Effect of Cross- or Unidirectional Rolling on the Microstructure, Corrosion Rate, and Hemolysis of Ternary Magnesium–Zinc–Gallium Alloys
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
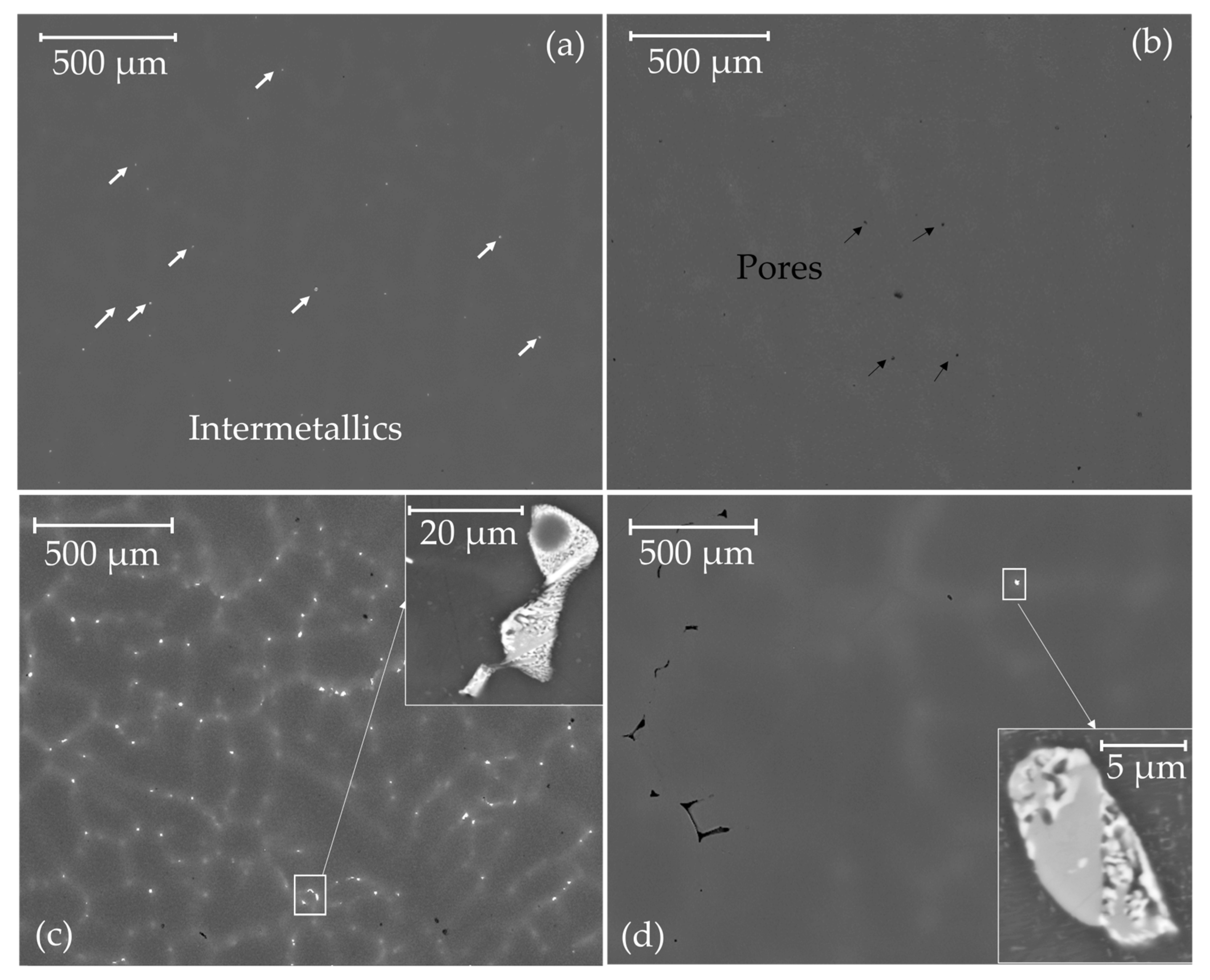
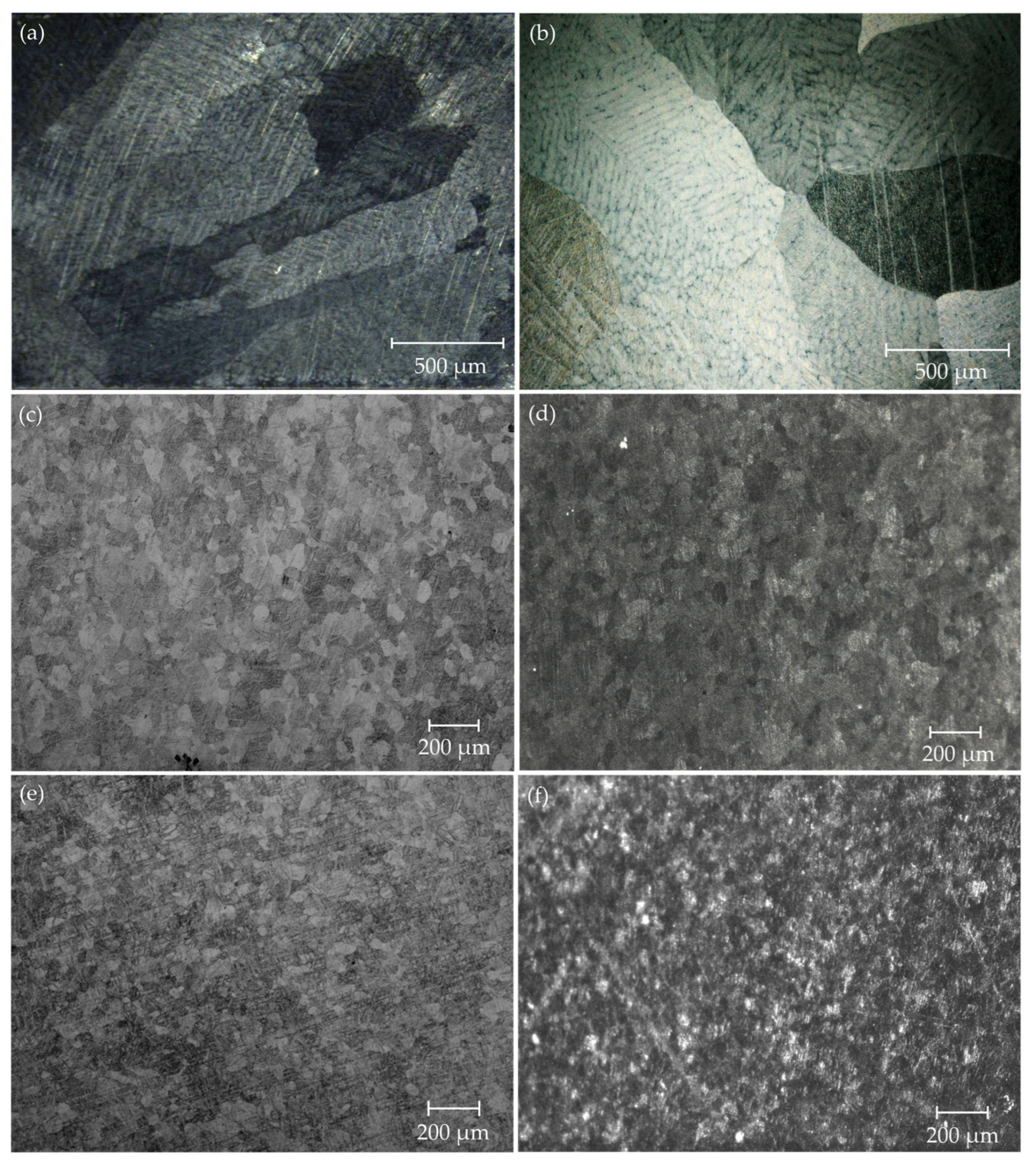
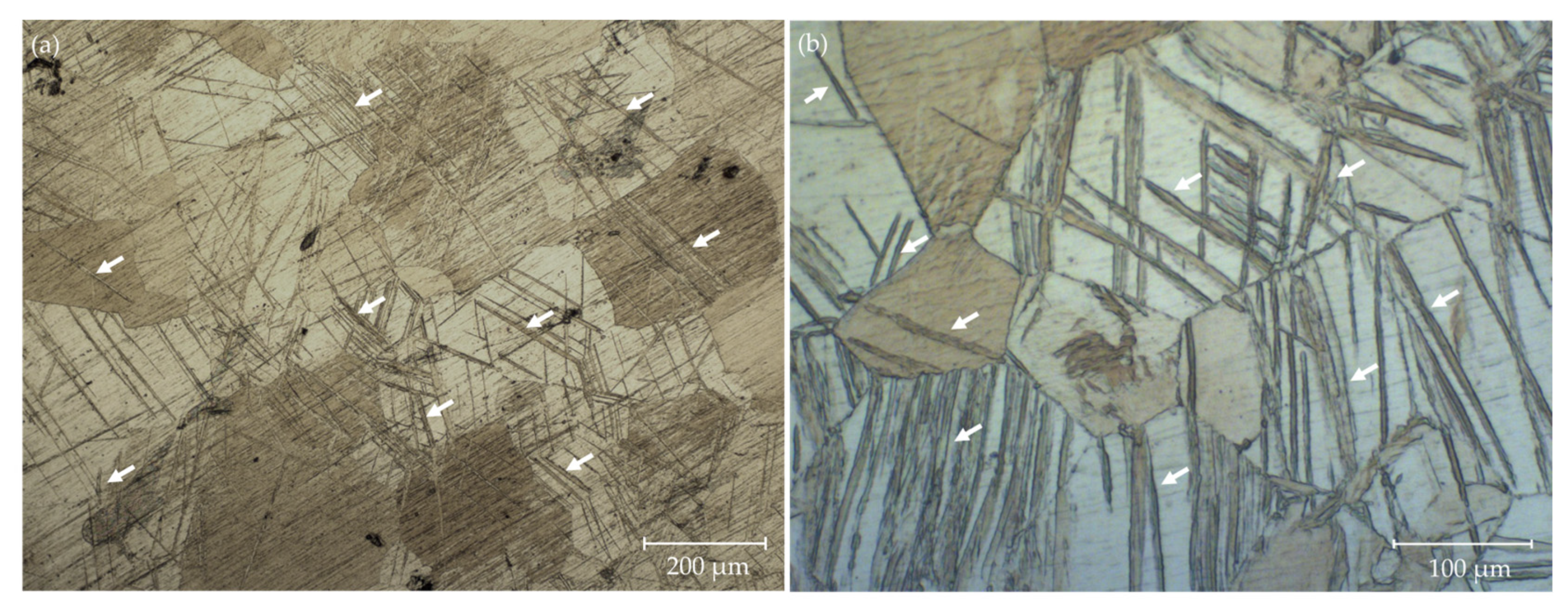
3.1. Microstructure
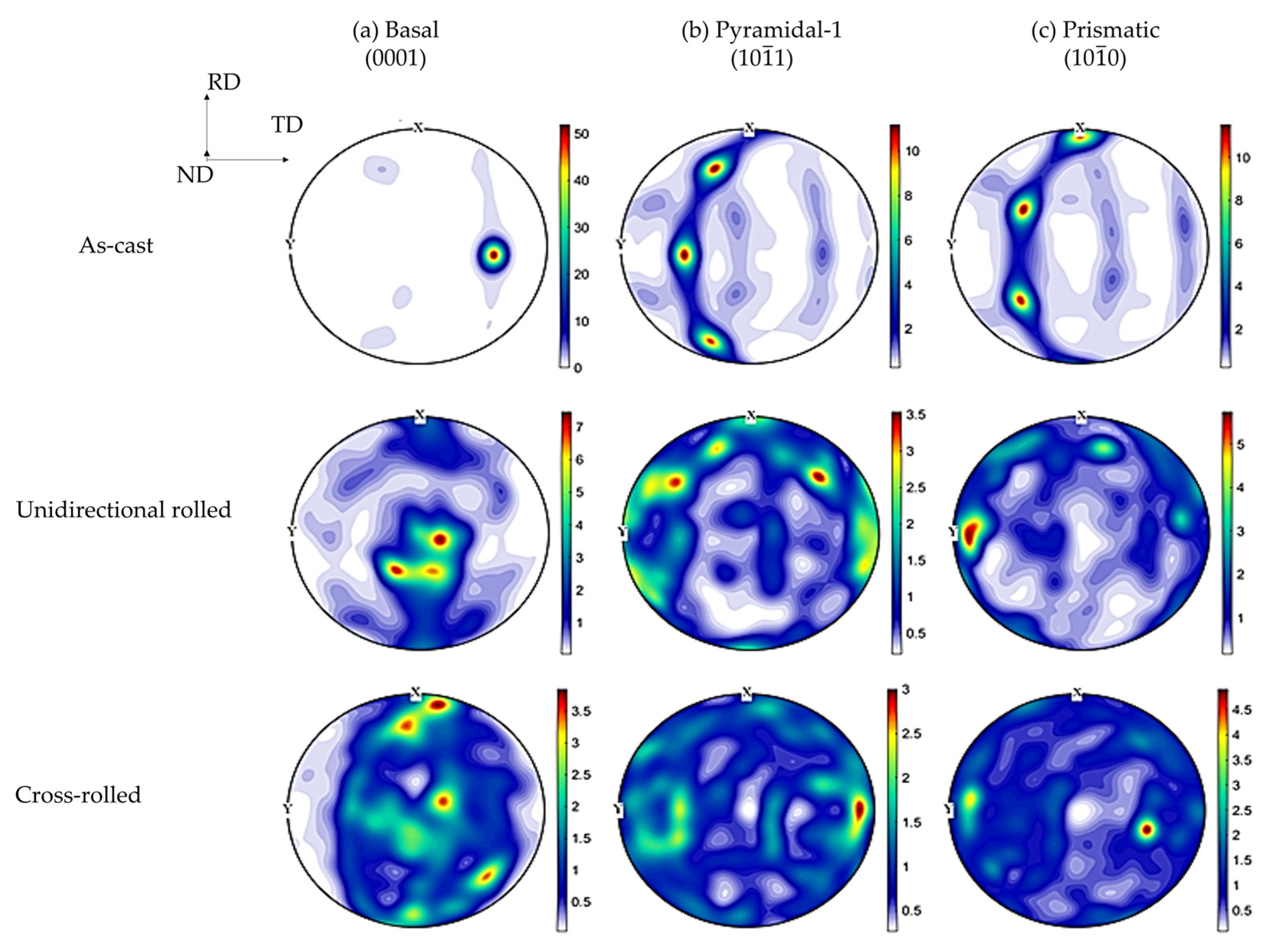
3.2. Texture
3.3. Corrosion
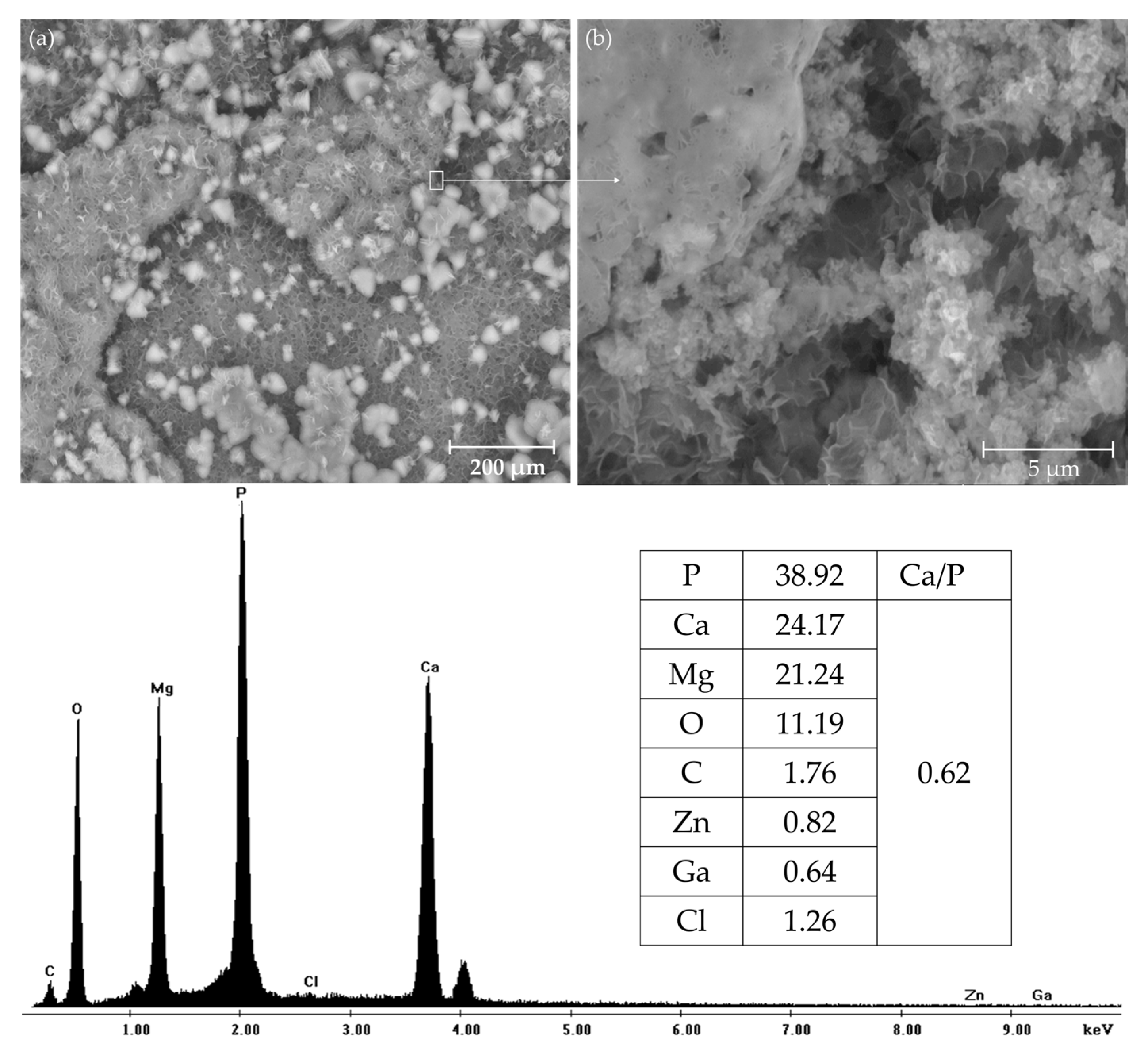
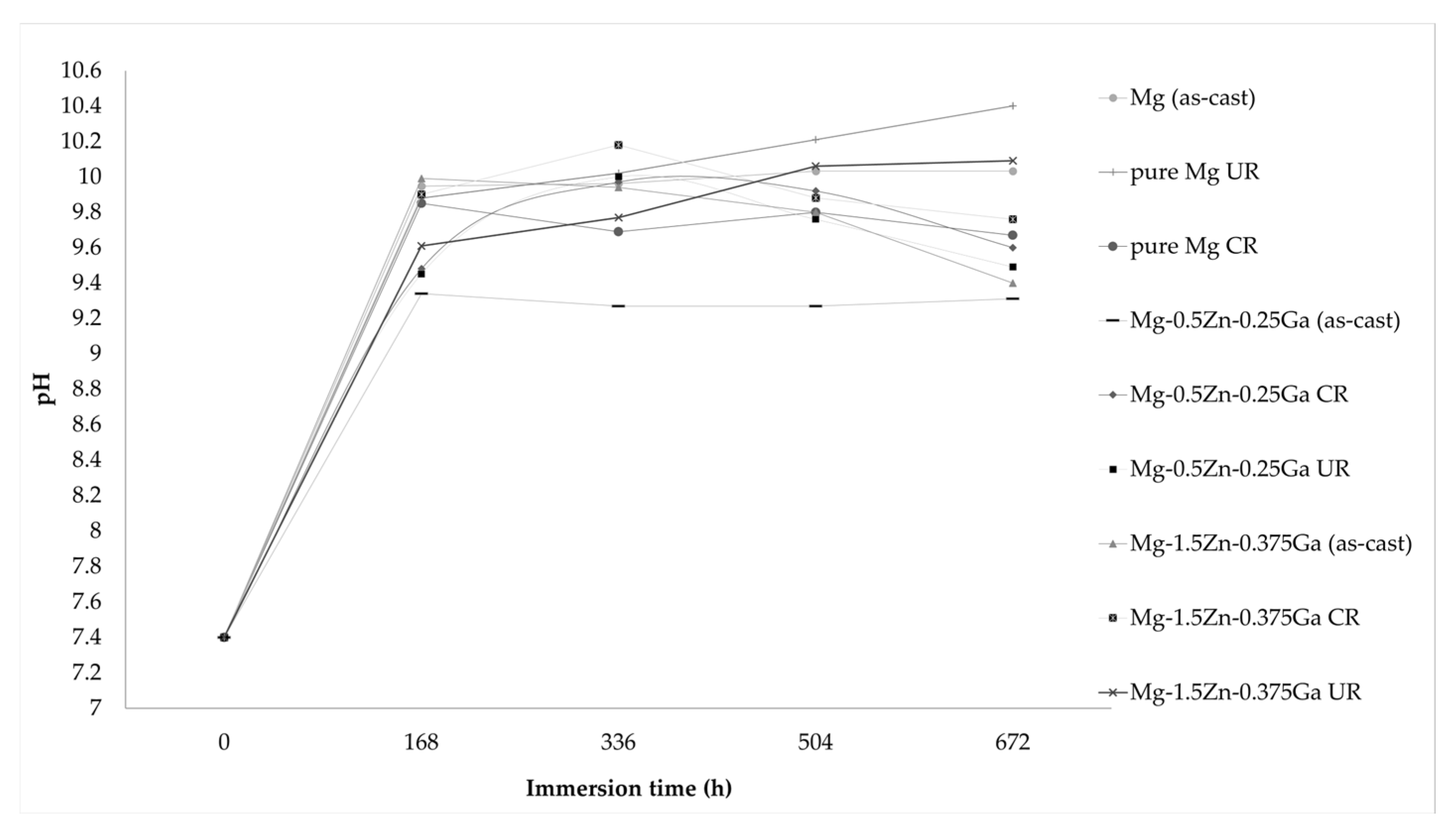
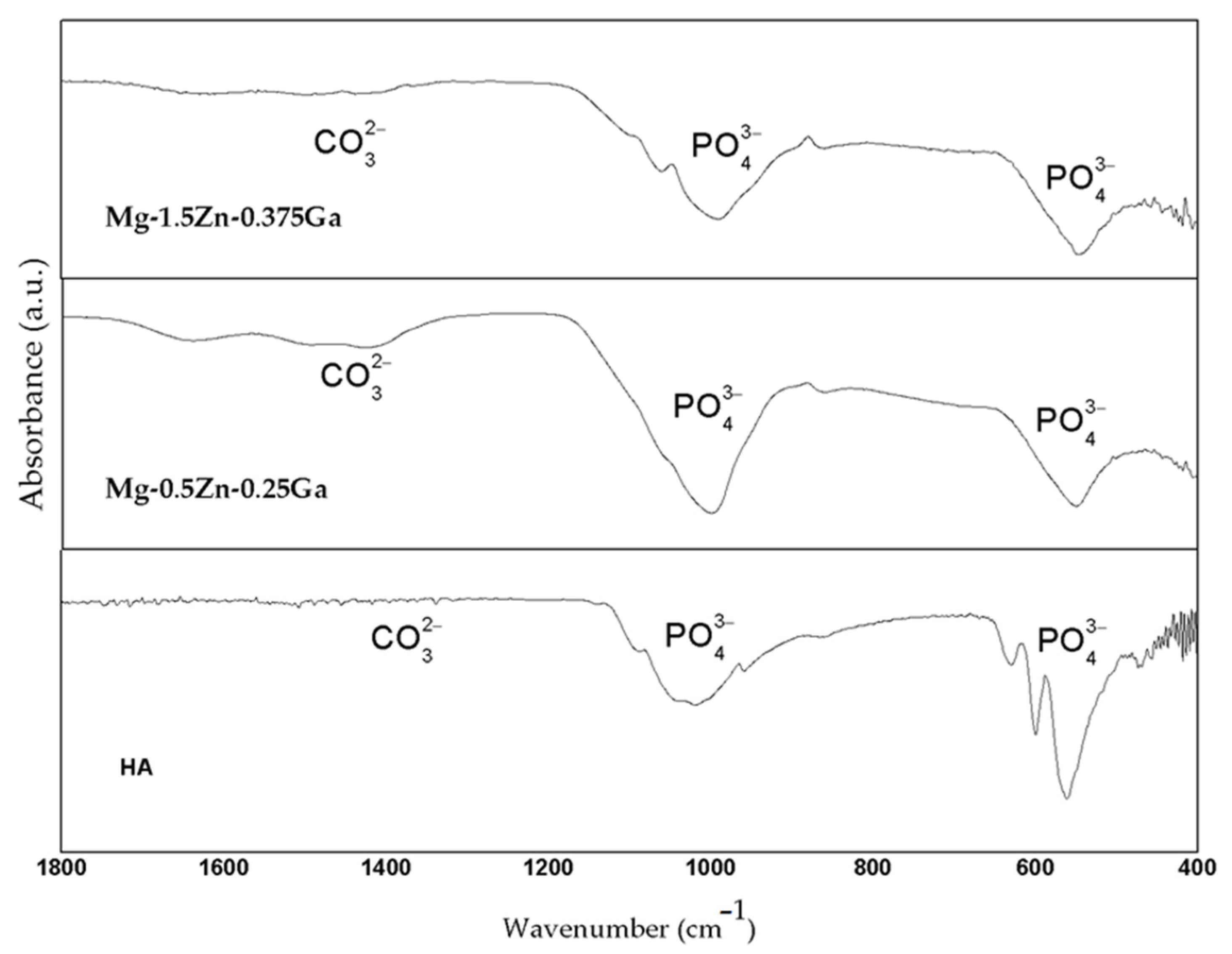
3.4. In Vitro Bioactivity Assessment
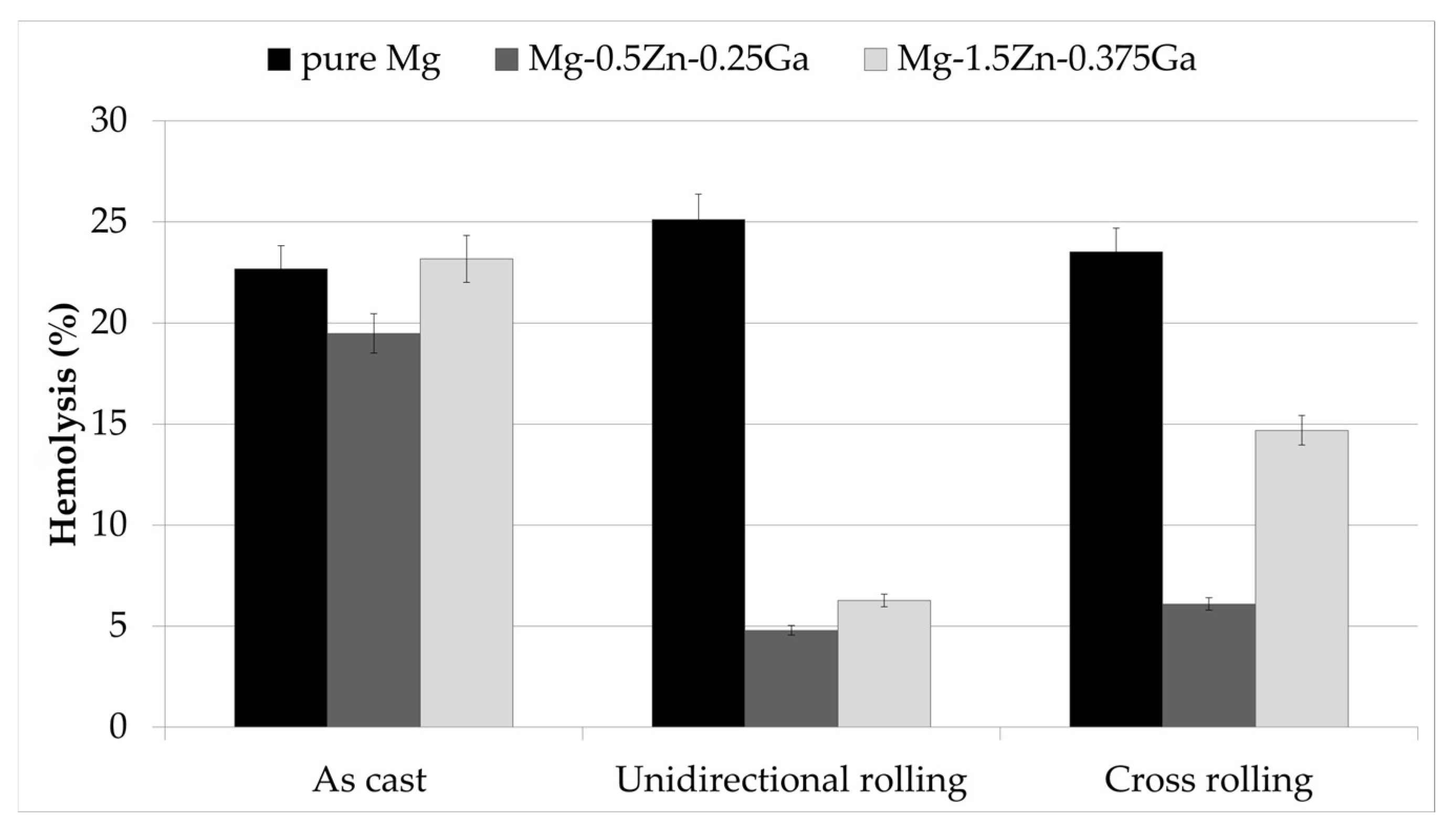
3.5. Hemolysis
4. Conclusions
- The feasibility of obtaining rolled biodegradable magnesium alloys (Mg-0.5Zn-0.25Ga and Mg-1.5Zn-0.375Ga) for biomedical applications has been demonstrated.
- After heat treating, the size of the (Mg, Ga)7Zn3 and (Mg, Zn)5Ga2 intermetallics decreased and were homogeneously distributed. Both ternary alloys were either unidirectionally or cross-rolled.
- After rolling, the alloys’ microstructure was considerably refined. A higher refinement was achieved after cross-rolling. This is due to the fact that cross-rolling induces severe plastic deformations, which cause the activation of pyramidal and/or prismatic slip systems, generating higher heterogeneity in the microstructure.
- The Mg-1.5Zn-0.375Ga alloy showed a finer microstructure than the Mg-0.5Zn-0.25Ga alloy due to both the severe plastic deformation that resulted after cross-rolling and the higher amount of alloying elements, which act as grain refiners.
- Texture intensity of the basal plane increases after unidirectional rolling, while lower texture intensity is observed for the cross-rolled alloy due to the activation of additional slip systems, such as the pyramidal and/or the prismatic systems.
- The lower corrosion rate was observed for the unidirectionally rolled alloys due to the developed basal texture. The Mg-1.5Zn-0.375Ga alloy showed a higher corrosion rate than the Mg-0.5Zn-0.25Ga alloy since the voids formed during heat treating were not fully eliminated during rolling.
- Both rolled alloys have been shown to be bioactive due to the formation of a Ca- and P-rich layer with a similar morphology to that of the apatite formed on the existing bioactive systems after immersion in SBF.
- The unidirectionally rolled Mg-0.5Zn-0.25Ga alloy demonstrated no hemolytic properties (4.7%).
- According to the results obtained in this work, this unidirectionally rolled alloy may be a potential biodegradable material for bone replacement and regeneration.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, J. 1-Metallic biomaterials: State of the art and new challenges. In Woodhead Publishing Series in Biomaterials; Balakrishnan, P.S.M.S., Thomas, S.B.T.-F.B.M., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 1–33. [Google Scholar] [CrossRef]
- Khan, A.R.; Grewal, N.S.; Zhou, C.; Yuan, K.; Zhang, H.-J.; Jun, Z. Recent advances in biodegradable metals for implant applications: Exploring in vivo and in vitro responses. Results Eng. 2023, 20, 101526. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, S.; Bai, J.; Yang, Y.; Wang, Y.; Zhou, Y.; Jiang, W.; Wang, J.; Zhu, J.; Zhu, C.; et al. Magnesium-based biomaterials for coordinated tissue repair: A comprehensive overview of design strategies, advantages, and challenges. J. Magnes. Alloys 2024, 12, 3025–3061. [Google Scholar] [CrossRef]
- Abraham, A.M.; Subramani, V. Effect of Magnesium as Biomaterial in Biodegrdation. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Joshi, C.P.; Rao, R.N.; Golla, C.B.; Özcan, M.; Prasad, P.S. Comprehensive review of Mg-based alloys: Mechanical, chemical and biological properties for prosthetic and orthopedic applications. J. Mater. Res. Technol. 2025, 35, 2487–2502. [Google Scholar] [CrossRef]
- Pradeep, R.C.; Karthik, B.N.B.; Balaji, N.S.; Saikiran, A.; Rajesh, K.A. Effect of nutrient-based alloying elements on biodegradable magnesium alloys: Evolution, challenges, and strategies for orthopaedic applications. Biomed. Eng. Adv. 2025, 9, 161. [Google Scholar] [CrossRef]
- Xu, L.; Liu, X.; Sun, K.; Fu, R.; Wang, G. Corrosion Behavior in Magnesium-Based Alloys for Biomedical Applications. Materials 2022, 15, 2613. [Google Scholar] [CrossRef]
- Ran, Z.; Dai, W.; Xie, K.; Hao, Y. Advances of biodegradable magnesium-based implants for orthopaedics. Life Res. 2022, 5, 7. [Google Scholar] [CrossRef]
- Dong, L.; Zhang, G.; Shen, Z.; Hong, X.; Xing, Y.; Wu, Y.; Yang, W.; Zhang, B.; Shi, Z. Degradation of WE43 Magnesium Alloy in Vivo and Its Degradation Products on Macrophages. ACS Omega 2025, 10, 17280–17295. [Google Scholar] [CrossRef]
- Liu, T.; Chen, X.; Wang, F.; Venezuela, J.; Wang, Y.; Shi, Z.; Chen, W.; Dargusch, M. Contrasting the mechanisms of high corrosion resistance in ultra-high purity Mg-Sr alloys in Hanks’ and NaCl solutions. Electrochim. Acta 2025, 518, 145716. [Google Scholar] [CrossRef]
- Kovacevic, S.; Ali, W.; Martínez-Pañeda, E.; Llorca, J. Phase-field modeling of pitting and mechanically-assisted corrosion of Mg alloys for biomedical applications. Acta Biomater. 2023, 164, 641–658. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, T.; Jiang, J.; Xue, W.; Wang, W.; Ni, J.; Zhang, X.; Liu, X. Advances in magnesium-based implants for biomedical applications: A comprehensive review and future perspectives. J. Magnes. Alloys 2025, 13, 2978–3003. [Google Scholar] [CrossRef]
- Tran, L.N.T.; González-Fernández, C.; Gomez-Pastora, J. Impact of Different Red Blood Cell Storage Solutions and Conditions on Cell Function and Viability: A Systematic Review. Biomolecules 2024, 14, 813. [Google Scholar] [CrossRef]
- Suh, J.S.; Suh, B.-C.; Bae, J.H.; Kim, Y.M. Machine learning-based design of biodegradable Mg alloys for load-bearing implants. Mater. Des. 2022, 225, 111442. [Google Scholar] [CrossRef]
- Bazhenov, V.; Koltygin, A.; Komissarov, A.; Li, A.; Bautin, V.; Khasenova, R.; Anishchenko, A.; Seferyan, A.; Komissarova, J.; Estrin, Y. Gallium-containing magnesium alloy for potential use as temporary implants in osteosynthesis. J. Magnes. Alloys 2020, 8, 352–363. [Google Scholar] [CrossRef]
- Hernández-Cortés, A.A.; Escobedo-Bocardo, J.C.; Cortés-Hernández, D.A.; Almanza-Robles, J.M. Effect of Gallium Content and Heat Treatment on the Microstructure and Corrosion Rate of Magnesium Binary Alloys. Metals 2019, 9, 990. [Google Scholar] [CrossRef]
- Wu, Z.; Hu, J.; Yu, L.; Wang, K.; Zhang, W.; Fan, H.; Deng, Z.; Wu, J.; Wang, K. Corrosion behavior investigation of gallium coating on magnesium alloy in simulated body fluid. J. Mater. Res. Technol. 2023, 27, 225–236. [Google Scholar] [CrossRef]
- Yang, Y.; He, C.; Yang, W.; Qi, F.; Xie, D.; Shen, L.; Peng, S.; Shuai, C. Mg bone implant: Features, developments and perspectives. Mater. Des. 2020, 185, 108259. [Google Scholar] [CrossRef]
- He, D.; Zhou, M.; Dai, Z.; Zhang, Z.; Shi, Q.; Dong, X.; Chen, G.; Han, Y.; Zha, Y.; Meng, H.; et al. Effects of Ga on the corrosion behaviors of severe plastic deformation strengthened biodegradable Mg-2Zn-xGa alloys. Corros. Sci. 2025, 257, 113265. [Google Scholar] [CrossRef]
- Sahu, M.R.; Yamamoto, A. An overview of the recent developments in biodegradable Mg-Zn alloy. J. Magnes. Alloys 2025, 13, 486–509. [Google Scholar] [CrossRef]
- Ji, C.; Ma, A.; Jiang, J.; Song, D.; Liu, H.; Guo, S. Research status and future prospects of biodegradable Zn-Mg alloys. J. Alloys Compd. 2024, 993, 174669. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, J.; Xie, J.; Tian, S.; Ren, R.; Fan, H.; Fang, Y.; Wang, X.; Wang, K.; Chen, T.; et al. Multifunctional biodegradable Mg-Zn heterostructured alloy for calvarial defect repair: Simultaneous skull/dural regeneration and immunomodulation. Chem. Eng. J. 2025, 521, 166490. [Google Scholar] [CrossRef]
- Chen, H.; Han, Y.; Liu, C.; Chen, Z. Effect of unidirectional rolling and cross-rolling on microstructure and mechanical anisotropy of Mg-Gd-Y-Ag-Zr plates. J. Alloys Compd. 2023, 960, 170680. [Google Scholar] [CrossRef]
- Hernández-Cortés, A.A.; Escobedo-Bocardo, J.C.; Cortés-Hernández, D.A.; Vazquez-Montiel, R.H.; Peralta-Montes, J.S.; Almanza-Robles, J.M. Microstructure, corrosion rate, and mechanical properties of unidirectionally and cross-rolled Mg-0.375Ga and Mg-0.750Ga alloys. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 110, 646–659. [Google Scholar] [CrossRef]
- Li, Z.; Chen, L.; Tang, J.; Sun, W.; Zhao, G.; Zhang, C. Improving mechanical anisotropy and corrosion resistance of extruded AA7075 alloy by warm cross rolling and annealing. J. Alloys Compd. 2021, 863, 158725. [Google Scholar] [CrossRef]
- Hernández-Cortés, A.A.; Escobedo-Bocardo, J.C.; Cortés-Hernández, D.A. Influence of the Alloying Elements on the Corrosion Behavior of As-Cast Magnesium–Gallium–Zinc Alloys in Simulated Body Fluid. Metals 2023, 13, 743. [Google Scholar] [CrossRef]
- ASTM G1-03(2011); Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. ASTM International: West Conshohocken, PA, USA, 2011. [CrossRef]
- ASTMF756-17; Standard Practice for Assessment of Hemolytic Properties of Materials. ASTM: West Conshohocken, PA, USA, 2017; p. 6. [CrossRef]
- Shi, D.; Ma, A.; Pérez-Prado, M.; Cepeda-Jiménez, C. Activation of second-order pyramidal slip and other secondary mechanisms in solid solution Mg-Zn alloys and their effect on tensile ductility. Acta Mater. 2022, 244, 118555. [Google Scholar] [CrossRef]
- Mirzadeh, H. Grain refinement of magnesium alloys by dynamic recrystallization (DRX): A review. J. Mater. Res. Technol. 2023, 25, 7050–7077. [Google Scholar] [CrossRef]
- Shah, S.; Liu, M.; Khan, A.; Ahmad, F.; Chaudry, U.M.; Khan, M.Y.; Abdullah, M.; Xu, S.; Peng, Z. Recrystallization aspects and factors affecting their roles in Mg alloys: A comprehensive review. J. Magnes. Alloys 2025, 13, 1879–1914. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Zhang, A.; Yu, H.; Kang, X.; Wang, C.; Song, Y.; Ni, J.; Zheludkevich, M.L.; Zhang, X. High-performance Mg–Zn alloy achieved by the ultrafine grain and nanoparticle design. Bioact. Mater. 2024, 41, 371–384. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, J.U.; Kim, Y.M.; Park, S.H. Microstructural evolution and grain growth mechanism of pre-twinned magnesium alloy during annealing. J. Magnes. Alloys 2021, 9, 1233–1245. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Lin, X.; Zhang, T.; Dang, C.; Wang, Y.; Huang, W.; Pan, F. Solidification texture dependence of the anisotropy of mechanical properties and damping capacities of an AZ31 Mg-based alloy fabricated via wire-arc additive manufacturing. J. Mater. Res. Technol. 2023, 25, 2589–2601. [Google Scholar] [CrossRef]
- Zecevic, M.; Beyerlein, I.J.; Knezevic, M. Activity of pyramidal I and II slip in Mg alloys as revealed by texture development. J. Mech. Phys. Solids 2018, 111, 290–307. [Google Scholar] [CrossRef]
- Liu, M.; Qiu, D.; Zhao, M.-C.; Song, G.; Atrens, A. The effect of crystallographic orientation on the active corrosion of pure magnesium. Scr. Mater. 2008, 58, 421–424. [Google Scholar] [CrossRef]
- Huang, Z.; Zou, J.; Yang, G.; Zhu, Y.; Lai, H.; Qi, C. Effect of Cross Rolling on the Corrosion Resistance of AZ31 Magnesium Alloy. Rev. Chim. 2020, 71, 176–182. [Google Scholar] [CrossRef]
- Xin, R.L.; Wang, M.Y.; Gao, J.C.; Liu, P.; Liu, Q. Effect of microstructure and texture on corrosion resistance of magnesium alloy. Mater. Sci. Forum 2009, 610-613, 1160–1163. [Google Scholar] [CrossRef]
- Drouet, C. Apatite Formation: Why it may not work as planned, and how to conclusively identify apatite compounds. BioMed Res. Int. 2013, 2013, 490946. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lu, T.; He, F.; Zhang, W.; Yuan, X.; Wang, X.; Ye, J. Physicochemical and cytological properties of poorly crystalline calcium-deficient hydroxyapatite with different Ca/P ratios. Ceram. Int. 2022, 48, 24765–24776. [Google Scholar] [CrossRef]
- Hilger, D.M.; Hamilton, J.G.; Peak, D. The Influences of Magnesium upon Calcium Phosphate Mineral Formation and Structure as Monitored by X-ray and Vibrational Spectroscopy. Soil Syst. 2020, 4, 8. [Google Scholar] [CrossRef]
- Liang, J.; He, Y.; Jia, R.; Li, S.; Duan, L.; Xu, S.; Mei, D.; Tang, X.; Zhu, S.; Wei, J.; et al. Enhancing the corrosion resistance of magnesium alloys with biodegradable poly(trimethylene carbonate) chemical modification coating. Mater. Today Adv. 2024, 21, 100460. [Google Scholar] [CrossRef]
- Chou, Y.-F.; Chiou, W.-A.; Xu, Y.; Dunn, J.C.; Wu, B.M. The effect of pH on the structural evolution of accelerated biomimetic apatite. Biomaterials 2004, 25, 5323–5331. [Google Scholar] [CrossRef] [PubMed]
- Wang, M. Springer Series in Biomaterials Science and Engineering; 2014; Volume 2, pp. 1–559. Available online: http://www.springer.com/series/10955 (accessed on 20 February 2025).
- Chen, Y.; Zhang, S.; Li, J.; Song, Y.; Zhao, C.; Wang, H.; Zhang, X. Influence of Mg2+ concentration, pH value and specimen parameter on the hemolytic property of biodegradable magnesium. Mater. Sci. Eng. B 2011, 176, 1823–1826. [Google Scholar] [CrossRef]
- Zhang, E. Phosphate treatment of magnesium alloy implants for biomedical applications. In Surface Modification of Magnesium and Its Alloys for Biomedical Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2015; Volume 2, pp. 23–57. [Google Scholar] [CrossRef]
- Ye, C.-H.; Xi, T.-F.; Zheng, Y.-F.; Wang, S.-Q.; Li, Y.-D. In vitro corrosion and biocompatibility of phosphating modified WE43 magnesium alloy. Trans. Nonferrous Met. Soc. China 2013, 23, 996–1001. [Google Scholar] [CrossRef]

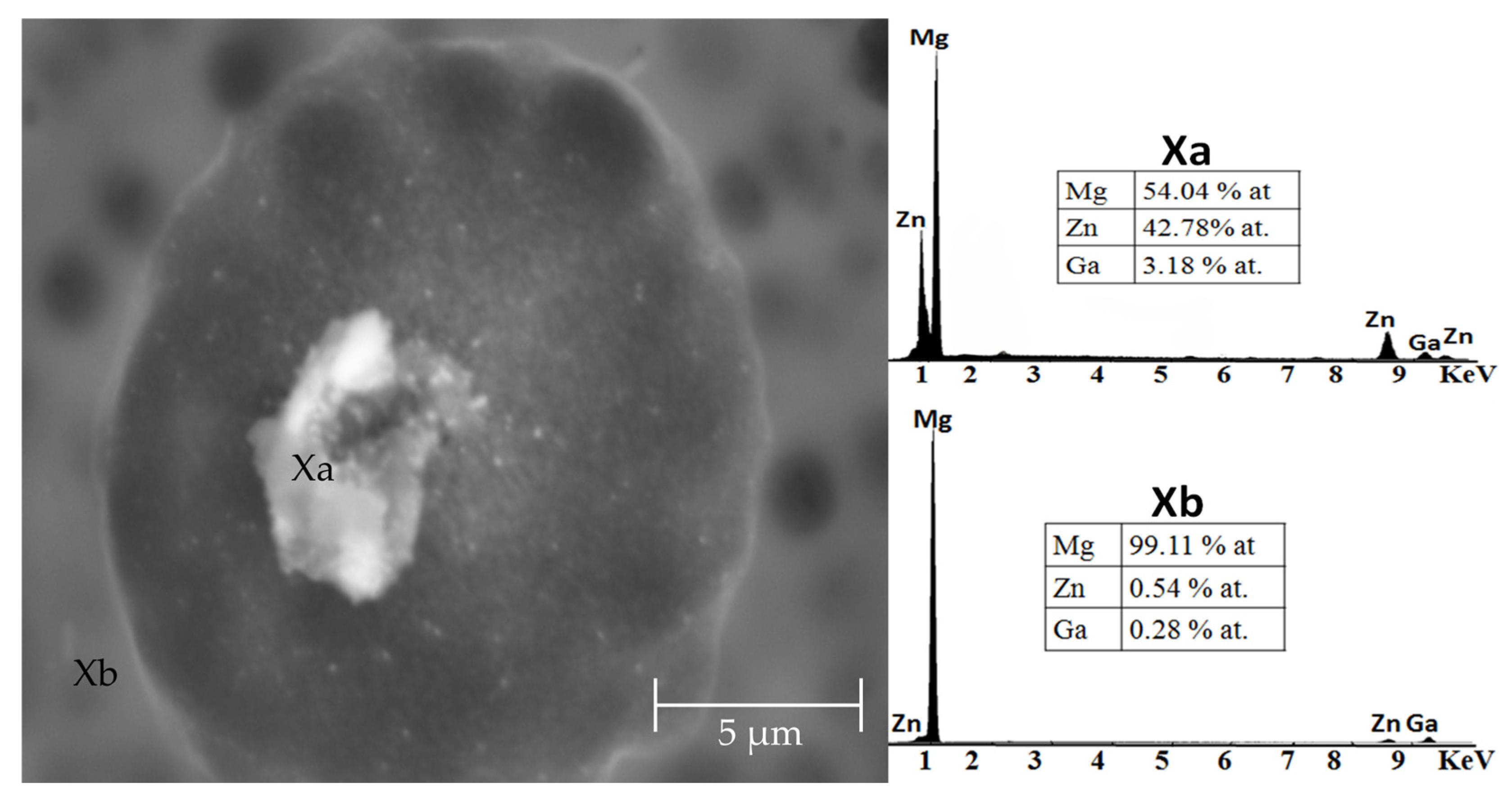
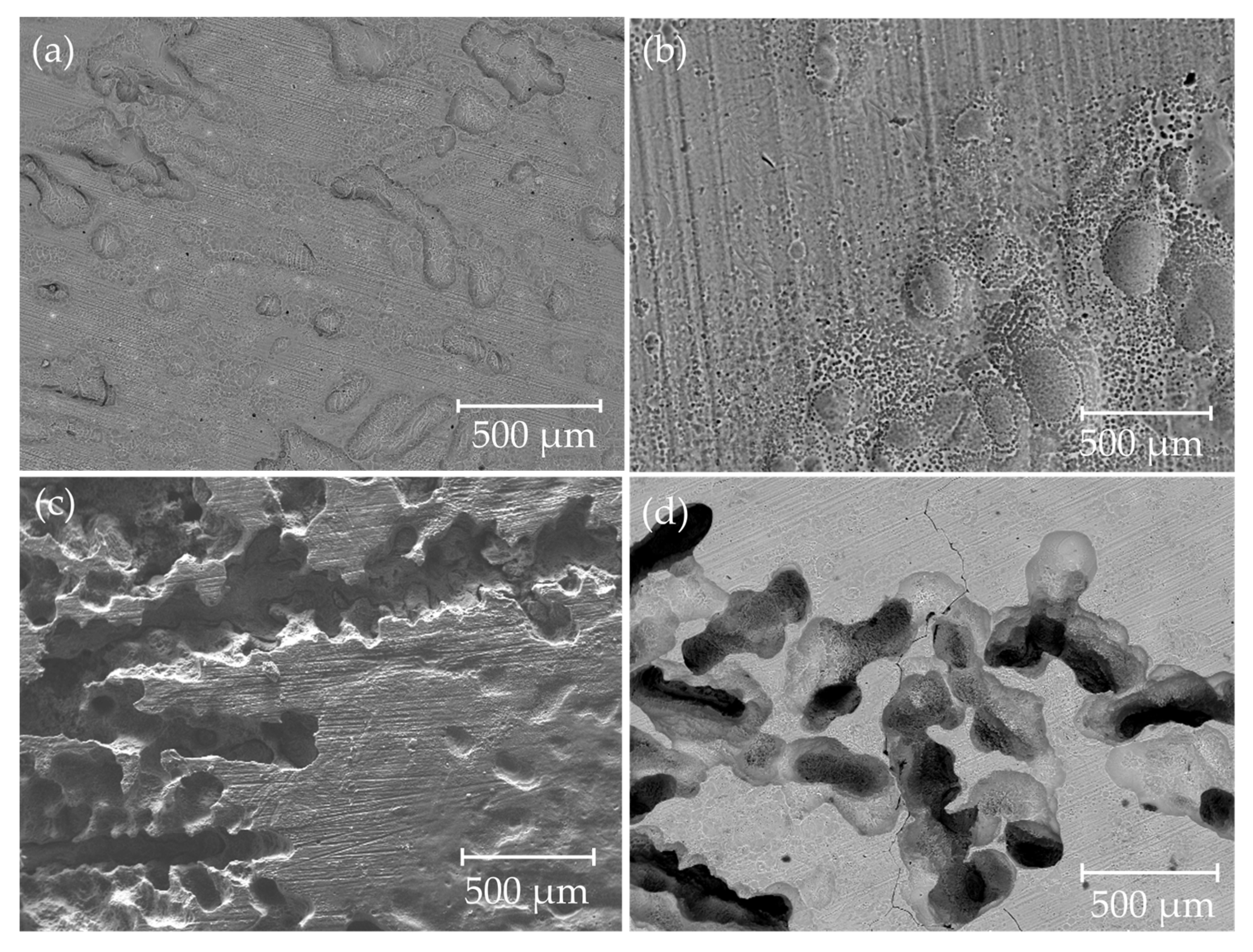
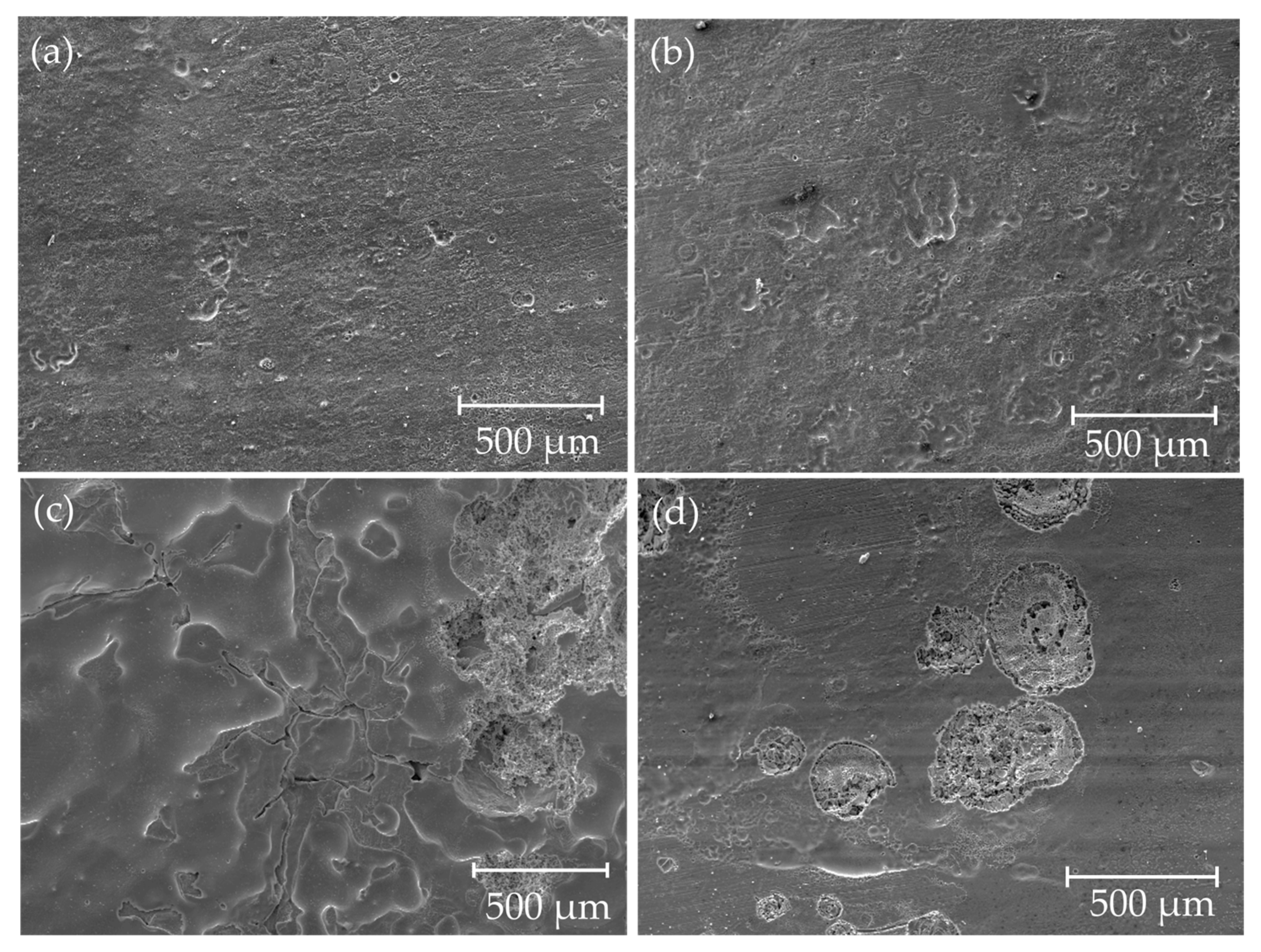
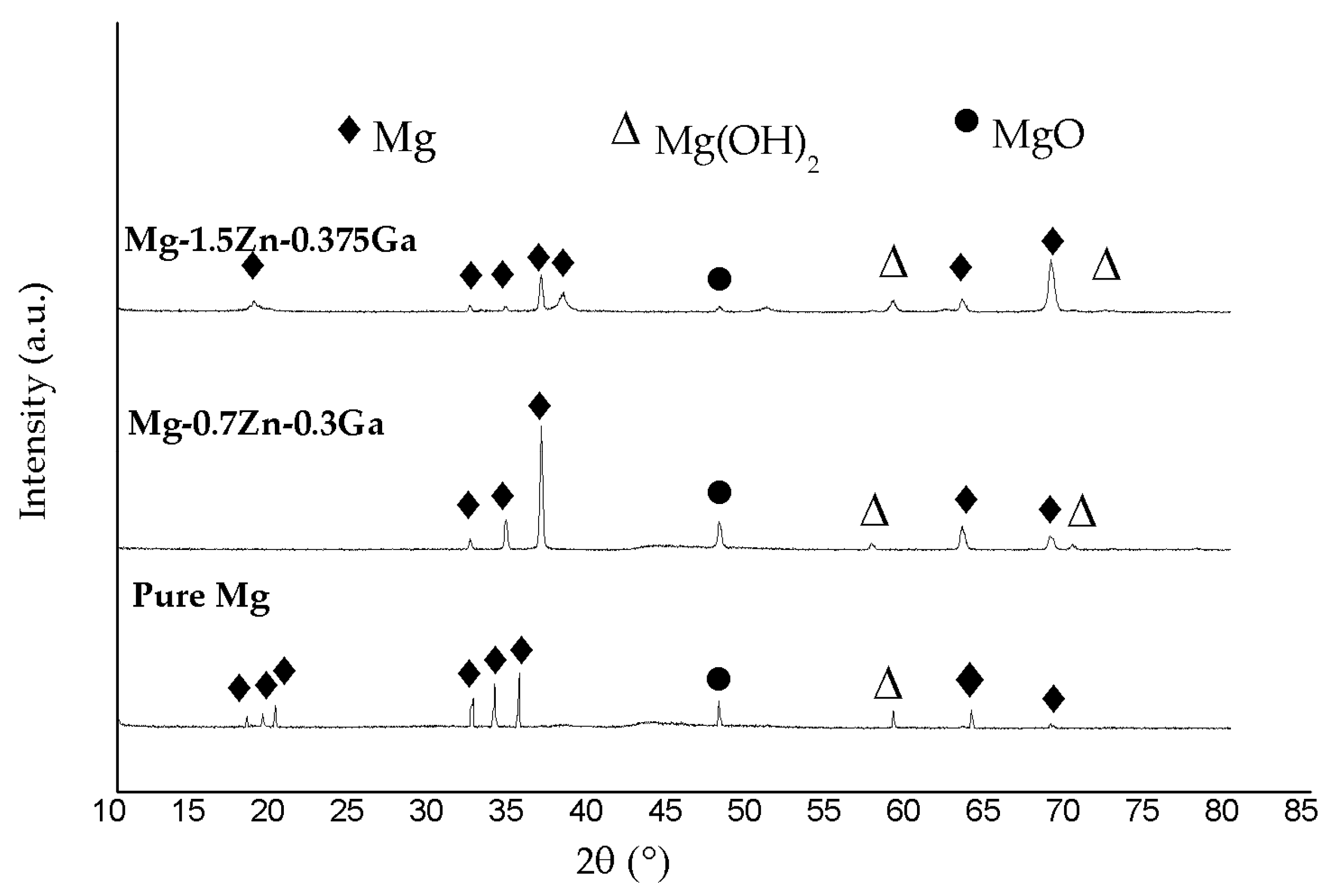

| Nominal Composition | Mg | Ga | Zn | Fe | Ni | Cu |
|---|---|---|---|---|---|---|
| pure Mg | 99.99 | 0 | 0 | ≤0.0002 | ≤0.0006 | ≤0.0002 |
| Mg-0.5Zn-0.25Ga | 99.21 | 0.25 | 0.522 | 0.003 | 0.002 | ≤0.0002 |
| Mg-1.5Zn-0.375Ga | 98.38 | 0.35 | 1.35 | 0.002 | 0.002 | ≤0.0002 |
| Nominal Composition | Average Grain Size (mm) | ||
|---|---|---|---|
| As-Cast | Unidirectional Rolled | Cross-Rolled | |
| Pure Mg | 530 (±196.48) | - | - |
| Mg-0.5Zn-0.25Ga | 534 (±181.5) | 90 (±22.45) | 42 (±27.93) |
| Mg-1.5Zn-0.375Ga | 477 (±129.14) | 64 (±17.22) | 27 (±11.3) |
| Alloy | Grain Size (μm) | Corrosion Rate (mm/Year) | Hemolysis (%) | |||
|---|---|---|---|---|---|---|
| UR | CR | UR | CR | UR | CR | |
| Mg-0.5Zn-0.25Ga | 90 | 42 | 0.8 | 0.95 | 4.7 | 5.6 |
| Mg-1.5Zn-0.375Ga | 64 | 27 | 1.4 | 1.85 | 6.0 | 14.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Cortés, A.A.; Escobedo-Bocardo, J.C.; Almanza-Robles, J.M.; Cortés-Hernández, D.A. Effect of Cross- or Unidirectional Rolling on the Microstructure, Corrosion Rate, and Hemolysis of Ternary Magnesium–Zinc–Gallium Alloys. Metals 2025, 15, 1165. https://doi.org/10.3390/met15111165
Hernández-Cortés AA, Escobedo-Bocardo JC, Almanza-Robles JM, Cortés-Hernández DA. Effect of Cross- or Unidirectional Rolling on the Microstructure, Corrosion Rate, and Hemolysis of Ternary Magnesium–Zinc–Gallium Alloys. Metals. 2025; 15(11):1165. https://doi.org/10.3390/met15111165
Chicago/Turabian StyleHernández-Cortés, Anabel Azucena, José C. Escobedo-Bocardo, José Manuel Almanza-Robles, and Dora Alicia Cortés-Hernández. 2025. "Effect of Cross- or Unidirectional Rolling on the Microstructure, Corrosion Rate, and Hemolysis of Ternary Magnesium–Zinc–Gallium Alloys" Metals 15, no. 11: 1165. https://doi.org/10.3390/met15111165
APA StyleHernández-Cortés, A. A., Escobedo-Bocardo, J. C., Almanza-Robles, J. M., & Cortés-Hernández, D. A. (2025). Effect of Cross- or Unidirectional Rolling on the Microstructure, Corrosion Rate, and Hemolysis of Ternary Magnesium–Zinc–Gallium Alloys. Metals, 15(11), 1165. https://doi.org/10.3390/met15111165







