Optimizing the High-Temperature Oxidation Resistance of Nb-Si-Based Alloys by Adding Different Ti/Mo/Hf Elements
Abstract
1. Introduction
2. Methods
2.1. Material Preparation
2.2. Measurement and Analysis Methods
3. Results and Discussion
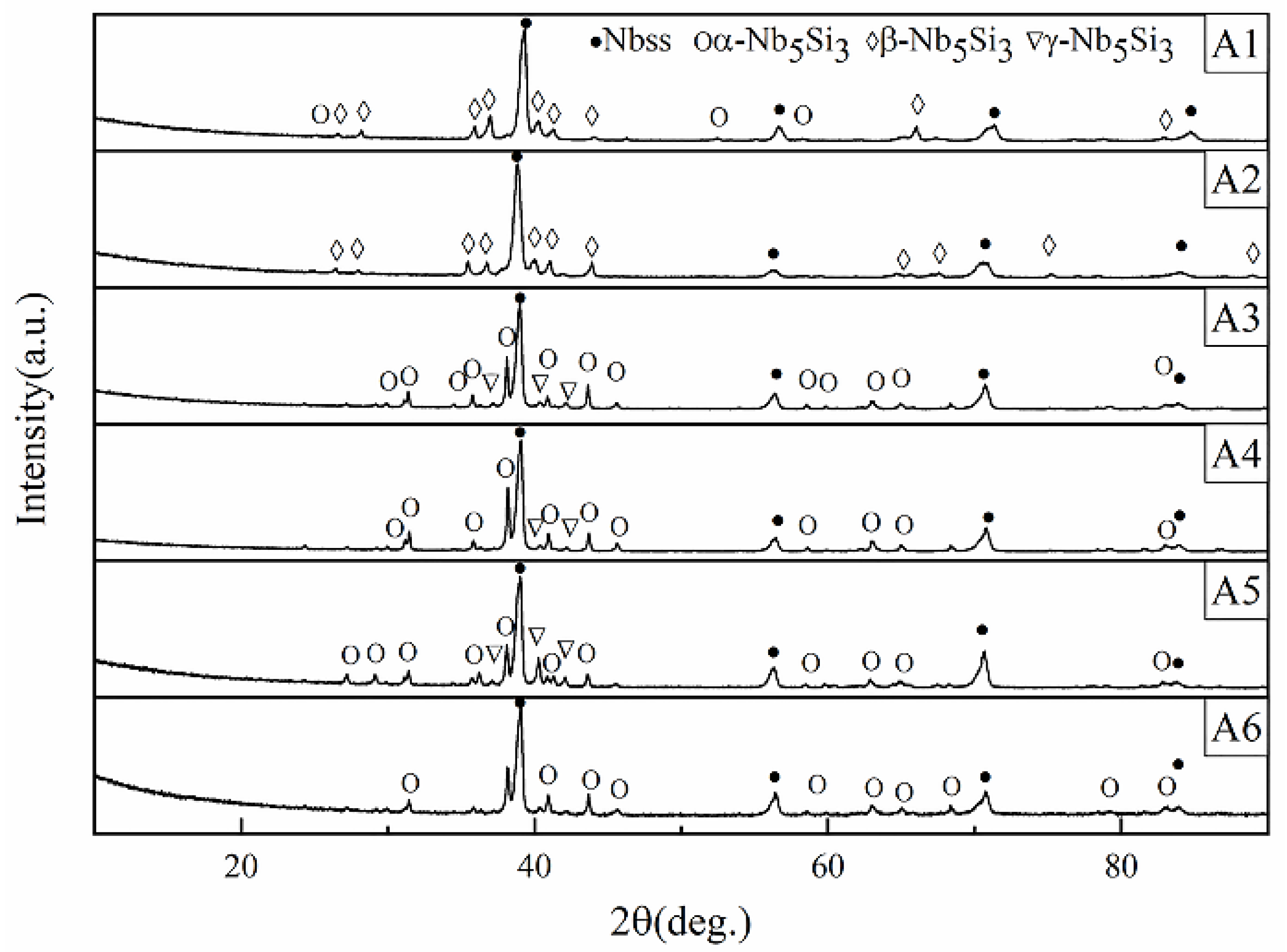
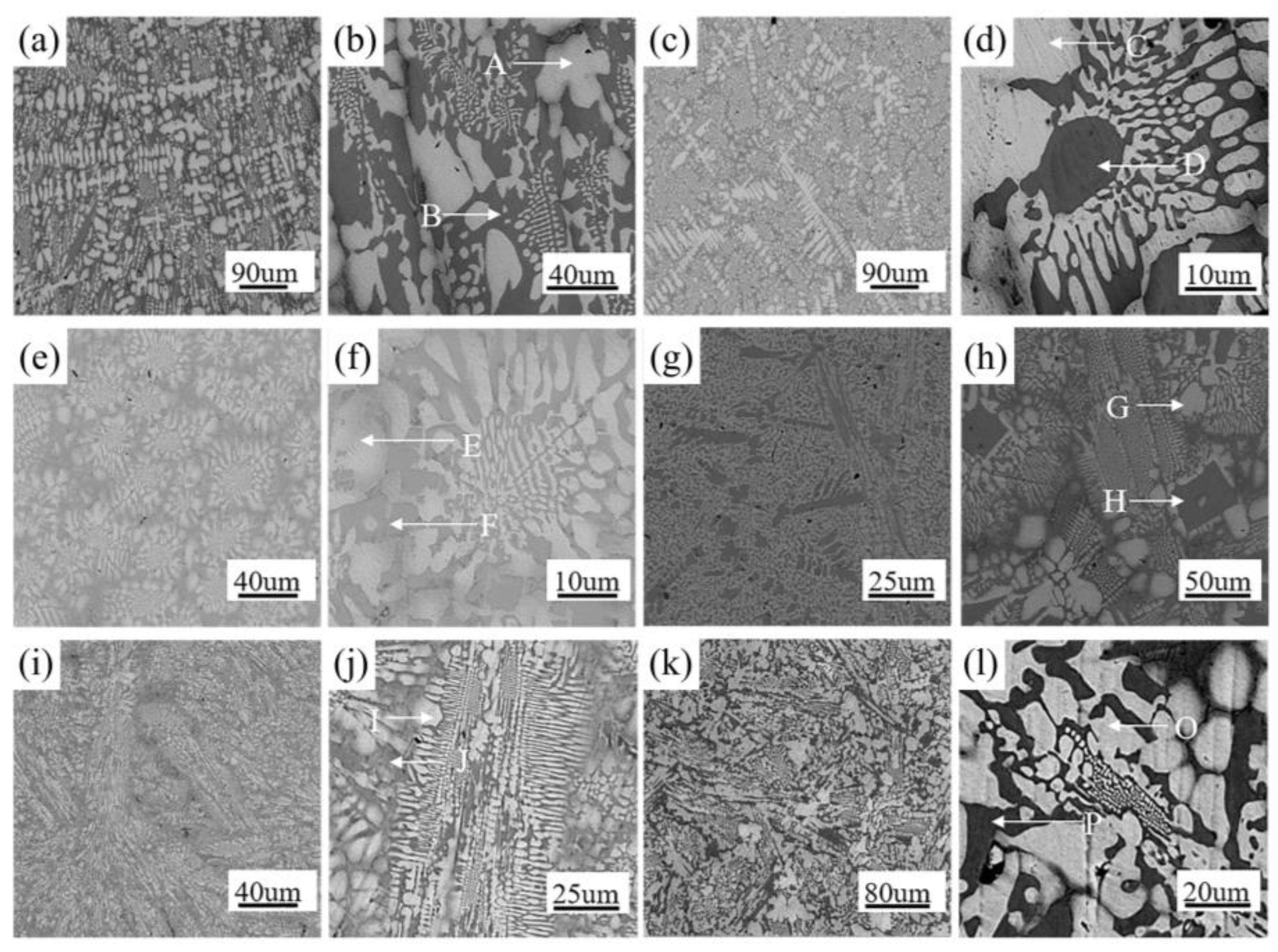
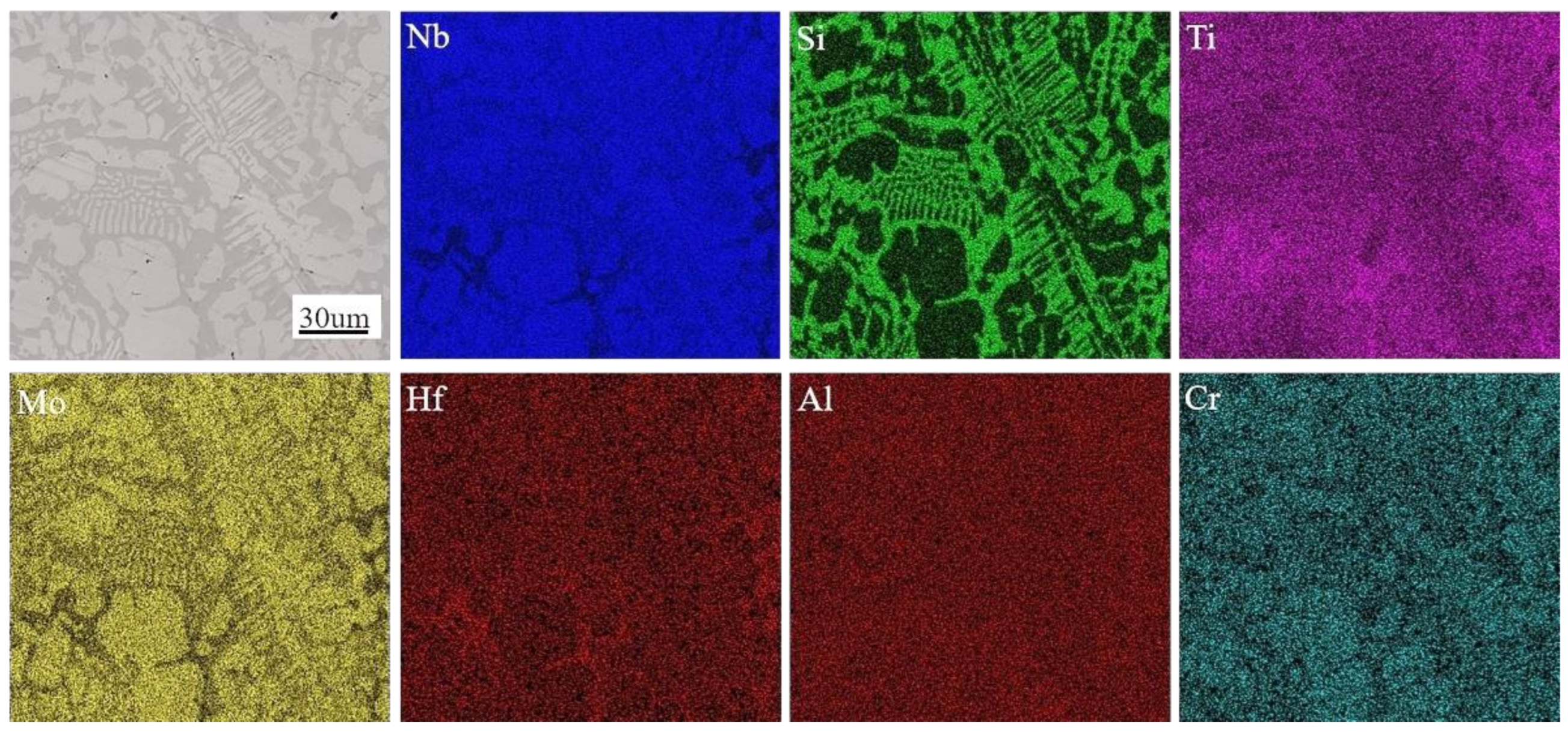
3.1. Microstructural Evolution Induced by Different Chemical Compositions in Nb-Si-Ti-Mo-Hf-Al-Cr Alloys
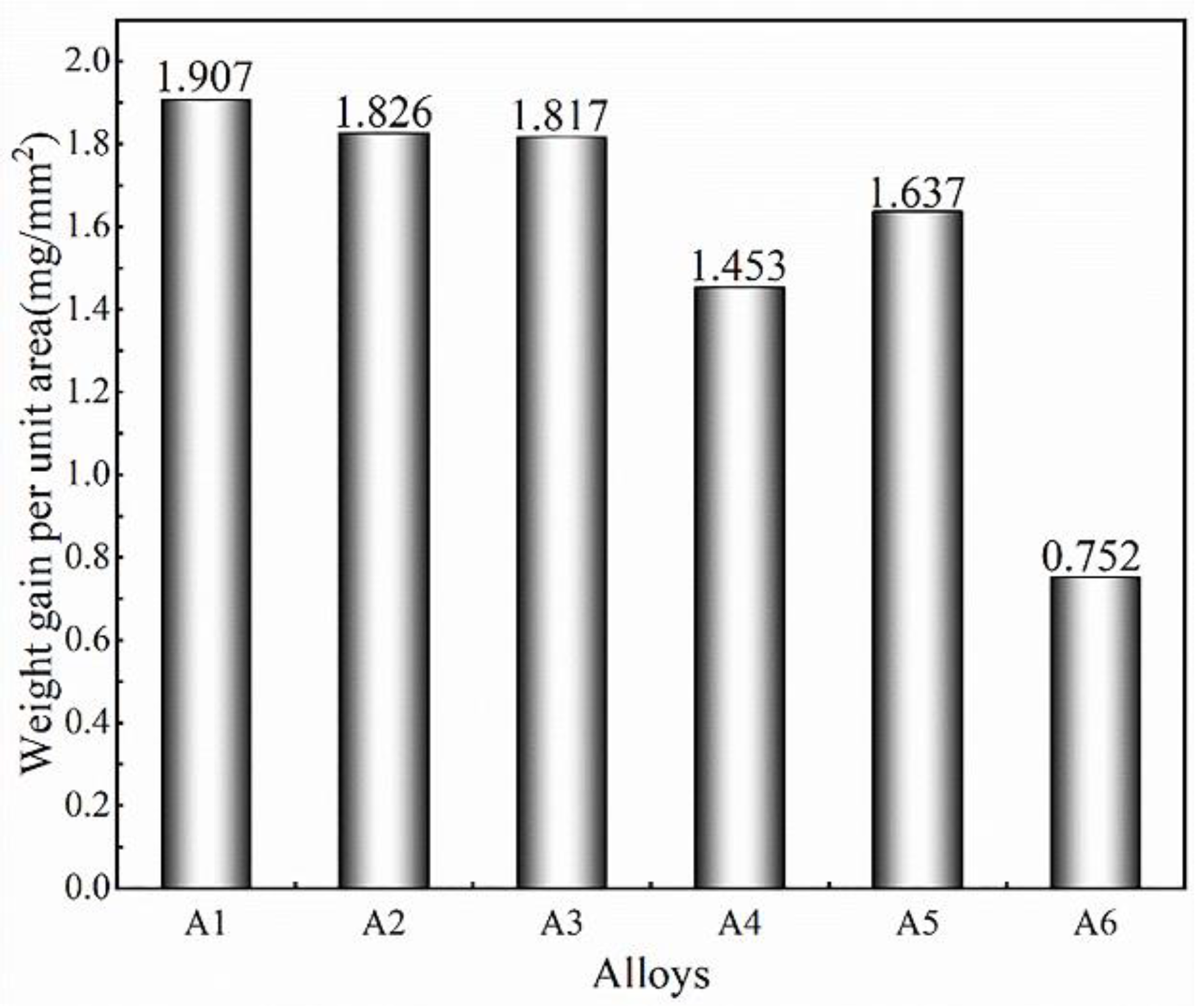
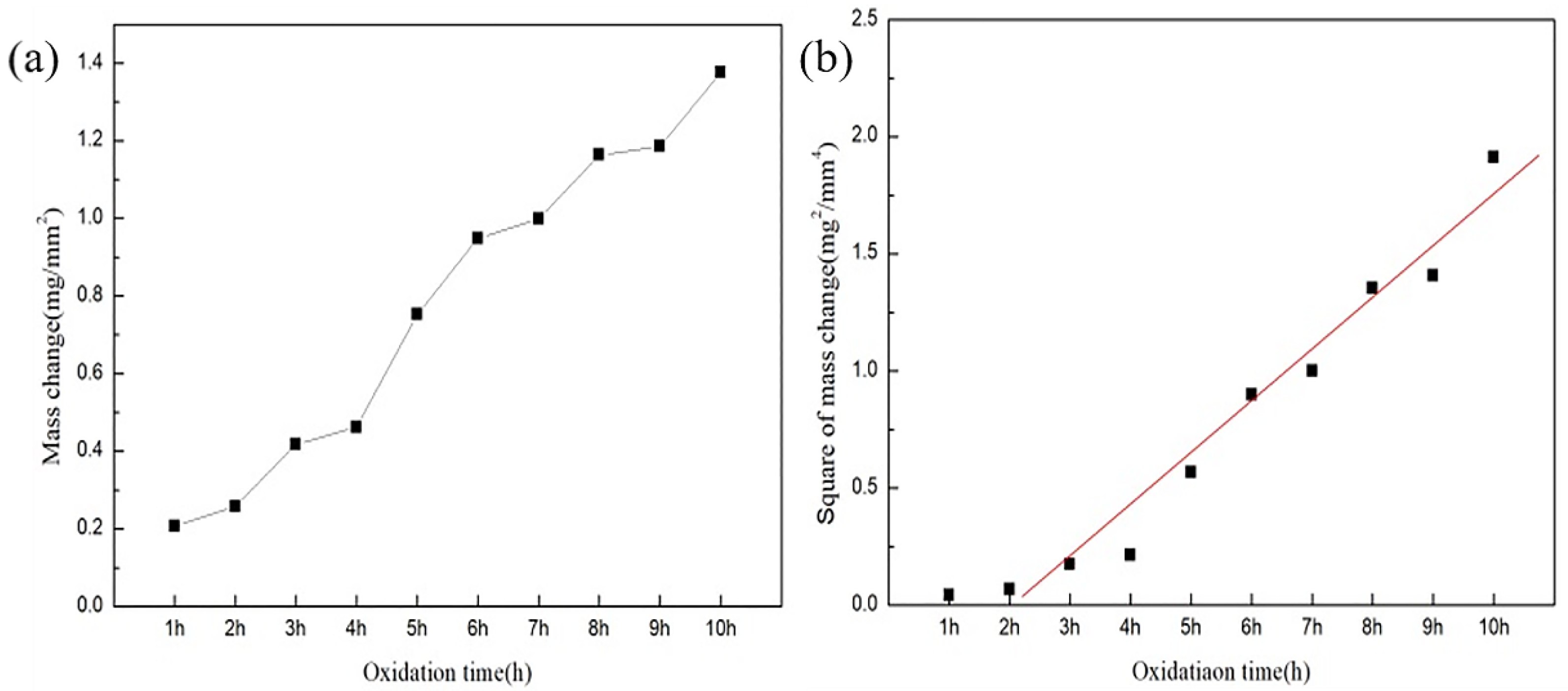
3.2. Oxidation Behaviour and Oxidation Kinetics of Alloys at 1523 K for Different Time Periods
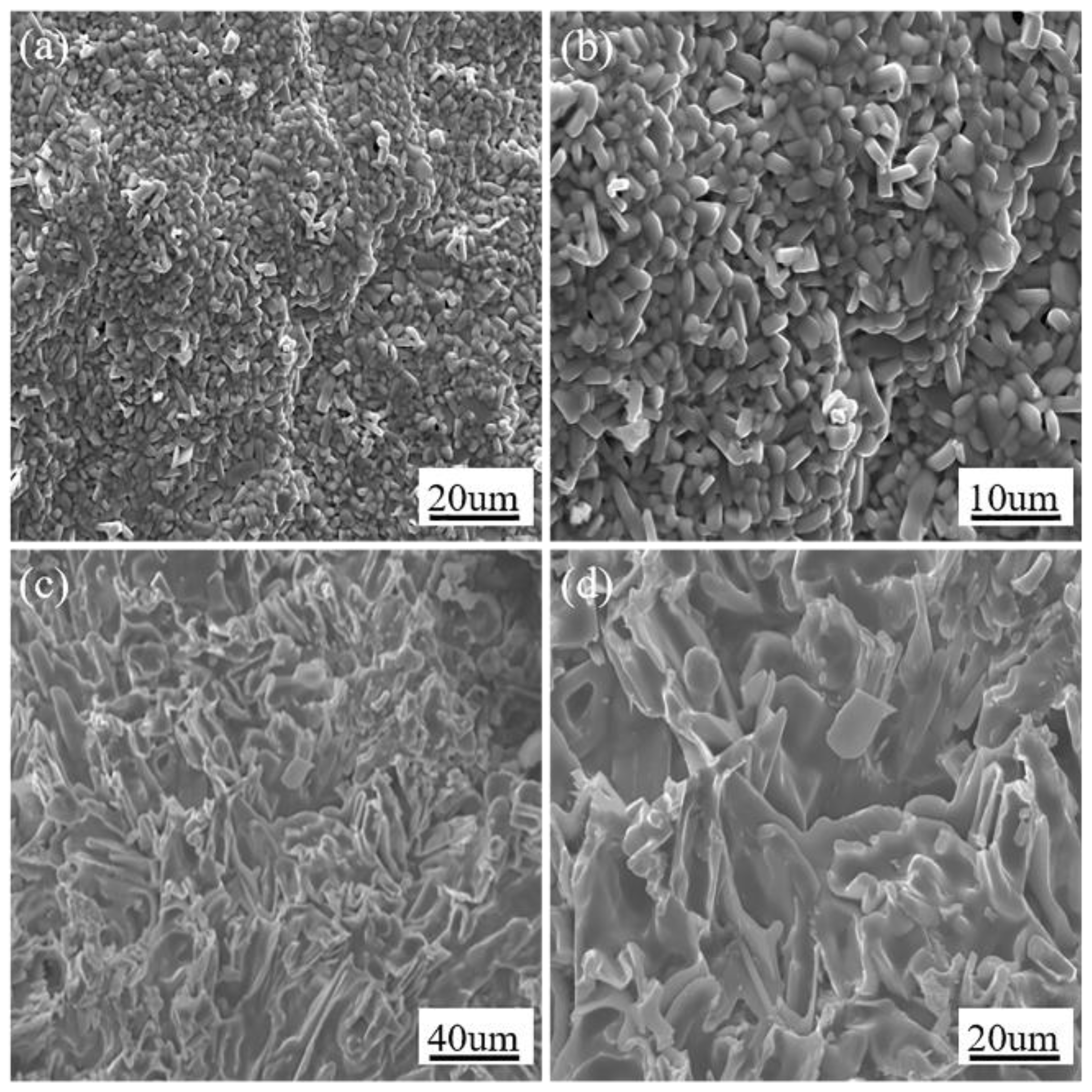
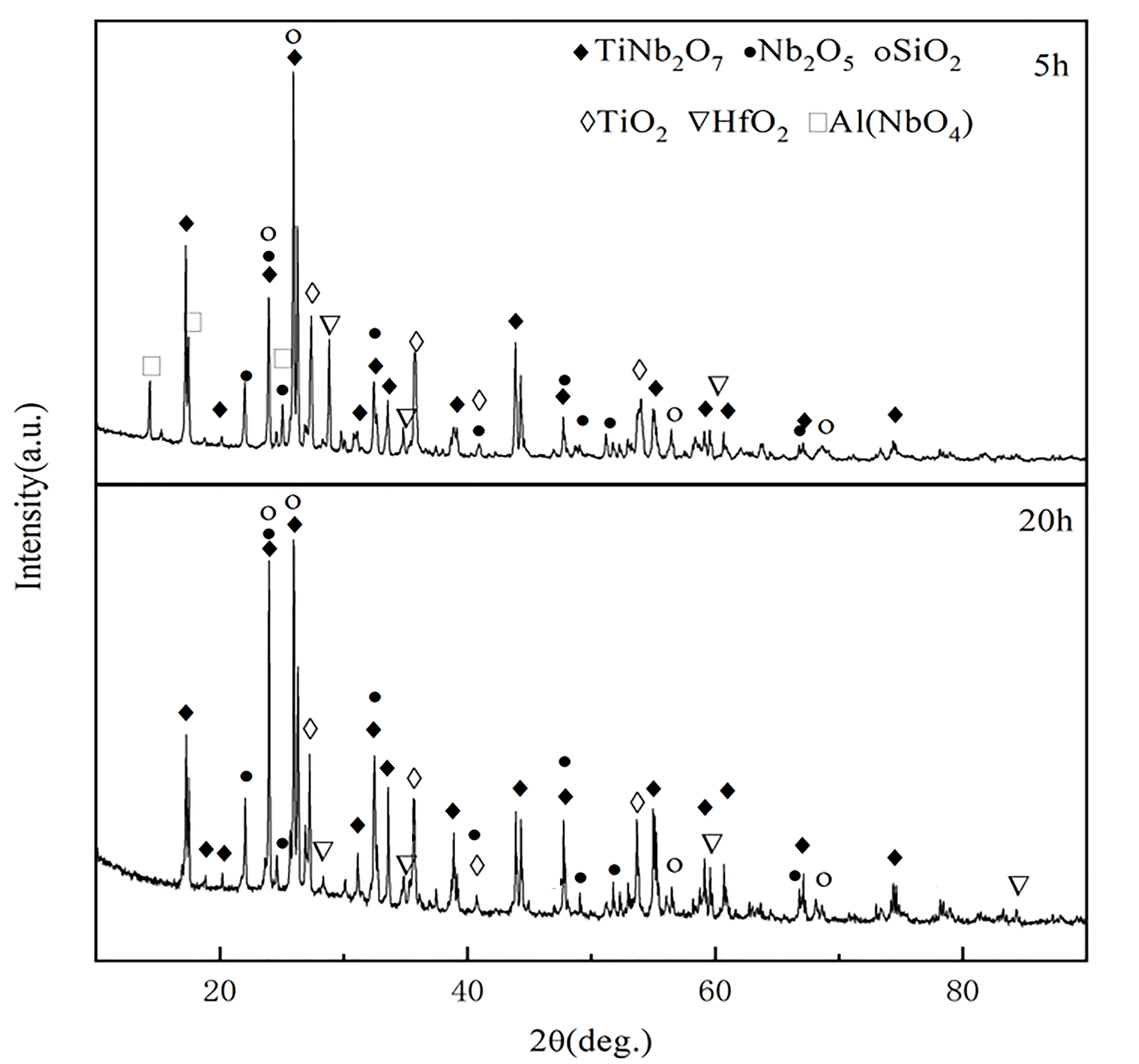
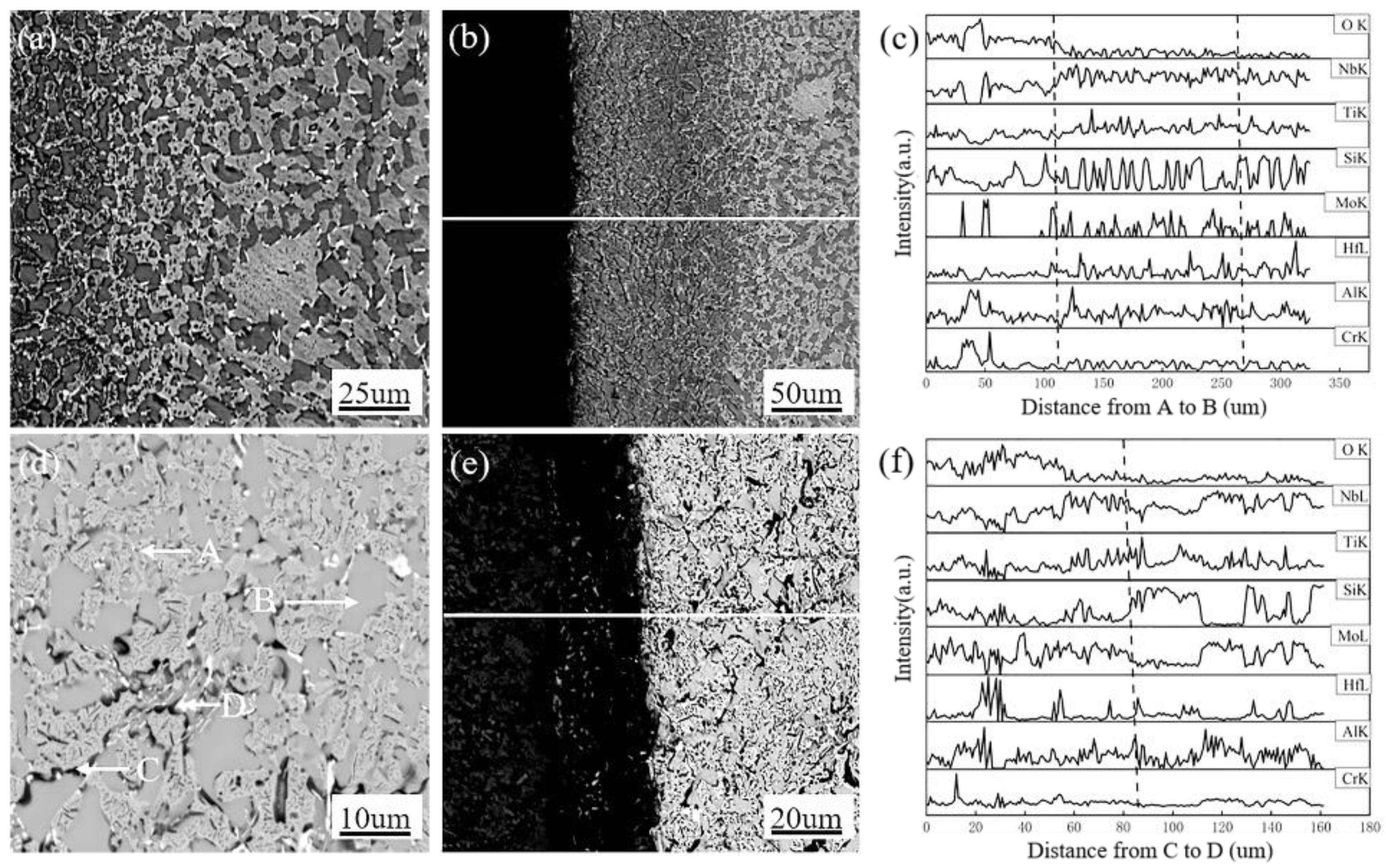
3.3. Morphologies and Microstructure of Oxide Scale and Interior Alloys
4. Conclusions
- (1)
- We mainly identified Nbss and Nb5Si3 and a small amount of metastable phase (Nb3Si) in the Nb-Si-Ti-Mo-Hf-Al-Cr alloy. These alloying elements (Ti, Hf, Mo) can eliminate the metastable phase. The Mo element has the function of stabilizing β-Nb5Si3, while the presence of Hf can refine the primary Nbss in the alloy, and the Ti element can promote the formation of α-Nb5Si3.
- (2)
- Nb-Si-Ti-Mo-Hf-Al-Cr alloy oxidizes at 1523 K at different times. The oxidation products are TiNb2O7, Nb2O5, SiO2, TiO2, and HfO2, respectively. During the oxidation process, a solid phase reaction occurs: Nb2O5 + TiO2→TiNb2O7. The formation of TiNb2O7 can effectively inhibit oxygen diffusion, while Hf consumes oxygen via a solid solution.
- (3)
- The oxidation resistance of Nb-Si-Ti-Mo-Hf-Al-Cr alloys is primarily affected by solid solution elements. When the alloy is oxidized, the Nb5Si3 shows extreme oxidation resistance, but O cracks quickly corrode the Nbss and are generated due to the deformation stress between the Nbss and Nb5Si3, which exacerbates the oxidation of the internal alloy. Hf can rapidly migrate to the Nbss/Nb5Si3 interface to form HfO2, which consumes O, preventing O from diffusing into the internal alloy and effectively improving the oxidation resistance of the alloy. Optimizing the Ti/Hf/Mo ratios synergistically can effectively improve the oxidation resistance and mechanical properties of alloys.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, B.; Zhou, X.; Shuitcev, A.; Zarinejad, M.; Tong, Y. Effects of Nb addition on the microstructure and martensitic transformation in NiTiHf-based high-temperature shape memory alloys. Intermetallics 2025, 182, 108790. [Google Scholar] [CrossRef]
- Zhu, E.; Xia, D.; Chen, P.; Han, Q.; Wang, X.; Liu, Z.; Jiang, K. Study on the Effect of Niobium on the High Temperature Oxidation Resistance of Ferritic Stainless Steel. Metals 2024, 14, 25. [Google Scholar] [CrossRef]
- Zhang, W.; Qiao, Y.; Guo, X.; Li, L.; Nan, X. Excellent oxidation resistance: A dense and well-bonded MoSi2/(Nb,X)Si2 composite coating on Nb-Si based alloy. Corros. Sci. 2025, 249, 112863. [Google Scholar] [CrossRef]
- Hou, Q.; Li, M.; Chen, Z.; Zhou, C. Effect of TiN diffusion barrier on interdiffusion between Mo–Si–B coating and Nb–Si based alloy. Mater. Chem. Phys. 2025, 337, 130584. [Google Scholar] [CrossRef]
- Huang, Y.; Jia, L.; Kong, B.; Guo, Y.; Wang, N. Microstructure and room temperature fracture toughness of Nb–Si-based alloys with Sr addition. Rare Met. 2024, 43, 3904–3912. [Google Scholar] [CrossRef]
- Yue, G.; Guo, X.; Qiao, Y.; Luo, F. Isothermal oxidation and interdiffusion behavior of MoSi2/WSi2 compound coating on Nb-Ti-Si based alloy. Appl. Surf. Sci. 2020, 504, 144477. [Google Scholar] [CrossRef]
- Yin, X.; Liang, J.; Zhang, X.; Wang, C.; Chen, S.; Shang, S.; Liu, C. Improving the mechanical properties and oxidation resistance of Nb-16Si-20Ti alloys with B4C addition. Int. J. Refract. Met. Hard Mater. 2024, 119, 106543. [Google Scholar] [CrossRef]
- Liu, W.; Huang, S.; Ye, C.; Jia, L.; Kang, Y.; Sha, J.; Chen, B.; Wu, Y.; Xiong, H. Progress in Nb-Si ultra-high temperature structural materials: A review. J. Mater. Sci. Technol. 2023, 149, 127–153. [Google Scholar] [CrossRef]
- Holgate, C.; Frey, C.; Endsley, M.; Suzuki, A.; Levi, C.; Pollock, T. Design of an alumina forming coating for Nb-base refractory alloys. Mater. Des. 2025, 251, 113652. [Google Scholar]
- Guo, Y.; Jia, L.; Su, H.; Zhang, F.; Peng, H.; Zhang, H. Surface Remelting-Mediated Improvement in Oxidation Resistance of Cr2Nb-Containing Nb-Si-Based Alloys at High Temperatures. Adv. Eng. Mater. 2019, 21, 1900425. [Google Scholar] [CrossRef]
- Li, Z.; Luo, L.; Wang, B.; Su, B.; Luo, L.; Wang, L.; Su, Y.; Guo, J.; Fu, H. Cooperative effects of Mo and B additions on the microstructure and mechanical properties of multi-elemental Nb-Si-Ti based alloys. Mater. Charact. 2023, 206, 113421. [Google Scholar]
- Geng, J.; Tsakiropoulos, P. A study of the microstructures and oxidation of Nb-Si-Cr-Al-Mo in situ composites alloyed with Ti, Hf and Sn. Intermetallics 2007, 15, 382–395. [Google Scholar] [CrossRef]
- Yu, J.; Chen, D.; Xu, F.; Wang, S.; Cui, X.; Gong, W.; Chen, R. Simultaneous improving high temperature oxidation resistance and room temperature toughness of Nb–Si based alloy via appropriate Ta content addition strategy. Int. J. Refract. Met. Hard Mater. 2024, 124, 106800. [Google Scholar]
- Zhang, W.; Qiao, Y.; Guo, X.; Li, L.; Nan, X. Microstructure characteristics and oxidation behavior of a dense Si-Cr-Fe-Ti coating with a multi-layer structure on Nb-Si based alloy by slurry sintering. Corros. Sci. 2025, 246, 112743. [Google Scholar] [CrossRef]
- Perrin, A.; Fernandez-Zelaia, P.; Ledford, C.; Lin, Y.; Berry, E.; Dehoff, R.; Kirka, M.; Yang, Y. Design of Silicide-Strengthened Nb–Si–Cr–(Mo) alloys for additive manufacturing. Mater. Des. 2025, 251, 113616. [Google Scholar] [CrossRef]
- Guo, Y.; He, J.; Li, Z.; Jia, L.; Su, H.; Zhang, J.; Zhang, H. Tuning microstructures and improving oxidation resistance of Nb-Si based alloys via electron beam surface melting. Corros. Sci. 2020, 163, 108281. [Google Scholar] [CrossRef]
- Grammenos, I.; Tsakiropoulos, P. Study of the role of Al, Cr and Ti additions in the microstructure of Nb-18Si-5Hf base alloys. Intermetallics 2010, 18, 242–253. [Google Scholar]
- Zelenitsas, K.; Tsakiropoulos, P. Study of the role of Al and Cr additions in the microstructure of Nb-Ti-Si in situ composites. Intermetallics 2005, 13, 1079–1095. [Google Scholar] [CrossRef]
- Su, Z.; Liu, B.; Zhang, Y.; Song, J.; Qian, B.; Liu, W.; Sun, S.; Qiu, J.; Dai, Y. Precision processing of Nb-Si alloy via water-jet guided laser: Realization of inhibited-oxidation and small-taper. Opt. Laser Technol. 2025, 187, 112853. [Google Scholar] [CrossRef]
- Tian, X.; Guo, X. Structure and oxidation behavior of Si-Y co-deposition coatings on an Nb silicide based ultrahigh temperature alloy prepared by pack cementation technique. Surf. Coat. Technol. 2009, 204, 313–318. [Google Scholar] [CrossRef]
- Wen, S.H.; Zhou, C.G.; Sha, J.B. Microstructural evolution and oxidation behaviour of Mo-Si-B coatings on an Nb-16Si-22Ti-7Cr-2Al-2Hf alloy at 1250 °C prepared by spark plasma sintering. Surf. Coat. Technol. 2018, 352, 320–329. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, X. Preparation and oxidation resistance of silicide/aluminide composite coatings on an Nb-Ti-Si based alloy. Surf. Coat. Technol. 2015, 274, 18–25. [Google Scholar] [CrossRef]
- Hou, Q.; Li, M.; Shao, W.; Zhou, C. Oxidation and interdiffusion behavior of Mo-Si-B coating on Nb-Si based alloy prepared by spark plasma sintering. Corros. Sci. 2020, 169, 108638. [Google Scholar] [CrossRef]
- Pang, J.; Wang, W.; Zhou, C. Microstructure evolution and oxidation behavior of B modified MoSi2 coating on Nb–Si based alloys. Corros. Sci. 2016, 105, 1–7. [Google Scholar]
- Wang, W.; Yuan, B.; Zhou, C. Formation and oxidation resistance of germanium modified silicide coating on Nb based in situ composites. Corros. Sci. 2014, 80, 164–168. [Google Scholar]
- Grammenos, I.; Tsakiropoulos, P. Study of the role of Hf, Mo and W additions in the microstructure of Nb-20Si silicide based alloys. Intermetallics 2011, 19, 1612–1621. [Google Scholar]
- Cheng, G.M.; He, L.L. Microstructure evolution and room temperature deformation of a unidirectionally solidified Nb-22Ti-16Si-3Ta-2Hf-7Cr-3Al-0.2Ho (at.%) alloy. Intermetallics 2011, 19, 196–201. [Google Scholar] [CrossRef]
- Gao, R.; Peng, H.; Guo, H.; Chen, B. Additively manufactured Nb-Ti-Si based alloy: As-built and heat-treated conditions. Add. Manuf. Lett. 2024, 11, 100242. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Chen, R.; Wang, X.; Su, Y.; Fu, H. Gadolinium doping induced the microstructure evolution and mechanical properties improvement of Nb-Si based in-situ superalloy. J. Alloys Compd. 2024, 1004, 175942. [Google Scholar] [CrossRef]
- Sun, Z.; Guo, X.; He, Y.; Guo, J.; Yang, Y.; Chang, Y. Investigation on the as-cast microstructure of Nb silicide based multicomponent alloys. Intermetallics 2010, 18, 992–997. [Google Scholar] [CrossRef]
- Tian, Y.X.; Guo, J.T.; Sheng, L.; Cheng, G.; Zhou, L.; He, L.; Ye, H. Microstructures and mechanical properties of cast Nb-Ti-Si-Zr alloys. Intermetallics 2008, 16, 807–812. [Google Scholar]
- He, D.; Feng, Q.; Fu, Y.; Zhang, M.; Chen, G.; Gao, P.; Li, C. Erosion behaviors of BaZrO3 and BaZrO3/Y2O3 dual-phase refractories within Nb–Si melts during vacuum induction melting. J. Mater. Res. Technol. 2024, 33, 9726–9734. [Google Scholar] [CrossRef]
- Cheng, J.; Yi, S.; Park, J.S. Oxidation behaviors of Nb-Si-B ternary alloys at 1100°C under ambient atmosphere. Intermetallics 2012, 23, 12–19. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Q.; Chen, R.; Wang, X.; Zou, Y.; Su, Y.; Fu, H. Formation of near core-shell-like structure and dual-phase nanoprecipitation behavior in Nb-Si-Ti based alloys. Compos. Part B Eng. 2024, 283, 111634. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, B.; Zhou, C. Formation and oxidation resistance of Hf and Al modified silicide coating on Nb-Si based alloy. Corros. Sci. 2014, 86, 304–309. [Google Scholar] [CrossRef]
- Xu, J.; Shi, Z.; Zhang, Z.; Huang, H.; Liu, X. Significant enhancement of high temperature oxidation resistance of pure titanium via minor addition of Nb and Si. Corros. Sci. 2020, 166, 108430. [Google Scholar]
- Geng, T.; Li, C.; Zhao, X.; Xu, H.; Guo, C.; Du, Z. Experimental study on the as-cast solidification of the Si-rich alloys of the Nb-Si-Mo ternary system. Intermetallics 2010, 18, 1007–1015. [Google Scholar] [CrossRef]
- Su, L.; Jia, L.; Jiang, K.; Zhang, H. The oxidation behavior of high Cr and Al containing Nb-Si-Ti-Hf-Al-Cr alloys at 1200 and 1250 °C. Int. J. Refract. Met. Hard Mater. 2017, 69, 131–137. [Google Scholar] [CrossRef]
- Nelson, J.; Ghadyani, M.; Utton, C.; Tsakiropoulos, P. A Study of the Effects of Al, Cr, Hf, and Ti Additions on the Microstructure and Oxidation of Nb-24Ti-18Si Silicide Based Alloys. Materials 2018, 11, 1579. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, X. Re-Melting Nb–Si-Based Ultrahigh-Temperature Alloys in Ceramic Mold Shells. Metals 2019, 9, 721. [Google Scholar] [CrossRef]
- Lefez, B.; Jouen, S.; Hannoyer, B.; Bacos, M.P.; Beucher, E. Oxidation behaviour of the 47Nb-16Si-25Ti-8Hf-2Al-2Cr alloy sheet and vibrational spectroscopy. Mater. High Temp. 2014, 26, 15–20. [Google Scholar]
- Esparza, N.; Rangel, V.; Gutierrez, A.; Arellano, B.; Varma, S.K. A comparison of the effect of Cr and Al additions on the oxidation behaviour of alloys from the Nb-Cr-Si system. Mater. High Temp. 2016, 33, 105–114. [Google Scholar] [CrossRef]
- Geng, J.; Tsakiropoulos, P.; Shao, G. Oxidation of Nb-Si-Cr-Al in situ composites with Mo, Ti and Hf additions. Mater. Sci. Eng. A 2006, 441, 26–38. [Google Scholar] [CrossRef]
- Jiang, W.; Shao, W.; Sha, J.; Zhou, C. Experimental studies and modeling for the transition from internal to external oxidation of three-phase Nb-Si-Cr alloys. Prog. Nat. Sci. Mater. Int. 2018, 28, 740–748. [Google Scholar]
- Sun, L.; Fu, Q.G.; Fang, X.Q.; Sun, J. A MoSi2-based composite coating by supersonic atmospheric plasma spraying to protect Nb alloy against oxidation at 1500 °C. Surf. Coat. Technol. 2018, 352, 182–190. [Google Scholar] [CrossRef]
- Andreev, D.; Vdovin, Y.; Yukhvid, V.; Golosova, O. Mo–Nb–Si–B Alloy: Synthesis, Composition, and Structure. Metals 2021, 11, 803. [Google Scholar] [CrossRef]
- Yang, T.; Guo, X. Effects of Nb Content on the Mechanical Alloying Behavior and Sintered Microstructure of Mo-Nb-Si-B Alloys. Metals 2019, 9, 653. [Google Scholar] [CrossRef]


| No. | Si | Ti | Mo | Hf | Al | Cr | Nb |
|---|---|---|---|---|---|---|---|
| A1 | 16 | 18 | 10 | 5 | 2 | 2 | Bal |
| A2 | 16 | 20 | 15 | 1 | 2 | 2 | Bal |
| A3 | 16 | 22 | 5 | 3 | 2 | 2 | Bal |
| A4 | 18 | 20 | 5 | 3 | 2 | 2 | Bal |
| A5 | 16 | 20 | 5 | 5 | 2 | 2 | Bal |
| A6 | 16 | 20 | 5 | 3 | 2 | 2 | Bal |
| Energy Spectrum Point | Nb | Si | Ti | Hf | Mo | Al | Cr |
|---|---|---|---|---|---|---|---|
| A | 39.7 | 19.1 | 19.0 | 3.0 | 15.4 | 1.4 | 2.4 |
| B | 38.2 | 39.3 | 11.9 | 5.2 | 4.7 | 0.5 | 0.3 |
| C | 45.5 | 2.8 | 17.2 | 2.6 | 27.5 | 2.0 | 2.5 |
| D | 29.1 | 36.3 | 24.4 | 3.3 | 4.3 | 1.4 | 1.4 |
| E | 45.2 | 3.8 | 17.0 | 4.4 | 9.7 | 1.5 | 18.5 |
| F | 21.4 | 38.0 | 25.1 | 7.8 | 1.8 | 1.4 | 4.4 |
| G | 61.4 | 5.2 | 13.9 | 2.0 | 14.4 | 1.6 | 1.5 |
| H | 42.0 | 39.4 | 10.6 | 3.3 | 2.5 | 1.8 | 0.4 |
| I | 55.4 | 6.1 | 18.3 | 3.4 | 12.4 | 2.5 | 2.1 |
| J | 37.8 | 38.8 | 11.0 | 5.2 | 4.8 | 1.7 | 0.7 |
| O | 58.5 | 4.7 | 18.2 | 2.6 | 11.7 | 2.0 | 2.3 |
| P | 38.8 | 36.2 | 14.1 | 3.9 | 5.8 | 0.8 | 0.5 |
| Oxidation Time | 1 h | 2 h | 3 h | 4 h | 5 h | 6 h | 7 h | 8 h | 9 h | 10 h |
|---|---|---|---|---|---|---|---|---|---|---|
| thickness (um) | 23 | 77.9 | 116.5 | 129.5 | 144.5 | 188.5 | 243 | 323 | 429 | 438 |
| Oxidation weight gain (mg/mm2) | 0.21 | 0.26 | 0.42 | 0.46 | 0.75 | 0.95 | 1.00 | 1.16 | 1.19 | 1.38 |
| Square of oxidation weight gain (mg2/mm4) | 0.04 | 0.07 | 0.18 | 0.21 | 0.57 | 0.90 | 1.00 | 1.36 | 1.41 | 1.90 |
| Spectrum Point | O | Nb | Si | Ti | Hf | Mo | Al | Cr |
|---|---|---|---|---|---|---|---|---|
| A | 2.8 | 38.7 | 37.1 | 11.8 | 3.4 | 4.2 | 1.5 | 0.5 |
| B | 11.0 | 59.2 | 4.7 | 7.3 | 1.0 | 12.0 | 1.7 | 3.3 |
| C | 14.2 | 29.9 | 23.3 | 22.0 | 2.6 | 4.4 | 2.6 | 1.1 |
| D | 19.2 | 21.3 | 27.5 | 12.5 | 15.5 | 1.9 | 1.5 | 0.5 |
| Element | Nb | Si | Ti | Hf | Mo | Al | Cr |
|---|---|---|---|---|---|---|---|
| Highest valence oxide | Nb2O5 | SiO2 | TiO2 | HfO2 | MoO3 | Al2O3 | Cr2O3 |
| Electronegativity | 1.41 | 1.91 | 1.38 | 1.16 | 1.47 | 1.61 | 1.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Shan, Z.; Luo, L.; Li, Z.; Liang, X.; Su, Y.; Yang, T.; Zang, Y.; Jin, D. Optimizing the High-Temperature Oxidation Resistance of Nb-Si-Based Alloys by Adding Different Ti/Mo/Hf Elements. Metals 2025, 15, 439. https://doi.org/10.3390/met15040439
Zhang Y, Shan Z, Luo L, Li Z, Liang X, Su Y, Yang T, Zang Y, Jin D. Optimizing the High-Temperature Oxidation Resistance of Nb-Si-Based Alloys by Adding Different Ti/Mo/Hf Elements. Metals. 2025; 15(4):439. https://doi.org/10.3390/met15040439
Chicago/Turabian StyleZhang, Youwei, Zhongde Shan, Lei Luo, Zhaobo Li, Xiao Liang, Yanqing Su, Tao Yang, Yong Zang, and Dehua Jin. 2025. "Optimizing the High-Temperature Oxidation Resistance of Nb-Si-Based Alloys by Adding Different Ti/Mo/Hf Elements" Metals 15, no. 4: 439. https://doi.org/10.3390/met15040439
APA StyleZhang, Y., Shan, Z., Luo, L., Li, Z., Liang, X., Su, Y., Yang, T., Zang, Y., & Jin, D. (2025). Optimizing the High-Temperature Oxidation Resistance of Nb-Si-Based Alloys by Adding Different Ti/Mo/Hf Elements. Metals, 15(4), 439. https://doi.org/10.3390/met15040439








