Abstract
This study explores the corrosion inhibition of C38 steel in a 1 M hydrochloric acid (HCl) solution using a novel benzoxazole-2-thione compound. The inhibitor was synthesized and structurally characterized by both 1H NMR (DMSO-d6/TMS) and 13C NMR spectroscopy. Electrochemical techniques, including Tafel polarization and electrochemical impedance spectroscopy, were employed to evaluate the inhibition performance. The results indicate that the benzoxazole-2-thione significantly reduces the corrosion rate, achieving a maximum inhibition efficiency of 95.25% at a concentration of 10−4 M. To gain deeper insights into the inhibition mechanism, theoretical methods such as density functional theory, Monte Carlo simulations, and molecular dynamics were applied to investigate the adsorption behavior of the compound on the steel surface. The adsorption process follows the Langmuir isotherm model, suggesting the coexistence of physisorption and chemisorption interactions. Surface morphology and elemental composition analyses using scanning electron microscopy coupled with energy-dispersive X-ray spectroscopy (SEM-EDX) confirm the formation of a protective inhibitor film on the steel surface.
1. Introduction
The construction sector widely uses C38 steel, which is deployed in industrial metallurgical processes due to its high physical and mechanical properties. Unfortunately, these metals are damaged by aggressive substances, such as acids, and corrode, leading to economic problems [1,2], as well as affecting the durability and safety of steel structures in atmospheric and marine conditions [3]. Many methods have been adopted, including using corrosion inhibitors.
Organic molecules reduce the rate of corrosion in corrosive media. By introducing them into the medium at a low concentration, these mainly heteroatomic compounds (containing N, S, O or P) have several bonds with metal surfaces, which allow them to interact with the surfaces based on the electronic configuration of the material and the reactive sites [4,5,6].
The various organic inhibitor classes include benzoxazole derivatives with a heterocyclic structure and functional groups, providing a significant adsorption capacity. Benzoxazole-2-thione is particularly promising, as the thione group enhances its ability to interact with metal surfaces, potentially increasing its inhibition efficacy.
Several studies have demonstrated the effectiveness of benzoxazole derivatives as corrosion inhibitors for steel in hydrochloric acid media. For instance, Yang et al. synthesized three benzoxazole compounds—2-(benzo[d]oxazol-2-yl)phenol (BOP), 6-(benzo[d]oxazol-2-yl)pyridin-2-ol (BOPO), and 2-(quinolin-2-yl)benzo[d]oxazole (QBO)—and showed that their inhibition efficiency for N80 steel in 1 M HCl increased at higher concentrations, with QBO revealing the highest performance due to its quinoline substituent enhancing adsorption [7]. Another study investigated a bis-benzoxazole derivative as an inhibitor for C38 steel in hydrochloric acid, revealing significant corrosion protection through strong adsorption on the metal surface [8]. Additionally, a substituted benzisoxazole derivative (FPBH) was evaluated for mild steel corrosion inhibition in 0.5 M HCl, achieving up to 94.5% efficiency by forming a protective adsorbed layer, as confirmed by electrochemical and theoretical analyses [9].
The experimental techniques employed were Tafel curves and spectroscopic impedance spectroscopy to study the synthesised molecule benzoxazole-2-thione as a corrosion inhibitor in HCl 1 M medium.
Quantum theoretical chemistry, the MC technique as well as MD simulation were adopted for determining the inhibitory properties arising from the interaction and adsorption phenomena involving the inhibitor and the substrate surface [10,11,12].
Electrochemical techniques are adopted to study corrosion inhibition mechanisms, which provide quantitative data on corrosion rates and the inhibitor effect. Additionally, the inhibition properties result from the interaction and adsorption phenomena. Therefore, quantum theoretical physical chemistry, the Monte Carlo technique, and the molecular dynamics simulation procedure are well suited for the study [13,14,15,16].
2. Materials and Methods
2.1. Benzoxazole-2-Thione Synthesis
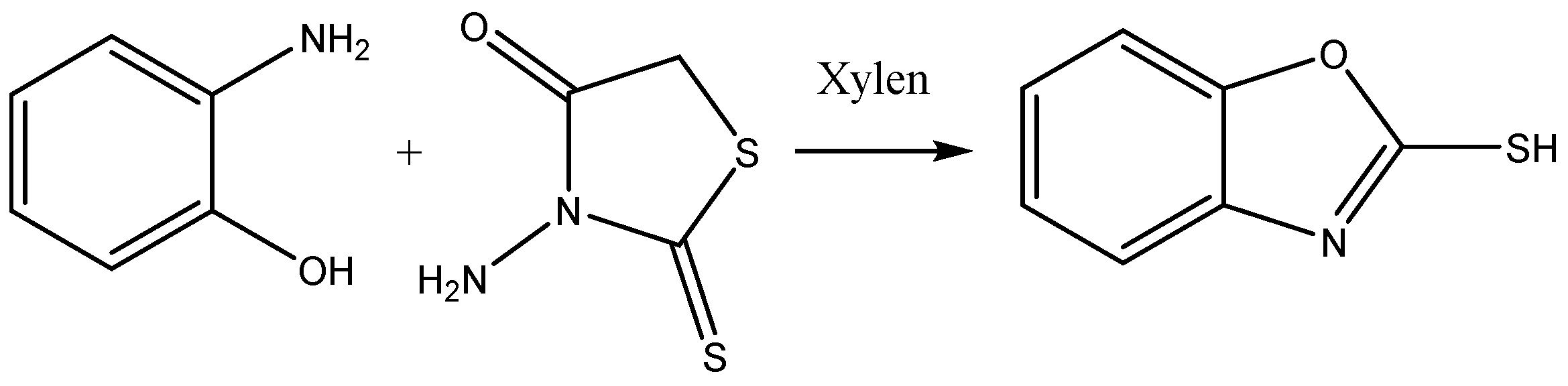
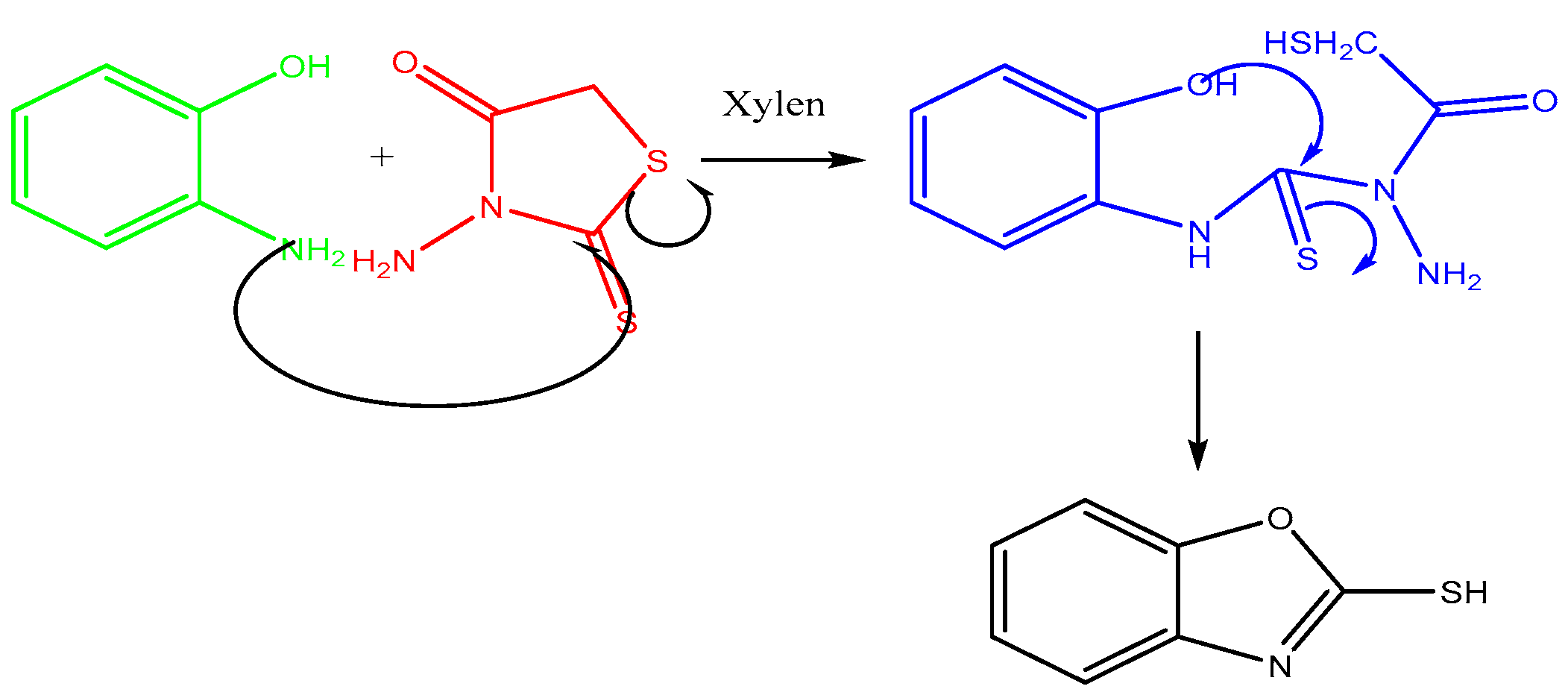
The mixture of benzoxazole-2-thiones (0.01 mol), rhodanine (0.01 mol) and 20 mL xylene is placed in a flask. The flask was then heated under reflux for 24 h, resulting in a white precipitate formation, which was then recovered, oven dried and purified by recrystallisation in ethanol. The final product is the compound benzoxazole-2-thione with the molecular formula C7H6NOS and a molar mass of 151 g/mol. The reaction is illustrated in Figure 1.
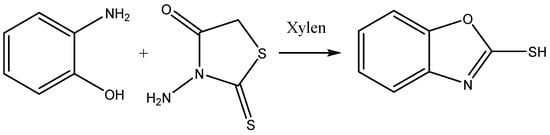
Figure 1.
Synthesis consists of benzoxazole-2-thione.
The synthesis of benzoxazole-2-thione was performed in two main steps. First, o-aminophenol was reacted with carbon disulfide (CS2) to form a thione intermediate, which was treated in the presence of rhodanine, which functions as a stabilizing agent and promotes cyclization to the final benzoxazole-2-thione.
The techniques used in our work are NMR, IR, melting point spectra (Köfer apparatus, uncorrected), IR spectra, NMR spectra, mass spectra, column chromatography (silica gel 60, 230–400 mesh), proton and carbon 13 NMR spectral data and the NMR spectrum of the composite.
2.2. Electrochemical Tests
The C38 steel was polished using abrasive paper, rinsed with distilled water, ultrasonically degreased and then dried [17,18].
The electrochemical tests were performed via a SP150 biological potentiostat linked by Ec-lab software V10.1 for collecting and processing the results (Figure 2). The setup adopted includes C38 steel as the working electrode, a saturated calomel electrode (SCE) as the reference and Pt wire as the counter electrode.
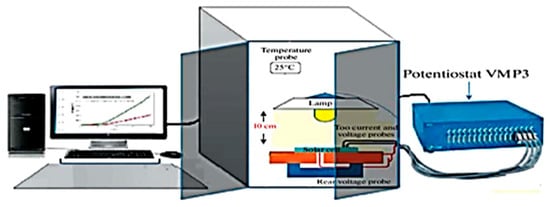
Figure 2.
Electrochemical device comprising a potentiostat connected to a computer.
The molecule was dissolved in 75 mL of 1M HCl at ambient temperature and employed as an inhibitor in the study over a concentration range of 10−5 to 10−4 Mol·L−1.
Before each test, relaxation for 30 min was necessary to stabilise the system. All electrochemical tests were carried out at 25 °C.
For the spectroscopic impedance, the frequency range adopted was between 100 kHz and 10 MHz. For the Tafel curves, the applied potential varies within a range of ±0.25 V with a scanning speed of 0.5 mV/sec.
Based on Equations (1) and (2), the inhibitory efficiency, respectively, can be calculated from the intensity potential and spectroscopic impedance plots [8].
Icorr, I′corr refer to the intensities of corrosion current determined from Tafel plots extrapolated without and with inhibitor [19].
Rct and R′ct represent the C38 steel charge transfer resistance without and with inhibitor, respectively [20].
2.3. Density Functional Theory (DFT) Method
In quantum chemistry, DFT (Density Functional Theory) is an advanced molecular modelling technique. It is particularly relevant for analyzing systems made up of several atoms. As a theoretical method, it is crucial to establish links between the effectiveness of the protection and the electronic characteristics of the product under investigation [21,22]. This approach offers the possibility of obtaining an in-depth understanding of the links between molecular structures, electronic interactions and inhibition capacities, opening up significant prospects in the composition of chemical products with specific characteristics.
The quantum chemistry program Gaussian 09 A.02 was used to perform the calculations via the B3LYP density function [23,24] as well as the 6-311G (d,p) basis sets [25]. In a computational chemistry study, this basis set was employed to optimize the molecular geometry and evaluate intrinsic properties of a molecule in the gas phase. Optimization involved iteratively adjusting atomic positions to minimize the total energy of the molecule according to the B3LYP/6-311G (d,p) model, resulting in a stable molecular structure. Subsequently, various intrinsic properties, such as molecular energy, geometric parameters, electronic properties, and vibrational frequencies, were calculated. These calculations were performed under gas phase conditions, assuming the molecule is isolated without solvent interactions, enabling a focused study of fundamental molecular behavior.
Various significant parameters related to the molecular electronic structure, whether local or global indicators, were calculated. The associated energies and the LUMO and HOMO frontier orbital distributions were determined. The energy gap between these LUMO-HOMO orbitals was also evaluated for assessing the role of the electronic characteristics of the molecule under study. Several quantum chemical descriptors of the molecular electronic structure, both local and global, were calculated.
These parameters include chemical hardness (), dipole moment (, chemical electronegativity ), and the electrophilicity index () of the inhibitor. The aim of this approach was to anticipate and predict the associated chemical properties.
Furthermore, a fraction of the transferred was analyzed to determine electron transfer within the molecule [26]. denote the inhibitor’s absolute electronegativity and hardness. For C38 steel, . These data were examined and analysed in relation to the efficacy of the inhibition process, indicating that studies should be carried out to assess the links between certain variables or factors and inhibition capacity.
To understand the inhibitor selectivity and impact of reactive sites on inhibitory effectiveness, an analysis of local reactivity was carried out. Molecular electrostatic potential (MEP) and Parr indices are two of the local parameters that were examined using the DFT method.
2.4. Molecular Dynamics and Monte Carlo Simulations
This method uses the BIOVIA Materials Studio 7.0 program to examine energetic interactions during adsorption of protector in their neutral states onto the C38 steel surface. The simulation of adsorption was carried out on the surface, specifically selected for its stability [27].
The compass force field was adopted to characterize how the C38 steel surface interacts with the inhibitor. The simulation was performed in a supercell of dimensions (15 × 15), with a void 30 Å thick positioned above the C38 steel filled with 200 molecules of water H2O, 9 molecules of HCl (H+, Cl−) and one inhibitor molecule. This configuration was placed in a simulation box (42.99 Å × 60.80 Å × 58.37 Å) using periodic boundary conditions. Electrostatic interactions were handled using Ewald and group methods [28,29] as follows:
Einter = Etot − (Einh + Esurf + H2O + HCl);
Etot: Simulation system total energy;
Einh: The inhibitor energy;
Esurf + H2O + HCl: The combined energy from the C38 steel surface as well as the (H2O + HCl) molecules. The bond energy: EBinding = −Einteraction.
3. Results
3.1. Identification Methods: 1H-NMR (DMSO-d6/TMS) and 13C NMR
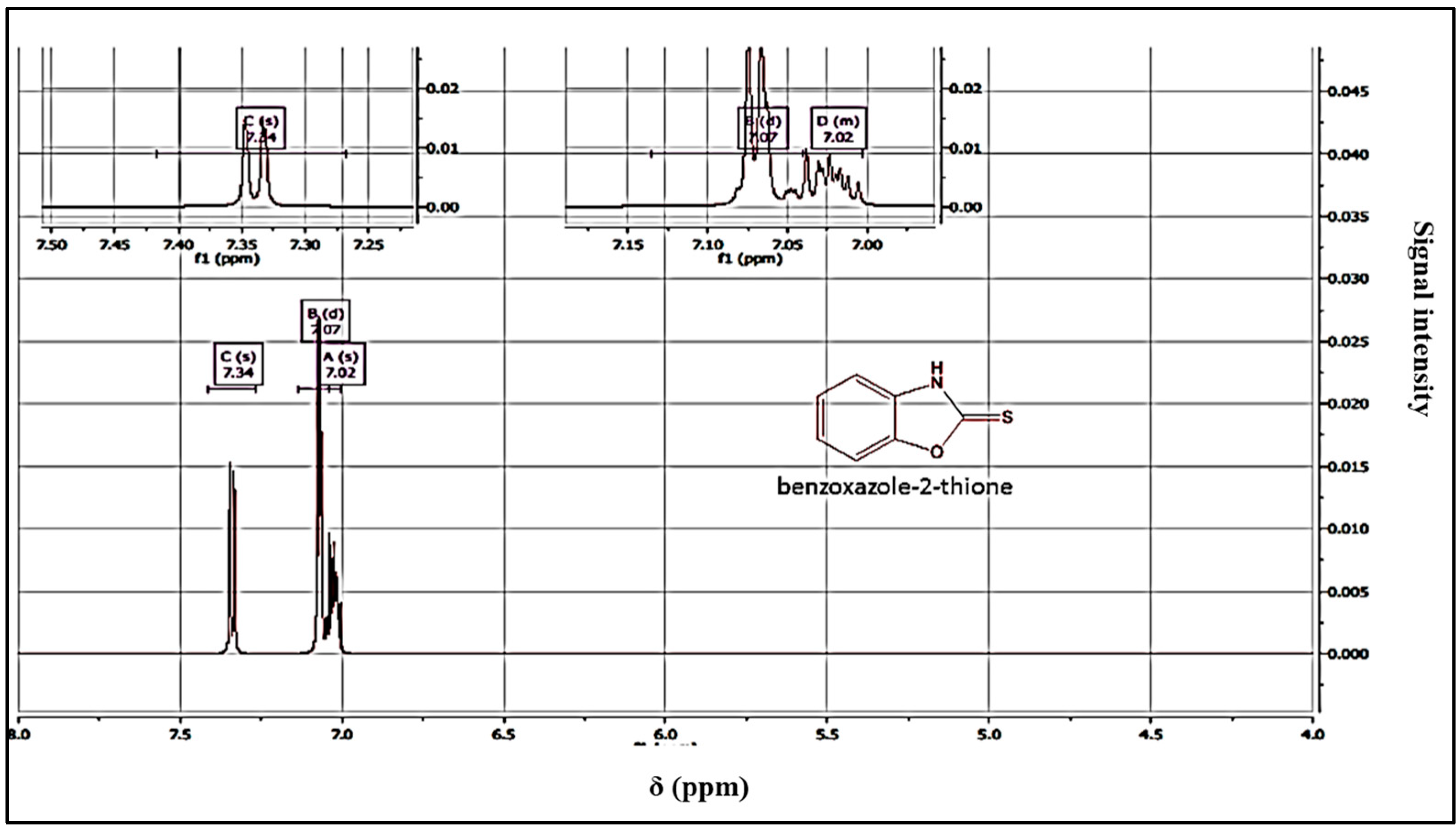
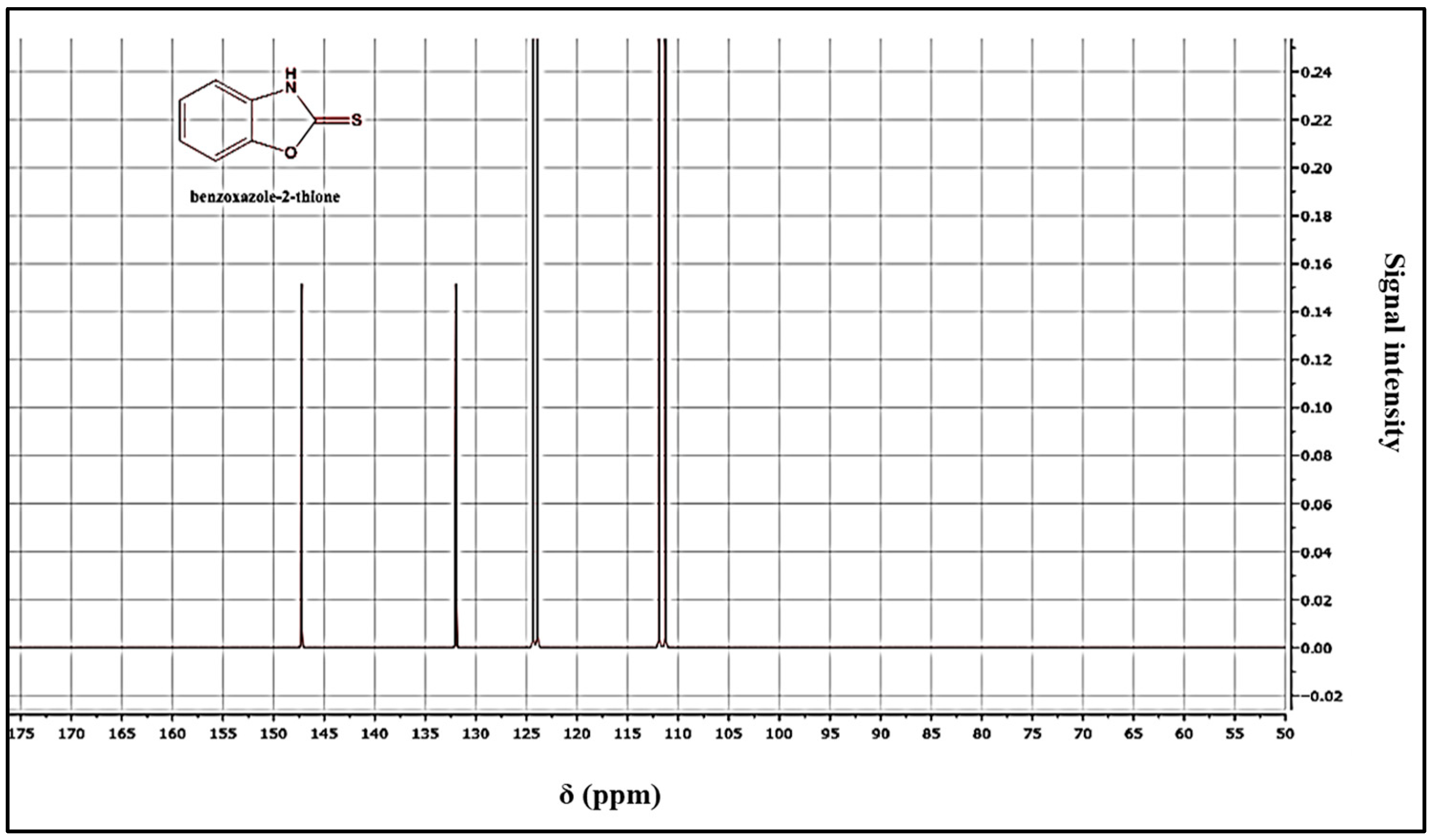
Yield = 89%; mp = 198 °C. 1HNMR (DMSO-d6): 7.02 (m, 2Har); 7.07 (m, 2Har); 7.34 (m, 2Har); 13CNMR (DMSO-d6): 111.43 (CH); 112.73 (CH); 124.43 (CH); 125.13 (CH); 132.36 (CH); 158.82 (C); 159.12 (C=S). HRMS, m/z: 151(M), calcd for C7 H6NOS: 151.02517, found: 151.0251.
This study presents an innovative method for synthesizing benzoxazole-2-thione derivatives. These starting compounds are of great interest because of their chemical reactivity and biological properties. A mixture of o-aminophenol and N-aminorhodanine was heated in xylene for 24 h.
The structure of the products was determined using nuclear magnetic resonance (NMR) and mass data. The 1H NMR spectra revealed the presence of the NH signal, while the 13C NMR spectra showed the C=S signal, confirming the structure of the products (Figure 3 and Figure 4). A possible mechanism for the formation of benzoxazole-2-thione is illustrated in Figure 5. The important step is the intermediate A stage, where cycling leads to the C=S group rather than the C=O group, resulting in the benzotriazepine product. In this reaction, only benzoxazole-2-thione was obtained.
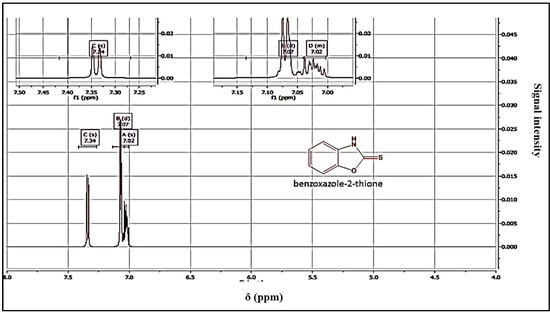
Figure 3.
1H NMR spectrum of benzoxazole-2-thione derivatives.
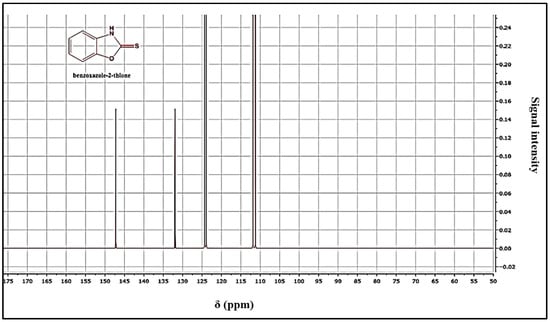
Figure 4.
13C NMR spectrum of benzoxazole-2-thione derivatives.
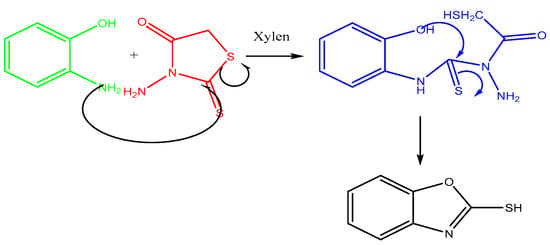
Figure 5.
Reaction mechanism of benzoxazole-2-thione synthesis.
3.2. Concentration Effect
3.2.1. Polarization Measurements
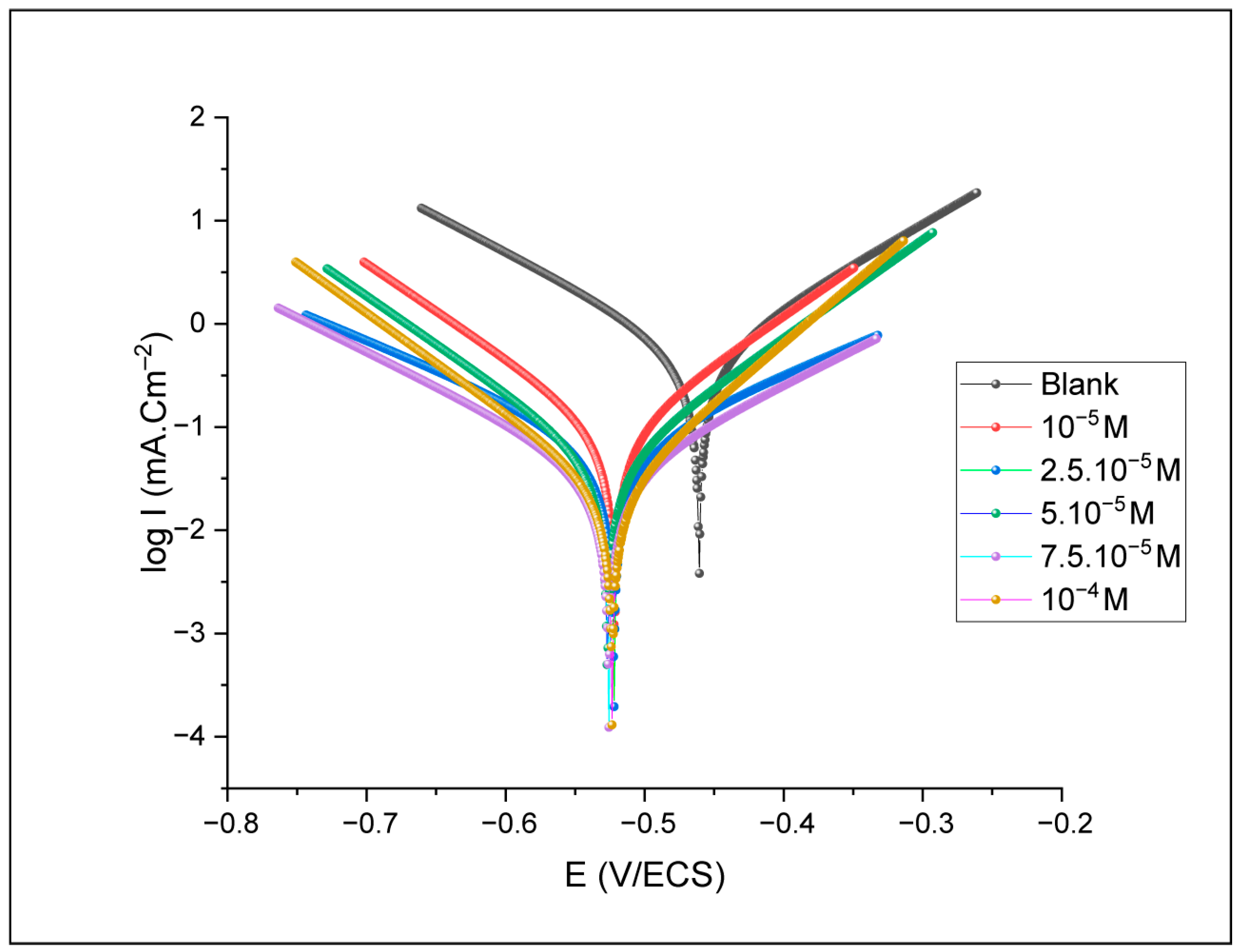
The Tafel plots provide various electrochemical factors, such as corrosion current density (Icorr), corrosion potential (Ecorr), cathodic (βc) and anodic (βa) slopes. Corrosion potential and current density mean values (Ecorr-M, Icorr-M) are obtained after three repetitions to ensure better reliability. The results obtained are illustrated in Figure 6.
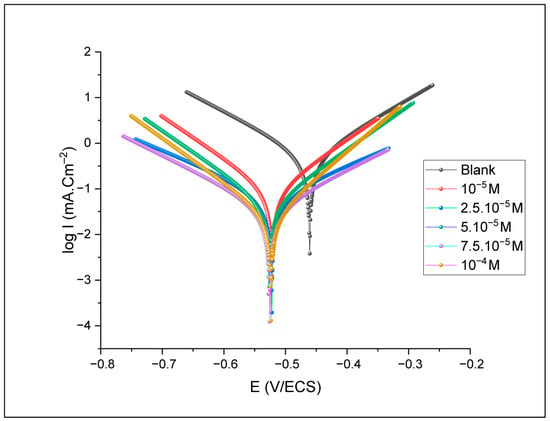
Figure 6.
Polarization curves at different concentrations of benzoxazole-2-thionein 1 M HCl solution.
The analysis indicates that increasing the Benzoxazole-2-Thione inhibitor (BOZS) concentration results in a shift towards lower current values, indicating a reduction in the corrosion rate. However, the curve’s shape suggests that the inhibitor interacts selectively with the active sites on the surface [30], thus reducing the corrosion process without altering the overall nature of the electrochemical process. The variation in corrosion potential indicates that the inhibitor affects the cathodic and anodic reactions simultaneously, reducing both the metallic dissolution and the release of hydrogen associated with the cathodic reactions [31] (Table 1).

Table 1.
Tafel results of the C38 steel with various concentrations of benzoxazole-2-thione.
The impact of the inhibitor on Tafel slopes confirms its mixed nature, influencing both oxidation and reduction processes [32]. The decrease in corrosion current density with inhibitor concentration increase leads to a significant rise in the efficiency, reaching a maximum of 95.25%.
The present investigation demonstrates a maximum inhibition efficiency at a remarkably low concentration of 10−4 M. This is particularly high compared to many commercial corrosion inhibitors, which typically achieve inhibition efficiencies ranging from 80% to over 90%, but often require significantly higher concentrations, typically in the 100–500 ppm range, to achieve optimum protection [33,34].
3.2.2. Electrochemical Impedance Spectroscopy EIS Method
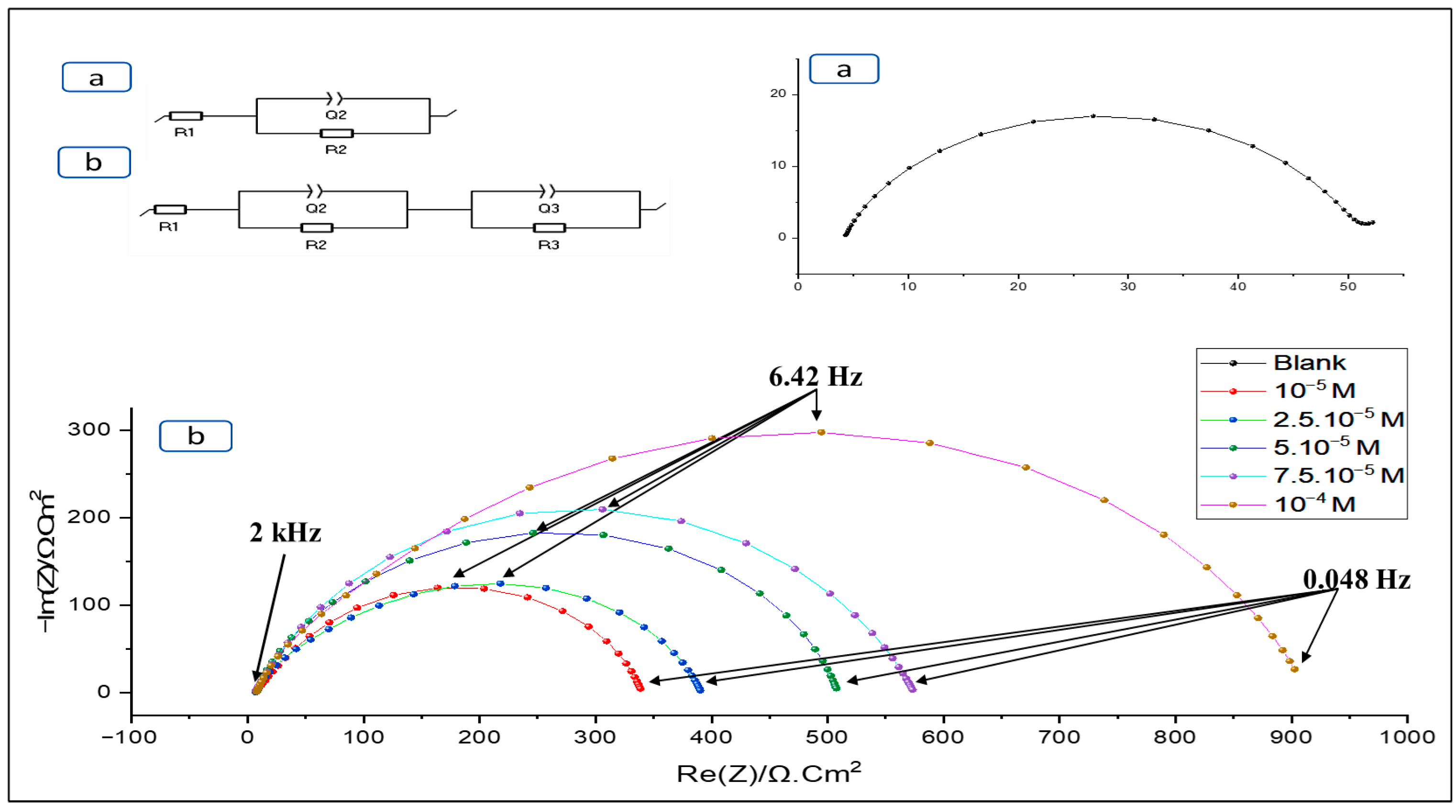
EIS is a method employed to study inhibition mechanisms. It provides information on the electrochemical processes involved at the metal-solution interface. Impedance data are analysed by modelling the results using equivalent circuits containing resistive and capacitive elements [35].
The electrical model adopted includes the electrolyte resistance (R1) and the resistance induced by the protective layer (R2). In addition, the Faraday resistance (R3) is associated with electrochemical charge transfer processes. The constant phase elements (Q2, Q3) are introduced to provide a better model of the frequency dispersion of the time constants [36,37].
Interpretation of the Nyquist diagram, obtained in a hydrochloric medium, reveals depressed semicircles, indicating the presence of a mechanism controlled by charge transfer (Figure 7). Adding the inhibitor BOZS has no effect on the overall shape of the Nyquist diagram, but results in a progressive increase in capacitive loop sizes as a function of concentration. This confirms the influence of the inhibitor on the formation and stabilisation of the protective layer [38].
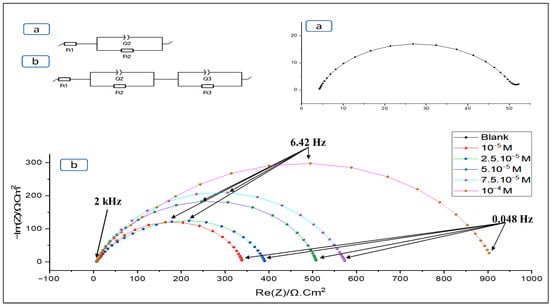
Figure 7.
Nyquist plots with (b) and without (a) inhibitor in hydrochloric acid.
As Table 2 illustrates, the resistance of charge transfer and inhibitory efficiency improved with inhibitor concentration increase, reaching a maximum of 94.7% corresponding to 10−4 M.

Table 2.
EIS results with and without benzoxazole-2-thionein in hydrochloric acid (1 M).
3.3. Adsorption Isotherms
The adsorption of inhibitors is a phenomenon based on their interactions with the metal. This process depends on the adsorbed H2O molecules’ replacement by inhibitor molecules. This process can be represented by Equation (3) [39,40]:
Orgaq + xH2Oads <=> Orgads + xH2Oaq
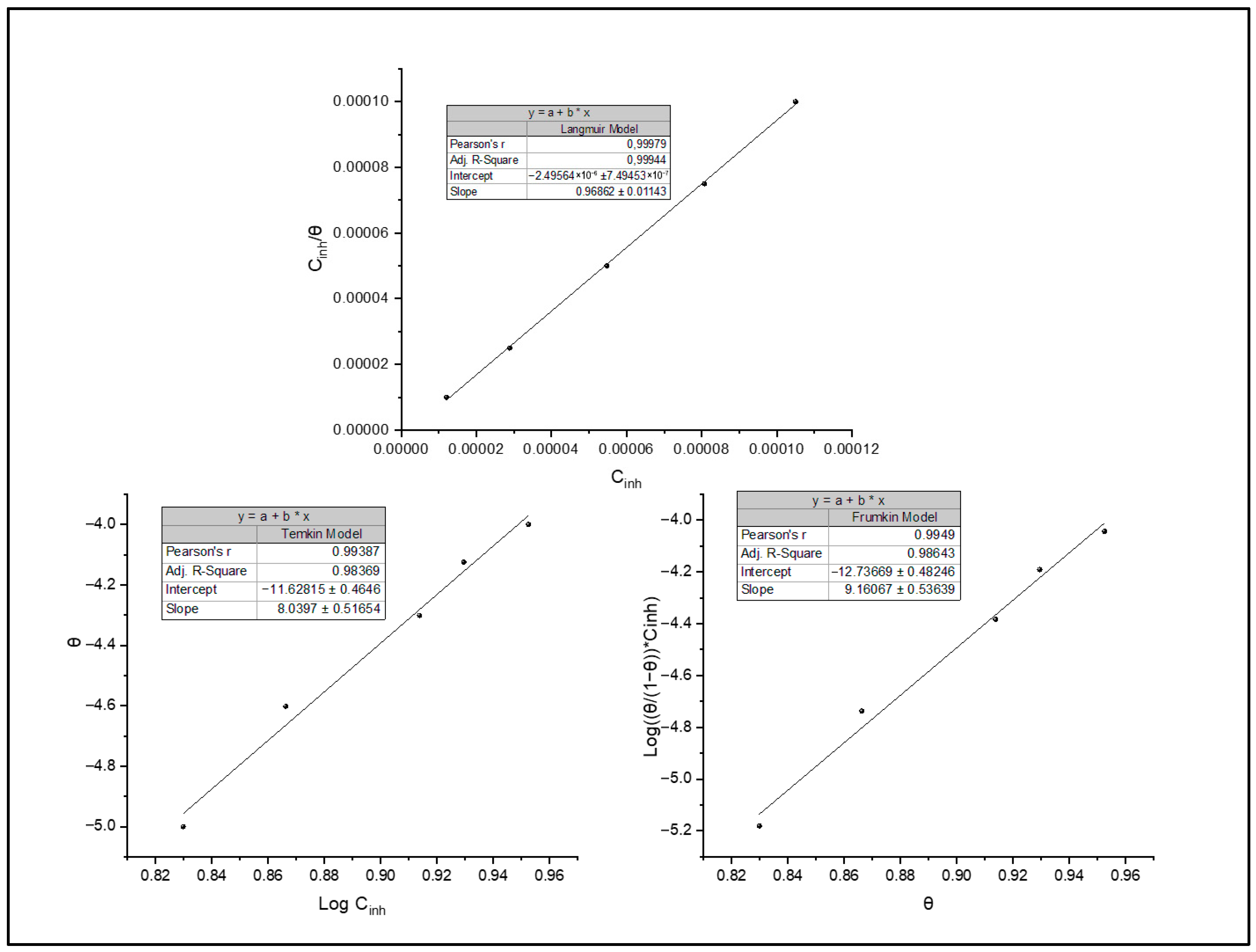
Various isothermal models have been adopted to estimate the inhibitor adsorption process, including Frumkin, Temkin and Langmuir. Langmuir’s model stands out for its ability to analyse the obtained data according to the correlation coefficient obtained (Figure 8). This model is based on several hypotheses, such as a uniform mono-molecular layer of the inhibitor formation onto the surface and the absence of interactions between the adsorbed molecules. It establishes a relationship between the inhibitor concentration in solution Cinh and the degree of surface coverage θ through Equation (4) [8,32]:
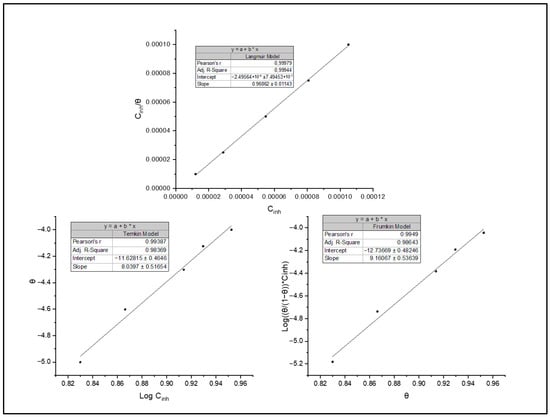
Figure 8.
Isotherms of adsorption: Langmuir, Temkin and Frumkin.
The constant of adsorption Kads and adsorption standard free energy of ΔG° ads are necessary parameters to understand the interactions. The adsorption free energy is calculated according to the following equation [39]:
ΔG°ads = −2.303 RT log (55.5 × Kads)
The results obtained indicate an adsorption constant of 3.538 L/mol, indicating a significant affinity between BOZS inhibitor and C38 steel surface. In addition, the value of ΔG°ads = −38.14 kJ/mol highlights significant interactions between BOZS molecules and the C38 steel’s active sites. The negativity of this value confirms the adsorption process spontaneity, underlining the tendency for deposition [41].
The adsorption process involves physisorption and chemisorption. Physisorption is based on van der Waals forces, favoured by the presence of charges on the surface and by interaction with ionic species in solution [42,43]. However, chemisorption involves more significant interactions, such as charge transfers, made possible by the vacant orbitals of transition metals and the heteroatoms of inhibitors, whose non-bonding electrons facilitate the formation of chemical bonds [44].
3.4. The Immersion Time Effect
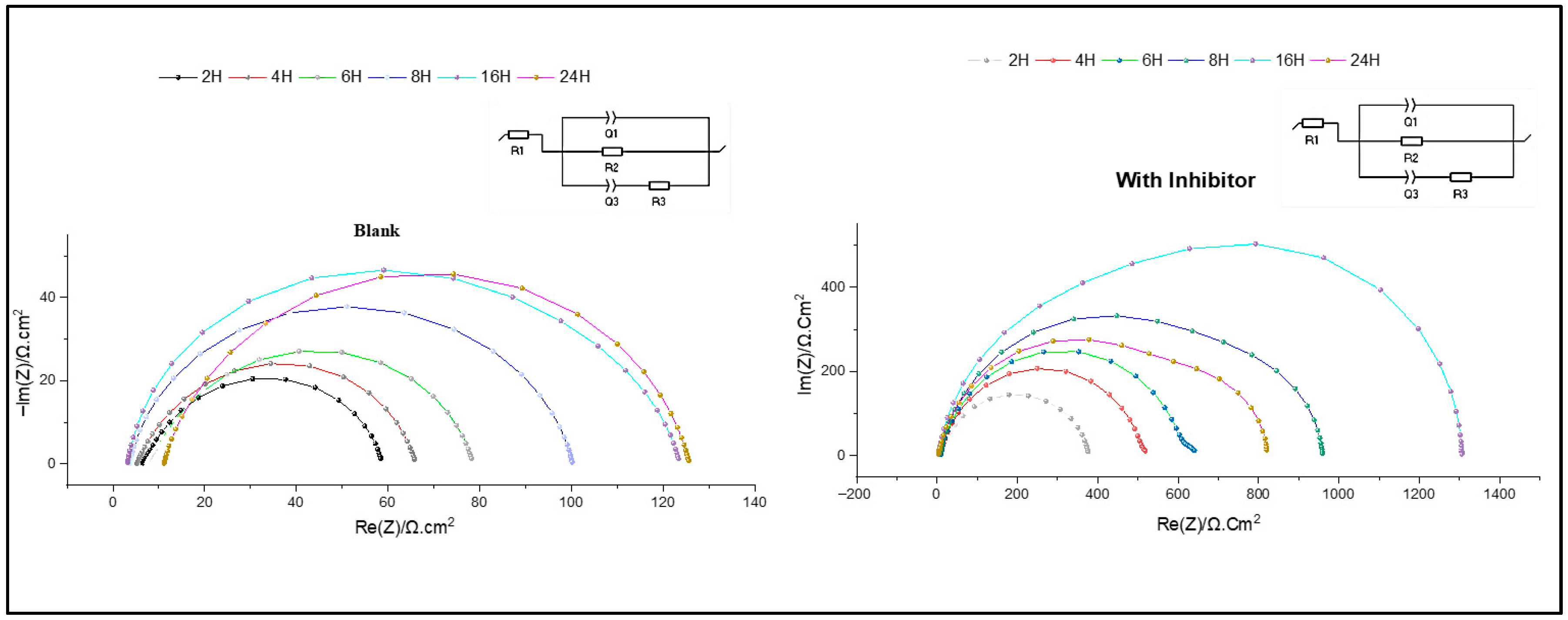
The stability of the formed protective layers is the basis for assessing the long-term effectiveness of corrosion inhibitors. Figure 9 depicts the impedance curves obtained for C38 steel during various immersion times, highlighting the formation and degradation of protective layers.
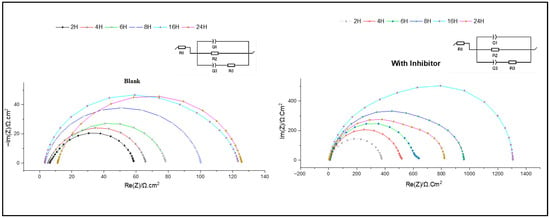
Figure 9.
Nyquist plots during 2 h, 4 h, 6 h, 8 h, 16 h and 24 h without and with inhibitor.
In this study, immersion times ranging from 2 h to 24 h were tested in a hydrochloric acid solution (1 M). The impedance curves, in the form of depressed semicircles, were similar, in the absence or presence of the inhibitor.
The electrochemical parameters were extracted using equivalent circuit modelling, as summarised in Table 3. These parameters include R1, representing the resistance of the solution, R2, corresponding to the formed protective layer resistance, and R3, which is related to the faradaic reaction. Furthermore, the phase constants Q2 and Q3 characterise the capacitive behaviour of the electrochemical double layer on the metal–solution interface [2].

Table 3.
EIS parameters results without and with 10−4 mol/l of benzoxazole-2-thione at different immersion times.
The results obtained indicate a significant decrease in charge transfer resistance (Rct) and inhibition efficiency (IE%) after 8 h of immersion, from 90.75% to 86.01%. Initially, Rct increased with immersion time, reaching a maximum after 16 h. This increase is attributed to the adsorption of inhibitor molecules and the replacement of water molecules by Cl− ions. However, over time, a decrease in Rct indicates inhibitor desorption, which may be due to prolonged exposure to an aggressive environment. This phenomenon is related to surface modifications, probably due to variations in surface hardness during immersion [45].
3.5. Surface Analysis
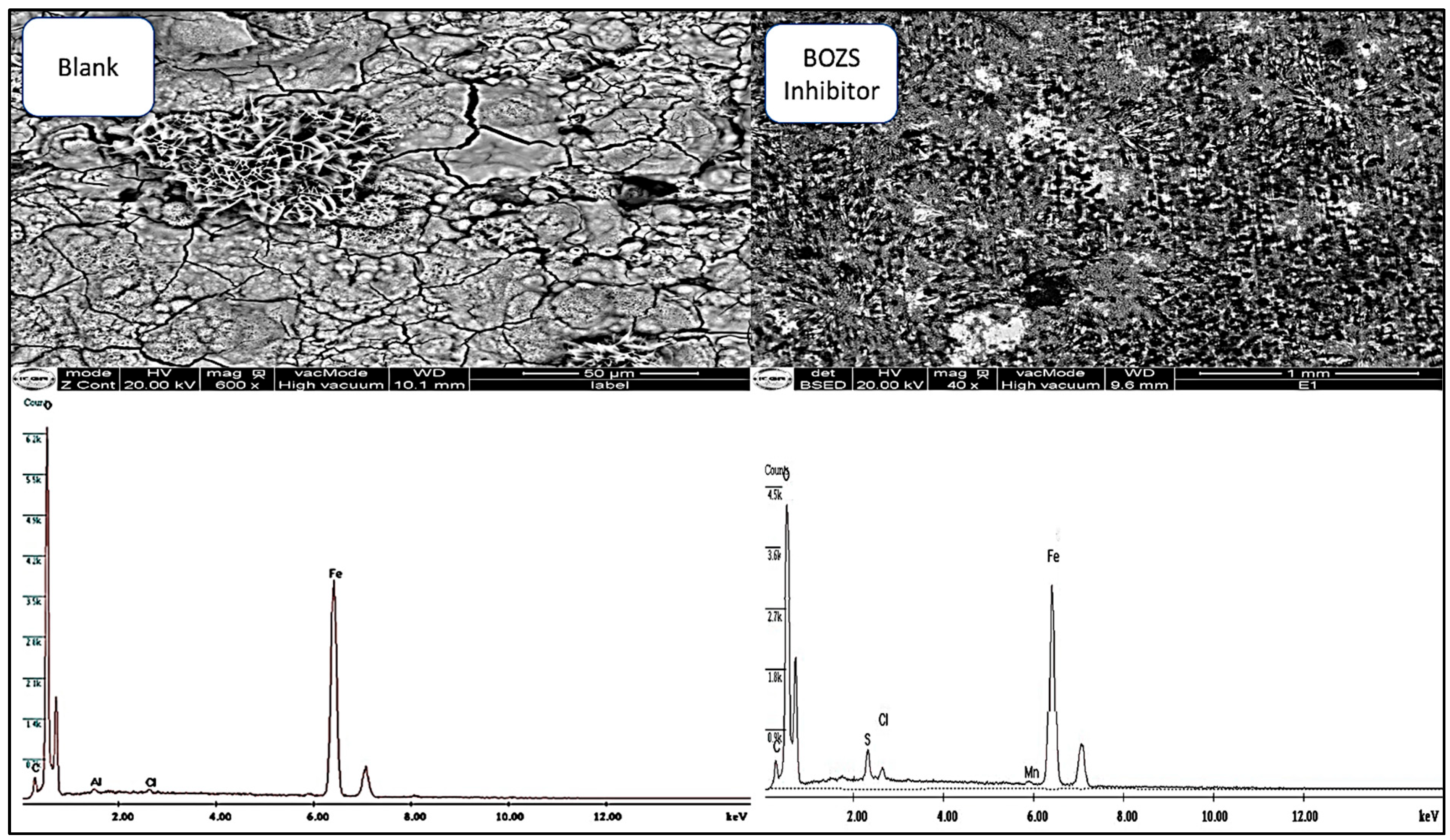
The study examines C38 steel surface analysis, using the SEM-EDX method. The analysis is based on the examination of the steel surface after immersion for 16 h in a hydrochloric acid solution, with and without BOZS inhibitor.
The results obtained on C38 steel without the inhibitor (Figure 10) revealed significant damage, in particular, the existence of pitting due to hydrochloric acid attack. However, when the BOZS inhibitor was added at a concentration of 10−4 mol/L, a significant surface improvement was observed, attributed to the adsorption of BOZS, and protective layer formation [46]. This observation is confirmed by the spectra of composition, which indicate a dissolution of the steel and a layer of iron hydroxide, oxide or hydrochloric acid salt formation [8].
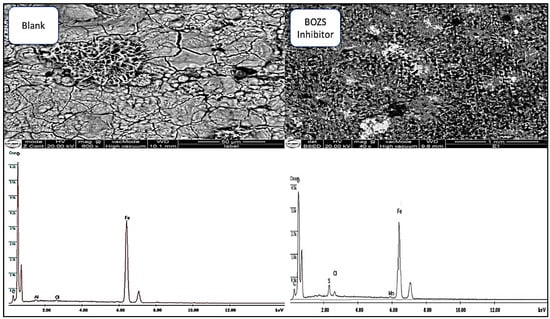
Figure 10.
SEM-EDS surface analysis in HCl solution without and with inhibitor (10−4 M) after 16 h of immersion.
The decrease in the oxygen peak after immersion suggest the formation of an active complex, inhibiting charge transfer and thus reducing the rate of corrosion. In addition, the appearance of a sulphur peak in the spectrum, in the presence of the inhibitor, indicates the BOZS adsorption and the formation of a protective layer [47,48,49].
3.6. Quantum Chemistry Calculations
3.6.1. MEP Descriptor
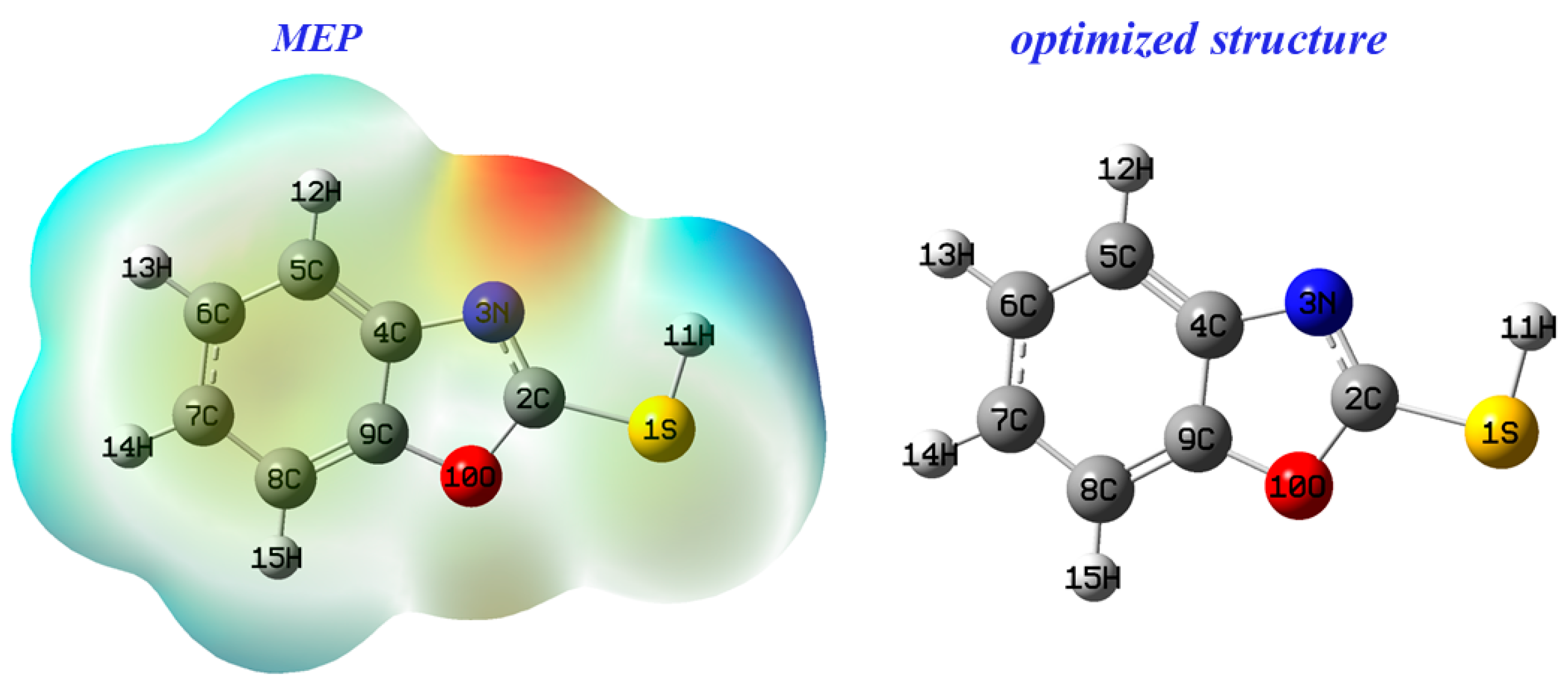
MEP is a quantum descriptor for predicting the reacting behavior of 2-mercaptobenzoxazole, and is linked to electronic density [50].
Figure 11 shows that areas with negative electrostatic dipoles are prone to electrophilic attack, while those with positive dipoles are sensitive to nucleophilic attack. The N3 nitrogen atom, highlighted in red for its high negative charge, is likely the target for electrophilic attack. Conversely, the S1 sulfur atom, indicated by a blue region, is potentially susceptible to nucleophilic attack.
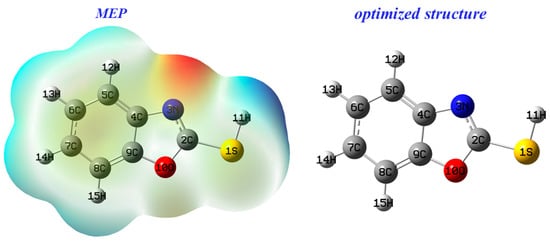
Figure 11.
Electrostatic potential map of benzoxazole-2-thione.
From Figure 11, the HOMO molecular orbital density is restricted on carbon atoms (C4, C7 and C9), with nitrogen and sulfur indicating that they are electron rich, while LUMO is mainly distributed on carbon atoms (C2, C5 and C8). Consequently, atoms (C4, C7, C9, N3 and S1) of benzoxazole-2-thiol tested can donate electrons, while atoms (C2, C5 and C8) cannot.
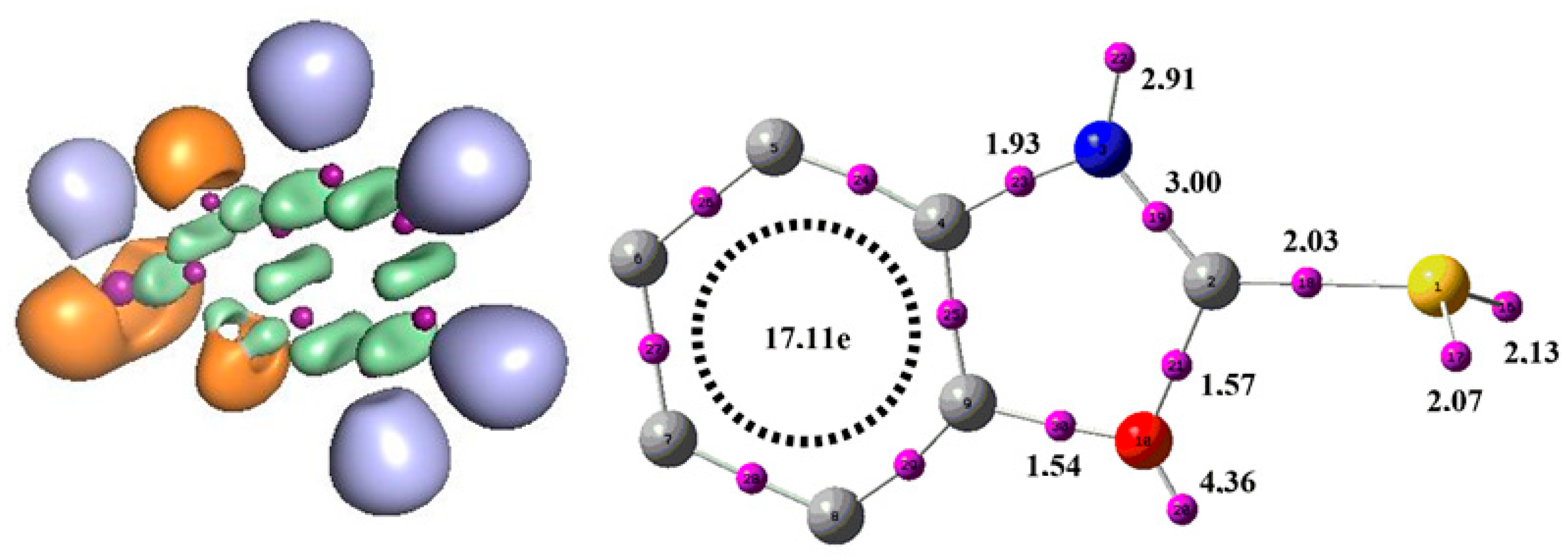
3.6.2. Electronic Localization Function (ELF)
From the ELF results presented in Figure 12, we can observe that the benzoxazole-2-thiol molecule contains 4 mono-synaptic basins, distributed on different atoms. Two monosynaptic basins on sulphur V(S) and V’(S) with a total population of 4.2e, corresponding to the two solitary pairs located on this atom. A mono-synaptic basin on the O atom with a population of 4.36e and a monosynaptic basin on nitrogen with a population of 2.91e. The existence of non-bonding doublets on these atoms explains the presence of monosynaptic basins. There are several disynaptic pools for this reagent, but the most important is the V(C2, N) basin, containing a population of 3.00e corresponding to a double bond, which gives an idea of the reactivity of this reagent. With regard to the reactivity of heteroatoms, a large population due to the presence of non-bonding doublets, associated with an excess of charges in the monosynaptic basins, contributes to the high chemical reactivity of heteroatoms. These factors provide important understandings into the availability of electrons for chemical reactions and could be employed to understand and predict the reactive behavior of molecules containing heteroatoms. This indicates that nitrogen exhibits high reactivity, as revealed by the results obtained for ELF calculations, confirmed by the indices.
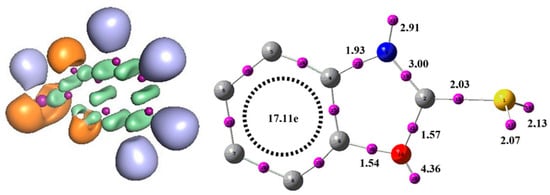
Figure 12.
Structure ELF du benzoxazole-2-thione.
3.6.3. Local Reactivity Indices
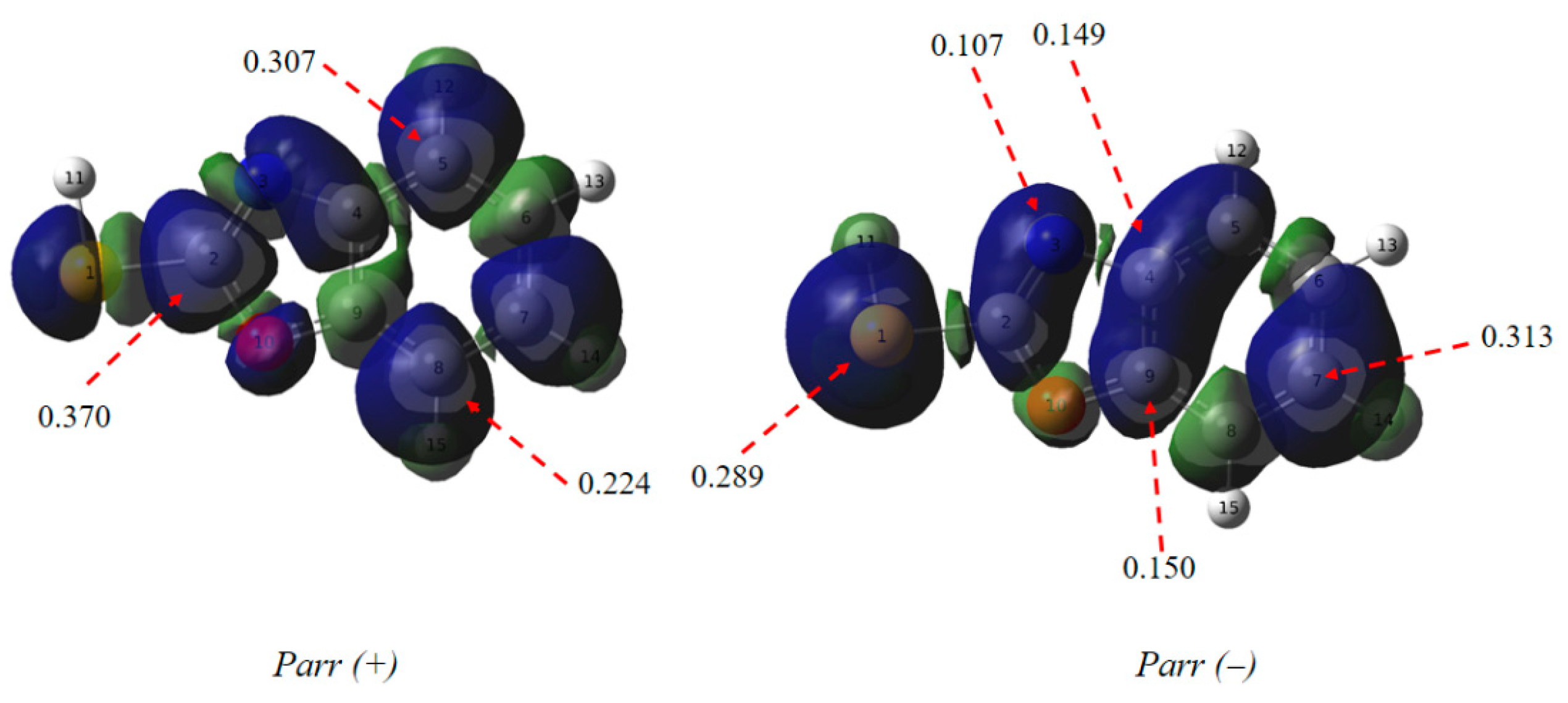
Introduction of Parr functions as dependable descriptors for identifying reactive sites in molecules during interactions with electrophiles or nucleophiles [51] These functions determine the site of electrophilicity, marked by Parr+(r) at its highest value in the electrophilic compound, and the site of nucleophilicity, indicated by Parr−(r) at its highest value in the nucleophilic benzoxazole-2-thiol (Figure 13).
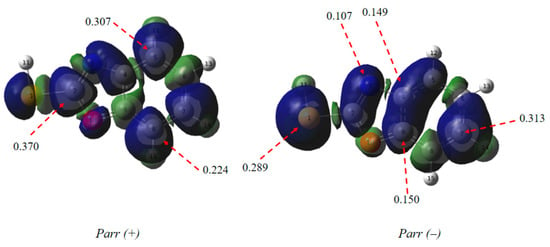
Figure 13.
Local reactivity descriptors calculated for the inhibitor.
The highest value is that of the sulphur atom, the nitrogen and the carbon atoms (C4, C7 and C9), therefore, they have a more pronounced nucleophilic character than C9 which has a high function. So the main interaction is between carbon C9 and the metal. The sites favorable to electrophilic attack for benzoxazole-2-thiol are the carbon atoms (C2, C5 and C8).
3.6.4. Molecular Frontier Orbitals
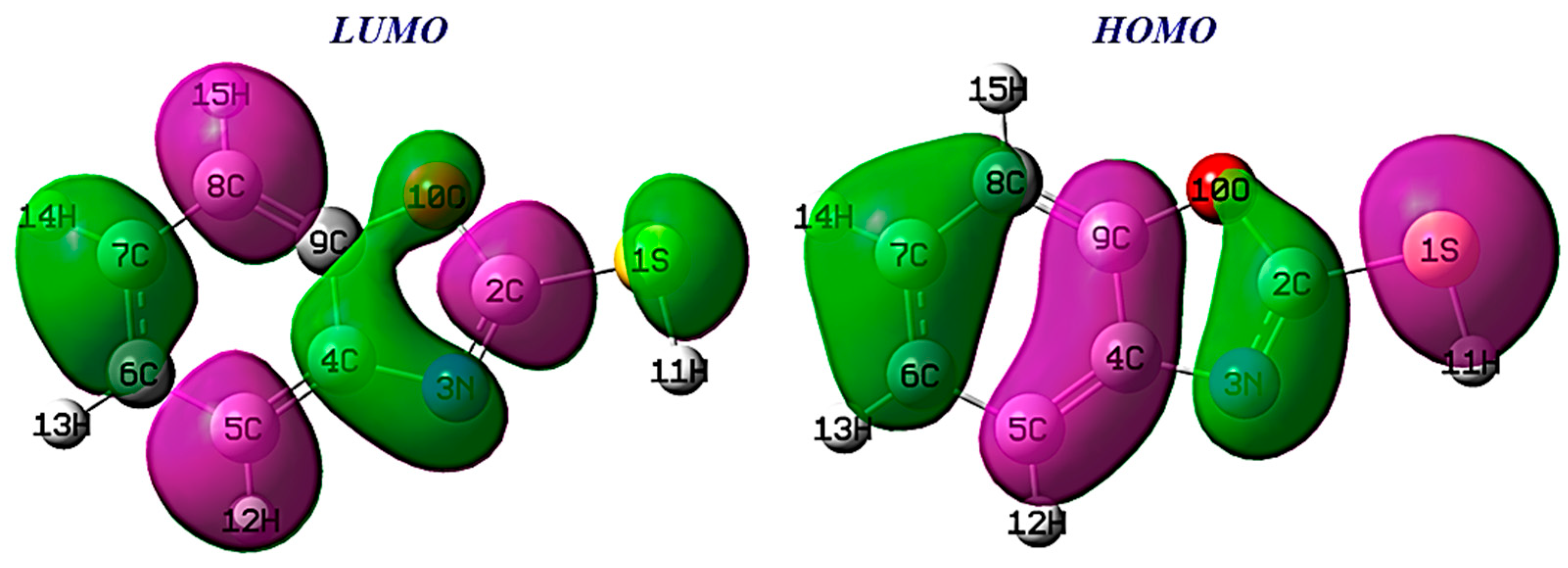
Understanding the reactivity of a molecule is crucial, and analyzing its frontier orbitals is a key aspect of this process [52]. The HOMO identifies electron/donating regions within the molecule, which are prone to interaction with electrophiles. Conversely, the LUMO identifies regions within benzoxazole-2-thiol that have a higher tendency to accept electrons.
From Figure 14, the HOMO benzoxazole-2-thiol orbital density is localized on carbon C4, C7 and C9, with nitrogen and sulfur indicating that they are electron-rich, while LUMO is mainly distributed on carbon atoms (C2, C5 and C8). Consequently, atoms (C4, C7, C9, N3 and S1) of the inhibitor can give electrons to atoms C2, C5 and C8.
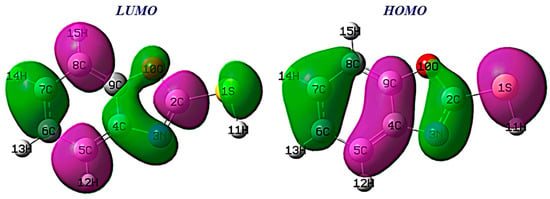
Figure 14.
HOMO and LUMO orbitals of benzoxazole-2-thion.
3.6.5. Analysis of Global Inhibitor Reactivity
Chemical descriptors of reactivity are spatially independent quantities, referred to as global descriptors. The quantum parameters of the molecule were calculated from HOMO and LUMO orbitals via B3LYP/DFT method.
According to Table 4, the EHOMO value of −6.417 eV indicates a high electron-donating ability towards the C38 steel surface. Additionally, the relatively low dipole moment (μ = 0.544 Debye) and small energy gap (E(LUMO-HOMO) = 5.428 eV) suggest high inhibition efficiency. The positive value of ΔN, less than 3.6 eV, shows a strong tendency for electron donation to the free d orbital of C38 steel. The tendency is maintained by the low molecular hardness. Furthermore, the low electrophilicity index (ω = 2.526 eV) of benzoxazole-2-thiol suggests a possible increase in efficiency.

Table 4.
Some quantum descriptors of the inhibitor in the isolated state calculated by B3LYP/6-311G (d) (values in (eV)).
3.6.6. Benzoxazole-2-Thiol—C38 Steel Complex
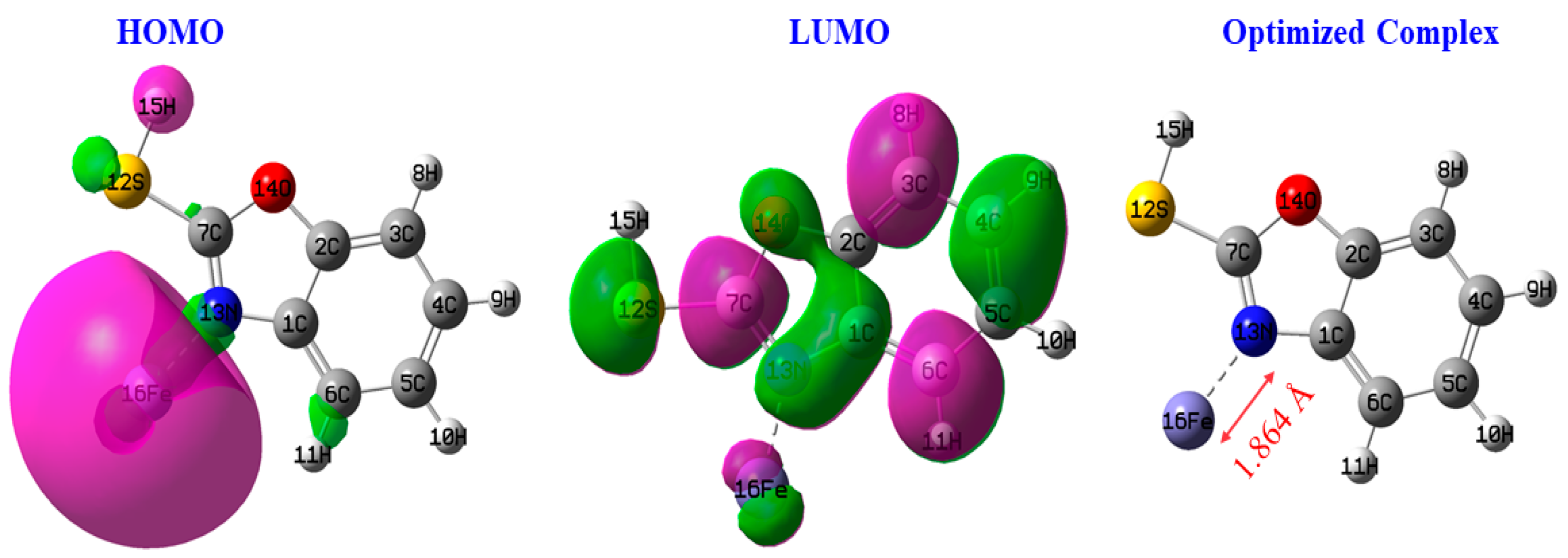
According to Figure 15, we observed a redistribution of the density of the orbitals in benzoxazole-2-thione in the presence of the iron atom compared with the isolated protector, particularly at the most reactive sites of the inhibitor. The modification of this molecular orbital structure of benzoxazole-2-thione is a sign of coupling between the adsorbate and the substrate, which also depends on the distance between the protector and the steel surface. We also note that the LUMO is highly condensed at the iron, showing that the inhibitor/iron complex could be the most reactive to electron gain via the iron atom.
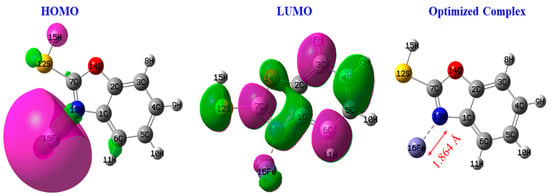
Figure 15.
HOMO and LUMO of the complex.
The distance between the surface iron atom and the nitrogen atom of benzoxazole-2-thione involved in the Steel-N bond formation is typical of covalent bonds (1.846 Å).
3.6.7. Protonated Inhibitor
DFT calculations are essential for understanding the effects of protonation of benzoxazole-2-thione molecules on their interactions with C38 steel surfaces. They provide detailed information on electronic properties and molecular interactions.
According to Table 5, the energy levels of HOMO and LUMO orbitals of the protonated species are lower than those of its neutral form. This suggests that the protonated benzoxazole-2-thione accepts electrons in a system where electrons go from C38 steel to benzoxazole-2-thione.

Table 5.
Quantum chemical parameters calculated for the protonated and neutral forms.
Term negative of ΔN suggests that electron transfer from C38 steel species to the protonated benzoxazole-2-thione is more favorable. This ability to capture electrons is reinforced by the high electronegativity value (χ = 8.714 eV) as well as the electrophilicity index (ω = 15.248 eV).
3.7. Molecular Dynamics and Monte Carlo Simulation Results
3.7.1. Monte Carlo Method
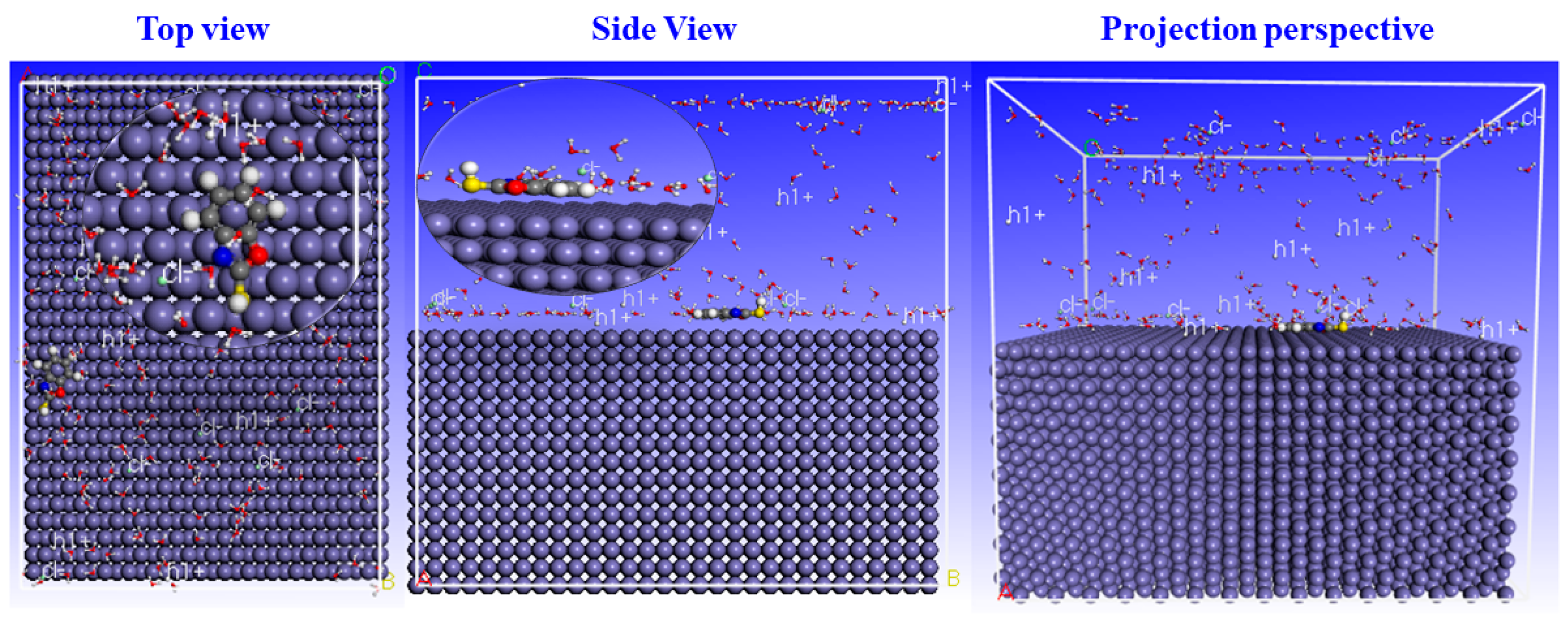
As shown in Figure 16, the adsorption energy of benzoxazole-2-thione on the C38 steel surface is negative. Consequently, adsorption of this organic compound is possible and favorable. Furthermore, a higher adsorption energy in absolute terms indicates greater inhibitor-substrate interaction. As a result, adsorption of the inhibitor on the Fe(110) metal surface is highly effective and can provide protection and form a barrier to iron corrosion.
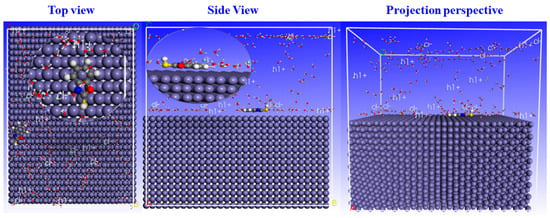
Figure 16.
Side and Top view configuration of benzoxazole-2-thione adsorption on C38 steel.
Figure 16 shows that benzoxazole-2-thione is adsorbed parallel to the iron surface. This indicates that strong interactions occur between benzoxazole-2-thione and iron atoms. The planar adsorption mode is due to the heteroatoms’ free electrons and the aromatic ring π electrons present in benzoxazole-2-thione; this orientation ensures a large adsorption surface and therefore a high inhibitory efficiency.
3.7.2. MD Simulations
The calculated interaction energy between the iron surface and benzoxazole-2-thione (Table 6) is equal to (−176.39 kcal/mol). The more negative the interaction energy, the more strongly benzoxazole-2-thione adsorbs spontaneously onto the iron surface. As a result, the binding energy of the inhibitor is higher, making the adsorption system more stable with higher inhibitor efficiency.

Table 6.
Binding and interaction energies between benzoxazole-2-thione and the C38 steel substrate surface.
Benzoxazole-2-thione can therefore better protect the iron surface against corrosion. The C38 steel surface can be protected by the formation of an inhibitory layer [53,54].
3.7.3. Examination of the Inhibitor/Surface Fe (110) Interaction: The RDF Approach
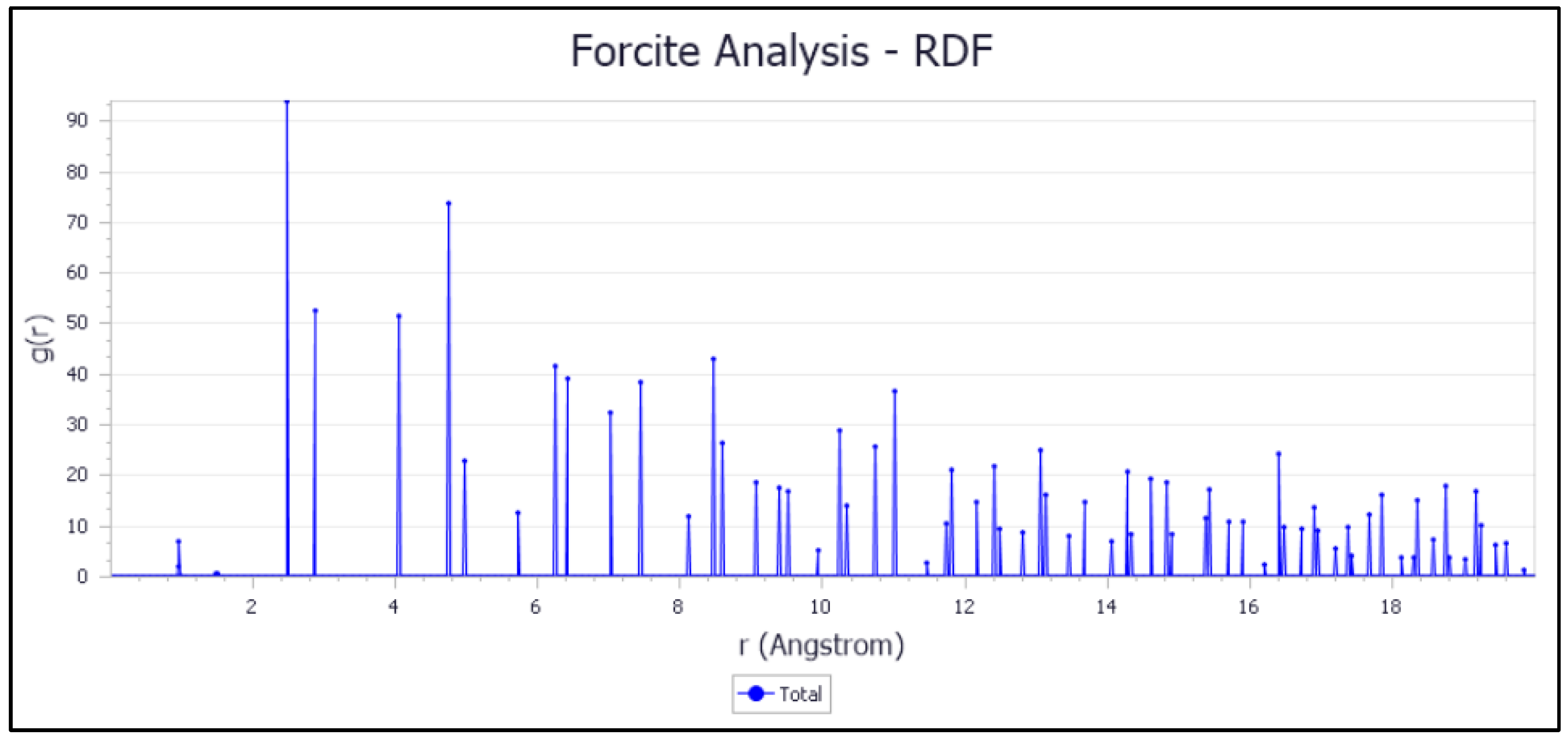
The analysis of the structure and interactions in different material systems can be carried out using the radial distribution function (RDF). RDF provides detailed information about the spatial organization of atoms and molecules, which is important for many applications in materials science, chemistry, and physics. In the context of corrosion inhibition, RDF analysis has critical atomic-scale insights into how inhibitor molecules arrange and interact with the Fe (110) surface.
RDF results allow us to identify preferred adsorption sites, characterize the strength and nature of inhibitor/metal interactions, and determine the molecular orientation of inhibitors on the steel surface. These details are essential for the inhibitors’ efficiency, which depends on their ability to form a stable, protective adsorbed layer that prevents corrosive agents from attacking the metal. The results obtained are presented in Figure 17.
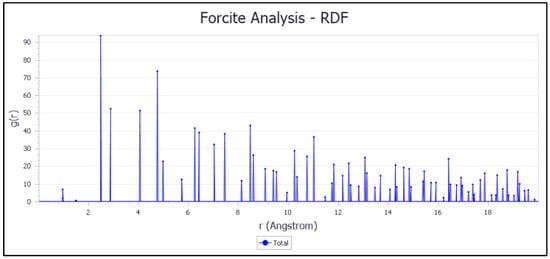
Figure 17.
RDF of inhibitor adsorption on the Fe (110) surface.
The most intense peak in the RDF analysis at a distance less than 3.5 Å suggests specific interactions between the iron (Fe) atoms of the surface and the inhibitor atoms, indicating the formation of chemical bonds. Thus, the adsorption type of the inhibitor molecule on the Fe (110) surface is chemisorption [55]. This results in a strong and specific interaction, which is essential for the stability and effectiveness of corrosion inhibition 0.
These conclusions highlight the importance of the nature of the surface and crystallographic orientation in the adsorption behavior of inhibitory molecules, such as the BOSZ reagent. A thorough understanding of these interactions can guide the corrosion protection development.
4. Conclusions
This study investigates the performance of benzoxazole-2-thione as an inhibitor for C38 steel in HCl medium. Electrochemical techniques, including potentiodynamic polarisation and electrochemical impedance spectroscopy, were employed to evaluate its effectiveness, which indicates that inhibition efficiency increases with BOZS concentration, reaching a peak of 95.25%. Immersion time tests revealed that the protective layer reached optimum stability after 16 h of immersion, with a gradual decline thereafter due to desorption effects. Also, the free energy of adsorption confirms a mixed mechanism of physisorption and chemisorption. The interactions inhibitor-surface, water and hydrochloric acid were studied by using computational methods such as density functional theory, Monte Carlo simulations and molecular dynamics. The high correlation between experimental and theoretical data validates the formation of a stable protective film on the steel surface.
Author Contributions
Conceptualization, N.L. (Najoua Labjar) and S.E.H.; methodology, M.O., K.B., S.J., A.B., N.L. (Najoua Labjar), H.N., M.E.I., M.S.-A., M.L., N.L. (Nabil Lahrache), A.T. and S.E.H.; software, M.O., N.L. (Najoua Labjar) and S.E.H.; validation, M.O., K.B., S.J., A.B., N.L. (Nabil Lahrache), H.N., M.E.I., M.S.-A., M.L., N.L. (Najoua Labjar), A.T. and S.E.H.; formal analysis, N.L. (Najoua Labjar) and S.E.H.; investigation, M.O., K.B. and S.J.; data curation; writing—original draft preparation, M.O., K.B., S.J., A.B., N.L. (Nabil Lahrache), H.N., M.E.I., M.S.-A., M.L., N.L. (Najoua Labjar) and A.T.; writing—review and editing, N.L. (Nabil Lahrache); visualization, M.O., K.B., S.J., A.B., N.L. (Nabil Lahrache), H.N., M.E.I., M.S.-A., M.L. and N.L. (Najoua Labjar); supervision, K.B., S.J., A.B., N.L. (Nabil Lahrache), H.N., M.E.I., M.S.-A., M.L., N.L. (Najoua Labjar) and A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are openly available in a public repository.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zarrok, H.; Saddik, R.; Oudda, H.; Hammouti, B.; Midaoui, A.E.; Zarrouk, A.; Benchat, N.; Touhami, M.E. 5-(2-Chlorobenzyl)-2,6-Dimethylpyridazin-3-One: An efficient Inhibitor of C38 Steel Corrosion in Hydrochloric Acid. Der Pharma Chem. 2011, 3, 272–282. [Google Scholar]
- Al-Sharabi, H.A.; Bouiti, K.; Bouhlal, F.; Labjar, N.; Dahrouch, A.; El Mahi, M.; Lotfi, E.M.; El Otmani, B.; Benabdellah, G.A.; El Hajjaji, S. Electrochemical and Thermodynamic Evaluation on Corrosion Inhibition of C38 in 1M HCl By the Rumex Ethanolic Extract. Int. J. Corros. Scale Inhib. 2022, 11, 382–401. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, Y.; Cao, Y.; Chen, F.; Luo, Y.; Xiao, X.; Deng, Y.; Liu, Y. Field testing, analytical, and numerical assessments on the fatigue reliability on bridge suspender by considering the coupling effect of multiple pits. Struct. Infrastruct. Eng. 2025, 1–16. [Google Scholar] [CrossRef]
- Garcia-Ochoa, E.; Guzmán-Jiménez, S.J.; Hernández, J.G.; Pandiyan, T.; Vásquez-Pérez, J.M.; Cruz-Borbolla, J. Benzimidazole ligands in the corrosion inhibition for carbon steel in acid medium: DFT study of its interaction on Fe30 surface. J. Mol. Struct. 2016, 1119, 314–324. [Google Scholar] [CrossRef]
- Hegazy, M.A.; El-Etre, A.Y.; El-Shafaie, M.; Berry, K.M. Novel cationic surfactants for corrosion inhibition of carbon steel pipelines in oil and gas wells applications. J. Mol. Liq. 2016, 214, 347–356. [Google Scholar] [CrossRef]
- Messali, M.; Lgaz, H.; Dassanayake, R.; Salghi, R.; Jodeh, S.; Abidi, N.; Hamed, O. Guar gum as efficient non-toxic inhibitor of carbon steel corrosion in phosphoric acid medium: Electrochemical, surface, DFT and MD simulations studies. J. Mol. Struct. 2017, 1145, 43–54. [Google Scholar] [CrossRef]
- Yang, D.; Feng, X.; Yan, N.; Wang, Y.; Lu, L.; Mei, P.; Chen, W.; Lai, L. Corrosion Inhibition Studies of Benzoxazole Derivates for N80 Steel in 1 M HCl Solution: Synthesis, Experimental, and DTF Studies. Open J. Yangtze Oil Gas 2022, 7, 101–123. [Google Scholar]
- Omari, M.; Bouiti, K.; Labjar, N.; Jebbari, S.; Barhoumi, A.; Ait Sir, H.; El Hajjaji, S.; Dahrouch, A.; Nasrellah, H.; El idrissi, M.; et al. Novel bisbenzoxazole derivative as an effective corrosion inhibitor for C38 steel in hydrochloric acid medium: Experimental and theoretical investigations. Can. Metall. Q. 2025, 64, 1193–1211. [Google Scholar] [CrossRef]
- Anusha, G.; Mishma, J.C.; Sinha, R.K.; Suvarna, A.S.; Gaonkar, S.L. New benzisoxazole derivative: A potential corrosion inhibitor for mild steel in 0.5 M hydrochloric acid medium-insights from electrochemical and density functional theory studies. Heliyon 2023, 9, e21014. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.A.; Hegazy, M.M.; Awad, M.K.; Shawky, M. Chemical, electrochemical, theoretical (DFT & MEP), thermodynamics and surface morphology studies of carbon steel during gas and oil production using three novel di-cationic amphiphiles as corrosion inhibitors in acidic medium. J. Mol. Liq. 2021, 337, 116541. [Google Scholar] [CrossRef]
- Obot, I.B.; Macdonald, D.D.; Gasem, Z.M. Density functional theory (DFT) as a powerful tool for designing new organic corrosion inhibitors. Part 1: An overview. Corros. Sci. 2015, 99, 1–30. [Google Scholar] [CrossRef]
- Faska, Z.; Majidi, L. DFT study on the adsorption mechanism of pulegone and pulegone oxide molecules in gas and aqueous phases as effective corrosion inhibitors in Molar Hydrochloric Acid. Moroc. J. Chem. 2018, 6, 283–293. [Google Scholar]
- Toghan, A.; Gadow, H.S.; Fawzy, A.; Alhussain, H.; Salah, H. Adsorption Mechanism, Kinetics, Thermodynamics, and Anticorrosion Performance of a New Thiophene Derivative for C-Steel in a 1.0 M HCl: Experimental and Computational Approaches. Metals 2023, 13, 1565. [Google Scholar] [CrossRef]
- AlJeilawi, O.; AlAni, H.; AlZahra, A.; AlSultani, K. Exploring the Potential of Quantum Chemical Calculations for Synthesized Quinazoline Derivatives as Superior Corrosion Inhibitors in Acidic Environment. Phys. Chem. Res. 2024, 12, 205–217. [Google Scholar] [CrossRef]
- Bougrine, K.; Saber, I.; Barebita, H.; Ferraa, S.; Ouakki, M.; Belfaquir, M.; El youbi, M.S. Potentials for Inhibiting Corrosion on Mild Steel using Various Borphosphate Glasses In 1 M HCl. Anal. Bioanal. Electrochem. 2025, 17, 1–17. [Google Scholar] [CrossRef]
- Kharbach, Y.; Qachchachi, F.Z.; Haoudi, A.; Tourabi, M.; Zarrouk, A.; Jama, C.; Olasunkanmi, L.O.; Ebenso, E.E.; Bentiss, F. Anticorrosion performance of three newly synthesized isatin derivatives on carbon steel in hydrochloric acid pickling environment: Electrochemical, surface and theoretical studies. J. Mol. Liq. 2017, 246, 302–316. [Google Scholar] [CrossRef]
- Quartarone, G.; Ronchin, L.; Vavasori, A.; Tortato, C.; Bonaldo, L. Inhibitive action of gramine towards corrosion of mild steel in deaerated 1.0M hydrochloric acid solutions. Corros. Sci. 2012, 64, 82–89. [Google Scholar] [CrossRef]
- Fouda, A.S.; Hassan, A.F.; Elmorsi, M.A.; Fayed, T.A.; Abdelhakim, A. Chalcones as Environmentally-Friendly Corrosion Inhibitors for Stainless Steel Type 304 in 1 M HCl Solutions. Int. J. Electrochem. Sci. 2014, 9, 1298–1320. [Google Scholar] [CrossRef]
- Ouadi, Y.E.; Lamsayah, M.; Bendaif, H.; Benhiba, F.; Touzani, R.; Warad, I.; Zarrouk, A. Electrochemical and theoretical considerations for interfacial adsorption of novel long chain acid pyrazole for mild steel conservation in 1 M HCl medium. Chem. Data Collect. 2021, 31, 100638. [Google Scholar] [CrossRef]
- Faustin, M.; Maciuk, A.; Salvin, P.; Roos, C.; Lebrini, M. Corrosion inhibition of C38 steel by alkaloids extract of Geissospermum laeve in 1M hydrochloric acid: Electrochemical and phytochemical studies. Corros. Sci. 2015, 92, 287–300. [Google Scholar] [CrossRef]
- Bammou, L.; Belkhaouda, M.; Salghi, R.; Benali, O.; Zarrouk, A.; Zarrok, H.; Hammouti, B. Corrosion inhibition of steel in sulfuric acidic solution by the Chenopodium Ambrosioides Extracts. J. Assoc. Arab Univ. Basic Appl. Sci. 2014, 16, 83–90. [Google Scholar] [CrossRef]
- Finšgar, M.; Lesar, A.; Kokalj, A.; Milošev, I. A comparative electrochemical and quantum chemical calculation study of BTAH and BTAOH as copper corrosion inhibitors in near neutral chloride solution. Electrochim. Acta 2008, 53, 8287–8297. [Google Scholar] [CrossRef]
- Gece, G. Drugs: A review of promising novel corrosion inhibitors. Corros. Sci. 2011, 53, 3873–3898. [Google Scholar] [CrossRef]
- Costa, D.; Marcus, P. Adsorption of Organic Inhibitor Molecules on Metal and Oxidized Surfaces Studied by Atomistic Theoretical Methods. In Molecular Modeling of Corrosion Processes, 1st ed.; Taylor, C.D., Marcus, P., Eds.; Wiley: Hoboken, NJ, USA, 2015; pp. 125–156. [Google Scholar] [CrossRef]
- Raghavachari, K. Perspective on “Density functional thermochemistry. III. The role of exact exchange”. Theor. Chem. Acc. Theory Comput. Model. Theor. Chim. Acta 2000, 103, 361–363. [Google Scholar] [CrossRef]
- El Faydy, M.; Galai, M.; El Assyry, A.; Tazouti, A.; Touir, R.; Lakhrissi, B.; Ebn Touhami, M.; Zarrouk, A. Experimental investigation on the corrosion inhibition of carbon steel by 5-(chloromethyl)-8-quinolinol hydrochloride in hydrochloric acid solution. J. Mol. Liq. 2016, 219, 396–404. [Google Scholar] [CrossRef]
- Sastri, V.S.; Ghali, E.; Elboujdaini, M.; Sastri, V.S. Corrosion Prevention and Protection: Practical Solutions; Wiley: Chichester, UK, 2007. [Google Scholar]
- Lukovits, I.; Kálmán, E.; Zucchi, F. Corrosion Inhibitors—Correlation between Electronic Structure and Efficiency. Corrosion 2001, 57, 3–8. [Google Scholar] [CrossRef]
- Finšgar, M. 2-Mercaptobenzimidazole as a copper corrosion inhibitor: Part I. Long-term immersion, 3D-profilometry, and electrochemistry. Corros. Sci. 2013, 72, 82–89. [Google Scholar] [CrossRef]
- El Hamdouni, Y.; Bouhlal, F.; Kouri, H.; Chellouli, M.; Benmessaoud, M.; Dahrouch, A.; Labjar, N.; El Hajjaji, S. Use of Omeprazole as Inhibitor for C38 Steel Corrosion in 1.0 M H3PO4 Medium. J. Fail. Anal. Prev. 2020, 20, 563–571. [Google Scholar] [CrossRef]
- Bouiti, K.; aldeen Al-sharabi, H.; Bouhlal, F.; Labjar, N.; Dahrouch, A.; Mahi, M.E.; Lotfi, E.M.; El Otmani, B.; Benabdellah, G.A.; El Hajjaji, S. Use of the ethanolic extract from Eriobotrya Japonica seeds as a corrosion inhibitor of C38 in a 1 M HCl medium. Int. J. Corros. Scale Inhib. 2022, 11, 1319–1334. [Google Scholar] [CrossRef]
- Km, S.; Praveen, B.M.; Devendra, B.K. A review on corrosion inhibitors: Types, mechanisms, electrochemical analysis, corrosion rate and efficiency of corrosion inhibitors on mild steel in an acidic environment. Results Surf. Interfaces 2024, 16, 100258. [Google Scholar] [CrossRef]
- Al-Sodani, K.A.A.; Maslehuddin, M.; Al-Amoudi, O.S.B.; Saleh, T.A.; Shameem, M. Efficiency of generic and proprietary inhibitors in mitigating Corrosion of Carbon Steel in Chloride-Sulfate Environments. Sci. Rep. 2018, 8, 11443. [Google Scholar] [CrossRef] [PubMed]
- Bouiti, K.; aldeen Al-sharabi, H.; Bensemlali, M.; Bouhlal, F.; Abidi, B.; Labjar, N.; Laasri, S.; El Hajjaji, S. Effect of temperature on corrosion inhibition by ethanolic extract of Eriobotrya Japonica seeds in chloride medium 1M. Eur. Phys. J. Appl. Phys. 2022, 97, 67. [Google Scholar] [CrossRef]
- Bouhlal, F.; Labjar, N.; Abdoun, F.; Mazkour, A.; Serghini-Idrissi, M.; Mahi, M.E.; Lotfi, E.M.; Skalli, A.; Hajjaji, S.E. Chemical and electrochemical studies of the inhibition performance of hydro-alcoholic extract of used coffee grounds (HECG) for the corrosion of C38 steel in 1M hydrochloric acid. Egypt. J. Pet. 2020, 29, 45–52. [Google Scholar] [CrossRef]
- Bouhouche, I.; Bouiti, K.; Chraka, A.; El Hamil, A.; Labjar, N.; Damour, H.; Dahrouch, A.; Nasrellah, H.; Benmessaoud, M.; El Hajjaji, S. Eco-conscious corrosion inhibitor synthesis via Soxhlet extraction: Experimental study in HCl 1M and theoretical analysis using DFT and MC simulations. Can. Metall. Q. 2025, 64, 605–623. [Google Scholar] [CrossRef]
- Bentiss, F.; Jama, C.; Mernari, B.; Attari, H.E.; Kadi, L.E.; Lebrini, M.; Traisnel, M.; Lagrenée, M. Corrosion control of mild steel using 3,5-bis(4-methoxyphenyl)-4-amino-1,2,4-triazole in normal hydrochloric acid medium. Corros. Sci. 2009, 51, 1628–1635. [Google Scholar] [CrossRef]
- Hossam, K.; Bouhlal, F.; Hermouche, L.; Merimi, I.; Labjar, H.; Chaouiki, A.; Labjar, N.; Malika, S.-I.; Dahrouch, A.; Chellouli, M.; et al. Understanding Corrosion Inhibition of C38 Steel in HCl Media by Omeprazole: Insights for Experimental and Computational Studies. J. Fail. Anal. Prev. 2021, 21, 213–227. [Google Scholar] [CrossRef]
- Al-Sharabi, H.A.; Bouhlal, F.; Bouiti, K.; Bensemlali, M.; Labjar, N.; Benabdellah, G.A.; Dahrouch, A.; Laasri, S.; El Mahi, M.; Lotfi, E.M.; et al. Study of the corrosion inhibition of C38 steel in a 1M HCl medium by the ethanolic extract of Rumex Nervosus Vahl leaves. EPJ Appl. Phys. 2022, 97, 76. [Google Scholar] [CrossRef]
- Bentiss, F.; Lebrini, M.; Vezin, H.; Chai, F.; Traisnel, M.; Lagrené, M. Enhanced corrosion resistance of carbon steel in normal sulfuric acid medium by some macrocyclic polyether compounds containing a 1,3,4-thiadiazole moiety: AC impedance and computational studies. Corros. Sci. 2009, 51, 2165–2173. [Google Scholar] [CrossRef]
- Suedile, F.; Robert, F.; Roos, C.; Lebrini, M. Corrosion inhibition of zinc by Mansoa alliacea plant extract in sodium chloride media: Extraction, Characterization and Electrochemical Studies. Electrochim. Acta 2014, 133, 631–638. [Google Scholar] [CrossRef]
- Larouj, M.; Lgaz, H.; Serrar, H.; Zarrok, H.; Bourazmi, H.; Zarrouk, A.; Elmidaoui, A.; Guenbour, A.; Boukhris, S.; Oudda, H. Adsorption properties and inhibition of carbon steel corrosion in hydrochloric acid solution by ethyl 3-hydroxy-8-methyl-4-oxo-6-phenyl-2-(p-toly)-4, 6-dihydropyrimido [2, 1-b][1, 3] thiazine-7-carboxylate. J. Mater. Environ. Sci. 2015, 6, 3251–3267. [Google Scholar]
- Bouiti, K.; Al-sharabi, H.A.; Bouhlal, F.; Abidi, B.; Labjar, N.; Bensemlali, M.; Hajjaji, S.E. Response surface methodology for optimizing corrosion inhibition: Investigating the synergistic effect of Eriobotrya japonica extract and potassium iodide. Euro-Mediterr. J. Environ. Integr. 2024, 9, 469–481. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, N.; Li, X.; Zhang, L.; Wu, L.; Huang, Y. Synergistic inhibition behavior between indigo carmine and cetyl trimethyl ammonium bromide on carbon steel corroded in a 0.5 M HCl solution. Appl. Surf. Sci. 2015, 357, 845–855. [Google Scholar] [CrossRef]
- Chaubey, N.; Savita; Singh, V.K.; Quraishi, M.A. Corrosion inhibition performance of different bark extracts on aluminium in alkaline solution. J. Assoc. Arab Univ. Basic Appl. Sci. 2017, 22, 38–44. [Google Scholar] [CrossRef]
- Rehioui, M.; Abbout, S.; Benzidia, B.; Hammouch, H.; Erramli, H.; Daoud, N.A.; Badrane, N.; Hajjaji, N. Corrosion inhibiting effect of a green formulation based on Opuntia Dillenii seed oil for iron in acid rain solution. Heliyon 2021, 7, e06674. [Google Scholar] [CrossRef] [PubMed]
- Oguzie, E.E.; Njoku, V.O.; Enenebeaku, C.K.; Akalezi, C.O.; Obi, C. Effect of hexamethylpararosaniline chloride (crystal violet) on mild steel corrosion in acidic media. Corros. Sci. 2008, 50, 3480–3486. [Google Scholar] [CrossRef]
- M’hiri, N.; Veys-Renaux, D.; Rocca, E.; Ioannou, I.; Boudhrioua, N.M.; Ghoul, M. Corrosion inhibition of carbon steel in acidic medium by orange peel extract and its main antioxidant compounds. Corros. Sci. 2016, 102, 55–62. [Google Scholar] [CrossRef]
- Akkermans, R.L.C.; Spenley, N.A.; Robertson, S.H. Monte Carlo methods in Materials Studio. Mol. Simul. 2013, 39, 1153–1164. [Google Scholar] [CrossRef]
- Guo, L.; Obot, I.B.; Zheng, X.; Shen, X.; Qiang, Y.; Kaya, S.; Kaya, C. Theoretical insight into an empirical rule about organic corrosion inhibitors containing nitrogen, oxygen, and sulfur atoms. Appl. Surf. Sci. 2017, 406, 301–306. [Google Scholar] [CrossRef]
- Chafi, M.; Byadi, S.; Barhoumi, A.; Limouni, W.; Tizliouine, A.; Jama, C.; El Hachemi Omari, L. Study of copper removal by modified biomaterials using the response surface methodology, DFT Calculation, and molecular dynamic simulation. J. Mol. Liq. 2022, 363, 119799. [Google Scholar] [CrossRef]
- Sulaiman, K.O.; Onawole, A.T.; Faye, O.; Shuaib, D.T. Understanding the corrosion inhibition of mild steel by selected green compounds using chemical quantum based assessments and molecular dynamics simulations. J. Mol. Liq. 2019, 279, 342–350. [Google Scholar] [CrossRef]
- Kavimani, M.; Balachandran, V.; Narayana, B.; Vanasundari, K.; Revathi, B. Topological analysis (BCP) of vibrational spectroscopic studies, docking, RDG, DSSC, Fukui functions and chemical reactivity of 2-methylphenylacetic acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 190, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Hsissou, R.; Abbout, S.; Seghiri, R.; Rehioui, M.; Berisha, A.; Erramli, H.; Assouag, M.; Elharfi, A. Evaluation of corrosion inhibition performance of phosphorus polymer for carbon steel in [1 M] HCl: Computational studies (DFT, MC and MD simulations). J. Mater. Res. Technol. 2020, 9, 2691–2703. [Google Scholar] [CrossRef]
- Guo, L.; Kaya, S.; Obot, I.B.; Zheng, X.; Qiang, Y. Toward understanding the anticorrosive mechanism of some thiourea derivatives for carbon steel corrosion: A combined DFT and molecular dynamics investigation. J. Colloid Interface Sci. 2017, 506, 478–485. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).