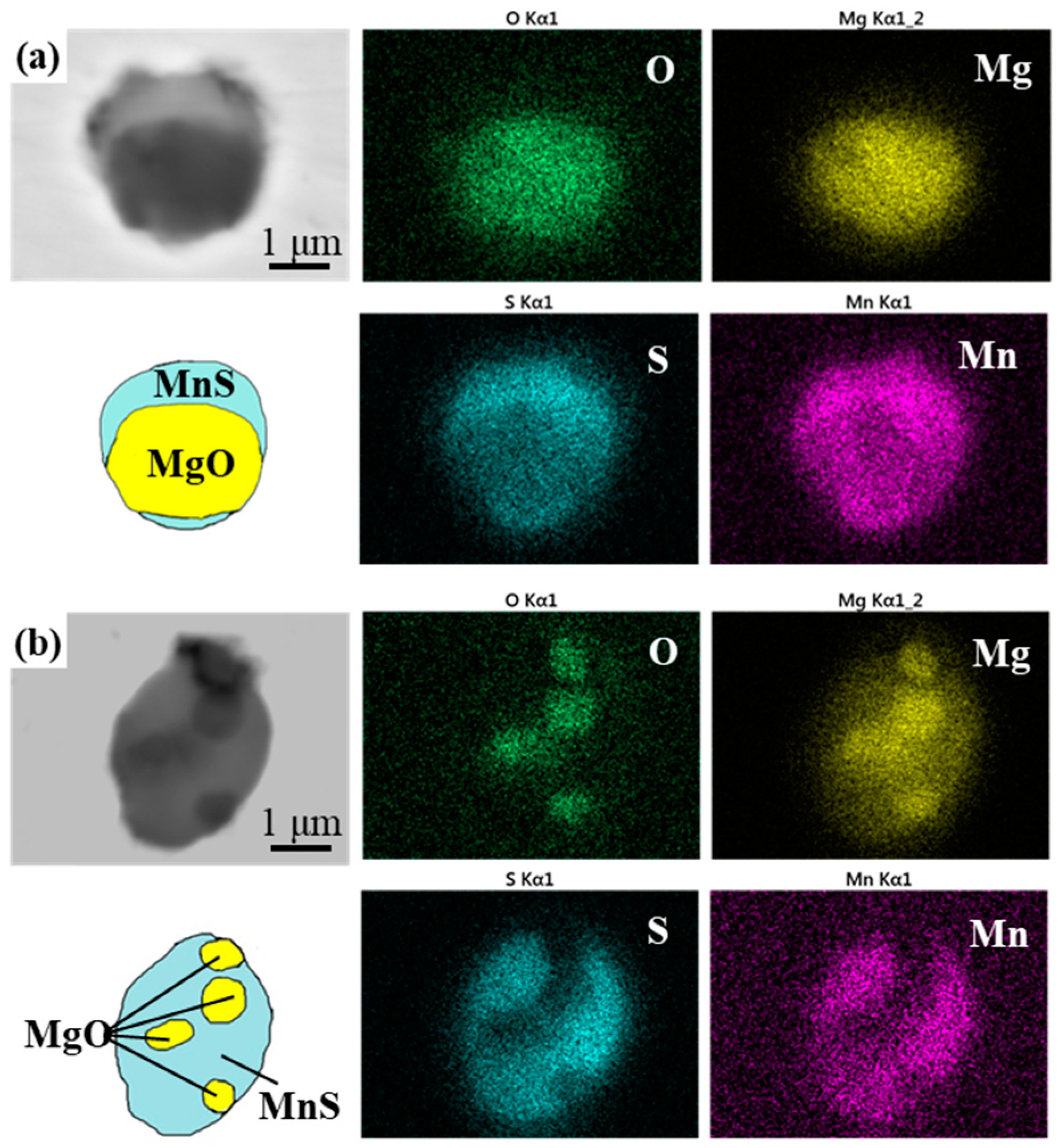
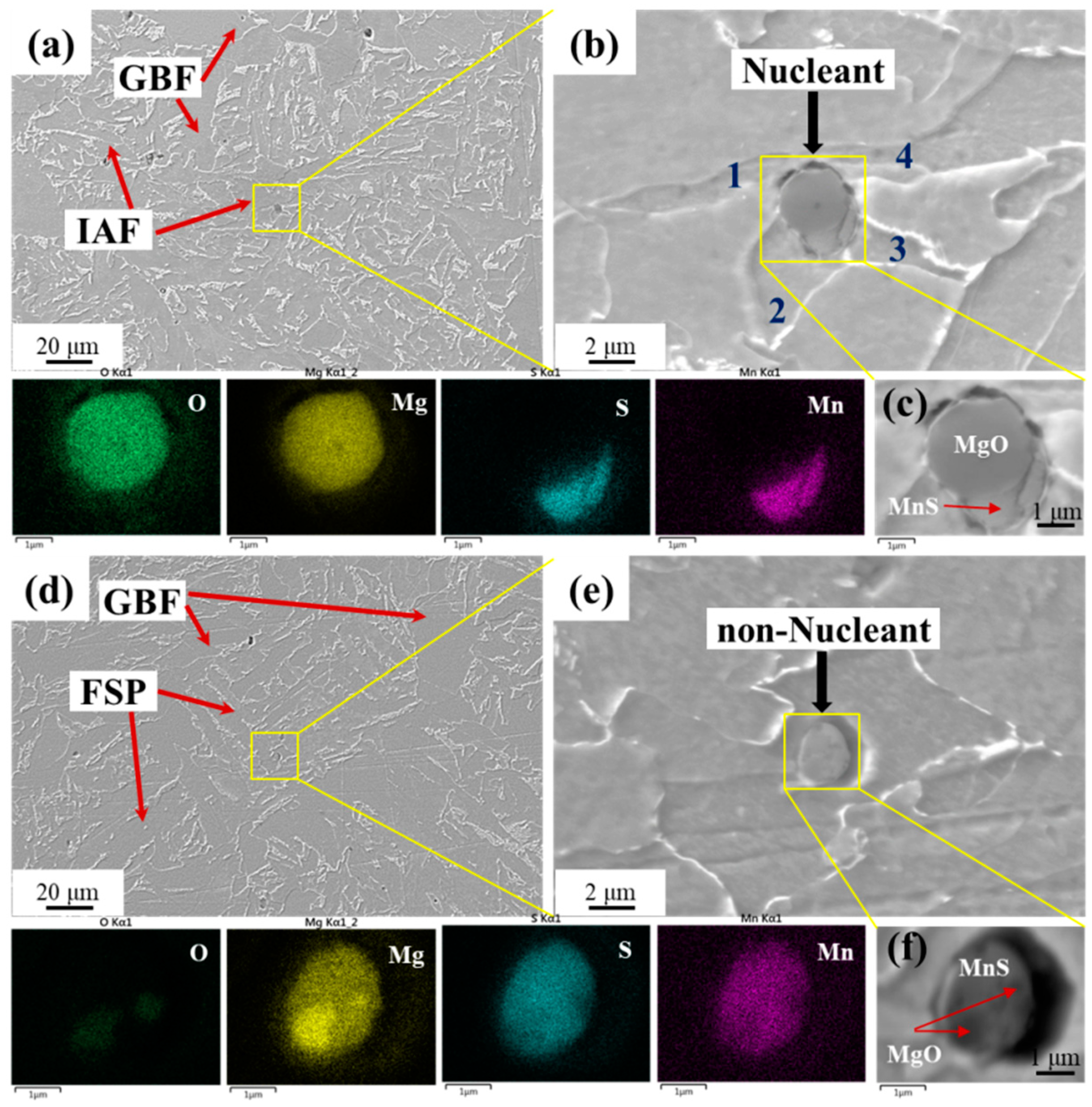
3.1. Morphology and Composition of Typical Inclusions
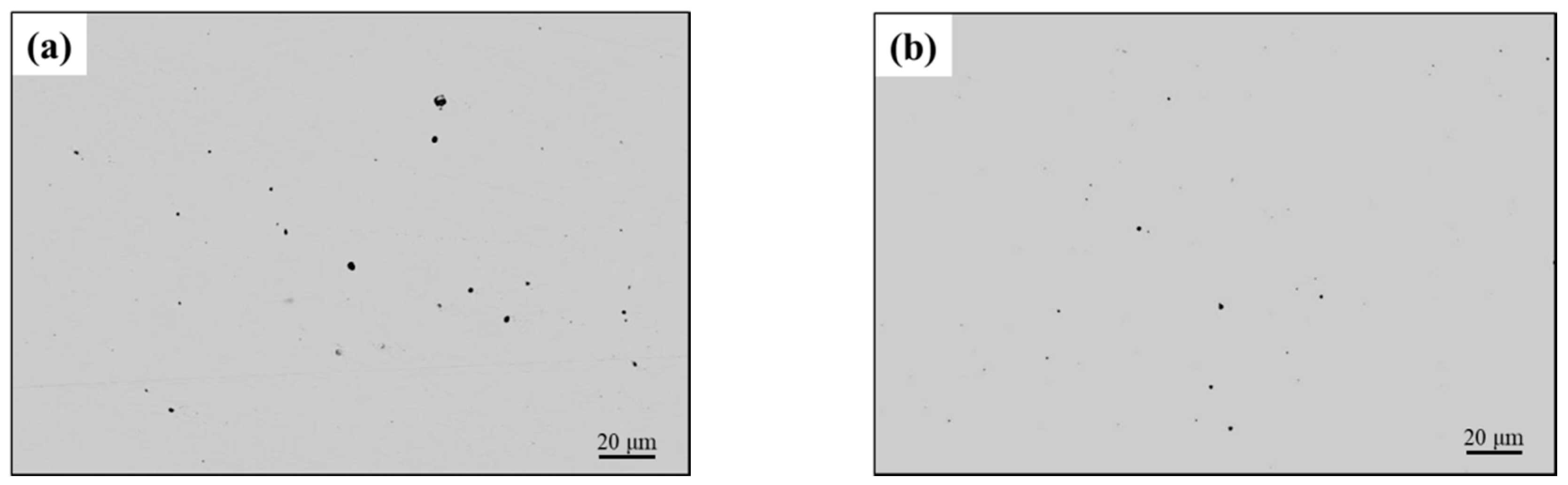
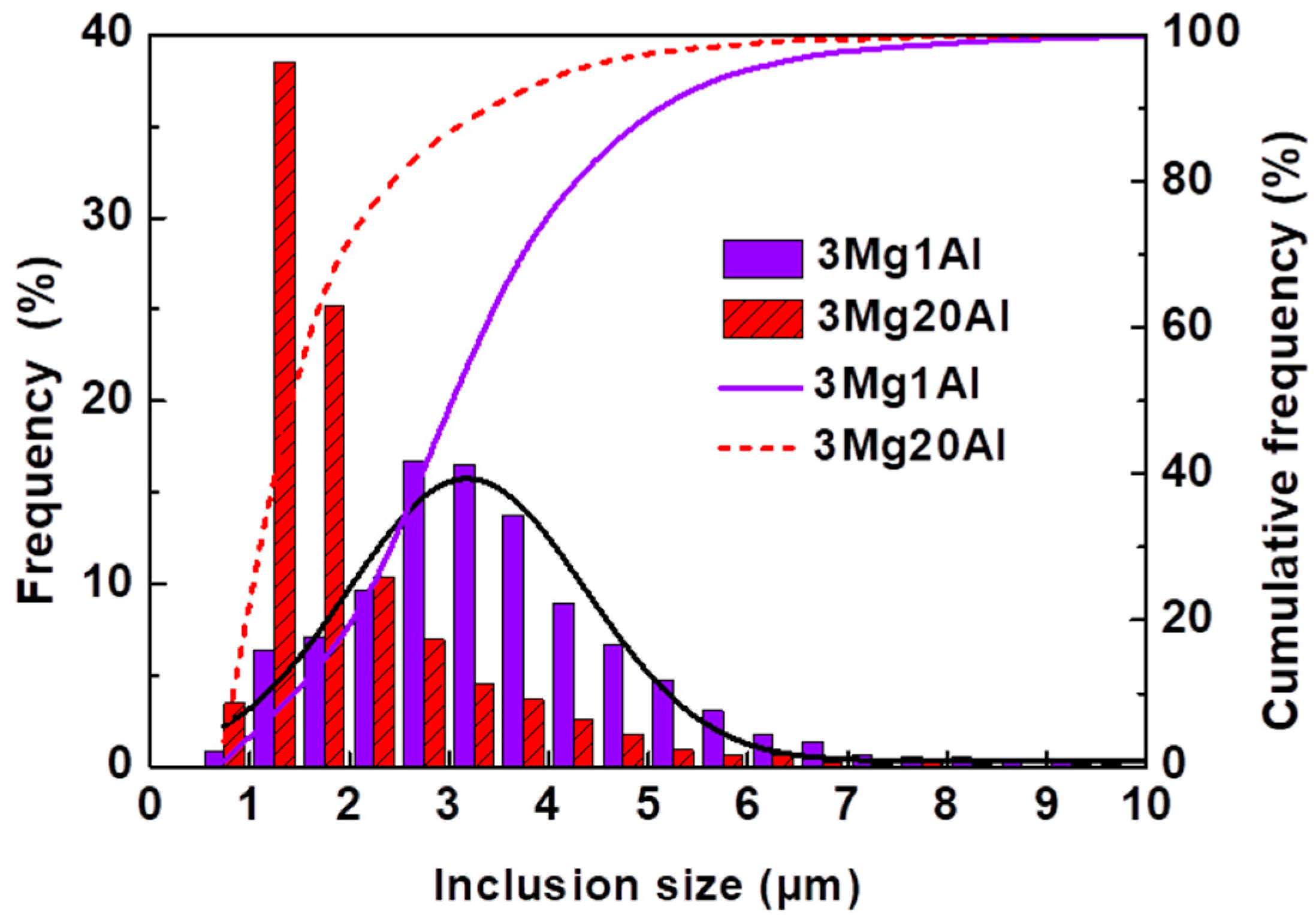
3.2. Number Density and Size Distribution of Inclusions
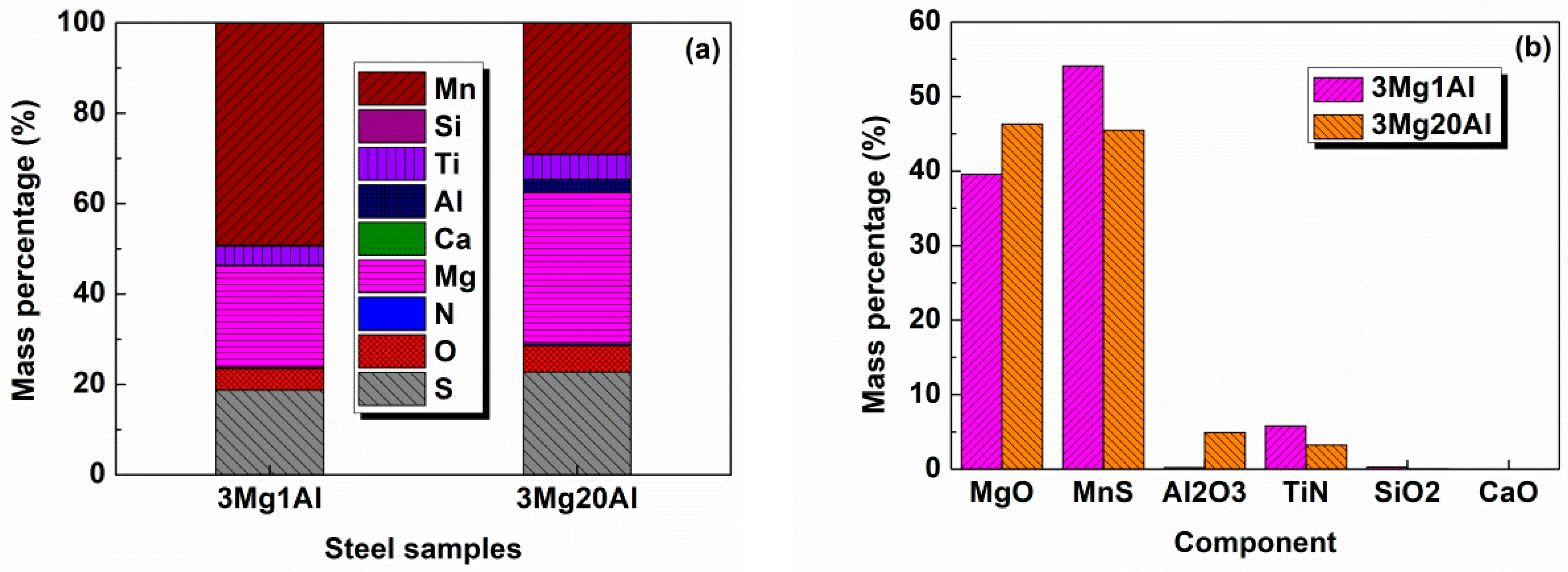
3.3. Composition of Inclusions
3.4. Types of Inclusions
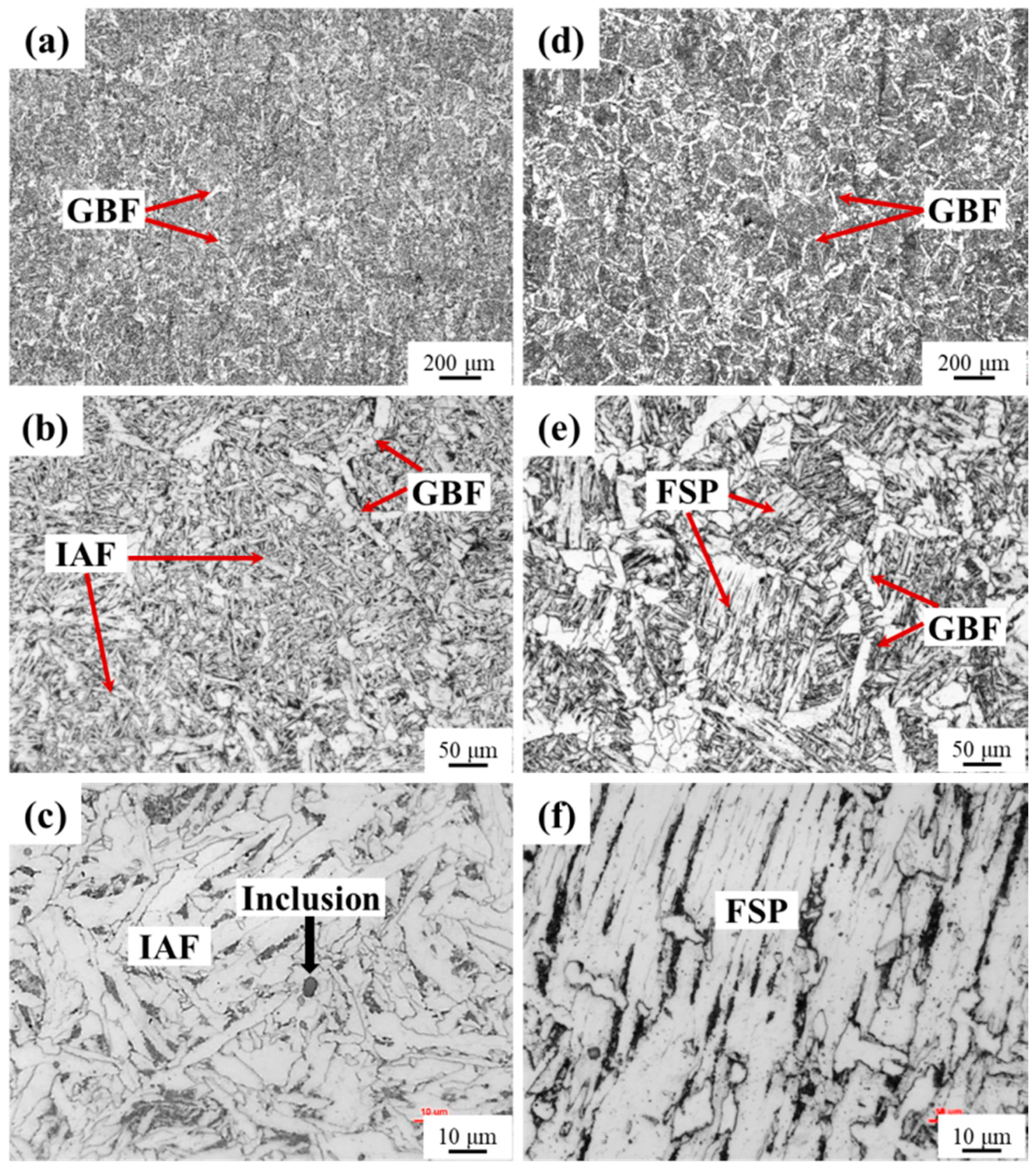
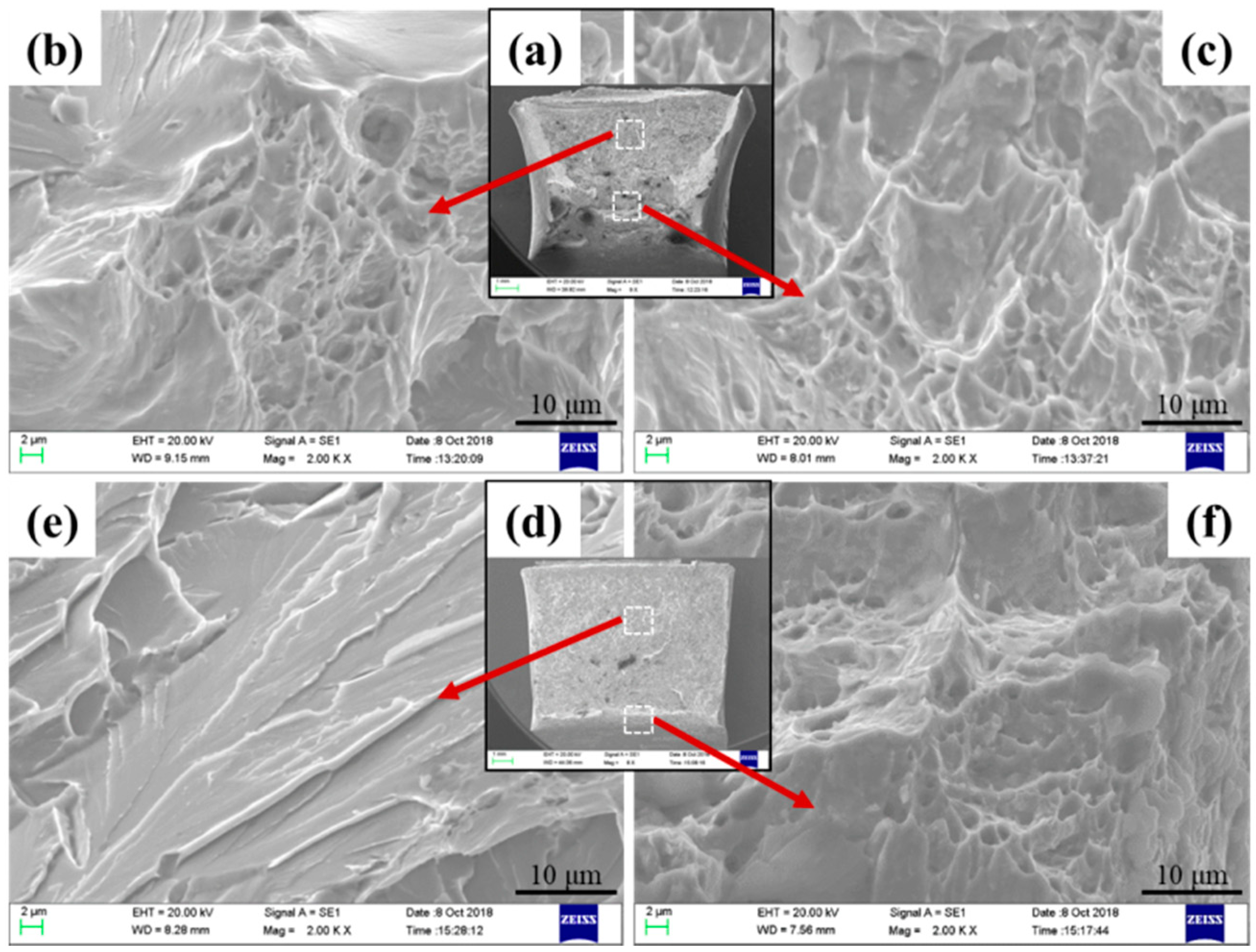
3.5. HAZ Microstructure and Toughness
4. Discussion
4.1. Effect of Al on Inclusions
4.2. Effect of Al on HAZ Microstructures
5. Conclusions
- The main inclusion types in 3Mg1Al without Al addition were MnS, MgO-MnS, MgO-MnS-TiN, and those in 3Mg20Al with Al addition were MgO-MnS, MgO-MnS-TiN, MgO-Al2O3-MnS. The number density of inclusions in 3Mg1Al, 96.65 mm−2, was similar to that in 3Mg20Al, 95.03 mm−2. However, the mean size of inclusions in the former, 3.47 μm, was larger than that in the latter, 2.03 μm.
- Although the chemistries of the main kind of oxysulfide complex inclusions in 3Mg1Al and 3Mg20Al were both MgO-MnS, the morphologies were quite different. The former consisted of a central single MgO particle and outside, the MnS phase. The latter comprised several small MgO particles entrapped by the MnS phase.
- Because the MgO-MnS complex inclusions in 3Mg1Al could nucleate intragranular acicular ferrites (IAFs) and these in 3Mg20Al were non-nucleant, the main intragranular microstructure in HAZs for 3Mg1Al was ductile IAFs, while that for 3Mg20Al was brittle ferrite side plates (FSPs). Therefore, the HAZ toughness of Mg deoxidized the steel plate without Al addition was much better than that with Al addition.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Takamura, J.; Mizoguchi, S. Roles of Oxides in Steels Performance. In Proceedings of the Sixth International Iron and Steel Congress, Nagaya, Japan, 21–26 October 1990. [Google Scholar]
- Kojima, A.; Kiyose, A.; Uemori, R.; Minagawa, M.; Hoshino, M.; Nakashima, T.; Ishida, K.; Yasui, H. Super high HAZ toughness technology with fine microstructure imparted by fine particles. Shinnittetsu Giho 2004, 2–5. [Google Scholar]
- Suzuki, S.; Ichimiya, K.; Akita, T. High tensile strength steel plates with excellent HAZ toughness for shipbuilding. JFE Tech. Rep. 2005, 5, 24–29. [Google Scholar]
- Yang, J.; Zhu, K.; Wang, R.Z.; Shen, J.G. Improving the toughness of heat affected zone of steel plate by use of fine inclusion particles. Steel Res. Int. 2011, 82, 552–556. [Google Scholar] [CrossRef]
- Sarma, D.S.; Karasev, A.V.; Jönsson, P.G. On the role of non-metallic inclusions in the nucleation of acicular ferrite in steels. ISIJ Int. 2009, 49, 1063–1074. [Google Scholar] [CrossRef]
- Kim, H.S.; Chang, C.H.; Lee, H.G. Evolution of inclusions and resultant microstructural change with Mg addition in Mn/Si/Ti deoxidized steels. Scripta Mater. 2005, 53, 1253–1258. [Google Scholar] [CrossRef]
- Chai, F.; Yang, C.F.; Su, H.; Zhang, Y.Q.; Xu, Z. Effect of magnesium on inclusion formation in Ti-killed steels and microstructural evolution in welding induced coarse-grained heat affected zone. J. Iron Steel Res. Int 2009, 16, 69–74. [Google Scholar] [CrossRef]
- Park, S.C.; Jung, I.H.; Oh, K.S.; Lee, H.G. Effect of Al on the evolution of non-metallic inclusions in the Mn-Si-Ti-Mg deoxidized steel during solidification: Experiments and thermodynamic calculations. ISIJ Int. 2004, 44, 1016–1023. [Google Scholar] [CrossRef]
- Li, X.B.; Min, Y.; Yu, Z.; Liu, C.J.; Jiang, M.F. Effect of Mg addition on nucleation of intra-granular acicular ferrite in Al-killed low carbon steel. J. Iron Steel Res. Int. 2016, 23, 415–421. [Google Scholar] [CrossRef]
- Xu, L.Y.; Yang, J.; Wang, R.Z.; Wang, Y.N.; Wang, W.L. Effect of Mg content on the microstructure and toughness of heat-affected zone of steel plate after high heat input welding. Metall. Mater. Trans. A 2016, 47, 3354–3364. [Google Scholar] [CrossRef]
- Xu, L.Y.; Yang, J.; Wang, R.Z.; Wang, W.L.; Wang, Y.N. Effect of Mg addition on formation of intragranular acicular ferrite in heat-affected zone of steel plate after high-heat-input welding. J. Iron Steel Res. Int. 2018, 25, 433–441. [Google Scholar] [CrossRef]
- Xu, L.Y.; Yang, J.; Wang, R.Z.; Wang, W.L.; Ren, Z.M. Effect of welding heat input on microstructure and toughness of heated-affected zone in steel plate with Mg deoxidation. Steel Res. Int. 2017, 88, 1700157. [Google Scholar] [CrossRef]
- Lou, H.; Wang, C.; Wang, B.; Wang, Z.; Li, Y.; Chen, Z. Inclusion Evolution behavior of Ti-Mg oxide metallurgy steel and its effect on a high heat input welding HAZ. Metals 2018, 8, 534. [Google Scholar] [CrossRef]
- Van, E.M.; Guo, M.; Zinngrebe, E.; Blanpain, B.; Jung, I. Evolution of non-metallic inclusions in secondary steelmaking: Learning from inclusion size distributions. ISIJ Int. 2013, 53, 1974–1982. [Google Scholar]
- Pervushin, G.V.; Suito, H. Effect of primary deoxidation products of Al2O3, ZrO2, Ce2O3 and MgO on TiN precipitation in Fe-10 mass% Ni alloy. ISIJ Int. 2001, 41, 748–756. [Google Scholar] [CrossRef]
- Lee, T.K.; Kim, H.J.; Kang, B.Y.; Hwang, S.K. Effect of inclusion size on the nucleation of acicular ferrite in welds. ISIJ Int. 2000, 40, 1260–1268. [Google Scholar] [CrossRef]
- Kimura, S.; Nakajima, K.; Mizoguchi, S. Behavior of alumina-magnesia complex inclusions and magnesia inclusions on the surface of molten low-carbon steels. Metall. Mater. Trans. B 2001, 32, 79–85. [Google Scholar] [CrossRef]
- Yang, J.; Xu, L.Y.; Zhu, K.; Wang, R.Z.; Zhou, L.J.; Wang, W.L. Improvement of HAZ toughness of steel plate for high heat input welding by inclusion control with Mg deoxidation. Steel Res. Int. 2015, 86, 619–625. [Google Scholar] [CrossRef]
- Karasev, A.V.; Suito, H. Characteristics of fine oxide particles produced by Ti/M (M = Mg and Zr) complex deoxidation in Fe-10 mass%Ni alloy. ISIJ Int. 2008, 48, 1507–1516. [Google Scholar] [CrossRef]
- Ohta, H.; Suito, H. Characteristics of particle size distribution of deoxidation products with Mg, Zr, Al, Ca, Si/Mn and Mg/Al in Fe-10 mass% Ni alloy. ISIJ Int. 2006, 46, 14–21. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Wu, T.; Wu, G.; Li, J. Effect of acid-Soluble aluminum on the evolution of non-metallic inclusions in spring steel. Metall. Mater. Trans. B 2017, 48, 943. [Google Scholar] [CrossRef]
- Ohta, H.; Suito, H. Effects of dissolved oxygen and size distribution on particle coarsening of deoxidation product. ISIJ Int. 2006, 46, 42–49. [Google Scholar] [CrossRef]
- Lee, J.L.; Pan, Y.T. Effect of sulfur content on the microstructure and toughness of simulated heat-affected zone in Ti-killed steels. Metall. Trans. A 1993, 24, 1399–1408. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, L.; Zhu, L. Effect of inclusion size and type on the nucleation of acicular ferrite in high strength ship plate steel. ISIJ Int. 2018, 58, 965–969. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Y.; Wang, S.; Zhang, Q.; Zhang, C. Research of microalloy elements to induce intragranular acicular ferrite in shipbuilding steel. Ironmaking Steelmaking 2017, 1–9. [Google Scholar] [CrossRef]
- Kang, Y.B.; Lee, H.G. Thermodynamic analysis of Mn-depleted near Ti oxide inclusions for intragranular nucleation of ferrite in steel. ISIJ Int. 2010, 50, 501–508. [Google Scholar] [CrossRef]
- Mabuchi, H.; Uemori, R.; Fujioka, M. The role of Mn depletion in intra-granular ferrite transformation in the heat affected zone of welded joints with large heat input in structural steels. ISIJ Int. 1996, 36, 1406–1412. [Google Scholar] [CrossRef]
- Zheng, C.C.; Wang, X.M.; Li, S.R.; Shang, C.J.; He, X.L. Effects of inclusions on microstructure and properties of heat-affected-zone for low-carbon steels. Sci. Chin. Technol. Sci. 2012, 55, 1556–1565. [Google Scholar] [CrossRef]
- Shi, M.H.; Zhang, P.Y.; Zhu, F.X. Toughness and microstructure of coarse grain heat affected zone with high heat input welding in Zr-bearing low carbon steel. ISIJ Int. 2014, 54, 188–192. [Google Scholar] [CrossRef]


| Steels | C | Si | Mn | P | S | Ti | Mg | Al | Als * | O | N |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3Mg1Al | 0.082 | 0.22 | 1.56 | 0.006 | 0.005 | 0.011 | 0.0027 | 0.001 | 0.001 | 0.0011 | 0.0032 |
| 3Mg20Al | 0.082 | 0.22 | 1.56 | 0.006 | 0.004 | 0.011 | 0.0027 | 0.020 | 0.019 | 0.0007 | 0.0032 |
| Steels | Area (mm2) | Number of Inclusions | Number Density (mm−2) | Mean Size (μm) |
|---|---|---|---|---|
| 3Mg1Al | 38.8 | 3750 | 96.65 | 3.47 |
| 3Mg20Al | 31.6 | 3003 | 95.03 | 2.03 |
| Steels | Impact Fracture | Impact Toughness (J) | |||
|---|---|---|---|---|---|
| Fibrous Zone (%) | Shear Lip Zone (%) | Radical Zone (%) | Individual Value | Mean | |
| 3Mg1Al | 56.03 | 21.95 | 22.01 | 195, 186, 222 | 201 |
| 3Mg20Al | 20.63 | 2.51 | 76.86 | 59, 91, 75 | 75 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Yang, J.; Wang, R. Influence of Al Content on the Inclusion-Microstructure Relationship in the Heat-Affected Zone of a Steel Plate with Mg Deoxidation after High-Heat-Input Welding. Metals 2018, 8, 1027. https://doi.org/10.3390/met8121027
Xu L, Yang J, Wang R. Influence of Al Content on the Inclusion-Microstructure Relationship in the Heat-Affected Zone of a Steel Plate with Mg Deoxidation after High-Heat-Input Welding. Metals. 2018; 8(12):1027. https://doi.org/10.3390/met8121027
Chicago/Turabian StyleXu, Longyun, Jian Yang, and Ruizhi Wang. 2018. "Influence of Al Content on the Inclusion-Microstructure Relationship in the Heat-Affected Zone of a Steel Plate with Mg Deoxidation after High-Heat-Input Welding" Metals 8, no. 12: 1027. https://doi.org/10.3390/met8121027
APA StyleXu, L., Yang, J., & Wang, R. (2018). Influence of Al Content on the Inclusion-Microstructure Relationship in the Heat-Affected Zone of a Steel Plate with Mg Deoxidation after High-Heat-Input Welding. Metals, 8(12), 1027. https://doi.org/10.3390/met8121027





