Abstract
The increasing global demand for copper has driven the search for more efficient and sustainable extraction methods, particularly due to the environmental concerns associated with conventional processes. This study investigated the leaching of copper minerals MC (Cu2O) and malachite (Cu2CO3(OH)2) using glycine as an organic ligand in an alkaline medium, and evaluated the efficiency of hydrogen peroxide (H2O2) and ozone (O3) as oxidizing agents. Chemical and mineralogical characterization using XRD, XRF, ICP, and SEM confirmed the predominance of MC and malachite, along with secondary phases such as hematite and calcite. Leaching experiments were carried out by varying glycine and oxidant concentrations at pH 10, with a reaction time of 240 min, agitation at 800 min−1, a solution volume of 300 mL, and a mineral sample concentration of 0.33 g·L−1. The results showed that O3 exhibited low efficiency due to its limited solubility, whereas H2O2 achieved dissolution rates of 69.7% for MC in 150 min and 96.3% for MMin just 60 min. The glycine-H2O2 system optimized reaction time by 84% compared to conventional methods, emerging as a sustainable alternative due to the low toxicity and biodegradability of glycine.
1. Introduction
Copper is essential in multiple industries due to its importance in technological applications, construction, and renewable energy [1,2,3]. However, its recovery through traditional methods, such as pyrometallurgy [4] and acid leaching [5,6], poses significant environmental and economic challenges [7,8,9]. Historically, copper recovery has relied on pyrometallurgical processes, which include smelting and refining of ores [10]. Although these methods have been effective for large-scale copper production, they present serious drawbacks such as high sulfur dioxide emissions, elevated energy consumption, and significant environmental impacts [11,12,13]. Additionally, the current decline in high-grade copper deposits has necessitated the exploration of sustainable and cost-effective alternatives for recovering copper from low-grade or difficult-to-process ores [14,15,16,17].
In response to this issue, more sustainable alternatives have been sought, particularly for the treatment of oxidized copper minerals such as cuprite (Cu2O) (MC) and malachite (Cu2CO3(OH)2 (MM). While sulfuric acid leaching has been widely used, this method generates acid mine drainage and liquid effluents that frequently exceed the thresholds set by international environmental standards, causing harm to surrounding water bodies, soils, and communities [18,19,20].
One of the most significant advancements in copper extraction technology has been the implementation of hydrometallurgical processes, particularly heap leaching, solvent extraction, and electrowinning [21,22,23]. Although these methods are more environmentally friendly compared to pyrometallurgy, they still face challenges such as high sulfuric acid consumption, reagent stability issues, and low copper recovery efficiency, especially when dealing with oxidized and low-grade ores [24,25].
In this context, glycine has emerged as an alternative lixiviant with environmentally friendly properties. This non-essential amino acid acts as a chelating agent, forming stable complexes with copper ions, especially under slightly alkaline conditions [26,27,28].
Unlike sulfuric acid, glycine does not generate contaminant by-products and minimizes the acidification of surrounding soils and water bodies. Moreover, its ability to regenerate and its low cost make it an economically viable solution [29,30,31].
Although its optimal performance is achieved under alkaline conditions, recent studies have demonstrated its ability to function effectively over a broad pH range, allowing the design of safer and more environmentally compatible processes [32,33,34].
Aligned with this sustainable approach, green oxidants such as hydrogen peroxide (H2O2) and ozone (O3) have been introduced to enhance metal dissolution without generating hazardous by-products. Both oxidants degrade into harmless compounds, reinforcing the environmental viability of glycine-based leaching systems [35,36,37].
This study aims to evaluate the leaching efficiency of MC and MM using glycine in combination with H2O2 and O3 under controlled alkaline conditions. A series of experiments was designed to analyze the influence of glycine concentration, oxidant dosage, pH, and temperature on copper dissolution. The ores and leaching residues were characterized by SEM–EDS, ICP, AAS, XRF, and XRD [38,39,40,41].
The results obtained not only contribute to a deeper understanding of the glycine–oxidant system but also provide a solid foundation for the development of cleaner, more efficient hydrometallurgical technologies aligned with the principles of sustainable mining [42,43,44].
2. Materials and Methods
2.1. Sample Preparation
The copper-bearing mineral samples used in this study were collected from two mining districts in Mexico, where processing plants primarily focus on extracting gold, silver, and base metals. The MC samples were obtained from Santa María de La Paz, San Luis Potosí, while the MM samples originated from Mapimí, Durango. The historical significance of these regions is reflected in the social development of nearby provinces, although they also exhibit environmental impacts associated with mining activities.
Particle size reduction was performed using a Fritsch Pulverisette 2 automatic mill with an agate mortar. The resulting powders were homogenized using the quartering method until a representative sample of 200 g was obtained. Granulometric analysis was performed using a wet sieving method with 100 g of sample, employing Tyler meshes of 60, 100, 140, 200, 270, 325, and 400 mesh sizes. The mass retained on each sieve was correlated with the copper content to determine its distribution within the sample.
2.2. Mineralogical and Elemental Characterization
Elemental chemical characterization of the samples was performed in two stages. First, the copper grade in each granulometric fraction was determined by atomic absorption spectroscopy (AAS), using a Perkin Elmer Analyst 200 spectrophotometer, PerkinElmer, PerkinElmer, Inc., Waltham, MA, USA. Subsequently, the total elemental composition was confirmed by inductively coupled plasma optical emission spectroscopy (ICP–OES), using a Perkin Elmer 8300 spectrometer PerkinElmer, Inc., Shelton, CT, USA, and by X-ray fluorescence (XRF), using a portable Bruker Titan S1 analyzer, Bruker Corporation, Billerica, MA, USA equipped with Chemplex spectrometric membranes. These techniques allowed the identification of both major and trace elements present in the samples.
Mineralogical characterization was carried out using X-ray diffraction (XRD) with a D8-Advance diffractometer, Bruker AXS GmbH, Karlsruhe, Germany, operating over a 2θ range of 10–80° at a scanning speed of 8°·min−1. This technique enabled the identification of the primary mineral matrix and secondary phases in the samples. The Match3 and DiffracEVA 5.1 software packages were used for mineral phase identification. In addition, selected samples were prepared for scanning electron microscopy coupled with energy-dispersive X-ray spectroscopy (SEM–EDS), JEOL Ltd., Akishima, Tokyo, Japan, using a JEOL IT-300 microscope operating at 30 kV. This technique provided information on particle morphology, distribution, and surface composition through secondary electron micrographs and their corresponding EDS spectra.
2.3. Thermodynamic Simulation
The simulation of the leaching system (Cu2+–NH2CH2COOH–H2O) was carried out using HSC Chemistry 6 software, with the aim of identifying the predominant copper species as a function of pH and redox potential (Eh). Pourbaix diagrams were constructed to determine the optimal conditions that favor metal dissolution and the formation of stable complexes.
2.4. Leaching Conditions
The experimental setup consisted of a two-neck, round-bottom flask with a standard 24/40 ground joint, placed in a water bath to ensure uniform thermal distribution. Magnetic stirring, as well as temperature and pH control, were performed using a Thermo Scientific Super Nuova hot plate, Thermo Fisher Scientific Inc., Waltham, MA, USA, equipped with a potentiometer and an epoxy-body electrode (Orion 3-Star, Thermo Fisher Scientific, Ecublens, Switzerland).
Leaching was carried out using glycine (NH2CH2COOH, purity > 98.5%), Meyer, Química Suastes, S.A. de C.V., Tlahuac CDMX, as the chelating agent. To ensure its stability and promote the formation of soluble complexes, the pH was adjusted to alkaline conditions using a 1 mol·L−1 sodium hydroxide solution (NaOH, purity 98.2%, J.T. Baker, Avantor Performance Materials, Phillipsburg, NJ, USA).
Two environmentally benign oxidizing agents were used: hydrogen peroxide (H2O2, purity 29–32%, Baker, Avantor Performance Materials, Radnor, PA, USA) and ozone (O3). In the H2O2 experiments, the oxidant was added once at the beginning of the reaction, with no subsequent additions. This approach, supported by previous studies [45,46], has proven effective even considering the partial decomposition of H2O2 in alkaline media—particularly for systems with oxidized copper minerals, such as those used in this study, which do not require progressive oxidation. In the case of ozone, an O3 generator model Wish-Ozone168A, OzoneWish, Guangzhou, China, was connected to a porous stone diffuser, with the flow rate controlled by a precision rotameter. The concentration of dissolved ozone was not monitored in order to simulate simplified operational conditions analogous to those in a pilot plant. The concentrations of glycine, oxidants, and operating parameters are summarized in Table 1.

Table 1.
Experimental Reagents and Conditions.
A sample mass of 0.165 g was dissolved in 0.5 L of leaching solution, corresponding to a pulp density of 0.33 g·L−1. This ratio was deliberately chosen to ensure that glycine would not act as a limiting reagent during the process, thus allowing for a clear evaluation of the impact of oxidizing agents and experimental variables on extraction efficiency. Although this does not reflect typical industrial operating conditions, the selected density is suitable for laboratory-scale tests aimed at identifying general performance trends. This will provide a foundation for future studies focused on kinetic modeling and process scaling, in which optimized solid-to-liquid ratios can be proposed to enhance process performance at the pilot plant level.
Aliquots were taken at intervals from 0 to 240 min and analyzed by atomic absorption spectrophotometer 2380 (PerkinElmer, Inc., Shelton, CT, USA, to quantify the copper concentration in solution. Copper recovery was calculated as a percentage using the following expression:
where [Cu]complexed represents the concentration of copper in solution, and [Cu]initial corresponds to the initial copper content present in the mineral sample.
3. Results
The following section presents the identification of the mineral phases present in the MC and MM samples, along with the confirmation of their elemental composition using the characterization techniques previously described. After verifying the copper content and its mineralogical associations, the thermodynamic simulation of the proposed leaching system is presented, followed by the results of the experimental tests conducted with varying concentrations of oxidizing agents.
3.1. Chemical Analysis
The mineral samples corresponding to MC and MM were subjected to acid digestion in triplicate, using a 3:1 mixture of HCl and HNO3. The resulting solutions (Table 2) were analyzed by atomic absorption spectroscopy (AAS) and inductively coupled plasma optical emission spectroscopy (ICP-OES), yielding consistent average results between both methods. According to the ICP analysis, the MM sample exhibited a copper grade of 21.6%, classifying it as a suitable ore for leaching processes assisted by mechanical or pneumatic agitation. In the case of the MC sample, a copper content of 12.7% was recorded, exceeding the minimum threshold required for tank or heap leaching techniques, thereby confirming its suitability for the proposed leaching system [47,48]. These values are consistent with previous studies on the mineral composition of the mining districts from which the samples were obtained [49].

Table 2.
Copper Content in Mineral Composites MC and MM.
Complementary elemental analysis using X-ray fluorescence (XRF) confirmed and expanded the compositional information of the samples. In the MC sample (Figure 1A), a copper content of 13.93% was recorded, along with an iron concentration approximately three times higher, as well as Ca (4.78%) and Zn (3.13%)—elements commonly associated with carbonates such as calcite, siderite, and hydrozincite. In the MM sample (Figure 1B), the iron content reached 27.76%, likely present as oxides or hydroxides (e.g., hematite, goethite). Trace elements such as Al, Si, Ca, Zn, S, and Pb were also detected at concentrations below 1.28%, associated with gangue minerals and secondary phases.
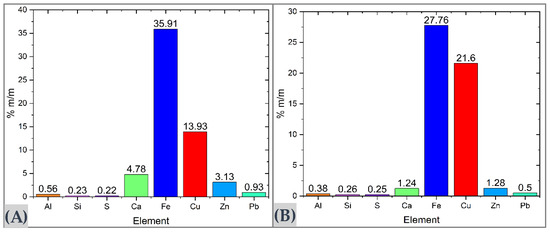
Figure 1.
Elemental composition of mineral composites analyzed by XRF. (A) MC sample. (B) MM sample.
3.2. Granulometric Analysis
The chemical analysis conducted on the sieved powders allowed for the calculation of copper distribution in both samples, with the aim of ensuring efficient metal liberation during the leaching process. Figure 2 shows that the highest copper distribution corresponds to particles retained between mesh sizes 60 and 100, with a copper distribution of 37.68% for the MC sample and 46.25% for the MM sample. The next significant copper fraction is associated with finer particles smaller than 37 microns. The remaining particles within the 100–140 mesh range exhibited copper distributions of less than 9.58% in both samples. Given this heterogeneous distribution, and to avoid excluding a substantial amount of copper present in the finer fractions, it was determined that using the entire sample as a composite mineral would ensure adequate metal liberation during the glycine leaching experiments [50].
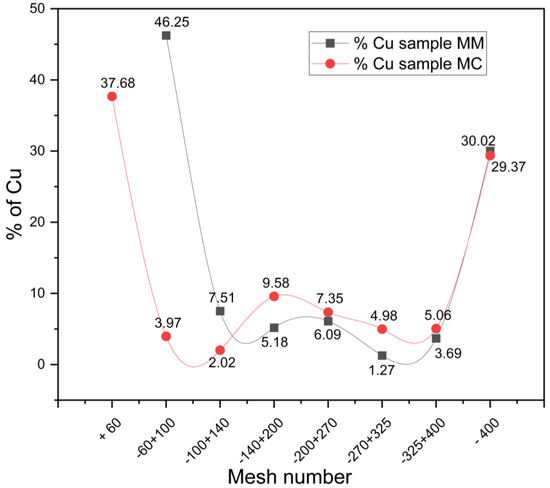
Figure 2.
Copper distribution (%) relative to mesh size for MC and MM samples.
3.3. Mineralogical Characterization
In Figure 3, the X-ray diffraction (XRD) pattern of the MC sample reveals the gangue minerals include calcite (CaCO3) (JCPDS 01-071-3699) as the predominant phase, along with other mineral species such as goethite (FeO(OH)) (JCPDS 01-081-0462), hematite (Fe2O3) (JCPDS 00-024-0072), and vesuvianite (Ca10Al4(Mg,Fe)2Si9O34(OH)4) (JCPDS 00-011-0145), which contribute to the high iron content in the sample. Paradsasvarite ((Zn,Cu)2(CO3)(OH)2) (JCPDS 00-067-0404) was also identified as a source of zinc associated with the target metal.
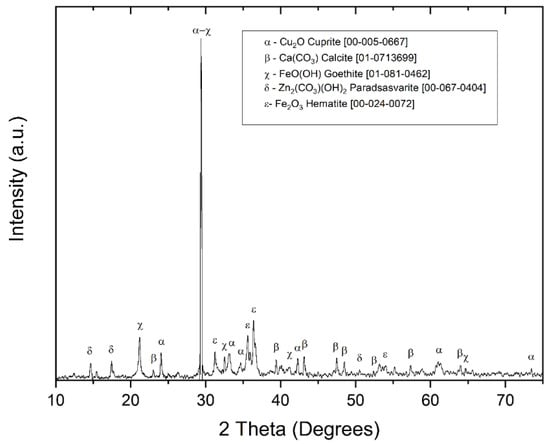
Figure 3.
X-ray diffraction (XRD) pattern for the MC sample composite.
The XRD analysis of the MM sample (Figure 4) identified copper carbonates as the primary mineral matrix, composed mainly of malachite (CuCO3(OH)2) (JCPDS 00-010-0399) and mcguinnessite ((Mg,Cu)2(CO3)(OH)2) (JCPDS 00-035-0481), both of which are considered suitable minerals for copper extraction [51]. Additional detected minerals include tetrahedrite (Cu14Sb13) (JCPDS 00-042-0560), goethite (FeO(OH)) (JCPDS 00-017-0535), hematite (Fe2O3) (JCPDS 00-024-0072), and calcite (CaCO3) (JCPDS S96-901-6707), which correlate with the iron and calcium contents identified in the chemical analysis.
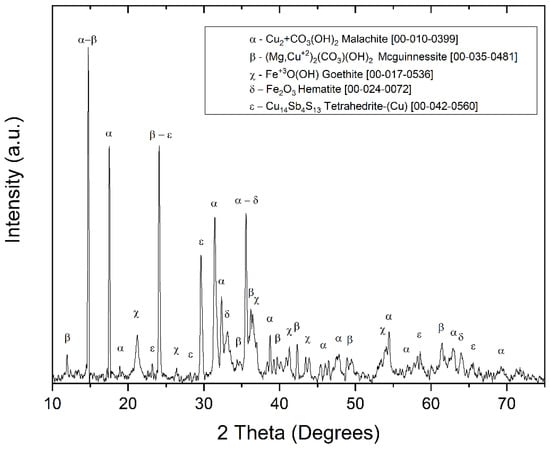
Figure 4.
X-ray diffraction (XRD) pattern for the MM sample composite.
3.4. Secondary Electron Micrography
The secondary electron micrographs of the MC compound particles (Figure 5A) revealed a heterogeneous particle size distribution, with irregular morphology, sharp edges, and rough surface textures. Energy-dispersive X-ray spectroscopy (EDS) analysis (Figure 5B) confirmed the presence of elements such as Cu, Fe, K, O, Ca, Zn, and Si, which is consistent with the chemical composition and mineral species identified by XRD, including cuprite (Cu2O), calcite (CaCO3), hematite (Fe2O3), goethite (FeO(OH)), and vesuvianite (Ca10Al4(Mg,Fe)2Si9O34(OH)4). The observed gold (Au) signal is attributed to the sample preparation process and was not considered part of the mineral association.
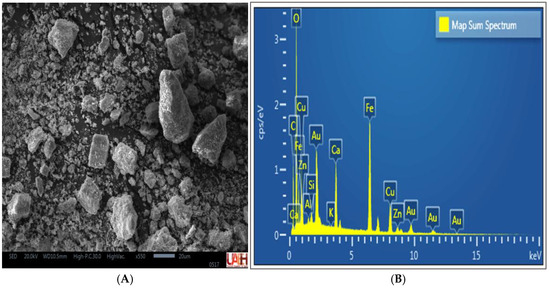
Figure 5.
SEM-EDS analysis of the MC composite. (A) Energy-dispersive spectrum. (B) Secondary electron micrograph.
The secondary electron micrographs of the MM compound particles (Figure 6A) exhibited a more homogeneous texture with smooth edges, corresponding to the laminar morphology typical of carbonate minerals. EDS analysis (Figure 6B) revealed the presence of Cu, Fe, O, C, and Ca, along with trace amounts of Al and Si, in agreement with the results obtained by XRD and XRF. These elements are associated with minerals such as malachite (CuCO3(OH)2), mcguinnessite ((Mg,Cu)2(CO3)(OH)2), hematite (Fe2O3), goethite (FeO(OH)), and calcite (CaCO3), confirming the mineral matrix of the compounds used.
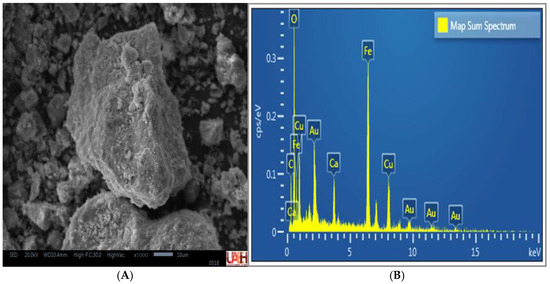
Figure 6.
SEM-EDS analysis of the MM composite. (A) Energy-dispersive spectrum (B) Secondary electron micrograph.
3.5. Thermodynamic Simulation of the Gly-Cu-H2O System
Pourbaix diagrams were constructed considering the predominance of the main metals (Cu and Fe) in both samples in relation to the organic ligand glycine (Gly) in an aqueous medium. The system elements were evaluated under saturation conditions at a reaction temperature of 30 °C.
In Figure 7A, the predominance area of the copper diglycinate complex (Cu(Gly)2), which is responsible for copper complexation in solution, is observed between −0.1 and 1.2 volts and within a pH range of 0 to 11.5, falling inside the water stability region. This confirms the need for oxidizing conditions in the experimental tests and the stability of the ligand under alkaline conditions (Figure 7B). No evidence of iron–glycine complex formation was found, indicating no competition in copper complexation despite the high concentrations of iron (Figure 7C).
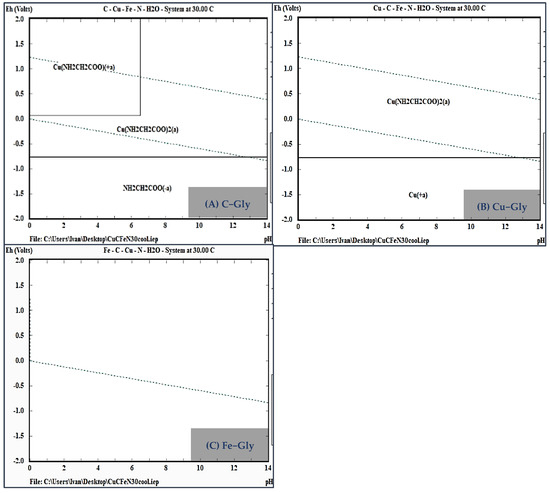
Figure 7.
Eh−pH diagram of the Gly−Mineral system at 30 °C. (A) C−Gly, (B) Cu−Gly, (C) Fe−Gly.
3.6. Comparative Study of Copper Leaching in Glycine-MM and Glycine-MC Systems
El The behavior of the system was analyzed as a function of the concentration of the organic ligand through a series of experiments ranging from 0.1 to 2 mol·L−1, while keeping all other parameters constant. For both mineral compounds, the dissolution curves exhibit a similar trend, with a pronounced conversion period during the first 60 min, followed by stabilization after 240 min of reaction.
Based on the chemical–mineralogical characterization and thermodynamic simulation, reactions were proposed to explain the formation of the copper diglycinate complex (Cu(NH2CH2COO)2). These reactions are based on mass balance, solubility under different pH levels, and copper oxidation states, with the aim of identifying the most stable complex under the proposed leaching system conditions [52].
In Figure 8A, which shows the dissolution of copper from the MC sample powders, no significant dissolution rate was observed at concentrations between 0.1 and 0.5 mol·L−1. Copper dissolution becomes favorable at concentrations above 0.7 mol·L−1, reaching a maximum of 44.66% copper in solution after 180 min at 1 mol·L−1. Beyond this concentration range, copper recovery decreases to 28.4% due to glycine saturation in the system. The initial stage involves the formation of metal hydroxides due to hydrolysis and the presence of oxygen during dynamic agitation, which increases copper availability for complexation with glycine and ensures the stable formation of the diglycinate complex (Equations (2) and (3)).
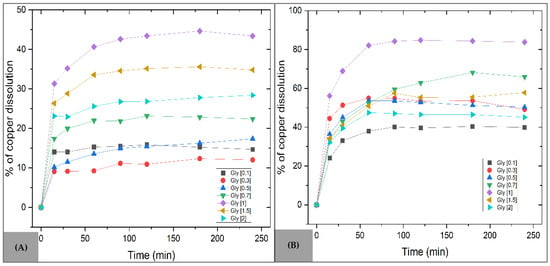
Figure 8.
Effect of glycine concentration on copper dissolution. Operating conditions: Temperature = 30 °C, pH = 10, s/l = 0.3 g/L, stirring = 800 rpm. (A) MC sample (B) MM sample.
For the MM compound (Figure 8B), an improvement in copper dissolution was observed with increasing glycine concentration, achieving the best results at 1 mol·L−1, with a maximum recovery of 84.77% in just 120 min. However, as seen in Figure 8A, higher concentrations did not result in a substantial increase in dissolution, indicating that beyond this point there is no clear dependency between the amount of dissolved copper and the concentration of the complexing agent. The easy protonation of carbonate ions facilitates the dissolution of malachite, ensuring copper availability for complexation with glycine. Sodium carbonate was identified as the residual solid [53], formed through ionic interactions with Na+ and Ca2+ cations [54].
The decrease in copper recovery at glycine concentrations above 1 mol·L−1 can be attributed to various physicochemical limitations of the system. These include the possible saturation of the medium with free glycine, which may shift the speciation equilibrium and hinder the formation of the Cu(Gly)2 complex. Additionally, it has been reported that high glycine concentrations slightly increase the viscosity of the solution, thereby reducing mass transfer efficiency at the solid-liquid interface [34]. Excess glycine may also induce competitive adsorption phenomena on the mineral surface, particularly in the presence of secondary ions such as Ca2+—originating from the NaOH used for pH adjustment—which interferes with copper availability for complex formation [52,55]. On the other hand, higher magnetic stirring speeds may improve dissolution by increasing the contact area between solid particles and the aqueous medium, thereby enhancing species diffusion and partially overcoming these limitations.
To confirm copper complexation from both mineral compounds, the leachates were analyzed using Fourier-transform infrared spectroscopy (FTIR). A control solution containing 1 mol·L−1 glycine was compared with the final aliquots from each mineral sample at the same ligand concentration. The results indicate the presence of the characteristic functional groups of glycine (NH2, CH2, COOH), confirming the purity of the reagent in the control solution. In the leachates from the MC and MM samples, a band at 1572 cm−1 corresponds to the carboxylate group, indicating the coordination of Cu2+ with glycine (Figure 9). Although the band positions for free glycine and the Cu–Gly complex do not vary significantly, the intensities of the free glycine bands decrease, suggesting its reaction with copper ions [56,57]; these findings support the formation of the copper–glycine complex.
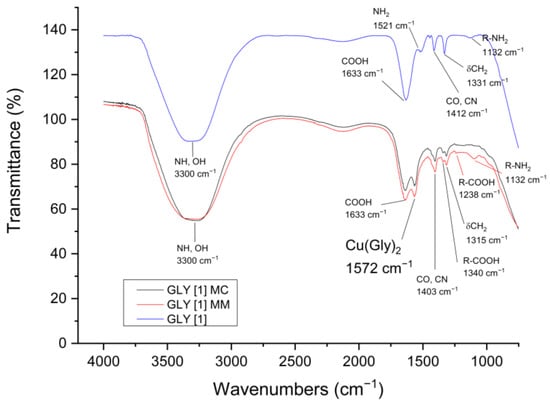
Figure 9.
Infrared spectra of Gly, Gly−MM, and Gly−MC samples.
3.7. Comparative Study of Oxidative Copper Leaching in the Glycine System
Having confirmed the formation of the glycine–copper (Gly–Cu) complex from both mineral compounds, the effect of different oxidizing agents (O3 and H2O2) on copper dissolution was evaluated. Particular attention was given to the use of ozone (O3) due to its viability as an environmentally friendly alternative: it produces no secondary residues upon decomposition, is effective in alkaline media (pH 10–12), and possesses a high oxidation potential of 2.07 V at 25 °C [38,58,59].
Figure 10A presents the copper dissolution curves for the MC sample with ozone addition. Within the studied concentration range, the effect of ozone was consistent, with maximum performance observed at a flow rate of 2 L min−1, achieving 51.92% dissolution in 210 min. This value represents a 7.26% improvement compared to the use of glycine alone, highlighting the role of ozone as a leaching enhancer. In the initial reaction stage (Equation (5)), ozone not only facilitates the formation of metal hydroxides but also releases oxygen, thereby increasing copper availability for complexation (Equation (6)).
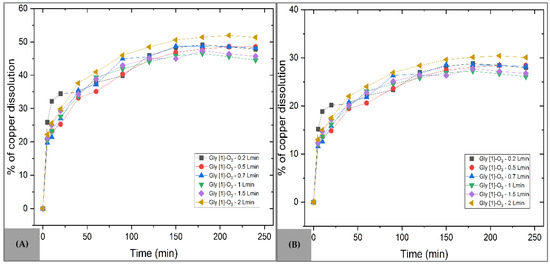
Figure 10.
Effect of O3 flow rate on copper dissolution with glycine. Operating conditions: Temperature = 30 °C, pH = 10, s/l = 0.3 g/L, stirring = 800 rpm, Gly [1] M. (A) MC sample (B) MM sample.
In contrast, for the MM sample (Figure 10B), the addition of ozone reduced the efficiency of the process, with only 30.41% Cu dissolution at a flow rate of 2 L min−1 after 210 min. This negative response can be attributed to the rapid decomposition of ozone in alkaline solutions, which limits its direct action on the mineral. Its low solubility and short half-life under these conditions compromise its effective availability during the reaction. It is worth noting that recent studies have explored strategies to stabilize ozone in aqueous media through ultrasound, co-oxidants, or the use of catalysts [60,61]. Equation (7) summarizes the proposed mechanism for this system.
To evaluate the oxidative effect of hydrogen peroxide (H2O2) on copper leaching from both mineral samples, experiments were conducted by varying only the concentration of this reagent. Figure 11A illustrates the progress of copper dissolution for the MC sample, showing that lower H2O2 concentrations favor higher leaching rates, particularly in the range of 0.5% to 1%. In this case, the initial reaction stage proceeds as shown in Equation (5), promoting the availability of cupric ions in the medium. However, the addition of H2O2 also results in increased formation of metal hydroxides due to its decomposition in aqueous solution (Equation (8)).
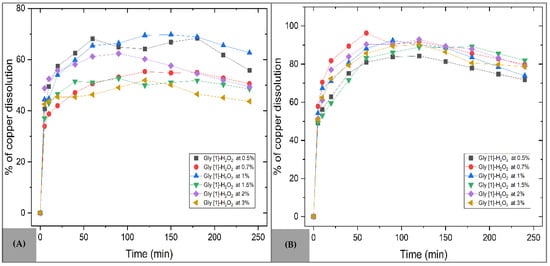
Figure 11.
Effect of H2O2 concentration on copper dissolution with glycine. Operating conditions: Temperature = 30 °C, pH = 10, s/l = 0.3 g/L, stirring = 800 rpm, Gly [1] M. (A) MC sample (B) MM sample.
The oxidizing environment facilitates the conversion of Cu+ to Cu2+, allowing the Cu2+ ion to associate with the glycinate ion [55,62], as represented in Equation (9), achieving a maximum copper dissolution of 69.76% at an H2O2 concentration of 1%.
In the case of the MM sample (Figure 11B), an even more efficient dissolution was observed, with a sharp increase during the first 60 min. A maximum copper dissolution of 96.29% was achieved at an H2O2 concentration of 0.7%, representing an 11.52% improvement compared to the use of glycine alone. This behavior is attributed to the generation of highly reactive hydroxyl radicals, which facilitate the dissolution of base metals. Additionally, H2O2 is compatible with a wide pH and temperature range and decomposes into environmentally benign products—water and oxygen (Equation (10))—positioning it as an efficient and sustainable oxidizing agent [37].
Additionally, the results clearly demonstrate that H2O2 plays a decisive role in copper leaching, achieving a maximum dissolution of 96.29% at a concentration of 0.7%, representing an 11.52% improvement compared to the use of glycine alone. These findings position hydrogen peroxide as the most effective oxidizing agent in this study, outperforming other systems based on tartrate or citric acid [63], and offering a promising approach for optimizing extraction strategies.
The dissolution curves revealed a behavior characterized by the absence of an induction period, followed by a rapid conversion stage that enabled high extraction levels in short reaction times. This pattern was particularly evident in the glycine-H2O2 combination applied to the MM sample. Such behavior is attributed to the oxidized nature of the minerals used (MM and MC), which react favorably in alkaline media, as well as to the high reactivity of the glycine-H2O2 system under the experimental conditions employed.
However, reductions in efficiency were also observed under certain conditions, such as the use of ozone or excessive glycine concentrations, highlighting the influence of multiple physicochemical factors in the leaching process. Accordingly, this study focused on performing a comparative evaluation of extractive efficiency and providing a comprehensive interpretation of the observed chemical behavior.
Although direct measurement of redox potential (Eh) was not conducted, it was assumed to be controlled by the precise dosage of oxidizing agents, considering their standard potentials of 0.94 V for H2O2 and 2.07 V for O3. The Eh-pH predominance diagrams generated by simulation confirmed that the experimental conditions remained within the stability field of the Cu(Gly)2 complex, supporting this indirect control strategy, as applied in previous studies [34,38].
Finally, the combination of glycine as a leaching agent with hydrogen peroxide not only optimizes copper recovery but also offers additional environmental benefits. Recent research has shown that residues generated from glycine-H2O2 leaching systems may exhibit phytostimulant properties, promoting plant germination. This feature suggests an integrated approach to more sustainable mining, combining metallurgical efficiency with potential for ecological restoration [64].
3.8. Residual Solids Characterization
The residual solids were analyzed by SEM–EDS to confirm the dissolution of the target elemental content. Figure 12 and Figure 13 present the energy-dispersive X-ray spectra obtained from particles of the MC and MM compounds after the leaching tests with the highest copper dissolution rates.
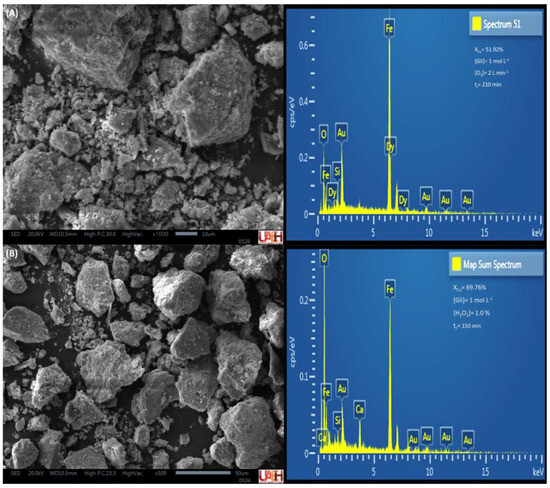
Figure 12.
Energy−dispersive spectra of MC residual solids, O3 (A), and H2O2 (B).
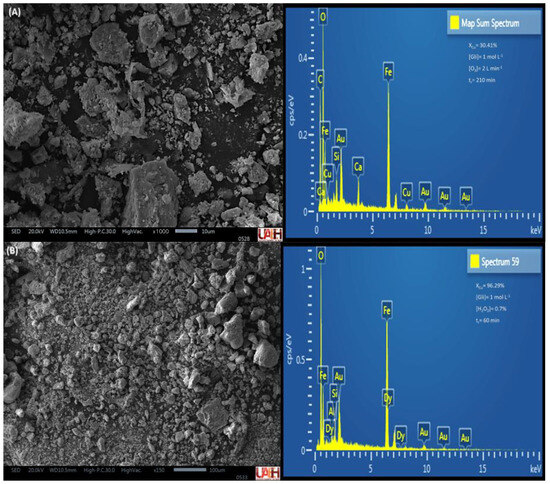
Figure 13.
Energy−dispersive spectra of MM residual solids, O3 (A), and H2O2 (B).
In Figure 12A, a predominant presence of iron is observed, which was not complexed by glycine. The remaining copper corresponds to a maximum leaching efficiency of 51.92%, indicating that 48.08% of the mineral powders remained unreacted. In contrast, in Figure 12B, the addition of H2O2 significantly reduced the copper content in the residual solids, rendering it undetectable in the compositional analysis.
Similarly, in the MM compound, copper was detected in the remaining particles after ozone-regulated leaching, along with Fe, Ca, and Si elements typically found in gangue minerals (Figure 13A). However, the residual solids resulting from leaching of the MM compound with H2O2 (Figure 13B) contained only 3.71% of unreacted copper, rendering it undetectable by energy-dispersive spectroscopy analysis.
Both systems align with the principles of green mining, offering stable, solid residues that are easily manageable and potentially reusable. These findings highlight the importance of sustainable processes in mineral leaching and underscore the value of optimizing systems to maximize metal extraction while minimizing environmental impacts.
4. Discussion
The results obtained provide a detailed understanding of the behavior of glycine-based leaching systems, as well as the impact of oxidizing agents on process efficiency. Through mineralogical, chemical, and thermodynamic analyses, the optimal conditions for copper dissolution from MC and MM were identified, two minerals that exhibit markedly different behaviors in the leaching medium.
4.1. Influence of Mineralogical Composition
The extractive viability of a copper deposit depends on the concentration and quality of the mineral species present. These deposits commonly host a variety of copper-bearing minerals, with MC being one of the most relevant due to its high copper content, making it a primary source for extraction [65]. Malachite, as a copper carbonate, also plays a key role by facilitating leaching processes during acidic hydrometallurgical stages. However, its frequent association with other carbonates, such as calcite, can alter reaction chemistry and hinder copper dissolution through conventional methods. In this context, the diverse mineralogical composition of the MC sample highlights the presence of key copper minerals, reinforcing its relevance to oxide-type copper deposits [66].
The mineralogical differences identified have a significant influence on the efficiency of the leaching process. MM exhibits greater susceptibility to dissolution in alkaline media due to the ease with which its carbonate ions are protonated, allowing for effective copper release. In contrast, MC requires a preliminary oxidative step to convert Cu+ into Cu2+ and thus facilitate dissolution [31,52]. These particularities underscore the importance of proper mineralogical characterization when exploring alternative leaching systems, employing complementary techniques such as XRD and SEM–EDS.
4.2. Stability of the Cu-Gly Complex and Selectivity of Glycine
The formation of the copper diglycinate complex (Cu(Gly)2), confirmed by infrared spectroscopy (Figure 9), represents one of the key features of the proposed system. This complex is highly stable under alkaline conditions, as demonstrated in the predominance diagrams (Figure 7), where no evidence of complexation with iron was observed, supporting the selectivity of glycine toward copper. This behavior has been previously reported in studies involving glycine applied to mixed ores and smelting residues [30,57]. Such selectivity is especially valuable when processing complex minerals containing coexisting metals such as Fe, Ca, or Zn. The absence of secondary complex formation prevents unnecessary reagent consumption and improves the purity of the resulting solution.
Moreover, electrochemical studies and X-ray photoelectron spectroscopy (XPS) have demonstrated that glycine facilitates copper dissolution [67]. According to the proposed reaction pathway, metallic copper is oxidized by ozone and hydrogen peroxide, generating cupric ions that subsequently form highly soluble and stable complexes with glycine [68].
4.3. Effect of Glycine Concentration
The copper dissolution curves (Figure 8) show that, for both MC and MM, process efficiency improves as glycine concentration increases, reaching an optimal point at 1 mol·L−1. At higher concentrations, recovery decreases due to system saturation and limited solubility [52]. This behavior is consistent with similar studies conducted on copper oxide-type minerals and electronic waste [3].
The copper dissolution curves (Figure 8) reveal a clear pattern in both samples: increasing the glycine concentration initially enhances process efficiency, up to an optimal point at 1 mol·L−1, beyond which the trend reverses. This behavior suggests the existence of an ideal operating window, beyond which excess ligand offers no additional benefit and may even negatively impact recovery.
Although both samples exhibit similar overall behavior, they differ in their sensitivity to increasing reagent concentration. The sample with a higher proportion of MM showed a more sustained response, while the one containing MC experienced a more pronounced decline after exceeding the optimal threshold. This distinction underscores the influence of mineralogy on the stability of the glycine–metal system.
Despite these variations, the consistency in the observed behavior allows 1 mol·L⁻1 to be established as an efficient reference concentration for glycine leaching in alkaline systems. Furthermore, it reinforces the importance of defining specific conditions for each mineral type in order to maximize process efficiency while avoiding unnecessary reagent consumption.
4.4. Comparison Between Oxidizing Agents: O3 vs. H2O2
The use of oxidizing agents such as ozone (O3) and hydrogen peroxide (H2O2) proved to be decisive for the performance of the alkaline glycine system, especially in compounds with distinct mineralogical characteristics. Although ozone presented operational limitations, its application remains relevant from an environmental perspective. The low efficiency observed may be attributed to its limited solubility and rapid decomposition in alkaline media, phenomena widely documented in the literature. Gurol and Singer [69] demonstrated that this degradation intensifies at high pH due to the generation of hydroxyl radicals (•OH), while Weavers and Hoffmann [70] noted that even under continuous supply, the limited mass transfer of ozone compromises its effectiveness as an oxidant. Therefore, the low performance observed is consistent with findings from systems applied to sulfide minerals.
Despite its constraints, ozone remains attractive as an eco-friendly option, as its decomposition does not produce polluting by-products. This has prompted research aimed at improving its effectiveness, such as the use of ultrasound to enhance mass transfer [71] or catalysts to promote the formation of reactive species [72]. These approaches open new possibilities for adapting ozone use to more demanding leaching processes, provided that system conditions are optimized.
In contrast, hydrogen peroxide exhibited more favorable behavior, attributed to its ability to generate highly reactive species that facilitate the oxidation of metallic compounds. Its compatibility with alkaline media and decomposition into harmless products has driven its adoption in sustainable industrial applications. Parvulescu et al. [73] highlighted its efficiency in catalytic water treatment, while Shi et al. [74] emphasized its usefulness in battery recycling processes, noting its safety, biodegradability, and chemical stability. In the context of this study, the incorporation of H2O2 not only improved system efficiency but also enabled significantly shorter operation times compared to traditional agents.
The glycine-H2O2 combination thus emerges as an effective and environmentally responsible alternative, with the potential to be integrated into sustainable mining frameworks. Işildar [75] emphasized the low toxicity and operational viability of glycine in hydrometallurgical processes, and previous studies such as that by Tanda et al. [52] had already demonstrated its effectiveness under controlled conditions. However, the approach presented in this work provides a novel contribution by applying an experimental design that systematically compares different oxidants in alkaline media at room temperature, without requiring additional redox adjustments.
Additionally, the validation of the combined use of glycine and H2O2 in high-grade oxidized minerals represents a significant advancement over studies focused on sulfide materials such as chalcopyrite [34]. The fact that this system generates residues with phytostimulant properties, as suggested by recent studies [64], reinforces its value not only from a metallurgical recovery standpoint but also in terms of environmental circularity. Thus, the findings presented here not only confirm previously reported trends such as the operational limitations of ozone [38,70] or the potential of the glycine–peroxide system in leaching applications [67] but also extend their applicability toward more sustainable and scalable contexts.
4.5. Solid Residue Analysis
SEM–EDS analysis of the post-leaching residues (Figure 12 and Figure 13) demonstrated the effectiveness of the system in copper removal. Following the use of H2O2, copper content was virtually undetectable, confirming the high efficiency of the process. Moreover, the resulting residues are chemically stable and have shown potential for agricultural applications as soil enhancers, due to their low heavy metal content and richness in carbonates and phosphates [64].
Although no direct phytotoxicity or biostimulation tests were performed in this study, various previous reports support the potentially beneficial use of the generated solid residues. Glycine, used as the leaching agent, has been reported as a biostimulant in crops, improving biomass production, photosynthesis, and enzymatic activity [76,77]. The oxidants used (H2O2 and O3) decompose into harmless by-products and leave no hazardous residues if properly stabilized [73]. Unlike acidic processes, glycine leaching operates in an alkaline medium that does not contribute to the acidification of surrounding soils or water bodies. Although the use of high-pH solutions can pose ecological risks, recent studies have shown that residues generated under alkaline conditions (pH 9.5) may even exhibit phytostimulant effects, as demonstrated by the Zucconi germination test [64].
This evidence supports the hypothesis that the residues generated in this investigation could be integrated into environmentally responsible management strategies and may even possess added value as products with phytostimulant potential or as soil enhancers, promoting a circular economy approach in both mining and agricultural contexts.
4.6. Economic Comparison of the Leaching Process
To estimate the cost associated with the leaching of oxidized copper ores using glycine and hydrogen peroxide, a theoretical scenario was considered for the treatment of one ton (1000 kg) of ore. According to Equations (9) and (10), two moles of glycine as a complexing agent and one mole of hydrogen peroxide as an oxidant are required per mole of copper.
Two scenarios were evaluated: MC with 21.6% copper and MM with 12.7%, corresponding to 216 kg and 127 kg of copper per ton of ore, or 3398.9 and 1998.4 moles of Cu, respectively. Based on these values, and considering the molecular weights of glycine (75.07 g·mol−1) and H2O2 (34.01 g·mol−1), the estimated requirements were 510.3 kg of glycine and 115.6 kg of H2O2 for MC, and 300.0 kg of glycine and 68.0 kg of H2O2 for malachite. With average market prices of 3.50 USD/kg for glycine and 0.65 USD/kg for H2O2, the total costs amounted to 1860.7 USD and 1094.3 USD, respectively.
For comparison, conventional systems were considered, including typical processes using sulfuric acid and ozone (for MC), and ammonia (for malachite). The estimated reagent consumption was 333 kg of H2SO4 and 82 kg of O3 for MC, and 136 kg of NH3 for malachite. Using reference prices of 0.30 USD/kg (H2SO4), 0.90 USD/kg (O3), and 0.75 USD/kg (NH3), the total costs were significantly lower: 173.5 USD for MC and 102.1 USD for malachite.
Although conventional acid leaching processes with sulfuric acid or other inorganic agents present considerably lower initial costs estimated at 173.5 USD for MC and 102.1 USD for MM compared to 1860.73 USD and 1094.32 USD for the glycine–H2O2 system, this difference does not necessarily translate to an overall economic advantage. Conventional systems generate acidic by-products, toxic effluents, and sludges that require specialized treatment and regulatory compliance, which increases operational and environmental remediation costs. In contrast, the proposed system using glycine and green oxidants such as H2O2 or O3 reduces the generation of hazardous residues and operates under mild conditions, lowering post-process management expenses. Moreover, glycine is a biodegradable reagent that can be recycled without significant loss of efficiency, thereby reducing long-term net consumption [78,79]. The oxidants employed decompose into harmless products (water and oxygen), avoiding additional loads in effluent treatment [73].
This approach not only promotes more environmentally sustainable operation, but also has the potential to offset its higher initial costs through medium- and long-term savings in waste management, regulatory compliance, and energy consumption. Therefore, the glycine–oxidant system emerges as an economically viable alternative when considering the total integrated cost of the process.
5. Conclusions
This study demonstrated that the leaching system using glycine as the lixiviant, combined with hydrogen peroxide (H2O2) and ozone (O3) as oxidizing agents, constitutes an effective and sustainable alternative for copper extraction from minerals such as MC and malachite. The results highlighted a significant optimization in dissolution rates achieved with H2O2, with recoveries of up to 96.3% for MM in just 60 min and 69.7% for MC in 150 min. Although O3 showed lower efficiency due to its limited solubility, its environmentally benign nature positions it as a complementary oxidant in processes focused on sustainability.
Glycine proved to be a non-toxic and biodegradable lixiviant with high selectivity toward copper, minimizing the dissolution of other metals and generating chemically stable solid residues. This significantly reduces the risks associated with landfill disposal. The system’s operational simplicity, low reagent costs, and the potential for glycine regeneration consolidate this method as an economically viable solution aligned with the goals of green mining.
Additionally, the applicability of the glycine–oxidant system for processing low-grade or complex ores is attributed to its capacity to maximize copper recovery while minimizing environmental impacts in the treatment of secondary and marginal deposits. The stability of the generated residues and the fully alkaline operating conditions eliminate the hazards associated with the use of strong acids, enhancing both occupational safety and the durability of mining infrastructure.
Author Contributions
J.I.M.: conceptualization, methodology, software, research, writing, preparation of the original draft, and funding. A.M.T.: conceptualization, methodology, writing—review and editing, and funding. M.R.: data analysis, data curation, supervision, and funding. N.T.: software, visualization, supervision, and funding. G.C.: methodology, software, visualization, and funding. U.M.F.: methodology, software, visualization, and funding. M.P.L.: validation, formal analysis, visualization and methodology, software, visualization, and funding. G.U.: methodology, software, visualization, and funding. J.C.J.: conceptualization, methodology, data curation, writing—review and editing, and editing, and funding. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors wish to thank the Universidad Autónoma del Estado de Hidalgo for the institutional support provided throughout this research. Additionally, we extend our gratitude to the Área Académica de Ciencias de la Tierra y Materiales for facilitating the necessary facilities and equipment to carry out this project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rówiński, E.; Pławecki, M. Structural and electrical properties of electrodeposited single junction of cuprous (I) oxide copper. arXiv 2016. [Google Scholar] [CrossRef]
- Van Yken, J.; Boxall, N.J.; Cheng, K.Y.; Nikoloski, A.N.; Moheimani, N.R.; Kaksonen, A.H. E-waste recycling and resource recovery: A review of technologies, barriers, and facilitators with a focus on Oceania. Metals 2021, 11, 1313. [Google Scholar] [CrossRef]
- Kumari, R.; Prabhakar, R.; Samadder, S.R. Enhanced copper extraction from waste printed circuit boards using glycine after supercritical methanol pre-treatment: Process optimization, leaching kinetics, and thermodynamic analysis. Waste Manag. 2025, 193, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Nazer, A.; Pavez, O.; Rojas, F.; Aguilar, C. A review of copper slag applications. In Proceedings of the Iberomet XI. X Conamet/Sam, Viña del Mar, Chile, 2–5 November 2010. [Google Scholar] [CrossRef]
- Alguacil, F.J. Copper recovery through leaching–solvent extraction–electrowinning: Towards the 21st century. Rev. De Met. 1998, 34, 499–506. [Google Scholar] [CrossRef]
- Herreros, O.; Bernal, N.; Quiroz, R.; Fuentes, G.; Viñals, J. Leaching of copper concentrates using NaCl and the soluble copper provided by the concentrate itself. Rev. De Met. 2005, 41, 384–392. [Google Scholar] [CrossRef]
- Guamán, F. A look at digital television through IPTV technologies over copper network with ADSL technology. Rev. De La Fac. De Cienc. Químicas 2017, 41–55. [Google Scholar]
- Yin, S.; Wang, L.; Kabwe, E.; Chen, X.; Yan, R.; An, K.; Wu, A. Copper bioleaching in China: Review and perspective. Minerals 2018, 8, 32. [Google Scholar] [CrossRef]
- Safari, H.; Rezaee, M.; Chelgani, S.C. Ecofriendly leaching agents for copper extraction—An overview of amino and organic acid applications. Green Smart Min. Eng. 2024, 1, 336–345. [Google Scholar] [CrossRef]
- Hong, J.; Chen, Y.; Liu, J.; Ma, X.; Qi, C.; Ye, L. Análisis del ciclo de vida de la producción de cobre: Un estudio de caso en China. Int. J. Life Cycle Assess 2018, 23, 1814–1824. [Google Scholar] [CrossRef]
- Reyes, A.M.; Pompa, N.P.; Montoya, M.D. Environmental impact assessment produced by residuals from La Mina Grande on the Cobre River. Rev. Cuba. De Química 2009, 21, 59–65. [Google Scholar]
- Schlesinger, M.E.; King, M.J.; Sole, K.C.; Davenport, W.G. Extractive Metallurgy of Copper; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 0-08-0440290. [Google Scholar]
- Habashi, F. Principles of Extractive Metallurgy; Routledge: Boca Raton, Fl, USA, 2017. [Google Scholar] [CrossRef]
- Prado, O.A. Situation and Perspectives of Metallic Mining in Argentina; Economic Commission for Latin America and the Caribbean (CEPAL): Santiago, Chile, 2005. [Google Scholar]
- Brierley, C.L. How will biomining be applied in future? Trans. Nonferrous Met. Soc. China 2008, 18, 1301–1310. [Google Scholar] [CrossRef]
- Martínez, L.O. Copper mining in the Norte Chico (traditional) and the perspective of medium and small producers. Si Somos Am. Rev. De Estud. Transfronterizos 2010, 10, 37–59. [Google Scholar]
- Perea, C.G.; Ihle, C.; Dyer, L.; Díaz Quezada, S.; Estay, H. Study of Copper Oxide Leaching in Alkaline Monosodium Glutamate Solution. Minerals 2024, 14, 714. [Google Scholar] [CrossRef]
- Lagos, G. Environmental impacts of mining in Chile. Ambiente Y Desarro. 1997, 4, 13–20. [Google Scholar]
- Watling, H.R. Chalcopyrite hydrometallurgy at atmospheric pressure: Review of acidic sulfate, sulfate–chloride, and sulfate–nitrate process options. Hydrometallurgy 2013, 140, 163–180. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2011; ISBN 978-92-4-154761-1. [Google Scholar]
- Biswas, A.K.; Davenport, W.G. Extractive Metallurgy of Copper: International Series on Materials Science and Technology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 20, ISBN 0-08-024736-9. [Google Scholar]
- Torres, D.A.G. Sulfuric Acid Consumption and Kinetics of Leaching from Oxidized Copper Minerals. Bachelor’s Thesis, Universidad de Chile, Santiago, Chile, 2011. [Google Scholar] [CrossRef]
- Trujillo, J.Y.; Cisternas, L.A.; Gálvez, E.D.; Mellado, M.E. Optimal design and planning of heap leaching process. Application to copper oxide leaching. Chem. Eng. Res. Des. 2014, 92, 308–317. [Google Scholar] [CrossRef]
- Araya, G.; Toro, N.; Castillo, J.; Guzmán, D.; Guzmán, A.; Hernández, P.; Jeldres, R.I.; Sepúlveda, R. Lixiviación de minerales de óxido de cobre mediante adición de ácido débil de fundiciones de cobre. Metales 2020, 10, 627. [Google Scholar] [CrossRef]
- Petersen, J.; Dixon, D.G. Modeling and optimization of heap bioleaching processes. In Biomining; Springer: Berlin/Heidelberg, Germany, 2007; pp. 153–176. [Google Scholar] [CrossRef]
- Deng, Z.; Oraby, E.; Li, H.; Eksteen, J. Extracción de cobre de calcopirita mediante soluciones alcalinas de glicina-amoníaco. Minerals 2022, 12, 1507. [Google Scholar] [CrossRef]
- Wu, J.; Ahn, J.; Lee, J. A Sustainable Complexation Leaching of Critical Metals from Spent Lithium-Ion Batteries by Glycine in a Neutral Solution. Min. Metall. Explor. 2024, 41, 1605–1617. [Google Scholar] [CrossRef]
- Mohammed, T.; Bezuidenhout, G.A.; Oraby, E.A.; Eksteen, J.J. Separación secuencial de cobalto, cobre y níquel de soluciones alcalinas de glicinato mediante extracción con disolventes. J. Sustain. Metall. 2024, 10, 2455–2468. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, D.; Liu, H.; Fan, G.; Peng, W.; Cao, Y. Selective complexation leaching of copper from copper smelting slag with an alkaline glycine solution: An effective recovery method of copper from secondary resources. Sep. Purif. Technol. 2023, 326, 124619. [Google Scholar] [CrossRef]
- Eksteen, J.J.; Oraby, E.A.; Tanda, B.C. A conceptual process for copper extraction from chalcopyrite in alkaline glycinate solutions. Miner. Eng. 2017, 108, 53–66. [Google Scholar] [CrossRef]
- Oraby, E.A.; Eksteen, J.J. Selective leaching of copper from a gold–copper concentrate in glycine solutions. Hydrometallurgy 2014, 65, 96–102. [Google Scholar]
- Gutiérrez, E.R.C.; Sarmiento, A.W.S. Evaluation of the basic medium leaching process for oxidized copper minerals. Rev. Investig. Altoandinas 2015, 17, 411–416. [Google Scholar]
- Hurtado, R.; Suazo, A. High-temperature silver leaching in complex pyrite ore. Rev. De La Soc. Química Del Perú 2019, 85, 97–108. [Google Scholar]
- Flores, D.J.; Graber, T.A.; Angel-Castillo, A.H.; Hernández, P.C.; Taboada, M.E. Uso de peróxido de hidrógeno como agente oxidante en la lixiviación de calcopirita: Una revisión. Metals 2025, 15, 531. [Google Scholar] [CrossRef]
- Celik, H. Extraction of gold and silver from a Turkish gold ore through thiourea leaching. Min. Metall. Explor. 2004, 21, 144–148. [Google Scholar] [CrossRef]
- Lira, J.M.G.; Aguilar, M.D.J.S.; Pedroza, F.R.C.; González, E.N.A. A comparison of alternative reagents to cyanide as gold leaching agents: A review. Cienc. Lat. Rev. Científica Multidiscip. 2023, 7, 2410–2434. [Google Scholar] [CrossRef]
- González, C.; Valbuena, A.; Celis, B.; Perentena, L.; Colina, M. Oxidative degradation of chitosan with hydrogen peroxide. Rev. Iberoam. De Polímeros Y Mater. 2015, 16, 43–68. [Google Scholar]
- Wang, J.; Faraji, F.; Ghahreman, A. Evaluation of ozone as an efficient and sustainable reagent for chalcopyrite leaching: Process optimization and oxidative mechanism. J. Ind. Eng. Chem. 2021, 104, 333–344. [Google Scholar] [CrossRef]
- Xie, F.; Chen, J.-N.; Wang, J.; Wang, W. Review of gold leaching in thiosulfate-based solutions. Trans. Nonferrous Met. Soc. China 2021, 31, 3506–3529. [Google Scholar] [CrossRef]
- Barros, K.S.; Vielmo, V.S.; Moreno, B.G.; Riveros, G.; Cifuentes, G.; Bernardes, A.M. Datos de composición química de las principales etapas de la producción de cobre a partir de minerales sulfurados en Chile: Una revisión para contribuir a los estudios de economía circular. Minerals 2022, 12, 250. [Google Scholar] [CrossRef]
- Bazán, V.; Sarquis, P.; Brandaleze, E. Characterization of a copper mineral in Argentina for matte production. Dyna 2011, 78, 220–228. [Google Scholar]
- Guo, S.; Zhou, X.; Song, S.; Mei, Y.; Zhao, J.; Fang, Y. Optimization of leaching conditions for removing sodium from sodium-rich coals by orthogonal experiments. Fuel 2017, 208, 499–507. [Google Scholar] [CrossRef]
- Oraby, E.A.; Eksteen, J.J. Gold and copper leaching from gold-copper ores and concentrates using a glycine-based process. Hydrometallurgy 2017, 169, 195–204. [Google Scholar]
- Behera, S.K.; Meena, H.; Chakraborty, S.; Meikap, B.C. Application of response surface methodology (RSM) for optimization of leaching parameters for ash reduction from low-grade coal. Int. J. Min. Sci. Technol. 2018, 28, 621–629. [Google Scholar] [CrossRef]
- Petrović, S.J.; Bogdanović, G.D.; Antonijević, M.M. Leaching of chalcopyrite with hydrogen peroxide in hydrochloric acid solution. Trans. Nonferrous Met. Soc. China 2018, 28, 1444–1455. [Google Scholar] [CrossRef]
- Karaca, H.; Ceylan, K. Chemical cleaning of Turkish lignites by leaching with aqueous hydrogen peroxide. Fuel Process. Technol. 1997, 50, 19–33. [Google Scholar] [CrossRef]
- Ji, G.; Liao, Y.; Wu, Y.; Xi, J.; Liu, Q. A review on hydrometallurgical leaching research of low-grade complex chalcopyrite. J. Sustain. Metall. 2022, 8, 964–977. [Google Scholar] [CrossRef]
- Ballester, A.; Felipe Verdeja, L.; Sancho, J. Extractive Metallurgy, 2nd ed.; Síntesis: Barcelona, Spain, 2000; Volume 1, ISBN 84-7738-802-4. [Google Scholar]
- Megaw, P.K.M.; Ruiz, J.; Titley, S.R. High-temperature, carbonate-hosted Ag-Pb-Zn(Cu) deposits of northern Mexico. Econ. Geol. 1988, 83, 1856–1885. [Google Scholar] [CrossRef]
- Shabani, M.A.; Irannajad, M.; Azadmehr, A.R. Investigación sobre la lixiviación de malaquita con ácido cítrico. Int. J. Miner. Met. Mater. 2012, 19, 782–786. [Google Scholar] [CrossRef]
- Mexicano, S.G. Mapimí Geological-Mining Map, G13-D14, Dgo., Scale 1: 50,000. Boletín: FGDC-STD-001-1998. Servicio Geologico Mexicano: Pachuca, Hidalgo, México, 2006.
- Tanda, B.C.; Eksteen, J.J.; Oraby, E.A. An investigation into the leaching behavior of copper oxide minerals in aqueous alkaline glycine solutions. Hydrometallurgy 2017, 167, 153–162. [Google Scholar] [CrossRef]
- Rey, E.O.C.M.J.d.P.Á.; Cervantes, M.L.R. Metallic Elements and Their Main Compounds, 1st ed.; Uned Universidad Nacional de Educacion a Distancia: Madrid, Spain, 2024; Volume 1, ISBN 9788436279559. [Google Scholar]
- Lide, D.R. Handbook of Chemistry and Physics, 89th ed.; CRC Press: Boston, MA, USA, 2008; Volume 1, ISBN 0-8493-0485-7. [Google Scholar]
- Toro, N.; Gálvez, E.; Robles, P.; Castillo, J.; Villca, G.; Salinas-Rodríguez, E. Uso de recursos hídricos alternativos en procesos de lixiviación de cobre en la industria minera chilena: Una revisión. Metals 2022, 12, 445. [Google Scholar] [CrossRef]
- Hamada, Y.Z.; Makoni, N.; Hamada, H. Cu2+ complexes with the simplest amino acid glycine (Gly). J. Nanomed. Res. 2017, 5, 00123. [Google Scholar] [CrossRef]
- Mestizo, P.D.; Narváez, D.M.; Pinzón-Ulloa, J.A.; Di Bello, D.T.; Franco-Ulloa, S.; Macías, M.A.; Groot, H.; Miscione, G.P.; Suescun, L.; Hurtado, J.J. Nuevos complejos con ligandos donantes de bases de Schiff de cumarina tetradentada de ONNO: Estructuras de rayos X, cálculos de DFT, dinámica molecular y posible actividad anticancerígena. Biometals 2021, 34, 119–140. [Google Scholar] [CrossRef]
- Havlik, T.; Skrobian, M. Acid leaching of chalcopyrite in the presence of ozone. Can. Metall. Q. 1990, 29, 133–139. [Google Scholar] [CrossRef]
- Umbarila-Ortega, M.F.; Prado-Rodríguez, J.S.; Agudelo-Valencia, R.N. Sulfide removal using ozone as an oxidizing agent in tannery wastewater. Rev. Fac. De Ing. 2019, 28, 25–38. [Google Scholar] [CrossRef]
- Sokić, M.; Marković, B.; Stanković, S.; Kamberović, Ž.; Štrbac, N.; Manojlović, V.; Petronijević, N. Kinetics of chalcopyrite leaching by hydrogen peroxide in sulfuric acid. Metals 2019, 9, 1173. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Y.; He, C.; Yin, R.; Liu, J.; Qiu, T. Ultrasound-enhanced catalytic ozonation oxidation of ammonia in aqueous solution. Int. J. Environ. Res. Public Health 2019, 16, 2139. [Google Scholar] [CrossRef]
- Zhao, L.; Ma, J.; Zhai, X. Enhanced mechanism of catalytic ozonation by ultrasound with orthogonal dual frequencies for the degradation of nitrobenzene in aqueous solution. Ultrason. Sonochem. 2010, 17, 84–91. [Google Scholar] [CrossRef]
- Gutiérrez, E.R.C. Copper recovery from malachite using tartrate solutions in the leaching process, Puno 2017. Rev. De Investig. 2020, 9, 35–46. [Google Scholar] [CrossRef]
- Barragán-Mantilla, S.P.; Gascó, G.; Almendros, P.; Méndez, A. Insights into the use of green leaching systems based on glycine for the selective recovery of copper. Miner. Eng. 2024, 206, 108534. [Google Scholar] [CrossRef]
- Torres, J.V. Capitalismo y minería global: Perspectivas latinoamericanas 1500–1914. Hist. Crítica 2023, 89, 43–76. [Google Scholar] [CrossRef]
- Mark, A.; Petersen, M.A.; Della Libera, M.; Jannas, R.R.; Maynard, S.R. Geología del depósito de oro y plata relacionado con el pórfido de Cerro San Pedro, San Luis Potosí, México. Soc. Econ. Geol. 2001, 217–230. [Google Scholar] [CrossRef]
- Seal, S.; Kuiry, S.C.; Heinmen, B. Effect of glycine and hydrogen peroxide on chemical–mechanical planarization of copper. Thin Solid Film. 2003, 423, 243–251. [Google Scholar] [CrossRef]
- Du, T.; Luo, Y.; Desai, V. The combinatorial effect of complexing agent and inhibitor on chemical–mechanical planarization of copper. Microelectron. Eng. 2004, 71, 90–97. [Google Scholar] [CrossRef]
- Gurol, M.D.; Singer, P.C. Kinetics of ozone decomposition: A dynamic approach. Environ. Sci. Technol. 1982, 16, 377–383. [Google Scholar] [CrossRef]
- Weavers, L.K.; Hoffmann, M.R. Sonolytic decomposition of ozone in aqueous solution. Environ. Sci. Technol. 1998, 32, 3941–3947. [Google Scholar] [CrossRef]
- Zhang, D.; Fu, L.; Liu, H.; Li, H.; Wang, S.; Zhang, M.; Zhu, M.; Zhang, L. High-efficiency leaching of chalcopyrite by ozone with ultrasonic promotion: Kinetics and mechanism. J. Mol. Liq. 2024, 401, 124682. [Google Scholar] [CrossRef]
- Li, H.; Hu, C.; He, X.; Wang, J.; Tian, S.; Zhu, X.; Mao, X. Mechanism and kinetics study of vanadium leaching from landfilled metallurgical residues by ultrasonic with ozonation enhancement in a low-acid medium. Ultrason. Sonochem. 2024, 109, 106998. [Google Scholar] [CrossRef]
- Parvulescu, V.I.; Epron, F.; Garcia, H.; Granger, P. Recent progress and prospects in catalytic water treatment. Chem. Rev. 2021, 122, 2981–3121. [Google Scholar] [CrossRef]
- Shi, R.; Wang, B.; Tang, D.; Wei, X.; Zhou, G. Towards high value-added recycling of spent lithium-ion batteries for catalysis application. Electrochem. Energy Rev. 2024, 7, 28. [Google Scholar] [CrossRef]
- Işildar, A. Metal Recovery from Electronic Waste: Biological Versus Chemical Leaching for Recovery of Copper and Gold; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Bartucca, M.L.; Cerri, M.; Del Buono, D.; Forni, C. Use of biostimulants as a new approach for the improvement of phytoremediation performance—A Review. Plants 2022, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- González-Quero, M.; Aguilar-Garrido, A.; Paniagua-López, M.; García-Huertas, C.; Sierra-Aragón, M.; Blasco, B. Physiological Response of Lettuce (Lactuca sativa L.) Grown on Technosols Designed for Soil Remediation. Plants 2024, 13, 3222. [Google Scholar] [CrossRef] [PubMed]
- Jamett, I.; Carrasco, P.; Olmos, M.; Hernández, P. Glycine/glutamate: “Green” alternatives to recover metals from minerals/residues—Review of current research. Minerals 2022, 13, 22. [Google Scholar] [CrossRef]
- Tanda, B.C.; Oraby, E.A.; Eksteen, J.J. Recovery of copper from alkaline glycine leach solution using solvent extraction. Sep. Purif. Technol. 2017, 187, 389–396. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).