Study of Selective Recovery of Lead- and Zinc-Based Products from Leachate After Alkaline Leaching of Copper Shaft Furnace Dust
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
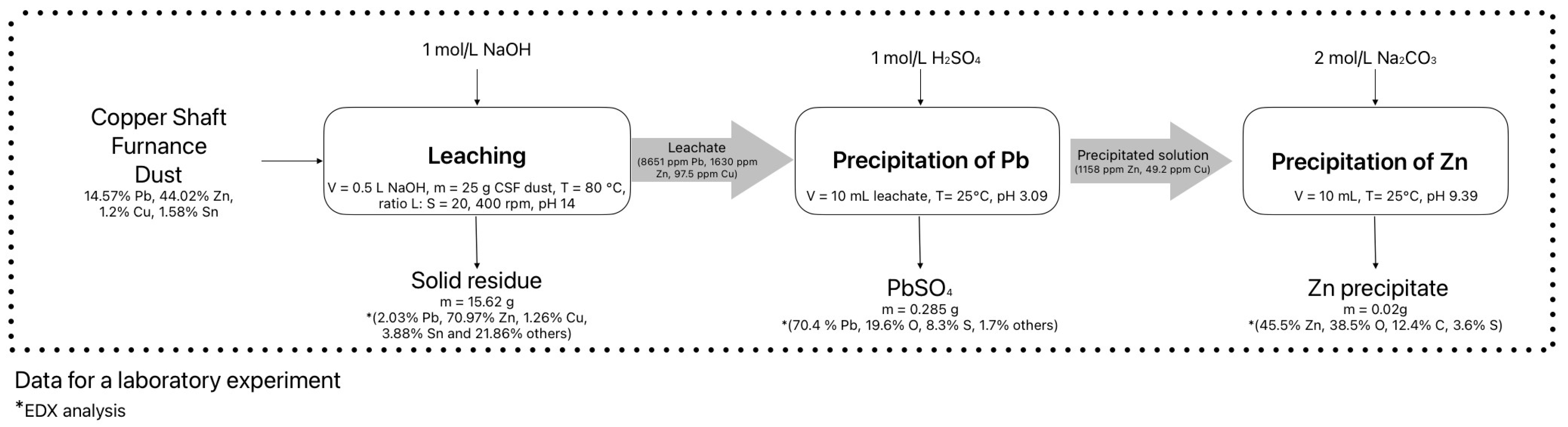
3.1. Alcaline Leaching
3.2. Chemical Precipitation
3.2.1. Lead Precipitation
3.2.2. Zinc Precipitation
4. Conclusions
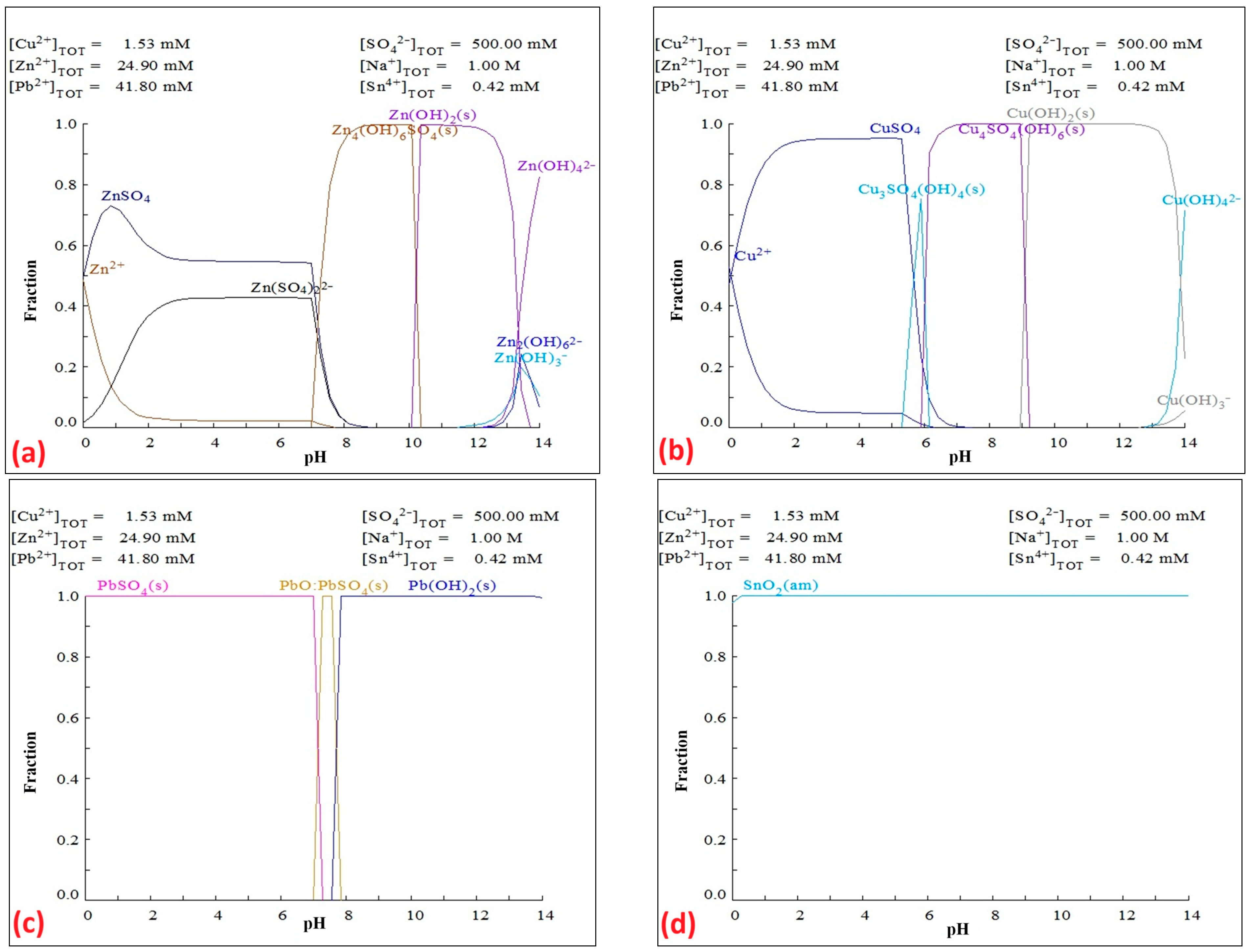
- For efficient precipitation of lead from an alkaline solution at pH = 14, lead forms the insoluble compound PbSO4 within the pH range 2 to 7.8 (Eh = 0.2 to –0.2 V) within the stability limits of water;
- For efficient precipitation of zinc from an acidic solution in the pH range 6–8, Zn is expected to precipitate as ZnCO3;
- If the solution also contains Cu, copper will precipitate in the form of Cu2CO3(OH)2(s) (pH 6–10) and as Cu(OH)2(s) (pH 10–13.5) at ambient temperatures and will be present as an impurity in the zinc precipitate.
- The optimal conditions for precipitating lead as PbSO4 from an alkaline leachate (pH 13.5) are a 1 mol/L H2SO4 solution at pH 3.09 and Eh 0.22 V at 25 °C;
- The optimal conditions for precipitating zinc from this solution (pH 3.09) are the use of 2 mol/L Na2CO3 as the precipitating agent at pH 9.39 and Eh −0.14 V at 25 °C.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, C.L.; Xu, M.; Cui, S.; Li, Z.; Fang, H.; Wang, P. Copper-induced ripple effects by the expanding electric vehicle fleet: A crisis or an opportunity. Resour. Conserv. Recycl. 2020, 161, 104861. [Google Scholar] [CrossRef]
- Klose, S.; Pauliuk, S. Sector-level estimates for global future copper demand and the potential for resource efficiency. Resour. Conserv. Recycl. 2023, 193, 106941. [Google Scholar] [CrossRef]
- Grohol, M.; Veeh, C. Study on the Critical Raw Materials for the EU 2023: Final Report; Publications Office of the European Union: Luxembourg, 2023; ISBN 978-92-68-00414-2. [Google Scholar]
- Cehlár, M.; Šimková, Z. Critical raw materials as a part of sustainable development. Multidiszcip. Tudományok 2021, 11, 12–23. [Google Scholar] [CrossRef]
- Schlesinger, M.E.; Sole, K.C.; Davenport, W.G.; Alvear Flores, G.R.F. Extractive Metallurgy of Copper, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Forsén, O.; Aromaa, J.; Lundström, M. Primary copper smelter and refinery as a recycling plant—A system integrated approach to estimate secondary raw material tolerance. Recycling 2017, 2, 19. [Google Scholar] [CrossRef]
- Orac, D.; Laubertova, M.; Piroskova, J.; Klein, D.; Bures, R.; Klimko, J. Characterization of dusts from secondary copper production. J. Min. Metall. Sect. B Metall. 2020, 56, 221–228. [Google Scholar] [CrossRef]
- European Waste Catalogue and Hazardous Waste List. Available online: https://www.umweltbundesamt.de/sites/default/files/medien/2503/dokumente/2014-955-eg-en.pdf (accessed on 1 November 2025).
- Okanigbe, D.O.; Popoola, A.P.I.; Adeleke, A.A. Characterization of Copper Smelter Dust for Copper Recovery. Procedia Manuf. 2017, 7, 121–126. [Google Scholar] [CrossRef]
- D’Inverno, G.; Carosi, L.; Romano, G. Meeting the challenges of the waste hierarchy: A performance evaluation of EU countries. Ecol. Indic. 2024, 160, 111641. [Google Scholar] [CrossRef]
- Oráč, D.; Klimko, J.; Klein, D.; Pirošková, J.; Liptai, P.; Vindt, T.; Miškufová, A. Hydrometallurgical recycling of copper anode furnace dust for a complete recovery of metal values. Metals 2022, 12, 36. [Google Scholar] [CrossRef]
- Havlik, T. Chapter 12—Leaching of Copper Sulphides. In Hydrometallurgy: Principles and Application, 1st ed.; Series in Metals and Surface Engineering; Woodhead Publishing: Cambridge, UK, 2008; ISBN 978-1-84569-407-4. [Google Scholar]
- Dosmukhamedov, N.; Zholdasbay, E.; Argyn, A. Extraction of Pb, Cu, Zn and As from Fine Dust of Copper Smelting Industry via Leaching with Sulfuric Acid. Sustainability 2023, 15, 15881. [Google Scholar] [CrossRef]
- Kadiyski, M.; Angelov, N.; Iliev, P.; Stefanov, E.; Semerdzhiev, T.; Sopotenska, I. Treatment of Copper Flue Dust From Flash Furnace Waste Heat Boiler for Impurities Control. J. Chem. Technol. Metall. 2024, 59, 1189–1198. [Google Scholar] [CrossRef]
- Laubertová, M.; Kollová, A.; Trpčevská, J.; Plešingerová, B.; Briančin, J. Hydrometallurgical treatment of converter dust from secondary copper production: A study of the lead cementation from acetate solution. Minerals 2021, 11, 1326. [Google Scholar] [CrossRef]
- Dhiman, S.; Ghosh, A.; Saravanan, V.; Jain, R. Hydrometallurgical separation of iron and copper from copper industrial dust waste and recovery of copper as copper oxide. Sustain. Chem. Clim. Action 2025, 7, 100120. [Google Scholar] [CrossRef]
- Laubertová, M.; Sisol, M.; Briančin, J.; Trpcevská, J.; Ružizičková, M. Recovery of Valuable Materials Based on Pb and Zn in the Hydrometallurgical Processing of Copper Shaft Furnace Dust. Materials 2025, 18, 1935. [Google Scholar] [CrossRef] [PubMed]
- Gamutan, J.; Koide, S.; Sasaki, Y.; Nagasaka, T. Selective dissolution and kinetics of leaching zinc from lime treated electric arc furnace dust by alkaline media. J. Environ. Chem. Eng. 2024, 12, 111789. [Google Scholar] [CrossRef]
- Okanigbe, D.O.; Popoola, A.P.I.; Adeleke, A.A.; Otunniyi, I.O.; Popoola, O.M. Investigating the impact of pretreating a waste copper smelter dust for likely higher recovery of copper. Procedia Manuf. 2019, 35, 430–435. [Google Scholar] [CrossRef]
- Che, J.; Zhang, W.; Deen, K.M.; Wang, C. Eco-friendly treatment of copper smelting flue dust for recovering multiple heavy metals with economic and environmental benefits. J. Hazard. Mater. 2024, 465, 133039. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, W.; Ma, B.; Che, J.; Xia, L.; Wen, P.; Wang, C. Recovering metals from flue dust produced in secondary copper smelting through a novel process combining low temperature roasting, water leaching and mechanochemical reduction. J. Hazard. Mater. 2022, 430, 128497. [Google Scholar] [CrossRef]
- Sabzezari, B.; Koleini, S.M.J.; Ghassa, S.; Shahbazi, B.; Chelgani, S.C. Microwave-leaching of copper smelting dust for Cu and Zn extraction. Materials 2019, 12, 1822. [Google Scholar] [CrossRef]
- Yang, T.; Fu, X.; Liu, W.; Chen, L.; Zhang, D. Hydrometallurgical Treatment of Copper Smelting Dust by Oxidation Leaching and Fractional Precipitation Technology. JOM 2017, 69, 1982–1986. [Google Scholar] [CrossRef]
- Youcai, Z.; Stanforth, R. Selective separation of lead from alkaline zinc solution by sulfide precipitation. Sep. Sci. Technol. 2001, 36, 2561–2570. [Google Scholar] [CrossRef]
- Hong, T.; Wei, Y.; Li, L.; Mumford, K.A.; Stevens, G.W. An investigation into the precipitation of copper sulfide from acidic sulfate solutions. Hydrometallurgy 2020, 192, 105288. [Google Scholar] [CrossRef]
- Tang, J.; Steenari, B.M. Solvent extraction separation of copper and zinc from MSWI fly ash leachates. Waste Manag. 2015, 44, 147–154. [Google Scholar] [CrossRef]
- Lenz, D.M.; Martins, F.B. Lead and zinc selective precipitation from leach electric arc furnace dust solutions. Matéria 2007, 12, 503–509. [Google Scholar] [CrossRef]
- Navarro, M.; May, P.M.; Hefter, G.; Königsberger, E. Solubility of CuO(s) in highly alkaline solutions. Hydrometallurgy 2014, 147–148, 68–72. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, P.; Yang, J.; Li, M.; Hu, Y.; Liang, S.; Wang, J.; Li, S.; Xiao, K.; Hou, H.; et al. A low-emission strategy to recover lead compound products directly from spent lead-acid battery paste: Key issue of impurities removal. J. Clean. Prod. 2019, 210, 1534–1544. [Google Scholar] [CrossRef]
- Roine, A. HSC Chemistry® Software, version 11; Outotec Research Oy: Pori, Finland, 2023.
- Picazo-Rodríguez, N.G.; Soria-Aguilar, M.D.J.; Martínez-Luévanos, A.; Almaguer-Guzmán, I.; Chaidez-Félix, J.; Carrillo-Pedroza, F.R. Direct acid leaching of sphalerite: An approach comparative and kinetics analysis. Minerals 2020, 10, 359. [Google Scholar] [CrossRef]
- McMahon, M.E.; Santucci, R.J.; Scully, J.R. Advanced chemical stability diagrams to predict the formation of complex zinc compounds in a chloride environment. RSC Adv. 2019, 9, 19905–19916. [Google Scholar] [CrossRef] [PubMed]
- Gajić, N.; Ranitović, M.; Marković, M.; Kamberović, Ž. Thermodynamics Study for Selective Leaching of Copper and Zinc from Complex Secondary Raw Materials Using Oxidative Sulfuric Acid Leaching. Metall. Mater. Data 2023, 1, 65–70. [Google Scholar] [CrossRef]





| Name | Content | Purity | Producer | City/State |
|---|---|---|---|---|
| NaOH | 98 (%) | Analytical | Centralchem | Bratislava/Slovakia |
| H2SO4 | 96 (%) | Analytical | Penta Chemicals Unlimited | Prague/Czech Republic |
| Na2CO3 | 99.2 (%) | Analytical | Chemapol | Prague/Czech Republic |
| Chemical Reaction | ΔG°T (kJ) | No. |
|---|---|---|
| 80 °C | ||
| −31.128 | (1) | |
| −78.120 | (2) | |
| −77.277 | (3) | |
| −50.080 | (4) |
| Chemical Reaction | ΔG°25 °C (kJ) | Ks | pH | No. |
|---|---|---|---|---|
| −219.449 | – | 1–5.6 | (5) | |
| −153.833 | – | 5.6–8.5 | (6) | |
| 94.095 | 3.263 × 10−17 | 5.6–8.5 | (7) | |
| −226.762 | – | 1–3.2 | (8) | |
| −190.229 | – | 3.3–9 | (9) | |
| 123.177 | 2.618 × 10−22 | 3.3–9 | (10) | |
| −203.974 | – | 2–7.8 | (11) | |
| 44.435 | 1.639 × 10−8 | 2–7.8 | (12) | |
| −165.560 | – | 1–9 | (13) |
| Sample | Solution | Precipitate Agent | pH (-) | Eh (V) | Pb (ppm) | Zn (ppm) | Cu (ppm) | Color ** |
|---|---|---|---|---|---|---|---|---|
| MRD82 | Initial Sample * | – | 13.05 | −0.350 | 8651 | 1630 | 97.5 | − |
| MRD86 | Pb precipitation | 1 mol/L H2SO4 | 3.09 | 0.225 | 10.2 | 1198 | 67.80 | White |
| MRD83 | Pb precipitation | 1 mol/L H2SO4 | 5.16 | 0.103 | 9.7 | 1158 | 49.20 | White |
| MRD84 | Pb precipitation | 1 mol/L H2SO4 | 7.75 | −0.047 | 6.8 | 60.20 | 1.60 | White |
| MRD85 | Pb precipitation | 1 mol/L H2SO4 | 12.00 | −0.295 | 335.6 | 24.50 | 2.70 | Blue–Green |
| Chemical Reaction | ΔG°25 °C (kJ) | Ks | pH | No. |
|---|---|---|---|---|
| −56.470 | – | 1.5–9.2 | (14) | |
| 56.470 | 1.276 × 10−10 | 1.5–9.2 | (15) | |
| −429.151 | – | 9.2–12.3 | (16) | |
| 73.734 | 1.205 × 10−13 | 9.2–12.3 | (17) | |
| −56.360 | – | 0.5–5.9 | (18) | |
| 56.360 | 1.334 × 10−10 | 0.5–5.9 | (19) | |
| −123.177 | – | 5.9–14 | (20) | |
| 55.252 | 2.618 × 10−22 | 5.9–14 | (21) |
| Sample | Solution | Precipitate Agent | pH (-) | Eh (V) | Pb (ppm) | Zn (ppm) | Cu (ppm) | Precipitate ** |
|---|---|---|---|---|---|---|---|---|
| MRD83 | IS for MRD94 * | – | 5.16 | 0.103 | 9.7 | 1158 | 49.2 | – |
| MRD94 | Zn precipitation | 2 mol/L Na2CO3 | 9.39 | −0.140 | 0.3 | 2.7 | 2.0 | Green/White |
| MRD92 | Zn precipitation | 2 mol/L Na2CO3 | 8.69 | −0.099 | 0.3 | 9.1 | 2.6 | Green |
| MRD93 | Zn precipitation | 2 mol/L Na2CO3 | 9.11 | −0.123 | 0.2 | 3.9 | 3.2 | Green |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ružičková, M.; Laubertová, M.; Marcin, M. Study of Selective Recovery of Lead- and Zinc-Based Products from Leachate After Alkaline Leaching of Copper Shaft Furnace Dust. Metals 2025, 15, 1362. https://doi.org/10.3390/met15121362
Ružičková M, Laubertová M, Marcin M. Study of Selective Recovery of Lead- and Zinc-Based Products from Leachate After Alkaline Leaching of Copper Shaft Furnace Dust. Metals. 2025; 15(12):1362. https://doi.org/10.3390/met15121362
Chicago/Turabian StyleRužičková, Michaela, Martina Laubertová, and Michal Marcin. 2025. "Study of Selective Recovery of Lead- and Zinc-Based Products from Leachate After Alkaline Leaching of Copper Shaft Furnace Dust" Metals 15, no. 12: 1362. https://doi.org/10.3390/met15121362
APA StyleRužičková, M., Laubertová, M., & Marcin, M. (2025). Study of Selective Recovery of Lead- and Zinc-Based Products from Leachate After Alkaline Leaching of Copper Shaft Furnace Dust. Metals, 15(12), 1362. https://doi.org/10.3390/met15121362








