Study on the Effect of Niobium on the High Temperature Oxidation Resistance of Ferritic Stainless Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results
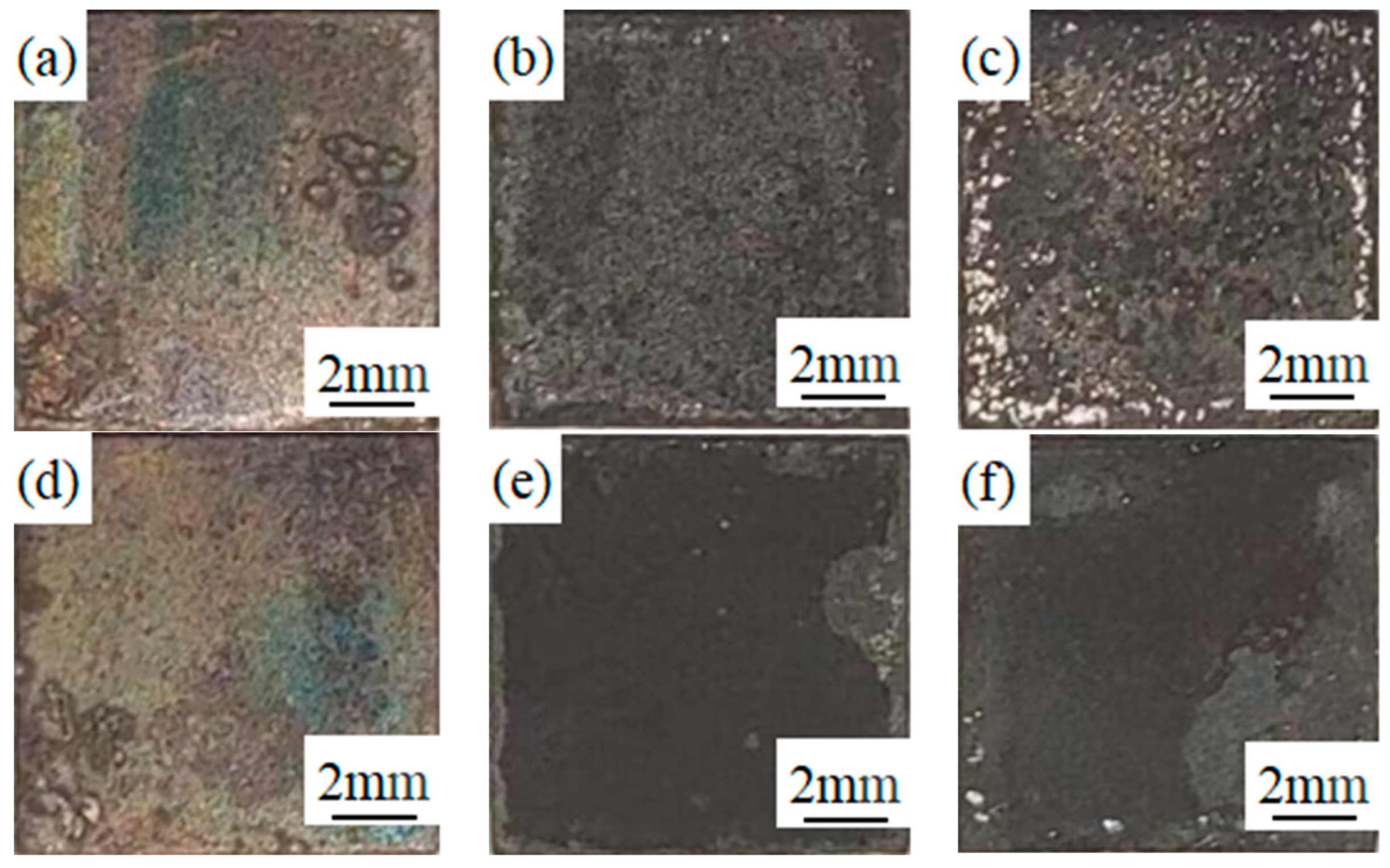
3.1. Surface High-Temperature Oxidation Morphology of Experimental Materials at Different Temperatures
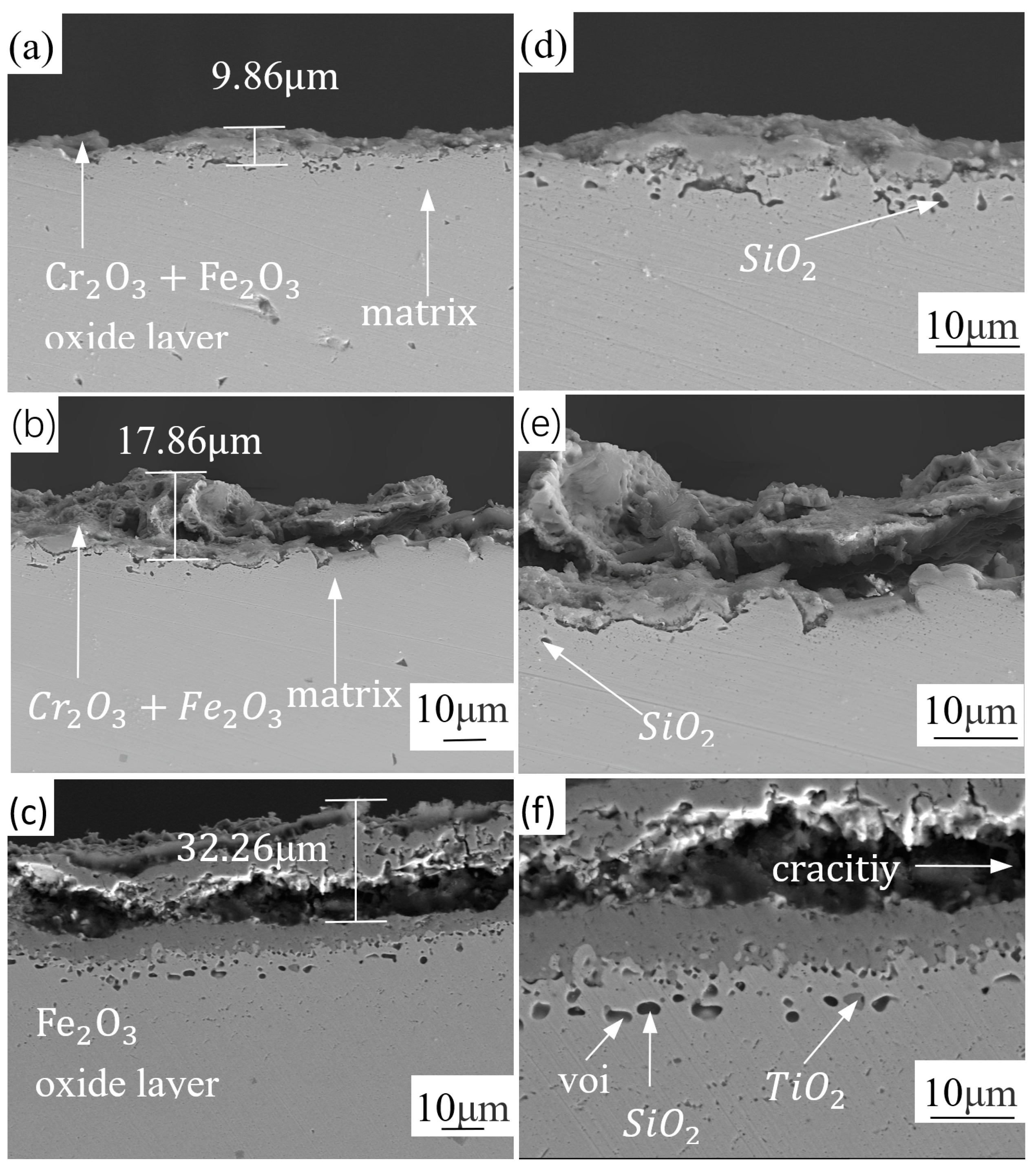
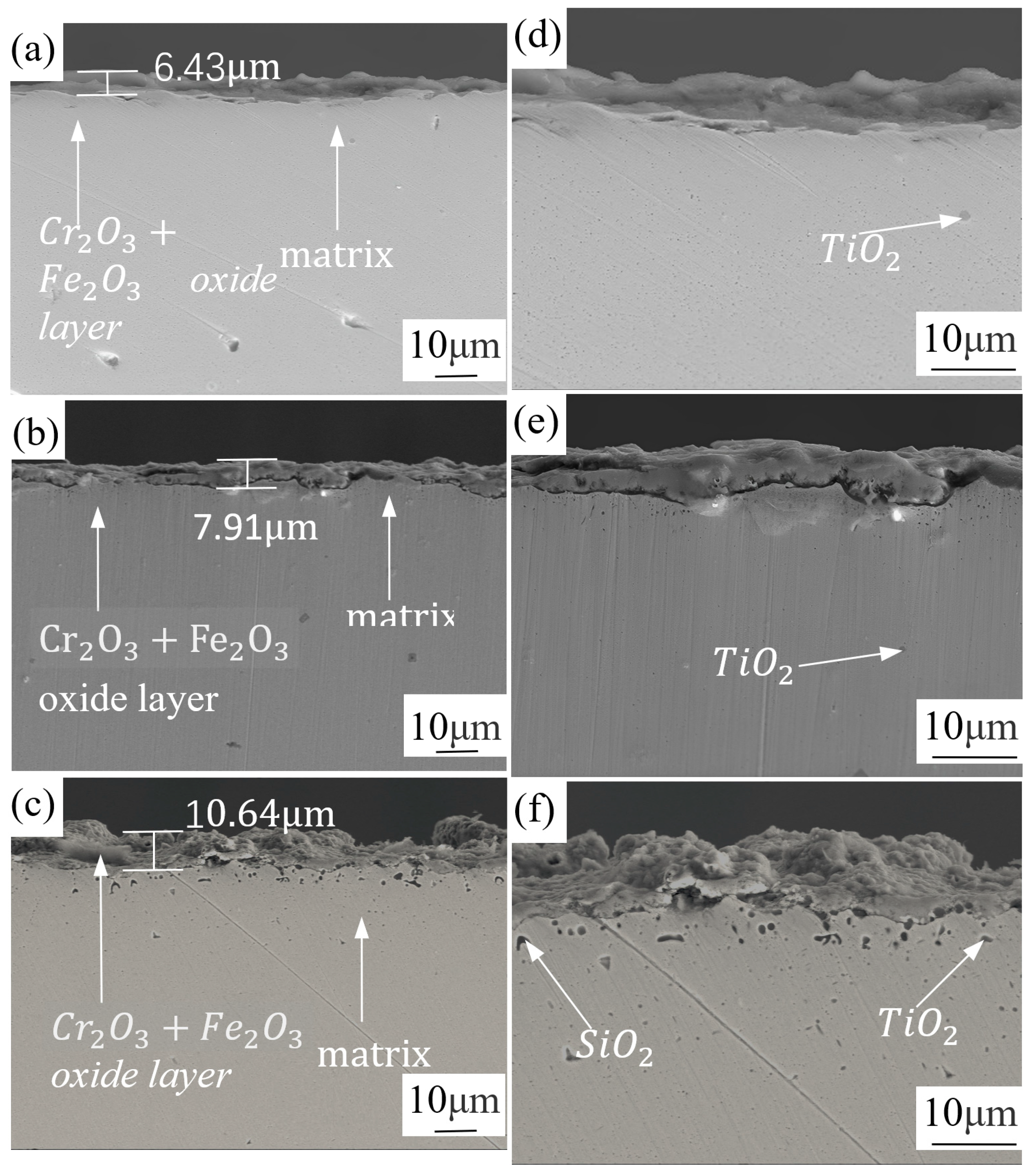
3.2. Analysis of Oxide Layer Thickness of Experimental Materials at Different Temperatures
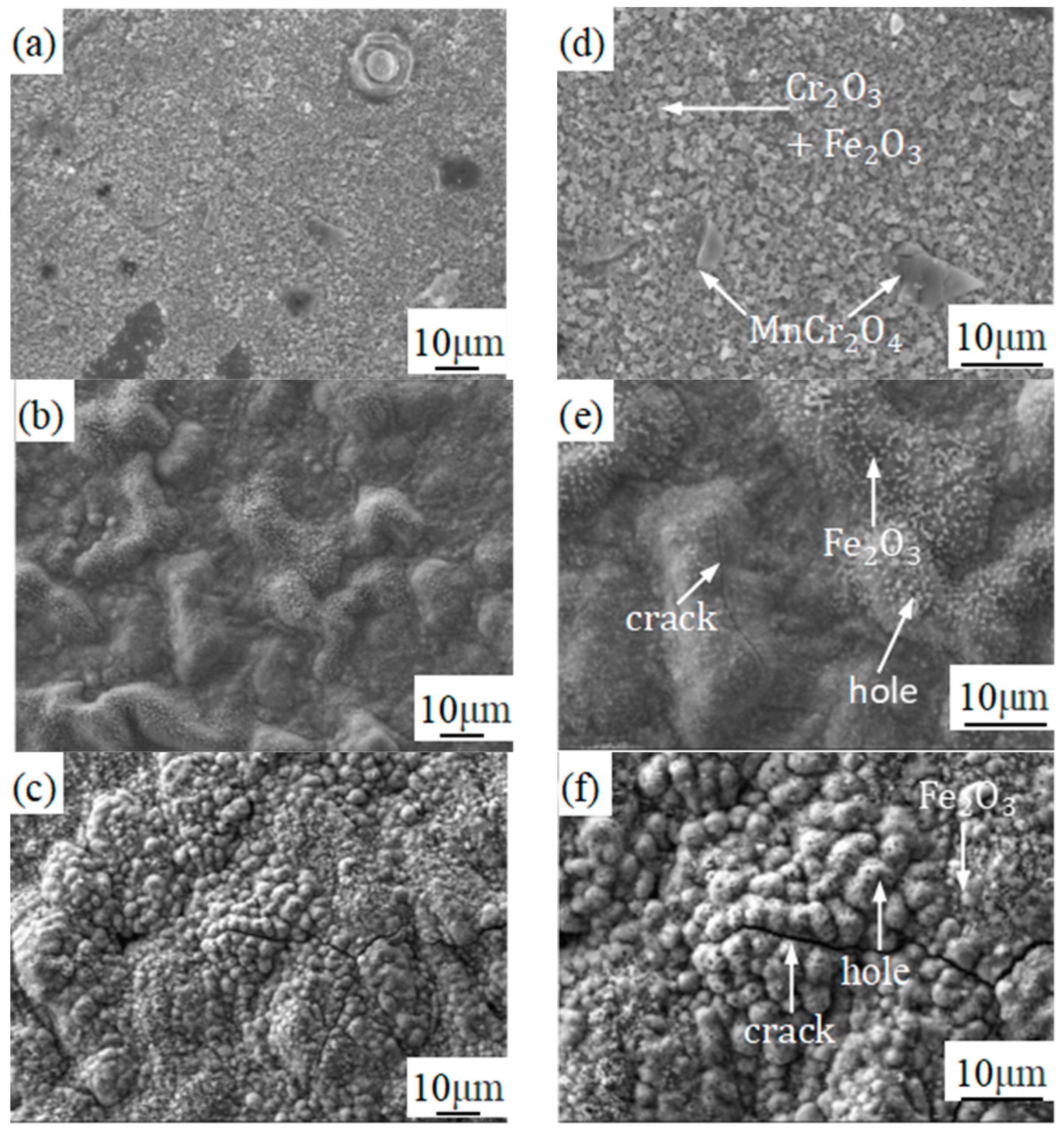
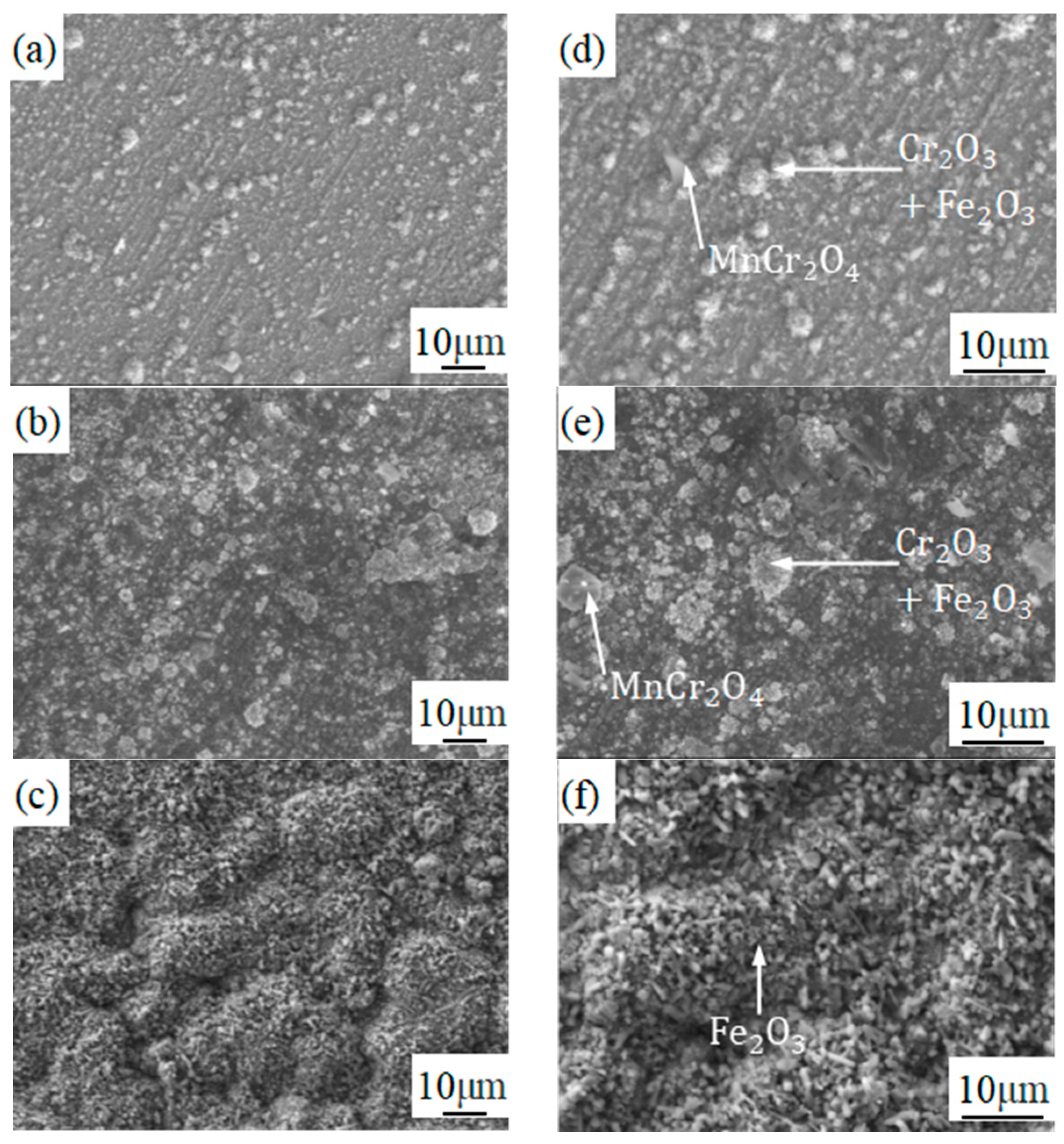
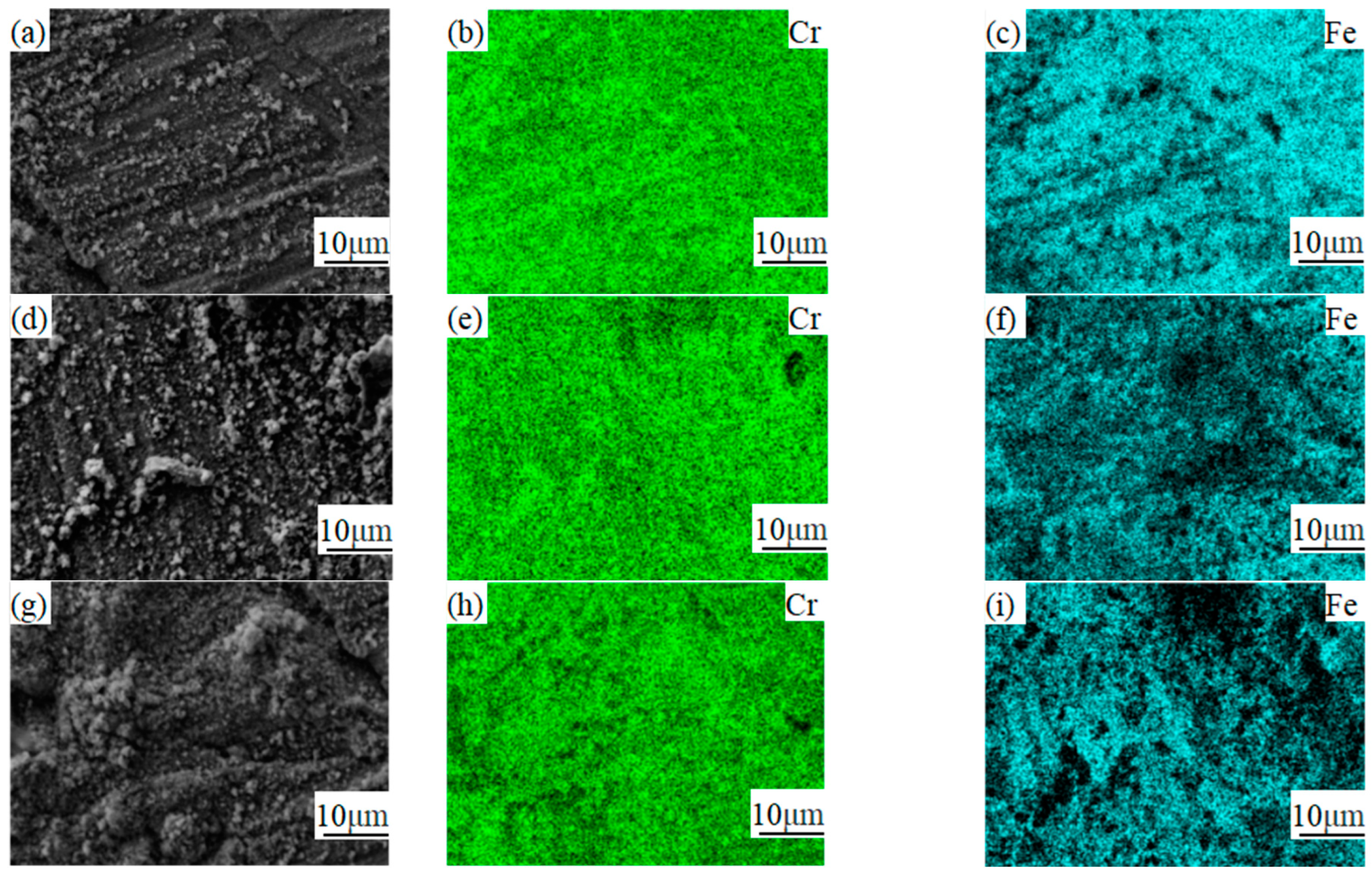
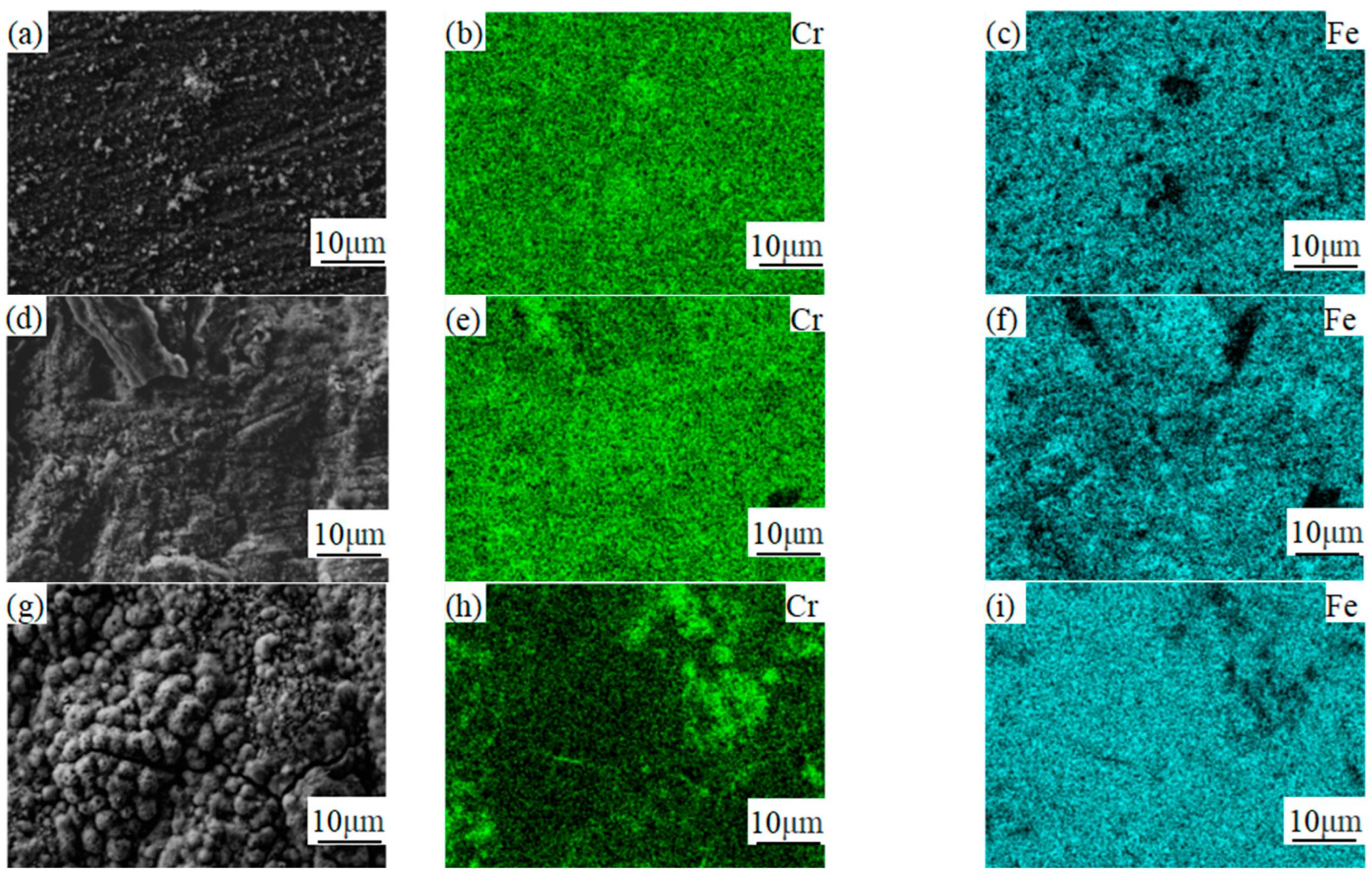
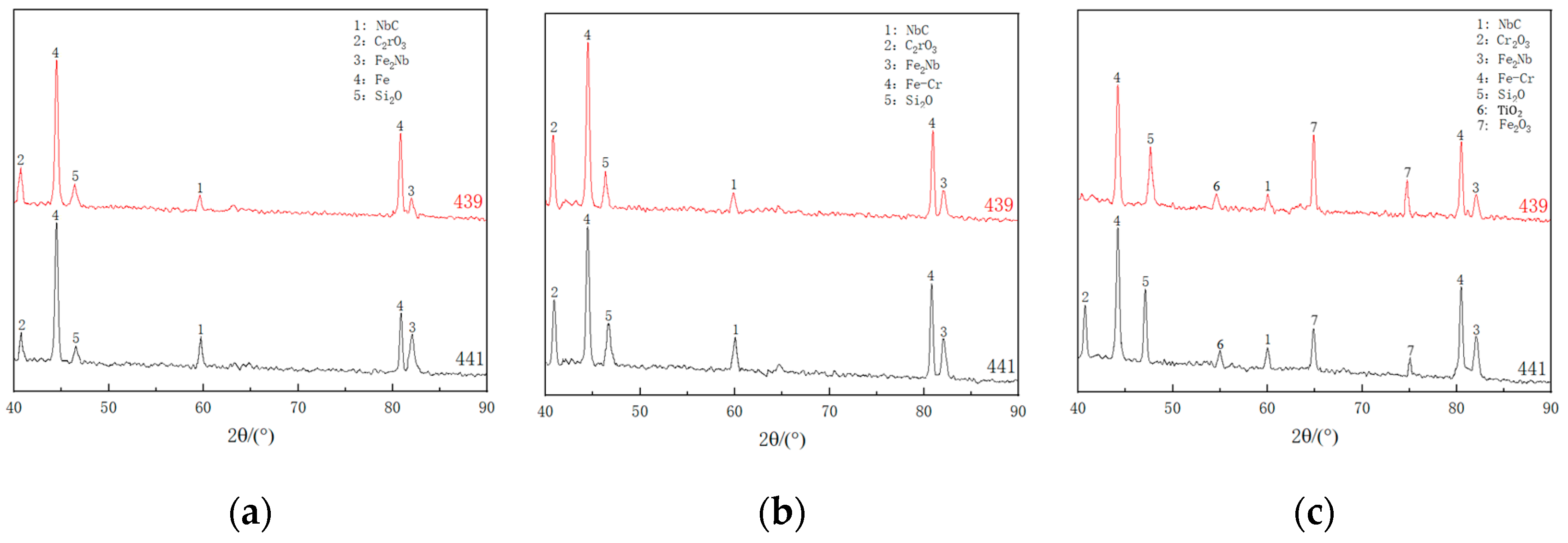
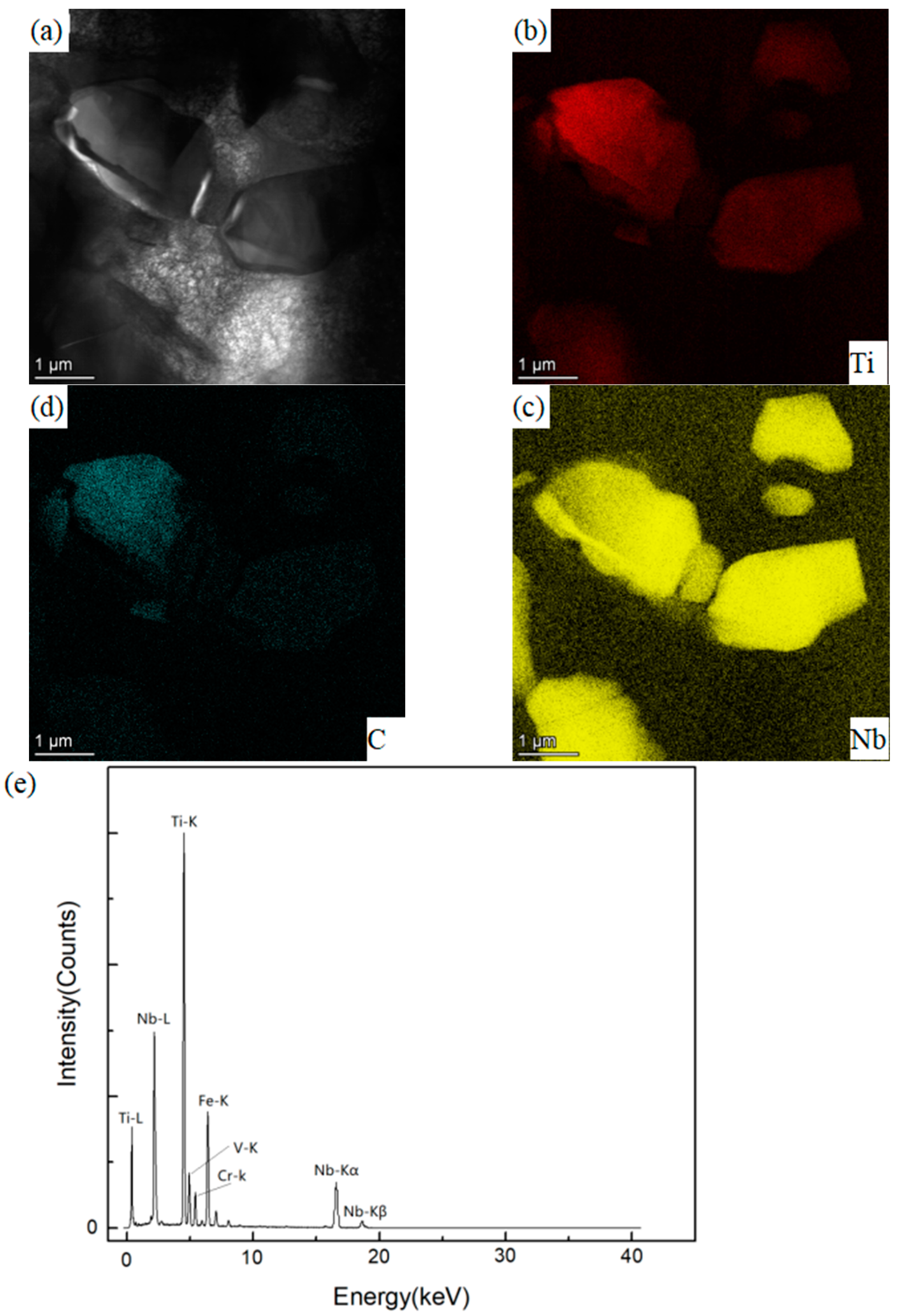
3.3. Analysis of Composition Distribution and Phase Structure of the Oxidized Surface at Different Temperatures
4. Discussion
5. Conclusions
- (1)
- As the oxidation temperature increases, the oxide layer of both types of stainless steel will increase. The oxide layer thickness of 441 stainless steel with higher niobium content is less than 439 stainless steel at the same temperature. The oxide layer of 441 stainless steel is intact at any temperature, while 439 stainless steel exhibits cracking and voids in the oxide layer at high temperatures.
- (2)
- The phase structure composition and oxidation morphology of the oxide layer vary at different temperatures. When the sample is oxidized at 700 °C, the oxidation layer of both types of ferritic stainless steel are Cr2O3 and Fe2O3. The oxidation layer of 439 stainless steel is dense and uniform, while 441 stainless steel is a discrete oxide particle. When the sample is oxidized at 800 °C, the oxidation products on the surface of 439 stainless steel are sintered together, and needle-like Fe2O3 structures appear on the oxide film, while the oxidation particles on the surface of 441 stainless steel grow and aggregate into a uniform oxide layer. When the sample is oxidized at 900 °C, the oxidation layer exhibits internal and external layering, with an outer layer of Fe2O3 shell structure and an inner layer of iron-containing oxides such as Fe2O3, Fe3O4, and FeO. However, 441 stainless steel has a needle-like Fe2O3 oxidation tumor on its surface but no cracking occurs, indicating a relatively complete oxidation layer.
- (3)
- The reason why 439 steel has poor high-temperature oxidation resistance is that (Nb, Ti) C precipitates coarsen at high temperatures, which has a destructive effect on high-temperature resistance. The reason why 441 steel has good high-temperature oxidation resistance is due to the precipitation of NbN and other niobium-containing precipitates at grain boundaries, which has the effect of refining grains and hindering the escape of Cr elements.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Oku, M. Development of Heat-resistant Ferritic Stainless Steel for Automotive Exhaust Systems Made by Nippon Steel. World Steel 2011, 5, 10–16. [Google Scholar]
- Di Schino, J.M.; Barteri, K.M. High temperature resistance of a high nitrogen and low nickel austenitic stainless steel. J. Mat. Sci. Lett. 2003, 22, 691–693. [Google Scholar]
- Dong, Z.; Chen, H.; Lang, Y. The effect of niobium and titanium on the high-temperature mechanical properties of ferritic stainless steel used in the hot end of automotive exhaust systems. Steel 2013, 48, 64–68. [Google Scholar]
- Liu, T.; Chen, L.; Bi, H.; Che, X. Effect of Mo on High-Temperature Fatigue Behavior of 15CrNbTi Ferritic Stainless Steel. Acta Metall. Sin. Engl. Lett. 2014, 27, 452–456. [Google Scholar] [CrossRef]
- Meng, Q.; Li, D.; Yang, J.; Liu, T. High temperature oxidation behavior of 310S heat-resistant steel. Mater. Eng. 2022, 50, 137–149. [Google Scholar]
- Zhang, M.; Han, Y.; Zu, G.; Sun, J.; Zhu, W.; Chen, H.; Ran, X. High-Temperature Oxidation Behavior of a Cu-Bearing 17Cr Ferritic Stainless Steel. Scanning 2020, 2020, 8847831. [Google Scholar] [CrossRef]
- Badin, V.; Diamanti, E.; Forêt, P.; Darque-Caretti, E. Characterization of oxide scales formed on ferritic stainless steel 441 at 1100 °C under water vapor. Oxid. Met. 2014, 82, 347. [Google Scholar] [CrossRef]
- Movahedi-Rad, A.; Pelaseyed, S.S.; Attarian, M.; Shokrallahzadeh, R. Oxidation behavior of AISI 321, AISI 316, and AISI 409 stainless steels: Kinetic, thermodynamic, and diffusion studies. J. Mater. Res. 2016, 31, 2088–2096. [Google Scholar] [CrossRef]
- Jacob, Y.P.; Haanappel, V.; Stroosnijder, M.F. The effect of gas composition on the isothermal oxidation behaviour of PM chromium. Corros. Sci. 2002, 44, 2027–2039. [Google Scholar] [CrossRef]
- Raj, K.A. On High-Temperature Materials: A Case on Creep and Oxidation of a Fully Austenitic Heat-Resistant Superalloy Stainless Steel Sheet. J. Mater. 2013, 2013, 124649. [Google Scholar]
- Cheng, L. Study on High Temperature Oxidation Behavior of Ferritic Stainless Steel. Ph.D. Thesis, Shenyang University, Shenyang, China, 2021. [Google Scholar]
- Duan, X.; Zhang, J.; Zhang, S.; Li, G.; Wei, Y. The effect of Nb and Ti on the high-temperature oxidation behavior of 444 ultra pure ferrite stainless steel. Spec. Steel 2021, 42, 7–11. [Google Scholar]
- Seo, H.S.; Yun, D.W.; Kim, K.Y. Oxidation behavior of ferritic stainless steel containing Nb, Nb-Si and Nb-Ti for SOFC interconnect. Int. J. Hydrogen Energy 2012, 38, 2432–2442. [Google Scholar] [CrossRef]
- Safikhani, A.; Esmailian, M.; Tinatiseresht, T.; Darban, G.B.D. High temperature cyclic oxidation behavior of ferritic stainless steel with addition of alloying elements Nb and Ti for use in SOFCs interconnect. Int. J. Hydrogen Energy 2016, 41, 6045–6052. [Google Scholar] [CrossRef]
- Haitao, Y.; Hongyun, B.; Xin, L.; Zhou, X. Precipitation and mechanical properties of Nb-modified ferritic stainless steel during isothermal aging. Mater. Charact. 2009, 60, 204. [Google Scholar]
- Peng, J.; Li, M.; Luo, S. Study on high-temperature oxidation behavior of B443NT ferritic stainless steel. Baosteel Technol. 2013, 22–25+31. [Google Scholar]
- Huang, H.; Yang, Y. Study on high-temperature oxidation behavior of niobium titanium double stabilized 430 stainless steel. J. Mater. Res. 2014, 28, 641–648. [Google Scholar]
- Fujita, N.; Ohmura, K.; Kikuchi, M.; Suzuki, T.; Funaki, S.; Hiroshige, I. Effect of Nb on high-temperature properties for ferritic stainless steel. Scr. Mater. 1996, 35, 705–710. [Google Scholar] [CrossRef]
- Cheng, X.W.; Jiang, Z.Y.; Monaghan, B.J.; Longbottom, R.J.; Wei, D.B.; Hee, A.C.; Jiang, L.Z. Degradation of ferritic stainless steels at 1200 °C in air. Mater. Corros. 2018, 69, 63–75. [Google Scholar] [CrossRef]
- Teng, S.; Qian, Z. Study on the high-temperature oxidation resistance of ultra-pure ferrite stainless steel. Gansu Metall. 2022, 44, 53–55. [Google Scholar]
- Qin, Z.; Taosha, L.; Wen, W.; Langlang, Z.; Yuefeng, J. High temperature oxidation kinetics and evolution of oxide film structure of nanocrystalline Ni-12Cr alloy at 800 °C. Chin. J. Corros. Prot. 2022, 42, 733–742. [Google Scholar]
- Chen, X.; Xiaochun, W.; Na, M.; Qianliang, S. 3DAP Study on the C Segregation Behavior of High Carbon High Alloy Steel During Deep Cooling Treatment. J. Met. 2015, 51, 325–332. [Google Scholar]
- Huang, Z.; Yang, Y. The effect of niobium titanium on the high-temperature precipitation behavior of the second phase in ferritic stainless steel. Met. Heat Treat. 2015, 40, 7–12. [Google Scholar]
- Xin, Z.; Wei, D.; Xunzheng, H. The effect of niobium titanium on the second phase precipitation and mechanical properties of 430 ferrite stainless steel. Baosteel Technol. 2015, 6–12. [Google Scholar]
- Chen, Y.; Zhan, J.; Ni, Q.; Li, M. Oxidation Behavior of 441 Ferritic Stainless Steel in Simulated Automotive Exhaust Gas Environment. Corros. Sci. Prot. Technol. 2019, 31, 174–180. [Google Scholar]
- Zhao, T.; Teng, L.; Jin, Y.; Zhang, J.; Yang, Y. Microstructure and mechanical properties of niobium containing ferritic stainless steel suitable for automotive exhaust manifolds. Shanghai Met. 2020, 42, 63–68. [Google Scholar]
- Hou, Q.; Jing, D.; Liao, Z.; Wang, K.; Xi, B.; Huang, Z. Effect of niobium on oxidation weight gain of 65SiCrV6 spring steel. Steel 2022, 1–15. [Google Scholar]
- Duan, X.; Zhang, J.; Wang, B.; Li, G. Behavior of Trace Elements during High Temperature Oxidation of 444 Ferritic Stainless Steel. In Proceedings of the 13th China Steel Annual Conference-7. Advanced Steel Materials and Their Applications, Online, 23–24 November 2022; pp. 443–449. [Google Scholar]



| C | Si | Mn | P | Cr | Ni | Al | Nb | Ti | N | |
|---|---|---|---|---|---|---|---|---|---|---|
| 439 | 0.010 | 0.37 | 0.13 | 0.015 | 17.59 | 0.10 | 0.065 | 0.17 | 0.20 | 0.0084 |
| 441 | 0.010 | 0.39 | 0.23 | 0.016 | 18.00 | 0.10 | 0.037 | 0.41 | 0.16 | 0.0085 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, E.; Xia, D.; Chen, P.; Han, Q.; Wang, X.; Liu, Z.; Jiang, K. Study on the Effect of Niobium on the High Temperature Oxidation Resistance of Ferritic Stainless Steel. Metals 2024, 14, 25. https://doi.org/10.3390/met14010025
Zhu E, Xia D, Chen P, Han Q, Wang X, Liu Z, Jiang K. Study on the Effect of Niobium on the High Temperature Oxidation Resistance of Ferritic Stainless Steel. Metals. 2024; 14(1):25. https://doi.org/10.3390/met14010025
Chicago/Turabian StyleZhu, En, Dianxiu Xia, Peidun Chen, Qing Han, Xuelin Wang, Zhiheng Liu, and Kun Jiang. 2024. "Study on the Effect of Niobium on the High Temperature Oxidation Resistance of Ferritic Stainless Steel" Metals 14, no. 1: 25. https://doi.org/10.3390/met14010025
APA StyleZhu, E., Xia, D., Chen, P., Han, Q., Wang, X., Liu, Z., & Jiang, K. (2024). Study on the Effect of Niobium on the High Temperature Oxidation Resistance of Ferritic Stainless Steel. Metals, 14(1), 25. https://doi.org/10.3390/met14010025







