Corrosion Resistance and Surface Conductivity of 446 Stainless Steel with Electrochemical Cr-Enrichment and Nitridation for Proton Exchange Membrane Fuel Cell (PEMFC) Bipolar Plates
Abstract
1. Introduction
2. Experimental Section
2.1. Material and Surface Modification
2.2. Interfacial Contact Resistance Measurement
2.3. Electrochemical Corrosion Measurements
2.4. Surface and Solution Analyses
3. Results
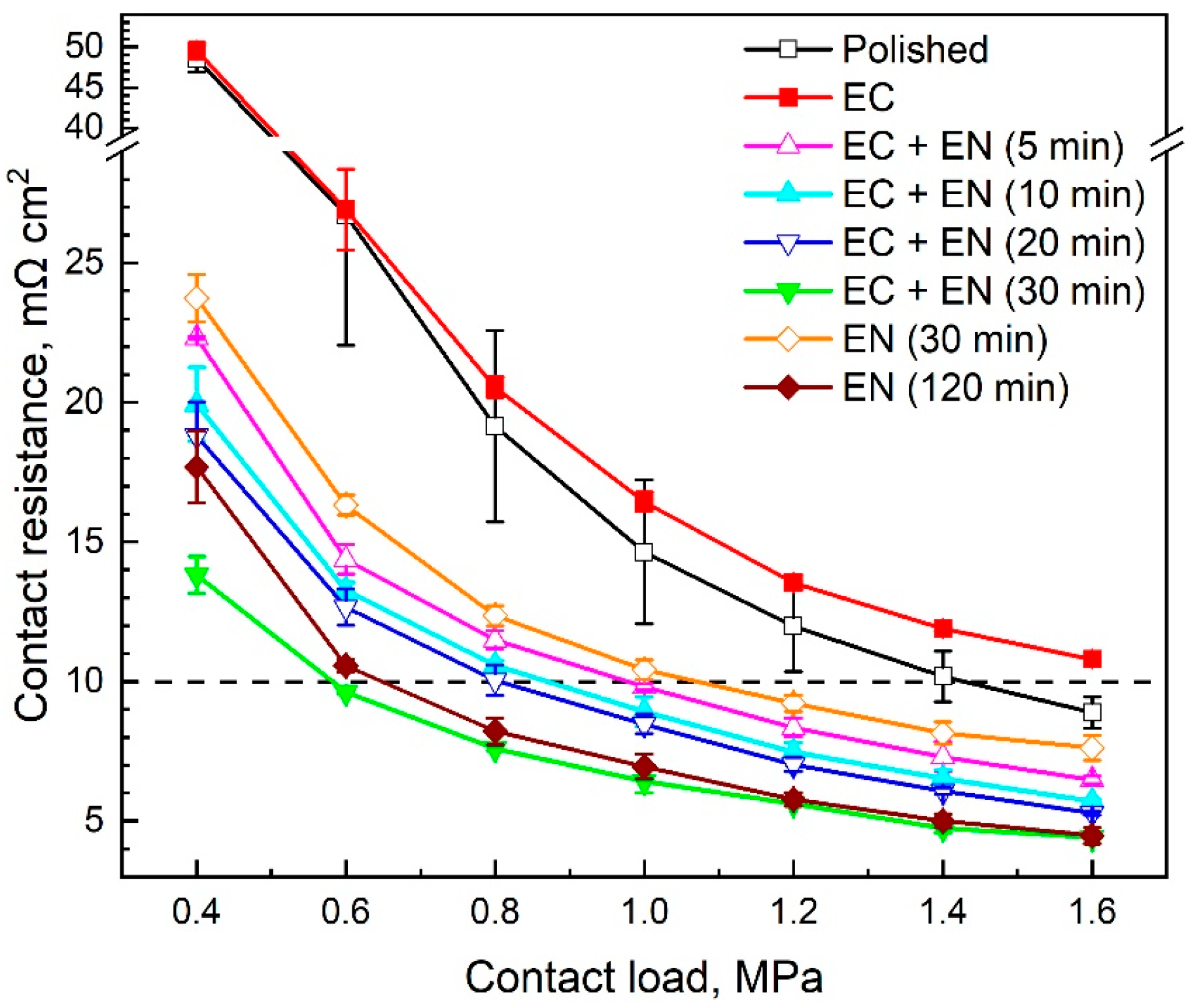
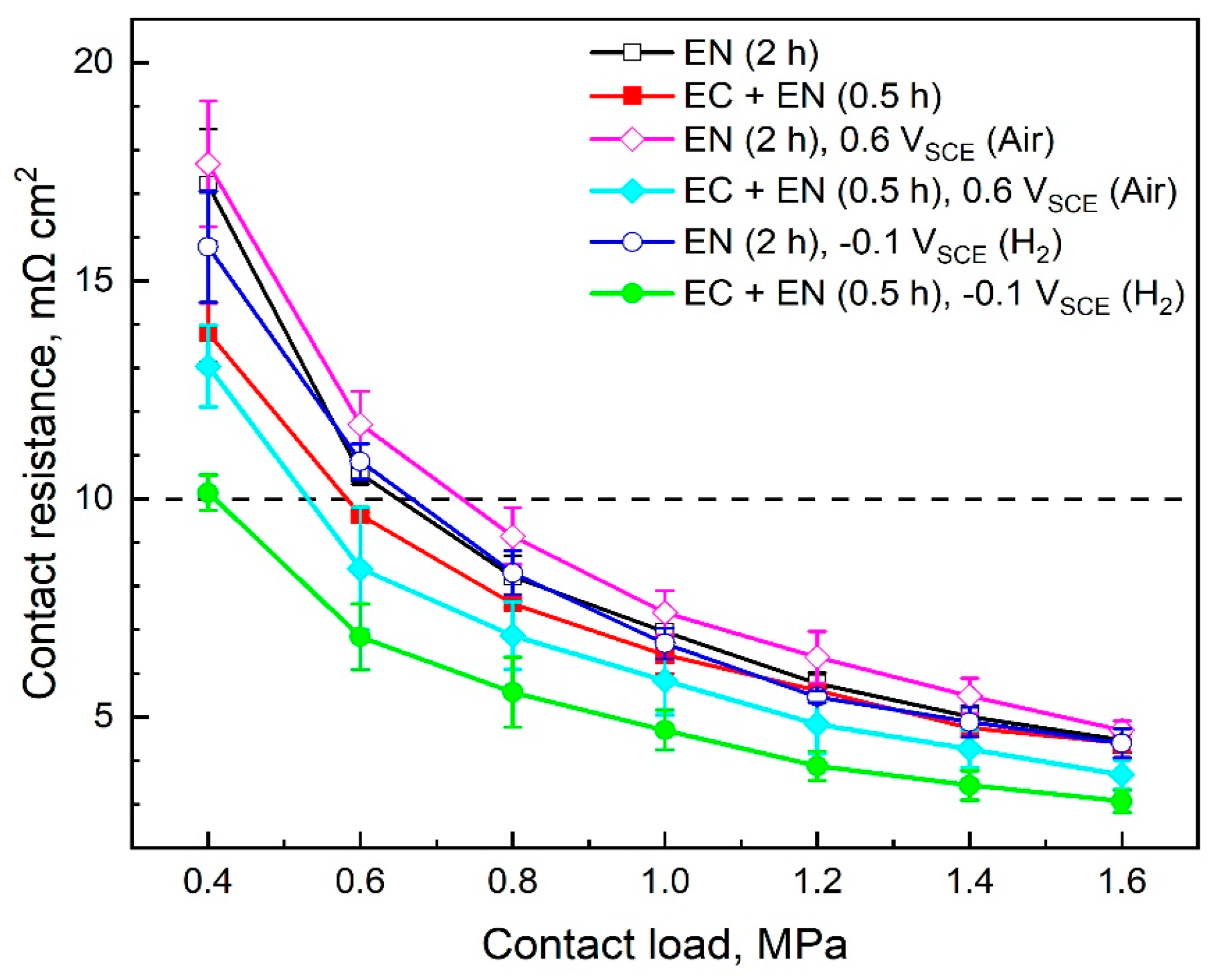
3.1. Surface Conductivity
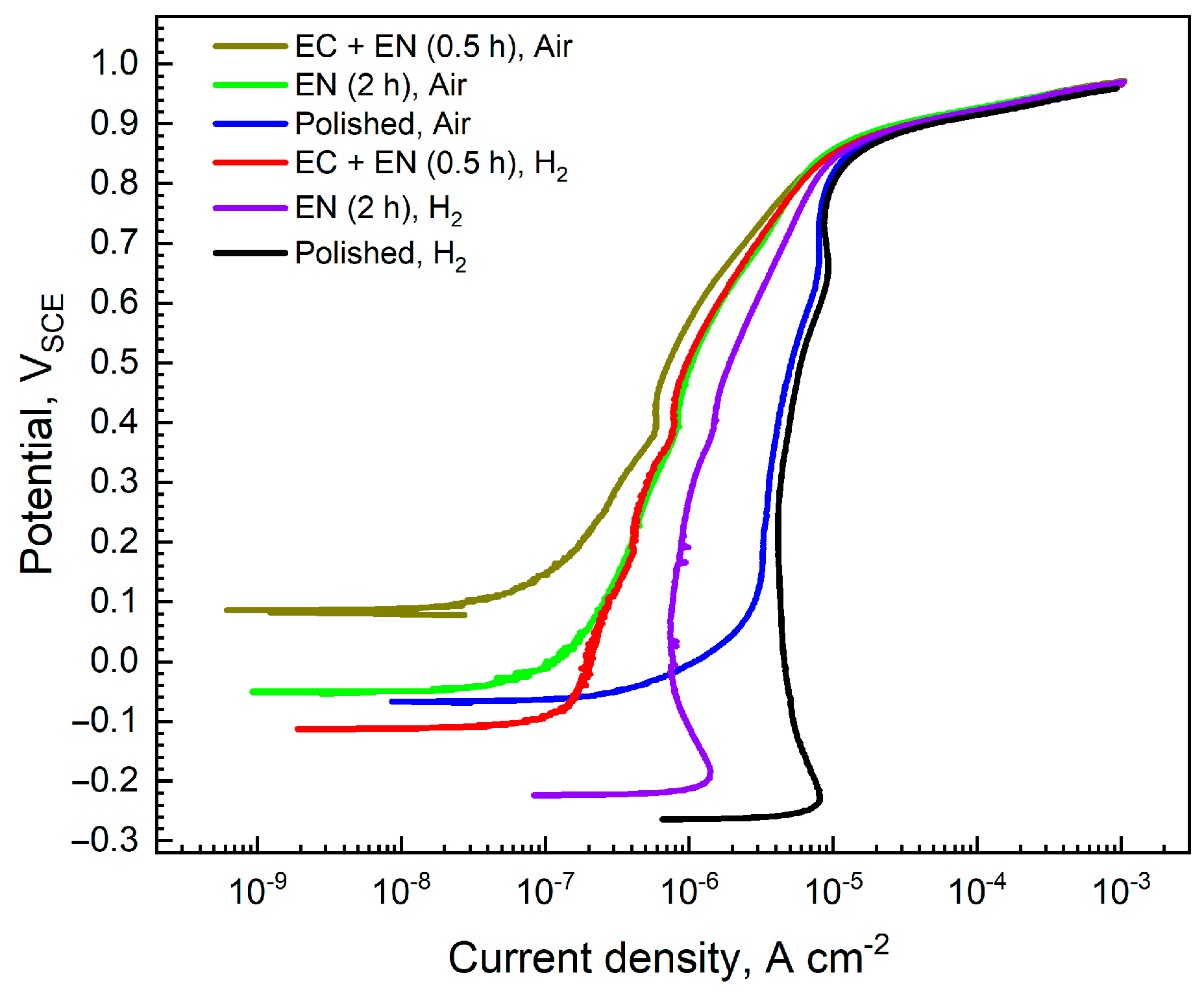
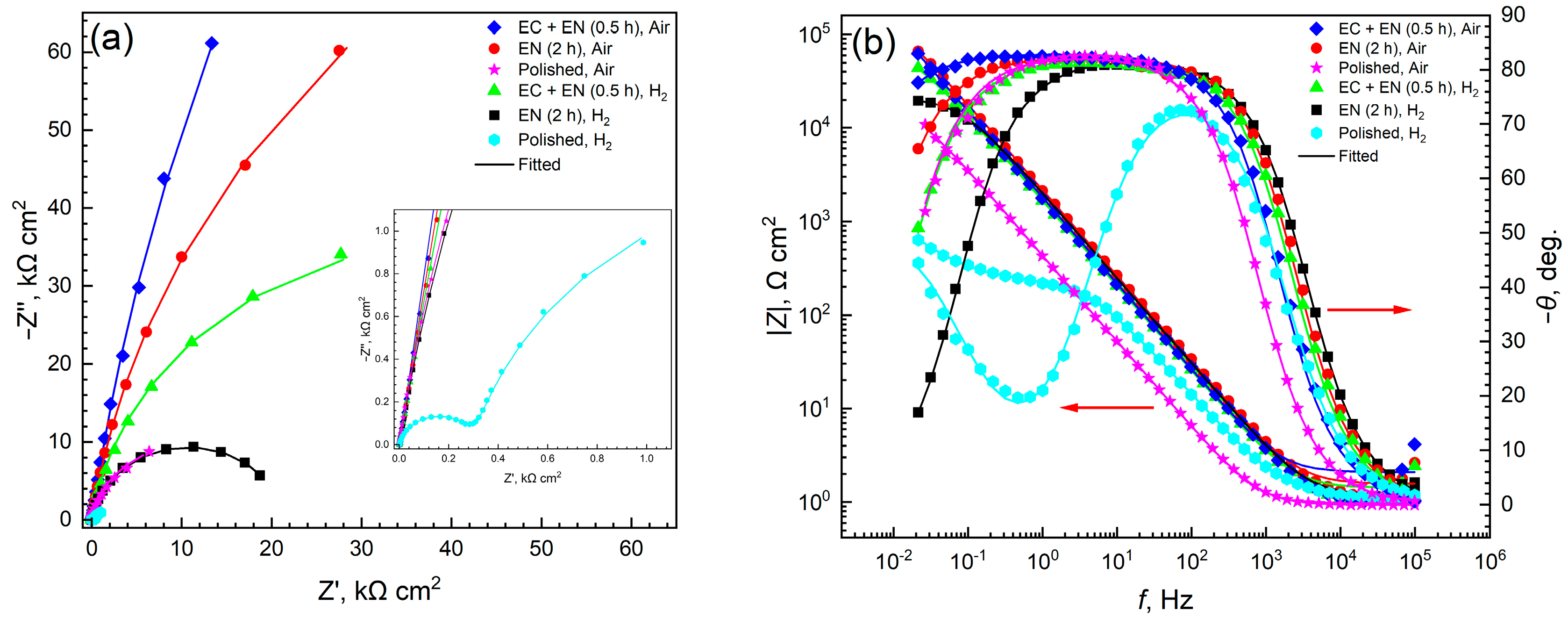
3.2. Electrochemical Corrosion Behavior
3.2.1. Anodic Polarization Curves
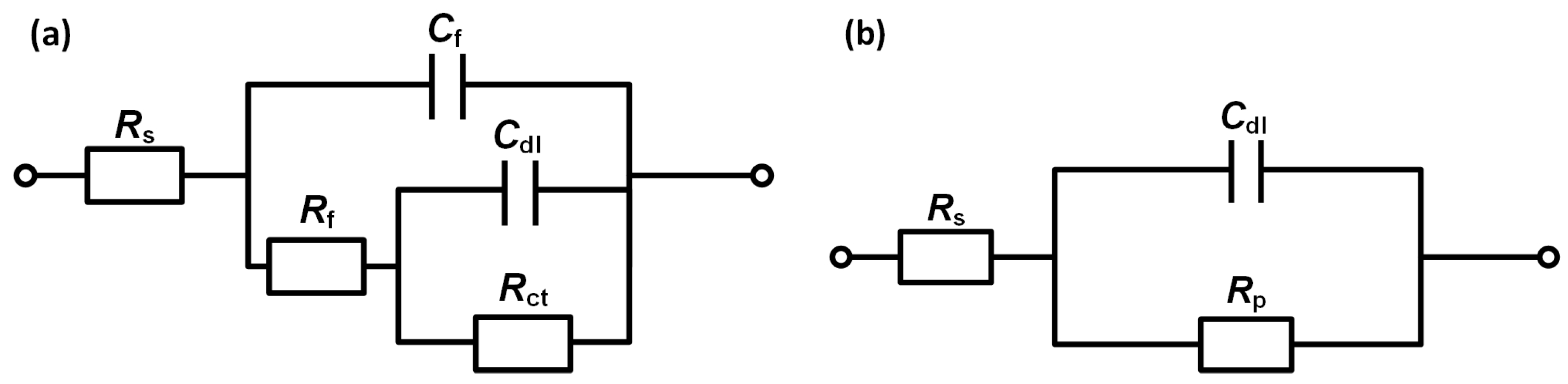
3.2.2. EIS Characteristics
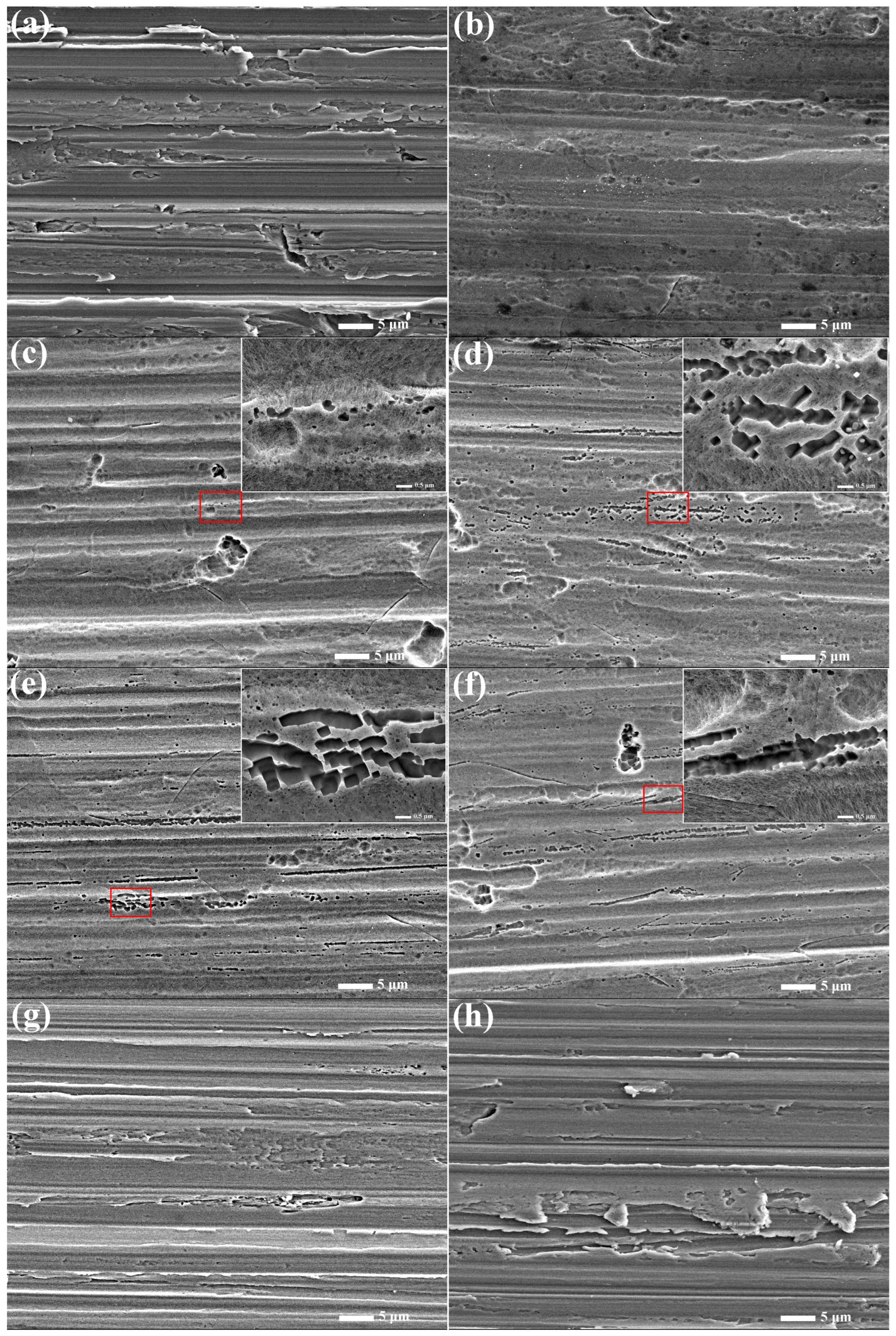
3.3. Surface Morphology
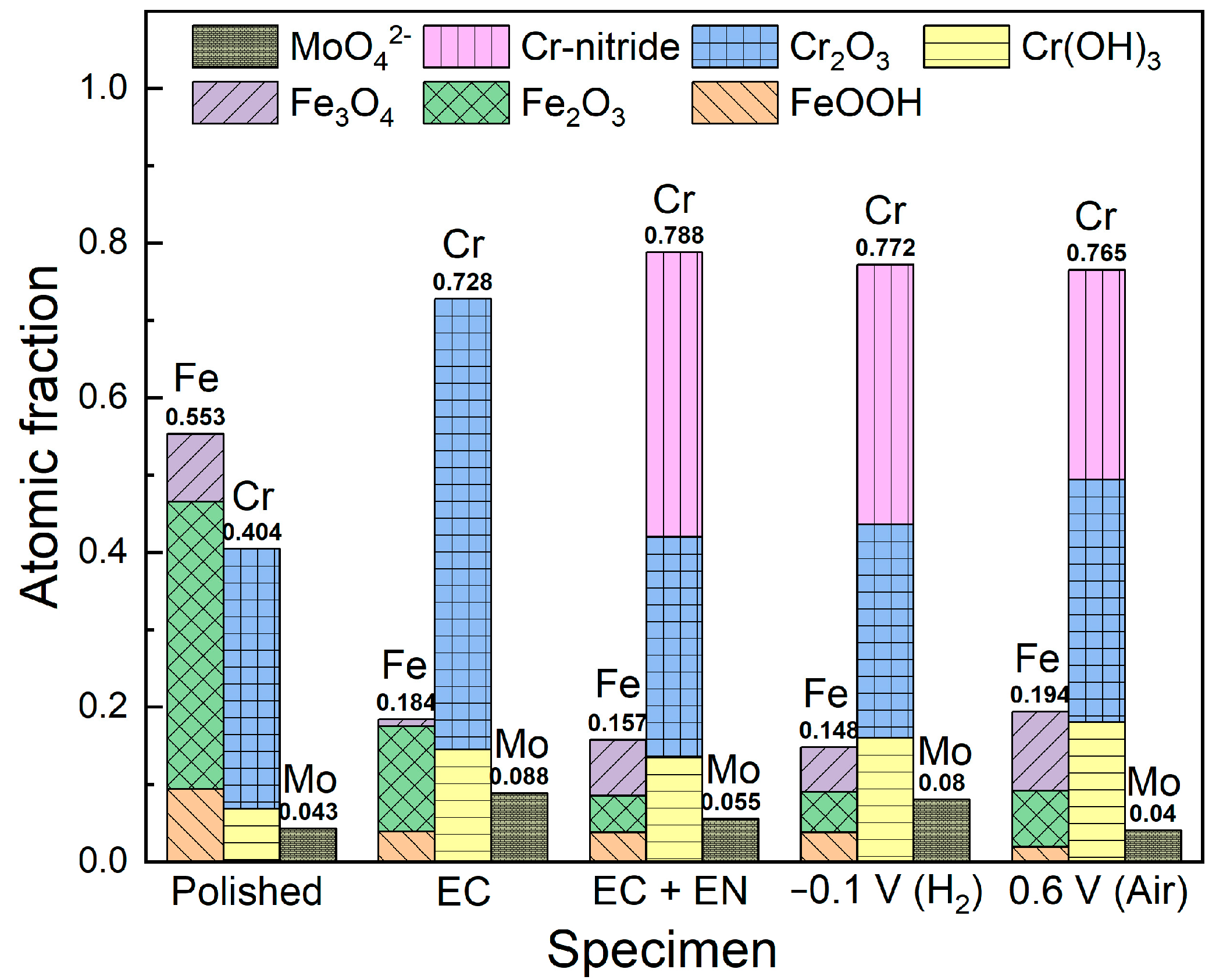
3.4. XPS Analysis of Different Specimen Surfaces
3.5. Release of Metallic Ions
4. Discussion
4.1. Surface Conductivity and Corrosion Resistance
4.2. Effect of Nitriding Time
5. Conclusions
- (1)
- The electrochemical Cr-rich pretreatment produces an oxide film with a Cr/Fe ratio of approximately 4.0 on the specimen surface. For the Cr-rich pretreated and nitrided specimens, the extension of the nitriding time from 5 to 30 min results in the enhancement of surface conductivity but the degradation of corrosion resistance to some extent. After 0.5 h of electrochemical nitridation, a thin mixed layer of oxides and nitrides forms on the specimen surface, which mainly consists of Cr-oxide and -nitride with atomic fractions of 0.42 and 0.37, respectively.
- (2)
- After 2 h of direct nitridation, the specimen displays an active corrosion state in the simulated anode environment. In contrast, after Cr-rich pretreatment and 0.5 h of nitridation, the specimen can spontaneously passivate in the simulated cathode and anode environments, which results in a significantly higher corrosion potential and polarization resistance as well as a lower passive current density. The Cr-rich pretreatment enhances the corrosion resistance of nitrided specimens under free corrosion conditions.
- (3)
- For the specimens with Cr-rich pretreatment and 0.5 h of nitridation, after 5 h of polarization at 0.6 VSCE in the simulated cathode environment, the stable current density and the ICR value at 1.4 MPa are approximately 0.26 μA cm−2 and 4.3 mΩ cm2, respectively. Whereas, after 5 h of polarization at −0.1 VSCE in the simulated anode environment, the specimens are in the cathodic polarization state. The stable current density and the ICR value at 1.4 MPa are approximately −0.39 μA cm−2 and 3.4 mΩ cm2, respectively. The Cr-rich pretreatment improves the corrosion resistance and surface conductivity of the nitrided specimens under typical PEMFC working potential conditions.
- (4)
- After 5 h of polarization tests at 0.6 and −0.1 VSCE in the simulated cathode and anode environments, the release amounts of Fe are significantly higher than those of Cr and Mo. There are many Cr-nitrides remaining on the surface film, with atomic fractions of about 0.27 and 0.34, respectively.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiao, K.; Xuan, J.; Du, Q.; Bao, Z.; Xie, B.; Wang, B.; Zhao, Y.; Fan, L.; Wang, H.; Hou, Z.; et al. Designing the next generation of proton-exchange membrane fuel cells. Nature 2021, 595, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Ogungbemi, E.; Ijaodola, O.; Khatib, F.N.; Wilberforce, T.; El Hassan, Z.; Thompson, J.; Ramadan, M.; Olabi, A.G. Fuel cell membranes–Pros and cons. Energy 2019, 172, 155–172. [Google Scholar] [CrossRef]
- Wilberforce, T.; Ijaodola, O.; Baroutaji, A.; Ogungbemi, E.; Olabi, A.G. Effect of Bipolar Plate Material on Proton Exchange Membrane Fuel Cell Performance. Energies 2022, 15, 1886. [Google Scholar] [CrossRef]
- Li, H.M.; Bi, F.F.; Dong, L.; Xu, Z.T.; Li, X.B.; Zhou, F.; Fan, C.Y.; Shi, W.Y.; Peng, L.F.; Lai, X.M. A novel ex-situ accelerated evaluation method for metallic bipolar plates in proton exchange membrane fuel cells. J. Power Sources 2023, 580, 233374. [Google Scholar] [CrossRef]
- Xuan, J.; Xin, Y.; Xu, L.; Guo, M.; Huang, L.; Zhang, Y.; Zhao, Y.; Liu, Y.; Li, L.; Xue, L.; et al. Effects of fluoride ions on corrosion performance and surface properties of SS304 in simulated PEMFC cathodic environments. Renew. Energy 2023, 212, 769–778. [Google Scholar] [CrossRef]
- Xuan, J.; Liu, Y.; Xu, L.; Bai, S.; Xin, Y.; Wang, L.; Zhang, G.; Su, Y.; Xue, L.; Li, L. Investigation of acidity on corrosion behavior and surface properties of SS304 in simulated PEMFC cathode environments. Int. J. Hydrogen Energy 2022, 47, 22938–22951. [Google Scholar] [CrossRef]
- Novalin, T.; Eriksson, B.; Proch, S.; Bexell, U.; Moffatt, C.; Westlinder, J.; Lagergren, C.; Lindbergh, G.; Wreland Lindström, R. Concepts for preventing metal dissolution from stainless-steel bipolar plates in PEM fuel cells. Energy Conv. Manag. 2022, 253, 115153. [Google Scholar] [CrossRef]
- Yang, L.X.; Liu, R.J.; Wang, Y.; Liu, H.J.; Zeng, C.L.; Fu, C. Corrosion and interfacial contact resistance of nanocrystalline β-Nb2N coating on 430 FSS bipolar plates in the simulated PEMFC anode environment. Int. J. Hydrogen Energy 2021, 46, 32206–32214. [Google Scholar] [CrossRef]
- Mani, S.P.; Agilan, P.; Kalaiarasan, M.; Ravichandran, K.; Rajendran, N.; Meng, Y. Effect of multilayer CrN/CrAlN coating on the corrosion and contact resistance behavior of 316L SS bipolar plate for high temperature proton exchange membrane fuel cell. J. Mater. Sci. Technol. 2022, 97, 134–146. [Google Scholar] [CrossRef]
- Wang, H.; Sweikart, M.A.; Turner, J.A. Stainless steel as bipolar plate material for polymer electrolyte membrane fuel cells. J. Power Sources 2003, 115, 243–251. [Google Scholar] [CrossRef]
- Meng, W.; Zhu, H.; Wang, X.; Li, G.; Fan, Y.; Sun, D.; Kong, F. Electrochemical Behavior and Surface Conductivity of C/TiC Nanocomposite Coating on Titanium for PEMFC Bipolar Plate. Metals 2022, 12, 771. [Google Scholar] [CrossRef]
- Leng, Y.; Ming, P.; Yang, D.; Zhang, C. Stainless steel bipolar plates for proton exchange membrane fuel cells: Materials, flow channel design and forming processes. J. Power Sources 2020, 451, 227783. [Google Scholar] [CrossRef]
- Forouzanmehr, M.; Reza Kashyzadeh, K.; Borjali, A.; Ivanov, A.; Jafarnode, M.; Gan, T.H.; Wang, B.; Chizari, M. Detection and Analysis of Corrosion and Contact Resistance Faults of TiN and CrN Coatings on 410 Stainless Steel as Bipolar Plates in PEM Fuel Cells. Sensors 2022, 22, 750. [Google Scholar] [CrossRef]
- Li, S.; Jin, R.; Li, S.; Wang, L.; Xie, Z.; Li, X.; Wang, Z. High corrosion resistance and conductivity of Al2O3/CrN coating for metal bipolar plates in PEMFCs: Al2O3 hinders CrN columnar crystals growth. Int. J. Hydrogen Energy 2024, 50, 805–816. [Google Scholar] [CrossRef]
- Cheng, H.; Luo, H.; Wang, X.; Pan, Z.; Zhao, Q.; Dong, C.; Li, X. Improving the performance of titanium bipolar plate in proton exchange membrane water electrolysis environment by nitrogen-chromium composite cathode plasma electrolytic deposition. Int. J. Hydrogen Energy 2023, 48, 38557–38568. [Google Scholar] [CrossRef]
- Haye, E.; Deschamps, F.; Caldarella, G.; Piedboeuf, M.-L.; Lafort, A.; Cornil, H.; Colomer, J.-F.; Pireaux, J.-J.; Job, N. Formable chromium nitride coatings for proton exchange membrane fuel cell stainless steel bipolar plates. Int. J. Hydrogen Energy 2020, 45, 15358–15365. [Google Scholar] [CrossRef]
- Chen, Q.; Su, M.; Pang, G.; Zhou, Q.; Liang, D.; Zhang, E. Improving the corrosion resistance and conductivity of 316L bipolar plates used for PEMFCs by applying Cr/CrN multilayer coating. Appl. Surf. Sci. 2025, 689, 162573. [Google Scholar] [CrossRef]
- Choi, J.-H.; Eun Kang, H.; Kim, D.-J.; Soo Yoon, Y. A Comprehensive Review of Stainless-Steel Bipolar Plate Coatings and Their Role in Mitigating Corrosion in Aggressive Proton-Exchange Membrane Fuel Cells Environments. Chem. Eng. J. 2024, 493, 152662. [Google Scholar] [CrossRef]
- Liu, R.; Jia, Q.; Zhang, B.; Lai, Z.; Chen, L. Protective coatings for metal bipolar plates of fuel cells: A review. Int. J. Hydrogen Energy 2022, 47, 22915–22937. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, X.; Meng, W.; Kong, F. Effects of Heat Treatment on Microstructures and Properties of Cold Rolled Ti-0.3Ni Sheets as Bipolar Plates for PEMFC. Metals 2022, 12, 792. [Google Scholar] [CrossRef]
- Pugal Mani, S.; Rajendran, N. Corrosion and interfacial contact resistance behavior of electrochemically nitrided 316L SS bipolar plates for proton exchange membrane fuel cells. Energy 2017, 133, 1050–1062. [Google Scholar] [CrossRef]
- Willenbruch, R.D.; Clayton, C.R.; Oversluizen, M.; Kim, D.; Lu, Y. An XPS and electrochemical study of the influence of molybdenum and nitrogen on the passivity of austenitic stainless steel. Corros. Sci. 1990, 31, 179–190. [Google Scholar] [CrossRef]
- Wang, H.; Teeter, G.; Turner, J.A. Modifying a stainless steel via electrochemical nitridation. J. Mater. Chem. A 2011, 21, 2064–2066. [Google Scholar] [CrossRef]
- Lv, J.; Liang, T. Improved corrosion resistance of 316L stainless steel by nanocrystalline and electrochemical nitridation in artificial saliva solution. Appl. Surf. Sci. 2015, 359, 158–165. [Google Scholar] [CrossRef]
- Lv, J.; Liang, T.; Luo, H. Effect of grain refinement and electrochemical nitridation on corrosion resistance of the 316L stainless steel for bipolar plates in PEMFCs environment. J. Power Sources 2015, 293, 692–697. [Google Scholar] [CrossRef]
- Jinlong, L.; Jin, H.; Tongxiang, L. The effect of electrochemical nitridation on the corrosion resistance of the passive films formed on the 2205 duplex stainless steel. Mater. Lett. 2019, 256, 126640. [Google Scholar] [CrossRef]
- Olsson, C.O.A.; Landolt, D. Passive films on stainless steels—Chemistry, structure and growth. Electrochim. Acta 2003, 48, 1093–1104. [Google Scholar] [CrossRef]
- Tian, H.; Wang, J.; Liu, Z.; Han, P. Effect of Nitrogen on the Corrosion Resistance of 6Mo Super Austenitic Stainless Steel. Metals 2024, 14, 391. [Google Scholar] [CrossRef]
- Villegas-Tovar, J.; Gaona-Tiburcio, C.; Lara-Banda, M.; Maldonado-Bandala, E.; Baltazar-Zamora, M.A.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Olguin-Coca, J.; Estupiñan-Lopez, F.; Almeraya-Calderón, F. Electrochemical Corrosion Behavior of Passivated Precipitation Hardening Stainless Steels for Aerospace Applications. Metals 2023, 13, 835. [Google Scholar] [CrossRef]
- Kim, D.; Clayton, C.; Oversluizen, M. On the question of nitrate formation by N-containing austenitic stainless steels. Mater. Sci. Eng. A 1994, 186, 163–169. [Google Scholar] [CrossRef]
- Clayton, C.R.; Halada, G.P.; Kearns, J.R. Passivity of high-nitrogen stainless alloys: The role of metal oxyanions and salt films. Mater. Sci. Eng. A 1995, 198, 135–144. [Google Scholar] [CrossRef]
- Halada, G.; Kim, D.; Clayton, C. Influence of nitrogen on electrochemical passivation of high-nickel stainless steels and thin molybdenum-nickel films. Corrosion 1996, 52, 36–46. [Google Scholar] [CrossRef]
- Wang, H.; Turner, J.A. Electrochemical nitridation of a stainless steel for PEMFC bipolar plates. Int. J. Hydrogen Energy 2011, 36, 13008–13013. [Google Scholar] [CrossRef]
- Wang, H.; Turner, J.A. Modifying a Stainless Steel for PEMFC Bipolar Plates via Electrochemical Nitridation. Fuel Cells 2013, 13, 917–921. [Google Scholar] [CrossRef]
- Mani, S.P.; Anandan, C.; Rajendran, N. Formation of a protective nitride layer by electrochemical nitridation on 316L SS bipolar plates for a proton exchange membrane fuel cell (PEMFC). RSC Adv. 2015, 5, 64466–64470. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, H.; Jin, X.; Zhang, N.; Li, M. Effect of potential on corrosion behavior and contact resistance of electrochemically nitrided 446 stainless steel in simulated cathode environment of proton exchange membrane fuel cell. J. Solid State Electrochem. 2025; in press. [Google Scholar] [CrossRef]
- Noh, J.; Laycock, N.; Gao, W.; Wells, D. Effects of nitric acid passivation on the pitting resistance of 316 stainless steel. Corros. Sci. 2000, 42, 2069–2084. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wu, Y.S.; Zhang, L.; Yu, Z.Y. Atomic force microscopy and X-ray photoelectron spectroscopy study on the passive film for Type 316L stainless steel. Corrosion 2001, 57, 540–546. [Google Scholar] [CrossRef]
- O’Laoire, C.; Timmins, B.; Kremer, L.; Holmes, J.D.; Morris, M.A. Analysis of the Acid Passivation of Stainless Steel. Anal. Lett. 2006, 39, 2255–2271. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Yang, Y.; Wu, Z.; Tan, L.; Ren, Y.; Yang, K. Enhancing Pitting Corrosion Resistance of Severely Cold-Worked High Nitrogen Austenitic Stainless Steel by Nitric Acid Passivation. J. Electrochem. Soc. 2019, 166, C365–C374. [Google Scholar] [CrossRef]
- Wang, Z.; Di-Franco, F.; Seyeux, A.; Zanna, S.; Maurice, V.; Marcus, P. Passivation-Induced Physicochemical Alterations of the Native Surface Oxide Film on 316L Austenitic Stainless Steel. J. Electrochem. Soc. 2019, 166, C3376–C3388. [Google Scholar] [CrossRef]
- Wang, Z.C.; Paschalidou, E.M.; Seyeux, A.; Zanna, S.; Maurice, V.; Marcus, P. Mechanisms of Cr and Mo Enrichments in the Passive Oxide Film on 316L Austenitic Stainless Steel. Front. Mater. 2019, 6, 232. [Google Scholar] [CrossRef]
- Lynch, B.; Wang, Z.; Ma, L.; Paschalidou, E.-M.; Wiame, F.; Maurice, V.; Marcus, P. Passivation-Induced Cr and Mo Enrichments of 316L Stainless Steel Surfaces and Effects of Controlled Pre-Oxidation. J. Electrochem. Soc. 2020, 167, 141509. [Google Scholar] [CrossRef]
- Maurice, V.; Peng, H.; Klein, L.H.; Seyeux, A.; Zanna, S.; Marcus, P. Effects of molybdenum on the composition and nanoscale morphology of passivated austenitic stainless steel surfaces. Faraday Discuss. 2015, 180, 151–170. [Google Scholar] [CrossRef]
- Lee, S.-J.; Lai, J.-J. The effects of electropolishing (EP) process parameters on corrosion resistance of 316L stainless steel. J. Mater. Process. Technol. 2003, 140, 206–210. [Google Scholar] [CrossRef]
- Acquesta, A.; Monetta, T. Green Approach for Electropolishing Surface Treatments of Additive Manufactured Parts: A Comprehensive Review. Metals 2023, 13, 874. [Google Scholar] [CrossRef]
- Lebedeva, O.; Kultin, D.; Zakharov, A.; Kustov, L. Advantages of Electrochemical Polishing of Metals and Alloys in Ionic Liquids. Metals 2021, 11, 959. [Google Scholar] [CrossRef]
- Han, Y.; Mei, J.; Peng, Q.; Han, E.-H.; Ke, W. Effect of electropolishing on corrosion of Alloy 600 in high temperature water. Corros. Sci. 2015, 98, 72–80. [Google Scholar] [CrossRef]
- Xu, Y.; Mao, Y.; Ijaz, M.H.; Ibrahim, M.E.; Le, S.; Wang, F.; Jiang, J.; Chi, D.; An, M.; Song, S.; et al. Review—Principles and Applications of Electrochemical Polishing. J. Electrochem. Soc. 2024, 171, 093506. [Google Scholar] [CrossRef]
- Mu, J.; Sun, T.; Leung, C.L.A.; Oliveira, J.P.; Wu, Y.; Wang, H.; Wang, H. Application of electrochemical polishing in surface treatment of additively manufactured structures: A review. Prog. Mater. Sci. 2023, 136, 101109. [Google Scholar] [CrossRef]
- Ur Rahman, Z.; Deen, K.M.; Cano, L.; Haider, W. The effects of parametric changes in electropolishing process on surface properties of 316L stainless steel. Appl. Surf. Sci. 2017, 410, 432–444. [Google Scholar] [CrossRef]
- Łyczkowska-Widłak, E.; Lochyński, P.; Nawrat, G. Electrochemical Polishing of Austenitic Stainless Steels. Materials 2020, 13, 2557. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Yokoyama, Y.; Shibano, T.; Sugiura, T.; Katori, N. Development of Electropolishing-Based Corrosion Resistance Technique for Ferritic Stainless Steel. SAE Int. J. Mater. Manuf. 2016, 9, 474–481. [Google Scholar] [CrossRef]
- Xu, R.; Jin, X.; Bi, H.; Zhang, Z.; Li, M. Effect of surface roughness on contact resistance and electrochemical corrosion behavior of 446 stainless steel in simulated anode environments for proton exchange membrane fuel cell. J. Solid State Electrochem. 2024, 28, 3087–3098. [Google Scholar] [CrossRef]
- Tan, Z.; Xu, R.; Bi, H.; Zhang, Z.; Li, M. Effects of potential on corrosion behavior and contact resistance of 446 stainless steel in simulated proton exchange membrane fuel cell cathode environment. J. Solid State Electrochem. 2023, 27, 1993–2003. [Google Scholar] [CrossRef]
- Chen, M.; Ding, J.C.; Kwon, S.-H.; Wang, Q.; Zhang, S. Corrosion resistance and conductivity of NbN-coated 316L stainless steel bipolar plates for proton exchange membrane fuel cells. Corros. Sci. 2022, 196, 110042. [Google Scholar] [CrossRef]
- Yan, P.; Ying, T.; Yang, Y.; Cao, F.; Li, Y.; Wang, J.; Zeng, X. Investigation of anodized Ta/Ag coating on magnesium bipolar plate for lightweight proton exchange membrane fuel cells. Corros. Sci. 2022, 197, 110086. [Google Scholar] [CrossRef]
- Inzelt, G.; Lewenstam, A.; Scholz, F. Handbook of Reference Electrodes; Springer: Heidelberg, Germany, 2013; pp. 112–118. [Google Scholar] [CrossRef]
- Wang, Y.; Northwood, D.O. Effects of O2 and H2 on the corrosion of SS316L metallic bipolar plate materials in simulated anode and cathode environments of PEM fuel cells. Electrochim. Acta 2007, 52, 6793–6798. [Google Scholar] [CrossRef]
- Hou, Z.; Xiu, S.; Sun, C.; Zou, X.; Yao, Y. Enhancing the surface integrity and SCC resistance of 304 austenitic stainless steel by pre-stress grinding. J. Mater. Res. Technol. 2023, 25, 2890–2902. [Google Scholar] [CrossRef]
- Yang, C.; Jiang, X.; Zhang, W.; Wang, X. Effect of surface nanocrystallization induced by mechanical grinding treatment on stress corrosion cracking behavior of 316 L austenitic stainless steel in boiling MgCl2 solution. Mater. Charact. 2024, 218, 114458. [Google Scholar] [CrossRef]
- Luo, H.; Dong, C.; Xiao, K.; Li, X. The passive behaviour of ferritic stainless steel containing alloyed tin in acidic media. RSC Adv. 2016, 6, 9940–9949. [Google Scholar] [CrossRef]
- Marcus, P.; Grimal, J.M. The anodic dissolution and passivation of NiCrFe alloys studied by ESCA. Corros. Sci. 1992, 33, 805–814. [Google Scholar] [CrossRef]
- Marcus, P.; Bussell, M.E. XPS study of the passive films formed on nitrogen-implanted austenitic stainless steels. Appl. Surf. Sci. 1992, 59, 7–21. [Google Scholar] [CrossRef]
- Lippitz, A.; Hübert, T. XPS investigations of chromium nitride thin films. Surf. Coat. Tech. 2005, 200, 250–253. [Google Scholar] [CrossRef]
- Zeng, H.; Yang, Y.; Zeng, M.; Li, M. Effect of dissolved oxygen on electrochemical corrosion behavior of 2205 duplex stainless steel in hot concentrated seawater. J. Mater. Sci. Technol. 2021, 66, 177–185. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, H.; Xin, S.; Hou, X.; Li, M. Electrochemical corrosion behavior of 2205 duplex stainless steel in hot concentrated seawater under vacuum conditions. Corros. Sci. 2020, 165, 108383. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, B.; Fu, Y.; Hu, X.; Cao, X.; Pan, Z.; Wei, Y.; Luo, H.; Li, X. Effect of cold deformation on corrosion behavior of selective laser melted 316L stainless steel bipolar plates in a simulated environment for proton exchange membrane fuel cells. Corros. Sci. 2022, 201, 110257. [Google Scholar] [CrossRef]
- Kong, D.; Ni, X.; Dong, C.; Zhang, L.; Man, C.; Yao, J.; Xiao, K.; Li, X. Heat treatment effect on the microstructure and corrosion behavior of 316L stainless steel fabricated by selective laser melting for proton exchange membrane fuel cells. Electrochim. Acta 2018, 276, 293–303. [Google Scholar] [CrossRef]
- Olefjord, I.; Wegrelius, L. The influence of nitrogen on the passivation of stainless steels. Corros. Sci. 1996, 38, 1203–1220. [Google Scholar] [CrossRef]
- Chao, K.L.; Liao, H.Y.; Shyue, J.J.; Lian, S.S. Corrosion Behavior of High Nitrogen Nickel-Free Fe-16Cr-Mn-Mo-N Stainless Steels. Metall. Mater. Trans. B. 2014, 45, 381–391. [Google Scholar] [CrossRef]
- Patterson, T.A.; Carver, J.C.; Leyden, D.E.; Hercules, D.M. A surface study of cobalt-molybdena-alumina catalysts using x-ray photoelectron spectroscopy. J. Phys. Chem. 1976, 80, 1700–1708. [Google Scholar] [CrossRef]
- Fajardo, S.; Llorente, I.; Jiménez, J.A.; Bastidas, J.M.; Bastidas, D.M. Effect of Mn additions on the corrosion behaviour of TWIP Fe-Mn-Al-Si austenitic steel in chloride solution. Corros. Sci. 2019, 154, 246–253. [Google Scholar] [CrossRef]
- Graham, M.J.; Bardwell, J.A.; Sproule, G.I.; Mitchell, D.F.; MacDougall, B.R. The growth and stability of passive films. Corros. Sci. 1993, 35, 13–18. [Google Scholar] [CrossRef]
- Kocijan, A.; Donik, Č.; Jenko, M. Electrochemical and XPS studies of the passive film formed on stainless steels in borate buffer and chloride solutions. Corros. Sci. 2007, 49, 2083–2098. [Google Scholar] [CrossRef]




| C | Si | Mn | P | S | Cu | Ni | Cr | Mo | N | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.01 | 0.43 | 0.21 | 0.023 | 0.005 | 0.14 | 2.08 | 27.38 | 3.67 | 0.017 | Bal. |
| Category | Electrochemical Workstation | Chemical Reagent | 446 Stainless Steel Sheet | Reference Electrode |
|---|---|---|---|---|
| Model/Type | PARSTAT MC 1000 | AR | - | 232-01 |
| Supplier | Princeton Applied Research (Oak Ridge, TN, USA) | Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) | Baoshan Iron & Steel Co., Ltd. (Shanghai, China) | INESA Scientific Instrument Co., Ltd. (Shanghai, China) |
| Specimen | Ecorr (vs. SCE) | ipeak (μA cm2) | i−0.1V (μA cm2) | i0.6V (μA cm2) |
|---|---|---|---|---|
| Polished, H2 | −0.26 | 8.14 | 5.28 | 8.20 |
| Polished, Air | −0.07 | - | - | 6.93 |
| EN (2 h), H2 | −0.22 | 1.40 | 0.95 | 2.91 |
| EN (2 h), Air | −0.05 | - | - | 1.66 |
| EC + EN (0.5 h), H2 | −0.11 | - | 0.07 | 1.59 |
| EC + EN (0.5 h), Air | 0.09 | - | - | 1.18 |
| EC + EN (0 min), H2 | 0.15 | - | - | 0.91 |
| EC + EN (5 min), H2 | 0.11 | - | - | 1.18 |
| EC + EN (10 min), H2 | 0.07 | - | - | 1.26 |
| EC + EN (20 min), H2 | 0.03 | - | - | 1.42 |
| Specimen (H2) | i5h (μA cm2) | Specimen (Air) | i5h (μA cm2) |
|---|---|---|---|
| Polished | −0.05 | Polished | 1.02 |
| EN (120 min) | −0.22 | EN (120 min) | 0.48 |
| EC + EN (5 min) | −0.40 | EC + EN (5 min) | 0.14 |
| EC + EN (30 min) | −0.39 | EC + EN (30 min) | 0.26 |
| Specimen | Rs Ω cm2 | Y0-dl Ω−1 cm−2 sn | αdl | Rp or Rct Ω cm2 | Y0-f Ω−1 cm−2 sn | αf | Rf Ω cm2 | χ2 |
|---|---|---|---|---|---|---|---|---|
| EC + EN (0.5 h), Air | 2.08 | 1.01 × 10−4 | 0.92 | 7.79 × 105 | - | - | - | 7.33 × 10−3 |
| EN (2 h), Air | 1.57 | 8.61 × 10−5 | 0.92 | 2.21 × 105 | - | - | - | 2.74 × 10−3 |
| Polished, Air | 1.13 | 4.27 × 10−4 | 0.92 | 2.17 × 104 | - | - | - | 6.55 × 10−4 |
| EC + EN (0.5 h), H2 | 1.41 | 1.11 × 10−4 | 0.91 | 8.12 × 104 | - | - | - | 2.81 × 10−3 |
| EN (2 h), H2 | 1.18 | 9.25 × 10−5 | 0.92 | 2.15 × 104 | - | - | - | 1.58 × 10−3 |
| Polished, H2 | 1.60 | 7.21 × 10−3 | 0.84 | 3.80 × 103 | 1.85 × 10−4 | 0.89 | 3.12 × 102 | 1.25 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, R.; Zhu, Y.; Zhu, R.; Li, M. Corrosion Resistance and Surface Conductivity of 446 Stainless Steel with Electrochemical Cr-Enrichment and Nitridation for Proton Exchange Membrane Fuel Cell (PEMFC) Bipolar Plates. Metals 2025, 15, 566. https://doi.org/10.3390/met15050566
Xu R, Zhu Y, Zhu R, Li M. Corrosion Resistance and Surface Conductivity of 446 Stainless Steel with Electrochemical Cr-Enrichment and Nitridation for Proton Exchange Membrane Fuel Cell (PEMFC) Bipolar Plates. Metals. 2025; 15(5):566. https://doi.org/10.3390/met15050566
Chicago/Turabian StyleXu, Ronghai, Yangyue Zhu, Ruigang Zhu, and Moucheng Li. 2025. "Corrosion Resistance and Surface Conductivity of 446 Stainless Steel with Electrochemical Cr-Enrichment and Nitridation for Proton Exchange Membrane Fuel Cell (PEMFC) Bipolar Plates" Metals 15, no. 5: 566. https://doi.org/10.3390/met15050566
APA StyleXu, R., Zhu, Y., Zhu, R., & Li, M. (2025). Corrosion Resistance and Surface Conductivity of 446 Stainless Steel with Electrochemical Cr-Enrichment and Nitridation for Proton Exchange Membrane Fuel Cell (PEMFC) Bipolar Plates. Metals, 15(5), 566. https://doi.org/10.3390/met15050566






