Corrosion Inhibition of C38 Steel in 1 M HCl Using Benzoxazole-2-Thione: Electrochemical, SEM-EDX, and Theoretical Studies
Abstract
1. Introduction
2. Materials and Methods
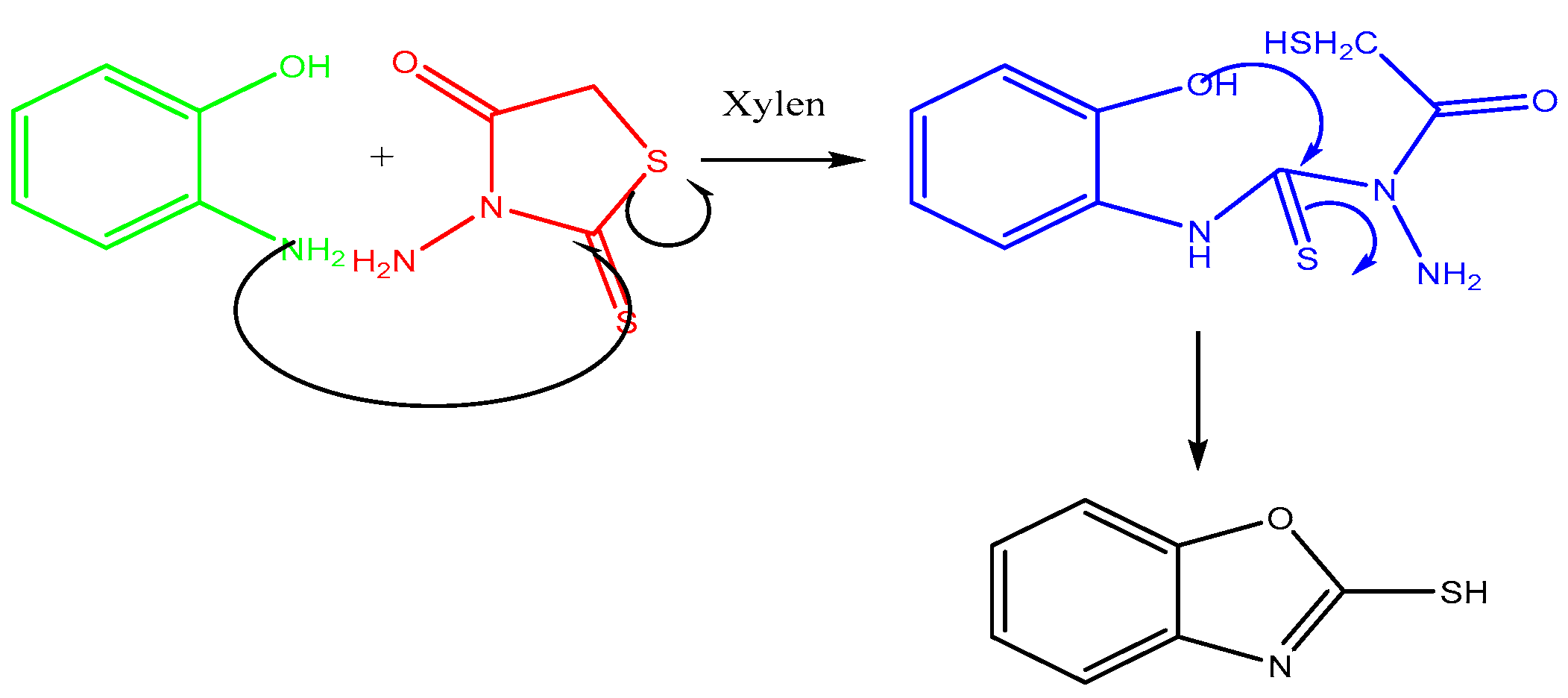
2.1. Benzoxazole-2-Thione Synthesis
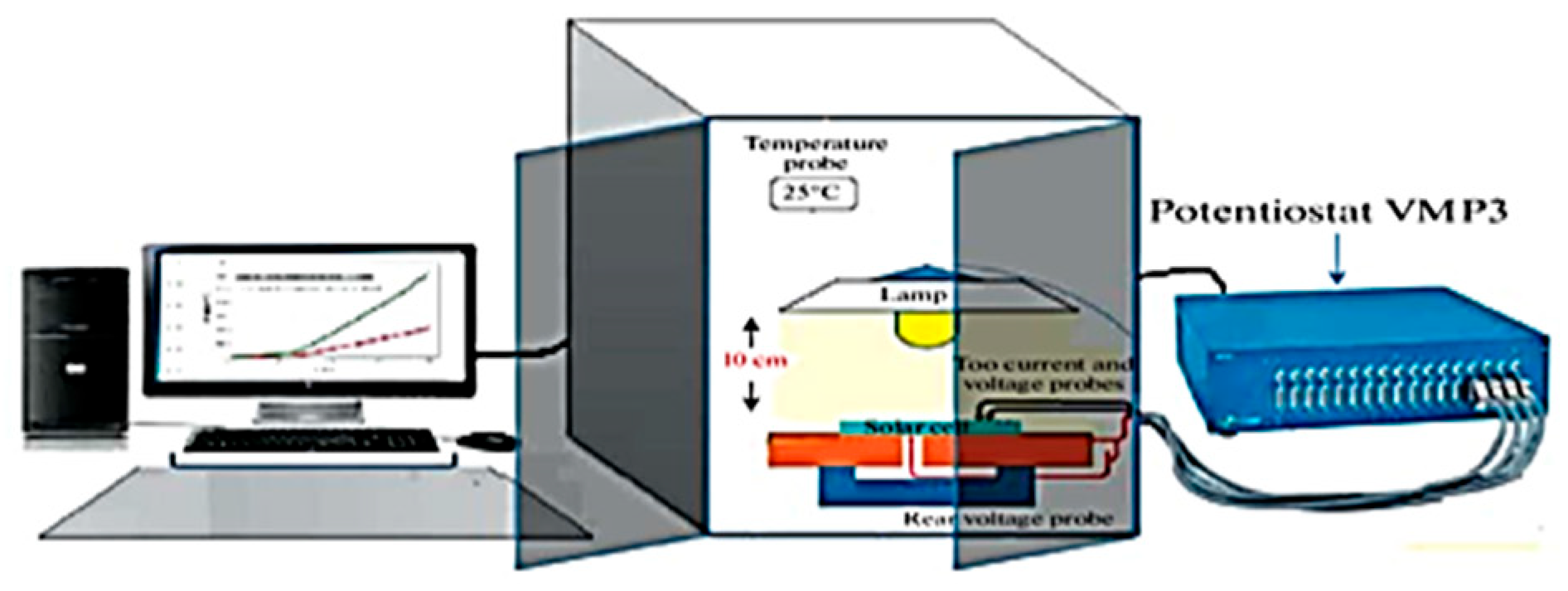
2.2. Electrochemical Tests
2.3. Density Functional Theory (DFT) Method
2.4. Molecular Dynamics and Monte Carlo Simulations
3. Results
3.1. Identification Methods: 1H-NMR (DMSO-d6/TMS) and 13C NMR
3.2. Concentration Effect
3.2.1. Polarization Measurements
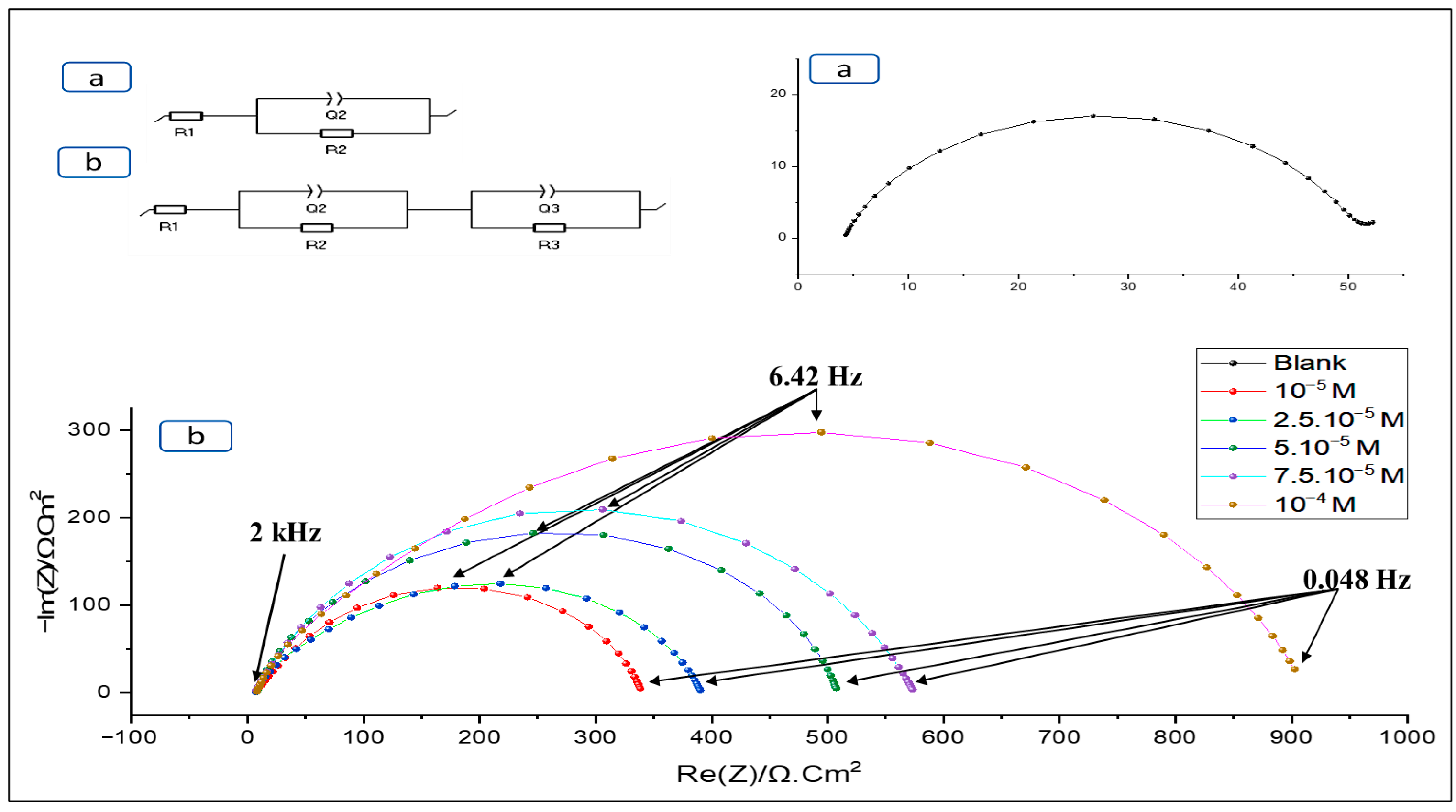
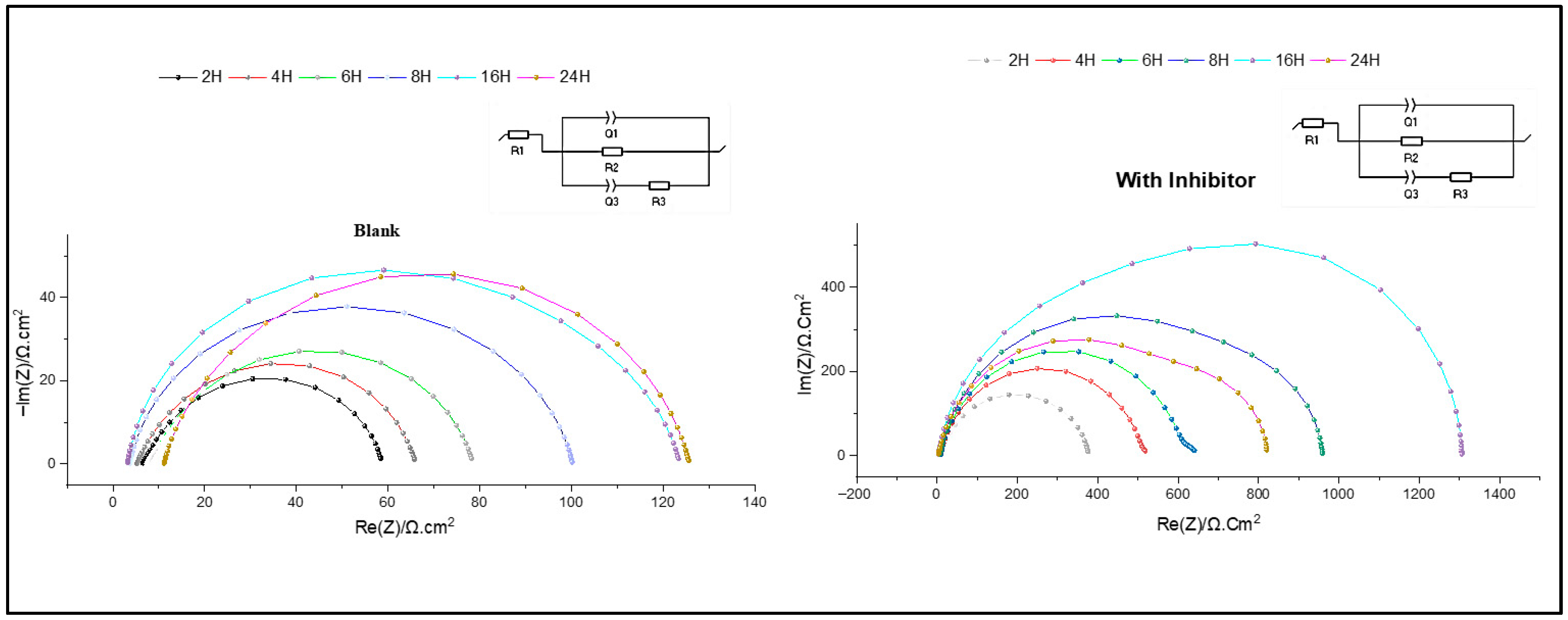
3.2.2. Electrochemical Impedance Spectroscopy EIS Method
3.3. Adsorption Isotherms
3.4. The Immersion Time Effect
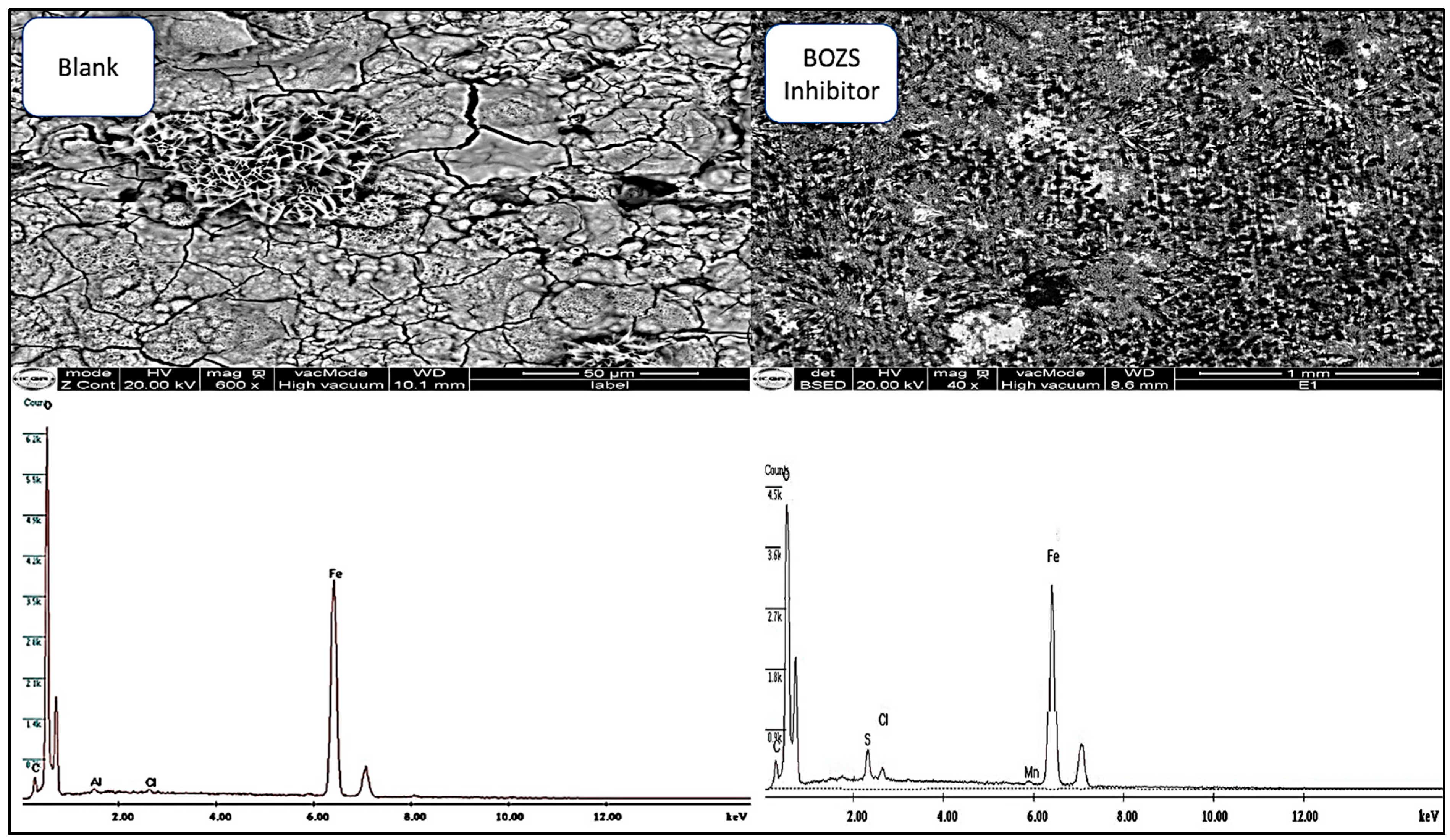
3.5. Surface Analysis
3.6. Quantum Chemistry Calculations
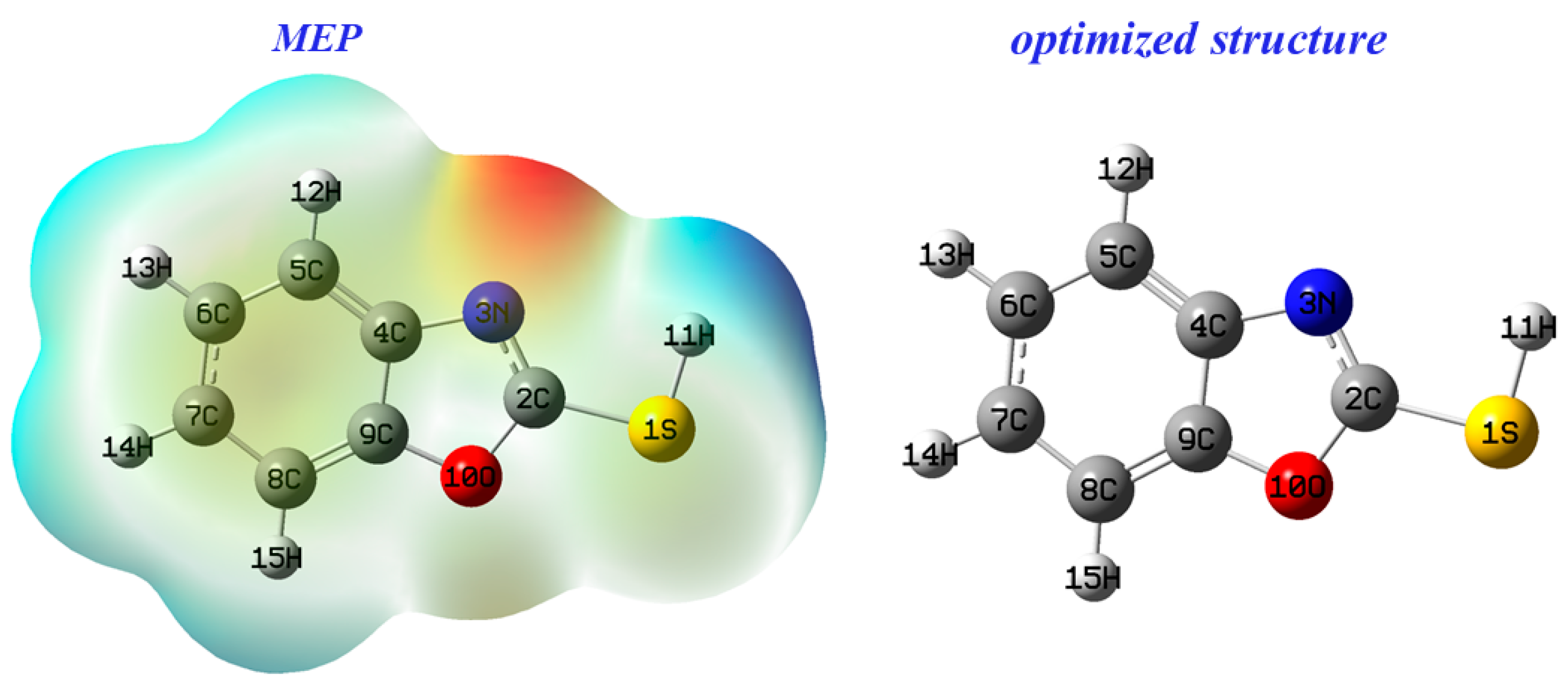
3.6.1. MEP Descriptor
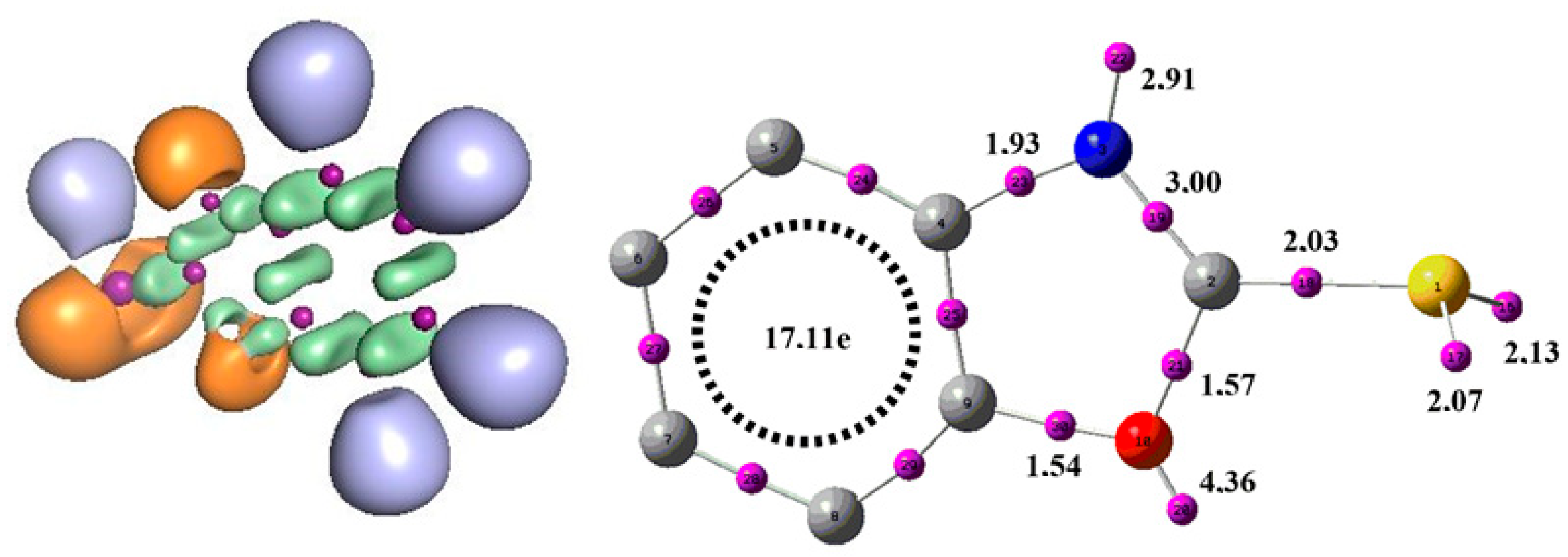
3.6.2. Electronic Localization Function (ELF)
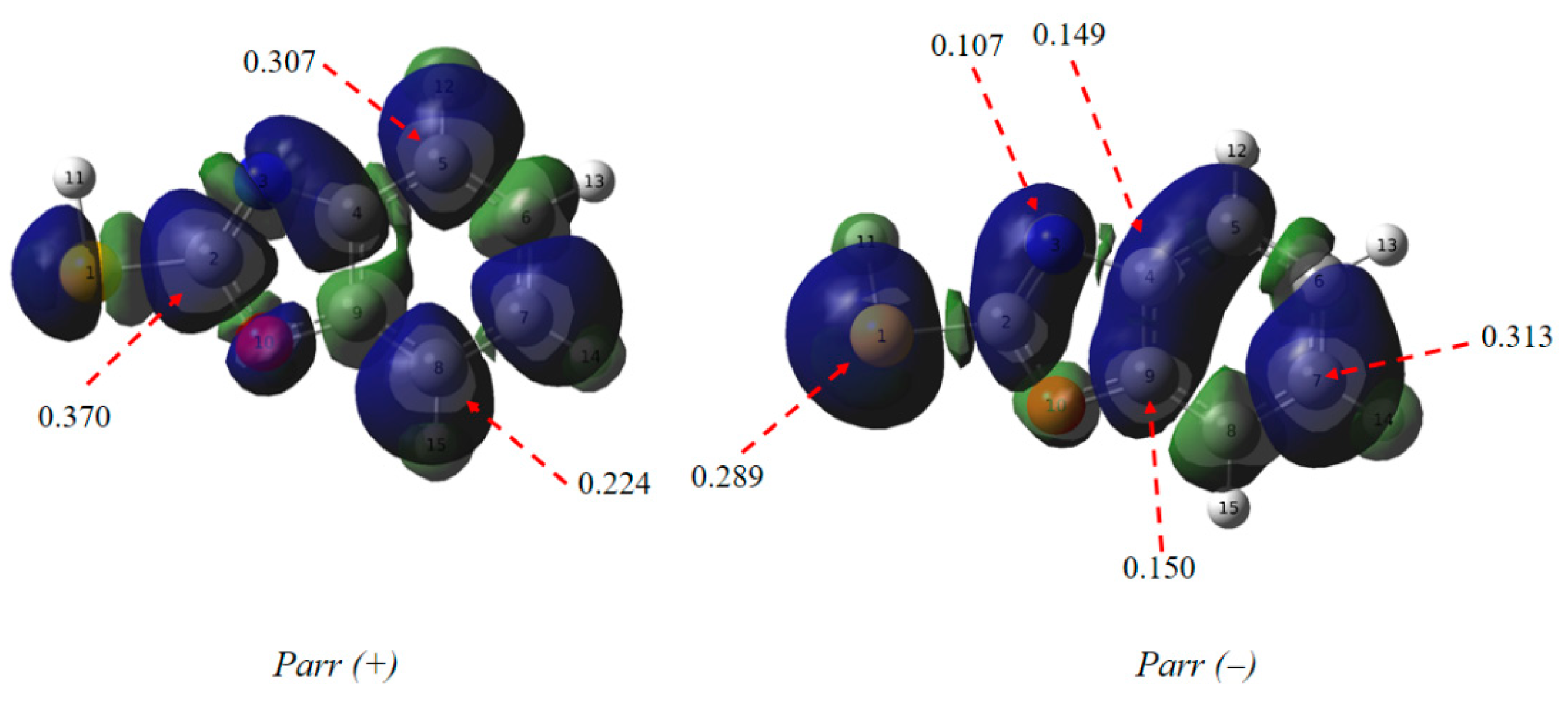
3.6.3. Local Reactivity Indices
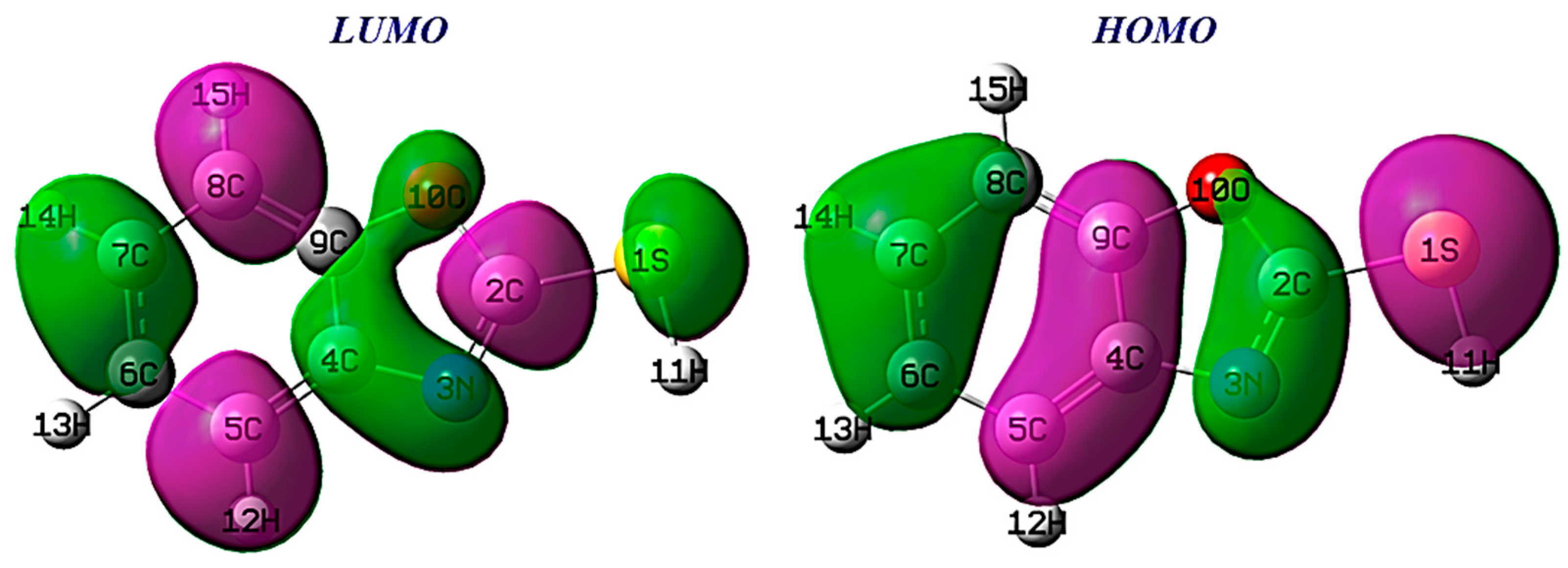
3.6.4. Molecular Frontier Orbitals
3.6.5. Analysis of Global Inhibitor Reactivity
3.6.6. Benzoxazole-2-Thiol—C38 Steel Complex
3.6.7. Protonated Inhibitor
3.7. Molecular Dynamics and Monte Carlo Simulation Results
3.7.1. Monte Carlo Method
3.7.2. MD Simulations
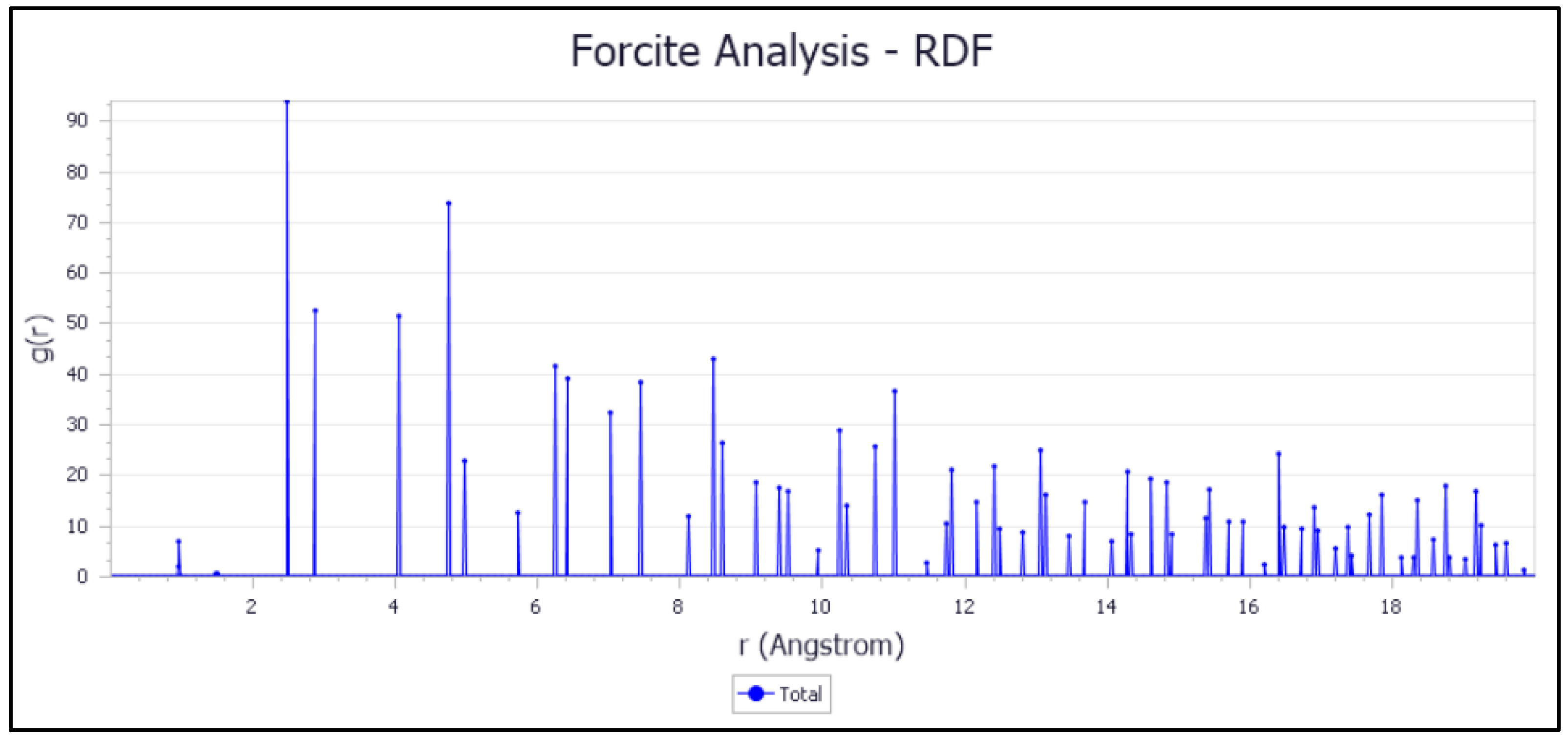
3.7.3. Examination of the Inhibitor/Surface Fe (110) Interaction: The RDF Approach
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zarrok, H.; Saddik, R.; Oudda, H.; Hammouti, B.; Midaoui, A.E.; Zarrouk, A.; Benchat, N.; Touhami, M.E. 5-(2-Chlorobenzyl)-2,6-Dimethylpyridazin-3-One: An efficient Inhibitor of C38 Steel Corrosion in Hydrochloric Acid. Der Pharma Chem. 2011, 3, 272–282. [Google Scholar]
- Al-Sharabi, H.A.; Bouiti, K.; Bouhlal, F.; Labjar, N.; Dahrouch, A.; El Mahi, M.; Lotfi, E.M.; El Otmani, B.; Benabdellah, G.A.; El Hajjaji, S. Electrochemical and Thermodynamic Evaluation on Corrosion Inhibition of C38 in 1M HCl By the Rumex Ethanolic Extract. Int. J. Corros. Scale Inhib. 2022, 11, 382–401. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, Y.; Cao, Y.; Chen, F.; Luo, Y.; Xiao, X.; Deng, Y.; Liu, Y. Field testing, analytical, and numerical assessments on the fatigue reliability on bridge suspender by considering the coupling effect of multiple pits. Struct. Infrastruct. Eng. 2025, 1–16. [Google Scholar] [CrossRef]
- Garcia-Ochoa, E.; Guzmán-Jiménez, S.J.; Hernández, J.G.; Pandiyan, T.; Vásquez-Pérez, J.M.; Cruz-Borbolla, J. Benzimidazole ligands in the corrosion inhibition for carbon steel in acid medium: DFT study of its interaction on Fe30 surface. J. Mol. Struct. 2016, 1119, 314–324. [Google Scholar] [CrossRef]
- Hegazy, M.A.; El-Etre, A.Y.; El-Shafaie, M.; Berry, K.M. Novel cationic surfactants for corrosion inhibition of carbon steel pipelines in oil and gas wells applications. J. Mol. Liq. 2016, 214, 347–356. [Google Scholar] [CrossRef]
- Messali, M.; Lgaz, H.; Dassanayake, R.; Salghi, R.; Jodeh, S.; Abidi, N.; Hamed, O. Guar gum as efficient non-toxic inhibitor of carbon steel corrosion in phosphoric acid medium: Electrochemical, surface, DFT and MD simulations studies. J. Mol. Struct. 2017, 1145, 43–54. [Google Scholar] [CrossRef]
- Yang, D.; Feng, X.; Yan, N.; Wang, Y.; Lu, L.; Mei, P.; Chen, W.; Lai, L. Corrosion Inhibition Studies of Benzoxazole Derivates for N80 Steel in 1 M HCl Solution: Synthesis, Experimental, and DTF Studies. Open J. Yangtze Oil Gas 2022, 7, 101–123. [Google Scholar]
- Omari, M.; Bouiti, K.; Labjar, N.; Jebbari, S.; Barhoumi, A.; Ait Sir, H.; El Hajjaji, S.; Dahrouch, A.; Nasrellah, H.; El idrissi, M.; et al. Novel bisbenzoxazole derivative as an effective corrosion inhibitor for C38 steel in hydrochloric acid medium: Experimental and theoretical investigations. Can. Metall. Q. 2025, 64, 1193–1211. [Google Scholar] [CrossRef]
- Anusha, G.; Mishma, J.C.; Sinha, R.K.; Suvarna, A.S.; Gaonkar, S.L. New benzisoxazole derivative: A potential corrosion inhibitor for mild steel in 0.5 M hydrochloric acid medium-insights from electrochemical and density functional theory studies. Heliyon 2023, 9, e21014. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.A.; Hegazy, M.M.; Awad, M.K.; Shawky, M. Chemical, electrochemical, theoretical (DFT & MEP), thermodynamics and surface morphology studies of carbon steel during gas and oil production using three novel di-cationic amphiphiles as corrosion inhibitors in acidic medium. J. Mol. Liq. 2021, 337, 116541. [Google Scholar] [CrossRef]
- Obot, I.B.; Macdonald, D.D.; Gasem, Z.M. Density functional theory (DFT) as a powerful tool for designing new organic corrosion inhibitors. Part 1: An overview. Corros. Sci. 2015, 99, 1–30. [Google Scholar] [CrossRef]
- Faska, Z.; Majidi, L. DFT study on the adsorption mechanism of pulegone and pulegone oxide molecules in gas and aqueous phases as effective corrosion inhibitors in Molar Hydrochloric Acid. Moroc. J. Chem. 2018, 6, 283–293. [Google Scholar]
- Toghan, A.; Gadow, H.S.; Fawzy, A.; Alhussain, H.; Salah, H. Adsorption Mechanism, Kinetics, Thermodynamics, and Anticorrosion Performance of a New Thiophene Derivative for C-Steel in a 1.0 M HCl: Experimental and Computational Approaches. Metals 2023, 13, 1565. [Google Scholar] [CrossRef]
- AlJeilawi, O.; AlAni, H.; AlZahra, A.; AlSultani, K. Exploring the Potential of Quantum Chemical Calculations for Synthesized Quinazoline Derivatives as Superior Corrosion Inhibitors in Acidic Environment. Phys. Chem. Res. 2024, 12, 205–217. [Google Scholar] [CrossRef]
- Bougrine, K.; Saber, I.; Barebita, H.; Ferraa, S.; Ouakki, M.; Belfaquir, M.; El youbi, M.S. Potentials for Inhibiting Corrosion on Mild Steel using Various Borphosphate Glasses In 1 M HCl. Anal. Bioanal. Electrochem. 2025, 17, 1–17. [Google Scholar] [CrossRef]
- Kharbach, Y.; Qachchachi, F.Z.; Haoudi, A.; Tourabi, M.; Zarrouk, A.; Jama, C.; Olasunkanmi, L.O.; Ebenso, E.E.; Bentiss, F. Anticorrosion performance of three newly synthesized isatin derivatives on carbon steel in hydrochloric acid pickling environment: Electrochemical, surface and theoretical studies. J. Mol. Liq. 2017, 246, 302–316. [Google Scholar] [CrossRef]
- Quartarone, G.; Ronchin, L.; Vavasori, A.; Tortato, C.; Bonaldo, L. Inhibitive action of gramine towards corrosion of mild steel in deaerated 1.0M hydrochloric acid solutions. Corros. Sci. 2012, 64, 82–89. [Google Scholar] [CrossRef]
- Fouda, A.S.; Hassan, A.F.; Elmorsi, M.A.; Fayed, T.A.; Abdelhakim, A. Chalcones as Environmentally-Friendly Corrosion Inhibitors for Stainless Steel Type 304 in 1 M HCl Solutions. Int. J. Electrochem. Sci. 2014, 9, 1298–1320. [Google Scholar] [CrossRef]
- Ouadi, Y.E.; Lamsayah, M.; Bendaif, H.; Benhiba, F.; Touzani, R.; Warad, I.; Zarrouk, A. Electrochemical and theoretical considerations for interfacial adsorption of novel long chain acid pyrazole for mild steel conservation in 1 M HCl medium. Chem. Data Collect. 2021, 31, 100638. [Google Scholar] [CrossRef]
- Faustin, M.; Maciuk, A.; Salvin, P.; Roos, C.; Lebrini, M. Corrosion inhibition of C38 steel by alkaloids extract of Geissospermum laeve in 1M hydrochloric acid: Electrochemical and phytochemical studies. Corros. Sci. 2015, 92, 287–300. [Google Scholar] [CrossRef]
- Bammou, L.; Belkhaouda, M.; Salghi, R.; Benali, O.; Zarrouk, A.; Zarrok, H.; Hammouti, B. Corrosion inhibition of steel in sulfuric acidic solution by the Chenopodium Ambrosioides Extracts. J. Assoc. Arab Univ. Basic Appl. Sci. 2014, 16, 83–90. [Google Scholar] [CrossRef]
- Finšgar, M.; Lesar, A.; Kokalj, A.; Milošev, I. A comparative electrochemical and quantum chemical calculation study of BTAH and BTAOH as copper corrosion inhibitors in near neutral chloride solution. Electrochim. Acta 2008, 53, 8287–8297. [Google Scholar] [CrossRef]
- Gece, G. Drugs: A review of promising novel corrosion inhibitors. Corros. Sci. 2011, 53, 3873–3898. [Google Scholar] [CrossRef]
- Costa, D.; Marcus, P. Adsorption of Organic Inhibitor Molecules on Metal and Oxidized Surfaces Studied by Atomistic Theoretical Methods. In Molecular Modeling of Corrosion Processes, 1st ed.; Taylor, C.D., Marcus, P., Eds.; Wiley: Hoboken, NJ, USA, 2015; pp. 125–156. [Google Scholar] [CrossRef]
- Raghavachari, K. Perspective on “Density functional thermochemistry. III. The role of exact exchange”. Theor. Chem. Acc. Theory Comput. Model. Theor. Chim. Acta 2000, 103, 361–363. [Google Scholar] [CrossRef]
- El Faydy, M.; Galai, M.; El Assyry, A.; Tazouti, A.; Touir, R.; Lakhrissi, B.; Ebn Touhami, M.; Zarrouk, A. Experimental investigation on the corrosion inhibition of carbon steel by 5-(chloromethyl)-8-quinolinol hydrochloride in hydrochloric acid solution. J. Mol. Liq. 2016, 219, 396–404. [Google Scholar] [CrossRef]
- Sastri, V.S.; Ghali, E.; Elboujdaini, M.; Sastri, V.S. Corrosion Prevention and Protection: Practical Solutions; Wiley: Chichester, UK, 2007. [Google Scholar]
- Lukovits, I.; Kálmán, E.; Zucchi, F. Corrosion Inhibitors—Correlation between Electronic Structure and Efficiency. Corrosion 2001, 57, 3–8. [Google Scholar] [CrossRef]
- Finšgar, M. 2-Mercaptobenzimidazole as a copper corrosion inhibitor: Part I. Long-term immersion, 3D-profilometry, and electrochemistry. Corros. Sci. 2013, 72, 82–89. [Google Scholar] [CrossRef]
- El Hamdouni, Y.; Bouhlal, F.; Kouri, H.; Chellouli, M.; Benmessaoud, M.; Dahrouch, A.; Labjar, N.; El Hajjaji, S. Use of Omeprazole as Inhibitor for C38 Steel Corrosion in 1.0 M H3PO4 Medium. J. Fail. Anal. Prev. 2020, 20, 563–571. [Google Scholar] [CrossRef]
- Bouiti, K.; aldeen Al-sharabi, H.; Bouhlal, F.; Labjar, N.; Dahrouch, A.; Mahi, M.E.; Lotfi, E.M.; El Otmani, B.; Benabdellah, G.A.; El Hajjaji, S. Use of the ethanolic extract from Eriobotrya Japonica seeds as a corrosion inhibitor of C38 in a 1 M HCl medium. Int. J. Corros. Scale Inhib. 2022, 11, 1319–1334. [Google Scholar] [CrossRef]
- Km, S.; Praveen, B.M.; Devendra, B.K. A review on corrosion inhibitors: Types, mechanisms, electrochemical analysis, corrosion rate and efficiency of corrosion inhibitors on mild steel in an acidic environment. Results Surf. Interfaces 2024, 16, 100258. [Google Scholar] [CrossRef]
- Al-Sodani, K.A.A.; Maslehuddin, M.; Al-Amoudi, O.S.B.; Saleh, T.A.; Shameem, M. Efficiency of generic and proprietary inhibitors in mitigating Corrosion of Carbon Steel in Chloride-Sulfate Environments. Sci. Rep. 2018, 8, 11443. [Google Scholar] [CrossRef] [PubMed]
- Bouiti, K.; aldeen Al-sharabi, H.; Bensemlali, M.; Bouhlal, F.; Abidi, B.; Labjar, N.; Laasri, S.; El Hajjaji, S. Effect of temperature on corrosion inhibition by ethanolic extract of Eriobotrya Japonica seeds in chloride medium 1M. Eur. Phys. J. Appl. Phys. 2022, 97, 67. [Google Scholar] [CrossRef]
- Bouhlal, F.; Labjar, N.; Abdoun, F.; Mazkour, A.; Serghini-Idrissi, M.; Mahi, M.E.; Lotfi, E.M.; Skalli, A.; Hajjaji, S.E. Chemical and electrochemical studies of the inhibition performance of hydro-alcoholic extract of used coffee grounds (HECG) for the corrosion of C38 steel in 1M hydrochloric acid. Egypt. J. Pet. 2020, 29, 45–52. [Google Scholar] [CrossRef]
- Bouhouche, I.; Bouiti, K.; Chraka, A.; El Hamil, A.; Labjar, N.; Damour, H.; Dahrouch, A.; Nasrellah, H.; Benmessaoud, M.; El Hajjaji, S. Eco-conscious corrosion inhibitor synthesis via Soxhlet extraction: Experimental study in HCl 1M and theoretical analysis using DFT and MC simulations. Can. Metall. Q. 2025, 64, 605–623. [Google Scholar] [CrossRef]
- Bentiss, F.; Jama, C.; Mernari, B.; Attari, H.E.; Kadi, L.E.; Lebrini, M.; Traisnel, M.; Lagrenée, M. Corrosion control of mild steel using 3,5-bis(4-methoxyphenyl)-4-amino-1,2,4-triazole in normal hydrochloric acid medium. Corros. Sci. 2009, 51, 1628–1635. [Google Scholar] [CrossRef]
- Hossam, K.; Bouhlal, F.; Hermouche, L.; Merimi, I.; Labjar, H.; Chaouiki, A.; Labjar, N.; Malika, S.-I.; Dahrouch, A.; Chellouli, M.; et al. Understanding Corrosion Inhibition of C38 Steel in HCl Media by Omeprazole: Insights for Experimental and Computational Studies. J. Fail. Anal. Prev. 2021, 21, 213–227. [Google Scholar] [CrossRef]
- Al-Sharabi, H.A.; Bouhlal, F.; Bouiti, K.; Bensemlali, M.; Labjar, N.; Benabdellah, G.A.; Dahrouch, A.; Laasri, S.; El Mahi, M.; Lotfi, E.M.; et al. Study of the corrosion inhibition of C38 steel in a 1M HCl medium by the ethanolic extract of Rumex Nervosus Vahl leaves. EPJ Appl. Phys. 2022, 97, 76. [Google Scholar] [CrossRef]
- Bentiss, F.; Lebrini, M.; Vezin, H.; Chai, F.; Traisnel, M.; Lagrené, M. Enhanced corrosion resistance of carbon steel in normal sulfuric acid medium by some macrocyclic polyether compounds containing a 1,3,4-thiadiazole moiety: AC impedance and computational studies. Corros. Sci. 2009, 51, 2165–2173. [Google Scholar] [CrossRef]
- Suedile, F.; Robert, F.; Roos, C.; Lebrini, M. Corrosion inhibition of zinc by Mansoa alliacea plant extract in sodium chloride media: Extraction, Characterization and Electrochemical Studies. Electrochim. Acta 2014, 133, 631–638. [Google Scholar] [CrossRef]
- Larouj, M.; Lgaz, H.; Serrar, H.; Zarrok, H.; Bourazmi, H.; Zarrouk, A.; Elmidaoui, A.; Guenbour, A.; Boukhris, S.; Oudda, H. Adsorption properties and inhibition of carbon steel corrosion in hydrochloric acid solution by ethyl 3-hydroxy-8-methyl-4-oxo-6-phenyl-2-(p-toly)-4, 6-dihydropyrimido [2, 1-b][1, 3] thiazine-7-carboxylate. J. Mater. Environ. Sci. 2015, 6, 3251–3267. [Google Scholar]
- Bouiti, K.; Al-sharabi, H.A.; Bouhlal, F.; Abidi, B.; Labjar, N.; Bensemlali, M.; Hajjaji, S.E. Response surface methodology for optimizing corrosion inhibition: Investigating the synergistic effect of Eriobotrya japonica extract and potassium iodide. Euro-Mediterr. J. Environ. Integr. 2024, 9, 469–481. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, N.; Li, X.; Zhang, L.; Wu, L.; Huang, Y. Synergistic inhibition behavior between indigo carmine and cetyl trimethyl ammonium bromide on carbon steel corroded in a 0.5 M HCl solution. Appl. Surf. Sci. 2015, 357, 845–855. [Google Scholar] [CrossRef]
- Chaubey, N.; Savita; Singh, V.K.; Quraishi, M.A. Corrosion inhibition performance of different bark extracts on aluminium in alkaline solution. J. Assoc. Arab Univ. Basic Appl. Sci. 2017, 22, 38–44. [Google Scholar] [CrossRef]
- Rehioui, M.; Abbout, S.; Benzidia, B.; Hammouch, H.; Erramli, H.; Daoud, N.A.; Badrane, N.; Hajjaji, N. Corrosion inhibiting effect of a green formulation based on Opuntia Dillenii seed oil for iron in acid rain solution. Heliyon 2021, 7, e06674. [Google Scholar] [CrossRef] [PubMed]
- Oguzie, E.E.; Njoku, V.O.; Enenebeaku, C.K.; Akalezi, C.O.; Obi, C. Effect of hexamethylpararosaniline chloride (crystal violet) on mild steel corrosion in acidic media. Corros. Sci. 2008, 50, 3480–3486. [Google Scholar] [CrossRef]
- M’hiri, N.; Veys-Renaux, D.; Rocca, E.; Ioannou, I.; Boudhrioua, N.M.; Ghoul, M. Corrosion inhibition of carbon steel in acidic medium by orange peel extract and its main antioxidant compounds. Corros. Sci. 2016, 102, 55–62. [Google Scholar] [CrossRef]
- Akkermans, R.L.C.; Spenley, N.A.; Robertson, S.H. Monte Carlo methods in Materials Studio. Mol. Simul. 2013, 39, 1153–1164. [Google Scholar] [CrossRef]
- Guo, L.; Obot, I.B.; Zheng, X.; Shen, X.; Qiang, Y.; Kaya, S.; Kaya, C. Theoretical insight into an empirical rule about organic corrosion inhibitors containing nitrogen, oxygen, and sulfur atoms. Appl. Surf. Sci. 2017, 406, 301–306. [Google Scholar] [CrossRef]
- Chafi, M.; Byadi, S.; Barhoumi, A.; Limouni, W.; Tizliouine, A.; Jama, C.; El Hachemi Omari, L. Study of copper removal by modified biomaterials using the response surface methodology, DFT Calculation, and molecular dynamic simulation. J. Mol. Liq. 2022, 363, 119799. [Google Scholar] [CrossRef]
- Sulaiman, K.O.; Onawole, A.T.; Faye, O.; Shuaib, D.T. Understanding the corrosion inhibition of mild steel by selected green compounds using chemical quantum based assessments and molecular dynamics simulations. J. Mol. Liq. 2019, 279, 342–350. [Google Scholar] [CrossRef]
- Kavimani, M.; Balachandran, V.; Narayana, B.; Vanasundari, K.; Revathi, B. Topological analysis (BCP) of vibrational spectroscopic studies, docking, RDG, DSSC, Fukui functions and chemical reactivity of 2-methylphenylacetic acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 190, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Hsissou, R.; Abbout, S.; Seghiri, R.; Rehioui, M.; Berisha, A.; Erramli, H.; Assouag, M.; Elharfi, A. Evaluation of corrosion inhibition performance of phosphorus polymer for carbon steel in [1 M] HCl: Computational studies (DFT, MC and MD simulations). J. Mater. Res. Technol. 2020, 9, 2691–2703. [Google Scholar] [CrossRef]
- Guo, L.; Kaya, S.; Obot, I.B.; Zheng, X.; Qiang, Y. Toward understanding the anticorrosive mechanism of some thiourea derivatives for carbon steel corrosion: A combined DFT and molecular dynamics investigation. J. Colloid Interface Sci. 2017, 506, 478–485. [Google Scholar] [CrossRef] [PubMed]



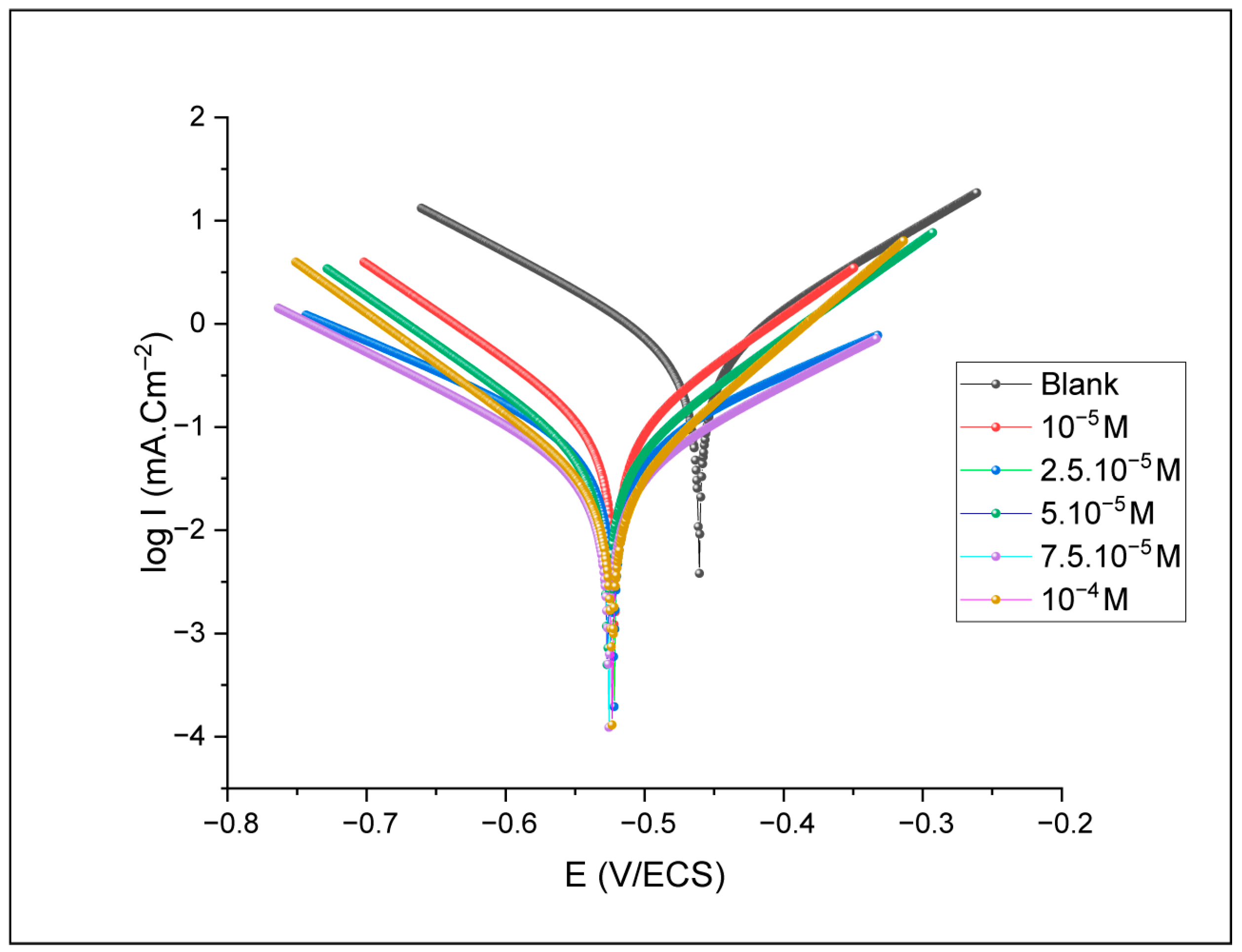



| Concentration (mol/L) | Icorr (µA/cm2) | Icorr-M (µA/cm2) | Standard Deviation (µA/cm2) | −Ecorr (mV/SCE) | −Ecorr-M (mV/SCE) | Standard Deviation (mV/SCE) | βa (mV) | −βc (mV) | IETP (%) |
|---|---|---|---|---|---|---|---|---|---|
| Blank | 520.046 | 517.93 | 2.116 | 460.741 | 457.883 | 2.858 | 128.4 | 142.2 | - |
| 10−5 | 88.513 | 86.791 | 1.722 | 521.573 | 523.331 | 1.758 | 107.9 | 109.1 | 82.98 |
| 2.5 × 10−5 | 69.564 | 71.393 | 1.829 | 522.033 | 523.711 | 1.678 | 180.9 | 178.2 | 86.62 |
| 5 × 10−5 | 44.809 | 43.91 | 0.899 | 526.751 | 524.428 | 2.323 | 104.8 | 107.1 | 91.38 |
| 7.5 × 10−5 | 36.665 | 34.782 | 1.883 | 525.787 | 522.901 | 2.886 | 149.1 | 149.7 | 92.95 |
| 10−4 | 24.682 | 22.921 | 1.761 | 523.375 | 522.682 | 0.693 | 86.9 | 103.1 | 95.25 |
| Concentration (mol/L) | R1 (Ohm × cm2) | R2 (Ohm × cm2) | R3 (Ohm × cm2) | Q2 (e−3 × F × cm−2) | n2 | Q3 (e−3× F × cm−2) | n3 | X2 × 10−3 | Rct (Ω × cm2) | IEEIS % |
|---|---|---|---|---|---|---|---|---|---|---|
| Blank | 4.116 | 48.19 | - | 3.4 | 0.8 | - | - | 2.3 | 48.19 | - |
| 10−5 | 6.585 | 308.9 | 24.64 | 0.282 | 0.8 | 1.194 | 0.7 | 1.6 | 333.54 | 85.55 |
| 2.5 × 10−5 | 6.711 | 336.1 | 48.7 | 0.126 | 0.8 | 0.140 | 0.7 | 5.2 | 384.8 | 87.48 |
| 5 × 10−5 | 7.825 | 465 | 36.24 | 0.168 | 0.8 | 0.138 | 0.8 | 3.9 | 501.24 | 90.38 |
| 7.5 × 10−5 | 6.09 | 427.4 | 141.4 | 0.0699 | 0.8 | 0.153 | 0.8 | 2.1 | 568.8 | 91.52 |
| 10−4 | 7.461 | 850.3 | 56.29 | 0.061 | 0.8 | 0.076 | 0.8 | 4.3 | 906.59 | 94.68 |
| Immersion Time | R1 (Ω × cm2) | R2 (Ω × cm2) | R3 (Ω × cm2) | Q2 (e−3 × F × cm−2) | n2 | Q3 (e−3 × F × cm−2) | n3 | Rct (Ω × cm2) | IE % |
|---|---|---|---|---|---|---|---|---|---|
| Blank | |||||||||
| 2 | 6.398 | 47.68 | 4.621 | 0.806 | 0.7 | 1.111 | 0.9 | 52.301 | - |
| 4 | 5.189 | 35 | 25.93 | 0.757 | 0.8 | 1.742 | 0.9 | 60.93 | - |
| 6 | 7.25 | 65.1 | 6.143 | 0.738 | 0.7 | 1.327 | 0.9 | 71.243 | - |
| 8 | 3.672 | 55.34 | 41.18 | 0.404 | 0.9 | 1.202 | 0.9 | 96.52 | - |
| 16 | 3.138 | 35.47 | 84.94 | 0.376 | 0.9 | 1.84 | 0.9 | 120.41 | - |
| 24 | 11.14 | 113.3 | 1.216 | 0.266 | 0.9 | 1.335 | 0.9 | 114.516 | - |
| BOZS Inhibitor | |||||||||
| 2 | 11.1 | 38.67 | 327.9 | 1.617 | 0.9 | 0.109 | 0.8 | 366.57 | 85.73 |
| 4 | 8.222 | 504 | 13.09 | 0.096 | 0.8 | 0.248 | 0.8 | 517.09 | 88.22 |
| 6 | 8.368 | 602.4 | 36.55 | 0.089 | 0.8 | 0.327 | 0.8 | 638.95 | 88.85 |
| 8 | 6.58 | 719.1 | 234 | 0.050 | 0.8 | 0.677 | 0.9 | 935.1 | 89.68 |
| 16 | 3.228 | 740.5 | 562.6 | 0.072 | 0.9 | 0.032 | 0.8 | 1303.1 | 90.75 |
| 24 | 3.117 | 602.3 | 216.6 | 0.028 | 0.8 | 0.392 | 0.9 | 818.9 | 86.01 |
| ----- | ∆Egap | ∆N | ||||||
|---|---|---|---|---|---|---|---|---|
| Inhibitor | −0.989 | −6.417 | 5.428 | 0.544 | 3.703 | 2.714 | 2.526 | 0.607 |
| ---- | ∆Egap | (D) | ∆N | |||||
|---|---|---|---|---|---|---|---|---|
| BOZS | −0.989 | −6.417 | 5.428 | 0.544 | 3.703 | 2.714 | 2.526 | 0.607 |
| BOZS H+ | −6.224 | −11.204 | 4.980 | 3.878 | 8.714 | 2.490 | 15.248 | −0.344 |
| ---- | |||||
|---|---|---|---|---|---|
| Inhibitor | −823,159.649 | −823,065.296 | 81.997 | −176.39 | 176.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omari, M.; Bouiti, K.; Jebbari, S.; Lahrache, N.; Barhoumi, A.; Labjar, N.; Hajjaji, S.E.; Said-Ahmed, M.; Lebrini, M.; Nasrellah, H.; et al. Corrosion Inhibition of C38 Steel in 1 M HCl Using Benzoxazole-2-Thione: Electrochemical, SEM-EDX, and Theoretical Studies. Metals 2025, 15, 810. https://doi.org/10.3390/met15070810
Omari M, Bouiti K, Jebbari S, Lahrache N, Barhoumi A, Labjar N, Hajjaji SE, Said-Ahmed M, Lebrini M, Nasrellah H, et al. Corrosion Inhibition of C38 Steel in 1 M HCl Using Benzoxazole-2-Thione: Electrochemical, SEM-EDX, and Theoretical Studies. Metals. 2025; 15(7):810. https://doi.org/10.3390/met15070810
Chicago/Turabian StyleOmari, Mohamed, Khalid Bouiti, Said Jebbari, Nabil Lahrache, Ali Barhoumi, Najoua Labjar, Souad El Hajjaji, Mahado Said-Ahmed, Mounim Lebrini, Hamid Nasrellah, and et al. 2025. "Corrosion Inhibition of C38 Steel in 1 M HCl Using Benzoxazole-2-Thione: Electrochemical, SEM-EDX, and Theoretical Studies" Metals 15, no. 7: 810. https://doi.org/10.3390/met15070810
APA StyleOmari, M., Bouiti, K., Jebbari, S., Lahrache, N., Barhoumi, A., Labjar, N., Hajjaji, S. E., Said-Ahmed, M., Lebrini, M., Nasrellah, H., Idrissi, M. E., & Tounsi, A. (2025). Corrosion Inhibition of C38 Steel in 1 M HCl Using Benzoxazole-2-Thione: Electrochemical, SEM-EDX, and Theoretical Studies. Metals, 15(7), 810. https://doi.org/10.3390/met15070810









