Corrosion Resistance Properties of As-Sintered 17-4 PH Samples Additive-Manufactured Through Binder Jetting
Abstract
1. Introduction
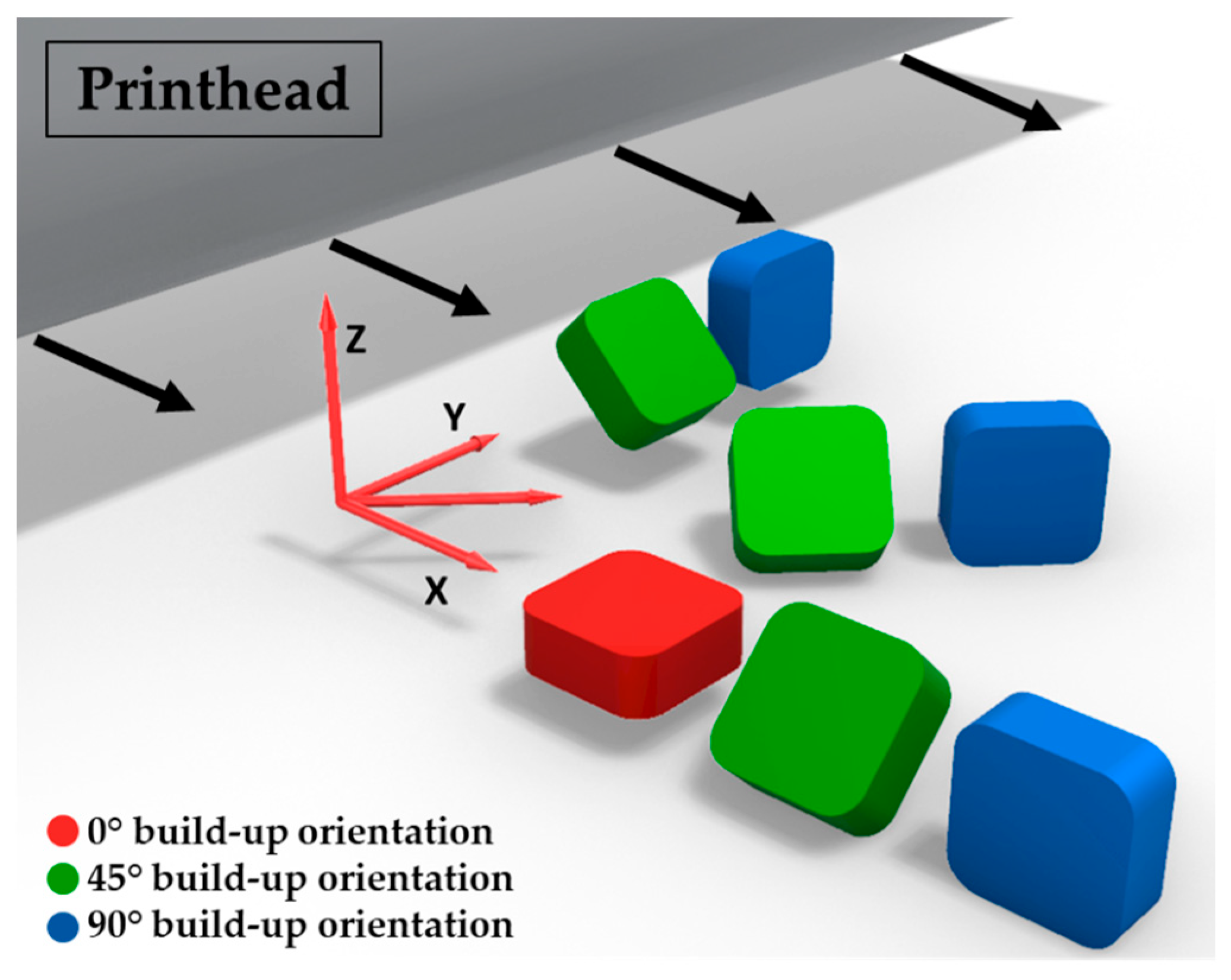
2. Materials and Methods
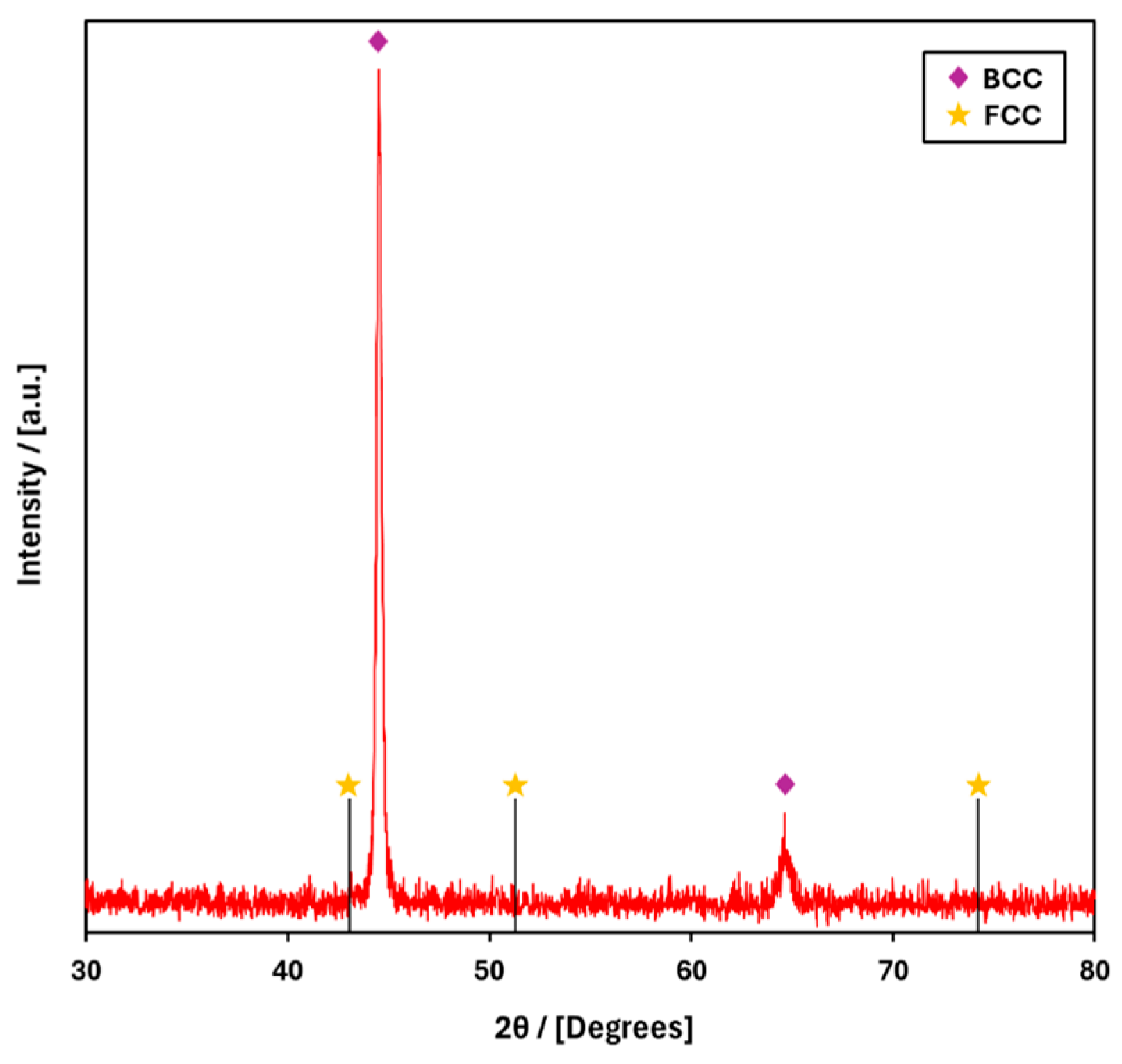
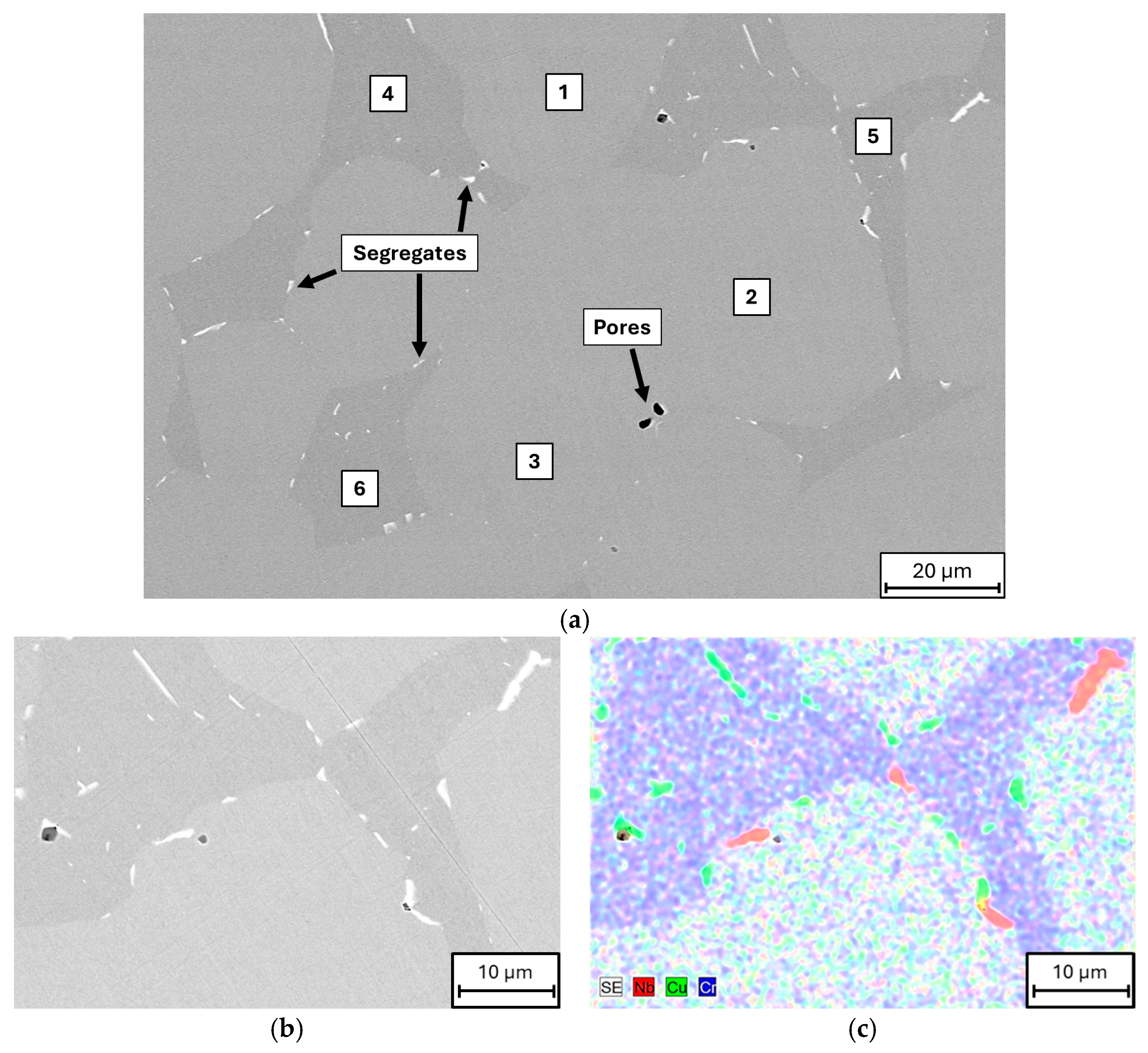
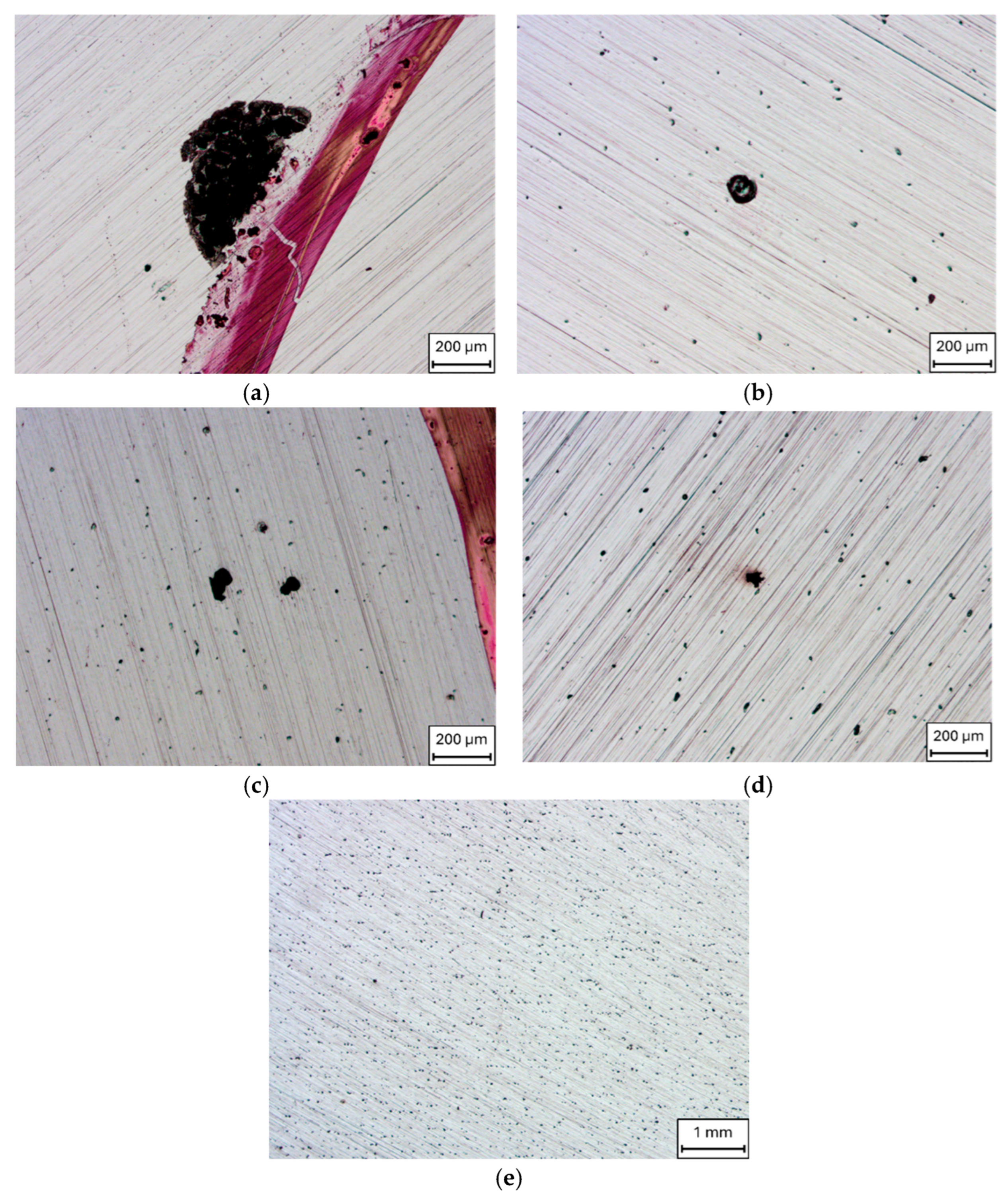
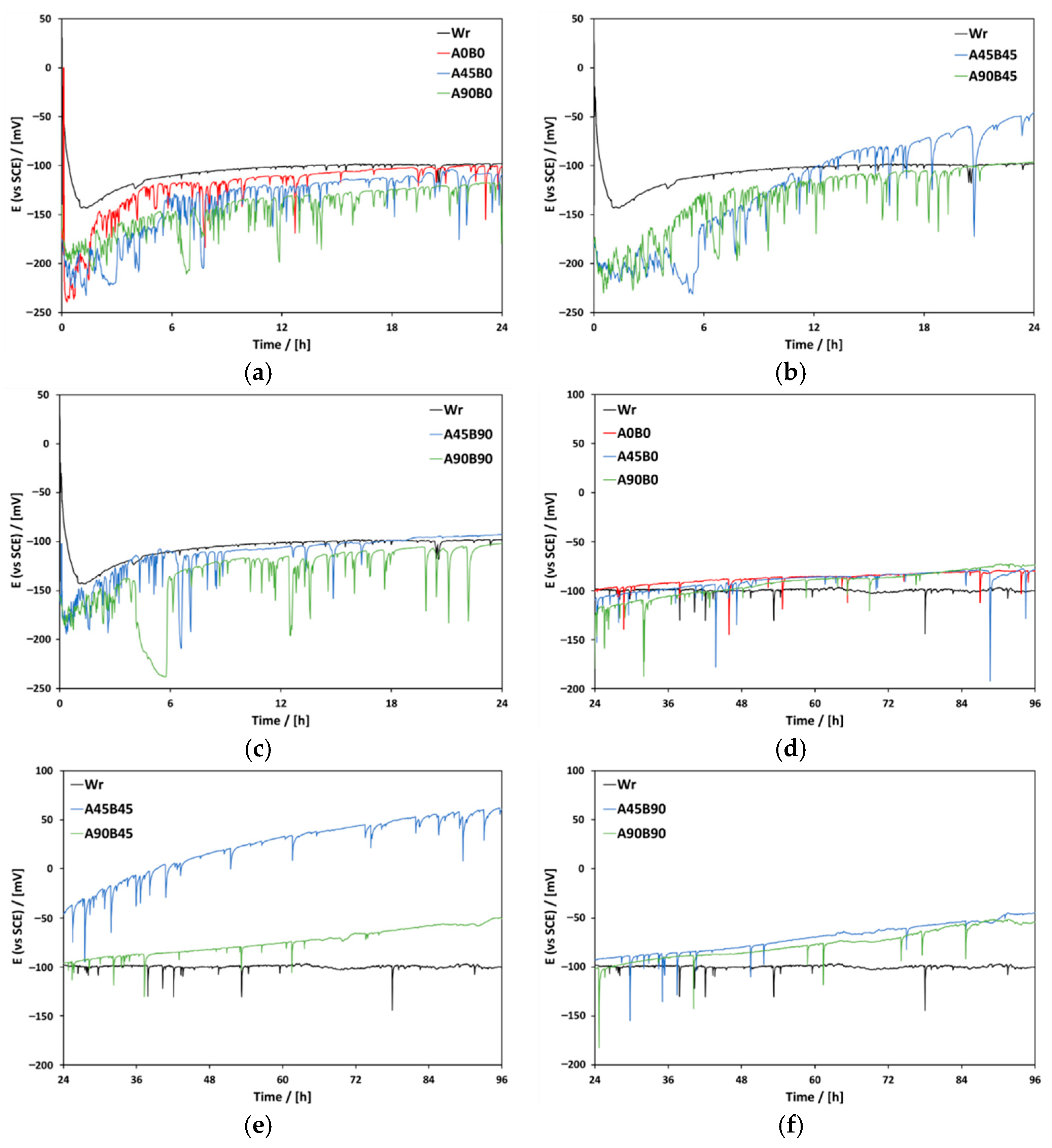
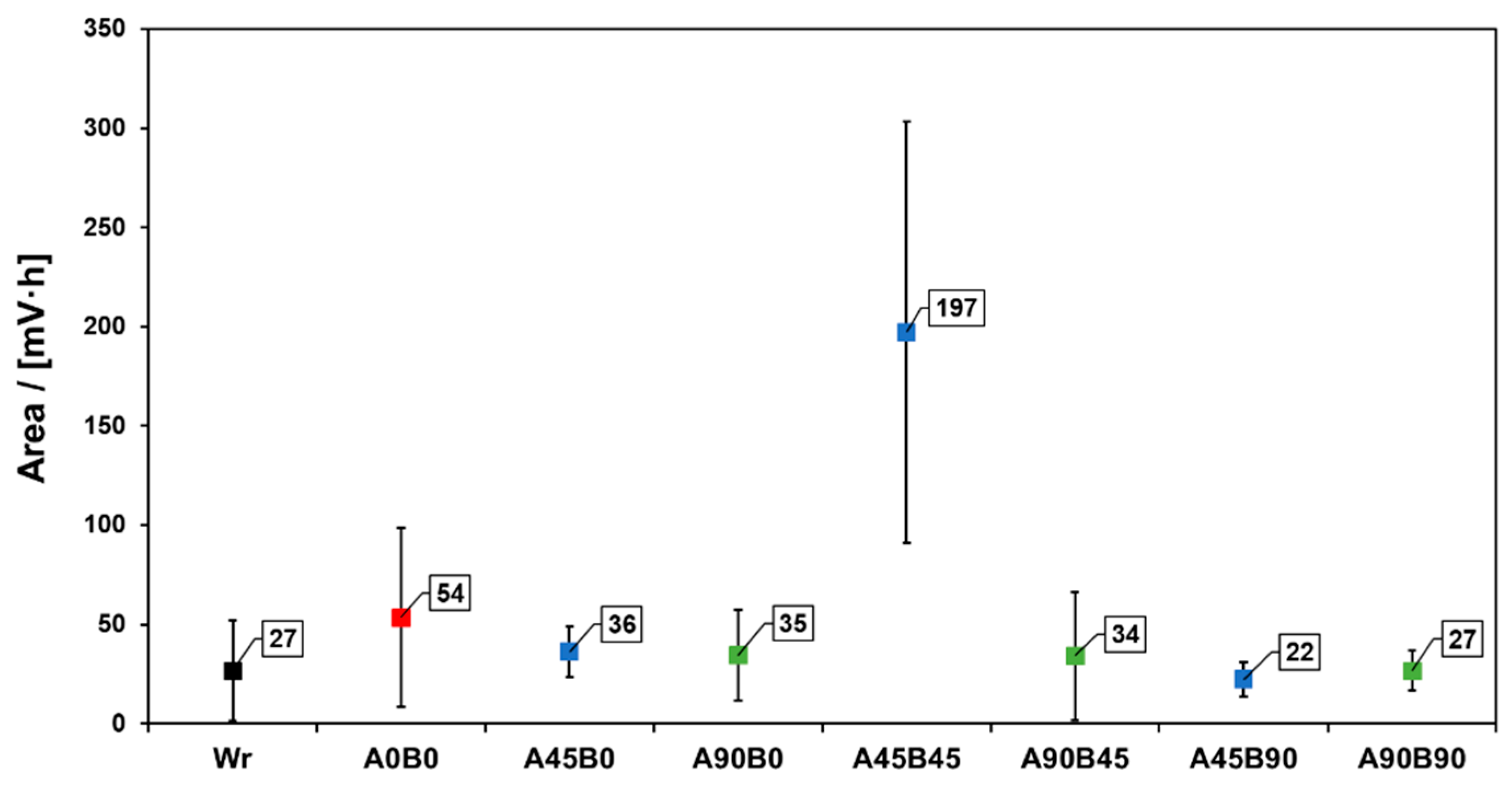
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vafadar, A.; Guzzomi, F.; Rassau, A.; Hayward, K. Advances in Metal Additive Manufacturing: A Review of Common Processes, Industrial Applications, and Current Challenges. Appl. Sci. 2021, 11, 1213. [Google Scholar] [CrossRef]
- Li, M.; Du, W.; Elwany, A.; Pei, Z.; Ma, C. Metal binder jetting additive manufacturing: A literature review. J. Manuf. Sci. Eng. Trans. ASME 2020, 142, 090801. [Google Scholar] [CrossRef]
- Dini, F.; Ghaffari, S.A.; Jafar, J.; Hamidreza, R.; Marjan, S. A review of binder jet process parameters; powder, binder, printing and sintering condition. Met. Powder Rep. 2020, 75, 95–100. [Google Scholar] [CrossRef]
- Kráľ, J.; Dzuro, T.; Debski, H. Applying Binder Jetting Technology to 316L Stainless Steel Materials and Testing Its Mechanical and Dimensional Properties Depending on the Printing Method. Materials 2024, 17, 4400. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, I.; Forcellese, A.; Forcellese, P.; Mancia, T.; Mignanelli, C.; Simoncini, M.; Verdini, T. Effect of Printing Orientation Angle and Heat Treatment on the Mechanical Properties and Microstructure of Binder-Jetting-Printed Parts in 17-4 PH Stainless Steel. Metals 2024, 14, 1220. [Google Scholar] [CrossRef]
- Ibrahim, B.; Lopez, L.; Kulkarni, S.; Jobes, D.; Forgiarini, M.; Barber, J.R.; Gordon, J.V. Increasing strength properties in sinter-based additive manufacturing of SS316L via metal material jetting of sub-micron powders. Addit. Manuf. 2024, 89, 104268. [Google Scholar] [CrossRef]
- Lecis, N.; Beltrami, R.; Mariani, M. Binder jetting 3D printing of 316 stainless steel: Influence of process parameters on microstructural and mechanical properties. Metall. Ital. 2021, 113, 31–41. [Google Scholar]
- Mirzababaei, S.; Paul, B.K.; Pasebani, S. Metal Powder Recyclability in Binder Jet Additive Manufacturing. Jom 2020, 72, 3070–3079. [Google Scholar] [CrossRef]
- Lanzutti, A.; Marin, E. The Challenges and Advances in Recycling/Re-Using Powder for Metal 3D Printing: A Comprehensive Review. Metals 2024, 14, 886. [Google Scholar] [CrossRef]
- Xu, M.; Guo, H.; Wang, Y.; Hou, Y.; Dong, Z.; Zhang, L. Mechanical properties and microstructural characteristics of 316L stainless steel fabricated by laser powder bed fusion and binder jetting. J. Mater. Res. Technol. 2023, 24, 4427–4439. [Google Scholar] [CrossRef]
- Alegre, J.M.; Díaz, A.; García, R.; Peral, L.B.; Lorenzo-Bañuelos, M.; Cuesta, I.I. Mechanical and Fatigue Properties of Ti-6Al-4V Alloy Fabricated Using Binder Jetting Process and Subjected to Hot Isostatic Pressing. Materials 2024, 17, 3825. [Google Scholar] [CrossRef]
- Meenashisundaram, G.K.; Xu, Z.; Nai, M.L.S.; Lu, S.; Ten, J.S.; Wei, J. Binder jetting additive manufacturing of high porosity 316L stainless steel metal foams. Materials 2020, 13, 3744. [Google Scholar] [CrossRef]
- Padmakumar, M. Additive Manufacturing of Tungsten Carbide Hardmetal Parts by Selective Laser Melting (SLM), Selective Laser Sintering (SLS) and Binder Jet 3D Printing (BJ3DP) Techniques. Lasers Manuf. Mater. Process. 2020, 7, 338–371. [Google Scholar] [CrossRef]
- Mostafaei, A.; Elliott, A.M.; Barnes, J.E.; Li, F.; Tan, W.; Cramer, C.L.; Nandwana, P.; Chmielus, M. Binder jet 3D printing—Process parameters, materials, properties, modeling, and challenges. Prog. Mater. Sci. 2021, 119, 100707. [Google Scholar] [CrossRef]
- Fang, X.; Zu, Y.; Ma, Q.; Hu, J. State of the art of metal powder bonded binder jetting printing technology. Discov. Mater. 2023, 3, 15. [Google Scholar] [CrossRef]
- Batmaz, R.; Zardoshtian, A.; Sabiston, T.D.; Tangestani, R.; Chakraborty, A.; Krutz, N.; Pendurti, S.; Natarajan, A.; Martin, E. An Investigation into Sinterability Improvements of 316L Binder Jet Printed Parts. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2022, 53, 915–926. [Google Scholar] [CrossRef]
- Mao, Y.; Li, J.; Li, W.; Cai, D.; Wei, Q. Binder jetting additive manufacturing of 316L stainless-steel green parts with high strength and low binder content: Binder preparation and process optimization. J. Mater. Process. Technol. 2021, 291, 117020. [Google Scholar] [CrossRef]
- Jiang, R.; Monteil, L.; Kimes, K.; Mostafaei, A.; Chmielus, M. Influence of powder type and binder saturation on binder jet 3D–printed and sintered Inconel 625 samples. Int. J. Adv. Manuf. Technol. 2021, 116, 3827–3838. [Google Scholar] [CrossRef]
- Berger, C.; Pötschke, J.; Scheithauer, U.; Michaelis, A. Influence of Various Binder Jet Printers on the Additive Manufacturing of Hardmetals. Crystals 2024, 14, 947. [Google Scholar] [CrossRef]
- Miyanaji, H.; Orth, M.; Akbar, J.M.; Yang, L. Process development for green part printing using binder jetting additive manufacturing. Front. Mech. Eng. 2018, 13, 504–512. [Google Scholar] [CrossRef]
- Vukkum, V.B.; Gupta, R.K. Review on corrosion performance of laser powder-bed fusion printed 316L stainless steel: Effect of processing parameters, manufacturing defects, post-processing, feedstock, and microstructure. Mater. Des. 2022, 221, 110874. [Google Scholar] [CrossRef]
- Voisin, T.; Shi, R.; Zhu, Y.; Qi, Z.; Wu, M.; Sen-Britain, S.; Zhang, Y.; Qiu, S.R.; Wang, Y.M.; Thomas, S.; et al. Pitting Corrosion in 316L Stainless Steel Fabricated by Laser Powder Bed Fusion Additive Manufacturing: A Review and Perspective. JOM 2022, 74, 1668–1689. [Google Scholar] [CrossRef]
- Voisin, T.; Forien, J.-B.; Perron, A.; Aubry, S.; Bertin, N.; Samanta, A.; Baker, A.; Wang, Y.M. New insights on cellular structures strengthening mechanisms and thermal stability of an austenitic stainless steel fabricated by laser powder-bed-fusion. Acta Mater. 2021, 203, 116476. [Google Scholar] [CrossRef]
- Melia, M.A.; Duran, J.G.; Koepke, J.R.; Saiz, D.J.; Jared, B.H.; Schindelholz, E.J. How build angle and post-processing impact roughness and corrosion of additively manufactured 316L stainless steel. Npj Mater. Degrad. 2020, 4, 21. [Google Scholar] [CrossRef]
- Sander, G.; Babu, A.P.; Gao, X.; Jiang, D.; Birbilis, N. On the effect of build orientation and residual stress on the corrosion of 316L stainless steel prepared by selective laser melting. Corros. Sci. 2021, 179, 109149. [Google Scholar] [CrossRef]
- Forcellese, P.; Mancia, T.; Simoncini, M.; Bellezze, T. Investigation on Corrosion Resistance Properties of 17-4 PH Bound Metal Deposition As-Sintered Specimens with Different Build-Up Orientations. Metals 2022, 12, 588. [Google Scholar] [CrossRef]
- Forcellese, P.; Mancia, T.; Simoncini, M.; Bellezze, T. Characterization of Microstructure and Localized Corrosion Resistance of Heat-Treated 17-4 PH Stainless Steel Fabricated by Material Extrusion. Metals 2025, 15, 137. [Google Scholar] [CrossRef]
- Forcellese, P.; Belegni, S.; Silvi, A.; Mancia, T.; Simoncini, M.; Bellezze, T. Study of corrosion resistance of 17-4 PH stainless steel as-sintered and heat treated samples manufactured by Bound Metal Deposition. La Metall. Ital. 2023, 114, 9–14. [Google Scholar]
- Suwanpreecha, C.; Manonukul, A. A Review on Material Extrusion Additive Manufacturing of Metal and How It Compares with Metal Injection Moulding. Metals 2022, 12, 429. [Google Scholar] [CrossRef]
- Huang, N.; Cook, O.J.; Argüelles, A.P.; Beese, A.M. Review of Process–Structure–Property Relationships in Metals Fabricated Using Binder Jet Additive Manufacturing. Metallogr. Microstruct. Anal. 2023, 12, 883–905. [Google Scholar] [CrossRef]
- Kaae, P.H.; Eikeland, E.Z. Corrosion Performance of Additively Manufactured Stainless Steel by Binder Jetting. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1249, 012051. [Google Scholar] [CrossRef]
- Gunarathne, C. Corrosion Evaluation of Metal Binder-Jetted Stainless Steel Parts: Influence of Printing Parameters on Performance. ECS Meet. Abstr. 2024, MA2024-01, 2811. [Google Scholar] [CrossRef]
- Atapour, M.; Wang, X.; Persson, M.; Odnevall Wallinder, I.; Hedberg, Y.S. Corrosion of Binder Jetting Additively Manufactured 316L Stainless Steel of Different Surface Finish. J. Electrochem. Soc. 2020, 167, 131503. [Google Scholar] [CrossRef]
- Wu, Y.; Blaine, D.; Marx, B.; Schlaefer, C.; German, R.M. Sintering densification and microstructural evolution of injection molding grade 17-4 PH stainless steel powder. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2002, 33, 2185–2194. [Google Scholar] [CrossRef]
- Huber, D.; Stich, P.; Fischer, A. Heat Treatment of 17–4 PH Stainless Steel Produced by Binder Jet Additive Manufacturing (BJAM) from N2-Atomized Powder. Prog. Addit. Manuf. 2022, 7, 187–199. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Z.; Chen, L.; Zhu, D.; Fu, Z. Optimization of Printing Parameters to Achieve High-Density 316L Stainless Steel Manufactured by Binder Jet 3D Printing. J. Mater. Eng. Perform. 2023, 32, 3602–3616. [Google Scholar] [CrossRef]
- Singh, G.; Missiaen, J.M.; Bouvard, D.; Chaix, J.M. Additive manufacturing of 17–4 PH steel using metal injection molding feedstock: Analysis of 3D extrusion printing, debinding and sintering. Addit. Manuf. 2021, 47, 102287. [Google Scholar] [CrossRef]
- Di Pompeo, V.; Santecchia, E.; Santoni, A.; Sleem, K.; Cabibbo, M.; Spigarelli, S. Microstructure and Defect Analysis of 17-4PH Stainless Steel Fabricated by the Bound Metal Deposition Additive Manufacturing Technology. Crystals 2023, 13, 1312. [Google Scholar] [CrossRef]
- Mirzababaei, S.; Paul, B.K.; Pasebani, S. Microstructure-property relationship in binder jet produced and vacuum sintered 316L. Addit. Manuf. 2022, 53, 102720. [Google Scholar] [CrossRef]
- Lecis, N.; Mariani, M.; Beltrami, R.; Emanuelli, L.; Casati, R.; Vedani, M.; Molinari, A. Effects of process parameters, debinding and sintering on the microstructure of 316L stainless steel produced by binder jetting. Mater. Sci. Eng. A 2021, 828, 142108. [Google Scholar] [CrossRef]
- Kumar, P.; Jayaraj, R.; Suryawanshi, J.; Satwik, U.R.; McKinnell, J.; Ramamurty, U. Fatigue strength of additively manufactured 316L austenitic stainless steel. Acta Mater. 2020, 199, 225–239. [Google Scholar] [CrossRef]
- Chen, L.; Chen, W.; Fu, Z.; Ding, G.; Chen, Z.; Zhu, D. Binder Jet 3D Printing of 316L Stainless Steel: Orthogonal Printing and Sintering Process Optimization. Adv. Eng. Mater. 2023, 25, 2200641. [Google Scholar] [CrossRef]
- Huber, D.; Vogel, L.; Fischer, A. The effects of sintering temperature and hold time on densification, mechanical properties and microstructural characteristics of binder jet 3D printed 17-4 PH stainless steel. Addit. Manuf. 2021, 46, 102114. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, W.; Chen, L.; Zhu, D.; Chen, Q.; Fu, Z. Influence of initial relative densities on the sintering behavior and mechanical behavior of 316 L stainless steel fabricated by binder jet 3D printing. Mater. Today Commun. 2022, 31, 103369. [Google Scholar] [CrossRef]
- Banas, J.; Mazurkiewicz, A. The effect of copper on passivity and corrosion behaviour of ferritic and ferritic–austenitic stainless steels. Mater. Sci. Eng. A 2000, 277, 183–191. [Google Scholar] [CrossRef]
- Shu, J.; Bi, H.; Li, X.; Xu, Z. The effect of copper and molybdenum on pitting corrosion and stress corrosion cracking behavior of ultra-pure ferritic stainless steels. Corros. Sci. 2012, 57, 89–98. [Google Scholar] [CrossRef]
- Li, Z.; Chen, J.; Xue, W.; Yin, C.; Song, J.; Xiao, K. Role of segregation behavior of Cu and Sb in the region of inclusions on initial corrosion. npj Mater. Degrad. 2023, 7, 29. [Google Scholar] [CrossRef]
- Hu, S.; Han, E.-H.; Liu, X. Atomic-scale evidence for the intergranular corrosion mechanism induced by co-segregation of low-chromium ferritic stainless steel. Corros. Sci. 2021, 189, 109588. [Google Scholar] [CrossRef]



| Cr | Ni | Cu | Si | C | Mn | Mo | Fe |
|---|---|---|---|---|---|---|---|
| 16.54 | 4.77 | 3.35 | 0.54 | 0.02 | 0.46 | 0.21 | Bal. |
| Cr | Ni | Cu | Nb | Si | Fe | ||
|---|---|---|---|---|---|---|---|
| Bright grains (martensite) | Average | 15.6 | 4.4 | 4.0 | 0.3 | 0.7 | 75.0 |
| Std. Dev. | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |
| Dark grains (ferrite) | Average | 22.2 | 1.9 | 0.9 | 0.3 | 1.0 | 73.7 |
| Std. Dev. | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forcellese, P.; Khan, W.A.; Mancia, T.; Simoncini, M.; Reiser, M.; Kouřil, M.; Bellezze, T. Corrosion Resistance Properties of As-Sintered 17-4 PH Samples Additive-Manufactured Through Binder Jetting. Metals 2025, 15, 1082. https://doi.org/10.3390/met15101082
Forcellese P, Khan WA, Mancia T, Simoncini M, Reiser M, Kouřil M, Bellezze T. Corrosion Resistance Properties of As-Sintered 17-4 PH Samples Additive-Manufactured Through Binder Jetting. Metals. 2025; 15(10):1082. https://doi.org/10.3390/met15101082
Chicago/Turabian StyleForcellese, Pietro, Wasiq Ali Khan, Tommaso Mancia, Michela Simoncini, Matěj Reiser, Milan Kouřil, and Tiziano Bellezze. 2025. "Corrosion Resistance Properties of As-Sintered 17-4 PH Samples Additive-Manufactured Through Binder Jetting" Metals 15, no. 10: 1082. https://doi.org/10.3390/met15101082
APA StyleForcellese, P., Khan, W. A., Mancia, T., Simoncini, M., Reiser, M., Kouřil, M., & Bellezze, T. (2025). Corrosion Resistance Properties of As-Sintered 17-4 PH Samples Additive-Manufactured Through Binder Jetting. Metals, 15(10), 1082. https://doi.org/10.3390/met15101082










