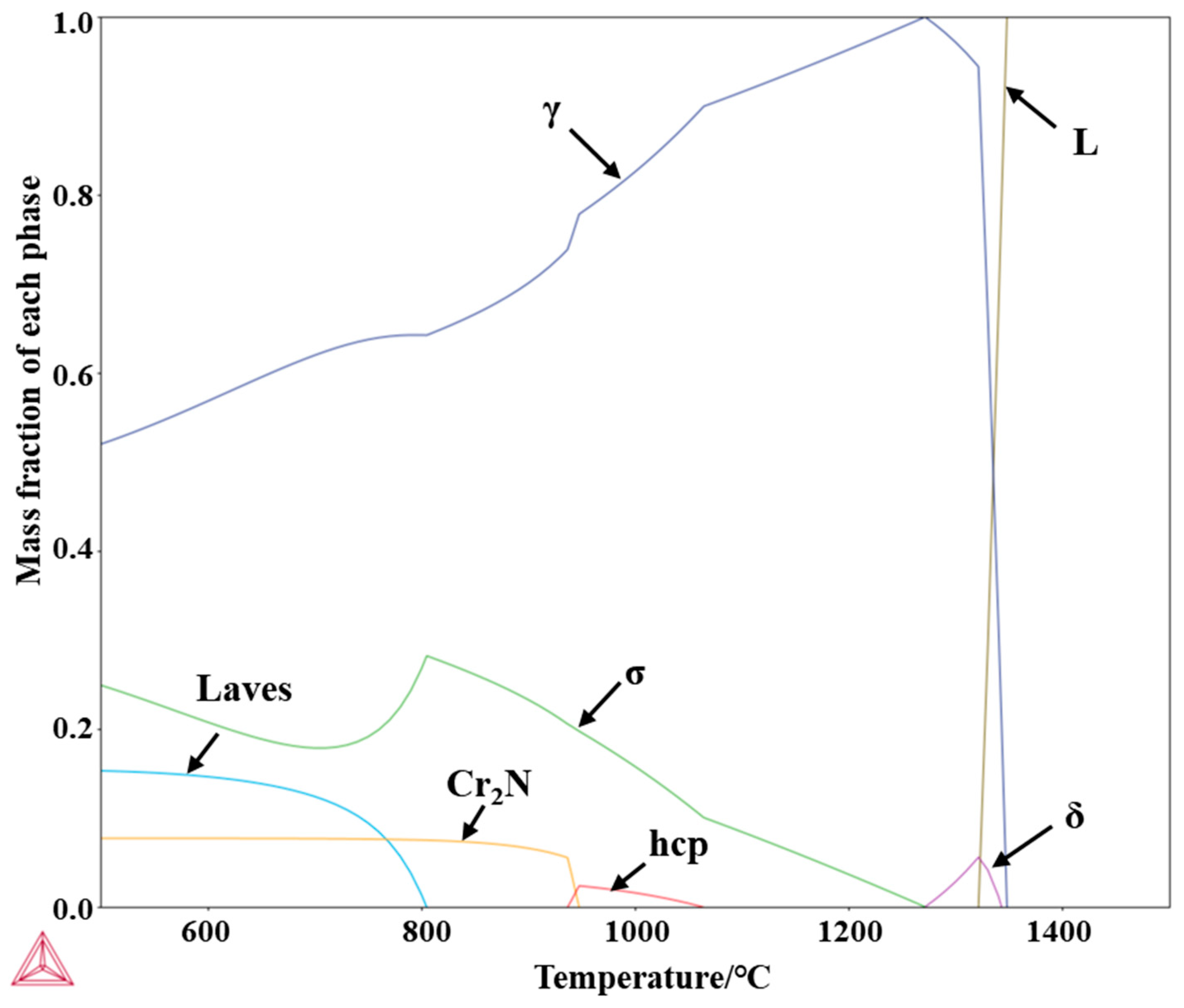
3.3. Microstructure
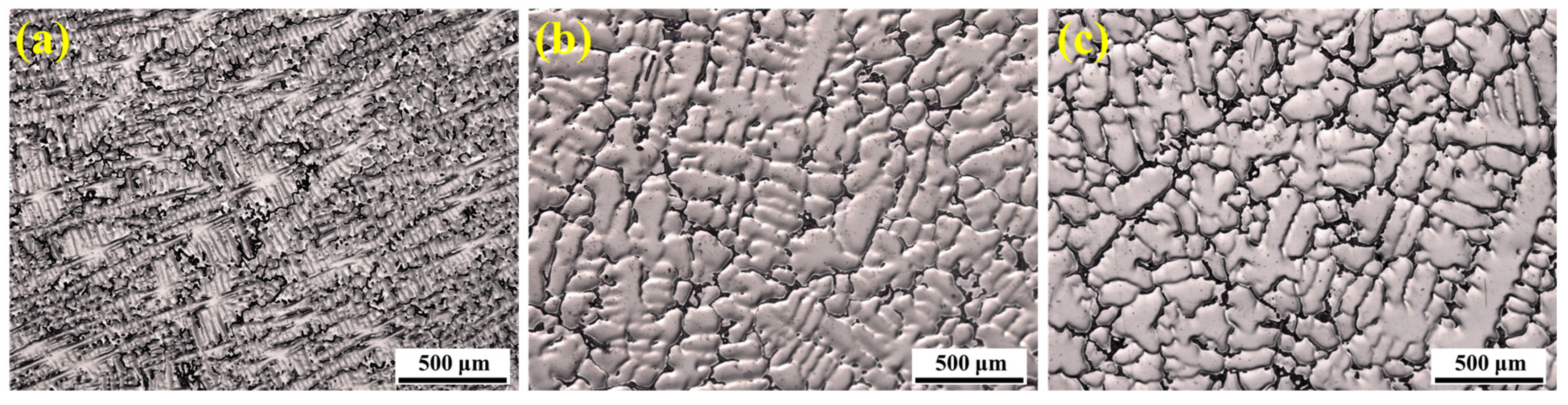
The dendritic microstructure at different locations of the S32654 ingot was observed using OM, as shown in
Figure 2. The results indicate that a large number of black precipitates exist between the dendrites. Significant differences were observed in the dendrite morphology and secondary dendrite arm spacing at different locations. At the edge of the ingot, the solidification rate was relatively high, resulting in a well-developed and fine dendritic structure with numerous secondary dendrite arms. At the midpoint of the ingot radius (R/2), the reduced solidification rate caused obvious coarsening of the secondary dendrite arms. The center of the ingot exhibited an equiaxed grain structure with a coarser solidification microstructure. Overall, from the edge to the center, as the cooling rate decreased, the morphology of the precipitates changed from discrete to more continuous, and the size of irregularly shaped precipitates significantly increased.
During the alloy-solidification process, the growth of dendrites significantly influences macrosegregation, microsegregation, and pore distribution, which ultimately affect the mechanical properties of the material [
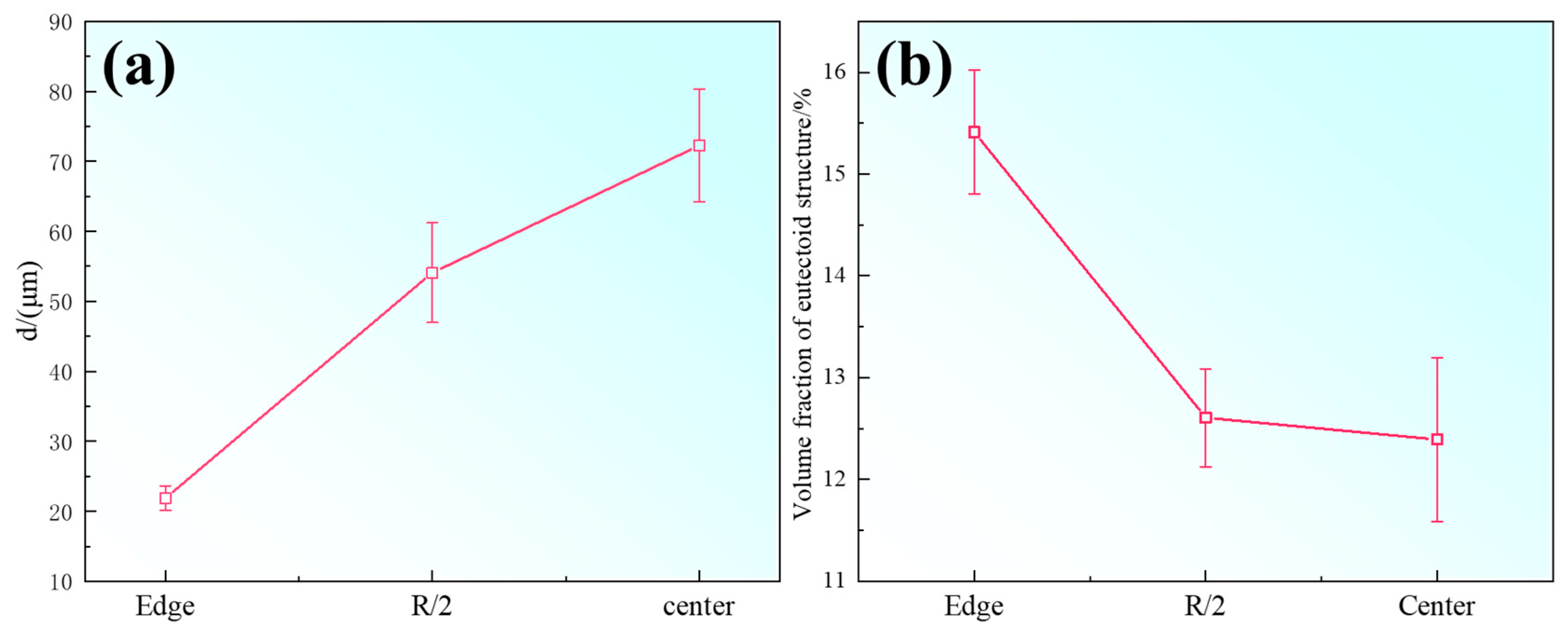
23]. The secondary dendrite arm spacing (SDAS) is an important indicator reflecting the degree of dendritic refinement. The SDAS at different locations of the ingot was measured, and the results are shown in
Figure 3a. As the cooling rate decreases from the ingot edge toward the center, the secondary dendrite arm spacing increases significantly. Generally, a smaller SDAS corresponds to a narrower range of elemental segregation, making it easier to achieve microstructural homogenization of the stainless steel through solution treatment. Additionally, microscopic shrinkage porosity and inclusion distributions tend to be finer and more uniform, which is crucial for improving the performance of stainless steel.
Meanwhile, the volume fraction of eutectoid phases at different ingot locations was also measured, with results shown in
Figure 3b. The volume fraction of eutectoid phases in the edge region is significantly higher than that at the center, showing an opposite trend to the variation of SDAS. Previous studies have shown [
24] that in SASS ingots, the σ phase is mainly formed by the eutectoid decomposition of austenite. Under rapid cooling conditions, although overall diffusion is limited, the small dendrite spacing impedes the uniform diffusion of alloying elements (such as Cr and Mo), leading to severe local segregation; the σ phase forms precisely in these severely segregated interdendritic regions. With extended time and slower cooling at the ingot center, sufficient diffusion occurs, homogenizing element distribution and thereby reducing the conditions favorable for σ phase precipitation.
The microstructure morphology of the σ phase is shown in
Figure 4. As illustrated in
Figure 4a, there is a distinct interface between the σ phase and the γ phase. The eutectoid structure exhibits a clear honeycomb morphology, while the γ
2 phase appears as a lamellar distribution.
Figure 4b shows two cracks that penetrate through the γ
2 phase and propagate along the σ phase, reflecting the hard and brittle nature of the σ phase. Its precipitation in the steel leads to material embrittlement. Konosu et al. [
25] observed hot cracks in stainless steel weldments and found that cracks propagated along the γ/σ interface, which is the initial fracture site with low ductility and also a key nucleation site for the σ phase. Further observations revealed that the γ
2 phase exhibits two distinct morphologies: one is a coral-like agglomeration (
Figure 4c), and the other is a lamellar distribution on the plane (
Figure 4d).
After deep etching, the SEM morphology of the eutectoid structure is shown in
Figure 4e,f. The eutectoid structure between dendrites, after etching, forms a network of pits, while the matrix remains relatively intact. From
Figure 4f, the corrosion traces of the lamellar eutectoid structure are evident; the σ phase has been etched away, whereas the γ
2 phase is preserved. To further investigate the phase composition of the eutectoid structure, EBSD characterization was performed on the SASS, with results shown in
Figure 4g–i. In these images, the σ phase is marked in blue, the γ austenite in green, and the γ
2 phase is distributed as granular particles on the surface of the σ phase, forming a honeycomb structure.
3.4. Segregation Behavior
Typically, the σ phase formed during solidification is significantly coarser than that formed by solid-state phase transformation. Due to its large size, even solution treatment cannot fully dissolve the solidification σ phase, and the residual σ phase markedly deteriorates the material’s hot workability. Therefore, reducing elemental segregation in the solidification microstructure and effectively controlling σ phase precipitation are key factors for the stable production of the S32654 alloy [
5].
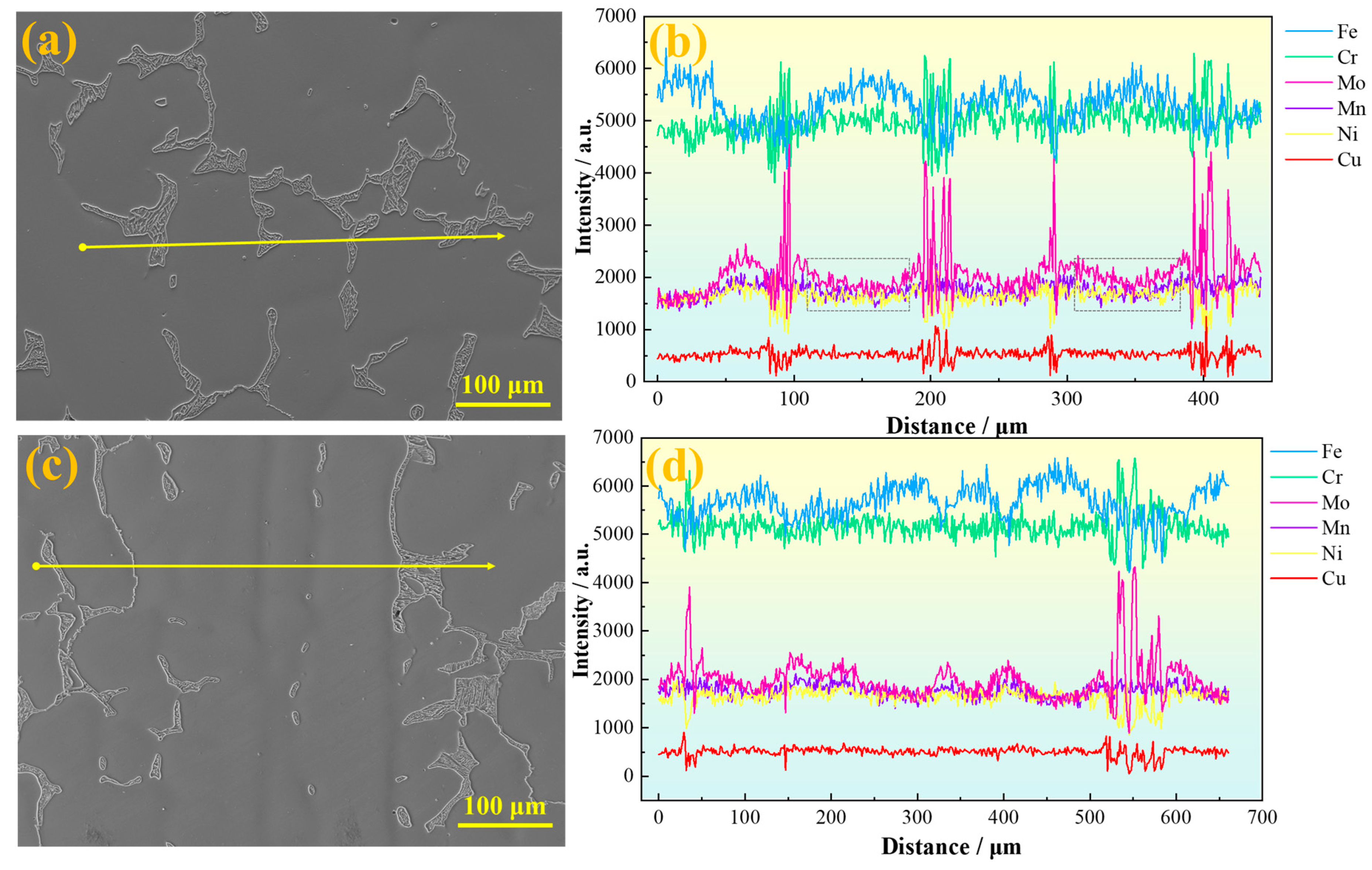
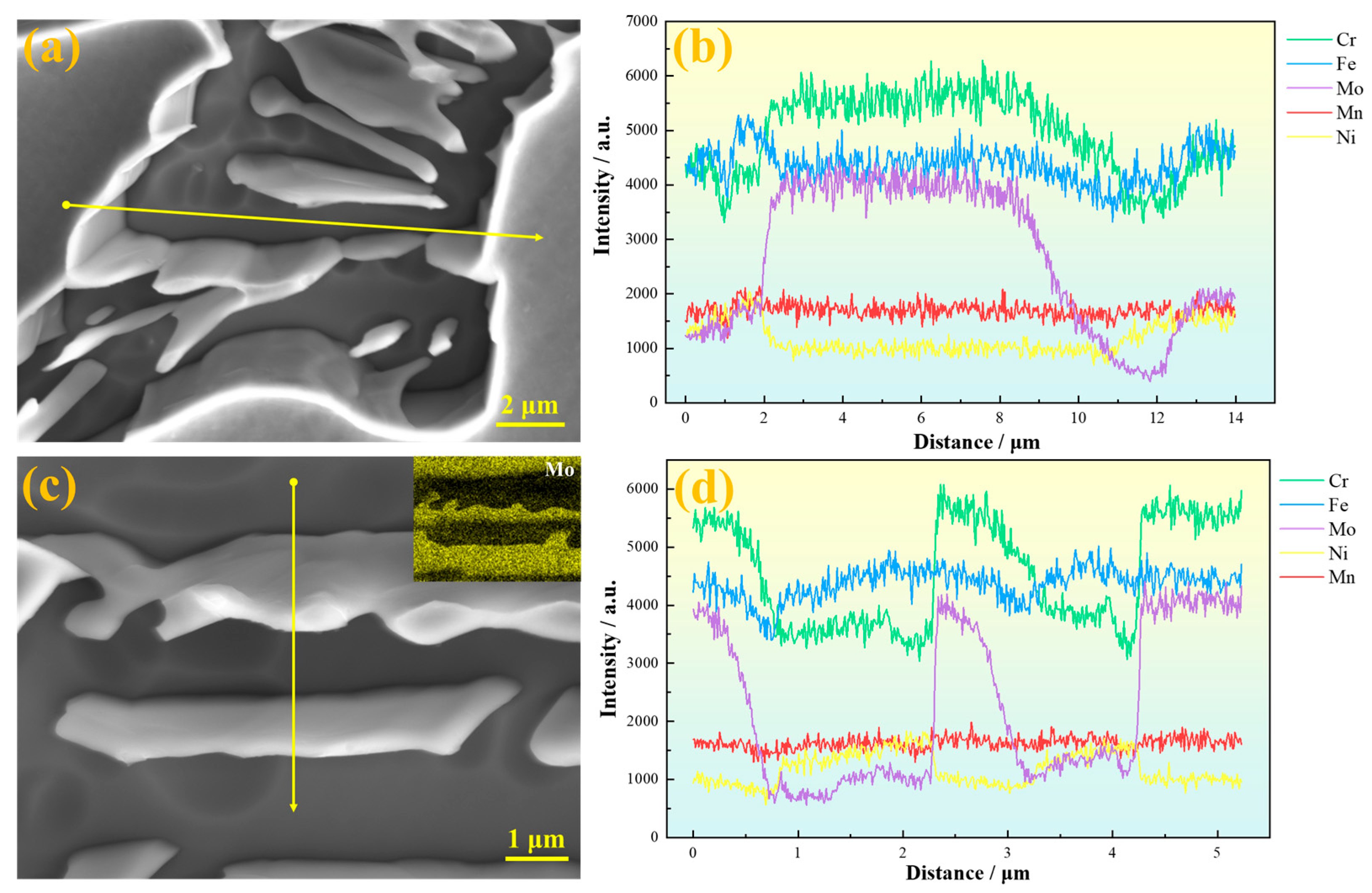
Figure 5a,b present line scan results parallel to the primary dendrite growth direction of the sample. Significant compositional fluctuations of various elements are observed in the secondary dendrite arm regions, especially around the eutectoid structure, where element contents vary drastically. Among these, Mo, Cr, and Fe exhibit pronounced fluctuations, while Mn shows relatively minor variations. As indicated by the gray box in
Figure 5b, the distribution curve of Mo on the austenite matrix shows a typical concave profile, with a lower concentration at the center and higher concentrations at both edges. This pattern results from Mo’s partition coefficient being less than 1: dendrites first solidify from the liquid, with the central dendrite region forming earliest, causing the lowest Mo concentration there. The interdendritic regions solidify later, retaining Mo-enriched residual liquid that eventually forms Mo-rich segregated areas. During dendrite lateral growth, solute elements like Mo are progressively rejected toward the dendrite sides and interdendritic zones, further intensifying segregation.
Figure 5c,d show line scan results perpendicular to the dendrite trunk direction. It can be seen that Mo exhibits obvious positive segregation in the interdendritic regions. Slight Mo enrichment is also observed in the austenite regions at the center of secondary dendrites, although eutectoid structures have not yet formed there. The greater the amplitude of Mo content fluctuation, the easier it is for eutectoid structures to be induced in the interdendritic areas. Since SASS inherently contains a high Mo content, and Mo tends to segregate in interdendritic regions, combined with the relatively low diffusion rates of other alloying elements in austenite, this further aggravates Mo segregation and ultimately promotes the precipitation of brittle σ phase [
26].
To further clarify the elemental distribution around the eutectoid structure, energy-dispersive spectroscopy (EDS) analysis was conducted, and the results are shown in
Figure 6. The line scan path in
Figure 6a crosses the austenitic matrix and σ phase, revealing a clear depletion of Cr and Mo elements around the σ phase, with Mo depletion being more pronounced. This elemental depletion significantly reduces the pitting corrosion resistance in these regions.
Figure 6c,d present line scan results within the eutectoid structure. A distinct compositional gradient is observed at the γ
2/σ phase interface. Cr and Mo elements are relatively uniformly distributed in the γ
2 phase, whereas their distributions fluctuate within the σ phase, indicating a relatively faster diffusion rate of Cr and Mo in the γ
2 phase. Furthermore, the EDS area scan shown in the upper right corner of
Figure 6c corroborates these findings, with the most pronounced contrast in Mo content between the γ
2 and σ phases, consistent with the line scan results.
The compositional inhomogeneity caused by dendritic segregation leads to microstructural differences, which significantly affect the material’s properties. To characterize the degree of microsegregation in the solidified structure of SASS, the segregation ratio at the center of the ingot was measured using EDS point scanning:
represents the average elemental concentration in the interdendritic region, while
denotes the average concentration in the dendrite arms, which is used to quantify the degree of microsegregation in the solidified structure. Twenty regions each were selected in the interdendritic and dendrite cores for compositional analysis to reduce experimental error. The measurement results are shown in
Figure 7a. The results indicate that the degree of elemental segregation follows the order: Mo > Ni > Mn > Fe > Cr. Among them, Mo exhibits the most severe segregation with a segregation ratio of 1.69. Besides Mo, Ni also shows significant segregation behavior, with a segregation ratio of 0.85, indicating notable Ni enrichment during solidification.
The average compositions of the σ phase, γ phase, and γ
2 phase in SASS were statistically analyzed, with the results shown in
Figure 7b. Here, C
γ2 and C
σ represent the elemental contents in the γ
2 phase and σ phase, respectively, and C
σ/C
γ2 denotes their content ratio. The results indicate that Mo exhibits the largest difference in content between the γ
2 and σ phases, with a C
σ/C
γ2 ratio reaching 3.19; next is Ni, with a C
σ/C
γ2 ratio of 0.49. Therefore, besides the diffusion behavior of Mo and Cr, the diffusion characteristics of Ni in the S32654 alloy should also not be overlooked. Comparing the compositions of austenite and γ
2 phases reveals that the γ
2 phase contains more Ni and Mn but less Cr and Mo. This suggests that during σ phase precipitation, a large amount of Mo and Cr preferentially segregate into the σ phase, accompanied by the depletion of Ni and Mn, leading to a relative increase in Ni content in the γ
2 phase. It is worth noting that due to the lower content of corrosion-resistant elements such as Cr and Mo in the newly formed γ
2 phase, its pitting resistance equivalent number (PREN) significantly decreases, resulting in relatively poor corrosion resistance of the γ
2 phase [
27].
3.5. In Situ Observation of Melting and Solidification Processes Under High Temperature Confocal Microscopy
To investigate the dissolution behavior of precipitates, nucleation during solidification, and the grain growth process in SASS, in situ heating observations of the S32654Y sample were conducted using HT-CLSM.
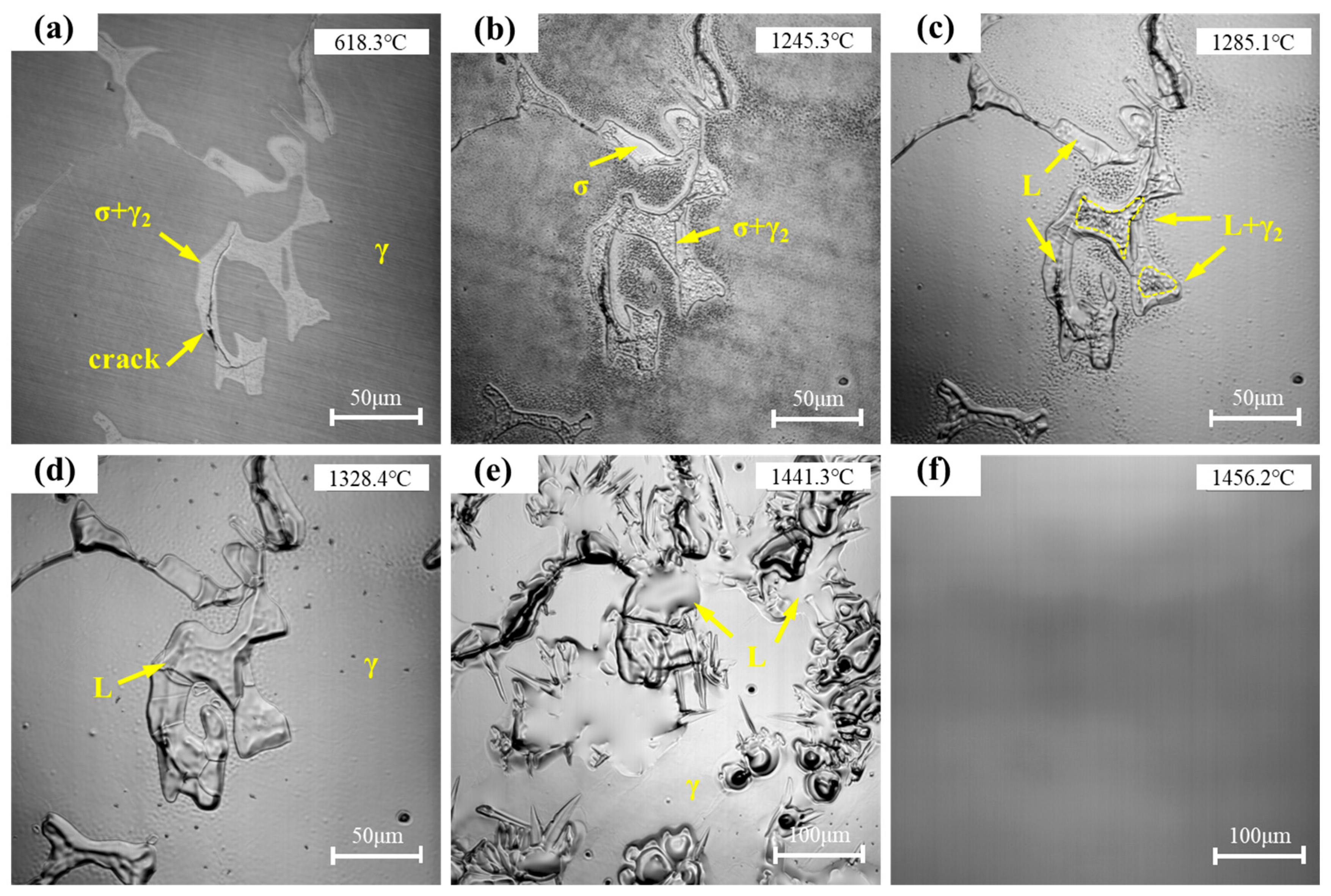
Figure 8 shows the representative microstructural evolution of the sample during heating. When the temperature reached 618.3 °C, numerous secondary phases were observed distributed on the austenite matrix (
Figure 8a). As the temperature increased to 1245.3 °C, the σ phase in the interdendritic regions began to dissolve (
Figure 8b); with further heating to 1285.1 °C, the γ
2 phase gradually started to dissolve (
Figure 8c); at 1328.4 °C, the γ
2 phase was completely dissolved into the matrix (
Figure 8d). Studies have shown that if the alloy is heated slowly and held below the eutectoid temperature for sufficient time, the secondary phases can gradually dissolve under solid-state conditions without melting. However, when the temperature rapidly rises and exceeds the eutectoid point, the secondary phases are prone to liquefaction. Subsequently, as the temperature increased to 1441.3 °C, the melt pool rapidly expanded, and the austenite matrix began to dissolve more rapidly (
Figure 8e); finally, at 1456.2 °C, the dendritic morphology completely disappeared, and the sample fully transformed into a liquid state (
Figure 8f). These results indicate that the σ phase and γ
2 phase have different dissolution temperature ranges, with the σ phase dissolving prior to the γ
2 phase, and the γ
2 phase dissolving significantly earlier than the melting of the austenite matrix.
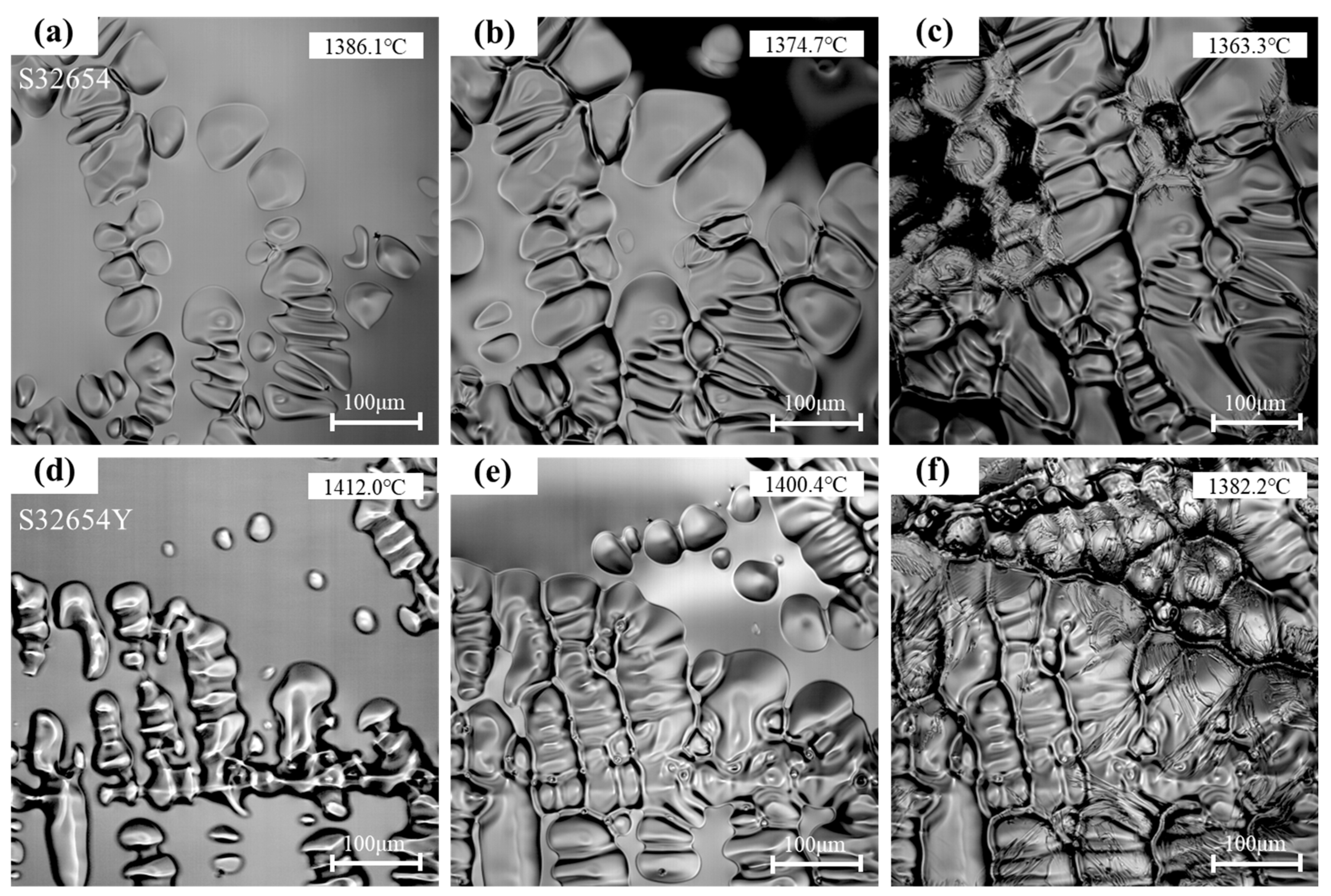
The solidification processes of the two samples were observed in situ using a HT-CLSM, as shown in
Figure 9 and
Figure 10. The corresponding temperature and time changes during solidification were synchronously recorded and marked in the figures. The HT-CLSM analysis results are summarized in
Table 2, where T
n represents the initial nucleation temperature, T
s the complete solidification temperature, ΔT the solidification temperature range, and t the time required for complete solidification. For the S32654 alloy, the molten steel began initial nucleation upon undercooling to 1410.6 °C (
Figure 9a). Twelve seconds after nucleation onset, a large number of austenite nuclei formed within the field of view, with a nucleation density of 103.2 nuclei/mm
2 (
Figure 9b). As solidification progressed, the nuclei rapidly grew and increased in number. During further cooling (
Figure 10a), austenite dendrites gradually formed and extended toward the liquid phase, with the solid phase fraction continuously increasing (
Figure 10b). Finally, when the temperature dropped to 1363.3 °C, the liquid phase completely transformed into solid, marking the end of the solidification process (
Figure 10c). The solidification temperature range of this alloy was 47.2 °C, and the total solidification time was 90.4 s.
In contrast, the S32654Y sample exhibited significant differences during solidification. As shown in
Figure 9d–f, initial solidification of this sample began at 1435.5 °C (
Figure 9d), with a nucleation temperature notably higher than that of the sample without rare earth additions. This indicates that microalloying with the rare earth element Y promotes the formation of the austenite phase. Studies have shown that rare earth elements can form fine inclusions at the early stages of solidification, providing nucleation sites that effectively enhance the heterogeneous nucleation of the austenite phase. Previous research [
12] points out that inclusions formed by rare earth elements typically possess good lattice matching, which is one of the key factors promoting austenite nucleation.
After 12 s of initial nucleation (
Figure 9e), the nucleation surface density of austenite reaches 193.5 nuclei/mm
2, and the grain size is 13.4 μm, indicating that the addition of rare earth Y can significantly promote the solidification nucleation of SASS and effectively reduce the grain size. As the temperature gradually decreases, the solid–liquid interface continuously advances into the interdendritic regions, with the residual liquid phase gradually diminishing and forming a connected network (
Figure 10d,e). Ultimately, the residual liquid in the interdendritic regions completely solidifies. The complete solidification temperature of S32654Y is 1384.1 °C, with a solidification temperature range of 51.4 °C and a solidification time of 102.1 s. The addition of rare earth Y broadens the solidification temperature range and extends the grain growth period, resulting in a more uniform and finer final solidification microstructure. Previous studies have confirmed that rare earth elements contribute to the refinement of the SASS solidification microstructure. For example, Ce inclusions exhibit good lattice matching with austenite, effectively promoting heterogeneous nucleation [
28].
As shown in
Figure 10c,f, both samples formed feather-like structures at the final stage of solidification. Obvious grain boundary migration phenomena can be observed, with fine lines indicating the traces left by migrating grain boundaries. The migrating grain boundaries (MGBs) formed during the final stage of solidification are a special type of grain boundary characterized by high interfacial energy, making them more prone to migration. During migration, grain boundaries tend to develop a crystallographically aligned and straight morphology, thereby reducing the system’s overall energy. In this process, new grain boundaries separate from the original subgrain boundaries (SGBs) [
29].
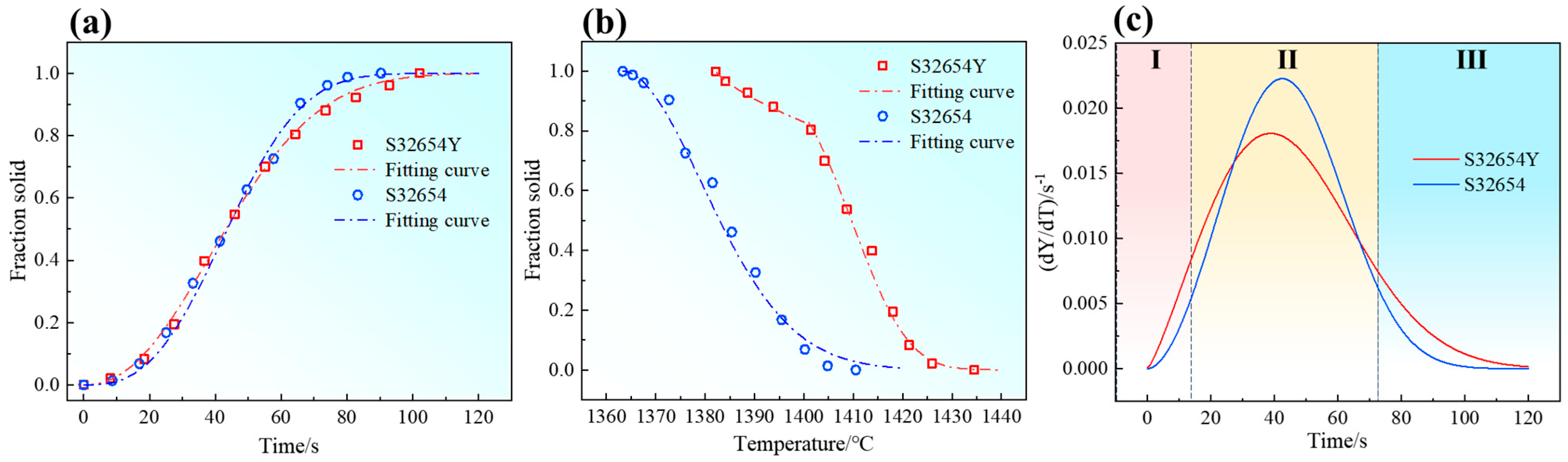
The relationship between the solid phase fraction and time during solidification is shown in
Figure 11a. To quantitatively describe the nucleation and growth behavior of the new phase, this study employs the Johnson–Mehl–Avrami–Kolmogorov (JMAK) theory for analysis. The JMAK equations are given in Equations (4) and (5), where the Avrami exponent n can be used to analyze the nucleation mechanism during solidification [
30]. The solidification rate constant k reflects the transformation rate. Based on Equation (4), the experimental data were fitted, and the fitting results are summarized in
Table 3.
In the equations,
t is the solidification time,
n is the Avrami exponent, and
k is the solidification rate constant. The Avrami exponents for the two samples are 2.78 and 2.38, respectively, both indicating a site-saturated nucleation mechanism. The relationship between the solid fraction and temperature is given by Equation (6), where
A and
B are constants,
Y is the solid fraction,
T is the temperature, and
Ts is the solidus temperature. The fitting results are shown in
Table 4.
Figure 11b,c show the solid fraction–time fitting curves and solidification rate–time curves, respectively. The solidification process of the samples can be divided into three stages: the initial solidification stage, the steady solidification stage, and the final solidification stage. In the initial stage, due to low undercooling, the phase transformation rate is relatively slow. As the temperature continues to decrease, undercooling increases, significantly enhancing the transformation rate and leading to rapid solidification. In the later stage, as Cr and Mo segregation becomes more pronounced, the compositional difference between the remaining liquid and solid phases increases markedly. The austenitic matrix must absorb more solute atoms enriched in the residual liquid, which significantly slows the solidification rate. The addition of rare earth Y accelerates the initial solidification process and increases the nucleation rate, but also prolongs the final solidification time. As a result, the duration of element segregation increases, leading to more severe segregation at the end of solidification.
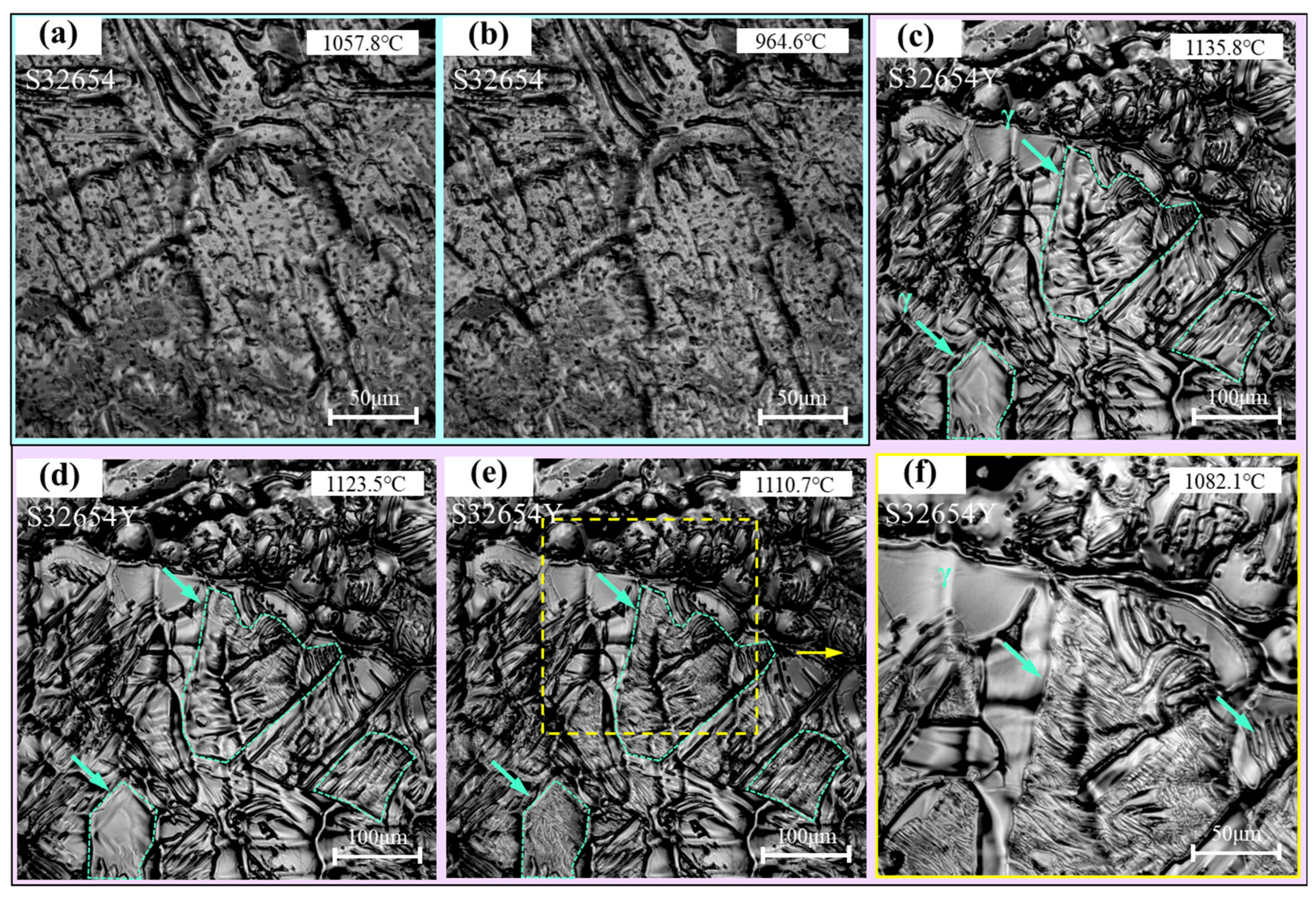
The solid-state phase transformation process of the samples is shown in
Figure 12. For the S32654 sample, when cooled to 964.6 °C (
Figure 12b), a sudden phase transformation occurs, resulting in the precipitation of a large amount of lamellar structures, primarily distributed between the feather-like structures. In contrast, for the S32654Y sample, as shown in
Figure 12c, no phase transformation is observed when the temperature drops to 1135.8 °C. Subsequently, when the temperature decreases to 1123.5 °C (
Figure 12d), lamellar structures begin to precipitate, as indicated by the green arrows. Further cooling to 1110.7 °C completes the precipitation of the lamellar structures, and the phase transformation occurs at a very fast rate.
Figure 12f is a magnified view of
Figure 12e, clearly showing the precipitation of a secondary phase between the feather-like structures, while the feather-like structures themselves remain unchanged. In addition, some of the secondary phase precipitates directly from the austenitic dendrites.
3.6. Formation Mechanism of the σ Phase
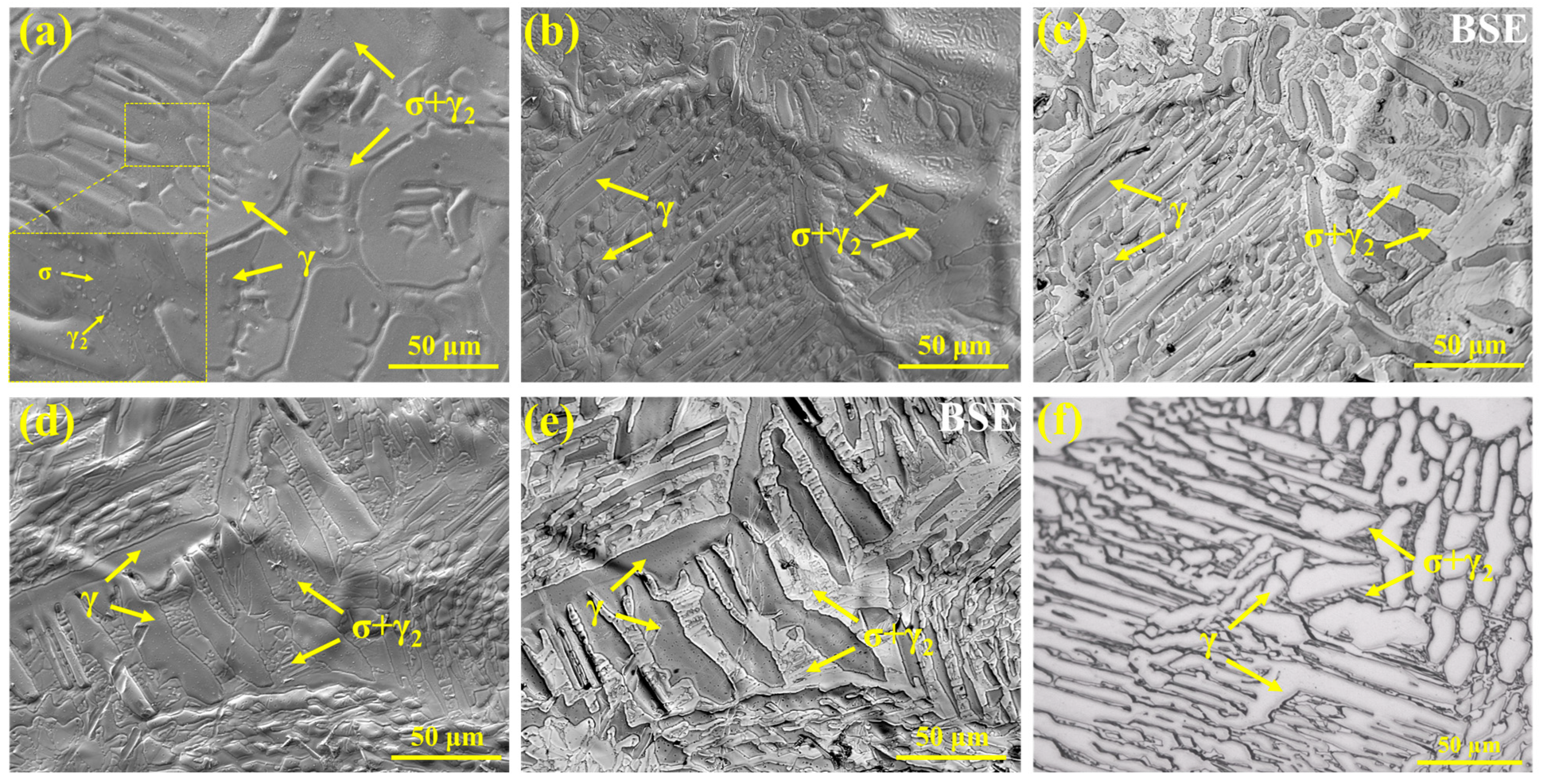
The microstructure of the samples observed using high-temperature in situ confocal microscopy is shown in
Figure 13. For the S32654 sample, a large number of dendritic structures can be seen on the surface, with the dendrites slightly protruding above the surface. Their morphology corresponds to the feather-like structures shown in
Figure 12 (
Figure 13a). Lamellar structures are distributed between the dendrites, appearing as depressions. As shown in
Figure 13b, the solidification microstructure of the S32654Y sample is finer and exhibits typical eutectoid characteristics, primarily distributed between the feather-like and granular structures. The particles within the eutectoid structure are γ
2 phase.
Figure 13c is the corresponding backscattered electron (BSE) image of
Figure 13b, revealing two distinct contrast regions: the brighter areas represent the σ phase, while the darker areas correspond to the austenite phase.
Figure 13d,e clearly show the morphological features of the austenitic dendrites and the interdendritic σ phase.
Figure 13f presents the microstructure of the S32654Y sample after metallographic preparation, showing a morphology highly consistent with the in situ microstructure observed in the lower left region of
Figure 13c, both consisting of austenitic dendrites and interdendritic eutectoid structures.
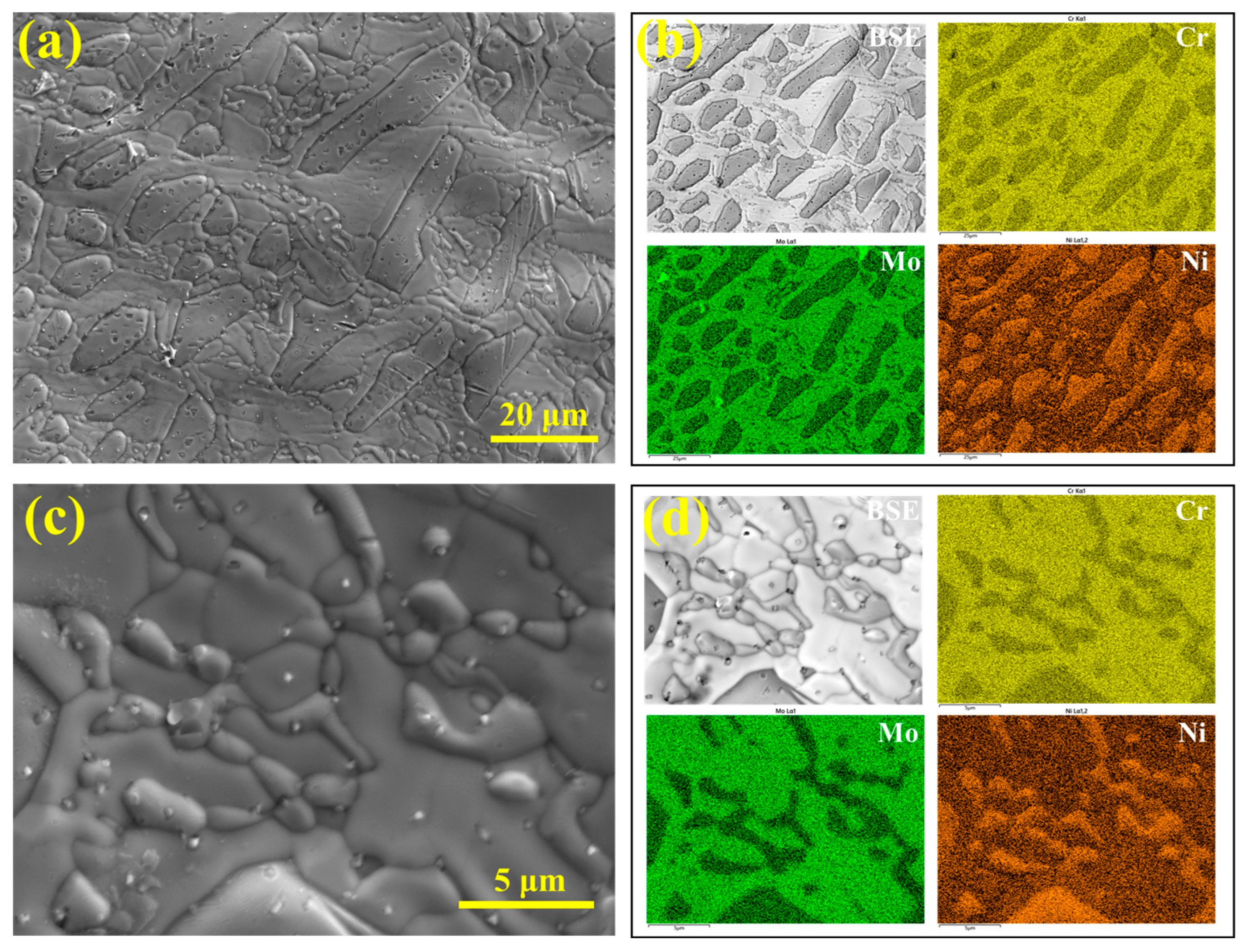
To further characterize the eutectoid microstructure, EDS was used to analyze its composition, as shown in
Figure 14a,b. The results indicate that the eutectoid structure is rich in Cr and Mo, while the granular austenite is enriched in Ni.
Figure 14c,d present the EDS analysis within the eutectoid structure, revealing that the granular phase corresponds to the Ni-rich γ
2 phase. Therefore, the σ phase is not directly precipitated from the enrichment of σ-forming elements in the liquid phase, but rather forms through a eutectoid reaction at the final stage of solidification. No ferrite phase was observed during solidification, indicating that the eutectoid decomposition of ferrite (δ → σ + γ
2) is not responsible for the formation of the σ phase.
Based on the phase composition analysis of super austenitic stainless steel, the σ phase is formed through a solid-state phase transformation of austenite at approximately 1124 °C (using the S32654Y sample as an example), specifically via the reaction γ → γ
2 + σ. The γ
2 phase is distributed in a lamellar or granular form within the gaps of the σ phase. Due to Cr and Mo depletion and Ni enrichment in the austenite near the σ phase region, the surrounding austenite becomes unstable and transforms into the γ
2 phase upon σ phase precipitation. This transformation originates from the high Cr and Mo content in the austenite formed from the residual liquid. During non-equilibrium solidification, Cr and Mo atoms lack sufficient time to diffuse and thus accumulate in the residual liquid, leading to significant compositional differences between the interdendritic regions (residual liquid) and the matrix after solidification. Grain boundary migration and volume expansion result in a “protrusion” phenomenon. The austenite in interdendritic regions becomes supersaturated; when the temperature drops to a critical point, these alloying elements can no longer remain dissolved in austenite, and a solid-state transformation occurs during cooling, releasing energy and forming the σ phase. This entire process can be described as L → L + γ → γ → γ + γ
2 + σ. This sequence aligns with thermodynamic calculations, indicating that upon cooling, the σ phase can directly precipitate from the γ matrix under thermodynamic equilibrium conditions. When the carbon and nitrogen contents are below the critical threshold, the σ phase precipitates directly at the austenite grain boundaries through a typical solid-state reaction [
17].
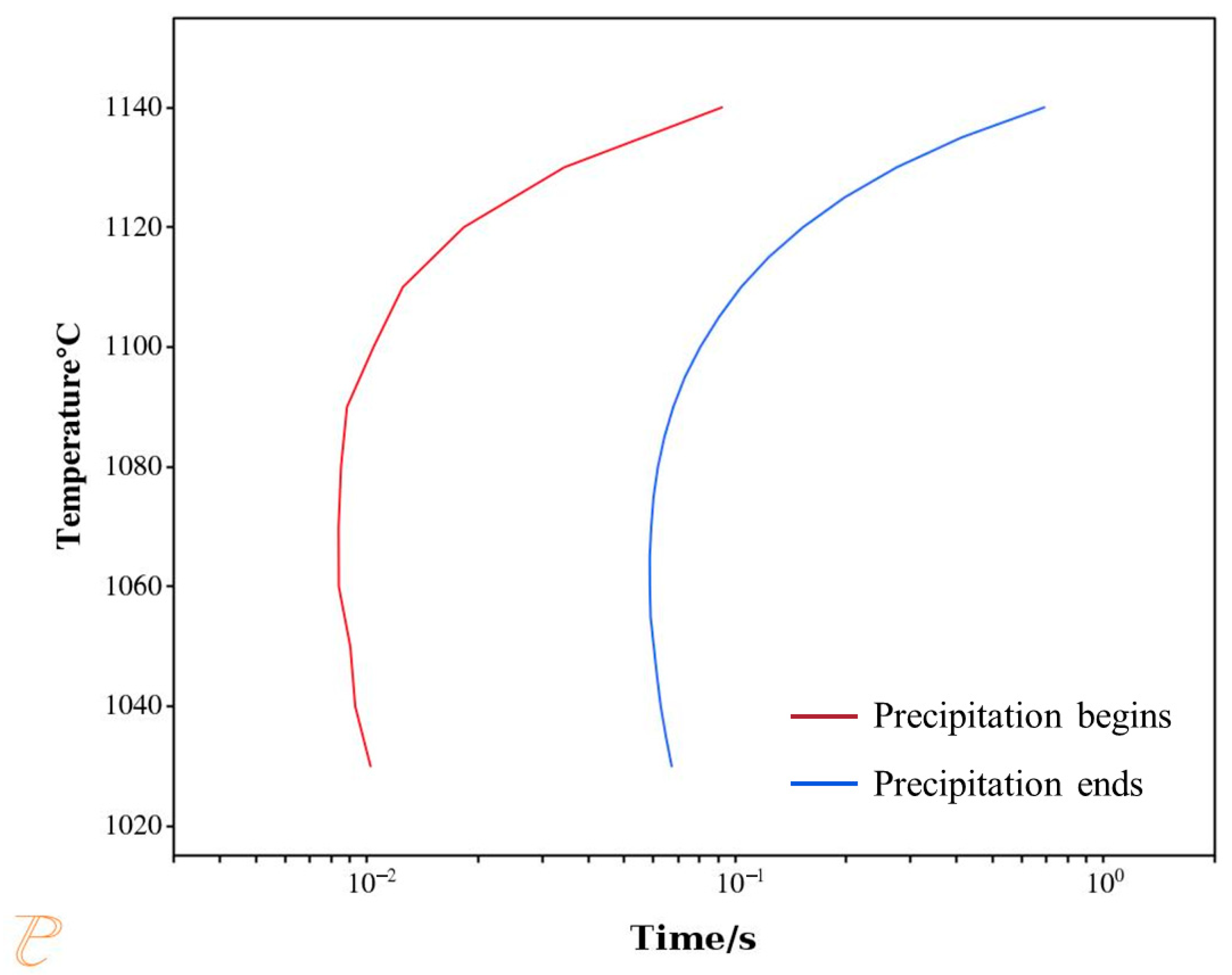
The kinetics of phase transformation play a crucial role in controlling the microstructure and properties of materials. The time required for isothermal transformations can be characterized using a Time–Temperature–Transformation (TTT) diagram.
Figure 15 presents the TTT curve for the precipitation of the σ phase from austenite. The red curve represents the onset time of σ phase precipitation, while the blue curve corresponds to the completion time of the precipitation reaction. As shown in the figure, the σ phase precipitates at a very fast rate—even a short residence time within the calculated temperature range can lead to its rapid formation. This result is consistent with the aforementioned in situ observations. Therefore, relying solely on rapid cooling is insufficient to effectively suppress σ phase precipitation. Additionally, the “nose temperature” for σ phase precipitation is 1070 °C, at which the precipitation rate reaches its maximum.
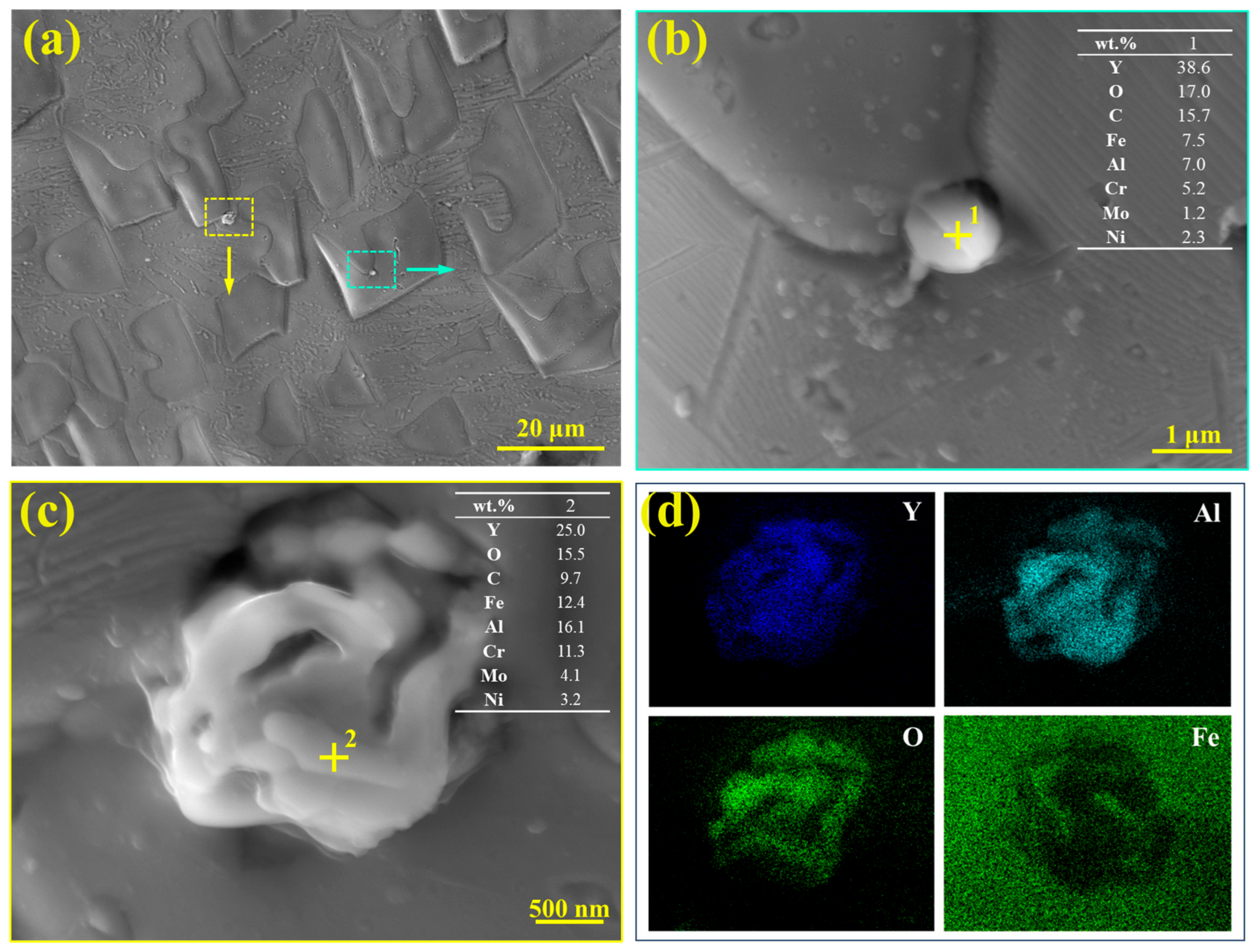
Further observations revealed the presence of some inclusions in the S32654Y sample, as shown in
Figure 16. Small-sized inclusions can be seen at the γ/σ phase interface.
Figure 16b,c show the high-magnification morphology of the inclusions and the results of EDS point analysis, respectively. The inclusion at Point 1 appears as a spherical particle, while that at Point 2 is in a clustered form. Based on their main elemental composition (Y, Al, and O), these inclusions can be identified as rare-earth-containing oxide inclusions. The EDS elemental mapping in
Figure 16d further confirms that the inclusions are mainly composed of Y, Al, and O, suggesting that their principal phase is likely YAlO
3. As reported in previous studies, rare earth additions typically promote the formation of inclusions with good lattice matching to the austenite matrix, which can serve as heterogeneous nucleation sites and thereby promote grain refinement [
28]. In addition, rare earth elements have high surface activity and tend to accumulate at the solid–liquid interface ahead of the solidification front, reducing the surface tension of the molten steel and suppressing grain growth. These inclusions may also act as potential heterogeneous nucleation sites for σ phase precipitation, influencing its transformation behavior.
3.7. The Effect of Rare Earth Element Y on Solidification Microstructure and Microhardness
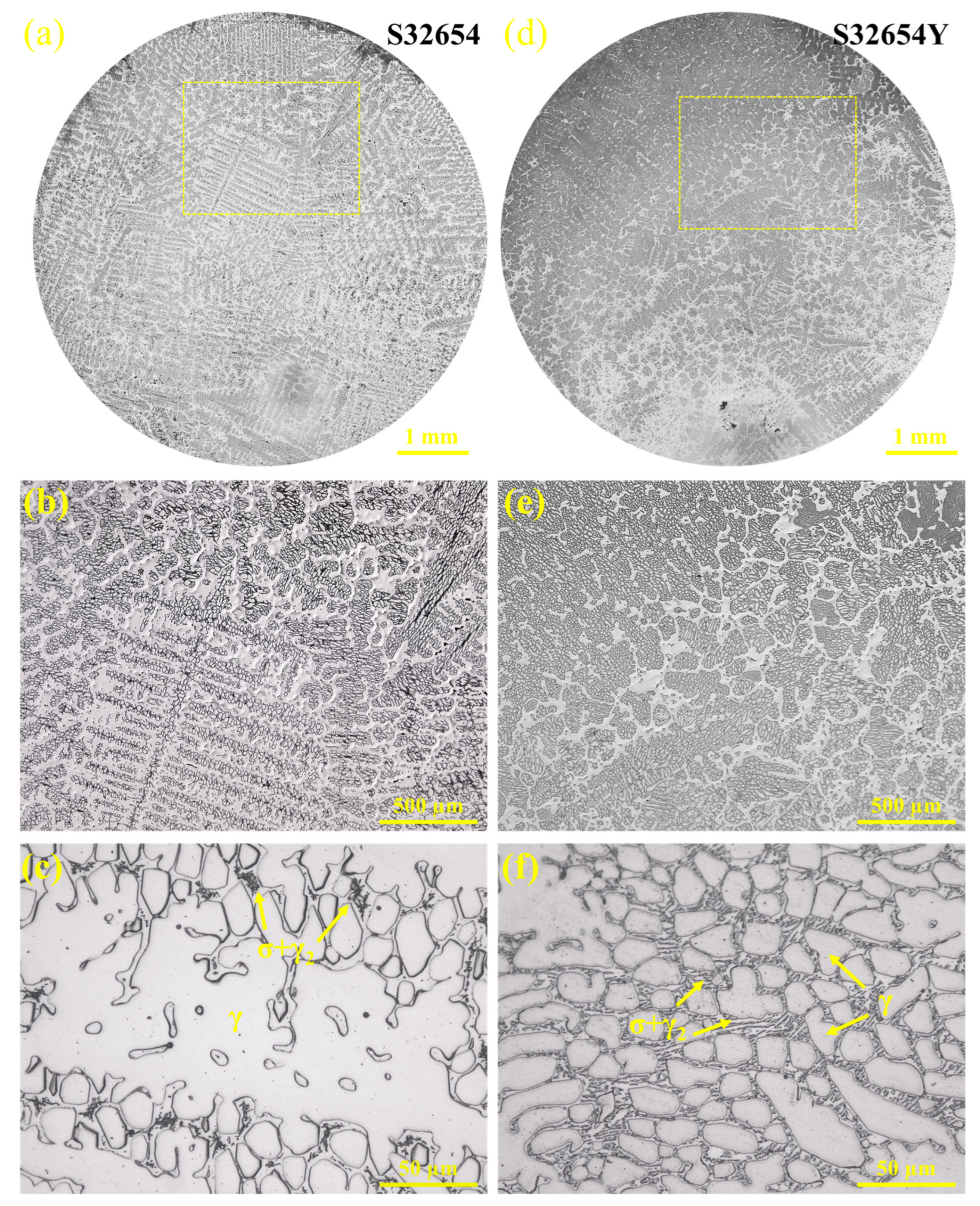
The metallographic structures of the samples observed via HT-CLSM are shown in
Figure 17. Significant differences are evident between the two samples.
Figure 17a displays an OM image of the entire cross-section of the S32654 sample, showing the formation of typical dendritic structures, with no other phases observed—confirming the in situ precipitation process of the σ phase.
Figure 17b, a magnified view of (a), clearly reveals the austenitic dendrites and the eutectoid structures between them.
Figure 17d presents the OM microstructure of the S32654Y sample, in which the structure is mainly composed of cellular crystals; the interiors of the cells contain granular austenite and eutectoid structures.
Statistical results in
Table 5 indicate that although rare earth element Y refines the austenite grains and secondary dendrite arm spacing, the eutectoid content increases. This is because Y extends the solidification temperature range and grain growth time, promoting the microsegregation of σ-phase-forming elements such as Cr and Mo. The addition of rare earth Y increases the number of heterogeneous nucleation sites, and raises both the initial solidification temperature and the σ phase precipitation temperature, thereby enhancing atomic diffusion during solidification and promoting the extensive precipitation of the σ phase in the subsequent eutectoid transformation. Related studies have shown that inclusions formed by rare earth elements also act as heterogeneous nucleation sites for the σ phase, and Y may alter the energy state of the matrix and lower the nucleation barrier for σ phase formation [
12]. Zhang S [
31] reported that rare earth Ce can increase the precipitation temperature of the σ phase and enhance the activity of Cr and Mo during its initial formation, thus facilitating σ phase nucleation.
Compared with mold-cast samples, the eutectoid content in HT-CLSM samples is higher. This is primarily due to the faster solidification rate in HT-CLSM samples, which prevents sufficient diffusion of solute elements according to thermodynamic equilibrium, leading to significant local enrichment of Cr, Mo, and other elements at grain boundaries and interdendritic regions. During the subsequent phase transformation stage, these highly concentrated enriched zones provide favorable compositional conditions for the formation of the σ phase.
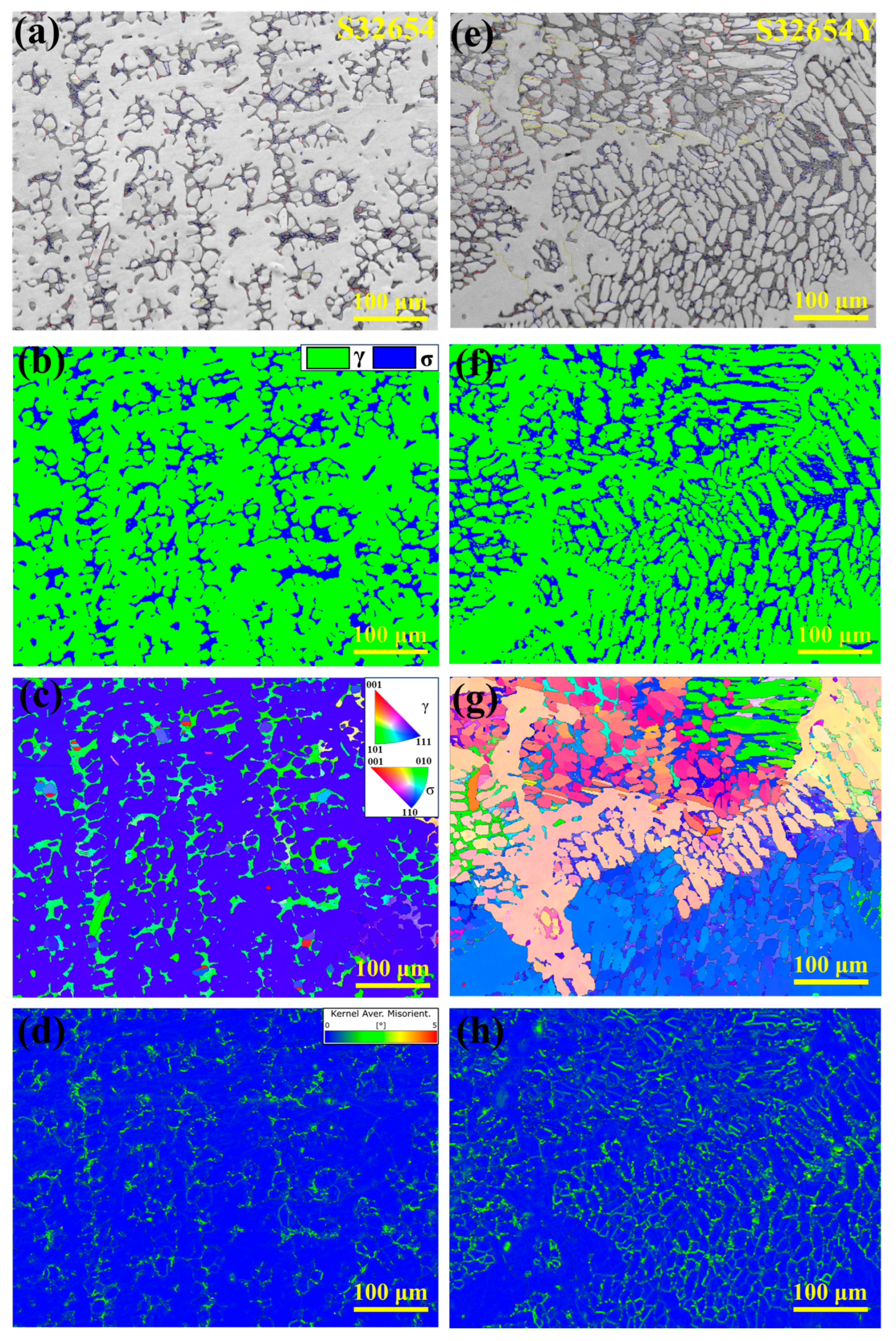
To further investigate the microstructural characteristics of the HT-CLSM samples, EBSD analysis was conducted on the central regions of both samples, as shown in
Figure 18. The grain boundary fractions and σ phase contents were quantitatively analyzed and are summarized in
Table 6. Understanding the nature of different boundaries or interfaces in SASS is crucial.
Figure 18a,e show the IQ maps of the two samples. In this study, grain boundaries with a misorientation angle greater than 15° are defined as high-angle grain boundaries (HAGBs), shown as red lines in the figures; those with misorientation between 2° and 15° are defined as low-angle grain boundaries (LAGBs), shown in blue; coincidence site lattice (CSL Σ3) boundaries are indicated in yellow.
Among the grain boundary characteristics of both samples, LAGBs dominate throughout the interfacial region, while the S32654Y sample exhibits a higher proportion of HAGBs.
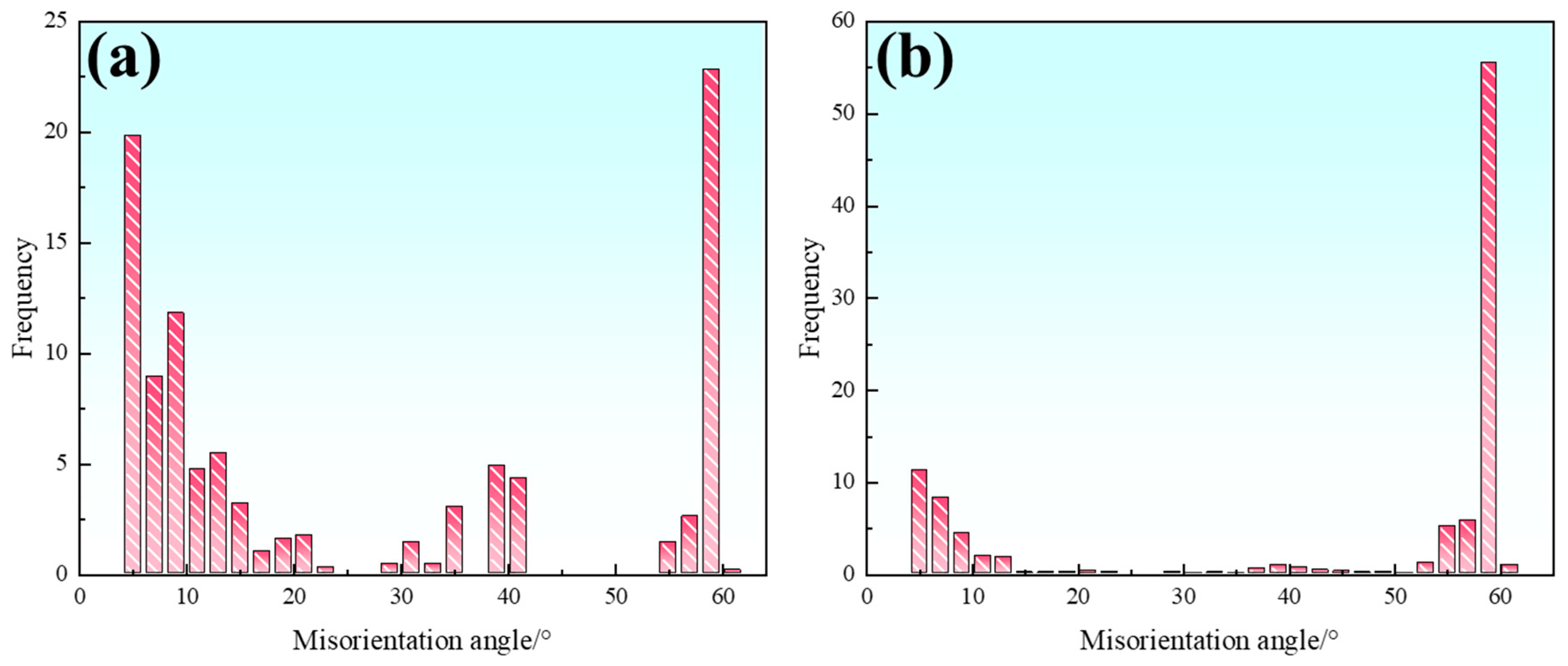
Figure 19 displays the statistical distribution of misorientation angles in the austenite phase of both samples, showing that the S32654Y sample contains a higher fraction of CSL Σ3 boundaries. CSL boundaries possess a high degree of atomic order and low grain boundary energy, thus offering better stability within the material. The addition of rare earth element Y increases local solute undercooling and induces interfacial energy fluctuations in the dendritic regions, thereby promoting the formation of twin dendrites. Moreover, CSL boundaries are considered to impede crack propagation to some extent, contributing to improved toughness in SASS.
As shown in
Figure 18b,f, the σ phase is mainly distributed in the interdendritic regions of austenite. The volume fraction of the σ phase in the S32654 sample is 15.1%, whereas in the S32654Y sample it reaches 20.4%, indicating that the addition of rare earth element Y promotes the precipitation of the σ phase during solidification. This is consistent with the previously reported statistical results of the eutectic phase fraction.
Figure 18c,g show the grain orientation maps (IPF maps), illustrating the distribution of grain orientations. The austenite in the S32654 sample exhibits relatively uniform orientation, presenting a single dendritic structure, whereas the S32654Y sample contains multiple dendrites with a clearly visible feather-like morphology, further confirming that rare earth Y effectively refines the solidification microstructure.
Figure 18d,h present the local average misorientation (KAM) maps, which indicate that strain is mainly concentrated in the σ phase regions, suggesting the presence of significant local stresses within the σ phase.
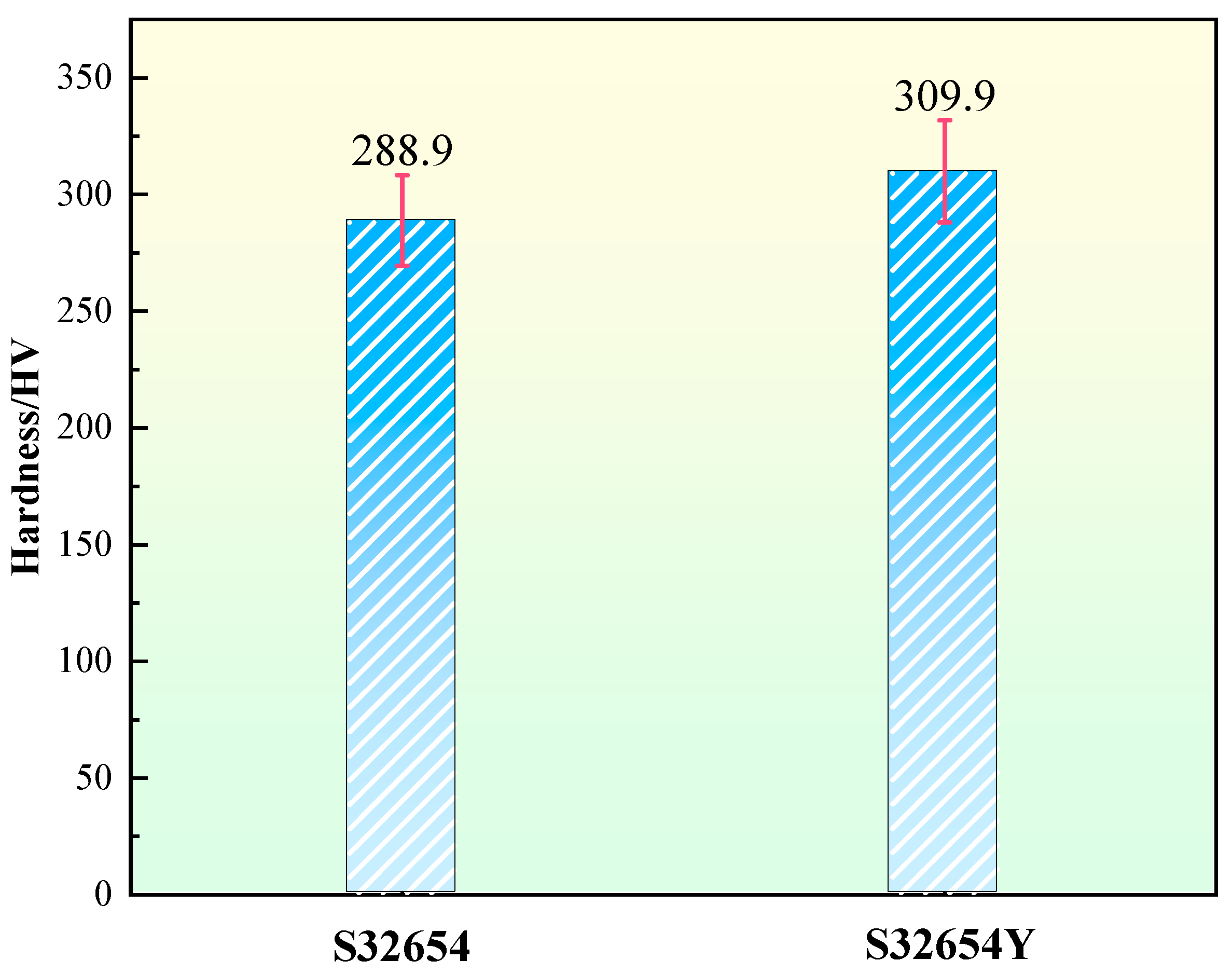
The microhardness of the two samples is shown in
Figure 20. It can be observed that after the addition of the rare earth element Y, the microhardness of the sample increased from 288.9 HV to 309.9 HV. The hardness improvement is mainly attributed to two factors: first, the σ phase itself has a hardness much higher than that of the austenitic matrix, and its precipitation significantly enhances the overall hardness of the material; second, the introduction of rare earth elements promotes the refinement of secondary dendrites in the microstructure, which also contributes to the increase in microhardness.
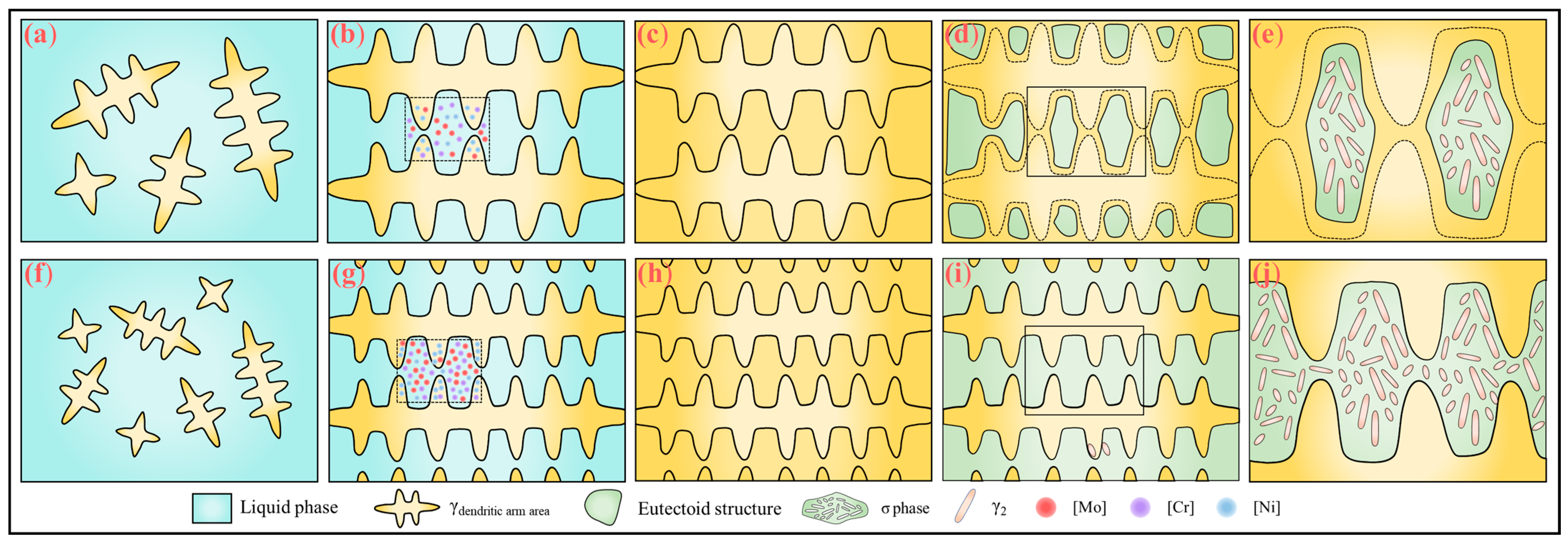
Figure 21 illustrates a schematic diagram of the mechanism by which the rare earth element Y affects the solidification microstructure and σ phase precipitation behavior in SASS. In the early stage of solidification (
Figure 21a,f), rare earth inclusions act as heterogeneous nucleation sites, facilitating the nucleation of austenite and raising the nucleation starting temperature. With the introduction of rare earth Y (
Figure 21b,g), the alloy’s solidification temperature range is broadened, and the grain growth time is extended, which intensifies the segregation of Cr and Mo elements in the interdendritic regions. At the end of solidification (
Figure 21c,h), the microstructure transforms into fully austenitic. During the subsequent eutectic transformation stage (
Figure 21d,i), the addition of rare earth elements raises the eutectic transformation temperature and promotes the precipitation of the σ phase. Meanwhile, rare earth inclusions may also serve as heterogeneous nucleation sites for the σ phase, leading to an increase in σ phase content. Additionally, due to dendrite refinement, the growth space for the σ phase is limited, resulting in a smaller σ phase size. In summary, through the synergistic effect of rare earth inclusions and solute Y, the solidification microstructure of the sample is refined, but the σ phase content increases (
Figure 21e,j).