Temperature-Correlated Characterization of EoL Lithium Cobalt Oxide Batteries with Microwave-Based Pyrometallurgical Recovery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Thermodynamic Analysis
2.2.2. Experimental Design
3. Results
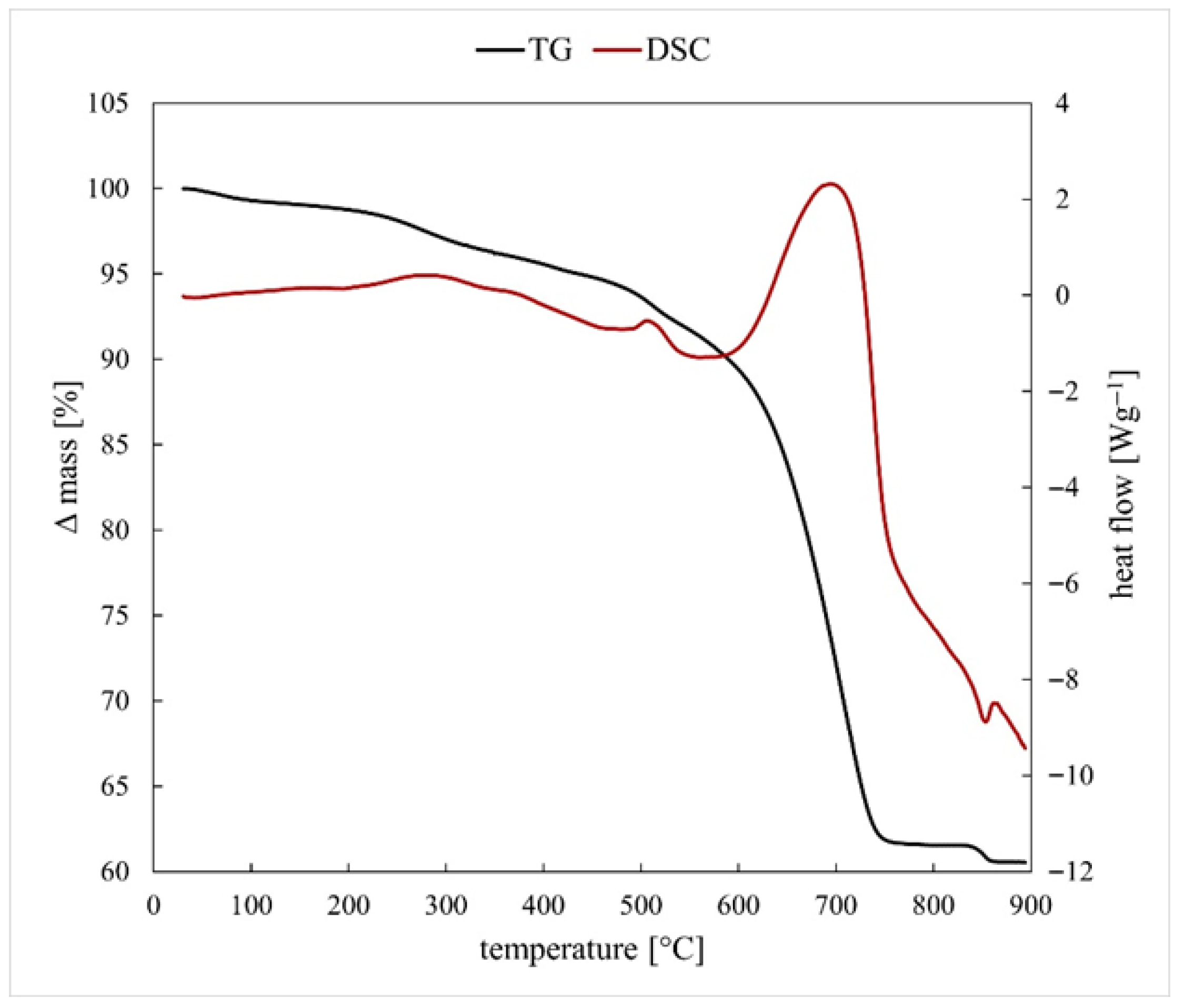
3.1. Thermodynamic Simulations
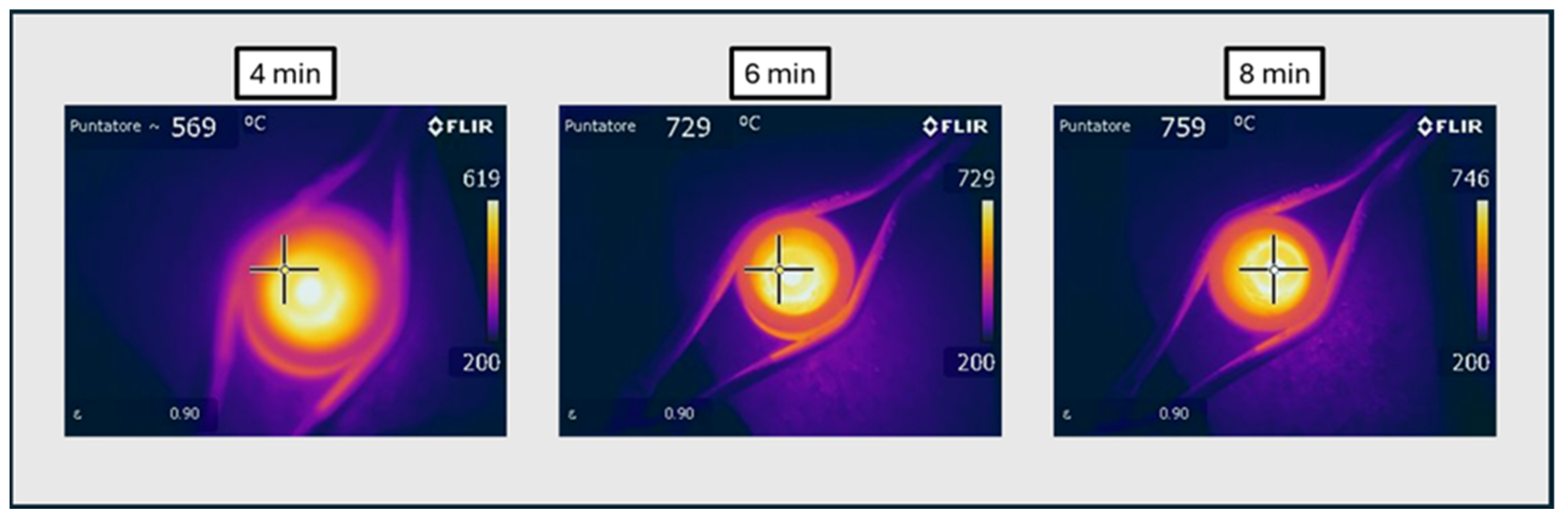
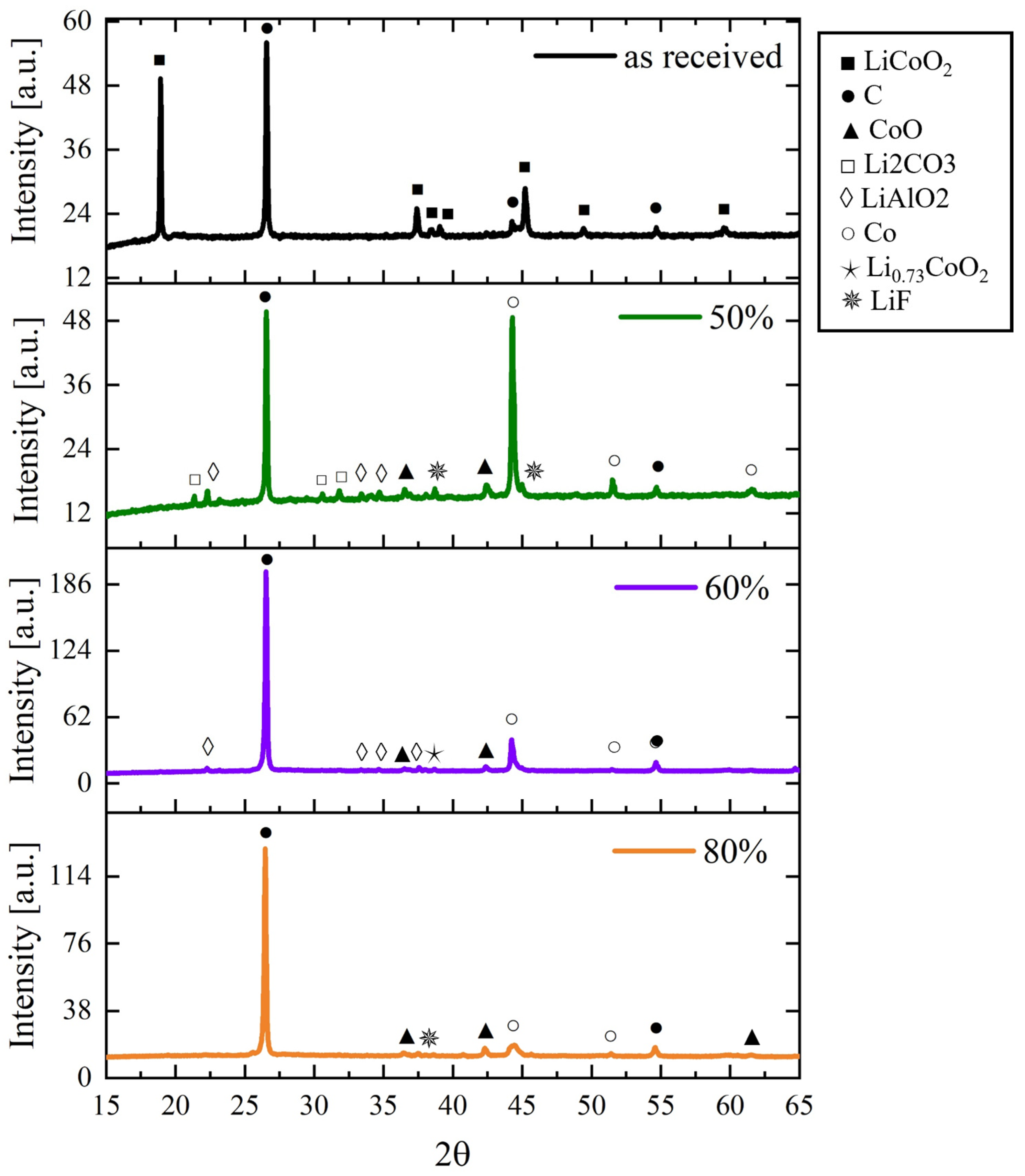
3.2. Microwave Tests
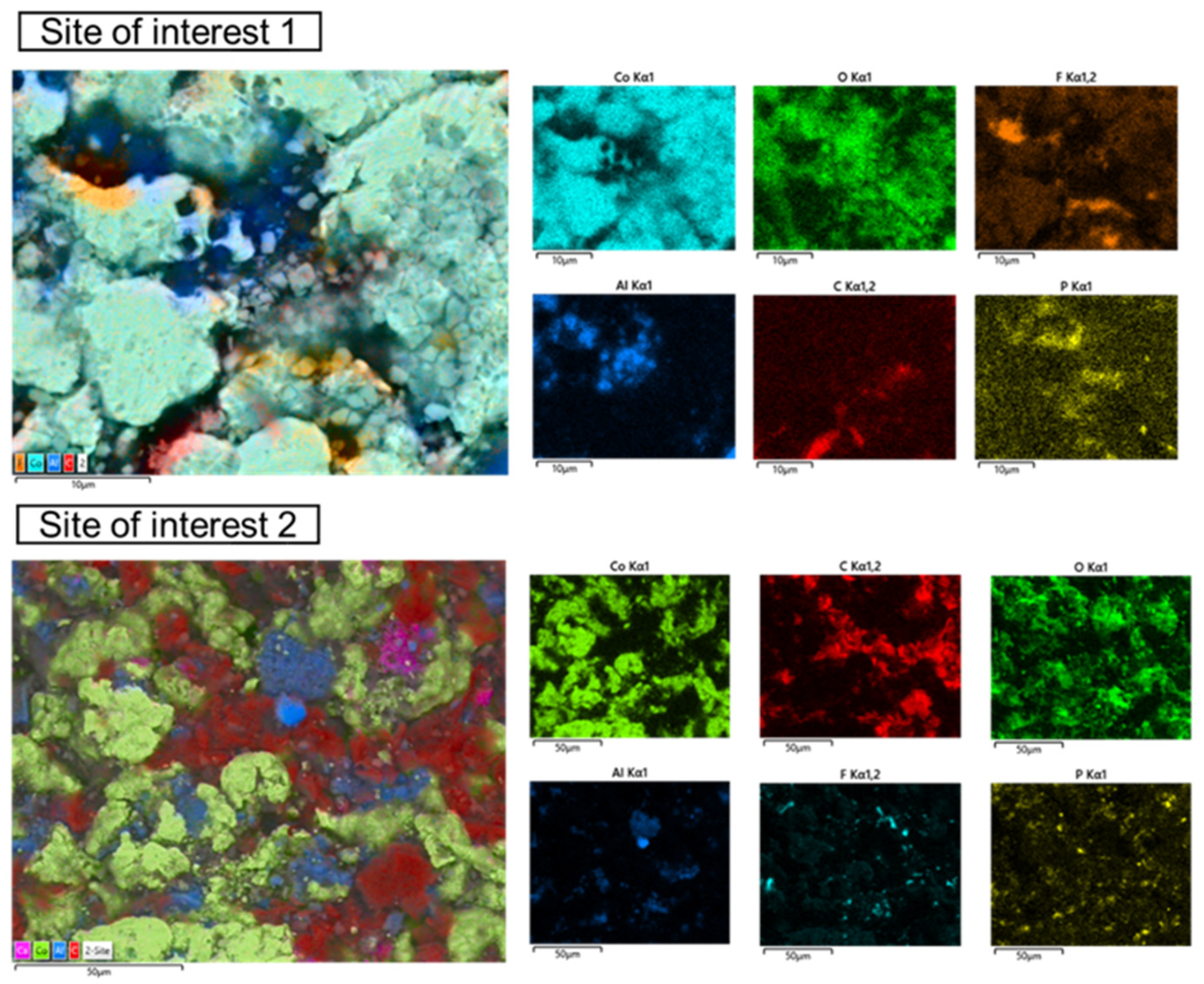
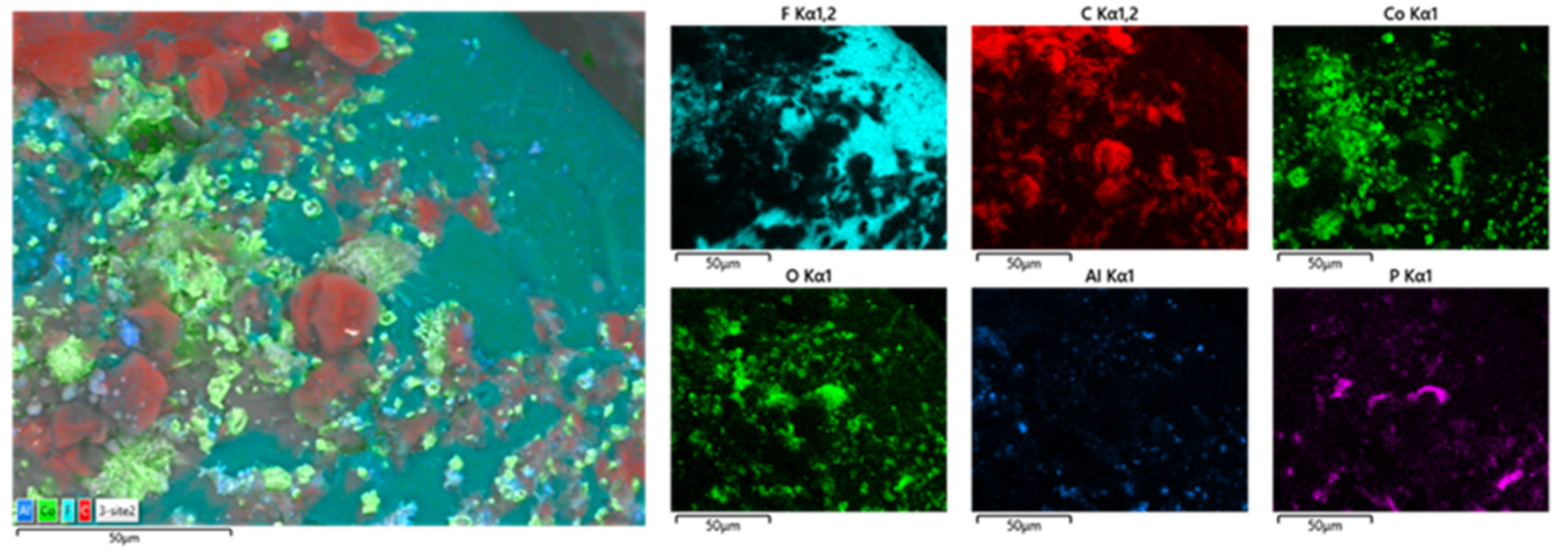
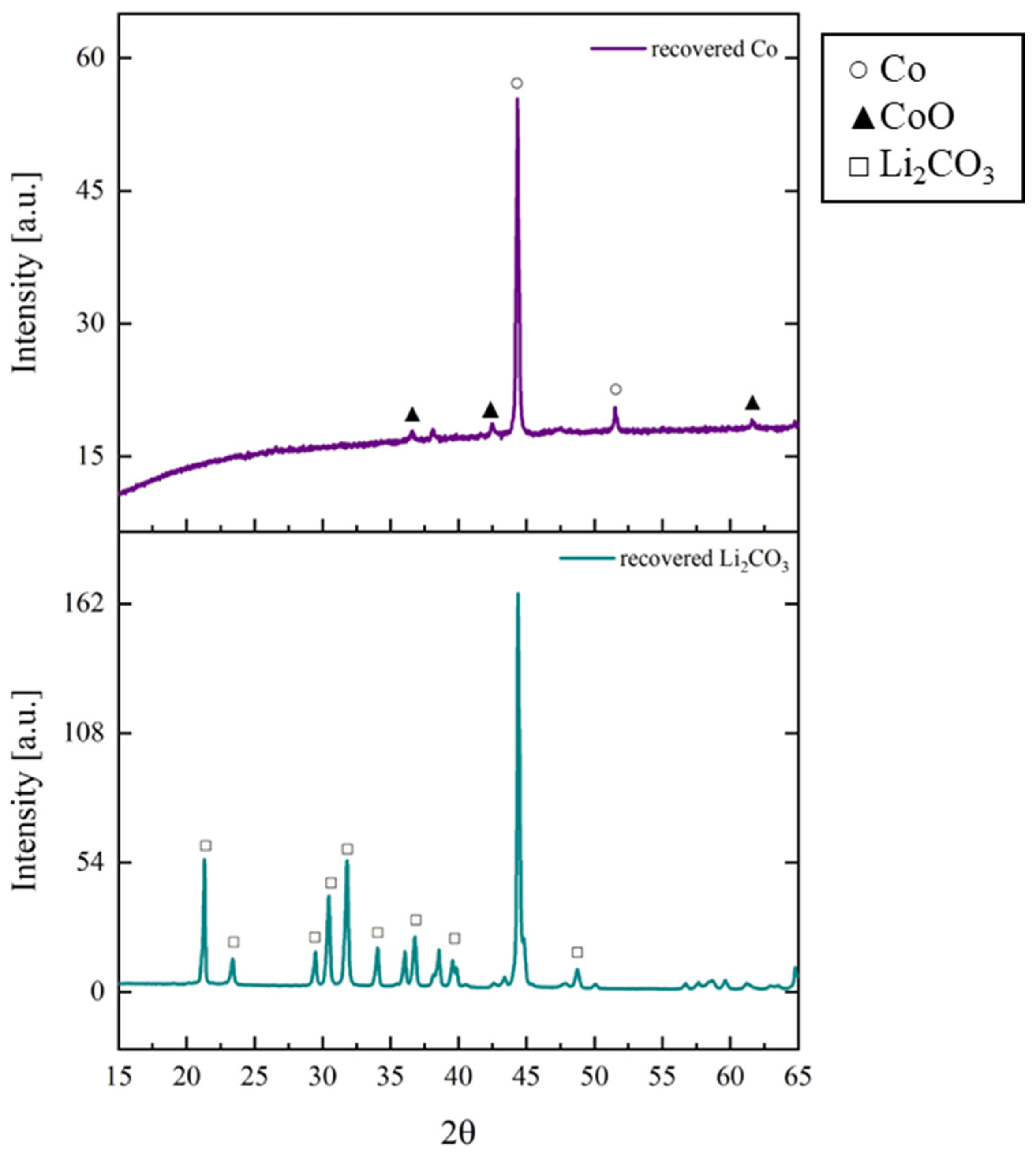
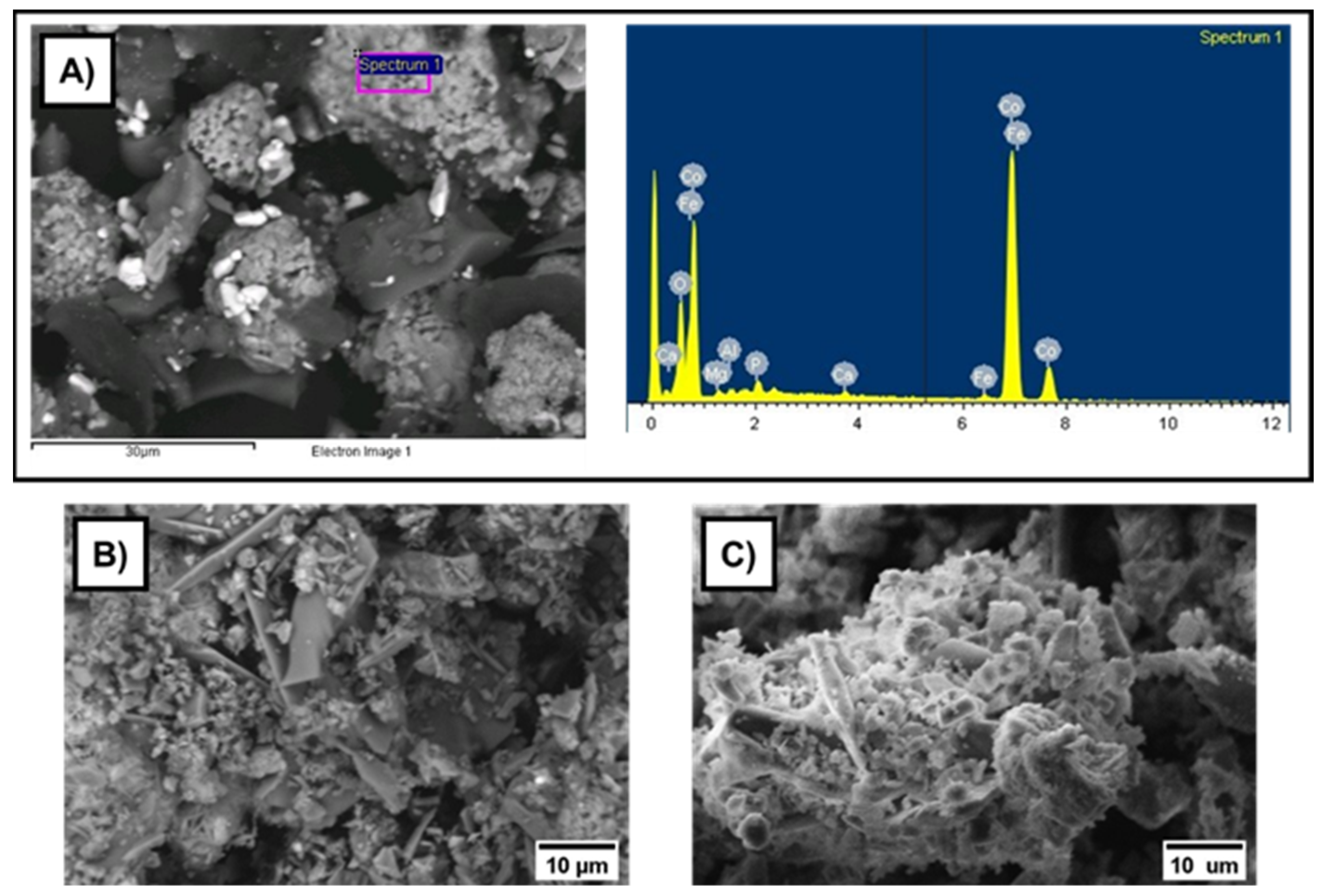
3.3. Wet Magnetic Separation Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| EOL | End Of Life |
| SEM | Scanning Electron Microscope |
| EDS | Energy Dispersive Spectroscopy |
| XRD | X-Ray Diffraction |
| LIB | Lithium-ion Battery |
| MW | Microwave |
| LCO | Lithium Cobalt Oxide |
| NMC | Nickel Manganese Cobalt |
| TGA | Thermogravimetric Analysis |
| DSC | Differential Scanning Calorimetry |
| PVDF | Polyvinylidene fluoride |
| ICDD | International Centre for Diffraction Data |
References
- Maisel, F.; Neef, C.; Marscheider-Weidemann, F.; Nissen, N.F. A forecast on future raw material demand and recycling potential of lithium-ion batteries in electric vehicles. Resour. Conserv. Recycl. 2023, 192, 106920. [Google Scholar] [CrossRef]
- Gangaja, B.; Nair, S.; Santhanagopalan, D. Reuse, Recycle, and Regeneration of LiFePO4 Cathode from Spent Lithium-Ion Batteries for Rechargeable Lithium- and Sodium-Ion Batteries. ACS Sustain. Chem. Eng. 2021, 9, 4711–4721. [Google Scholar] [CrossRef]
- Zhu, A.; Bian, X.; Han, W.; Cao, D.; Wen, Y.; Zhu, K.; Wang, S. The application of deep eutectic solvents in lithium-ion battery recycling: A comprehensive review. Resour. Conserv. Recycl. 2023, 188, 106690. [Google Scholar] [CrossRef]
- Suffia, S.; Dutta, D. Applications of deep eutectic solvents in metal recovery from E-wastes in a sustainable way. J. Mol. Liq. 2024, 394, 123738. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Bai, Y.; Duan, Y.; Zhang, B.; Liu, C.; Sun, X.; Feng, M.; Mu, T. Significant Improvement in Dissolving Lithium-Ion Battery Cathodes Using Novel Deep Eutectic Solvents at Low Temperature. ACS Sustain. Chem. Eng. 2021, 9, 12940–12948. [Google Scholar] [CrossRef]
- Swain, B. Recovery and recycling of lithium: A review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Asadi Dalini, E.; Karimi, G.; Zandevakili, S.; Goodarzi, M. A Review on Environmental, Economic and Hydrometallurgical Processes of Recycling Spent Lithium-ion Batteries. Miner. Process. Extr. Metall. Rev. 2021, 42, 451–472. [Google Scholar] [CrossRef]
- Liang, Z.; Cai, C.; Peng, G.; Hu, J.; Hou, H.; Liu, B.; Liang, S.; Xiao, K.; Yuan, S.; Yang, J. Hydrometallurgical Recovery of Spent Lithium Ion Batteries: Environmental Strategies and Sustainability Evaluation. ACS Sustain. Chem. Eng. 2021, 9, 5750–5767. Available online: https://pubs.acs.org/doi/full/10.1021/acssuschemeng.1c00942 (accessed on 5 September 2025). [CrossRef]
- Padwal, C.; Pham, H.D.; Jadhav, S.; Do, T.T.; Nerkar, J.; Hoang, L.T.M.; Kumar Nanjundan, A.; Mundree, S.G.; Dubal, D.P. Deep Eutectic Solvents: Green Approach for Cathode Recycling of Li-Ion Batteries. Adv. Energy Sustain. Res. 2022, 3, 2100133. [Google Scholar] [CrossRef]
- Holzer, A.; Windisch-Kern, S.; Ponak, C.; Raupenstrauch, H. A Novel Pyrometallurgical Recycling Process for Lithium-Ion Batteries and Its Application to the Recycling of LCO and LFP. Metals 2021, 11, 149. [Google Scholar] [CrossRef]
- Kwon, O.-s.; Sohn, I. Fundamental thermokinetic study of a sustainable lithium-ion battery pyrometallurgical recycling process. Resour. Conserv. Recycl. 2020, 158, 104809. [Google Scholar] [CrossRef]
- Makuza, B.; Tian, Q.; Guo, X.; Chattopadhyay, K.; Yu, D. Pyrometallurgical options for recycling spent lithium-ion batteries: A comprehensive review. J. Power Sources 2021, 491, 229622. [Google Scholar] [CrossRef]
- Pan, C.; Shen, Y. Pyrometallurgical recycling of spent lithium-ion batteries from conventional roasting to synergistic pyrolysis with organic wastes. J. Energy Chem. 2023, 85, 547–561. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, Z.; Lin, X.; Zhang, Y.; He, Y.; Cao, H.; Sun, Z. A Mini-Review on Metal Recycling from Spent Lithium Ion Batteries. Engineering 2018, 4, 361–370. [Google Scholar] [CrossRef]
- Cornelio, A.; Galli, E.; Scaglia, M.; Zanoletti, A.; Zacco, A.; Bonometti, A.; Magugliani, G.; Mossini, E.; Macerata, E.; Federici, S.; et al. Recovery of NMC-lithium battery black mass by microwave heating processes. Energy Storage Mater. 2024, 72, 103703. [Google Scholar] [CrossRef]
- Zhu, A.; Bian, X.; Han, W.; Wen, Y.; Ye, K.; Wang, G.; Yan, J.; Cao, D.; Zhu, K.; Wang, S. Microwave-ultra-fast recovery of valuable metals from spent lithium-ion batteries by deep eutectic solvents. Waste Manag. 2023, 156, 139–147. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, B.; Zhang, L.; Guo, S. Microwave-absorbing properties of cathode material during reduction roasting for spent lithium-ion battery recycling. J. Hazard. Mater. 2020, 384, 121487. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, W.; Jia, K.; Li, S.; Jia, P.; Wang, W.; Song, Z.; Zhao, X.; Mao, Y.; Chen, S. Progress on the Microwave-Assisted Recycling of Spent Lithium Battery Graphite. Processes 2023, 11, 1451. [Google Scholar] [CrossRef]
- Bresolin, B.-M.; Zanoletti, A.; Bontempi, E. Recent improvements in salt-assisted and microwave-assisted recovery methods for sustainable metal extraction from NCM cathodes in spent lithium-ion batteries: A review. Sep. Purif. Technol. 2025, 363, 131918. [Google Scholar] [CrossRef]
- Scaglia, M.; Cornelio, A.; Zanoletti, A.; Corte, D.L.; Biava, G.; Alessandri, I.; Forestan, A.; Alba, C.; Depero, L.E.; Bontempi, E. Microwave-Assisted Recovery of Spent LiCoO2 Battery from the Corresponding Black Mass. Batteries 2023, 9, 536. [Google Scholar] [CrossRef]
- Pindar, S.; Dhawan, N. Evaluation of in-situ microwave reduction for metal recovery from spent lithium-ion batteries. Sustain. Mater. Technol. 2020, 25, e00201. [Google Scholar] [CrossRef]
- Fahimi, A.; Alessandri, I.; Cornelio, A.; Frontera, P.; Malara, A.; Mousa, E.; Ye, G.; Valentim, B.; Bontempi, E. A microwave-enhanced method able to substitute traditional pyrometallurgy for the future of metals supply from spent lithium-ion batteries. Resour. Conserv. Recycl. 2023, 194, 106989. [Google Scholar] [CrossRef]
- Pindar, S.; Dhawan, N. Carbothermal Reduction of Spent Mobile Phones Batteries for the Recovery of Lithium, Cobalt, and Manganese Values. JOM J. Miner. Met. Mater. Soc. 2019, 71, 4483–4491. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Xu, Z. Environmentally-friendly oxygen-free roasting/wet magnetic separation technology for in situ recycling cobalt, lithium carbonate and graphite from spent LiCoO2/graphite lithium batteries. J. Hazard. Mater. 2016, 302, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Pindar, S.; Dhawan, N. Comparison of Microwave and Conventional Indigenous Carbothermal Reduction for Recycling of Discarded Lithium-Ion Batteries. Trans. Indian Inst. Met. 2020, 73, 2041–2051. [Google Scholar] [CrossRef]
- Siddique, I.J.; Salema, A.A. Unraveling the metallic thermocouple effects during microwave heating of biomass. Energy 2023, 267, 126529. [Google Scholar] [CrossRef]
- Nuraeni, B.A.; Avarmaa, K.; Prentice, L.H.; Rankin, W.J.; Rhamdhani, M.A. Recovery of Cobalt and Lithium by Carbothermic Reduction of LiCoO2 Cathode Material: A Kinetic Study. Metall. Mater. Trans. B 2023, 54, 602–620. [Google Scholar] [CrossRef]
- Zhang, G.; Yuan, X.; Tay, C.Y.; He, Y.; Wang, H.; Duan, C. Selective recycling of lithium from spent lithium-ion batteries by carbothermal reduction combined with multistage leaching. Sep. Purif. Technol. 2023, 314, 123555. [Google Scholar] [CrossRef]
- Perdana, I.; Aprilianto, D.R.; Fadillah, F.A.; Fadli, R. Lithium recovery from mixed spent LFP-NMC batteries through atmospheric water leaching. Nat. Sci. Rep. 2025, 15, 2591. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, L.; Dai, J.; Zhou, L.; Zhang, M.; Xie, C.; Hao, H.; Hou, B.; Bao, Y.; Yin, Q. Crystallization of Lithium Carbonate from Aqueous Solution: New Insights into Crystal Agglomeration. Ind. Eng. Chem. Res. 2019, 58, 18448–18455. [Google Scholar] [CrossRef]
- Taborga, P.; Brito, I.; Graber, T.A. Effect of additives on size and shape of lithium carbonate crystals. J. Cryst. Growth 2017, 460, 5–12. [Google Scholar] [CrossRef]
- Karapin-Springorum, L.; Sarycheva, A.; Dopilka, A.; Cha, H.; Ihsan-Ul-Haq, M.; Larson, J.M.; Kostecki, R. An infrared, Raman, and X-ray database of battery interphase components. Sci. Data 2025, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhang, X.; Zheng, X.; Lin, X.; Cao, H.; Zhang, Y.; Sun, Z. Lithium Carbonate Recovery from Cathode Scrap of Spent Lithium-Ion Battery: A Closed-Loop Process. Environ. Sci. Technol. 2017, 51, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
| Sample Name | Graphite wt% | Graphite [g] | LCO [g] | Power [W] | Time [s] |
|---|---|---|---|---|---|
| 50G11 | 50 | 0.2 | 0.2 | 950 | 660 |
| 60G6 | 60 | 0.24 | 0.16 | 950 | 360 |
| 80G8 | 80 | 0.32 | 0.08 | 950 | 480 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitacco, E.; Ragazzini, M.; Bernardini, C.; Ghadimi, M.; Pigato, M.; Forzan, M.; Brunelli, K. Temperature-Correlated Characterization of EoL Lithium Cobalt Oxide Batteries with Microwave-Based Pyrometallurgical Recovery. Metals 2025, 15, 1302. https://doi.org/10.3390/met15121302
Pitacco E, Ragazzini M, Bernardini C, Ghadimi M, Pigato M, Forzan M, Brunelli K. Temperature-Correlated Characterization of EoL Lithium Cobalt Oxide Batteries with Microwave-Based Pyrometallurgical Recovery. Metals. 2025; 15(12):1302. https://doi.org/10.3390/met15121302
Chicago/Turabian StylePitacco, Emma, Marco Ragazzini, Caterina Bernardini, Mehran Ghadimi, Mirko Pigato, Michele Forzan, and Katya Brunelli. 2025. "Temperature-Correlated Characterization of EoL Lithium Cobalt Oxide Batteries with Microwave-Based Pyrometallurgical Recovery" Metals 15, no. 12: 1302. https://doi.org/10.3390/met15121302
APA StylePitacco, E., Ragazzini, M., Bernardini, C., Ghadimi, M., Pigato, M., Forzan, M., & Brunelli, K. (2025). Temperature-Correlated Characterization of EoL Lithium Cobalt Oxide Batteries with Microwave-Based Pyrometallurgical Recovery. Metals, 15(12), 1302. https://doi.org/10.3390/met15121302








