Abstract
The anodic oxidation of titanium implants is a practical, cost-effective method to enhance implant success, especially due to rising hypersensitivity concerns. This study investigated the electrochemical behavior, surface characteristics, and biocompatibility of anodized commercially pure titanium (cpTi, grade IV). Anodization is performed on polished, cleaned cpTi sheet samples in 1 M H2SO4 using a constant voltage of 15 V for 15 and 45 min. The color of the oxide layer is evaluated using the CIELab color space, while composition is analyzed by a scanning electron microscope (SEM) equipped with an energy dispersive spectrometer (EDS). Additionally, X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS) are performed to identify and monitor the phase transformations of the formed titanium oxides. Corrosion measurements are performed in 9 g L−1 NaCl, pH = 7.4, and show the excellent corrosion stability of the anodized samples in comparison with pure titanium. The biological response is assessed by determining mitochondrial activity and gene expression in human fibroblasts. Anodized surfaces, particularly Ti-45, promote higher mitochondrial activity and the upregulation of adhesion-related genes (N-cadherin and Vimentin) in human gingival fibroblasts, indicating improved biocompatibility and the potential for enhanced early soft tissue integration.
1. Introduction
The American Dental Association recommended titanium and various titanium alloys in 2001 as sustainable alternatives to conventional noble and base metal alloys [1]. Regarding the surface characteristics of dental implants, when exposed to air, titanium develops a thin natural protective oxide layer with an average thickness of ~10 nm on its surface [2]. Several surface modification techniques have been successfully introduced to increase the thickness of this layer and generate bioactive TiO2 [3]. Those coatings are developed to perform multiple functions, such as improving the healing process, reducing the prevalence of periimplantitis, and increasing the long-term effectiveness of the implant. After implant placement, direct contact of peri-implant tissue with the titanium surface induces a host immune response, a phenomenon crucial for proper tissue integration. In addition, the permanent implantation of the protein in the desired position would promote cell adhesion and cell differentiation and new tissue formation around the implant [4]. Contemporary findings suggest that a thin layer of proteins is seen between the titanium implant surface and osseous tissue [5]. These proteins’ activities are particularly important for therapeutic success during immediate and early implant loading protocols [6]. A few seconds after implant placement, cells secrete a range of substances as a type of cell-to-cell communication [7]. A few days after surgery, fibroblasts usually travel to the wound and produce extracellular matrix and protective elements such as collagen, elastin, and proteoglycans [8]. The release of messenger molecules causes the proliferation phase to begin. In reaction to low tissue oxygen concentrations at the wound site (~50 mmHg pO2), cells create hypoxia-induced factors [9]. The most important is VEGF, which interacts with the cells that surround blood vessels. At that moment, angiogenesis, a process responsible for connecting new vessels to the preexisting vascular system, takes place.
Despite the formation of a natural or anodized TiO2 layer, which is widely recognized as the primary factor enhancing the corrosion resistance of titanium and its alloys, localized corrosion phenomena such as pitting and crevice corrosion remain significant challenges [10,11,12,13,14,15]. These forms of corrosion can lead to the release of metal ions, potentially compromising the biocompatibility and mechanical integrity of titanium-based implants due to cytotoxic effects [16,17]. Therefore, evaluating the susceptibility of titanium and its alloys to localized corrosion in physiological environments is critical. In order to improve the corrosion stability of Ti and its alloys, oxidation (anodization) is usually performed. Anodic oxidation is a process governed by the dynamic equilibrium between oxide film growth and its dissolution, both of which are strongly influenced by the electrolyte composition and temperature. This method also offers the possibility to overcome the disadvantage of titanium’s grayish appearance by adjusting the color of the oxide that forms. The applied voltage can vary to form a thicker titanium oxide (TiO2) layer, resulting in a more desirable color on the Ti surface [18,19,20].
Many different compositions of the anodizing solutions based on inorganic acids and bases, organic acids, organic solvents, etc., have been used [11,18,19]. In the literature, compact oxide films and nanostructured materials, including disordered porous films, are formed through anodization utilizing different electrolytes such as H2SO4, H3PO4, NaOH, CH3COOH, Na2HPO4, and NH4Cl, and self-organized porous or nanotubular films can be obtained in the presence of fluoride ions from NH4F, HF, and NaF [11,12,20,21]. Also, different organic-based electrolytes containing ethylene glycol, glycerol, diethylene glycol, citric acid, and oxalic acid are used, but mainly for nanotube formation [22,23,24]. It is also worth mentioning that adjusting the anodization variables, such as the type of electrolyte, concentration of the electrolyte, pH, temperature, applied voltage or current, and duration, can influence the color of the anodized samples and the physical and chemical characteristics of the oxide layer that develops on titanium and its alloys [25].
Depending on the electrolyte and voltage, practically the whole spectrum of color can be obtained; more details can be found in [17,19,26,27,28,29]. For example, Khadiri et al. [30] investigated anodization in an applied voltage ranging from 20 to 35 V in a 5 M phosphoric acid solution. A red-violet shade is observed at 20 V, which shifts to a dark blue at 25 V and then transits to a light blue at 35 V. By anodizing cpTi in 1 M KOH solution at 25 °C for 1 min at 5, 10, 20, 30, and 40 V, variations in the color are varieties of gold, violet, dark, blue, and light blues [31]. The corrosion current in simulated body fluid (SBF) depends on the applied anodizing voltage. For the voltages of 5 V, 10 V, 20 V, 30 V, and 40 V, the determined corrosion currents are 4.35 μA, 2.6 μA, 2.75 μA, 3.42 μA, and 2.65 μA, respectively. Numerous studies have investigated the corrosion behavior of commercially pure titanium (cpTi) and its alloys mainly in simulated body fluids, such as Ringer and Hanks solutions. For instance, Tamilselvi et al. [32] reported corrosion potentials, Ecorr, of −387 mV and −401 mV, with corrosion current densities, jcorr, of 5.97 × 10−7 A cm−2 and 4.84 × 10−7 A cm−2 for cpTi and Ti-6Al-4V, respectively, in saline solutions. Under an impressed potential of +500 mV for 1 h, these values improved to 3.05 × 10−8 A cm−2 and 1.06 × 10−8 A cm−2, respectively, due to the formation of a protective TiO2 passive film. Similarly, Jáquez-Muñoz et al. [12] evaluated the electrochemical corrosion behavior of Ti-cp2, Ti-6Al-2Sn-4Zr-2Mo, Ti-6Al-4V, and Ti Beta-C anodized in 1 M H2SO4 and H3PO4 solutions at a constant current density of 0.025 A cm−2, and tested in 3.5 wt% NaCl and H2SO4 environments. Non-anodized alloys exhibited jcorr values in the range of 10−6 A cm−2, while anodized samples showed improved corrosion resistance, with jcorr values decreasing from 10−7 A cm−2 to 10−9 A cm−2 and Ecorr values shifting approximately 0.2 V to the more positive value. The authors noted that anodization produced a more homogeneous and protective oxide layer, particularly an α-phase-dominated layer on alloys like Ti-6Al-4V, whereas β-phase-rich alloys, such as Ti Beta-C, formed less uniform oxide layers, resulting in relatively poorer corrosion performance. Additionally, Almeraya-Calderón et al. [11] reported a jcorr of 7.16 × 10−6 A cm−2 and an Ecorr of −421 mV vs. SCE for Ti-cp2 in Ringer solution, with a passive range of 1.154 V. Surface preparation techniques also significantly influence corrosion behavior. Milošev et al. [13] demonstrated that grinding, diamond polishing, and chemo-mechanical polishing of Ti-6Al-4V in 0.9 wt.% NaCl and in artificial saliva reduced the surface roughness, which is correlated with enhanced polarization resistance, as confirmed using electrochemical impedance spectroscopy.
Even though many electrolytes for anodization are used, the one based on sulfuric acid is dominant in practice [19,33]. The electrolyte can be a moderately concentrated sulfuric acid (1 to 2 M is appropriate). Kumar et al. [33] investigated anodization in sulfuric acid in a range of concentrations of 0.5–5 M, applying a voltage from 10 V to 90 V, and proposed that the optimal concentration is 1 M H2SO4 and a voltage of 15 V. They also observed that when the voltage exceeds 10 V, the homogeneity improved continuously, but is lost when voltage approaches 20 V. To obtain a fine nanoporous surface with remarkable homogeneity, anodization at 15 V is performed, which justifies the statement that 15 V is the optimum voltage to obtain a precise nanoporous surface. Capek et al. [34] investigated the Ti anodization in 1 M H2SO4 by varying the temperature from 6 °C to 2 °C and 36 °C, and applying a voltage of 5 V, 10 V, and 15 V. Using the ellipsometry for the samples anodized for 4 min, 60 min, and 180 min with 5 V and 15 V, they determined a thickness of ~10 nm for an anodization voltage of 5 V and an exponential increase in thickness from 30 nm to 110 nm for an anodization voltage of 15 V.
However, the reported corrosion parameters vary widely due to differences in surface preparation, alloy microstructure, and testing conditions, such as the composition and pH of solutions like Ringer and Hanks solutions [11,35]. This variability underscores the need for standardized corrosion testing protocols.
Therefore, this study aims to systematically investigate the corrosion behavior of commercially pure and anodized titanium in a 9 g L−1 NaCl environment, with a focus on the effects of anodization on corrosion resistance and its relevance to biomedical applications. Given that the microstructure, composition, thickness, and phase distribution of the oxide layer influence soft tissue integration, this study aimed to evaluate the effect of anodically oxidized cpTi surfaces on human fibroblasts’ biocompatibility.
2. Materials and Methods
2.1. Anodization and Characterization of Oxidized Titanium
2.1.1. Anodization
For anodization, a cpTi plate (in the rest of the text, cpTi was denoted as Ti), 0.127 mm thick (Alfa Aesar, Ward Hill, MA, USA), with the dimensions of 1 cm × 1 cm with a neck of 0.2 cm × 3 cm, was used. The samples were ground with SiC abrasive papers (1000–4000 grit) and polished with 0.05 μm Al2O3 on a polishing cloth, followed by degreasing in acetone and DI water in an ultrasonic bath. As has been well investigated, anodization was performed in 1 M H2SO4 (Merck KGaA, Darmstadt, Germany) at room temperature with a constant voltage of 15 V for 15 min and 45 min [33,34]. As a constant-voltage power supply, the ISKRA MA 4153 with voltage regulation from 0 to 25 V and a maximum current of 0.4 A (Iskar, Kranj, Slovenia) was used. Anodization was performed in a 100 cm3 beaker, with two platinum 2 cm × 2 cm counter electrodes. After the immersion of the Ti electrode in the solution, the voltage was gradually increased from 0 V to 15 V for 30 s. The voltage was kept constant for 15 min and 45 min, and the voltage and current were recorded with a digital voltmeter, ISO-Tech IDM 73 (Iso-Tech, Corby, Northamptonshire, UK), interfaced to a PC via RS-232, with a step size of 10 s. The potential was measured using a saturated calomel electrode (SCE) with a Gamry 1010E (Gamry, Warminster, PA, USA) potentiostat/galvanostat. After anodization, the samples were removed from the solution and washed with distilled water and isopropanol. In the following text, base Ti and Ti anodized for 15 min and 45 min are denoted as Ti-0, Ti-15, and Ti-45, respectively.
2.1.2. Color Measurements
The color of the anodized samples, as well as the color of the non-oxidized Ti plate, were measured with a Datacolor Spectro 700 (DATACOLOR, Basel, Switzerland) spherical spectrophotometer under the following conditions: D65, 10°, and SPI. The final result, i.e., the color coordinates, was obtained by averaging from three measurements at three different locations on each sample based on the CIELab color system. The images of samples were taken using a Canon 77D camera (Canon, Tokyo, Japan).
2.1.3. SEM and EDS Characterization
For the non-electrochemical characterization, only pure Ti, Ti-0, and Ti-45 were used to avoid a significant number of figures. The morphology and chemical composition of the samples were analyzed using a scanning electron microscope (SEM) JEOL JSM-6610LV (JEOL, Tokyo, Japan) at 20 kV, coupled to an Energy Dispersive Spectrometer (EDS) model, X-Max Large Area Analytical Silicon Drift connected with INCA Energy 350 (Oxford Instruments, Concord, MA, USA).
2.1.4. X-Ray Diffraction (XRD) Measurements
X-ray diffraction was performed on a Philips PW 1050 diffractometer (Philips, Amsterdam, The Netherlands) with Cu–Kα1,2 radiation using a Ni filter at room temperature. The diffraction data were collected with a scanning step width of 0.05° and 3 s time per step in a 2θ range from 10° to 80°.
2.1.5. X-Ray Photoelectron Spectroscopy (XPS) Measurements
X-ray photoelectron spectroscopy (XPS) measurements were carried out using a SPECS system equipped with an XP50M X-ray source (Oxford Instruments, Oxford, UK), a Focus 500 X-ray monochromator (Incoatec GmbH, Geesthacht, Germany), and a PHOIBOS 100/150 analyzer (SPECS GmbH, Berlin, Germany). The system employed an Al Kα anode (photon energy of 1486.74 eV), operating at 12.5 kV and 32 mA. Survey scans were acquired over a binding energy range of 0 to 1000 eV using a constant pass energy of 40 eV, a step size of 0.5 eV, and a dwell time of 0.2 s in FAT mode. High-resolution spectra for Ti 2p and O 1s regions were recorded with a pass energy of 20 eV, a step size of 0.1 eV, and a dwell time of 2 s. The base pressure in the analysis chamber was maintained at 8 × 10−9 mbar. All binding energies were calibrated concerning the C 1s peak at 284.8 eV.
2.2. Corrosion Measurements
The corrosion measurements were performed according to the modified ISO 10271:2011 [36] and ASTM F 2129-15 standards [37]. All the experiments were conducted in a 9 g L−1 (0.9 wt.%) NaCl (Merck KGaA, Darmstadt, Germany) solution at pH = 7.4 adjusted with 0.1 M NaOH (Merck KGaA, Darmstadt, Germany), as recommended by ISO 10271:2011 [36], at room temperature. The three-compartment glass cell with a volume of 200 cm3 using the saturated calomel electrode (SCE) as a reference, and a lead plate of 1 cm × 6 cm inserted in a micro-porous polypropylene bag to avoid the formation of hypochlorite that could disturb measurements were used. We modified the standards by the means that during the establishment of the open circuit potentials, simultaneous linear polarization measurements were conducted at ±10 mV vs. Eocp, and the polarization resistance, Rp, was determined. Also, both standards recommend that the polarization curve starts at −100 mV in cathodic directions vs. Eocp, which is an insufficiently small cathodic polarization from which the Tafel slope could be properly determined. Also, the standard ISO 10271:2011 [36] did not consider a reverse scan as mandatory, from which the repassivation potential can be obtained. In that manner, we performed a cyclic polarization test, but not to +0.8 V as recommended by ASTM F 2129-15 [37], considering that the breakdown potential for Ti and its alloys is at much higher potentials. Also, both standards recommended experiments in electrolytes that are purged with an inert gas to remove dissolved oxygen. As oxygen reduction was considered the dominant cathodic reaction under the given conditions for application in the mouth, for example, a prosthesis, where oxygen is present, the obtained results according to the standards will be unrealistic. For the problems of using those two standards, please refer to [35,38]. Hereafter, after 1 h of exposure in 9 g L−1 NaCl, the cyclic polarization curve was recorded with a sweep rate of 1 mV s−1 starting from −0.5 V with conditioning during 300 s, followed by a forward scan to the breakdown potential and increase in the current density to ~100 μA cm−2, and then applying a backward scan to −0.5 V. The imaginary and real capacitance were determined at the open circuit potential in the frequency range from 10 kHz to 0.1 mHz. All corrosion investigations were performed using a Gamry 1010E (Gamry, Warminster, PA, USA) potentiostat/galvanostat.
2.3. Biocompatibility
2.3.1. Mitochondrial Activity Assay (MTT Assay)
Human gingival fibroblasts (HGFs) were isolated from the gingival tissue of a healthy 18-year-old donor following the protocol described in a previous study [39] previously approved by the ethics committee (No. 36/7), Faculty of Dental Medicine, University of Belgrade, and carried out in compliance with the Declaration of Helsinki. Cells after the third passage were used in the experiment.
The experimental samples were first placed into 24-well plates and sterilized under ultraviolet light for 15 min on each side. Complete culture medium (Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 100 U/mL penicillin–streptomycin (all reagents were from Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA)) was added to the plates. The next day, the samples were transferred to new 24-well plates. A total of 1 × 104 HGFs were seeded onto each sample and cultured under standard conditions (37 °C, 5% CO2, humidified atmosphere). Cell viability was assessed after 7 days of culture. The culture medium was removed and replaced with an MTT solution (0.5 mg mL−1; Sigma-Aldrich, St. Louis, MO, USA). The samples were incubated for 4 h. Subsequently, the MTT solution was discarded, and dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA) was added. The plates were shaken at 250 rpm for 20 min at 37 °C in the dark. The solutions were transferred into a 96-well plate, and the absorbance was measured at 540 nm using a microplate reader (RT-2100c, Rayto, Shenzhen, China). Mitochondrial activity was expressed as a percentage relative to the control group. The experiment was performed in triplicate, in two independent experiments.
2.3.2. Gene Expression
Samples were prepared with the same protocol used for the MTT assay. HGF cells were seeded onto samples at a density of 5 × 104 cells per sample and cultured under standard conditions (37 °C, 5% CO2, in a humidified incubator). After 7 days of incubation, total RNA was extracted using TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA), in accordance with the manufacturers protocol. The RNA concentration was quantified using a microvolume spectrophotometer, BioSpec-nano UV–Vis Spectrophotometer (Shimadzu Scientific Instruments, Columbia, MD, USA). For cDNA synthesis, 1 μg of total RNA was reverse-transcribed using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, USA) and oligo(dT) primers. Target gene amplification was performed with the SensiFAST™ SYBR® Hi-ROX Kit (Bioline, London, UK). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as the internal control for normalization. The primer sequences used for quantitative PCR (qPCR) are listed as (5′–3′) and are presented in Table 1.

Table 1.
The primer sequences used for quantitative PCR (qPCR).
PCR reactions were carried out in a final volume of 15 μL, comprising SYBR Green Master Mix, 200 nmol L−1 of each primer, and 2 μL of synthesized cDNA. Each sample was run in duplicate across three independent experiments. qPCR was performed using the CFX96 real-time PCR system (Bio-Rad Laboratories, Hercules, CA, USA). A melting curve analysis followed each run to verify the amplification specificity. Cycle threshold (Ct) values, defined as the number of cycles required for the fluorescent signal to cross a preset threshold, were recorded. Relative mRNA expression was quantified using the 2−∆Ct method, with the gene expression levels of N-cadherin, VEGF-A, and Vimentin normalized to GAPDH. The experiment was performed in triplicate, in two independent experiments.
2.3.3. Contact Angle Measurements
Surface wettability was measured under room temperature (22.5 ± 0.2 °C) conditions. Samples (Ti, Ti-15, and Ti-45) were set onto a measurement settle, and a 2 µL drop of deionized water, as a polar component, was placed onto the film’s surface using a micropipette (Finnpipette, Thermo Fisher, Helsinki, Finland) at a distance of 4 mm and an angle of 90°. The measurements of the non-polar components diiodomethane and ethylene glycol were conducted similarly. Contact angles (θ) were measured 1 s after the liquid achieved contact with the substrate surface by measuring the angle between the plane tangent to the drop and the plane of the underlying surface. The analyzer’s setup consists of a Canon 77D camera with a Canon ultrasonic micro-lens f100 mm (Tokyo, Japan) and a position stand. The data were analyzed using ImageJ contact angle software (version 1.5t, National Institutes of Health, LOCI, University of Wisconsin, Madison, WI, USA).
2.3.4. Statistical Analysis
For the assessment of the biocompatibility tests, the software package GraphPad Prism ver. 9 was used (GraphPad Software, Inc., San Diego, CA, USA). The Kolmogorov–Smirnov test was used to assess whether the data followed a normal distribution. For biocompatibility testing, the Kruskal–Wallis test was used. The values are presented as the means ± SDs. The results of contact angle measurements were subjected to a statistical analysis using descriptive measurements and the Kruskal–Wallis test. A p value less than 0.05 was considered significant.
3. Results and Discussion
3.1. Anodization and Characterization of Oxidized Titanium
3.1.1. Titanium Anodization
In Figure 1, the dependence of the voltage, potentials, and current densities during the anodization of Ti is shown. At a constant applied voltage of 15 V, the observed lower electrode potential of ~13 V is associated with a high overpotential for the hydrogen evolution reaction on the cathode. The current density initially rises rapidly between 7 mA cm−2 and 5 mA cm−2 and then decreases to ~1.5 mA cm−2. A decrease in the current over time under constant voltage is associated with the formation of a low-conducting oxide film on the Ti surface. By integrating the current density over time, a charge of 1.59 C cm−2 is determined for the sample anodized for 15 min, and 4.64 C cm−2 for the sample anodized for 45 min. Although oxygen evolution and a change in the color of the electrolyte are observed during anodization, the maximum oxide thicknesses, dt, can be estimated from the given charge values, Q, using the modified Faraday’s law:
where Q is the passed charge in C cm−2; M(TiO2) is 79.9 g mol−1 and ρ(TiO2) = 4.23 g cm−3, the molar mass and density of TiO2; and η is the current efficiency, which in this case is equal to one. Using Equation (1), the theoretical thicknesses of 0.75 μm and 2.25 μm are calculated. But, Capek et al. [34], using ellipsometry, determined the oxide thickness of Ti oxide film anodized at 15 V in 1 M H2SO4 for ~40 nm for 15 min and around 70 nm for 45 min. As pointed out by Prando et al. [40], in 0.5 M H2SO4, the passed charge of 1 C cm−2 corresponds to an oxide film thickness of about 560 nm in the absence of any parasitic reactions. In our case, the charge is 1.59 C cm−2 for 15 min, and 4.64 C cm−2 for 45 min of anodization, which corresponds to thicknesses of 0.890 μm and 2.6 μm, which are in good agreement with the thicknesses determined using Equation (1).
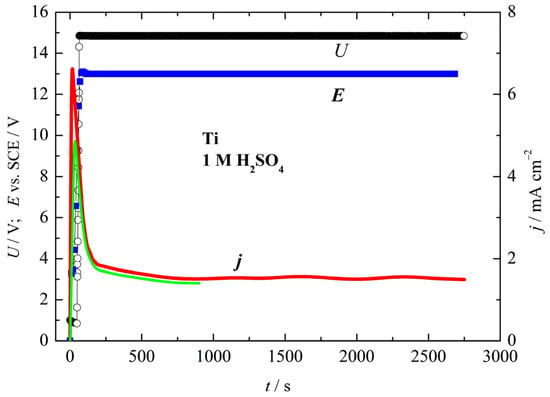
Figure 1.
The dependence of the anodization voltage, U (black line), and potential, E (blue line) over time (left axis), and the dependence of the current densities (green curve for 15 min and red curve for 45 min) over time (right axis) for anodizing Ti in a 1 M H2SO4 solution.
The anodization process in acidic media proceeds via an overall simplified reaction:
Ti + 2H2O = TiO2 + 4H+ + 4e−
Zhang et al. and Sul et al. [41,42] give some more plausible mechanisms for the anodization of Ti that include the formation of non-stoichiometric oxy-hydrated TiO2−x(OH)2x, where x is between zero and two, or TiOx(OH)4−2x, where x is between zero and one. Both compounds represent hydrated titanium dioxide (TiO2), where x is a variable that determines the ratio of hydroxide (OH) groups to oxygen (O) atoms. These non-stoichiometric compounds describe a range of titanium compounds that are part of the “titanic acid” family, including hydrated titanium dioxide and various titanium hydroxide species. The mechanisms could be represented via the following plausible reactions:
or
Ti = Ti4+ + 4e−
Ti4+ + (4 − x)H2O = TiOx(OH)4−2x + 4H+
Ti + 2H2O = TiO2 + 4H+ + 4e−
TiO2 + xH2O = TiO2−x(OH)2x
As “x” varies, these equations describe a process where water (H2O) is removed from the original titanium hydroxide, resulting in a compound with a formula of TiOx(OH)4−2x or TiO2−x(OH)2x, where the ratio of oxygen to hydroxide groups changes. The product of TiOx(OH)4−2x or TiO2−x(OH)2x hydrolysis could be different compounds like metatitanic TiO(OH)2 or H2TiO3, orthotitanic H4TiO4, pyrotitanic H2Ti2O, and so forth.
However, in addition to the reactions described in Equation (2), alternative processes such as the oxygen evolution reaction may also occur. Moreover, Ti3+ can form a soluble hydrated complex, [Ti(H2O)6]3+ [43,44], which can significantly reduce the current efficiency.
2H2O = 4H+ + O2 + 4e−
Ti + 6H2O = [Ti(H2O)6]3+ + 3e−
Hence, we chose a different approach to estimate the formed oxide thickness. Assuming that TiO2 without UV light could be treated as an insulator, the Ti and solution can represent the conducting plates of the capacitors, and knowing that, the capacitance, C, is given as
where ε0 is the electric permeability of vacuum, 8.854 × 10−14 F cm−1; εr is the relative electric permeability of the oxide; S is the surface area, 1 cm2; and d is the real thickness. Theoretically, the ideal capacitance is frequency independent, but in the electrochemical measurements, the impedance of the capacitance is frequency dependent according to the following equation:
Consequently, in Figure 2a, the dependence of real and imaginary capacitance over the logarithm of the frequency at open circuit potential is shown. It should be noted that the Ti-0 imaginary part shows only one time constant at 1000 Hz, while Ti-15 and Ti-45 show two time constants at 3800 Hz and 2500 Hz, and 1.2 Hz and 4.2 Hz, respectively. This could be interpreted as the existence of naturally formed and electrochemically grown oxide films. The real capacitance part in Figure 2a significantly increases from high to medium frequencies, and then much more slowly to low frequencies. At high frequencies, the data are not unreliable, due to the occurrence of electrochemical double layer, parasitic capacitances, etc. So, if we look at the real part of Equation (8), it can be written as
or
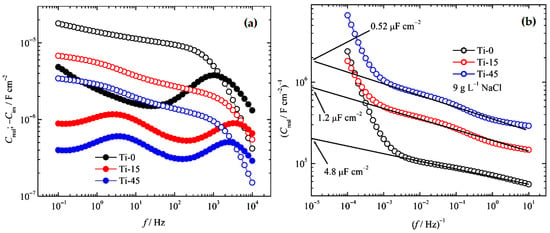
Figure 2.
(a) The dependence of the real capacitance (open symbols) and imaginary capacitance (full symbols) on frequency. (b) The dependence of the reciprocal values of the real capacitance on the reciprocal value of frequency.
Representing log (Creal)−1 over the log (f)−1, in the case when f → ∞, log (f)−1 → 0, Equation (12) becomes
Therefore, impedance becomes the reciprocal value of the capacitance. Because all the slopes are practically parallel (Figure 2a), we can suppose that 100 kHz could be taken as the infinitive, and the determined capacitances for Ti-0, T-15, and Ti-45 are 4.8 μF cm−2, 1.2 μF cm−2, and 0.52 μF cm−2, respectively. It is known from the literature that the thickness, d, of naturally formed oxide on Ti and its alloys is around 10 nm [2,45].
Consequently, from Equation (9), it can be calculated the constant k, nm F cm−2, connecting the thicknesses of the naturally formed oxide and the determined capacitance as follows:
The estimated value of k is 4.7 × 10−12 nm F cm−2. Thus, the thickness of the anodized samples can be calculated by dividing the constant k by the capacitance. For Ti-15, the estimated thickness is ~40 ± 15 nm, and for Ti-45, it is 90 ± 30 nm, which is in excellent agreement with the experimentally determined values by Capek et al. [34]. Accordingly, it can be concluded that the current efficiency for anodization is very low, as was also observed by Prando et al. [40]. For example, under the galvanostatic oxidation of Ti with a current density of 5 mA cm−2, the current efficiency was <10%.
In our case, we can only evaluate the current efficiency by dividing the estimated thickness by the theoretical thickness. For Ti-15, the estimated current efficiency is in the order of 4.5%, while for Ti-45 it is around 3.5%. It is plausible that a compact film is formed only during the initial period of oxidation, and after the rest of the time, during slow oxide film growth, the observed current is accompanied by oxygen evolution reaction, which is visually observed, and the dissolution of Ti as Ti3+.
With the estimated thickness, using Equation (9), the relative electric permeability of the oxide could be calculated. For Ti-15, εr is 54.2 ± 20, and for Ti-45, εr is 52.8 ± 17. Schneider et al. [46] investigated anodic thin oxide films on titanium for capacitor applications and observed that capacitance decreases with film thickness, from 5.5 µF cm−2 for an anodic film of 5 nm (excluding naturally formed oxide) to 1.5 µF cm−2 for an anodic film of 26 nm. From the determined capacitance and thickness of the oxide film, they determined the relative dielectric permittivity in the range of 50 to 55, independent of the film thickness and applied potential for anodization. Lui et al. [47] anodized Ti in 1 M H2SO4 and 1 M H3PO4, and using electrochemical impedance spectroscopy to estimate capacitance and real film thickness measured by TEM, the relative dielectric permittivity of the anodic films formed after anodization at 50 V in H2SO4 and H3PO4 were calculated to be ~77.6 and ~62.4, respectively.
Even though we did not determine the roughness of the samples, it is well elaborated in the literature that anodization increases it, which is beneficial for cell attachment and biological performance [48].
3.1.2. Color Measurements
In Figure 3, the images of the Ti samples are shown. It can be seen that the non-oxidized sample had a grey color, the sample anodized for 15 min was uniformly dark green, and the sample anodized for 45 min was dark purple. But considering that the color appearance is dependent on the spectator’s eye, the color is determined by spectrophotometry.
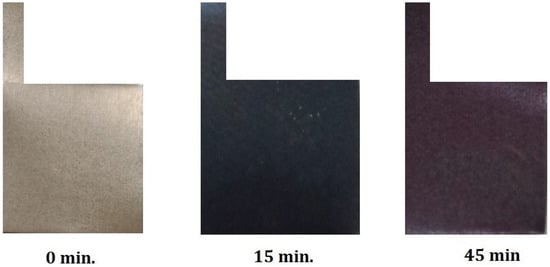
Figure 3.
Appearance of the color after the anodization of Ti in 1 M H2SO4 for 15 min and 45 min.
The results of the color measurements, as well as the approximate color appearance of the samples, are shown in Table 2. The L*a*b* values of the untreated sample correspond to a predominantly neutral gray tone with a slight yellow tint. The L* coordinate (brightness) decreases with the duration of electrochemical anodization, and so the sample becomes darker as the oxide layer becomes thicker. The a* coordinate values (red–green) increase with the duration of anodization, i.e., a shift toward the red end of the color axis. However, the values of the b* coordinate (yellow–blue) decrease more for the sample anodized for 15 min compared to the sample anodized for 45 min, resulting in a greenish appearance of the Ti-15 sample and a reddish appearance of the Ti-45 sample. The approximate color appearance calculated from the obtained parameters is in good agreement with the images given in Figure 3.

Table 2.
The results of the color measurements of the pure Ti and Ti anodized for 15 min and 45 min, with the software-simulated color appearance.
The origin of the color of anodized samples could be explained as follows. Because of its white color, TiO2 needs surface oxygen vacancies, like Ti–OH and Ti–H bonding, and Ti3+ species are assumed to transform pristine white TiO2 into colors like black, blue, gray, yellow, brown, etc. The outstanding performance of colored TiO2, for example in photocatalysis, is attributed mostly to defect species such as Ti3+ [49].
3.1.3. EDS and SEM Analyses
Figure 4a,d show the EDS spectra for Ti-0 and Ti-45 determined from the SEM images shown in Figure 4b,e. It can be seen that among Ti and O, carbon is present but is considered surface contamination and the remains of grinding with SiC paper. The determined compositions for Ti-0 are 89.4 wt.% of Ti and 7.1 wt.% of O, while for Ti-45, they are 79.5 wt.% of Ti and 18.0 wt.% of O. This indicates that on anodized Ti, an oxide film is formed. At low magnifications in Figure 4b,e, the homogenous surface can be observed, with the appearance of small black dots. The black dots observed on the polished Ti surfaces in SEM images often indicate the presence of surface imperfections or embedded particles. These imperfections can be due to the soft and ductile nature of Ti that is easily deformed by abrasive particles during grinding and polishing processes. Embedded particles could originate from grinding with SiC abrasive papers [50]. At higher magnifications, Figure 4c,f practically do not show significant differences between samples. The matrix of the Ti material during grinding and polishing is mainly removed in the ductile mode. Typically, the flaws that occur in the surface layer can be categorized into the following groups: (i) voids and micro-cracks, (ii) fractures or crushed areas, (iii) pulled-out sections, and (iv) smearing [51].
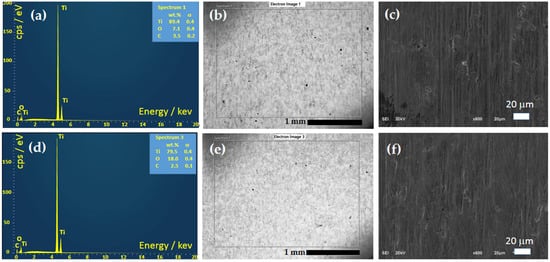
Figure 4.
EDS and SEM images of the Ti-0 (a–c) and Ti-45 anodized in 1 M H2SO4 for 45 min (d–f).
Considering C as an impurity, by converting wt.% to at.% of Ti and O in Table 3, it can be seen that the oxygen content increases significantly after anodization, from 17.0 at.% to 37.8 at.%, and the Ti content decreases from 71.5 at.% to 55.8 at.%. But, from the manual of Oxford Instruments used for EDS, the penetration depth of rays at 20 kV is ~1 μm; as a result, the titanium content could also originate from the bulk of the materials, and thus the composition of the oxide is discussable under these conditions.

Table 3.
The summarized EDS analysis of the surfaces of Ti-0 and Ti-45 shown in Figure 4c,e.
3.1.4. XRD Analysis
X-ray patterns of the titanium sheet before and after anodization for 45 min are presented in Figure 5. The diffractograms follow the symmetry of the α-Ti phase, a hexagonal close-packed (hcp) structure. No additional peaks associated with the crystalline oxide phase are observed after anodization. XRD cannot confirm the presence of titanium oxide due to its low crystallinity and/or thin layer.
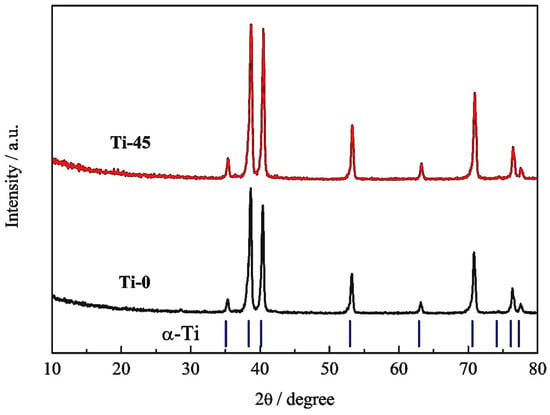
Figure 5.
XRD pattern of the titanium before (Ti-0) and after anodization (Ti-45) in 1 M H2SO4.
The Le Bail whole pattern decomposition method [52] is used to calculate the lattice parameters (Table 4). The fit is performed in the hexagonal space group P63/mmc. XFIT-95 software (Dec 23 2010), utilizing a Fundamental Parameters convolution approach to generate line profiles, was employed for the determination of the crystallite size [53]. The lattice parameters and crystallite sizes before and after anodization show slight differences. After anodization, the cell parameters decrease and align more closely with the literature data for alpha titanium (PDF# 00-089-2762). This can be attributed to the surface effect of the pristine titanium sheet and the formation of an oxide layer after anodization. Specifically, before anodization, the X-rays were scattered from both the surface and the interior of the titanium sheet. The surface may have higher concentrations of defects and experience greater strain, leading to a slight expansion of the lattice compared to the interior of the metal. The observed decrease in lattice parameters after anodization can be considered indirect evidence of oxide formation. The formation of an oxide layer eliminates the surface effects of the titanium sheet by rearranging titanium atoms into a more organized structure. This enhanced structural organization results in a decrease in the lattice parameters and an increase in the crystallite size from 38 nm to 42 nm, both of which are observed after anodization. Khadiri et al. [30] also did not observe a crystalline structure by anodizing cpTi in 5 M H3PO4 in the voltage range from 20 V to 35 V. Also, small anatase peaks are detected in 1 M H2SO4 only at the anodization voltage of 60 V [54].

Table 4.
Lattice parameters determined by the Le Bail method, and the determined crystallite size of Ti-0 and the anodized sample Ti-45.
3.1.5. XPS Analysis
X-ray photoelectron spectroscopy (XPS) measurements are used to determine the elemental composition and oxidation states of the surface of the samples. Figure 6 displays the XPS survey spectra for two titanium-containing samples: Ti-0 and Ti-45. Both spectra show the presence of titanium (Ti), oxygen (O), and carbon (C). The determined composition is shown in Table 5. The carbon signal is most likely due to surface contamination from organic compounds adsorbed from air during sample preparation, handling, or storage.
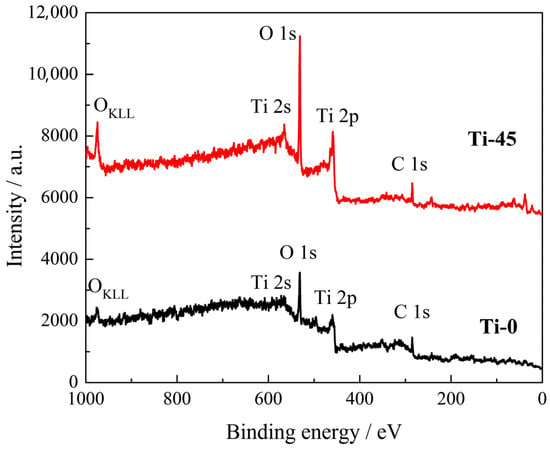
Figure 6.
Survey XPS spectra of pure titanium, Ti-0, and the sample anodized for 45 min, Ti-45, in 1 M H2SO4.

Table 5.
The determined at.% of the detected elements from the XPS measurements in Figure 6.
The relative increase in the peaks connected with Ti and O is significant for the anodized sample, as shown in Figure 6. The recalculated at.% based only on Ti and O for the sample Ti-0 is 37.7 at% of Ti and 62.3 at.% of O, neglecting carbon as an impurity. That corresponds to a stoichiometry of ~Ti0.4O0.6 and could represent the ~Ti2O3 phase, but it is deficient in oxygen for the formation of TiO2. For the anodized sample, Ti-45, the recalculated composition is 23.6 at.% of Ti and 76.4 at.% of O; so, the O to Ti atomic ratio is ~3.2, indicating a complex structure of the formed oxide film, for example, a hydrated mixture of Ti3+ and Ti4+ like TiO(OH) × TiO2(OH)2.
High-resolution XPS spectra of the Ti 2p and O 1s regions for the two titanium samples are shown in Figure 7.
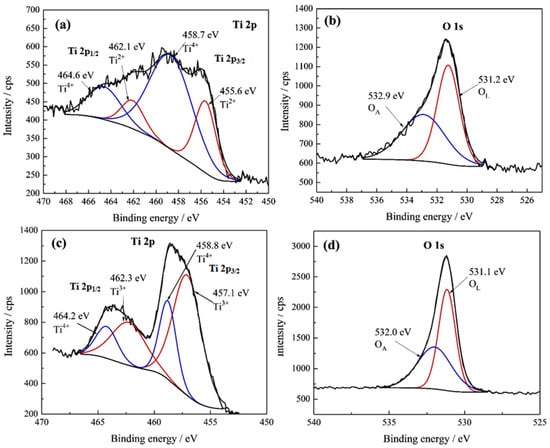
Figure 7.
High-resolution XPS spectra of (a) Ti 2p for pure titanium Ti-0, (b) O 1s for Ti-0, (c) Ti 2p for the sample anodized for 45 min, Ti-45, and (d) O 1s for Ti-45.
The Ti 2p spectrum for Ti-45 (Figure 7c) shows a clear doublet of Ti 2p3/2 and Ti 2p1/2. Peaks at 457.1 and 462.3 eV can be assigned to the Ti3+ oxidation state, while the peaks at 458.8 and 464.2 eV can be attributed to the Ti4+ oxidation state. Ti3+ is commonly associated with compounds like Ti2O3, where titanium exists in an intermediate oxidation state. The combination of Ti3+ and Ti4+ suggests an even more reduced sample compared to the previous one, possibly indicating a sub-stoichiometric oxide or a surface rich in oxygen vacancies [55]. For Ti-45, its O 1s spectrum (Figure 7d) also contains two peaks at 531.1 and 532.0 eV, which can be ascribed to lattice and adsorbed oxygen, respectively. The relative B.E. position of this adsorbed oxygen peak varies between the samples, reflecting differences in surface reactivity, hydration, or contamination between them. In Table 6, the summarized data of well-established XPS positions of different titanium oxidation states are given based on a comprehensive analysis by Biesinger et al. [56] of the selected literature values compiled from the NIST database [57]. Considering that the data presented in Table 6 are determined for pure oxides, our results, taking into account reported standard deviations, are reasonably close to the reported values.

Table 6.
Peak positions of Ti 2p3/2 with the standard deviations, ΔE = E(Ti 2p1/2) − E(Ti 2p3/2) peak splitting with the standard deviations [56,57], and the determined peak positions of Ti-0 and Ti-45.
Trivalent titanium (Ti3+) can coexist with the more common tetravalent titanium (Ti4+) in the oxide layer during titanium anodization. While the primary goal of anodization is to create a protective layer of TiO2, the process can induce the localized reduction of Ti4+ to Ti3+, especially in areas with defects. During this process, some titanium atoms in the oxide layer can be reduced from the 4+ oxidation state (Ti4+) to the 3+ oxidation state (Ti3+). Reasons for the formation of Ti3+ are mostly defects in the oxide layer, such as oxygen vacancies (missing oxygen atoms), that can lead to localized areas where titanium is reduced. Also, the specific electrochemical conditions during anodization, such as the voltage and electrolyte composition, can influence the amount of Ti3+ formation [49,58].
3.2. Corrosion Measurements
Figure 8a shows the dependence of the open circuit potentials, Eocp, of the investigated electrodes in 9 g L−1 NaCl. The anodized samples show a small increase in the potential after the immersion in the electrolyte from ~0 V to 75 mV. In contrast, pure Ti shows a significant nonlinear increase in the open-circuit potential over time from −0.375 V to ~0 V. The polarization resistance, Rp, for the pure sample gradually increases from ~50 kΩ cm−2 to 250 kΩ cm−2, as can be seen in Figure 8b. The anodized sample shows an initial decrease in the polarization resistance and stabilizes around 2.0 MΩ cm−2 for Ti-15 and 3.5 MΩ cm−2 for Ti-45. Such behavior could be explained as follows. The naturally formed oxide film on pure Ti is likely inhomogeneous. Upon contact with the electrolyte, the solution penetrates through its pores and reacts with the underlying Ti surface, forming an additional oxide. This process leads to an increase in the open-circuit potential and polarization resistance. In contrast, for the anodized samples, the electrolyte initially fills the pores of the oxide layer, which results in a temporary decrease in the film’s polarization resistance.
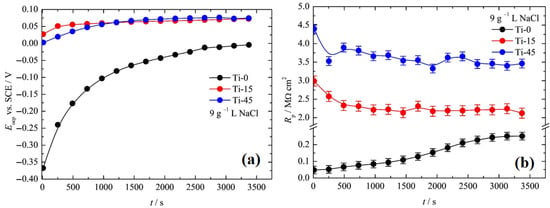
Figure 8.
(a) The dependence of the open circuit potentials, Eocp, over time, and (b) the dependence of the polarization resistance, Rp, over time, for Ti-0, Ti-15, and Ti-45.
The anodic linear polarization curves of the investigated samples are shown in Figure 9. Pure Ti shows some increase in the current density already at the potential of ~0.150 V in the range from 5 μA cm−2 to 10 μA cm−2, followed by an additional increase in the current density above 1 V. A rapid increase in the current density is observed above potentials of ~1.3 V. Anodized samples do not shows any feature below ~1.35 V, where the increase in the current is observed for Ti-0 sample. It should be also mentioned that a fast increase in the current density is at significantly higher potentials than the thermodynamic potential for the oxygen evolution reaction:
of 0.548 V vs. SCE at pH 7.4.
4H2O = O2 + 4OH− + 4e−
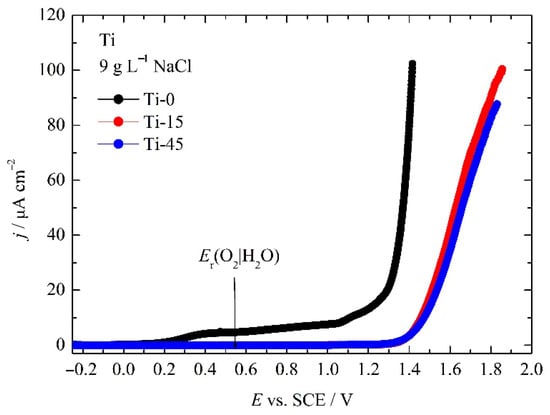
Figure 9.
The anodic linear polarization curve of the investigated samples in 9 g L−1 NaCl.
In Figure 10a, the Evans diagram of the investigated pure and anodized samples is shown. Starting at −0.5 V, the linear part of the cathodic part of the polarization curves over two magnitudes of current density is observed. The slopes of the line, ranging from −177 mV dec−1 for pure Ti to ~−115 mV dec−1 for anodized samples, could be associated with Tafel behavior, most probably with an oxygen reduction reaction (ORR). Increasing the potential practically means that the same corrosion potentials are reached at around −0.15 V. Pure Ti does not form a passive film near the corrosion potential, but active dissolution occurs up to the potentials of 0.3 V when passive behavior is reached. The breakdown potential occurs at ~0.97 V. Anodized samples show similar behavior in the cathodic branches, but after reaching the corrosion potentials, practically passivity begins. Before reaching the breakdown potential, Ti-15 in the passive range shows some increase in the current that could be associated with changes in the oxide film structure. Researchers often express a metal pitting resistance, labeled Rpit, with the equation Rpit = ΔE = |Ecorr − Epit|. The gap between Ecorr and Epit shows how easily pitting can start on that surface [51]. When ΔE is very small, the material becomes more vulnerable, and tiny shifts in potential push the metal toward severe pitting. In our case, ΔE is higher than 1 V, indicating excellent resistance to pitting corrosion. From the intercept of the cathodic Tafel lines and corrosion potentials, the corrosion current densities are estimated. To determine whether the samples undergo repassivation after the breakdown potentials, cyclic polarization curves are recorded and presented in Figure 10b.
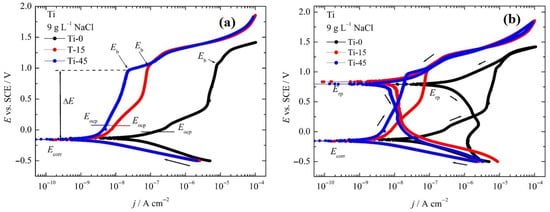
Figure 10.
(a) The Evans diagram and (b) the cyclic polarization curves of the investigated pure and anodized samples in 9 g L−1 NaCl at a scan rate v = 1 mV s−1. Arrows indicate the direction of the potential scan.
The reverse scan is recorded after reaching ~100 μA cm−2, and, interestingly, for all three samples, hysteresis characteristics for pitting corrosion are not observed [59]. Also, in the passive region, the occurrence of metastable pitting is not observed. Thus, it is plausible that instead of pitting corrosion, oxygen evolution occurs. The repassivation potential, Erp, of all three samples occurs at practically the same potential of ~0.8 V.
All the determined corrosion parameters are summarized in Table 5. The open-circuit potential after one hour is 0 V for Ti-0, 69 mV for Ti-15, and 75 mV for Ti-45, indicating that a delicate balance between the oxygen reduction reaction (ORR) as the cathodic reaction and the dissolution of base metals or oxides as the anodic reaction exists. When the overpotential, η, is minimal (±10 mV) and the electrochemical system is in a state of equilibrium, the traditional Stern–Geary equation for the polarization resistance, Rp, can be expressed with the following equation:
From this equation, the current densities at open-circuit potentials can be estimated. From Table 7, it can be seen that the calculated current density, jp,c, at open-circuit potentials is in very good agreement with values of jocp determined from Figure 10a. It is worth mentioning that for the blank Ti samples, jocp is more than an order of magnitude higher than for anodized samples. The determined corrosion potentials, Ecorr, are more negative than the open-circuit potentials, which could be explained that under cathodic polarization, the delicate balance between ORR and corrosion reactions is disturbed. The corrosion current density, jcorr, determined by Tafel extrapolation, is also an order of magnitude smaller for anodized than for pure Ti. Passive currents show a tremendous improvement for the anodized sample. For pure Ti, the passive current density is in the range of ~5.5 μA cm−2, while for Ti-15, it is in the range of 7 nA cm−2 to 80 nA cm−2, and for Ti-45, it is in the range of 4 nA cm−2 to 20 nA cm−2. The breakdown potentials for all samples are higher than 1 V. An interesting discussion about the breakdown potential is elaborated by Rosenbloom et al. [35]. Authors relying solely on in vitro tests have developed conservative acceptance criteria, which could be applied broadly to all materials tested according to ASTM F 2129-15 [37]. This benchmark stems from published in vivo measurements showing that most in vivo biological resting potentials lie below roughly +0.3 V vs. SCE. Any component destined for living tissue must tolerate potentials higher than those normally reached in the body. Because a single corrosion failure may have severe clinical and financial effects, adding an extra safety margin is only practical. For these reasons, the authors conclude that a material demonstrating a breakdown potential at or above +0.6 mV vs. SCE can reasonably be regarded as corrosion-safe for medical use. Therefore, all our samples satisfied these recommendations. From the data presented in Table 7, it is obvious that anodized samples possess superior corrosion properties to pure Ti.

Table 7.
Summarized corrosion parameters of the investigated samples in 9 g L−1 NaCl.
SEM and EDS Analyses After Corrosion
After the cyclic polarization test, the pure Ti and Ti anodized for 45 min were investigated by EDS and SEM, as shown in Figure 11. Comparing the result of EDS analysis before cyclic polarization, shown in Table 3, and after polarization, shown in Table 8, it can be observed that for pure Ti, there is no change in the Ti content of 71.5 at.%, accompanied by an increase in the O content from 17.0 at.% to 20.2 at.%, indicating a change in the oxide structure during polarization. In contrast, for Ti-45, the Ti content slightly decreases from 55.8 at.% to 54.5 at.%, while the O content decreases from 37.8 at.% to 34.9 at.%, suggesting a different surface behavior during polarization.
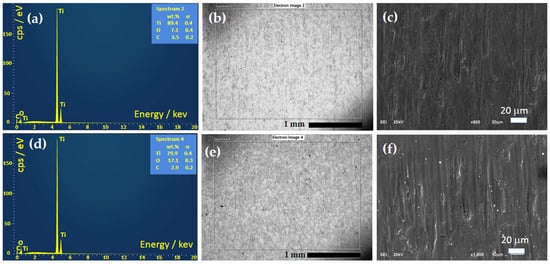
Figure 11.
EDS spectra and SEM images of pure Ti-0 (a–c) and Ti-45 (d–f) after cyclic polarization, as shown in Figure 10b.

Table 8.
EDS analysis of Ti-0 and Ti-45 after the cyclic polarization test shown in Figure 10b.
This could be explained by the slight dissolution of Ti through oxide pores; reactions with oxygen, OH−, and Cl−; and the formation of unstable TiOCl2 that immediately hydrolyzes and forms TiO2 on the surface. The small white spots that are visible in Figure 11f could be the formed TiO2, which explains the increase in the oxygen content in the EDS. Also, from Figure 11b,e, it can be seen that the black spots visible in Figure 4b,e for pure Ti, are practically completely removed, but for Ti-45, they are still slightly visible, probably due to occlusion with the oxide film formed during anodization. Also, from the SEM analysis, it can be concluded that pitting corrosion does not occur and that after the breakdown potentials, only an oxygen evolution reaction occurs.
3.3. Biocompatibility
3.3.1. MTT Assay
The mitochondrial activity of oral fibroblasts seeded on Ti samples and cultured for 7 days is approximately equal to the control in the Ti-15 group (98.6%), and significantly higher in the Ti-45 group (123.9%), as shown in Figure 12. The MTT assay, a widely used colorimetric method, reflects the metabolic competence of cells exposed to the anodized cpTi alloy surface. Assessing cell viability after 7 days of culture provides insights into the material’s ability to support long-term cell survival and proliferation, which are crucial for successful implant integration. As presented in Figure 12, the mitochondrial activity observed in the experimental groups is comparable to or significantly higher than control group, depending on the anodization period. Those findings imply the good long-term biocompatibility and stability of anodized cpTi in a biological environment.
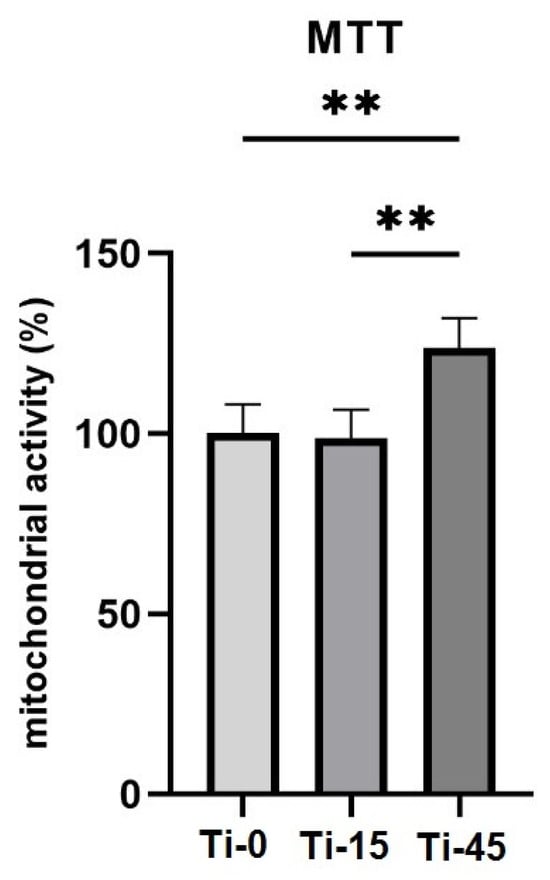
Figure 12.
Cell viability for the three groups, polished Ti-0, anodized T-15 min, and Ti-45, at 7 days of culture; Kruskal–Wallis test, ** p < 0.01.
3.3.2. Gene Expression
To further evaluate the biological responses and functional and phenotypic changes in HGF seeded and cultured for 7 days on the anodized cpTi alloy, a gene expression analysis was performed. The selected markers relevant to cell adhesion, cytoskeletal organization, and angiogenesis, as key indicators of fibroblast behavior and tissue integration with biomaterials, were analyzed, as shown in Figure 13. N-cadherin and Vimentin are significantly upregulated in both experimental groups, Ti-15 and Ti-45. In contrast, VEGF-A expression is downregulated in the experimental groups compared to the cells grown on the polished Ti surface.
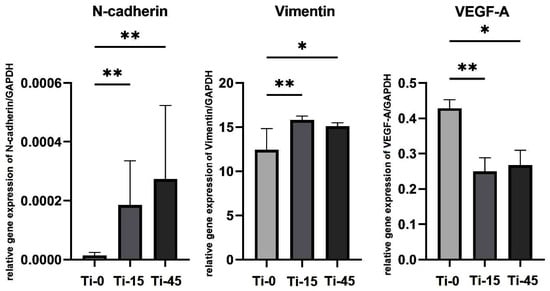
Figure 13.
Relative gene expression of N-cadherin, Vimentin, and VEGF-A obtained after 7 days of HGF culture on polished Ti (Ti-0), 15 min anodized Ti (Ti-15), and 45 min anodized Ti (Ti-45); Kruskal–Wallis test, * p < 0.05 and ** p < 0.01.
3.3.3. Wettability and Surface Energy
The contact angle measurements of the reference liquids on the samples are presented in Table 9. Comparing the contact angle measurements between groups (polished Ti and 15 min and 45 min anodized Ti), the Kruskal–Wallis test showed that anodization has a statistically significant effect on the decrease in the contact angle, p = 0.019. For all three samples, it is observed that the contact angle decreases with increasing anodization time, which indicates better wettability of anodized titanium than base titanium, shown in Figure 14 as an example.

Table 9.
Descriptive statistics of the wettability measurements of the investigated samples in distilled water, diiodomethane, and ethylene glycol.

Figure 14.
Example of a contact angle for the Ti sample with a moderate wettability.
For the determination of the surface energy of the samples, we used the Owens–Wendt method [60]. It involves measuring the contact angles of at least two liquids with known surface tensions and dividing the surface energy into polar and dispersive force components. The Owens–Wendt method categorizes these forces into: dispersive (or London) forces, weak, short-range forces arising from temporary fluctuations in the electron distribution; and polar (or specific) forces, stronger forces arising from permanent dipoles, hydrogen bonding, or acid–base interactions. The calculations are performed using the online program Surface Energy Calculator [61].
The calculated values of surface energy that increase from 58.8 mJ m−2 for Ti-0 to 65.1 mJ m−2 for Ti-45, as can be seen in Figure 15, are in agreement with the literature data of ~60 mJ m−2 for pure titanium [62]. It should also be mentioned that the dominant forces are dispersive or London forces.
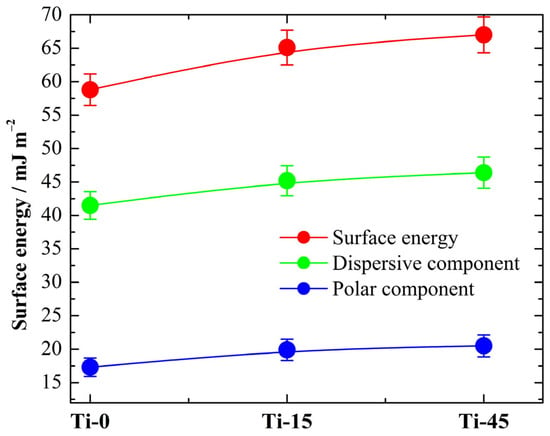
Figure 15.
The determined surface energy and dispersive and polar components from the contact angle measurements shown in Table 9 for the investigated pure and anodized Ti.
Materials with higher surface energy tend to be more easily wetted and bonded to, while those with low surface energy are more resistant to wetting and bonding. The surface energy of titanium (Ti) and its oxides is a crucial property, especially for biomedical applications like dental and orthopedic implants. It influences cell behavior, adhesion, and overall biocompatibility. Hereafter, anodization increases the surface energy and could have a beneficial effect in dental applications.
3.3.4. Biological Activity
The results of several in vitro studies demonstrated that anodized titanium increased the levels of cell proliferation and maturation [18,20,63].
N-cadherin is considered one of the main factors in guiding cell-to-cell interactions during fibroblast differentiation, as it is well-documented that N-cadherin regulates the formation and renewal of junctions through the cell’s microtubule network [64]. Our results showed significantly higher expression of this gene, which indicates the potential for greater tissue deposition on the titanium implant surface. Similar results were confirmed in previous research [19].
As far as the ECM Vimentin protein, the results were similar. Vimentin is engaged in cell–matrix adhesion, both with cell spreading and migration. It has been reported that it successfully regulates the directional migration of fibroblasts [65]. Our results demonstrated that the anodization process could increase the ability of fibroblasts to spread on an anodized titanium surface, and the difference was statistically significant in comparison to machined and polished titanium.
In contrast, the results revealed a different expression pattern for VEGF-A. Previous research has shown that VEGF-A, a key pro-angiogenic factor during wound healing, is primarily produced in response to hypoxic conditions [66]. The downregulation of VEGF-A in our experimental samples could be explained by changes in the surface chemistry after anodization. It is assumed that the surface oxide layer formed during oxidation in sulfuric acid would have reduced angiogenesis. Anodic oxidation could stimulate adherent fibroblast cell phenotypes rather than angiogenic phenotypes. Also, the downregulation of VEGF-A could be related to the loss of oxygen from TiO2. The reduction of Ti4+ to Ti3+ is associated with a reduced potential for angiogenesis, indicating that the primary defects on TiO2 surfaces are due to Ti3+ defects and oxygen vacancies [67]. Given that oxygen is crucial for angiogenesis, our findings suggest that VEGF stimulates signals for the formation of new blood vessels, thereby enhancing oxygen delivery.
Reduced VEGF-A expression indicates a lack of migratory pro-angiogenic factors, potentially leading to decreased angiogenesis in the initial healing stages. N-cadherin, vimentin, and VEGF-A play distinct but interconnected roles, especially in tissue remodeling, angiogenesis, and fibroblast behavior. N-cadherin and vimentin support fibroblast mobility, structure, and ECM deposition [68,69], while VEGF-A predominantly stimulates endothelial cell proliferation and migration [16]. Anodized titanium surfaces have been reported to upregulate adhesion-related proteins in human gingival fibroblasts, suggesting a phenotypic shift toward extracellular matrix production and structural support. This indicates that titanium surfaces may direct fibroblasts toward tissue remodeling functions rather than promoting angiogenic signaling [70].
Considering the cell type examined in our research, this composition appears nontoxic to fibroblast cells, as previously reported [71]. However, it may decrease the potential for angiogenesis, which might attenuate tissue healing, as the gene expression analysis reveals that the presence of Ti3+ has a suppressive effect on angiogenesis-related genes, which could potentially attenuate the healing process.
In this study, we prepared model surfaces with the anodized substrate to achieve an oxide layer with excellent chemical stability. Titanium oxides with low roughness, produced through oxidation, exhibit low friction, making them suitable for long-term fluid flow and enhanced heat exchange [72,73]. In line with that, these surfaces also promote the nonspecific adsorption of proteins, cells, and other biomolecules, which expands their applications, particularly in dental implantology [74]. Moderately hydrophilic surfaces (contact angle of around 40°) support optimal cell attachment [75], a trend also observed in studies using polymer thin films with contact angles between 20° and 60° [76]. It is also reported that hydrophilic surfaces can promote cell differentiation [77].
Moreover, hydrophilic behavior is desirable for application as coatings of orthopedic implants, because good wettability promotes better tissue integration [78]. The results of our study justified that anodic oxidation significantly increased the hydrophilicity of the titanium surface.
4. Conclusions
- cpTi is successfully anodized in 1 M H2SO4 at a constant voltage of 15 V for 15 min and 45 min.
- The thickness of anodized samples is determined by a newly developed method by the analysis of frequency-dependent capacitance.
- For 15 min of anodization, the thickness was estimated to ~40 ± 15 nm, and for 45 min, it was 90 ± 30 nm.
- From the EDS, XRD, and XPS analyses, it is confirmed that the oxide layer is very complex.
- Anodized samples have a superior corrosion stability in 9 g L−1 NaCl than pure cpTi.
- By the SEM analysis, after cyclic polarization, it is concluded that all three samples do not undergo pitting corrosion, and that the oxygen evolution reaction is the main reaction.
- Anodized samples enhance the surface hydrophilicity.
- The calculated values of surface energy increase from 58.8 mJ m−2 for Ti-0 to 65.1 mJ m−2 for Ti-45.
- Anodized samples produce a surface-driven stimulation of human gingival fibroblasts by activating their adhesion and spreading mechanisms.
Author Contributions
Conceptualization, B.N.G., A.S.P. and M.M.L.; methodology, B.N.G., M.M.L. and D.M.; validation, B.N.G., A.S.P. and M.M.L.; formal analysis, B.N.G., A.S.P., M.M.L. and D.M.; investigation, A.S.P., M.M.L., D.M., L.R., D.J., P.Ž. and B.N.G.; resources, M.M.L. and B.N.G.; data curation, A.S.P., M.M.L., D.M., L.R., D.J., P.Ž. and B.N.G.; writing—original draft preparation, B.N.G., A.S.P., M.M.L. and D.M.; writing—review and editing, B.N.G., A.S.P. and M.M.L.; visualization, B.N.G., A.S.P. and M.M.L.; supervision, B.N.G. and M.M.L.; project administration, A.S.P.; funding acquisition, B.N.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Education, Science and Technological Development, Serbia, grant number 451-03-137/2025-03/200135.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the Faculty of Dental Medicine, University of Belgrade (protocol code 36/7, approval date: 11 March 2020).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We are grateful to Maja Radetić from the Department of Textile Engineering of the Faculty of Technology and Metallurgy, University of Belgrade, Serbia, for her help with the sample color measurements.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Eichmiller, F.C. Titanium applications in dentistry. J. Am. Dent. Assoc. 2003, 134, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Prando, D.; Brenna, A.; Diamanti, M.V.; Beretta, S.; Bolzoni, F.; Ormellese, M.; Pedeferri, M. Corrosion of titanium: Part 2: Effects of surface treatments. J. Appl. Biomater. Funct. Mater. 2018, 16, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Long, S.; Zhu, J.; Jing, Y.; He, S.; Cheng, L.; Shi, Z. A comprehensive review of surface modification techniques for enhancing the biocompatibility of 3D-Printed titanium implants. Coatings 2023, 13, 1917. [Google Scholar] [CrossRef]
- Williams, D.F. Biocompatibility pathways and mechanisms for bioactive materials: The bioactivity zone. Bioact. Mater. 2021, 10, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Barberi, J.; Spriano, S. Titanium and protein adsorption: An overview of mechanisms and effects of surface features. Materials 2021, 14, 1590. [Google Scholar] [CrossRef] [PubMed]
- Romanos, G. Biomolecular cell-signaling mechanisms and dental implants: A review on the regulatory molecular biologic patterns under functional and immediate loading. Int. J. Oral. Maxillofac. Implant. 2016, 31, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Pandey, C.; Rokaya, D.; Bhattarai, B.P. Contemporary concepts in osseointegration of dental implants: A review. Bio. Med. Res. Int. 2022, 2022, 6170452. [Google Scholar] [CrossRef] [PubMed]
- Tracy, L.E.; Minasian, R.A.; Caterson, E.J. Extracellular matrix and dermal fibroblast function in the healing wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ju, L.S.; Irudayaraj, J. Oxygenated wound dressings for hypoxia mitigation and enhanced wound healing. Mol. Pharm. 2023, 20, 3338–3355. [Google Scholar] [CrossRef] [PubMed]
- Gaona-Tiburcio, C.; Jáquez-Muñoz, J.M.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Lara-Banda, M.; Lira-Martinez, M.A.; Reyes-Blas, H.; Baltazar-Zamora, M.Á.; Landa-Ruiz, L.; Lopez-Leon, L.D.; et al. Corrosion behavior of titanium alloys (Ti CP2, Ti-6Al-2Sn-4Zr-2Mo, Ti-6Al-4V and Ti Beta-C) with anodized and exposed in NaCl and H2SO4 solutions. Metals 2024, 14, 160. [Google Scholar] [CrossRef]
- Almeraya-Calderón, F.; Jáquez-Muñoz, J.M.; Lara-Banda, M.; Zambrano-Robledo, P.; Cabral-Miramontes, J.A.; Lira-Martínez, A.; Estupinán-López, F.; Tiburcio, C.G. Corrosion behavior of titanium and titanium alloys in Ringer’s solution. Int. J. Electrochem. Sci. 2022, 17, 220751. [Google Scholar] [CrossRef]
- Jáquez-Muñoz, J.; Gaona-Tiburcio, C.; Chacón-Nava, J.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Delgado, A.; Rios, J.F.-D.L.; Bocchetta, P.; Almeraya-Calderón, F. Electrochemical corrosion of titanium and titanium alloys anodized in H2SO4 and H3PO4 solutions. Coatings 2022, 12, 325. [Google Scholar] [CrossRef]
- Milošev, I.; Sačer, D.; Kapun, B.; Rodič, P. The effect of metallographic preparation on the surface characteristics and corrosion behaviour of TI-6AL-4V alloy in simulated physiological solutions. J. Electrochem. Soc. 2024, 171, 111503. [Google Scholar] [CrossRef]
- Wu, B.; Tang, Y.; Wang, K.; Zhou, X.; Xiang, L. Nanostructured Titanium Implant Surface Facilitating Osseointegration from Protein Adsorption to Osteogenesis: The Example of TiO2 NTAs. Int. J. Nanomed. 2022, 17, 1865–1879. [Google Scholar] [CrossRef] [PubMed]
- Eisenbarth, E.; Velten, D.; Müller, M.; Thull, R.; Breme, J. Biocompatibility of β-stabilizing elements of titanium alloys. Biomaterials 2004, 25, 5705–5713. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, L. A Review on biomedical titanium alloys: Recent progress and prospect. Adv. Eng. Mater. 2019, 21, 1801215. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Che, S.; Yang, Z.; Tian, Y. Chemical leveling mechanism and oxide film properties of additively manufactured Ti–6Al–4V Alloy. J. Mater. Sci. 2019, 54, 13753–13766. [Google Scholar] [CrossRef]
- Vattanasup, C.; Kuntiyaratana, T.; Rungsiyakull, P.; Chaijareenont, P. Color formation on titanium surface treated by anodization and the surface characteristics: A review. Dent. J. 2023, 44, 13–21. [Google Scholar] [CrossRef]
- Wang, T.; Wang, L.; Lu, Q.; Fan, Z. Changes in the esthetic, physical, and biological properties of a titanium alloy abutment treated by anodic oxidation. J. Prosthet. Dent. 2018, 121, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Gulati, K.; Prideaux, M.; Kogawa, M.; Lima-Marques, L.; Atkins, G.J.; Findlay, D.M.; Losic, D. Anodized 3D-printed titanium implants with dual micro- and nano-scale topography promote interaction with human osteoblasts and osteocyte-like cells. J. Tissue Eng. Regen. Med. 2016, 11, 3313–3325. [Google Scholar] [CrossRef] [PubMed]
- Regonini, D.; Bowen, C.R.; Jaroenworaluck, A.; Stevens, R. A review of growth mechanism, structure and crystallinity of anodized TiO2 nanotubes. Mater. Sci. Eng. R Rep. 2013, 74, 377–406. [Google Scholar] [CrossRef]
- Niu, D.; Han, A.; Cheng, H.; Ma, S.; Tian, M.; Liu, L. Effects of organic solvents in anodization electrolytes on the morphology and tube-to-tube spacing of TiO2 nanotubes. Chem. Phys. Lett. 2019, 735, 136776. [Google Scholar] [CrossRef]
- Kieser, T.A.; Kunst, S.R.; Morisso, F.D.P.; Machado, T.C.; Oliveira, C.T. Anodização de titânio em ácido cítrico. Res. Soc. Dev. 2022, 11, e25311830872. [Google Scholar] [CrossRef]
- Pilipenko, A.; Maizelis, A.; Pancheva, H.; Zhelavska, Y. Electrochemical oxidation of VT6 titanium alloy in oxalic acid solutions. Chem. Chem. Technol. 2020, 14, 221–226. [Google Scholar] [CrossRef]
- İzmir, M.; Ercan, B. Anodization of titanium alloys for orthopedic applications. Front. Chem. Sci. Eng. 2018, 13, 28–45. [Google Scholar] [CrossRef]
- Karambakhsh, A.; Afshar, A.; Ghahramani, S.; Malekinejad, P. Pure commercial titanium color anodizing and corrosion resistance. J. Mater. Eng. Perform. 2011, 20, 1690–1696. [Google Scholar] [CrossRef]
- Wadhwani, C.P.K.; O’Brien, R.; Kattadiyil, M.T.; Chung, K.-H. Laboratory technique for coloring titanium abutments to improve esthetics. J. Prosthet. Dent. 2015, 115, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Wadhwani, C.; Brindis, M.; Kattadiyil, M.T.; O’Brien, R.; Chung, K.-H. Colorizing titanium-6aluminum-4vanadium alloy using electrochemical anodization: Developing a color chart. J. Prosthet. Dent. 2017, 119, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Ross, N. Anodizing color coded anodized Ti6Al4V medical devices for increasing bone cell functions. Int. J. Nanomed. 2013, 8, 109–117. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khadiri, M.; Elyaagoubi, M.; Idouhli, R.; Koumya, Y.; Zakir, O.; Benzakour, J.; Benyaich, A.; Abouelfida, A.; Outzourhit, A. Electrochemical study of anodized titanium in phosphoric acid. Adv. Mater. Sci. Eng. 2020, 2020, 5769071. [Google Scholar] [CrossRef]
- Kurniawan, K.F.; Ulfah, I.M.; Kozin, M. Effect of anodic oxidation voltages on the color and corrosion resistance of commercially pure titanium (CP-Ti). J. Evrimata 2023, 1, 18–23. [Google Scholar] [CrossRef]
- Tamilselvi, S.; Murugaraj, R.; Rajendran, N. Electrochemical impedance spectroscopic studies of titanium and its alloys in saline medium. Mater. Corros. 2007, 58, 113–120. [Google Scholar] [CrossRef]
- Kumar, A.; Kushwaha, M.K. Surface modification of titanium alloy by anodic oxidation method to improve its biocompatibility. Curr. Sci. 2021, 120, 907. [Google Scholar] [CrossRef]
- Capek, D.; Gigandet, M.-P.; Masmoudi, M.; Wery, M.; Banakh, O. Long-time anodisation of titanium in sulphuric acid. Surf. Coat. Technol. 2007, 202, 1379–1384. [Google Scholar] [CrossRef]
- Rosenbloom, S.N.; Corbett, R.A. An Assessment of ASTM F 2129 Electrochemical testing of small medical implants-lessons learned. In Proceedings of the CORROSION 2007, Nashville, TN, USA, 11–15 March 2007. [Google Scholar] [CrossRef]
- ISO 10271:2011; Dentistry-Corrosion Test Methods for Metallic Materials. International Organization for Standardization: Geneva, Switzerland, 2011.
- ASTM F 2129-15; Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements to Determine the Corrosion Susceptibility of Small Implant Devices. ASTM International: West Conshohocken, PA, USA, 2015.
- Grgur, B.N.; Lazić, V.; Stojić, D.; Rudolf, R. Electrochemical testing of noble metal dental alloys: The influence of their chemical composition on the corrosion resistance. Corr. Sci. 2021, 184, 109412. [Google Scholar] [CrossRef]
- Lazić, M.M.; Majerič, P.; Lazić, V.; Milašin, J.; Jakšić, M.; Trišić, D.; Radović, K. Experimental investigation of the biofunctional properties of Nickel–Titanium alloys depending on the type of production. Molecules 2022, 27, 1960. [Google Scholar] [CrossRef] [PubMed]
- Prando, D.; Fajardo, S.; Pedeferri, M.; Ormellese, M. Titanium anodization efficiency through real-time gravimetric measurement of oxygen evolution. J. Electrochem. Soc. 2020, 167, 061507. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, Z.Z.; Xia, Y.; Huang, Z.; Lefler, H.; Zhang, T.; Sun, P.; Free, M.L.; Guo, J. A novel chemical pathway for energy efficient production of Ti metal from upgraded titanium slag. Chem. Eng. J. 2015, 286, 517–527. [Google Scholar] [CrossRef]
- Sul, Y.-T.; Johansson, C.B.; Jeong, Y.; Albrektsson, T. The electrochemical oxide growth behaviour on titanium in acid and alkaline electrolytes. Med. Eng. Phys. 2001, 23, 329–346. [Google Scholar] [CrossRef] [PubMed]
- Uudsemaa, M.; Tamm, T. Calculations of hydrated titanium ion complexes: Structure and influence of the first two coordination spheres. Chem. Phys. Lett. 2001, 342, 667–672. [Google Scholar] [CrossRef]
- Kallies, B.; Meier, R. Electronic structure of 3d [M(H2O)6]3+ Ions from ScIII to FeIII: A quantum mechanical study based on DFT computations and natural bond orbital analyses. Inorg. Chem. 2001, 40, 3101–3112. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Thompson, G.E. Formation of porous anodic oxide film on titanium in phosphoric acid electrolyte. J. Mater. Eng. Perform. 2014, 24, 59–66. [Google Scholar] [CrossRef]
- Schneider, M.; Schroth, S.; Schilm, J.; Michaelis, A. Micro-EIS of anodic thin oxide films on titanium for capacitor applications. Electrochim. Acta 2008, 54, 2663–2671. [Google Scholar] [CrossRef]
- Liu, Z.J.; Zhong, X.; Walton, J.; Thompson, G.E. Anodic film growth of titanium oxide using the 3-electrode electrochemical technique: Effects of oxygen evolution and morphological characterizations. J. Electrochem. Soc. 2015, 163, E75–E82. [Google Scholar] [CrossRef]
- Alipal, J.; Lee, T.C.; Koshy, P.; Abdullah, H.Z.; Idris, M.I. Evolution of anodised titanium for implant applications. Heliyon 2021, 7, e07408. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, R.; Kait, C.F.; Chia, H.Y.; Isa, M.H.; Huei, L.W.; Sahrin, N.T.; Khan, N. Manipulation of the Ti3+/Ti4+ ratio in colored titanium dioxide and its role in photocatalytic degradation of environmental pollutants. Surf. Interfaces 2022, 32, 102146. [Google Scholar] [CrossRef]
- Spajić, I.; Rodič, P.; Šekularac, G.; Lekka, M.; Fedrizzi, L.; Milošev, I. The effect of surface preparation on the protective properties of Al2O3 and HfO2 thin films deposited on cp-titanium by atomic layer deposition. Electrochim. Acta 2020, 366, 137431. [Google Scholar] [CrossRef]
- Zhao, B.; Ding, W.F.; Dai, J.B.; Xi, X.X.; Xu, J.H. A comparison between conventional speed grinding and super-high speed grinding of (TiCp+TiBw)/Ti–6Al–4V composites using vitrified CBN wheel. Int. J. Adv. Manuf. Technol. 2014, 72, 69–75. [Google Scholar] [CrossRef]
- Bail, A.L. Whole powder pattern decomposition methods and applications: A retrospection. Powder Diffr. 2005, 20, 316–326. [Google Scholar] [CrossRef]
- Cheary, R.W.; Coelho, A. A fundamental parameters approach to X-ray line-profile fitting. J. Appl. Crystallogr. 1992, 25, 109–121. [Google Scholar] [CrossRef]
- Vera, M.L.; Colaccio, Á.; Rosenberger, M.R.; Schvezov, C.E.; Ares, A.E. Influence of the electrolyte concentration on the smooth TiO2 anodic voatings on Ti-6Al-4V. Coatings 2017, 7, 39. [Google Scholar] [CrossRef]
- Milićević, N.; Novaković, M.; Potočnik, J.; Milović, M.; Rakočević, L.; Abazović, N.; Pjević, D. Influencing surface phenomena by Au diffusion in buffered TiO2-Au thin films: Effects of deposition and annealing processing. Surf. Interfaces 2022, 30, 101811. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Naumkin, A.V.; Kraut-Vass, A.; Gaarenstroom, S.W.; Powell, C.J. NIST X-ray Photoelectron Spectroscopy Database (SRD 20), Version 5.0; Division of the National Institute of Standards and Technology (NIST): Gaithersburg, MD, USA, 2023. [CrossRef]
- Peng, W.-C.; Chen, Y.-C.; He, J.-L.; Ou, S.-L.; Horng, R.-H.; Wuu, D.-S. Tunability of p- and n-channel TiOx thin film transistors. Sci. Rep. 2018, 8, 9255. [Google Scholar] [CrossRef] [PubMed]
- Esmailzadeh, S.; Aliofkhazraei, M.; Sarlak, H. Interpretation of cyclic potentiodynamic polarization test results for study of corrosion behavior of metals: A review. Prot. Met. Phys. Chem. Surf. 2018, 54, 976–989. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Steven Abbott TCNF. 2012. Available online: https://www.stevenabbott.co.uk/abbottapps/SEC/index.html (accessed on 15 July 2025).
- Hieda, J.; Sakaguchi, A.; Nakano, M.; Akasaka, H.; Ohtake, N. Relationships between surface energy and charge of surface-modified titanium and HAp formation. App. Surf. Sci. 2018, 465, 509–516. [Google Scholar] [CrossRef]
- Kim, M.-H.; Park, K.; Choi, K.-H.; Kim, S.-H.; Kim, S.; Jeong, C.-M.; Huh, J.-B. Cell adhesion and in vivo osseointegration of Sandblasted/Acid Etched/Anodized dental implants. Int. J. Mol. Sci. 2015, 16, 10324–10336. [Google Scholar] [CrossRef] [PubMed]
- Mary, S.; Charrasse, S.; Meriane, M.; Comunale, F.; Travo, P.; Blangy, A.; Gauthier-Rouvière, C. Biogenesis of N-Cadherin-dependent cell-cell contacts in living fibroblasts is a microtubule-dependent kinesin-driven mechanism. Mol. Biol. Cell 2002, 13, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Miron-Mendoza, M.; Poole, K.; DiCesare, S.; Nakahara, E.; Bhatt, M.P.; Hulleman, J.D.; Petroll, W.M. The role of vimentin in human corneal fibroblast spreading and myofibroblast transformation. Cells 2024, 13, 1094. [Google Scholar] [CrossRef] [PubMed]
- Bao, P.; Kodra, A.; Tomic-Canic, M.; Golinko, M.S.; Ehrlich, H.P.; Brem, H. The role of vascular endothelial growth factor in wound healing. J. Surg. Res. 2008, 153, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Zhang, R.; Sun, J.; Liu, C. Oxidation mechanism of biomedical titanium alloy surface and experiment. Int. J. Corros. 2020, 2020, 1678615. [Google Scholar] [CrossRef]
- Jiang, D.; Christ, S.; Correa-Gallegos, D.; Ramesh, P.; Gopal, S.K.; Wannemacher, J.; Mayr, C.H.; Lupperger, V.; Yu, Q.; Ye, H.; et al. Injury triggers fascia fibroblast collective cell migration to drive scar formation through N-cadherin. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Shen, Y.; Mohanasundaram, P.; Lindström, M.; Ivaska, J.; Ny, T.; Eriksson, J.E. Vimentin coordinates fibroblast proliferation and keratinocyte differentiation in wound healing via TGF-β-slug signaling. Proc. Natl. Acad. Sci. USA 2016, 113, E4320–E4327. [Google Scholar] [CrossRef] [PubMed]
- Crenn, M.-J.; Dubot, P.; Mimran, E.; Fromentin, O.; Lebon, N.; Peyre, P. Influence of aanodized titanium surfaces on the behavior of gingival cells in contact with: A systematic review of in vitro studies. Crystals 2021, 11, 1566. [Google Scholar] [CrossRef]
- Jo, D.H.; Kim, J.H.; Son, J.G.; Song, N.W.; Kim, Y.I.; Yu, Y.S.; Lee, T.G.; Kim, J.H. Anti-Angiogenic Effect of Bare Titanium Dioxide Nanoparticles on Pathologic Neovascularization without Unbearable Toxicity. Nanomedicine 2014, 10, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Jokanović, V.; Čolović, B.; Nenadović, M.; Petkoska, A.T.; Mitrić, M.; Jokanović, B.; Nasov, I. Ultra-high and near-zero refractive indices of magnetron sputtered thin-film metamaterials based on TIXOY. Adv. Mater. Sci. Eng. 2016, 2016, 4565493. [Google Scholar] [CrossRef]
- Yeniyol, S.; Bölükbaşı, N.; Bilir, A.; Çakır, A.F.; Yeniyol, M.; Ozdemir, T. Relative contributions of surface roughness and crystalline ttructure to the biocompatibility of titanium nitride and titanium oxide coatings deposited by PVD and TPS coatings. Int. Sch. Res. Not. 2013, 2013, 783873. [Google Scholar] [CrossRef]
- Čolović, B.; Kisić, D.; Jokanović, B.; Rakočević, Z.; Nasov, I.; Petkoska, A.T.; Jokanović, V. Wetting properties of titanium oxides, oxynitrides and nitrides obtained by DC and pulsed magnetron sputtering and cathodic arc evaporation. Mater. Sci. Pol. 2019, 37, 173–181. [Google Scholar] [CrossRef]
- Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yan, C.; Zheng, Z. Functional polymer surfaces for controlling cell behaviors. Mater. Today 2018, 21, 38–59. [Google Scholar] [CrossRef]
- Al-Azzam, N.; Alazzam, A. Micropatterning of cells via adjusting surface wettability using plasma treatment and graphene oxide deposition. PLoS ONE 2022, 17, e0269914. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, C.P.; Krishnan, K.K.; Sudeep, U.; Ramachandran, K.K. Osteogenic and antibacterial properties of TiN-Ag coated Ti-6Al-4V bioimplants with polished and laser textured surface topography. Surf. Coat. Technol. 2023, 474, 130058. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).


