Advances in Integrated Extraction of Valuable Components from Ti-Bearing Slag
Abstract
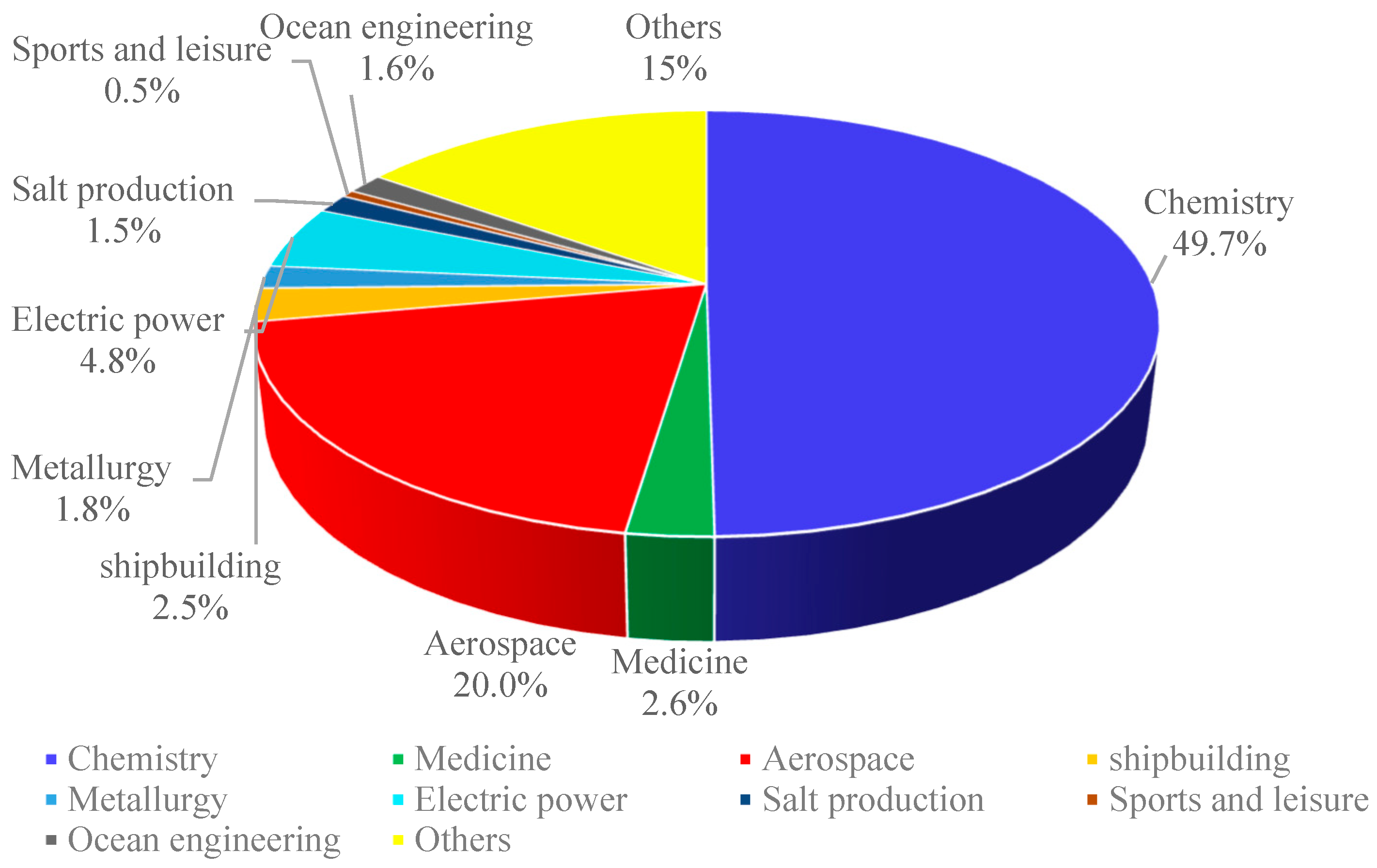
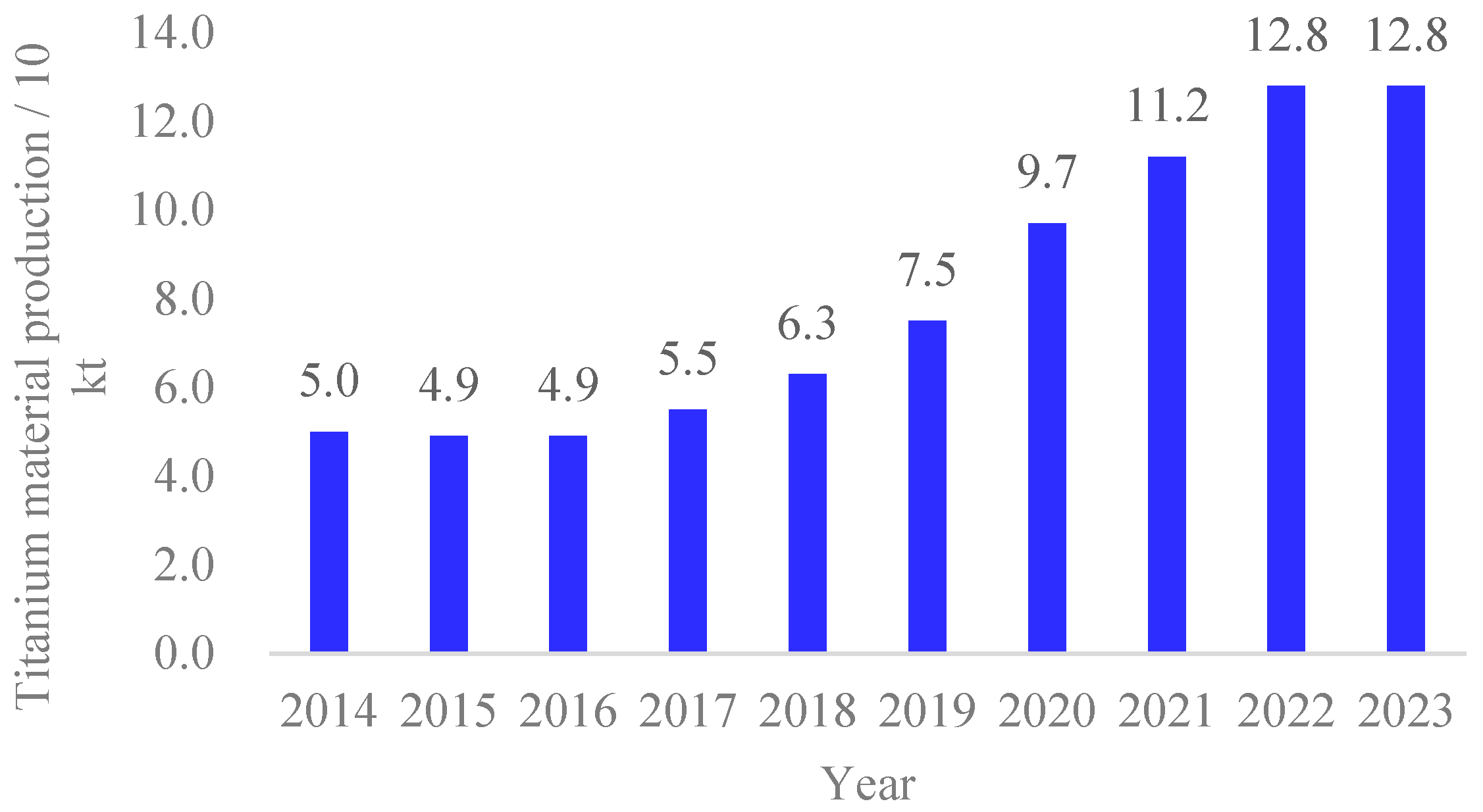
1. Introduction
2. Classification, Properties, and Components of Ti-Bearing Slags (TBS)
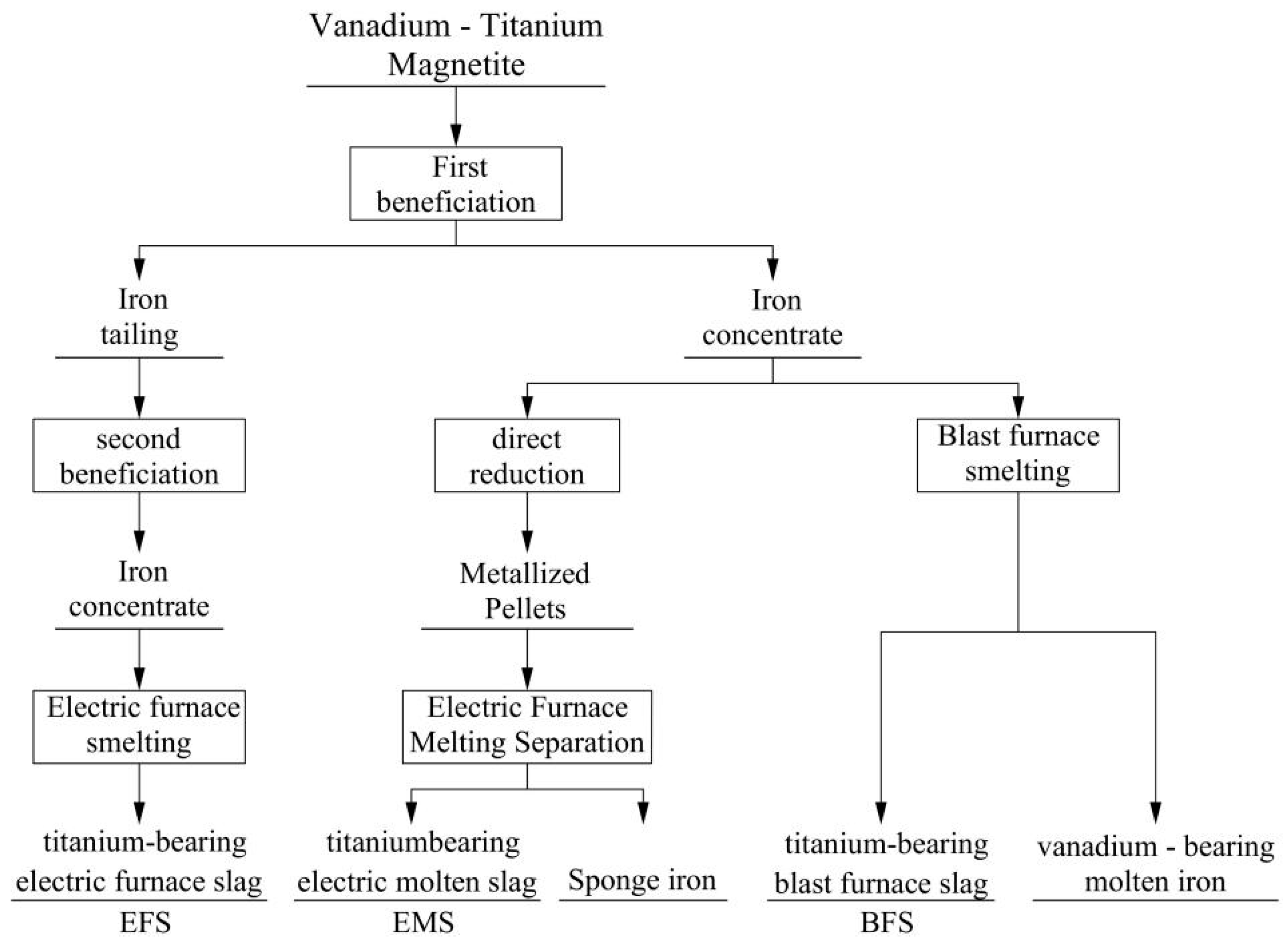
2.1. Classification and Properties of TBS
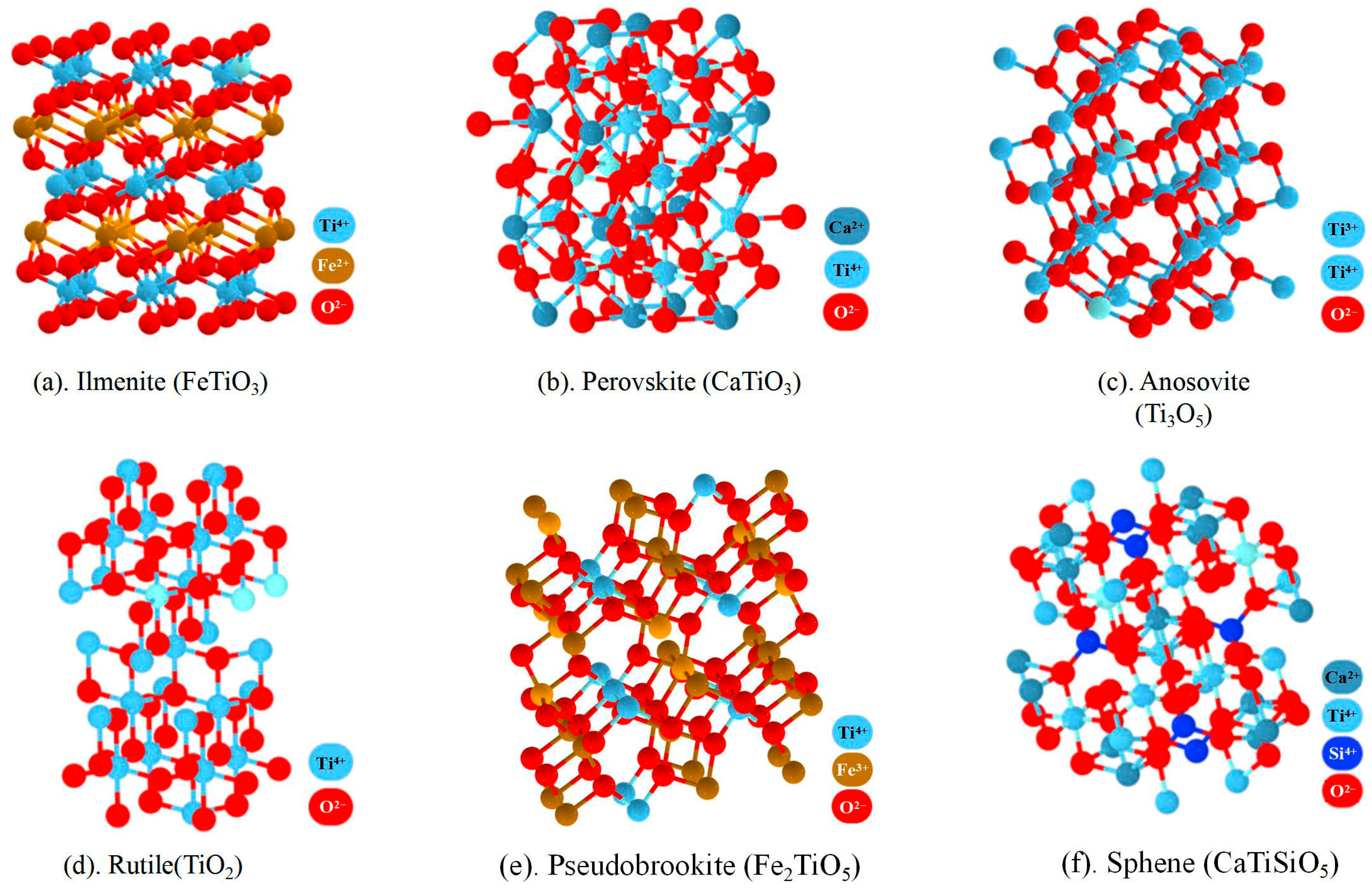
2.2. The Chemical Components and Mineral Phases of TBS
3. Analysis of the Current Technologies for Ti Extraction from TBS
3.1. Hydrometallurgical Leaching
3.2. Pyrometallurgical Titanium Extraction Methods
3.2.1. Carbothermal Reduction
3.2.2. Metallothermic Reduction
3.3. Molten Electrolysis Technology for Titanium Extraction
3.4. Selective Precipitation Titanium Extraction Technology
3.5. Comparative Analysis of Titanium Extraction Processes from TBS
4. Innovative Insights on Industrial Titanium Extraction
5. Conclusions
- (1)
- TBS is a multi-component byproduct wherein titanium is primarily locked within refractory minerals. This intricate structure necessitates aggressive extraction conditions, often leading to high energy consumption and environmental concerns.
- (2)
- Comparative analysis of mainstream technologies reveals a performance–energy trade-off. Hydrometallurgical methods are improved by activation roasting, while pyrometallurgical routes benefit from cleaner gaseous reductants to lower energy intensity. For molten electrolysis, liquid metal cathodes not only prevent dendritic growth but also resolve electrolytic deposition and polarization. In selective separation, techniques with supergravity integration and optimized crystal growth kinetics enable high-purity products, albeit with sensitivity to process parameters.
- (3)
- Future development must focus on low-carbon processes (use of hydrogen for pyrometallurgical treatment) and the synergistic recovery of all valuable elements via hydrometallurgical treatment. The proposed strategy of “Online conditioning–mineral phase reconstruction–directional crystallization–optimized liberation” is recommended to harness the slag’s waste heat, prevent inhomogeneous crystallization, and enable efficient titanium recovery on an industrial scale.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| TBS | Ti-bearing Slags |
| BFS | blast furnace slag |
| EMS | electric molten slag |
| EFS | electric furnace slag |
| MOE | molten oxide electrolysis |
| LMC | Liquid metal cathodes |
| EMS | electromagnetic stirring |
References
- Tebaldo, V.; Gautier di Confiengo, G.; Duraccio, D.; Faga, M.G. Sustainable Recovery of Titanium Alloy: From Waste to Feedstock for Additive Manufacturing. Sustainability 2024, 16, 330. [Google Scholar] [CrossRef]
- Chong, X.X.; Luan, W.L.; Wang, F.X.; Qiu, T.D.; Zhang, W.Y. Overview of global titanium resources status and titanium consumption trend in China. Conserv. Util. Miner. Resour. 2020, 40, 162–170. [Google Scholar]
- Hu, K. Research on the Physicochemical Properties and Structure of High Titania Slag. Ph.D. Thesis, Chongqing University, Chongqing, China, 2021. [Google Scholar]
- Jena, K.D.; Xu, Y.; Hayat, M.D.; Zhang, W.; Cao, P. Aiming at low-oxygen titanium powder: A review. Powder Technol. 2021, 394, 1195–1217. [Google Scholar] [CrossRef]
- An, Z.S.; Chen, Y.; Zhao, W.; Huai, J. Report on China Titanium Industry Progress in 2023. Titan. Ind. Prog. 2024, 41, 41–48. [Google Scholar]
- U.S. Geological Survey. Mineral Commodity Summaries 2024; U.S. Geological Survey: Reston, VA, USA, 2024; p. 212. [CrossRef]
- Tang, Q.X.; Gan, C.D.; Yang, J.Y.; Huang, Y. Dynamics of vanadium and response of inherent bacterial communities in vanadium–titanium magnetite tailings to beneficiation agents, temperature, and illumination. Environ. Pollut. 2023, 330, 121743. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, J.L.; Li, H.; Huo, D.X.; Xie, S.S. Resource Recycling progress of Panzhihua Titanium Bearing Blast Furnace Slag. Multipurp. Util. Miner. Resour. 2018, 39, 12–15. [Google Scholar] [CrossRef]
- Zhang, S.S.; Wang, Z.Y.; Hu, P.; Rao, J.T.; Zhang, J.L.; Pang, J. Distribution behavior of vanadium and titanium between hot metal and high titanium slag relevant to HIsmelt smelting condition. J. Mater. Res. Technol. 2022, 19, 4517–4524. [Google Scholar] [CrossRef]
- Wang, Z.X.; Peng, Z.H.; Li, Y.; Zhu, Y.Z.; Xie, K.Q. Selective sulfuric acid cyclic leaching of vanadium from the calcification roasting pellets of vanadium titanomagnetite. J. Mater. Res. Technol. 2023, 23, 778–790. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.Q.; Liu, X.M.; Zhang, B.M.; Chi, Q.H.; Li, D.P. Spatial distribution of the critical mineral resource element titanium in China and its influencing factors. J. Geochem. Explor. 2024, 256, 107334. [Google Scholar] [CrossRef]
- Li, W.; Wang, N.; Fu, G.Q.; Chu, M.S.; Zhu, M.Y. Influence of TiO2 addition on the oxidation induration and reduction behavior of Hongge vanadium titanomagnetite pellets with simulated shaft furnace gases. Powder Technol. 2018, 326, 137–145. [Google Scholar] [CrossRef]
- Cai, Y.F.; Song, N.N.; Yang, Y.F.; Sun, L.M.; Hu, P.; Wang, J.S. Recent progress of efficient utilization of titanium-bearing blast furnace slag. Int. J. Miner. Metall. Mater. 2022, 29, 22–31. [Google Scholar] [CrossRef]
- Wang, H.R.; Zhang, Y.L.; An, Z.Q.; Zhao, S.Q. Hydrometallurgical process for recovering titanium from titanium-bearing blast furnace slag in Panzhihua Steel Plant. Nonferrous Met. Sci. Eng. 2016, 7, 21–24. [Google Scholar]
- Liu, G.; Zhang, C.; Zhang, S.P.; Guo, C.; Liu, S.M.; Li, M.; Wu, Y.P. Effect and mechanism of calcination on improving the hydration activity of titanium extraction slag. Constr. Build. Mater. 2024, 447, 138144. [Google Scholar] [CrossRef]
- Han, Y.; Kim, S.; Go, B.; Lee, S.; Park, S.; Jeona, H. Optimized magnetic separation for efficient recovery of V and Ti enriched concentrates from vanadium-titanium magnetite ore: Effect of grinding and magnetic intensity. Powder Technol. 2021, 391, 282–291. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Lu, Z.Y.; Hou, L.; Chen, J.K. Preparation and properties of reactive powder concrete by using titanium slag aggregates. Constr. Build. Mater. 2020, 234, 117342. [Google Scholar] [CrossRef]
- Krivenko, P.; Kovalchuk, O.; Pasko, A.; Croymans, T.; Hult, M.; Lutter, G.; Schroeyers, W.; Vandevenne, N.; Schreurs, S. Development of alkali activated cements and concrete mixture design with high volumes of red mud. Constr. Build. Mater. 2017, 151, 819–826. [Google Scholar] [CrossRef]
- Pradhan, P.; Dey, S.; Manna, K.; Panda, A.B.; Pal, S. In situ synthesis of a photocatalyst using TiO2 QDs-immobilized functionalized galactomannan for degradation of organic pollutant. J. Water Process. Eng. 2025, 69, 106617. [Google Scholar] [CrossRef]
- Hu, G. Study on Structural Regulation and Photocatalytic Behavior of Ti-bearing Blast Furnace Slag. Ph.D. Thesis, Chongqing University, Chongqing, China, 2021. [Google Scholar]
- Swami, M.C.; Dabade, B.M. Seawater’s impact on the mechanical properties of SiO2 and TiO2 fillers in glass reinforced epoxy composites. Mater. Today Proc. 2024, 103, 251–255. [Google Scholar] [CrossRef]
- Janczarek, M.; Klapiszewski, Ł.; Jędrzejczak, P.; Klapiszewska, I.; Ślosarczyk, A.; Jesionowski, T. Progress of functionalized TiO2-based nanomaterials in the construction industry: A comprehensive review. Chem. Eng. J. 2022, 430, 132062. [Google Scholar] [CrossRef]
- Rodriguez, M.H.; Rosales, G.D.; Pinna, E.G.; Tunez, F.M.; Toro, N. Extraction of Titanium from Low-Grade Ore with Different Leaching Agents in Autoclave. Metals 2020, 10, 497. [Google Scholar] [CrossRef]
- Matsanga, N.; WaKalenga, M.; Nheta, W. An Overview of Thermochemical Reduction Processes for Titanium Production. Minerals 2025, 15, 17. [Google Scholar] [CrossRef]
- Zhang, L.F.; Ding, M.T.; He, C.P.; Lin, H.; Lei, Y.Y.; He, Y.Q. Extracting Titanium from Titanium-containing Blast Furnace Slag by Pyrometallurgy. China Resour. Compr. Util. 2020, 38, 94–96. [Google Scholar]
- Xu, Y.; Li, D.D.; Yuan, M.; Cai, Y.Q. Effect of Temperature Conditions and Additives on Selective Precipitation Process of Perovskite Crystals. Iron Steel Vanadium Titan. 2020, 41, 86–95. [Google Scholar]
- Bian, Z.Z. Recovery of Valuable Metals from Vanadium Titanomagnetite Concentrate by Ammonium Salt Roasting. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2021. [Google Scholar]
- Dubenko, A.V.; Nikolenko, M.V.; Pasenko, O.O.; Kostyniuk, A.; Likozar, B. Intensification of Sulfuric Acid Leaching of Altered Ilmenite via Adding Fluoride Activator. Processes 2021, 9, 1922. [Google Scholar] [CrossRef]
- Feng, Q.S.; Lv, M.R.; Mao, L.; Duan, B.H.; Yang, Y.C.; Chan, G.Y.; Lu, X.G.; Li, C.H. Research Progress of Titanium Sponge Production: A Review. Metals 2023, 13, 408. [Google Scholar] [CrossRef]
- Takeda, O.; Ouchi, T.; Okabe, T.H. Recent Progress in Titanium Extraction and Recycling. Metall. Mater. Trans. B 2020, 51, 1315–1328. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Zhang, X.; Huang, B.; Yang, Y.S.; Zhao, Z.W. Electrochemical reduction processes and extraction of titanium with different valence states in molten CaO-CaF2 slags. J. Alloys Compd. 2024, 1002, 175379. [Google Scholar] [CrossRef]
- Dai, W.; Qin, B.; Liu, Y.X.; Yan, H.W.; Ma, W.H. Electrolysis of rich Titanium Slag in Molten CaCl2 with a Liquid Zn Cathode. Int. J. Electrochem. Sci. 2020, 15, 11493–11508. [Google Scholar] [CrossRef]
- Yang, S.P.; Liu, S.M.; Guo, S.J.; Zhang, T.T.; Li, J.H. Fusion Separation of Vanadium-Titanium Magnetite and Enrichment Test of Ti Element in Slag. Materials 2022, 15, 6795. [Google Scholar] [CrossRef]
- Ma, Z.; Zhao, Z.W.; Guo, X.Q.; Guo, W.T. Selective precipitation and non-isothermal crystallization kinetics of britholite from low grade REE-bearing slag. J. Rare Earths 2023, 41, 1812–1818. [Google Scholar] [CrossRef]
- Hu, Q.Q.; Ma, D.L.; Zhou, K.; Liu, Y.J.; You, Y.; You, Z.X.; Lv, X.W. Phase transformation and slag evolution of vanadium–titanium magnetite pellets during softening–melting process. Powder Technol. 2022, 396, 710–717. [Google Scholar] [CrossRef]
- Xu, C.B.; Zhang, Y.M.; Liu, T.; Huang, J. Characterization and Pre-Concentration of Low-Grade Vanadium-Titanium Magnetite Ore. Minerals 2017, 7, 137. [Google Scholar] [CrossRef]
- Chen, F.; Li, H.; Guo, Y.F.; Liu, K.; Wang, S.; Yang, L.Z.; Wen, Y.K.; Zhang, M. Reduction disintegration behavior and crystallographic transformation of vanadium–titanium magnetite pellets during hydrogen-based reduction. J. Mater. Res. Technol. 2025, 34, 152–163. [Google Scholar] [CrossRef]
- Qian, G.Y.; Wang, H.; Pang, S.; Sun, Y.W.; Guo, J.W.; Wang, D.; Zhou, J.; Sheng, Z.L.; Wang, Z. New insights into the application of non-consumable electrode electroslag remelting refining: Enhanced removal of inclusions in Ti-Si alloy based the reduction of Ti-bearing blast furnace slag by SoG-Si scraps. J. Clean. Prod. 2025, 490, 144684. [Google Scholar] [CrossRef]
- Li, Y.Y.; Yan, B.J. A novel approach for pre-concentrating titanium from Ti-bearing blast furnace slag. Sep. Sci. Technol. 2023, 58, 1679–1688. [Google Scholar] [CrossRef]
- Li, Y. Physical Chemistry Study on Recovery of Valuable Elements in Ti-Bearing Electric Arc Furnace Molten Slag. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2015. [Google Scholar]
- Li, J.H.; Qiu, K.H.; Gong, Y.H. Developments of Comprehensive Utilization and Extraction Technology of Ti Component form Ti-bearing Blast Furnace Slag of Panzhihua Iron and Steel Co. Sichuan Chem. Ind. 2010, 13, 21–25. [Google Scholar]
- Yu, W. Commercial-scale test on Ti slag smelting from Ti concentrate. Rare Mttals Cem. Carbides 2004, 32, 29–32. [Google Scholar]
- Zhang, R.; Hou, Y.L.; Fan, G.Q.; Huang, D.J.; Ding, X.M.; Dang, J. Gas-based reduction and carbonization of titanium minerals in titanium-bearing blast furnace slag: A combined thermodynamic, experimental and DFT study. Int. J. Hydrogen Energy 2022, 47, 7586–7599. [Google Scholar] [CrossRef]
- Wang, J.X.; Yue, D.; Wen, L.Y.; Hu, L.W.; Lv, X.M. Experimental characterization of chemical mineral composition and morphology of titanium in carbonized slag particles. J. Mater. Res. Technol. 2025, 34, 1571–1581. [Google Scholar] [CrossRef]
- Lv, N.N.; Qiu, Y.C.; Shi, J.J.; Su, C. Effect of ZrO2 on the phase relations for CaO-SiO2-TiO2 system related to efficient extraction of titanium from titania-bearing slag. Calphad 2024, 85, 102698. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, Q.Y.; Lv, G.Z.; Dou, Z.H.; Zhang, T.A. Enhancement and mechanistic study of ilmenite aeration leaching for synthetic rutile production using a horizontal reactor. Powder Technol. 2025, 455, 120740. [Google Scholar] [CrossRef]
- Kim, Y.; Nam, C.; Kim, S.; Jeon, H. Rheological properties and structure of molten FeO-TiO2-B2O3 ilmenite smelting slag. J. Non-Cryst. Solids 2021, 552, 120308. [Google Scholar] [CrossRef]
- Yang, H.Z.; Sun, B.Y.; Zhu, Y.F.; Yin, D.Q.; Yao, J.Y. Critical role of surficial activity in the sintering process of TiO2 nanoparticles by molecular dynamics simulation. Powder Technol. 2022, 398, 117071. [Google Scholar] [CrossRef]
- Passi, M.; Pal, B. A review on CaTiO3 photocatalyst: Activity enhancement methods and photocatalytic applications. Powder Technol. 2021, 388, 274–304. [Google Scholar] [CrossRef]
- Yang, Y.T.; Chen, B.H.; Pal, A.; Li, C.H.; Lin, Z.H.; Huang, M.H. Lattice variations in CaTiO3 cubes and cuboids and their use in photocatalytic benzimidazole formation. J. Mater. Chem. A 2025, 13, 10475–10486. [Google Scholar] [CrossRef]
- Deng, P.; Chen, X.M.; Liu, D.C.; Jiang, W.L.; Li, L. The electronic structures and chemical stability of anosovite M3O5 (M: Mg, Fe, Ti): DFT + U and ab initio molecular dynamics study. J. Solid State Chem. 2022, 307, 122734. [Google Scholar] [CrossRef]
- Ren, S.; Su, Z.H.; Liu, W.Z.; Sun, Y.L.; Li, X.M.; Yang, J. Ti3O5 and Al2TiO5 Crystals Flotation Characteristics from Ti-bearing Blast Furnace Slag: A Density Functional Theory and Experimental Study. Crystals 2020, 10, 838. [Google Scholar] [CrossRef]
- Hu, Q.Q.; Xin, R.; Gao, X.D.; Wang, Y.; You, Y.; You, Z.X.; Lv, X.W. Reduction disintegration behavior of vanadium titanomagnetite pellets in H2-CO-CO2-N2 mixtures. Powder Technol. 2023, 413, 118073. [Google Scholar] [CrossRef]
- Jiang, S.; Xiong, S.B.; Wu, H.B.; Zhao, D.Y.; You, X.M.; Xu, Y.H. In Situ Reconstruction of Hole-Selective Perovskite Heterojunction with Graded Energetics Toward Highly Efficient and Stable Solar Cells. Adv. Energy Mater. 2023, 13, 2300983. [Google Scholar] [CrossRef]
- Lv, W.; Lv, X.W.; Zhang, Y.Y.; Li, S.P.; Tang, K.; Song, B. Isothermal oxidation kinetics of ilmenite concentrate powder from Panzhihua in air. Powder Technol. 2017, 320, 239–248. [Google Scholar] [CrossRef]
- Zhu, F.X. Extraction of Titanium from the Vanadium Titanomagnetite and the Preparation of Ti-Al Alloys. Ph.D. Thesis, Cheng Du University of Technology, Chengdu, China, 2023. [Google Scholar]
- Wan, X.B.; Shi, J.J.; Klemettinen, L.; Chen, M.; Taskinen, P.; Jokilaakso, A. Equilibrium phase relations of CaO-SiO2-TiO2 system at 1400 ℃ and oxygen partial pressure of 10−10 atm. J. Alloys Compd. 2020, 847, 156472. [Google Scholar] [CrossRef]
- Lv, X.D.; Chen, D.; Xin, Y.T.; Lv, W.; Dang, J.; Lv, X.W. Isothermal kinetics of carbothermic reduction of ilmenite concentrate with the addition of sodium carbonate. Powder Technol. 2021, 392, 14–22. [Google Scholar] [CrossRef]
- Zuo, C.Y.; Yang, S.L.; Cao, Z.Q.; Yu, H.T.; Wei, X.H. Excellent energy storage and hardness performance of Sr0.7Bi0.2TiO3 ceramics fabricated by solution combustion-synthesized nanopowders. Chem. Eng. J. 2022, 442, 136330. [Google Scholar] [CrossRef]
- Jing, J.F.; Guo, Y.F.; Wang, S.; Chen, F.; Yang, L.Z.; Qiu, G.Z. Recent Progress in Electric Furnace Titanium Slag Progressing and Utilization: A Review. Crystals 2022, 12, 958. [Google Scholar] [CrossRef]
- Zhang, Q.S.; Gao, L.K.; Chen, X.M.; Rao, B.; Wang, F.W. Research progress of titanium extraction technology from titanium bearing blast furnace slag. Ind. Miner. Process. 2023, 52, 28–36. [Google Scholar]
- Sukmara, S.; Suyanti; Adi, W.A.; Manaf, A. Mineral analysis and its extraction process of ilmenite rocks in titanium-rich cumulates from Pandeglang Banten Indonesia. J. Mater. Res. Technol. 2022, 17, 3384–3393. [Google Scholar] [CrossRef]
- Kim, J.; Azimi, G. Selective Precipitation of Titanium, Magnesium, and Aluminum from the Steelmaking Slag Leach Liquor. Resour. Conserv. Recy. 2022, 180, 106177. [Google Scholar] [CrossRef]
- Zhu, L.G.; Kong, L.X.; Yang, B.; Xu, B.Q. Production of low-oxygen titanium powder by thermochemical and electrochemical processes: Current state and perspectives. J. Mater. Res. Technol. 2025, 36, 1522–1535. [Google Scholar] [CrossRef]
- Nie, W.L.; Wen, S.M.; Liu, D.W.; Hu, T.; Zhang, L.B. Innovative application of two-stage sulfuric acid leaching for efficient recovery of Ti from titanium-bearing electric furnace slag. J. Environ. Chem. Eng. 2023, 11, 109174. [Google Scholar] [CrossRef]
- Yu, S.S.; Ao, X.Q.; Liang, L.J.; Mao, X.Y.; Guo, Y. Recovery of rare earth elements from sedimentary rare earth ore via sulfuric acid roasting and water leaching. J. Rare Earths 2025, 43, 805–814. [Google Scholar] [CrossRef]
- Yuan, K.; He, S.Q.; Yu, B.; Qian, S.Y.; Wu, X.Y.; Li, W.Y.; Zhao, C.M. Synergistic Leaching of Titanium Aluminum and Magnesium Components during Dilute Acid Pressure Treatment of High-Titanium Blast Furnace Slag. Molecules 2024, 29, 3336. [Google Scholar] [CrossRef]
- Sui, Q.Q.; Dou, Z.H.; Zhang, T.A.; Yang, Z.N. Study on the one-step acid conversion of the alkali conversion product of high titanium slag to prepare TiO2 of high purity. Hydrometallurgy 2022, 211, 105887. [Google Scholar] [CrossRef]
- Nie, W.L.; Wen, S.M.; Feng, Q.C.; Liu, D.; Zhou, Y.W. Mechanism and kinetics study of sulfuric acid leaching of titanium from titanium-bearing electric furnace slag. J. Mater. Res. Technol. 2020, 9, 1750–1758. [Google Scholar] [CrossRef]
- Sadeghi, M.H.; Esfahany, M.N. Development of a Safe and Environmentally Friendly Sulfate Process for the Production of Titanium Oxide. Ind. Eng. Chem. Res. 2022, 61, 1786–1796. [Google Scholar] [CrossRef]
- Tian, C.X. Optimization of titanium dioxide production by clean continuous acidolysis of ilmenite using response surface methodology. Sustain. Chem. Pharm. 2024, 42, 101821. [Google Scholar] [CrossRef]
- Xiang, Q.J.; Quan, X.J.; Li, L.; Wang, H.B.; Chen, X.H.; Li, P. Formation rules and emission reduction method of sublimed sulfur in acidolysis exhaust gas of titanium concentrate. Inorg. Chem. Ind. 2024, 56, 96–103. [Google Scholar]
- Li, P.; Quan, X.J.; Li, L.; Wang, H.B.; Chen, X.H.; Qi, X.Q.; Li, G.; Xiang, Q.M. Study on the occurrence state of substances in sublimation sulfur from the tail gas of acid hydrolysis of titanium concentrate. Iron Steel Vanadium Titan. 2024, 45, 84–90. [Google Scholar]
- Stopić, S.; Kostić, D.; Damjanović, V.; Perušić, M.; Filipović, R.; Nikolić, N.; Friedrich, B. Recovery of Titanium and Aluminum from Secondary Waste Solutions via Ultrasonic Spray Pyrolysis. Metals 2025, 15, 701. [Google Scholar] [CrossRef]
- Sorkhe, Y.A.; Bavafa, P.; Buldu, A.I.; Yılmaz, B.S.; Derin, B.; Arslan, C. Synthesis of anatase powder by ilmenite digestion followed by selective thermal decomposition and leaching. Ceram. Int. 2025, 51, 366–378. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Liu, W.Z.; Zhang, G.Q.; Tang, S.W.; Yue, H.R.; Liang, B.; Luo, D.M. Recovery of titanium, aluminum, magnesium and separating silicon from titanium-bearing blast furnace slag by sulfuric acid curing-leaching. Int. J. Miner. Metall. Mater. 2022, 29, 1705–1714. [Google Scholar] [CrossRef]
- Yan, Z.P.; Zhang, Y.; Zhang, S.L.; Zhang, Y.; Sun, P.; Song, Z.W.; Safdar, F.; Qi, T. Preparation of titanium mineral from vanadium titanomagnetite concentrates by hydrogen reduction and acid leaching. Trans. Nonferrous Met. Soc. China 2022, 32, 3099–3109. [Google Scholar] [CrossRef]
- Han, F.; Wang, M.J.; Liu, W.; Song, W.J. Recovery of sulfuric acid and iron from titanium dioxide waste acid by membrane electrolysis combined with selective electrodialysis. Sep. Purif. Technol. 2024, 344, 127199. [Google Scholar] [CrossRef]
- He, S.Q. Study on Principle and Chemical Kinetics of Valuable Components Extraction and Separation from Panzhihua High Titanium-Blast Furnace Slag. Ph.D. Thesis, Southwest University of Science and Technology, Mianyang, China, 2020. [Google Scholar]
- Zhou, L.S.; Peng, T.J.; Sun, H.J.; Wang, S.Y. Thermodynamics analysis and experiments on Ti bearing blast furnace slag leaching enhanced by sulfuric acid roasting. RSC Adv. 2022, 12, 34990–35001. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.Y.; Lu, R.F.; Zhang, T.; Lü, L.; Chen, Y.X.; Tang, S.W. Chemical dehydration coupling multi-effect evaporation to treat waste sulfuric acid in titanium dioxide production process. Chin. J. Chem. Eng. 2020, 28, 1162–1170. [Google Scholar] [CrossRef]
- Zhang, W.G.; Zhang, T.A.; Cai, L.L.; Lv, G.Z.; Cao, X.J. Preparation of Doped Iron Phosphate by Selective Precipitation of Iron from Titanium Dioxide Waste Acid. Metals 2020, 10, 789. [Google Scholar] [CrossRef]
- Wang, M.H.; Zhao, Z.; Jia, P.A.; Wang, Y.P.; Chen, C.; Wang, Z.J. Macrokinetics of Hydrolysis Reaction of Titanium Ion Solution. Chem. React. Eng. Technol. 2023, 39, 439–443. [Google Scholar]
- Ju, J.R.; Feng, Y.L.; Li, H.R.; Liu, S.L.; Xu, C.L. Separation and recovery of V, Ti, Fe and Ca from acidic wastewater and vanadium-bearing steel slag based on a collaborative utilization process. Sep. Purif. Technol. 2021, 276, 119335. [Google Scholar] [CrossRef]
- Wang, D.S.; Lv, X.M.; Hou, Y.Q. Postmortem analysis of MgO-C bricks used in smelting furnace for fabricating TiC-bearing slag. J. Mater. Res. Technol. 2024, 31, 1171–1183. [Google Scholar] [CrossRef]
- Bai, C.Y.; Deng, Y.; Zhou, Q.; Deng, G.; Yang, T.T.; Yang, Y.Y. Effect of different curing methods on the preparation of carbonized high-titanium slag based geopolymers. Constr. Build. Mater. 2022, 342, 128023. [Google Scholar] [CrossRef]
- Wang, J.X.; Wang, Z.; Tu, J.G.; Zhu, J.; Wang, M.Y.; Jiao, S.Q. Purification of titanium oxycarbonitride by leaching from titanium slag with carbothermal reduction. Hydrometallurgy 2023, 215, 105984. [Google Scholar] [CrossRef]
- Zhang, R.; Dang, J.; Liu, D.; Lv, Z.P.; Fan, G.Q.; Hu, L.W. Reduction of perovskite-geikielite by methane-hydrogen gas mixture: Thermodynamic analysis and experimental results. Sci. Total Environ. 2020, 699, 134355. [Google Scholar] [CrossRef]
- Safdar, F.; Zhang, Y.; Zheng, S.L.; Chen, X.; Sun, P.; Zhang, Y.; Li, P. Recovery of TiO2-enriched material from vanadium titano-magnetite concentrates by partial carbon reduction and mild acid leaching. Hydrometallurgy 2020, 193, 105324. [Google Scholar] [CrossRef]
- Han, J.Q.; Zhang, J.; Zhang, J.H.; Chen, X.; Zhang, L.; Tu, G.F. Extraction of vanadium and enrichment of titanium from modified Ti-bearing blast furnace slag. Hydrometallurgy 2021, 201, 105577. [Google Scholar] [CrossRef]
- Fan, G.Q.; Hou, Y.L.; Huang, D.J.; Dang, J.; Zhang, R.; Xiang, J.Y.; Lv, X.W.; Ding, X.M. Synthesis of Ti (C, N, O) ceramic from rutile at low temperature by CH4-H2-N2 gas mixture. Int. J. Refract. Met. Hard Mater. 2021, 101, 105659. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Zheng, K.; Jiang, J.X.; Zhang, S.Y.; Xu, G. Effect of operating parameters on high-temperature selective enrichment and precipitation of titanium component in Ti-bearing blast furnace slag and the precipitation mechanism of perovskite. J. Mater. Res. Technol. 2021, 15, 2686–2696. [Google Scholar] [CrossRef]
- Wang, C.; Lei, Y.; Ma, W.H.; Qiu, P. An approach for simultaneous treatments of diamond wire saw silicon kerf and Ti-bearing blast furnace slag. J. Hazard. Mater. 2021, 401, 123446. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.Q.; Wang, M.; Dang, J.; Zhang, R.; Lv, Z.P.; He, W.C.; Lv, X.W. A novel recycling approach for efficient extraction of titanium from high-titanium-bearing blast furnace slag. Waste Manag. 2021, 120, 626–634. [Google Scholar] [CrossRef]
- Fan, G.Q. Fundamental Research on the Synthesis of Titanium Carbonitride under CH4-H2-N2 Atmosphere at Low Temperature. Ph.D. Thesis, Chongqing University, Chongqing, China, 2022. [Google Scholar]
- Sui, Y.L.; Guo, Y.F.; Jiang, T.; Qiu, G.Z. Separation and recovery of iron and titanium from oxidized vanadium titano-magnetite by gas-based reduction roasting and magnetic separation. J. Mater. Res. Technol. 2019, 8, 3036–3042. [Google Scholar] [CrossRef]
- Islam, I.; Tandersen, D.; Taimullah, A.; Sata, Y.A.; Hendrawan, Y.; Hidayat, T.; Zulhan, Z. Hydrogen plasma smelting reduction for fast production of green ferronickel. Int. J. Hydrogen Energy 2024, 94, 300–309. [Google Scholar] [CrossRef]
- Samal, S.K.; Mishra, B.; Mishra, S.C. Carboaluminothermic Production of Ferrotitanium from Ilmenite Through Thermal Plasma. J. Sustain. Metall. 2020, 6, 563–575. [Google Scholar] [CrossRef]
- Jayasankar, K.; Mandal, A.; Pany, A. Synthesis of Fe-TiC In-Situ Composites by Plasma Smelting of Ilmenite. Mater. Manuf. Process. 2011, 26, 1330–1334. [Google Scholar] [CrossRef]
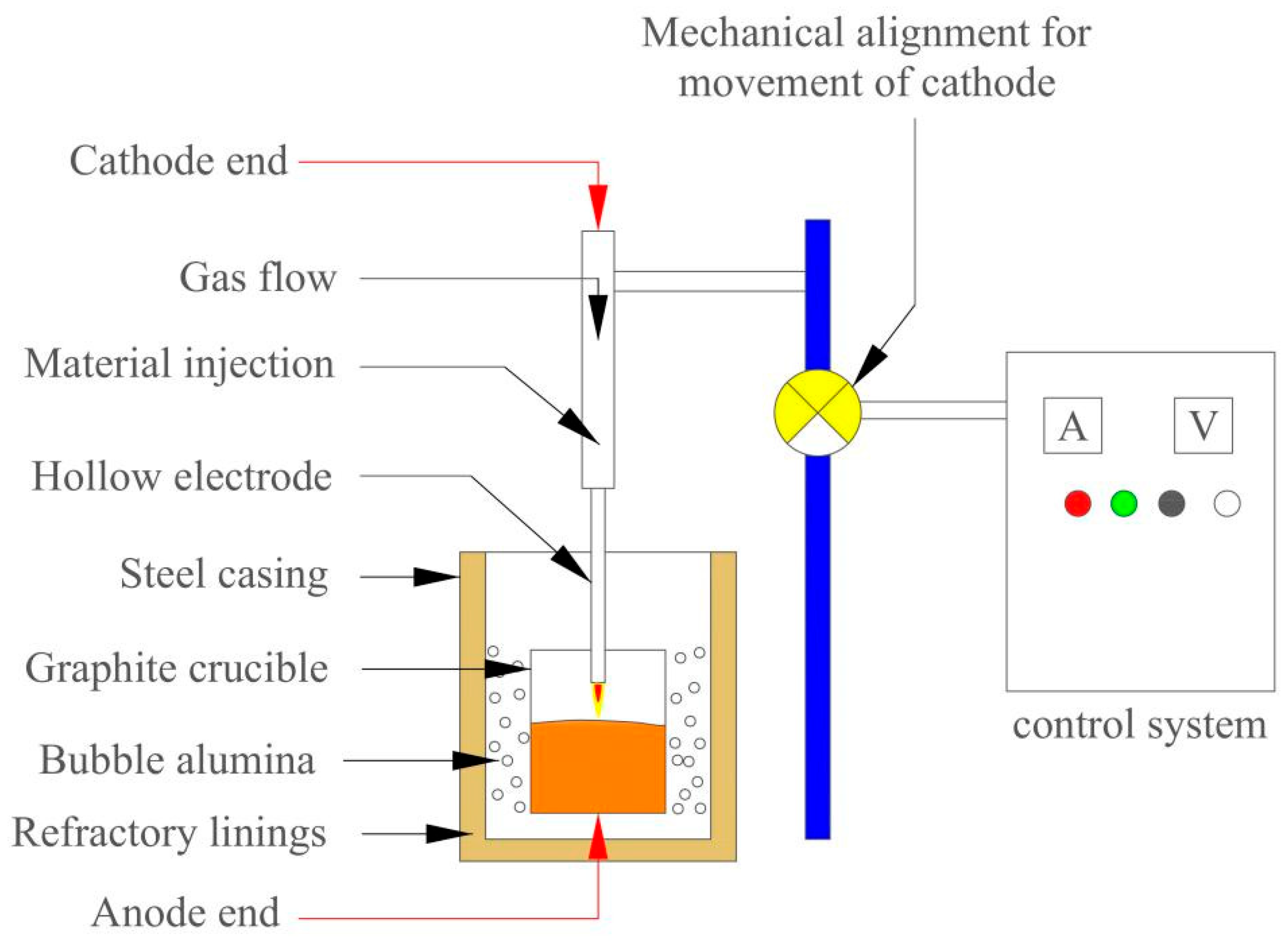
- Li, X. Basic Research on Deep Nitrogen Control Process of Full Scrap Steel Arc Furnace Based on Hollow Electrode Composite Gas Injection. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2024. [Google Scholar]
- Chen, C. Basic Research on the Preparation of Low-Oxygen and Low-Aluminum Ferrotitanium by Multi-Stage and Depth Reduction. Ph.D. Thesis, Northeastern University, Shenyang, China, 2018. [Google Scholar]
- Gao, F.; Nie, Z.R.; Yang, D.P.; Sun, B.X.; Liu, Y.; Gong, X.Z.; Wang, Z.H. Environmental impacts analysis of titanium sponge production using Kroll process in China. J. Clean. Prod. 2018, 174, 771–779. [Google Scholar] [CrossRef]
- Lee, B.P.; Choi, J.S.; Ko, J.W.; Park, J.G.; Min, D.J.; Sohn, I. Metallothermic reduction of Sc2O3 using Al-Ca alloys equilibrated with CaO-CaCl2 flux. J. Mater. Res. Technol. 2024, 28, 327–334. [Google Scholar] [CrossRef]
- You, J.; Yang, J.H.; Dong, W.X.; Wang, Y.W.; Wang, Y. Preparation of ferrotitanium alloy by aluminothermic reduction of perovskite. Process Saf. Environ. Prot. 2024, 187, 305–311. [Google Scholar] [CrossRef]
- GB/T 3282-2012; Ferrotitanium. China Standards Press: Beijing, China, 2012.
- Li, Z.C.; Lei, Y.; Ma, W.H.; Zhang, Y.K.; Wang, C. Preparation of high-purity TiSi2 and eutectic Si-Ti alloy by separation of Si–Ti alloy for clean utilization of Ti-bearing blast furnace slag. Sep. Purif. Technol. 2021, 265, 118473. [Google Scholar] [CrossRef]
- Che, Y.S.; Mai, G.P.; Li, S.L.; He, J.L.; Song, J.X.; Yi, J.H. Kinetic mechanism of magnesium production by silicothermic reduction of CaO·MgO in vacuum. Trans. Nonferrous Met. Soc. China 2020, 30, 2812–2822. [Google Scholar] [CrossRef]
- Vorobkalo, N.; Baisanov, A.; Makhambetov, Y.; Mynzhasar, Y.; Nurgali, N. Technological research of process for producing titanium rich slag and complex titanium-containing ferroalloy. Heliyon 2023, 9, e18989. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.Y.; Pei, G.S.; Lv, W.; Liu, S.L.; Lv, X.W.; Qiu, G.B. Preparation of synthetic rutile from reduced ilmenite through the aeration leaching process. Chem. Eng. Process. Process Intensif. 2020, 147, 107774. [Google Scholar] [CrossRef]
- Subasinghe, H.C.S.; Ratnayake, A.S. Processing of ilmenite into synthetic rutile using ball milling induced sulphurisation and carbothermic reduction. Miner. Eng. 2021, 173, 107197. [Google Scholar] [CrossRef]
- Feng, E.K.; Gao, D.J.; Wang, Y.T.; Yu, F.S.; Wang, C.X.; Wen, J.W.; Gao, Y.Q.; Huang, G.Y.; Xu, S.M. Sustainable recovery of titanium from secondary resources: A review. J. Environ. Manag. 2023, 339, 117818. [Google Scholar] [CrossRef]
- Zhu, X.F.; Li, Q.; Zhang, Y.; Fang, Z.G.; Zheng, S.L.; Sun, P.; Xia, Y. Mini-review on the preparation of titanium metal by the thermochemical processes. Chin. J. Process. Eng. 2019, 19, 456–464. [Google Scholar]
- Dong, Z.W. Study on Preparing Low Oxygen Content Titanium Alloy Powder by Metal Thermal Reduction and Its Forming Process. Ph.D. Thesis, Central South University, Changsha, China, 2023. [Google Scholar]
- Cheng, G.J.; Han, T.; Zhang, X.F.; Xue, X.X.; Yang, H.; Bai, R.G.; Zhang, W.J. A novel method of enhancing valuable element recovery for ultra-high-titanium magnetite. J. Clean. Prod. 2023, 410, 137184. [Google Scholar] [CrossRef]
- Gao, Z.X.; Yang, S.T.; Xue, X.X.; Yang, H.; Cheng, G.J. Extraction method for valuable elements of low-grade vanadia-titania magnetite. J. Clean. Prod. 2020, 250, 119451. [Google Scholar] [CrossRef]
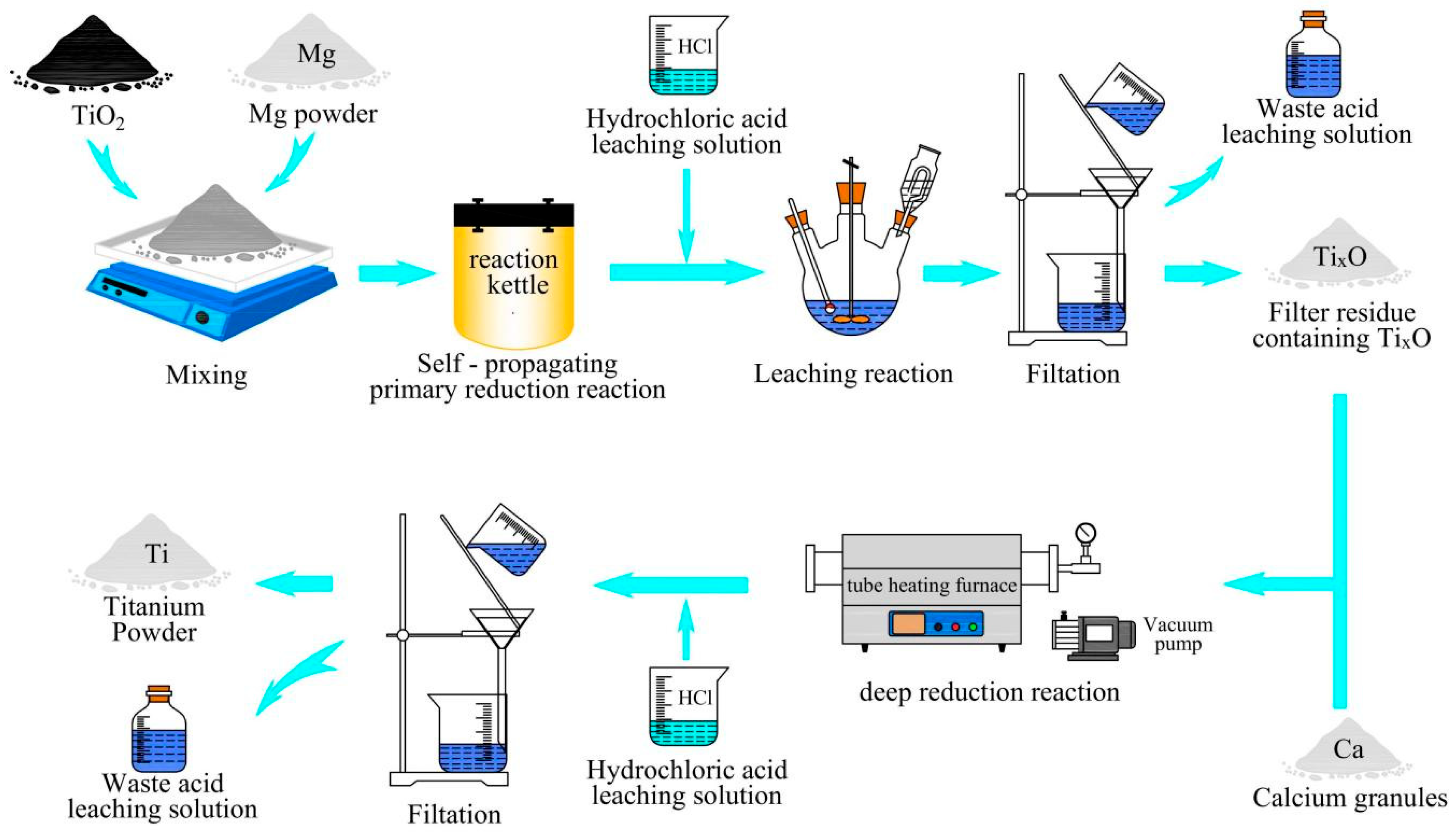
- Fan, S.G.; Dou, Z.H.; Zhang, T.A.; Yan, J.S. Self-propagating reaction mechanism of Mg-TiO2 system in preparation process of titanium powder by multi-stage reduction. Rare Met. 2021, 40, 2645–2656. [Google Scholar] [CrossRef]
- Zhu, L.G.; Bai, C.L.; Kong, L.X.; Yang, B.; Xu, B.Q. Preparation of low-oxygen titanium powder by magnesiothermic reduction of TiO2 assisted by MgCl2-HoCl3 molten salt. Trans. Nonferrous Met. Soc. China 2024, 34, 3749–3761. [Google Scholar] [CrossRef]
- Fan, S.G. Basic Research on Preparation of Titanium Powder by Multi-stage Reduction. Ph.D. Thesis, Northeastern University, Shenyang, China, 2020. [Google Scholar]
- Gonzalez, M.; Freitas, S.; Zhang, C.; Simpson, M.F. Metallothermic reduction of Cerium Chloride in molten Salt using Li, Na, and Ca Metal. J. Nucl. Mater. 2024, 596, 155086. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Zhang, J.L.; Zhao, B.J.; Liu, Z.J. Extraction of titanium resources from the titanium-containing waste slag: Thermodynamic analysis and experimental verification. Calphad 2020, 71, 102211. [Google Scholar] [CrossRef]
- Zhu, L.G.; Kong, L.X.; Bai, C.L.; Xu, B.Q.; Yang, B. Preparation of low-oxygen titanium powder by magnesiothermic reduction of TiO2 in KCl-MgCl2-YCl3 molten salt. J. Mater. Res. Technol. 2023, 25, 4929–4941. [Google Scholar] [CrossRef]
- Ma, T.X.; Yang, Y.; Liu, P.J.; Lv, X.W.; Hu, M.L. Research progress on electrolytic processes in molten salt to prepare titanium. J. Chongqing Univ. 2019, 42, 50–56. [Google Scholar]
- Yang, Y.H.; Yan, H.W.; Liu, Z.W.; Guo, S.H.; Zhang, J.; Xia, R. Titanium metal production via molten salt electrolysis of TiO2 using the NaF-K2TiF6 system under vacuum. Vacuum 2024, 230, 113721. [Google Scholar] [CrossRef]
- Chen, G.Z.; Fray, D.J.; Farthing, T.W. Direct electrochemical reduction of titanium dioxide to titanium in molten calcium chloride. Nature 2000, 407, 361–364. [Google Scholar] [CrossRef]
- Chen, Y.F.; Wang, M.Y.; Lv, A.J.; Zhao, Z.H.; An, J.L.; Zhang, J.T.; Tu, J.G.; Jiao, S.Q. Green preparation of vanadium carbide through one-step molten salt electrolysis. Ceram. Int. 2021, 47, 28203–28209. [Google Scholar] [CrossRef]
- Tian, D.H. The Electrochemical Extraction of Titanium by Casting Anode via USTB Process. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2020. [Google Scholar]
- Malek, B.; Serp, J.; Doreau, F.; Miguirditchian, M.; Vandenhende, M.; Pradeilles, N.; Lepetitcorps, Y.; Maitre, A. Production of Metallic Titanium by Electrowinning in Molten Salts of Titanium Oxycarbide Anode. Mater. Proc. 2021, 5, 63. [Google Scholar]
- Jiao, H.D.; Liu, M.J.; Wang, Z.; Lin, M.P.; Qu, Z.L.; Song, J.X.; Jiao, S.Q. Upcycling of Titanium by Molten Salt Electrorefining. ACS Sustain. Chem. Eng. 2023, 11, 5764–5772. [Google Scholar] [CrossRef]
- Wang, J.T.; Zhao, M.Y.; Gao, R.Y.; Pan, Z.H.; Wang, C.; Zou, K.G.; Xu, H.B.; Peng, G.L.; Chen, H.T.; Li, M.Y. Observation and study of electrodeposition behavior of Sn-Ag eutectic alloys in DLM process. Mater. Chem. Phys. 2025, 337, 130592. [Google Scholar] [CrossRef]
- Lv, H.Z.; Zhang, L.W.; Xi, X.L.; Nie, Z.R. Study on separation and purification of titanium alloys (TC4-6Al-4V) by molten salt electrolysis. Sep. Purif. Technol. 2025, 354, 129213. [Google Scholar] [CrossRef]
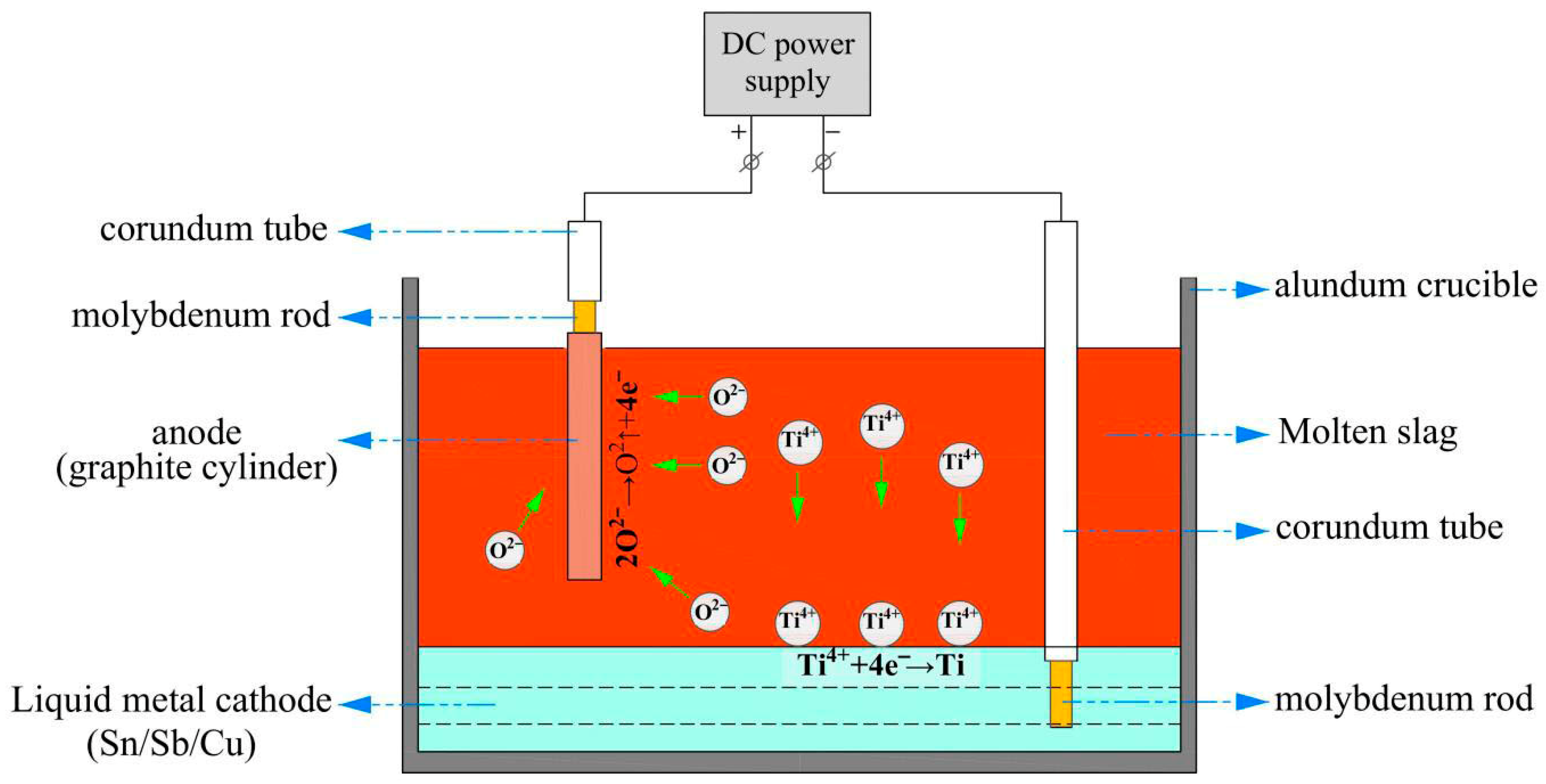
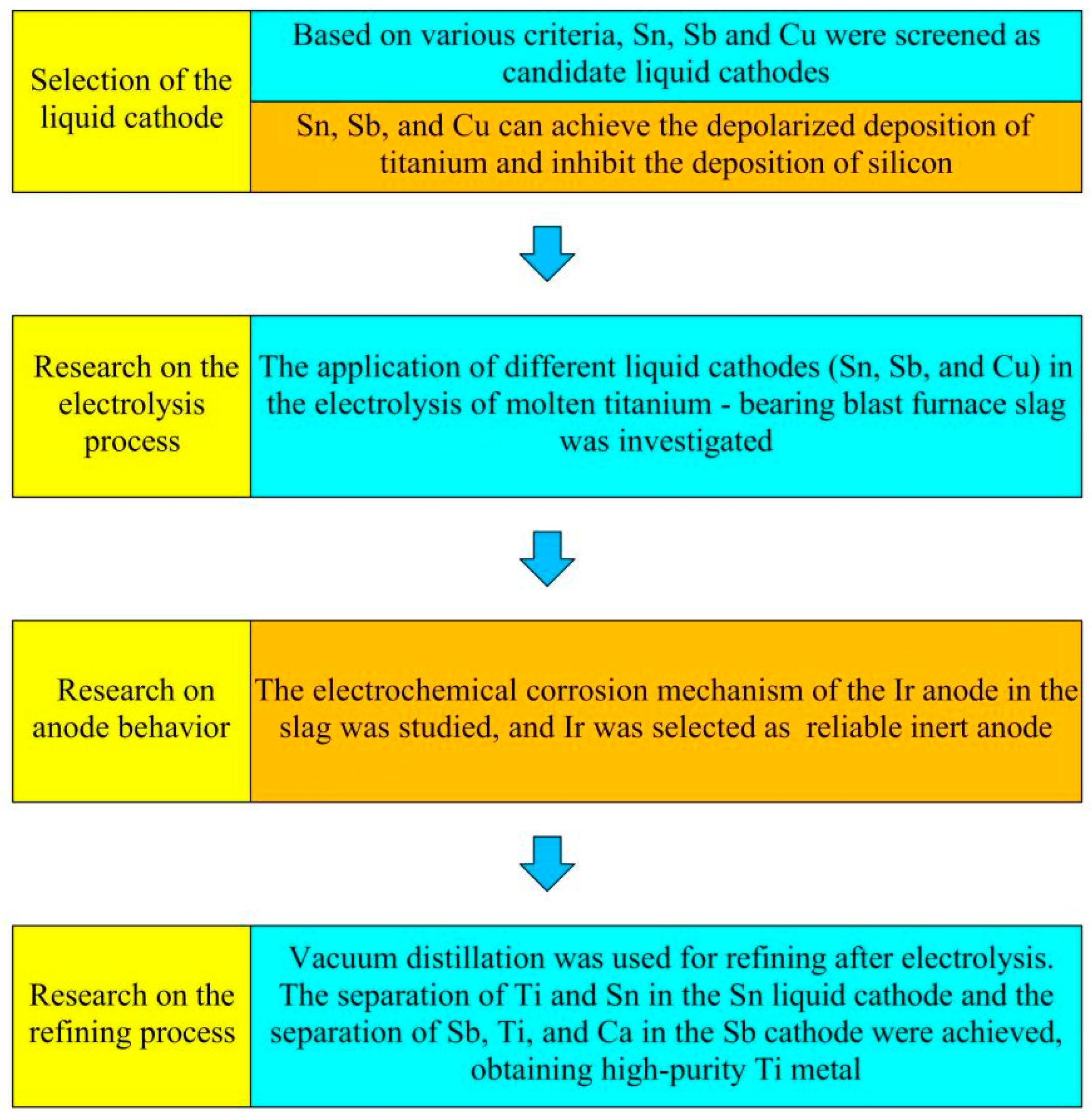
- Pu, Z.H.; Luo, Y.W.; Wang, W.; Zhang, G.H.; Wang, M.Y.; Tu, J.G.; Zhu, H.M.; Jiao, S.Q. A universal study of liquid metal cathodes for direct extraction of titanium within a closed loop. J. Clean. Prod. 2022, 368, 133135. [Google Scholar] [CrossRef]
- Li, S.J.; Gao, Y.; Wang, W.; Tian, D.H.; Pu, Z.H.; Jiao, H.D.; Jiao, S.Q. The diffusion dynamic of Ti in liquid cathodes during molten oxide electrolysis. Scripta Mater. 2025, 259, 116548. [Google Scholar] [CrossRef]
- Pu, Z.H.; Jiao, H.D.; Mi, Z.S.; Wang, M.Y.; Jiao, S.Q. Selective extraction of titanium from Ti-bearing slag via the enhanced depolarization effect of liquid copper cathode. J. Energy Chem. 2020, 42, 43–48. [Google Scholar] [CrossRef]
- Bai, X.Y.; Shang, X.J.; Wan, H.L.; Che, Y.S.; Yang, B.; He, J.L.; Song, J.X. Sustainable recycling of titanium from TiO2 in spent SCR denitration catalyst via molten salt electrolysis. J. Energy Chem. 2021, 58, 557–563. [Google Scholar] [CrossRef]
- Jiao, H.D. Electrode Process of Titanium Ions at Liquid Metal Cathodes. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2018. [Google Scholar]
- Pu, Z.H. Fundamental Research on the Electrolytic Preparation of Titanium and Its Alloys from Molten Titanium-Bearing Blast Furnace Slag. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2022. [Google Scholar]
- Pu, Z.H.; Wang, W.; Wang, Z.; Kou, M.Y.; Luo, Y.W.; Ge, J.B.; Tao, X.; Wang, M.Y.; Jiao, S.Q. A recyclable method for titanium extraction and oxygen evolution from Ti-bearing slags. Fundam. Res. 2024, 4, 86–94. [Google Scholar] [CrossRef]
- Li, L.Y.; Sun, R.Y.; Yuan, R.; Lin, M.P.; Wang, L.L.; Wang, Q.; Tu, J.G.; Jiao, H.D.; Jiao, S.Q. Rational design of pulse-electrolysis protocols promotes molten-salt electrorefining. Chem. Eng. J. 2024, 500, 157424. [Google Scholar] [CrossRef]
- Xie, J.; Ye, Q.; Yang, F.; Qian, G.M.; Zhang, H.J. Selective enrichment and extraction of well-catalyzed perovskites on melting-microwave heating process from blast furnace slag. J. Clean. Prod. 2023, 420, 138377. [Google Scholar] [CrossRef]
- Lan, X.; Gao, J.T.; Xue, K.R.; Xu, H.H.; Guo, Z.C. A new finding and technology for selective separation of different REEs from CaO-SiO2-CaF2-P2O5-Fe3O4-RE2O3 system. Sep. Purif. Technol. 2022, 293, 121121. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Sun, L.F.; Zhao, H.; Xiang, T.Y.; Qiu, J.Y.; Jiang, M.F. Isothermal phase diagram of CaO-SiO2-Nb2O5-5wt% Fe2O3-TiO2 system at 1200 °C. Ceram. Int. 2022, 48, 31636–31651. [Google Scholar] [CrossRef]
- Ilatovskaia, M.; Fabrichnaya, O. Thermodynamic assessment of the Al2O3-MgO-TiO2 system. J. Alloys Compd. 2019, 790, 1137–1148. [Google Scholar] [CrossRef]
- Chen, M.; Shi, J.J.; Taskinen, P.; Jokilaakso, A. Experimental determination of the 1300 °C and 1400 °C isotherms for CaO-SiO2-TiO2-10 wt% Al2O3 system in air. Ceram. Int. 2020, 46, 9183–9191. [Google Scholar] [CrossRef]
- Chen, Y.; Li, M.; Wang, S.S.; Zhu, L.L.; Zhang, X.B.; He, S.P.; Wang, Q.Q. Effect of MgO on solidification and crystallization properties of ultrahigh-basicity mold flux. Mater. Chem. Phys. 2022, 276, 125403. [Google Scholar] [CrossRef]
- Liu, C.J.; Qiu, J.Y.; Liu, Z.Y.; Zhu, D.Y.; Wang, Y.G. Phase equilibria in the system CaO-SiO2-Nb2O5-La2O3 at 1473 K with pO2 = 10−15.47 atm. Ceram. Int. 2020, 46, 7711–7718. [Google Scholar] [CrossRef]
- Chen, M.; Wan, X.B.; Taskinen, P.; Sukhomlinov, D.; Shi, J.J.; Michallik, R.; Jokilaakso, A. Phase equilibria in TiO2-rich part of the MgO-CaO-TiO2 system at 1500–1600 °C. Ceram. Int. 2022, 48, 20116–20125. [Google Scholar] [CrossRef]
- Qi, J.; Liu, C.J.; Jiang, M.F. Crystallization characteristics of CaO-Al2O3-SiO2-Li2O-B2O3 mold flux with different values of w(CaO)/w(Al2O3). Ceram. Int. 2021, 47, 34396–34404. [Google Scholar] [CrossRef]
- Zhu, S.; Hu, J.G.; Zhang, C.H.; Li, S.; An, N. Study on optimization and mechanism of mechanical activation process of titanium-bearing blast furnace slag. J. Mater. Res. Technol. 2022, 19, 3130–3144. [Google Scholar] [CrossRef]
- Ma, H.B.; Jiao, K.X.; Zhang, J.L.; Zong, Y.B.; Zhang, J. The influence of basicity and TiO2 on the crystallization behavior of high Ti-bearing slags. Cryst. Eng. Comm. 2020, 22, 361–370. [Google Scholar] [CrossRef]
- Gong, W.P.; Liu, Y.Z.; Luo, Z.H. Heat capacity of samarium titanates and phase equilibria of Sm2O3-TiO2 system. J. Alloys Compd. 2021, 860, 158429. [Google Scholar] [CrossRef]
- Xu, R.Z.; Wang, Z. Influence of Al2O3 on viscous behavior and structure characteristic of TiO2-bearing molten slags. Ceram. Int. 2024, 50, 24016–24024. [Google Scholar] [CrossRef]
- Cui, Y.T.; Wei, S.T.; Zhang, X.B.; He, S.P.; Wang, Q.Q. Effect of BaO/Al2O3 ratio and Li2O content on viscosity and crystallization of BaO-Al2O3-TiO2-based mold slags. Ceram. Int. 2024, 50, 5464–5472. [Google Scholar] [CrossRef]
- Liu, W.G.; Qin, J.H.; Xing, X.D.; Wang, J.S.; Zuo, H.B. Viscosity and structure evolution of CaO-SiO2-MgO-Al2O3-BaO slag with the CaO/SiO2 mass ratio of 0.9. Ceram. Int. 2021, 47, 33483–33489. [Google Scholar] [CrossRef]
- Guo, Y.F.; Jing, J.F.; Wang, S.; Chen, F.; Yang, L.Z.; Qiu, G.Z. Effect of CaO/MgO on rheological properties and structure of CaO-MgO-SiO2-Al2O3-50% TiO2 slag with SiO2/Al2O3 = 1.0. Ceram. Int. 2024, 50, 38732–38740. [Google Scholar] [CrossRef]
- Han, J.Q. Technology Study on Rutile Transformation, Settling, and Separation of Titanium Components in Titanium-Bearing Mixed Molten Slag. Ph.D. Thesis, Northeastern University, Shenyang, China, 2021. [Google Scholar]
- Han, J.Q.; Zhang, J.; Zhang, J.H.; Chen, X.; Zhang, L.; Tu, G.F. Recovery of Fe, V, and Ti in modified Ti-bearing blast furnace slag. Trans. Nonferrous Met. Soc. China 2022, 32, 333–344. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, L.; Li, Y.H.; Li, X. Crystallization behavior and growing process of rutile crystals in Ti-bearing blast furnace slag. High Temp. Mater. Process. 2016, 35, 787–797. [Google Scholar] [CrossRef]
- Lu, Y.; Gao, J.T.; Wang, F.Q.; Guo, Z.C. Separation of Anosovite from Modified Titanium-Bearing Slag Melt in a Reducing Atmosphere by Supergravity. Metall. Mater. Trans. B 2016, 48, 749–753. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, H.H.; Hou, Y.; Zhang, S.; Dang, J.; Lv, X.W. Thermophysical properties, crystallization behavior and structure of CaO-SiO2-MgO-Al2O3-TiO2-FeO slag with varying TiO2 contents. Ceram. Int. 2024, 50, 39069–39079. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Q.; Zhang, B.; Zhang, T.A.; Dou, Z.H.; Song, S.J. Structural interpretation of viscous flow and crystallization behavior of ultra-high titanium molten slag. Ceram. Int. 2025, 51, 22277–22286. [Google Scholar] [CrossRef]
- Ma, H.B.; Jiao, K.X.; Zhang, J.L.; Zong, Y.B.; Zhang, J.; Meng, S. Viscosity of CaO-MgO-Al2O3-SiO2-TiO2-FeO slag with varying TiO2 content: The Effect of Crystallization on Viscosity Abrupt Behavior. Ceram. Int. 2021, 47, 17445–17454. [Google Scholar] [CrossRef]
- Du, Y. Mineral Phase Transformation and Selective Super-Gravity Separation of Titanium in Titanium Bearing Slag. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2021. [Google Scholar]
- Du, Y.; Gao, J.T.; Lan, X.; Guo, Z.C. Recovery of rutile from Ti-Bearing blast furnace slag through phase transformation and super-gravity separation for dielectric material. Ceram. Int. 2020, 46, 9885–9893. [Google Scholar] [CrossRef]
- Du, Y.; Gao, J.T.; Lan, X.; Guo, Z.C. Replacement behavior of Ti and Mg and selective separation of MgxTi3-xO5(x = 1, 0.9, 0.75, 0.6) from Ti-bearing electric furnace smelting slag via super-gravity. Ceram. Int. 2022, 48, 7918–7925. [Google Scholar] [CrossRef]
- Zhang, W.; Ou, C.G.; Yuan, Z.G. Precipitation and growth behaviour of metatitanic acid particles from titanium sulfate solution. Powder Technol. 2017, 315, 31–36. [Google Scholar] [CrossRef]
- Gu, H.Z.; Wu, J.J.; Wei, K.X.; Ma, W.H. Synergetic recovery of Ti and Fe from Ti-bearing blast furnace slag and red mud by diamond wire saw silicon waste. Process Saf. Environ. Prot. 2024, 190, 1301–1310. [Google Scholar] [CrossRef]
- Zhang, T.; Tan, Y.Q.; Xu, Y.L.; Liu, C. A thermal-initiated monomer binder enhancing green strength with low binder saturation for binder jetting additive manufacturing of cemented carbide. Int. J. Refract. Met. Hard Mater. 2024, 118, 106494. [Google Scholar] [CrossRef]
- Wang, Y.F.; Kalscheur, J.; Su, Y.Q.; Hensen, J.M.; Vlachos, D.G. Real-time dynamics and structures of supported subnanometer catalysts via multiscale simulations. Nat. Commun. 2021, 12, 5430. [Google Scholar] [CrossRef]
- Momeni, K.; Ji, Y.Z.; Wang, Y.X.; Paul, S.; Neshani, S.; Yilmaz, D.E.; Shi, Y.K.; Zhang, D.; Jiang, J.-W.; Park, H.S.; et al. Multiscale computational understanding and growth of 2D materials: A review. npj Comput. Mater. 2020, 6, 22. [Google Scholar] [CrossRef]
- Ferreira, F. Multiscale modelling of twisted bilayers of 2D materials. Nat. Rev. Phys. 2022, 4, 632. [Google Scholar] [CrossRef]
- Li, L.J.; Zhang, C.D.; Liu, Y.; Zhao, S.X.; Zhao, J.Q.; Xu, K. Research progress of direct reduction of vanadium titanium magnetite and resource utilization of titanium-bearing slag. China Metall. 2025, 35, 44–54. [Google Scholar]
- Wu, Q.F.; He, F.; Li, J.J.; Kim, H.S.; Wang, Z.J.; Wang, J.C. Phase-selective recrystallization makes eutectic high-entropy alloys ultra-ductile. Nat. Commun. 2022, 13, 4697. [Google Scholar] [CrossRef]
- Yin, X.M.; Wang, Y.X.; Bai, X.J.; Wang, Y.M.; Chen, L.H.; Xiao, C.L. Rare earth separations by selective borate crystallization. Nat. Commun. 2017, 8, 14438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, S.J.; Yan, D.B.; Yan, D.B.; Zhang, Y.C. Thermodynamic Simulation of the Melting Characteristics of Titanium-containing High Furnace Slag. Xinjiang Iron Steel 2024, 3, 12–14. [Google Scholar]
- Pang, J.; Kong, L.X.; Zhu, L.G.; Xu, B.Q.; Xu, J.J.; Bai, C.L.; Yang, B. Preparation of ultralow-oxygen titanium by direct reduction of TiO2. Trans. Nonferrous Met. Soc. China 2024, 34, 681–693. [Google Scholar] [CrossRef]
- Li, F.; Huang, H.; Zong, X.; Wang, K.; Liu, H.; Liu, X.; Ding, X. Simulation Study on Directional Solidification of Titanium-Aluminum Alloy Based on Liquid Metal Cooling Method. Metals 2025, 15, 366. [Google Scholar] [CrossRef]
- Tian, J.Y.; Gao, Y.Q.; Ye, X.Y.; Huang, F.; Zhao, Y.W.; Zhang, T. Optimizing particle translation and self-rotation by adjusting gravity-driven hydrocyclone inclination angle for separation and activation of granular sludge. J. Water Process Eng. 2023, 54, 104030. [Google Scholar] [CrossRef]
- Amosah, M.E.; Zhou, J.; Galvin, K.P. Fourth generation gravity separation using the Reflux Classifier. Miner. Eng. 2025, 224, 109216. [Google Scholar] [CrossRef]
- Zhou, E.H.; Yan, G.H.; Weng, X.Y.; Zhang, Z.X.; Zhao, P.F.; Zhang, B. A novel and low cost coal separation process: Combination of deep screening classification and gravity separation. Powder Technol. 2020, 367, 568–575. [Google Scholar] [CrossRef]
- Gu, H.Z.; Cao, J.; Wu, J.J.; Xu, M.; Ma, W.H. Recovery of Ti-bearing blast furnace slag and diamond wire saw silicon powder waste by alloying and electromagnetic separation technique. J. Clean. Prod. 2022, 359, 132080. [Google Scholar] [CrossRef]
- Li, W.B.; Liu, X.; Liu, D.Q.; Han, Y.X. Mineralogical reconstruction of Titanium-Vanadium hematite and magnetic separation mechanism of titanium and iron minerals. Adv. Powder Technol. 2022, 33, 103408. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Li, X.M.; Wang, S.Z. Effect of B2O3 on the Melting Characteristics and Slag-Matte Separation of Nickel Flash Furnace Melting. JOM 2023, 75, 3532–3544. [Google Scholar] [CrossRef]
- Wang, S.Q.; Yuan, Z.Y.; Yu, N.Y. Design and Optimization of a Waste Heat Recovery Organic Rankine Cycle System with a Steam-Water Dual Heat Source. Energy Technol. 2023, 11, 2201499. [Google Scholar] [CrossRef]
- Oyejobi, D.O.; Firoozi, A.A.; Fernández, D.B.; Avudaiappan, S. Integrating circular economy principles into concrete technology: Enhancing sustainability through industrial waste utilization. Results Eng. 2024, 24, 102846. [Google Scholar] [CrossRef]
- Jiao, H.D.; Song, W.L.; Chen, H.S.; Wang, M.Y.; Jiao, S.Q.; Fang, D.N. Sustainable recycling of titanium scraps and purity titanium production via molten salt electrolysis. J. Clean. Prod. 2020, 261, 121314. [Google Scholar] [CrossRef]
- Wang, B.; Qiu, J.Y.; Guo, Q.H.; Gong, Y.; Xu, J.L.; Yu, G.S. Numerical Simulations of Solidification Characteristics of Molten Slag Droplets in Radiant Syngas Coolers for Entrained-Flow Coal Gasification. ACS Omega 2021, 6, 20388–20397. [Google Scholar] [CrossRef]
- Ding, B.; Liao, Q.; Zhu, X.; Wang, H. Deep insight into phase transition and crystallization of high temperature molten slag during cooling: A review. Appl. Therm. Eng. 2021, 184, 116260. [Google Scholar] [CrossRef]
- He, W.C.; Lü, X.W.; Ding, C.Y.; Yan, Z.M. Oxidation pathway and kinetics of titania slag powders during cooling process in air. Int. J. Miner. Metall. Mater. 2021, 28, 981–990. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, J.F.; Jia, X.L.; Xing, T.; Liu, C.J.; Jiang, M.F. Mineral phase reconstruction of Bayan Obo tailings by smelting reduction and crystallization control of molten slag containing niobium, rare earth and titanium. Miner. Eng. 2024, 214, 108780. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, Q.S.; Wang, H.; Sun, H.Y. Influence of the redox conditions on the crystallization behavior of anosovite in Ti-bearing titanomagnetite smelting slag. Results Chem. 2021, 3, 100136. [Google Scholar] [CrossRef]
- Zielińska, M.; Yang, H.; Madej, Ł.; Malinowski, Ł. Influence of electromagnetic field on stirring energy in selected metallurgical equipment. Steel Res. Int. 2024, 95, 2300534. [Google Scholar] [CrossRef]
- Li, Z.W.; Chen, H.; Liu, Z. Effect of electromagnetic stirring method on flow characteristics of A356 aluminum alloy melt. Int. J. Lightweight Mater. Manuf. 2025, 8, 321–330. [Google Scholar] [CrossRef]
- Luo, H.; Zhang, X.; Wang, Y.; Jiang, B.; Deng, Z.X.; Liu, J.Y. Multi-physics coupled numerical simulation study to optimize process parameters for electromagnetic stirring of semi-solid A356 aluminum alloy under the influence of skin effect. Int. Commun. Heat Mass Transfer 2024, 157, 107834. [Google Scholar] [CrossRef]
- Goharkhah, M.; Salarian, A.; Ashjaee, M.; Shahabadi, M. Convective heat transfer characteristics of magnetite nanofluid under the influence of constant and alternating magnetic field. Powder Technol. 2015, 274, 258–267. [Google Scholar] [CrossRef]
- Wen, J.C.; Cheng, J.H.; Mine, X.D.; Huang, Y.C.; Liu, Y. Strengthened solid solution and homogenized eutectic structure of Al-based multi-component alloy via electromagnetic stirring. J. Alloys Compd. 2024, 1002, 175537. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, W.; Wang, Z.L.; Li, L. Investigation of a jet and stirring synergistic flotation column combining two-phase flow and particle tracking simulation methods with experiments. Powder Technol. 2025, 453, 120628. [Google Scholar] [CrossRef]
- Xue, B.T.; Yang, L.Z.; Guo, Y.F.; Chen, F.; Wang, S.; Zhang, F.Q.; Yang, Z.S. Design and Construction of a Laboratory-Scale Direct-Current Electric Arc Furnace for Metallurgical and High-Titanium Slag Smelting Studies. Metals 2021, 11, 732. [Google Scholar] [CrossRef]
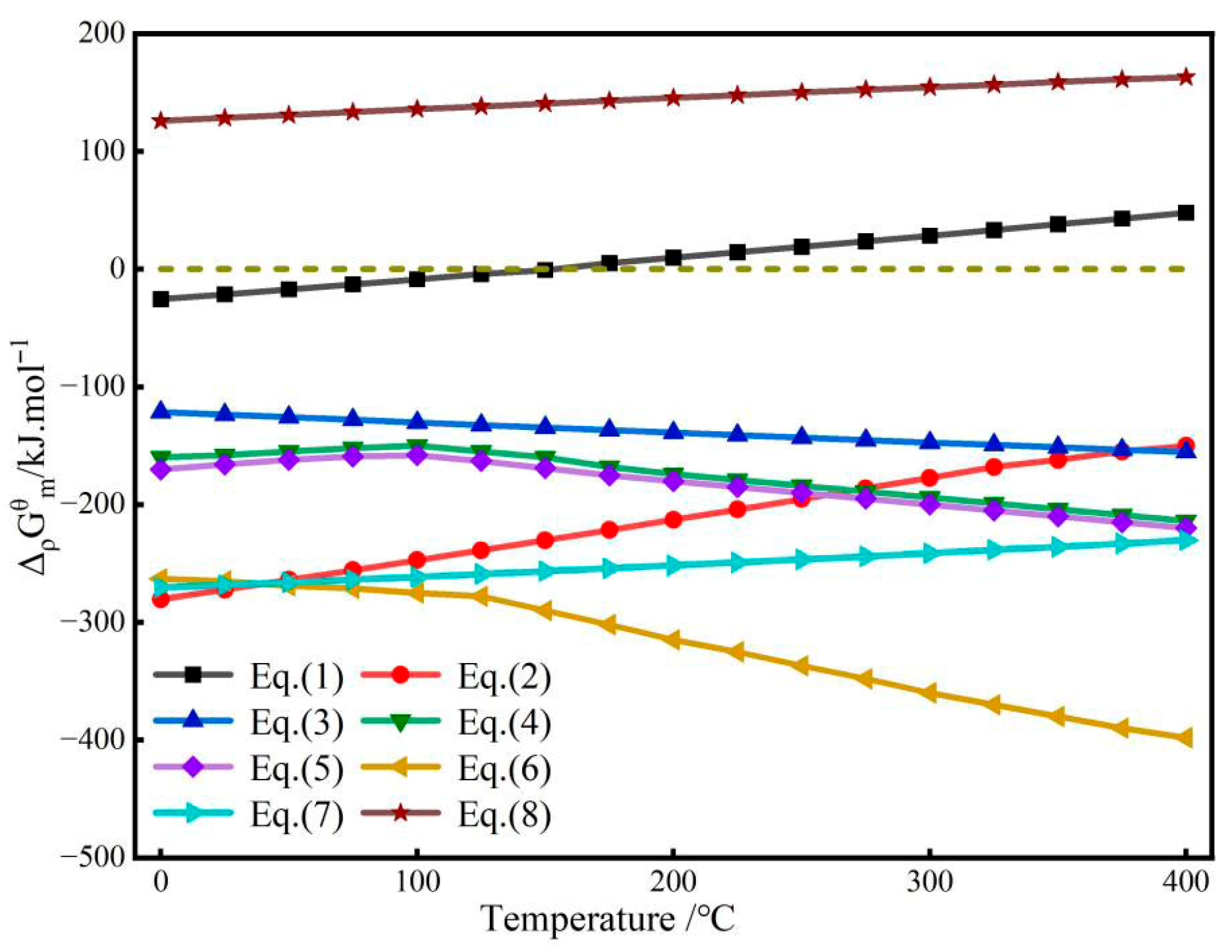
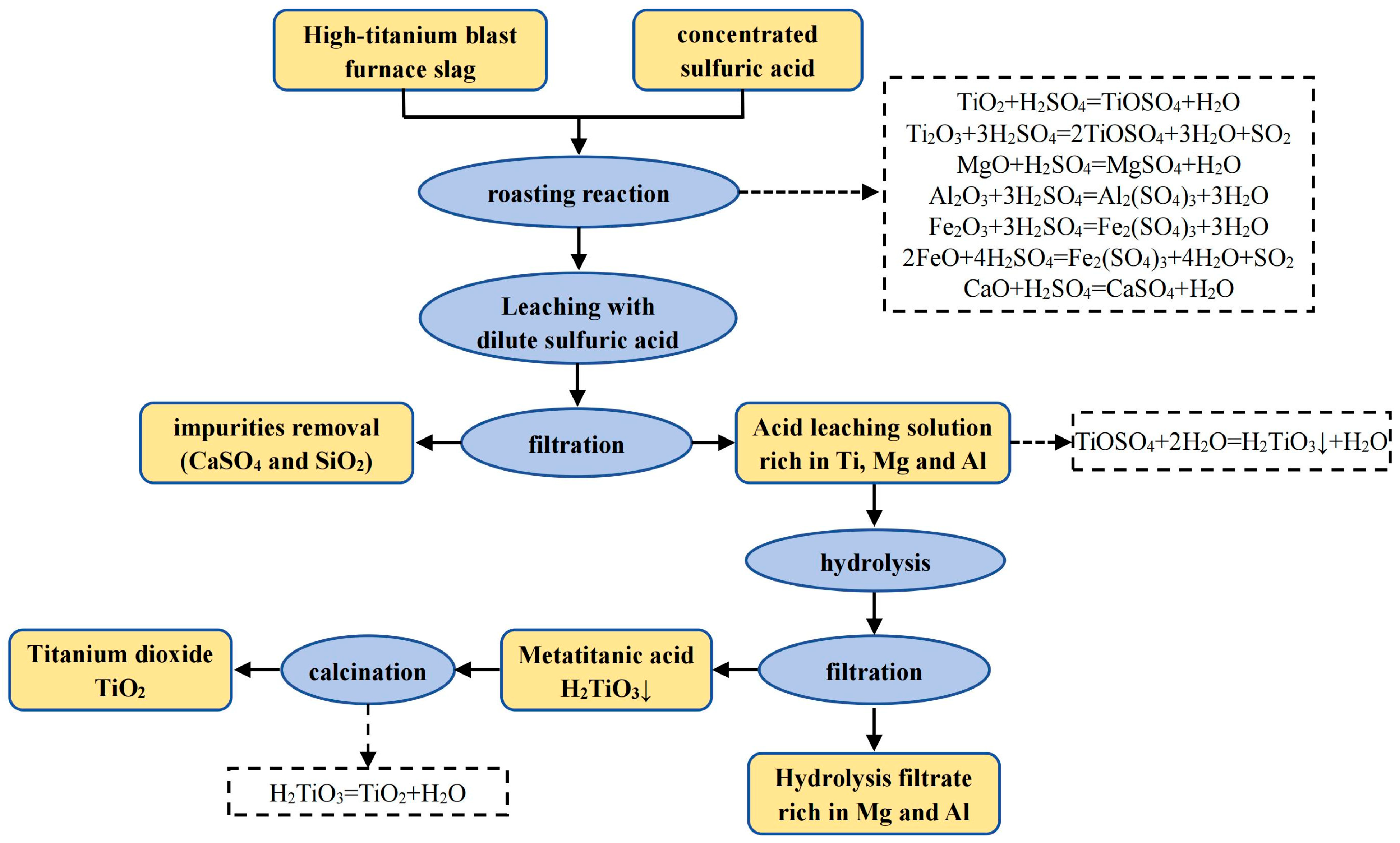

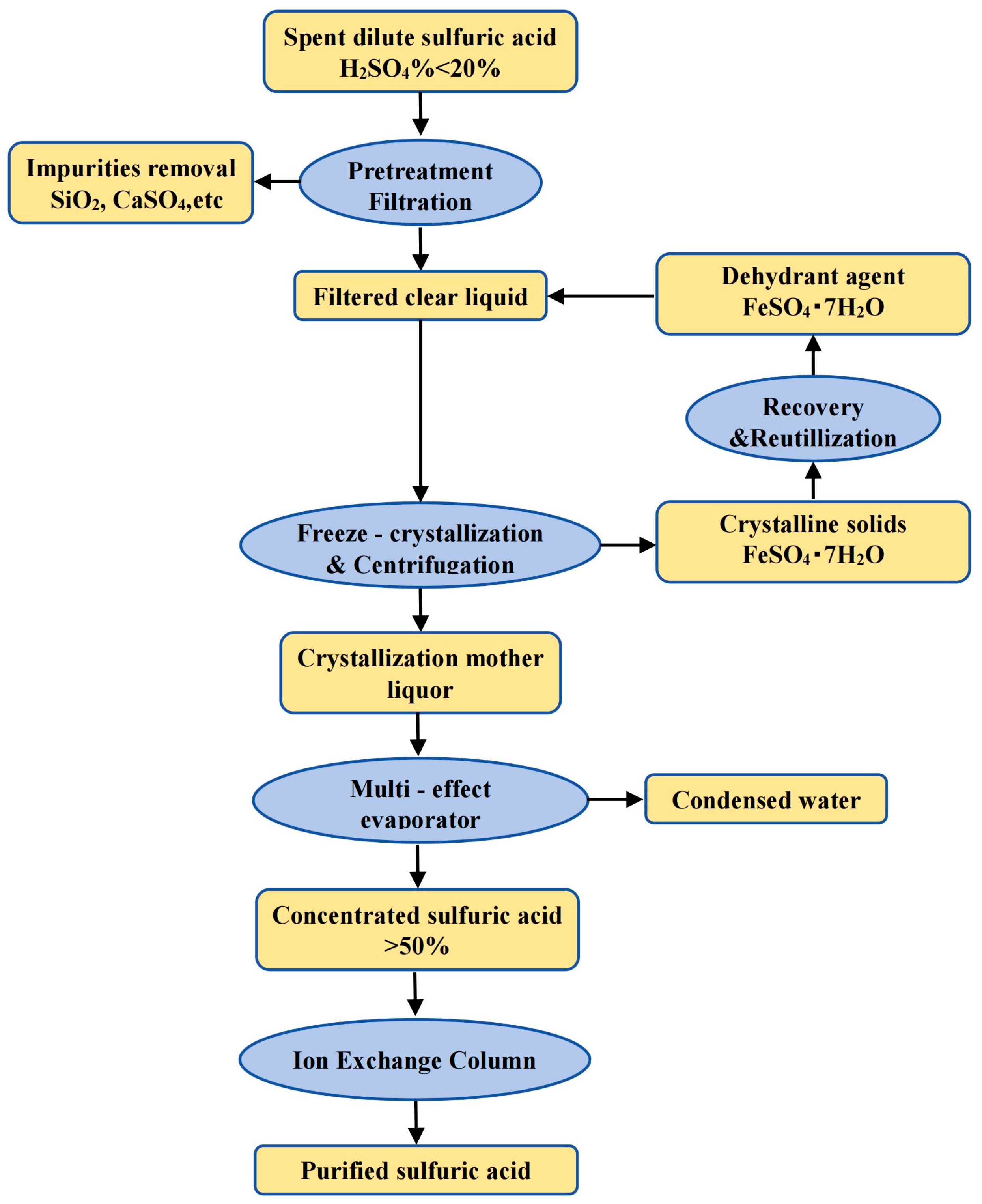
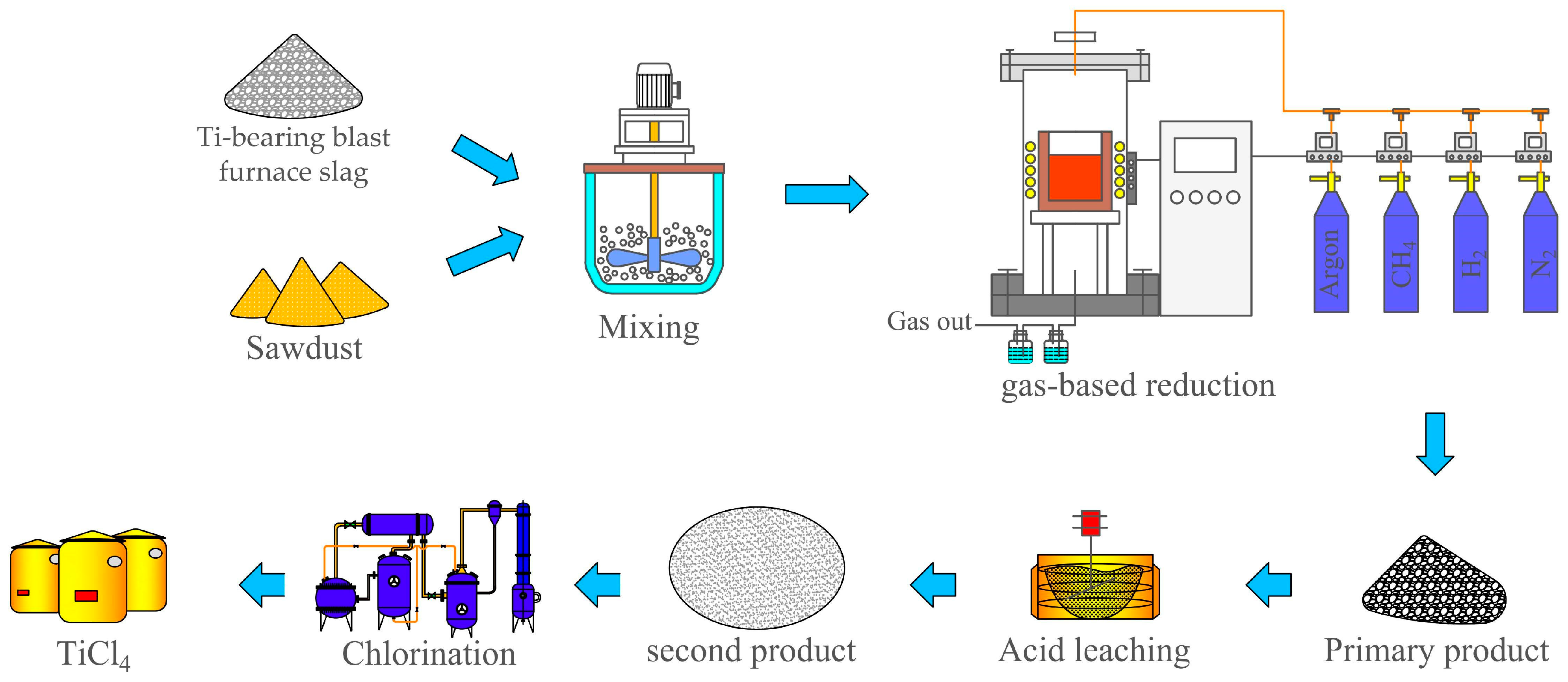
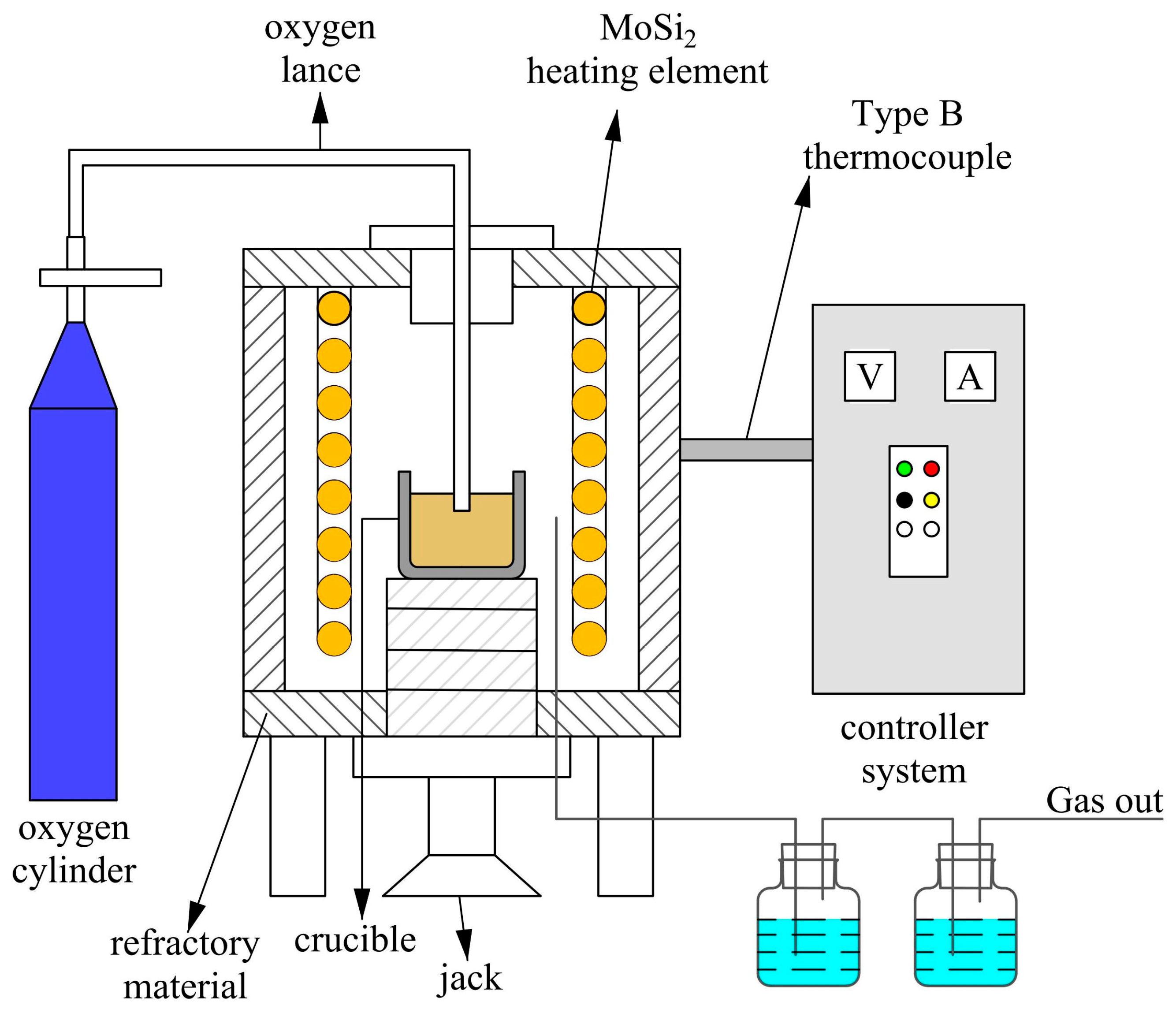
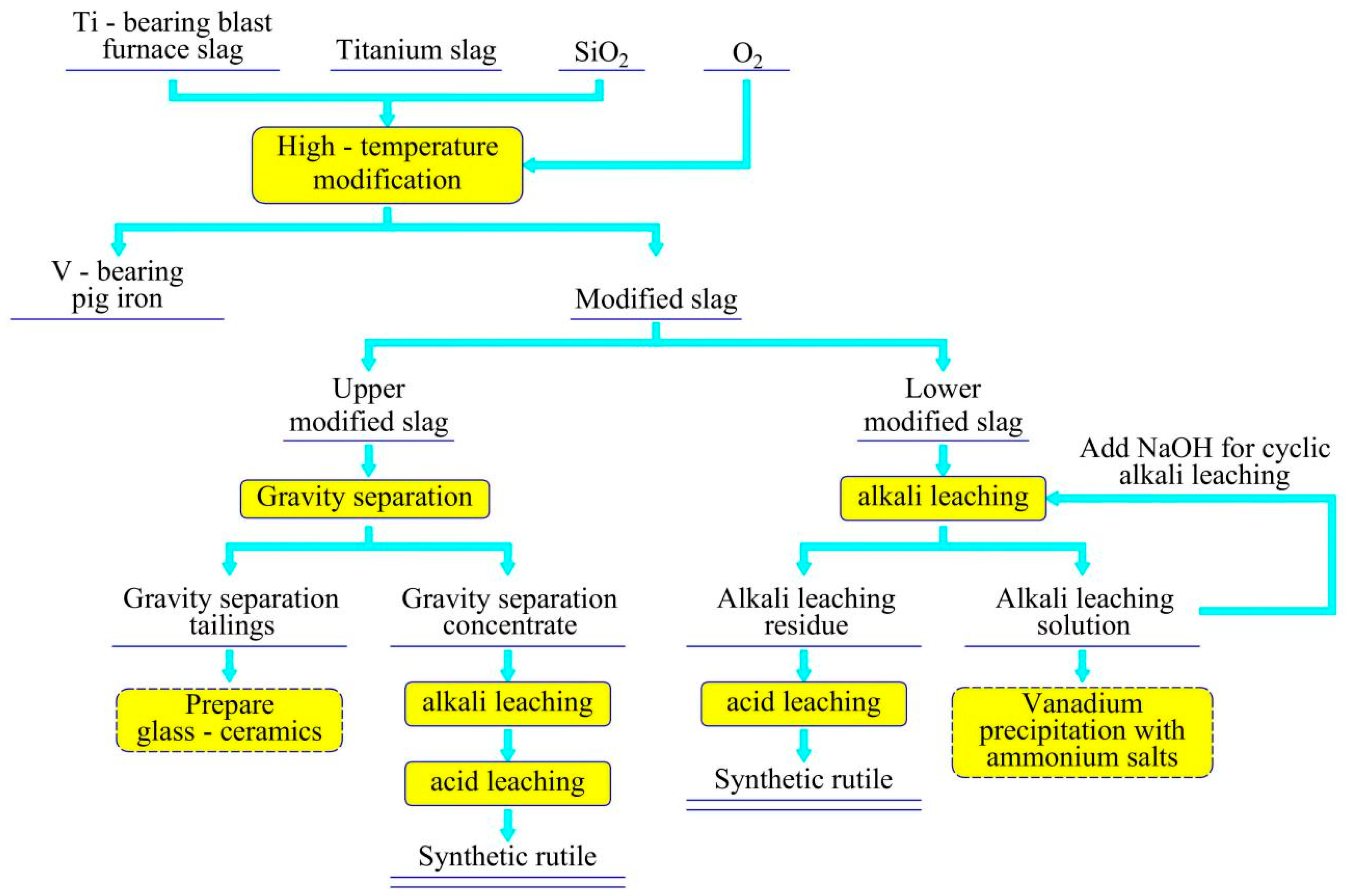

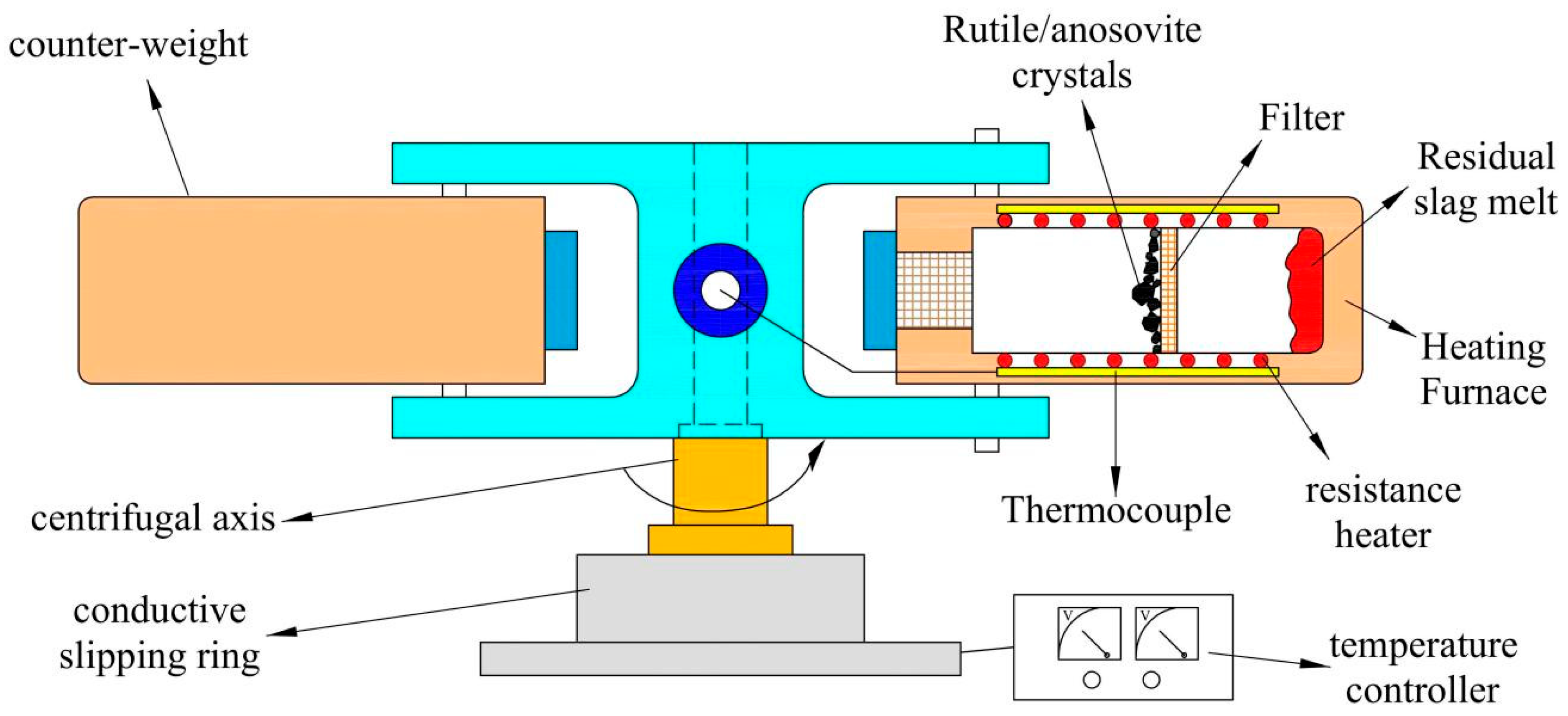
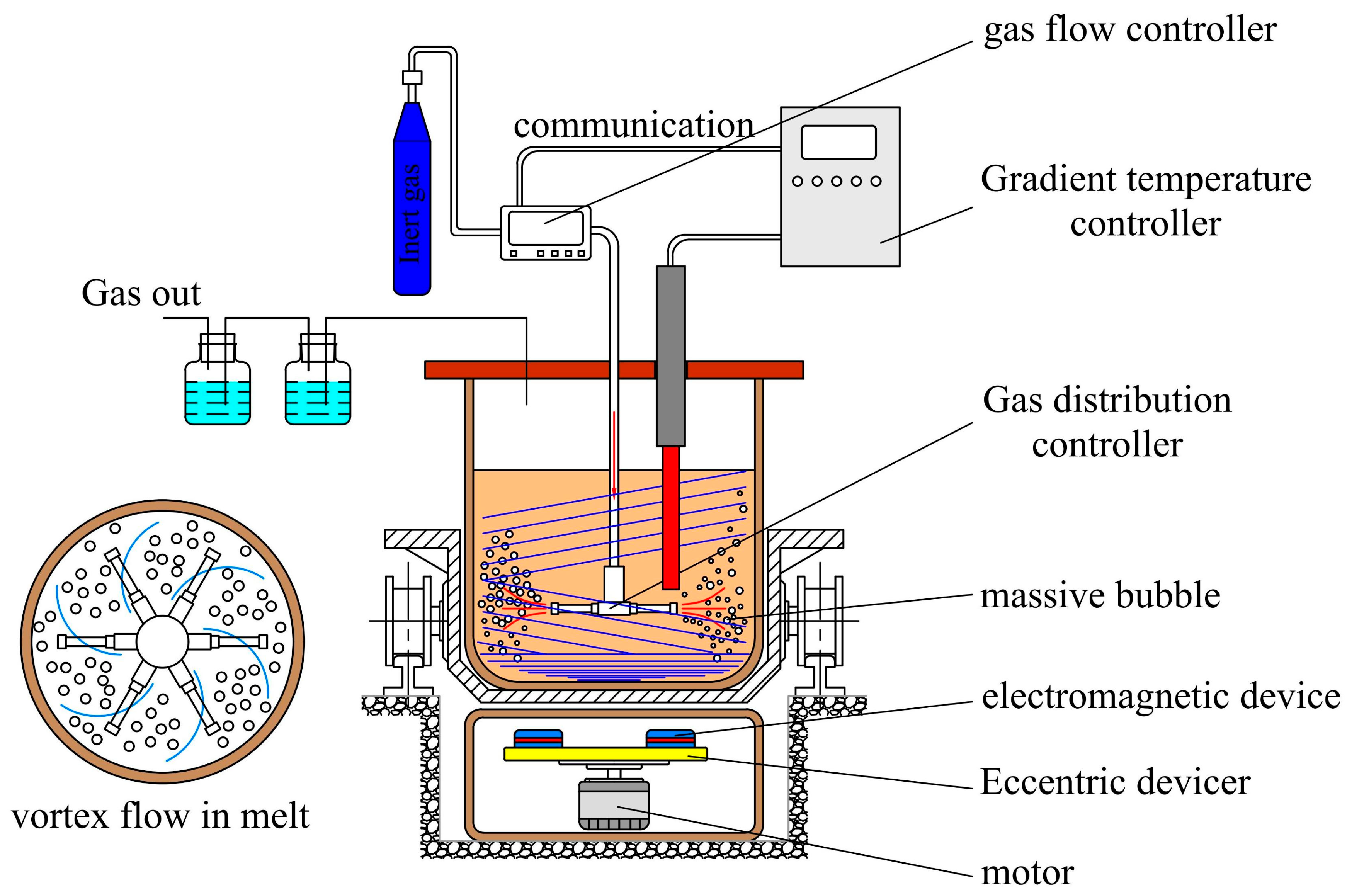
| Classification | TiO2 Content wt.% | Properties |
|---|---|---|
| BFS | 20–29% | generated during blast furnace ironmaking; large production; low grade of TiO2 content; complex mineral composition; intricate phase structures; great difficulty in comprehensive utilization. |
| EFS | ≥75% | generated during electric furnace slag; high grade of TiO2 content; critical feedstock for both sulfate-process titanium dioxide production and chloride-process sponge titanium manufacturing. |
| EMS | 45–68% | obtained via direct reduction-iron extraction followed by electric furnace melting separation of vanadium–titanium magnetite; high titanium grade; low impurity content; superior potential for value-added utilization. |
| Type | TiO2 | Al2O3 | MgO | CaO | SiO2 | TFe | MFe | FeO | V2O5 | Ti2O3 | TiC | MnO2 | MnO | P2O5 | S | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EMS | 45.32 | 12.26 | 7.58 | 2.91 | 13.10 | 11.09 | 0.27 | 14 | 2.00 | 0.167 | 0.3 | - | 1.44 | - | - | - |
| BFS | 23.35 | 11.09 | 7.06 | 28.64 | 25.44 | 2.82 | - | - | 0.20 | - | - | 0.75 | - | 0.022 | 0.12 | 0.50 |
| EFS | 76.03 | 2.40 | 7.39 | 1.72 | 3.20 | 8.46 | - | 6.15 | 0.215 | 5.07 | - | - | - | - | 0.25 | 0.335 |
| Phase | Molecular Formula | Density (p/cm)−3 | Crystal Structure | Geometric Morphology | Key Unit Cell Parameters |
|---|---|---|---|---|---|
| Ilmenite | FeTiO3 | 4.20–5.20 | Trigonal Crystal System | Tabular, granular | a = 5.09 Å, c = 14.07 Å |
| Perovskite | CaTiO3 | 4.10 | Cubic Crystal System | Spindle shaped | a = 3.85 Å |
| Anosovite | Ti3O5 | 4.68–4.79 | Orthorhombic Crystal System | Bundled | a = 9.12 Å b = 3.78 Å c = 5.03 Å |
| Rutile | TiO2 | 4.20–4.30 | Tetragonal Crystal System | Tetragonal prismatic, acicular | a = 4.59 Å c = 2.96 Å |
| Pseudobrookite | Fe2TiO5 | 4.49 | Orthorhombic Crystal System | Acicular | a = 9.74 Å b = 3.74 Å c = 5.09 Å |
| Sphene | CaTiSiO5 | 3.50 | Monoclinic Crystal System | Wedge shaped, tabular | a = 6.55 Å b = 8.70 Å c = 7.43 Å β = 119.3° |
| Extraction Method | TBS Composition (TiO2 wt.%) | Optimal Conditions | Ti Recovery/Outcome | Key Advantages | Key Challenges | Ref. |
|---|---|---|---|---|---|---|
| Hydrometallurgical Leaching | ||||||
| Conventional H2SO4 | 20–29% (BFS) | Acid conc. < 90%, T ~ boiling point | ~80% (estimated) | Simple process, wide adaptability | High acid consumption, severe corrosion, waste acid, and gypsum colloids | [66,67,68,69,70] |
| Activation Roasting-Leaching | 20–29% (BFS) | Acid conc. > 92%, Act. Roasting Temp.130 °C, Act. Roasting Temp.40 min, Acid-to-slag = 1.4 | >82.85% | Prevents gypsum colloids, improves filtration, and lowers acid use | Spent acid treatment required, process complexity | [71,79] |
| Pyrometallurgical Reduction | ||||||
| Carbothermal Reduction | ≥75% (EFS) | 1600–1800 °C, Coke reductant | 80–90% | Abundant reductant, high capacity | Excessive energy (>4.32 GJ/t), high CO2 emissions (≥1.5 t/t) | [85,86,87] |
| Gaseous Reduction (H2/CO/CH4) | 22–25% (BFS) | 1200 °C, H2/CO/CH4 | 97.71% conversion | Lower temp, lower energy consumption (↓37%), superior kinetics | Requires a gas handling system, process control | [94,95,96] |
| Molten Electrolysis | ||||||
| Liquid Metal Cathode (MOE) | ~22–25% (BFS) | Current density: ~0.5–0.8 A/cm2, T: 900–1000 °C, Cathode: Sn or Cu | 92–98% | Prevents dendritic growth, suppresses Si co-deposition | Requires downstream separation | [131,132,133,134,135] |
| MOE + Vacuum Distillation | ~22–25% (BFS) | Electrolysis: (as above); Distillation: 1500 °C/1 Pa/4 h (Sn) or 1100 °C/1 Pa/3 h (Sb) | >98% purity | Produces high-purity Ti metal or alloy | High energy consumption for distillation | [136,137] |
| Selective Precipitation and Separation | ||||||
| Rutile Transformation-Gravity Sedimentation | ~22–25% (BFS, Modified) | SiO2 addition: 5–10%, Oxidation time: 126 s, Controlled cooling | 98.73% | Forms separable rutile crystals, lower energy than supergravity | Requires precise composition and thermal control, slower separation speed | [155,156,157,158,159,160,161] |
| Supergravity Separation | ~22–25% (BFS, Modified) | temp.1300 °C, super-gravity G = 800, duration 6 min | 95.37% purity | High separation efficiency, high-purity product | Sensitive to temperature fluctuations, high energy use | [162,163,164,165] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Du, P.; Zhang, J.; Zhao, S.; Gao, M.; Wang, Q.; Tian, T.; Li, L.; Long, Y. Advances in Integrated Extraction of Valuable Components from Ti-Bearing Slag. Metals 2025, 15, 1080. https://doi.org/10.3390/met15101080
Li C, Du P, Zhang J, Zhao S, Gao M, Wang Q, Tian T, Li L, Long Y. Advances in Integrated Extraction of Valuable Components from Ti-Bearing Slag. Metals. 2025; 15(10):1080. https://doi.org/10.3390/met15101080
Chicago/Turabian StyleLi, Chenhui, Peipei Du, Jiansong Zhang, Suxing Zhao, Minglei Gao, Qianhua Wang, Tielei Tian, Lanjie Li, and Yue Long. 2025. "Advances in Integrated Extraction of Valuable Components from Ti-Bearing Slag" Metals 15, no. 10: 1080. https://doi.org/10.3390/met15101080
APA StyleLi, C., Du, P., Zhang, J., Zhao, S., Gao, M., Wang, Q., Tian, T., Li, L., & Long, Y. (2025). Advances in Integrated Extraction of Valuable Components from Ti-Bearing Slag. Metals, 15(10), 1080. https://doi.org/10.3390/met15101080







