Abstract
Monocrystalline silicon, despite its widespread use as a photoelectrode material, is hindered by inherent drawbacks, such as high surface reflectivity, vulnerability to oxide passivation, and instability in aqueous electrolytes. To address these, a micropyramidal texture is fabricated on the silicon surface via wet chemical etching. A heterojunction photoanode was constructed by sequentially depositing NiSe2 and WO3 onto the textured silicon using chemical bath deposition, forming NiSe2/WO3@SiMPs. The photoanode demonstrates optimal photoelectrochemical performance at a NiSe2 to WO3 mass ratio of 9:1. Under simulated solar illumination (AM 1.5 G, 100 mW cm−2), it achieves a photocurrent of 5.62 mA cm−2 at 1.23 V (vs. RHE), and a maximum photocurrent of 13.6 mA cm−2 at 2.0 V (vs. RHE), markedly outperforming the individual components NiSe2@SiMPs (8.23 mA cm−2) and WO3@SiMPs (0.95 mA cm−2) at 2.0 V (vs. RHE). Electrochemical impedance spectroscopy (EIS) results show a markedly lower charge transfer resistance (Rct) for the NiSe2/WO3@SiMPs (8.16 Ω) compared to the single-phase counterparts NiSe2@SiMPs (121.48 Ω) and WO3@SiMPs (902.23 Ω), indicating more efficient charge separation. In addition, the photocurrent remains steady for about 10 h without significant degradation. This work presents a promising strategy for improving the photoelectrochemical water splitting efficiency of silicon-based photoelectrodes through rational heterostructure engineering.
1. Introduction
Electrocatalytic hydrogen evolution via water splitting is an environmentally sustainable approach and currently represents the primary technology for green hydrogen production. However, its high energy demand poses a significant barrier to large-scale commercial implementation [1,2,3]. Therefore, the development of efficient photoanodes for photoelectrochemical (PEC) water splitting is critical for effective solar-to-hydrogen energy conversion. However, a major challenge lies in designing semiconductor photoelectrodes with an appropriate bandgap, broad photoresponse, high surface activity, and long-term operational stability. Although Pt- and Ir-based materials are widely recognized for their superior catalytic performance in water splitting, their high cost and limited availability severely constrain large-scale deployment [4,5]. Consequently, there is an urgent need to develop alternative photoelectrocatalysts based on Earth-abundant, non-noble metals that offer both high catalytic activity and robust stability [6,7,8].
Among various candidates, crystalline silicon (Si) has garnered significant attention due to its narrow bandgap (~1.12 eV), which aligns well with the solar spectrum [9,10]. However, planar silicon suffers from high surface reflectivity, low surface area, and poor chemical stability in aqueous environments that limit its PEC efficiency [11]. A promising strategy to overcome these limitations is the formation of heterojunctions with other semiconductors to enhance charge separation and surface activity. Tungsten trioxide (WO3), an n-type semiconductor with a suitable band structure and favorable photoelectrochemical properties, is an excellent candidate for constructing heterojunctions [12]. For instance, Li and co-workers developed a direct Z-scheme photoelectrocatalytic electrode by integrating oxygen-deficient WO3−x nanowires with TiO2 nanorod arrays, achieving efficient PEC water splitting under UV–visible light irradiation [13]. Furthermore, the energy band alignment between silicon and WO3 facilitates heterojunction formation, i.e., silicon has a valence band at approximately 0.62 eV, close to the conduction band edge of WO3, promoting effective interfacial charge transfer [14]. However, the inherent slow electron transport in WO3 remains a bottleneck, limiting the catalytic efficiency of WO3/Si heterostructures. Overcoming this limitation is essential for realizing their full potential in PEC applications [15,16].
Selenium-based compounds, particularly nickel diselenide (NiSe2), are intrinsically paramagnetic metals with high electrical conductivity, making them promising candidates for oxygen evolution reaction (OER) applications [17,18]. For instance, Jafari et al. fabricated a NiSe2/WO3 composite heterostructure on nickel foam, which exhibited outstanding durability and catalytic activity in 1.0 M KOH, achieving a current density of 10 mA cm−2 at an overpotential of 260 mV and a Tafel slope of approximately 54 mV dec−1 [19]. In such a heterojunction, the two materials are staggered, promoting spatial separation of photogenerated electrons and holes. This spatial charge separation effectively reduces recombination rates, thereby enhancing the catalytic efficiency of the composite material [20].
Herein, photoelectrodes incorporating both p–n and Z-scheme heterojunctions were fabricated by anchoring WO3 and NiSe2 onto a micropyramidal-textured silicon substrate (Scheme 1). The synergistic interaction among the multiple components optimizes the electronic structure, enhances charge and mass transfer, and improves system stability. As a result, the catalytic activity of both WO3/SiMPs and NiSe2/SiMPs is significantly enhanced. This multi-heterojunction photoanode presents an improved efficiency for photoelectrochemical water splitting, providing a new approach for green hydrogen production.
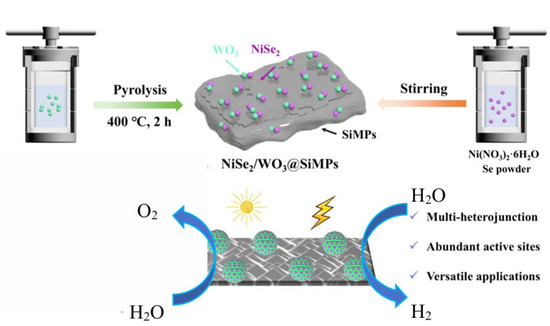
Scheme 1.
Preparation of NiSe2/WO3@ SiMPs composites for photoelectrocatalytic hydrogen production from water splitting.
2. Materials and Methods
2.1. Materials
Monocrystalline silicon (Si) was purchased from Jingxin Electronics (Danyang, China). All reagents were analytical grade and used without further purification. Selenium powder (Se), sodium tungstate (Na2WO4·2H2O), ammonium chloride (NH4Cl), hydrogen chloride (HCl), and nickel nitrate hexahydrate (Ni(NO3)2·6H2O) were bought from Sinopharm Chemical Reagent (Shanghai, China). Deionized (DI) water was utilized for the preparation of aqueous solutions.
2.2. Preparation of WO3
Na2WO4·2H2O (2.0 g, 6 mmol) was dissolved in water (60 mL) and stirred for 10 min, then HCl (1 M) solution was added dropwise to adjust the pH value of the solution until the pH was less than 1. After that, NH4Cl (13 g, 0.25 mol) was added and stirred for 30 min. The solution was transferred to a 100 mL hydrothermal kettle, and the reaction was carried out at 180 °C for 12 h. After the reaction was completed, the products were cooled naturally, washed with water for 3 times, and dried at 60 °C in vacuum. Finally, the obtained product was calcined in a muffle furnace at a heating rate of 5 °C/min until the temperature reached 400 °C and was kept for 2 h.
2.3. Preparation of NiSe2
Ni(NO3)2·6H2O (580 mg, 2 mmol) was dissolved in water (50 mL) and stirred for 30 min to form a solution to prepare sample A. Then NaOH (8.0 mg, 0.2 mmol) and Se powder (460 mg, 6 mmol) were dissolved in 10 mL of deionized water with magnetic stirring to form solution B. Subsequently, solution A was mixed with solution B, subjected to ultrasound for 30 min, then transferred to a 100 mL bottle and reacted at 200 °C for 10 h. After naturally cooling, the product was washed with distilled water and ethanol three times. Finally, the sample was dried at 60 °C for 12 h in a drying oven.
2.4. Preparation of NiSe2/WO3@SiMPs
Si micropyramid (SiMP) was constructed by a wet chemical etching method according to our previous studies [21] with some modification. NiSe2 and WO3 mixtures were dispersed in an ethylene glycol solution (5 mL) when the ratio of NiSe2 to WO3 was 10:0, 0:10, 8:2, 9:1, and 9.5:0.5, respectively. Then the sample solution was loaded on the surface of micropyramidal silicon wafers by the drip coating method. The NiSe2/WO3@SiMPs composites were obtained after vacuum drying.
2.5. Preparation of Working Electrodes
The back side of the NiSe2/WO3@SiMPs composites is evenly coated with a layer of indium balls, and then the copper wire is fixed to the back of the material with tin wire, and finally the back and sides of the NiSe2/WO3@SiMPs composites are wrapped with silicon-sealed kraft glue to ensure that only the load surface of the silicon wafer is in contact with the electrolyte.
2.6. Characterizations
The crystal phases of the composites were identified by a D/max-g B X-ray diffractometer (XRD, Rigaku, Japan) with Cu Ka radiation (λ = 1.54178 Å). The morphology of the as-prepared samples was detected by scanning electron microscope (FEI Nova Nano SEM 230, Thermo Fisher Scientific, Waltham, MA, USA). The elemental distributions were observed by electron diffraction X-ray (EDS) spectrometer coupled with the scanning electron microscope. Transmission electron microscopy (TEM, Helios 5 CX, Thermo Fisher, USA) was used to further analyze the microstructures. The surface elemental chemical states were collected by X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi, Thermo Fisher Scientific, USA) with reference to the C 1s peak at 284.8 eV. UV–vis absorbance spectra were probed by employing a UV–Visible spectrophotometer (UV-2700, Shimadzu, Japan), with BaSO4 as the reflectance standard.
2.7. PEC Measurements
PEC performance was evaluated by an electrochemical workstation (CHI 660E, Chenhua, Shanghai, China) with a typical three-electrode cell. The platinum was used as the counter electrode (cathode) and Ag/AgCl as the reference electrode, with the prepared samples as the working electrode (anode), respectively. A 350 W xenon lamp (Parx-350, Automation Technology, Nanjing, China) was used as the light source for PEC measurements with an AM 1.5 G and an illumination density of 100 mW cm−2. The Mott–Schottky test was performed in KOH solution (1 M). The Na2SO4 solution (0.5 M) serves as the electrolyte solution. The J-V curves were collected by LSV mode (linear sweeping voltammetry) with a scan rate of 50 mV s−1. Under illumination, electrochemical impedance spectroscopy (EIS) of the samples was performed at a frequency range from 100 MHz to 1 Hz, and an AC amplitude of 5 mV. All potentials reported were calculated and, unless otherwise stated, referred to reversible hydrogen electrode (RHE) in Equation (1).
According to the J-V curve, the external bias photon-current conversion efficiency (ABPE) is obtained by Equation (2) [22].
where Jp is the photocurrent density (mA cm−2) at the corresponding applied potential, is the light power density (100 mW cm−2), and is the applied external potential V (vs. RHE).
3. Results and Discussion
3.1. Characterizations of NiSe2/WO3@SiMPs Composites
XRD analyses for the crystal structures of NiSe2/WO3@SiMPs (9:1) were performed by an X-ray diffractometer. As shown in Figure 1a, the strong diffraction peak at 69.3° of the sample is attributed to the silicon (400) crystal plane, according to the standard card of monocrystalline silicon (JCPDS No. 80-0018). The peaks of the composite that appeared at 25.86, 29.95, 33.58, 36.90, 42.86, 50.74, and 55.52° can be attributed to the (111), (200), (210), (211), (220), (311), and (321) planes of NiSe2, respectively (JCPDS No. 88-1711). Meanwhile, the diffraction peaks at 24.33, 33.58, 37.64, 42.84, 52.13, and 58.32° match the (110), (111), (210), (300), (310), and (400) planes of WO3, respectively (JCPDS No. 33-1387). It is worth noting that the characteristic peaks of the composites show deviations compared to the peaks of the standard comparison cards, which can be attributed to the presence of heterojunctions in the samples [23].
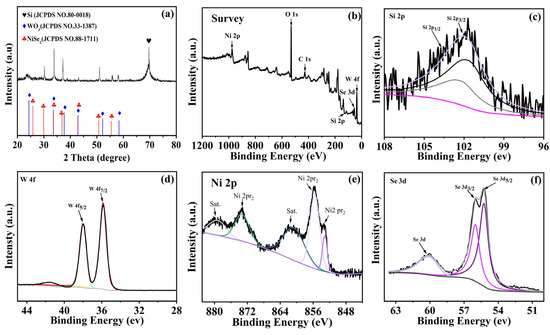
Figure 1.
XRD spectra (a) of NiSe2/WO3@SiMPs composites. Full XPS spectrum of NiSe2/WO3@SiMPs (b), XPS spectra of Si 2p (c), W 4f (d), Ni 2p (e), and Se 3d (f).
Figure 1b presents the XPS analysis of the NiSe2/WO3@SiMPs (9:1) composite. As shown in the full survey spectrum (Figure 1b), the sample comprises Ni, O, C, Si, Se, and W elements. The C 1s peaks were calibrated using charge correction. In the high-resolution Si 2p spectrum (Figure 1c), two fitted peaks at binding energies of 96.63 and 101.46 eV correspond to elemental silicon, confirming the presence of Si without significant oxidation [24]. The W 4f spectrum (Figure 1d) exhibits two well-defined peaks at 35.73 and 37.87 eV, assigned to W 4f5/2 and W 4f7/2, respectively, indicating that tungsten is present predominantly in the W6+ oxidation state [25]. The O 1s region (Figure S1) can be deconvoluted into two peaks at 530.48 and 530.98 eV, typically associated with lattice oxygen and surface adsorbed oxygen species, respectively. The Ni 2p spectrum (Figure 1e) shows characteristic peaks at 853.37 and 861.78 eV for Ni 2p3/2, and at 873.54 eV for Ni 2p1/2. Additionally, two low-intensity satellite peaks are observed, which are consistent with previously reported Ni2+ features [26]. As depicted in Figure 1f, the Se 3d spectrum reveals peaks at 55.25 and 56.00 eV, assigned to Se 3d5/2 and Se 3d3/2, respectively [27,28]. A distinct peak at 58.93 eV corresponds to elemental selenium (Se 3d), suggesting the presence of residual unreacted Se [29].
The morphology and crystalline characteristics of the composite were investigated using SEM and HR-TEM. As shown in Figure 2a, a well-defined micropyramidal structure was successfully fabricated on the surface of monocrystalline silicon. This structure enhances light absorption and offers an increased surface area for the effective loading of NiSe2 and WO3. Figure 2b reveals that the as-prepared WO3@SiMPs exhibit a lamellar morphology, while NiSe2, shown in Figure 2c, forms nano-scale spherical clusters. This spherical morphology facilitates improved contact and interaction with the lamellar WO3. Figure 2d reveals significant differences in the NiSe2/WO3@SiMPs (9:1) morphology, mainly showing three distinctive phases: (i) micropyramidal structure as substrate based on monocrystalline silicon, (ii) nanocluster domains of nanometer size based on NiSe2 and, (iii) nanosheet structures based on WO3, as confirmed by SEM-EDS analysis (Figure 2e). Elemental distribution was further examined by energy-dispersive X-ray spectroscopy (EDS) mapping conducted via SEM. As shown in Figure 2e, Si, W, O, Se, and Ni elements are homogeneously distributed across the selected region, confirming the uniform composition of the composite.
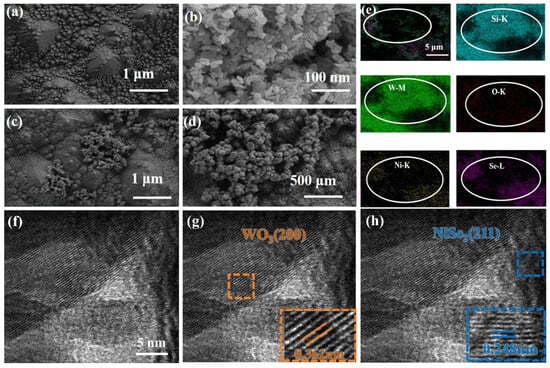
Figure 2.
SEM of SiMPs (a), WO3@SiMPs (b), NiSe2@SiMPs (c), NiSe2/WO3@SiMPs (d), and EDS mapping of NiSe2/WO3@SiMPs (e). HR-TEM image (f–h) of NiSe2/WO3@SiMPs.
The NiSe2/WO3@SiMPs (9:1) composite was investigated using HR-TEM. As shown in Figure 2f–h, well-defined lattice fringes are observed in the NiSe2 nanoclusters, with an interplanar spacing of approximately 0.248 nm, which corresponds to the (211) crystallographic plane of NiSe. In addition, lattice fringes with a spacing of 0.362 nm are identified and attributed to the (200) plane of WO3. These observations confirm that the NiSe2 nanospheres adhere to the surface of the WO3 nanosheets (Figure 2g,h), enabling intimate interfacial contact between the two phases. This contact will be beneficial to the improvement of water splitting activity [30], with the conclusion firmly supported by electrochemical data (Figure 3a,b).
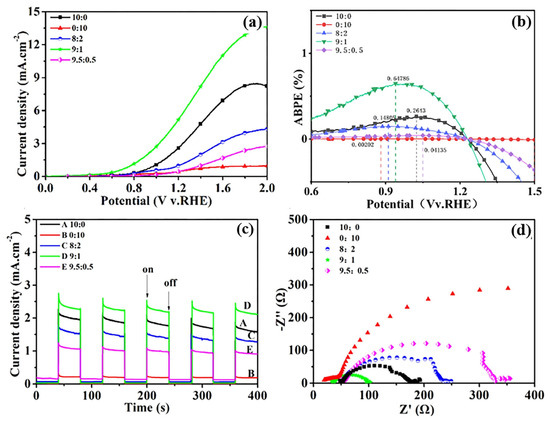
Figure 3.
Photocurrent density−voltage plots (a), applied bias photon to electron conversion efficiency (b), I−T curves (at 0.7 VRHE) (c), and Nyquist diagrams (d) of NiSe2/WO3@SiMPs composites with different ratios.
3.2. PEC Performance
UV–visible diffuse reflectance spectroscopy was conducted to investigate the optical absorption properties of NiSe2/WO3@SiMPs (9:1), WO3@SiMPs, and NiSe2@SiMPs. As shown in Figure S2, the NiSe2/WO3@SiMPs composite exhibits significantly enhanced light absorption in both the ultraviolet and visible regions compared to the individual WO3@SiMPs and NiSe2@SiMPs. This broadened and intensified absorption is expected to improve the utilization of solar energy, thereby contributing to the superior photocatalytic performance of the heterostructure.
The photoelectrochemical (PEC) water splitting performance of NiSe2/WO3@SiMPs with varying NiSe2 to WO3 ratios (10:0, 0:10, 8:2, 9:1, and 9.5:0.5) was systematically evaluated. The J–V curves of these composites are shown in Figure 3a. The onset potentials were determined to be 0.072 V (10:0), 0.133 V (0:10), 0.222 V (8:2), −0.002 V (9:1), and 0.238 V (9.5:0.5) vs. RHE. Notably, the onset potential of the 9:1 composite was cathodically shifted to −0.002 V, indicating a lower energy requirement for water oxidation, and exhibits high photocurrent of 5.62 mA cm−2 at 1.23 V (vs. RHE) in 0.5 M Na2SO4 solution under AM 1.5 G light illumination, compared with the recently reported heterostructure-based photoelectrodes (Table S1) [31,32,33,34,35,36,37,38,39,40,41,42]. In terms of the maximum photocurrent density at 2.0 V (vs. RHE), the values are 8.23 mA cm−2 (10:0), 0.95 mA cm−2 (0:10), 4.31 mA cm−2 (8:2), 13.59 mA cm−2 (9:1), and 2.73 mA cm−2 (9.5:0.5). Among them, NiSe2/WO3@SiMPs (9:1) exhibits the highest photocurrent density of 13.6 mA cm−2 at 2.0 V (vs. RHE), clearly outperforming the individual components NiSe2@SiMPs (8.23 mA cm−2) and WO3@SiMPs (0.95 mA cm−2). Figure 3b further presents the maximum applied bias photo-to-current efficiency (ABPE) for the corresponding samples, with values of 0.264% (10:0), 0.002% (0:10), 0.148% (8:2), 0.647% (9:1), and 0.041% (9.5:0.5). Again, the 9:1 composite achieves the highest efficiency, consistent with its superior photocurrent performance. The transient photocurrent responses under chopped light illumination at 0.7 VRHE (Figure 3c) reveal a rapid and reproducible photoresponse for all samples at 40 s intervals. The 9:1 sample not only demonstrates the strongest photocurrent response but also shows a decline in performance when the NiSe2 content exceeds this ratio. This suggests that an excessive amount of NiSe2 may lead to a decreased spectral range of light absorption (Figure S2) and reduced photocatalytic efficiency. Electrochemical impedance spectroscopy (EIS) was performed to investigate the interfacial charge transfer properties. As shown in the Nyquist plots (Figure 3d), the charge transfer resistances (Rct) were measured to be 121.48 Ω (10:0), 902.23 Ω (0:10), 64.26 Ω (8:2), 8.16 Ω (9:1), and 272.96 Ω (9.5:0.5). The 9:1 sample exhibits the lowest charge transfer resistances (Rct), confirming improved separation and mobility of photogenerated charge carriers [20]. Notably, the photocurrent density, applied bias photo-to-current efficiency (ABPE), and photocurrent transient of the NiSe2/WO3@SiMPs (9:1) photoanode were all prominent among other ratios (Figure 3a–c), which is attributed to the synergistic effect of a composite with an intimate interfacial contact, as evidenced by SEM/TEM. The intimate heterojunction interface facilitates improved charge separation and transfer [35,36]. This is directly supported by EIS measurements (Figure 3d). The composite exhibits the most efficient charge separation and transport, which further supports its outstanding PEC performance.
The long-term stability of WO3@SiMPs (0:10) and NiSe2/WO3@SiMPs (9:1) photoelectrodes was evaluated at 0.7 VRHE under simulated solar irradiation (AM 1.5 G). As depicted in Figures S3 and S4, in the first 10 min, the photocurrent of NiSe2/WO3@SiMPs (9:1) decreased significantly. This behavior should be a stabilization process, which is consistent with the literature reports [43]. Then the photocurrent remains steady for about 10 h, while the WO3@SiMPs electrode without NiSe2 deposition shows a low photocurrent. This indicates that NiSe2 mainly improves the activity of PEC water splitting in the composite.
3.3. PEC Mechanism
The Mott–Schottky tests were performed on NiSe2 and Si. The intercept of the straight part of the curve from the X-axis is approximately equal to the value of the flat potential (Ef), and the obtained value can be converted into a normal hydrogen electrode (NHE) at pH = 7 by Equation (3). According to Figure 4a,b, the Ef (vs. NHE) values of NiSe2 and Si were calculated to be 0.307 and 0.185 eV using Equation (3), respectively. Correcting Ef by 0.2 eV yields an EVB value of about 0.507 and 0.385 eV for NiSe2 and Si relative to NHE, respectively. As can be seen from Figure 4a, the slope of the Mott–Schottky curve for NiSe2 is negative, indicating that NiSe2 is a p-type semiconductor [32]. In addition, using a Tauc plot (Figure 4c,d), the Eg values of NiSe2 and Si are 0.848 and 1.187 eV, respectively [40,44]. The ECB of NiSe2 and Si was calculated to be −0.341 and −0.802 eV by Equation (4). The Eg value of WO3 is 2.7 eV, with an EVB value of about 3.0 eV [45].
E (NHE pH = 7) = EAg/AgCl − 0.059 (7 − pH of electrolyte) + 0.198
Evb = Ecb + Eg
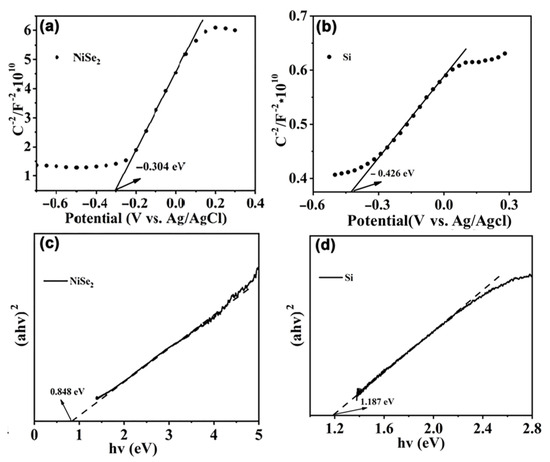
Figure 4.
Mott–Schottky plots at frequency of 1 kHz in KOH solution (1 M) of (a) NiSe2, (b) Si. Tauc plots of (c) NiSe2, (d) Si.
The photoelectrochemical mechanism of water splitting on a NiSe2/WO3@SiMPs composite was investigated. As shown in Figure 5, under light illumination, the three semiconductors in the photoanode can be excited to produce carriers. Due to the contact between WO3 and NiSe2 (as shown in Figure 2f–h), a p-n heterojunction is formed between NiSe2 and WO3. Thus, the electrons and holes of the two semiconductors continue to flow to balance the Fermi level, the photoelectrons in the NiSe2 conduction band (CB) are transferred to the CB of WO3, the photogenerated holes in the valence band (VB) of WO3 are transferred to the VB of NiSe2, and O2 is formed by reacting with water on the surface of the photoanode. This p-n junction enhanced charge separation and transfer behavior. When they are loaded on silicon, a Z-scheme heterojunction between WO3 and Si is formed, the photogenerated electrons in the WO3 CB are further transferred to the VB of Si, which annihilate the holes in the VB of Si and electrons in CB of WO3 [46], and the photogenerated electrons in Si could be transported to the back contact and then to the counter electrode for hydrogen evolution. Therefore, the multi-heterojunctions among different semiconductors (NiSe2, WO3, and Si) effectively inhibit the recombination of electrons and holes, which results in excellent photoelectrocatalytic performance. This synergy is supported by the observed PEC performance for NiSe2/WO3@SiMPs with varying NiSe2 to WO3 ratios (Figure 3).
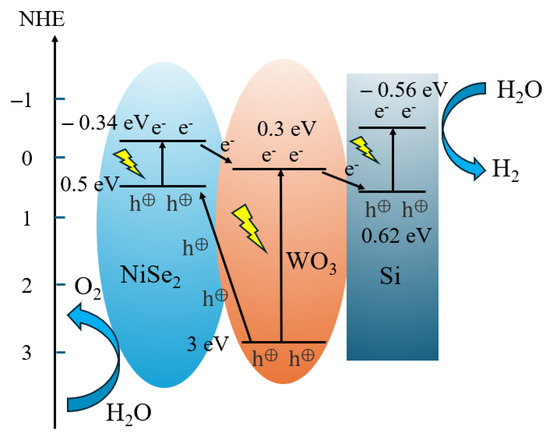
Figure 5.
Schematic diagrams of charge transfer mechanism in NiSe2/WO3@SiMPs composites.
4. Conclusions
In summary, lamellar WO3 and NiSe2 nanoclusters were synthesized via a solvothermal method and uniformly deposited onto the surface of silicon micropyramids using a drop-casting technique. NiSe2/WO3@SiMPs photoanodes were successfully constructed and characterized for efficient PEC water splitting. Among the prepared composites, the optimized NiSe2/WO3@SiMPs (9:1) exhibited a remarkable photocurrent density of 13.6 mA cm−2 at 2.0 V (vs. RHE), a cathodically shifted onset potential of −0.002 V (vs. RHE), and a maximum photon-current conversion efficiency of 0.647%, while the photocurrent remains steady for about 10 h of continuous illumination. This remarkably enhanced PEC performance compared to its individual components is attributed to the synergistic effect of a composite with intimate interfacial contact, as evidenced by SEM/TEM. The intimate heterojunction interface facilitates improved charge separation and transfer. This is directly supported by our electrochemical data: (i) the dramatic decrease in charge transfer resistance in EIS measurements, (ii) the increased photocurrent density in PEC tests, and (iii) the favorable band alignment and increased charge carrier density revealed by Mott–Schottky analysis. This work demonstrates the promoting effect of constructing multiple heterojunction pairs of WO3 and NiSe2 on the surface of silicon wafers to improve the PEC water splitting ability, which can also be extended to improve the photoelectrochemical properties of other semiconductor materials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/met15111164/s1, Figure S1: XPS spectra of O 1s; Figure S2:The UV-vis absorption spectra of NiSe2/WO3@SiMPs, WO3@SiMPs and NiSe2@SiMPs; Figure S3: The long-term stability of WO3@SiMPs and NiSe2/WO3@SiMPs photoelectrodes at 0.7 VRHE under AM 1.5G light illumination; Figure S4: XRD (a) and SEM (b) of NiSe2/WO3@SiMPs after stability testing; Table S1: PEC performances comparison of recently reported heterostructure photoelectrodes.
Author Contributions
Data curation, Writing-Original draft preparation, L.Z.; Formal analysis, Investigation, Validation, J.L. (Jie Li); Formal analysis, Investigation, Supervision, J.L. (Jialu Liu); Methodology, Supervision, Z.Z.; Validation, Writing-Reviewing and Editing, Y.C.; Conceptualization, Writing-Reviewing and Editing, P.Y. and H.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Natural Science Foundation of Hunan Province (No. 2024JJ7210, Department of Science and Technology of Hunan Province), the Scientific Research Project of Hunan Education Department of China (No. 23C0780, Hunan Provincial Department of Education), and the Youth Student Basic Research Projects of the Hunan Provincial Natural Science Foundation (No. 2025JJ60904, Department of Science and Technology of Hunan Province).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sanati, S.; Morsali, A.; Garcia, H. First-row transition metal-based materials derived from bimetallic metal-organic frameworks as highly efficient electrocatalysts for electrochemical water splitting. Energy Environ. Sci. 2022, 15, 3119–3151. [Google Scholar] [CrossRef]
- Panchenko, V.A.; Kovalev, A.A.; Litti, Y.V.; Daus, Y.V. Prospects for the production of green hydrogen: Review of countries with high potential. Int. J. Hydrogen Energy 2023, 48, 4551–4571. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, R.; Lv, Z.; Liu, J.; Zhou, H.; Xu, C. Green hydrogen: A promising way to the carbon-free society. Chin. J. Chem. Eng. 2022, 43, 2–13. [Google Scholar] [CrossRef]
- Zheng, T.; Liu, Y.; Li, Y.; Yang, L.; Ren, H.; Wang, X.; Fuji, R.; Kitao, O.; Nakamura, T.; Sasaki, S. Panchromatic Pt/TiO2-based photocatalysts sensitized with carboxylated chlorin dyads for water splitting hydrogen evolution. Appl. Surf. Sci. 2023, 619, 156570. [Google Scholar] [CrossRef]
- Stratakes, B.M.; Miller, A.J.M. H2 evolution at an electrochemical “underpotential” with an iridium-based molecular photoelectrocatalyst. ACS Catal. 2020, 10, 9006–9018. [Google Scholar] [CrossRef]
- Faraji, M.; Yousefi, M.; Yousefzadeh, S.; Zirak, M.; Naseri, N.; Jeon, T.H.; Choi, W.; Moshfegh, A.Z. Two-dimensional materials in semiconductor photoelectrocatalytic systems for water splitting. Energy Environ. Sci. 2019, 12, 59–95. [Google Scholar] [CrossRef]
- Farooq, U.; Ahmad, T.; Naaz, F.; Islam, S. Review on metals and metal oxides in sustainable energy production: Progress and perspectives. Energy Fuels 2023, 37, 1577–1632. [Google Scholar] [CrossRef]
- Yao, T.; An, X.; Han, H.; Chen, J.Q.; Li, C. Photoelectrocatalytic materials for solar water splitting. Adv. Energy Mater. 2018, 8, 1800210. [Google Scholar] [CrossRef]
- Sun, K.; Shen, S.; Liang, Y.; Burrows, P.E.; Mao, S.S.; Wang, D. Enabling silicon for solar-fuel production. Chem. Rev. 2014, 114, 8662–8719. [Google Scholar] [CrossRef]
- Jing, S.; Jiang, H.; Hu, Y.; Li, C. Graphene supported mesoporous single crystal silicon on Cu foam as a stable lithium-ion battery anode. J. Mater. Chem. A 2014, 2, 16360–16364. [Google Scholar] [CrossRef]
- Lichterman, M.F.; Sun, K.; Hu, S.; Zhou, X.; McDowell, M.T.; Shaner, M.R.; Richter, M.H.; Crumlin, E.J.; Carim, A.I.; Saadi, F.H.; et al. Protection of inorganic semiconductors for sustained, efficient photoelectrochemical water oxidation. Catal. Today 2016, 262, 11–23. [Google Scholar] [CrossRef]
- Wu, P.; Liu, Z.; Chen, D.; Zhou, M.; Wei, J. Flake-like NiO/WO3 p-n heterojunction photocathode for photoelectrochemical water splitting. Appl. Surf. Sci. 2018, 440, 1101–1106. [Google Scholar] [CrossRef]
- Lin, S.; Ren, H.; Wu, Z.; Sun, L.; Zhang, X.; Lin, Y.; Zhang, K.; Lin, C.; Tian, Z.; Li, J. Direct Z-scheme WO3 nanowire-bridged TiO2 nanorod arrays for highly efficient photoelectrochemical overall water splitting. J. Energy Chem. 2021, 59, 721–729. [Google Scholar] [CrossRef]
- Hamann, T.W.; Lewis, N.S. Control of the stability, electron-transfer kinetics, and pH-dependent energetics of Si/H2O interfaces through methyl termination of Si (111) surfaces. J. Phys. Chem. B 2006, 110, 22291–22294. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, C.; Arunachalam, P.; Ramachandran, K.; Al-Mayouf, A.M.; Karuppuchamy, S. Recent advances in semiconductor metal oxides with enhanced methods for solar photocatalytic applications. J. Alloys Compd. 2020, 828, 154281. [Google Scholar] [CrossRef]
- Liu, G.; Wang, X.; Wang, X.; Han, H.; Li, C. Photocatalytic H2 and O2 evolution over tungsten oxide dispersed on silica. J. Catal. 2012, 293, 61–66. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Zhang, X.; Liu, D.; Srinivas, K.; Ma, F.; Wang, B.; Yu, B.; Wu, Q.; Chen, Y. Facile and scalable synthesis of heterostructural NiSe2/FeSe2 nanoparticles as efficient and stable binder-free electrocatalyst for oxygen evolution reaction. Int. J. Hydrogen Energy 2021, 46, 35198–35208. [Google Scholar] [CrossRef]
- Yang, Y.; Kang, Y.; Zhao, H.; Dai, X.; Cui, M.; Luan, X.; Zhang, X.; Nie, F.; Ren, Z.; Song, W. An interfacial electron transfer on tetrahedral NiS2/NiSe2 heterocages with dual--phase synergy for efficiently triggering the oxygen evolution reaction. Small 2020, 16, 1905083. [Google Scholar] [CrossRef]
- Jafari, F.; Gholivand, M.B. Investigation of the oxygen evolution reaction at the NiSe2/WO3 nanocomposite catalyst. Mater. Today Chem. 2023, 29, 101432. [Google Scholar] [CrossRef]
- Rosa, D.; Remmani, R.; Bavasso, I.; Bracciale, M.P.; Di Palma, L. Biochar supported Fe–TiO2 composite for wastewater treatment: Solid-state synthesis and mechanistic insights. Chem. Eng. Sci. 2025, 317, 122076. [Google Scholar] [CrossRef]
- Zhong, Z.; Chen, W.; Chen, X.; Li, J.; Yang, H.; Zhang, L.; Yang, P. Nanospherical Fe3O4 modified NixSy@SiMPs composite materials improve photoelectrochemical Performance. J. Mater. Sci. Mater. Electron. 2024, 35, 244–253. [Google Scholar]
- Guo, H.S.; Zhao, W.F.; Ge, Q.M.; Jiang, N.; Liu, M.; Cong, H. Photoelectrochemical water splitting improved by a cucurbit[7]uril-induced ternary heterojunction. Chem. Eng. J. 2025, 505, 159340. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.; Liu, S.; Zhang, X.; Zhang, Z.; Li, Y.; Li, G.; Dimitrov, D. Direct Z-scheme g-C3N4/WO3 photocatalyst with atomically defined junction for H2 production. Appl. Catal. B Environ. 2019, 254, 76–85. [Google Scholar] [CrossRef]
- Christodoulou, X.; Velasquez-Orta, S.B. Microbial electrosynthesis and anaerobic fermentation: An economic evaluation for acetic acid production from CO2 and CO. Environ. Sci. Technol. 2016, 50, 11234–11242. [Google Scholar] [CrossRef]
- Yang, W.; Wang, J.; Si, C.; Peng, Z.; Zhang, Z. Tungsten diselenide nanoplates as advanced lithium/sodium ion electrode materials with different storage mechanisms. Nano Res. 2017, 10, 2584–2598. [Google Scholar] [CrossRef]
- Wang, S.; Li, W.; Xin, L.; Wu, M.; Sun, W.; Lou, X. Pollen-inspired synthesis of porous and hollow NiO elliptical microstructures assembled from nanosheets for high-performance electrochemical energy storage. Chem. Eng. J. 2017, 321, 546–553. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, C.; Liu, H.; Feng, L. Efficient synergism of NiSe2 nanoparticle/NiO nanosheet for energy-relevant water and urea electrocatalysis. Appl. Catal. B Environ. 2020, 276, 119165. [Google Scholar] [CrossRef]
- Guo, K.; Yang, F.; Cui, S.; Chen, W.; Mi, L. Controlled synthesis of 3D hierarchical NiSe microspheres for high-performance supercapacitor design. RSC Adv. 2016, 6, 46523–46530. [Google Scholar]
- Chen, S.; Huang, Y.; Li, H.; Wang, F.; Xu, W.; Zheng, D.; Lu, X. One-Pot Synthesis of NiSe2 with layered structure for nickel-zinc battery. Molecules 2023, 28, 1098. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Z.; Liu, Z. Novel WO3/Sb2S3 heterojunction photocatalyst based on WO3 of different morphologies for enhanced efficiency in photoelectrochemical water splitting. ACS Appl. Mater. Interfaces 2016, 15, 9684–9691. [Google Scholar]
- Liu, L.; Ruan, M.; Wang, C.; Liu, Z. Optimization of the BiO8 polar group of BiVO4 by Cl−-embedded modification to manipulate bulk-surface carrier separation for achieving efficient Piezo-PEC water oxidation. Appl. Catal. B Environ. 2024, 354, 124117. [Google Scholar] [CrossRef]
- Chen, X.; Ruan, M.; Wang, C.; Zhong, T.; Liu, Z. Phase engineering to construct In2S3 heterophase junctions and abundant active boundaries and surfaces for efficient Pyro-PEC performance in CdS/In2S3. J. Mater. Chem. A 2024, 12, 15440–15452. [Google Scholar] [CrossRef]
- Fang, G.; Liu, Z.; Han, C.; Wang, P.; Ma, X.; Lv, H.; Huang, C.; Cheng, Z.; Tong, Z. Promising CoFe-NiOOH ternary polymetallic cocatalyst for BiVO4-based photoanodes in photoelectrochemical water splitting. ACS Appl. Energy Mater. 2021, 4, 3842–3850. [Google Scholar] [CrossRef]
- Song, K.; Hou, H.; Gong, C.; Gao, F.; Zhang, D.; Zhi, F.; Yang, W.; He, F. Enhanced solar water splitting of BiVO4 photoanodes by in situ surface band edge modulation. J. Mater. Chem. A 2022, 10, 22561–22570. [Google Scholar] [CrossRef]
- Wei, P.; Wen, Y.; Lin, K.; Li, X. 2D/3D WO3/BiVO4 heterostructures for efficient photoelectrocatalytic water splitting. Int. J. Hydrogen Energy 2021, 46, 27506–27515. [Google Scholar] [CrossRef]
- Wang, H.; Xia, Y.; Wen, N.; Shu, Z.; Jiao, X.; Chen, D. Surface states regulation of sulfide-based photoanode for photoelectrochemical water splitting. Appl. Catal. B Environ. 2022, 300, 120717. [Google Scholar] [CrossRef]
- Bai, P.; Xie, J.; Wang, H.; Kang, X.; Wang, X. α-Co(OH)2 with interlayer anions for photocatalytic/photoelectrocatalytic water splitting. Appl. Surf. Sci. 2023, 640, 158305. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, W.; Cheng, L.; Jiang, L.; Deng, X.; Yan, J.; Yang, H. Integration of Fe3O4 nanospheres and micropyramidal textured silicon wafer with improved photoelectrochemical performance. J. Mater. Sci. Mater. Electron. 2021, 32, 5176–5185. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, D.; Xie, H.; Liu, Y.; Meng, C.; Zhang, P.; Fan, F.; Li, R.; Li, C. Modulating oxygen vacancies in lead chromate for photoelectrocatalytic water splitting. Adv. Mater. 2023, 35, 2300914. [Google Scholar] [CrossRef]
- Sun, H.; Hua, W.; Liang, S.; Li, Y.; Wang, J. A self-adaptive semiconductor-liquid junction for highly active and stable solar water splitting. J. Mater. Chem. A 2022, 10, 20414–20423. [Google Scholar] [CrossRef]
- Fu, Y.M.; Dong, C.L.; Zhou, W.; Lu, Y.R.; Huang, Y.C.; Liu, Y.; Guo, P.H.; Zhao, L.; Chou, W.C.; Shen, S.H. A ternary nanostructured α-Fe2O3/Au/TiO2 photoanode with reconstructed interfaces for efficient photoelectrocatalytic water splitting. Appl. Catal. B Environ. 2020, 260, 118206. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, D.; Li, D.; Xiao, M.; Deng, Y.; Han, Y.; Tao, R.; Fan, X.; Liu, K. A novel p-type CoSe2 co-catalyst cooperated with hematite for boosting photoelectrochemical water splitting. Fuel 2024, 362, 130931. [Google Scholar] [CrossRef]
- Acharya, L.; Nayak, S.; Pattnaik, S.P.; Acharya, R.; Parida, K. Resurrection of boron nitride in pn type-II boron nitride/B-doped-g-C3N4 nanocomposite during solidstate Z-scheme charge transfer path for the degradation of tetracycline hydrochloride. J. Colloid Interface Sci. 2020, 566, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Du, R.; Zhang, L.; Zhu, H.; Zhang, H.; Wang, P. Carbon-layer-protected cuprous oxide nanowire arrays for efficient water reduction. ACS Nano 2013, 7, 1709–1717. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hu, Y.; Meng, S.; Fu, X. Study on the separation mechanisms of photogenerated electrons and holes for composite photocatalysts g-C3N4-WO3. Appl. Catal. B Environ. 2014, 150, 564–573. [Google Scholar] [CrossRef]
- Murillo-Sierra, J.C.; Hernández-Ramírez, A.; Zhao, Z.; Martínez-Hernández, A.; Gracia-Pinilla, M.A. Construction of direct Z-scheme WO3/ZnS heterojunction to enhance the photocatalytic degradation of tetracycline antibiotic. J. Environ. Chem. Eng. 2021, 9, 105111. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).