Corrosion Performance of Welded Joints for E40 Marine Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Welding
2.3. Characterization Methods
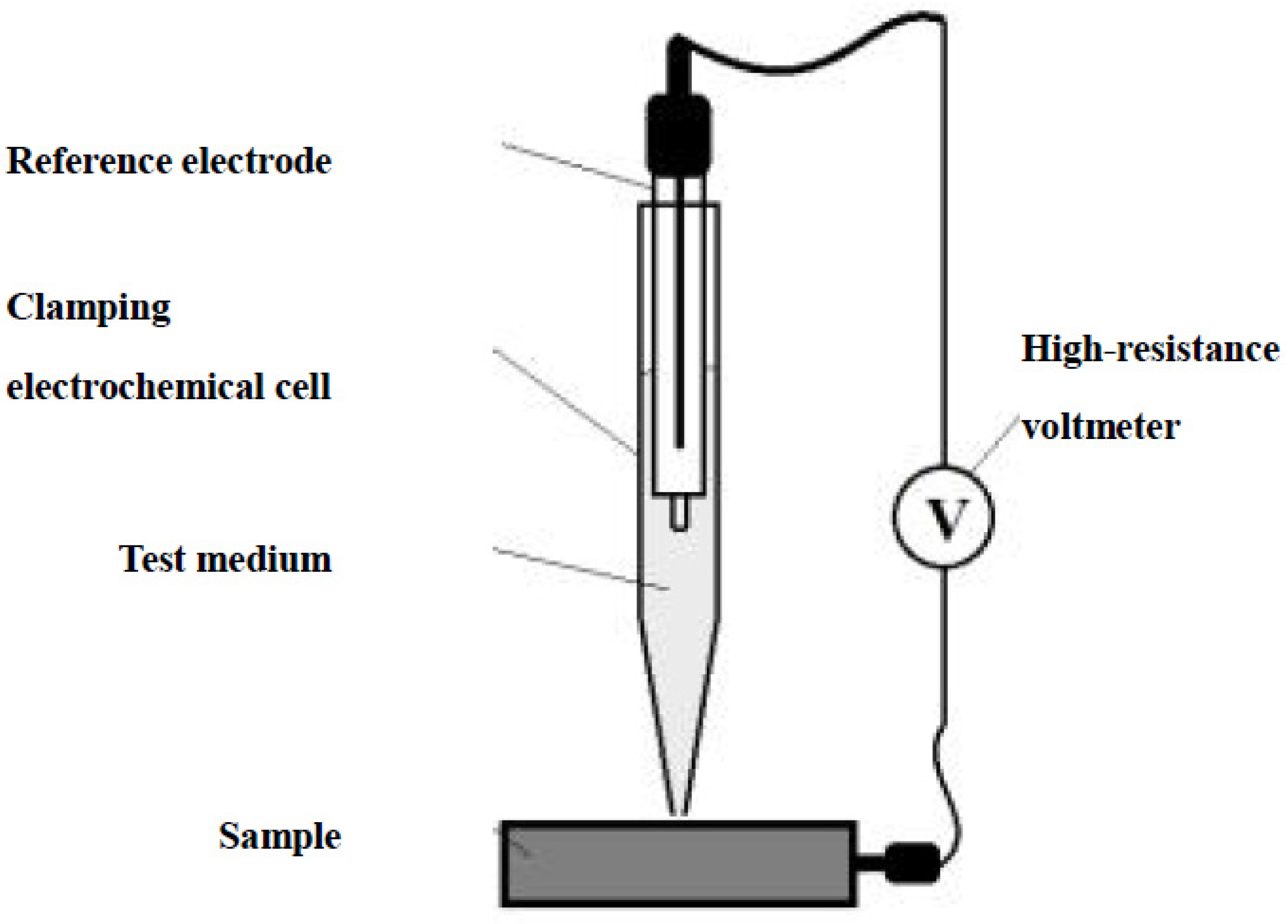
2.3.1. Electrochemical Tests
2.3.2. Metallographic Tests
3. Results
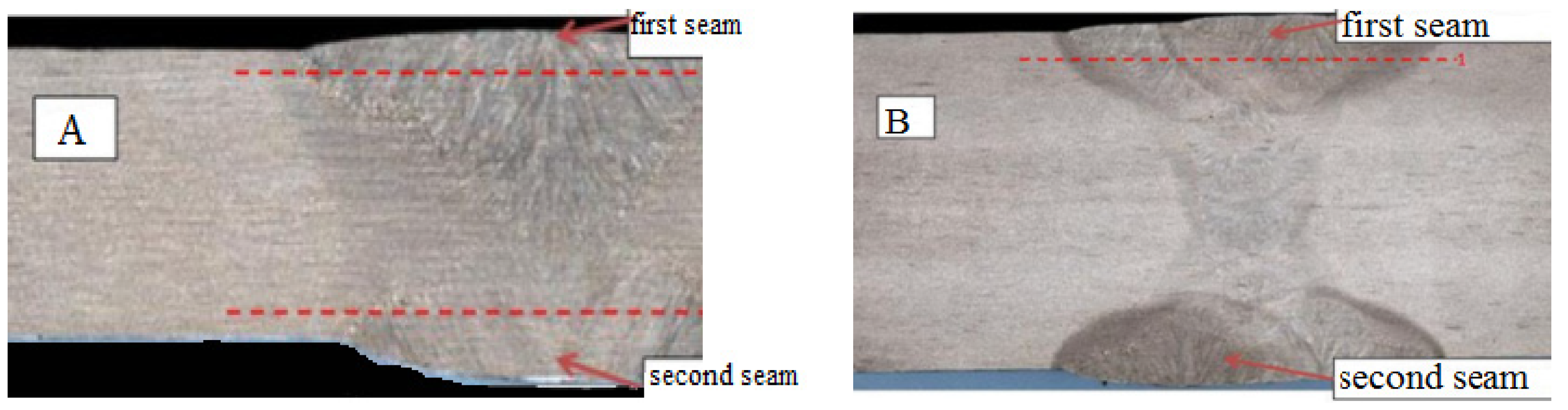
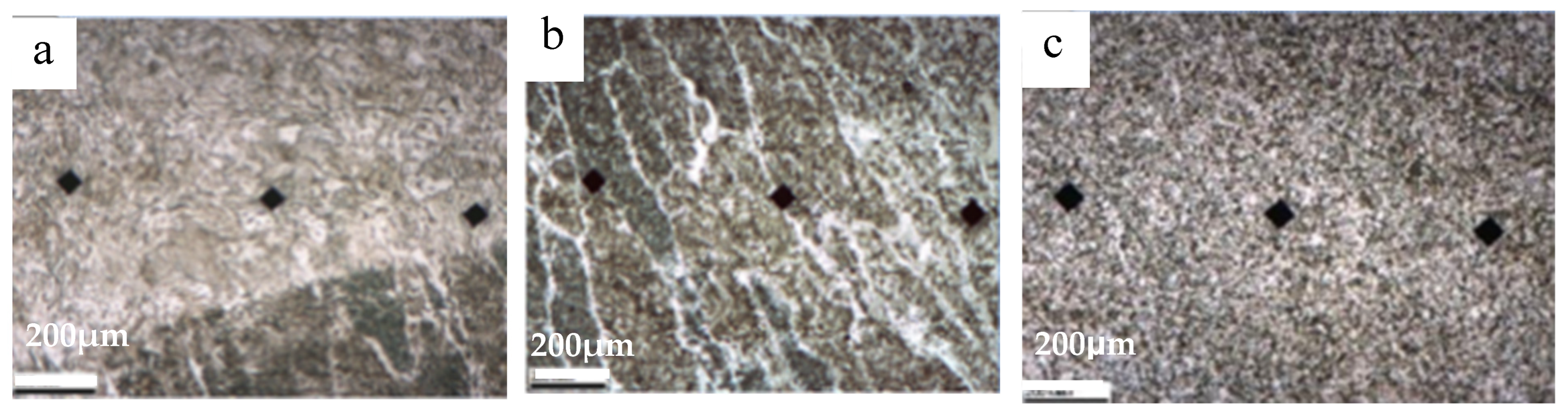
3.1. Microstructure and Microhardness of Welded Joints
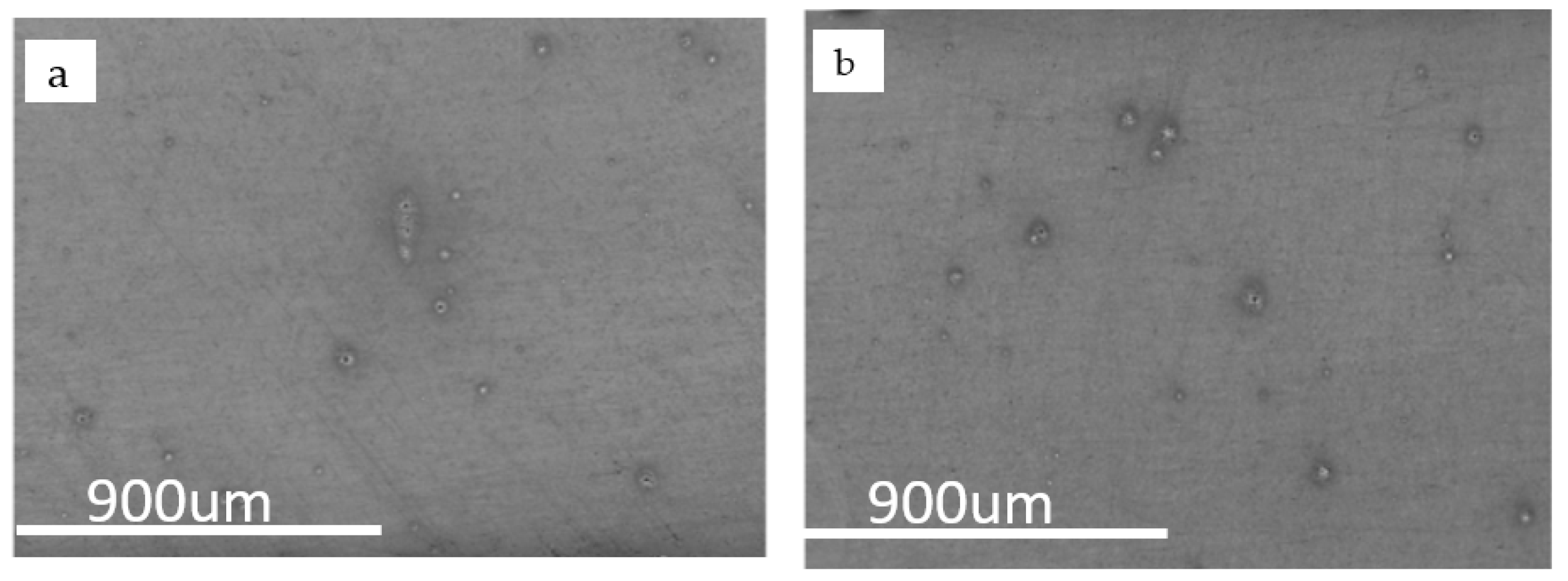
3.2. Corrosion Rate in a Moving Environment Simulator
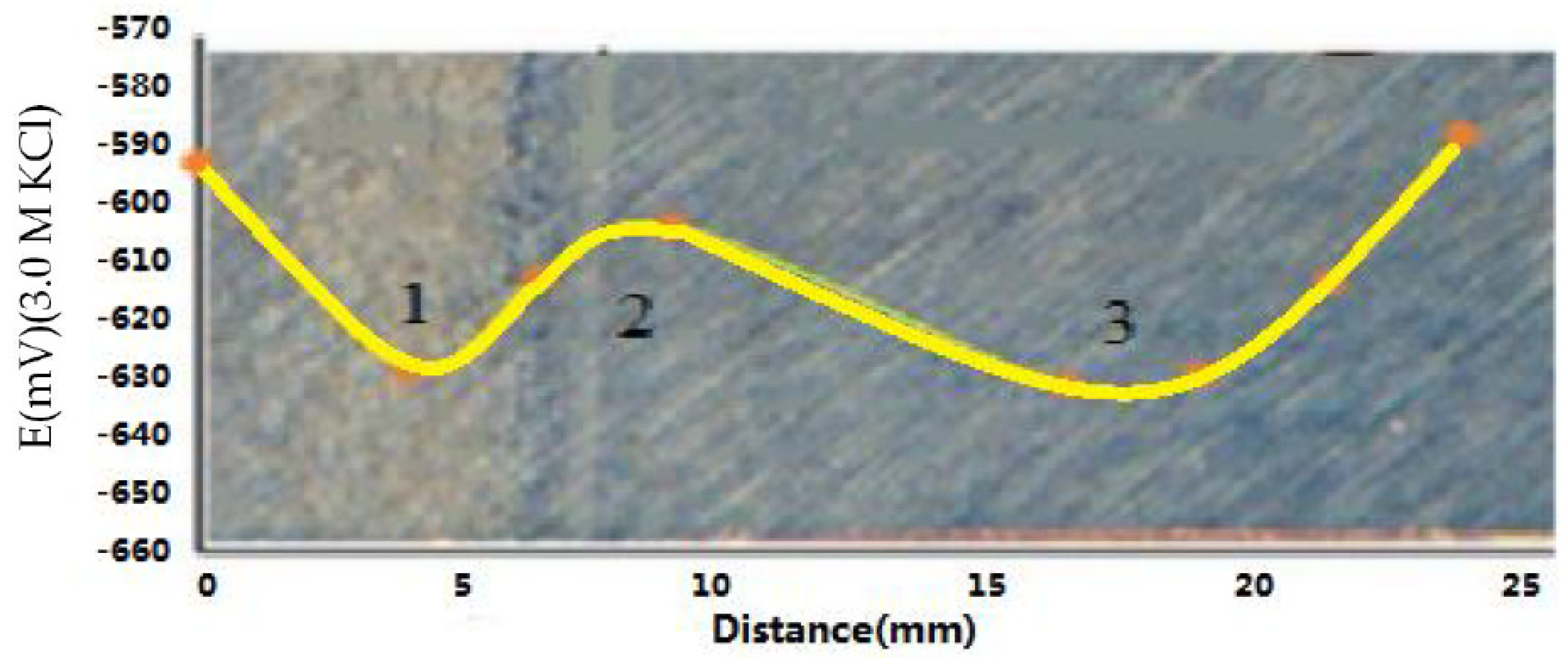
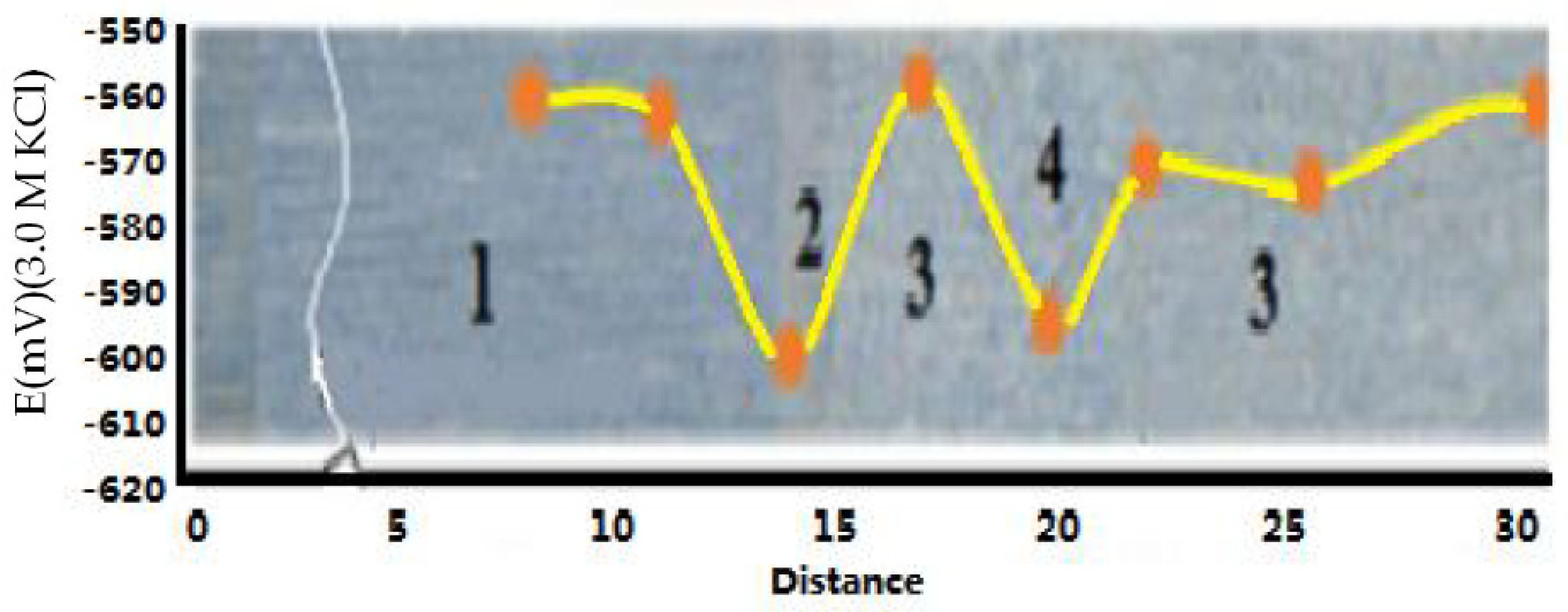
3.3. Free Corrosion Potential of Welded Joints
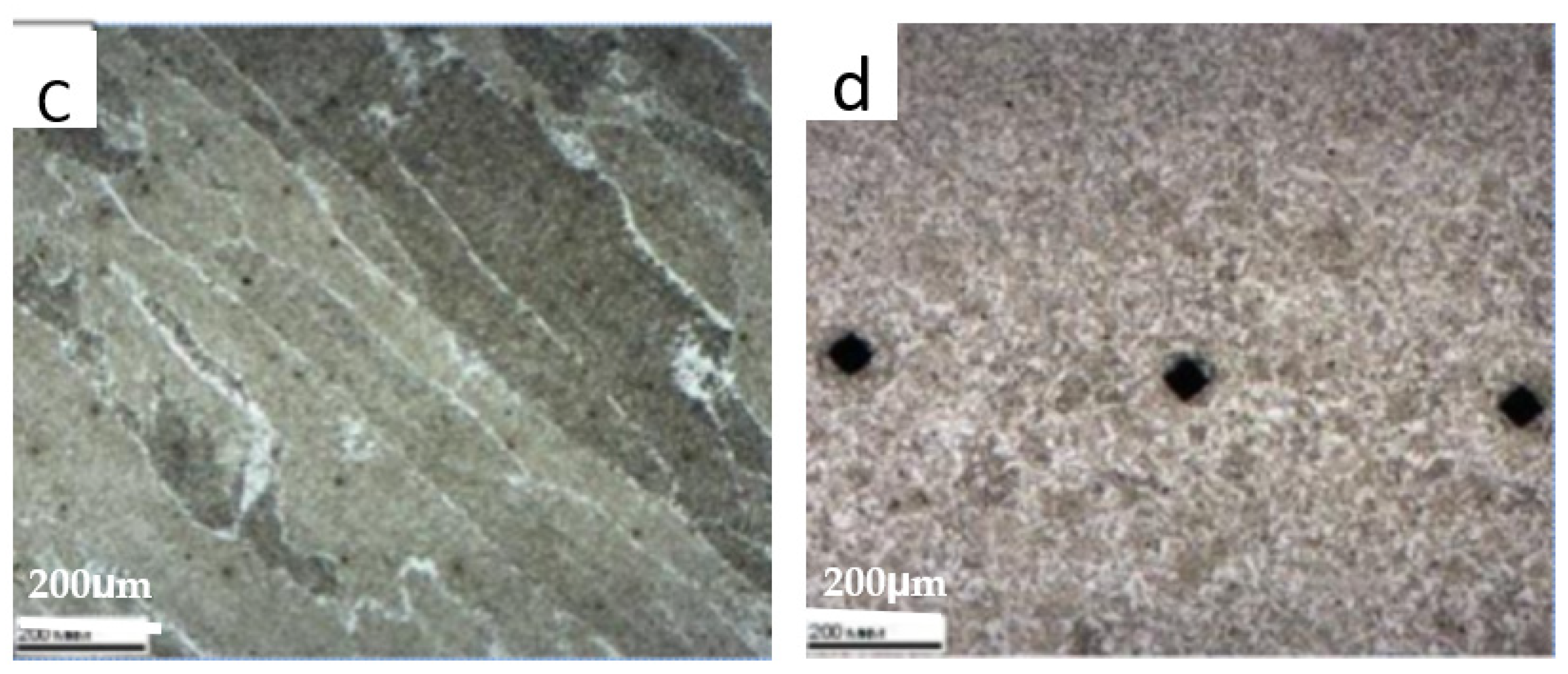
3.4. HAZ Microstructure
4. Conclusions
- (1)
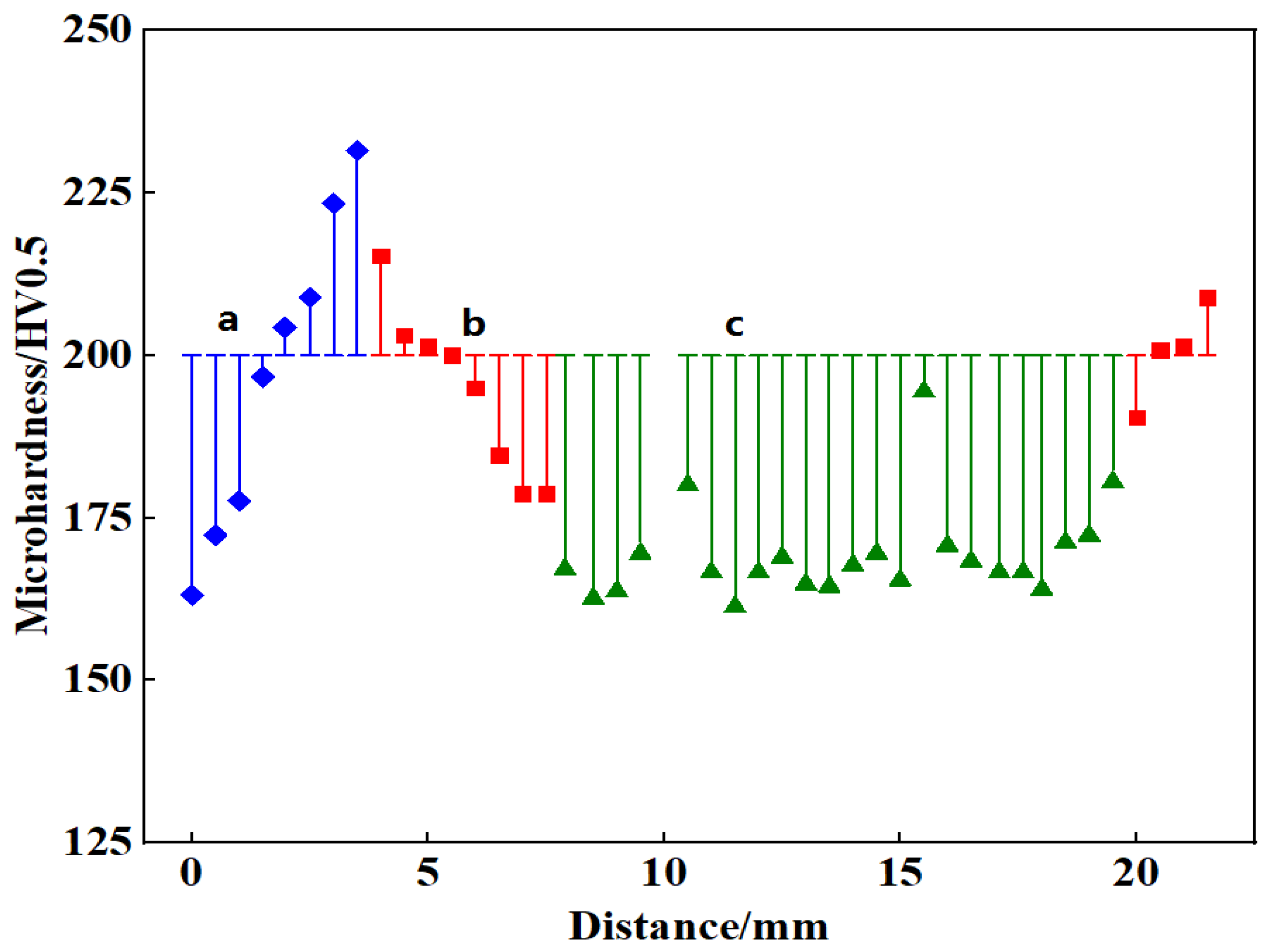
- The microhardness distribution of welded joints was compared. The microhardness of Steel A increased in the weld area, reaching a maximum value of 230 HV near the fusion line characterized by a bainitic structure. The microhardness of the welded seam of Steel B increased from 170 HV in the base metal to 240 HV in the middle of the seam. The maximum microhardness was measured in the recrystallization area.
- (2)
- The corrosion rate of the matrix was higher than that of other parts of the weld, due to the presence of corrosive non-metallic inclusions in the substrate. The average corrosion rates of Steels A and B were 0.85 and 1.19 mm/year in the HAZ, respectively. The serious corrosion was due to the lower free corrosion potential.
- (3)
- The free corrosion potentials of different parts of the welds were measured. The free corrosion potential difference of the adjacent area of Steel B was more than 40 mV, which implied a significant electrochemical difference that reduced the corrosion resistance. In contrast, the potential difference of Steel A was small. The recrystallization area had the highest microhardness, and local corrosion defects occurred due to recrystallization. The weld fusion line had the lowest free corrosion potential due to the precipitation of sub-micron ions.
- (4)
- EDS and XRD analyses of the precipitates identified Mg, Si, and Ca oxide components in addition to Ti and Nb carbonitrides. Nanometer non-metallic inclusions were observed in the matrix, which was coated with precipitates of Ti and Nb carbonitrides. Such composite precipitates may further reduce the corrosion resistance in the HAZ.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Wu, H.; Sun, Y. Effect of the inclusion features on mechanical properties of steel. Ironmak. Steelmak. 2021, 49, 405–411. [Google Scholar] [CrossRef]
- Hoar, T.P. Corrosion Control at Welded Joints in Constructional Steels. Br. Corros. J. 1967, 2, 46–48. [Google Scholar] [CrossRef]
- Baker, R.G.; Whitman, J.G. Practical Corrosion Problems in Relation to Welded Joints. I. Special Characteristics of the Welding Process Which can Cause Corrosion Problems. Brit. Corros. J. 1967, 2, 34–40. [Google Scholar] [CrossRef]
- Xu, S.; Wang, H.; Li, A.; Wang, Y.; Su, L. Effects of corrosion on surface characterization and mechanical properties of butt-welded joints. J. Constr. Steel Res. 2016, 126, 50–62. [Google Scholar] [CrossRef]
- Li, M.; Li, W.Y.; Yang, X.W.; Alexopoulos, N.D. Corrosion performance and mechanical properties of friction stir welded AA2024-T3 joints under different corrosion solution exposure. Mater. Corros. 2017, 68, 970–976. [Google Scholar] [CrossRef]
- Sinhmar, S.; Dwivedi, D. Enhancement of mechanical properties and corrosion resistance of friction stir welded joint of AA2014 using water cooling. Mater. Sci. Eng. A 2017, 684, 413–422. [Google Scholar] [CrossRef]
- Qi, K.; Li, R.; Wang, G.; Li, G.; Liu, B.; Wu, M. Microstructure and Corrosion Properties of Laser-Welded SAF 2507 Super Duplex Stainless Steel Joints. J. Mater. Eng. Perform. 2018, 28, 287–295. [Google Scholar] [CrossRef]
- Bai, D.; Liu, F.; Zhang, H.; Liu, J. Study on corrosion behavior of high nitrogen steel welded joint assisted by ultrasonic vibration. Mater. Lett. 2022, 317, 132101. [Google Scholar] [CrossRef]
- Cheng, M.; He, P.; Lei, L.; Tan, X.; Wang, X.; Sun, Y.; Li, J.; Jiang, Y. Comparative studies on microstructure evolution and corrosion resistance of 304 and a newly developed high Mn and N austenitic stainless steel welded joints. Corros. Sci. 2021, 183, 109338. [Google Scholar] [CrossRef]
- Wang, J.F.; Ma, J.K.; Liu, Y.; Zhang, T.; Wu, S.; Sun, Q. Influence of Heat Input on Microstructure and Corrosion Resistance of Underwater Wet-Welded E40 Steel Joints. J. Mater. Eng. Perform. 2020, 29, 6987–6996. [Google Scholar] [CrossRef]
- Bocchi, S.; Cabrini, M.; D’urso, G.; Giardini, C.; Lorenzi, S.; Pastore, T. The influence of process parameters on mechanical properties and corrosion behavior of friction stir welded aluminum joints. J. Manuf. Process. 2018, 35, 1–15. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, J.; Zhang, J.; Yu, X.; Li, Q.; Wang, Z.; Fan, D. Microstructure and corrosion characterization of weld metal in stainless steel and low carbon steel joint under different heat input. Mater. Today Commun. 2021, 29, 102948. [Google Scholar] [CrossRef]
- Wu, W.; Hu, S.; Shen, J. Microstructure, mechanical properties and corrosion behavior of laser welded dissimilar joints between ferritic stainless steel and carbon steel. Mater. Des. 2015, 65, 855–861. [Google Scholar] [CrossRef]
- Montes, O.F.; Garcés, R.S.; Reyes, F.A.R.; Robledo, P.d.C.Z.; López, F.H.E.; Calderón, F.A. Behaviour on corrosion of API X70 steel welded using the process of double submerged arc welding immersed in corrosive media. Weld. Int. 2018, 32, 651–659. [Google Scholar] [CrossRef]
- Li, M.; Wu, H.; Sun, Y. Influence of Non-metallic Inclusions on Corrosive Properties of Polar Steel. Front. Mater. 2021, 8, 602851. [Google Scholar] [CrossRef]
- Yan, Y.; Deng, H.; Xiao, W.; Ou, T.; Cao, X. Corrosion behaviour of X80 pipeline steel welded joint in H2O-saturated supercritical-CO2 environment. Int. J. Electrochem. Sci. 2020, 15, 1713–1726. [Google Scholar] [CrossRef]
- Ma, H.; Liu, Z.; Du, C.; Wang, H.; Li, X.; Zhang, D.; Cui, Z. Stress corrosion cracking of E690 steel as a welded joint in a simulated marine atmosphere containing sulphur dioxide. Corros. Sci. 2015, 100, 627–641. [Google Scholar] [CrossRef]
- Song, J.; Li, Z.; Xiao, K.; Luo, H.; Chen, J.; Yu, W.; Zhang, X.; Dong, C. Study on the local corrosion behaviour and mechanism of bogie steel welded joints. Corros. Sci. 2022, 208, 110709. [Google Scholar] [CrossRef]
- Rajesh, S.; Badheka, V. Influence of heat input/multiple passes and post weld heat treatment on strength electrochemical characteristics of friction stir weld joint. Mater. Manuf. Process. 2018, 33, 156–164. [Google Scholar] [CrossRef]
- Gu, Y.; Lu, N.; Xu, Y.; Shi, Y.; Zhang, G.; Sun, Q. Microstructure characteristics of Q345R-steel welded joints and their corrosion behavior in a hydrofluoric acid environment. J. Nucl. Mater. 2023, 574, 154214. [Google Scholar] [CrossRef]
- Dou, X.; He, Z.; Zhang, X.; Liu, Y.; Liu, R.; Tan, Z.; Zhang, D.; Li, Y. Corrosion behavior and mechanism of X80 pipeline steel welded joints under high shear flow fields. Colloids Surf. A Physicochem. Eng. Asp. 2023, 665, 131225. [Google Scholar] [CrossRef]
- Rodionova, I.G.; Baklanova, O.N.; Filippov, G.A.; Reformatskaya, I.I.; Podobaev, A.N.; Zinchenko, S.D.; Filatov, M.V.; Efimov, S.V.; Tishkov, Y.V.; Golovanov, A.V.; et al. The role of non-metallic inclusions in accelerating the local corrosion of metal products made of plain-carbon and low-alloy steels. Metallurgist 2005, 49, 125–130. [Google Scholar] [CrossRef]
- Rodionova, I.G.; Baklanova, O.N.; Zaitsev, A.I. On the role of non-metallic inclusions in the acceleration of local corrosion of oil-field pipelines made of carbon and low-alloy steels. Russ. Metall. 2004, 5, 13–18. [Google Scholar]
- Li, H.; Qiao, J.; Jiang, X.; Chen, J.; Wu, Y. Tissue and corrosion properties of laser welded joints of high strength steel. Trans. China Weld. Instit. 2015, 36, 75–78. [Google Scholar]
- Li, Y.; Yang, M.; Huang, S.; Wu, K. Study on corrosion resistance of X90 pipeline steel welded joint. Electric Weld. Mach. 2017, 47, 37–42. [Google Scholar]
- Zinchenko, S.D.; Lamukhin, A.M.; Filatov, M.; Efimov, S.; Rodionova, I.; Zaitsev, A.; Baklanova, O. Development of recommendations on making tube steels produced at the severstal combine cleaner with respect to corrosion-active non-metallic inclusions. Metallurgist 2005, 4, 131–137. [Google Scholar] [CrossRef]
- Eliyan, F.F.; Alfantazi, A. Effect of bicarbonate concentration on corrosion of high strength steel. Corros. Eng. Sci. Technol. 2014, 50, 178–185. [Google Scholar] [CrossRef]
- Wang, J.; Guo, P.; Wang, X.; Di, H.; Zhao, Y. Study on microstructure and corrosion resistance of laser welded joint of X100 pipeline steel. Appl. Laser 2017, 37, 835–840. [Google Scholar]
- Yan, M.; Wei, B.; Xu, J.; Li, Y.; Hu, Y.; Cai, Z.; Sun, C. Insight into sulfate-reducing bacteria corrosion behavior of X80 pipeline steel welded joint in a soil solution. J. Mater. Res. Technol. 2023, 24, 5839–5863. [Google Scholar] [CrossRef]
- Moshtaghi, M.; Loder, B.; Safyari, M.; Willidal, T.; Hojo, T.; Mori, G. Hydrogen trapping and desorption affected by ferrite grain boundary types in shielded metal and flux-cored arc weldments with Ni addition. Int. J. Hydrogen Energy 2022, 47, 20676–20683. [Google Scholar] [CrossRef]


| Sample | C | Mn | Si | S | P | Cr | Ti | Nb | Al |
|---|---|---|---|---|---|---|---|---|---|
| A | 0.06 | 1.48 | 0.18 | 0.002 | 0.012 | 0.15 | 0.005 | 0.012 | 0.042 |
| B | 0.12 | 1.56 | 0.24 | 0.003 | 0.016 | 0.11 | 0.013 | 0.036 | 0.018 |
| Sample | Average Depth of Corrosion, mm | Average Corrosion Rate, mm/year | ||||
|---|---|---|---|---|---|---|
| Base Metal | HAZ | Seam Metal | Base Metal | HAZ | Seam Metal | |
| A | 0.032 | 0.023 | 0.034 | 1.16 | 0.85 | 1.23 |
| B | 0.045 | 0.033 | 0.030 | 1.64 | 1.19 | 1.10 |
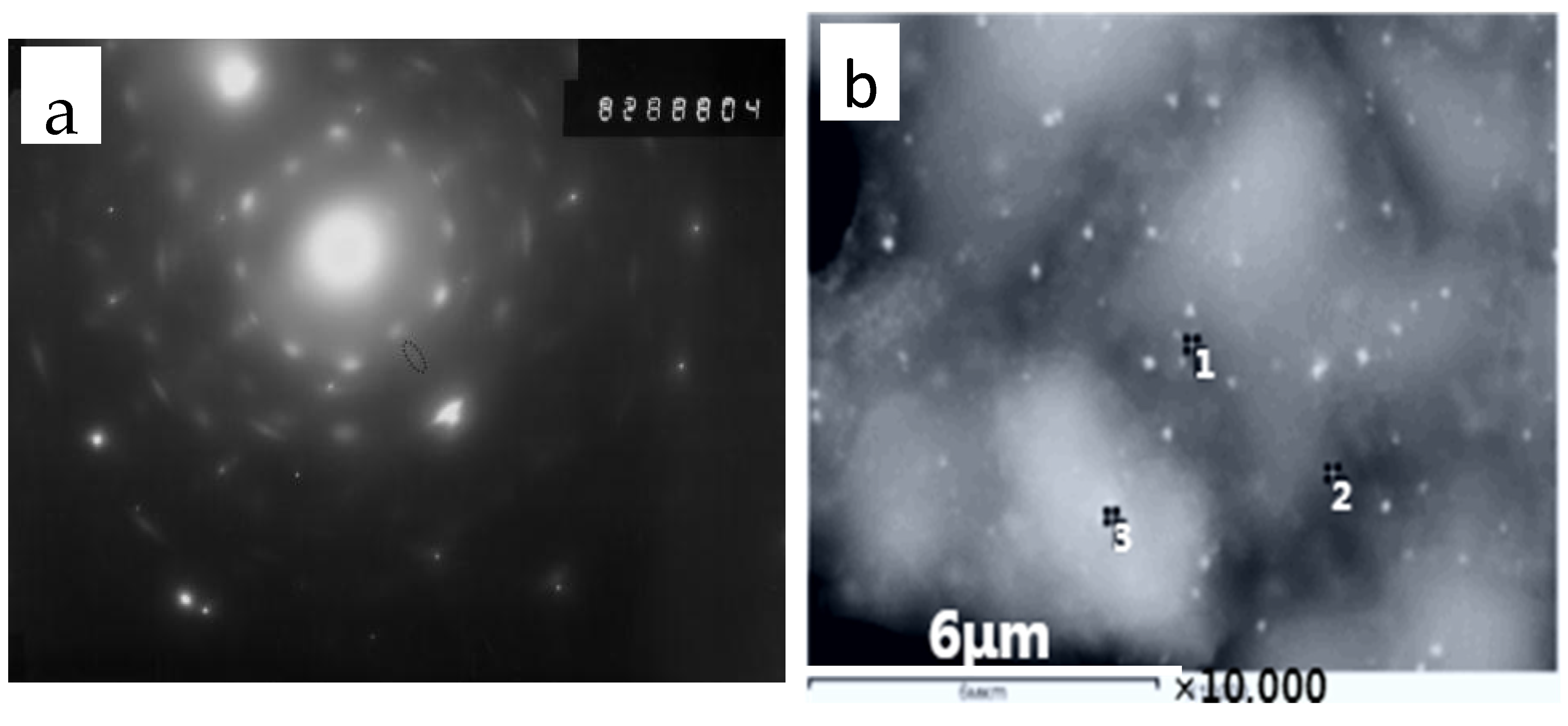
| Spectrum | C | O | Mg | Al | Si | Ca | Ti | Mn | Fe | Nb |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 20.51 | 12.42 | 0.00 | 1.19 | 0.42 | 0.36 | 0.39 | 1.12 | 60.81 | 2.15 |
| 2 | 41.68 | 13.82 | 0.00 | 0.97 | 1.35 | 0.22 | 0.00 | 0.89 | 38.46 | 1.69 |
| 3 | 17.19 | 9.27 | 0.45 | 1.46 | 0.55 | 0.22 | 0.00 | 1.40 | 68.51 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Wu, H.; Sun, Y. Corrosion Performance of Welded Joints for E40 Marine Steel. Metals 2023, 13, 1528. https://doi.org/10.3390/met13091528
Li M, Wu H, Sun Y. Corrosion Performance of Welded Joints for E40 Marine Steel. Metals. 2023; 13(9):1528. https://doi.org/10.3390/met13091528
Chicago/Turabian StyleLi, Ming, Huajie Wu, and Yanhui Sun. 2023. "Corrosion Performance of Welded Joints for E40 Marine Steel" Metals 13, no. 9: 1528. https://doi.org/10.3390/met13091528
APA StyleLi, M., Wu, H., & Sun, Y. (2023). Corrosion Performance of Welded Joints for E40 Marine Steel. Metals, 13(9), 1528. https://doi.org/10.3390/met13091528






