Characterization of Different Types of Screen-Printed Carbon Electrodes Modified Electrochemically by Ceria Coatings
Abstract
1. Introduction
2. Materials and Methods
3. Results
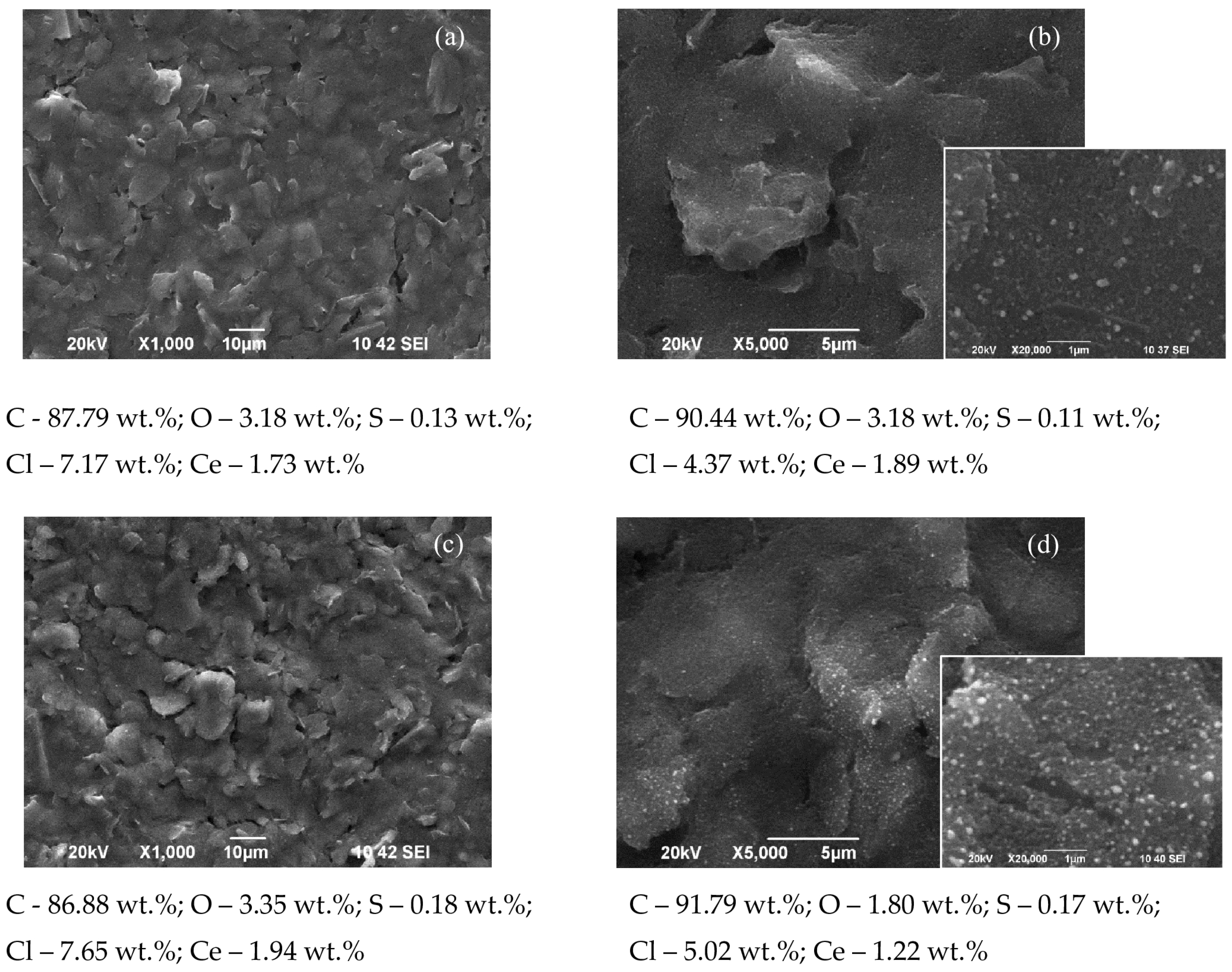
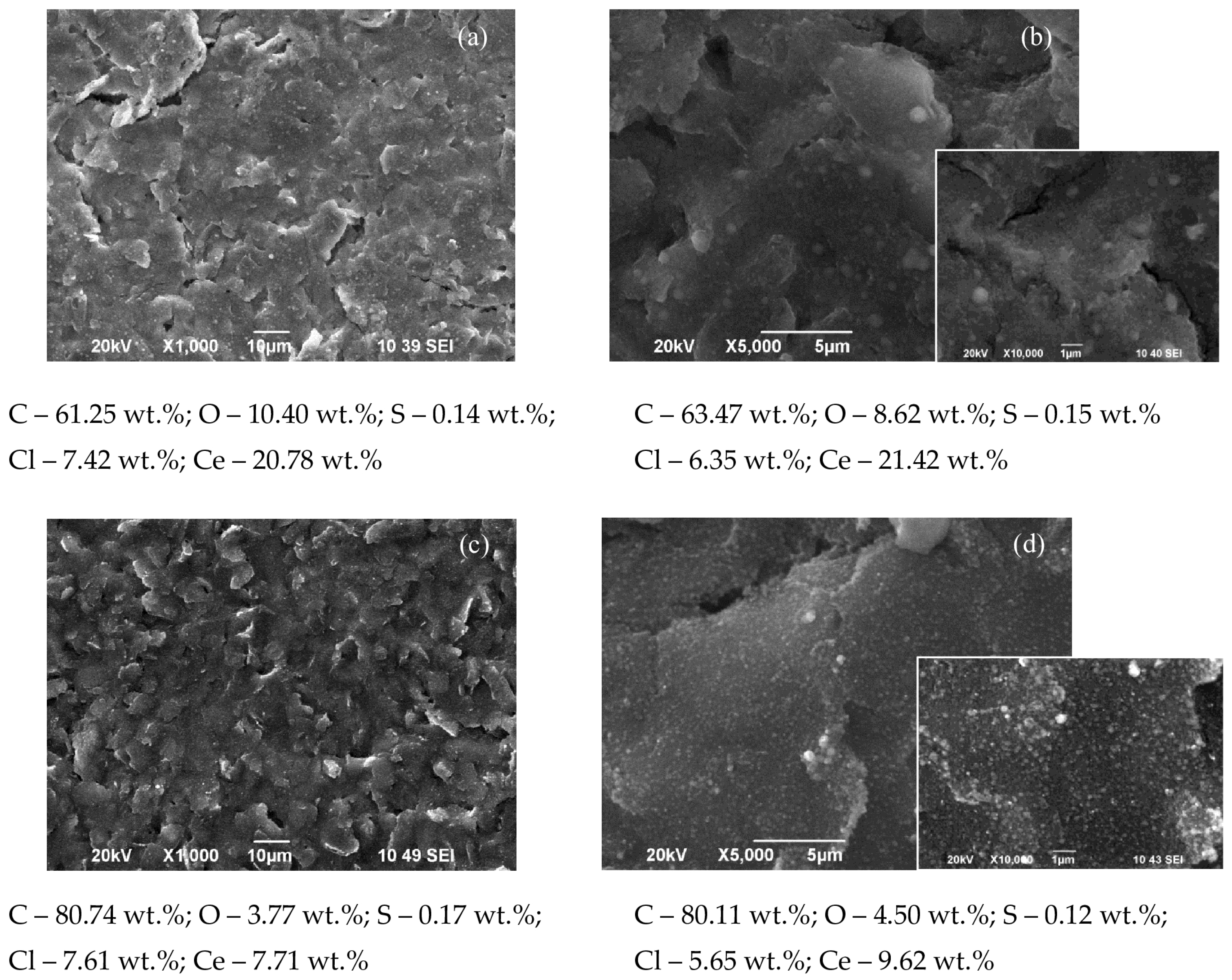
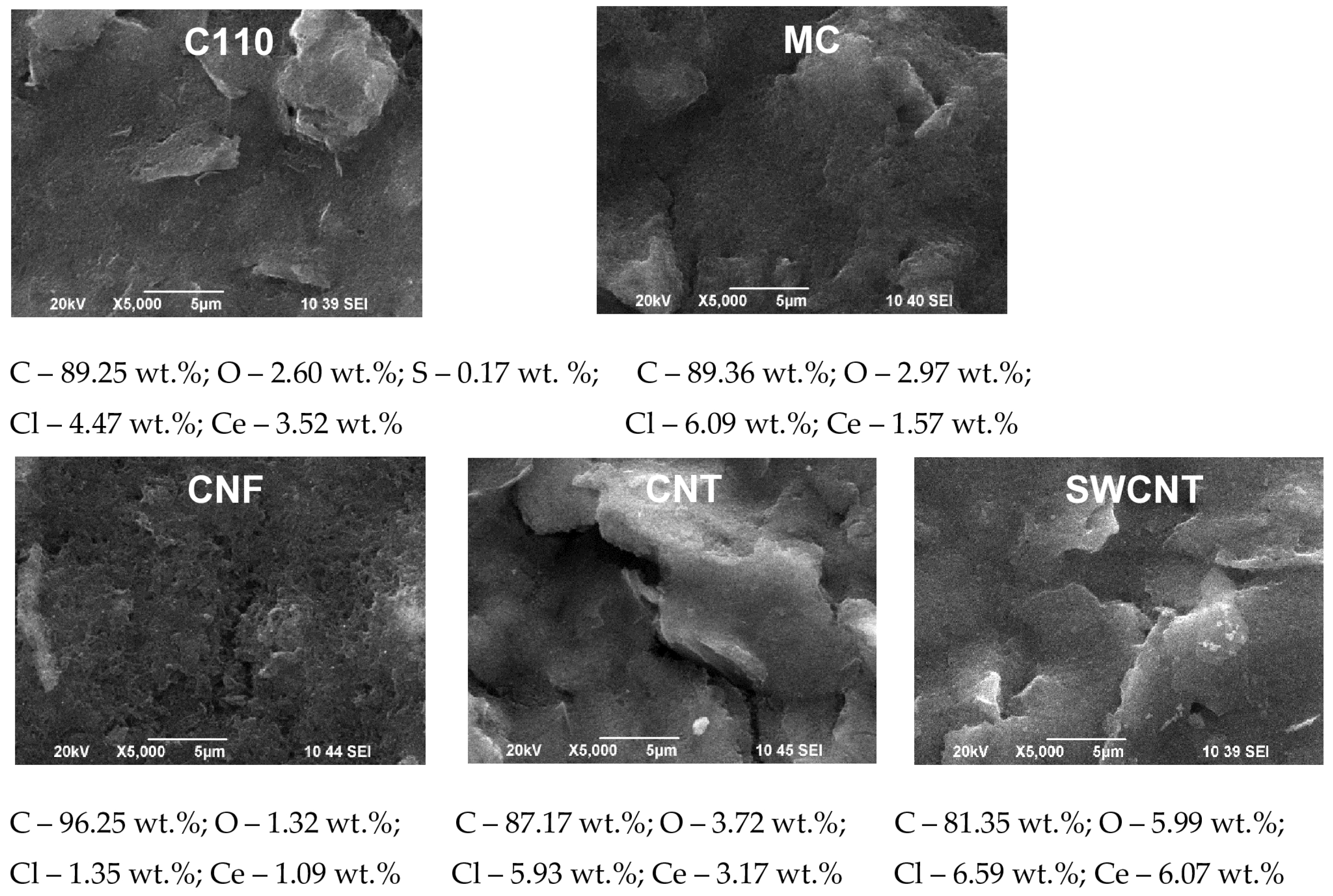
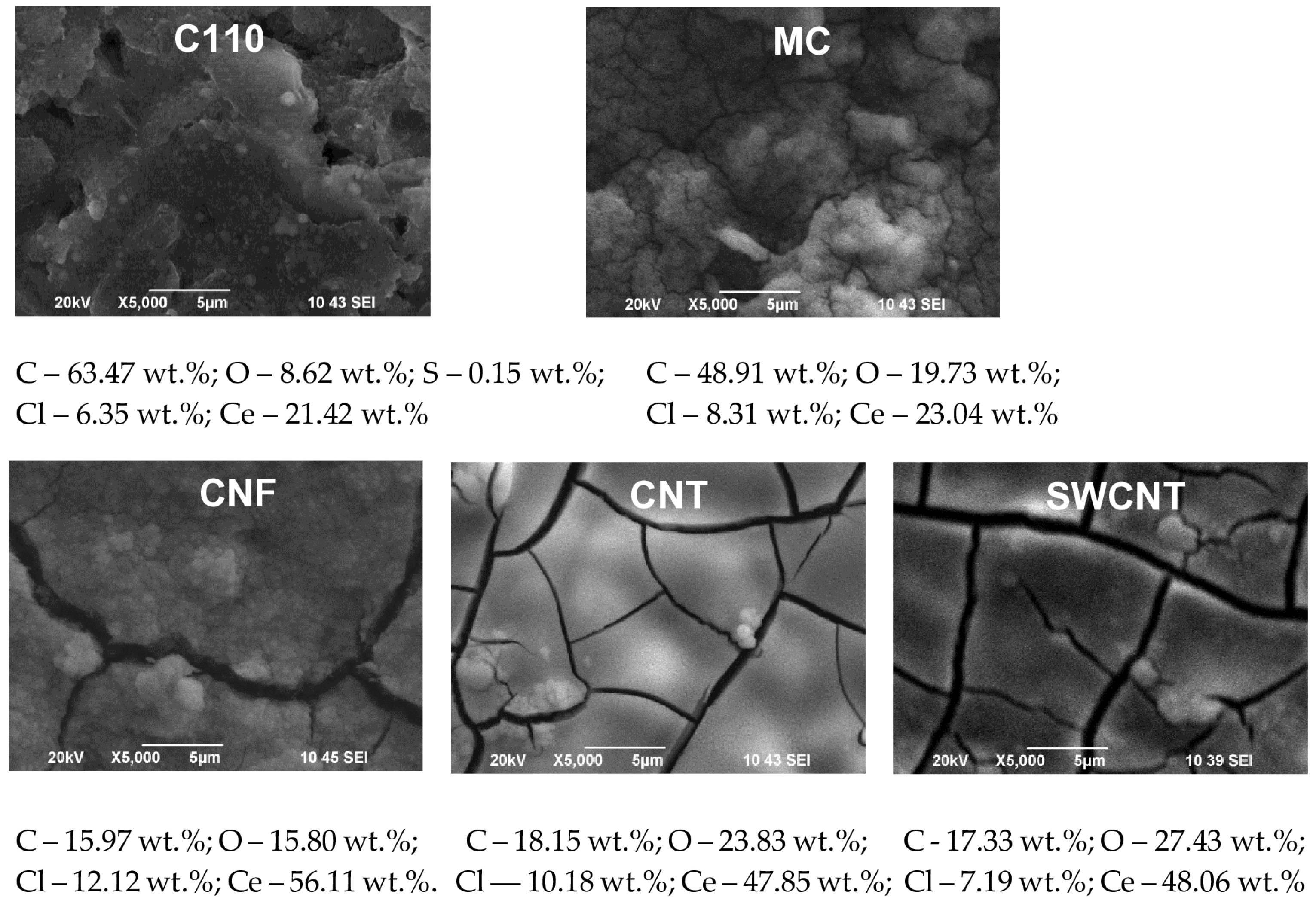
3.1. SEM and EDS Investigations
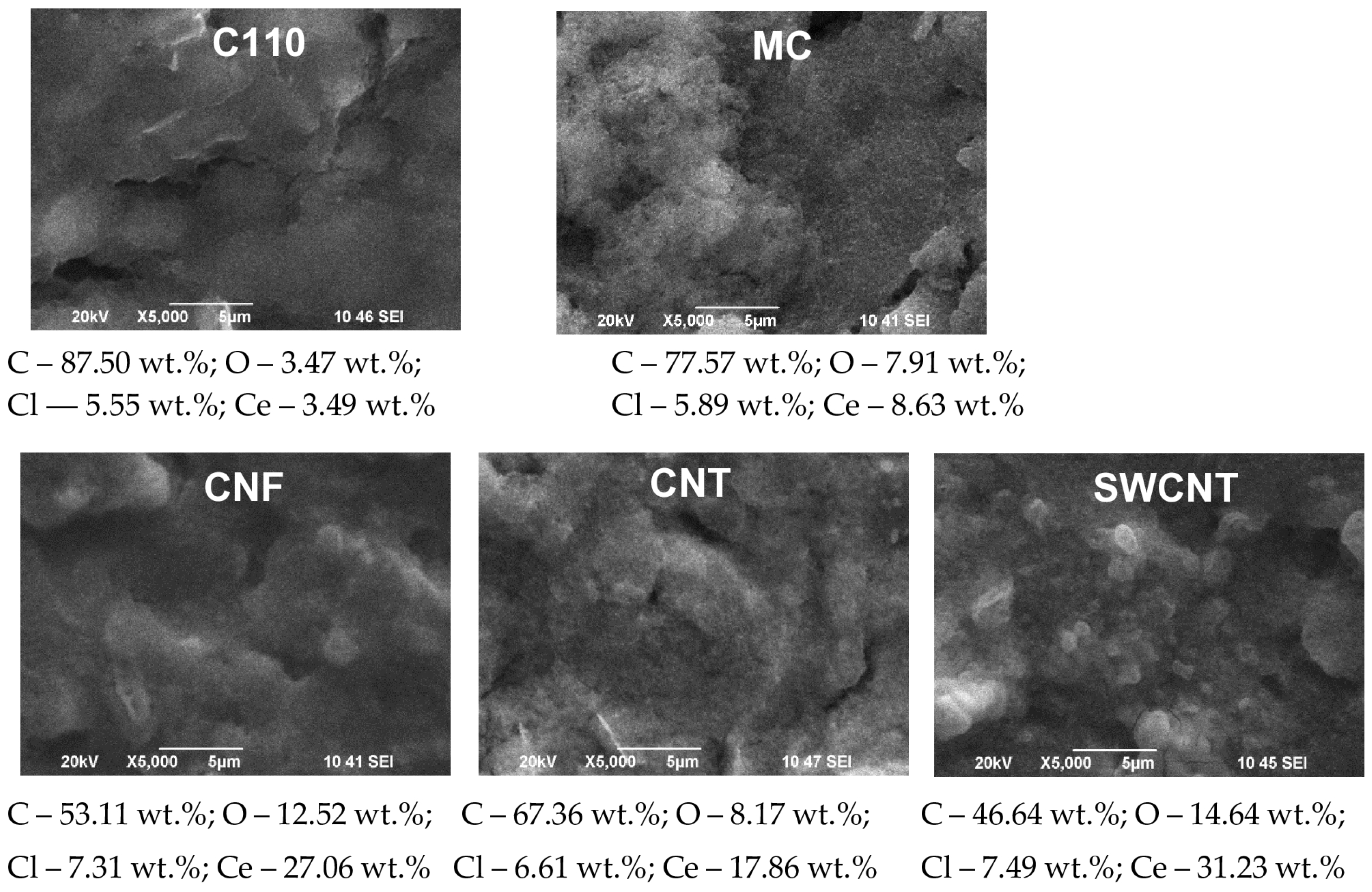
- Current density 0.5 mA·cm−2 and electrodeposition time 40 min (Figure 3): The morphology and surface structure of the SPCEs treated in this way practically reproduce those of the untreated substrates. Quantities of cerium oxide deposited were established for: C110 (Ce—3.52%), CNT (Ce—3.17%), and SWCNT (Ce—6.07%). Although a relatively lower quantity of cerium oxide registered on the CNF electrode (Ce—1.09%), it attracts attention with its highly developed surface morphology and structure. With a lower quantity of cerium (1.57%), in this mode, the MC system is also characterized;
- Current density 0.5 mA·cm−2 and electrodeposition time 80 min (Figure 4): The change in the morphology, structure, and chemical composition of the surface of the such treated SPCE is drastic. The quantity of cerium in the deposited layer is: C110—11.9%; MC—23.04%; CNF—56.11%; CNT—47.85%; SWCNT—48.06%. Accordingly, the modifying surface layer of cerium oxide on C110 is made up of uniformly dispersed spheroidal agglomerates with a diameter of ~0.05–0.2 µm. The surface cerium oxide layer formed on MC is made up of many times larger (diameter ~1–3 µm), densely arranged spheroidal agglomerates, containing cracks with a width of ~0.01–0.05 µm. The morphology and structure of the modified CNF are similar to MC, with fewer but wider cracks, reaching over 1 µm. The electrodeposited cerium oxide layer on the CNT is a dense and smooth coating containing regularly formed cracks with a width of ~0.1–0.5 µm. Similar conclusions are drawn for the modified SWCNT;
- Registered differences may be related to the thicker cerium oxide coating deposited at the higher (1 mA·cm−2) current density, evidence for which is the established higher cerium quantity in the studied SPCEs systems: C110 (Ce—3.49%), MC (Ce—8.63%), CNF (Ce—27.06%), CNT (Ce—17.8%); SWCNT (Ce—31.23%);
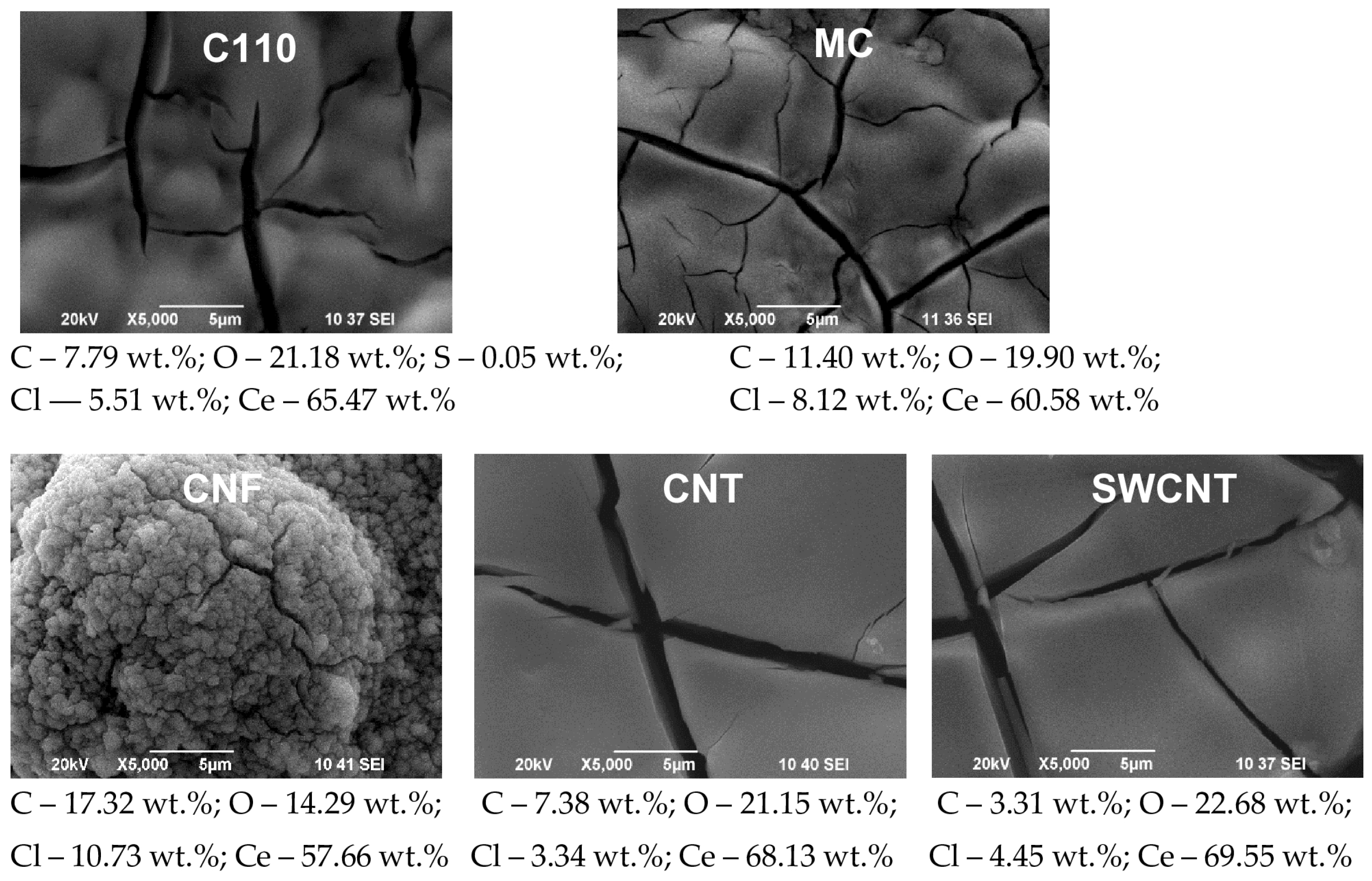
- Current density 1 mA·cm−2 and electrodeposition time 80 min (Figure 6): The change in the morphology, structure, and chemical composition of the SPCEs treated in this way is even more pronounced. The quantity of registered cerium is respectively: C110 (Ce—65.47%), MC (60.58%), CNF (57.66%), CNT (Ce—68.13%); SWCNT (Ce—69.55%). Accordingly, the modifying surface layer of cerium oxide on C110 is made up of a smooth, dense coating containing regularly formed cracks with a width of ~0.2–1.5 µm. Similar—dense and cracked—are the coatings also deposited on MC, CNT, and SWCNT electrodes. Significantly different in its morphology and structure is the cerium oxide coating deposited on a CNF substrate. It is made up of spheres with a highly developed surface, the diameter of which varies in the range of ~1–7 µm.
3.2. XPS Investigations
- At the lower cathode current density (0.5 mA·cm−2), the maximum recorded value for Ce, reaching 11.2 at.%, was recorded on SPCE MC. It is important to note that this sample also recorded the highest value for the Ce4+ concentration—63 at.% (Table 1);
- Relatively high values of Ce, specifically for the concentration of Ce4+, were also found in SPCEs CNF, CNT, SWCNT, and C110;
- At the higher cathode current density (1 mA·cm−2), the maximum recorded values of Ce concentration, reaching 14.7 at.% and 11.3 at.%, are registered on SPCEs SWCNT and CNT, respectively. And in these samples, the highest value for the Ce4+ concentration was established—64 at.% (Table 2);
- Also noteworthy, with regard to the relatively high value of Ce, specifically the concentration of Ce4+, are the results characterizing SPCEs C110 and MC, at a cathode current density of 1 mA·cm−2.
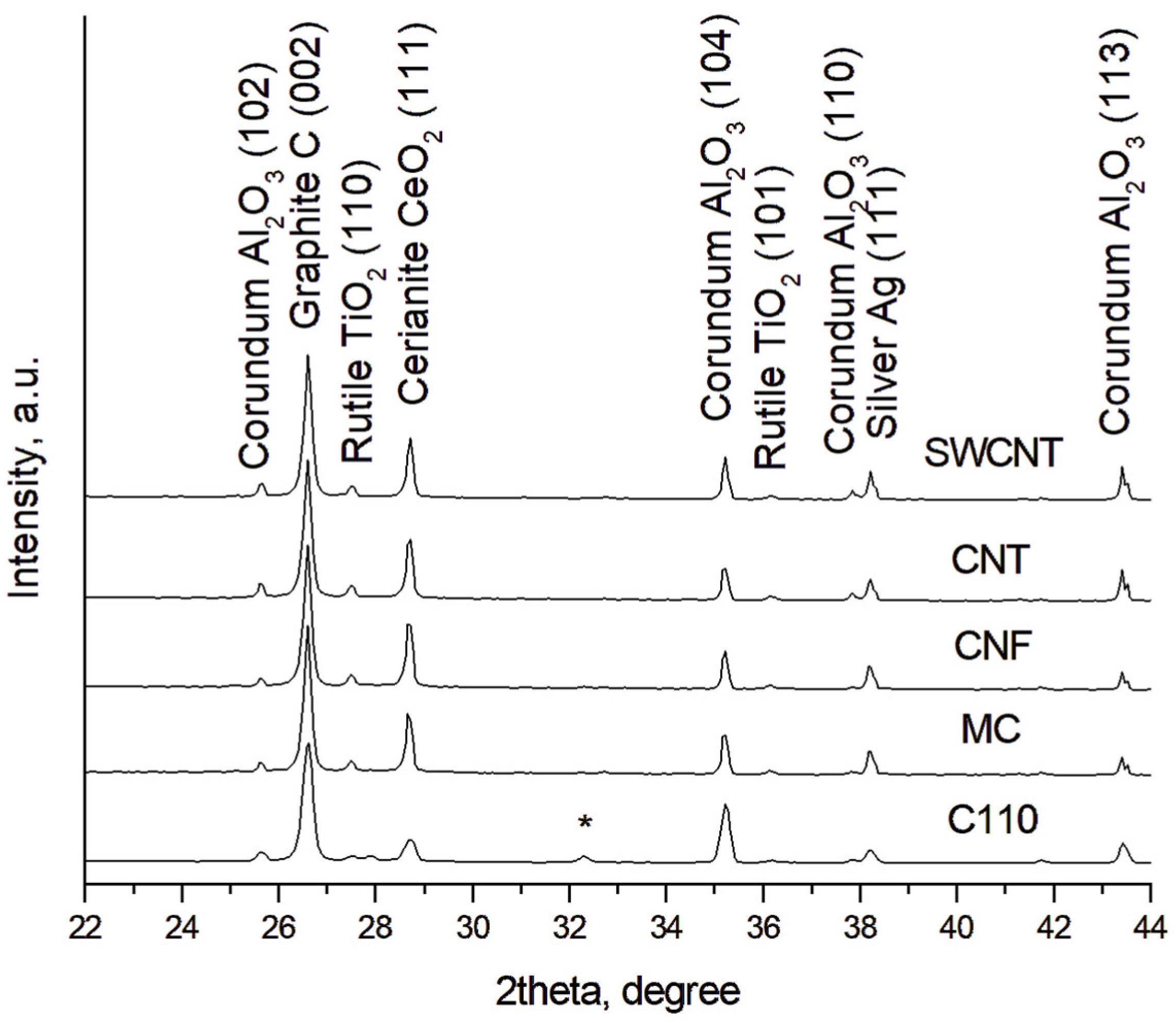
3.3. XRD Investigations
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SPCEs | Screen-printed carbon electrodes |
| C110 | Graphite |
| CNT | Carbon nanotubes |
| SWCNT | Single-walled carbon nanotubes |
| CNF | Carbon nanofibers |
| MC | Mesoporous carbon |
| SEM | Scanning electron microscope |
| EDS | Energy dispersive X-ray Spectroscopy |
| XRD | X-ray diffraction |
| XPS | X-ray photoelectron spectroscopy |
References
- Beitollahi, H.; Mohammadi, S.Z.; Safaei, M.; Tajik, S. Applications of Electrochemical Sensors and Biosensors Based on Modified Screen-Printed Electrodes: A Review. Anal. Methods 2020, 12, 1547–1560. [Google Scholar] [CrossRef]
- Antuña-Jiménez, D.; González-García, M.B.; Hernández-Santos, D.; Fanjul-Bolado, P. Screen-Printed Electrodes Modified with Metal Nanoparticles for Small Molecule Sensing. Biosensors 2020, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Ahammad, A.J.S.; Jin, J.-H.; Ahn, S.J.; Lee, J.-J. A Comprehensive Review of Glucose Biosensors Based on Nanostructured Metal-Oxides. Sensors 2010, 10, 4855–4886. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Beitollahi, H.; Amini, M.K.; Mirkhalaf, F.; Abdollahi-Alibeik, M. New Strategy for Simultaneous and Selective Voltammetric Determination of Norepinephrine, Acetaminophen and Folic Acid Using ZrO2 Nanoparticles-Modified Carbon Paste Electrode. Sens. Actuators B Chem. 2010, 151, 243–249. [Google Scholar] [CrossRef]
- Perry Murray, E.; Tsai, T.; Barnett, S.A. A Direct-Methane Fuel Cell with a Ceria-Based Anode. Nature 1999, 400, 649–651. [Google Scholar] [CrossRef]
- Uzunoglu, A.; Kose, D.A.; Stanciu, L.A. Synthesis of CeO2-Based Core/Shell Nanoparticles with High Oxygen Storage Capacity. Int. Nano Lett. 2017, 7, 187–193. [Google Scholar] [CrossRef]
- Stoukides, M. Solid-Electrolyte Membrane Reactors: Current Experience and Future Outlook. Catal. Rev. Sci. Eng. 2000, 42, 1–70. [Google Scholar] [CrossRef]
- Sun, C.; Li, H.; Chen, L. Nanostructured Ceria-Based Materials: Synthesis, Properties, and Applications. Energy Environ. Sci. 2012, 5, 8475–8505. [Google Scholar] [CrossRef]
- Asati, A.; Santra, S.; Kaittanis, C.; Nath, S.; Perez, J.M. Oxidase-like Activity of Polymer-Coated Cerium Oxide Nanopartieles. Angew. Chem. Int. Ed. 2009, 48, 2308–2312. [Google Scholar] [CrossRef] [PubMed]
- Tsud, N.; Acres, R.G.; Iakhnenko, M.; Mazur, D.; Prince, K.C.; Matolín, V. Bonding of Histidine to Cerium Oxide. J. Phys. Chem. B 2013, 117, 9182–9193. [Google Scholar] [CrossRef]
- Qiu, J.-D.; Cui, S.-G.; Liang, R.-P. Hydrogen Peroxide Biosensor Based on the Direct Electrochemistry of Myoglobin Immobilized on Ceria Nanoparticles Coated with Multiwalled Carbon Nanotubesby a Hydrothermal Synthetic Method. Microchim. Acta 2010, 171, 333–339. [Google Scholar] [CrossRef]
- Ibrahim, H.; Temerk, Y. Sensitive Electrochemical Sensor for Simultaneous Determination of Uric Acid and Xanthine in Human Biological Fluids Based on the Nano-Boron Doped Ceria Modified Glassy Carbon Paste Electrode. J. Electroanal. Chem. 2016, 780, 176–186. [Google Scholar] [CrossRef]
- Ibrahim, H.; Temerk, Y. A Novel Electrochemical Sensor Based on B Doped CeO2 Nanocubes Modified Glassy Carbon Microspheres Paste Electrode for Individual and Simultaneous Determination of Xanthine and Hypoxanthine. Sens. Actuators B Chem. 2016, 232, 125–137. [Google Scholar] [CrossRef]
- Temerk, Y.; Ibrahim, H. A New Sensor Based on in Doped CeO2 Nanoparticles Modified Glassy Carbon Paste Electrode for Sensitive Determination of Uric Acid in Biological Fluids. Sens. Actuators B Chem. 2016, 224, 868–877. [Google Scholar] [CrossRef]
- Ibrahim, H.; Temerk, Y.; Farhan, N. Electrochemical Sensor for Individual and Simultaneous Determination of Guanine and Adenine in Biological Fluids and in DNA Based on a Nano-In-Ceria Modified Glassy Carbon Paste Electrode. RSC Adv. 2016, 6, 90220–90231. [Google Scholar] [CrossRef]
- Ibrahim, M.; Temerk, Y.; Ibrahim, H. Fabrication of a New Biosensor Based on a Sn Doped Ceria Nanoparticle Modified Glassy Carbon Paste Electrode for the Selective Determination of the Anticancer Drug Dacarbazine in Pharmaceuticals. RSC Adv. 2017, 7, 32357–32366. [Google Scholar] [CrossRef]
- Andreeva, D.; Ivanov, I.; Ilieva, L.; Sobczak, J.W.; Avdeev, G.; Tabakova, T. Nanosized Gold Catalysts Supported on Ceria and Ceria-Alumina for WGS Reaction: Influence of the Preparation Method. Appl. Catal. A Gen. 2007, 333, 153–160. [Google Scholar] [CrossRef]
- Metrohm. Available online: https://www.metrohm.com/bg_bg/search.html?q=electrodes&cat=electrode (accessed on 13 May 2025).
- Cherneva, S.; Guergova, D.; Iankov, R.; Stoychev, D. Investigation of Mechanical Properties of Mono- and Multi-Layer Alumina and Ceria Films Using Finite Element Modeling and Nanoindentation Experiments. J. Eng. Mater. Technol. Trans. ASME 2019, 141, 011006. [Google Scholar] [CrossRef]
- Nikolova, D.; Stoyanova, E.; Stoychev, D.; Stefanov, P.; Marinova, T. Anodic Behaviour of Stainless Steel Covered with an Electrochemically Deposited Ce2O3-CeO2 Film. Surf. Coat. Technol. 2006, 201, 1559–1567. [Google Scholar] [CrossRef]
- Stoyanova, E.; Guergova, D.; Stoychev, D.; Avramova, I.; Stefanov, P. Passivity of OC404 Steel Modified Electrochemically with CeO2-Ce2O3 Layers in Sulfuric Acid Media. Electrochim. Acta 2010, 55, 1725–1732. [Google Scholar] [CrossRef]
- Guergova, D.; Stoyanova, E.; Stoychev, D.; Avramova, I.; Atanasova, G.; Stefanov, P. Corrosion Stability of Stainless Steel, Modified Electrochemically with Ce2O3-CeO2 Films, in 3.5% NaCl Media. Bulg. Chem. Commun. 2011, 43, 150–157. [Google Scholar]
- Avramova, I.; Suzer, S.; Guergova, D.; Stoychev, D.; Stefanov, P. CeOx/Al2O3 Thin Films on Stainless Steel Substrate - Dynamical X-ray Photoelectron Spectroscopy Investigations. Thin Solid Films 2013, 536, 63–67. [Google Scholar] [CrossRef]
- Guergova, D.; Stoyanova, E.; Stoychev, D.; Avramova, I.; Stefanov, P. Self-Healing Effect of Ceria Electrodeposited Thin Films on Stainless Steel in Aggressive 0.5 Mol/L NaCl Aqueous Solution. J. Rare Earths 2015, 33, 1212–1227. [Google Scholar] [CrossRef]
- Putz, H.; Brandenburg, K. Match!—Phase Analysis Using Powder Diffraction. Available online: http://www.crystalimpact.com/match/ (accessed on 20 February 2025).
- Gražulis, S.; Daškevič, A.; Merkys, A.; Chateigner, D.; Lutterotti, L.; Quirós, M.; Serebryanaya, N.R.; Moeck, P.; Downs, R.T.; Le Bail, A. Crystallography Open Database (COD): An Open-Access Collection of Crystal Structures and Platform for World-Wide Collaboration. Nucleic Acids Res. 2012, 40, 420–427. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Greczynski, G. Binding Energy Referencing in X-ray Photoelectron Spectroscopy: Expanded Data Set Confirms That Adventitious Carbon Aligns to the Sample Vacuum Level. Appl. Surf. Sci. 2024, 670, 160666. [Google Scholar] [CrossRef]
- Jafari, S.; Linford, M.; Sherwood, P. Insight Note. Complications of Magnesium Achromatic X-rays in XPS: A Historical and Practical Perspective. Surf. Interface Anal. 2025, 57, 221–225. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. The Same Chemical State of Carbon Gives Rise to Two Peaks in X-ray Photoelectron Spectroscopy. Sci. Rep. 2021, 11, 11195. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Fang, D. A Review on C1s XPS-Spectra for Some Kinds of Carbon Materials. Fuller. Nanotub. Carbon Nanostructures 2020, 28, 1048–1058. [Google Scholar] [CrossRef]
- Belmonte, T.; Thiébaut, J.M.; Mézerette, D. Role of Active Species in Surface Cleaning by an Ar-N2 Atmospheric Pressure Post-Discharge. J. Phys. D. Appl. Phys. 2002, 35, 1919–1926. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. Impact of Sample Storage Type on Adventitious Carbon and Native Oxide Growth: X-ray Photoelectron Spectroscopy Study. Vacuum 2022, 205, 111463. [Google Scholar] [CrossRef]
- Zhang, B.-B.; Ming, J.; Li, H.-Q.; Song, X.-N.; Wang, C.-K.; Hua, W.; Ma, Y. Structural Characterization of Nitrogen-Doped γ-Graphynes by Computational X-ray Spectroscopy. Carbon N. Y. 2023, 214, 118301. [Google Scholar] [CrossRef]
- Lee, J.-W.; Jeong, S.-P.; You, N.-H.; Moon, S.-Y. Tunable Synthesis of Predominant Semi-Ionic and Covalent Fluorine Bonding States on a Graphene Surface. Nanomaterials 2021, 11, 942. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.V.; Toro-Gonzalez, M.; Molina-Higgins, M.C.; Castano, C.E. Facile Radiolytic Synthesis of Ruthenium Nanoparticles on Graphene Oxide and Carbon Nanotubes. Mater. Sci. Eng. B 2016, 205, 28–35. [Google Scholar] [CrossRef]
- Biesinger, M.C. Accessing the Robustness of Adventitious Carbon for Charge Referencing (Correction) Purposes in XPS Analysis: Insights from a Multi-User Facility Data Review. Appl. Surf. Sci. 2022, 597, 153681. [Google Scholar] [CrossRef]
- Yang, C.-C.; Tsai, M.-H.; Yang, Z.-R.; Yang, Y.-W.; Tseng, Y.-C.; Wang, C.-H. An Effective Charge Neutralization Enabled by Graphene Overlayer in Ambient Pressure XPS Measurements of Insulators. Adv. Mater. Interfaces 2023, 10, 2201926. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, J.; Claypool, J.B.; O’Keefe, M.J. Structure and Corrosion Behavior of Sputter Deposited Ce-Al-o Coating on Al 2024-T3 Alloy Substrates. J. Electrochem. Soc. 2016, 163, C198–C204. [Google Scholar] [CrossRef]
- Burroughs, P.; Hamnett, A.; Orchard, A.F.; Thornton, G. Satellite Structure in the X-ray Photoelectron Spectra of Some Binary and Mixed Oxides of Lanthanum and Cerium. J. Chem. Soc. Dalt. Trans. 1976, 1686–1698. [Google Scholar] [CrossRef]
- Pardo, A.; Feliú, S., Jr.; Merino, M.C.; Arrabal, R.; Matykina, E. The Effect of Cerium and Lanthanum Surface Treatments on Early Stages of Oxidation of A361 Aluminium Alloy at High Temperature. Appl. Surf. Sci. 2007, 254, 586–595. [Google Scholar] [CrossRef]
- Jeong, H.-W.; Yun, G.-N.; Bae, Y.-S.; Oh, K.-R.; Hwang, Y.K. Exploring the Role of PdO and Oxygen Vacancies of Pd/CeO2 in Bicarbonate Hydrogenation. J. CO2 Util. 2025, 92, 103028. [Google Scholar] [CrossRef]
- Holgado, J.P.; Alvarez, R.; Munuera, G. Study of CeO2 XPS Spectra by Factor Analysis: Reduction of CeO2. Appl. Surf. Sci. 2000, 161, 301–315. [Google Scholar] [CrossRef]
- Lopinski, G.P.; Kodra, O.; Kunc, F.; Kennedy, D.C.; Couillard, M.; Johnston, L.J. X-ray Photoelectron Spectroscopy of Metal Oxide Nanoparticles: Chemical Composition, Oxidation State and Functional Group Content. Nanoscale Adv. 2025, 7, 1671–1685. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Lee, C.-T. Surface Analysis of (NH4)2Sx-Treated InGaN Using X-ray Photoelectron Spectroscopy. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 2001, 19, 1734–1738. [Google Scholar] [CrossRef]
- Hoch, L.B.; Wood, T.E.; O’Brien, P.G.; Liao, K.; Reyes, L.M.; Mims, C.A.; Ozin, G.A. The Rational Design of a Single-Component Photocatalyst for Gas-Phase CO2 Reduction Using Both UV and Visible Light. Adv. Sci. 2014, 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Klingenhof, M.; Gao, C.; Koketsu, T.; Weiser, G.; Pi, Y.; Liu, S.; Sui, L.; Hou, J.; Li, J.; et al. Facilitating Alkaline Hydrogen Evolution Reaction on the Hetero-Interfaced Ru/RuO2 through Pt Single Atoms Doping. Nat. Commun. 2024, 15, 1447. [Google Scholar] [CrossRef]
- Wyckoff, R.W.G. Fluorite Structure. In Crystal Structures 1; Interscience Publishers: New York, NY, USA, 1963; pp. 239–444. [Google Scholar]
- Breinlich, C.; Essen, J.M.; Barletta, E.; Wandelt, K. Growth, Structure and Electronic Properties of Ultrathin Cerium Oxide Films Grown on Pt(111). Thin Solid Films 2011, 519, 3752–3755. [Google Scholar] [CrossRef]
- Nagata, H.; Yoshimoto, M.; Koinuma, H.; Min, E.; Haga, N. Type-B Epitaxial Growth of CeO2 Thin Film on Si(111) Substrate. J. Cryst. Growth 1992, 123, 1–4. [Google Scholar] [CrossRef]


| Type of SPCE | Time of Deposition | C 1s, at. % | O, at. % | S, at. % | Cl, at. % | Ce 3d, at. % (Total Ce3+ + Ce4+) | Ce4+ (% of Ce Total) |
|---|---|---|---|---|---|---|---|
| C110 | 40 min | 72.6 | 23.1 | 1.8 | 1.8 | 0.7 | 36% |
| 80 min | 65.4 | 21.0 | 1.0 | 8.5 | 4.2 | 71% | |
| MC | 40 min | 69.4 | 18.4 | 1.2 | 7.5 | 3.6 | 48% |
| 80 min | 50.4 | 27.0 | 1.5 | 9.9 | 11.2 | 63% | |
| CNF | 40 min | 71.2 | 16.4 | 0.9 | 5.7 | 5.7 | 62% |
| 80 min | 52.7 | 26.2 | 1.6 | 11.0 | 8.5 | 38% | |
| CNT | 40 min | 65.7 | 19.9 | 1.5 | 7.8 | 5.1 | 61% |
| 80 min | 52.2 | 27.4 | 1.6 | 9.6 | 9.3 | 49% | |
| SWCNT | 40 min | 53.7 | 28.5 | 0.5 | 9.3 | 7.9 | 74% |
| 80 min | 52.6 | 31.9 | 1.2 | 5.6 | 8.8 | 48% |
| Type of SPCE | Time of Deposition | C 1s, at. % | O 1s, at. % | S, at. % | Cl 2p, at. % | Ce 3d, at. % (Total Ce3+ + Ce4+) | Ce4+ (% of Ce Total) |
|---|---|---|---|---|---|---|---|
| C110 | 40 min | 59.2 | 22.0 | 1.1 | 10.0 | 7.8 | 61% |
| 80 min | 41.0 | 36.1 | 2.0 | 11.7 | 9.3 | 79% | |
| MC | 40 min | 49.4 | 32.8 | 1.1 | 8.1 | 8.7 | 65% |
| 80 min | 48.6 (36.1) | 35.7 (41.8) | 0 | 7.9 (9.3) | 7.8 (9.1) | 83% | |
| CNF | 40 min | 60.8 | 24.2 | 1.2 | 5.9 | 7.9 | 45% |
| 80 min | 51.8 | 28.0 | 1.0 | 9.7 | 9.6 | 52% | |
| CNT | 40 min | 47.1 | 30.4 | 1.1 | 10.2 | 11.3 | 62% |
| 80 min | 57.9 (39.5) | 29.6 (42.5) | 1.0 (1.4) | 3.2 (4.6) | 8.3 (11.9) | 64% | |
| SWCNT | 40 min | 37.5 | 37.1 | 1.3 | 9.4 | 14.7 | 64% |
| 80 min | 65.1 (35.4) | 26.8 (49.7) | 1.2 (2.2) | 2.3 (4.3) | 4.5 (8.4) | 44% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreeva, R.; Tsanev, A.; Avdeev, G.; Stoychev, D. Characterization of Different Types of Screen-Printed Carbon Electrodes Modified Electrochemically by Ceria Coatings. Metals 2025, 15, 741. https://doi.org/10.3390/met15070741
Andreeva R, Tsanev A, Avdeev G, Stoychev D. Characterization of Different Types of Screen-Printed Carbon Electrodes Modified Electrochemically by Ceria Coatings. Metals. 2025; 15(7):741. https://doi.org/10.3390/met15070741
Chicago/Turabian StyleAndreeva, Reni, Aleksandar Tsanev, Georgi Avdeev, and Dimitar Stoychev. 2025. "Characterization of Different Types of Screen-Printed Carbon Electrodes Modified Electrochemically by Ceria Coatings" Metals 15, no. 7: 741. https://doi.org/10.3390/met15070741
APA StyleAndreeva, R., Tsanev, A., Avdeev, G., & Stoychev, D. (2025). Characterization of Different Types of Screen-Printed Carbon Electrodes Modified Electrochemically by Ceria Coatings. Metals, 15(7), 741. https://doi.org/10.3390/met15070741







