On the Optimization of T6 Heat Treatment Parameters of a Secondary Al-Si-Cu-Mg Foundry Aluminum Alloy: A Microstructural and Mechanical Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thermal Analysis
2.3. Heat Treatments
2.4. Tensile and Hardness Testing
2.5. TG-DTA Simultaneous Thermal Analysis
2.6. Microstructural Analysis
3. Results
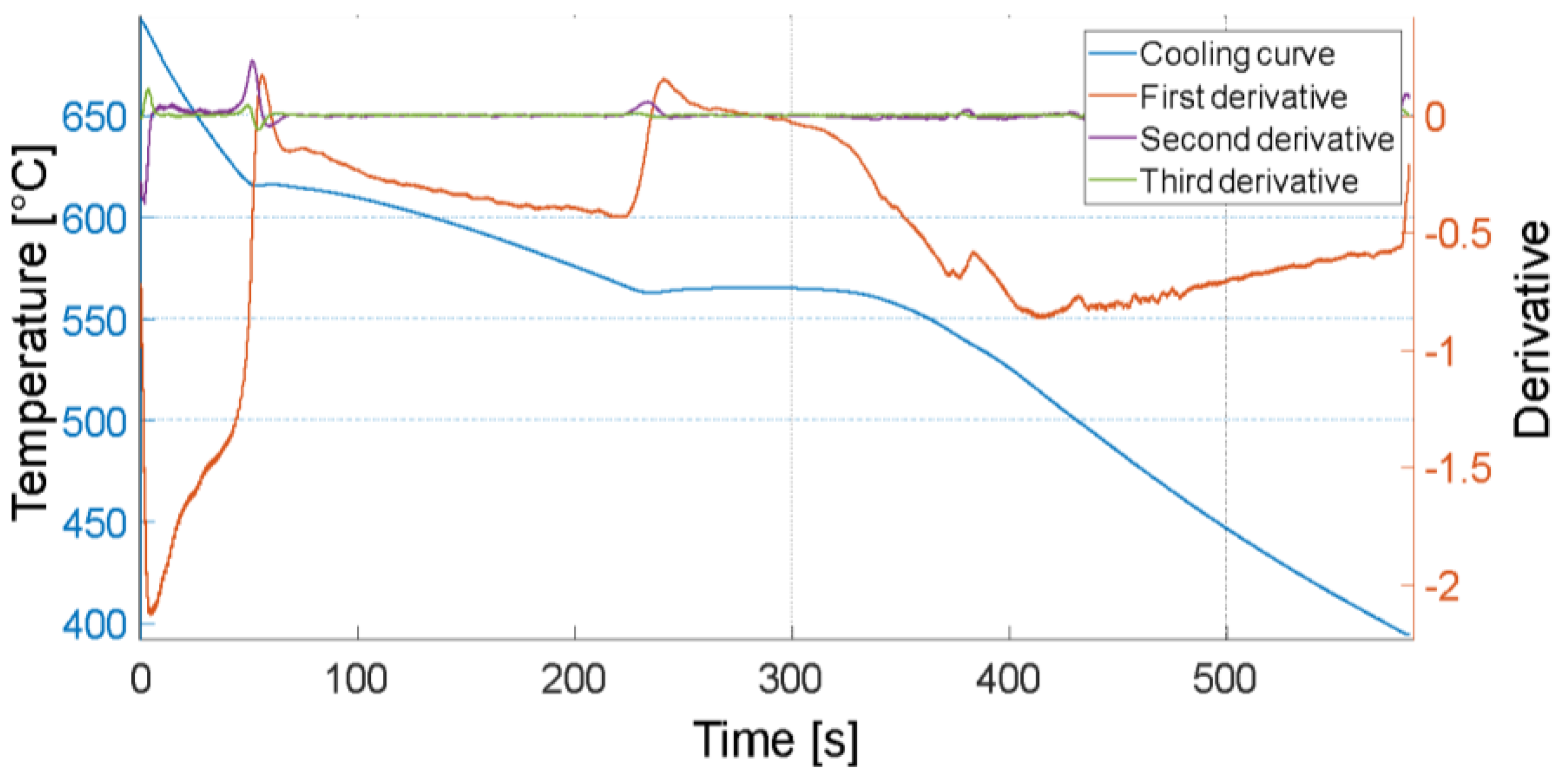
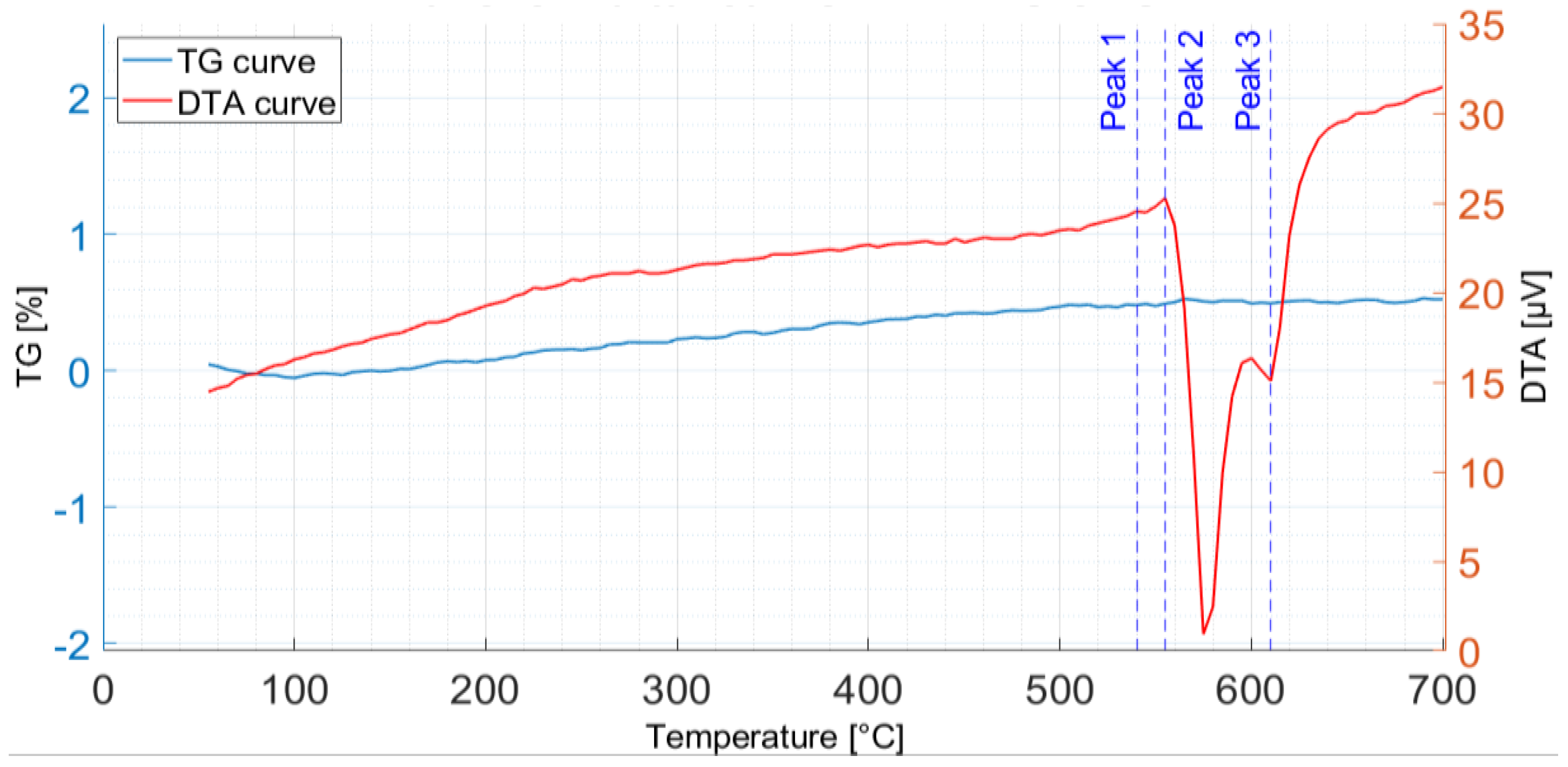
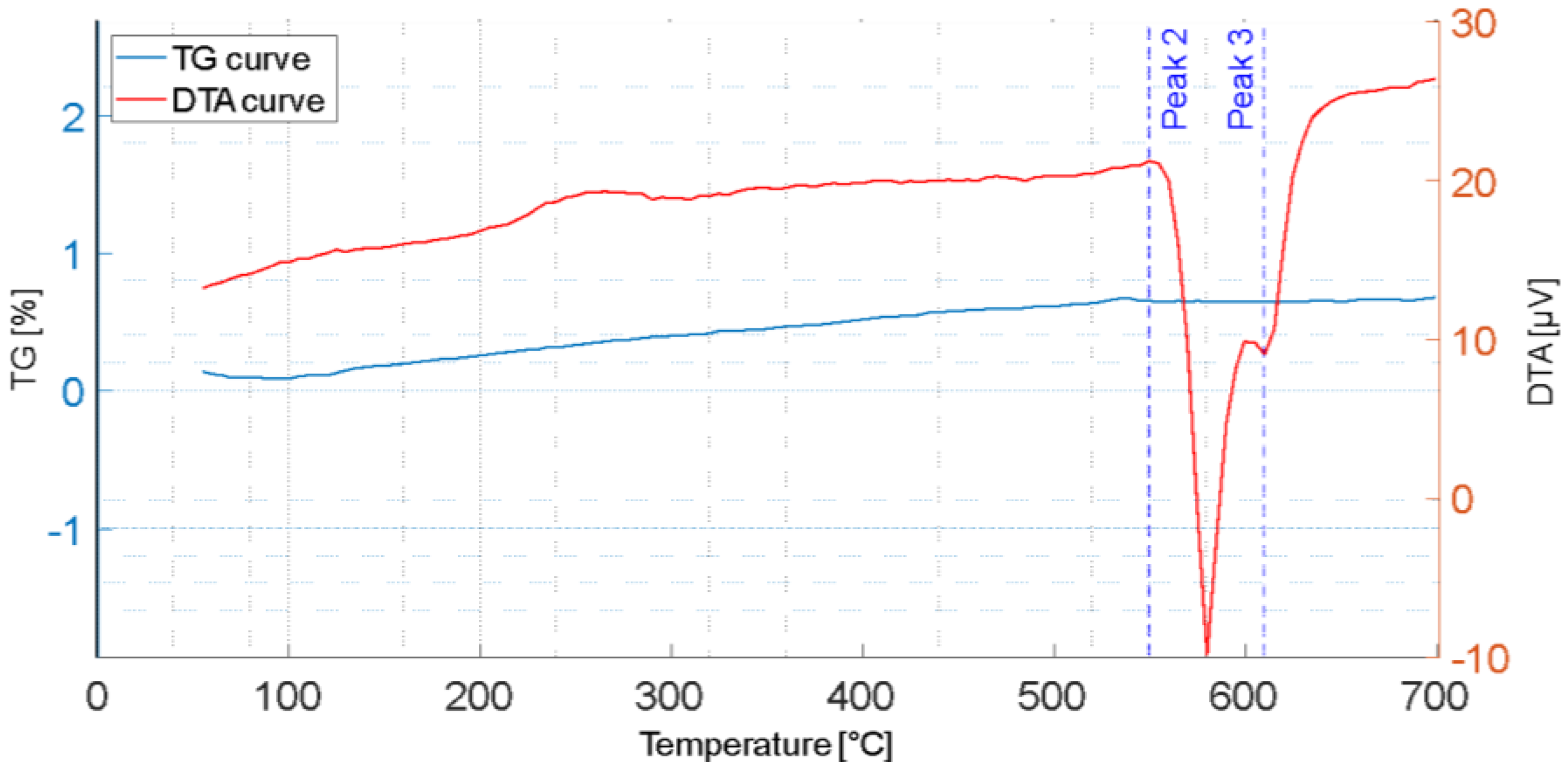
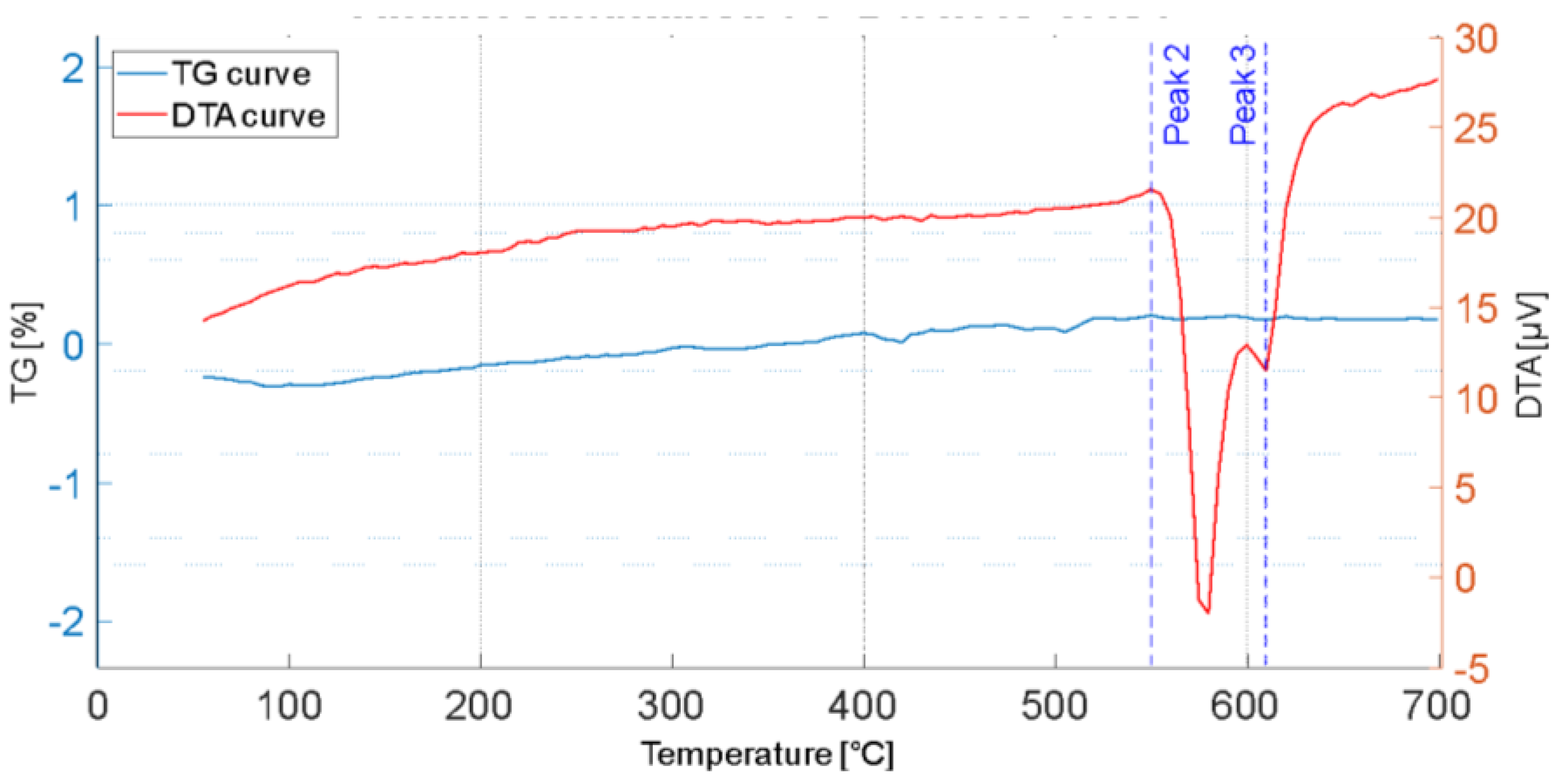
3.1. Thermal Analysis
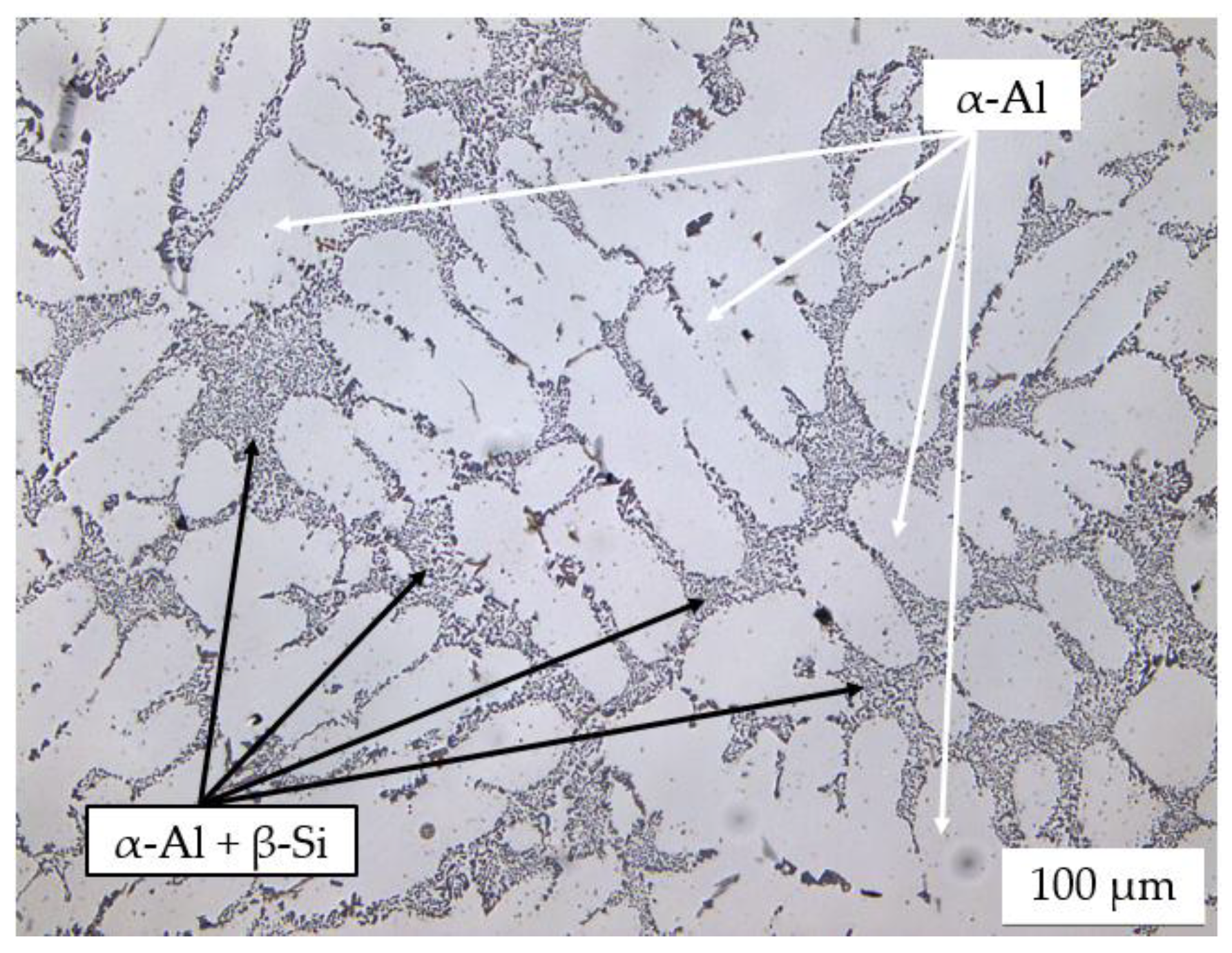
3.2. Microstructural Analysis
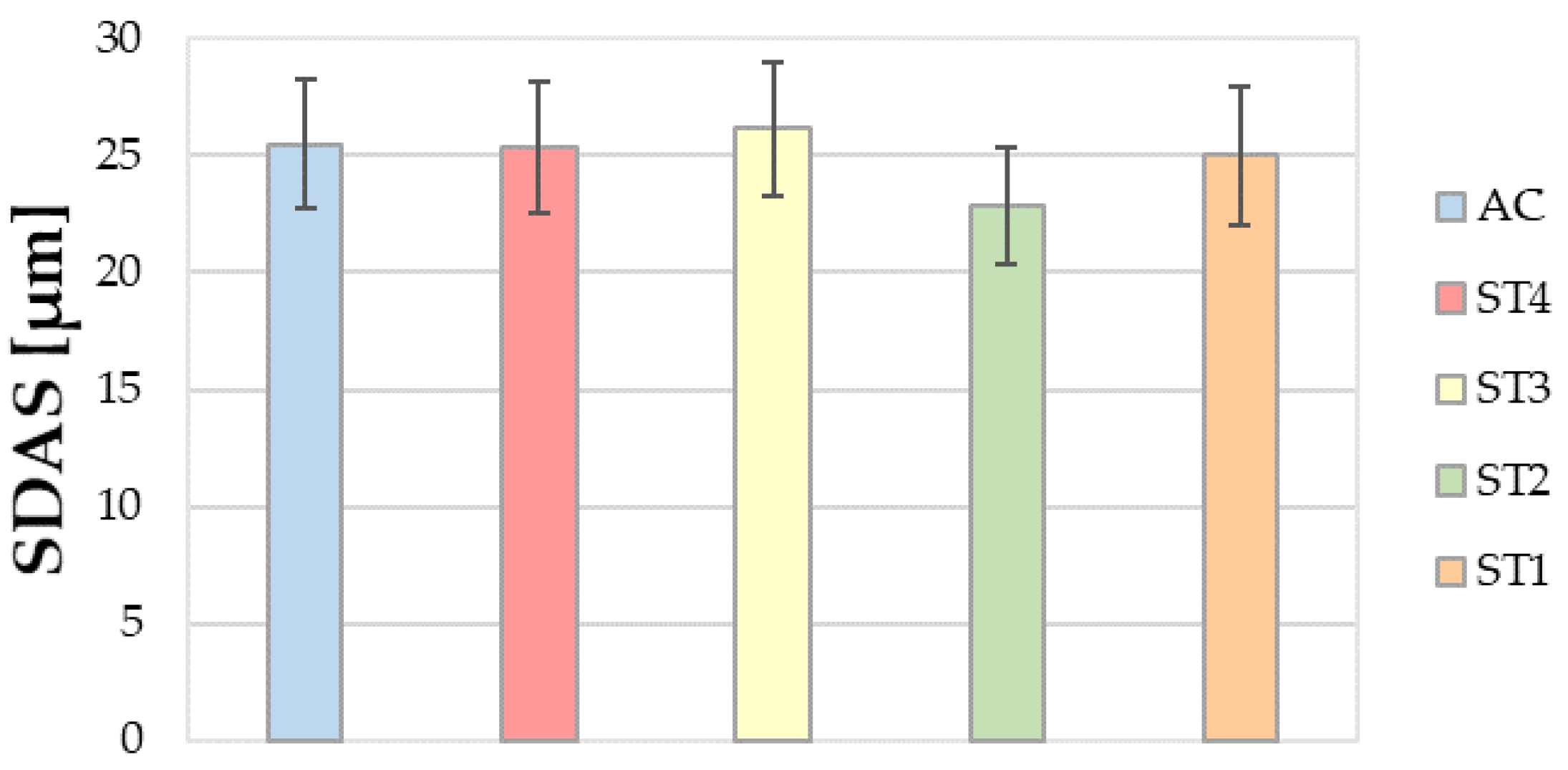
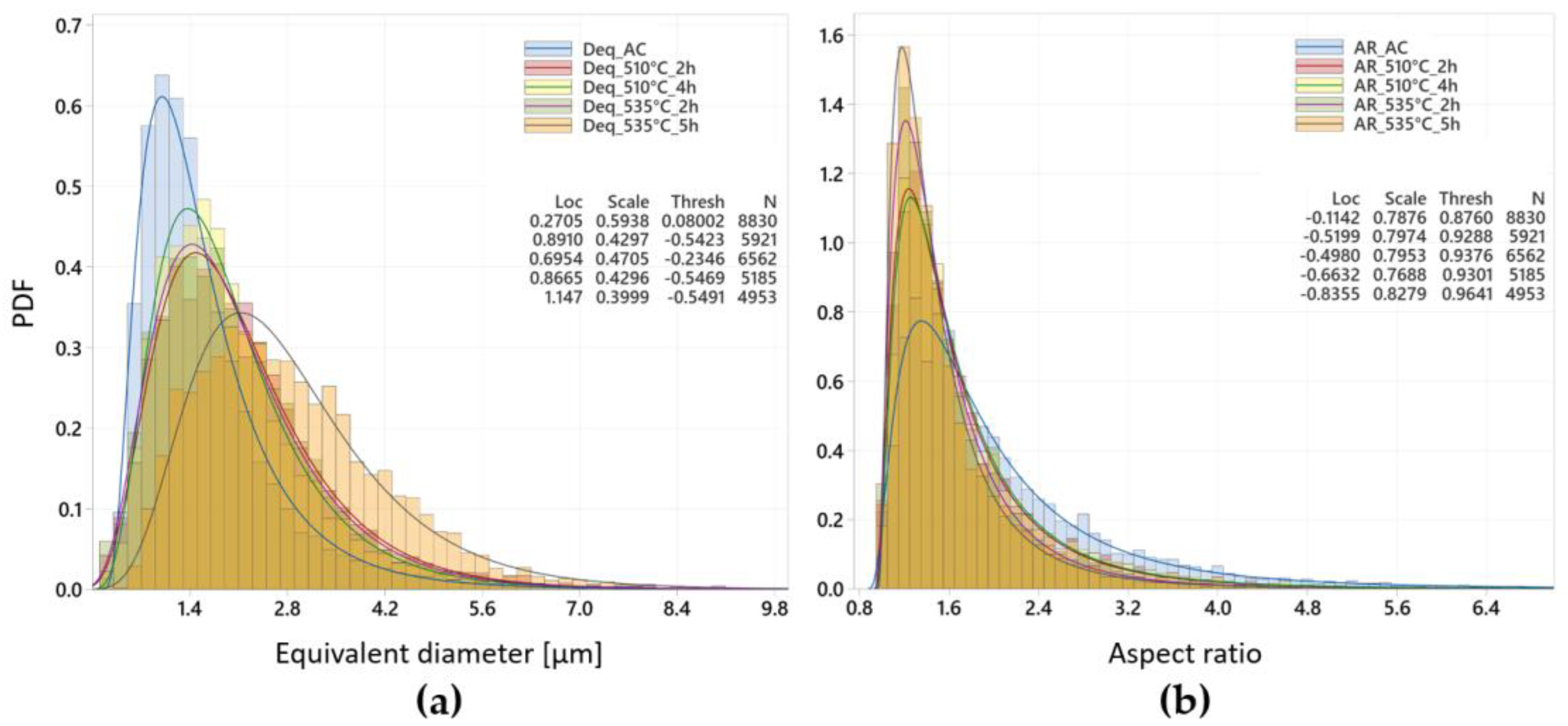
3.2.1. SDAS Measurements
3.2.2. Quantitative Analysis of Eutectic Si Particles
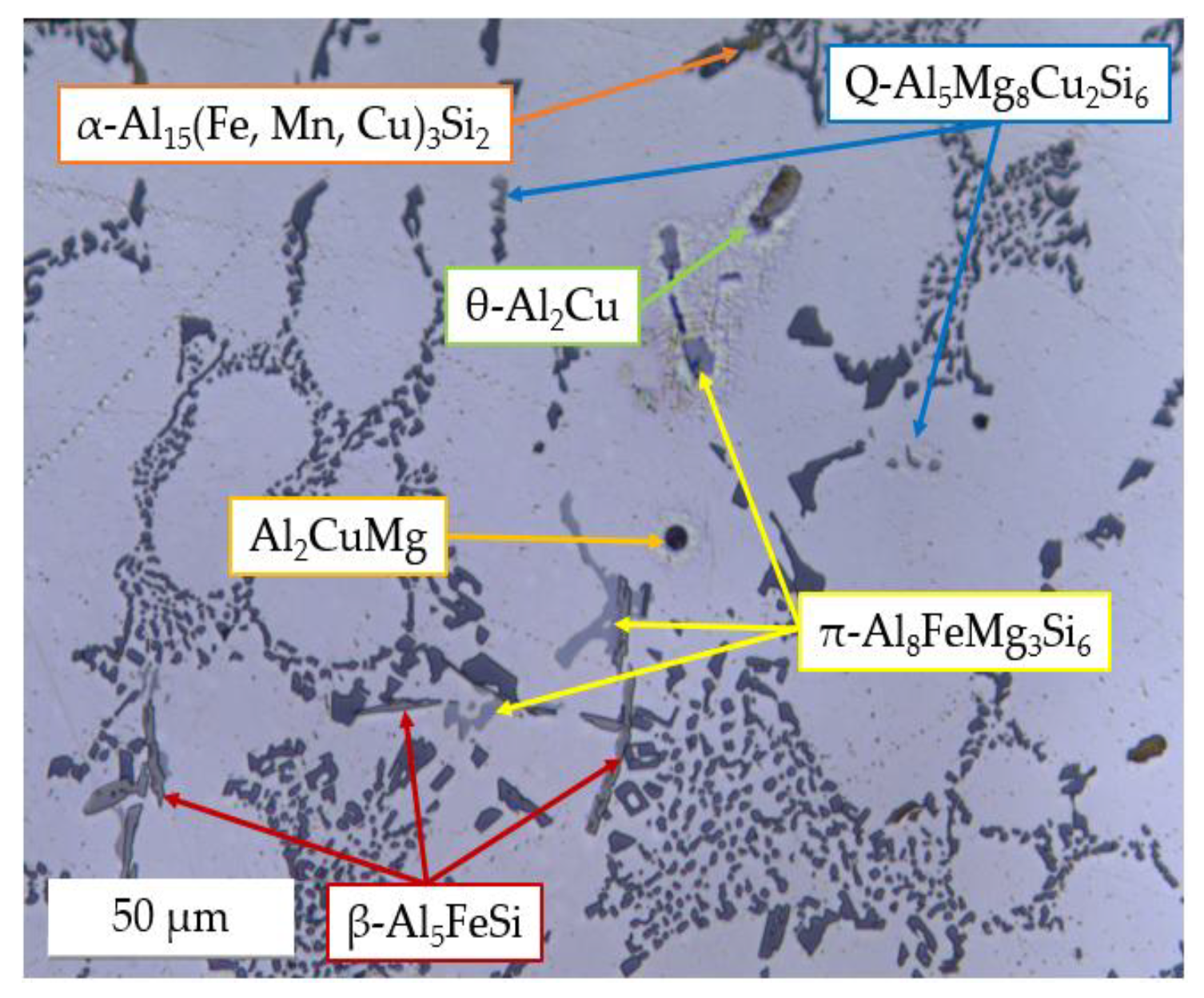
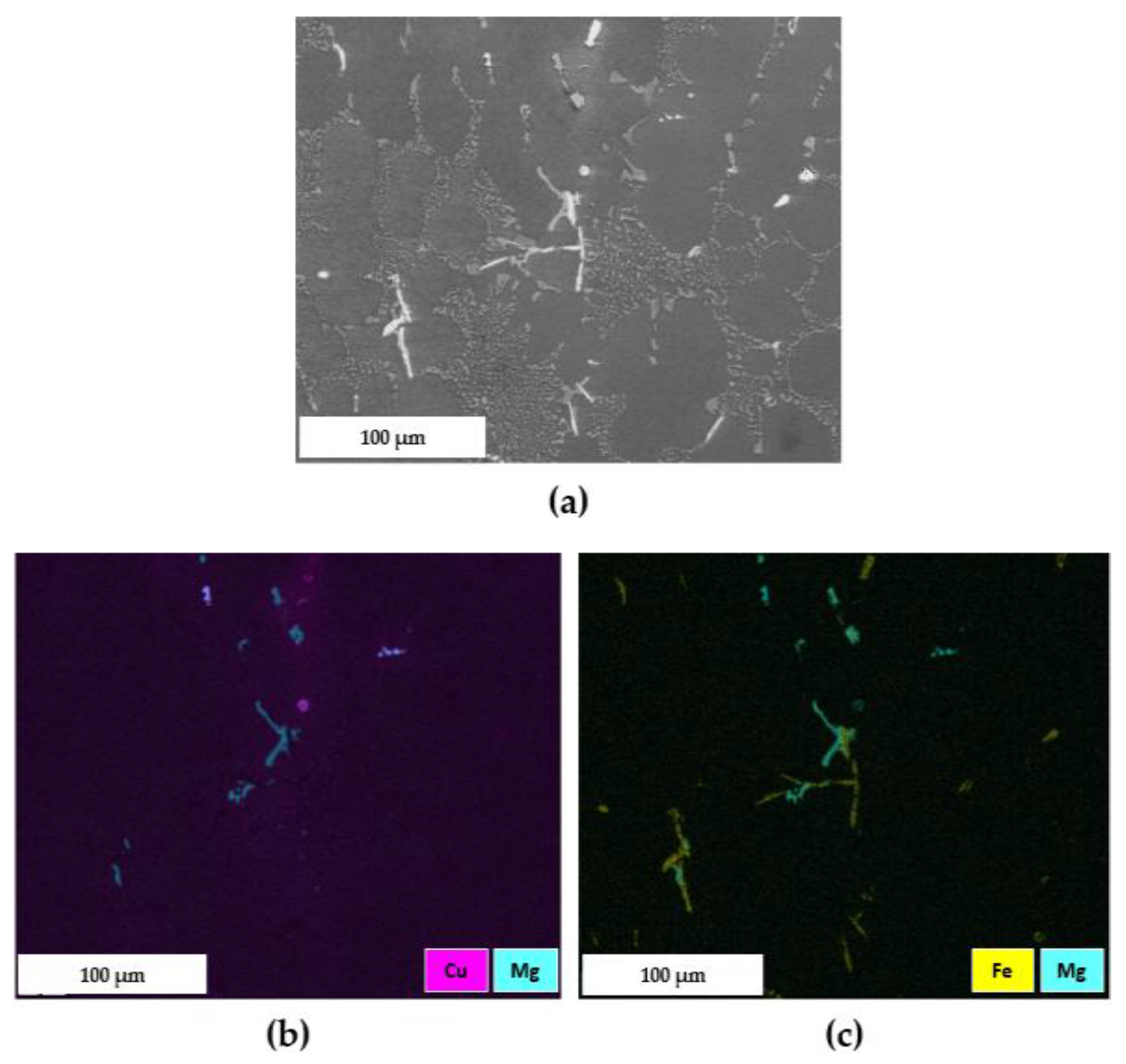
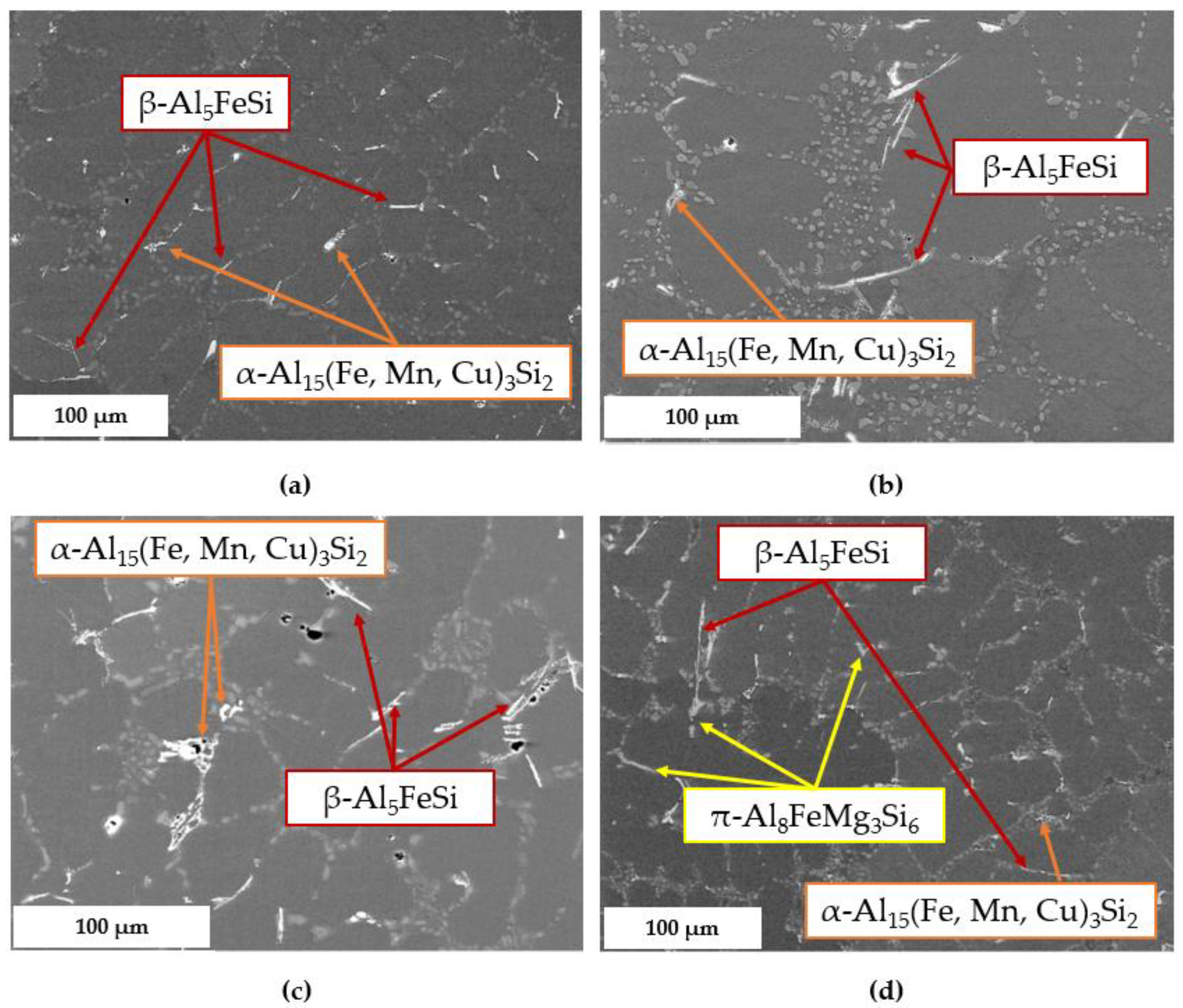
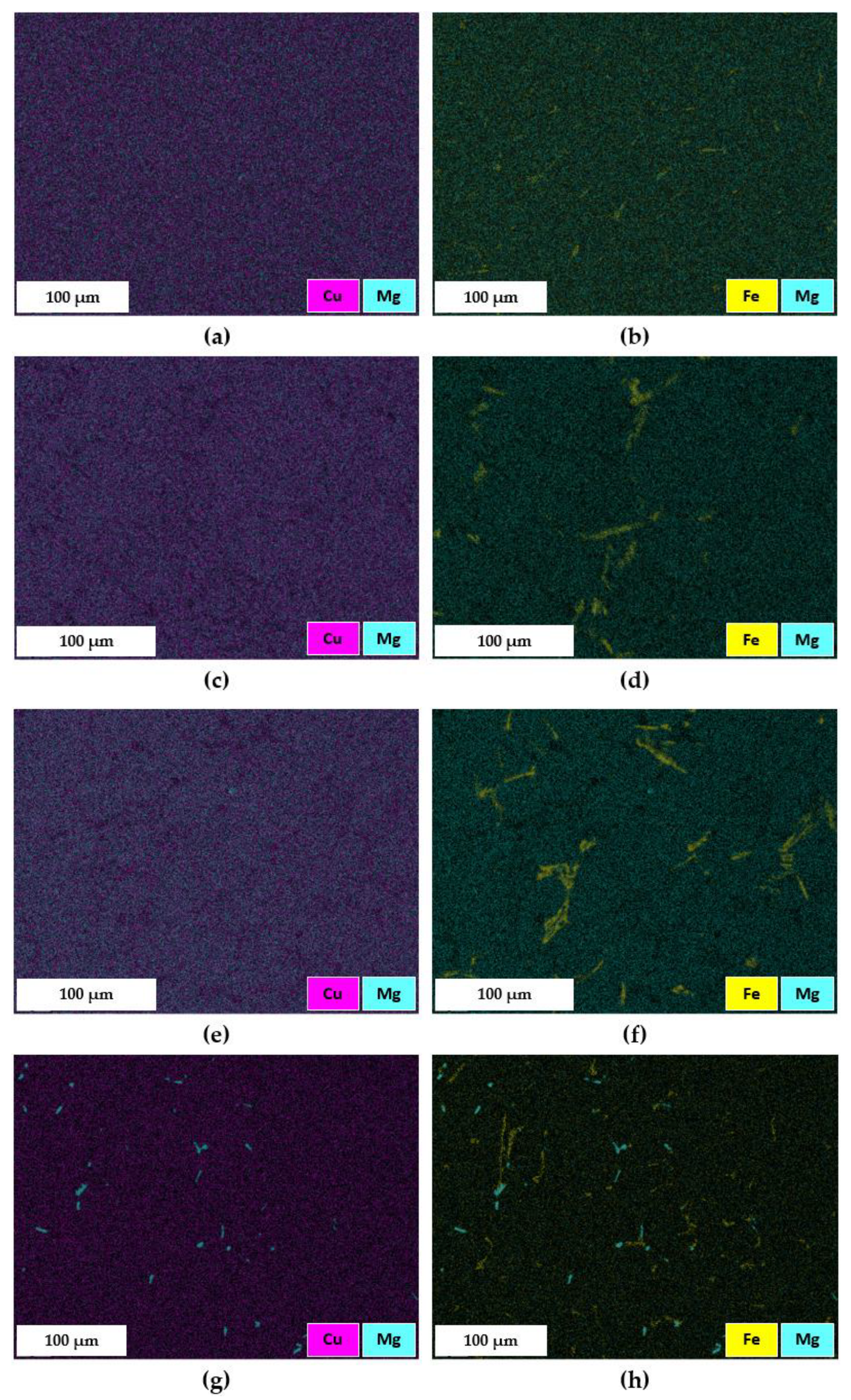
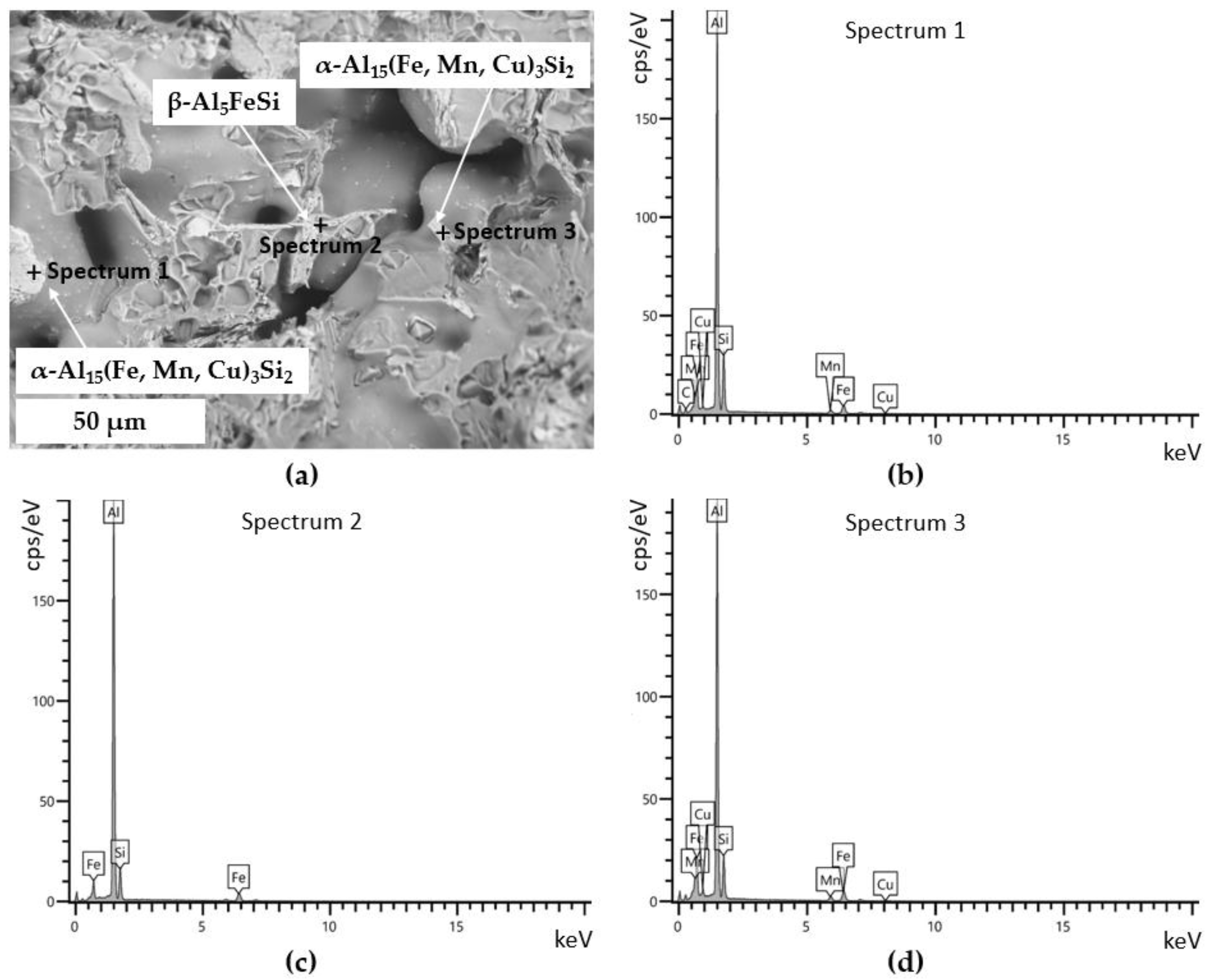
3.2.3. Secondary Intermetallic Phases
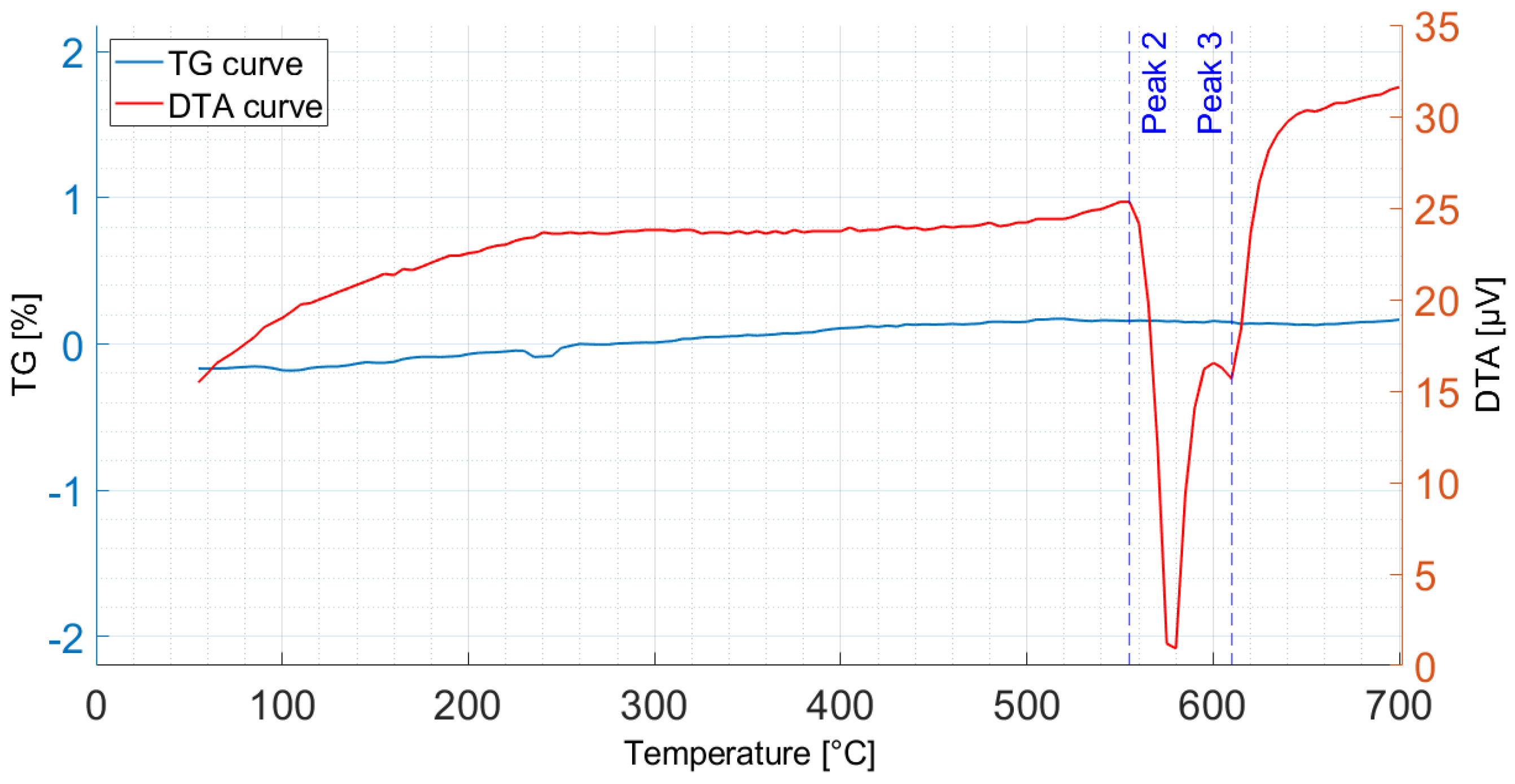
3.3. Simultaneous Thermogravimetric and Differential Thermal Analysis
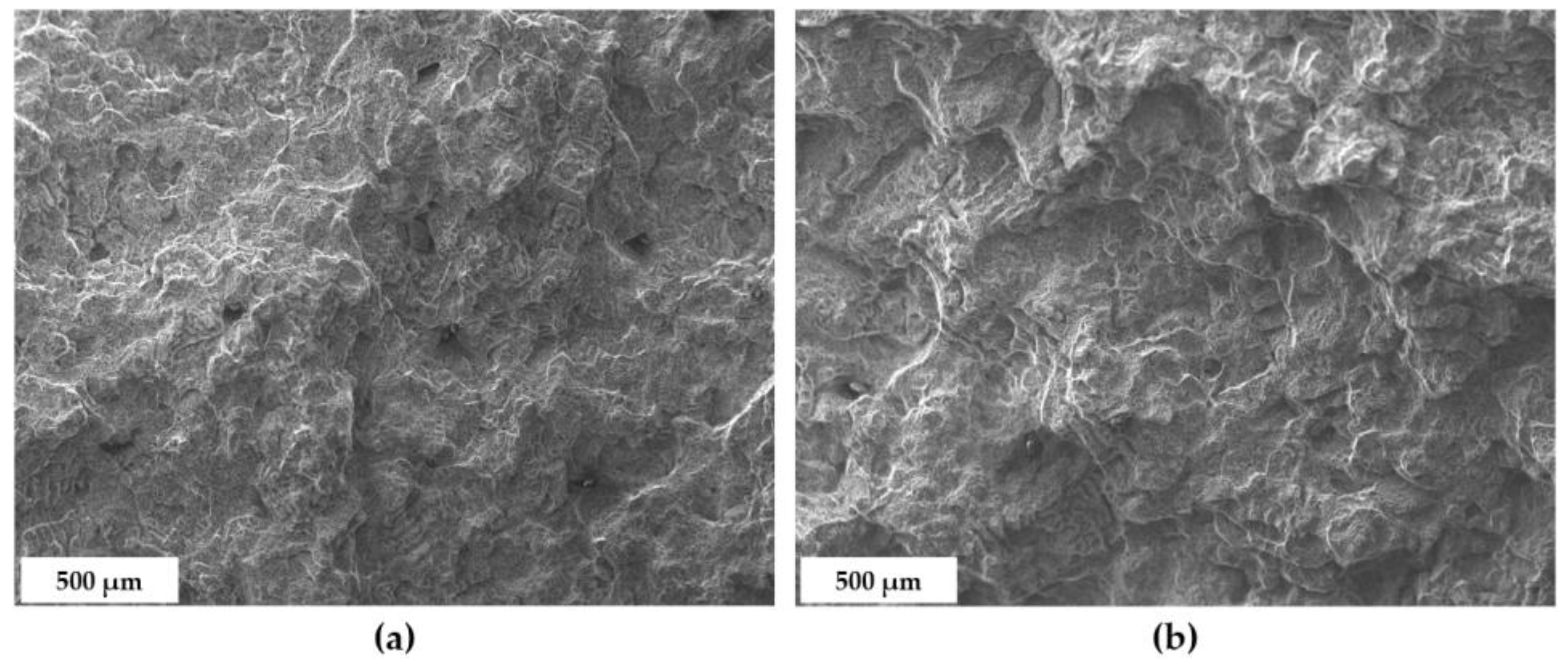
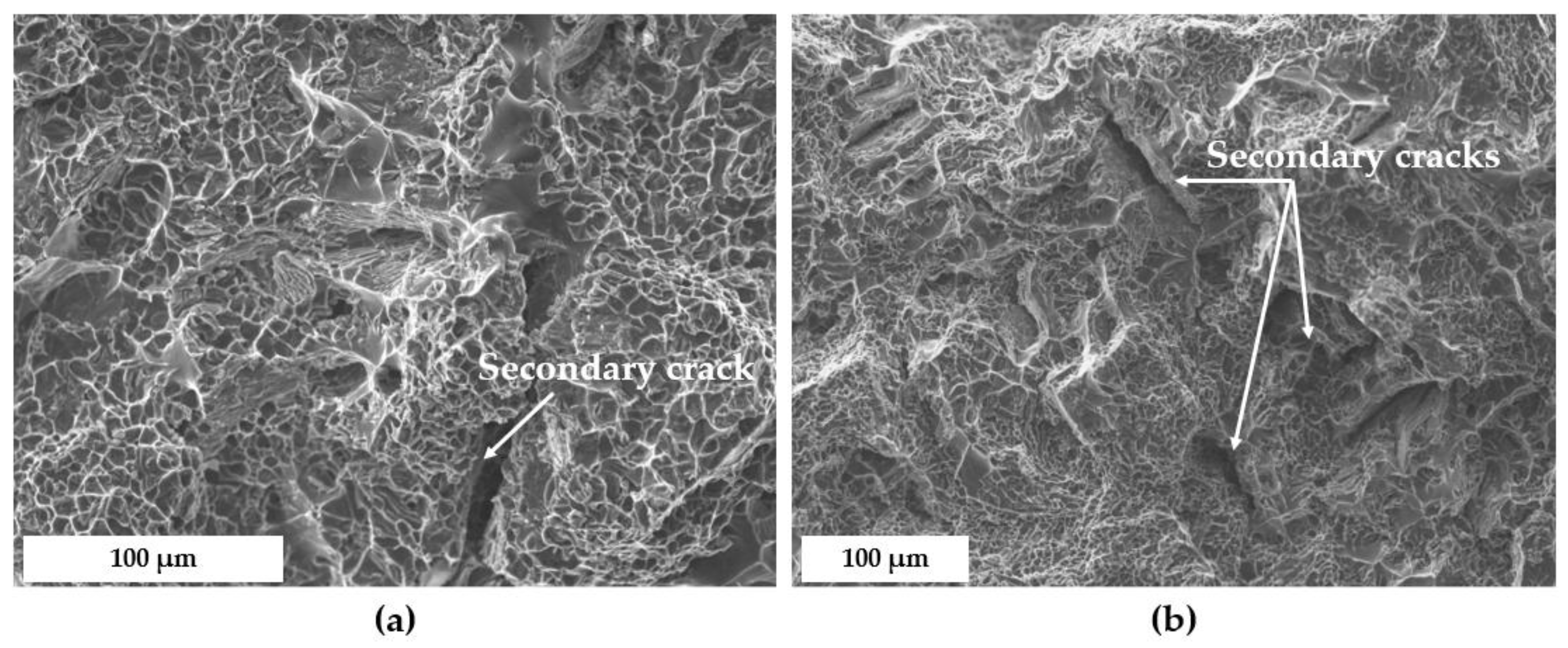
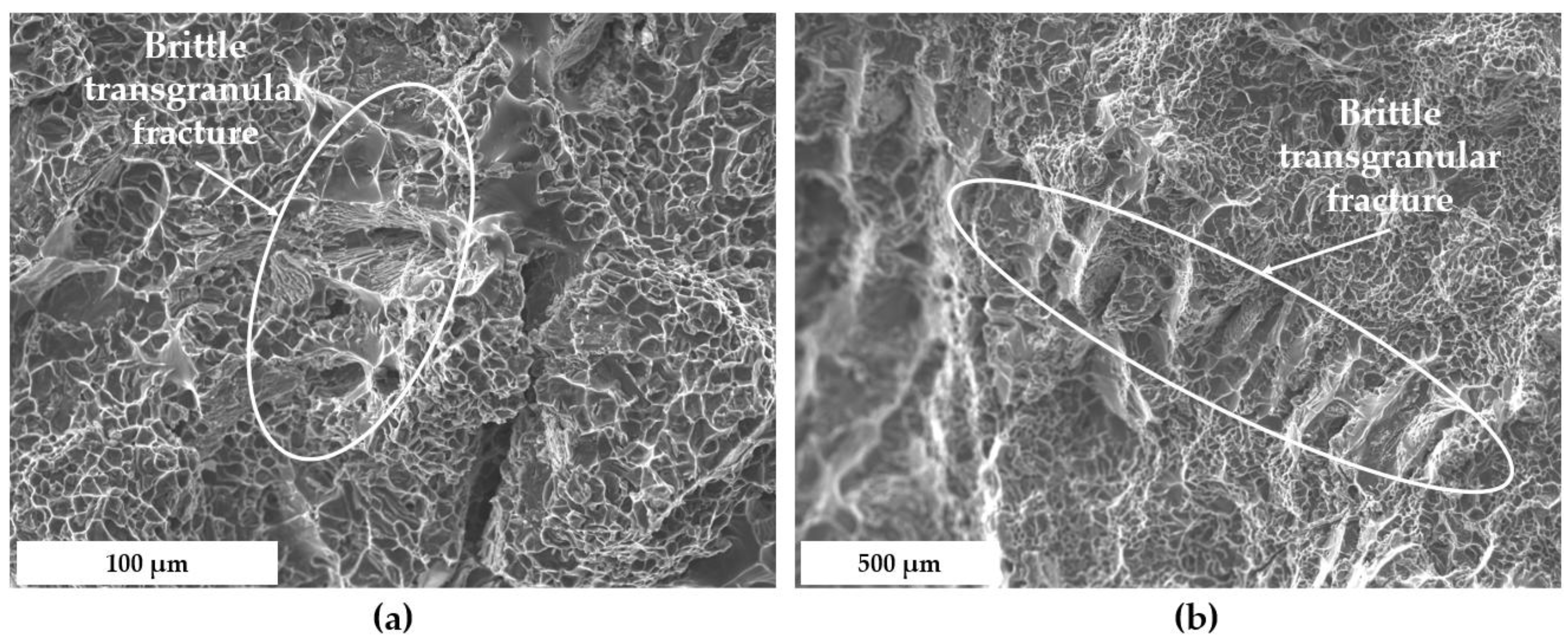
3.4. Fractographic Analysis
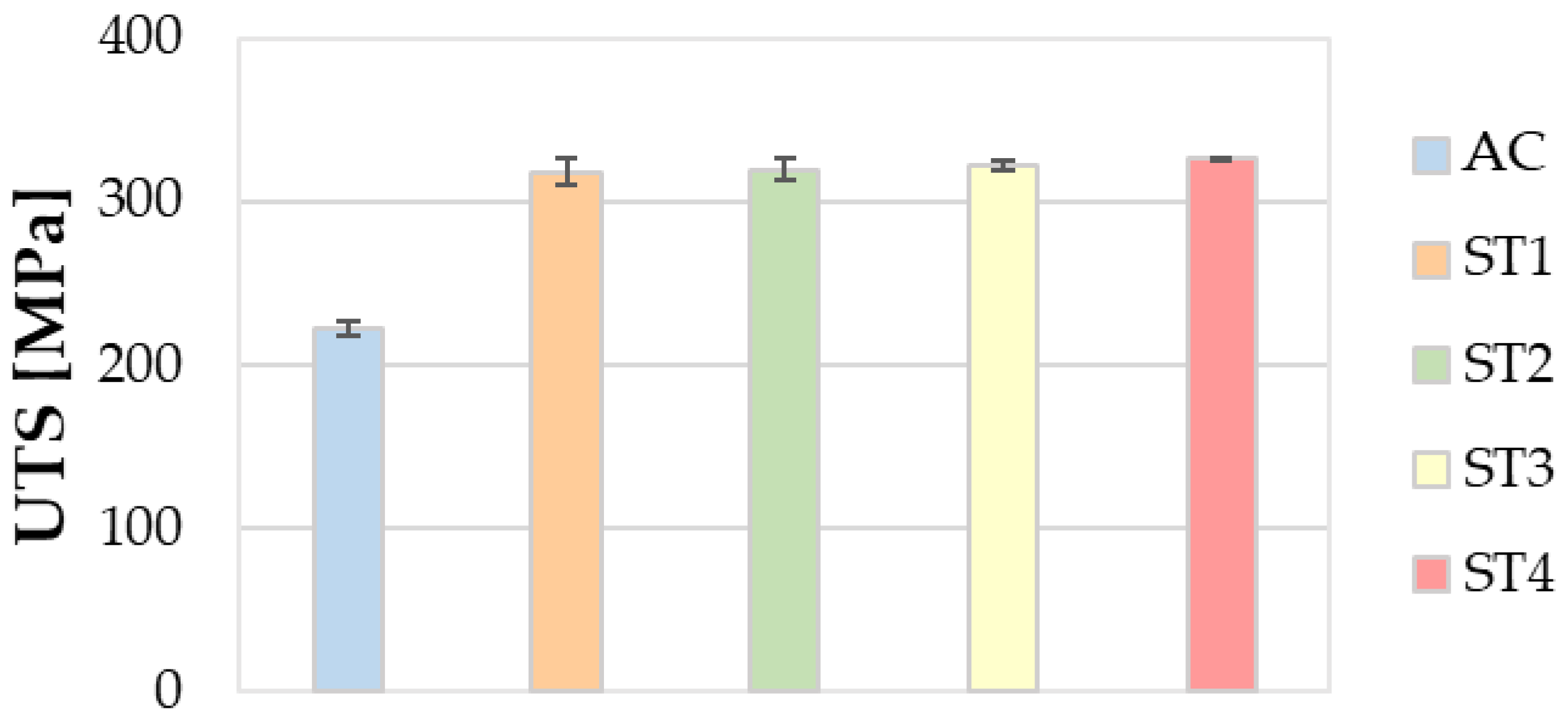
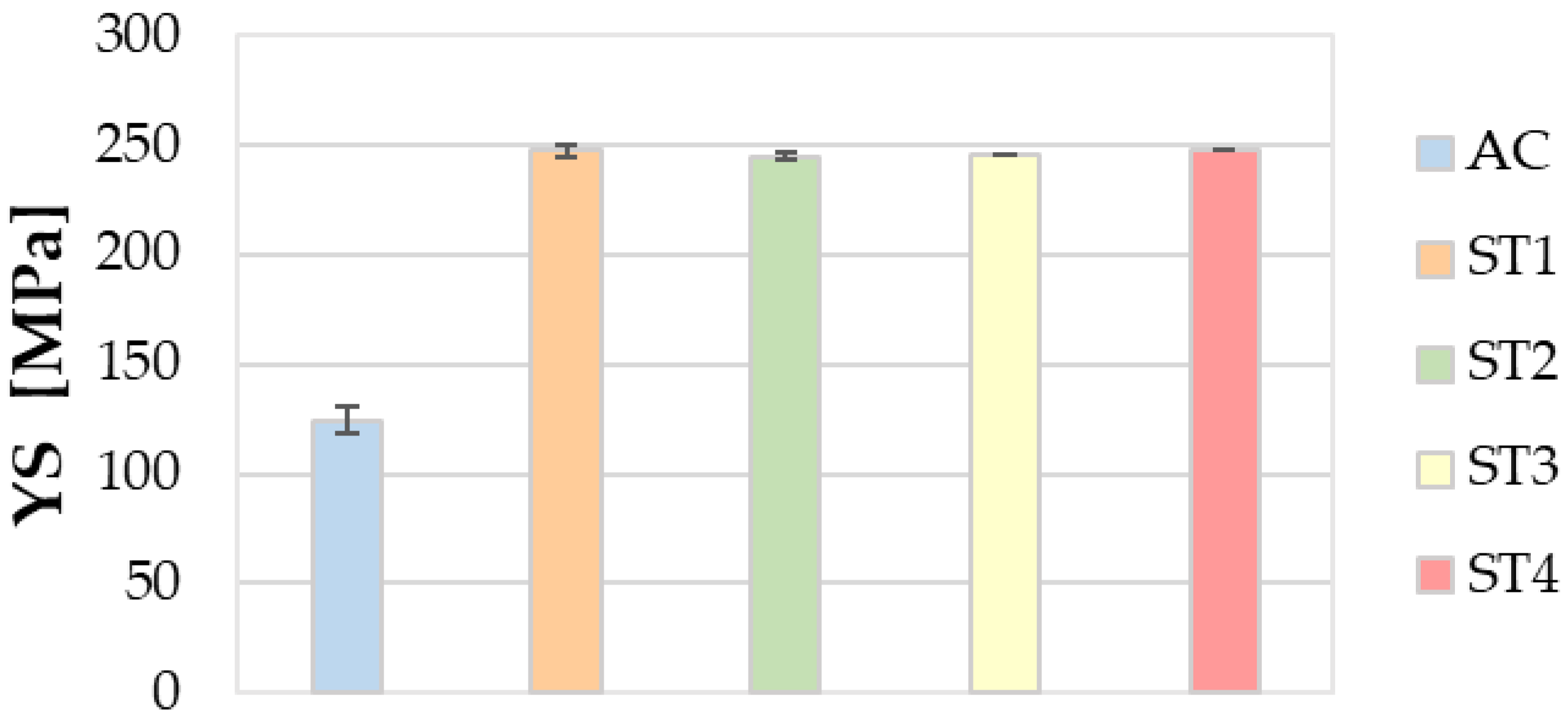
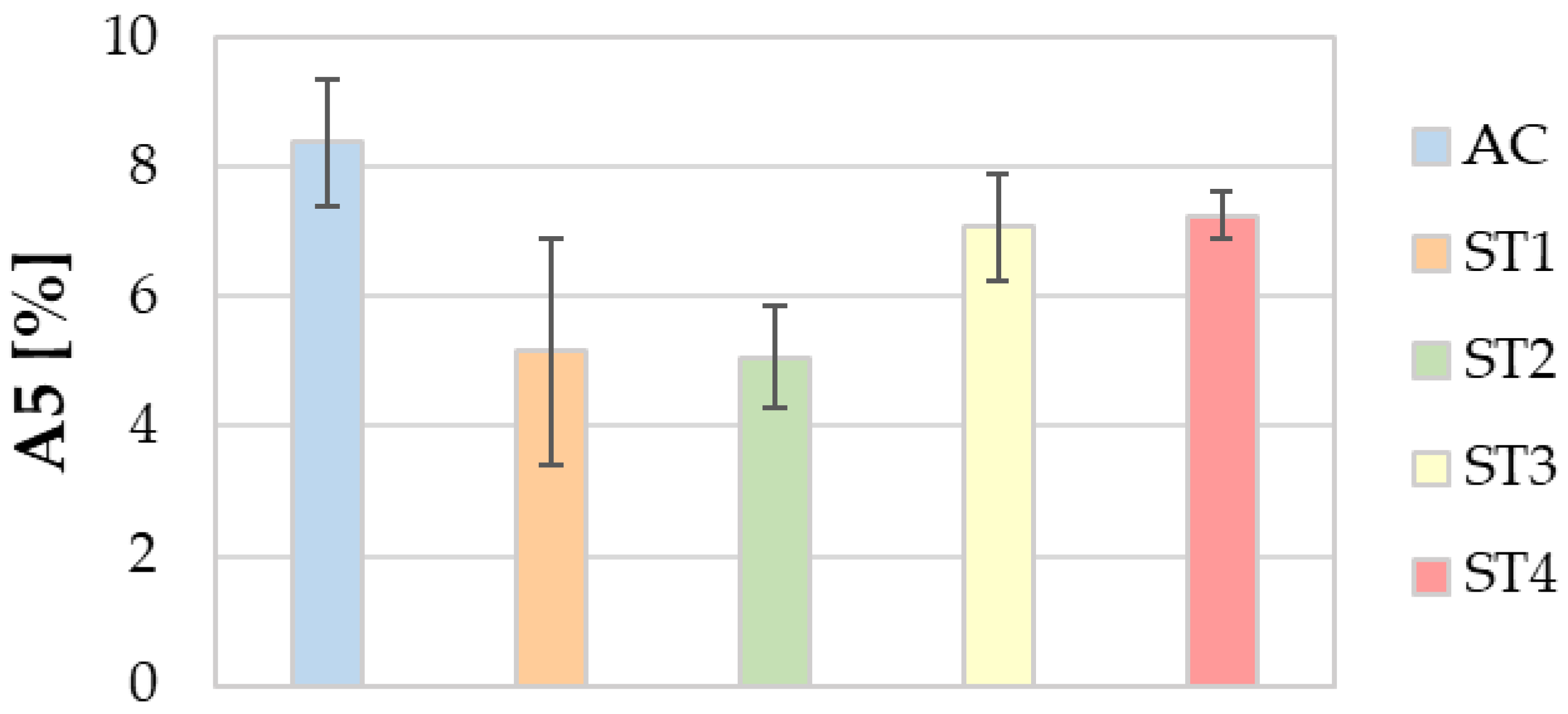
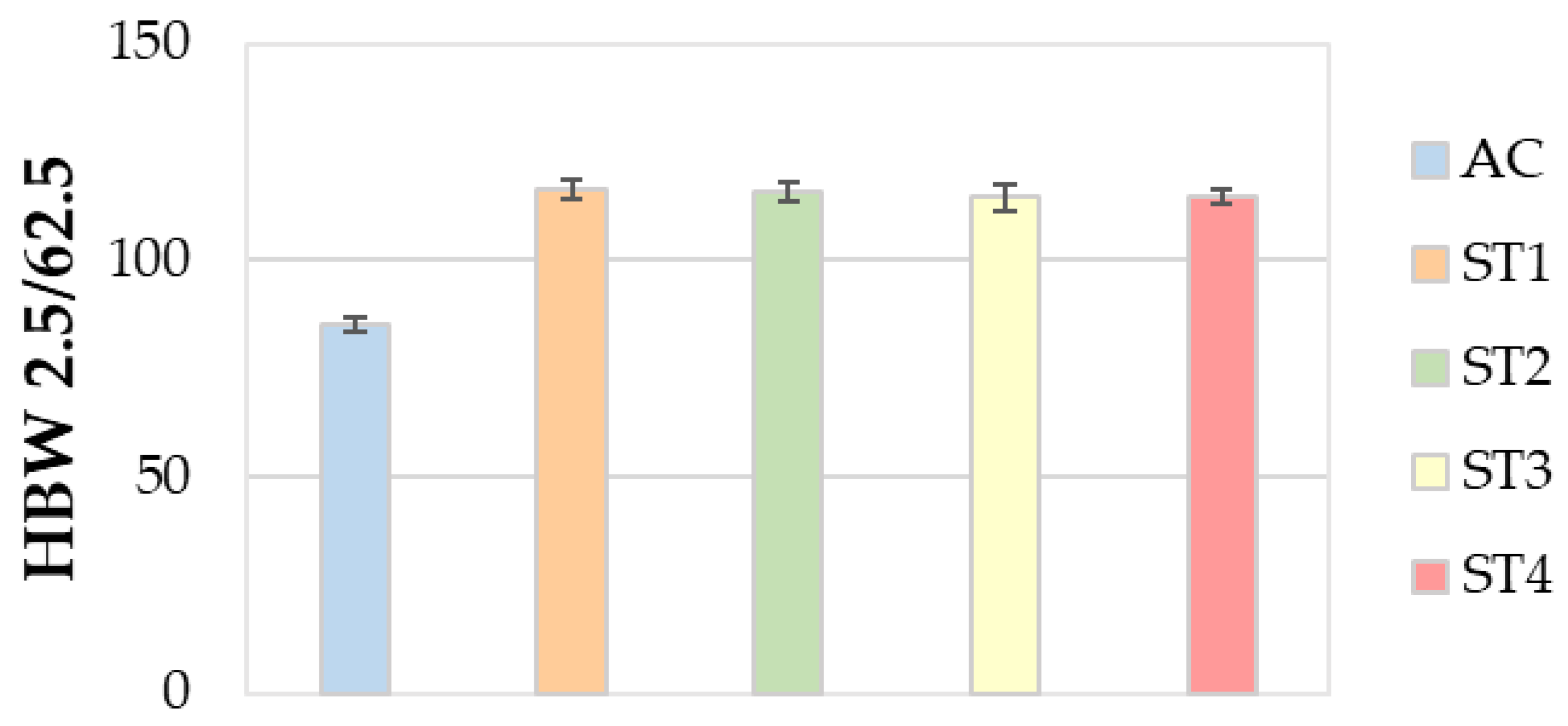
3.5. Mechanical Properties
4. Conclusions
- Concerning the solidification sequence of the examined alloy, thermal analysis proved the formation of the primary α-Al phase, the α-Al + β-Si eutectic structure, the Fe-rich β-Al5FeSi and α-Al15(Fe, Mn, Cu)3Si2 intermetallics, the π-Al8FeMg3Si6 and β-Mg2Si precipitates, and the low-melting post-eutectic θ-Al2Cu and Q-Al5Mg8Cu2Si6 phases. Except for the β-Mg2Si, the presence of these phases within the as-cast microstructure was also confirmed by light and scanning electron microscopy.
- The DTA analyses showed that the proposed SHT parameters are effective in dissolving the secondary phases formed during the first solidification, thus enhancing the mechanical properties after the subsequent AA.
- The EDS maps proved that the ST4 treatment, carried out at 510 °C for 2 h, caused only a partial dissolution of the π-Al8FeMg3Si6 phase into the α-Al matrix. Nevertheless, the strengthening effect was guaranteed by the dissolution of (Mg2Si), (Al2Cu) or (Al5Mg8Cu2Si6) intermetallic phases, thus to the synergic effect of Mg and Cu in the subsequent precipitation hardening during AA.
- Regarding the mechanical properties, the ST4 treatment guarantees a relevant enhancement of both the tensile strength and hardness, without leading to a remarkable decrease in ductility.
- Finally, the ST4 treatment helps to improve productivity and lower energy and/or gas consumption in furnaces, along with environmental emissions, thanks to the reduction in times and temperatures of solution heat treatment, with respect to the ST1 one performed at 535 °C for 5 h.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Backerud, L.; Chai, G.; Tamminen, J. Solidification Characteristics of Aluminium Alloys: Foundry Alloys 2; Skanaluminium: Stockholm, Sweden, 1990; ISBN 0874331196/9780874331196. [Google Scholar]
- Li, Z.; Samuel, A.M.; Samuel, F.H. Effect of alloying elements on the segregation and dissolution of CuAl2 phase in Al-Si-Cu 319 alloys. J. Mater. Sci. 2003, 38, 1203–1218. [Google Scholar] [CrossRef]
- Samuel, E.H.; Samuel, A.M.; Doty, H.W. Factors controlling the type and morphology of Cu-containing phases in 319 Al alloy. AFS Trans. 1996, 30, 893–901. [Google Scholar]
- Samuel, A.M.; Ouellet, P.; Samuel, F.H.; Doty, H.W. Microstructural interpretation of thermal analysis of commercial 319 Al alloy with Mg and Sr additions. AFS Trans. 1997, 105, 951–962. [Google Scholar]
- Stunovà, B.B. Strontium as a structure modifier for non-binary Al–Si alloy. Acta Polytech. 2012, 52, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, H.; Han, X.; Shao, B.; Li, L.; Cui, J. The analysis of strontium modification on microstructure and mechanical properties of Al-25%Mg2Si in situ composite. J. Mater. Eng. Perform. 2017, 26, 4415–4423. [Google Scholar] [CrossRef]
- Sjölander, E.; Seiffedine, S. The heat treatment of Al-Si-Cu-Mg casting alloys. J. Mater. Process. Technol. 2010, 210, 1249–1259. [Google Scholar] [CrossRef]
- Sjölander, E.; Seiffedine, S. Optimization of solution treatment of cast Al-7Si-0.3Mg and Al-8Si-3Cu-0.5Mg alloys. Metall. Mater. Trans. A 2013, 45, 1916–1927. [Google Scholar] [CrossRef]
- Shivkumar, S.; Wang, L.; Apelian, D. Molten metal processing of advanced cast aluminum alloys. JOM 1991, 43, 26–32. [Google Scholar] [CrossRef]
- Cao, X.; Campbell, J. Morphology of β-Al5FeSi phase in Al-Si cast alloys. Mater. Trans. 2006, 47, 1303–1312. [Google Scholar] [CrossRef]
- Mahta, M.; Emamy, M.; Daman, A.; Keyvani, A.; Campbell, J. Precipitation of Fe-rich intermetallics in Cr- and Co-modified A413 alloy. Int. J. Cast Met. Res. 2005, 18, 73–79. [Google Scholar] [CrossRef]
- Mondolfo, L.F. Aluminum Alloys: Structure and Properties; Butterworth: London, UK, 1976; ISBN 1483144828/9781483144825. [Google Scholar]
- Rometsch, P.A.; Arnberg, L.; Zhang, D.L. Modelling dissolution of Mg2Si and homogenisation in Al–Si–Mg casting alloys. Int. J. Cast Met. Res. 1999, 12, 197–220. [Google Scholar] [CrossRef]
- Dons, A.L.; Pedersen, L.; Brusethaug, S. Modelling the microstructure of heat treated AlSi foundry alloys. Aluminium 2000, 76, 294–297. [Google Scholar]
- Taylor, J.A.; St John, D.H.; Barresi, J.; Couper, M.J. Influence of Mg content on the microstructure and solid solution chemistry of Al–7%Si–Mg casting alloys during solution treatment. Mater. Sci. Forum 2000, 331–337, 277–282. [Google Scholar] [CrossRef]
- Wang, Q.G.; Davidson, C.J. Solidification and precipitation behaviour of Al–Si–Mg casting alloys. J. Mater. Sci. 2001, 36, 739–750. [Google Scholar] [CrossRef]
- Samuel, A.M.; Gauthier, J.; Samuel, F.H. Microstructural aspects of the dissolution and melting of Al2Cu phase in Al–Si alloys during solution heat treatment. Metall. Mater. Trans. A 1996, 27, 1785–1798. [Google Scholar] [CrossRef]
- Javidani, M.; Larouche, D.; Chen, X.G. Evolution of intermetallic phases in multicomponent Al-Si foundry alloys containing different Cu, Mg and Fe content. AFS Trans. 2014, 14, 1–9. [Google Scholar]
- Crowell, N.; Shivkumar, S. Solution treatment effects in cast Al–Si–Cu alloys. AFS Trans. 1995, 107, 721–726. [Google Scholar]
- ISO 6892-1:2019; Metallic Materials—Tensile Testing—Part 1: Method of Test at Room Temperature. ISO: Geneva, Switzerland, 2019.
- ASTM E8/E8M-21; Standard Test Methods for Tension Testing of Metallic Materials. ASTM International: West Conshohocken, PA, USA, 2021. [CrossRef]
- Stefanescu, D.M. Thermal analysis—Theory and applications in metalcasting. Int. J. Met. 2015, 9, 7–22. [Google Scholar] [CrossRef]
- Djurdjevic, M.B.; Odanovic, Z.; Talijan, N. Characterization of the solidification path of AlSi5Cu(1–4 wt.%) alloys using cooling curve analysis. Aluminum: Shaping and Forming. JOM 2011, 63, 51–57. [Google Scholar] [CrossRef]
- Fortini, A.; Lattanzi, L.; Merlin, M.; Garagnani, G.L. Comprehensive evaluation of modification level assessment in Sr-modified aluminium alloys. Int. J. Met. 2018, 12, 697–711. [Google Scholar] [CrossRef]
- Djurdjevic, M.B.; Vicario, I.; Huber, G. Review of thermal analysis applications in aluminium casting plants. Rev. Metal. 2014, 50, e021. [Google Scholar] [CrossRef]
- ISO 6506-1:2014; Metallic Materials—Brinell Hardness Test—Part 1: Test Method. ISO: Geneva, Switzerland, 2014.
- Boettinger, W.J.; Kattner, U.R.; Moon, K.W.; Perepezko, J.H. Chapter Five: DTA and heat-flux DSC measurements of alloy melting and freezing. In Methods for Phase Diagram Determination; Zhao, J.-C., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2007; pp. 151–221. ISBN 978-0-08-044629-5. [Google Scholar]
- Wang, Q.G. Microstructural effects on the tensile and fracture behavior of aluminum casting alloys A356/357. Metall. Mater. Trans. A 2003, 34, 2887–2899. [Google Scholar] [CrossRef]
- Shabestari, S.G.; Ghodrat, S. Assessment of modification and formation of intermetallic compounds in aluminum alloy using thermal analysis. Mater. Sci. Eng. A 2007, 467, 150–158. [Google Scholar] [CrossRef]
- Apelian, D.; Shivkumar, S.; Sigworth, G. Fundamental aspects of heat treatment of cast Al–Si–Mg alloys. AFS Trans. 1989, 137, 727–742. [Google Scholar]
- Choi, S.W.; Kim, Y.M.; Lee, K.M.; Cho, H.S.; Hong, S.K.; Kim, Y.C.; Kang, C.S.; Kumai, S. The effects of cooling rate and heat treatment on mechanical and mechanical and thermal characteristics of Al–Si–Cu–Mg foundry alloys. J. Alloys Compd. 2014, 617, 654–659. [Google Scholar] [CrossRef]
- Lasa, L.; Rodriguez-Ibabe, J.M. Evolution of the main intermetallic phases in Al-Si-Cu-Mg casting alloys during solution treatment. J. Mater. Sci. 2004, 39, 1343–1355. [Google Scholar] [CrossRef]
- Ganesh, M.R.S.; Reghunath, N.; Levin, M.J.; Prasad, A.; Doondi, S.; Shankar, K.V. Strontium in Al–Si–Mg Alloy: A Review. Met. Mater. Int. 2022, 28, 1–40. [Google Scholar] [CrossRef]
- Javidani, M. Effect of Cu, Mg and Fe on Solidification Processing and Microstructure Evolution of Al-7Si Based Foundry Alloys. Ph.D. Thesis, Université Laval, Québec, QC, Canada, 2015. Available online: http://hdl.handle.net/20.500.11794/26146 (accessed on 16 January 2025).
- Li, Y.J.; Brusethaug, S.; Olsen, A. Influence of Cu on the Mechanical Properties and Precipitation Behavior of AlSi7Mg0.5 Alloy During Aging Treatment. Scr. Mater. 2006, 54, 99–103. [Google Scholar] [CrossRef]
- Klančnik, G.; Medved, J.; Mrvar, P. Differential Thermal Analysis (DTA) and Differential Scanning Calorimetry (DSC) as a Method of Material Investigation. RMZ Mater. Geoenviron. 2010, 57, 127–142. [Google Scholar]
- Beder, M.; Akçay, S.B.; Varol, T.; Çuvalci, H. The Effect of Heat Treatment on the Mechanical Properties and Oxidation Resistance of AlSi10Mg Alloy. Arab. J. Sci. Eng. 2024, 49, 15335–15346. [Google Scholar] [CrossRef]
- Hunt, W.H., Jr.; Brockenbrough, J.R.; Magnusen, P.E. An Al-Si-Mg composite model system: Microstructural effects on deformation and damage evolution. Scr. Metall. Mater. 1991, 25, 15–20. [Google Scholar] [CrossRef]
- Sokolowski, J.H.; Sun, X.C.; Byczynski, G.; Northwood, D.O.; Penrod, D.E.; Thomas, R.; Esseltine, A. Removal of Copper-Phase Segregation and the Subsequent Improvement in Mechanical Properties of Cast 319 Aluminum Alloys by a Two-Stage Solution Heat Treatment. J. Mater. Process. Technol. 1995, 53, 385–392. [Google Scholar] [CrossRef]
- Sangchan, A.; Plookphol, T.; Wannasin, J.; Wisutmethangoon, S. Effect of Strontium on Microstructure and Mechanical Properties of Semi-Solid A356 Al Alloy. Adv. Mater. Res. 2014, 893, 353–356. [Google Scholar] [CrossRef]


| Al | Si | Cu | Mg | Fe | Mn | Ti | Sr | Cr | B |
|---|---|---|---|---|---|---|---|---|---|
| Balance | 6.82 | 0.44 | 0.34 | 0.22 | 0.11 | 0.043 | 0.026 | 0.0091 | 0.0024 |
| Designation | Solution Heat Treatment | Quenching | Artificial Aging |
|---|---|---|---|
| ST1 | 535 °C—5 h | 70 °C | 165 °C—4 h |
| ST2 | 535 °C—2 h | 70 °C | 165 °C—4 h |
| ST3 | 510 °C—4 h | 70 °C | 165 °C—4 h |
| ST4 | 510 °C—2 h | 70 °C | 165 °C—4 h |
| AC | ST2 | ST3 | ST4 |
|---|---|---|---|
| 62.7 | 84.0 | 62.0 | 65.1 |
| Transformation | Temperature [°C] |
|---|---|
| (1) Liquid → α-Al | 625–620 (liquidus) |
| (2) Liquid → α-Al + β-Si + β-Al5FeSi + α-Al15(Fe, Mn, Cu)3Si2 | 570–565 |
| (3) Liquid → α-Al + β-Si + π-Al8FeMg3Si6 + β-Mg2Si | 540 |
| (4) Liquid → α-Al + β-Si + θ-Al2Cu + Q-Al5Mg8Cu2Si6 | 515–510 (solidus) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merlin, M.; Antonioli, L.; Bin, F.; Morales, C.; Soffritti, C. On the Optimization of T6 Heat Treatment Parameters of a Secondary Al-Si-Cu-Mg Foundry Aluminum Alloy: A Microstructural and Mechanical Characterization. Metals 2025, 15, 742. https://doi.org/10.3390/met15070742
Merlin M, Antonioli L, Bin F, Morales C, Soffritti C. On the Optimization of T6 Heat Treatment Parameters of a Secondary Al-Si-Cu-Mg Foundry Aluminum Alloy: A Microstructural and Mechanical Characterization. Metals. 2025; 15(7):742. https://doi.org/10.3390/met15070742
Chicago/Turabian StyleMerlin, Mattia, Lorenzo Antonioli, Federico Bin, Cindy Morales, and Chiara Soffritti. 2025. "On the Optimization of T6 Heat Treatment Parameters of a Secondary Al-Si-Cu-Mg Foundry Aluminum Alloy: A Microstructural and Mechanical Characterization" Metals 15, no. 7: 742. https://doi.org/10.3390/met15070742
APA StyleMerlin, M., Antonioli, L., Bin, F., Morales, C., & Soffritti, C. (2025). On the Optimization of T6 Heat Treatment Parameters of a Secondary Al-Si-Cu-Mg Foundry Aluminum Alloy: A Microstructural and Mechanical Characterization. Metals, 15(7), 742. https://doi.org/10.3390/met15070742









