Characterization of Various Stainless Steels Containing Gadolinium as Thermal Neutron Absorbing and Shielding Materials
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Alloy Design and Production
2.2. Microstructure Characterization
2.3. Mechanical Properties of the Experimental Alloys
2.4. Electrochemical Corrosion Test
3. Results and Discussion
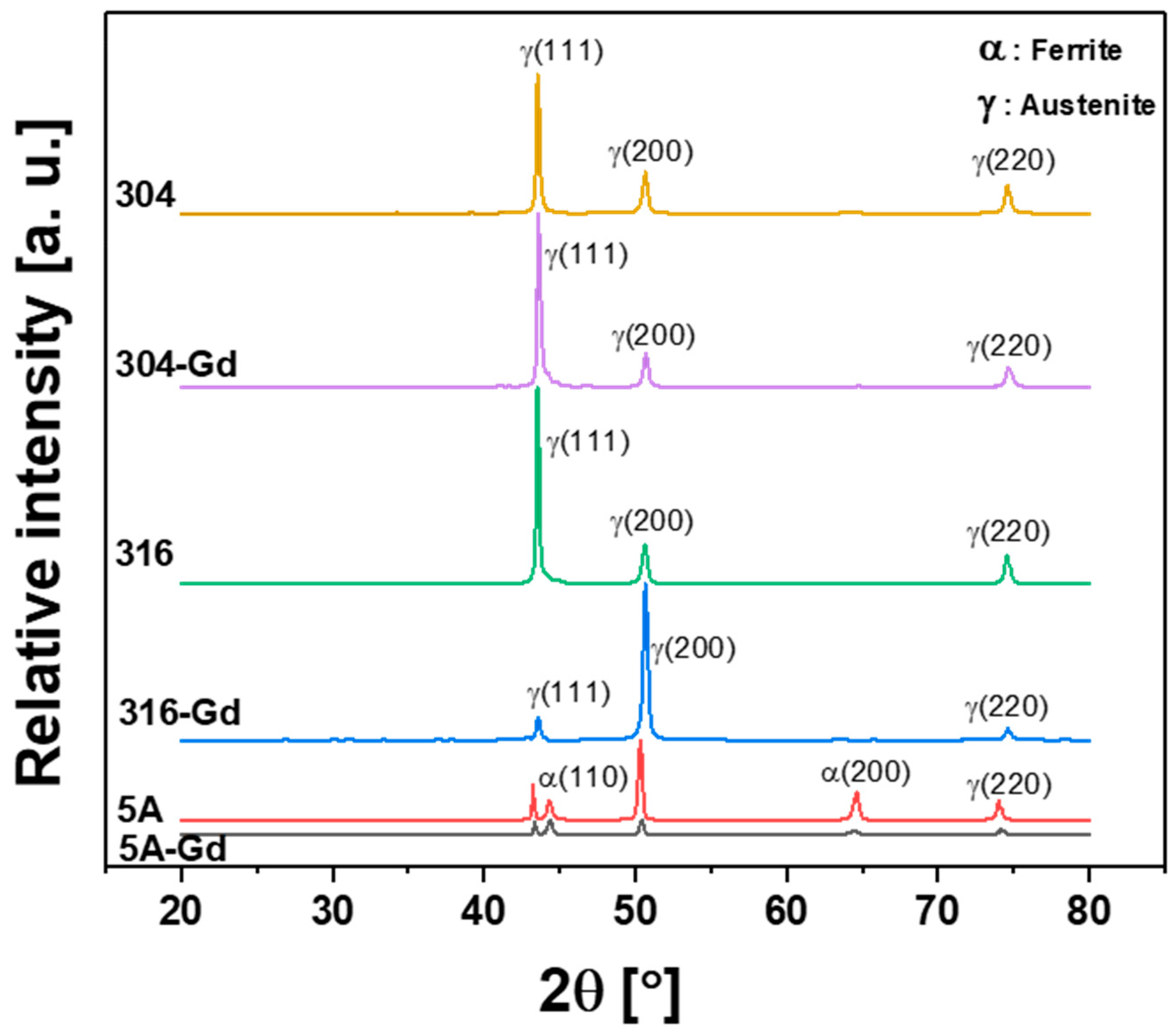
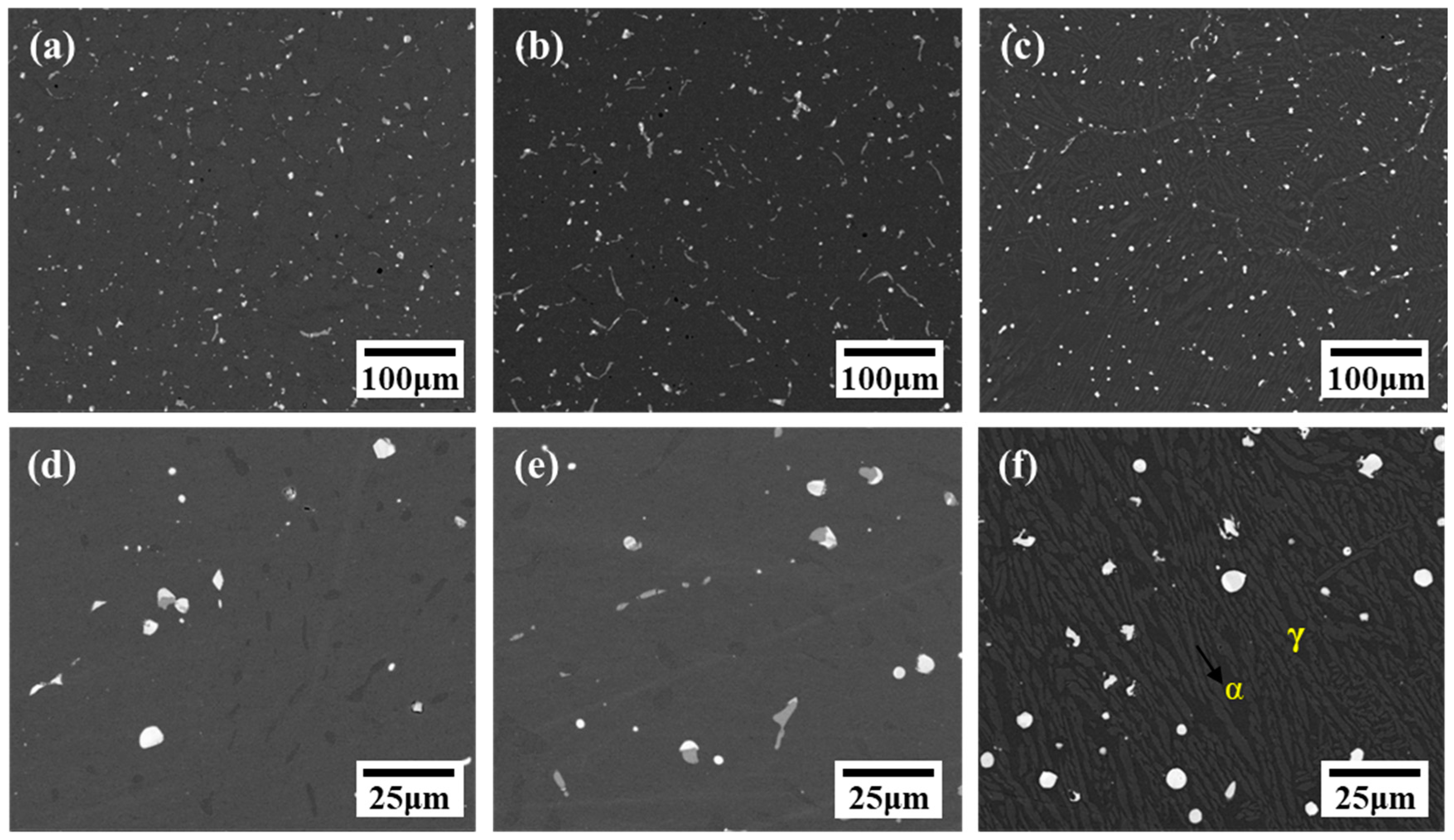
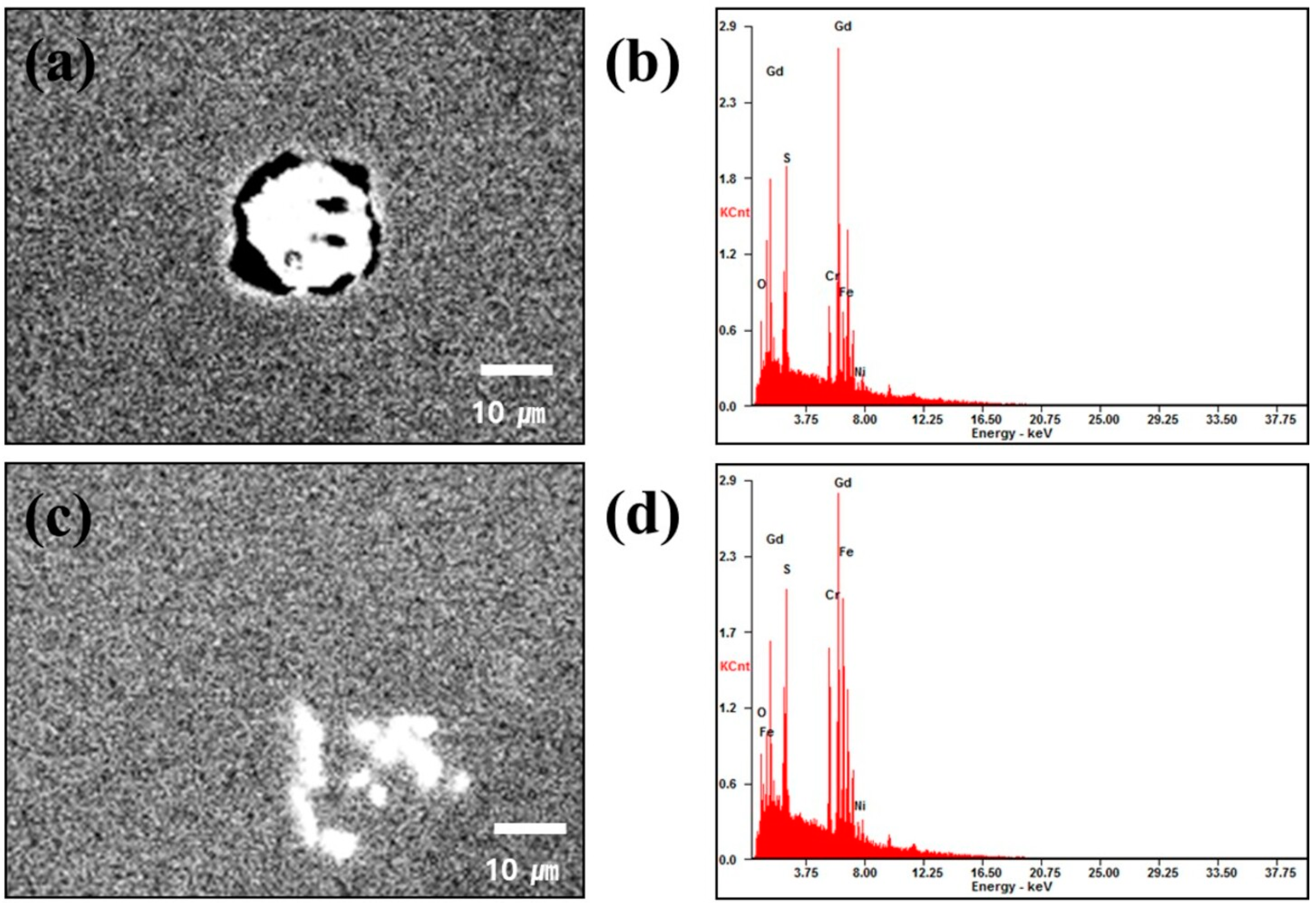
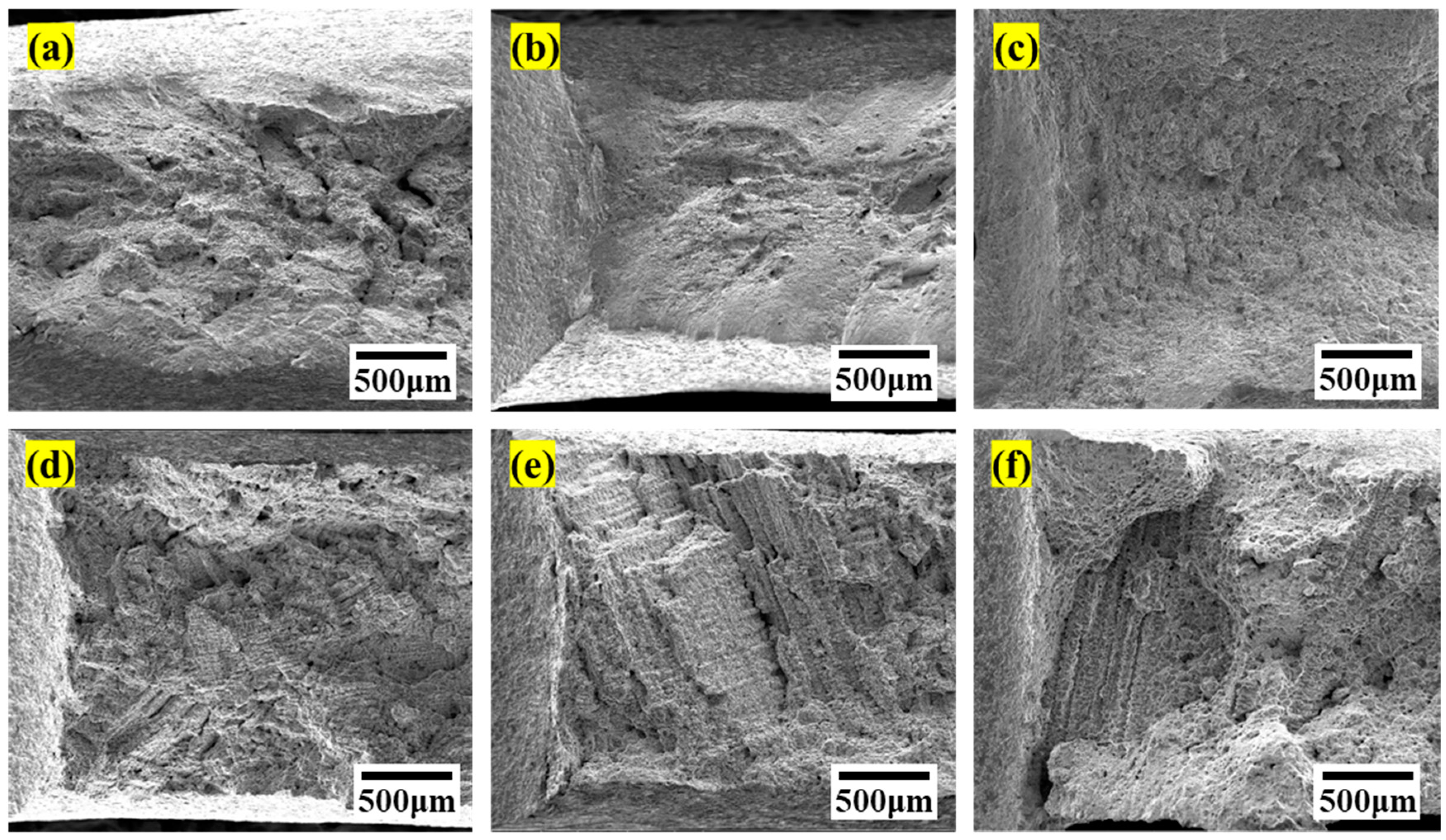
3.1. Microstructural Characterization
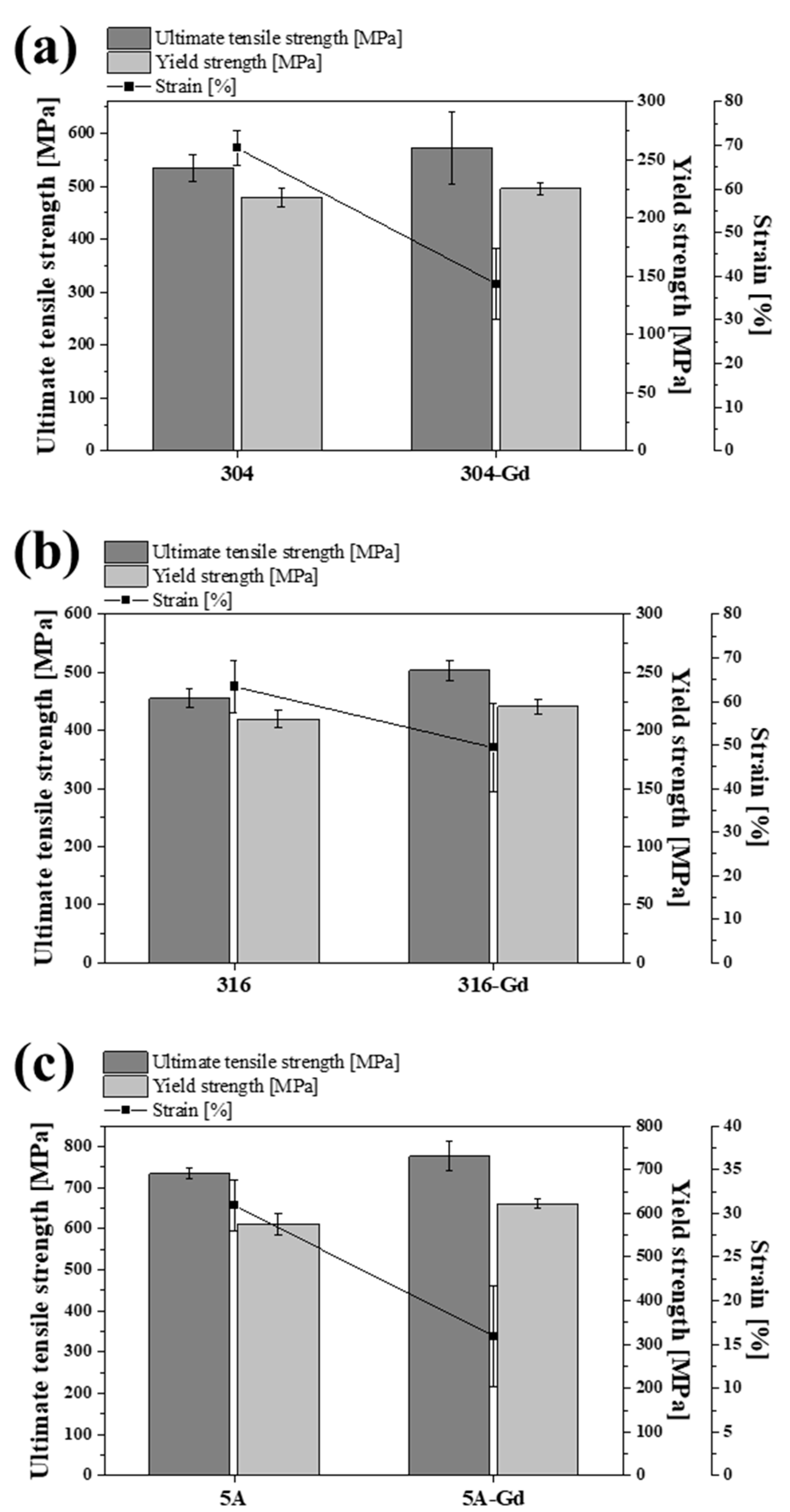
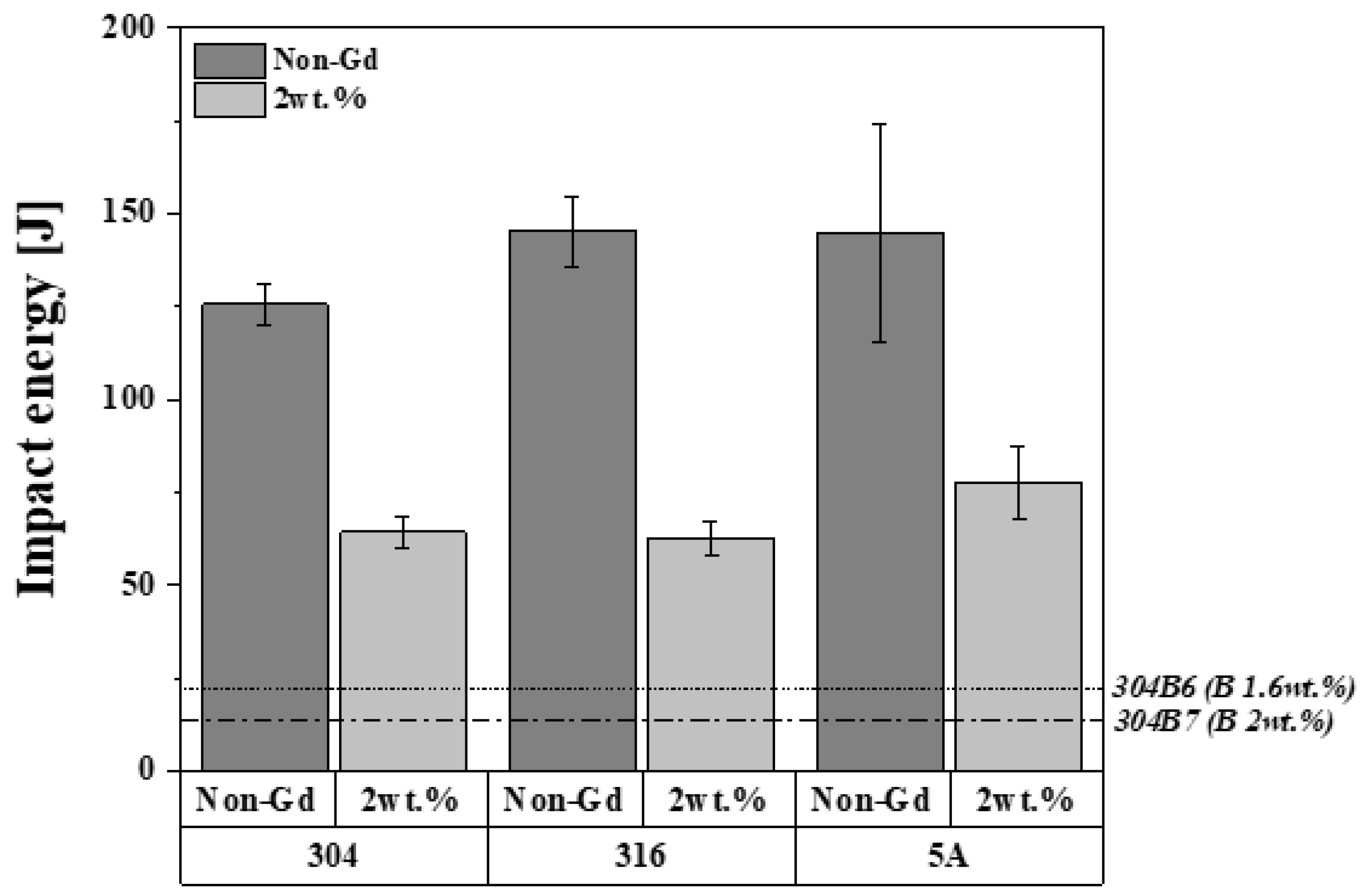
3.2. Mechanical Properties
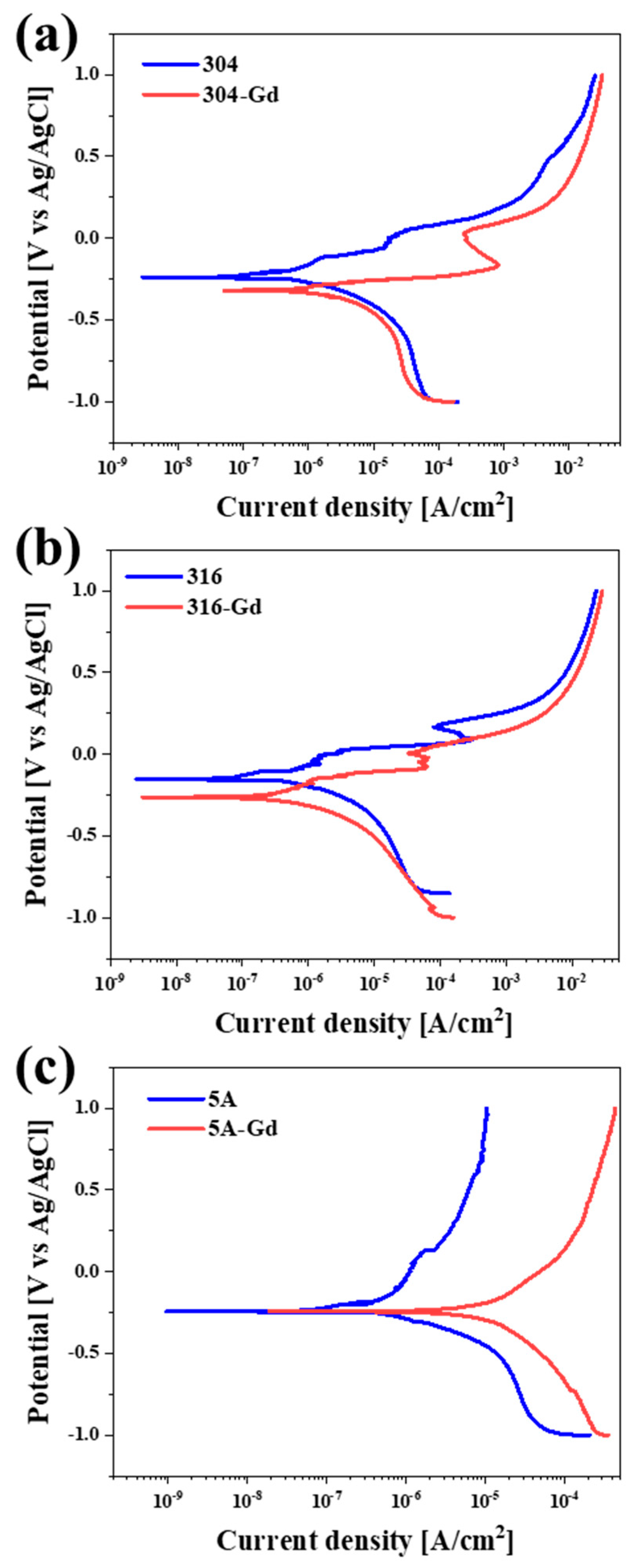
3.3. Corrosion Resistances of the Alloys
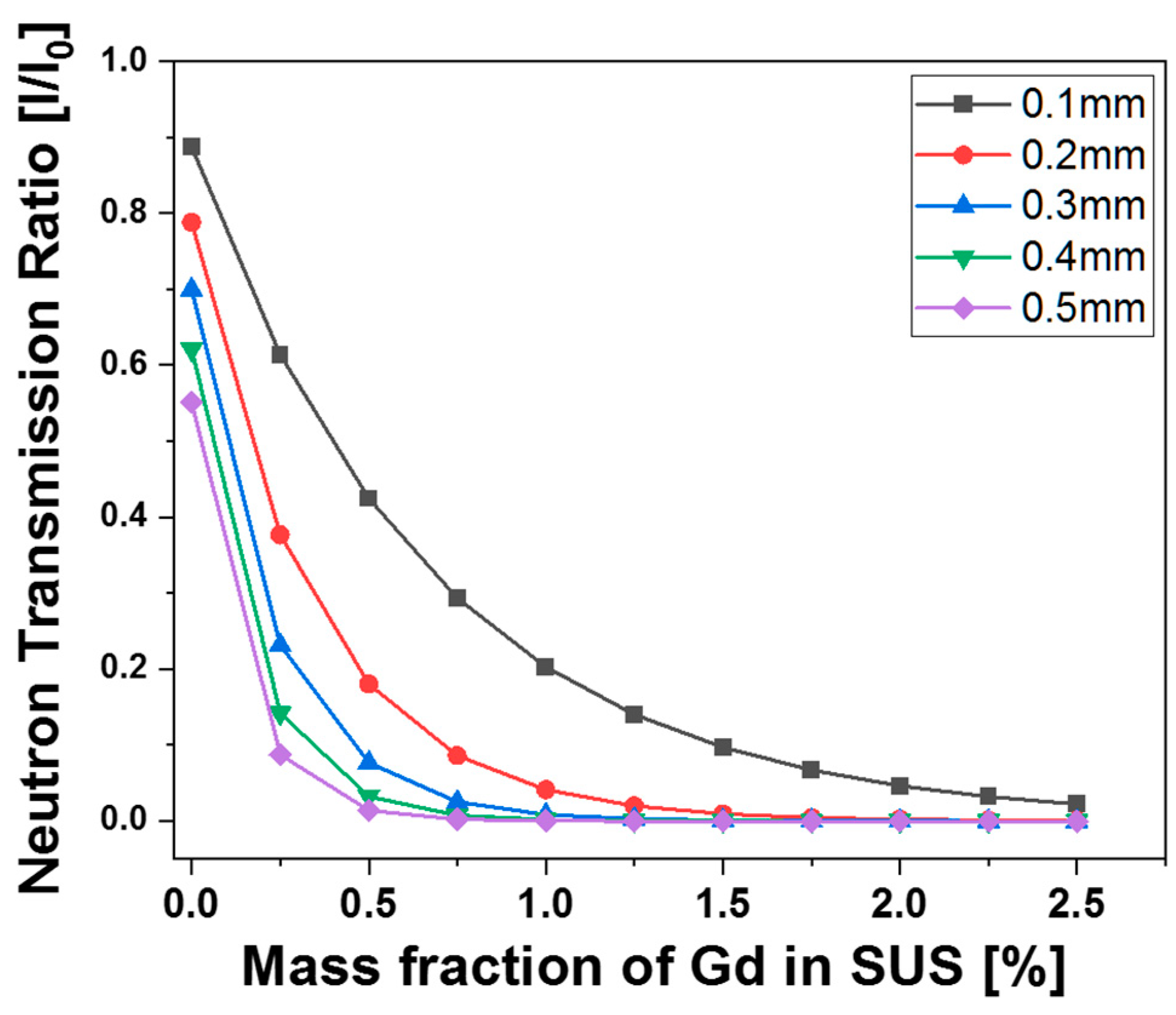
3.4. Thermal-Neutron-Absorbing Properties of the Alloys
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, S.-W.; Ahn, J.-H.; Moon, B.-M.; Kim, D.; Oh, S.; Kim, Y.-J.; Jung, H.-D. Preliminary study on FeGd alloys as binary alloys and master alloys for potential spent nuclear fuel (SNF) application. Mater. Des. 2020, 194, 108906. [Google Scholar] [CrossRef]
- Moon, B.-M.; Lee, S.-W.; Kim, M.-J.; Jung, S.-R.; Kim, Y.-J.; Jung, H.-D. The effect of gadolinium on the microstructures and Charpy impact properties of super duplex stainless steels. Metals 2018, 8, 474. [Google Scholar] [CrossRef]
- Taylor, R.; Mathers, G.; Banford, A. The development of future options for aqueous recycling of spent nuclear fuels. Prog. Nucl. Energy 2023, 164, 104837. [Google Scholar] [CrossRef]
- Qi, Z.; Yang, Z.; Li, J.; Guo, Y.; Yang, G.; Yu, Y.; Zhang, J. The advancement of neutron-shielding materials for the transportation and storage of spent nuclear fuel. Materials 2022, 15, 3255. [Google Scholar] [CrossRef] [PubMed]
- Karley, D.; Shukla, S.K.; Rao, T.S. Microbiological assessment of spent nuclear fuel pools: An in-perspective review. J. Environ. Chem. Eng. 2022, 10, 108050. [Google Scholar] [CrossRef]
- Holdsworth, A.F.; Eccles, H.; Sharrad, C.A.; George, K. Spent Nuclear Fuel—Waste or Resource? The Potential of Strategic Materials Recovery during Recycle for Sustainability and Advanced Waste Management. Waste 2023, 1, 249–263. [Google Scholar] [CrossRef]
- Gan, B.; Liu, S.; He, Z.; Chen, F.; Niu, H.; Cheng, J.; Tan, B.; Yu, B. Research progress of metal-based shielding materials for neutron and gamma rays. Acta Metall. Sin. (Engl. Lett.) 2021, 34, 1609–1617. [Google Scholar] [CrossRef]
- Qi, Z.-D.; Yang, Z.; Yang, X.-G.; Wang, L.-Y.; Li, C.-Y.; Dai, Y. Performance study and optimal design of Gd/316L neutron absorbing material for Spent Nuclear Fuel transportation and storage. Mater. Today Commun. 2023, 34, 105342. [Google Scholar] [CrossRef]
- Jung, Y.; Lee, M.; Kim, K.; Ahn, S. 10B (n, α) 7Li reaction-induced gas bubble formation in Al–B4C neutron absorber irradiated in spent nuclear fuel pool. J. Nucl. Mater. 2020, 533, 152077. [Google Scholar] [CrossRef]
- Jiang, L.; Xu, Z.; Fei, Y.; Zhang, Q.; Qiao, J.; Wu, G. The design of novel neutron shielding (Gd + B4C)/6061Al composites and its properties after hot rolling. Compos. Part B Eng. 2019, 168, 183–194. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, W.; Lee, D.; Lemaire, M.; Lee, H.-J.; Sohn, D.-S.; Kwon, H. Development of integral type spent fuel pool storage rack with gadolinium and europium-containing structure materials. Ann. Nucl. Energy 2019, 130, 107–117. [Google Scholar] [CrossRef]
- Hur, D.H.; Jeon, S.-H.; Han, J.; Park, S.-Y.; Chun, Y.-B. Effect of gadolinium addition on the corrosion behavior and oxide properties of titanium in boric acid solution at 50 °C. J. Mater. Res. Technol. 2022, 21, 3051–3061. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, L.; Zhang, Q.; Qiao, J.; Gong, D.; Wu, G. The design of a novel neutron shielding B4C/Al composite containing Gd. Mater. Des. 2016, 111, 375–381. [Google Scholar] [CrossRef]
- Cong, S.; Ran, G.; Li, Y.; Chen, Y. Ball-milling properties and sintering behavior of Al-based Gd2O3–W shielding materials used in spent-fuel storage. Powder Technol. 2020, 369, 127–136. [Google Scholar] [CrossRef]
- Lister, T.; Mizia, R.; Pinhero, P.; Trowbridge, T.; Delezene-Briggs, K. Studies of the corrosion properties of Ni-Cr-Mo-Gd neutron-absorbing alloys. Corrosion 2005, 61, 706–717. [Google Scholar] [CrossRef]
- Mizia, R.E.; Lister, T.E.; Pinhero, P.J.; Trowbridge, T.L.; Hurt, W.L.; Robino, C.V.; Stephens, J.J., Jr.; DuPont, J.N. Development and testing of an advanced neutron-absorbing gadolinium alloy for spent nuclear fuel storage. Nucl. Technol. 2006, 155, 133–148. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.; Wan, S.; Zhang, T. Design, fabrication and comprehensive properties of the novel thermal neutron shielding Gd/316L composites. Fusion Eng. Des. 2021, 171, 112566. [Google Scholar] [CrossRef]
- Yang, X.; Song, L.; Chang, B.; Yang, Q.; Mao, X.; Huang, Q. Development of Gd-Si-O dispersed 316L stainless steel for improving neutron shielding performance. Nucl. Mater. Energy 2020, 23, 100739. [Google Scholar] [CrossRef]
- Wang, H.; Wang, T.; Peng, J. Effect of gadolinium addition on microstructure, mechanical properties and corrosion resistance of 316L austenitic stainless steels. Phys. Met. Metallogr. 2021, 122, 1640–1647. [Google Scholar] [CrossRef]
- Baik, Y.; Choi, Y.; Moon, B.M.; Sohn, D.S.; Bogdanov, S.G.; Pirogov, A.N. Effect of gadolinium addition on the corrosion, wear, and neutron absorbing behaviors of duplex stainless steel sheet. Phys. Met. Metallogr. 2015, 116, 1135–1142. [Google Scholar] [CrossRef]
- Ahn, J.-H.; Jung, H.-D.; Im, J.-H.; Jung, K.H.; Moon, B.-M. Influence of the addition of gadolinium on the microstructure and mechanical properties of duplex stainless steel. Mater. Sci. Eng. A 2016, 658, 255–262. [Google Scholar] [CrossRef]
- Jung, M.Y.; Baik, Y.; Choi, Y.; Sohn, D.-S. Corrosion and mechanical properties of hot-rolled 0.5% Gd-0.8% B-stainless steels in a simulated nuclear waste treatment solution. Nucl. Eng. Technol. 2019, 51, 207–213. [Google Scholar] [CrossRef]
- Schmidt, M.; Del Corso, G.; Klankowski, K.; Lherbier, L.; Novotnak, D. Review of the Development and Testing of a New Family of Boron and Gadolinium-bearing Dual Thermal Neutron Absorbing Alloys-13026. In Proceedings of the WM2013: Waste Management Conference: International Collaboration and Continuous Improvement, Phoenix, AZ, USA, 24–28 February 2013; pp. 24–28. [Google Scholar]
- Saeed, A. Developed borated austenitic stainless steel alloys as nuclear reactor control rods. Nucl. Eng. Des. 2023, 413, 112515. [Google Scholar] [CrossRef]
- Kang, M.-H.; Cheon, K.-H.; Jo, K.-I.; Ahn, J.-H.; Kim, H.-E.; Jung, H.-D.; Jang, T.-S. An asymmetric surface coating strategy for improved corrosion resistance and vascular compatibility of magnesium alloy stents. Mater. Des. 2020, 196, 109182. [Google Scholar] [CrossRef]
- Lister, T.; Mizia, R.E.; Erickson, A.; Matteson, B. General and Localized Corrosion of Borated Stainless Steels. In Proceedings of the NACE CORROSION, New Orleans, LA, USA, 16–20 March 2008; p. NACE-08590. [Google Scholar]
- Lister, T.; Mizia, R.; Erickson, A.; Trowbridge, T. Electrochemical Corrosion Testing of Neutron Absorber Materials; Idaho National Lab. (INL): Idaho Falls, ID, USA, 2007. [Google Scholar]
- Robino, C.; Cieslak, M. High-temperature metallurgy of advanced borated stainless steels. Metall. Mater. Trans. A 1995, 26, 1673–1685. [Google Scholar] [CrossRef]
- He, J. The Effect of Boron Content, Temperature, and Neutron Fluence on the Mechanical Properties of the Modified Type 304 Stainless Steels with Boron; The Pennsylvania State University: State College, PA, USA, 1995. [Google Scholar]
- Wan, S.; Wang, W.; Chen, H.; Zhou, J.; Zhang, Y.; Liu, R.; Feng, R. 155/157Gd areal density: A model for design and fabrication of Gd2O3/316L novel neutron shielding composites. Vacuum 2020, 176, 109304. [Google Scholar] [CrossRef]
- Ha, H.-Y.; Lee, T.-H.; Jo, H.-H.; Lee, J.; Jang, J.H. Corrosion behavior of (Fe, Ni)–Gd intermetallic compounds in FeNi-based neutron-absorbing steels. J. Nucl. Mater. 2023, 578, 154367. [Google Scholar] [CrossRef]
- Florez, R.; Loaiza, A.; Giraldo, C.H.C.; Colorado, H.A. Calcium silicate phosphate cement with samarium oxide additions for neutron shielding applications in nuclear industry. Prog. Nucl. Energy 2021, 133, 103650. [Google Scholar] [CrossRef]
- Castley, D.; Goodwin, C.; Liu, J. Computational and experimental comparison of boron carbide, gadolinium oxide, samarium oxide, and graphene platelets as additives for a neutron shield. Radiat. Phys. Chem. 2019, 165, 108435. [Google Scholar] [CrossRef]
- Jing, H.; Geng, L.; Qiu, S.; Zou, H.; Liang, M.; Deng, D. Research progress of rare earth composite shielding materials. J. Rare Earths 2023, 41, 32–41. [Google Scholar] [CrossRef]


| Alloys | Fe | Cr | Ni | Mo | Mn | Si | N | Gd |
|---|---|---|---|---|---|---|---|---|
| 304 (Aimed) | Bal. | 18 | 10 | - | 1.3 | 0.8 | 0.1 | 2 |
| 304 | Bal. | 18.4 | 9.21 | - | 1.01 | 0.45 | 0.13 | - |
| 304-Gd | Bal. | 18.1 | 9.44 | - | 1.13 | 0.62 | 0.12 | 1.76 |
| 316 (Aimed) | Bal. | 18 | 12 | 2.4 | 1.3 | 0.8 | 0.1 | 2 |
| 316 | Bal. | 19.3 | 11.6 | 1.9 | 1.4 | 0.91 | 0.12 | - |
| 316-Gd | Bal. | 18.3 | 10.9 | 2.12 | 1.19 | 0.77 | 0.14 | 2.13 |
| 5A (Aimed) | Bal. | 25 | 7 | 4.5 | 1.3 | 0.8 | 0.3 | 2 |
| 5A | Bal. | 25.4 | 7.17 | 4.23 | 1.16 | 0.65 | 0.25 | - |
| 5A-Gd | Bal. | 25.8 | 7.63 | 4.34 | 1.22 | 0.78 | 0.28 | 1.96 |
| UTS (MPa) | YS (MPa) | E (%) | |
|---|---|---|---|
| 304 | 534.2 ± 24.7 | 217.5 ± 7.4 | 69.3 ± 1.7 |
| 316 | 455.4 ± 16.3 | 210.0 ± 8.6 | 63.4 ± 2.7 |
| 5A | 734.7 ± 10.1 | 576.1 ± 10.3 | 30.9 ± 2.6 |
| 304-Gd | 572.6 ± 67.3 | 225.4 ± 6.8 | 38.3 ± 3.3 |
| 316-Gd | 504.0 ± 15.6 | 220.9 ± 5.1 | 49.4 ± 4.6 |
| 5A-Gd | 776.5 ± 24.3 | 622.4 ± 4.9 | 15.9 ± 5.1 |
| 304B6 (1.6 wt.% B) | 515 | 205 | 9–20 |
| 304B7 (2 wt.% B) | 515 | 205 | 6–17 |
| Alloys | Impact Energy (J) |
|---|---|
| 304 | 125.6 ± 5.1 |
| 316 | 145.4 ± 9.3 |
| 5A | 144.8 ± 26.1 |
| 304-Gd | 64.3 ± 3.8 |
| 316-Gd | 62.8 ± 4.9 |
| 5A-Gd | 77.7 ± 9.7 |
| 304B6 (1.6 wt.% B) | 22 |
| 304B7 (2 wt.% B) | 14 |
| Alloys | Ecorr (mV vs. Ag/AgCl) | Icorr (μA/cm2) | βc (mV/decade) | βa (mV/decade) | Rp (kΩ∙cm2) |
|---|---|---|---|---|---|
| 304 | −229.1 | 0.55 | 328.1 | 233.9 | 107.2 |
| 316 | −180.6 | 0.19 | 128.8 | 109.9 | 133.1 |
| 5A | −220.1 | 0.38 | 145.6 | 375.5 | 119.7 |
| 304-Gd | −318.4 | 1.35 | 313.0 | 153.1 | 33.2 |
| 316-Gd | −299.1 | 2.68 | 387.5 | 501.0 | 35.4 |
| 5A-Gd | −245.8 | 4.84 | 498.4 | 305.8 | 17.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, S.; Ahn, J.-H.; Jung, R.; Kim, H.-J.; Chu, Y.; Choi, D.H.; Lee, H.; Jung, H.-D. Characterization of Various Stainless Steels Containing Gadolinium as Thermal Neutron Absorbing and Shielding Materials. Metals 2024, 14, 16. https://doi.org/10.3390/met14010016
Oh S, Ahn J-H, Jung R, Kim H-J, Chu Y, Choi DH, Lee H, Jung H-D. Characterization of Various Stainless Steels Containing Gadolinium as Thermal Neutron Absorbing and Shielding Materials. Metals. 2024; 14(1):16. https://doi.org/10.3390/met14010016
Chicago/Turabian StyleOh, SeKwon, Ji-Ho Ahn, Rockhoon Jung, Hyun-Jong Kim, Younghwan Chu, Dae Hyun Choi, Hyun Lee, and Hyun-Do Jung. 2024. "Characterization of Various Stainless Steels Containing Gadolinium as Thermal Neutron Absorbing and Shielding Materials" Metals 14, no. 1: 16. https://doi.org/10.3390/met14010016
APA StyleOh, S., Ahn, J.-H., Jung, R., Kim, H.-J., Chu, Y., Choi, D. H., Lee, H., & Jung, H.-D. (2024). Characterization of Various Stainless Steels Containing Gadolinium as Thermal Neutron Absorbing and Shielding Materials. Metals, 14(1), 16. https://doi.org/10.3390/met14010016








