The Research on Carbon Deoxygenation of Molten Steel and Its Application in the Converter Steelmaking Process
Abstract
1. Introduction
2. Experimental Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
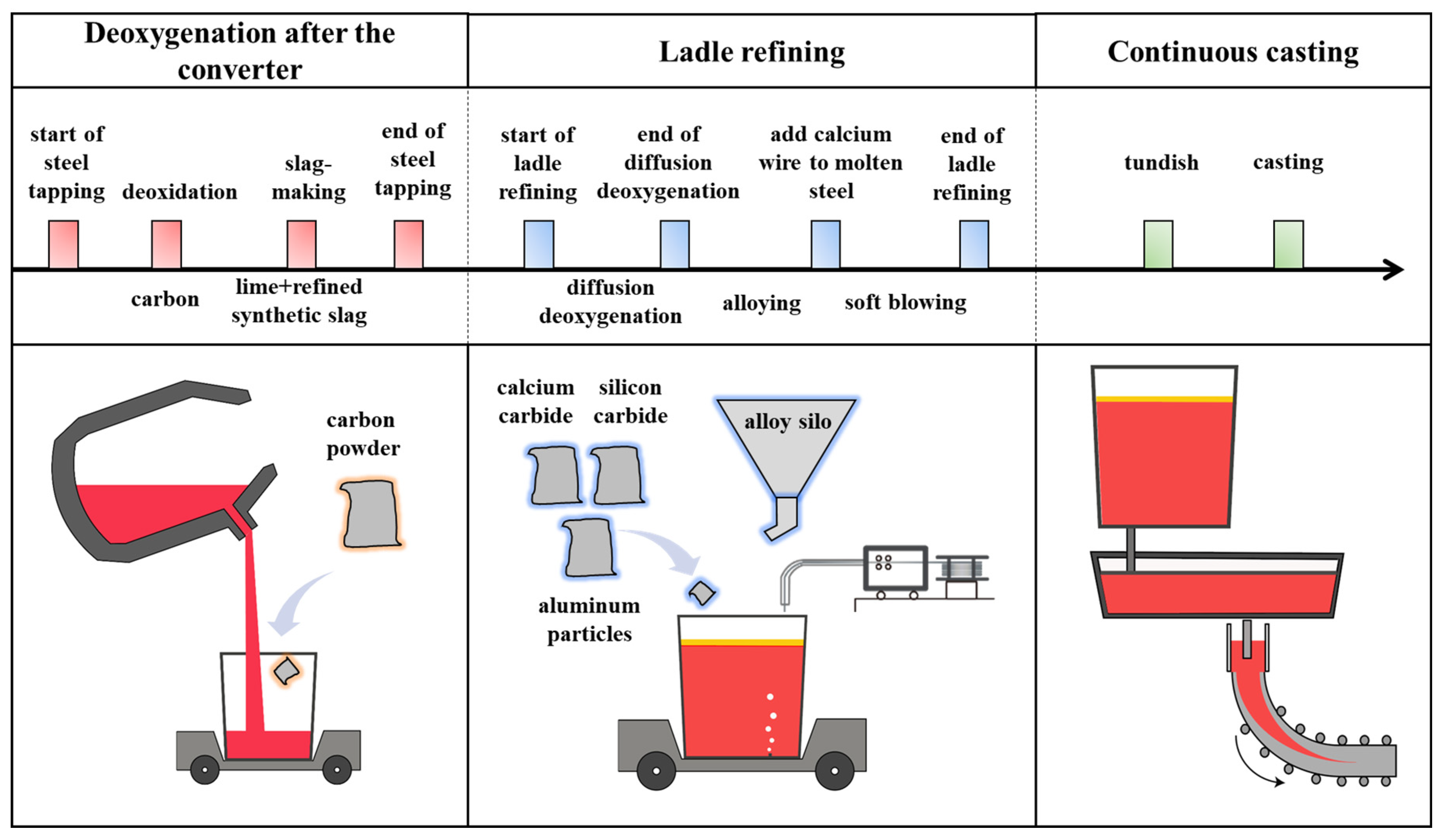
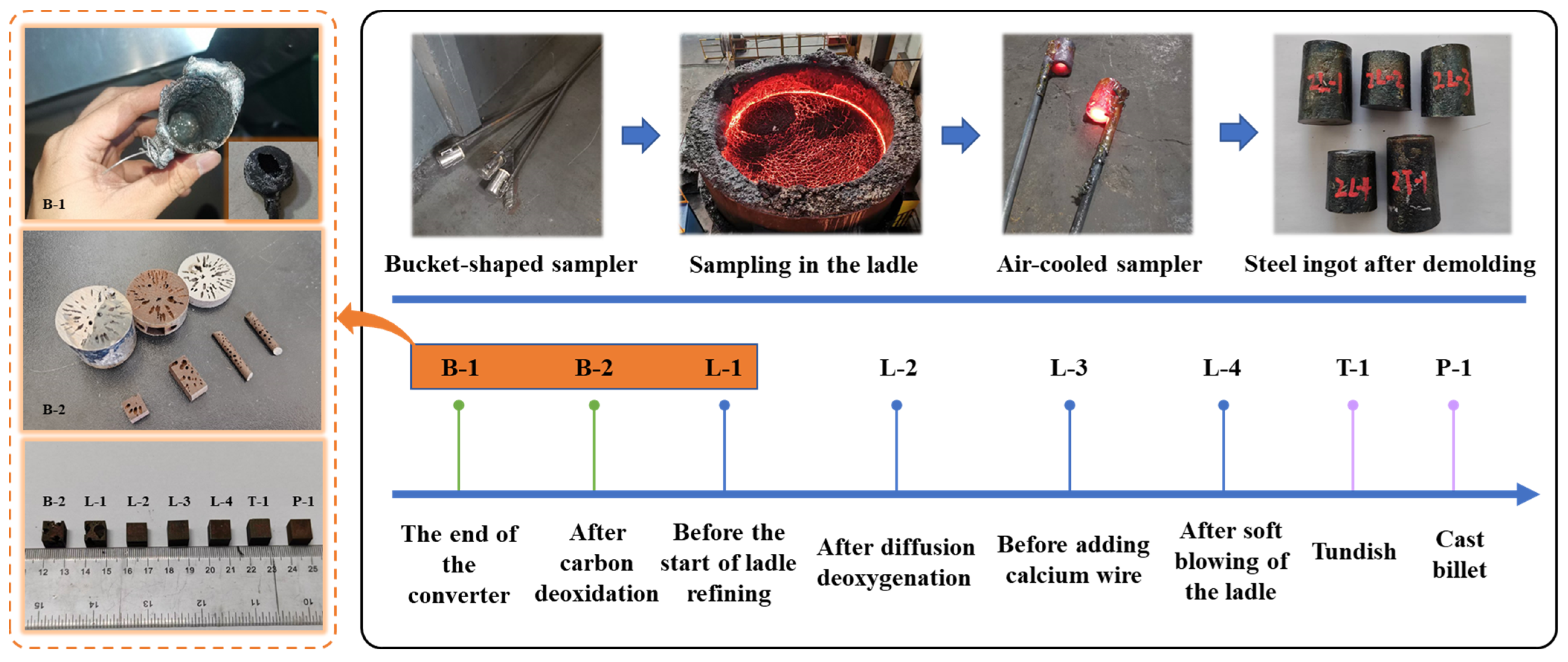
2.2.1. Deoxygenation Process and Sampling Plan
2.2.2. Sample Testing Method
3. Results and Discussion
3.1. Theoretical Calculation of the Carbon Deoxygenation Process
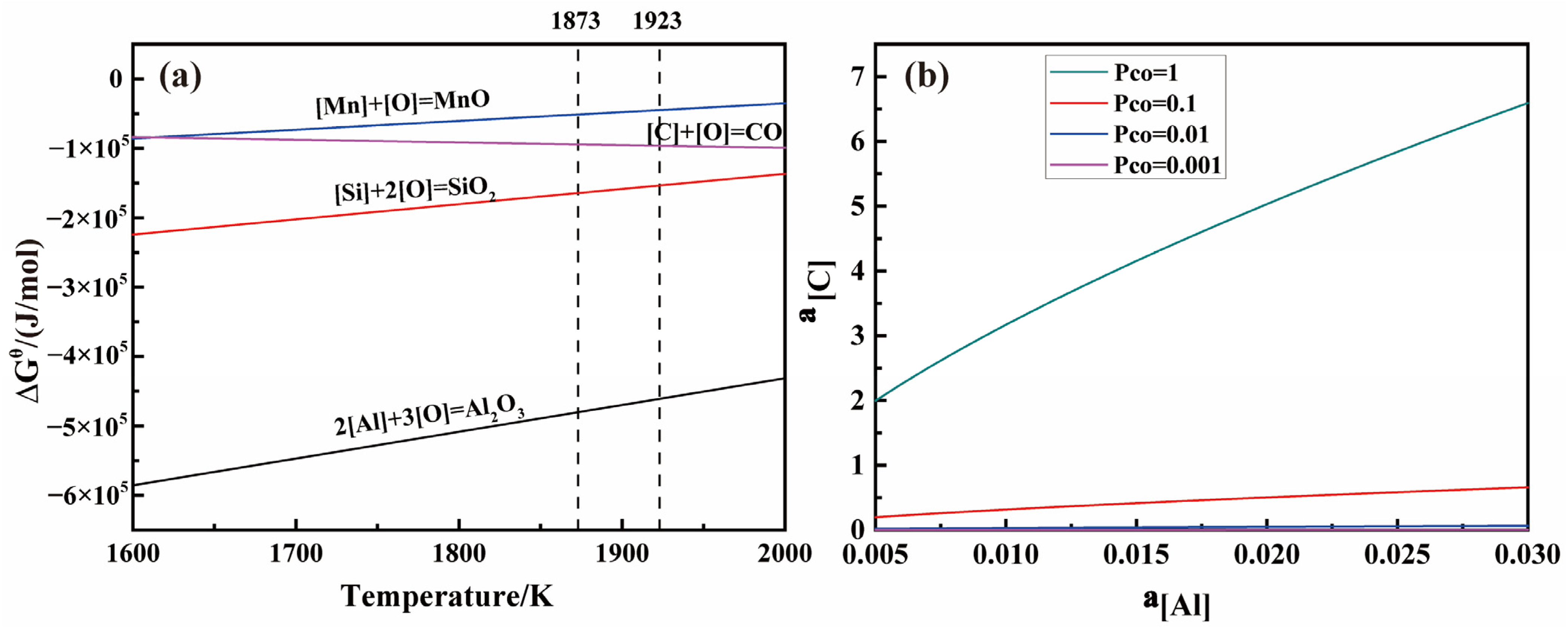
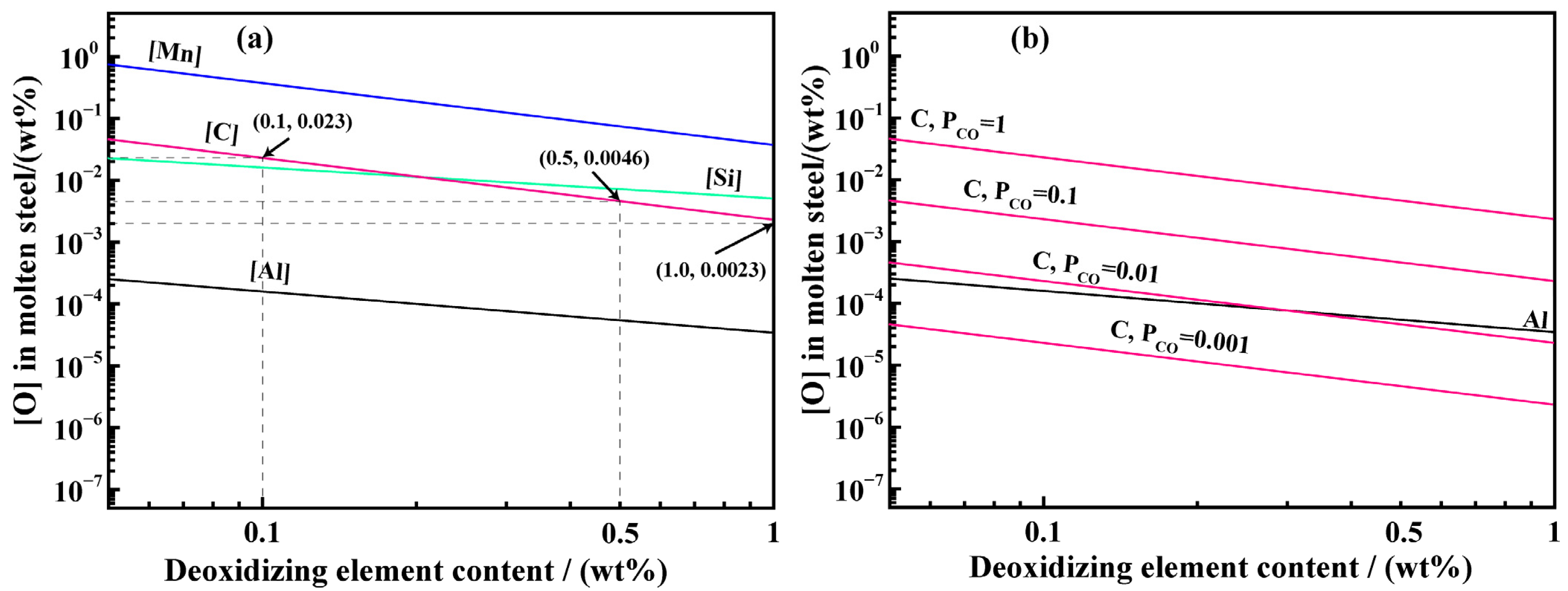
3.1.1. Analysis of the Deoxygenation Ability of Different Elements
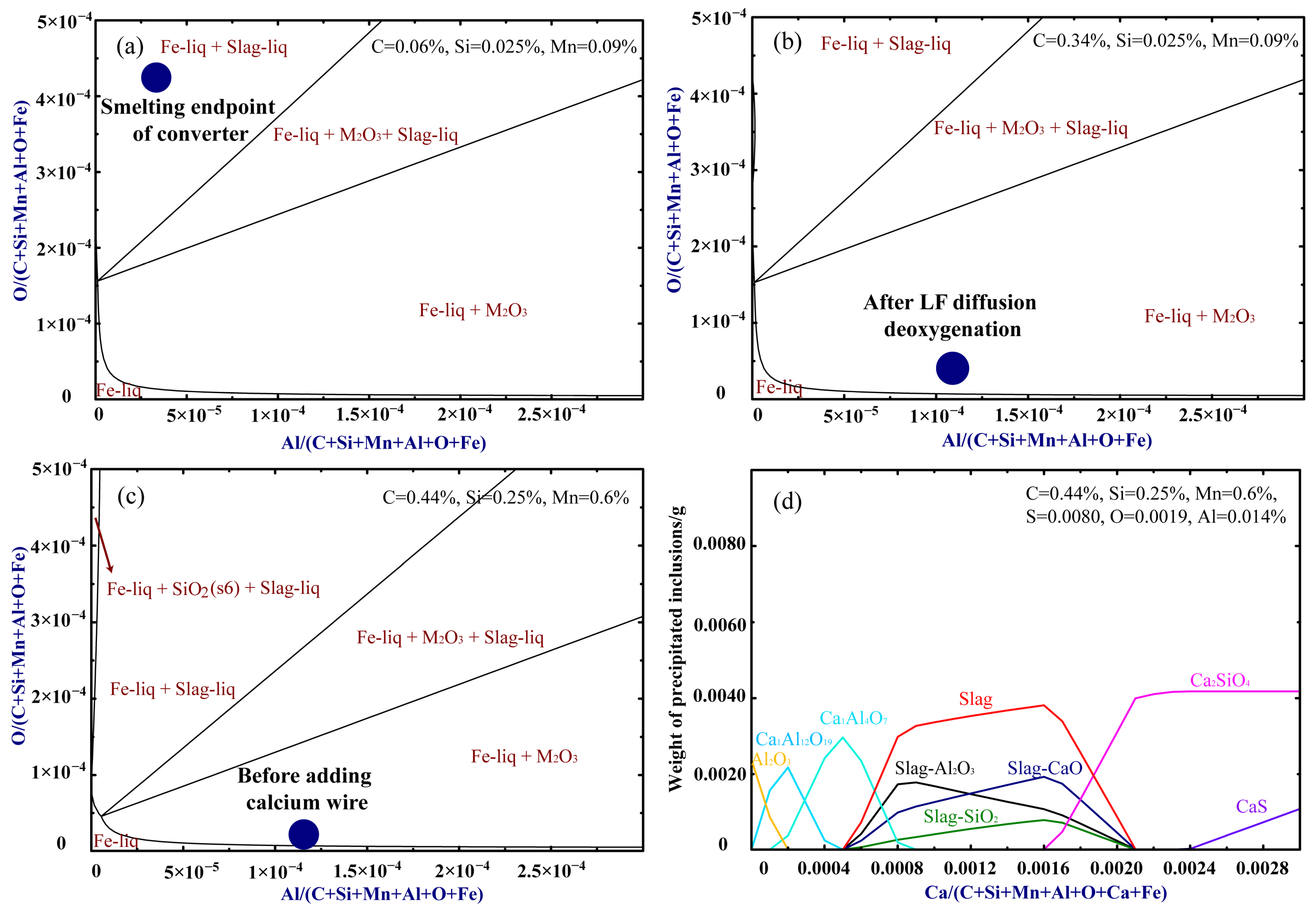
3.1.2. Limit Analysis of Carbon Deoxygenation Reaction
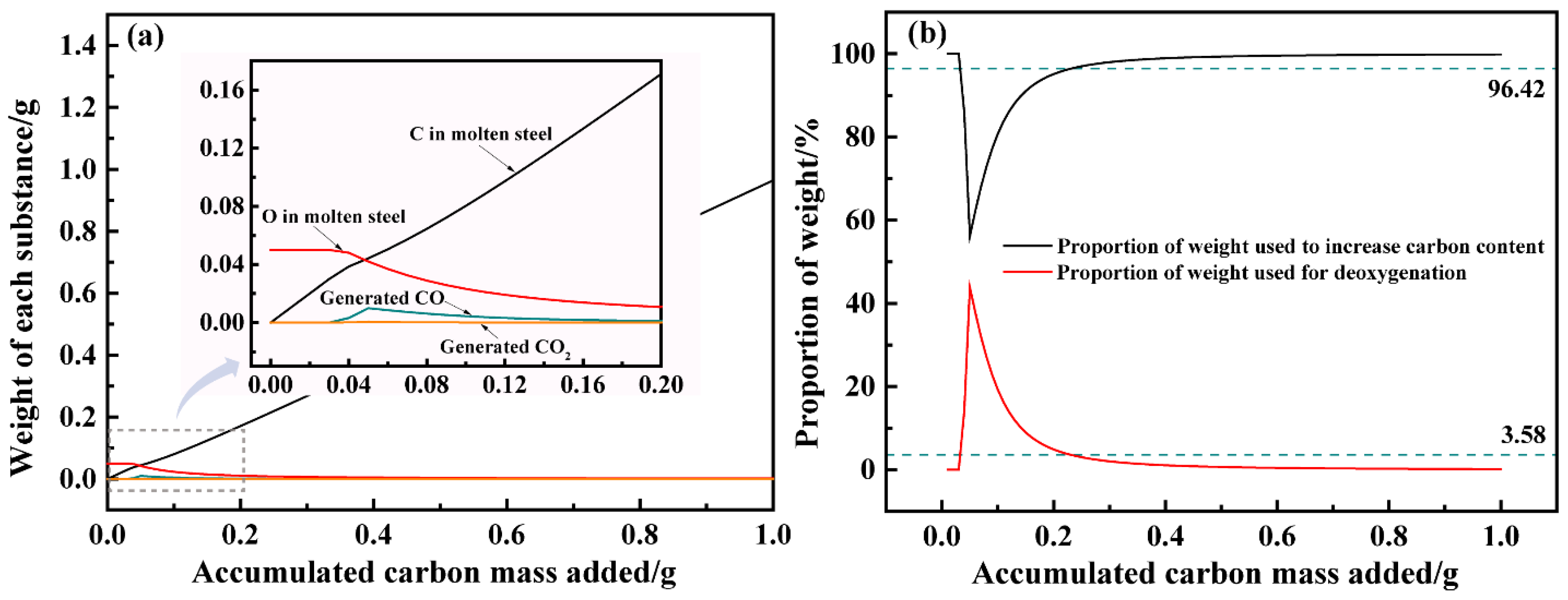
3.1.3. The Allocation Pathway of Carbon in the Carbon Deoxygenation Process
3.2. Application Effect of the Carbon Deoxidation Process in 45 Steel
3.2.1. Changes in Oxygen and Nitrogen Content in Steel
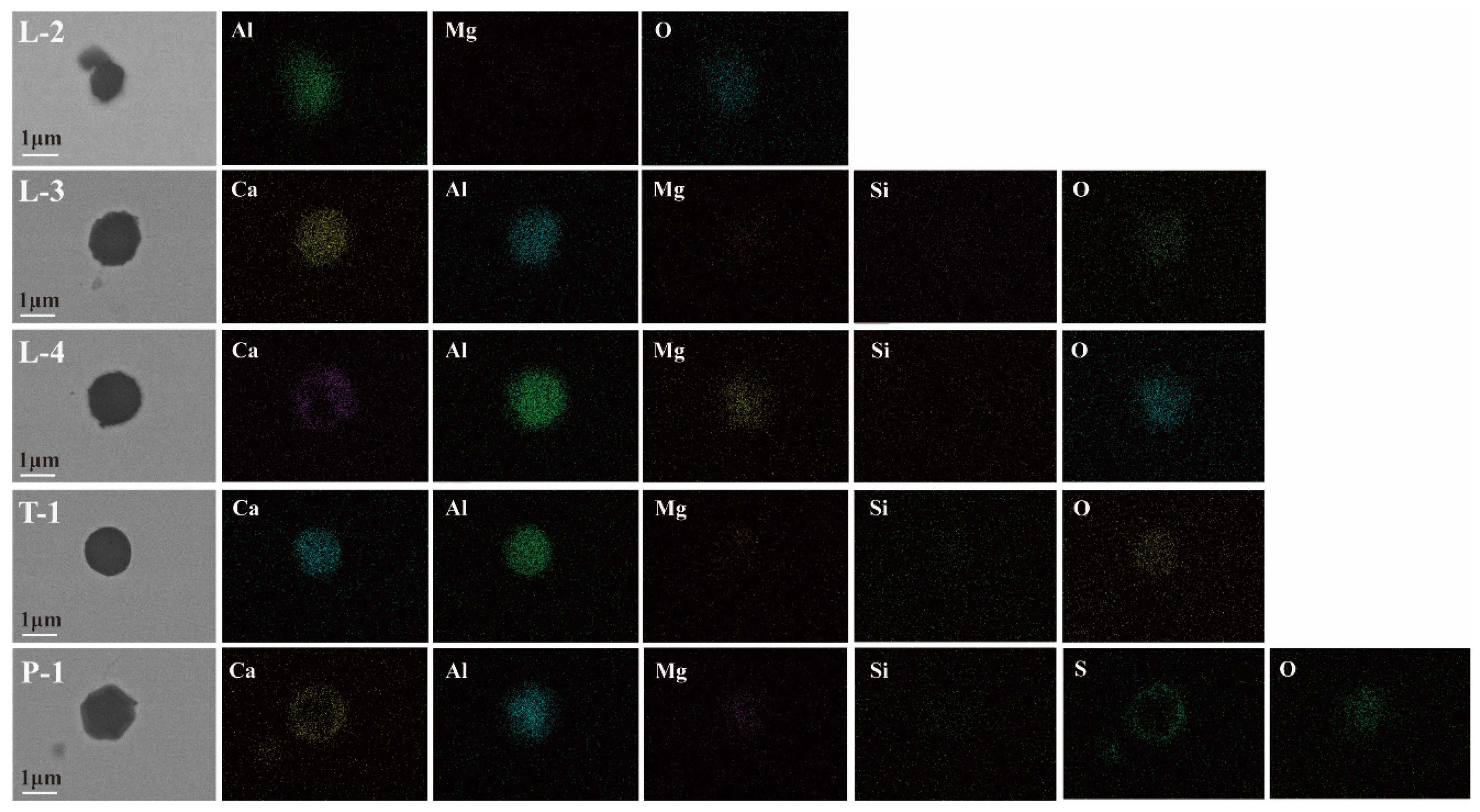
3.2.2. Study on the Morphology and Evolution of Inclusions in the Carbon Deoxidation Process
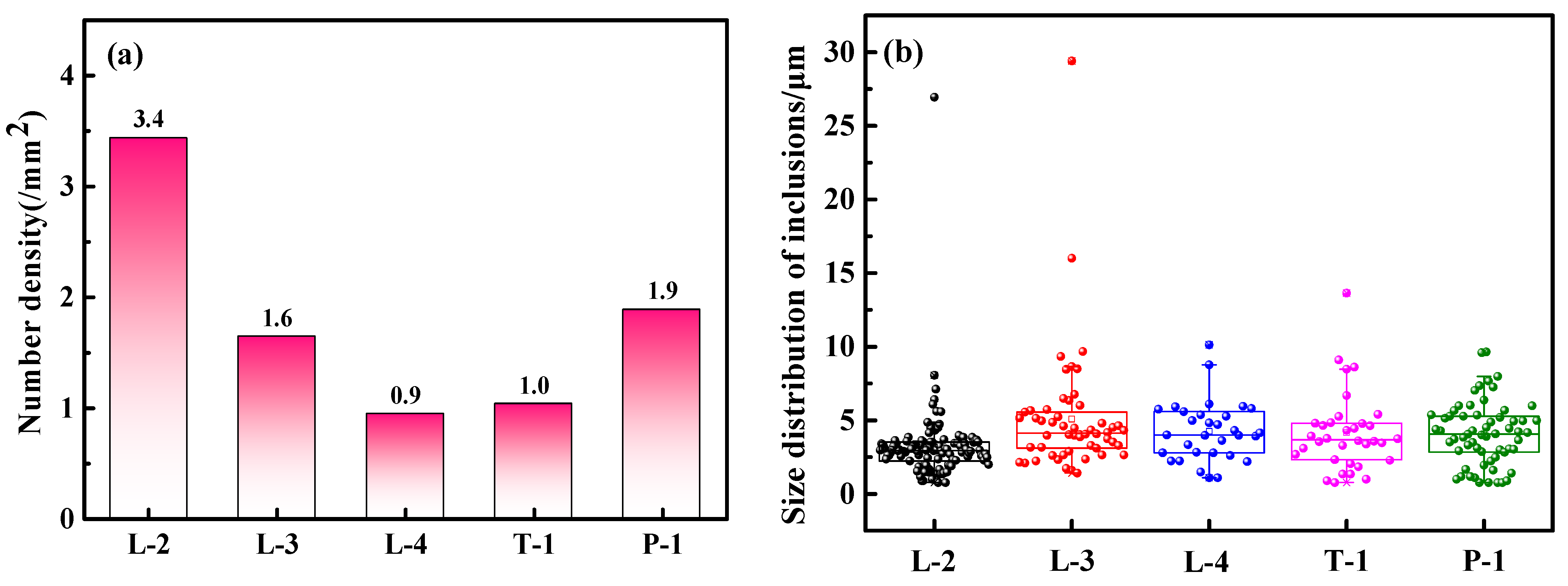
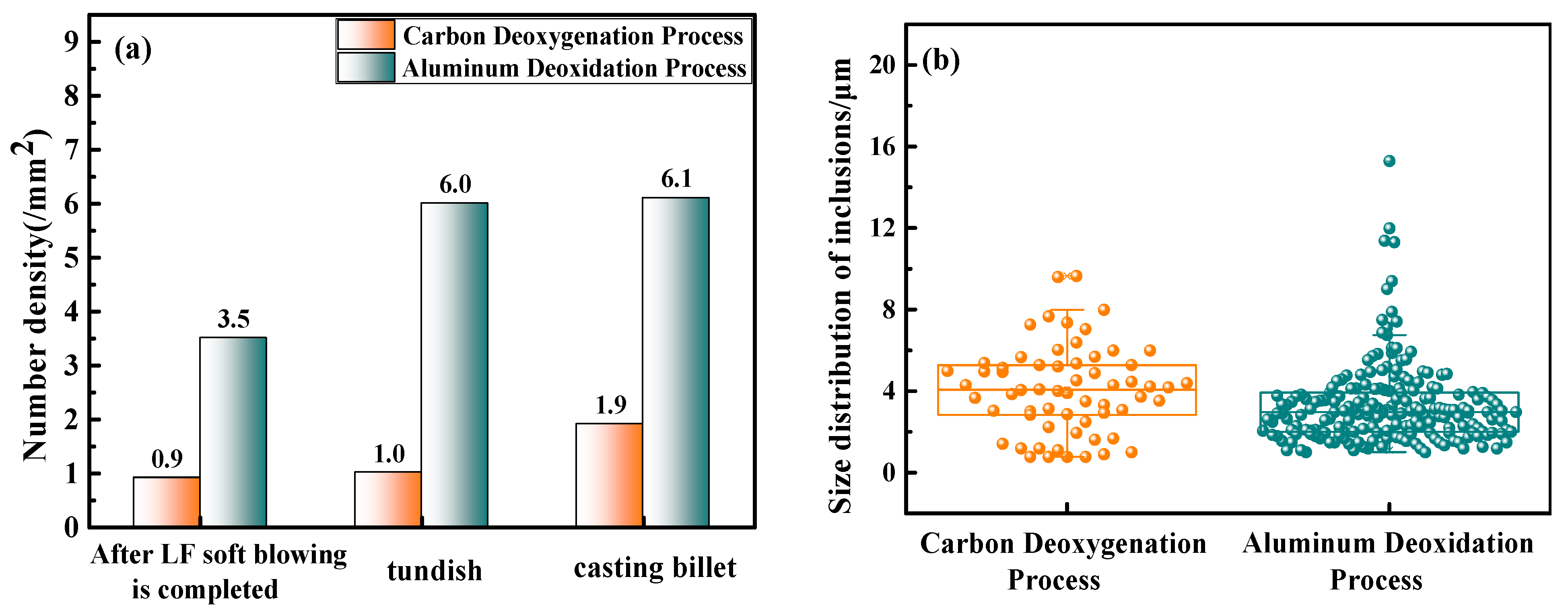
3.2.3. Comparison of the Cleanliness of Cast Billets Produced by Different Deoxidation Processes
3.2.4. Comparison of Deoxygenation Costs for Different Deoxygenation Processes
4. Conclusions
- At the steelmaking temperature, the priority of deoxidation element reaction in the molten steel is [Al] > [Si] > [C] > [Mn]. Therefore, when carbon deoxidation is carried out during steel tapping, carbon must be added to the molten steel before alloy elements such as Al and Si. Under normal pressure, the carbon deoxidation effect is greatly affected by the carbon content of the molten steel. Without vacuum treatment conditions, carbon deoxidation cannot be used as the final deoxidation method, and other such methods still need to be combined to complete the final deoxidation of molten steel.
- After reaching carbon oxygen balance in oxygen-rich molten steel, the added carbon participates in both carbonization and deoxidation. When the carbon content of the molten steel ranges from 0.038% to 0.12%, the proportion of carbon added for deoxidation is relatively high. As the dissolved oxygen content in the molten steel decreases, the carbon deoxidation rate slows down. Therefore, in actual production, measures need to be taken in the later stage of carbon deoxidation to promote the deoxidation effect.
- In the carbon deoxidation process, the final deoxidation of molten steel in LF is still completed by the Al element. Compared with the aluminum deoxidation process, the carbon deoxidation process has shown certain advantages in oxygen and nitrogen control. In addition, the evolution law of inclusions in the carbon deoxidation process can be summarized as Al-Si-Mn-O→Al-(Ca)-(Mg)-O→Ca-Al-(Mg)-(Si)-O.
- Compared with the aluminum deoxidation process, the number of inclusions in the cast billet produced by the carbon deoxidation process is reduced by 68.8%, and there are fewer large-sized inclusions in the billet. In addition, the carbon deoxidation process eliminates the addition of aluminum blocks after the converter, reducing the cost of deoxidants by CNY 15.47/ton of steel. In summary, the carbon deoxidation process has a better inclusion control effect and lower deoxidation costs.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, B.X.; Fan, K.Y.; Yin, F.X.; Feng, J.H.; Ji, P.G. Effect of caliber rolling reduction ratios on the microstructure and mechanical properties of 45 medium carbon steel. Mater. Sci. Eng. A 2020, 774, 138954. [Google Scholar] [CrossRef]
- Van Ende, M.A.; Guo, M.X.; Proost, J.; Blanpain, B.; Wollants, P. Formation and morphology of Al2O3 inclusions at the onset of liquid Fe deoxidation by Al addition. ISIJ Int. 2011, 51, 27–34. [Google Scholar] [CrossRef]
- Abdulsalam, M.; Jacobs, M.; Webler, B.A. Automated detection of non-metallic inclusion clusters in aluminum-deoxidized steel. Metall. Mater. Trans. B 2021, 52, 3970–3985. [Google Scholar] [CrossRef]
- Yang, S.-F.; Li, J.-S.; Wang, Z.-F.; Li, J.; Lin, L. Modification of MgO·Al2O3 spinel inclusions in Al-killed steel by Ca-treatment. Int. J. Miner. Metall. Mater. 2011, 18, 18–23. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, L.F.; Ren, Y.; Chen, W.; Liu, F.G. Formation and prevention of nozzle clogging during the continuous casting of steels: A review. ISIJ Int. 2024, 64, 1–20. [Google Scholar] [CrossRef]
- Gutiérrez, E.; Barreto, J.d.J.; Garcia-Hernandez, S.; Morales, R.; González-Solorzano, M.G. Decrease of nozzle clogging through fluid flow control. Metals 2020, 10, 1420. [Google Scholar] [CrossRef]
- Bharath, V.; Auradi, V.; Kumar, G.B.V.; Nagaral, M.; Chavali, M.; Helal, M.; Sami, R.; Aljuraide, N.I.; Hu, J.W.; Galal, A.M. Microstructural evolution, tensile failure, fatigue behavior and wear properties of Al2O3 reinforced Al2014 alloy T6 heat treated metal composites. Materials 2022, 15, 4244. [Google Scholar] [CrossRef]
- Alalkawi, H.J.M.; Hamdany, A.A.; Alasadi, A.A. Influence of nanoreinforced particles (Al2O3) on fatigue life and strength of aluminium based metal matrix composite. Al-Khwarizmi Eng. J. 2017, 13, 91–99. [Google Scholar] [CrossRef]
- Xiao, W.; Bao, Y.-P.; Gu, C.; Wang, M.; Liu, Y.; Huang, Y.-S.; Sun, G.-T. Ultrahigh cycle fatigue fracture mechanism of high-quality bearing steel obtained through different deoxidation methods. Int. J. Miner. Metall. Mater. 2021, 28, 804–815. [Google Scholar] [CrossRef]
- Li, Z.; Qiu, J.; Chen, Y.; Li, C.; He, F.; Zhao, K.; Lu, X.; Liu, R.; Ju, D.; Zheng, C.; et al. Physical and numerical simulation for optimization of bottom blowing arrangement of 160-ton ladle. Metall. Res. Technol. 2025, 122, 118. [Google Scholar] [CrossRef]
- Li, Z.; Ouyang, W.; Wang, Z.; Zheng, R.; Bao, Y.; Gu, C. Physical simulation study on flow field characteristics of molten steel in 70t ladle bottom argon blowing process. Metals 2023, 13, 639. [Google Scholar] [CrossRef]
- Pereira, J.A.M.; Rocha, V.C.D.; Yoshioka, A.; Bielefeldt, W.V.; Vilela, A.C.F. Analysis of secondary refining slag parameters with focus on inclusion cleanliness. Mater. Res. 2018, 21, e20180296. [Google Scholar] [CrossRef]
- Reis, B.H.; Bielefeldt, W.V.; Vilela, A.C.F. Efficiency of inclusion absorption by slags during secondary refining of steel. ISIJ Int. 2014, 54, 1584–1591. [Google Scholar] [CrossRef]
- Liu, C.; Huang, F.; Wang, X. The effect of refining slag and refractory on inclusion transformation in extra low oxygen steels. Metall. Mater. Trans. B 2016, 47, 999–1009. [Google Scholar] [CrossRef]
- Devi, S.; Singh, R.K.; Sen, N.; Pradhan, N. Study of calcium treatment in steel ladles for the modification of alumina inclusions to avoid nozzle clogging during casting. In Proceedings of the Materials Science Forum, Chennai, India, 20–21 February 2020; Trans Tech Publications Ltd.: Baech, Switzerland, 2020; Volume 978, pp. 12–20. [Google Scholar]
- Kumar, S.; Keshari, K.K.; Deva, A.; Singh, R.K.; Roy, S.; Kumar, V.; Toppo, S.; Abhishek, K.; Pradhan, N. Abrupt casting failures due to sub entry nozzle clogging in calcium treated aluminum killed steel. J. Fail. Anal. Prev. 2023, 23, 221–233. [Google Scholar] [CrossRef]
- Wang, Z.; Bao, Y. Development and prospects of molten steel deoxidation in steelmaking process. Int. J. Miner. Metall. Mater. 2024, 31, 18–32. [Google Scholar] [CrossRef]
- Watanabe, T. Deoxidation of stainless steel by carbon in laboratory-scale vacuum induction melting. J. Vac. Sci. Technol. 1970, 7, S144–S148. [Google Scholar] [CrossRef]
- Xue, Z.; Li, Z.; Zhang, J.; Gao, Y. Study on Deoxidization by Carbon in VIM Refining. Iron Steel 2023, 39, 12–14. [Google Scholar]
- Xue, Z.L.; Qi, J.H.; Zhou, G.F.; Jin, Y. Deoxidizing Law of Aluminum-Containing Steel During VIM. J. Lron Steel Res. 2007, 19, 18–20. [Google Scholar]
- Li, D.M.; Jiang, J.P. Industrial Testing Research on Vacuum Carbon Deoxidization for large Rotor Steel of Power station. Heavy Mach. Technol. 2000, 2, 32–36. [Google Scholar]
- Dong, X.L.; Gao, Y.Q.; Gao, Y.; Pang, Z.X. Application of Vacuum Carbon Deoxidation Purification Technologies to Manufacturing Large Steel Ingots. Foundry 2013, 62, 843–845. [Google Scholar]
- Ba, J.T.; Gao, J.J. Application of VOD Technology in Aluminum Deoxidation Steel. Heavy Cast. Forg. 2015, 3, 46–48. [Google Scholar]
- Xin, X.Q.; Li, J.J.; Liu, Q.; Qi, Y.X.; Xue, L.L. Application and Research on Vacuum Carbon Deoxidization Technique of 17CrNiMo6 Carburizing Gear Steel. Heavy Cast. Forg. 2019, 2, 6–7. [Google Scholar]
- GB/T 2001-2013; Coke-Determination of Proximate Analysis. Standards Press of China: Beijing, China, 2013.
- GB/T 2286-2017; Determination of Total Sulfur Composition of Coke. Standards Press of China: Beijing, China, 2017.
- Guo, H.J. Physical Chemistry of Metallurgy; Higher Education Press: Beijing, China, 2021. [Google Scholar]
- Xiao, W. Study on Key Process and Inclusion Control of GCr15 Bearing Steel Based on Non Aluminum Deoxidation. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2021. [Google Scholar]
- Zhu, M.; Deng, Z. Evolution and control of non-metallic inclusions in steel during secondary refining process. Acta Met. Sin 2021, 58, 28–44. [Google Scholar]


| Process Node | C | Si | Mn | P | S | Alt | |
|---|---|---|---|---|---|---|---|
| BOF smelting endpoint | lower limit | 0.08 | - | - | - | - | - |
| upper limit | 0.15 | - | - | 0.020 | - | - | |
| Before LF refining begins | lower limit | 0.35 | 0.10 | 0.45 | - | - | - |
| upper limit | 0.40 | 0.20 | 0.55 | - | - | - | |
| Finished product | target value | 0.46 | 0.22 | 0.62 | ≤0.025 | ≤0.012 | 0.010 |
| Testing Items | Fixed Carbon | Ash | Volatile | Moisture | S |
|---|---|---|---|---|---|
| Mass fraction/wt% | 92.14 | 5.68 | 1.62 | 0.34 | 0.22 |
| Deoxidation Element | Reaction Equation | Variation Value of Standard Gibbs Free Energy (J/mol) |
|---|---|---|
| Al | ||
| Si | ||
| Mn | ||
| C |
| Composition of Molten Steel | C/wt% | Si/wt% | Mn/wt% | Al/wt% | O/×10−6 |
|---|---|---|---|---|---|
| Smelting endpoint of the converter | 0.06 | 0.025 | 0.09 | 0.0038 | 432 |
| After LF diffusion deoxygenation | 0.34 | 0.025 | 0.09 | 0.0128 | 49 |
| Before adding calcium wire | 0.44 | 0.250 | 0.60 | 0.0140 | 19.3 |
| Different Deoxidation Processes | Deoxidant | Cost Per Ton of Steel/CNY | |||||
|---|---|---|---|---|---|---|---|
| Types | Aluminum Blocks | Calcium Carbide | Silicon Carbide | Aluminum Particles | Carbon Powder | ||
| Unit Price/(CNY/kg) | 20.02 | 3.92 | 4.54 | 20.52 | 2.64 | ||
| Aluminum deoxidation | Consumption/(kg/t) | 0.75 | 1.38 | 0.50 | 0.25 | 4.89 | 40.74 |
| Carbon deoxidation | 0 | 0.59 | 1.18 | 0.29 | 4.12 | 24.74 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, F.; Bao, Y. The Research on Carbon Deoxygenation of Molten Steel and Its Application in the Converter Steelmaking Process. Metals 2025, 15, 648. https://doi.org/10.3390/met15060648
Gao F, Bao Y. The Research on Carbon Deoxygenation of Molten Steel and Its Application in the Converter Steelmaking Process. Metals. 2025; 15(6):648. https://doi.org/10.3390/met15060648
Chicago/Turabian StyleGao, Fang, and Yanping Bao. 2025. "The Research on Carbon Deoxygenation of Molten Steel and Its Application in the Converter Steelmaking Process" Metals 15, no. 6: 648. https://doi.org/10.3390/met15060648
APA StyleGao, F., & Bao, Y. (2025). The Research on Carbon Deoxygenation of Molten Steel and Its Application in the Converter Steelmaking Process. Metals, 15(6), 648. https://doi.org/10.3390/met15060648





