Interfacial Reactions and Fracture Behavior of Ti Alloy-Ag28Cu Brazing Joints: Influence of Titanium Alloy Composition
Abstract
1. Introduction
- (1)
- Dissolution of Ti and alloying elements in the solder alloy
- (2)
- Diffusion of Cu from the solder into the base material
- (3)
- Formation of Ti-Cu-rich intermetallic phases
- (4)
- Depletion of Cu in the melt, especially in the vicinity of the base material
- (5)
- Isothermal solidification of Ag as a consequence of Cu depletion
- (6)
- Precipitation of intermetallic phases from the residual melt
- (7)
- Eutectoid β-Ti → α-Ti + Ti2Cu reaction in the Cu-enriched diffusion zone
2. Materials and Methods
3. Results
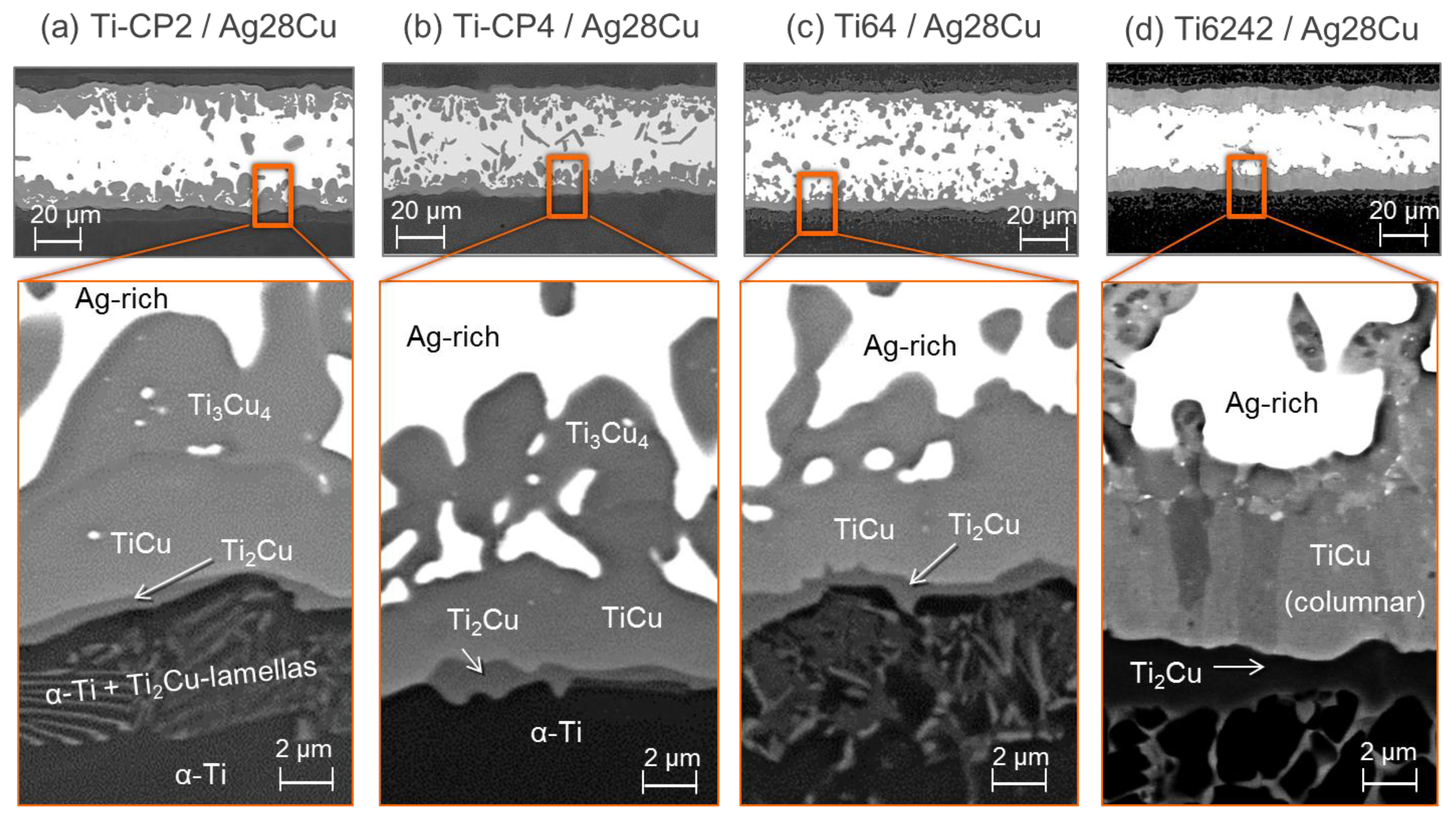
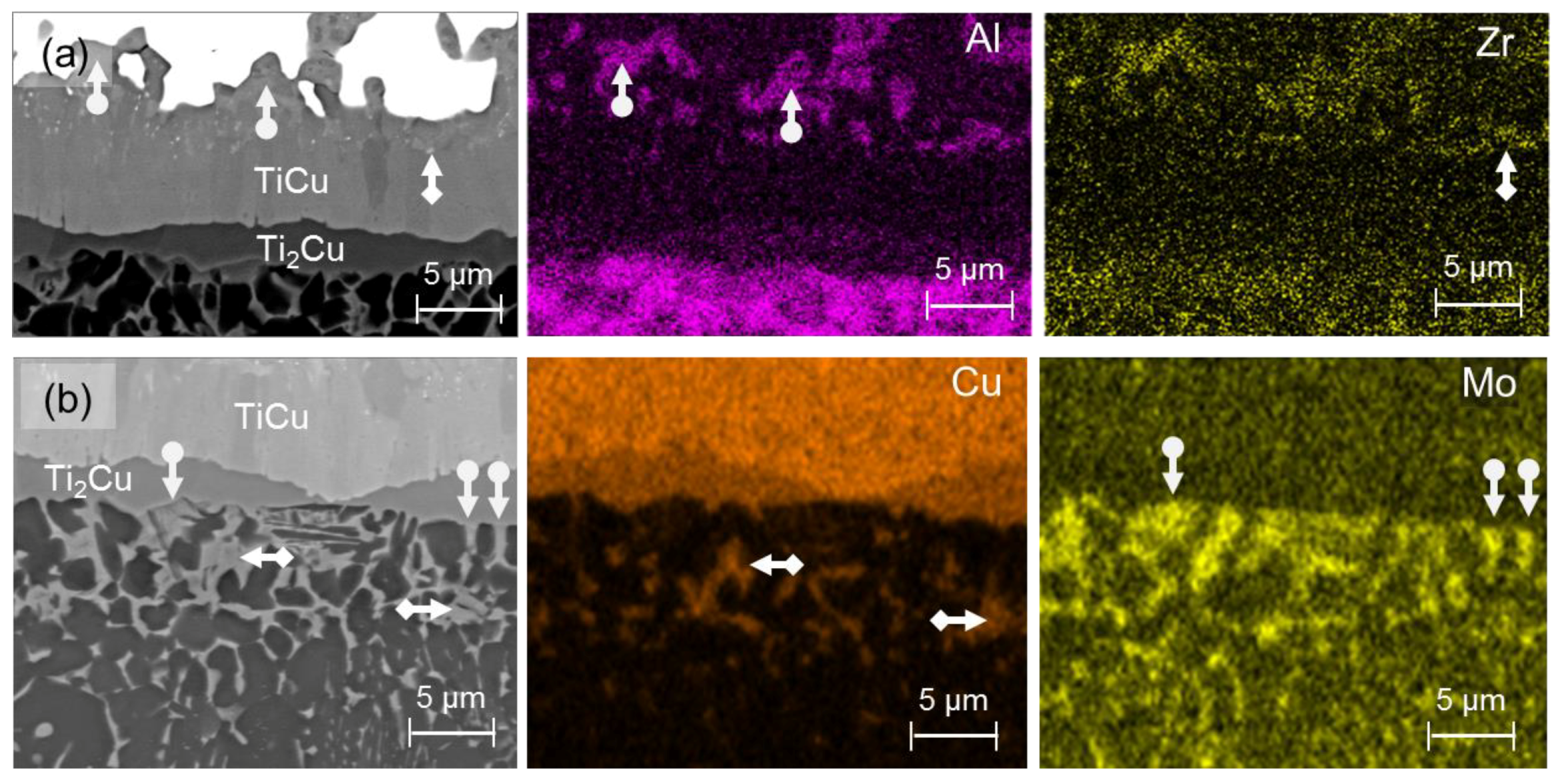
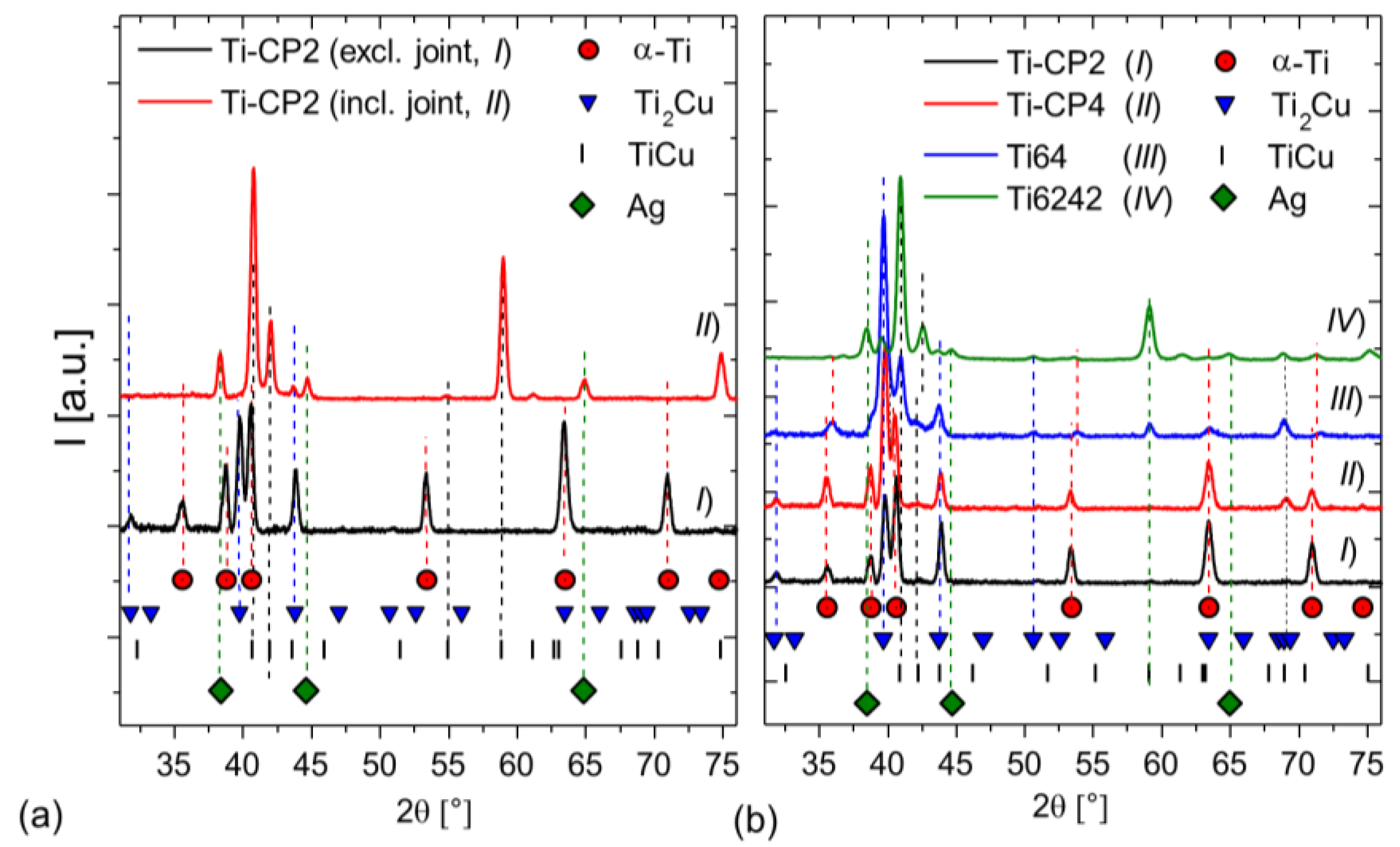
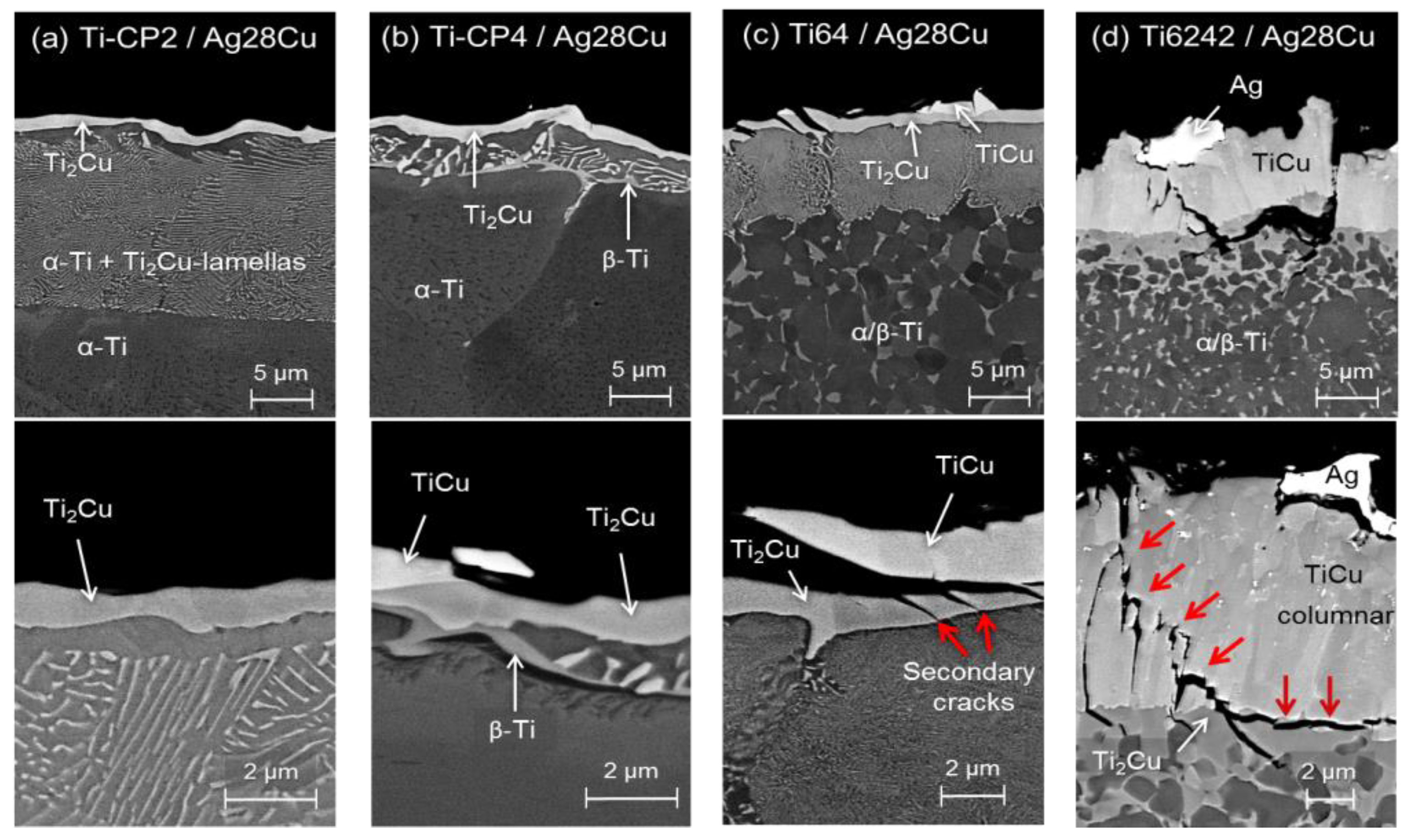
3.1. Microstructure of the Brazing Joints
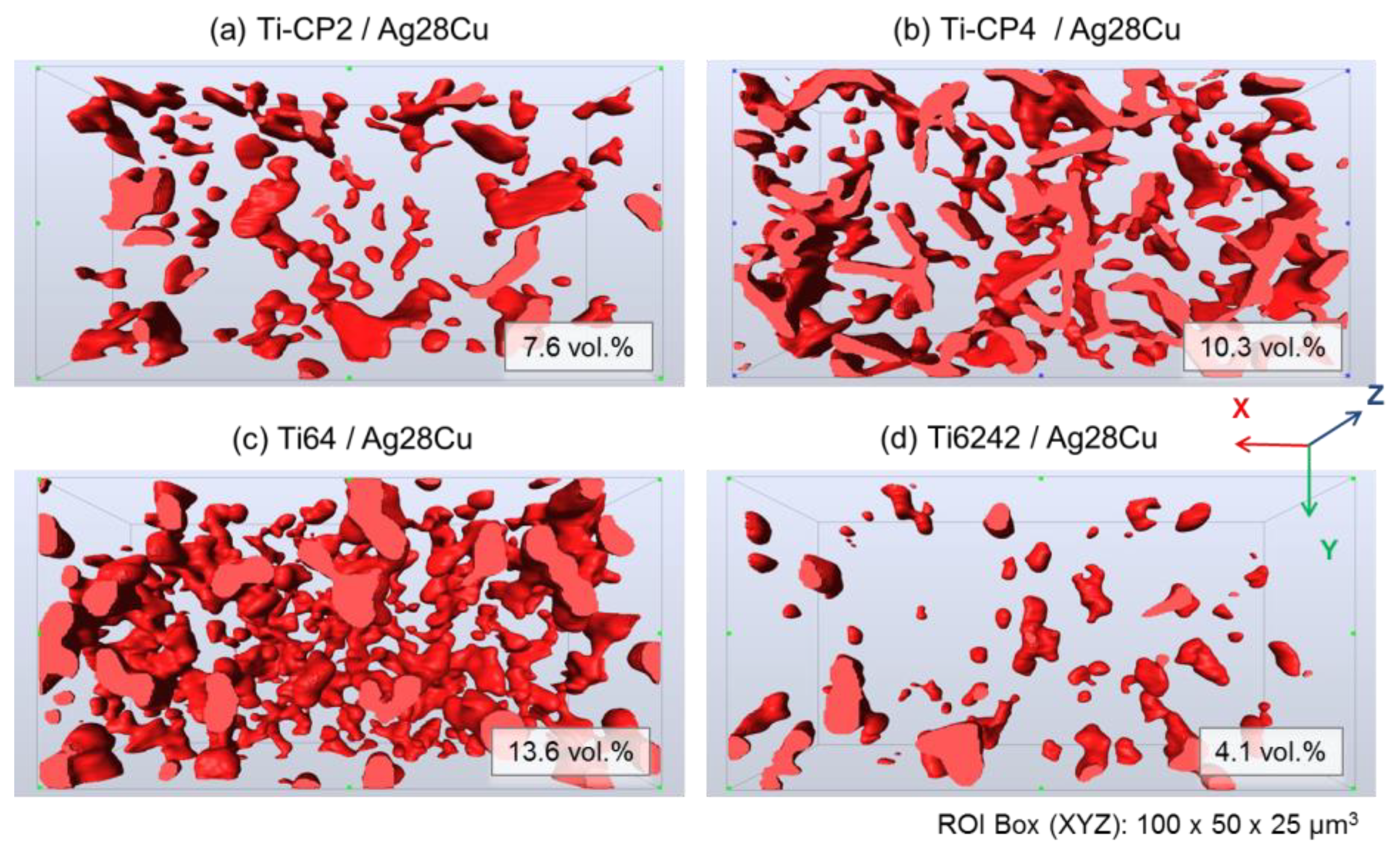
3.2. Characterization of Joints by Synchrotron X-Ray Microtomography (SXCT)
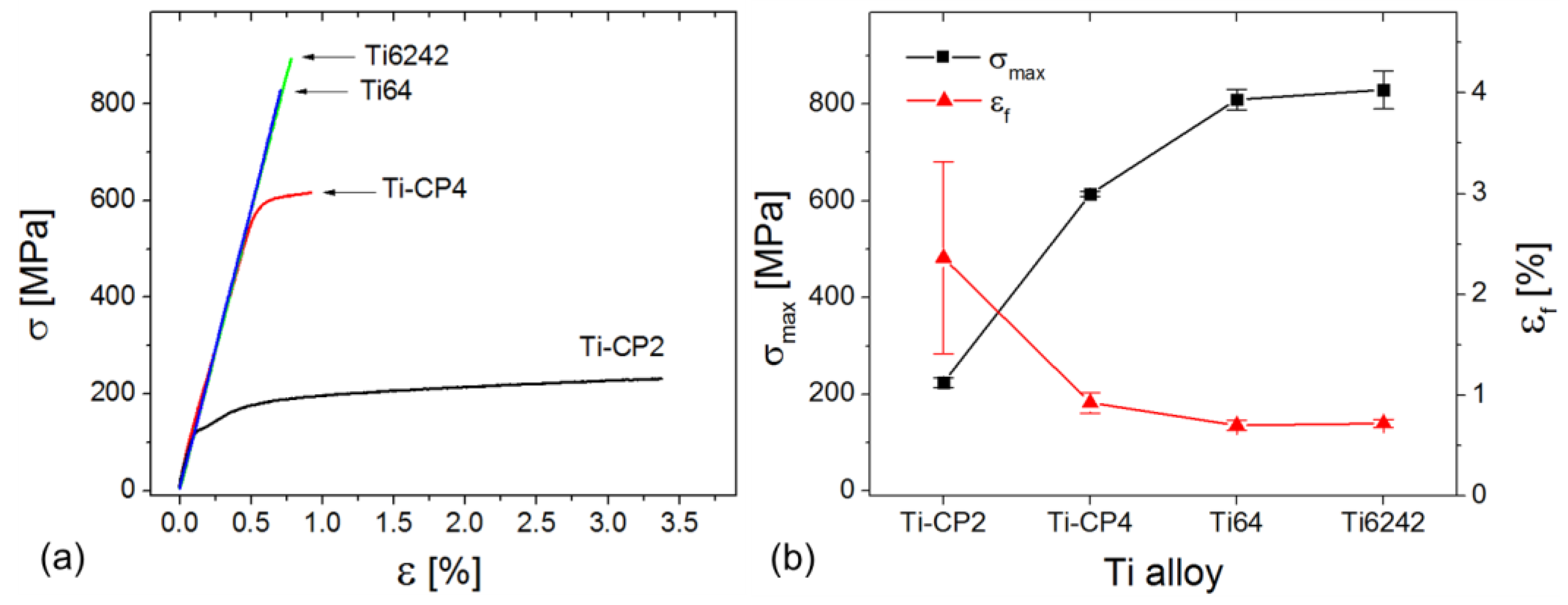
3.3. Tensile Tests
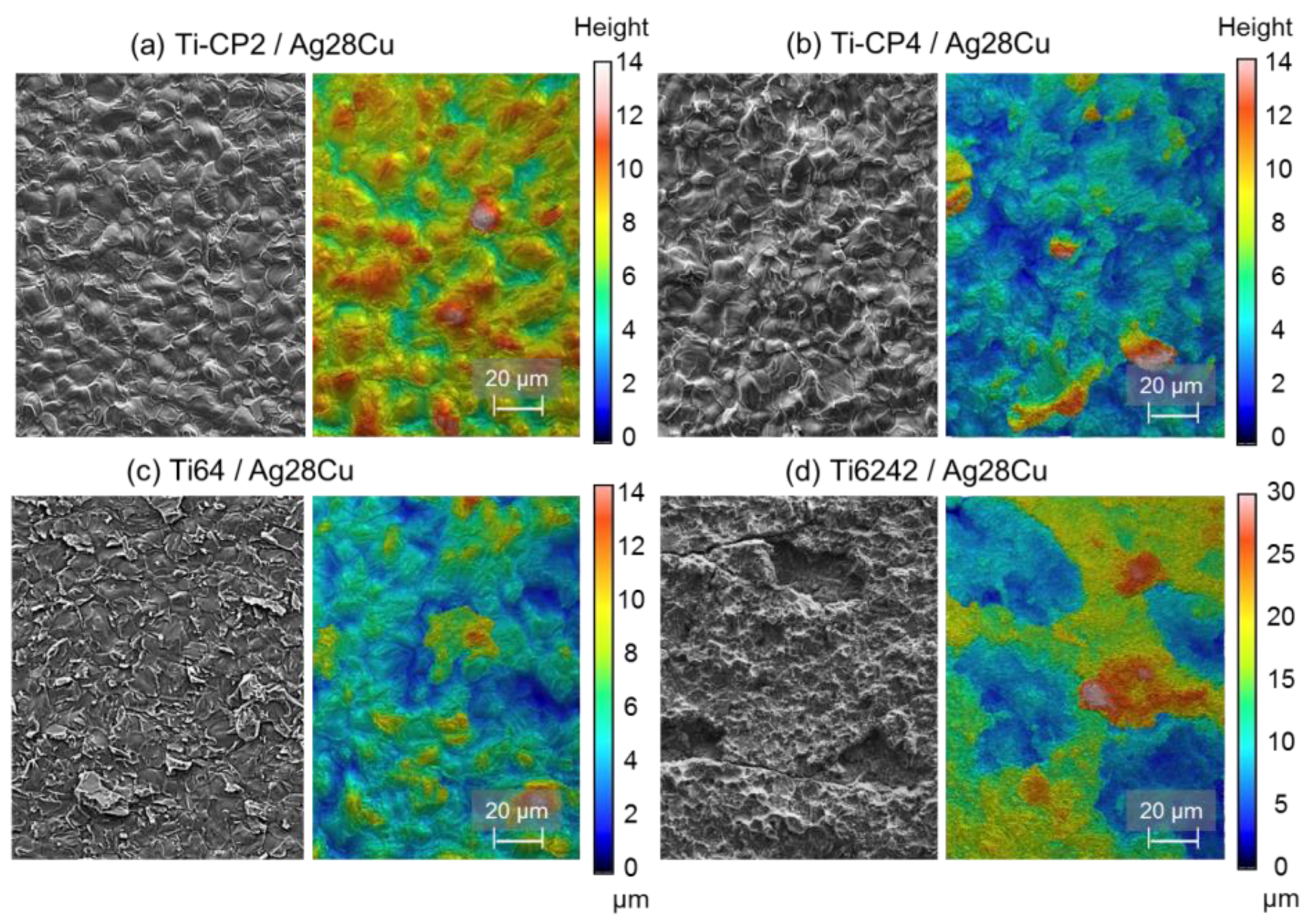
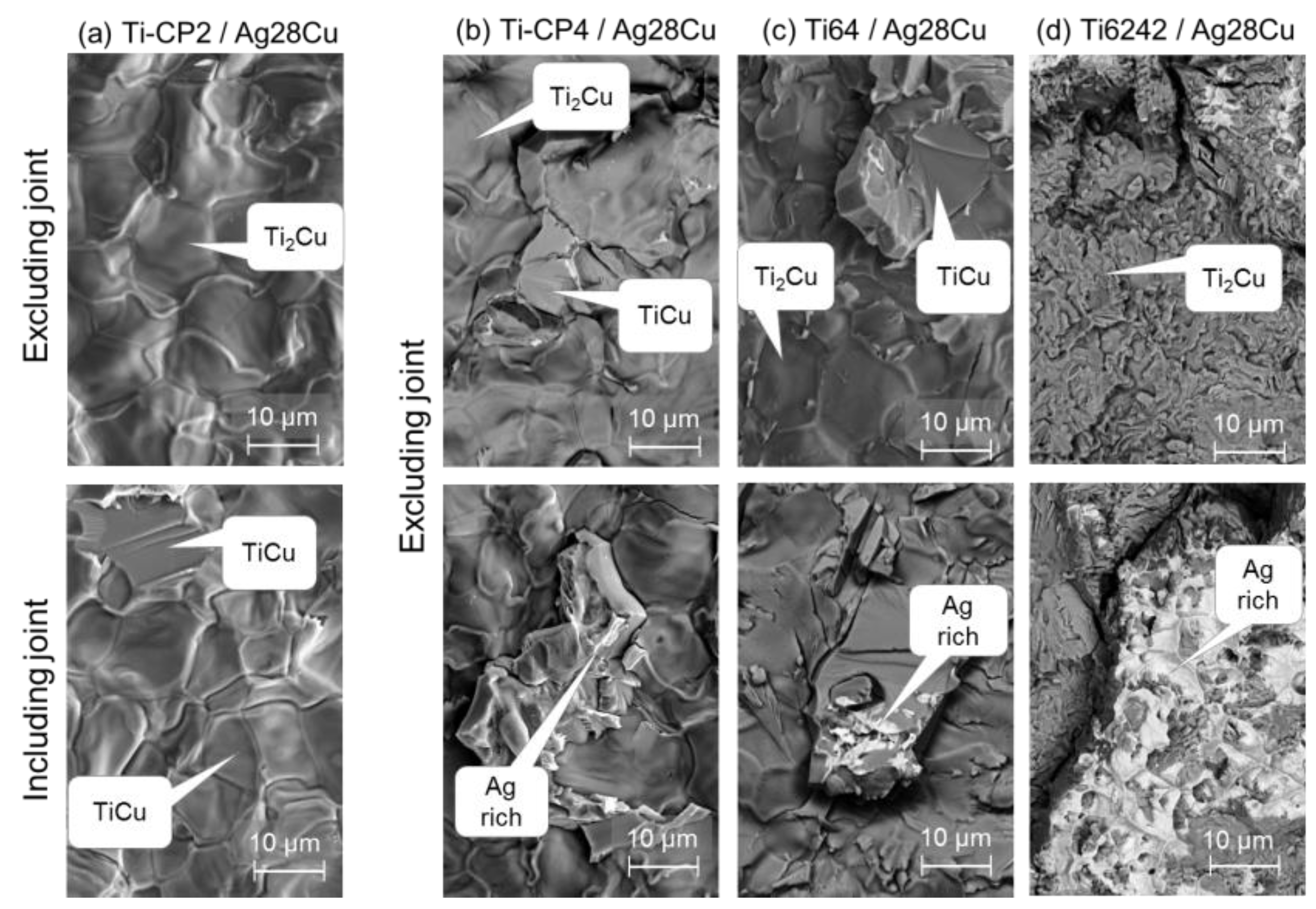
3.4. Fractography
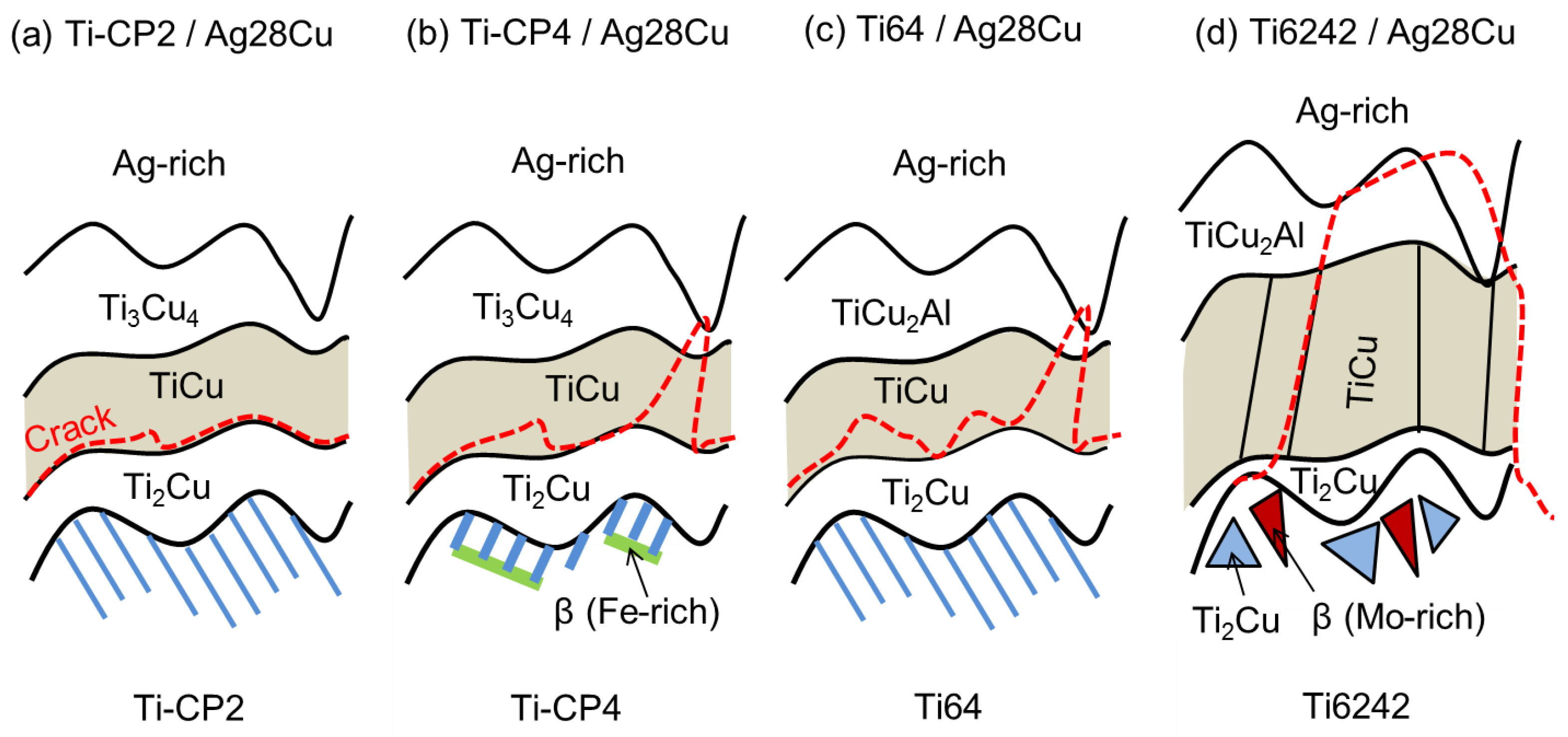
4. Discussion
4.1. Brazing Reaction Products
4.2. Strength and Failure Mechanisms of the Joints
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kocian, F.; Ebel, P.-B.; Drees, B.; Schulze, K.; Hausmann, J.; Voggenreiter, H. Hybrid structures in aero engines. CEAS Aeronaut. J. 2015, 6, 217–228. [Google Scholar] [CrossRef]
- Sandin, T. What´s happening with aerospace brazing. Weld. J. 2013, 92, 56–58. [Google Scholar]
- Laik, A.; Shirzadi, A.A.; Sharma, G.; Tewari, R.; Jayakumar, T.; Dey, G.K. Microstructure and Interfacial Reactions During Vacuum Brazing of Stainless Steel to Titanium Using Ag-28 pct Cu Alloy. Metall. Mater. Trans. A 2015, 46, 771–782. [Google Scholar] [CrossRef]
- Lee, J.G.; Lee, M.-K. Microstructure and mechanical behavior of a titanium-to-stainless steel dissimilar joint brazed with Ag-Cu alloy filler and an Ag interlayer. Mater. Charact. 2017, 129, 98–103. [Google Scholar] [CrossRef]
- Shapiro, A.; Rabinkin, A. State of the Art of Titanium-based Brazing Filler Metals. Weld. J. 2003, 82, 36–43. [Google Scholar]
- Raghavan, V. Ag-Al-Cu (Silver-Aluminum-Copper). J. Phase Equilib. 2008, 29, 256–258. [Google Scholar] [CrossRef]
- Shiue, R.K.; Wu, S.K.; Chan, C.H. The interfacial reactions of infrared brazing Cu and Ti with two silver-based braze alloys. J. Alloys Compd. 2004, 372, 148–157. [Google Scholar] [CrossRef]
- Andrieux, J.; Dezellus, O.; Bosselet, F.; Sacerdote-Peronnet, M.; Sigala, C.; Chiriac, R.; Viala, J.C. Details on the Formation of Ti2Cu3 in the Ag-Cu-Ti System in the Temperature Range 790 to 860 °C. J. Phase Equilib. Diffus. 2008, 29, 156–162. [Google Scholar] [CrossRef]
- Andrieux, J.; Dezellus, O.; Bosselet, F.; Viala, J. Low-Temperature Interface Reaction Between Titanium and the Eutectic Silver-Copper Brazing Alloy. J. Phase Equilib. Diffus. 2009, 30, 40–45. [Google Scholar] [CrossRef]
- Laik, A.; Shirzadi, A.A.; Tewari, R.; Kumar, A.; Jayakumar, T.; Dey, G.K. Microstructure and Interfacial Reactions During Active Metal Brazing of Stainless Steel to Titanium. Metall. Mater. Trans. A 2013, 44, 2212–2225. [Google Scholar] [CrossRef]
- Gussone, J.; Reinhard, C.; Kasperovich, G.; Gherekhloo, H.; Merzouk, T.; Hausmann, J. In-situ investigation of microcrack formation and strains in Ag–Cu-based multi-metal matrix composites analysed by synchrotron radiation. Mater. Sci. Eng. A 2014, 612, 102–114. [Google Scholar] [CrossRef]
- Leyens, C.; Hausmann, J.; Kumpfert, J. Continuous Fiber Reinforced Titanium Matrix Composites: Fabrication, Properties, and Applications. Adv. Eng. Mater. 2003, 5, 399–410. [Google Scholar] [CrossRef]
- Donachie, M.J. Titanium: A Technical Guide, 2nd ed.; ASM International: Materials Park, OH, USA, 2000; pp. 1–381. [Google Scholar]
- Kubaschewski, O. Ag-Cu-Ti (Silver-Copper-Titanium). In Ternary Alloy Systems. Non-Ferrous Metal Systems. Part 3: Selected Soldering and Brazing Systems; Effenberg, G., Ilyenko, S., Eds.; Springer: Berlin, Germany, 2007; Volume 11, pp. 63–74. [Google Scholar]
- Murray, J.L. The Cu−Ti (Copper-Titanium) system. Bull. Alloy Phase Diagr. 1983, 4, 81–95. [Google Scholar] [CrossRef]
- Okamoto, H. Cu-Ti (Copper-Titanium). J. Phase Equilib. 2002, 23, 549–550. [Google Scholar] [CrossRef]
- Liu, X.J.; Wang, C.P.; Ohnuma, I.; Kainuma, R.; Ishida, K. Phase equilibria and phase transformation of the body-centered cubic phase in the Cu-rich portion of the Cu–Ti–Al system. J. Mater. Res. 2008, 23, 2674–2684. [Google Scholar] [CrossRef]
- Schmid-Fetzer, R. Al-Cu-Ti (Aluminium-Copper-Titanium). In Ternary Alloy Systems. Light Metal Systems. Part 2: Selected Systems from Al-Cu-Fe to Al-Fe-Ti; Effenberg, G., Ilyenko, S., Eds.; Springer: Berlin, Germany, 2005; Volume 11, pp. 156–173. [Google Scholar]
- Boyer, R.; Welsch, G.; Collings, E.W. Materials Properties Handbook: Titanium Alloys; ASM International: Materials Park, OH, USA, 1994; pp. 1–1169. [Google Scholar]
- Leyens, C.; Peters, M. Titanium and Titanium Alloys: Fundamentals and Applications; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Turchanin, M.A.; Velikanova, T.Y.; Agraval, P.G.; Abdulov, A.R.; Dreval’, L.A. Thermodynamic assessment of the Cu-Ti-Zr system. III. Cu-Ti-Zr system. Powder Metall. Met. Ceram. 2008, 47, 586–606. [Google Scholar] [CrossRef]
- Storchak-Fedyuk, A.M.; Artyukh, L.V.; Grytsiv, A.V.; Agraval, P.G.; Turchanin, M.A.; Velikanova, T.Y. Phase Equilibria in the Cu–Ti–Zr System at 750°C. II. The Isothermal Section with Copper Content from 50 to 100 at.%. Powder Metall. Met. Ceram. 2017, 56, 220–230. [Google Scholar] [CrossRef]
- Wang, C.P.; Liu, H.; Yang, M.J.; Jiang, H.X.; Zhang, H.Y.; Liu, X.J. Experimental Investigation of Phase Equilibria in the Cu–Mo–Ti Ternary System. J. Phase Equilib. 2018, 39, 35–43. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, Q.; Chen, Z.; Wei, C.; Cai, G.-M.; Jiang, L.; Jin, Z.; Zhao, J.-C. Measurement of interdiffusion and impurity diffusion coefficients in the bcc phase of the Ti–X (X = Cr, Hf, Mo, Nb, V, Zr) binary systems using diffusion multiples. J. Mater. Sci. 2017, 52, 3255–3268. [Google Scholar] [CrossRef]
- Bania, P.J. Beta titanium alloys and their role in the titanium industry. JOM 1994, 46, 16–19. [Google Scholar] [CrossRef]
- Technical Data Sheet UMICORE. Available online: http://technicalmaterials.umicore.com/Brazetec/en/datenblaetter/TD_BrazeTec_7200_EN.pdf (accessed on 24 September 2018).

| Alloy | Ti | Al | V | Sn | Zr | Mo | Si | N | C | H | Fe | O |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ti-CP2 | bal. | - | - | - | - | - | - | 0.03 | 0.08 | 0.02 | 0.30 | 0.25 |
| Ti-CP4 | bal. | - | - | - | - | - | - | 0.05 | 0.08 | 0.02 | 0.50 | 0.40 |
| Ti64 | bal. | 6.5 | 4.5 | - | - | - | - | 0.05 | 0.1 | 0.01 | 0.30 | 0.20 |
| Ti6242 | bal. | 6.5 | - | 2.25 | 4.5 | 2.25 | 0.01 | 0.05 | 0.05 | 0.01 | 0.25 | 0.15 |
| Ti-Alloy | Phase | Ti | Cu | Ag | Al | Mo | Zr | Sn |
|---|---|---|---|---|---|---|---|---|
| Ti-CP2 | Ti2Cu | 66.0 | 32.0 | 2.0 | - | - | - | - |
| TiCu | 49.2 | 47.6 | 3.2 | - | - | - | - | |
| Ti3Cu4 | 41.1 | 57 | 1.9 | - | - | - | - | |
| Ti-CP4 | Ti2Cu | 66.2 | 31.7 | 2.1 | - | - | - | - |
| TiCu | 49.1 | 48 | 2.9 | - | - | - | - | |
| Ti3Cu4 | 41.8 | 56.3 | 1.9 | - | - | - | - | |
| Ti64 | Ti2Cu | 62.6 | 30.8 | 2 | 4.6 | - | - | - |
| TiCu | 48.1 | 48 | 2.9 | 1 | - | - | - | |
| Ti3Cu4 | 41 | 57.1 | 1.9 | - | - | - | - | |
| TiCu2Al | 24.6 | 65.1 | 2 | 8.3 | - | - | - | |
| Ti6242 | Ti2Cu | 62.4 | 31.0 | 1.5 | 3.7 | - | 1.4 | - |
| TiCu | 49.2 | 43.3 | 4 | 1.3 | 0.4 | 0.9 | 0.9 | |
| TiCu2Al | 24.7 | 56.8 | 2.9 | 12.0 | - | 3.2 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gussone, J.; Kasperovich, G.; Haubrich, J.; Requena, G. Interfacial Reactions and Fracture Behavior of Ti Alloy-Ag28Cu Brazing Joints: Influence of Titanium Alloy Composition. Metals 2018, 8, 830. https://doi.org/10.3390/met8100830
Gussone J, Kasperovich G, Haubrich J, Requena G. Interfacial Reactions and Fracture Behavior of Ti Alloy-Ag28Cu Brazing Joints: Influence of Titanium Alloy Composition. Metals. 2018; 8(10):830. https://doi.org/10.3390/met8100830
Chicago/Turabian StyleGussone, Joachim, Galina Kasperovich, Jan Haubrich, and Guillermo Requena. 2018. "Interfacial Reactions and Fracture Behavior of Ti Alloy-Ag28Cu Brazing Joints: Influence of Titanium Alloy Composition" Metals 8, no. 10: 830. https://doi.org/10.3390/met8100830
APA StyleGussone, J., Kasperovich, G., Haubrich, J., & Requena, G. (2018). Interfacial Reactions and Fracture Behavior of Ti Alloy-Ag28Cu Brazing Joints: Influence of Titanium Alloy Composition. Metals, 8(10), 830. https://doi.org/10.3390/met8100830







