Austenite Decomposition and Precipitation Behavior of Plastically Deformed Low-Si Microalloyed Steel
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
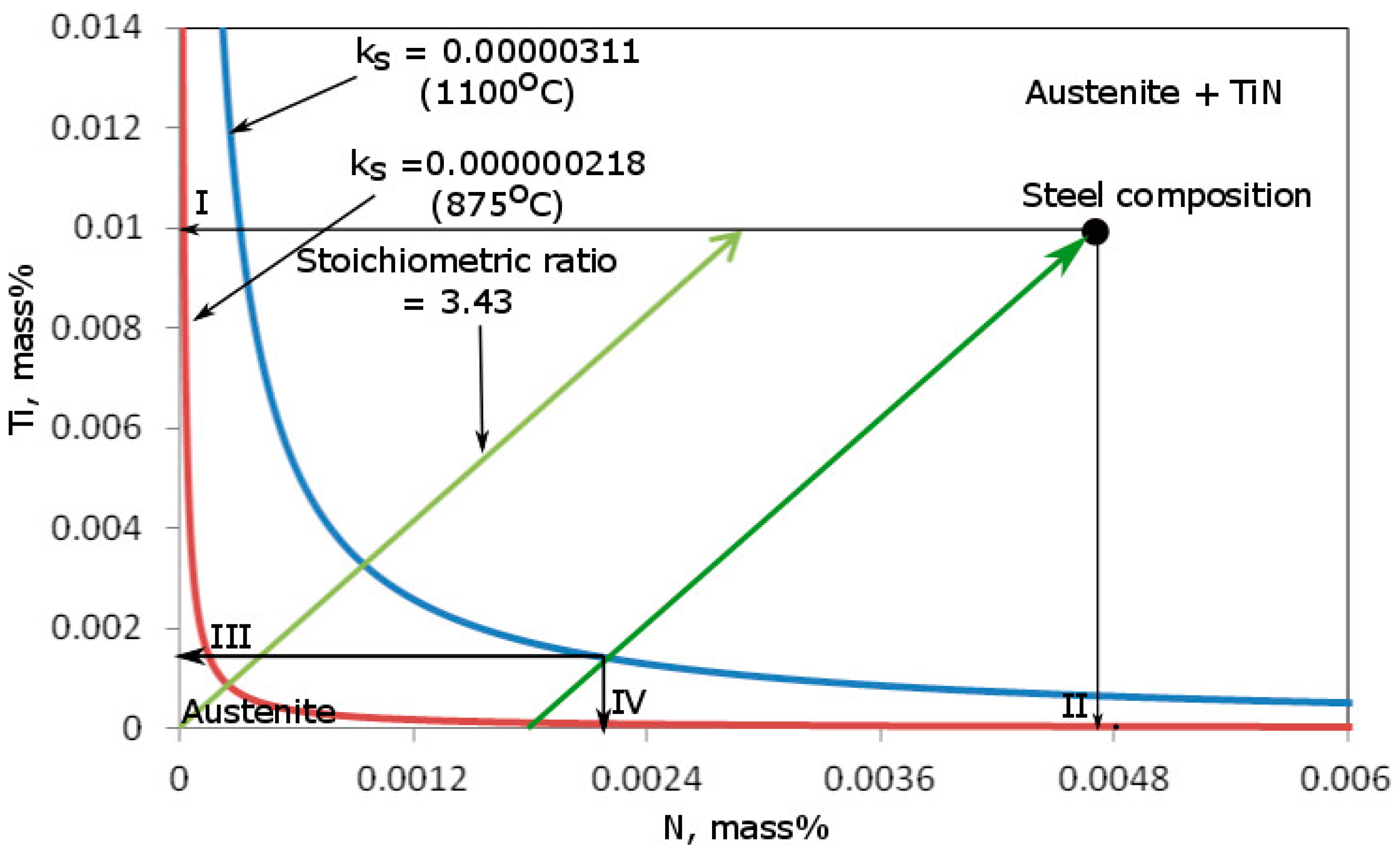
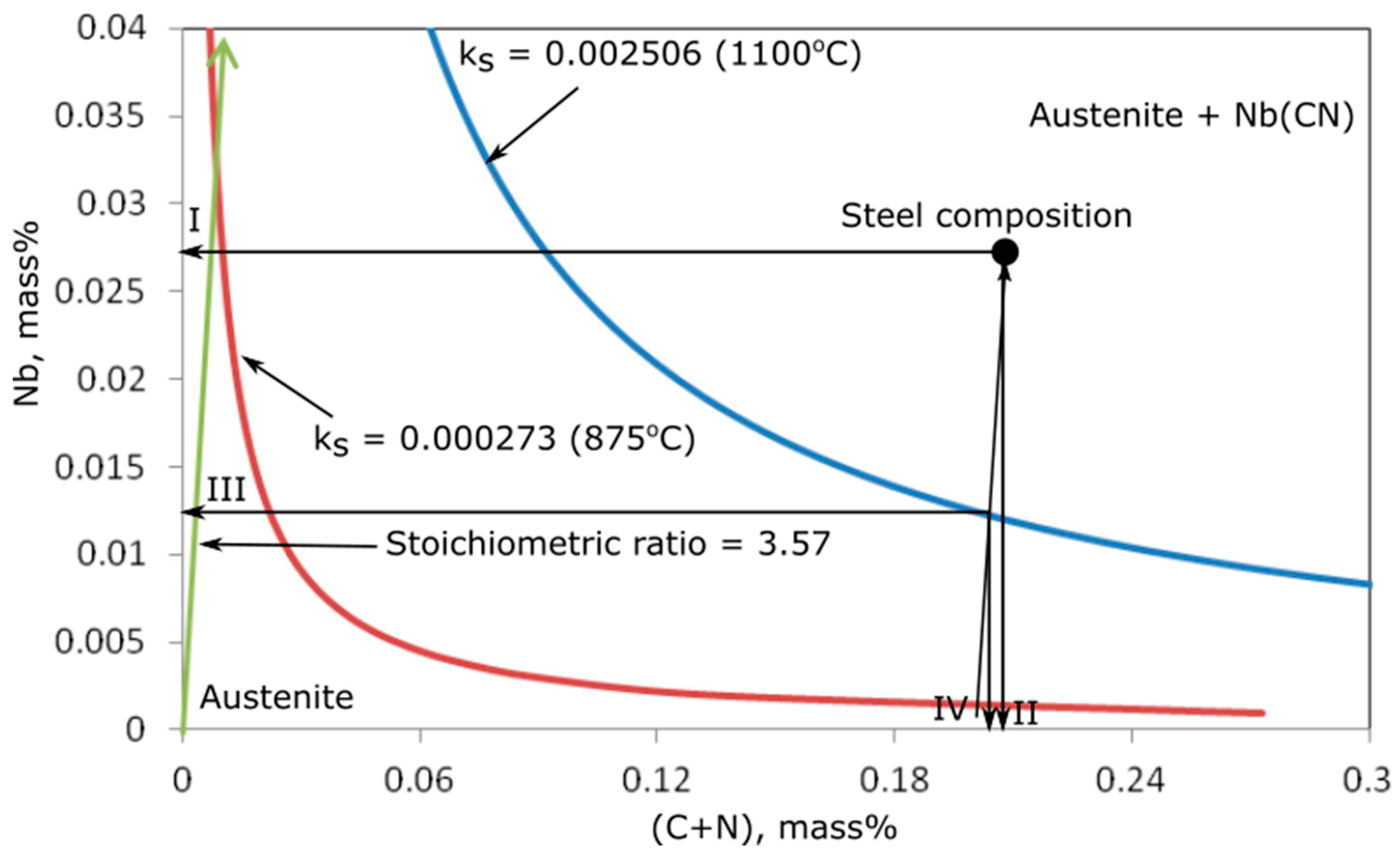
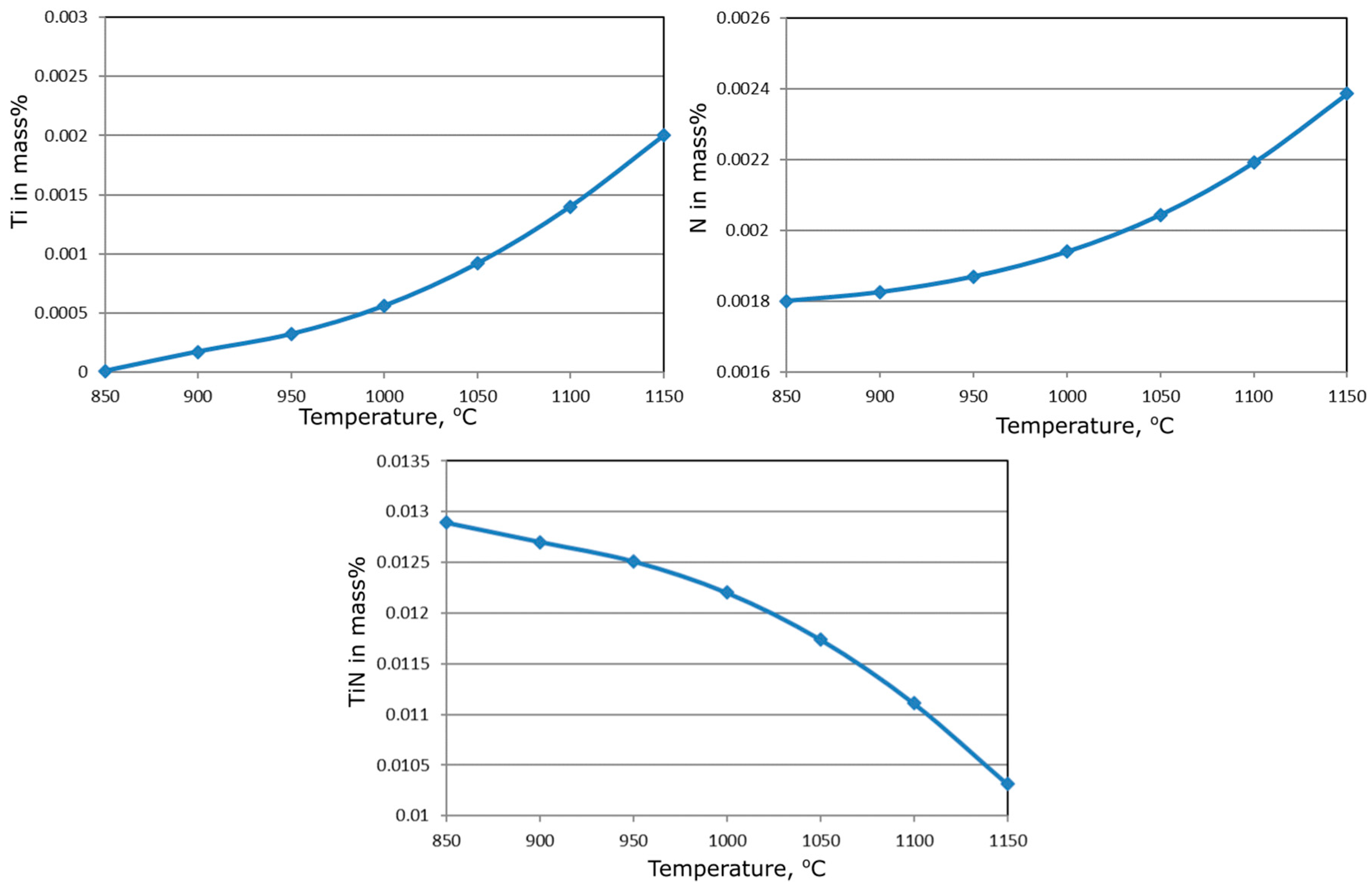
3.1. Thermodynamic Calculations of Precipitation Processes
3.2. Thermodynamic Calculations of Phase Transformations
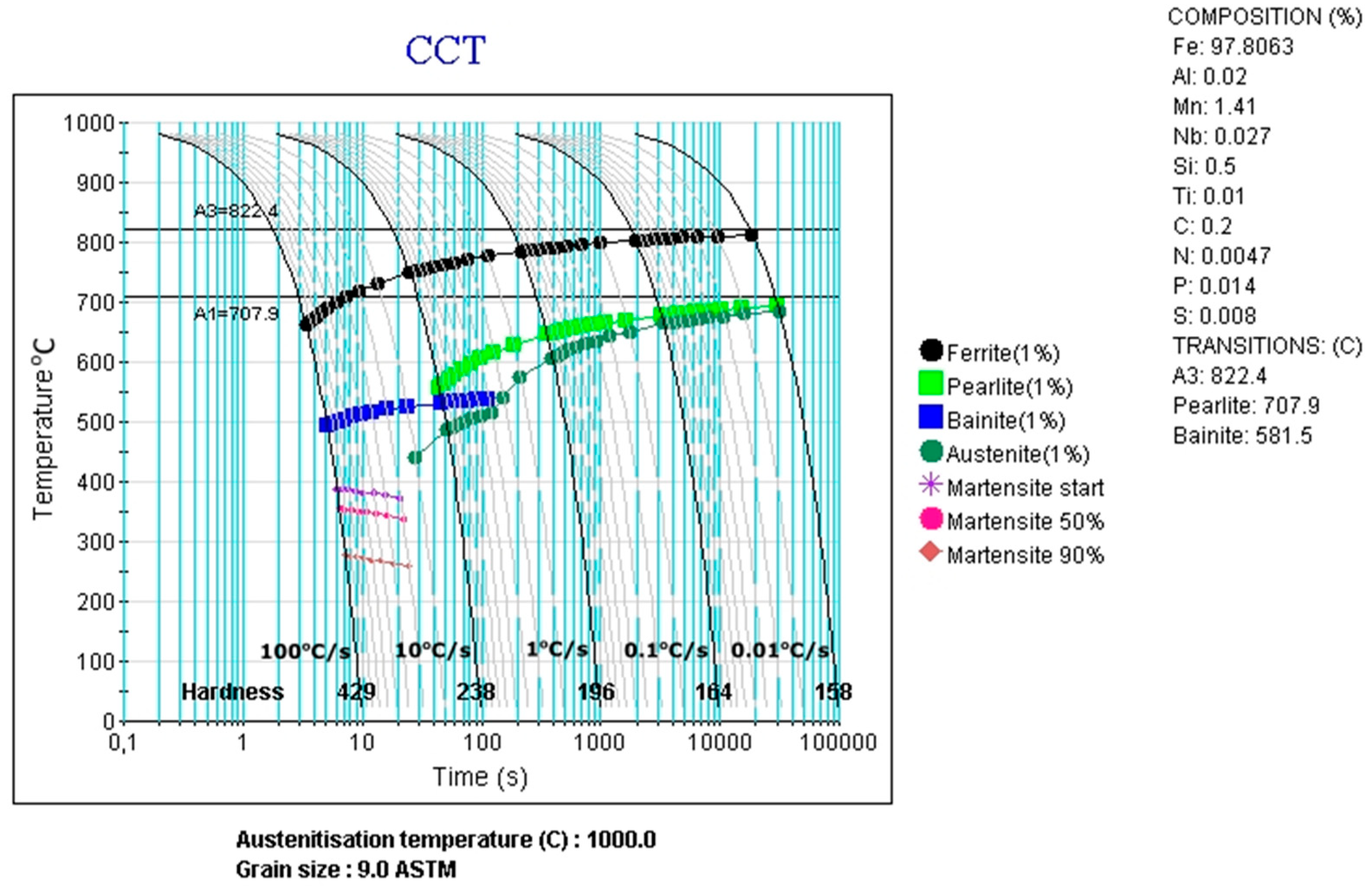
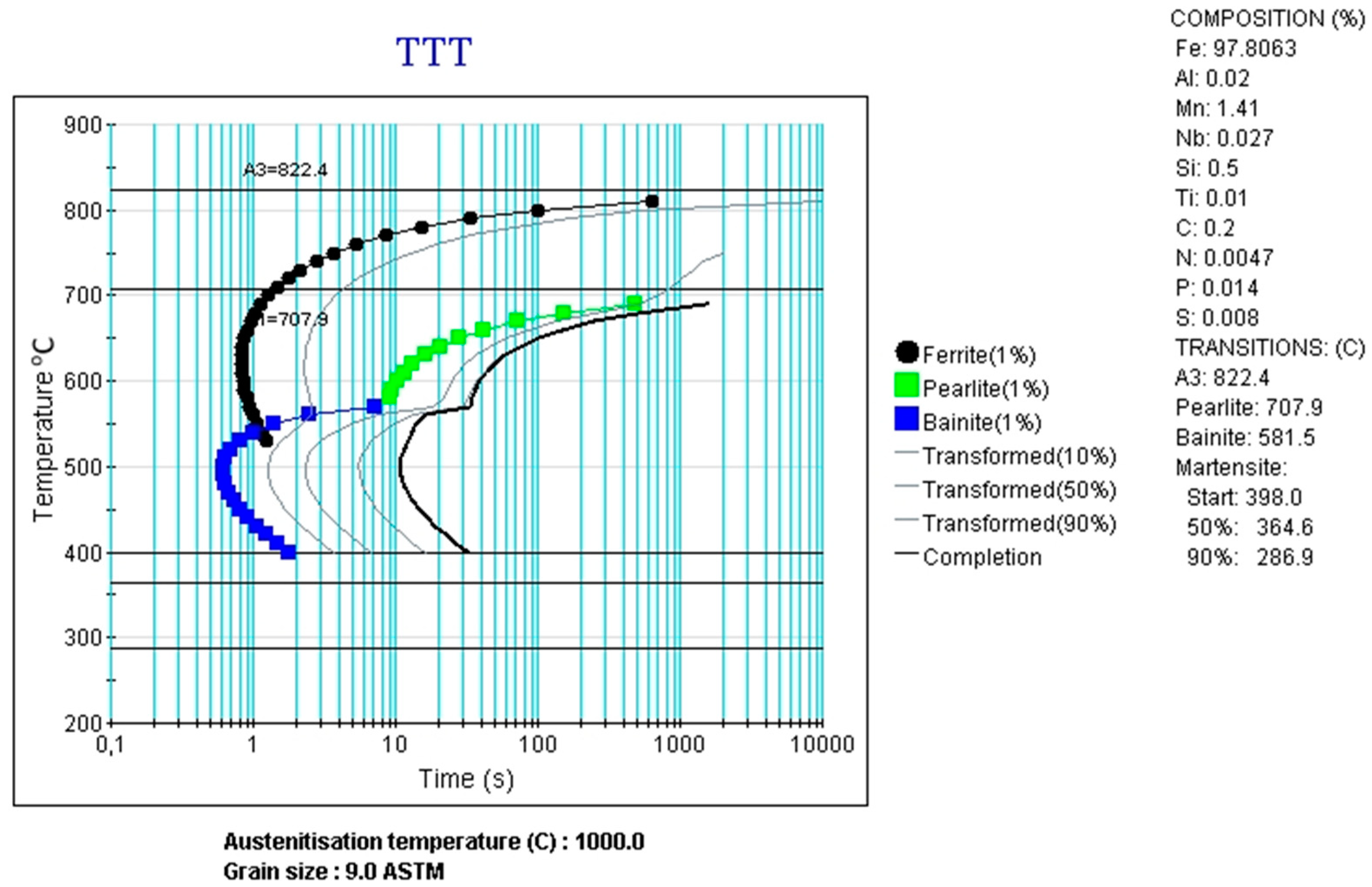
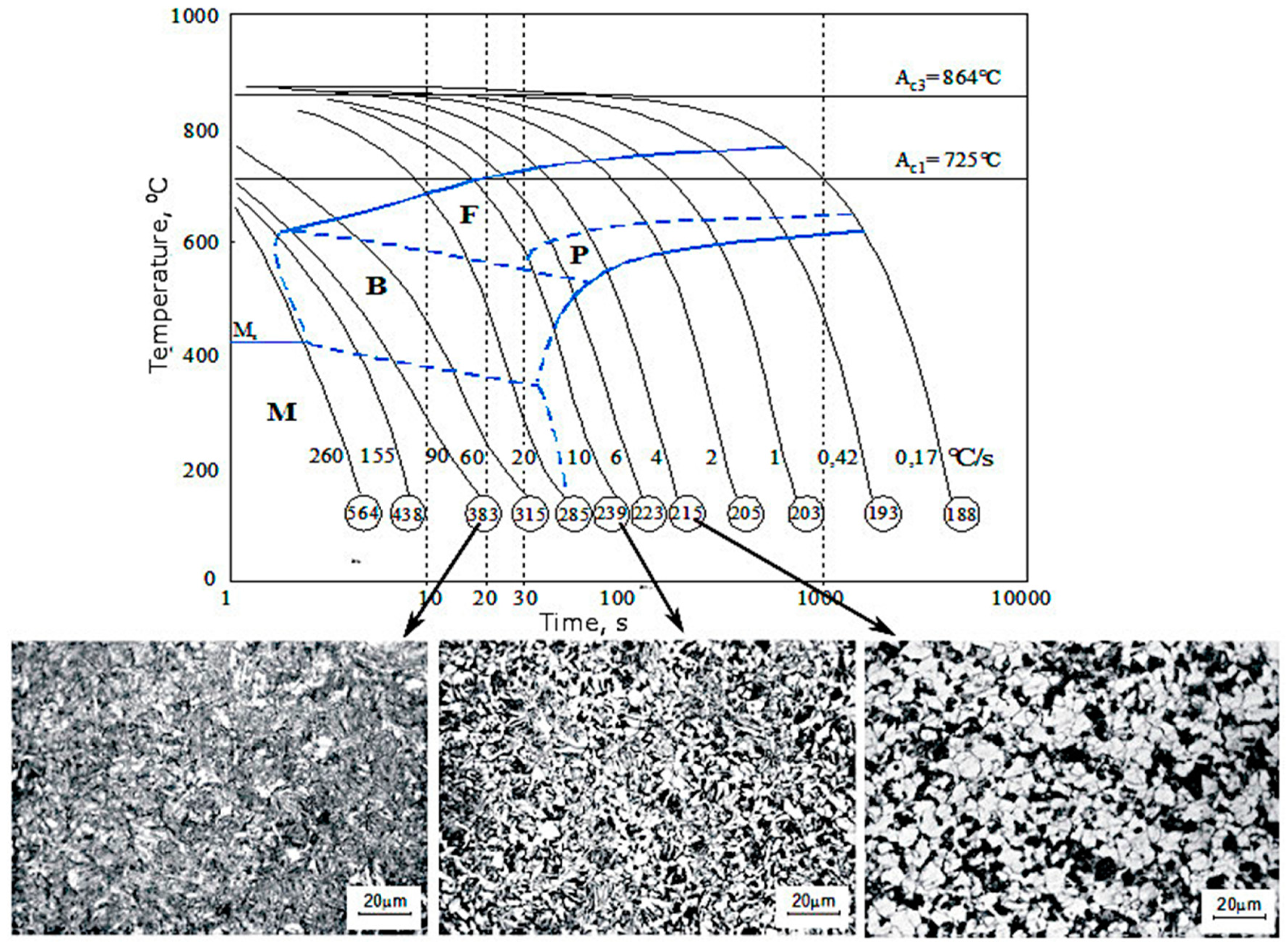
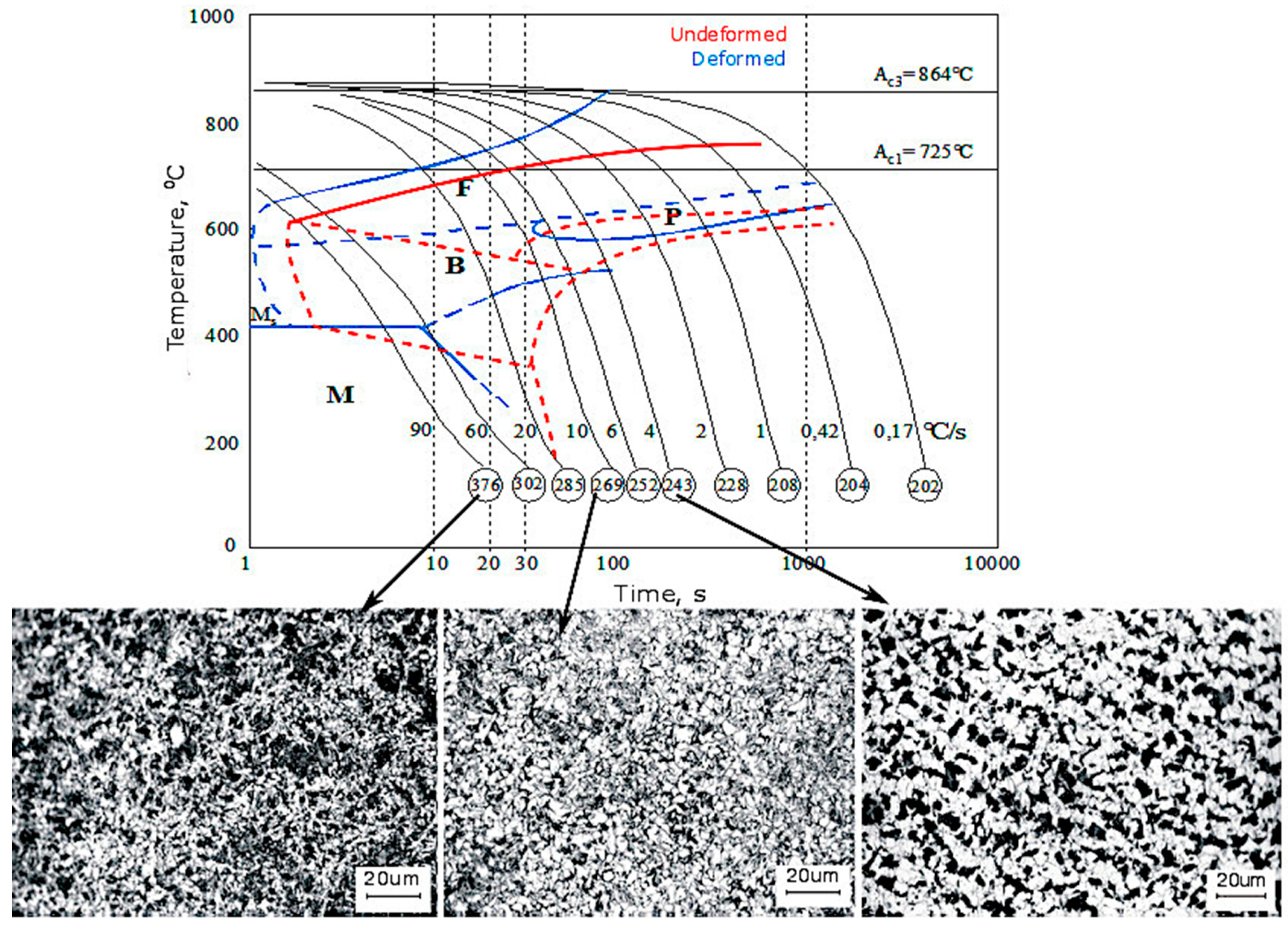
3.3. CCT and DCCT Diagrams
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baker, T.N. Microalloyed steels. Ironmak. Steelmak. 2016, 43, 264–307. [Google Scholar] [CrossRef]
- Muszka, K.; Dymek, S.; Majta, J.; Hodgson, P. Microstructure and properties of a C-Mn steel subjected to heavy plastic deformation. Arch. Metall. Mater. 2010, 55, 641–645. [Google Scholar]
- Smith, D.; Joris, O.P.J.; Sankaran, A.; Weekes, H.E.; Bull, D.J.; Prior, T.J.; Dye, D.; Errandonea, D.; Proctor, J.E. On the high-pressure phase stability and elastic properties of β-titanium alloys. J. Phys. Condens. Matter 2017, 29, 155401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, L.; Fu, R.Y.; Krizan, D.; De Cooman, B.C. Continuous cooling transformation diagrams and properties of micro-alloyed TRIP steels. Mater. Sci. Eng. A 2006, 438–440, 296–299. [Google Scholar] [CrossRef]
- Grajcar, A.; Różański, M.; Kamińska, M.; Grzegorczyk, B. Effect of gas atmosphere on the non-metallic inclusions in laser-welded TRIP steel with Al and Si additions. Mater. Technol. 2016, 50, 945–950. [Google Scholar] [CrossRef]
- Pereloma, E.V.; Al-Harbi, F.; Gazder, A.A. The crystallography of carbide-free bainites in thermo-mechanically processed low Si transformation-induced plasticity steels. J. Alloys Compd. 2014, 615, 96–110. [Google Scholar] [CrossRef]
- Kaijalainen, A.; Vahakuopus, N.; Somani, M.; Mehtonen, S.; Porter, D.; Komi, J. The effects of finish rolling temperature and niobium microalloying on the microstructure and properties of a direct quenched high-strength steel. Arch. Metall. Mater. 2017, 62, 619–626. [Google Scholar] [CrossRef]
- Grajcar, A.; Radwański, K. Microstructural comparison of the thermomechanically treated and cold deformed Nb-microalloyed TRIP steel. Mater. Technol. 2014, 48, 679–683. [Google Scholar]
- Caballero, F.B.; Roelofs, H.; Hasler, S.; Capadevila, C.; Chao, J.; Cornide, J.; Garcia-Mateo, C. Influence of bainite morphology on impact toughness of continuously cooled cementite free bainitic steels. Mater. Sci. Technol. 2012, 28, 95–102. [Google Scholar] [CrossRef]
- Ranjan, R.; Beladi, H.; Singh, S.B.; Hodgson, P.D. Thermo-mechanical processing of TRIP-aided steels. Metall. Mater. Trans. A 2015, 46, 3232–3247. [Google Scholar] [CrossRef]
- Adamczyk, J.; Grajcar, A. Structure and mechanical properties of DP-type and TRIP-type sheets obtained after the thermomechanical processing. J. Mater. Process. Technol. 2005, 162–163, 267–274. [Google Scholar] [CrossRef]
- Tsukatani, I.; Hashimoto, S.; Inoue, T. Effects of silicon and manganese addition on mechanical properties of high-strength hot-rolled sheet steel containing retained austenite. ISIJ Int. 1991, 31, 992–1000. [Google Scholar] [CrossRef]
- Kawulok, R.; Schindler, I.; Kawulok, P.; Rusz, S.; Opela, P.; Kliber, J.; Solowski, Z.; Cmiel, K.M.; Podolinsky, P.; Malis, M.; et al. Transformation kinetics of selected steel grades after plastic deformation. Metalurgija 2016, 55, 357–360. [Google Scholar]
- Grajcar, A. Thermodynamic analysis of precipitation processes in Nb-Ti-microalloyed Si-Al TRIP steel. J. Therm. Anal. Calorim. 2014, 118, 1011–1020. [Google Scholar] [CrossRef]
- De Ardo, A.J.; Garcia, J.E.; Hua, M.; Garcia, C.I. A new frontier in microalloying: Advanced high strength, coated sheet steels. Mater. Sci. Forum 2005, 500–501, 27–38. [Google Scholar] [CrossRef]
- Nasr El-Din, H. Effect of cold deformation and multiphase treatment conditions on the characterization of low-carbon, low-silicon multiphase steel. Steel Grips 2005, 3, 196–205. [Google Scholar]
- Radwanski, A. Structural characterization of low-carbon multiphase steels merging advanced research methods with light optical microscopy. Arch. Civ. Mech. Eng. 2016, 16, 282–293. [Google Scholar] [CrossRef]
- Opiela, M. Thermodynamic analysis of the precipitation of carbonitrides in microalloyed steels. Mater. Technol. 2015, 49, 395–401. [Google Scholar] [CrossRef]
- Kurc-Lisiecka, A.; Lisiecki, A. Laser welding of the new grade of advanced high-strength steel Domex 960. Mater. Technol. 2017, 51, 199–204. [Google Scholar]
- Opiela, M.; Grajcar, A. Hot deformation behavior and softening kinetics of Ti-V-B microalloyed steels. Arch. Civ. Mech. Eng. 2012, 12, 327–333. [Google Scholar] [CrossRef]
- Kucerova, L.; Opatova, K.; Kana, J.; Jirkova, H. High versatility of niobium alloyed AHSS. Arch. Metall. Mater. 2017, 62, 1485–1491. [Google Scholar] [CrossRef]
- Uranga, P.; Lopez, B.; Rodriguez-Ibabe, J.M. Microstructural modelling of Nb microalloyed steels during thin slab direct rolling processing. Steel Res. Int. 2007, 78, 199–209. [Google Scholar] [CrossRef]
- Krizan, D.; Spiradek-Hahn, K.; Pichler, A. Relationship between microstructure and mechanical properties in Nb-V microalloyed TRIP steel. Mater. Sci. Technol. 2015, 31, 661–668. [Google Scholar] [CrossRef]
- Gladman, T. The Physical Metallurgy of Microalloyed Steels; The Institute of Materials, University Press: Cambridge, UK, 1997. [Google Scholar]
- Jung, J.; Lee, S.J.; Kim, S.; De Cooman, B.C. Effect of Ti additions on micro-alloyed Nb TRIP steel. Steel Res. Int. 2011, 82, 857–865. [Google Scholar] [CrossRef]
- Irvine, K.J.; Pickering, F.B.; Gladman, T. Grain refined C-Mn steels. J. Iron Steel Inst. 1967, 205, 161–182. [Google Scholar]
- Gorka, J. Microstructure and properties of the high-temperature HAZ of thermo-mechanically treated S700MC high-yield-strength steel. Mater. Technol. 2016, 50, 617–621. [Google Scholar] [CrossRef]
- Jimenez-Melero, E.; van Dijk, N.H.; Zhao, L.; Sietsma, J.; Offerman, S.E.; Wright, J.P.; van der Zwaag, S. Martensitic transformation of individual grains in low-alloyed TRIP steels. Scr. Mater. 2007, 56, 421–424. [Google Scholar] [CrossRef]
- Siciliano, F.; Jonas, J.J. Mathematical modeling of the hot strip rolling of microalloyed Nb, multiply-alloyed Cr-Mo, and plain C-Mn steels. Metall. Mater. Trans. A 2000, 31A, 511–530. [Google Scholar] [CrossRef]
- Siciliano, F.; Poliak, E.I. Modeling of the resistance to hot deformation and the effects of microalloying in high-Al steels under industrial conditions. Mater. Sci. Forum 2005, 500–501, 195–202. [Google Scholar] [CrossRef]
- Nowotnik, A.; Siwecki, T. The effect of TMCP parameters on the microstructure and mechanical properties of Ti-Nb microalloyed steel. J. Microsc. 2010, 237, 258–262. [Google Scholar] [CrossRef]
- Saunders, N.; Guo, Z.; Li, X.; Miodownik, A.P.; Schille, J.P. Using JMatPro to model materials properties and behavior. JOM 2003, 55, 60–65. [Google Scholar] [CrossRef]


| C | Mn | Si | Al | S | P | Nb | Ti | N |
|---|---|---|---|---|---|---|---|---|
| 0.2 ± 0.01 | 1.41 ± 0.03 | 0.50 ± 0.03 | 0.020 ± 0.002 | 0.008 ± 0.001 | 0.014 ± 0.002 | 0.027 ± 0.001 | 0.010 ± 0.001 | 0.0047 ± 0.0002 |
| Temperature [K] | Log ks | ks |
|---|---|---|
| 1423 | −5.30193 | 0.00000499 |
| 1373 | −5.50666 | 0.00000311 |
| 1323 | −5.72686 | 0.00000188 |
| 1273 | −5.96437 | 0.00000109 |
| 1223 | −6.22129 | 0.00000060 |
| 1173 | −6.50012 | 0.00000032 |
| 1123 | −6.80378 | 0.00000016 |
| Temperature [K] | Log ks | ks |
|---|---|---|
| 1423 | −2.43025 | 0.003713 |
| 1373 | −2.60105 | 0.002506 |
| 1323 | −2.78476 | 0.001641 |
| 1273 | −2.98291 | 0.00104 |
| 1223 | −3.19725 | 0.000635 |
| 1173 | −3.42987 | 0.000372 |
| 1123 | −3.68321 | 0.000207 |
| Temperature | Log ks | ks | [Ti], mass% | [N], mass% | (TiN), mass% | |
|---|---|---|---|---|---|---|
| [°C] | [K] | |||||
| 1150 | 1423 | −5.30193 | 0.00000499 | 0.00200 | 0.002386 | 0.010314 |
| 1100 | 1373 | −5.50666 | 0.00000311 | 0.00140 | 0.002191 | 0.011109 |
| 1050 | 1323 | −5.72686 | 0.00000188 | 0.00092 | 0.002043 | 0.011739 |
| 1000 | 1273 | −5.96437 | 0.00000109 | 0.00056 | 0.001939 | 0.012201 |
| 950 | 1223 | −6.22129 | 0.00000060 | 0.000321 | 0.001869 | 0.012510 |
| 900 | 1173 | −6.50012 | 0.00000032 | 0.000173 | 0.001825 | 0.012702 |
| 850 | 1123 | −6.80378 | 0.00000016 | 0.0000086 | 0.001800 | 0.012891 |
| Temperature | Log ks | ks | [Nb], mass% | [C,N], mass% | (NbCN), mass% | |
|---|---|---|---|---|---|---|
| [°C] | [K] | |||||
| 1150 | 1423 | −2.43025 | 0.003713 | 0.0390 | 0.211679 | 0 |
| 1100 | 1373 | −2.60105 | 0.002506 | 0.0250 | 0.208265 | 0.003735 |
| 1050 | 1323 | −2.78476 | 0.001641 | 0.0164 | 0.205846 | 0.015390 |
| 1000 | 1273 | −2.98291 | 0.001040 | 0.0100 | 0.204198 | 0.022802 |
| 950 | 1223 | −3.19725 | 0.000635 | 0.0059 | 0.203120 | 0.027980 |
| 900 | 1173 | −3.42987 | 0.000372 | 0.0033 | 0.202447 | 0.031253 |
| 850 | 1123 | −3.68321 | 0.000207 | 0.00175 | 0.202046 | 0.033204 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grajcar, A.; Morawiec, M.; Zalecki, W. Austenite Decomposition and Precipitation Behavior of Plastically Deformed Low-Si Microalloyed Steel. Metals 2018, 8, 1028. https://doi.org/10.3390/met8121028
Grajcar A, Morawiec M, Zalecki W. Austenite Decomposition and Precipitation Behavior of Plastically Deformed Low-Si Microalloyed Steel. Metals. 2018; 8(12):1028. https://doi.org/10.3390/met8121028
Chicago/Turabian StyleGrajcar, Adam, Mateusz Morawiec, and Wladyslaw Zalecki. 2018. "Austenite Decomposition and Precipitation Behavior of Plastically Deformed Low-Si Microalloyed Steel" Metals 8, no. 12: 1028. https://doi.org/10.3390/met8121028
APA StyleGrajcar, A., Morawiec, M., & Zalecki, W. (2018). Austenite Decomposition and Precipitation Behavior of Plastically Deformed Low-Si Microalloyed Steel. Metals, 8(12), 1028. https://doi.org/10.3390/met8121028





